Abstract
Background and Aims
For women with inflammatory bowel disease [IBD], it is not very well known how IBD or IBD treatment affects their breast milk components. We aimed to investigate whether breast milk composition differs in healthy control [HC] versus IBD mothers in terms of antibodies, cytokines, and metabolite,s to identify potential impact of IBD breast milk on neonatal immune system.
Methods
Breast milk specimens from HC [n = 17] and IBD [n = 31 for Crohn’s disease [CD]; and n = 41 for ulcerative colitis [UC]; were collected at 3 and 6 months postpartum [PP3] and [PP6], respectively. Faecal samples were also collected. Cytokines and immunoglobulins [IgA/IgG/IgE] were analysed by multiplex Meso Scale Discovery [MSD] and commercial kits. Moreover, breast milk metabolites were analysed by 1H nuclear magnetic resonance [NMR].
Results
We found that breast milk from IBD mothers showed significantly lower levels of IgA, sugar metabolite [lactose], and 2-aminobutyrate. In contrast, we observed that breast milk from mothers with IBD had increased levels of pro-inflammatory cytokines and higher energy metabolites [lactate and succinate] than milk from healthy mothers. In addition, we noticed that the type of treatment [5-aminosalicylic acid versus biologics] influenced the milk cytokines and metabolites profile.
Conclusions
The reduction in immunoprotective components of IBD breast milk such as sIgA and lactose theoretically may modulate the potential protective effects of breastfeeding. On the other hand, presence of higher levels of pro-inflammatory cytokines, lactate, and succinate may predispose the offspring to an inflammatory condition or impact on the gut microbiome. Better understanding of the role of succinate in infants and its potential effects on microbiome or mucosal immunity merits further investigations.
Keywords: Inflammatory bowel disease, human breast milk, metabolites
1. Introduction
Inflammatory bowel disease [IBD] is categorised into Crohn’s disease [CD] and ulcerative colitis [UC], with complex aetiology.1 Although the cause of IBD is not fully understood, genetic susceptibility, environmental factors, and more importantly dysbiosis of gut microbiome, are contributing factors in IBD pathogenesis.2,3 Both disorders are associated with significant morbidity and have enormous impact on patients’ quality of life and their ability to function, which highlights the need for better understanding of the disease pathogenesis.
Many women with IBD are in the peak of their reproductive years and they are likely to go through pregnancy and childbirth during their illness.4 To achieve good pregnancy outcome, women with IBD who are planning to get pregnant or are pregnant must be treated and monitored using a multidisciplinary approach that involves gastroenterologists, obstetricians, and dietitians who specialise in high-risk pregnancies.
However, the impact of IBD itself or medications to treat IBD on the growing fetus and the newborn is not fully understood. Thus, selection of safe and effective medications for IBD mothers during pregnancy and nursing is very crucial for both the mother and the offspring. As there is a risk of continued disease activity or flare of disease in the postpartum period, mothers are advised to continue medications in the breastfeeding period [the Toronto Consensus Guidelines]. Currently, a wide range of biological medications, immunosuppressants, and corticosteroid drugs are used to treat IBD and are considered low-risk throughout pregnancy and breastfeeding.6–9 However, some of the medications used to manage IBD merit further investigations for their safety during preconception, pregnancy, and lactation. Due to the strong evidence that active IBD increases the likelihood of adverse pregnancy outcomes, therapeutic interventions during pregnancy are still considered as the primary option for protecting both mother and fetus.10,11 One of the major concerns for parents with IBD is the possible impact of IBD and its medications on the growing fetus and breastfed infant. A large body of evidence supports the protective role of breastfeeding against IBD by influencing offspring gut microbiome establishment and development of the innate mucosal immunity.12,13
Human breast milk is enriched with bioactive substances including proteins, peptides, carbohydrates, lipids, and other immune or non-immune metabolites which play crucial roles not only in providing passive immunity, but also in shaping the formation of neonatal immune system.14 A complex network of cytokines and immunoglobulins in human milk play a significant role in compensating for the developmental delay of the neonatal immune system and in preventing the occurrence of immune-mediated diseases.15,16 Cytokines and antibodies in human breast milk have been shown to differ, depending on various factors. This includes the mother’s immune status and the metabolite components which come from both maternal blood and de novo synthesis in the mammary glands, with potential consequences on the infant’s health.17,18 In agreement, we have shown that immunisation during pregnancy results in induction of a substantial levels of antibodies and cytokines, which are not only detectable in the serum of mothers but also transferable via milk to the offspring.19,20 The biological and metabolite components of milk can modulate the immune system of the newborn, and therefore the excretion levels of cytokines or medications in milk should be taken into consideration for breastfeeding. Multiple studies have investigated the presence of different levels of medications in breast milk of IBD patients and their potential impacts on infants born to these mothers.21,22 However, to our knowledge, no comprehensive report on quantitative analysis of milk metabolites in IBD versus healthy subjects exists. Thus, in this study we have investigated metabolic profile, cytokines, and antibody levels in healthy versus IBD subjects at 3 and 6 months postpartum. In addition, fecal calprotectin in infants [delivery, PP3, PP6, and PP12] and mothers [PP3, PP6, and PP12] was quantified. Therefore, the objective of this study was to establish possible relationships between metabolite composition and immune profiling [cytokines and antibodies] in breast milk from healthy versus IBD mothers.
2. Material and Methods
2.1. Study population
Human breast milk was collected from healthy or IBD adult women at 3 and 6 months postpartum [PP3 and PP6]. Samples were processed and kept frozen until use. The appropriate institutional review boards at the University of Alberta approved the studies [Pro00056685]. All study participants gave written informed consent to participate in this study [Table 1].
Table 1.
Study participants’ information.
| Disease type | Ulcerative colitis [n = 41] | ||||
|---|---|---|---|---|---|
| Medication group | No medication [n = 2] |
5-ASA [n = 15] |
Imuran [n = 4] |
Biologics [n = 16] |
Topical [n = 4] |
| Gestation [weeks] | 38.93 | 39.17 | 35.68 | 39.55 | 39.89 |
| % C-section | 100 [2] | 27 [4] | 50 [2] | 31 [5] | 75 [3] |
| % Anti-TNF | 0 | 0 | 0 | 75 [12] | 0 |
| % Anti-integrin | 0 | 0 | 0 | 25 [4] | 0 |
| % Monotherapy | 0 | 0 | 0 | 63 [10] | 0 |
| Disease type | Crohn’s disease [n = 31] | ||||
| Medication group |
No medication
[n = 3] |
5-ASA
[n = 3] |
Imuran
[n = 5] |
Biologics
[n = 18] |
Topical
[n = 2] |
| Gestation [weeks] | 37.67 | 39.095 | 38.14 | 38.85 | 33 |
| % C-section | 33 [1] | 33 [1] | 40 [2] | 100 [18] | 100 [2] |
| % Anti-TNF | 0 | 0 | 0 | 100 [18] | 0 |
| % Monotherapy | 0 | 0 | 0 | 33 [6] | 0 |
Healthy group had gestation age ≥37.9 weeks, 30% C-section, and were without any treatments.
5-ASA, 5-aminosalicylic aid; C-section, caesarean section.
2.2. Antibody [IgA/IgG/IgE] measurement
IgA [assay range: 1.03–750 ng/mL], IgG [assay range: 0.69–500 ng/mL], and IgE [assay range: 0.69–500 ng/mL] levels were separately quantified in human breast milk, using an ELISA kit and following the manufacturer’s instructions [Bethyl Laboratories, Montgomery, USA].
2.3. Cytokines quantification
A total of 89 human breast samples were assayed using the V-PLEX proinflammatory Panel 1 human kit [Meso Scale Discovery [MSD]], cytokines panel 1 human kit [MSD], and Th17 panel human kit [MSD], according to the manufacturer’s instructions. These kits are highly sensitive multiplex enzyme-linked immunosorbent assays [ELISA] for quantitatively measuring cytokine levels including interferon γ [IFN-γ], interleukin [IL]-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, tumour necrosis factor α [TNF-α], IL-17, IL-21, IL-22, IL-23, IL-27, IL-1α, IL-5, and IL-12p40 from a small sample volume [25 μL], using an electrochemiluminescent detection method. All the test samples were analysed in duplicate, and the data were acquired using a SECTOR S 6000 plate reader [MSD]. The mean concentration of each cytokine was expressed as pg/mL. By using these kits, the minimum detectable concentrations of IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, TNF-α, IL-17, IL-21, IL-22, IL-23, IL-27, IL-1α, IL-5, and IL-12p40 were 0.20, 0.04, 0.09, 0.02, 0.06, 0.04, 0.03, 0.11, 0.24, 0.04, 5.86, 1.65, 2.78, 4.55, 38.7, 0.09, 0.22, and 0.39 pg/mL, respectively. Moreover, concentrations of human breast milk TGF-β1 levels were measured using Duoset ELISA Development Systems [R&D Systems] according to the manufacturer’s instructions.
2.4. Nuclear magnetic resonance analysis
Breast milk samples were prepared for nuclear magnetic resonance [NMR] analysis by removing them from -80°C storage and defrosting at room temperature. Each sample [450 μL] was centrifuged at 11000 rpm for 25 min at room temperature, and the aqueous top layer carefully removed and filtered through a 3-kDa molecular weight cut-off filter [Amicon Ultra-0.5; Millipore] composed of low-protein-binding regenerated cellulose, to remove lipids and proteins. The 160 μL supernatant was transferred to a fresh Eppendorf tube after centrifugation, and 40 μL of internal standard buffer containing 250 mM phosphate buffer with 5.0 mM [4-dimethyl-4-silapentane-1-sulfonic acid] DSS and 10% D2O were added. Then the mixture was vortexed and centrifuged at 10000 rpm for 5 min at 4°C, followed by gently transferring the solution into a 3-mm Bruker NMR tube and sorting it at 4°C until NMR acquisition [within 24 h of sample preparation]. The resulting1H–NMR spectra were processed and analysed using the Chenomx NMR Suite Professional software package [version 7.6, Chenomx Inc., Edmonton, AB, Canada], which allows for qualitative and quantitative analysis of NMR spectra by manually fitting spectral signatures using an internal database. Before spectral analysis, the singlet produced by DSS was used as an internal standard for both chemical shift referencing [set to 0 ppm] and for metabolite quantification.
2.4.1. Faecal calprotectin measurement
The frozen faecal samples were collected from infants/mothers and processed using a CALEX Cap Stool Extraction Device. Then the faecal calprotectin [FCP] concentration was measured using an fCAL ELISA Calprotectin kit [Bühlmann Laboratories AG].
2.5. Statistical analysis
Statistical analyses were performed using GraphPad Prism Software, version 7.0. All the data were expressed as mean ± standard error of the mean [SEM] of the corresponding parameter. To detect differences between treated and control groups, Student’s t test was applied. Two-way analysis of variance [ANOVA], followed by Tukey’s test for multiple comparisons, were performed to check for significant differences when more than two groups were compared.
3. Results
3.1. Lower IgA antibody in breast milk of IBD compared with healthy mothers
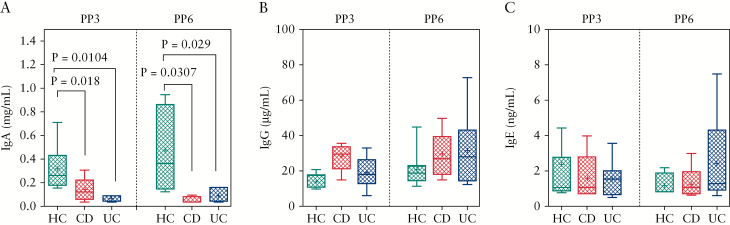
To determine if there were differences in the immunoglobulin levels in the breast milk of IBD versus healthy mothers, we measured breast milk IgA, IgG, and IgE levels by ELISA. Breast milk samples from 14 healthy mothers [7 PP3, 7 PP6], 11 mothers with CD [5 PP3, 6 PP6], and 18 mothers with UC [11 PP3, 7 PP6] were used for these studies. Compared with healthy mothers, IgA content was reduced in mothers with UC at PP3 [p = 0.0104] and PP6 [p = 0.029] [Figure 1A]. Similarly, IgA content was decreased at PP3 in CD mothers [p = 0.018], and this decrease persisted at PP6 [p = 0.0307] when compared with healthy mothers [Figure 1A]. In contrast, no differences were observed in IgG and IgE contents between the groups, irrespective of PP3 or PP6 [Figure 1B, C].
Figure 1.
Antibody levels in breast milk from IBD versus healthy subjects. [A] IgA, [B] IgG, and [C] IgE showing antibodies levels in breast milk samples of healthy mothers [HC] and mothers with Crohn’s disease [CD] or ulcerative colitis [UC], determined by ELISA assays at 3 [PP3] or 6 months [PP6] postpartum. IBD, inflammatory bowel disease; PP3, 3 months postpartum; PP6, 6 months postpartum.
3.2. No significant difference in pro-inflammatory cytokines in breast milk from mothers with IBD compared with healthy mothers
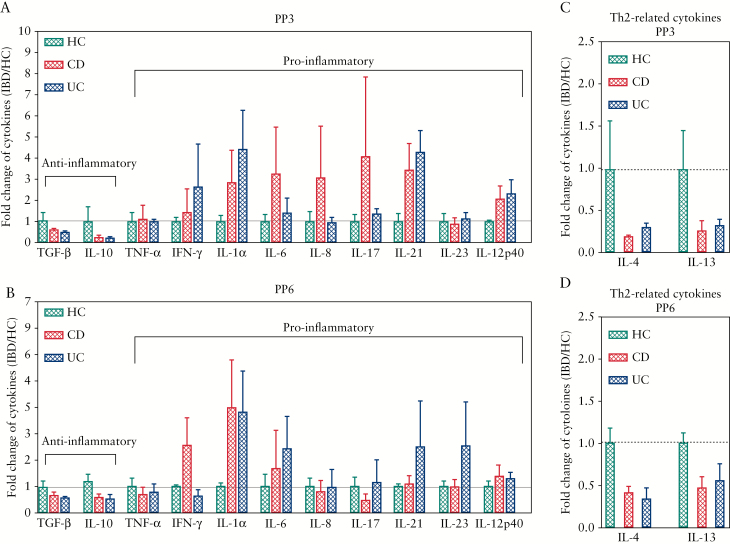
The role of cytokines in initiation, progression, and resolution of IBD is well documented.23 We have previously shown that the passive transfer of maternal cytokines may modulate the immune system of the newborn,20 and we therefore aimed to measure the levels of pro- and anti-inflammatory cytokines in the breast milk of IBD versus healthy mothers. As shown in Figure 2, the cytokine levels were positive [above the detection threshold] in 76.7% of cases for IL-10 and IL-17, and in 41.9% for IL-21. The other cytokine quantifications were positive in at least 86% of the samples. No statistically significant differences in cytokines were observed between groups due to heterogeneity of human subjects; however, as expected, the pro-inflammatory cytokines such as IFN-γ, IL-1α, IL-6, IL-17, IL-21, and IL-12 exhibited a higher trend in IBD versus healthy mothers at PP3 [Figure 2A]. Similarly, we noted that IFN-γ, IL-1α, and IL-6 were higher in CD patients, whereas IL-1, IL-6, IL-21, IL-23 appeared to be elevated in UC compared to healthy mothers at PP6 [Figure 2B]. In contrast, IBD patients’ breast milk appeared to have lower levels of anti-inflammatory cytokines [TGF-β1 and IL-10] when compared with healthy mothers at both PP3 and PP6 time points, but this difference was not significant [Figure 2A and B]. Moreover, we found that the breast milk from IBD patients seemed to have lower Th2-related cytokines, such as IL-4 and IL-13, compared with healthy mothers at both time points, but without any significant difference [Figure 2C and D]. Taken together, these results suggest although no significant differences were identified in cytokine levels between healthy and IBD status, mothers with IBD showed an increasing trend for pro-inflammatory cytokines and decreasing trend for anti-inflammatory cytokines at both PP3 than PP6.
Figure 2.
Pro-inflammatory and anti-inflammatory cytokines levels in breast milk. [A] Data showing pro-inflammatory and anti-inflammatory cytokines in breast milk of HC and IBD mothers [CD and UC] at PP3. [B] Data showing pro-inflammatory and anti-inflammatory cytokines in breast milk of HC and IBD mothers [CD and UC] at PP6. [C] Graph showing IL-4 and IL-13 levels in breast milk of HC versus IBD mothers at PP3 or [D] PP6. Milk samples were analysed by using V-PLEX human kits [Meso Scale Discovery, Gaithersburg, MD, USA], and the results were expressed by the fold change [IBD/HC] for each cytokine. IBD, inflammatory bowel disease, CD, Crohn’s disease; UC, ulcerative colitis; PP3, 3 months postpartum; PP6, 6 months postpartum.
3.3. Heatmap analysis of breast milk metabolites compositions
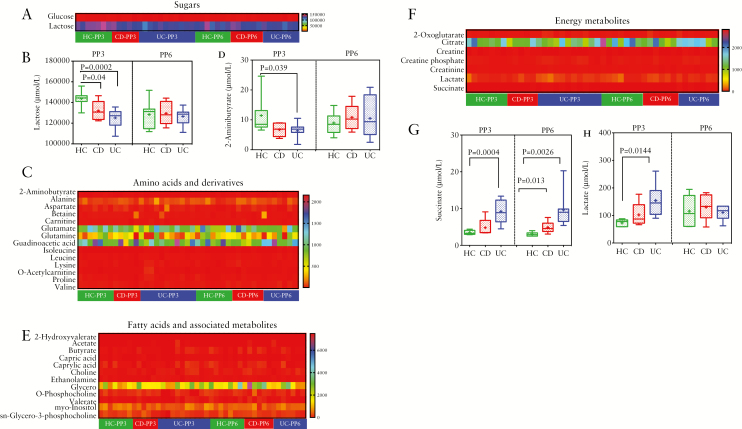
Next, to compare the metabolite composition in IBD versus healthy mothers, we analysed 38 breast milk metabolites using H-NMR.1 To distinguish the different metabolites, we clustered metabolites into functional categories including sugars [n = 2], amino acids and derivatives [n = 14], fatty acids and associated metabolites [n = 12], energy metabolites [n = 7], and others [n = 3]. As shown on the heatmap in Figure 3A, we found lactose was the most abundant compound compared with other metabolites in the breast milk, and was reduced in mothers with either CD [p = 0.04] or UC [p = 0.0002] at PP3 [Figure 3B].
Figure 3.
Heatmaps analysis of milk metabolomic studies. [A] Heatmap data showing comparison of sugars in breast milk of HC versus IBD [B] Graph showing lactose levels in HC versus IBD breast milk samples at PP3 and PP6. [C] Heatmap analysis showing amino acids and derivatives in milk samples of HC versus IBD mothers. [D] Data showing comparison of 2-aminobutyrate levels in breast milk from HC and IBD mothers. [E] Heatmap showing comparison of fatty acids and associated metabolites in milk of HC versus IBD mothers. [F] Heatmap comparison of energy metabolites in breast milk of HC versus IBD mothers. [G] Graph showing changes in breast milk succinate and [H] lactate of HC versus IBD mothers are PP3 and PP6. The heatmap graphically represents individual changes of concentration intensity. The colour red represents the relatively lower abundance, and the colour blue represents the relatively higher abundance, of each metabolite. The insert is the bar plot of the significant difference between the HC and IBD among the certain metabolites. IBD, inflammatory bowel disease; HC, healthy controls; PP3, 3 months postpartum; PP6, 6 months postpartum.
However, at 6 months postpartum no significant difference was detected for lactose between the groups [Figure 3B]. Another prominent cluster separating breast milk of HC and IBD was amino acids and derivatives. Among the identified amino acids and derivatives, glutamate, glutamine, and guadinoacetic acid showed an increasing trend in breast milk of both healthy and IBD mothers, though no significant differences were found [Figure 3C]. Moreover, 2-aminobutyrate showed a decreasing trend in IBD compared with healthy controls at PP3, with statistical significance in UC versus healthy mothers. However, by 6 months postpartum this difference was no longer present [Figure 3D]. Moreover, the complexities of fatty acids and associated metabolites, which reflect the hydrolysis of triglycerides, were found to be indicative of human breast milk. These fatty acids include saturated fatty acids [SFAs], monounsaturated fatty acids [MUFAs], and polyunsaturated fatty acids [PUFAs]. As revealed by our study, no differences were found in fatty acids and associated metabolites in the milk between healthy mothers and mothers with IBD [Figure 3E]. Finally, when we compared the energy metabolites in milk from IBD versus healthy mothers, we found that IBD samples exhibited an opposite trend compared with the above metabolites [Figure 3F]. The level of lactate appeared to be higher in IBD versus healthy mothers’ milk at PP3, though it reached significance only in the UC group [p = 0.0144]; however, no difference was observed between the groups at PP6 [Figure 3G]. Notably, as shown in Figure 3H, the levels of succinate in UC milk samples was significantly higher compared with healthy mothers not only at PP3 [p = 0.0004] but also at PP6 [p = 0.0026]. Although milk samples from CD mothers appeared to have higher succinate at PP3, this difference was not significant [Figure 3H]. Interestingly, succinate levels in breast milk of CD mothers only increased at PP6 [Figure 3H].
3.4. The effects of type of medication in IBD mothers on their milk metabolites
We further investigated whether 5-ASA or biologics medications changes the metabolomic components of breast milk in IBD mothers. To determine whether the type of IBD medication influences cytokine levels in breast milk of mothers with IBD, we clustered UC patients based on their medications. Given that 5-ASA and biologics accounted for the majority of medications, we performed a subgroup analysis of only 5-ASA or only biologics users [Table 1].
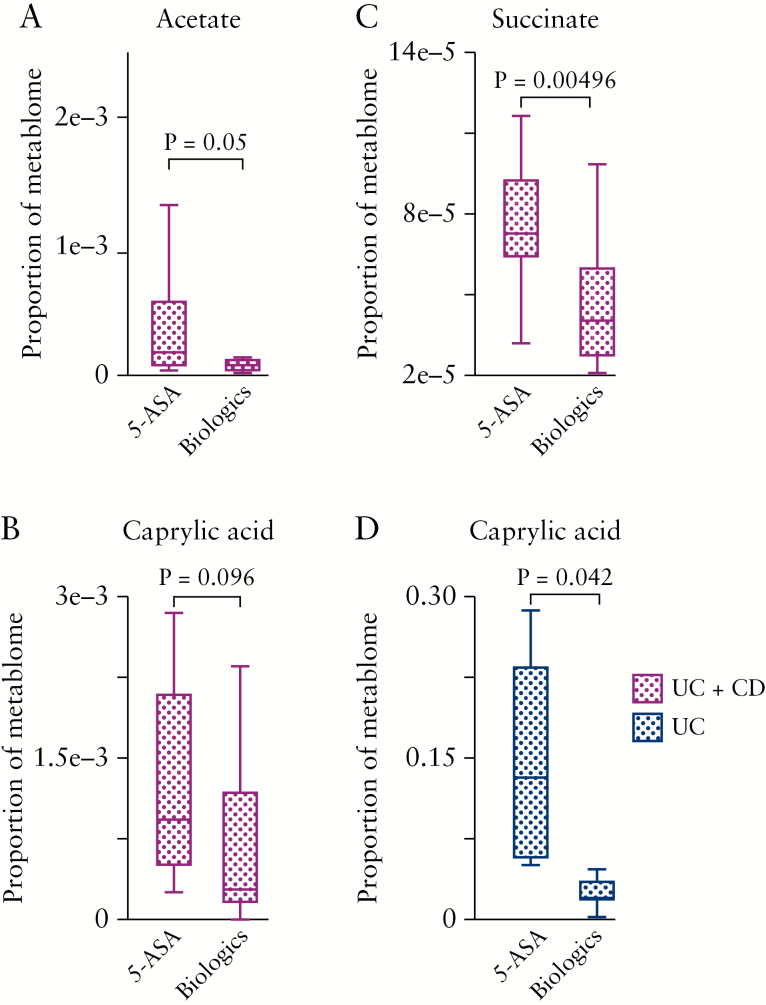
Interestingly, we observed that patients on 5-ASA, regardless of the clinical presentation of IBD [CD or UC], had significantly higher levels of acetate, succinate, and caprylic acid in their breast milk compared with patients who were on biologics [Figure 4A–C]. Of note, the difference in caprylic acid levels in breast milk of UC mothers on 5-ASA, compared with biologics, was significantly pronounced [Figure 4D].
Figure 4.
The effects of treatment on breast milk metabolities. [A] Proportion of acetate; [B] proportion of succinate;[C] proportion of caprylic acid; in breast milk of IBD patients on 5-ASA versus biologics. [D] Proportion of caprylic acid in breast milk of UC patients on 5-ASA versus biologics. For UC + CD group, we had 5-ASA [n = 8] and biologics [n = 16], and for the UC-only group we had 5-ASA [n = 7] and biologics [n = 7] milk specimens. The biologics group is inclusive of exposures to either anti-TNF or anti-integrin. IBD, inflammatory bowel disease, CD, Crohn’s disease; UC, ulcerative colitis, 5-ASA, 5-amino-salicylic acid.
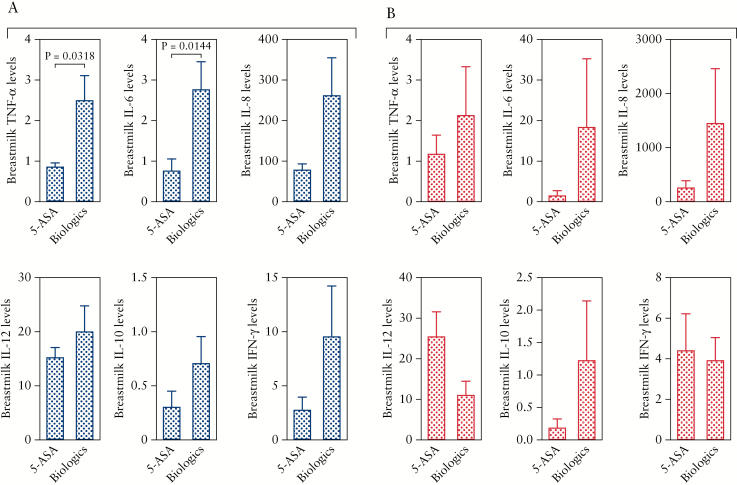
3.5. The impact of medications in IBD mothers on cytokine profile in their breast milk
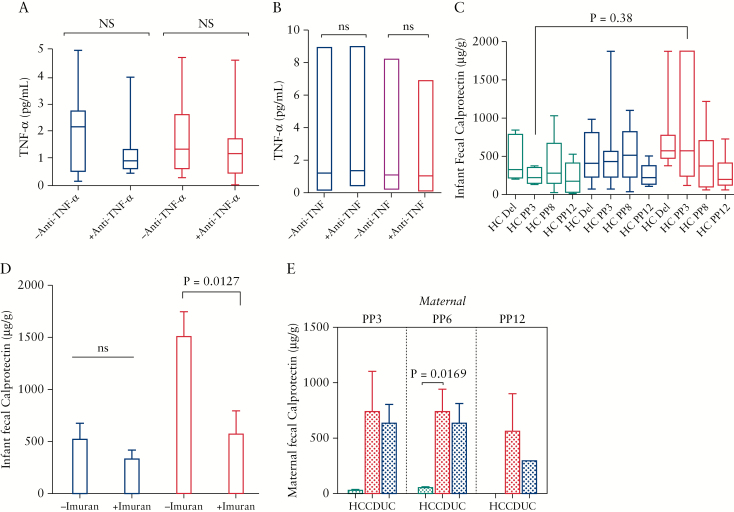
As we discussed above, we divided patients based on their medications into two groups [5-ASA and biologics]. We found that UC patients on biologics had overall higher levels of pro-inflammatory cytokines. Although there was a trend for higher IL-12, IL-8, and IFN-γ in this group, it did not reach significance [Figure 5A]. This difference reached significance only for TNF-α and IL-6 cytokines in UC patients [Figure 5A]. In contrast, reduction of cytokine levels by 5-ASA in CD patients was not the case, and we observed variable effects as shown in Figure 5B. Another important observation was that CD patients appear to have much higher levels of cytokines in their milk when they are on biologics compared with UC patients. As shown in Figure 5A and B, IL-6, IL-8, and IL-12p40 are more abundant in CD versus UC patients. In addition, the effects of anti-TNF-α treatment on TNF-α levels in the breast milk and serum of IBD mothers was analysed. Interestingly, we did not find any significant reduction in the TNF-α levels in the breast milk or serum of anti-TNF-α treated mothers [Figure 6A and B]. In addition, we did not observe any association between disease activity (e.g. Harvey Bradshaw Index [HBI], partial Mayo score [PMayo], or C-reactive protein (CRP)] and cytokine levels in studied subjects [data not shown].
Figure 5.
The impact of treatment on breast milk cytokines. [A] Showing cytokines [TNF-α, IL-6, IL-8, IL-12p40, IL-10, and IFN-γ] in breast milk of mothers with UC either on 5-ASA [n = 15] or biologics [n = 16] treatments. [A] Showing cytokines [TNF-α, IL-6, IL-8, IL-12p40, IL-10, and IFN-γ] in breast milk of mothers with CD on either 5-ASA [n = 3] or biologics [n = 18] treatments. Cytokines are measured as pg/ml. CD, Crohn’s disease; UC, ulcerative colitis; 5-ASA, 5-aminosalicylic acid.
Figure 6.
The effects of anti-TNF-α treatment on TNF-α levels in serum, and FCP concentration in infants and mothers. [A] Data showing TNF-α levels in the milk, and [B] serum of mothers on either anti-TNF-α or other treatments. [C] Showing concentrations of FCP in meconium, and faecal samples of infants born to healthy, UC and CD mothers at PP3, PP6 and PP12. [D] Plots indicating FCP levels in faecal samples of infants [PP3] born to mothers with UC or CD on imuran versus other treatments. [E] Graphs showing FCP levels in faecal samples of healthy mothers versus CD or UC at PP3, PP6 and PP12. CD, Crohn’s disease; UC, ulcerative colitis; FCP, faecal calprotectin; PP3, 3 months postpartum; PP6, 6 months postpartum.
3.6. The impact of disease or medication on the faecal calprotectin concentration in infants
The faecal calprotectin [FCP] concentration in the meconium and faecal samples of newborns at birth, PP3, PP6 and PP12, and the FCP of their mothers at PP3, PP6 and PP12, was measured. We found that only infants born to CD mothers had significantly higher FCP levels at PP3 compared with infants born to healthy mothers [Figure 6C]. In addition, the effects of mother’s medication on infants FCP concentration was investigated. We observed that different medications had no significant impact on the FCP concentrations in the infant’s faecal specimens at different time points [e.g. birth, PP3, PP6 and PP12] except imuran, which significantly reduced the levels of FCP in faecal samples of infants born to CD mothers at PP3 [Figure 6D]. However, this was not the case for infants born to UC mothers [Figure 6D]. Finally, FCP concentration in mother’s faecal specimens was quantified and, despite higher levels in IBD mothers compared with healthy controls, this difference reached significance for only CD mothers at PP6 [Figure 6E].
4. Discussion
Breastfeeding is highly recommended as the primary source of nutrients and bioactive molecules for neonates, and the American Academy of Pediatrics recommends breastfeeding for at least 6 months after birth.24,25 However, many women who require medications are hesitant to nurse their infants and tend to reduce or discontinue breastfeeding earlier than women who are not receiving medication, or even refuse to breastfeed. To date, several studies have explored detectable drug levels in IBD mothers’ breast milk,6,21,26 and the general consensus is that breastfeeding by IBD mothers is safe.27,28 However, breastfeeding is discouraged for some medications such as methotrexate, ciclosporin, metronidazole, and ciprofloxacin.28 Therefore, symptomatic IBD mothers who require treatment must consult with health care providers about potential risks of breastfeeding. Despite some reports, it is still unclear whether IBD and/or IBD medications during gestation or lactation would affect the immune components in breast milk. Here, we have demonstrated that breast milk from mothers with IBD and from healthy mothers differs in immune parameters such as antibodies, cytokines, and metabolites composition. Our study sheds novel light on differential components of breast milk from IBD compared with healthy mothers, and explains how these differences may impact on the immune system of the offspring.
Breastfeeding by mothers affected by IBD has been the subject of debate and controversy. Several epidemiological studies have reported that IBD mothers receiving therapy should not be discouraged from nursing their children and nursing may even provide a protective effect against disease flare in the postpartum year,6,22 whereas other studies have not found such associations.29,30
Breastfeeding is known to provide significant health benefits to infants, including the transmission of protective antibodies,31 cytokines,15,32 immunoreactive biochemicals and leukocytes.20,33 Unlike most mammals, human breast milk antibodies that are transferred from mother to infant do not enter the child’s circulation in large amounts.34 Secretory IgA is considered the most abundant antibody subtype present in secretions including milk, providing mucosal immunity to the infant.35 Similarly in our study, IgA was identified as the most enriched milk antibody in healthy mothers, followed by IgG and IgE antibodies. Faecal IgA level is considered as a marker of intestinal immunity and its deficiency has been associated with chronic gastrointestinal inflammation.36,37 In accordance with these reports, IBD mothers had lower levels of IgA in their breast milk than healthy mothers. This suggests that IBD mothers’ breast milk suffers from lower levels of such a protective factor that plays a crucial role in the maintenance of microbiome and neutralisation of invasive pathogens in the gut and respiratory tract.20,38 Whether lower milk IgA in IBD mothers compromises intestinal immunity of their offspring warrants further investigations. Although other larger antibodies, such as IgG [160 kDa] and IgE [196 kDa], can be transferred to the neonate via breast milk and exhibit important immunological roles in newborns,39,40 we did not detect any significant difference in their levels between IBD and healthy mothers.
Besides protective antibodies, human breast milk contains an array of cytokines. Breast milk cytokines are important in the development of the gastrointestinal tract of the suckling.41,42 In addition, elevation of cytokines in breast milk may stimulate the immune system of the offspring as a protective mechanism against infections.20,43 However, overexpression of pro-inflammatory cytokines in milk could lead to excessive stimulation/activation of the neonatal immune system, and subsequently onset of autoimmune inflammatory conditions.44
Our study showed a trend to lower TGF-β1 concentration in breast milk of mothers with IBD compared with healthy mothers, although not significant, which may influence the development of the newborn’s immune response. Recently, we have shown that IBD mothers have lower CD71+ erythroid cells compared with healthy controls during pregnancy.45 Since CD71+ erythroid cells constitutively produce TGF-β, lower frequency of these cells may result in lower TGF-β in these patients. Even though TGF-β levels in milk were not different between IBD and healthy mothers, we suggest that IBD mothers might have have lower TGF-β expression at the site of inflammation.45 TGF-β is an important family of growth factors involved in developing oral tolerance and regulating most mechanisms triggered by antigen feeding, as well as in maintaining intestinal immune homeostasis.46,47
Breast milk constitutes an important source of TGF-β1 for the neonate because its expression is absent in the neonatal intestine before Day 10, after which it increases progressively.46 In keeping with our observations, other reports indicated that mothers with other inflammatory disease, such as allergic conditions, have lower levels of TGF-β1 in their breast milk.42,48 Likewise, we detected lower levels of IL-10 in breast milk of IBD mothers in our study which could theoretically limit its protective role in the newborn’s immune system, but this did not reach significance. IL-10, a potent anti-inflammatory cytokine, is found in human breast milk and is produced by mammary cells. However, it is also present in lymphocytes and macrophages in milk.20,49 IL-10 plays a crucial role in maintaining gut homeostasis and dampens the Th1 response, thereby inhibiting pro-inflammatory cytokines’ function. In contrast, breast milk of IBD mothers was characterised by an increase in pro-inflammatory cytokines [IFN-γ, IL-1α, IL-6, IL-8, IL-17, IL-21, and IL-12p40] at PP3 and IFN-γ, IL-1α, IL-6, IL-17, IL-21, Il-23 and IL-12p40 at PP6, compared with healthy mothers. However, these differences were not statistically significant. Novel biologics that target cytokines or their signalling pathways are becoming widely used in IBD patients.
Based on our data, it appears that biologics exhibit lower inhibitory effects on the cytokine levels in breast milk compared with 5-ASA. This may reflect the immune system plasticity, that blockade of one cytokine can drive the induction of others.50 Among these pro-inflammatory cytokines, IFN-γ, IL-1α, IL-6, IL-8, and IL-21 are predominantly abundant in IBD mothers’ breast milk. Thus, alterations in human milk pro-inflammatory cytokines levels may reflect differential trafficking of immune cells or cytokines into the mammary glands of IBD patients.51 Interestingly, we did not observe any significant difference in TNF-α levels among the groups since it is the main target of biologics, but a trend to lower Th2 type cytokines [IL-4 and IL-13] in breast milk of IBD patients compared with healthy mothers was evident. Even though Crohn’s disease and ulcerative colitis are driven by a Th1/Th17 and mainly a Th2 type immune response, respectively,23 this was not reflected in the milk. As we and others have reported, newborns have an immunosuppressive environment52,53 which is required for swift adaptation to the gut microbiome.54 As a result, the neonatal immune system is characterised by functionally suppressed neutrophils and antigen-presenting cells activity, attenuated responses to pattern-recognition receptor stimulation, and a skewed Th2 type immune response,55 which favours the establishment of the microbe-host homeostasis during early colonisation of skin and mucosal surfaces.51,54,56,57 Thus, elevated pro-inflammatory cytokines in breast milk of IBD mothers may result in upregulation of Toll-like receptor expression and subsequently immune activation in intestinal epithelial cells.51 Such changes may interfere with intestinal tolerance during initial bacterial colonisation in the suckling infant.
Finally, in this study we used 1H-NMR-based metabolomics to evaluate whether IBD or the type of treatment would affect breast milk metabolism. Our results showed that only four metabolites among 38 metabolites were significantly different between IBD and healthy mothers. Among the milk components measured, the lactose concentration made the major contribution [> 93%] and hence may be the main driving force underlying the nursing effect. It is well known that lactose is first hydrolysed into its glucose and galactose moieties by lactase, located in the mucosa of the small intestine, which can modulate gut microbiota and immune responses during the suckling period by inducing the expression of IL-37 in colonic epithelial cells, monocytes, and macrophages.58–60
Our results demonstrated significantly decreased levels of lactose in IBD mothers’ breast milk at PP3, which suggested that the lower lactose in IBD milk could dampen the gut health of neonates by decreasing the growth of potentially beneficial bacteria and promoting potential pathobionts.58 Moreover, in this study we showed that succinate, a tricarboxylic acid [TCA] cycle metabolite, was less abundant in healthy mothers’ than in IBD mothers’ breast milk. Although initially succinate was reported to be involved in adenosine triphosphate [ATP] generation in mitochondria, its role outside metabolism has recently emerged as an inflammatory signal molecule.61 Some studies have shown that succinate can increase expression of genes that facilitate angiogenesis, metastasis, and glycolysis, ultimately leading to tumour progression.62 Similar to its role in tumours, succinate can impair prolyl hydroxylase domain enzyme activity in macrophages and directly or indirectly via reactive oxygen species [ROS] induce hypoxia-induced factor 1-alpha [HIF-1α] stabilisation.63 Thus, succinate acts as an inflammatory signal and chemokine for migration of monocyte-derived macrophages and dendritic cells [DCs].64In addition, succinate in synergy with Toll-like receptors [TLRs]-3 and 7 ligands enhances pro-inflammatory cytokine production by DCs.64 However, how it gets accumulated in immune cells is not very well understood.65
More importantly, succinate levels and other metabolites may modulate glucose metabolism and subsequently affect gut microbiota. For instance, a high succinate level has been reported to be associated with impaired glucose metabolism and specific modifications in the gut bacteria in obese individuals.66 It has also been reported that succinate promotes Clostridium difficile infection following microbiota disturbance.67 Some bacterial species, such as Prevotellaceae and Veillonellaceae, are considered as succinate-producing bacteria and others. like Odoribacteraceae and Clostridaceae, as succinate-consuming bacteria.66 Increased abundance in Veillonellaceae and decreased in Clostridaceae is strongly associated with disease status in CD patients.68 Thus, we speculate that IBD mothers’ gut microenvironment might favour the growth of succinate=producing commensals but prevent the growth of succinate-consuming bacteria. Subsequently succinate gets released into the circulation because of the compromised intestinal integrity, and can be detected in the milk. Therefore, the potential impact of milk succinate on modulating the gut microbiota merits further investigation and will be the focus of our future studies.
Another interesting observation of our study was lower 2-aminobutyrate in breast milk of IBD mothers at PP3. There is a possibility that lipopolysaccharide [LPS] induces succinate accumulation by reduction of aminobutyric acid,69 which is reflected in lower 2-aminobutyrate in breast milk of IBD mothers. Thus, our observations suggest that increased succinate may enhance monocytes/macrophages and DC recruitment in the mammary glands of IBD patients, as a potential mechanism for increased pro-inflammatory cytokines in breast milk. Based on the literature, the effect of TCA intermediates intake has only been studied in adults,70 so lack of evidence restrains us from understanding the role of high abundance of TCA metabolite [succinate] in breast milk and the impact of ingesting TCA metabolite-rich breast milk on infant growth. Nevertheless, it is still plausible to speculate that the higher concentration of TCA metabolite in breast milk may be an unfavourable factor for the newborn immune development, due to its inflammatory role. Whether the over-consumption of TCA metabolite is safe for the growth of human infants needs further investigation.
Furthermore, we found significantly higher lactate content in breast milk of UC patients at PP3 compared with healthy subjects. Lactate, as the main metabolite of many fermented products, has been reported to be higher in faecal specimens of UC patients.70,71 Recent reports indicate a more beneficial role for lactate, with immunomodulatory properties. Therefore, further studies are required to better understand the impact of higher lactate in breast milk on neonatal immune system and microbiome.
In conclusion, human breast milk is a complex biological mixture and our report is the first to compare the differences between breast milk composition from healthy mothers and from mothers with IBD in terms of antibodies, cytokines, and metabolites components. The results revealed that IBD mothers’ breast milk contained attenuated IgA levels, increased pro-inflammatory cytokines expression, and a transformed content of certain metabolites components compared with breast milk from healthy mothers. It might be speculated that such differences may influence the protective effects of breastfeeding on infant’s health; however, further prospective studies on larger study cohorts are needed to reveal whether such differences in breast milk composition ultimately predispose the offspring to developing IBD and/or other immunological disorders.
Several limitations of our study should be acknowledged including, for instance, small sample size especially when patients were subgrouped based on their medications. It is always challenging when longitudinal studies are conducted, due to the lack of patient adherence. In addition, we were unable to perform measurement of drug levels in the breast milk of IBD patients in our studies. The potential impact of drugs type/level in milk on its composition should be taken in consideration. Therefore, we support conducting such studies on larger cohorts, with particular attention to addressing the potential impact of milk differences on the infant microbiome.
Funding
This work was supported by a Foundation Scheme grant from the Canadian Institutes of Health Research [CIHR], a New Investigator Salary Award from CIHR, and a New Investigator grant in Maternal, Reproductive, Child and Youth Health from CIHR/Women and Children’s Health Research Institute [WCHRI] [all to SE]. In addition, WCHRI supported this study through an innovation grant [to VH and SE]. We also thank China Scholarship Council [CSC] for the financial support [to XM].
Conflict of Interest
The authors have no financial, professional, or personal conflicts of interest to declare.
Acknowledgements
We also appreciate the support of Dr Rshmi Khurana and Janie Tyrell. Without study volunteers this work was impossible, so we appreciate their contribution and support.
Author Contributions
XM performed antibody ELISA assays, analysed cytokine and metabolomics data, structured the data, and wrote the first draft of manuscript. GD contributed to milk samples processing, performing MSD cytokine ELISAs and data analysis. PK contributed by performing 1H-NMR-based metabolomics assays. YE contributed by conducting MSD cytokine ELISAs. RW analysed the impact of medication on cytokines and metabolites. RS and LM were actively involved in patient recruitment and sample and clinical data collection. VN and NH were involved in performing MSD cytokines assays. M provided intellectual support and guidance. VH, as a clinician, recruited all of the study participants, provided all the clinical data, and assisted in clinical data analysis. HC co-supervised XM. LD, as a clinician, also contributed to patient recruitment. SE proposed and designed the study, supervised all of the research, analysed some of the data, and wrote the manuscript.
References
- 1. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017;152:313–21.e2. [DOI] [PubMed] [Google Scholar]
- 2. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho JH, Abraham C. Inflammatory bowel disease genetics: Nod2. Annu Rev Med 2007;58:401–16. [DOI] [PubMed] [Google Scholar]
- 4. Jung SA. Concerns in pregnancy and childbirth of women with inflammatory bowel disease. Intest Res 2016;14:107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol 2018;15:39–49. [DOI] [PubMed] [Google Scholar]
- 6. Lahat A, Shitrit AB, Naftali T, et al. Vedolizumab levels in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis 2018;12:120–3. [DOI] [PubMed] [Google Scholar]
- 7. Kattah MG, Milush JM, Burt T, et al. Anti-TNF and thiopurine therapy in pregnant IBD patients does not significantly alter a panel of B-cell and T-cell subsets in 1-year-old infants. Clin Transl Gastroenterol 2018;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grosen A, Julsgaard M, Kelsen J, Christensen LA. Infliximab concentrations in the milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis 2014;8:175–6. [DOI] [PubMed] [Google Scholar]
- 9. Kane S, Ford J, Cohen R, Wagner C. Absence of infliximab in infants and breast milk from nursing mothers receiving therapy for Crohn’s disease before and after delivery. J Clin Gastroenterol 2009;43:613–6. [DOI] [PubMed] [Google Scholar]
- 10. Huang VW, Chang HJ, Kroeker KI, et al. Management of inflammatory bowel disease during pregnancy and breastfeeding varies widely: a need for further education. Can J Gastroenterol Hepatol 2016;2016:6193275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nielsen OH, Maxwell C, Hendel J. IBD medications during pregnancy and lactation. Nat Rev Gastroenterol Hepatol 2014;11:116–27. [DOI] [PubMed] [Google Scholar]
- 12. Penders J, Thijs C, van den Brandt PA, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 2007;56:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turfkruyer M, Verhasselt V. Breast milk and its impact on maturation of the neonatal immune system. Curr Opin Infect Dis 2015;28:199–206. [DOI] [PubMed] [Google Scholar]
- 14. Vieira Borba V, Sharif K, Shoenfeld Y. Breastfeeding and autoimmunity: Programing health from the beginning. Am J Reprod Immunol 2018, Jan. doi: 10.1111/aji.12778. [DOI] [PubMed] [Google Scholar]
- 15. Garofalo R. Cytokines in human milk. J Pediatr 2010;156:S36–40. [DOI] [PubMed] [Google Scholar]
- 16. Tomicić S, Johansson G, Voor T, Björkstén B, Böttcher MF, Jenmalm MC. Breast milk cytokine and IgA composition differ in Estonian and Swedish mothers relationship to microbial pressure and infant allergy. Pediatr Res 2010;68:330–4. [DOI] [PubMed] [Google Scholar]
- 17. Smilowitz JT, O’Sullivan A, Barile D, German JB, Lönnerdal B, Slupsky CM. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr 2013;143:1709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He Y, Lawlor NT, Newburg DS. Human milk components modulate toll-like receptor-mediated inflammation. Adv Nutr 2016;7:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elahi S, Buchanan RM, Babiuk LA, Gerdts V. Maternal immunity provides protection against pertussis in newborn piglets. Infect Immun 2006;74:2619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elahi S, Thompson DR, Van Kessel J, et al. The protective role of passively transferred maternal cytokines against bordetella pertussis infection in newborn piglets. Infect Immun 2017, Mar 23. doi: 10.1128/IAI.01063-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matro R, Martin CF, Wolf D, Shah SA, Mahadevan U. Exposure concentrations of infants breastfed by women receiving biologic therapies for inflammatory bowel diseases and effects of breastfeeding on infections and development. Gastroenterology 2018;155:696–704. [DOI] [PubMed] [Google Scholar]
- 22. Moffatt DC, Ilnyckyj A, Bernstein CN. A population-based study of breastfeeding in inflammatory bowel disease: initiation, duration, and effect on disease in the postpartum period. Am J Gastroenterol 2009;104:2517–23. [DOI] [PubMed] [Google Scholar]
- 23. Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011;140:1756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartick MC, Schwarz EB, Green BD, et al. Suboptimal breastfeeding in the United States: Maternal and pediatric health outcomes and costs. Matern Child Nutr 2017;13:e12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stough CO, Khalsa AS, Nabors LA, et al. Predictors of exclusive breastfeeding for 6 months in a national sample of us children. Am J Health Promot 2018. doi: 10.1177/0890117118774208. [DOI] [PubMed] [Google Scholar]
- 26. Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNFα drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY study. Am J Gastroenterol 2018;113:396–403. [DOI] [PubMed] [Google Scholar]
- 27. Nielsen OH. New strategies for treatment of inflammatory bowel disease. Front Med [Lausanne] 2014;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaspersen JD, Pedersen JN, Hansted JG, et al. Generic structures of cytotoxic liprotides: nano-sized complexes with oleic acid cores and shells of disordered proteins. Chembiochem 2014;15:2693–702. [DOI] [PubMed] [Google Scholar]
- 29. Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis 2011;5:555–8. [DOI] [PubMed] [Google Scholar]
- 30. Agrawal A, Hotte N, Lumb R, et al. Maternal IBD and IBD therapies may influence breast milk pro-inflammatory cytokines and the infant’s fecal calprotectin. Gastroenterology 2017;152:S759. [Google Scholar]
- 31. Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr 2005;135:1–4. [DOI] [PubMed] [Google Scholar]
- 32. Murphy J, Pfeiffer RM, Lynn BCD, et al. Pro-inflammatory cytokines and growth factors in human milk: an exploratory analysis of racial differences to inform breast cancer etiology. Breast Cancer Res Treat 2018;172:209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jain L, Vidyasagar D, Xanthou M, Ghai V, Shimada S, Blend M. In vivo distribution of human milk leucocytes after ingestion by newborn baboons. Arch Dis Child 1989;64:930–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Donovan SM. Role of human milk components in gastrointestinal development: Current knowledge and future needs. J Pediatr 2006;149:S49–61. [Google Scholar]
- 35. Stelwagen K, Carpenter E, Haigh B, Hodgkinson A, Wheeler TT. Immune components of bovine colostrum and milk. J Anim Sci 2009;87:3–9. [DOI] [PubMed] [Google Scholar]
- 36. Albers R, Antoine JM, Bourdet-Sicard R, et al. Markers to measure immunomodulation in human nutrition intervention studies. Br J Nutr 2005;94:452–81. [DOI] [PubMed] [Google Scholar]
- 37. Cunningham-Rundles C. Physiology of IgA and IgA deficiency. J Clin Immunol 2001;21:303–9. [DOI] [PubMed] [Google Scholar]
- 38. Kelly D, King T, Aminov R. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat Res 2007;622:58–69. [DOI] [PubMed] [Google Scholar]
- 39. Alasil SM. Breastfeeding as a tool that empowers infant immunity through maternal vaccination. J Vaccines Vaccin 2015. doi: 10.4172/2157–7560.1000271. [Google Scholar]
- 40. Hochwallner H, Alm J, Lupinek C, et al. Transmission of allergen-specific IgG and IgE from maternal blood into breast milk visualized with microarray technology. J Allergy Clin Immunol 2014;134:1213–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-beta 1 null mice. Science 1994;264:1936–8. [DOI] [PubMed] [Google Scholar]
- 42. Brenmoehl J, Ohde D, Wirthgen E, Hoeflich A. Cytokines in milk and the role of TGF-beta. Best Pract Res Clin Endocrinol Metab 2018;32:47–56. [DOI] [PubMed] [Google Scholar]
- 43. Kelleher SL, Lönnerdal B. Immunological activities associated with milk. Adv Nutr Res 2001;10:39–65. [DOI] [PubMed] [Google Scholar]
- 44. Wöckel A, Abou-Dakn M, Beggel A, et al. Inflammatory breast diseases during lactation: Health effects on the newborn a literature review. Mediat Inflamm 2008. doi: 10.1155/2008/298760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dunsmore G, Koleva P, Ghobakhloo N, et al. Lower abundance and impaired function of CD71+ erythroid cells in inflammatory bowel disease patients during pregnancy. J Crohns Colitis 2018.doi: 10.1093/ecco-jcc/jjy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oddy WH, McMahon RJ. Milk-derived or recombinant transforming growth factor-beta has effects on immunological outcomes: a review of evidence from animal experimental studies. Clin Exp Allergy 2011;41:783–93. [DOI] [PubMed] [Google Scholar]
- 47. Olivares M, Albrecht S, De Palma G, et al. Human milk composition differs in healthy mothers and mothers with celiac disease. Eur J Nutr 2015;54:119–28. [DOI] [PubMed] [Google Scholar]
- 48. Oddy WH, Rosales F. A systematic review of the importance of milk TGF-beta on immunological outcomes in the infant and young child. Pediatr Allergy Immunol 2010;21:47–59. [DOI] [PubMed] [Google Scholar]
- 49. Peloquin JM, Goel G, Villablanca EJ, Xavier RJ. Mechanisms of pediatric inflammatory bowel disease. Annu Rev Immunol 2016;34:31–64. [DOI] [PubMed] [Google Scholar]
- 50. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–42. [DOI] [PubMed] [Google Scholar]
- 51. Zambruni M, Villalobos A, Somasunderam A, et al. Maternal and pregnancy-related factors affecting human milk cytokines among Peruvian mothers bearing low-birth-weight neonates. J Reprod Immunol 2017;120:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elahi S. New insight into an old concept: role of immature erythroid cells in immune pathogenesis of neonatal infection. Front Immunol 2014;5:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Harbeson D, Ben-Othman R, Amenyogbe N, Kollmann TR. Outgrowing the immaturity myth: the cost of defending from neonatal infectious disease. Front Immunol 2018;9:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elahi S, Ertelt JM, Kinder JM, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013;504:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol 2014;35:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Adkins B. Neonatal immunology: responses to pathogenic microorganisms and epigenetics reveal an “immunodiverse” developmental state. Immunol Res 2013;57:246–57. [DOI] [PubMed] [Google Scholar]
- 57. Currie AJ, Curtis S, Strunk T, et al. Preterm infants have deficient monocyte and lymphocyte cytokine responses to group B streptococcus. Infect Immun 2011;79:1588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bian G, Ma S, Zhu Z, et al. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ Microbiol 2016;18:1566–77. [DOI] [PubMed] [Google Scholar]
- 59. Cederlund A, Kai-Larsen Y, Printz G, et al. Lactose in human breast milk an inducer of innate immunity with implications for a role in intestinal homeostasis. PLoS One 2013;8:e53876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Toscano M, De Grandi R, Grossi E, Drago L. Role of the human breast milk-associated microbiota on the newborns’ immune system: a mini review. Front Microbiol 2017;8:2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013;496:238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 2005;7:77–85. [DOI] [PubMed] [Google Scholar]
- 63. Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol 2008;28:718–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rubic T, Lametschwandtner G, Jost S, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol 2008;9:1261–9. [DOI] [PubMed] [Google Scholar]
- 65. Mills E, O’Neill LA. Succinate: a metabolic signal in inflammation. Trends Cell Biol 2014;24:313–20. [DOI] [PubMed] [Google Scholar]
- 66. Serena C, Ceperuelo-Mallafré V, Keiran N, et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J 2018;12:1642–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ferreyra JA, Wu KJ, Hryckowian AJ, Bouley DM, Weimer BC, Sonnenburg JL. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 2014;16:770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014;15:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Qian L, Zhao A, Zhang Y, et al. Metabolomic approaches to explore chemical diversity of human breast-milk, formula milk and bovine milk. Int J Mol Sci 2016;17:2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hove H, Mortensen PB. Influence of intestinal inflammation [IBD] and small and large bowel length on fecal short-chain fatty acids and lactate. Dig Dis Sci 1995;40:1372–80. [DOI] [PubMed] [Google Scholar]
- 71. Vernia P, Caprilli R, Latella G, Barbetti F, Magliocca FM, Cittadini M. Fecal lactate and ulcerative colitis. Gastroenterology 1988;95:1564–8. [DOI] [PubMed] [Google Scholar]