Abstract
Background
Our research was designed to investigate the relationship of spleen tyrosine kinase (Syk) and inflammatory factors with coronary heart disease (CHD) and the risk factors of CHD.
Material/Methods
In our study, 226 patients were enrolled, from October 2017 to March 2018. Clinical and biochemical data were collected. We collected samples of peripheral blood monocytes (PBMs) from the enrolled patients. The patients were divided in 4 groups: patients without coronary artery disease (control group), patients with stable angina pectoris (SAP group), patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS group), and patients with ST-segment elevation acute myocardial infarction group (STEMI group). We detect the protein levels of Syk and inflammatory factors expression by western blot.
Results
Our results found the protein levels of Syk and inflammatory factors expression in the NSTE-ACS and STEMI groups were higher than those in the SAP and control groups. The protein levels of Syk and inflammatory factors expression in the SAP group were higher than those in the control group. Moreover, there were many risk factors significantly associated with Syk. Besides that, these risk factors were also independent risk factors of CHD.
Conclusions
Our results found that the level of Syk was associated with the severity of CHD. From our study, we found that higher levels of Syk and inflammatory factors protein were associated with worse results of the CHD. For the first time, Syk was reported to be a promising therapeutic factor for CHD patients.
MeSH Keywords: Coronary Disease, Inflammation, Risk Factors
Background
Coronary heart disease (CHD) is a multifactorial disease, characterized by a chronic inflammatory process occurring primarily at the atherosclerotic plaque [1]. Many researchers have found that inflammation was very important in the initiation and progression of atherosclerosis among these diseases including CHD [2,3]. However, it depends on various metabolic and inflammatory factors, as well as the genetic background of the patient [4]. Although CHD mortality has decreased obviously over the past decades in developed and underdeveloped countries, it remains the main cause of death and disability in the world [5,6]. Many CHD patients often present with a painless cardiovascular event, without classical clinical manifestations. We hoped in this study to find some relatively inexpensive, suitable and widely available inflammatory factors which would be able to predict the severity of CHD.
Monocytes play an important role in the inflammatory process of CHD. Inflammatory response can induce secretion of a variety of inflammatory factors, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-12, matrix metalloproteinase (MMP)-9, and monocyte chemoattractant protein (MCP)-1 [7–10]. These factors will exacerbate inflammatory response in the plaques [11–14].
Spleen tyrosine kinase (Syk), a cytoplasmic tyrosine kinase, is a member of the non-receptor-type protein tyrosine kinase family. Syk is known to be mainly expressed in hematopoietic cells, but its expression has also been reported in epithelial and endothelial cells [15–17]. The reported roles of Syk include, but are not limited to, relaying adaptive immune receptor signaling, cellular adhesion, innate immune recognition, platelet activation, and vascular development [18]. Several research studies have found that Syk is involved in many diseases, such as cancer, infectious diseases, rheumatoid arthritis, allergic asthma, thrombocytopenic purpura, etc. [19]. As we know, Syk plays a major role in the initiation and the progression of inflammation. Syk, as an inflammatory factor has gained more and more focus. However, research on the correlation between Syk and CHD is not well established. Finding the correlation between Syk and CHD could help in be better understanding of the level of Syk related to the severity of patients with CHD. The aim of our research was to study whether Syk and inflammatory factors are correlated with CHD. Our research detected the expression levels and the possible correlations between the Syk and inflammatory factors in different CHD patients. Syk may help predict the acute coronary events before clinical manifestations of the disease appear. On the other hand, Syk may be a promising therapeutic factor for the CHD.
Cardiovascular risk factors contain many classical risk factors, including serum concentrations of cholesterol, blood pressure, age, sex, diabetes mellitus, dyslipidemia, smoking status, and depression [20–29]. These risk factors increase the risk of CHD. However, there are no studies showing the relationship of Syk and CHD risk factors. We proposed to research the relationship of Syk and cardiovascular risk factors in different CHD patients. There were still many CHD patients without traditional risk factors, so we must find other risk factors for patients with CHD. Our study found some risk factors that have a predictive value of severity in CHD patients.
Increased understanding of the role of the inflammatory response in atherosclerosis may lead to the discovery of more effective diagnostic and treatment methods and ultimately improve the prognosis of patients with CHD. Our research found that high Syk and inflammatory factors levels were related to an increased severity of CHD. From our laboratory results, we found that the levels of Syk and inflammatory factors might be predicting the severity of different CHD patients, because there were significant positive correlations between them. We hoped that our finding will encourage other investigations on these biomarkers in CHD patients.
Material and Methods
Patients
Our study recruited patients with or without CHD from Heze Municipal Hospital from October 2017 to March 2018. The patients were 31 to 83 years of age. Coronary angiography was performed for every patient, and different postures were chosen if necessary. The results of coronary angiography in the 4 group patients were analyzed by 2 experienced doctors. The exclusion criteria included lung disease, kidney disease, liver disease, heart failure, cancer, or inability to participate in the research. The research was approved by the Medical Ethics Committee of Heze Municipal Hospital. All patients’ data, including demographics, diseases history, and laboratory test were obtained from hospital medical records.
Patients were divided into 4 groups according to the guidelines of the American College of Cardiology/American Heart Association (ACC/AHA) [30]. There were 52 patients without coronary artery disease (control group), 58 patients with stable angina pectoris (SAP group), 60 patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS group), and 56 patients with ST-segment elevation acute myocardial infarction (STEMI group). The baseline characteristics of the 4 groups of patients are shown in Table 1.
Table 1.
Clinical and biochemical characteristics of the patients in the four group patients.
| Parameters | Control (n=52) | SAP (n=58) | NSTE-ACS (n=67) | STEMI (n=49) | p Value |
|---|---|---|---|---|---|
| Age (year) | 60.35±1.40 | 60.05±1.32 | 61.50±1.30 | 62.00±1.35 | 0.698 |
| Male [n (%)] | 18 (34.6%) | 39 (67.2%) | 46 (68.7%) | 34 (69.4%) | 0.000 |
| Drinking [n (%)] | 5 (9.6%) | 15 (25.9%) | 24 (35.8%) | 16 (32.7%) | 0.009 |
| Smoking [n (%)] | 4 (7.7%) | 24 (41.4%) | 35 (52.2%) | 25 (51.0%) | 0.000 |
| Diabetes [n (%)] | 5 (9.6%) | 5 (8.6%) | 11 (16.4%) | 10 (20.4%) | 0.233 |
| Hypertension [n (%)] | 14 (26.9%) | 19 (32.8%) | 30 (44.8%) | 14 (28.6%) | 0.206 |
| SBP (mmHg) | 134.56±2.80 | 138.59±2.65 | 139.85±2.61 | 137.54±2.70 | 0.562 |
| DBP (mmHg) | 76.90±1.65 | 79.69±1.56 | 80.12±1.53 | 76.23±1.59 | 0.206 |
| Heart rate (beats/min) | 72.58±1.29 | 71.72±1.22 | 73.38±1.20 | 71.11±1.24 | 0.575 |
| Scr (μmol/L) | 63.89±2.02 | 67.97±1.91 | 70.18±1.88 | 66.16±1.94 | 0.132 |
| Hcy (μmol/L) | 10.63±0.82 | 11.97±0.78 | 10.94±0.76 | 9.61±0.79 | 0.206 |
| TC (mmol/L) | 4.40±0.15 | 4.26±0.15 | 4.49±0.14 | 4.07±0.15 | 0.206 |
| WBC (109/L) | 6.28±0.58 | 7.22±0.55 | 7.82±0.54 | 8.16±0.56 | 0.011 |
| TG (mmol/L) | 1.52±0.19 | 1.45±0.18 | 1.87±0.18 | 1.70±0.19 | 0.359 |
| HDL (mmol/L) | 1.32±0.05 | 1.32±0.05 | 1.07±0.05 | 1.12±0.05 | 0.000 |
| LDL (mmol/L) | 2.83±0.13 | 2.77±0.12 | 2.95±0.12 | 2.66±0.13 | 0.414 |
| ALT (U/L) | 26.77±3.79 | 33.59±3.59 | 34.80±3.53 | 35.48±3.65 | 0.331 |
| FIB (g/L) | 2.59±2.06 | 6.70±1.95 | 2.96±1.92 | 2.72±1.99 | 0.039 |
| CK (U/L) | 80.96±87.82 | 95.07±83.15 | 80.17±81.75 | 960.20±84.62 | 0.000 |
| CK-MB (U/L) | 15.81±9.18 | 16.17±8.69 | 15.70±8.54 | 107.00±8.84 | 0.000 |
| AST (U/L) | 23.23±8.99 | 28.21±8.51 | 27.52±8.37 | 108.20±8.67 | 0.000 |
| UA (mmol/L) | 290.31±11.26 | 310.66±10.66 | 317.98±10.48 | 283.14±10.85 | 0.073 |
| FPG (mmol/L) | 5.27±0.30 | 5.45±0.29 | 5.81±0.28 | 6.26±0.29 | 0.044 |
| NT-proBNP (pg/ml) | 146.46±100.23 | 307.19±94.90 | 481.98±93.31 | 562.61±96.58 | 0.014 |
| LVIDd (mm) | 46.50±0.55 | 47.03±0.52 | 47.22±0.51 | 47.46±0.53 | 0.630 |
| LVEF (%) | 61.08±0.92 | 60.31±0.87 | 58.95±0.86 | 57.93±0.89 | 0.065 |
| LVFS (%) | 33.39±0.59 | 32.86±0.56 | 31.47±0.55 | 31.39±0.57 | 0.030 |
| The number of vessel disease (n) | 0.06±0.09 | 1.29±0.08 | 2.40±0.08 | 2.61±0.08 | 0.000 |
| The number of stent placement (n) | 0.02±0.11 | 0.71±0.11 | 1.83±0.11 | 1.80±0.11 | 0.000 |
Normally distributed continuous variables are presented as mean ±SD. Categorical variables are presented as frequencies. SBP – systolic blood pressure; DBP – diastolic blood pressure; Scr – serum creatinine; Hcy – homocysteine; TC – total cholesterol; WBC – white blood cells; TG – triglyceride; HDL – high-density lipoprotein; LDL – low-density lipoprotein; ALT – alanine aminotransferase; FIB – fibrinogen; CK – creatine kinase; CK-MB – creatine kinase-MB; AST – aspartate aminotransferase; UA – uric acid; FPG – fasting blood glucose; NT-proBNP – N-terminal pro brain natriuretic peptide; LVIDd – left ventricular internal diameter at end-diastole; LVEF – left ventricular ejection fraction; LVFS – left ventricular fractional shortening.
Separation of peripheral blood monocytes
All blood samples were taken after an overnight 12 hours fasting period. Blood samples were collected from every patient on admission to the hospital. We obtained about 15 mL of peripheral blood from each patient. The blood samples with the same volume of phosphate-buffered saline (PBS) were poured into 50 mL conical centrifuge tubes and mixed thoroughly at room temperature. Thereafter, 9 mL of lymphocyte separation liquid was added to the diluted cell suspension. The mixture was centrifuged at 2500 rpm for 30 minutes at 20°C. Following this, the mononuclear cell layer was transferred into a new centrifuge tube and washed 3 times with excess PBS. The monocular cells were incubated with 2 mL of high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and then transferred to a 6-well cell culture plate. The plate containing the cells was incubated horizontally for 2 hours in a 37°C, 5% CO2-humidified incubator. The peripheral blood monocytes (PBMs) were obtained from the bottom of the plate after 2 hours of incubation according to their characteristic of adherence. The purity of monocular cells in this study was 92% and cell viability was 95%.
Western blot
Cells were washed twice with ice-cold PBS. Proteins were then extracted using Cell Lysis buffer. Nuclear and whole cell extracts containing protein were subjected to 10% sodium sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Then, the gel was transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% non-fat milk in tris-buffered saline with tween (TBST) at room temperature for 2 hours and incubated overnight at 4°C with the following first antibody: rabbit-anti Syk, rabbit-anti IL-6, rabbit-anti IL-12, rabbit-anti MMP-9, rabbit-anti MCP-1. They were washed with TBST and then probed with a second antibody conjugated with horseradish peroxidase at room temperature for 1 hour. Using an enhanced chemiluminescence kit, immunoreactive bands were detected by an automatic gel imaging machine.
Statistics
Continuous variables were expressed as the mean ± standard deviation (SD). Independent sample t-test was used to compare the means ± SD between 2 groups. Descriptive statistics were performed using one-way analysis of variance (ANOVA) tests. One-way ANOVA was used to compare with the expression level of Syk and the expression levels of inflammatory factors in the 4 groups of patients, followed by Tukey’s multiple comparisons test. Pearson correlation analysis was carried out to analysis the correlation between the expression level of Syk and the expression levels of inflammatory factors in the 4 groups of patients. The multivariate logistic regression analysis was carried out for the analysis of the risk factors of the 4 groups of patients. The multivariate linear regression analysis was used for the analysis of the independent risk factors of the 4 groups of patients. Pearson correlation analysis was carried out to analysis the correlation between the expression level of Syk and the independent risk factors in the 4 groups of patients. All analyses were conducted using SPSS 17.0, and a value of P<0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 226 patients met the research criteria and were included in this research. The mean age of the patients was 61 year, and the proportion of male patients was 39.3%. Besides, the mean level of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were 138 mm Hg and 78 mm Hg, respectively. The mean baseline heart rate was 72 beats per minute. There were 77 patients (34.5%) who had a history of hypertension, and 31 patients (13.7%) who had diabetes mellitus. There were 88 patients (38.9%) identified as smokers, and 60 patients (26.5%) identified as drinkers. Except for demographics and disease history of patients, related laboratory test, including white blood cells (WBC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (Scr), homocysteine (Hcy), fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), creatine kinase (CK), creatine kinase-MB (CK-MB), uric acid (UA), fibrinogen (FIB), N-terminal pro brain natriuretic peptide (NT-proBNP), left ventricular internal diameter at end-diastole (LVIDd), left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), the number of vessel disease, the number of stent placement were also recorded in detail. The data were summarized in Table 1.
Clinical and biochemical characteristics
As shown in Table 1, there were distinct differences among the 4 groups of patients with regard to sex, drinking, smoking, WBC, HDL, FIB, FPG, CK, CK-MB, AST, NT-proBNP, LVFS, the number of vessel disease, and the number of stent placement. As shown in Table 1, there were no distinct differences among the 4 groups of patients in age, diabetes, hypertension, SBP, DBP, heart rate, Scr, Hcy, TC, TG, LDL, ALT, UA, LVIDd and LVEF.
Logistic regression analysis of the risk factors in the four group patients
The 4 groups of patients were regarded as dependent variables, and age, sex, drinking, smoking, diabetes, hypertension, SBP, DBP, heart rate, WBC, HDL, FIB, CK, CK-MB, AST, Scr, Hcy, TC, TG, LDL, ALT, UA, FPG, NT-proBNP, LVIDd, LVEF, LVFS, the number of vessel disease, and the number of stent placement were taken as independent variables. Logistic regression analysis was conducted, and the results showed that sex, drinking, smoking, WBC, FIB, FPG, CK, CK-MB, AST, NT-proBNP, LVFS, the number of vessel disease, and the number of stent placement were statistically significant differences risk factors in the 4 groups of patients (Table 2).We also found that for age, diabetes, hypertension, SBP, DBP, heart rate, Scr, Hcy, TC, TG, LDL, ALT, UA, HDL, LVIDd and LVEF, there was no significant differences in risk factors between the 4 groups of patients (Table 2).
Table 2.
Logistic regression analysis results of the risk factors in the four groups patients.
| Parameters | B | S.E. | Wald | P |
|---|---|---|---|---|
| Male [n (%)] | 1.434 | 0.334 | 18.403 | 0.000 |
| Drinking [n (%)] | 1.469 | 0.498 | 8.705 | 0.003 |
| Smoking [n (%)] | 2.416 | 0.542 | 19.863 | 0.000 |
| WBC (109/L) | 0.869 | 0.252 | 11.880 | 0.001 |
| HDL (mmol/L) | −0.400 | 0.233 | 2.942 | 0.086 |
| FIB (g/L) | 0.567 | 0.235 | 5.799 | 0.016 |
| CK (U/L) | 0.530 | 0.176 | 9.070 | 0.003 |
| CK-MB (U/L) | 0.487 | 0.212 | 5.276 | 0.022 |
| AST (U/L) | 0.615 | 0.213 | 8.304 | 0.004 |
| FPG (mmol/L) | 0.368 | 0.207 | 3.171 | 0.045 |
| NT-proBNP (pg/ml) | 0.683 | 0.198 | 11.887 | 0.001 |
| LVFS (%) | −0.483 | 0.170 | 8.084 | 0.004 |
| The number of vessel disease (n) | 6.114 | 0.931 | 43.132 | 0.000 |
| The number of stent placement (n) | 5.060 | 1.031 | 24.103 | 0.000 |
WBC – white blood cells; HDL – high-density lipoprotein; FIB – fibrinogen; CK – creatine kinase; CK-MB – creatine kinase-MB; AST – aspartate aminotransferase; FPG – fasting blood glucose; NT-proBNP – N-terminal pro brain natriuretic peptide; LVFS – left ventricular fractional shortening.
Multiple linear regression analysis of independent risk factors in the 4 groups
For the 4 groups of patients, dependent variables were and sex, drinking, smoking, WBC, FIB, FPG, CK, CK-MB, AST, NT-proBNP, LVFS; the number of vessel disease and the number of stent placement were taken as independent variables. Multiple linear regression analysis was conducted, and the results showed that sex, drinking, smoking, WBC, FIB, CK, CK-MB, AST, NT-proBNP, LVFS, the number of vessel disease, and the number of stent placement were all independent risk factors of the 4 groups of patients (Table 3). We also found that FPG was not an independent risk factor in the 4 groups of patients (Table 3).
Table 3.
Multiple linear regression analysis results of independent risk factors in the four group patients.
| Parameters | B | S.E. | Beta | T | P |
|---|---|---|---|---|---|
| Male [n (%)] | 0.256 | 0.055 | 0.296 | 4.647 | 0.000 |
| Drinking [n (%)] | 0.200 | 0.062 | 0.210 | 3.209 | 0.002 |
| Smoking [n (%)] | 0.302 | 0.054 | 0.350 | 5.598 | 0.000 |
| WBC (109/L) | 0.134 | 0.037 | 0.237 | 3.655 | 0.000 |
| FIB (g/L) | 0.093 | 0.038 | 0.163 | 2.466 | 0.014 |
| CK (U/L) | 0.086 | 0.027 | 0.205 | 3.140 | 0.002 |
| CK-MB (U/L) | 0.072 | 0.030 | 0.156 | 2.371 | 0.019 |
| AST (U/L) | 0.082 | 0.026 | 0.203 | 3.098 | 0.002 |
| FPG (mmol/L) | 0.063 | 0.035 | 0.119 | 1.799 | 0.073 |
| NT-proBNP (pg/ml) | 0.099 | 0.027 | 0.239 | 3.689 | 0.000 |
| LVFS (%) | −0.088 | 0.030 | −0.193 | −2.940 | 0.004 |
| The number of vessel disease (n) | 0.268 | 0.016 | 0.737 | 16.321 | 0.000 |
| The number of stent placement (n) | 0.239 | 0.023 | 0.570 | 10.381 | 0.000 |
WBC – white blood cells; FIB – fibrinogen; CK – creatine kinase; CK-MB – creatine kinase-MB; AST – aspartate aminotransferase; FPG – fasting blood glucose; NT-proBNP – N-terminal pro brain natriuretic peptide; LVFS – left ventricular fractional shortening.
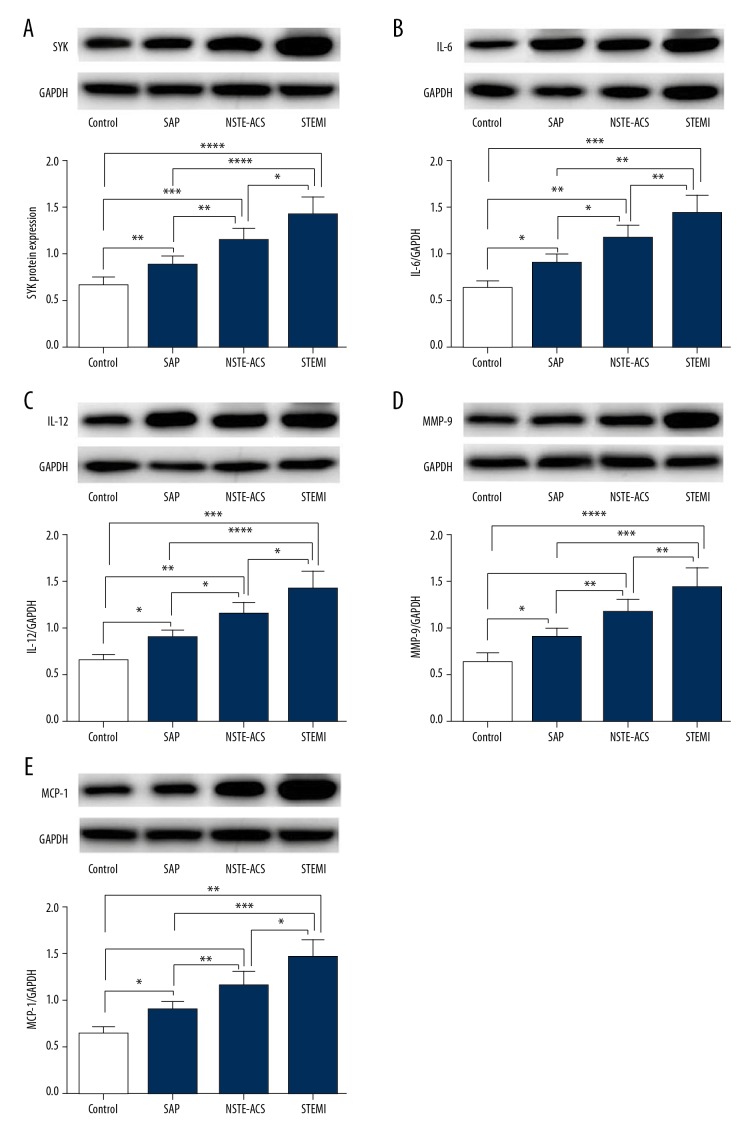
Western blot analysis
Western blot result showed that the protein expressions of Syk, IL-6, IL-12, MMP-9, and MCP-1 were higher in the STEMI group than in the NSTE-ACS group, the SAP group and the control group (Figure 1). The protein expressions of Syk, IL-6, IL-12, MMP-9, and MCP-1 were higher in the NSTE-ACS group than in the SAP group and the control group (Figure 1). The protein expression levels of Syk, IL-6, IL-12, MMP-9 and MCP-1 in the SAP group were higher than in the control group (Figure 1).
Figure 1.
The protein expression levels of Syk, IL-6, IL-12, MMP-9 and MCP-1 in the peripheral blood monocytes of the 4 groups of patients by western blotting. Peripheral blood monocytes were obtained from patients with STEMI, NSTEACS, SAP, and control groups. (A) The protein expression levels of Syk in the peripheral blood monocytes of the 4 groups were analyzed by western blotting and quantitative. (B) The protein expression levels of IL-6 in the peripheral blood monocytes of the 4 groups were analyzed by western blotting and quantitative. (C) The protein expression levels of IL-12 in the peripheral blood monocytes of the 4 groups were analyzed by western blotting and quantitative. (D) The protein expression levels of MMP-9 in the peripheral blood monocytes of the four groups were analyzed by western blotting and quantitative. (E) The protein expression levels of MCP-1 in the peripheral blood monocytes of the 4 groups were analyzed by western blotting and quantitative. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001 compared to control. The quantitative data were expressed as the mean ±SEM of at least 3 independent experiments. Control – patients without coronary stenosis; NSTE-ACS – non-ST-segment elevation acute coronary syndrome; SAP – stable angina pectoris; STEMI – ST segment elevation myocardial infarction.
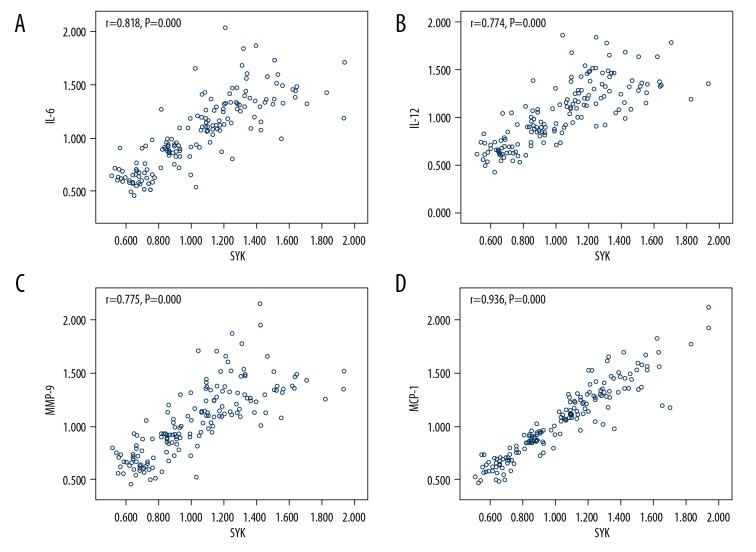
Pearson correlation analysis of the correlation between Syk and inflammatory factors in the 4 group patients
Pearson correlation analysis showed that the expression level of Syk had a positive correlation with the expression levels of IL-6, IL-12, MMP-9, and MCP-1 in 4 groups of patients (Figure 2).
Figure 2.
Analysis of the correlation between Syk and inflammatory factors. (A) There is a positive correlation between Syk and IL-6 in patients with or without coronary heart disease (CHD). (B) There is a positive correlation between Syk and IL-12 in patients with or without CHD. (C) There is a positive correlation between Syk and MMP-9 in patients with or without CHD. (D) There is a positive correlation between Syk and MCP-1 in patients with or without CHD.
Pearson correlation analysis of the correlation between Syk and independent risk factors in the 4 groups of patients
The independent risk factors were sex, drinking, smoking, WBC, FIB, CK, CK-MB, AST, NT-proBNP, LVFS, the number of vessel disease, and the number of stent placement. Pearson correlation analysis showed that the expression level of Syk had a positive correlation with smoking, WBC, CK, CK-MB, AST, NT-proBNP, the number of vessel disease, and the number of stent placement in the 4 groups of patients (Table 4). Pearson correlation analysis showed that the expression level of Syk had a negative correlation with the expression levels in male and with LVFS in the 4 groups of patients (Table 4).
Table 4.
Pearson correlation analysis results between Syk and independent risk factors in the four group patients.
| Syk | r | P |
|---|---|---|
| Male [n (%)] | −0.215 | 0.005 |
| Drinking [n (%)] | 0.127 | 0.101 |
| Smoking[n (%)] | 0.276 | 0.000 |
| WBC (109/L) | 0.148 | 0.046 |
| FIB (g/L) | −0.036 | 0.646 |
| CK (U/L) | 0.428 | 0.000 |
| CK-MB (U/L) | 0.429 | 0.000 |
| AST (U/L) | 0.445 | 0.000 |
| NT-proBNP (pg/ml) | 0.173 | 0.027 |
| LVFS (%) | −0.176 | 0.023 |
| The number of vessel disease (n) | 0.716 | 0.000 |
| The number of stent placemen (n) | 0.536 | 0.000 |
WBC – white blood cells; HDL – high-density lipoprotein; FIB – fibrinogen; CK – creatine kinase; CK-MB – creatine kinase-MB; AST – aspartate aminotransferase; FPG – fasting blood glucose; NT-proBNP – N-terminal pro brain natriuretic peptide; LVFS – left ventricular fractional shortening.
Discussion
As we know, Syk plays a major role in the initiation and the progression of inflammation. Nonetheless, the expression and clinical significance of Syk in patients with CHD is unknown. Our study first indicated that the protein levels of Syk and inflammatory factors increased with the increase severity of CHD. We also found many risk factors associated with the CHD. For the first time, we identified a potential therapeutic target related to the patients with CHD.
In our study, we investigated the relationships of Syk with the impact factors in patients with CHD. Our results showed that the protein level of Syk was significantly associated with the risk factors of CHD. Our study also found that Syk and the inflammatory factors were associated with the severity of CHD, especially for the high-risk CHD patients. The increase severity of CHD in patients was associated with the increase of the protein levels of Syk and inflammatory factors. Therefore, the levels of Syk and the inflammatory factors may be considered as an assessment measure for clinical outcomes.
From our study, we found that the expression levels of Syk and inflammatory factors were higher in patients with STEMI than in patients with NSTE-ACS, which were also higher than that in patients in the SAP group and the control group. Similarly, the levels of Syk and inflammatory factors measured by western blot were also higher in patients with NSTE-ACS than in patients in the SAP group and the control group. Hence, we concluded that the expression levels of Syk and inflammatory factors in patients with CHD increased with the severity of CHD increase. Thus, patients with high Syk and inflammatory factors levels have a higher risk of developing CHD and more severe coronary artery stenosis than others. We speculated that patients with high expression levels of Syk and related inflammatory factors had lower stability of atherosclerotic plaques, increased probability of plaque rupture, higher risk of CHD, and more severe coronary artery stenosis. We speculated that Syk was an important factor in regulating the level of inflammatory response, and inhibition of Syk could reduce the inflammatory response of atherosclerosis and thus stabilize the vulnerable atherosclerotic plaques.
In this study, we found that the expression level of Syk was significantly correlated with the risk factors of CHD, which suggests that we could use these highly correlated risk factors to monitor the inflammatory response of coronary atherosclerosis, and thus to monitor the acute cardiovascular events in patients with CHD. In this study, we have shown that sex, drinking, smoking, WBC, FIB, CK, CK-MB, AST, NT-proBNP, LVFS, the number of vessel disease, and the number of stent placement are strongly associated with Syk. In our study, LDL, FPG, and LVEF were not statistically associated with Syk. Whether the change of Syk expression level can reflect clinical outcome then alteration in treatment, remains uncertain. It was quite a surprise finding that the LDL level was no distinctly different between the control group of patients and CHD patients. We thought this might have something to do with the fact that some of the enrolled patients were already on statins and some were already on a low-fat diet.
This study showed that the number of white blood cells in the blood circulation is positively correlated with the severity of CHD, suggesting that white blood cells are related to inflammation and plaque instability in the body. This study showed that the higher the severity of CHD, the higher the level of FIB in the body and the higher the level of inflammation in the body, which may be related to the fact that FIB can stimulate the proliferation and migration of vascular smooth muscle cells, increase the blood viscosity, slow down the blood flow, accelerate the process of arteriosclerosis, and lead to severe arterial stenosis. Myocardial enzyme spectrum is an important hematological indicator of myocardial injury, such as AST, CK, and CK-MB, which are often used for the diagnosis of clinical severity in patients with CHD. This study found that elevated NT-proBNP concentration was associated with the severity of CHD. Myocardial ischemia may lead to the synthesis and release of NT-proBNP in proportion to myocardial ischemia. Therefore, NT-proBNP concentration can reflect the range and severity of ischemic injury. Color Doppler ultrasonography can dynamically show the internal structure of the heart, the rhythm of the heart and blood flow. The changes of patients’ myocardium can be seen through color Doppler ultrasound, and the myocardial movement and cardiac function of patients with CHD can be observed through the visual display of color Doppler ultrasound. Therefore, in future clinical work, we can predict the occurrence of cardiovascular events by detecting the changes of these factors.
In our study, we found Syk was one important factor regulating inflammation levels. Moreover, downregulation of Syk may reduce the inflammatory response and also reduce the incidence of cardiovascular events. Syk may be a better therapeutic target for the patients with CHD. A further investigation on heterogeneous effects of Syk changes is needed. Several limitations of this study must be acknowledged upon evaluating the results of this research. Firstly, the sample size was small so that a larger sample size is needed to replicate our findings. Secondly, only 4 inflammatory factors were investigated in our study. More representative inflammatory factors should be assessed in additional studies. Thirdly, we did not collect long-term outcomes to show the adverse outcomes rates in this study. Lastly, the mechanism of the association of Syk with inflammatory factors was not studied. This is the first time Syk has been reported to be related the progression of diseases, so further elucidation is required to explain its pathway.
Conclusions
Syk plays an important role in the occurrence and development of inflammatory responses in coronary atherosclerosis. As an emerging inflammatory marker, Syk has attracted more and more attention. Our study emphasized the importance of Syk and inflammatory factors associated with the severity of CHD. In addition, our findings may reveal a new therapeutic approach compared with traditional drug therapies that use statins, aspirin, clopidogrel, angiotensin converting enzyme inhibitors, and other drugs that play a therapeutic role mainly through mechanisms other than inflammation. Introduction of Syk into cardiovascular system research will provide new potential targets for the research of cardiovascular system diseases.
Footnotes
Source of support: Departmental sources
References
- 1.Wu MY, Li CJ, Hou MF, Chu PY. New insights into the role of inflammation in the pathogenesis of atherosclerosis. In J Mol Sci. 2017;18:20–34. doi: 10.3390/ijms18102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka K, Sata M. [Atherosclerosis: Progress in diagnosis and treatments. Topics: II. Atherosclerosis – promoting factors; Pathogenesis and pathophysiology; 3. From basic research: focusing on large and peripheral vessels]. Nihon Naika Gakkai Zasshi. 2013;102:305–12. doi: 10.2169/naika.102.305. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 3.Plasschaert H, Heeneman S, Daemen MJ. Progression in atherosclerosis: Histological features and pathophysiology of atherosclerotic lesions. Top Magn Reson Imaging. 2009;20:227–37. doi: 10.1097/RMR.0b013e3181ea2869. [DOI] [PubMed] [Google Scholar]
- 4.Vanhoutte PM, Shimokawa H, Feletou M, Tang EHC. Endothelial dysfunction and vascular disease – a 30th anniversary update. Acta Physiologica (Oxford, England) 2017;219:22–96. doi: 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- 5.Roth GA, Johnson J, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth GA, Huffman MD, Moran AE, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667–78. doi: 10.1161/CIRCULATIONAHA.114.008720. [DOI] [PubMed] [Google Scholar]
- 7.Cascieri MA. The potential for novel anti-inflammatory therapies for coronary artery disease. Nature. 2002;1:122–30. doi: 10.1038/nrd723. [DOI] [PubMed] [Google Scholar]
- 8.Ginhoux F, Jung S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 9.Su D, Li Z, Li X, et al. Association between serum interleukin-6 concentration and mortality in patients with coronary artery disease. Mediators Inflamm. 2013;2013:172–78. doi: 10.1155/2013/726178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan ZB, Fu XL, Li W, et al. Effect of notchl, 2, 3 genes silicing on NF-kappaB signaling pathway of macrophages in patients with atherosclerosis. Biomed Pharmacother. 2016;84:666–73. doi: 10.1016/j.biopha.2016.09.078. [DOI] [PubMed] [Google Scholar]
- 12.Hansson GK, Libby P. The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 13.Frostegard J, Ulfgren AK, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: Dominance of pro-inflammatory (Th1) and macrophage stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 14.Sakakura K, Nakano M, Otsuka F, et al. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013;22:399–411. doi: 10.1016/j.hlc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Kurosaki T. Functional dissection of BCR signaling pathways. Curr Opin Immunol. 2000;12:276–81. doi: 10.1016/s0952-7915(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 16.Inatome R, Yanagi S, Takano T, Yamamura H. A critical role for Syk in endothelial cell proliferation and migration. Biochem Biophys Res Commun. 2001;286:195–99. doi: 10.1006/bbrc.2001.5355. [DOI] [PubMed] [Google Scholar]
- 17.Correll PH, Paulson RF, Wei X. Molecular regulation of receptor tyrosine kinases in hematopoietic malignancies. Gene. 2006;374:26–38. doi: 10.1016/j.gene.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geahlen RL. Getting Syk: Spleen tyrosine kinase as a therapeutic target. Trends Pharmacol Sci. 2014;35:414–22. doi: 10.1016/j.tips.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luscher TF, von Eckardstein A, Simic B. Therapeutic targets to raise HDL in patients at risk or with coronary artery disease. Curr Vasc Pharmacol. 2012;10:720–24. doi: 10.2174/157016112803520972. [DOI] [PubMed] [Google Scholar]
- 21.de Araujo Goncalves P, Garcia-Garcia HM, Carvalho MS, Dores H. Diabetes as an independent predictor of high atherosclerotic burden assessed by coronary computed tomography angiography: The coronary artery disease equivalent revisited. Int J Cardiovasc Imaging. 2013;29:1105–14. doi: 10.1007/s10554-012-0168-4. [DOI] [PubMed] [Google Scholar]
- 22.Mahalle N, Kulkarni MV, Naik SS. Is hypomagnesaemia a coronary risk factor among Indians with coronary artery disease? J Cardiovasc Dis Res. 2012;3:280–86. doi: 10.4103/0975-3583.102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatzis G, Tousoulis D, Papageorgiou N, et al. Combined effects of smoking and interleukin-6 and C-reactive protein genetic variants on endothelial function, inflammation, thrombosis and incidence of coronary artery disease. Int J Cardiol. 2014;176:254–57. doi: 10.1016/j.ijcard.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 24.Alkandari JR, Andersen LB, Bauman AE, et al. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet. 2012;380:219–29. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charniot JC, Khani-Bittar R, Albertini JP, et al. Interpretation of lipoprotein-associated phospholipase A2 levels is influenced by cardiac disease, comorbidities, extension of atherosclerosis and treatments. Int J Cardiol. 2013;168:132–38. doi: 10.1016/j.ijcard.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 26.Gao W, He HW, Wang ZM, et al. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis. 2012;11:55. doi: 10.1186/1476-511X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, Rossouw J, Margolis KL. Smoking cessation, weight change, and coronary heart disease among postmenopausal women with and without diabetes. JAMA. 2013;310:94–96. doi: 10.1001/jama.2013.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koitabashi N, Kurabayashi M. [Stroke and cardiovascular disease related with hypertriglyceridemia]. Nihon Rinsho. 2013;71:1606–10. [in Japanese] [PubMed] [Google Scholar]
- 29.Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13:544–52. doi: 10.1007/s11886-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannon CP, Brindis RG, Chaitman BR, et al. 2013ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Acute Coronary Syndromes and Coronary Artery Disease Clinical Data Standards) Circulation. 2013;127:10–52. doi: 10.1161/CIR.0b013e3182831a11. [DOI] [PubMed] [Google Scholar]