Abstract
Cannabis sativa and its extracts have been used for centuries, both medicinally and recreationally. There is accumulating evidence that exogenous cannabis and related cannabinoids improve symptoms associated with inflammatory bowel disease [IBD], such as pain, loss of appetite, and diarrhoea. In vivo, exocannabinoids have been demonstrated to improve colitis, mainly in chemical models. Exocannabinoids signal through the endocannabinoid system, an increasingly understood network of endogenous lipid ligands and their receptors, together with a number of synthetic and degradative enzymes and the resulting products. Modulating the endocannabinoid system using pharmacological receptor agonists, genetic knockout models, or inhibition of degradative enzymes have largely shown improvements in colitis in vivo. Despite these promising experimental results, this has not translated into meaningful benefits for human IBD in the few clinical trials which have been conducted to date, the largest study being limited by poor medication tolerance due to the Δ9-tetrahydrocannabinol component. This review article synthesises the current literature surrounding the modulation of the endocannabinoid system and administration of exocannabinoids in experimental and human IBD. Findings of clinical surveys and studies of cannabis use in IBD are summarised. Discrepancies in the literature are highlighted together with identifying novel areas of interest.
Keywords: Inflammatory bowel disease, cannabis, cannabinoids
1. Introduction
The use of cannabis, whether for medicinal or recreational purposes, dates back to ancient civilisation, featuring in Chinese medicine almost 5000 years ago and also described in Egyptian, Greek, Indian, and Middle Eastern cultures.1 Within Western medicine, the earliest work was performed by William O’Shaughnessy in the mid 1800s.2 He defined effects of Indian hemp on healthy animals and in human cases of rheumatism, hydrophobia, cholera, tetanus, and infantile convulsions.3 Despite medicinal use for millennia, work continues to try to understand the mechanistic role of cannabinoids in gastrointestinal disease, including human inflammatory bowel disease and animal models of intestinal inflammation.
2. The endocannabinoid system
The major active ingredient of Cannabis sativa, and that which causes the psychotropic effects, is Δ9-tetrahydrocannabinol [THC], and was only isolated from the plant in 19644 when technology advanced to allow isolation and characterisation of individual components of mixtures. However, the plant contains up to 100 cannabinoid constituents including cannabidiol [CBD].5 For some years after the identification of THC, the potential target[s] of action of cannabinoids remained elusive.
The discovery of cannabinoid receptor 1 [CB1] in the 1980s as both an abundant G-protein coupled receptor [GPR] in the human brain,6 and also at lower levels in immune cells,7–12 was followed by cannabinoid receptor 2 [CB2]. This was identified in the human HL60 promyelocytic leukaemia cell line and has 44% homology to CB1.13 CB2 has been described in human immune cell subsets,8,11,12 particularly those of a myeloid lineage.14 Since the identification of CB1/2, other putative cannabinoid receptors have been identified, including GPR18,15 GPR55,16 GPR119,17 transient receptor potential channels,18,19 and peroxisome proliferator-activated receptors.19
Subsequently, endogenous ligands for these receptors, through which THC exerts its actions, were identified. Anandamide [AEA] is a partial agonist of CB1 and CB2, with 2-arachidonoylglycerol [2AG], a monoacylglycerol, acting as a full agonist at both these receptors although with greater potency for CB1 than CB2.19,20 Other bioactive lipids related to these classical endocannabinoids have been described, including N-acyl-ethanolamines (palmitoylethanolamine [PEA], oleoylethanolamine [OEA]) and other monoacylglycerols (2-oleoylglycerol [2OG], 2-palmitoylglycerol [2PG]). They may also bind to other cannabinoid-related receptors21 to exert actions independent of CB1/2. They may act to modulate 2AG signalling through an ‘entourage effect’,22,23 although whether this is to potentiate or inhibit is dependent on the experimental model used. Interestingly, gut microbes have been shown to produce lipid ligands with similarities to the wider endocannabinoids to signal through GPRs, including GPR119, to modulate host physiology particularly with glucose handling.24
2AG is synthesised from the action of diacylglycerol lipases [DAGL] α and β on diacylglycerols [DAGs] containing the arachidonate moiety. Metabolism of 2AG may proceed through hydrolysis, oxidation by COX2 or LOX enzymes, or epoxidation by components of the cytochrome P450 system,25,26 to produce arachidonic acid and eicosanoids [prostaglandins and leukotrienes]. In the murine brain, monoacylglycerol lipase [MGLL] accounts for 85% of 2AG hydrolytic activity, with further contributions by αβ-hydrolase domain 6 [ABHD6] and ABHD12.27 Fatty acid amide hydrolase [FAAH], the chief hydrolytic enzyme for AEA,28 also contributes slightly to 2AG hydrolysis. The contribution of MGLL to prostaglandin production downstream of 2AG appears to vary by tissue type—MGLL regulates prostaglandin production in murine liver and lung, and PLA2G4A in gut and spleen, with contributions from both enzymes in brain tissue.29 MGLL has also been demonstrated to hydrolyse prostaglandin glycerol esters [produced from 2AG by COX2 oxidation] and so may have more substrates than just monoacylglycerols.30
The cannabinoid receptors, together with their endogenous ligands and synthetic/degradative enzymes, form the endocannabinoid system [ECS] through which THC and other exocannabinoids act.
3. Inflammatory bowel disease
IBD can be classified into Crohn’s disease [CD] and ulcerative colitis [UC], based on characteristic clinical, radiological, endoscopic, and histological features. Although incompletely understood, the aetiology is thought to represent a complex interaction between genetic background, intestinal microbiota, environmental factors, and host immune response, resulting in persistent and dysregulated inflammation.
The incidence of IBD has increased in Western populations31,32 although it may be plateauing.33 However, this is not the case in the Asia-Pacific region where both incidence and prevalence are increasing.34 The increased incidence cannot be accounted for by substantial changes in host genetics, with environmental factors likely to be of critical importance [reviewed in 35 and briefly summarised in Table 1]. Although there has been an explosion in therapies for IBD, there remains an unmet clinical need for novel therapies and improved understanding of disease biology.
Table 1.
Effect of environmental factors on rates of IBD.
| Factor | General effect on rates of IBD |
|---|---|
| South to North migration | Increase |
| East to West migration | Increase |
| Smoking | Reduction [UC]; increase [CD] |
| Appendectomy | Reduction [UC]; increase [CD] |
| Antibiotics in childhood | Increase [Western populations]; reduction [Asia] |
| Improved hygiene/sanitation | Increase |
| COX inhibition | Increase [NSAIDs, aspirin]; reduction [COX2-inhibitors] |
| Reduced dietary fibre | Increase [CD] |
| High ω-6:ω-3 PUFA | Increase |
| Vitamin D deficiency | Increase |
| Stress | Increase |
| Increased physical activity | Reduction |
COX, cyclo-oxygenase; PUFA, polyunsaturated fatty acids; NSAID, non steroidal anti-inflammatory drug; CD, Crohn’s disease; UC, ulcerative colitis.
Data extracted from reference 35.
Genetic association studies have identified approximately 200 loci associated with IBD, with many shared between CD and UC.36,37 Differences in susceptibility alleles exist between ethnic backgrounds with, for instance, NOD2 and IL23R variants over-represented in European populations compared with East Asian populations.34,37 More recently, using high-density genotyping, 45 variants have been fine-mapped as potentially causal for IBD,38 and this approach may aid better understanding of disease mechanisms. Grouping of susceptibility loci using ontology pathway analysis has highlighted broad mechanisms of relevance in the immunological response in IBD, and this has informed subsequent study. This includes autophagy [ATG16L1, IRGM, LRRK2], endoplasmic reticulum stress [XBP1], innate immune cell sensing [NOD2], T cell tolerance [IL10, IL10R], IL23-pathways [IL23R, IL12B], lymphocyte trafficking [CCL7, IL8], and epithelial barrier function [MUC1, MUC3].39–42 Another development in the field of IBD genetics is evidence that genetic associations may be related to disease prognosis in addition to, or instead of, disease susceptibility.43 This might provide a novel avenue to stratify patients and target therapies accordingly.
4. Single nucleotide polymorphisms in ECS components in IBD
Single nucleotide polymorphisms [SNPs] in some ECS genes have been investigated in human IBD [Table 2]. The Q63R mutation in the CNR2 gene, encoding CB2, impairs endocannabinoid-induced inhibition of T cell proliferation.44 In an Italian paediatric IBD cohort, this mutation was associated with a more severe disease phenotype and shorter time to relapse for UC,45 but these were not replicated in a Turkish cohort of adult patients.46 This may be due to either age or ethnic differences between patients. The G1359A mutation in CNR1, encoding CB1, has been shown to have a lower prevalence in patients with UC than in controls, and to be associated with lower body mass index [BMI] and later age of onset in CD.47 The C385A substitution in FAAH results in reduced FAAH expression, but there are no differences in genotype prevalence in IBD.48,49 However, there is a possible association of the AA genotype with a penetrating phenotype and increased extra-intestinal manifestations in CD, and earlier age of onset in UC.48 Interestingly, the FAAH mutation is more prevalent in patients with diarrhoea-predominant or mixed-picture irritable bowel syndrome,50 suggesting effects on motility, secretion or pain perception.
Table 2.
Single nucleotide polymorphisms of components of the ECS studied in human IBD.
| Genotype | |||||
|---|---|---|---|---|---|
| FAAH C385A | CC | CA | AA | Associations | |
| Storr 2008 | CD [n = 202] | 67.3% | 31.2% | 1.5% | No differences in prevalence between groups. Phenotype not assessed in this study |
| Controls [n = 206] | 63.6% | 35.0% | 1.5% | ||
| Storr 2009 | CD [n = 435] | 65.8% | 30.1% | 4.1% | No differences in prevalence between groups. AA associated with more EIMs and penetrating phenotype in CD; earlier age of onset in UC. |
| UC [n = 167] | 65.3% | 32.9% | 1.8% | ||
| Controls [n = 406] | 61.6% | 34.5% | 3.9% | ||
| CNR1 G1359A | GG | GA | AA | ||
| Storr 2010 | CD [n = 216] | 53.3% | 39.8% | 6.9% | Lower prevalence of AA in UC versus controls. AA associated with lower body mass index and later age of onset of CD. |
| UC [n = 166] | 58.4% | 38.0% | 3.6% | ||
| Controls [n = 197] | 52.3% | 37.0% | 10.7% | ||
| CNR2 Q63R | QR | RR | |||
| Yonal 2014 | CD [n = 101] | 10.9% | 38.6% | 50.5% | No differences in prevalence or phenotype in this study. |
| UC [n = 101] | 6.9% | 43.6% | 49.5% | ||
| Controls [n = 101] | 11.9% | 37.6% | 50.5% | ||
| Striscuglui 2016 | CD [n = 112] | 2.7% | 48.2% | 49.1% | Paediatric cohort. RR genotype more prevalent in IBD than controls and associated with more severe disease activity at diagnosis. In UC, associated with higher risk of relapse. |
| UC [n = 105] | 18.1% | 38.1% | 43.8% | ||
| Controls [n = 600] | 16.0% | 51.7% | 32.3% | ||
Prevalence of genotypes are displayed alongside associations with disease phenotype. Data extracted from references 45–49.
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; ECS, endocannabinoid system; EIM, extra-intestinal manifestations.
5. ECS tone in IBD
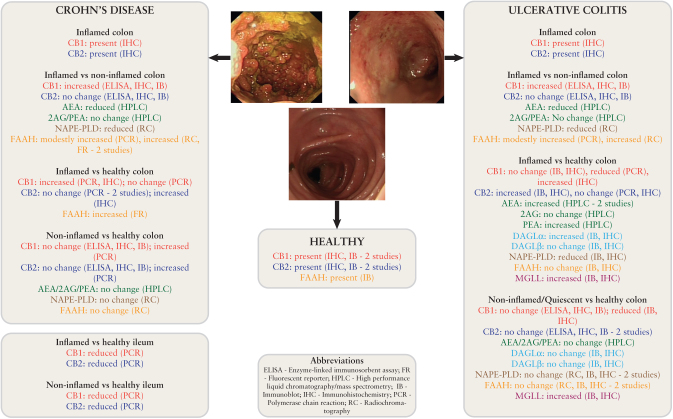
There is disagreement on the tone of the ECS between studies of human IBD [summarised in Figure 1]. CB1 has been identified in the colonic epithelium [particularly crypts51], some plasma cells of the lamina propria, smooth muscle, and the submucosal myenteric plexus.52 Conversely, CB2 localises in the absorptive and goblet cells of the epithelium, Paneth cells, and some subepithelial macrophages and plasma cells.51,52 CB2 was expressed at slightly higher levels than CB1 in one study.53 The effect of inflammation on expression of CB1 and CB2 is less clear. Increases in both CB1 and 2 in both CD and ‘acute phase IBD’ [a combination of UC and IBD-unclassified]52 have been observed. The authors of this study suggest that the changes in CB1 may be an effect of goblet cell depletion in epithelial architecture rather than a true increase in protein abundance. In other work, increases in either CB154,55 or alternatively in CB251 alone have been observed. A recent study of CB1 and CB2 gene expression in Crohn’s disease, the largest to date, demonstrated consistent detection of expression albeit at low levels in inflamed, non-inflamed, and healthy samples. A difference in expression patterns was seen between disease affecting the ileum, where both CB1 and 2 were reduced in inflamed/non-inflamed samples, and colon, where an increase was seen in CB1 and 2 in the non-inflamed but not inflamed samples.56
Figure 1.
A summary of existing studies of the endocannabinoid tone in human ileum and colon in health and IBD. Studies of the ECS in human IBD have yielded conflicting results dependent on the technique used, the site of sampling [ileum vs colon], and the comparison used [inflamed vs non-inflamed vs healthy]. Only one study has assessed the synthetic [DAGL] and key hydrolytic [MGLL] enzyme involved in 2AG metabolism. No studies have examined the presence of ABHD6 or 12. Data extracted from references 48, 51–59. IBD, inflammatory bowel disease; ECS, endocannabinoid system.
Similar controversy exists for levels of endocannabinoids themselves. AEA has been shown to be increased in inflamed samples from UC patients,57,58 yet reduced in another study.54 It is more plausible that AEA levels are indeed decreased in inflammation as it has been shown that there is reduced expression of the synthetic enzyme NAPE-PLD and increased expression of the hydrolytic enzyme FAAH in colonic inflammation.51,54,59,60 PEA is increased in inflammation in one study58 but does not change in another,54 and the two studies assessing 2AG levels both demonstrate no change in inflammation.54,57
To date, only one study has attempted to define levels of MGLL and the DAGL enzymes in human IBD.51 MGLL was localised to the central portion of epithelial cells, polymorphonuclear cells of the lamina propria, and the myenteric plexus, and was shown to be increased in inflammation. Previously MGLL has been shown to be widely distributed in the rat intestine, increasing in expression from duodenum to distal colon.61 DAGLα was similarly increased in inflammation, but no difference was seen in DAGLβ.
There are a multitude of potential explanations for the differences between these studies. In particular, the patients studied were not always well phenotyped for disease severity and extent, and were frequently grouped together. Furthermore, the potential role of medications such as systemic immunosuppression in altering expression levels was not explored. In many studies, comparisons were made between inflamed mucosa in an IBD patient and healthy mucosa from a different person. ECS tone has been shown to be affected by diurnal variation62 and ethnicity,63 not to mention the likely confounder of any comorbidities such as irritable bowel syndrome, in which the involvement of the ECS is well documented.64 Definitive annotation of ECS component distribution in the intestine will benefit from development of more sensitive and specific antibodies.
Interestingly, CBD reduces iNOS and S100B expression in cultured colonic biopsies from patients with UC, indicating effects on enteric glial cell activation. These effects were dependent on PPARγ, being abrogated by a PPARγ antagonist.65 Furthermore, fractions of THC have recently been extracted and assayed for effects on colonic IL-6/8 in ex vivo biopsies, with the greatest benefit seen with Δ9-tetrahydrocannabinolic acid [THCA] which does not have psychoactive effects.66 THCA has not yet been used in clinical trials.
6. In vivo studies
6.1. Cannabinoid receptors
One of the earliest studies of the effect of cannabinoid receptor modulation identified that CB1-/- mice exhibited more severe colitis following either dinitrobenzene sulphonic acid [DNBS] or dextran sulphate sodium [DSS] administration.67 Subsequently this finding was confirmed in trinitrobenzene sulphonic acid [TNBS] colitis and extended to demonstrate that CB2-/- and CB1-/-/CB2-/- double knockout mice also display worsened colitis.68 Although not reaching statistical significance, there was an additive effect of double knockout on macroscopic colonic inflammation but not histological inflammation. The effects of CB2-/- have also been confirmed in DSS colitis. The authors also demonstrated that murine peritoneal macrophages stimulated ex vivo with lipopolysaccharide [LPS]/DSS upregulated components of the NLRP3 inflammasome, that this was exacerbated in CB2-/- cells, and was improved by the CB2 agonist HU308.69
The findings of genetic knockout studies have largely been confirmed by use of CB agonists/antagonists. DNBS colitis is worsened by prophylactic administration of the CB1 antagonist SR141716A,67 with DSS and oil-of-mustard colitis improved by prophylactic ACEA, a CB1 agonist.70 Similarly, the CB2 agonists JWH133,70 HU308,69 or ALICB45971 are beneficial in ameliorating chemical colitis models when administered prophylactically. ALICB459 is particularly appealing from a translational medicine perspective, as it was effective with oral rather than intraperitoneal administration. CB2 agonists improve, and CB2 antagonists worsen, chemical colitis when administered therapeutically, and that this is CB2-dependent.72–74
Although most studies have used chemical colitis models, JWH133 ameliorates colitis in the IL10-/- model where mice develop spontaneous colitis by 12 weeks of age.73 GP-1a, purported to be a CB2 agonist, improves ileitis when administered retro-orbitally in the TNFΔARE model of Crohn’s-like ileitis. However, recent evidence would suggest that this compound may in fact be an inverse agonist of both CB1 and CB2 in vitro,75 and so it remains to be confirmed whether CB2 agonism or inverse agonism is effective here.
Complementing the results from double knockout studies, use of less selective agonists also display benefits in colitis. WIN55,212 is an agonist of both CB1 and CB2 and ameliorates colitis whether used prophylactically or therapeutically in TNBS and DSS models.76–78 AM841 [CB1 agonist] improves colitis in a cannabinoid receptor-dependent manner, with the effect lost in CB1-/- and CB2-/- mice.79 The same is true for the CB1 agonist, HU210,67 which also has effects on sustaining intestinal barrier function in a TLR4-independent manner.80 Interestingly the effect of non-selective cannabinoid receptor activation may be through central rather than peripheral mechanisms. CB13 is an agonist of both CB1 and CB2, with poor penetration of the central nervous system.81 However, this compound was not effective in murine TNBS or DSS colitis when administered intraperitoneally, but was effective when injected intracerebroventricularly.79 Equally ineffective was the peripherally restricted, non-selective agonist, SAB378.76
Two studies have suggested interplay between cannabinoid signalling and p38 MAPK in the modulation of colitis severity. In the first,77 Mk2-/- mice, who lack a downstream substrate of p38, exhibit a less severe colitis in response to DSS. This study also demonstrated that WIN55,212 impairs phosphorylation of p38 in response to DSS in both wild-type and Mk2-/- mice. Subsequently a similar result has been obtained by using the p38 inhibitor SB203580.78 One mechanism therefore, by which colitis is ameliorated by cannabinoids, may be through effects on MAPK pathways.
6.2. Endocannabinoid lipid ligands
A recent study investigated rectal 2AG administration in chemical colitis.82 Here the authors used carbon nanotubes linked to 2AG, with the aim of reducing rapid hydrolysis and improving the overall pharmacological profile. A single dose administered rectally 2 days before the instillation of TNBS in rats, and then a second dose 8 days after instillation, resulted in improvement of colitis. No effect was seen of free 2AG or of the carbon nanotubes alone.
In addition to the effect of 2AG on colitis, intraperitoneal AEA improves TNBS colitis.83 More evidence exists for the role of PEA in colitis, possible acting by inhibiting the induction of angiogenesis that is usually seen in chemical colitis.84 Intraperitoneal injection of PEA is beneficial in both established chemical colitis and when administered prophylactically.85 The effects are dose-dependent and require PPARα but not PPARγ.86 This study demonstrated a reduction in TLR4 and S100B expression on enteric glial cells and a reduction in MAPK signalling, as potential mechanisms of action. The effect of PEA is also dependent upon CB2 and GPR55.87 Adelmidrol is a PEA analogue which is effective orally in established colitis in a PPARγ- but not PPARα-dependent manner, contrary to the earlier study.88
6.3. Hydrolytic enzymes
6.3.1. MGLL
To date, only one study has assessed the role of MGLL inhibition in colitis.89 Rectal administration of TNBS was used to induce colitis, and the small molecule MGLL inhibitor JZL184 was administered prophylactically. Both macroscopic and microscopic colitis were ameliorated. This was associated with a reduction in mucosal and systemic pro-inflammatory cytokines such as IL-6, TNFα, and IL-12. MGLL inhibition also reduced LPS-induced endotoxaemia, which may suggest effects on mucosal barrier function. Certainly, THC and CBD have beneficial effects on intestinal permeability induced by EDTA in unstimulated Caco-2 [colonic carcinoma] cells.90 The effects of 2AG and AEA in this model were dependent on apical [worsened permeability] versus basolateral [improved permeability] administration. The same is true for JZL184 administration in unstimulated Caco-2 cells.91 When cytokines were administered to Caco-2 cells to mimic inflammation, JZL184 worsened permeability when applied apically either at the same time as the cytokines92 or after inflammation had been induced.91 The benefits of MGLL inhibition on TNBS colitis involved both CB1 and CB2, as inhibition of either abrogated the effects of JZL184.89 However the CB2 antagonist used, AM630, has recently been shown to have off-target effects,75 and so it would be useful to have data using alternative CB2 antagonists to confirm the role of this receptor in mediating the effects seen. It is also worth noting that the dose of JZL184 used here is high [32 mg/kg daily in divided doses] and is within the dose to desensitise CB1,93 although it is not clear whether this happens within the 3-day time frame used in this model.
6.3.2. FAAH
Although only one in vivo study has been performed using MGLL inhibition, several studies have investigated FAAH inhibition. First, FAAH-/- mice exhibit less severe chemical colitis.67 Use of pharmacological FAAH inhibitors corroborates the findings from the genetic study with amelioration of disease49,57,94,95 in a CB1- and CB2-dependent manner.49 A combined FAAH/COX inhibitor, ARN2508, was effective in a CB1- and PPARα-dependent manner.96 Inhibition using FAAH-II not only improved colitis and reduced pro-inflammatory cytokine production, but also impaired infiltration by immune cells and affected expression of micro-RNAs in mesenteric lymph nodes and Peyer’s patches of colitic mice97 as a potential mechanism of action. One study has suggested that therapeutic FAAH inhibition may have additional benefits over prophylactic administration, although this remains to be replicated.98 Despite several studies demonstrating a benefit of FAAH inhibition, findings are not unanimous. One study demonstrated that PF3845 is effective at ameliorating colitis in the TNBS model but not the DSS model.99 The beneficial effects on TNBS were not replicated by a second group.85
Inhibition of N-acylethanolamine-hydrolysing acid amidase [NAAA] results in increased levels of PEA and not AEA, and improvement in colitis.85 This corroborates the studies performed using PEA.
6.4. Other ECS targets
It is not well understood to what extent the non-CB1, non-CB2 cannabinoid receptors have roles in colitis. The atypical cannabinoid O-1602 is a derivative of CBD and is known to bind to GPR55. Although it exerts anti-inflammatory effects on both DSS and TNBS colitis, this is independent of GPR55 as genetic knockout of this receptor, and indeed of both CB1 and CB2, does not alter the effect of O-1602.100 It is not known therefore by which pathways this compound acts on in colitis. The GPR55 antagonist CID16020046 and GPR55-/- mice exhibit less severe colitis in response to DSS or TNBS, contrary to genetic knockout or pharmacological inhibition of CB1/2.101 Therefore, whereas CB1/2 are likely to have anti-inflammatory effects, GPR55 triggers a pro-inflammatory cascade.
Inhibition of AEA reuptake using the endocannabinoid membrane transport inhibitor VDM11 has also been shown to improve experimental colitis.49,57
6.5. Exocannabinoids
Further to the studies modulating endocannabinoid levels, administration of exocannabinoids in experimental colitis has largely demonstrated similar benefit. Β-caryophyllene is available orally and acts via CB2 and PPARγ to limit colitis.102 Similarly, αβ-amyrin ameliorates both TNBS103 and DSS104 colitis and acts, at least in part, through cannabinoid receptors. The phytocannabinoid CBD has been most extensively studied. It reduces intestinal inflammation induced by LPS as measured by TNFα.65 A reduction in inducible nitric oxide synthase [iNOS] expression has been demonstrated by CBD which also reduces IL-1β and increases IL-10 levels.105 The effect of CBD is maintained when administered intraperitoneally or rectally but not orally.106 It has also been shown to potentiate the anti-inflammatory effects of THC in chemical colitis.107 Synthetic derivatives such as abnormal CBD ameliorate colitis independently of CB1/2 and possibly through GPR18,108 whereas the highly concentrated CBD, known as CBD botanical drug substance [a major ingredient of nabiximols], was effective orally and intraperitoneally unlike pure CBD.109 Reference has been made to benefit of CBD in the IL10-/- mouse in a related publication,110 but this study has not been published independently. Despite these promising findings, CBD has been shown to worsen LPS-induced pulmonary inflammation in mice111 and so some caution should be maintained.
Other exocannabinoids which have been beneficial at ameliorating colitis include MFF [an extract of medicinal cannabis],112 cannabigerol,113 and cannabichromene.114
Of all experiments within the published literature assessing modulation of ECS components on experimental ileitis/colitis, few have used non-chemical models. These include IL10-/- colitis [one published study,73 and one referenced within the text of another110] and TNFΔARE ileitis.56 Several studies have used oil of mustard, croton oil, and LPS to induce colitis [detailed within a recent systematic review115] but these models are not classically felt to be experimental models of human IBD, although they may provide insight into intestinal inflammation in general.
Better understanding of the molecular wiring of the endocannabinoid system in the mammalian intestine may enable development of more specific models capable of interrogating the potential protective effects of this pathway via genetic ablation approaches. Further investigation of ECS modulation in other models of IBD and intestinal inflammation, such as Citrobacter rodentium and Helicobacter hepaticus, would be informative. The development of a first-in-class MGLL inhibitor for human clinical trials, ABX-1431 [Abide Therapeutics1], provides opportunity to investigate this in human IBD if preclinical study was supportive.
7. Clinical studies and trials in human IBD
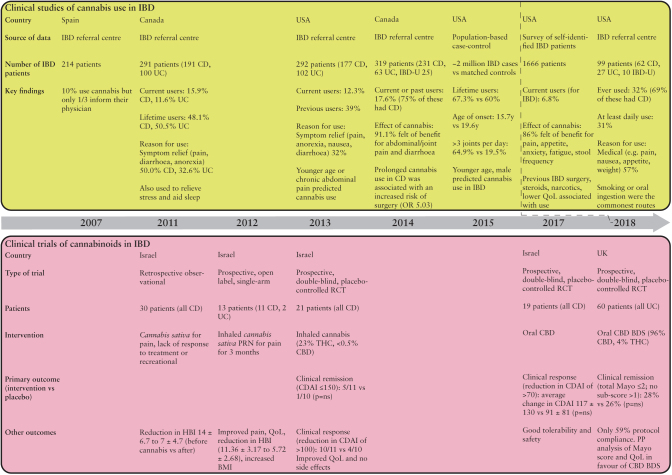
Several questionnaire-based studies have confirmed current use of cannabis in 6.8–15.9% of adult patients with IBD, with lifetime use in 48.1–67.3% of patients.116–120 A Canadian study found that 17.6% of IBD patients were current or previous users of cannabis specifically for IBD.60 Among an adolescent cohort, 32% of patients with IBD had ever used marijuana, 57% for medicinal purposes.121 The most common reasons given for cannabis use were to alleviate abdominal pain, diarrhoea, or anorexia60,117,118,120–122 and use is higher in patients with previous surgery or chronic analgesic requirements.117,118,120 Improvements in quality of life have also been demonstrated118 along with a reduction in Harvey-Bradshaw Index.122 A large population-based survey confirmed a younger age of onset of cannabis use in patients with IBD and overall heavier consumption.119 Another group has identified an association between prolonged cannabis use [>6 months] in CD and a higher incidence of previous surgery (odds ratio 5.030 [95% confidence interval 1.449–17.459]).60 The studies are summarised in Figure 2.
Figure 2.
A summary of clinical studies and trials of cannabis and cannabinoids in human IBD. Studies consistently demonstrate use of cannabis in patients with IBD, frequently for symptom relief. As yet, no clinical trials of cannabinoids in IBD have met their primary endpoints but demonstrate improvements in symptoms, quality of life, and clinical severity scores. Data extracted from references 60, 116–124, 126, 128. IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; OR, odds ratio; HBI, Harvey-Bradshaw Index; CDAI, Crohn’s Disease Activity Index; QoL, quality of life; CBD, cannabidiol; CBD BDS, cannabidiol botanical drug substance; THC, tetrahydrocannabinol; PP, per protocol; RCT, randomised-controlled trial; ns, not significant.
An open-label, single-arm study of inhaled THC used ‘as required’ for pain in IBD patients [n = 13] demonstrated effects on analgesia, improved quality of life, and increased body mass index.123 A reduction in Harvey-Bradshaw Index from 11.36 to 5.72 was seen in patients with CD [11/13] and a slight decrease in partial Mayo score for patients with UC [2/13]. Subsequently a prospective, placebo-controlled trial of inhaled THC [23% THC, <0.5% CBD] in CD failed to meet its primary endpoint of increased clinical remission.124 However, effects were seen on clinical response as assessed by the CD Activity Index [CDAI]—a scoring system based on largely clinical parameters with no objective assessment of inflammation.125 Patients reported improved quality of life and reduced pain, which likely accounts for the reduction in CDAI without there necessarily being an effect on inflammation. Alternatively, the choice of patients with medically refractory disease in this trial may mask subtle benefits.
The same group performed a double-blind randomised controlled trial [RCT] of oral CBD in CD.126 There were no safety issues identified, but they failed to meet the primary endpoint of a reduction in CDAI after 8 weeks. Whereas this may represent a failure of CBD to exert anti-inflammatory effects in human IBD, the randomisation procedure resulted in 6/10 [60%] of those treated with CBD versus 0/9 [0%] of placebo being current smokers. Smoking is well known to be associated with a more difficult-to-treat disease, and this may mask an effect of CBD.
Inhaled Cannabis sativa containing THC has been trialled in patients with extensive or left-sided UC refractory to medications, including thiopurines and biological agents, and published in abstract form.127 The placebo arm was inhaled Cannabis sativa from which THC had been extracted. This demonstrated no effect of THC on C-reactive protein [CRP] or calprotectin levels compared with placebo. A modest effect was seen on disease activity index and Mayo endoscopic subscore [reduction from 2 to 1 in the intervention group, p <0.01]. Improvements were seen in terms of abdominal pain, appetite, and general satisfaction, with no clear safety signals.
A double-blind, placebo-controlled, RCT of GW42003 [approximately 96% CBD, 4% THC] in active UC has recently been published.128 The trial recruited 60 patients with mild-moderate UC, excluding isolated proctitis, and remains the largest clinical trial of cannabinoids in human IBD to date. Participants were required to be on either no or stable dose of 5-aminosalicylic acid [5ASA] before entry. Importantly, this trial incorporated endoscopic evaluation and measurement of CRP and faecal calprotectin as objective measures of inflammation, alongside clinical scoring. The trial failed to meet the primary endpoint of clinical remission, but a reduction in Mayo score and improvement in quality of life were favoured by GW42003. However, only 59% of those treated complied with protocol, due to adverse effects likely due to the THC component. There is a need for cannabinoids which do not have neuropsychiatric side effects—THCA, discussed earlier, may be beneficial in this regard.
It is interesting to hypothesise why the experimental data are not yet translating into meaningful improvements for patients. This may simply represent immunological differences between species; or that chemical experimental colitis models are insufficient to accurately model human IBD; or that the route of drug administration is wrong; or that the inclusion criteria for patients in some of these trials generally selected for patients with more advanced, and therefore inherently more difficult to treat, disease. Replication of experimental findings in alternative animal models such as T cell transfer or IL10-/-/Helicobacter hepaticus should be encouraged. Interestingly, a recent study has shown that the Jurkat cell line [T cell line] is more resistant to the effects of CBD when cultured at physiological normoxia [12%] than at 21%.129 Given that the intestine is relatively hypoxic,130 especially in the context of active inflammation, this may also explain why exocannabinoids have so far failed to live up to expectations from preclinical work.
8. Conclusion
Research into the distribution and function of the endocannabinoid system in IBD and models of intestinal inflammation is increasing. There is accumulating evidence that enhancing signalling through cannabinoid receptors 1 and 2 has anti-inflammatory potential in the intestine in vivo. This was the subject of a recent systematic review and meta-analysis,115 although this paper did not include studies of cannabinoid receptor antagonists and, as mentioned above, did include studies of LPS, oil of mustard, and croton oil-induced colitis. Critically though, this article confirms the bias towards chemical models of colitis. Although cannabis use is fairly common in patients with IBD, particularly to relieve symptoms, the limited number of trials of exocannabinoids in IBD have not met their primary endpoints [Figure 2].
Before novel therapies targeting endocannabinoids, rather than exocannabinoids, can be translated into the clinical setting for IBD, it is essential that sufficient preclinical work is completed. There is an urgent need for better reagents to interrogate the system in vitro and in vivo. Antibodies are often non-specific,131,132 and small molecules do not necessarily target the receptor appropriately,75 potentially resulting in misleading results. Many ECS enzymes, including MGLL, FAAH, and DAGL, are serine hydrolases. Activity-based protein profiling [ABPP] can be used not only to profile activity of these enzymes in cells and tissues, but also identify off-target effects of inhibitors on other enzymes within this family,133 but has not yet been employed in human IBD.
The development of single-cell techniques opens up the possibility of better understanding ECS tone in individual cells. It is entirely plausible that the ECS functions in the gut in a similar way to the central nervous system where signals are sent between cells to modify neurotransmission.134 Improved understanding of the effect of inflammation on the ECS in different cell types might lead to a better understanding of how to translate this into meaningful therapies. At present though, it is difficult to make firm recommendations on the benefit or risk of cannabinoids in the management of the inflammation associated with human IBD.
It should not be overlooked, however, that a beneficial effect of cannabinoids on symptom control in patients with IBD is possible. There are well-documented effects of ECS modulation on gastrointestinal motility. Polymorphisms in FAAH50 and CB1135 have been associated with subtypes of irritable bowel syndrome in humans, and in vivo administration of CB1 antagonists reverses the inhibition of gastrointestinal motility seen with cannabinoid agonists.136 Dronabinol, a non-selective cannabinoid agonist, reduces gastric emptying, with a gender bias towards females.137 In addition to roles in gastric emptying, cannabinoids [including dronabinol, nabilone, and nabiximols] exert an anti-emetic effect likely mediated through central effects on CB1 and possibly CB2.138 The beneficial effects on nausea and vomiting may be lost, however, when cannabis is used chronically—resulting in the cannabis hyperemesis syndrome. This has many features similar to cyclic vomiting syndrome, a condition which has associations with CB1 polymorphisms.139 Although the exact mechanisms are poorly understood, it is reproducibly observed that symptoms may be relieved by hot bathing.140
The ECS, cannabinoids, and modulation of pain, including in visceral hypersensitivity associated with chronic stress, are inextricably linked and have been the subject of many reviews [including141 and142]. Indeed, nabiximols [a combination of THC and CBD] is licensed for the treatment of spasticity and spasms in multiple sclerosis, with some effects on pain in this condition.143 To this end and with relevance for IBD, a phase 2a clinical trial of olorinab [APD371], a full CB2 agonist, for visceral pain in Crohn’s disease is under way but has not yet reported [ClinicalTrials.gov Identifier: NCT03155945]. Any benefit of cannabis, cannabinoids, and ECS modulation in IBD has to be carefully balanced against the potential myriad negative, including neuropsychiatric, side effects.
Relevant to the rise in addiction to prescribed and illicit opiates, and the associated adverse health outcomes, there are valuable preclinical data suggesting overlap between the endocannabinoid and opioid systems. The MGLL inhibitor MJN110 and morphine act synergistically via μ-opioid and cannabinoid receptors to relieve neuropathic pain, but without the unwanted side effects of reduced gastrointestinal motility and cannabimimetic side effects.144 MGLL-/- mice are hypersensitive to the μ-opioid agonist, loperamide145 and CB2 agonists have been shown to induce μ-opioid receptor transcription in Jurkat cells.146 Modulation of the ECS may increase sensitivity to opioids and therefore may be a strategy to reduce opioid requirements if the evidence translates to human disease.
As calls for medicinal cannabis for treatment of epilepsy and other conditions intensify, it is all the more pressing that we better understand the effects of cannabinoids on human diseases—not just to identify novel applications, whether for symptomatic relief or as anti-inflammatory agents, but also to reduce the risk of exposing our patients to harm.
Acknowledgments
We acknowledge the support of the Oxford NIHR Biomedical Research Centre.
Footnotes
Abide Therapeutics. http://abidetx.com/news/abide-therapeutics-announces-first-subject-dosed-in-first-in-human-clinical-study-of-abx-1431-an-investigational-endocannabinoid-system-modulator/; Accessed 23 July, 2018.
Funding
This work was supported by an NIHR Research Professorship, United Kingdom Medical Research Council, and by a Wellcome Investigator Award [AS] and by Abide Therapeutics [AS, TA].
Conflict of Interest
TA and AS have received research funding from Abide Therapeutics. Abide Therapeutics have not contributed to any aspect of this manuscript.
Author Contributions
TA: concept, writing, approval of final manuscript. AS: concept, revised manuscript for intellectual content, approval of final manuscript.
References
- 1. Friedman D, Sirven JI. Historical perspective on the medical use of cannabis for epilepsy: Ancient times to the 1980s. Epilepsy Behav 2017;70:298–301. [DOI] [PubMed] [Google Scholar]
- 2. O’Shaughnessy WB. Extract from a memoir on the preparations of the indian hemp, or gunjah, [cannabis indica] their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. J Asiat Soc Bengal 1839;93:732–45. [PMC free article] [PubMed] [Google Scholar]
- 3. O’Shaughnessy WB. On the preparations of the indian hemp, or gunjah - cannabis indica, their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov Med J Retrosp Med Sci 1843;5:363–9. [PMC free article] [PubMed] [Google Scholar]
- 4. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 1964;86:1646. [Google Scholar]
- 5. Mechoulam R, Hanuš LO, Pertwee R, Howlett AC. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci 2014;15:757–64. [DOI] [PubMed] [Google Scholar]
- 6. Herkenham M, Lynn AB, Little MD, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 1990;87:1932–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouaboula M, Rinaldi M, Carayon P, et al. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem 1993;214:173–80. [DOI] [PubMed] [Google Scholar]
- 8. Galiègue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 1995;232:54–61. [DOI] [PubMed] [Google Scholar]
- 9. Daaka Y, Friedman H, Klein TW. Cannabinoid receptor proteins are increased in Jurkat, human T-cell line after mitogen activation. J Pharmacol Exp Ther 1996;276:776–83. [PubMed] [Google Scholar]
- 10. Sugamura K, Sugiyama S, Nozaki T, et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 2009;119:28–36. [DOI] [PubMed] [Google Scholar]
- 11. Krishnan G, Chatterjee N. Endocannabinoids alleviate proinflammatory conditions by modulating innate immune response in muller glia during inflammation. Glia 2012;60:1629–45. [DOI] [PubMed] [Google Scholar]
- 12. de Campos-Carli SM, Araújo MS, de Oliveira Silveira AC, et al. Cannabinoid receptors on peripheral leukocytes from patients with schizophrenia: Evidence for defective immunomodulatory mechanisms. J Psychiatr Res 2017;87:44–52. [DOI] [PubMed] [Google Scholar]
- 13. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993;365:61–5. [DOI] [PubMed] [Google Scholar]
- 14. Graham ES, Angel CE, Schwarcz LE, Dunbar PR, Glass M. Detailed characterisation of CB2 receptor protein expression in peripheral blood immune cells from healthy human volunteers using flow cytometry. Int J Immunopathol Pharmacol 2010;23:25–34. [DOI] [PubMed] [Google Scholar]
- 15. Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME. Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br J Pharmacol 2014;171:3908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryberg E, Larsson N, Sjögren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 2007;152:1092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansen KB, Rosenkilde MM, Knop FK, et al. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab 2011;96:E1409–17. [DOI] [PubMed] [Google Scholar]
- 18. De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 2011;163:1479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB₁ and CB₂. Pharmacol Rev 2010;62:588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodriguez de Fonseca F, Del Arco I, Bermudez-Silva FJ, et al. The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol 2005;40:2–14. [DOI] [PubMed] [Google Scholar]
- 21. Lambert DM, Di Marzo V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic?Curr Med Chem 1999;6:757–73. [PubMed] [Google Scholar]
- 22. Ben-Shabat S, Fride E, Sheskin T, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol 1998;353:23–31. [DOI] [PubMed] [Google Scholar]
- 23. Murataeva N, Dhopeshwarkar A, Yin D, et al. Where’s my entourage? The curious case of 2-oleoylglycerol, 2-linolenoylglycerol, and 2-palmitoylglycerol. Pharmacol Res 2016;110:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen LJ, Esterhazy D, Kim SH, et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 2017;549:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev 2011;111:5899–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zelasko S, Arnold WR, Das A. Endocannabinoid metabolism by cytochrome P450 monooxygenases. Prostaglandins Other Lipid Mediat 2015;116-117:112–23. [DOI] [PubMed] [Google Scholar]
- 27. Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol 2007;14:1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996;384:83–7. [DOI] [PubMed] [Google Scholar]
- 29. Nomura DK, Morrison BE, Blankman JL, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 2011;334:809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Savinainen JR, Kansanen E, Pantsar T, et al. Robust hydrolysis of prostaglandin glycerol esters by human monoacylglycerol lipase [MAGL]. Mol Pharmacol 2014;86:522–35. [DOI] [PubMed] [Google Scholar]
- 31. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 32. Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis 2007;13:481–9. [DOI] [PubMed] [Google Scholar]
- 33. Loftus CG, Loftus EV Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis 2007;13:254–61. [DOI] [PubMed] [Google Scholar]
- 34. Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis 2010;11:134–47. [DOI] [PubMed] [Google Scholar]
- 35. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015;12:205–17. [DOI] [PubMed] [Google Scholar]
- 36. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium [IIBDGC] Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu JZ, van Sommeren S, Huang H, et al. ; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015;47:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang H, Fang M, Jostins L, et al. ; International Inflammatory Bowel Disease Genetics Consortium Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 2017;547:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011;474:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Limbergen J, Radford-Smith G, Satsangi J. Advances in IBD genetics. Nat Rev Gastroenterol Hepatol 2014;11:372–85. [DOI] [PubMed] [Google Scholar]
- 41. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016;13:13–27. [DOI] [PubMed] [Google Scholar]
- 43. Lee JC, Biasci D, Roberts R, et al. ; UK IBD Genetics Consortium Genome-wide association study identifies distinct genetic contributions to prognosis and susceptibility in Crohn’s disease. Nat Genet 2017;49:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sipe JC, Arbour N, Gerber A, Beutler E. Reduced endocannabinoid immune modulation by a common cannabinoid 2 [CB2] receptor gene polymorphism: possible risk for autoimmune disorders. J Leukoc Biol 2005;78:231–8. [DOI] [PubMed] [Google Scholar]
- 45. Strisciuglio C, Bellini G, Miele E, et al. Cannabinoid receptor 2 functional variant contributes to the risk for pediatric inflammatory bowel disease. J Clin Gastroenterol 2018;52:e37–43. [DOI] [PubMed] [Google Scholar]
- 46. Yonal O, Eren F, Yılmaz Y, Atuğ Ö, Över HH. No association between the functional cannabinoid receptor type 2 Q63R variants and inflammatory bowel disease in Turkish subjects. Turk J Gastroenterol 2014;25:639–43. [DOI] [PubMed] [Google Scholar]
- 47. Storr M, Emmerdinger D, Diegelmann J, et al. The cannabinoid 1 receptor [CNR1] 1359 G/A polymorphism modulates susceptibility to ulcerative colitis and the phenotype in Crohn’s disease. PLoS One 2010;5:e9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Storr M, Emmerdinger D, Diegelmann J, et al. The role of fatty acid hydrolase gene variants in inflammatory bowel disease. Aliment Pharmacol Ther 2009;29:542–51. [DOI] [PubMed] [Google Scholar]
- 49. Storr MA, Keenan CM, Emmerdinger D, et al. Targeting endocannabinoid degradation protects against experimental colitis in mice: involvement of CB1 and CB2 receptors. J Mol Med [Berl] 2008;86:925–36. [DOI] [PubMed] [Google Scholar]
- 50. Camilleri M, Carlson P, McKinzie S, et al. Genetic variation in endocannabinoid metabolism, gastrointestinal motility, and sensation. Am J Physiol Gastrointest Liver Physiol 2008;294:G13–9. [DOI] [PubMed] [Google Scholar]
- 51. Marquéz L, Suárez J, Iglesias M, Bermudez-Silva FJ, Rodríguez de Fonseca F, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS One 2009;4:e6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wright K, Rooney N, Feeney M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology 2005;129:437–53. [DOI] [PubMed] [Google Scholar]
- 53. Harvey BS, Nicotra LL, Vu M, Smid SD. Cannabinoid CB2 receptor activation attenuates cytokine-evoked mucosal damage in a human colonic explant model without changing epithelial permeability. Cytokine 2013;63:209–17. [DOI] [PubMed] [Google Scholar]
- 54. Di Sabatino A, Battista N, Biancheri P, et al. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol 2011;4:574–83. [DOI] [PubMed] [Google Scholar]
- 55. Stintzing S, Wissniowski TT, Lohwasser C, Alinger B, Neureiter D, Ocker M. Role of cannabinoid receptors and RAGE in inflammatory bowel disease. Histol Histopathol 2011;26:735–45. [DOI] [PubMed] [Google Scholar]
- 56. Leinwand KL, Jones AA, Huang RH, et al. Cannabinoid receptor-2 ameliorates inflammation in murine model of Crohn’s disease. J Crohns Colitis 2017;11:1369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. D’Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J 2006;20:568–70. [DOI] [PubMed] [Google Scholar]
- 58. Darmani NA, Izzo AA, Degenhardt B, et al. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: review of the available pre-clinical data, and first human studies. Neuropharmacology 2005;48:1154–63. [DOI] [PubMed] [Google Scholar]
- 59. Nicotra LL, Vu M, Harvey BS, Smid SD. Prostaglandin ethanolamides attenuate damage in a human explant colitis model. Prostaglandins Other Lipid Mediat 2013;100-101:22–9. [DOI] [PubMed] [Google Scholar]
- 60. Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm Bowel Dis 2014;20:472–80. [DOI] [PubMed] [Google Scholar]
- 61. Duncan M, Thomas AD, Cluny NL, et al. Distribution and function of monoacylglycerol lipase in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 2008;295:G1255–65. [DOI] [PubMed] [Google Scholar]
- 62. Hanlon EC, Tasali E, Leproult R, et al. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J Clin Endocrinol Metab 2015;100:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kantae V, Nahon KJ, Straat ME, et al. Endocannabinoid tone is higher in healthy lean South Asian than white Caucasian men. Sci Rep 2017;7:7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Storr MA, Yüce B, Andrews CN, Sharkey KA. The role of the endocannabinoid system in the pathophysiology and treatment of irritable bowel syndrome. Neurogastroenterol Motil 2008;20:857–68. [DOI] [PubMed] [Google Scholar]
- 65. De Filippis D, Esposito G, Cirillo C, et al. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS One 2011;6:e28159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nallathambi R, Mazuz M, Ion A, et al. Anti-inflammatory activity in colon models is derived from δ9-tetrahydrocannabinolic acid that interacts with additional compounds in cannabis extracts. Cannabis Cannabinoid Res 2017;2:167–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 2004;113:1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Engel MA, Kellermann CA, Burnat G, Hahn EG, Rau T, Konturek PC. Mice lacking cannabinoid CB1-, CB2-receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid [TNBS]-induced colitis. J Physiol Pharmacol 2010;61:89–97. [PubMed] [Google Scholar]
- 69. Ke P, Shao BZ, Xu ZQ, et al. Activation of cannabinoid receptor 2 ameliorates DSS-induced colitis through inhibiting NLRP3 inflammasome in macrophages. PLoS One 2016;11:e0155076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol 2006;291:G364–71. [DOI] [PubMed] [Google Scholar]
- 71. El Bakali J, Muccioli GG, Body-Malapel M, et al. Conformational restriction leading to a selective CB2 cannabinoid receptor agonist orally active against colitis. ACS Med Chem Lett 2015;6:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor [CB2] protects against experimental colitis. Inflamm Bowel Dis 2009;15:1678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Singh UP, Singh NP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor-2 [CB2] agonist ameliorates colitis in IL-10[-/-] mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol Appl Pharmacol 2012;258:256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tourteau A, Andrzejak V, Body-Malapel M, et al. 3-Carboxamido-5-aryl-isoxazoles as new CB2 agonists for the treatment of colitis. Bioorg Med Chem 2013;21:5383–94. [DOI] [PubMed] [Google Scholar]
- 75. Soethoudt M, Grether U, Fingerle J, et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun 2017;8:13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cluny NL, Keenan CM, Duncan M, Fox A, Lutz B, Sharkey KA. Naphthalen-1-yl-[4-pentyloxynaphthalen-1-yl]methanone [SAB378], a peripherally restricted cannabinoid CB1/CB2 receptor agonist, inhibits gastrointestinal motility but has no effect on experimental colitis in mice. J Pharmacol Exp Ther 2010;334:973–80. [DOI] [PubMed] [Google Scholar]
- 77. Li YY, Yuece B, Cao HM, et al. Inhibition of p38/Mk2 signaling pathway improves the anti-inflammatory effect of WIN55 on mouse experimental colitis. Lab Invest 2013;93:322–33. [DOI] [PubMed] [Google Scholar]
- 78. Feng YJ, Li YY, Lin XH, Li K, Cao MH. Anti-inflammatory effect of cannabinoid agonist WIN55, 212 on mouse experimental colitis is related to inhibition of p38MAPK. World J Gastroenterol 2016;22:9515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fichna J, Bawa M, Thakur GA, et al. Cannabinoids alleviate experimentally induced intestinal inflammation by acting at central and peripheral receptors. PLoS One 2014;9:e109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lin S, Li Y, Shen L, et al. The anti-inflammatory effect and intestinal barrier protection of HU210 differentially depend on TLR4 signaling in dextran sulfate sodium-induced murine colitis. Dig Dis Sci 2017;62:372–86. [DOI] [PubMed] [Google Scholar]
- 81. Dziadulewicz EK, Bevan SJ, Brain CT, et al. Naphthalen-1-yl-[4-pentyloxynaphthalen-1-yl]methanone: a potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration. J Med Chem 2007;50:3851–6. [DOI] [PubMed] [Google Scholar]
- 82. Hassanzadeh P, Arbabi E, Atyabi F, Dinarvand R. Application of carbon nanotubes as the carriers of the cannabinoid, 2-arachidonoylglycerol: Towards a novel treatment strategy in colitis. Life Sci 2017;179:66–72. [DOI] [PubMed] [Google Scholar]
- 83. Engel MA, Kellermann CA, Rau T, Burnat G, Hahn EG, Konturek PC. Ulcerative colitis in AKR mice is attenuated by intraperitoneally administered anandamide. J Physiol Pharmacol 2008;59:673–89. [PubMed] [Google Scholar]
- 84. Sarnelli G, D’Alessandro A, Iuvone T, et al. Palmitoylethanolamide modulates inflammation-associated vascular endothelial growth factor [VEGF] signaling via the Akt/mTOR pathway in a selective peroxisome proliferator-activated receptor alpha [PPAR-α]-dependent manner. PLoS One 2016;11:e0156198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alhouayek M, Bottemanne P, Subramanian KV, et al. N-Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis. FASEB J 2015;29:650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Esposito G, Capoccia E, Turco F, et al. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 2014;63:1300–12. [DOI] [PubMed] [Google Scholar]
- 87. Borrelli F, Romano B, Petrosino S, et al. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br J Pharmacol 2015;172:142–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cordaro M, Impellizzeri D, Gugliandolo E, et al. Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of inflammatory bowel disease. Mol Pharmacol 2016;90:549–61. [DOI] [PubMed] [Google Scholar]
- 89. Alhouayek M, Lambert DM, Delzenne NM, Cani PD, Muccioli GG. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J 2011;25:2711–21. [DOI] [PubMed] [Google Scholar]
- 90. Alhamoruni A, Lee AC, Wright KL, Larvin M, O’Sullivan SE. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther 2010;335:92–102. [DOI] [PubMed] [Google Scholar]
- 91. Karwad MA, Couch DG, Theophilidou E, et al. The role of CB1 in intestinal permeability and inflammation. FASEB J 2017;31:3267–77. [DOI] [PubMed] [Google Scholar]
- 92. Alhamoruni A, Wright KL, Larvin M, O’Sullivan SE. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br J Pharmacol 2012;165:2598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kinsey SG, Wise LE, Ramesh D, et al. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther 2013;345:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Andrzejak V, Muccioli GG, Body-Malapel M, et al. New FAAH inhibitors based on 3-carboxamido-5-aryl-isoxazole scaffold that protect against experimental colitis. Bioorg Med Chem 2011;19:3777–86. [DOI] [PubMed] [Google Scholar]
- 95. Tourteau A, Leleu-Chavain N, Body-Malapel M, et al. Switching cannabinoid response from CB[2] agonists to FAAH inhibitors. Bioorg Med Chem Lett 2014;24:1322–6. [DOI] [PubMed] [Google Scholar]
- 96. Sasso O, Migliore M, Habrant D, et al. Multitarget fatty acid amide hydrolase/cyclooxygenase blockade suppresses intestinal inflammation and protects against nonsteroidal anti-inflammatory drug-dependent gastrointestinal damage. FASEB J 2015;29:2616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shamran H, Singh NP, Zumbrun EE, et al. Fatty acid amide hydrolase [FAAH] blockade ameliorates experimental colitis by altering microRNA expression and suppressing inflammation. Brain Behav Immun 2017;59:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhao X, Liang P, Liu J, et al. Elevation of arachidonoylethanolamide levels by activation of the endocannabinoid system protects against colitis and ameliorates remote organ lesions in mice. Exp Ther Med 2017;14:5664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sałaga M, Mokrowiecka A, Zakrzewski PK, et al. Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase [FAAH]. J Crohns Colitis 2014;8:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schicho R, Bashashati M, Bawa M, et al. The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm Bowel Dis 2011;17:1651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stančić A, Jandl K, Hasenöhrl C, et al. The GPR55 antagonist CID16020046 protects against intestinal inflammation. Neurogastroenterol Motil 2015;27:1432–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bento AF, Marcon R, Dutra RC, et al. β-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARγ pathway. Am J Pathol 2011;178:1153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Vitor CE, Figueiredo CP, Hara DB, Bento AF, Mazzuco TL, Calixto JB. Therapeutic action and underlying mechanisms of a combination of two pentacyclic triterpenes, alpha- and beta-amyrin, in a mouse model of colitis. Br J Pharmacol 2009;157:1034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Matos I, Bento AF, Marcon R, Claudino RF, Calixto JB. Preventive and therapeutic oral administration of the pentacyclic triterpene α,β-amyrin ameliorates dextran sulfate sodium-induced colitis in mice: the relevance of cannabinoid system. Mol Immunol 2013;54:482–92. [DOI] [PubMed] [Google Scholar]
- 105. Borrelli F, Aviello G, Romano B, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med [Berl] 2009;87:1111–21. [DOI] [PubMed] [Google Scholar]
- 106. Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology 2012;89:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jamontt JM, Molleman A, Pertwee RG, Parsons ME. The effects of Delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br J Pharmacol 2010;160:712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Krohn RM, Parsons SA, Fichna J, et al. Abnormal cannabidiol attenuates experimental colitis in mice, promotes wound healing and inhibits neutrophil recruitment. J Inflamm [Lond] 2016;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pagano E, Capasso R, Piscitelli F, et al. An orally active cannabis extract with high content in Cannabidiol attenuates chemically-induced intestinal inflammation and hypermotility in the mouse. Front Pharmacol 2016;7:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Malfait AM, Gallily R, Sumariwalla PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A 2000;97:9561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Karmaus PW, Wagner JG, Harkema JR, Kaminski NE, Kaplan BL. Cannabidiol [CBD] enhances lipopolysaccharide [LPS]-induced pulmonary inflammation in C57BL/6 mice. J Immunotoxicol 2013;10:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wallace JL, Flannigan KL, McKnight W, Wang L, Ferraz JG, Tuitt D. Pro-resolution, protective and anti-nociceptive effects of a cannabis extract in the rat gastrointestinal tract. J Physiol Pharmacol 2013;64:167–75. [PubMed] [Google Scholar]
- 113. Borrelli F, Fasolino I, Romano B, et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol 2013;85:1306–16. [DOI] [PubMed] [Google Scholar]
- 114. Romano B, Borrelli F, Fasolino I, et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol 2013;169:213–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Couch DG, Maudslay H, Doleman B, Lund JN, O’Sullivan SE. The use of cannabinoids in colitis: a systematic review and meta-analysis. Inflamm Bowel Dis 2018;24:680–97. [DOI] [PubMed] [Google Scholar]
- 116. García-Planella E, Marín L, Domènech E, et al. Use of complementary and alternative medicine and drug abuse in patients with inflammatory bowel disease. Med Clin [Barc] 2007;128:45–8. [DOI] [PubMed] [Google Scholar]
- 117. Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2011;23:891–6. [DOI] [PubMed] [Google Scholar]
- 118. Ravikoff Allegretti J, Courtwright A, Lucci M, Korzenik JR, Levine J. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Weiss A, Friedenberg F. Patterns of cannabis use in patients with Inflammatory Bowel Disease: a population based analysis. Drug Alcohol Depend 2015;156:84–9. [DOI] [PubMed] [Google Scholar]
- 120. Kerlin AM, Long M, Kappelman M, Martin C, Sandler RS. Profiles of patients who use marijuana for inflammatory bowel disease. Dig Dis Sci 2018;63:1600–4. [DOI] [PubMed] [Google Scholar]
- 121. Hoffenberg EJ, McWilliams SK, Mikulich-Gilbertson SK, et al. Marijuana use by adolescents and young adults with inflammatory bowel disease. J Pediatr 2018;199:99–105. [DOI] [PubMed] [Google Scholar]
- 122. Naftali T, Lev LB, Yablecovitch D, Yablekovitz D, Half E, Konikoff FM. Treatment of Crohn’s disease with cannabis: an observational study. Isr Med Assoc J 2011;13:455–8. [PubMed] [Google Scholar]
- 123. Lahat A, Lang A, Ben-Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: a pilot prospective study. Digestion 2012;85:1–8. [DOI] [PubMed] [Google Scholar]
- 124. Naftali T, Bar-Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol 2013;11:1276–80.e1. [DOI] [PubMed] [Google Scholar]
- 125. Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol 2016;13:567–79. [DOI] [PubMed] [Google Scholar]
- 126. Naftali T, Mechulam R, Marii A, et al. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig Dis Sci 2017;62:1615–20. [DOI] [PubMed] [Google Scholar]
- 127. Naftali T, Bar Lev Schlieder L, Sklerovsky Benjaminov F, et al. P398 cannabis induces clinical and endoscopic improvement in moderately active ulcerative colitis [UC]. J Crohn’s Colitis 2018;12[Suppl 1]:S306. [Google Scholar]
- 128. Irving PM, Iqbal T, Nwokolo C, et al. A randomized, double-blind, placebo-controlled, parallel-group, pilot study of cannabidiol-rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflamm Bowel Dis 2018;24:714–24. [DOI] [PubMed] [Google Scholar]
- 129. Kalenderoglou N, Macpherson T, Wright KL. Cannabidiol reduces leukemic cell size but is it important?Front Pharmacol 2017;8:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 2010;7:281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Grimsey NL, Goodfellow CE, Scotter EL, Dowie MJ, Glass M, Graham ES. Specific detection of CB1 receptors; cannabinoid CB1 receptor antibodies are not all created equal!J Neurosci Methods 2008;171:78–86. [DOI] [PubMed] [Google Scholar]
- 132. Marchalant Y, Brownjohn PW, Bonnet A, Kleffmann T, Ashton JC. Validating antibodies to the cannabinoid CB2 receptor: antibody sensitivity is not evidence of antibody specificity. J Histochem Cytochem 2014;62:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bachovchin DA, Cravatt BF. The pharmacological landscape and therapeutic potential of serine hydrolases. Nat Rev Drug Discov 2012;11:52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 2015;16:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Camilleri M, Kolar GJ, Vazquez-Roque MI, Carlson P, Burton DD, Zinsmeister AR. Cannabinoid receptor 1 gene and irritable bowel syndrome: phenotype and quantitative traits. Am J Physiol Gastrointest Liver Physiol 2013;304:G553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Pinto L, Izzo AA, Cascio MG, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology 2002;123:227–34. [DOI] [PubMed] [Google Scholar]
- 137. Esfandyari T, Camilleri M, Ferber I, Burton D, Baxter K, Zinsmeister AR. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil 2006;18:831–8. [DOI] [PubMed] [Google Scholar]
- 138. Sharkey KA, Darmani NA, Parker LA. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol 2014;722:134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wasilewski A, Lewandowska U, Mosinska P, et al. Cannabinoid receptor type 1 and mu-opioid receptor polymorphisms are associated with cyclic vomiting syndrome. Am J Gastroenterol 2017;112:933–9. [DOI] [PubMed] [Google Scholar]
- 140. Sorensen CJ, DeSanto K, Borgelt L, Phillips KT, Monte AA. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment a systematic review. J Med Toxicol 2017;13:71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sharkey KA, Wiley JW. The role of the endocannabinoid system in the brain-gut axis. Gastroenterology 2016;151:252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther 2010;126:21–38. [DOI] [PubMed] [Google Scholar]
- 143. Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep 2018;18:50. [DOI] [PubMed] [Google Scholar]
- 144. Wilkerson JL, Niphakis MJ, Grim TW, et al. The selective monoacylglycerol lipase inhibitor MJN110 produces opioid-sparing effects in a mouse neuropathic pain model. J Pharmacol Exp Ther 2016;357:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Taschler U, Eichmann TO, Radner FP, et al. Monoglyceride lipase deficiency causes desensitization of intestinal cannabinoid receptor type 1 and increased colonic μ-opioid receptor sensitivity. Br J Pharmacol 2015;172:4419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Börner C, Höllt V, Kraus J. Cannabinoid receptor type 2 agonists induce transcription of the mu-opioid receptor gene in Jurkat T cells. Mol Pharmacol 2006;69:1486–91. [DOI] [PubMed] [Google Scholar]