Abstract
This phase I dose-escalation/expansion study evaluated isatuximab (anti-CD38 monoclonal antibody) monotherapy in patients with relapsed/refractory multiple myeloma (RRMM). Patients progressing on or after standard therapy received intravenous isatuximab (weekly [QW] or every 2 weeks [Q2W]). The primary objective was to determine the maximum tolerated dose (MTD) of isatuximab. Overall, 84 patients received ≥ 1 dose of isatuximab. The MTD was not reached; no cumulative adverse reactions were noted. The most frequent adverse events were infusion reactions (IRs), occurring in 37/73 patients (51%) following introduction of mandatory prophylaxis. IRs were mostly grade 1/2, occurred predominantly during Cycle 1, and led to treatment discontinuation in two patients. CD38 receptor occupancy reached a plateau of 80% with isatuximab 20 mg/kg (highest dose tested) and was associated with clinical response. In patients receiving isatuximab ≥ 10 mg/kg, overall response rate (ORR) was 23.8% (15/63), including one complete response. In high-risk patients treated with isatuximab 10 mg/kg (QW or Q2W), ORR was 16.7% (3/18). Median (range) duration of response at doses ≥ 10 mg/kg was 25 (8–30) weeks among high-risk patients versus 36 (6–85) weeks for other patients. In conclusion, isatuximab demonstrated a manageable safety profile and clinical activity in patients with RRMM.
Subject terms: Myeloma, Cancer
Introduction
Over the past two decades, the development of novel chemotherapeutics, specific kinase inhibitors, and monoclonal antibodies (mAbs) has changed the treatment landscape for patients with hematologic malignancies1,2. In multiple myeloma (MM), agents including proteasome inhibitors (e.g., bortezomib, carfilzomib) and immunomodulatory drugs (e.g., lenalidomide, pomalidomide) have improved survival outcomes compared with previous cytotoxic regimens3–6. However, most patients with MM will still relapse following treatment with these agents, and the prognosis is particularly poor for those with recurrent disease following proteasome inhibitor and immunomodulatory drug treatment7.
Positive clinical data have recently emerged for mAbs directed against surface antigens on malignant plasma cells8,9. Both the anti-SLAMF7 mAb elotuzumab and the anti-CD38 mAb daratumumab received US Food and Drug Administration approval in 2015. These antibodies exert their cytotoxic effects through complement-dependent cellular cytotoxicity, antibody-dependent cellular cytotoxicity, and/or antibody-dependent cellular phagocytosis10. Elotuzumab demonstrated potent anti-MM activity in combination with lenalidomide and dexamethasone in patients with early relapsed/refractory MM (RRMM), yet showed no objective response as a single-agent11. In contrast, daratumumab received accelerated approval based on results from a phase II monotherapy and single-arm study showing an overall response rate (ORR) of 29.2% and median duration of response (DOR) of 7.4 months in heavily pretreated patients with RRMM12. Subsequently, two randomized phase III trials have demonstrated improved anti-MM activity when combining daratumumab with standard doublet therapies: bortezomib plus dexamethasone (CASTOR study: daratumumab/bortezomib/dexamethasone versus bortezomib/dexamethasone13) and lenalidomide plus dexamethasone (POLLUX study: daratumumab/lenalidomide/dexamethasone versus lenalidomide/dexamethasone14) in patients progressing after 1–3 prior lines of therapy. Both phase III studies showed marked improvements in progression-free survival (PFS) and ORR with triplet therapy (daratumumab/bortezomib/dexamethasone versus bortezomib/dexamethasone: hazard ratio for progression or death = 0.39; ORR 82.9% versus 63.2%, respectively; daratumumab/lenalidomide/dexamethasone versus lenalidomide/dexamethasone: hazard ratio for progression = 0.37; ORR 92.9% versus 76.4%, respectively). A phase Ib study of daratumumab in combination with pomalidomide and dexamethasone has also shown potent activity (ORR 59.2%) in patients who had received ≥ 2 prior lines of therapy, and these studies led to full US Food and Drug Administration approval for daratumumab as monotherapy and in these combinations for RRMM15.
Isatuximab is a novel immunoglobulin G1 kappa anti-CD38 mAb that binds selectively to a specific epitope on CD38. Preclinical studies suggest that isatuximab can target tumor cells through a combination of mechanisms, including antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, complement-dependent cellular cytotoxicity, and immune cell depletion/inhibition of immunosuppressive cells16,17. However, isatuximab appears unique among anti-CD38 mAbs as it can also induce direct apoptosis without cross-linking16. Furthermore, isatuximab is a potent inhibitor of CD38 enzymatic activity, which can impact on Ca2+ signaling16. Single-agent isatuximab has demonstrated anti-tumor activity in xenograft models of non-Hodgkin’s lymphoma, MM, and acute lymphoid leukemia16,18. Based on these encouraging preclinical data, a phase I, first-in-human study was initiated to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of isatuximab monotherapy in patients with RRMM (n = 84) and other hematologic malignancies (n = 5). Only patients with RRMM are included in this report.
Methods
Eligibility
Initially, patients with RRMM and other hematologic malignancies were included in the study; however, based on early clinical activity and high CD38 expression in MM, the protocol was amended during dose-escalation (at 10 mg/kg weekly [QW]) to enroll only patients with a confirmed diagnosis of RRMM who had progressed on/after standard therapy, including an immunomodulatory drug and a proteasome inhibitor. CD38 expression was also removed from entry criteria. Patients had confirmed diagnosis of MM and had progressed on or after standard therapy. Eligible patients were ≥ 18 years old, had good performance status (Karnofsky performance status ≥ 60), good baseline organ function (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and bilirubin ≤ 2.5 × upper limit of normal; serum creatinine ≤ 2 × upper limit of normal), and adequate bone marrow function (absolute neutrophil count ≥ 1.0 × 109/l, platelet count ≥ 75 × 109/l, and hemoglobin ≥ 9 g/dl). Key exclusion criteria included: prior treatment with anti-CD38-directed therapy; another concomitant or prior malignancy; active HIV, AIDS, or hepatitis B or C infection; known central nervous system disease; pregnancy or breast-feeding; or known intolerance to infused protein products.
Study design and treatment
This was a phase I, multicenter, open-label, dose-escalation study of single-agent isatuximab conducted in the USA, Spain, and France. Isatuximab was administered intravenously every 2 weeks (Q2W) or QW, in 2-week cycles, until disease progression, intolerable toxicity, or withdrawal of consent.
Isatuximab dose-escalation was planned from 0.0001 to 20 mg/kg (Supplementary Fig. S1). Following dose-escalation, two expansion cohorts (ECs) (EC1: standard-risk and high-risk patients; EC2: only high-risk patients) were added at 10 mg/kg Q2W. Another dosing cohort, evaluating the highest isatuximab dose (20 mg/kg QW), was added after efficacy and pharmacokinetic data became available from EC1. Premedication against infusion-related reactions (IRRs) was made mandatory from the 3 mg/kg Q2W cohort onward. Following the dose-escalation phases, two expansion cohorts (EC1: standard-risk and high-risk patients; EC2: only high-risk patients) of 18 patients each were added at 10 mg/kg Q2W. High-risk RRMM was defined as: abnormal genotype (del[17p], > 3 copies of 1q21, t[4;14], or t[14;16]) by cytogenetics or interphase fluorescence in situ hybridization; disease relapse within 6 months of autologous stem cell transplantation; or high-risk gene-expression profile (defined by investigator). For further details on dose-escalation and premedication see Supplementary Methods.
Study objectives
The primary objective was to determine the maximum tolerated dose (MTD; highest dose at which dose-limiting toxicities [DLTs] occurred in < 2 of 6 patients, assessed during the first 4 weeks of treatment) of isatuximab. Secondary objectives were evaluation of safety/tolerability, pharmacokinetics/pharmacodynamics, and preliminary efficacy of isatuximab.
Dose-limiting toxicities
A DLT was initially defined as an isatuximab-related occurrence of any of the following events: grade ≥ 3 non-hematologic toxicity; grade 4 neutropenia or grade 4 thrombocytopenia lasting > 5 days; grade ≥ 2 allergic reaction or hypersensitivity (i.e., infusion reactions [IRs]); or any other toxicity deemed by the investigators or sponsor to be dose-limiting. The DLT definition was amended at the 3 mg/kg Q2W cohort to eliminate grade ≤ 2 IR as part of the DLT definition, as patients experiencing a grade 2 IR before the end of the infusion were able to complete isatuximab dosing with appropriate management.
Safety and efficacy assessments
Safety was evaluated continuously by physical examination, laboratory tests, and reports of adverse events (AEs) using National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Isatuximab-related AEs beginning shortly after infusion were recorded as IRs and individual symptoms were recorded as AEs of special interest. Clinical responses were assessed according to the European Group for Blood and Marrow Transplant (EBMT) response criteria19. Disease responses were assessed every 28 days. Efficacy was assessed by ORR (at least partial response [PR]) and clinical benefit rate (CBR) (at least minimal response).
Treatment-emergent adverse events (TEAEs) were defined as adverse events (AEs) that developed, worsened (investigator’s judgment), or became serious during the on-treatment phase. IRs were considered adverse events of special interest and were followed closely. Additional safety assessments included: laboratory tests (hematology, serum chemistries, urinalyses, anti-drug antibodies), and pulmonary and cardiac evaluations.
Responses were assessed according to disease type. MM responses were classified according to the EBMT criteria19, with overall response rate (ORR) defined as attainment of at least partial response, and clinical benefit rate defined as attainment of at least minimal response; very good partial response is not included in the EBMT criteria.
Pharmacokinetic/pharmacodynamic assessment
Isatuximab plasma concentrations were determined using a validated enzyme-linked immunoabsorption assay with a lower limit of quantification of 0.5 ng/ml. Individual pharmacokinetic parameters were estimated by non-compartmental analysis. Receptor occupancy (RO) was derived from receptor density assessed by a quantitative flow cytometry assay (see Supplementary Methods for further details).
Statistical considerations
All analyses were performed on the all-treated population (patients who received ≥1 dose [even if incomplete] of isatuximab). Continuous data were summarized using descriptive statistics; categorical and ordinal data were summarized using number/percentage of patients. No statistical hypotheses were generated or power calculations performed. PFS (time from first dose to disease progression or death, whichever is first) was derived as a post-hoc variable and analyzed for patients treated at doses ≥ 10 mg/kg with the Kaplan–Meier method with patients from the EC2 cohort analyzed separately.
Study oversight
The protocol was approved by ethics committees at each institution and the study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization guidelines. All patients provided written informed consent.
Results
Patient characteristics
Initially, patients with RRMM and other hematologic malignancies (three non-Hodgkin’s lymphoma, two chronic lymphocytic leukemia) were included in the study. Only patients with RRMM are included in the analysis presented here. Eighty-four patients with RRMM were treated between June 2010 and December 2014. Four RRMM patients were treated in the accelerated dose-escalation cohorts, 36 in the basic dose-escalation cohorts, 37 in the expansion-phase cohorts (EC1, n = 19; EC2, n = 18), and 7 in the 20 mg/kg QW cohort. The 0.3 and 3 mg/kg cohorts were each expanded to six patients due to DLTs consistent with IRs (see below), and three patients whose disease progressed before completion of Cycle 2, were replaced for DLT evaluation (in the absence of DLTs). All 84 patients were included in the all-treated population.
Patient demographics and select baseline disease characteristics are summarized in Table 1. Overall, patients had received a median (range) of 5 (1–13) prior lines of therapy, and 62% had received prior carfilzomib or pomalidomide. In the high-risk EC2 cohort, the median (range) number of prior lines was 5.5 (2–8), and 72% had received prior carfilzomib or pomalidomide. Eighty-nine percent of high-risk patients had abnormal cytogenetics (del[17p] [44%], 1q21 gain [56%], or t[4;14] [22%]).
Table 1.
Baseline demographics and disease characteristics
| Characteristics | All (n = 84) | Isatuximab dose | ||
|---|---|---|---|---|
| ≤ 5 mg/kg (n = 21) | 10 mg/kg (n = 49) | 20 mg/kg (n = 14) | ||
| Age | ||||
| Median (range), years | 64 (40–81) | 64 (41–77) | 62.9 (40–81) | 63.5 (49–74) |
| ≥ 65 years, n (%) | 38 (45) | 9 (43) | 24 (49) | 5 (36) |
| Male/female, n (%) | 49 (58)/35 (42) | 12 (57)/9 (43) | 28 (57)/21 (43) | 9 (64)/5 (36) |
| ECOG PS, n (%) | ||||
| 0 | 11 (13) | 1 (5) | 8 (16) | 2 (14) |
| 1 | 58 (69) | 15 (71) | 32 (65) | 11 (79) |
| 2 | 15 (18) | 5 (24) | 9 (18) | 1 (7) |
| Median time since diagnosis, years (range) | 5.84 (1.2–22.8) | 4.91 (1.8–9.9) | 5.85 (1.2–22.8) | 5.99 (3.0–12.9) |
| MM subtype, n (%) | ||||
| IgA | 15 (18) | 6 (29) | 7 (14) | 2 (14) |
| IgD | 1 (1) | 0 | 1 (2) | 0 |
| IgG | 44 (52) | 7 (33) | 27 (55) | 10 (71) |
| IgM | 1 (1) | 0 | 1 (2) | 0 |
| Light-chain (κ + λ) | 23 (27) | 8 (38) | 13 (27) | 2 (14) |
| ISS stage at baseline, n (%) | ||||
| I | 29 (35) | 9 (43) | 15 (31) | 5 (36) |
| II | 30 (36) | 7 (33) | 19 (39) | 4 (29) |
| III | 23 (27) | 4 (19) | 14 (29) | 5 (36) |
| Missing | 2 (2) | 1 (5) | 1 (2) | 0 |
| Median BM PCs, % (range) | 40 (0–100) | 37 (0–90) | 40 (0.8–100) | 46 (0–85) |
| Albumin < 35 g/l, n (%) | 31 (37) | 7 (33) | 22 (45) | 2 (14) |
| B2M ≥ 5.5 mg/l, n (%) | 23 (27) | 4 (19) | 14 (29) | 5 (36) |
| EM plasmacytoma at baseline, n (%) | 12 (14) | 4 (19) | 7 (14) | 1 (7) |
| Median no. of prior treatment lines (range) | 5 (1–13) | 6 (2–13) | 5 (1–13) | 4.5 (2–7) |
| Prior SCT, n (%) | 68 (81) | 18 (86) | 36 (73) | 14 (100) |
| Prior treatments, n (%) | ||||
| Bortezomib | 83 (99) | 21 (100) | 49 (100) | 13 (93) |
| Carfilzomib | 36 (43) | 4 (19) | 25 (51) | 7 (50) |
| Lenalidomide | 79 (94) | 19 (90) | 48 (98) | 12 (86) |
| Pomalidomide | 34 (40) | 4 (19) | 23 (47) | 7 (50) |
| PI and IMiD | 84 (100) | 21 (100) | 49 (100) | 14 (100) |
B2M β2 microglobulin, BM bone marrow, ECOG PS Eastern Cooperative Oncology Group performance status, EM extramedullary, Ig immunoglobulin, IMiD immunomodulatory drug, ISS International Staging System, MM multiple myeloma, PC plasma cell, PI proteasome inhibitor, SCT stem cell transplantation
Treatment regimen defined as ≥ 1 planned cycle of a single-agent or combination therapy, irrespective of whether therapy is given as part of a planned sequence
Treatment line defined as ≥ 1 planned cycle of single-agent or combination therapy, or a sequence of treatments in a planned manner20
Treatment and disposition
All patients have discontinued treatment due to disease progression (n = 72 [85.7%]), other reasons (including patient preference) (n = 8 [9.5%]), or AEs (n = 4 [4.8%]).
Overall, the median (range) duration of isatuximab exposure was 11 (2–120) weeks, with a median of 5 (1–56) 2-week cycles administered. In patients treated at 10 and 20 mg/kg, the median duration of exposure was 14.4 weeks and 14.9 weeks, respectively. Median relative dose intensity of isatuximab was 98% and was consistent among dose levels. The median infusion time for the first and subsequent infusions was 3.50 and 2.60 h, respectively, for isatuximab 10 mg/kg, and 5.76 and 4.0 h, respectively, for isatuximab 20 mg/kg.
Safety
The MTD was not reached. DLTs were observed in two patients during Cycle 1 (one each in the 0.3 and 3 mg/kg cohorts). Both were grade 2 IRs that were part of the original DLT definition (see Supplementary Information). Both patients completed the first infusion, did not discontinue therapy due to the IRs, and did not experience IRs in subsequent cycles. Following these events, the protocol was amended to remove grade ≥ 2 IRs from the DLT definition, as these events were not dose-dependent and had no sequelae. The protocol was also modified at this point to mandate premedication for IR prophylaxis. After the introduction of mandatory prophylactic treatment, 36/73 patients (49.3%) experienced AEs consistent with IRs. Of the patients (treated at the ≤ 0.3 mg/kg doses) who received the first dose of isatuximab before the institution of mandatory prophylaxis, IRs occurred in 3/7 patients (43%). Overall, IRs that were reported as AEs of special interest were grade 1/2 in 94% of patients (Fig. 1). At the isatuximab doses ≥ 10 mg/kg, 47.6% of patients experienced IRs with the first infusion, and 8.3% with subsequent infusions. IRs tended to resolve the same day either spontaneously or with treatment. The most common symptoms ( ≥ 5%) reported during IRs were chills, dyspnea (12% each), nausea (11%), headache (8%), chest discomfort (7%), and pyrexia (6%), all of which were grade 1/2 in intensity. Two patients discontinued treatment due to grade 4 IRs, one at 20 mg/kg Q2W (grade 4 apnea, definitely related to diphenhydramine and possibly related to isatuximab), the other at 10 mg/kg Q2W (grade 4 hypertension). Isatuximab infusions were interrupted in 29.8% of patients; the most common AE resulting in dose interruption was IR (27.0%). Only three patients had dose interruptions in subsequent infusions (all at 10 mg/kg).
Fig. 1.
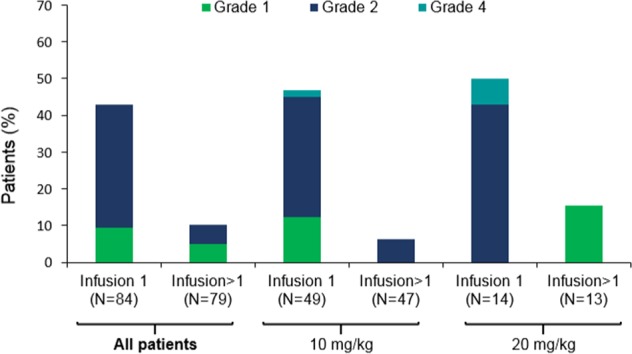
Infusion reactions according to number of infusions and dose level
Overall, the most common (> 10%) TEAEs, excluding IRRs and hematological TEAEs, were fatigue (37%), nausea (32%), upper respiratory tract infection (24%), and cough (23%) (Table 2). Grade 3/4 isatuximab-related TEAEs were reported in 17% of patients (Supplementary Table S1). Serious TEAEs were reported in 43% of patients. The most frequent grade 3/4 hematologic laboratory abnormalities during treatment were lymphopenia (34%), anemia (20%), thrombocytopenia (17%), and neutropenia (12%); the frequency of these abnormalities did not appear to be dose-dependent (Table 2). Grade 3/4 liver and renal abnormalities (laboratory assessment) occurred as follows: elevated aspartate aminotransferase, 4%; elevated alanine aminotransferase, 4%; elevated alkaline phosphatase, 1%; elevated creatinine, 5%.
Table 2.
Most commona TEAEs (regardless of relationship with study treatment)
| TEAE | All patients (n = 84), n (%) | Isatuximab dose All grades/grade 3/4, no. of patients |
|||
|---|---|---|---|---|---|
| All grades | Grade 3/4 | ≤ 5 mg/kg (n = 21) | 10 mg/kg (n = 49) | 20 mg/kg (n = 14) | |
| Any TEAE | 83 (99) | 49 (58) | 21/13 | 49/26 | 13/9 |
| Most common TEAEs | |||||
| Fatigue | 31 (37) | 3 (4) | 10/0 | 16/1 | 5/2 |
| Nausea | 27 (32) | 0 | 6/0 | 19/0 | 2/0 |
| Cough | 19 (23) | 0 | 5/0 | 11/0 | 3/0 |
| URTI | 20 (24) | 0 | 3/0 | 13/0 | 4/0 |
| Back pain | 17 (20) | 3 (4) | 2/0 | 14/2 | 1/1 |
| Diarrhea | 17 (20) | 0 | 3/0 | 11/0 | 3/0 |
| Vomiting | 14 (17) | 0 | 2/0 | 10/0 | 2/0 |
| Dyspnea | 16 (19) | 1 (1) | 3/0 | 10/1 | 3/0 |
| Headache | 15 (18) | 1 (1) | 6/1 | 6/0 | 3/0 |
| Pyrexia | 16 (19) | 2 (2) | 6/1 | 9/1 | 1/0 |
| Bone pain | 12 (14) | 3 (4) | 4/1 | 8/2 | 0 |
| Decreased appetite | 12 (14) | 0 | 1/0 | 9/0 | 2/0 |
| Chills | 11 (13) | 0 | 5/0 | 6/0 | 0 |
| Pneumonia | 6 (7) | 6 (7) | 2/1 | 5/5 | 0/0 |
| Laboratory abnormalities a,b | |||||
| Anemia | 80 (98) | 16 (20) | 20/4 | 48/11 | 12/1 |
| Lymphopenia | 65 (79) | 28 (34) | 15/8 | 40/18 | 10/2 |
| Leukopenia | 63 (77) | 7 (9) | 16/1 | 38/6 | 9/0 |
| Thrombocytopenia | 53 (64) | 14 (17) | 11/5 | 33/6 | 9/3 |
| Neutropenia | 37 (45) | 10 (12) | 7/1 | 25/9 | 5/0 |
| AST increased | 36 (43) | 3 (4) | 7/0 | 22/3 | 7/0 |
| ALT increased | 24 (29) | 3 (4) | 4/0 | 15/3 | 5/0 |
| ALP increased | 16 (19) | 1 (1) | 3/0 | 10/1 | 3/0 |
| Creatinine increased | 48 (58) | 4/5 | 9/2 | 31/2 | 8/0 |
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, TEAE treatment-emergent adverse event, URTI upper respiratory tract infection aAdverse events occurring in ≥ 10% of patients (all grades) or > 5% (grade 3/4), excluding infusion reactions
bFor laboratory abnormalities, percentages are calculated based on the number of evaluable patients for each parameter
Dose delay due to an AE occurred in 24 patients, most frequently due to infection (n = 14). Three patients experienced > 5 days of infusion delay due to an isatuximab-related AE: grade 3 neutropenia at Cycle 2 (10 mg/kg Q2W), two episodes of grade 2 upper respiratory tract infection for the same patient at Cycles 16 and 20 (10 mg/kg QW), and grade 3 pneumonia at Cycle 17 (3 mg/kg). Four patients discontinued treatment due to TEAEs: two patients with IRs described above, and one patient each due to grade 2 bone pain (5 mg/kg; not isatuximab-related), and fatal renal failure (10 mg/kg Q2W; not isatuximab-related). There were 11 other deaths, all occurring > 30 days after the last isatuximab dose and attributed to progressive disease (n = 10) or to reasons not related to isatuximab (bacterial meningitis/sepsis, n = 1).
Pharmacokinetics and pharmacodynamics
In the accelerated dose-escalation cohorts, isatuximab was not detectable ( < 0.5 ng/ml). At higher doses, isatuximab pharmacokinetics showed moderate to high total variability (coefficient of variation 14–81% of isatuximab exposure) (Supplementary Fig. S2). Isatuximab pharmacokinetics appeared to be nonlinear, as exposure (area under the plasma concentration–time curve [AUC] 2 weeks) increased in a greater than dose-proportional manner up to 20 mg/kg Q2W (Table 3). These data suggest the presence of target-mediated drug disposition. Some accumulation was observed following dosing at 10 or 20 mg/kg QW or Q2W, with accumulation highest at 20 mg/kg QW (at Cycle 3 AUC1 week the accumulation ratio [geometric mean] was 1.7 and 2.6 for 10 mg/kg QW and 20 mg/kg QW, respectively) (Supplementary Table S2).
Table 3.
Isatuximab plasma pharmacokinetic parameters at Cycle 1
| Parameters | Isatuximab dose and schedule | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.3 mg/kg Q2W | 1 mg/kg Q2W | 3 mg/kg Q2W | 5 mg/kg Q2W | 10 mg/kg QW | 10 mg/kg Q2W | 20 mg/kg QW | 20 mg/kg Q2W | |
| No. of patients with evaluable PK | 6 | 3 | 4 | 2 | 3 | 20 | 6 | 3 |
| Infusion duration, h | 2.53 | 4.38 | 4.53 | 4.28 | 2.30 | 2.32 | 4.88 | 5.88 |
| tmax, h | 2.49 | 4.35 | 6.99 | 7.65 | 2.25 | 4.75 | 4.30 | 5.87 |
| Cmax, μg/ml | 2.09 (31) | 13.5 (45) | 55.3 (28) | 135 | 183 (20) | 180 (40) | 356 (29) | 469 (28) |
| AUClast, μg h/ml | 16.5 (73) | 674a | 3120 (14) | 14 200 | 17 400 (23)c | 22 200 (50) | 32 200 (33)c | 49 900 (53) |
| AUC1 week, μg h/ml | 16.5 (73) | 460 (81) | 3110 (14) | 9 180 | 17 000 (22) | 14 400 (44) | 31 700 (31) | 33 300 (46) |
| AUC2 week, μg h/ml | NC | NC | NC | 13 100 | NC | 21 000 (54)b | NC | 49 900 (53) |
AUC area under the plasma concentration–time curve, Clast last measurable plasma concentration, Cmax maximum plasma concentration, NC not calculated, PK pharmacokinetics, Q2W every 2 weeks, tmax time taken to reach Cmax
Data are mean (coefficient of variation %), except for infusion duration and tmax, which are medians.
an = 2
bn = 5
cn = 18
The relationship between isatuximab plasma concentration and RO at the end of Cycle 2 is described by the maximum effect attributable to the drug (Emax) model (Fig. 2a), with an Emax of approximately 80%. The plateau for RO (i.e., Emax) was consistently reached for concentrations corresponding to 20 mg/kg Q2W. RO varied at < 20 mg/kg and ranged from approximately 40 to 80% at 10 mg/kg Q2W. Notably, RO was ≥ 70% in patients who achieved a PR or better (Fig. 2b). With the association between isatuximab concentration and RO established, and pharmacokinetic exposure highest and least variable at 20 mg/kg, the 20 mg/kg QW cohort was added.
Fig. 2. Relationship between receptor occupancy (RO), isatuximab concentration, and response.
Relationship between a RO and isatuximab concentration and b RO, isatuximab concentration, and response. Emax = 81.3%, EC50 = 0.019 µg/ml, γ = 0.595. C isatuximab concentration, EC50 half maximal effective concentration, Emax maximum effect, PR partial response, QW every week, Q2W every 2 weeks, RO receptor occupancy
Efficacy
Objective responses were observed at doses ≥ 1 mg/kg (i.e., when RO became detectable). In patients treated with 1–5 mg/kg (n = 11) the ORR was 18.2% and the CBR was 27.3%. In patients treated with ≥ 10 mg/kg (n = 63), the ORR was 23.8% and the CBR was 30.2% (Fig. 3a). A complete response (CR) was observed in 1 patient (10 mg/kg QW)—a 76-year-old man with lambda light-chain disease who had received four prior lines of therapy; his best response to the last two lines, including alkylating agents and bortezomib, was disease progression. Disease assessment showed PR at Cycles 2 and 4, then CR until last disease assessment at Cycle 42. In the high-risk cohort, the ORR was 16.7% and CBR 27.8% (PR recorded in 3/16 patients with adverse cytogenetics, including one patient with high-risk gene-expression profiling). In patients with extramedullary disease, ORR was 25% (3/12 patients). A waterfall plot of M-protein changes is shown in Fig. 3b; 11 patients attained an M-protein decrease of > 90%. For patients treated at doses ≥ 10 mg/kg (excluding EC2 cohort), median PFS was 3.7 months (95% CI 2.56 to 5.78) (Table 4). For patients treated at 10 mg/kg Q2W in the EC2 cohort median PFS was 2.9 (95% CI 1.87 to 5.49) (Table 4).
Fig. 3. Summary of response data in patients with relapsed/refractory multiple myeloma.
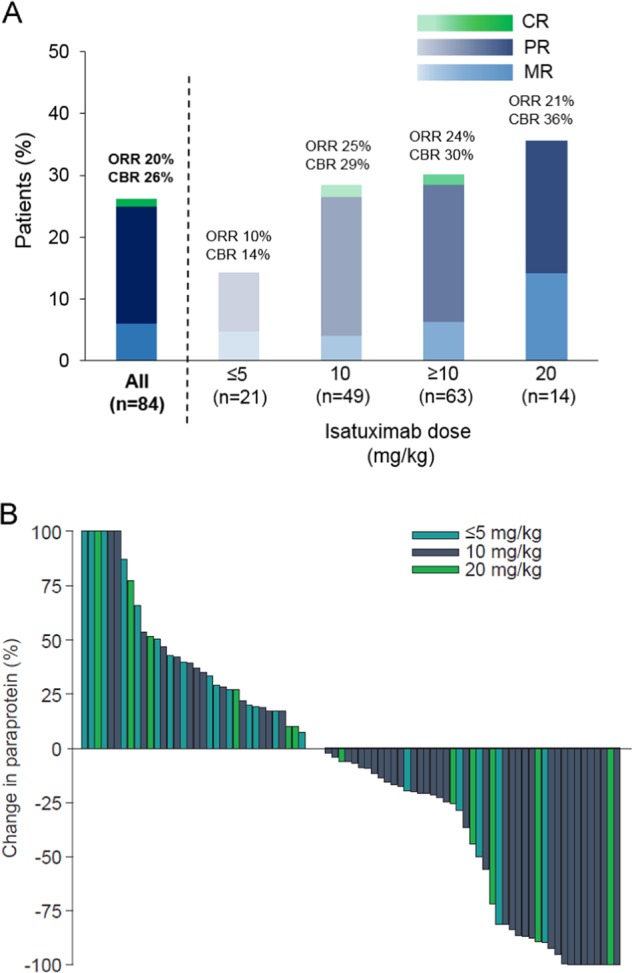
a Response histogram by isatuximab dose level, using European Group for Blood and Marrow Transplant criteria; b Waterfall plot of paraprotein change by isatuximab dose level in patients treated at isatuximab ≥ 1 mg/kg. Response not evaluable in three patients (1 at 10 mg/kg and 2 at 20 mg/kg). CBR clinical benefit rate, CR complete response, MR minimal response, ORR overall response rate, PR partial response
Table 4.
Progression-free survival—Kaplan–Meier estimates
| ≥10 mg/kg (excluding EC2) (N = 45) | HR cohort (EC2) (N = 18) | |
|---|---|---|
| Number (%) of events | 33 (73.3) | 16 (88.9) |
| Number (%) of patients censored | 12 (26.7) | 2 (11.1) |
| Kaplan–Meier estimates 25th quantile (95th CI) (months) |
2.1 (1.12–2.89) | 1.6 (0.85–2.79) |
| Median (95% CI) (months) | 3.7 (2.56–5.78) | 2.9 (1.87–5.49) |
| 75th quantile (95th CI) (months) | 9.2 (5.06–16.33) | 5.9 (2.99–12.71) |
| Probability of surviving (95% CI) | ||
| 2 months | 0.760 (0.630–0.890) | 0.611 (0.386–0.836) |
| 4 Months | 0.471 (0.313–0.628) | 0.438 (0.205–0.670) |
| 6 Months | 0.342 (0.185–0.499) | 0.250 (0.042–0.458) |
| 8 Months | 0.308 (0.153–0.463) | 0.125 (0.000–0.286) |
| 12 Months | 0.137 (0.015–0.258) | 0.125 (0.000–0.286) |
| 18 Months | 0.068 (0.000–0.181) | 0.063 (0.000–0.181) |
In patients responding to isatuximab ≥ 10 mg/kg, median (range) time to first response was 4.29 (3.9–48.0) weeks. For patients treated at 10 mg/kg outside the high-risk cohort (n = 31), median (range) DOR ( ≥ PR) was 36.14 (6.1–85.3) weeks. Median (range) DOR in patients who received 10 mg/kg in the high-risk cohort was 25.29 (8.0–30.0) weeks.
Discussion
This phase I study demonstrated that isatuximab monotherapy was generally well tolerated up to 20 mg/kg in patients with RRMM. IRs were the most common treatment-related AEs; these were grade 1/2 in 95% of patients, and occurred predominantly with the first infusion. Infusion interruption due to IRs occurred in 27% of patients, and only two patients discontinued treatment due to IRs.
Assessment of pharmacokinetics demonstrated that isatuximab exposure was nonlinear, as a result of target-mediated drug disposition. The initial pharmacokinetic/pharmacodynamic results from the dose-escalation cohorts and the first EC demonstrated the importance of RO, as only patients who attained RO ≥ 70% achieved a clinical response. As the plateau for RO was not consistently reached at doses up to 10 mg/kg, an additional dose cohort at 20 mg/kg QW was included. Overall, the pharmacokinetic parameters, including RO, were more favorable at this dose.
Isatuximab monotherapy demonstrated notable clinical activity with an ORR of 24% at isatuximab doses ≥ 10 mg/kg in this heavily pretreated RRMM population. Specifically, patients in this study had received a median of five prior lines of therapy, and over 60% had received pomalidomide or carfilzomib. Although there were no clear response differences observed between the 10 and 20 mg/kg dosing cohorts, the pharmacokinetic analyses suggest that the 20 mg/kg dose provides more consistent target saturation, especially in patients with bulky disease. Thus, the 20 mg/kg dose level has been further evaluated in subsequent monotherapy trials. Overall, the responses were rapid and durable, with a median time to first response of 4 weeks and a median DOR of approximately 25 weeks. An improvement in response quality from PR to CR was observed in one patient, although it should be noted that responses were assessed according to EBMT rather than International Myeloma Working Group criteria20, such that very good PR, an intermediate between PR and CR, was not assessed. In 2010, when this trial was initiated, the EBMT criteria were commonly used in MM clinical trials21,22.
This phase I study also reports promising results in patients with high-risk disease, as defined by cytogenetics or gene-expression profiling, suggesting that isatuximab therapy or immunotherapy is agnostic to previously defined adverse prognostic features. Clinical data for other mAb therapies, including elotuzumab11 and daratumumab12, further confirm that this therapeutic class is risk agnostic. The responses observed in this high-risk population were durable and notable because patients with high-risk disease generally have a shorter DOR23. However, only a small number of patients were included in the high-risk expansion cohort, thus further investigations are warranted, including the use of isatuximab in combination with other anti-MM therapies in patients with high-risk disease.
The response rates observed in this study are similar to those observed with other single-agent therapies approved for use in RRMM, such as carfilzomib, pomalidomide, and daratumumab12,24–26. For example, results from the phase II daratumumab monotherapy study in heavily pretreated patients with advanced MM are comparable with those reported here; in the daratumumab 16 mg/kg arm, median ORR was 29% and median DOR was 32.2 weeks, compared with 24% and 25 weeks, respectively, at isatuximab doses ≥ 10 mg/kg (including the EC2 cohort)12. The median PFS for patients treated at doses ≥ 10 mg/kg (excluding the EC2 cohort) was also consistent with what was reported for daratumumab (3.7 months). As expected, the median PFS for patients treated at 10 mg/kg Q2W in the dedicated HR risk cohort was shorter (Fig. 4). The overall incidence of IRRs with isatuximab monotherapy (47.2%) appears higher than the incidence of IRRs reported in a phase II study of daratumumab monotherapy (42%)12, although grade 3/4 IRRs occurred less frequently with isatuximab (grade 3, 0; grade 4, 2.2%) than with daratumumab (grade 3, 5%; grade 4, 0). Prophylactic therapies to prevent IRs are mandatory for both agents, with the difference that post-infusion medications are required to reduce the risk of delayed IRs with daratumumab.
Fig. 4.
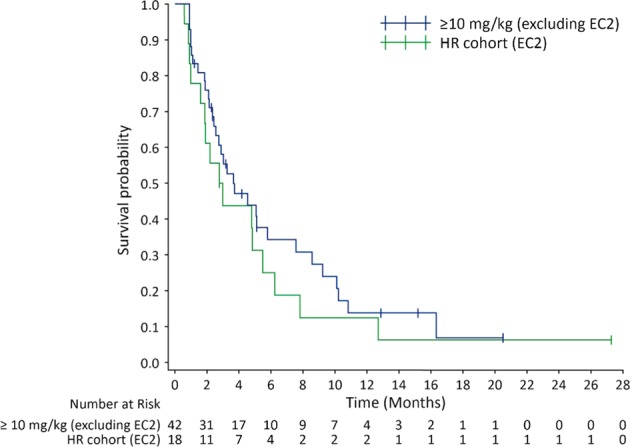
Progression-free survival Kaplan–Meier plot
Emerging data suggest that mAbs are most effective when used in combination with other therapies10. Elotuzumab monotherapy showed no objective response27, but potent activity with lenalidomide/dexamethasone11. The recent phase III data from the POLLUX (daratumumab/lenalidomide/dexamethasone14) and CASTOR (daratumumab/bortezomib/dexamethasone13) studies suggest an even greater benefit with using daratumumab in these combinations in early disease relapse (first to third relapse). The addition of isatuximab to lenalidomide/dexamethasone has shown clinical promise in heavily pretreated patients with RRMM28. There have been no studies that have attempted to investigate the optimum sequencing of antibody therapies, and whether particular agents show improved efficacy with certain combinations or lines of therapy remains unclear.
Three isatuximab phase III studies are currently ongoing; one evaluating isatuximab together with pomalidomide and dexamethasone in patients who have received at least 2 prior lines, a second combining isatuximab with carfilzomib and dexamethasone in patients who have received 1–3 prior lines, and a third study evaluating isatuximab in combination with bortezomib, lenalidomide, and dexamethasone in patients with newly diagnosed MM who are ineligible for transplant. Additional studies are under way for both isatuximab and daratumumab, as well as for elotuzumab, evaluating various combinations in front-line, relapsed disease, and smoldering MM. Whether one agent will emerge as superior in a particular treatment combination or setting is yet to be seen. With regard to CD38 antibodies, isatuximab and daratumumab bind to different and unique epitopes on CD38, and in vitro studies reveal differences in their mode of action, most notably that isatuximab promotes apoptosis without cross-linking16. Whether these differences will translate into differences in clinical activity, or whether patients who are refractory to one anti-CD38 antibody will respond to another, is yet unknown.
In conclusion, isatuximab monotherapy was generally well tolerated and demonstrated preliminary efficacy in the treatment of RRMM. Although an MTD was not reached, the optimum monotherapy dose/schedule was selected as 20 mg/kg weekly for four doses followed by Q2W dosing. An ongoing study is evaluating isatuximab monotherapy and includes patients who have failed to respond to prior daratumumab treatment (NCT02514668). Additional studies are planned that will evaluate isatuximab in combination with other immuno-oncology drugs, including checkpoint blockers. CD38 receptors are present on T-regulatory cells and other immunosuppressive cells, and emerging data suggest that CD38 therapy can stimulate cytotoxic T-cell responses29. The unique binding differences between the CD38 antibodies may, perhaps, prove most important when these agents are combined with other immuno-oncology agents. Further studies are necessary to identify the combination with the highest benefit/risk profile to patients with RRMM. Phase III studies are evaluating isatuximab (10 mg/kg) in combination with pomalidomide and dexamethasone, as well as with carfilzomib and dexamethasone in RRMM. Additional studies are ongoing to help identify the optimal combination and/or clinical setting in which to use isatuximab therapy.
Supplementary information
Supplemental Fig. S1 Study design (online only)
Supplemental Fig. S2 Individual isatuximab plasma pharmacokinetic profiles following the first intravenous infusion of isatuximab (online only)
Acknowledgements
We would like to thank investigators Michel Attal, Lionel Karlin, Robert Kaufmann, Philippe Moreau, John Morris, Enrique Ocio, Joshua Richter, and Jesus San Miguel for their valued contribution to the study. We also thank the following individuals from Sanofi for their respective contributions: Chrisie Ding and Diane Stash (trial management); Kathryn Corzo, Franck Dubin, and Corina Oprea (trial design and management); Anthony Hamlett (statistical analysis); and Dorothee Semiond (pharmacokinetic analysis). This study was funded by Sanofi. Editorial assistance was provided by Neil Harrison and Louise Wright of Adelphi Communications Ltd, funded by Sanofi.
Authors' contributions
Conception and design: T.M., S.S., M.G., E.C., J.M., K.H. Collection and assembly of data: T.M., S.S., M.G., J.M. Data analysis and interpretation: T.M., K.H., E.C., H.G., J.M.
Conflict of interest
Dr. Thomas Martin has received research funding from Sanofi. Dr. Stephen Strickland has received honoraria for an advisory role from Amgen, Alexion, Boehringer Ingelheim, Baxalta, CTI Biopharma, Sunesis, and Daiichi-Sankyo. He has also received research funding from Astellas, Boehringer Ingelheim, Celator, Celgene, Cyclacel, GlaxoSmithKline, Karyopharm Therapeutics, Novartis, Sanofi, and Sunesis. Dr. Martha Glenn declares that she has no conflict of interest. Eric Charpentier, Karl Hsu, and Hélène Guillemin are employees of Sanofi. Dr. Joseph Mikhael has received funding from Abbvie, Celgene, and Sanofi.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41408-019-0198-4).
References
- 1.Lichtman MA. Battling the hematological malignancies: the 200 years’ war. Oncologist. 2008;13:126–138. doi: 10.1634/theoncologist.2007-0228. [DOI] [PubMed] [Google Scholar]
- 2.Plawny L, Ries F. Emerging new anticancer biological therapies in 2013 (haematological malignancies) Curr. Opin. Oncol. 2014;26:363–370. doi: 10.1097/CCO.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eshaghian S, Berenson JR. Multiple myeloma: improved outcomes with new therapeutic approaches. Curr. Opin. Support. Palliat. care. 2012;6:330–336. doi: 10.1097/SPC.0b013e3283565c56. [DOI] [PubMed] [Google Scholar]
- 5.Rajkumar SV. Doublets, triplets, or quadruplets of novel agents in newly diagnosed myeloma? Hematology Am. Soc. Hematol. Educ. Program. 2012;2012:354–361. doi: 10.1182/asheducation-2012.1.354. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo A, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N. Engl. J. Med. 2012;366:1759–1769. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 7.Kumar SK, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherbenou DW, et al. The development of potential antibody-based therapies for myeloma. Blood Rev. 2015;29:81–91. doi: 10.1016/j.blre.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Donk NW, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol. Rev. 2016;270:95–112. doi: 10.1111/imr.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonial S, Durie B, Palumbo A, San-Miguel J. Monoclonal antibodies in the treatment of multiple myeloma: current status and future perspectives. Leukemia. 2016;30:526–535. doi: 10.1038/leu.2015.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lonial S, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 12.Lonial S, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387:1551–1560. doi: 10.1016/S0140-6736(15)01120-4. [DOI] [PubMed] [Google Scholar]
- 13.Palumbo A, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016;375:754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016;375:1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 15.Chari A, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130:974–981. doi: 10.1182/blood-2017-05-785246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deckert J, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38 + hematologic malignancies. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2014;20:4574–4583. doi: 10.1158/1078-0432.CCR-14-0695. [DOI] [PubMed] [Google Scholar]
- 17.Feng X, et al. Targeting CD38 suppresses induction and function of T regulatory cells to mitigate immunosuppression in multiple myeloma. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2017;23:4290–4300. doi: 10.1158/1078-0432.CCR-16-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hann, B. C. T. & Wang D. (editors). Isatuximab, an anti-CD38 antibody, shows anti-tumor activity in a preclinical model of multiple myeloma. American Association for Cancer Research (AACR) Annual Meeting (Washington, DC, USA, 2013).
- 19.Blade J, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br. J. Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 20.Rajkumar SV, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng W, et al. Bortezomib in combination with dexamethasone and subsequent thalidomide for newly-diagnosed multiple myeloma: a Chinese experience. Leuk. Res. 2009;33:1615–1618. doi: 10.1016/j.leukres.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Sucak G, et al. Abnormal protein bands in patients with multiple myeloma after haematopoietic stem cell transplantation: does it have a prognostic significance? Hematol. Oncol. 2010;28:180–184. doi: 10.1002/hon.936. [DOI] [PubMed] [Google Scholar]
- 23.Lonial S, Anderson KC. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia. 2014;28:258–268. doi: 10.1038/leu.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson PG, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826–1832. doi: 10.1182/blood-2013-11-538835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel DS, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lokhorst HM, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N. Engl. J. Med. 2015;373:1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 27.Zonder JA, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012;120:552–559. doi: 10.1182/blood-2011-06-360552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin T, et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;129:3294–3303. doi: 10.1182/blood-2016-09-737700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krejcik J, et al. Daratumumab depletes CD38 + immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128:384–394. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1 Study design (online only)
Supplemental Fig. S2 Individual isatuximab plasma pharmacokinetic profiles following the first intravenous infusion of isatuximab (online only)



