Abstract
Introduction
Gadolinium-based contrast agents (GBCA) have been used to enhance magnetic resonance imaging (MRI) since 1985. Recently, the media and online groups have voiced concerns about gadolinium deposition in patients with normal renal function based on “elevated” urinary gadolinium levels. The determination of increased urinary gadolinium levels is based on reference ranges developed in individuals with normal renal function who were never exposed to GBCA. Studies suggest an elevated gadolinium urinary elimination greater than 72 h post GBCA scan. We evaluated urine gadolinium concentrations over a 30-day period in patients administered GBCA.
Methods
In this prospective, observational pilot study, we enrolled subjects between 18 and 65 years of age with normal renal function who received GBCA for the first time. Urinary gadolinium was measured at days zero (prior to GBCA exposure), 3, 10, and 30 after GBCA exposure. We compared urinary gadolinium levels after GBCA exposure to the current reference range and calculated an estimated duration of “elevated” gadolinium urine levels in the average patient.
Results
All 13 subjects had 24-h urinary gadolinium levels higher than 0.7 μg/24 h with means of 1944 (± 1432) μg/24 h on day 3, 301 (± 218) μg/24 h on day 10, and 34 (± 33) μg/24 h on day 30. Based on calculated urinary gadolinium elimination kinetics, we estimate urinary gadolinium levels will often remain above the current reference range for > 50 days.
Conclusion
The current reference range of 0.7 μg/24 h for 24 h urinary gadolinium is not applicable to patients for at least 30 days following GBCA exposure.
Keywords: Gadolinium, Contrast, Urinary elimination
Introduction
Gadolinium-based contrast agents (GBCA) have been used to enhance magnetic resonance imaging (MRI) since 1985 [1]. In 2006, GBCA were implicated in causing nephrogenic systemic fibrosis in patients with renal impairment [2, 3] leading to a US Food and Drug Administration (FDA) ordered black box warning for several of these agents in 2007. In 2014, gadolinium deposits were found in the basal ganglia of patients with normal renal function after GBCA enhanced MRI scans [4]. Although there was no disease associated with these deposits, a new FDA warning was published [5]. Recently, laypeople with normal renal function began attributing various symptoms to “gadolinium toxicity” based on “elevated” urinary gadolinium levels months after undergoing MRI with GBCA [6–9]. However, the current reference range for 24-h (24hr) urinary gadolinium is derived from levels found in healthy individuals without a history of exposure to GBCA.
Early pharmacokinetic studies estimated that up to 100% of the GDCA dose given intravenously is excreted in the urine within 72-h [10, 11]. However, Lancelot recently suggested a second, delayed phase of elimination [12] leading us to hypothesize that gadolinium may be present in the urine for a prolonged period. With prolonged elimination, the applicability of the current 24-h urinary gadolinium reference range in patients with a history of GBCA exposure would be questionable.
Our primary objective was to determine the presence of gadolinium in the urine of healthy patients with normal renal function at 3, 10, and 30 days after first GBCA exposure. Our secondary objectives were to compare urinary gadolinium levels after GBCA exposure to the reference range, determine an estimated gadolinium urinary elimination half-life, and estimate the time needed for urinary gadolinium levels to fall below the current reference range value.
Methods
This is a prospective, observational pilot study designed using the STROBE checklist [13]. The study was reviewed and approved by the host institutional review board (IRB) and conducted at an 875-bed tertiary care facility. Recruitment occurred between September 2017 and January 2018; subjects were recruited through Radiology and Emergency Departments using scheduling lists and electronic medical records under an IRB-approved partial waiver of authorization for screening purposes. All patients between the ages of 18 and 65 years with normal kidney function, able to give informed consent, and receiving GBCA for the first time were considered eligible. Normal kidney function was defined as a creatinine level equal or below 1.5 mg/dL and a glomerular filtration rate (GFR) equal or above 59 mL/min/1.73 m2. Patients under 60 years of age without a history of diabetes, hypertension, or renal dysfunction were assumed to have normal renal function and did not require additional screening prior to GBCA administration in accordance with American College of Radiology recommendations [14]. In all other cases, a creatinine assessment was performed if there was not one in the preceding 6 months. The IRB did not permit serum creatinine determinations on patients who did not require them. Patients with a history of bipolar disorder, personality disorder, depression, schizoaffective disorder, schizophrenia, fibromyalgia, peripheral neuropathy, strokes, transient ischemic attacks, chronic pain, cerebral palsy, migraines, dermatitis, rheumatoid arthritis, systemic lupus erythematosus, Raynaud’s phenomenon, scleroderma, or dermatomyositis were also excluded
Urinary gadolinium and creatinine testing were performed at Mayo Medical Laboratories: a Clinical Laboratory Improvement Amendments (CLIA) accredited lab in Rochester, MN. Gadolinium analysis was performed using inductively coupled mass spectrometry (ICP-MS). Total gadolinium (free and chelated) was reported in μg/24 h. Creatinine was measured by an enzymatic colorimetric assay based on the conversion of creatinine to sarcosine. The gadolinium to creatinine ratio was reported on baseline (spot) and 24-h urine samples in μg gadolinium per gram of creatinine (μg Gd/g Cr). Total elemental urinary elimination is best estimated with a full 24-h collection rather than a spot sample [15].
Upon enrollment and before MRI with GBCA administration, spot urine samples were obtained and tested for gadolinium and creatinine. Any subjects with initial spot urinary creatinine-adjusted gadolinium levels that were more than two times the comparable reference 24-h creatinine-adjusted normal gadolinium level (0.8 μg Gd/g Cr) were to be excluded, but no enrolled subjects met or were excluded by these criteria. Full 24-h urine collections were performed on days 3, 10, and 30 after GBCA exposure. Samples were shipped from the subjects’ residences to the primary institution using a courier service. All samples were stored at 4 °C until processing and then frozen at − 20 °C until testing was performed.
The primary study outcome was to determine the mean and standard deviation of 24-h urinary gadolinium elimination both absolute (μg/24 h) and adjusted for creatinine (μg Gd/g Cr) at days 3, 10, and 30 after GBCA enhanced MRI. Our secondary outcomes were to compare 24-h urinary gadolinium elimination at days 3, 10, and 30 to the creatinine adjusted reference range of < 0.8 μg Gd/g Cr, determine an estimate of urinary gadolinium elimination half-life, and calculate an estimated time for urinary gadolinium levels to fall below the creatinine adjusted 24 h reference range value. Since subjects would receive different GBCA agents due to differing diagnoses and radiologists, we expected variable urinary gadolinium levels based on clearance of specific agents [1].
Determination of the mean elimination kinetics of gadolinium was planned to use all three data/time points (3, 10, and 30 days) using standard pharmacokinetic linear fit of the natural logarithm of each subject’s levels. However, due to published evidence of the biphasic elimination of gadolinium and concerns about bone persistence [12], a secondary analysis was planned to determine values for the elimination constant (Ke) for days 3 to 10, and 10 to 30 days and compare them to determine if these differed (paired sample t test, p < 0.05 significant). This analysis was performed because if there was biphasic elimination, this would provide a better estimation of the total time for a gadolinium level to return to the “normal” range by employing the terminal phase constant and not a mixture of two phases. The average extrapolated expected time until urinary clearance of gadolinium below the normal level was determined based on the mean concentrations at 10 days (minus outliers) and the average Ke elimination between 10 and 30 days in order to estimate the time expected for a urinary gadolinium level to return to the “normal” range.
The single sample with collection delayed by 1 day (i.e., 24-h collection completed on day four rather than day three) was included in half-life calculations with the true collection time (i.e., day four) but grouped with the closest set (i.e., days three) for the average concentration determinations at each time point.
Due to lack of pre-existing data for a power calculation, the target sample size (n = 12) was based on “the rule of 12” for pilot studies measuring continuous variables [16]. Data was recorded in Research Electronic Data Capture (REDCap) tools [17] hosted at the study site and statistical analyses was performed using a blinded, encrypted secure spreadsheet (Microsoft® Excel version 15.35, 2017) with the Real Statistics Resource Pack software (www.real-statistics.com). Values in parenthesis (±) represent one standard deviation.
Results
A total of 902 patients were screened between September 2017 and January 2018, 68 met inclusion criteria, and 13 subjects were enrolled without any drop-outs (see Fig. 1 and Table 1). All but one subject had a GFR of > 59 mL/min/1.73 m2 reported within 1 year of the MRI contrast study with the remaining subject (< 60 years of age) having a GFR > 59 mL/min/1.73 m2 determined within 16 months of the study. Eight (62%) subjects received gadobutrol (Gadavist®) with an average dose volume of 9.4 mL (1470 mg gadolinium), range 7–12 mL. Four (31%) subjects received gadopentetate dimeglumine (Magnevist®) with an average dose volume of 18.5 mL (1450 mg gadolinium), range 14–20 mL. One subject received 10 mL (390 mg gadolinium) of gadoxetate disodium (Eovist®). All subjects completed baseline spot urine and 24-h urinary collections at days 3, 10, and 30 after receiving GBCA; one subject collected the 3-day sample 1 day late, on day four.
Fig. 1.
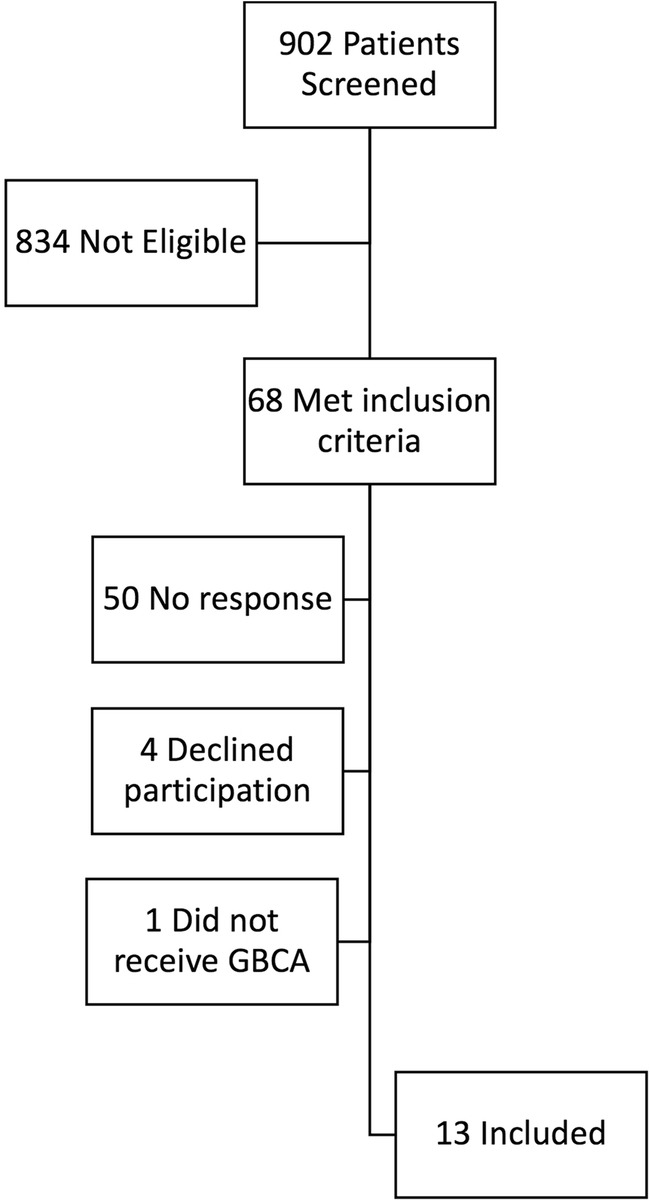
Summary of subject numbers during screening and enrollment
Table 1.
Subjects’ baseline characteristics
| Age, (range) | 44 years, (24–64 years) |
| Sex | Female n = 7 (54%) Male n = 6 (46%) |
| BMI, (range) | 28.7 kg/m2, (21.2–44.8 kg/m2) |
| Race | Caucasian n = 8 (62%) Black or African American n = 4 (31%) Unknown n = 1 (8%) |
| Reason for MRI | Musculoskeletal n = 3 (23%) Gastrointestinal/Genitourinary n = 6 (46%) Cancer n = 3 (23%) Other n = 1 (8%) |
| Concurrent medications | NSAIDS n = 6 (46%) Proton Pump Inhibitor n = 3 (23%) Selective serotonin uptake inhibitor n = 2 (15%) Gabapentin n = 2 (15%) Benzodiazepine n = 2 (15%) Opioid n = 1 (8%) |
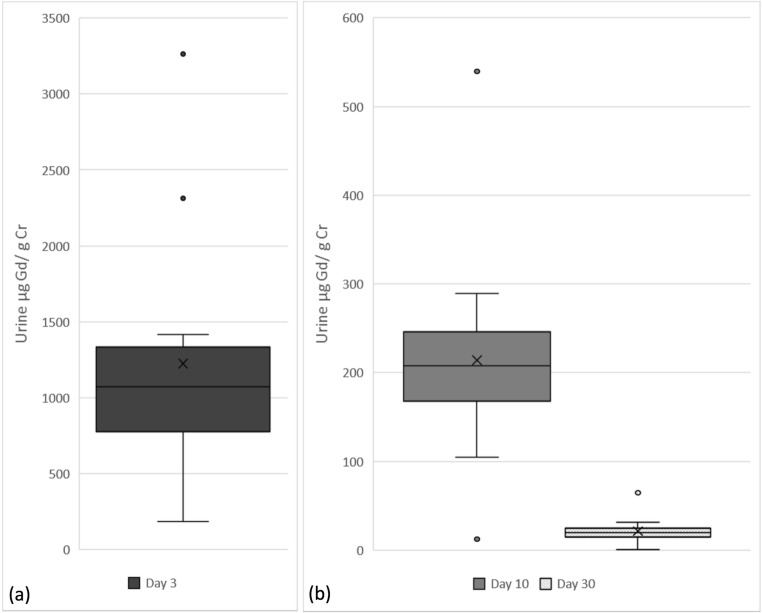
The average gadolinium to creatinine ratio was 0.3 μg (± 0.3) μg Gd/g at baseline (spot), 1225 (± 796) μg Gd/g Cr at day 3, 214 (± 120) μg Gd/g Cr at day 10, and 22 (± 15) μg Gd/g Cr at day 30; see Fig. 2 for the 3-, 10-, and 30-day values. The average 24 h gadolinium was 1944 (± 1432) μg/24 h on day 3, 301 (± 218) μg/24 h on day 10, and 34 (± 33) μg/24 h on day 30. See Table 2 for the individual subject’s urinary volume, creatinine, and gadolinium measurements. For subjects who received gadobutrol, their average dose was 18.3 mg Gd/kg [range 16.3–23.7]; their gadolinium to creatinine ratios were 1549 (± 828) μg Gd/g Cr at day 3, 257 (± 121) μg Gd/g Cr at day 10, and 27 (± 16) μg Gd/g Cr at day 30. For subjects who received gadopentetate dimeglumine, their average dose was 21.0 mg Gd/kg [range 16.7–23.8]; their gadolinium to creatinine ratios were 837 (± 301) μg Gd/g Cr at day three, 177 (± 55) μg Gd/g Cr at day 10, and 16 (± 7) μg Gd/g Cr at day 30.
Fig.2.
a Urinary gadolinium levels (μg Gd/g Cr) at day 3; b Urine gadolinium levels (μg Gd/g Cr) day 10 and 30. Box plot with means indicated by x, median and interquartile range (IQR) depicted as the box, whiskers indicating data within 1.5 the IQR, and outlier data points with dots
Table 2.
Individual subjects’ gadolinium samples. *Day 3 collection was on day 4
| Baseline (spot) | Day 3 | Day 10 | Day 30 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Creatinine (mg/dL) | Gd (μg/g Cr) | Volume (mL) | Creatinine (mg/dL) | Gd (μg) | Volume (mL) | Creatinine (mg/dL) | Gd (μg) | Volume (mL) | Creatinine (mg/dL) | Gd (μg) |
| 1 | 219 | 0.1 | 2000 | 123 | 5693 | 2000 | 84 | 429 | 1230 | 118 | 46 |
| 2 | 255 | < 0.1 | 1660 | 105 | 1927 | 1719 | 87 | 331 | 2200 | 69 | 30 |
| 3 | 203 | < 0.1 | 730 | 140 | 188 | 994 | 144 | 18 | 1275 | 161 | 0.9 |
| 4 | 117 | < 0.1 | 1822 | 86 | 1671 | 1326 | 143 | 329 | 700 | 226 | 40 |
| 5 | 30 | 0.7 | 1314 | 124 | 1726 | 1833 | 94 | 337 | 1875 | 92 | 28 |
| 6 | 17 | 1.2 | 1786 | 91 | 2298 | 1975 | 51 | 210 | 1100 | 53 | 12 |
| 7 | 186 | < 0.1 | 777 | 136 | 452 | 772 | 184 | 148 | 900 | 138 | 10 |
| 8 | 58 | < 0.1 | 2454 | 75 | 1397 | 3546 | 52 | 304 | 3025 | 96 | 38 |
| 9 | 198 | < 0.1 | 1200* | 111* | 1429* | 475 | 180 | 146 | 751 | 179 | 25 |
| 10 | 134 | < 0.1 | 2075 | 83 | 2068 | 950 | 102 | 280 | 895 | 76 | 16 |
| 11 | 31 | 0.4 | 2119 | 43 | 1145 | 1720 | 61 | 218 | 1371 | 77 | 24 |
| 12 | 185 | < 0.1 | 1763 | 104 | 1458 | 586 | 163 | 226 | 1841 | 107 | 31 |
| 13 | 108 | < 0.1 | 2054 | 57 | 3824 | 1772 | 98 | 937 | 2999 | 70 | 136 |
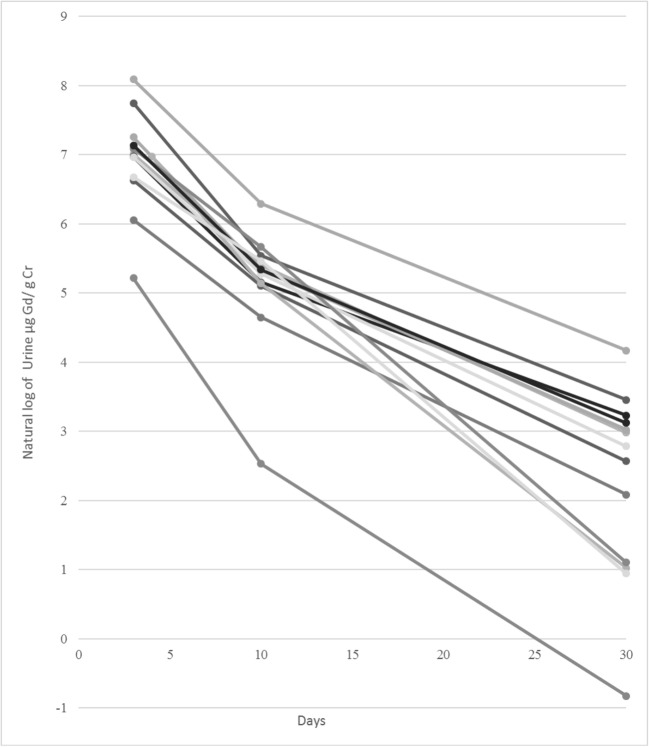
The average elimination constant (Ke) for urinary gadolinium from three to 10 days was 0.255 (±0.056) and for 10 to 30 days was 0.121 (± 0.018); this difference was significant (p < 0.0001). This corresponds to a half-life of 2.7 days and 5.7 days respectively. Figure 3 demonstrates the urinary gadolinium elimination kinetics between days 3 and 10 and days 10 and 30 for the 13 subjects. The estimated time for urinary gadolinium levels to become undetectable (i.e., less than 0.8 μg Gd/g Cr), based on the half-life between days 10 and 30 and using the 10-day mean was 57 days with an upper limit estimated at 84 days. This upper limit calculation utilized the means plus 1.96 standard deviations for the 10-day average and Ke; it excluded the one outlier subject that received gadoxetate disodium for the day 10 mean.
Fig. 3.
Natural log urinary gadolinium (μg Gd/g Cr) vs. number of days since GBCA administration for all subjects
Discussion
After exposure to GBCA, all subjects had total urine gadolinium levels above the current reference range of 0.7 μg/24 h at days 3, 10, and 30, and all subjects had gadolinium to creatinine ratios above 0.8 μg Gd/g Cr at days 3 and 10 with only one having a gadolinium to creatinine ratio below 0.8 μg Gd/g Cr at 30 days. In fact, at day 30 all but one subject had a 24-h Gd/Cr ratio that was at least 10 times the normal Gd/Cr ratio of 0.8 μg Gd/g Cr.
Our kinetic findings of two statistically different elimination constants also support Lancelot’s previous observations that GBCA have at least a two-phase urinary elimination process; a rapid early phase and a delayed, slower elimination phase [12]. In our study, we calculated an estimated average of 57 days for the urinary gadolinium to creatinine ratio to reach below the current reference range following GBCA exposure and possibly much longer (i.e., 80+ days). The identification that all subjects in this study had total urinary gadolinium levels above the current reference range and that the estimated clearance of gadolinium is greater than 50 days calls into question the applicability of the current reference range for patients with a history of GBCA exposure. To interpret urinary gadolinium levels following GBCA MRI, researchers need to develop a new reference range applicable to this population.
Limitations
Our limitations include a small sample size of a relatively healthy population, which was appropriate for a pilot study but prevents development of a comprehensive reference range. Another limitation includes the possibility of unrecognized renal disease in subjects, as all but two of them were less than 60 years old and thus may not have had a recent serum creatinine performed if they had undiagnosed co-morbidities such as diabetes or hypertension. However, all subjects were evaluated by a health care provider prior to the GBCA-enhanced MRI being ordered and screening to identify patients with renal impairment followed American College of Radiology recommendations [14].
Our study conclusively demonstrated that current reference ranges are inappropriate for patients administered GBCA within 10 days of an MRI as 100% of subjects demonstrated a μg Gd/g Cr of at least 15 times the upper limit of the current reference range of 0.8 μg Gd/g Cr. Even at 30 days post GBCA 93% of subjects demonstrated a μg Gd/g Cr of at least 10 times the upper limit. Our kinetic calculations are limited by the infrequent urinary gadolinium measurements. With the dramatic change in the elimination constant between days 3–10 and days 10–30, additional urinary measurement would make elimination determinations more accurate and improve our understanding of exactly when the two-phase elimination changes. Also, since none of our subjects had urinary gadolinium levels below the 0.7 μg/24 h reference range at day 30, additional measurements after day 30 may have been beneficial to directly measure at what point urinary gadolinium levels returns to “normal” in the majority of patients. The selection of patients without renal disease limits the generalizability to a larger heterogeneous population; however since gadolinium is, in part, renally eliminated, we would expect decreased gadolinium elimination in patients with renal dysfunction, not increased elimination. Regarding the exclusion of psychologic, neurologic, and rheumatologic disease states, our intention was to evaluate the gadolinium elimination in a healthy population, as a pilot study, and compare it to the existing reference range. We have no reason to believe the patients excluded due to these pre-existing conditions had altered elimination of gadolinium but felt this was appropriate for answering the primary and secondary objectives of this study.
Another limitation was the administration of different GBCA at different doses to the study subjects. With rates of dissociation and elimination differing between linear, macrocyclic, ionic, and non-ionic contrast agents, kinetic calculations should be done separately for each GBCA. Specifically, no conclusions can be made about the elimination of gadoxetate sodium as 50% is hepatically eliminated (n = 1 in this study). In addition, our study is limited by our measurement of total gadolinium rather than speciation of free and chelated gadolinium. Since transmetallation is thought to be the pathophysiology behind gadolinium deposition [6, 18], speciation of free and chelated urinary gadolinium may be of clinical utility.
Finally, the clinical utility of measuring urinary gadolinium levels is yet to be established. There have been no peer-reviewed publications linking urinary gadolinium levels to disease and no publications that indicate urinary gadolinium levels are representative of total body burden. The authors could find no studies in patients with nephrogenic systemic fibrosis corelating urine gadolinium levels with disease severity. With increasing concerns of the safety of GBCA in patients with normal renal function, it is important for clinicians to understand the clinical utility of urinary gadolinium testing and interpretation of these results. Our study shows that gadolinium levels remain elevated for greater than 1 month after GBCA MRI. Treatment (i.e., chelation) of elevated urinary gadolinium in patients with normal renal function, is not warranted based on elevated levels alone due to limited evidence of gadolinium deposition causing disease in patients with normal renal function and the lack of evidence supporting the efficacy of chelation in reducing body burden or symptoms [6, 19, 20].
Conclusion
In summary, our study provides information on medium- to long-term kinetics of gadolinium excretion in patients with normal renal function following GBCA administration. The current reference range for urinary gadolinium levels does not apply to patients who have received a GBCA MRI for at least a month.
Acknowledgments
Funding Sources
Funding for this project was provided by the Emergency Medicine Foundation/Medical Toxicology Foundation Research Grant.
Previous Presentation of Data
This data has not previously been presented.
Compliance with Ethical Standards
Conflict of Interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Runge VM, Ai T, Hao D, Hu X. The developmental history of the gadolinium chelates as intravenous contrast media for magnetic resonance. Investig Radiol. 2011;46(12):807–816. doi: 10.1097/RLI.0b013e318237913b. [DOI] [PubMed] [Google Scholar]
- 2.Grobner T. Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 3.Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359–2362. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 4.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834–841. doi: 10.1148/radiol.13131669. [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration. FDA Drug Safety Communication: FDA identifies no harmful effects to date with brain retention of gadolinium-based contrast agents for MRIs; review to continue. 2017. https://www.fda.gov/Drugs/DrugSafety/ucm559007.htm. Accessed 14 Dec 2017.
- 6.Greenberg SA. Zinc transmetallation and gadolinium retention after MR imaging: case report. Radiology. 2010;257(3):670–673. doi: 10.1148/radiol.10100560. [DOI] [PubMed] [Google Scholar]
- 7.Grimm H and Williams S. Gadolinium retention from contrast MRIs in 70 cases with normal renal function – 24 -hour urine test results. 2017. https://gadoliniumtoxicity.com/contrast-mri-gadolinium-retention-70-cases-final/. Accessed 11 July 2018.
- 8.Burke LM, Ramalho M, AlObaidy M, Chang E, Jay M, Semelka RC. Self-reported gadolinium toxicity: a survey of patients with chronic symptoms. Magn Reson Imaging. 2016;34(8):1078–1080. doi: 10.1016/j.mri.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M, Ramalho M. Gadolinium deposition disease: initial description of a disease that has been around for a while. Magn Reson Imaging. 2016;34(10):1383–1390. doi: 10.1016/j.mri.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Le Mignon M-M, Chambon C, Warrington S, Davies R, Bonnemain B. Gd-DOTA. Pharmacokinetics and tolerability after intravenous injection into healthy volunteers. Investig Radiol. 1990;25:933–937. doi: 10.1097/00004424-199008000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Staks T, et al. Pharmacokinetics, dose proportionality, and tolerability of gadobutrol after single intravenous injection in healthy volunteers. Investig Radiol. 1994;29:709–715. doi: 10.1097/00004424-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Lancelot E. Revisiting the pharmacokinetic profiles of gadolinium-based contrast agents: differences in long-term biodistribution and excretion. Investig Radiol. 2016;51(11):691–700. doi: 10.1097/RLI.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 14.ACR Committee on Drugs and Contrast Media. ACR manual on contrast media. 11th ed. 2018. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed 15 Nov 2018.
- 15.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4(4):287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data captuer (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakral C, Abraham JL. Gadolinium-induced nephrogenic systemic fibrosis is associated with insoluble Gd deposits in tissues: in vivo transmetallation confirmed by microanalysis. J Cutan Pathol. 2009;36(12):1244–1254. doi: 10.1111/j.1600-0560.2009.01283.x. [DOI] [PubMed] [Google Scholar]
- 19.Leung N, Pittelkow MR, Lee CU, Good JA, Hanley MM, Moyer TP. Chelation of gadolinium with deferoxamine in a patient with nephrogenic systemic fibrosis. NDT Plus. 2009;2(4):309–311. doi: 10.1093/ndtplus/sfp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maximova N, Gregori M, Zennaro F, Sonzogni A, Simeone R, Zanon D. Hepatic gadolinium deposition and reversibility after contrast agent-enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology. 2016;281(2):418–426. doi: 10.1148/radiol.2016152846. [DOI] [PubMed] [Google Scholar]