Abstract
Oesophageal atresia with or without tracheo-oesophageal fistula, ileal atresia and Hirschsprung’s disease are surgical malformations of the gastrointestinal tract typically diagnosed early in the neonatal period and varying in severity and prognosis. This report describes a full-term male newborn presenting simultaneous oesophageal atresia with distal tracheo-oesophageal fistula, ileal atresia and Hirschsprung’s disease. In addition to the complex types of gastrointestinal malformations involved, the combination of ileal atresia and Hirschsprung’s disease, as well as ganglion cells distal to intestinal atresia, resulted in a challenging diagnosis. Despite a successful outcome, the patient presented increased morbidity and prolonged hospitalisation. We highlight some important findings that may aid the early diagnosis of Hirschsprung’s disease in this clinical setting. To our knowledge, the association of oesophageal atresia/tracheo-oesophageal fistula, ileal atresia and Hirschsprung’s disease has not been previously reported.
Keywords: congenital disorders, neonatal and paediatric intensive care, paediatric surgery
Background
Oesophageal atresia with or without tracheo-oesophageal fistula, ileal atresia and Hirschsprung’s disease are three independent congenital surgical abnormalities of the gastrointestinal tract that are typically diagnosed early in the neonatal period and vary in severity and prognosis.
Oesophageal atresia/tracheo-oesophageal fistula is present in 1/2500–1/4500 live births,1 ileal atresia in 1/5000 live births2 and Hirschsprung’s disease in 1/5000 live births,3 whereas the combination of these disorders is much less frequent.
At this time there are approximately 25 cases of ileal atresia associated with Hirschsprung’s disease4–9 and one case of an oesophageal abnormality associated with Hirschsprung’s disease10 reported in the literature. However, as far as we know, there are no reports of oesophageal anomalies associated with Hirschsprung’s disease and/or ileal atresia.
A correct and timely diagnosis of these associations is essential to minimise potential morbidity and even mortality.
In a clinical phenotype combining tracheo-oesophageal abnormalities and neonatal intestinal obstruction, the former malformation is usually diagnosed by increased oral secretions and inability to pass an orogastric tube after birth with confirmatory X-ray demonstrating tube coiling11 preoperatively, whereas for the latter condition one must consider several differential diagnoses.
Common causes of intestinal obstruction in a newborn, with an incidence of 1/2000 live births, include intestinal atresia, Hirschsprung’s disease, meconium ileus and malrotation.12 In addition to plain films of the abdomen, most infants require a contrast enema to evaluate the aetiology and extent of bowel obstruction before surgery. Distended small bowel loops and microcolon are non-specific findings observed in radiographs of several causes of neonatal intestinal obstruction, including both intestinal atresia and total colonic aganglionosis.9
After intestinal anastomosis to repair ileal atresia, the overlapping signs and symptoms of anastomotic dysfunction with those of associated Hirschsprung’s disease may pose a diagnostic challenge. Diagnosis of Hirschsprung’s disease is established by rectal biopsy and requires histopathological demonstration of absence of ganglion cells and often presence of hypertrophic nerves trunks in the distal rectum.3
These patients are candidates for corrective surgeries. However, the association of gastrointestinal malformations carries an increased risk for postoperative morbidity6 and even mortality of the child, especially in cases of an unidentified phenotype combined with atypical findings.
Developing a better understanding of the processes linking these uncommon clinical associations may offer insight into their early detection and clinical management.
This paper presents the case of an infant with oesophageal atresia/tracheo-oesophageal fistula concurrent with ileal atresia and Hirschsprung’s disease who also had ganglion cells in the small bowel distal to ileal atresia. Here, we discuss the diagnostic challenges presented by these atypical findings along with the impact on the outcome. We also highlight possible signs for early diagnosis and subsequent correct management.
Case presentation
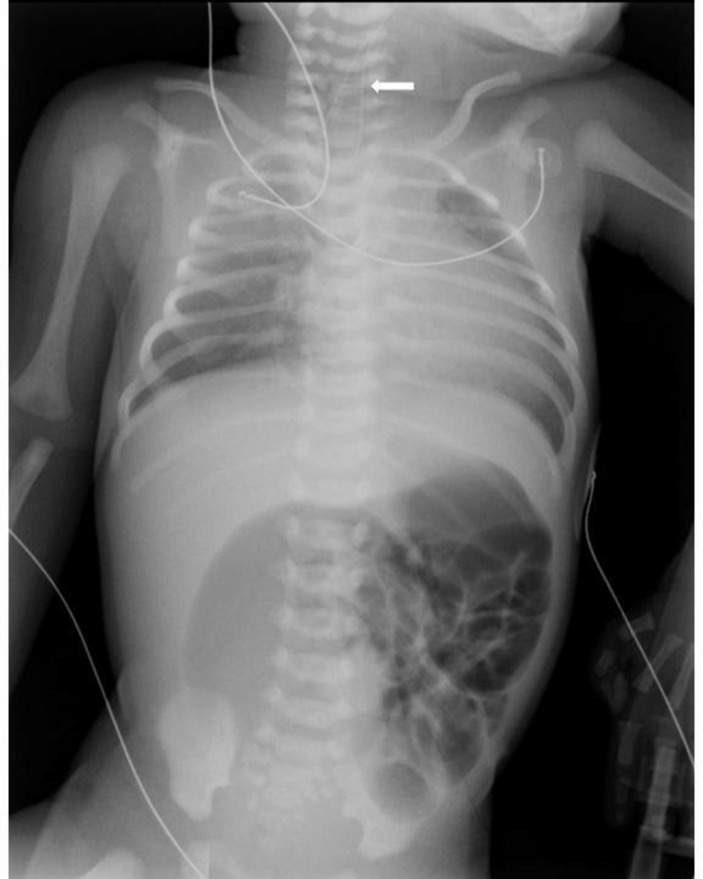
A full-term, 2710 g male infant was born at 39 weeks through spontaneous vaginal delivery. Routine antenatal ultrasounds found intrauterine growth restriction, left kidney agenesis, persistent left superior vena cava and suspicion of intestinal atresia. There was a history of polyhydramnios. Parents were not consanguineous and there was no relevant family history of congenital malformations on either parent’s side. At birth, Apgar scores were 5, 10 and 10 at 1, 5 and 10 min, respectively. Physical examination showed increased oral secretions, difficult breathing requiring supplemental oxygen through face-mask ventilation and abdominal distension. An orogastric tube could not be passed into the stomach, and a chest and abdominal X-ray diagnosed oesophageal atresia with distal tracheo-oesophageal fistula and intestinal obstruction (figure 1), later confirmed during surgery. No other dysmorphic findings were present. Antibiotics were then started and a replogle tube was placed into the upper oesophageal pouch for continuous suctioning of the saliva. Vertebral defects, Anal atresia, Cardiac anomalies, Tacheo-Esophageal fistula, Renal anomalies, and Limb anomalies (VACTERL) workup included renal ultrasound, which confirmed renal agenesis, and echocardiography, thus revealing interventricular communication and patent ductus arteriosus.
Figure 1.
One-day-old male infant with a triad of oesophageal atresia with distal tracheo-oesophageal fistula, ileal atresia and Hirschsprung’s disease. The thoracoabdominal radiograph shows an orogastric tube in the midoesophagus (arrow) and air-filled distended loops of bowel, with no distal gas.
The patient underwent exploratory laparotomy, performed on the first day of life. Perioperative bronchoscopy confirmed a distal tracheo-oesophageal fistula. Laparotomy revealed gastric distension and a type IIIa ileal atresia with an intestinal perforation of the proximal ileal pouch and a microcolon. A gastrostomy and a diverting ileostomy were created. Due to clinical instability, the abdominal oesophagus was encircled by a silicone rubber 6F tube placed around the gastro-oesophageal junction simulating a Pringle manoeuvre. Additionally, both ends of the tube were brought through the abdominal incision to provide extracorporeal occlusion of the abdominal oesophagus, thus preventing aspiration and overdistension of the stomach due to the tracheo-oesophageal fistula. Eight days later, the patient underwent thoracoscopy and tracheo-oesophageal fistula closure, without primary oesophageal anastomosis due to a long-gap oesophageal atresia diagnosis.
Postoperatively, the patient was kept on enteral feedings by gastrostomy and suctioning of the upper oesophageal pouch.
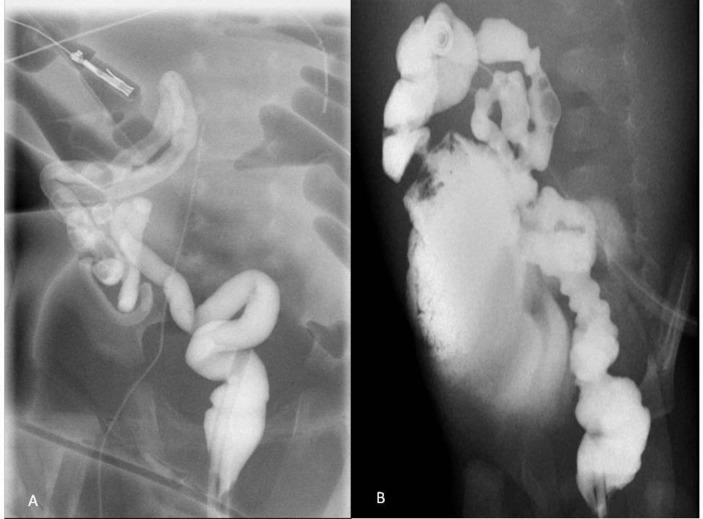
A water-soluble contrast enema was performed on the 27th day of life to determine colon anatomy and revealed a non-fixed coiled microcolon in the right abdominal quadrants (figure 2A). A daily saline rectal enema was then initiated to enlarge the colon. Subsequently, an ileocaecal anastomosis was performed at 7 weeks of life; a microcolon was still present.
Figure 2.
Repeated contrast enemas demonstrated a foreshortened non-fixed colon coiled in the right abdominal quadrants. (A) At 27 days of age and (B) at 4 months of age, with contrast progression through the anastomosis.
Meanwhile, histopathology revealed normal ganglion cells at both ends of the resected ileal stomas and of the appendix specimen.
Over the next couple of months, the patient remained obstructed after enterostomy reversal, presenting signs of gastrointestinal dysfunction which included severe constipation. During this period when the infant was at 4 months of age, repeated gastrointestinal contrast studies showed a persistent microcolon raising the suspicion of Hirschsprung’s disease (figure 2B). One month later, rectal suction biopsies, performed at the time of a right thoracotomy for oesophageal atresia repair, confirmed Hirschsprung’s disease showing the absence of ganglion cells.
Regarding oesophageal malformation repair, a primary oesophageal anastomosis was performed when the patient was at 5 months of age and included a thoracic tube. A leak occurred on the sixth postoperative day but healed completely on the next few days and could not be found in oesophageal contrast study on the 20th postoperative day. The thoracic tube was then removed, and oral feeding was started.
At 7 months of age the patient was taken back to the operation room for elective repair of Hirschsprung’s disease. During this surgery, multiple frozen biopsies of the gastrointestinal tract revealed total colonic aganglionosis and, as a result, the patient underwent total colectomy with direct ileoanal anastomosis.
Over the next 4 postoperative months, in addition to enteral feeds, parenteral nutrition support was required due to persistent diarrhoea and poor weight gain.
Outcome and follow-up
The patient was transferred to a district general hospital for maintenance care at 13 months of age without parenteral nutrition. Subsequently, he was discharged home after 1 month’s inpatient stay with normal bowel function and receiving full diet orally.
The gastrostomy tube was removed and surgical suture of persistent gastrocutaneous fistula was undertaken at 3 years of age uneventfully.
At the present, the patient is at 3 years of age and is doing very well with no concerns.
Discussion
We present a rare association of gastrointestinal malformations, namely oesophageal atresia/tracheo-oesophageal fistula, ileal atresia and Hirschsprung’s disease in an infant who had increased postoperative morbidity and a prolonged in-hospital stay. Despite this, the patient was successfully managed.
Our case was particularly challenging due to (a) unusual combination of gastrointestinal malformations; (b) coexistence in the combination of complex types of disease; (c) association of ileal atresia and Hirschsprung’s disease and (d) ganglion cells distal to ileal atresia.
In addition to the rarity of this combination, our infant presented with complex types of gastrointestinal malformations, including long-gap oesophageal atresia and VACTERL association11 (oesophageal atresia with tracheo-oesophageal fistula, renal agenesis and congenital heart abnormality), perforated ileal atresia with a mesenteric gap and long-segment aganglionosis. Adding extra complexity, our patient also had ganglion cells distal to the intestinal atresia and in the appendix, which, to our knowledge, is an unusual finding and has only been previously reported two times in the English literature.4
Unlike oesophageal atresia and intestinal atresia, which are commonly recognised soon after birth, studies suggest that the diagnosis of Hirschsprung’s disease coexisting with ileal atresia is usually late and, in cases of unrecognised aganglionosis at the time of atresia repair, there is a significant risk of anastomotic dehiscence.4 6 13 14 Our result is consistent with these studies, in which belated recognition of Hirschsprung’s disease occurred. Likewise, the diagnostic delay negatively affected the outcome.4 6 13 14 In our case some findings may explain this delay, namely a false negative diagnosis for Hirschsprung’s disease that was initially supported by the presence of ganglion cells in the distal ileum and appendix. We hypothesise whether a correct interpretation of persistent microcolon on repeat contrast enemas14 and surgeries could have led to earlier rectal biopsy and thus to earlier diagnosis and treatment of Hirschsprung’s disease; despite the fact that the latter sign has rarely been described. Fishman et al 14 emphasise the importance of a non-fixed, coiled, pelvic or central abdominal colon determined by contrast enema radiography or operative exploration as a key to early diagnosis. In these cases, rectal biopsies should be performed at the initial operation to avoid failure after the restoration of intestinal continuity.14
Presence of ganglion cells distal to ileal atresia has also been rarely reported in the literature.4 Theories postulating an early first-trimester vascular accident with gut atresia and failure of the caudal migration of the myenteric plexus as a possible cause of aganglionosis with associated intestinal atresia cannot explain ganglion cells distal to ileal atresia.4 15 The most plausible hypothesis for this association may involve the following in utero sequence of events: first, early trimester aganglionosis due to failure of the caudal migration of the myenteric plexus, which generally occurs between the fifth and 12th weeks of gestational life16; subsequently, occurrence of volvulus of a dilated ganglionic small bowel, emerging proximal to pre-existing aganglionic bowel,16 and finally, ischaemic volvulus resulting in ileal atresia with ganglion cells at both ends.
Despite the complexity of this case, a successful outcome was achieved by combining an innovative approach, such as temporary occlusion of the tracheo-oesophageal fistula, with the use of surgical options currently available for the repair of the involved malformations.
Conclusion
In this study, we characterise a complex case presented with a rare combination of three well-known surgical gastrointestinal malformations. As far as we know, the simultaneous occurrence of oesophageal atresia with tracheo-oesophageal fistula, ileal atresia and total colonic aganglionosis has not been previously described in the literature.
Some signs, including persistent microcolon, may guide to a timely rectal biopsy and lead to a definitive diagnosis of Hirschsprung’s disease concomitant with ileal atresia.
Learning points.
Association between oesophageal atresia/tracheo-oesophageal fistula, ileal atresia, Hirschsprung’s disease and ganglion cells distal to intestinal atresia is uncommon.
Hirschsprung’s disease in this clinical setting can be challenging to diagnose.
This rare combination may have a negative impact on the outcome.
For children failing to achieve normal intestinal function following repair of ileal atresia, Hirschsprung ’s disease, although rare, is an important treatable cause that must remain as a differential diagnosis. An early rectal suction biopsy should be undertaken to exclude the rare combination of Hirschsprung’s disease and ileal atresia.
Footnotes
Contributors: LPS was the primary writer for the case report. LPS and DC draft the manuscript. CC and MFL were the consultant surgeons operating on the patient. MFL critically reviewed and finalised the report. All the above authors were involved in the review and approval of the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Parental/guardian consent obtained.
References
- 1.Pinheiro PF, Simões e Silva AC, Pereira RM. Current knowledge on esophageal atresia. World J Gastroenterol 2012;18:3662–72. 10.3748/wjg.v18.i28.3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhibou H, Bassir A, Sami N, et al. . [Antenatal diagnosis and management of ileal atresia]. Pan Afr Med J 2016;24:240 10.11604/pamj.2016.24.240.9807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein AM, Thapar N, Karunaratne TB, et al. . Clinical aspects of neurointestinal disease: pathophysiology, diagnosis, and treatment. Dev Biol 2016;417:217–28. 10.1016/j.ydbio.2016.03.032 [DOI] [PubMed] [Google Scholar]
- 4.Janik JP, Wayne ER, Janik JS, et al. . Ileal atresia with total colonic aganglionosis. J Pediatr Surg 1997;32:1502–3. 10.1016/S0022-3468(97)90576-5 [DOI] [PubMed] [Google Scholar]
- 5.Daher P, Raffoul L, Riachy E, et al. . Uncommon co-occurrence of ileal atresia and total colonic aganglionosis in two unrelated newborns. Pediatr Surg Int 2012;28:85–7. 10.1007/s00383-011-2967-4 [DOI] [PubMed] [Google Scholar]
- 6.Goslin B, Brown A, Robertson D. Gastroschisis, ileal atresia, and Hirschsprung’s disease in a newborn: the first reported case. J Pediatr Surg 2012;47:2134–6. 10.1016/j.jpedsurg.2012.09.040 [DOI] [PubMed] [Google Scholar]
- 7.Wisbach GG, Vazquez WD. Ileal atresia, malrotation and Hirschsprung’s disease: a case report. J Pediatr Surg Case Rep 2013;1:e3–5. 10.1016/j.epsc.2012.12.002 [DOI] [Google Scholar]
- 8.McMurran AE, McMahon SV, Walker GM. Intestinal associations of a single umbilical artery. Arch Dis Child Fetal Neonatal Ed 2015;100:F263 10.1136/archdischild-2014-307582 [DOI] [PubMed] [Google Scholar]
- 9.Mandell GA, Biyyam DR, Jorgensen S, et al. . Total aganglionosis associated with ileal atresia and malrotation. Appl Radiol 2015. [Google Scholar]
- 10.Knod JL, Bondoc AJ, Garrison AP, et al. . Concurrent esophageal atresia with tracheoesophageal fistula and Hirschsprung disease. J Pediatr Surg Case Rep 2015;3:499–500. 10.1016/j.epsc.2015.09.015 [DOI] [Google Scholar]
- 11.Solomon BD, Baker LA, Bear KA, et al. . An approach to the identification of anomalies and etiologies in neonates with identified or suspected VACTERL (vertebral defects, anal atresia, tracheo-esophageal fistula with esophageal atresia, cardiac anomalies, renal anomalies, and limb anomalies) association. J Pediatr 2014;164:451–7. 10.1016/j.jpeds.2013.10.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juang D, Snyder CL. Neonatal bowel obstruction. Surg Clin North Am 2012;92:685–711. 10.1016/j.suc.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 13.Lally KP, Chwals WJ, Weitzman JJ, et al. . Hirschsprung’s disease: a possible cause of anastomotic failure following repair of intestinal atresia. J Pediatr Surg 1992;27:469–70. 10.1016/0022-3468(92)90339-9 [DOI] [PubMed] [Google Scholar]
- 14.Fishman SJ, Islam S, Buonomo C, et al. . Nonfixation of an atretic colon predicts Hirschsprung’s disease. J Pediatr Surg 2001;36:202–4. 10.1053/jpsu.2001.20053 [DOI] [PubMed] [Google Scholar]
- 15.Gupta M, Beeram MR, Pohl JF, et al. . Ileal atresia associated with Hirschsprung disease (total colonic aganglionosis). J Pediatr Surg 2005;40:e5–e7. 10.1016/j.jpedsurg.2005.05.064 [DOI] [PubMed] [Google Scholar]
- 16.Moore SW, Rode H, Millar AJW, et al. . Intestinal atresia and Hirschsprung’s disease. Pediatr Surg Int 1990;5:182 10.1007/BF00179658 [DOI] [Google Scholar]