Abstract
The objective of this paper was to compare the effect of recombinant follicle-stimulating hormone (rFSH) and urinary follicle-stimulating hormone (uFSH) on pregnancy rates and live birth rates with the gonadotropin-releasing hormone (GnRH) antagonist protocol in China. This retrospective study was conducted from January 2014 through August 2017. Patients treated with uFSH had significantly higher levels of luteinizing hormone (3.79 mIU/ml vs. 3.09 mIU/ml) and progesterone (0.93 ng/ml vs. 1.16 ng/ml) on the day of human chorionic gonadotropin (HCG) administration, and they also had higher pregnancy rates (24.19% vs. 22.86%). There was no significant difference in the rate of live births. In the logistic regression results of the rFSH group, the pregnancy rate was positively correlated with the level of luteinizing hormone, with an odds ratio (OR) of 1.09 (95% confidence interval [CI]: 1.00–1.18; P = 0.048). In the uFSH group, the pregnancy rate was negatively correlated with the progesterone level on the day of HCG administration, with an OR of 0.47 (95% CI: 0.27–0.77; P = 0.004). Our research concluded that uFSH performed better than rFSH in terms of pregnancy rates when it was associated with the GnRH antagonist protocol. Meanwhile, no significant differences in the rate of live births were observed between the two groups.
Introduction
One in six couples worldwide will experience at least one infertility problem during their reproductive years1, and the majority will benefit from assisted reproductive technology (ART). Since gonadotropin-releasing hormone (GnRH) antagonists have become available since the 1980s, and more and more researchers have paid attention to these drugs. Studies have shown that GnRH antagonists have many advantages over other GnRH analogues. For instance, some researchers have established that the GnRH antagonist protocol has also been more effective than the long GnRH agonist protocol in reducing gonadotropin consumption and the incidence of ovarian hyperstimulation syndrome (OHSS), with a shorter duration of stimulation2–5. Advantages of antagonists are the shorter duration of analogue treatment, the shorter duration of stimulation with follicle-stimulating hormone (FSH) and the lower risk of developing OHSS6. Meta-analysis results have suggested that GnRH antagonists can significantly increase the clinical pregnancy rate (CPR) and decrease the premature luteinisation rate in controlled ovarian hyperstimulation/intrauterine insemination cycles7.
More recently, the majority of studies on the GnRH antagonist protocol are related to progesterone, supplemented luteinizing hormone (LH), modified stimulated cycle, and so on. A study suggested that sustained FSH stimulation increases the level of progesterone during the in vitro fertilization (IVF) cycle. Moreover, serum progesterone prematurely appears to be interrelated to poor reproductive outcomes8. Research has revealed that the modified natural cycle has been shown to yield a higher live birth rate compared to the high-dose FSH cycle in the GnRH antagonist protocol9. Another study revealed no important difference in live birth rates with supplemental LH during IVF treatment in women who are over 35 years of age10. Another study confirmed that the modified FSH stimulated cycle shows a higher pregnancy rate than the normal FSH stimulated cycle in patients with a poor ovarian response11. However, papers have rarely compared the effect of recombinant follicle-stimulating hormone (rFSH) and urinary follicle-stimulating hormone (uFSH) on pregnancy rates and live birth rates with the GnRH antagonist protocol in a retrospective study.
Moreover, many studies have compared uFSH with rFSH with the GnRH agonist protocol regarding numbers of oocytes, pregnancy rates, live birth rates, and so on. Generally, these studies suggested that rFSH had an inferior performance in older patients than uFSH with a lower dose of FSH, but no evidence has shown that rFSH has clinical advantages for CPRs of different urinary-derived FSH gonadotropins when used with the GnRH antagonist protocol12,13. When choosing a GnRH analogue protocol, other factors should be considered, such as costs, patient acceptability, availability, and drug safety13. In addition, the manufacture of human FSH using recombinant deoxyribonucleic acid (DNA) technology (rFSH) is independent of urine collection and also guarantees a high availability of a biochemically pure FSH preparation (specific activity > 10 000 IU FSH/mg) that is free from urinary protein contaminants. It has the characteristics of high purity and low immunogenicity13. The uFSH pharmaceutical preparations are extracted from the urine of postmenopausal women. Therefore, the main component of these preparations is FSH, but there is also a small amount of LH14. The incidence of OHSS is lower after therapy with uFSH versus rFSH14. Further, these two drugs differ with respect to drug concentration peak times in the blood, half-life period, and prices. In summary, GnRH antagonists have unique advantages, the effects of rFSH and uFSH on assisted reproduction have been studied in other GnRH analogues, and the effects of rFSH and uFSH on assisted reproduction are insufficient studies in GnRH antagonists. Therefore, we speculated that the effects of rFSH and uFSH on assisted reproduction may be different under the GnRH antagonist protocol, which is worth studying and of great significance.
The purpose of our study was to compare the effect of rFSH and uFSH on pregnancy rates and other IVF outcomes using the logistic regression model, unlike the previous model that used the GnRH agonist protocol and mostly white individuals. By evaluating a large number of variables in common patients and selecting significant correlation variables for logistic regression model analysis, the results can be used to assess what factors ultimately determine the success of ART in general and when applied to the general population.
Result
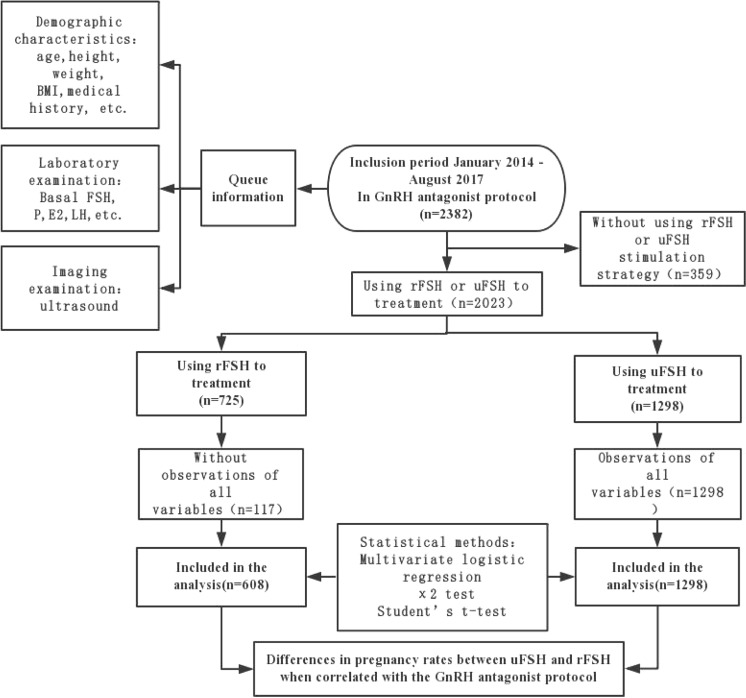
A total of 476 treatments without observations of all variables were not included in the final analysis. Of the 1906 treatment cycles eligible for analysis, 32% (608 treatments) were used in the rFSH group and 1298 treatments were used in the uFSH group (Fig. 1).
Figure 1.
Flowchart of patient disposition throughout the study. Retrospective, Eligibility and follow-up GnRH: gonadotropin-releasing hormone rFSH: recombinant follicle-stimulating hormone uFSH: urinary follicle-stimulating hormone FSH: follicle-stimulating hormone P: progesterone E2: estradiol LH: luteinizing hormone.
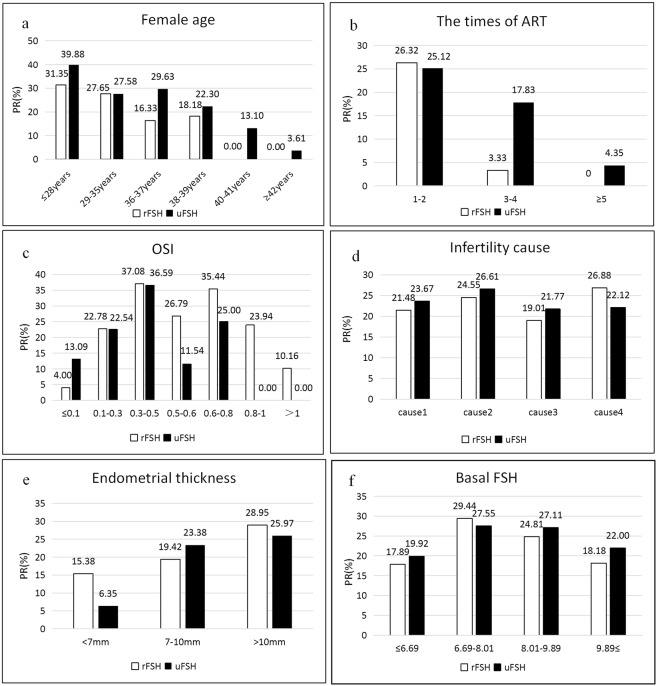
Figure 2 shows the relationship between some relevant variables (e.g., age and endometrial thickness) and pregnancy rates in the rFSH and uFSH groups. According to the women’s age, we divided the patients into six groups. When women were older than 39 years and younger than 42 years, there was a significant reduction in the success rates of pregnancy compared to the previous age group (28 to 38 years old). Meanwhile, when women were older than 39 years, pregnancy success rates were zero in the rFSH group. Moreover, in any age group, the uFSH group had better pregnancy rates than the rFSH group (Fig. 2a). At the same time, patients who received ART were divided into three groups. If the patient received ART more than 5 times, pregnancy success rates were only 4.35% in the uFSH group (Fig. 2b).
Figure 2.
Some factors related to pregnancy rate. PR: pregnancy rate Cause1: ovulation factor Cause2: non-ovulation factor Cause3: ovulation factor plus non-ovulation factor Cause4: unexplained, etc.
The ovarian sensitivity index (OSI) measures ovarian response. Its formula is: OSI = number of oocytes retrieved/the dose of FSH15. When the OSI was 0.3–0.5 or 0.6–0.8, the pregnancy success rates were 37.08% and 35.44%, respectively, in the rFSH group (Fig. 2c). The cause of infertility was divided into four variables. Patients with ovulation factors plus non-ovulation factors had the lowest pregnancy rate (19.01%) in the rFSH group. However, in the uFSH group, patients with non-ovulation factors had the highest pregnancy rate (Fig. 2d). When endometrial thickness was <7 mm, the pregnancy rate was 6.35% in the uFSH group. However, the pregnancy rate with an endometrium >10 mm was 25.97% in the uFSH group (Fig. 2e).
Cycle characteristics of patients undergoing oocyte retrieval are shown in Table 1. The rFSH group was characterized by significantly higher cancelled cycles (P < 0.001), number of metaphase II oocytes (P < 0.001), number of oocytes fertilized (P < 0.001), number of oocytes retrieved (P < 0.001), oestradiol levels on the day of human chorionic gonadotropin (HCG) administration (P < 0.001), progesterone level on the day of HCG administration (P < 0.001), number of follicles on the day of HCG administration (P < 0.001) and thicker endometrium (P = 0.002) compared with the uFSH group with GnRH antagonist cycles (Table 1). The proportions of embryos transferred (P = 0.017) and the rates of clinical pregnancy (P < 0.001) were significantly lower in the rFSH group compared with the uFSH group (Table 1). In addition, there was no significant difference in live birth rates between the two groups (Table 1).
Table 1.
Cycle characteristics of patients who occurred to oocyte retrieval in the recombinant FSH and the uFSH GnRH antagonist groups.
| rFSH(608) | uFSH(1298) | p | |
|---|---|---|---|
| Embryos transferred | 298 (49.01) | 714 (55.01) | 0.017b |
| No transferred | 310 (50.99) | 584 (44.99) | |
| The result of ART | |||
| Cancelled cycles | 297 (48.85) | 491 (37.83) | <0.001b |
| Pregnancy failure | 172 (28.29) | 493 (37.98) | |
| Clinical pregnancy | 139 (22.86) | 314 (24.19) | |
| Live birth | 124 (20.39) | 253 (19.49) | 0.693b |
| NO live birth | 484 (79.61) | 1045 (80.51) | |
| Number of MII oocytes | 10.74 (6.50) | 5.49 (3.43) | <0.001a |
| Number of oocytes fertilized | 7.01 (5.00) | 3.62 (2.66) | <0.001a |
| Number of oocytes retrieved | 12.24 (7.19) | 6.18 (3.81) | <0.001a |
| LH on day of HCG (mIU/ml) | 3.09 (2.60) | 3.79 (6.91) | 0.001a |
| Estradiol on day of HCG (pg/ml) | 3330.85 (2307.01) | 1783.19 (1122.22) | <0.001a |
| Progesterone on day of HCG (ng/ml) | 1.16 (0.64) | 0.93 (0.48) | <0.001a |
| Endometrial thickness (mm) | 11.04 (2.51) | 10.66 (2.52) | 0.002a |
| Number of follicles on HCG day | 10.32 (5.60) | 5.82 (3.00) | <0.001a |
Data are mean(SD) or number (percentage).
aTwo-sample t-test.
bPearsonχ2 test.
ART: assisted reproductive technology.
MII: metaphase II.
LH: luteinizing hormone.
HCG: human chorionic gonadotropin.
There was a significant difference in low, medium, and high antral follicle count (AFCs) in the rFSH group compared with the uFSH group (P < 0.001). Regarding the number of AFCs ≥ 19, there were no differences between the two groups (Table 2).
Table 2.
Patient and cycle characteristics by stimulation protocol and AFC category.
| Antral follicle count | MII oocytes in rFSH | MII oocytes in uFSH | p |
|---|---|---|---|
| 1–9(I) | 6.67 (3.48) | 5.17 (3.14) | <0.001a |
| 10–13(II) | 10.21 (4.20) | 6.85 (3.77) | <0.001a |
| 14–18(III) | 11.75 (5.89) | 9.03 (4.44) | 0.017a |
| ≥19(IV) | 15.04 (6.98) | 13.55 (5.16) | 0.395a |
Data are mean (SD).
aTwo-sample t-test.
Comparing the patient characteristics in cycles that did and did not lead to pregnancy (Table 3), the cause of infertility was a categorical variable with three categories, and there were no significant differences in the pregnancy rates. Significant differences were found in age (P < 0.001), times of ART (P < 0.001), embryos transferred (P < 0.001), duration of infertility (P < 0.001), progesterone level on the day of HCG administration (P < 0.001), and endometrial thickness (P < 0.001) in the two groups.
Table 3.
Baseline characteristics in cycles that did or did not result in pregnancy after treatment by antagonist protocol.
| Pregnancy (482) | No pregnancy (1519) | p | |
|---|---|---|---|
| Age (years) | |||
| ≤28 y | 149 (30.91%) | 285 (18.76%) | <0.001 |
| ≤35 y | 210 (43.57%) | 560 (36.87%) | |
| ≤37 y | 56 (11.62%) | 160 (10.53%) | |
| ≤39 y | 42 (8.71%) | 159 (10.47%) | |
| ≤41 y | 19 (3.94%) | 153 (10.07%) | |
| ≥42 y | 6 (1.24%) | 202 (13.3%) | |
| The times of ART | |||
| 1–2 | 456 (94.61%) | 1349 (88.81%) | <0.001 |
| 3-4 | 25 (5.19%) | 145 (9.55%) | |
| ≥5 | 1 (0.21%) | 25 (1.65%) | |
| OSI | <0.001 | ||
| ≤0.1 | 26 (5.39%) | 194 (12.81%) | |
| ≤0.3 | 199 (41.29%) | 698 (46.07%) | |
| ≤0.5 | 172 (35.68%) | 302 (19.93%) | |
| ≤0.6 | 19 (3.94%) | 66 (4.36%) | |
| ≤0.8 | 35 (7.26%) | 67 (4.42%) | |
| ≤1 | 18 (3.73%) | 62 (4.09%) | |
| ≤1.2 | 5 (1.04%) | 36 (2.38%) | |
| >1.2 | 8 (1.66%) | 90 (5.94%) | |
| Basal FSH | 8.38 (2.49) | 8.68 (3.47) | 0.04 |
| Number of oocytes retrieved | 8.4 (4.16) | 8.47 (6.95) | 0.768 |
| Number of MII oocytes | 7.57 (3.83) | 7.51 (5.88) | 0.788 |
| Number of embryos transferred | 1.8 (0.48) | 0.69 (0.90) | <0.001 |
| Duration of infertility (years) | 3.81 (3.22) | 4.57 (3.97) | <0.001 |
| Cause of female infertility | |||
| cause1 | 101 (20.95%) | 358 (23.57%) | 0.063 |
| cause2 | 235 (48.76%) | 641 (42.2%) | |
| cause3 | 138 (28.63%) | 479 (31.53%) | |
| cause4 | 8 (1.66%) | 41 (2.7%) | |
| Estradiol on day of HCG (pg/ml) | 2215 (1218.36) | 2435.07 (2001.18) | 0.004 |
| Progesterone on day of HCG (ng/ml) | 0.86 (0.30) | 1.06 (0.60) | <0.001 |
| LH on day of HCG (mIU/ml) | 3.25 (2.46) | 3.73 (6.55) | 0.017 |
| Endometrial thickness (mm) | |||
| <7 | 8 (1.66%) | 87 (5.85%) | <0.001 |
| 7–10 | 160 (33.20%) | 579 (38.96%) | |
| >10 | 314 (65.15%) | 820 (55.18%) | |
| Antral follicle count | 9.59 (6.96) | 9.21 (7.52) | 0.3 |
| BMI (kg/m2) | 21.9 (2.85) | 22.22 (2.84) | 0.035 |
Data are mean(SD) or number (percentage).
BMI: body mass index (kg/m2);
OSI: ovarian sensitivity index.
Cause1: ovulation factor.
Cause2: non-ovulation factor.
Cause3: ovulation factor plus non-ovulation factor.
Cause4: unexplained,etc.
Gn: gonadotropin.
aTwo-sample t-test.
bPearsonχ2 test.
By analysing the data in the previous table, we selected the significant variables for regression analysis. We finally found the best model that contains eight predictors, as shown in Table 4. The probability of pregnancy rate was significantly correlated with age (odds ratio [OR] 0.91, 95% confidence interval [CI]: 0.89–0.93; P < 0.001), embryos transferred (OR 4.88, 95% CI: 4.08–5.91; P < 0.001), endometrial thickness (OR 1.13, 95% CI: 1.08–1.19; P < 0.001), and progesterone level on the day of HCG administration (OR 0.56, 95% CI: 0.39–0.80; P = 0.002) (Table 4). The results for the rFSH group (i.e., the predictive factors for pregnancy rate after ART) along with their ORs, CIs, and P-values are presented in Table 5. This model shows that age (OR 0.91, 95% CI: 0.86–0.96; P < 0.001), embryos transferred (OR 6.90, 95% CI: 4.88–10.22; P < 0.001), endometrial thickness (OR 1.20, 95% CI: 1.10–1.31; P < 0.001), and LH level on the day of HCG administration (OR 1.09, 95% CI: 1.00–1.18; P = 0.048) differ significantly from pregnancy rate (Table 5).
Table 4.
Logistic Regression Analysis of GnRH antagonist protocol in clinical pregnancy.
| OR (25–95%) | p | |
|---|---|---|
| Age(years) | 0.91 (0.89–0.93) | <0.001 |
| Embryos transferred | 4.88 (4.08–5.91) | <0.001 |
| Duration of infertility (years) | 0.97 (0.93–1.00) | 0.071 |
| Progesterone on day of HCG (ng/ml) | 0.56 (0.39–0.80) | 0.002** |
| Endometrial thickness (mm) | 1.13 (1.08–1.19) | <0.001 |
| Total GnRHant dose | 0.93 (0.86–1.01) | 0.075 |
| LH on day of HCG (mIU/ml) | 1.01 (0.98–1.03) | 0.252 |
| Cause1 | * | |
| cause2 | 1.33 (0.96–1.84) | 0.084 |
| cause3 | 1.34 (0.94–1.91) | 0.102 |
| cause4 | 0.64 (0.27–1.55) | 0.341 |
Table 5.
Logistic Regression Analysis of GnRH antagonist protocol of recombinant FSH in clinical pregnancy.
| OR (25–95%) | p | |
|---|---|---|
| Age (years) | 0.91 (0.86–0.96) | <0.001 |
| Embryos transferred | 6.90 (4.88–10.22) | <0.001 |
| Duration of infertility (years) | 0.93 (0.85–1.01) | 0.074 |
| Progesterone on day of HCG (ng/ml) | 0.74 (0.38–1.18) | 0.302 |
| Endometrial thickness (mm) | 1.20 (1.10–1.31) | <0.001 |
| Estradiol on day of HCG (pg/ml) | 1.00 (1.00–1.00) | 0.253 |
| LH on day of HCG (mIU/ml) | 1.09 (1.00–1.18) | 0.048* |
| Recombinant FSH | 1.00 (0.96–1.03) | 0.833 |
We also observed that age (OR 0.91, 95% CI: 0.88–0.93; P < 0.001), number of embryos transferred (OR 4.03, 95% CI: 3.27–5.04; P < 0.001), progesterone level on the day of HCG administration (OR 0.47, 95% CI: 0.27–0.77; P = 0.004), and endometrial thickness (OR 1.11, 95% CI: 1.04–1.18; P = 0.001) differ significantly from pregnancy rate in the uFSH group (Table 6).
Table 6.
Logistic Regression Analysis of GnRH antagonist protocol of uFSH in clinical pregnancy.
| OR (25–95%) | p | |
|---|---|---|
| Age (years) | 0.91 (0.88–0.93) | <0.001 |
| Embryos transferred | 4.03 (3.27–5.04) | <0.001 |
| Duration of infertility (years) | 0.97 (0.93–1.02) | 0.233 |
| Progesterone on day of HCG (ng/ml) | 0.47 (0.27–0.77) | 0.004** |
| Endometrial thickness (mm) | 1.11 (1.04–1.18) | 0.001** |
| Total GnRHant dose | 0.90 (0.80–1.01) | 0.065 |
| Estradiol on day of HCG (pg/ml) | 1.00 (1.00–1.00) | 0.182 |
| Urinary FSH | 1.02 (0.99–1.05) | 0.306 |
Discussion
When associated with the GnRH antagonist protocol, our research revealed that uFSH was better than rFSH in terms of pregnancy rates. Moreover, we did not find a significant difference in the live birth rates between the two groups.
Our results support the conclusion of Youssef et al. They analysed 394 cycles with GnRH agonists, revealing that gonadotropin affects CPRs in ART cycles11. In our research, we analysed 1906 cycles. We selected patients using the GnRH antagonist protocol. Moreover, we also found that the uFSH group had a higher embryo transfer rate and lower cancelled cycle rate than the rFSH group, which is an interesting result. Similar findings were shown in another study16. Moreover, we speculate that this may be due to the use of uFSH reducing the incidence of OHSS, while rFSH has a higher rate of egg retrieval and may also increase the incidence of OHSS. However, further research is needed to confirm this speculation. In addition, there was no difference in live birth rates between the two groups. Relative studies showed that rFSH alone or in combination with LH had little impact on the outcome of a single ART cycle17. These different results might be due to differences in patient selection, protocol, route of administration, dose of gonadotropin, and study design.
Moreover, our results show that rFSH is more effective than uFSH in inducing metaphase II oocytes, fertilized oocytes, and follicles on the day of HCG administration. This conclusion is similar with some other studies. A meta-analysis strongly recommended that highly purified human menopausal gonadotropin (HMG) produced less oocytes with a higher total dose per cycle in ART compared with rFSH18. Some previous studies found that the number of oocytes retrieved was not enough to measure ovarian response. Because of the high ovarian response in women, the use of low-dose FSH could still obtain a high live birth rate19. For women with a low ovarian response, live birth rates did not increase when normal numbers of oocytes were obtained by prolonged stimulation or increased FSH doses20,21. To solve this problem, this study used OSI, which simultaneously considered the number of oocytes retrieved and FSH doses in measuring ovarian response15. In our study, we analysed OSI and pregnancy rates based on the work of previous researchers. We found that OSI and pregnancy rate had a normal distribution trend. Studies have shown that the incidence of OHSS increases with increases of the OSI value15. Therefore, we speculated that when the OSI value was low, there was low ovarian sensitivity and low pregnancy rates. When the OSI value was high, the incidence of OHSS increased and pregnancy rates decreased. Of course, this conclusion requires more studies to be proved.
By grouping the number of AFCs, we found that, as expected in the fourth group, the number of metaphase II oocytes was higher in the rFSH group compared to that in the uFSH group. It may be because rFSH is more conducive to the generation of metaphase II oocytes.
The logistic regression results of the GnRH antagonist protocol revealed that the progesterone level on the day of HCG administration was negatively correlated with the pregnancy rate. Some authors suggest that premature progesterone elevation has a negative impact on the pregnancy rate8,22,23. Moreover, the cause of premature progesterone elevation might be due to enhanced FSH stimulation in ART cycles24. In addition, the administration of HCG/LH activity would reduce this risk25. Several studies show that endometrial thickness is one of the independent variables predictive of clinical pregnancy26,27. This is in line with our findings. However, a meta-analysis including 22 studies with 10 724 in vitro fertilization-intracytoplasmic sperm injection (IVF-ICSI) treatment cycles revealed that the ability to determine the pregnancy rate through endometrial thickness is limited after IVF-ICSI treatment28.
By comparing the logistic regression results between the rFSH and uFSH groups, we found that age, number of embryos transferred, and endometrial thickness were significantly associated with pregnancy rates in both models. Studies have shown that uFSH is superior to rFSH when age is >39 years12. However, we found that the uFSH group had higher pregnancy rates than the rFSH group at any age, but more studies are needed to confirm this conclusion. In addition, the difference between the two groups was that the pregnancy rate was also positively correlated with the level of LH in the rFSH group. The combination of LH and rFSH leads to higher pregnancy rates29,30, which suggests that in order to improve the pregnancy rate, LH should be added later to the rFSH group with the GnRH antagonist protocol. However, the time and dose of LH administration needs to be further studied.
In the uFSH group, the pregnancy rate was negatively correlated with progesterone levels on the day of HCG administration, dose of GnRH antagonist, and duration of infertility. This suggests that in order to improve the pregnancy rate, relevant measures should be taken to reduce premature progesterone levels and the dose of GnRH antagonist.
This is a retrospective study. First, our data comes from clinical practice, and the results of the analysis are more in line with the actual situation faced by doctors. Second, it makes up for a gap in the literature. Past studies about two gonadotropins mainly were associated with the GnRH agonist protocol. However, our study focused on the GnRH antagonist protocol in China.
This research suggests some directions for future research, including continuing investigation to provide a clearer picture of the role of rFSH/uFSH in elderly patients, patients with a poor ovarian reserve, or both. Moreover, the cost, safety, and patient acceptability issues can also be studied. Such research would help to better answer the differences between the two drugs.
In conclusion, our research revealed that uFSH performed better than rFSH in terms of pregnancy rates when it was associated with the GnRH antagonist protocol. Meanwhile, no significant differences in the live birth rates were observed between the two groups. There are some differences in the pharmacological characteristics and prices of these two drugs. uFSH has the dual effects of FSH and LH, and the incidence of OHSS may be lower. rFSH contains a higher concentration of FSH and has a higher egg retrieval rate, which may also increase the incidence of OHSS. In addition, the price of rFSH is higher than that of uFSH. Therefore, the final therapeutic schedule was a combination of the patient’s age, duration of infertility, willingness to pay, ovarian response, willingness to accept higher cycle cancellation rates and possible risks of OHSS.
Materials and Methods
Study design
This study was conducted from January 2014 through August 2017 at the Reproductive Medicine Centre of Tongji Hospital, PR China. A total of 2382 infertile women who were stimulated with the GnRH antagonist protocol and had normal menstrual cycles were included in the study. Patients who underwent IVF/ICSI therapy used their own oocytes, and the embryos transferred were fresh or frozen. IVF guidelines considered ovarian, tubal, and male factors. Because the drug mainly acted on women, male factors were excluded. Other factors included endometriosis, uterine fibroids, chromosomal abnormalities, and unexplained elements. All data acquisition, management, and analyses were performed by the Data Analysis Centre of Tongji Hospital. All patients provided written informed consent to participate in the study, and we had access to information that could identify individual participants during or after data collection. According to the Institutional Review Board (IRB) of Tongji Hospital, our study was not subjected to ethics review, because all women participating in the study received routine IVF treatment in the hospital and no additional intervention or sampling was performed, as described by Yuzheng et al.31.
Study data acquisition
All data were retrospectively collected for the purpose of investigating the clinical pregnancy outcome of patients undergoing IVF/ICSI treatment. Patients were willing and able to accomplish the protocol requirements for the duration of the study. Data were collected from all patients before ovarian stimulation. They included medical history, age, duration of infertility, types of infertility, and female cause of infertility. Meanwhile, female height and weight were collected by medical measurement and the body mass index (BMI) was calculated. Other indicators, including AFC, insemination method, number of oocytes retrieved, and doses of FSH, were also obtained by the professional medical technician.
Study procedures
Ovarian stimulation was performed by using the GnRH antagonist protocol. After subcutaneous injection of 0.25 mg GnRH antagonist, 150 IU/day rFSH (32%) or uFSH (68%) was administered to the patient until the day of HCG administration. When a leading follicle reached 18 mm, 10000 IU HCG was administered. In two patient groups, oocyte retrieval was conducted through transvaginal ultrasound-guided aspiration 34–36 h after HCG administration. The total number of oocytes retrieved was recorded, including the number that were mature and immature.
Subsequently, fertilization methods include IVF, ICSI, IVF and ICSI, and early ICSI remediation. In addition to early ICSI remission, fertilization success was assessed 18 h after insemination. Fertilization was initially checked 6 h after insemination. If the oocytes were not fertilized, early rescue ICSI was performed immediately32.
Embryo transfer was performed on days 2 or 3 after oocyte retrieval, with no more than three embryos. In case the patient had OHSS, embryo transfer was cancelled. Daily vaginal progesterone was supplemented in the luteal phase, 2 applications/day, beginning on the day of oocyte retrieval until a urine pregnancy test 17 days later. The standard of positive pregnancy test is a beta HCG level of ≥5 mIU/mL; after 3 weeks, women were monitored through foetal ultrasound.
Study outcomes
The primary outcome was clinical pregnancy, which was defined as clinical signs of pregnancy through ultrasonographic visualization of one or more gestational sacs. In addition to intrauterine pregnancy, clinical records of ectopic pregnancy are also included33. The other outcomes include the number of oocytes retrieved, number of metaphase II oocytes, and levels of oestradiol and progesterone.
Statistical methods
The characteristics of patients with clinical pregnancy and without clinical pregnancy were compared. Continuous variables are expressed as the mean and standard deviation (SD), and categorical variables are expressed as percentage. Among the group comparisons, parametric tests were used for the normal distribution variables, while nonparametric tests were used the non-normal distribution variables.
Student’s t-tests were performed to evaluate the statistical relations between the subgroups. Meanwhile, the χ2 test was used to evaluate the significance of the proportion of the categorical variables. Multivariate logistic regression was conducted to predict the association between clinical pregnancy and potential factors such as age, embryos transferred, endometrial thickness, duration of infertility, and cause of female infertility. Because the multivariate logistic regression analysis can control the mixed effects of variables, it is widely used in most studies and proven to be effective in predicting IVF outcomes in clinical practice research34–36. A P-value < 0.05 was considered statistically significant. All statistical analyses were done using R language.
Acknowledgements
We gratefully acknowledge all staff of the Reproductive Medicine Center of Tongji Hospital for their support and cooperation. This study is supported by the National Natural Science Foundation of China (NSFC) (Grant no. 81672085, 71871169,71373188, 81372804, U1333115, 30901586). The Chinese medical association of clinical medicine special funds for scientific research projects (17020400709).
Author Contributions
W.P., H.T., L.J., C.H., J.X., W.P., D.Y., R.W., Y.L., W.H. and S.L. took part in study design, the collection and analysis of data, interpretation of results, writing of the manuscript. Revision of the manuscript and the final approval of the version to be published: all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Pan, Haiting Tu and Lei Jin contributed equally.
References
- 1.Gameiro S, et al. ESHRE guideline: routine psychosocial care in infertility and medically assisted reproduction—a guide for fertility staff. J. Hum Reprod. 2015;30:2476–2485. doi: 10.1093/humrep/dev177. [DOI] [PubMed] [Google Scholar]
- 2.Gordts S, et al. A prospective an domized study comparing a GnRH-antagonist versus a GnRH-agonist short protocol for ovarian stimulation in patients referred for IVF. J. Facts Views & Vision in Obgyn. 2012;4:82. [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Inany, H. G. et al. Gonadotropin-releasing hormone antagonists for assisted reproductive technology. J. Obstetrics & Gynecology. 118 (2011). [DOI] [PubMed]
- 4.Wang, R .L., Lin, S. R.,Wang, Y., Qian, W. P. & Zhou, L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: A systematic review and meta-analysis. J. PLos One. 12 (2017). [DOI] [PMC free article] [PubMed]
- 5.Xiao, J. S., Su, C. M. & Zeng, X. T. Comparisons of GnRH Antagonist versus GnRH Agonist Protocol in Supposed Normal Ovarian Responders Undergoing IVF: A Systematic Review and Meta-Analysis. J. PLos One. 9 (2014). [DOI] [PMC free article] [PubMed]
- 6.Al-Inany, H. G. et al. Gonadotropin-releasing hormone antagonists for assisted reproductive technology. J. Cochrane Database Syst Rev. 4 (2016). [DOI] [PMC free article] [PubMed]
- 7.Luo, S., Li, S. W., Jin, S., Li,Y. &Zhang,Y. Y. Effectiveness of GnRH antagonist in the management of subfertile couples undergoing controlled ovarian stimulation and intrauterine insemination: a meta-analysis. J. PLos One. 9 (2013). [DOI] [PMC free article] [PubMed]
- 8.Lawrenz B, Beligotti F, Engelmann N, Gates D, Fatemi HM. Impact of gonadotropin type on progesterone elevation during ovarian stimulation in GnRH antagonist cycles. J. Hum Reprod. 2016;31:2554–2560. doi: 10.1093/humrep/dew213. [DOI] [PubMed] [Google Scholar]
- 9.Lainas TG, et al. Live birth rates after modified natural cycle compared with high-dose FSH stimulation using GnRH antagonists in poor responders. J. Hum Reprod. 2015;30:2321–2330. doi: 10.1093/humrep/dev198. [DOI] [PubMed] [Google Scholar]
- 10.Vuong TN, Phung HT, Ho MT. Recombinant follicle-stimulating hormone and recombinant luteinizing hormone versus recombinant follicle-stimulating hormone alone during GnRH antagonist ovarian stimulation in patients aged ≥ 35 years: a randomized controlled trial. J. Hum Reprod. 2015;30:1188–1195. doi: 10.1093/humrep/dev038. [DOI] [PubMed] [Google Scholar]
- 11.Youssef MA, et al. A mild ovarian stimulation strategy in women with poor ovarian reserve undergoing IVF: a multicenter randomized non-inferiority trial. J. Hum Reprod. 2017;32:112–118. doi: 10.1093/humrep/dew282. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed MA, et al. Urinary follicle-stimulating hormone (FSH) is more effective than recombinant FSH in older women in a controlled randomized study. J. Fertil Steril. 2006;85:1398–403. doi: 10.1016/j.fertnstert.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 13.Alinany H, Aboulghar M, Mansour R, Serour G. Meta-analysis of recombinant versus urinary-derived FSH: an update. J. Hum Reprod. 2003;18:305–13. doi: 10.1093/humrep/deg088. [DOI] [PubMed] [Google Scholar]
- 14.Bergh C, et al. Recombinant human follicle stimulating hormone (r-hFSH; Gonal-F) versus highly purified urinary FSH (Metrodin HP): results of a randomized comparative study in women undergoing assisted reproductive techniques. J. Hum Reprod. 1997;12:2133–2139. doi: 10.1093/humrep/12.10.2133. [DOI] [PubMed] [Google Scholar]
- 15.Huber M, Hadziosmanovic N, Berglund L, Holte J. Using the ovarian sensitivity index to define poor, normal, and high response after controlled ovarian hyperstimulation in the long gonadotropin-releasing hormone agonist protocol: suggestions for a new principle to solve an old problem. J. Fertil Steril. 2013;100:1270–+. doi: 10.1016/j.fertnstert.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 16.Pacchiarotti A, Aragona C, Gaglione R, Selman H. Efficacy of a combined protocol of urinary and recombinant follicle-stimulating hormone used for ovarian stimulation of patients undergoing ICSI cycle. J. Journal of Assisted Reproduction and Genetics. 2007;24:400–405. doi: 10.1007/s10815-007-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humaidan P, et al. Efficacy and safety of follitropin alfa/lutropin alfa in ART: a randomized controlled trial in poor ovarian responders. J. Hum Reprod. 2017;32:544–555. doi: 10.1093/humrep/dew360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehert, P., Schertz, J. C. & Ezcurra, D. Recombinant human follicle-stimulating hormone produces more oocytes with a lower total dose per cycle in assisted reproductive technologies compared with highly purified human menopausal gonadotropin: a meta-analysis. J. Reproductive Biology & Endocrinology. 8 (2010). [DOI] [PMC free article] [PubMed]
- 19.Verberg MFG, et al. The clinical significance of the retrieval of a low number of oocytes following mild ovarian stimulation for IVF: a meta-analysis. J. Hum Reprod Update. 2009;15:5–12. doi: 10.1093/humupd/dmn053. [DOI] [PubMed] [Google Scholar]
- 20.Saldeen P, Kallen K, Sundstrom P. The probability of successful IVF outcome after poor ovarian response. J. Acta Obstet Gynecol Scand. 2007;86:457–461. doi: 10.1080/00016340701194948. [DOI] [PubMed] [Google Scholar]
- 21.Holte J, et al. Antral follicle counts are strongly associated with live-birth rates after assisted reproduction, with superior treatment outcome in women with polycystic ovaries. J. Fertil Steril. 2011;67:29–30. doi: 10.1016/j.fertnstert.2011.06.071. [DOI] [PubMed] [Google Scholar]
- 22.Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. J. Hum Reprod Update. 2013;19:433–457. doi: 10.1093/humupd/dmt014. [DOI] [PubMed] [Google Scholar]
- 23.Lu X, et al. Elevated progesterone on the trigger day does not impair the outcome of Human Menotrophins gonadotropin and Medroxy progesterone acetate treatment cycles. J.Scientific Reports. 2016;6:311–312. doi: 10.1038/srep31112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyrou D, et al. The relationship of premature progesterone rise with serum estradiol levels and number of follicles in GnRH antagonist/recombinant FSH-stimulated cycles. J. European Journal of Obstetrics Gynecology & Reproductive Biology. 2012;162:165–168. doi: 10.1016/j.ejogrb.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Werner MD, et al. Defining the ‘sweet spot’ for administered luteinizing hormone-to-follicle-stimulating hormone gonadotropin ratios during ovarian stimulation to protect against a clinically significant late follicular increase in progesterone: an analysis of 10,280 first in vitro fertilizationcycles. J. Fertil Steril. 2014;102:1312–1317. doi: 10.1016/j.fertnstert.2014.07.766. [DOI] [PubMed] [Google Scholar]
- 26.Ma, N. Z. et al. Influence of endometrial thickness on treatment outcomes following in vitro fertilization/intracytoplasmic sperm injection. J. Reproductive Biology & Endocrinology. 15 (2017). [DOI] [PMC free article] [PubMed]
- 27.Yuan X, et al. Endometrial thickness as a predictor of pregnancy outcomes in 10787 fresh IVF-ICSI cycles. J. Reproductive Biomedicine Online. 2016;33:197–205. doi: 10.1016/j.rbmo.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Kasius A, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. J. Hum Reprod Update. 2014;20:530–541. doi: 10.1093/humupd/dmu011. [DOI] [PubMed] [Google Scholar]
- 29.Santi, D., Casarini, L., Alviggi, C. & Simoni, M. Efficacy of Follicle-Stimulating Hormone (FSH) Alone, FSH + Luteinizing Hormone, Human Menopausal Gonadotropin or FSH + Human Chorionic Gonadotropin on Assisted Reproductive Technology Outcomes in the “Personalized” Medicine Era: A Meta-analysis. J. Front Endocrinol. 9 (2018). [DOI] [PMC free article] [PubMed]
- 30.Lehert P, et al. Recombinant human follicle-stimulating hormone (r-hFSH) plus recombinant luteinizing hormone versus r-hFSH alone for ovarian stimulation during assisted reproductive technology: systematic review and meta-analysis. J. Reproductive Biology and Endocrinology. 2014;12:1–14. doi: 10.1186/1477-7827-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y, et al. Hormonal replacement treatment improves clinical pregnancy in frozen-thawed embryos transfer cycles: a retrospective cohort study. J. American Journal of Translational Research. 2013;6:85–90. [PMC free article] [PubMed] [Google Scholar]
- 32.Ji JJ, et al. The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. J. Hum Reprod. 2013;28:2728–2734. doi: 10.1093/humrep/det303. [DOI] [PubMed] [Google Scholar]
- 33.Zegers-hochschild F, et al. The International Glossary on Infertility and Fertility Care, 2017. J. Fertil Steril. 2017;108:393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Chen ZJ, et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. J. New England Journal of Medicine. 2016;375:523–533. doi: 10.1056/NEJMoa1513873. [DOI] [PubMed] [Google Scholar]
- 35.Wei DM, et al. Effect of pretreatment with oral contraceptives and progestins on IVF outcomes in women with polycystic ovary syndrome. J. Hum Reprod. 2017;32:354–361. doi: 10.1093/humrep/dew325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarze JE, Crosby JA, Zegers-Hochschild F. Addition of neither recombinant nor urinary luteinizing hormone was associated with an improvement in the outcome of autologous in vitro fertilization/intracytoplasmaticsperm injection cycles under regular clinical settings: a multi-center observational analysis. J. Fertil Steril. 2016;106:1714–1718. doi: 10.1016/j.fertnstert.2016.09.003. [DOI] [PubMed] [Google Scholar]