Abstract
Background
The autotaxin/lysophosphatidic acid axis is involved in diverse biological processes including neurodevelopment, inflammation, and immunological functioning. The lysophosphatidic acid 1 receptor has been implicated in the pathophysiology of major depressive disorder and in the mechanism of action of antidepressants. However, it is unclear whether central or peripheral autotaxin levels are altered in patients with major depressive disorder.
Methods
Serum autotaxin levels were measured by an enzyme-linked immunosorbent assay in 37 patients with major depressive disorder diagnosed using DSM-IV-TR who underwent electroconvulsive therapy and were compared with those of 47 nondepressed controls matched for age and sex between January 2011 and December 2015. Patient serum levels of autotaxin before and after electroconvulsive therapy were also compared. In a separate sample set, cerebrospinal fluid autotaxin levels were compared between 26 patients with major depressive disorder and 27 nondepressed controls between December 2010 and December 2015. A potential association was examined between autotaxin levels and clinical symptoms assessed with the Hamilton Depression Rating Scale.
Results
Before electroconvulsive therapy, both serum and cerebrospinal fluidautotaxin levels were significantly lower in major depressive disorder patients than in controls (serum: P = .001, cerebrospinal fluid: P = .038). A significantly negative correlation between serum, but not cerebrospinal fluid, autotaxin levels and depressive symptoms was observed (P = .032). After electroconvulsive therapy, a parallel increase in serum autotaxin levels and depressive symptoms improvement was observed (P = .005).
Conclusion
The current results suggest that serum autotaxin levels are reduced in a state-dependent manner. The reduction of cerebrospinal fluid autotaxin levels suggests a dysfunction in the autotaxin/lysophosphatidic acid axis in the brains of patients with major depressive disorder.
Keywords: autotaxin, lysophosphatidic acid, major depressive disorder, cerebrospinal fluid, electroconvulsive therapy
Significance Statement.
The autotaxin (ATX)/lysophosphatidic acid (LPA) axis is involved in diverse biological processes including neurodevelopment, inflammation, neurological, and immunological functioning. The LPA1 receptor has been implicated in the pathophysiology of depressive disorder and in the mechanism of action of antidepressants. The current study showed decreased levels of ATX, which produces LPA, the endogenous LPA1 receptor ligand, in both serum and cerebrospinal fluid (CSF) in patients with major depressive disorders (MDD) compared with ATX levels of nondepressed controls. Furthermore, serum levels of ATX increased following acute electroconvulsive therapy, which is a highly effective acute treatment for severe forms of depression. These findings indicate that a decline in ATX/LPA axis function, as reflected by reduced expression of ATX, could be associated with the pathophysiology of MDD and that serum and CSF ATX measurements could be used as biomarkers for evaluating the efficacy of treatments in some MDD patients.
Introduction
Autotaxin (ATX) is a secreted enzyme that produces the lipid mediator lysophosphatidic acid (LPA) from extracellular lysophosphatidyl choline(Umezu-Goto et al., 2002)—it is the primary enzyme responsible for LPA production(van Meeteren et al., 2006). Lysophosphatidic acid exerts its effect through at least 6 LPA G-protein-coupled receptors (LPAR)(Yung et al., 2015) and is involved in numerous biological processes, including neuroprogenitor cell function(Yung et al., 2011), myelination(Anliker et al., 2013), synaptic transmission(Trimbuch et al., 2009), and the brain’s immunologic response(Schilling et al., 2004), which, in turn, influences neural development, function, and behavior. Autotaxin is widely expressed in brain as well as a number of peripheral tissues(van Meeteren et al., 2006)—the expression of LPAR is tightly linked with ATX(Frugier et al., 2011).
LPA has been implicated in a number of neurophysiological functions and neuropathologies(Yung et al., 2015). For example, LPAR1 knock-out mice exhibit depressive-like behaviors, including hippocampal-dependent cognitive alteration (Santin et al., 2009), hypoactivity (Castilla-Ortega et al., 2013), and anhedonia (Moreno-Fernandez et al., 2017). Decreased neurogenesis within the hippocampus of these mice was also observed (Matas-Rico et al., 2008). Interestingly, antidepressants bind to LPAR1 expressed in brain astrocytes(Kajitani et al., 2016; Olianas et al., 2016), activation of which leads to expression of glial cell line-derived neurotropic factor (Kajitani et al., 2016), an important mediator of major depressive disorder (MDD)(Takebayashi et al., 2006; Uchida et al., 2011; Tsybko et al., 2017). These findings suggest a possible association between LPAR1 and the pathophysiology of MDD, which, in turn, could be targeted for the treatment of MDD. The linkage between the dysfunction of LPAR1 and that of the ATX/LPA axis has not been established, but the existence of such a linkage has been strongly suggested from preclinical findings. Specifically, the association between the ATX/LPA axis and MDD has yet to be clinically defined.
The current study examined whether ATX is altered in patients with MDD and if the alteration reflects the severity of depressive symptoms. While postmortem brain has been used for molecular research of psychiatric disorders, including MDD, CSF and blood from living patients are preferred in order to understand the actual relationship between bio-molecules of interest and ongoing symptoms. In human serum, ATX is highly stable and does not greatly vary among healthy subjects(Nakamura et al., 2008). By contrast, direct measurement of LPA is challenging due to its instability(Aoki et al., 2002). Nonetheless, blood levels of ATX and LPA are significantly correlated(Hosogaya et al., 2008). Thus, levels of ATX could directly reflect LPA levels and therefore serve as a proxy for ATX/LPA axis activity. The current study measured serum and CSF levels of ATX in MDD patients.
Findings from preclinical studies indicate that the pathophysiology of MDD is mediated in part by an abnormality of the ATX/LPA axis and that currently available therapeutic interventions appear to modulate ATX functioning(Matas-Rico et al., 2008; Santin et al., 2009; Castilla-Ortega et al., 2013; Olianas et al., 2016; Kajitani et al., 2016; Moreno-Fernandez et al., 2017). To determine if in fact modulation of ATX in MDD patients is associated with symptom attenuation, the current study measured ATX levels in patients who underwent a therapeutic course of ECT.
METHODS
Patients
Serum samples were collected at the Department of Psychiatry of the National Hospital Organization Kure Medical Center (NHOKMC), Hiroshima, Japan between January 2011 and December 2015 (sample Set 1). Patients diagnosed as having MDD were recruited among inpatients who were scheduled for ECT, based on the guidelines of the American Psychiatric Association (American Psychiatric Association, 2001), at NHOKMC (n = 37, Set 1; Table 1). Serum samples were collected just before the first ECT session (Pre-ECT; before any ECT session) and 2 weeks after the final ECT session (Post-ECT). Forty-seven subjects, with no history of past or current mental disorders, were recruited as nondepressed controls matched for age and gender. Most MDD patients at Pre-ECT received antidepressant pharmacotherapy: mirtazapine (15–45 mg/d; n = 16), duloxetine (20–60 mg/d; n = 9), mianserin (10–60 mg/d; n = 6), paroxetine (10–40 mg/d; n = 5), escitalopram (10–40 mg/d; n = 5), nortriptyline (100 mg/d; n = 5), trazodone (25–50 mg/d; n = 3), imipramine (50 mg/d; n = 2), sulpiride (50 mg/d; n = 2), sertraline (100 mg/d; n = 1), and amoxapine (100 mg/d; n = 1). Fifteen patients received a combination of 2 antidepressant drugs (paroxetine and mianserin, 3; paroxetine and mirtazapine, 1; duloxetine and mirtazapine, 4; sertraline and mirtazapine, 1; escitalopram and mirtazapine, 1; imipramine and trazodone, 1; imipramine and mirtazapine, 1; nortriptyline and mianserin, 1; nortriptyline and trazodone, 1; nortriptyline and mirtazapine, 1), and 3 received a combination of 3 antidepressant drugs (escitalopram, mirtazapine, and sulpiride, 2; duloxetine, mirtazapine, and mianserin, 1). The mean imipramine (IMI) dose equivalence according to a previous report(Inada and Inagaki, 2015) at Pre-ECT was 225.7±151.6 mg/d. Three patients did not receive any antidepressant while undergoing the course of ECT.
Table 1.
Patient Characteristics
| Serum Samples (Set 1) | CSF Samples (Set 2) | MDD Set1 vs Set2 | |||||
|---|---|---|---|---|---|---|---|
| MDD at Pre-ECT | Controls | P value | MDD | Controls | P value | ||
| (n = 37) | (n = 47) | (n = 26) | (n = 27) | P value | |||
| Gender (female) | 22 (59.5%) | 28 (59.6%) | 0.991a | 13 (50.0%) | 13 (48.2%) | 0.893a | .457c |
| Age (years) | 58.7 ± 13.2 | 58.9 ± 11.4 | 0.953b | 41.2 ± 7.3 | 41.9 ± 9.1 | 0.689b | <.001d |
| Age of onset (years) | 53.9 ± 14.2 | 30.2 ± 10.6 | <.001d | ||||
| Duration of current episode (months) | 5.7 ± 6.2 | ND | – | ||||
| Psychotic features | 15 (40.5%) | ND | – | ||||
| Catatonic features | 7 (18.9%) | ND | – | ||||
| Number of episodes | 1.5 ± 1.9 | 1.9 ± 1.2 | .168d | ||||
| Number of ECT sessions per course | 10.0 ± 3.5 | ND | – | ||||
| Total HDRS | 24.4 ± 8.3 | 11.8 ± 7.1 | <.001d | ||||
| IMI equivalence (mg/d) | 225.4 ± 151.6 | 207.8 ± 109.6 | .923d | ||||
Abbreviations: CSF, cerebrospinal fluid; ECT, electroconvulsive therapy; HDRS, Hamilton Depression Rating Scale-17; IMI, imipramine; MDD, major depressive disorder; ND, not detected.
Serum samples (Set 1) and CSF samples (Set 2) were collected from separate facilities. Data shown as either mean ± SD or number (n) and percent of total (%).
aComparison between MDD and control group by chi-square test.
bComparison between MDD and control group by Mann-Whitney U-test.
cComparison between MDD in Set 1 and Set 2 by chi-square test.
dComparison between MDD in Set 1 and Set 2 by Mann-Whitney U-test.
In Set 2 (Table1), 26 MDD patients and 27 nondepressed control subjects, matched for age and gender, were recruited through advertisements in a free local magazine and an announcement at the NCNP website, between December 2010 and December 2015. From these subjects, CSF was collected once.
Eight MDD patients received antidepressant pharmacotherapy: milnacipran (75–100 mg/d; n = 2), sertraline (50–100 mg/d; n = 2), paroxetine (20–40 mg/d; n = 2), sulpiride (100–300 mg/d; n = 2), duloxetine (60 mg/d; n = 1), escitalopram (10 mg/d; n = 1), mirtazapine (30 mg/d; n = 1), mianserin (30 mg/d; n = 1), or imipramine (150 mg/d; n = 1). Three patients received a combination of 2 antidepressant drugs (paroxetine and mirtazapine, 1; sertraline and sulpiride, 1; escitalopram and milnacipran, 1), and 1 received a combination of 3 antidepressant drugs (milnacipran, mianserin, and sulpiride). The mean IMI dose equivalence was 207.8 ± 109.6 mg/d. Eighteen patients did not receive any antidepressants.
In both sample sets, a consensus diagnosis was made by either trained psychologists or psychiatrists according to the DSM-IV-TR(American Psychiatric Association, 2000), on the basis of the Mini-International Neuropsychiatric Interview, Japanese version(Sheehan et al., 1998; Otsubo et al., 2005), additional unstructured interviews, and information from medical histories. Exclusion criteria included a past or current history of other psychiatric disorders such as bipolar disorder, schizoaffective disorder or adjustment disorder, significant neurological illness, or any other significant medical illness, including inflammatory diseases such as liver disease, fibrosis, cancer, kidney disease, arthritis, and Alzheimer’s disease, as these conditions could be due to changes in the ATX/LPA axis(Umemura et al., 2006; Benesch et al., 2016; Shimizu et al., 2016). In Set 1, a total of 6 psychiatric patients displayed co-morbidities, including: 2 with generalized anxiety disorder, 2 with somatization disorder, 1 with panic disorder, and 1 with obsessive-compulsive disorder. The severity of the comorbidities was mild. All patients in Set 2 had no comorbidities. As for Set 1, if a patient received 2 or more acute ECT courses during the study period, the serum sample was collected at only the first acute ECT course.
Nondepressed subjects were screened based on either a clinical interview or a structured interview by trained psychologists or psychiatrists using the Mini-International Neuropsychiatric Interview, Japanese version. The person who assessed them was not involved in the subsequent analysis of the data. With all participants, their past medical history, current illness, if any, and current medications were reviewed. Furthermore, hepatic enzyme, renal function, lipid, inflammatory indices, and nutritional status of nondepressed subjects in Set 1 were screened by a blood test. As for Set 2, although subjects in Set 2 were not screened by a blood test, most subjects were younger than those in Set 1. Thus, rather than subject the patients to an unnecessary medical test, we determined that an interview for their past medical history, current illness, and current medication, if any, was sufficient.
Clinical symptoms in MDD patients were scored using the 17-item Hamilton Depression Rating Score [HDRS]. The 17 items of the HDRS were assigned to the following 5 subscales: core symptom (items 1, 2, 7, 8, 10, 13); sleep (items 4, 5, 6); activity (items 7, 8); psychic anxiety (items 9, 10); and somatic anxiety (items 11, 12, 13) in accordance with a previous report(Seretti et al., 1999). For Set 1, each patient’s symptoms were assessed at Pre-ECT session (baseline) and at Post-ECT by the same trained psychiatrist. The psychiatrist was not aware that patient ATX levels would be measured in serum or CSF and had no role in patient selection. Responders to ECT were defined as demonstrating a 50% decrease in HDRS score. For Set 2, each patient’s symptoms were assessed by a trained psychologists or psychiatrists at the time of lumbar puncture. While there were 10 patients with moderate or higher depression state (HDRS ≥ 13), there were 10 patients with mild depression state (HDRS ≤ 13) and 6 patients with remission state (HDRS ≤ 7).
After procedures were fully explained, written informed consent was obtained from all subjects. The current study was approved by the Ethics Committee of NHOKMC (27-09) and the Ethics Committee of NCNP (A2014-141).
Electroconvulsive Therapy (ECT) Procedures
ECT was performed according to previously described procedures(Shibasaki et al., 2016; Itagaki et al., 2017). Before undergoing ECT, each patient was screened for general health through laboratory tests such as blood test and urinalysis, and physical examination to exclude significant medical illness or clinical symptoms. Anesthesia was induced with i.v. thiamylal sodium (2–3 mg/ kg) and suxamethonium chloride (0.5–1 mg/kg, i.v.). A Thymatron System IV brief pulse square wave apparatus (Somatics Inc., Lake Bluff, IL) was used to induce electroconvulsions. Bilateral frontal-temporal electrodes were used as all ECT patients presented as severe, life-threatening cases(American Psychiatric Association, 2001). Only one adequate seizure was required for each session, which was defined as an electroencephalographic seizure lasting more than 25 seconds with a high amplitude, slow wave, and postictal suppression. The initial stimulus dose was determined using the half-age method(Petrides and Fink, 1996). If an adequate electroencephalographic seizure occurred in one session, the same stimulus energy was used at the next session. The maximum number of stimulations for each treatment session was 2. Electroconvulsive therapy was administered a maximum of 3 times per week and continued until the patient became asymptomatic or the attending psychiatrist determined that the patient had obtained the maximum benefit within 3 to 15 sessions (mean: 10.0 ± 3.5; Table 1). After the purpose and the ECT procedure were described in detail, written informed consent was obtained from patients or caregivers of patients prior to initiating ECT. During the ECT course, medications were permitted and titrated as needed.
Collection of Serum and CSF Samples
After an overnight fast, venous blood (Set 1) was taken in the morning (between 7:00 and 8:00 am) before (Pre-ECT) and after (Post-ECT) ECT at NHOKMC. Blood samples were drawn into anticoagulant-free tubes and kept at room temperature for 1 hour. Serum was separated by centrifugation at 3000 rpm for 15 minutes at 4°C and stored at −80°C until assay.
Cerebrospinal fluid (Set 2) was obtained by lumbar puncture between 10:00 am and 4:00 pm. Following application of local anesthetic, CSF was obtained by lumbar puncture at either L3–4 or L4–5 using an atraumatic pencil point needle (Uniever 22G, 75 mm, Unisis Corp, Tokyo, Japan). Eight mL of CSF was collected and immediately chilled on ice. The CSF was centrifuged at 4000 g for 10 minutes at 4°C, and the supernatant was dispensed into 0.5-mL aliquots in tubes and stored at −80°C until assay. Selected CSF samples were thawed (in ice-cold water), dispensed, and refrozen. CSF samples contaminated with blood were excluded from further analysis. While more depressed patients could have been sampled for CSF, it is challenging to subject severely depressed patients to a spinal tap.
Detection of ATX by Enzyme-Linked Immunosorbent Assay
All serum and CSF samples were brought to room temperature before use in an enzyme-linked immunosorbent assay. Serum and CSF ATX levels were determined with a Quantikine human ATX immunoassay (R&D Systems, Minneapolis, MN) following the manufacturer’s procedure. Quantification was performed with a Multi-Spectrophotometer Viento Microplate Reader (Sumitomo Dainippon Pharma Co, Osaka, Japan). The person performing the ATX assays at NHOKMC was unaware of the treatment status of the patients from whom the samples were obtained.
Statistical Analysis
Data are shown as mean ± SD. Tests for normality were performed using the Shapiro-Wilk and the Kolmogorov-Smirnov tests, and data were analyzed with nonparametric tests (SPSS version 22.0 for Windows, IBM Japan Corporation, Tokyo, Japan). Comparisons of parameters between patients with MDD and nondepressed controls were evaluated using the Mann-Whitney U-test. A chi-square test was used for categorical variables. A linear regression analysis was performed to evaluate possible relationships between serum and CSF ATX levels and clinical parameters, diagnosis, and clinical symptoms, controlled for gender. Repeated-measures ANOVA was used to compare the parameters between Pre-ECT and Post-ECT, controlled for gender. Statistical significance was defined as a 2-tailed P < .05.
RESULTS
Clinical Data
Patient characteristics of MDD patients and nondepressed controls for Set 1 and Set 2 are presented in Table 1. Distribution by gender within and between Set 1 and Set 2 did not significantly differ. However, patients in Set 1 were significantly older, and their onset of illness was earlier compared with subjects in Set 2. Patients with MDD in Set 1 had significantly higher HDRS compared with MDD patients in Set 2. There was no statistically significant difference in IMI equivalence doses.
Relationship Between Serum and CSF Levels of ATX and Gender in All Samples
The mean serum ATX level for females was significantly higher than that of males (213.4 ± 51.7 vs 151.8 ± 32.8 ng/mL, P < .001). The mean CSF ATX level for females was also significantly higher than that of males (281.1 ± 33.5 vs 257.8 ± 26.8 ng/mL, P = .007). These results indicate a gender-based difference in ATX levels in both serum and CSF, which was confirmed by linear regression analysis (Table 2; serum: nonstandardized coefficient β = −61.6, P < .001; CSF: nonstandardized coefficient β = −23.6, P = .005). Because of the gender-based difference, further analyses of ATX levels were performed adjusting for gender.
Table 2.
Multivariate Linear Regression Analyses Between Serum and CSF ATX Levels, Diagnosis
| Serum ATX at Pre-ECT (Set 1) | CSF ATX (Set 2) | |||||
|---|---|---|---|---|---|---|
| Nonstandardized Coefficient β | (95% CI) | P value | Nonstandardized Coefficient β | (95% CI) | P value | |
| Constant | 227.034 | (212.535–241.534) | <.001* | 289.670 | (275.585–303.754) | <.001* |
| Diagnosis (control = 0/MDD = 1) | -30.998 | (-49.598 –-12.397) | .001* | -17.186 | (-33.350–-1.022) | .038* |
| Gender (female = 0/male = 1) | -61.606 | (-80.419–-42.793) | <.001* | -23.599 | (-39.763–7.435) | .005* |
| R2 = 0.398, P < .001 | R2 = 0.205, P = .003 | |||||
Abbreviations: CSF, cerebrospinal fluid; ATX, autotaxin; MDD, major depressive disorder. Serum samples (Set 1) and CSF samples (Set 2) were collected from separate facilities. The horizontal bars represent the mean values adjusted for gender. *P < .05.
Relationship Between Serum and CSF Levels of ATX and Other MDD Patient Characteristics
No significant correlations were observed between either serum or CSF ATX levels and clinical characteristics such as age, age of onset, duration of current episode, number of episodes, body weight, body mass index, smoking history, use of nonsteroidal antiinflammatory drugs, and IMI equivalence dose (Set 1 and 2; data not shown).
There was no significant association of serum ATX levels with hepatic enzyme, lipid, inflammatory indices, or nutritional status, such as GOT, GPT, γ-GTP, total bilirubin, total cholesterol, low-density lipoprotein, high-density lipoprotein, triglyceride, C-reactive protein, leukocyte count, total protein, or albumin in the MDD group (Set 1; data not shown).
Serum and CSF Levels of ATX in the MDD Group Compared With the Control Group
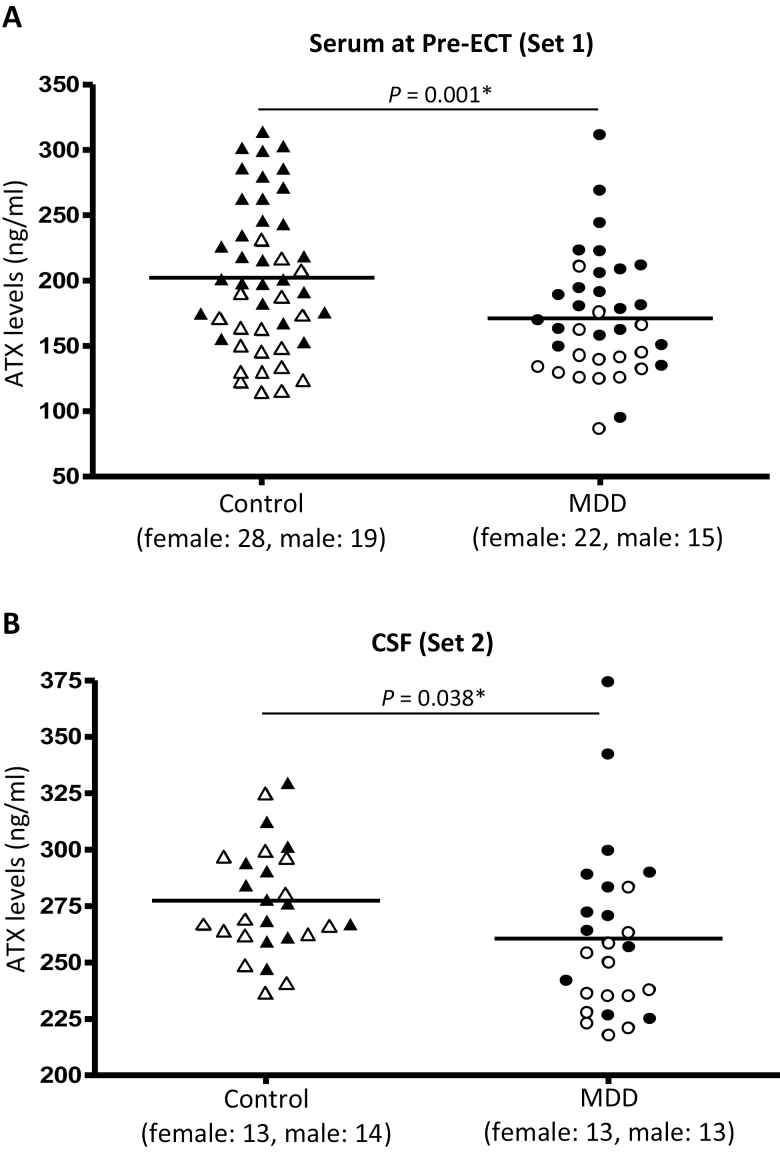
The mean serum levels of ATX in MDD patients at Pre-ECT and nondepressed controls in Set 1 were 171.1 ± 46.3 and 202.1 ± 56.3 ng/mL, respectively (Figure 1). The mean CSF levels of ATX in MDD patients and nondepressed controls in Set 2 were 260.7 ± 37.6 ng/mL and 277.4 ± 24.0 ng/mL, respectively (Figure 1). Serum ATX levels in patients with MDD were significantly lower than those of nondepressed controls as demonstrated by linear regression analysis adjusted for gender (Table 2; nonstandardized coefficient β = −30.998, P = .001). CSF ATX levels in MDD patients were also significantly lower than those of nondepressed controls (Table 2; nonstandardized coefficient β = −17.186, P = .038).
Figure 1.
Scatter plot of serum levels of autotaxin (ATX) (A) before a course of electroconvulsive therapy (ECT) and cerebrospinal fluid (CSF) levels of ATX (B) in control group (female: ▲; male: △) and major depressive disorder (MDD) group (female: ●; male: ○). Serum samples (Set 1) and CSF samples (Set 2) were collected from separate facilities. The horizontal bars represent the mean values adjusted for gender. *P < .05.
Correlation Between Serum and CSF Levels of ATX and Clinical Symptomatic Scores in MDD Patients
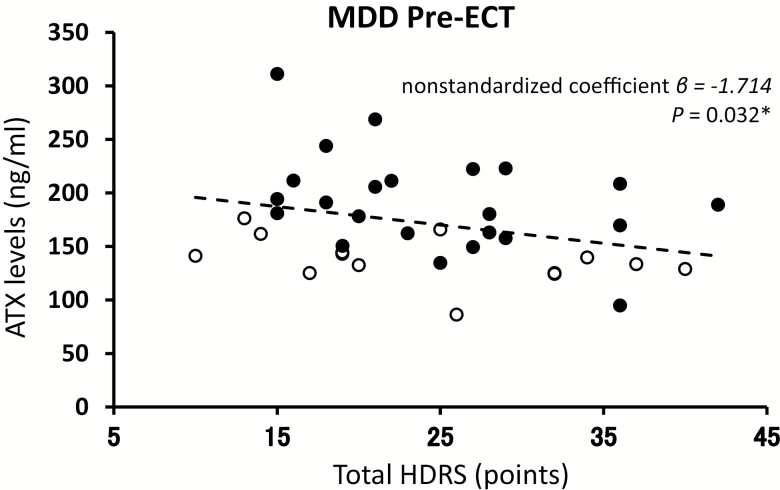
Correlation coefficients were calculated between serum and CSF levels of ATX and HDRS for each MDD group using linear regression analysis, adjusting for gender and age (Figure 2). In the serum samples from MDD patients (Set 1) before undergoing ECT, there was a significant negative correlation between serum levels of ATX and total HDRS score (Figure 2; nonstandardized coefficient β = −1.714, P = .032). Furthermore, a negative correlation between serum levels of ATX and subscale HDRS scores (activity) was observed (nonstandardized coefficient β = −9.491, P = .033). Higher serum levels of ATX were associated with a trend in lower subscale HDRS scores (core symptom, somatic anxiety, delusion, nonstandardized coefficient β = −3.722, −6.542, and −6.602; P = .064, .053, and .095, respectively). On the other hand, in Set 2, CSF levels of ATX did not correlate with total HDRS score (nonstandardized coefficient β = −1.341, P = .186) as well as subscale HDRS scores (data not shown).
Figure 2.
Correlation between serum levels of autotaxin (ATX) before a course of electroconvulsive therapy (ECT) and total Hamilton Depression Rating Scale-17 (HDRS) score in patients with major depressive disorder (MDD). The correlation coefficient was calculated by linear regression. Female values (●), n = 22; male values (○), n = 15. The dashed line represents the approximate correlation curve adjusted for gender. *P < .05.
Alterations of Serum Levels of ATX in the MDD Group Over the Course of ECT
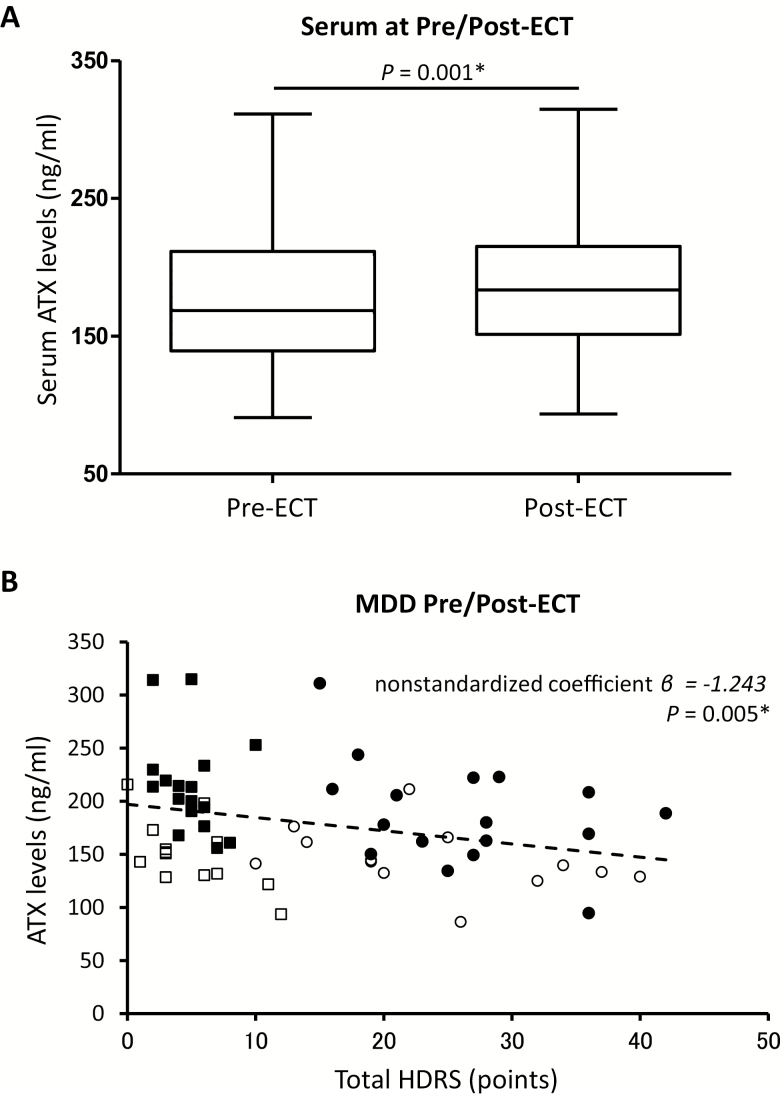
In Set 1, 30 of 37 serum samples from MDD patients (female: 17, male: 13) were collected after ECT. A total of 7 serum samples were eliminated from analysis: 2 patients withdrew their consent, 4 patients were temporarily discharged, and 1 had a fever. Among the 30 patients, HDRS scores for depressive symptoms were significantly decreased following ECT (Pre-ECT: 24.4 ± 8.3, Post-ECT: 5.0 ± 2.9; P < .001). Twenty-eight of 30 patients with MDD (93.3%) responded to ECT with a 50% or more decrease in HDRS score. There was no significant difference in the IMI equivalence dose between Pre-ECT and Post-ECT (Pre-ECT: 207.1 ± 167.1 mg/d and Post-ECT: 184.2 ± 97.1 mg/d, P = .409). Of the 37 patients that completed their course of ECT, serum samples from 30 patients were obtained. There were no statistically significant differences, including age, gender, HDRS score before and after ECT, and the number of ECT sessions received by each patient, between the 7 patients whose serum was not obtained and the 30 patients from whom serum was obtained (data not shown). Serum levels of ATX before ECT also did not differ between these 2 groups (data not shown).
The mean serum levels of ATX before and after ECT were 169.6 ± 46.3 and 186.9 ± 51.4 ng/mL, respectively. There was a statistically significant increase in serum levels of ATX in the MDD group over the course of ECT (Figure 3A; P = .001, repeated-measures ANOVA, adjusted for gender). Notably, 2 nonresponders to ECT showed slightly decreased rather than increased ATX levels after ECT. Correlation coefficients were calculated between serum levels of ATX and HDRS scores, combining results before and after ECT. There was a significant negative correlation between increased serum levels of ATX and decreased total HDRS score after ECT (Figure 3B; nonstandardized coefficient β = −1.243, P = .005).
Figure 3.
(A) Serum levels of autotaxin (ATX) before and after a course of electroconvulsive therapy (ECT) in the major depressive disorder (MDD) group, Set 1. (B) Correlation between serum levels of ATX and total Hamilton Depression Rating Scale-17 (HDRS) score at Pre/Post-ECT in the MDD group, Set 1. The correlation coefficient was calculated by linear regression. Pre-ECT female values (●), n = 17; male values (○), n = 13. Post-ECT female values (■), n = 17; male values (□), n = 13. The horizontal bars represent mean values. The dashed line represents the approximate correlation curve adjusted for gender. *P < .05.
Discussion
Both serum and CSF ATX were significantly reduced in MDD patients, and Pre-ECT ATX serum levels and depressive symptoms were negatively correlated. Furthermore, serum levels of ATX increased following ECT. In the current study, antidepressants did not appear to directly affect ATX levels as no correlations were observed between ATX levels and IMI dose equivalence (serum: nonstandardized coefficient β = 0.065, P = .154, CSF: nonstandardized coefficient β = −0.140, P = .159) and between IMI dose equivalence before and after ECT. Also, in rat brain, chronic antidepressant treatment did not affect either mRNA or protein expression of ATX (supplementary Figure 1). The current findings indicate that alterations of ATX, which produces LPA, in part, could underlie the pathophysiology and symptomology of MDD. However, there was no significant correlation in ATX CSF levels with severity of depression (HDRS), and the reason for this might be due to the limited number of patients and the inclusion of patients with either mild depression or patients who were in remission.
Autotaxin is abundantly expressed in brain glial cells, which play an important role in the pathophysiology of depression. Autotaxin mRNA is widely expressed in adult tissue, particularly in the brain(Fuss et al., 1997). In the adult brain, ATX is restricted to early and differentiating oligodendrocyte lineage cells and reactive astrocytes and choroid plexus epithelial and leptomeningeal cells(Savaskan et al., 2007). Brain glial cells such as oligodendrocyte and astrocytes are suggested as important sources of ATX(Savaskan et al., 2007; Zhang et al., 2014). Brain imaging and postmortem evaluation of the brain from MDD patients showed robust glial abnormalities such as decreased oligodendrocyte and astrocyte density and reduced expression of genes related to oligodendrocyte and astrocyte function(Tham et al., 2011; Wang et al., 2017). In addition, preclinical animal models of depression exposed to chronic stress showed morphological and numerical decreases in cortical, hippocampal, and amygdalar oligodendrocytes(Banasr et al., 2007; Czeh et al., 2007) and astrocytes(Banasr et al., 2010; Gong et al., 2012; Rajkowska and Stockmeier, 2013; Sanacora and Banasr, 2013), which support findings from brains of MDD patients(Rajkowska and Stockmeier, 2013; Rajkowska et al., 2015). These results suggest a potential linkage between MDD and a reduction of glia, which is a key source of ATX and could explain the reason for the decreased levels of ATX in MDD patients. By contrast, electroconvulsive seizure treatment in rats increased the number of newly divided cells expressing oligodendrocyte markers in the frontal cortex(Madsen et al., 2005). Electroconvulsive seizure also significantly increased the expression of glial fibullary acidic protein, a marker of reactive astrocytes, in the rat frontal limbic system(Kragh et al., 1993). These preclinical findings in combination with the current clinical findings suggest that amelioration of MDD by ECT could be mediated by an induction of the proliferation of oligodendrocyte and reactive astrocytes, thereby increasing ATX levels.
Another possibility is that chronic stress could be involved in the regulation of ATX expression. MDD is characterized by abnormal physiological responses to chronic stress. One likely source of serum ATX is adipose tissue(Ferry et al., 2003). Hypercortisolemia has been attributed to hypothalamic–pituitary–adrenal (HPA) axis dysregulation, a consistent biological response to chronic stress in MDD(Kunugi et al., 2006). Chronic treatment with prednisolone, which induces hypercortisolemia and changes in the HPA axis, decreases serum levels of ATX in humans by changing adipose tissue ATX expression(Sumida et al., 2013). This finding suggests reduced expression of ATX levels in MDD could be mediated through and a dysfunctional HPA axis. It is possible that a dysfunctional HPA axis could have mediated the decreased ATX observed in MDD patients in the current study, but measurement of serum or CSF cortisol will be needed to show a relationship between these parameters and alteration of ATX levels in MDD patients.
To place the current findings into perspective, there are a few limitations that should be mentioned. While the total sample size of the current study was not very large, significant correlations were nonetheless observed. It is possible that some of the changes in serum ATX levels following ECT could be due in part to a placebo response, but there were only 2 patients who did not respond to ECT, so this possibility could not be tested in the current study. While withholding treatment in the current study would have been unethical, examination of more patients in which ECT failed to alleviate symptoms would determine the extent, if any, of a placebo effect on ATX expression. Secondly, ATX, but not LPA, was measured. Measurement of LPA levels and its species requires the use of an enzymatic cycling assay and liquid chromatography/mass spectrometry, respectively(Kurano et al., 2015). Furthermore, the currently obtained samples, both serum and CSF, may not be used to measure LPA since sampling conditions were not under stringent conditions needed to ensure LPA stability(Hosogaya et al., 2008). Although there is a strong association between peripherally expressed ATX and LPA in humans(Watanabe et al., 2007; Masuda et al., 2008), the correlation between ATX and LPA levels in MDD patients is unclear. The correlation between peripheral blood and CSF ATX levels is not clear, though there could be a linkage between CSF and peripheral ATX levels as the current study showed decreased levels of ATX in both serum and CSF. An exact correlation may be difficult to obtain as there are technical difficulties with measuring LPA levels and there are a number of LPA species(Kurano et al., 2015). Further confirming the suitability of ATX levels as a surrogate to LPA activity could also be performed in preclinical models of depression by demonstrating a relationship between ATX and LPA levels in the periphery and in the CSF from the same MDD patient. Finally, only one factor in a biological cascade was measured without exploring a possible relationship between ATX levels and inflammation/immunological indices, such as cytokines. If inflammation, via the ATX/LPA axis mediates MDD, then future studies will need to demonstrate a relationship between ATX levels and inflammation/immunological indices, perhaps by utilizing fluids from the same patient.
The current study confirmed previous findings that the serum concentration of ATX in women is significantly higher than that of men(Nakamura et al., 2008). Since ATX is essential for stabilization of blood vessels and angiogenesis(Tanaka et al., 2006; van Meeteren et al., 2006) and is produced in adipocytes, higher levels in females could reflect basic biological differences or the higher body fat ratio found in women compared with men(Blaak, 2001). On the other hand, no significant difference was observed in CSF ATX concentrations between nondepressed males and females using a 2-site immunoenzymetric assay(Nakamura et al., 2009). In the current study, not only serum but also CSF ATX levels in women were significantly higher than those in men. This discrepancy could be due to a limited number of patients (n = 53) and a difference in the quantification method used in the current study compared with previous studies. There was no correlation between age and either serum or CSF ATX levels in females (serum: nonstandardized coefficient β = −0.182, P = .757; CSF: nonstandardized coefficient β = −0.629, P = .926), and between postmenopausal and serum or CSF ATX levels in females (serum: nonstandardized coefficient β = 6.226, P = .737; CSF: nonstandardized coefficient β = 9.712, P = .613), suggesting that there is no correlation between ATX levels and menopausal status. However, a future study should address potential relationships between menstrual and menopausal status and ATX levels.
In conclusion, the current study found, for the first time, that both serum and CSF levels of ATX were significantly decreased in MDD patients compared with nondepressed controls matched for age and gender. Also, there were significant correlations between depressive symptoms and serum levels of ATX. A course of ECT in MDD patients significantly increased serum levels of ATX. The findings suggest that alteration of ATX levels indicate changes in levels of lysophospholipids such as LPA, which occurs as a cellular response to MDD and could reflect the severity of depressive symptoms, which are, in turn, sensitive to ECT. Thus, the current findings lend support to the possibility that a decline in ATX, leading to decreased LPA, could be associated with the pathophysiology of MDD, and serum and CSF ATX measurement could be used as a biomarker for evaluating the efficacy of treatments in some MDD patients. However, in the current study, LPA levels were not directly quantified, given current methodological limitations. Future studies, utilizing readily accessible quantification methods, should be able to demonstrate a direct relationship between levels of ATX and LPA in MDD patients.
Funding
This work was supported by a Grants-in-Aid from the Japan Society for the Promotion of Science (15K09819 to M.T., 16K19796 to N.K., 16K18883 to M.O.-T., 15H02552 to Shigeto Y.); an Intramural Research Grant for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry (24-11, 27-1 to H.K.); Grant-in-Aid for Scientific Research (A) from the Ministry of Education, Science, Sports and Culture (25253075 to H.K.), and Health and Labour Sciences Research Grants for Comprehensive Research on Persons with Disabilities from Japan Agency for Medical Research and Development (AMED) (15dk0310061h0001, 16dk0307062h0001 to H.K.); Integrated Research on Depression, Dementia and Development Disorders from AMED (18dm0107093h0003 to Shigeto Y.).
Supplementary Material
Acknowledgments
We are grateful to our colleagues for their tireless and expert assistance: Dr Hideo Kobayakawa, Dr Keigo Nakatsu, Dr Takashi Iwamoto, and Dr Motonobu Nakamura. We also thank Dr Yasutaka Fujita for establishing the patients’ database, Professor Junko Tanaka and Tomoyuki Akita for advice on statistical analysis, and Dr Aldric Hama for editorial assistance.
Interest Statement
None.
References
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR, 4th ed. Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association (2001) The practice of electroconvulsive therapy: recommendation for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd ed. Washington, DC: American Psychiatric Press. [Google Scholar]
- Anliker B, Choi JW, Lin ME, Gardell SE, Rivera RR, Kennedy G, Chun J (2013) Lysophosphatidic acid (LPA) and its receptor, LPA1, influence embryonic schwann cell migration, myelination, and cell-to-axon segregation. Glia 61:2009–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H (2002) Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 277:48737–48744. [DOI] [PubMed] [Google Scholar]
- Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS (2007) Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry 62:496–504. [DOI] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G (2010) Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch MG, Tang X, Venkatraman G, Bekele RT, Brindley DN (2016) Recent advances in targeting the autotaxin-lysophosphatidate-lipid phosphate phosphatase axis in vivo. J Biomed Res 30:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaak E. (2001) Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 4:499–502. [DOI] [PubMed] [Google Scholar]
- Castilla-Ortega E, Rosell-Valle C, Blanco E, Pedraza C, Chun J, Rodríguez de Fonseca F, Estivill-Torrús G, Santín LJ (2013) Reduced wheel running and blunted effects of voluntary exercise in LPA1-null mice: the importance of assessing the amount of running in transgenic mice studies. Neurosci Res 77:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B, Müller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E (2007) Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology 32:1490–1503. [DOI] [PubMed] [Google Scholar]
- Ferry G, Tellier E, Try A, Grés S, Naime I, Simon MF, Rodriguez M, Boucher J, Tack I, Gesta S, Chomarat P, Dieu M, Raes M, Galizzi JP, Valet P, Boutin JA, Saulnier-Blache JS (2003) Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem 278:18162–18169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugier T, Crombie D, Conquest A, Tjhong F, Taylor C, Kulkarni T, McLean C, Pébay A (2011) Modulation of LPA receptor expression in the human brain following neurotrauma. Cell Mol Neurobiol 31:569–577. [DOI] [PubMed] [Google Scholar]
- Fuss B, Baba H, Phan T, Tuohy VK, Macklin WB (1997) Phosphodiesterase I, a novel adhesion molecule and/or cytokine involved in oligodendrocyte function. J Neurosci 17:9095–9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Sun XL, Wu FF, Su CJ, Ding JH, Hu G (2012) Female early adult depression results in detrimental impacts on the behavioral performance and brain development in offspring. CNS Neurosci Ther 18:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogaya S, Yatomi Y, Nakamura K, Ohkawa R, Okubo S, Yokota H, Ohta M, Yamazaki H, Koike T, Ozaki Y (2008) Measurement of plasma lysophosphatidic acid concentration in healthy subjects: strong correlation with lysophospholipase D activity. Ann Clin Biochem 45:364–368. [DOI] [PubMed] [Google Scholar]
- Inada T, Inagaki A (2015) Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci 69:440–447. [DOI] [PubMed] [Google Scholar]
- Itagaki K, Takebayashi M, Shibasaki C, Kajitani N, Abe H, Okada-Tsuchioka M, Yamawaki S (2017) Factors associated with relapse after a response to electroconvulsive therapy in unipolar versus bipolar depression. J Affect Disord 208:113–119. [DOI] [PubMed] [Google Scholar]
- Kajitani N, Miyano K, Okada-Tsuchioka M, Abe H, Itagaki K, Hisaoka-Nakashima K, Morioka N, Uezono Y, Takebayashi M (2016) Identification of lysophosphatidic acid receptor 1 in astroglial cells as a target for glial cell line-derived neurotrophic factor expression induced by antidepressants. J Biol Chem 291:27364–27370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh J, Bolwig TG, Woldbye DP, Jørgensen OS (1993) Electroconvulsive shock and lidocaine-induced seizures in the rat activate astrocytes as measured by glial fibrillary acidic protein. Biol Psychiatry 33:794–800. [DOI] [PubMed] [Google Scholar]
- Kunugi H, et al. (2006) Assessment of the dexamethasone/CRH test as a state-dependent marker for hypothalamic-pituitary-adrenal (HPA) axis abnormalities in major depressive episode: a multicenter study. Neuropsychopharmacology 31:212–220. [DOI] [PubMed] [Google Scholar]
- Kurano M, Suzuki A, Inoue A, Tokuhara Y, Kano K, Matsumoto H, Igarashi K, Ohkawa R, Nakamura K, Dohi T, Miyauchi K, Daida H, Tsukamoto K, Ikeda H, Aoki J, Yatomi Y (2015) Possible involvement of minor lysophospholipids in the increase in plasma lysophosphatidic acid in acute coronary syndrome. Arterioscler Thromb Vasc Biol 35:463–470. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Yeh DD, Valentine GW, Duman RS (2005) Electroconvulsive seizure treatment increases cell proliferation in rat frontal cortex. Neuropsychopharmacology 30:27–34. [DOI] [PubMed] [Google Scholar]
- Masuda A, Nakamura K, Izutsu K, Igarashi K, Ohkawa R, Jona M, Higashi K, Yokota H, Okudaira S, Kishimoto T, Watanabe T, Koike Y, Ikeda H, Kozai Y, Kurokawa M, Aoki J, Yatomi Y (2008) Serum autotaxin measurement in haematological malignancies: a promising marker for follicular lymphoma. Br J Haematol 143:60–70. [DOI] [PubMed] [Google Scholar]
- Matas-Rico E, García-Diaz B, Llebrez-Zayas P, López-Barroso D, Santín L, Pedraza C, Smith-Fernández A, Fernández-Llebrez P, Tellez T, Redondo M, Chun J, De Fonseca FR, Estivill-Torrús G (2008) Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol Cell Neurosci 39:342–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Fernández RD, Pérez-Martín M, Castilla-Ortega E, Rosell Del Valle C, García-Fernández MI, Chun J, Estivill-Torrús G, Rodríguez de Fonseca F, Santín LJ, Pedraza C (2017) maLPA1-null mice as an endophenotype of anxious depression. Transl Psychiatry 7:e1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Igarashi K, Ide K, Ohkawa R, Okubo S, Yokota H, Masuda A, Oshima N, Takeuchi T, Nangaku M, Okudaira S, Arai H, Ikeda H, Aoki J, Yatomi Y (2008) Validation of an autotaxin enzyme immunoassay in human serum samples and its application to hypoalbuminemia differentiation. Clin Chim Acta 388:51–58. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ohkawa R, Okubo S, Yokota H, Ikeda H, Yatomi Y, Igarashi K, Ide K, Kishimoto T, Masuda A, Yamamoto T, Tsuji S, Saito N, Kurokawa M, Okudaira S, Aoki J (2009) Autotaxin enzyme immunoassay in human cerebrospinal fluid samples. Clin Chim Acta 405:160–162. [DOI] [PubMed] [Google Scholar]
- Olianas MC, Dedoni S, Onali P (2016) LPA1 mediates antidepressant-induced ERK1/2 signaling and protection from oxidative stress in glial cells. J Pharmacol Exp Ther 359:340–353. [DOI] [PubMed] [Google Scholar]
- Otsubo T, Tanaka K, Koda R, Shinoda J, Sano N, Tanaka S, Aoyama H, Mimura M, Kamijima K (2005) Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci 59:517–526. [DOI] [PubMed] [Google Scholar]
- Petrides G, Fink M (1996) The “half-age” stimulation strategy for ECT dosing. Convuls Ther 12:138–146. [PubMed] [Google Scholar]
- Rajkowska G, Mahajan G, Maciag D, Sathyanesan M, Iyo AH, Moulana M, Kyle PB, Woolverton WL, Miguel-Hidalgo JJ, Stockmeier CA, Newton SS (2015) Oligodendrocyte morphometry and expression of myelin-related mRNA in ventral prefrontal white matter in major depressive disorder. J Psychiatr Res 65:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Stockmeier CA (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14:1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Banasr M (2013) From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry 73:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santin LJ, Bilbao A, Pedraza C, Matas-Rico E, López-Barroso D, Castilla-Ortega E, Sánchez-López J, Riquelme R, Varela-Nieto I, de la Villa P, Suardíaz M, Chun J, De Fonseca FR, Estivill-Torrús G (2009) Behavioral phenotype of malpa1-null mice: increased anxiety-like behavior and spatial memory deficits. Genes Brain Behav 8:772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan NE, Rocha L, Kotter MR, Baer A, Lubec G, van Meeteren LA, Kishi Y, Aoki J, Moolenaar WH, Nitsch R, Bräuer AU (2007) Autotaxin (NPP-2) in the brain: cell type-specific expression and regulation during development and after neurotrauma. Cell Mol Life Sci 64:230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T, Stock C, Schwab A, Eder C (2004) Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur J Neurosci 19:1469–1474. [DOI] [PubMed] [Google Scholar]
- Seretti A, Cusin C, Lattuada E, Di Bella D, Catalano M, Smeraldi E (1999) Serotonin transporter gene (5-HTTLPR) is not associated with depressive symptomatology in mood disorders. Mol Psychiatry 4:280–283. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33;quiz 34. [PubMed] [Google Scholar]
- Shibasaki C,, Takebayashi M,, Itagaki K,, Abe H,, Kajitani N,, Okada-Tsuchioka M,, Yamawaki S. (2016) Altered serum levels of matrix metalloproteinase-2, -9 in response to electroconvulsive therapy for mood disorders. Int J Neuropsychopharmacol 19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Furuichi K, Toyama T, Yamahana J, Ohkawa R, Igarashi K, Aoki J, Kaneko S, Yatomi Y, Wada T (2016) Serum autotaxin levels are associated with proteinuria and kidney lesions in japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy. Intern Med 55:215–221. [DOI] [PubMed] [Google Scholar]
- Sumida H, Nakamura K, Yanagida K, Ohkawa R, Asano Y, Kadono T, Tamaki K, Igarashi K, Aoki J, Sato S, Ishii S, Shimizu T, Yatomi Y (2013) Decrease in circulating autotaxin by oral administration of prednisolone. Clin Chim Acta 415:74–80. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hisaoka K, Nishida A, Tsuchioka M, Miyoshi I, Kozuru T, Hikasa S, Okamoto Y, Shinno H, Morinobu S, Yamawaki S (2006) Decreased levels of whole blood glial cell line-derived neurotrophic factor (GDNF) in remitted patients with mood disorders. Int J Neuropsychopharmacol 9:607–612. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H (2006) Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 281:25822–25830. [DOI] [PubMed] [Google Scholar]
- Tham MW, Woon PS, Sum MY, Lee TS, Sim K (2011) White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J Affect Disord 132:26–36. [DOI] [PubMed] [Google Scholar]
- Trimbuch T, et al. (2009) Synaptic PRG-1 modulates excitatory transmission via lipid phosphate-mediated signaling. Cell 138:1222–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsybko AS, Ilchibaeva TV, Popova NK (2017) Role of glial cell line-derived neurotrophic factor in the pathogenesis and treatment of mood disorders. Rev Neurosci 28:219–233. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, Suzuki T, Miyata N, Watanabe Y (2011) Epigenetic status of gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron 69:359–372. [DOI] [PubMed] [Google Scholar]
- Umemura K, Yamashita N, Yu X, Arima K, Asada T, Makifuchi T, Murayama S, Saito Y, Kanamaru K, Goto Y, Kohsaka S, Kanazawa I, Kimura H (2006) Autotaxin expression is enhanced in frontal cortex of alzheimer-type dementia patients. Neurosci Lett 400:97–100. [DOI] [PubMed] [Google Scholar]
- Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H (2002) Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 158:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradère JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J (2006) Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol 26:5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Jie W, Liu JH, Yang JM, Gao TM (2017) An astroglial basis of major depressive disorder? An overview. Glia 65:1227–1250. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J, Hama K, Okudaira S, Tanaka M, Tomiya T, Yanase M, Tejima K, Nishikawa T, Arai M, Arai H, Omata M, Fujiwara K, Yatomi Y (2007) Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J Clin Gastroenterol 41:616–623. [DOI] [PubMed] [Google Scholar]
- Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW, Kingsbury MA, Chun J (2011) Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med 3:99ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YC, Stoddard NC, Mirendil H, Chun J (2015) Lysophosphatidic acid signaling in the nervous system. Neuron 85:669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.