Abstract
Rationale
Protracted use of methamphetamine (mAMPH) can result in long-term impairments in cognitive function in humans. A previous study reported reversal-specific learning impairments in rats after a binge administration of mAMPH. Several studies show that extended exposure to mAMPH may confer protection against cognitive impairments and the insult to monoamine systems typically observed after larger binge doses.
Objectives
To explore this issue, we compared the effects of escalating and single doses of mAMPH (and saline, SAL) on retention, reversal learning, and post-mortem analysis of dopamine and serotonin transporters, DAT and SERT.
Methods
Rats learned to discriminate equiluminant stimuli and then were treated with either: (1) 4 weeks of mAMPH increasing by 0.3 mg/kg, culminating in 6 mg/kg (mAMPHescal); (2) 4 weeks of SAL with a single dose of 6 mg/kg on the last day of treatment (mAMPHsingle); or (3) 4 weeks of SAL. Following treatment, rats were tested on retention and reversal learning, with subsequent analysis of DAT and SERT binding across subregions of the striatum and frontoparietal cortex, respectively.
Results
Retention of the pretreatment discrimination was not significantly impaired in either mAMPH treatment group. A significant decrease in ventrolateral striatal DAT binding was observed only in the mAMPHsingle group and frontoparietal SERT was unaffected by either mAMPH treatment. Both treatment groups demonstrated attenuated reversal learning, particularly on measures of accuracy and effort.
Conclusions
These results show that extended and single-dose pretreatment with mAMPH similarly and selectively affect reversal learning, even in the absence of significant DAT or SERT changes.
Keywords: Inhibitory control, Cognitive flexibility, Psychostimulants, Dopamine transporter, Serotonin transporter
Introduction
Use of the highly addictive psychostimulant methamphetamine (mAMPH) can result in cognitive inflexibility in humans (Hoffman et al. 2006; Verdejo-Garcia et al. 2006) and experimental animals (Izquierdo et al. 2010; Parsegian et al. 2011; Groman et al. 2012). A lack of cognitive or inhibitory control is thought to contribute to an increased vulnerability to abuse drugs (Perry and Carroll 2008; Robinson and Berridge 2003). Long-term drug abuse, in turn, appears to result in decreased inhibitory control (Perry and Carroll 2008; Salo et al. 2002), typifying the complex and circuitous relationship between drug use and cognitive dysfunction (Izquierdo and Jentsch 2012; Lucantonio et al. 2012). Monoaminergic changes in frontostriatal circuitry have been observed after experimenter-administered regimens of mAMPH in animals. Specifically, striatal dopamine (DA) depletions occur readily after single-day, moderate-to-high dose “binge” mAMPH treatment regimens (Ricaurte et al. 1980; Wagner et al. 1980) and single mAMPH injections (Fukumura et al. 1998), with serotonergic changes in the frontoparietal cortex also found in animals treated similarly with mAMPH (O’Dell et al. 2012; Schmidt et al. 1985).
Escalating or sensitizing dosing regimens, characterized by small doses that then gradually increase in frequency and/or size, have been used to model human users' initial escalation phase of drug use (Simon et al. 2002). Importantly, these administration patterns can result in very different behavioral (Belcher et al. 2008) and neurochemical (Graham et al. 2008; Segal et al. 2003) profiles when compared to single-day dosing regimens. These administration patterns have also been known to confer a protective effect against the mAMPH-induced neurotoxicity commonly seen after single-day binge-type regimens (Graham et al. 2008; Segal et al. 2003) and the behavioral sequelae of these mAMPH treatment regimens can occur even in the absence of any significant DA or serotonin changes (Belcher et al. 2006; Kosheleff et al. 2011; cf. Groman et al. 2012). Furthermore, impairments on an object recognition task can be attenuated or prevented altogether when a single-day binge regimen is preceded by an escalating regimen (Belcher et al. 2008; Clark et al. 2007). Less is known, however, about how these different administration patterns might affect cognitive flexibility or inhibitory control.
Reversal learning tasks, in which the main requirement is to respond to a reversal in reward contingency, are practical and confirmed assays of inhibitory control. Monoamine systems in orbitofrontal cortex and striatum that are recruited in reversal tasks are also affected by mAMPH (Izquierdo and Jentsch 2012; Lucantonio et al. 2012). Brief, moderate-to-high doses of mAMPH are known to result in impairments on several measures of reversal: spatial reversal (White et al. 2009), response reversal (Cheng et al. 2007; cf. Daberkow et al. 2008), and visual discrimination reversal (Izquierdo et al. 2010) learning. However, no study has directly compared the effects of single-day doses of mAMPH on reversal learning to those found after gradually escalating, prolonged administration of mAMPH. Consequently, we chose to examine three groups treated with either a single exposure to mAMPH, extended mAMPH, or saline (SAL) on reversal learning. Behavioral testing was followed by quantification of DAT in the striatum and SERT (Shepard et al. 2004) in several areas of frontoparietal cortex, including regions known to be directly affected by mAMPH (Meredith et al. 2005; Nordahl et al. 2003) and involved in cognitive flexibility (Kesner and Churchwell 2011; Ragozzino 2002; Ragozzino et al. 2002).
Methods
Subjects
Thirty-one male Long–Evans rats (Charles River Laboratories, Raleigh, NC) weighing between 275 and 300 g at the beginning of the study were individually housed during food restriction, given water ad libitum and maintained at a 12-h light/12-h dark cycle, with the temperature at 22 °C. Body weights were monitored daily. Behavioral testing took place between 0800 and 1600 hours during the rats' inactive period, consistent with previous and ongoing studies in our lab (Izquierdo et al. 2010; Kosheleff et al. 2011). All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council of the National Academies 2011) and approved by the Institutional Animal Care and Use Committee at California State University, Los Angeles.
Apparatus
Operant conditioning chambers [35 cm (length)×28 cm (width)×34 cm (height)] (#80004, Lafayette Instrument Co., Lafayette, IN) were housed within sound- and light-attenuating cubicles (#83018DDP, Lafayette Instrument Co.). Each chamber was equipped with a houselight, tone generator, and an LCD touchscreen (EloTouch, Menlo Park, CA) in lieu of the wall opposing the pellet dispenser. The pellet dispenser delivered single 45-mg dustless sucrose pellets (BioServ, Frenchtown, NJ). Custom software (Ryklin Software Inc., NY) controlled touchscreen stimuli presentation, tone generation, houselight illumination, and pellet dispensation.
Behavioral training
General
All behavioral training and testing took place five times per week, one to two times per day, with each session lasting a maximum of 45 min. Methodological details are the same as outlined in Izquierdo et al. (2010), with the exception of the added tally of omissions in the present study (see details below). Time spent in each phase was not determined a priori, but was instead dependent on each individual animal's performance. Criterion for advancement for each phase of pretraining was 60 correct nose pokes to the stimulus within 45 min, on each of two consecutive days. During discrimination learning, posttreatment discrimination retention, and reversal learning testing, criterion for advancement to the next stage of testing was a minimum of 85 % correct trials for two consecutive days, with a minimum of 60 correct nose pokes. Only small areas (2.5-cm diameter circles) on the touchscreens underlying the stimuli were sensitive to nose poking, while all other areas remained unresponsive.
Food restriction and acclimation to food rewards
When rats reached a minimum body weight of 275 g, they were food-restricted to no less than 85 % of their free-feeding body weight to ensure motivation to work for food, while water was available ad libitum. On each of the 2 days prior to the start of testing, rats were fed 20 sucrose pellets in their home cage to accustom them to the food reward.
Autoshaping and pretraining
Autoshaping began with the display of white graphic stimuli on the black background of the touchscreen, the disappearance of which was paired with the onset of a “reinforcer event”: a sucrose pellet, a 1-second (s) tone, and a 1-s illumination of the houselight. Criterion for autoshaping occurred when rats ate 60 sucrose pellets within 30 min for each of two consecutive days. Pretraining consisted of different stages, in which the rats progressively learned to nose poke a stimulus (i.e., a white circle), initiate a trial, and were punished for nose poking the incorrect (blank) window on the touchscreen. Punishment consisted of the absence of a reinforcer event and an inability to initiate a new trial for 5 s. A “correction trial” occurred after a punishment and consisted of the same left/right presentation of stimuli (in this case, white circle and blank screen) until the rat nose poked correctly. If the rat did not nose poke either stimulus within 40 s of its appearance onscreen, an omission was recorded, in which the following occurred: (1) the trial timed out, (2) a punishment occurred, (3) an omission tallied, and (4) a new trial initiated.
Visual discrimination learning
With the exception of added omission tally in the present study, visual discrimination learning was identical to procedures outlined in Izquierdo et al. (2010). Briefly, two novel equiluminescent stimuli (SA and SB) were presented concurrently with predetermined reinforcement contingencies, used to allow either a reward event as a result of nose poking the correct stimulus (S+) or a punishment as a result of nose poking the incorrect stimulus (S−). Stimuli presentation (i.e., left/right presentation of the S+) occurred pseudorandomly. Stimulus assignment (SA+SB− or SA−SB+) was counterbalanced across treatment groups. Following successful completion of this phase (85 % correct trials, minimum of 60 correct responses, for each of two consecutive days), rats were removed from food restriction 5 days prior to drug treatment to ensure a healthy body weight and to mitigate any possible interaction with mAMPH.
Drug treatment
Rats were given injections of D-mAMPH (Sigma, St. Louis, MO; s.c.) or physiological SAL solution (1 ml/kg, s.c.) on a clean room procedure table in their housing room, five times per week for 4 weeks, between 1200 and 1500 hours. A 4-week treatment regimen was chosen for its similarity to other mAMPH- and amphetamine-escalating protocols (Featherstone et al. 2008; Fletcher et al. 2007; Madden et al. 2005; Segal and Kuczenski 1997). There were three treatment groups: (1) mAMPHescal group (n=16) received mAMPH, starting at 0.3 mg/kg and escalating in 0.3 mg/kg increments per day, culminating in 6 mg/kg, (2) mAMPHsingle group (n=9) received SAL for 4 weeks with a single dose of 6 mg/kg mAMPH only on the last day of treatment, and (3) the SAL group (n=6) received SAL for the duration of treatment. The largest dose of 6 mg/kg was chosen because it was found to be well-tolerated by our intermittently food-restricted animals. In addition to monitoring body weight, food consumption was assessed daily throughout treatment.
Posttreatment testing
Retention and reversal learning
Following 3–5 days of rest after treatment with mAMPH, rats were put back on food restriction and tested for retention of the initial discrimination contingencies, using procedures identical to the visual discrimination learning phase, above. Upon reaching criterion on this phase (85 % correct trials for each of two consecutive days), the rats were tested on a reversal of the reward contingencies. Parameters for the reversal phase were identical to the visual discrimination learning (above), with the exception that the reward contingencies were reversed.
[125I]RTI-55 binding to DAT and SERT
Rats were euthanized between 6 and 8 weeks after mAMPH or SAL treatment with an overdose of sodium pentobarbital (250 mg/kg, i.p.) and decapitated, and their brains were removed and frozen at −20 °C by immersion in isopentane. Twenty-micrometer-thick coronal sections were cut on a cryostat at the level of the anterior striatum (AP coordinates +1.9 to +1.0 mm, relative to bregma) and prefrontal cortex (AP coordinates +3.7 to +2.8 mm, according to Paxinos and Watson (2005)), thaw-mounted on Vectabond-treated glass slides and stored at −20 °C until used for autoradiographic determination of DAT or SERT binding, respectively. For the determination of striatal DAT binding, warmed slides removed from the −20 °C freezer were preincubated in a solution of assay buffer (10 mM NaPO4, 120 mM NaCl, and 100 mM sucrose) for 5 min to remove endogenous ligands that could interfere with subsequent radioligand binding. After preincubation, the sections were incubated in a solution of assay buffer containing 25 pM [125I]RTI-55 for 2 h. The preincubation and incubation media contained 100 nM fluoxetine to block [125I]RTI-55 binding to SERT (Boja et al. 1992). The sections were then rinsed twice for 2 min each at 4 °C in assay buffer then once for 10 s in 4 °C distilled water. The rinsed slides were then rapidly dried under a stream of heated air. Determinations of frontoparietal cortex SERT were performed in much the same way as those for striatal DAT except that fluoxetine was omitted. The dried slides and [14C]-containing autoradiographic standards were apposed to Hyperfilm MP (GE Healthcare) for 48 h before development. Our analyses were limited to quantification of DAT in striatum and SERT in frontoparietal cortex because the binding of [125I]RTI-55 to SERT in striatum and DAT in frontoparietal cortex was below threshold for accurate measurements. Under the assay conditions used, the binding of [125I]RTI-55 to striatal DAT constitutes >99 % of the its total binding, while the binding of [125I]RTI-55 to frontoparietal SERT constitutes >94 % of the total in that region.
Quantification of [125I]RTI-55 binding was done using an MCID image analyzer (InterFocus Imaging; Cambridge, England). Image densities were converted to [125I]RTI-55 binding levels using a calibration curve based on images of the standard slides packed with each film. Regional densities of binding were obtained by outlining the desired structures on their respective [125I]RTI-55 images. Values obtained represented mean measurements taken from both hemispheres in a total of four sections per animal. For DAT analysis, the images were first divided into caudate–putamen (CP) and whole nucleus accumbens septi samples. The CP was then subdivided into four subregions: dorsomedial, dorsolateral, ventromedial, and ventrolateral parts, which were separately quantified for binding. For SERT analysis, samples encompassing all cortical layers were taken in infralimbic, prelimbic, cingulate, motor, somatosensory, insular, and orbitofrontal cortical regions.
Data analyses
Data were analyzed using StatView software. Statistical significance was noted when p values were equal to or less than 0.05, and a trend towards significance was noted when p values were 0.06–0.08. Data were collected for three phases: (1) initial visual discrimination learning, (2) posttreatment retention, and (3) posttreatment reversal learning. Performance was analyzed on five measures: (1) sessions to criterion, (2) accuracy (i.e., percent of correct trials of total trials), (3) perseveration (i.e., number of consecutive errors), (4) attention (i.e., number of omissions or trials initiated, but no nose poke), and (5) effort (i.e., total trials or nose pokes committed, correct or incorrect).
Overall learning analyses were conducted using individual ANOVAs for mean performance on discrimination, retention, and reversal learning phases on the measures described above. “Sessions to criterion” represents the number of sessions required to meet the criterion of two consecutive sessions of 85 % correct trials and does not include the criterion run. To assess learning over time, analyses were conducted for the first 14 sessions for discrimination and first 20 sessions of retention and reversal learning, using repeated-measures ANOVAs (rmANOVA). These periods were selected for analyses because the slowest learner reached criterion in these time frames. If a subject reached criterion before the 14- or 20-session time frame, the mean of the last two sessions (the criterion run) was carried forward for this subject (Izquierdo et al. 2010). Early reversal learning across session was also analyzed using rmANOVAs, as was rat body weight and food consumption across the day of treatment. DAT and SERT values, reported as the percentage of the average SAL-treated values for each region, were also analyzed using rmANOVA. When ANOVAs and rmANOVAs resulted in significant main effects and interactions, respectively, Bonferroni post hoc pairwise comparisons were conducted.
Results
Pretreatment visual discrimination learning
Rats learned the visual discrimination task in an average of 3.94 (±0.60) sessions. An ANOVA revealed no group differences in sessions to criterion (SAL=4.0±1.27, mAMPHsingle=4.56±1.23, and mAMPHescal=3.56±0.84). There were no significant differences on any other measure of performance in the destined treatment groups, including percent correct, correction trials, omissions, or total trials committed. Treatment group means are shown in Table 1.
Table 1.
Discrimination and posttreatment retention and reversal performance
| Sessions to criteriona | Percent correctb | Correction trialsc | Omissionsd | Total trialse | ||
|---|---|---|---|---|---|---|
| Discrimination | SAL | 4.00±1.27 | 86.06±3.34 | 2.94±0.81 | 11.05±3.43 | 66.04±1.59 |
| mAMPHsingle | 4.56±1.23 | 89.79±1.74 | 2.16±0.43 | 7.30±3.31 | 59.83±2.67 | |
| mAMPHescal | 3.56±0.84 | 86.55±2.68 | 2.41±0.57 | 4.12±1.25 | 66.88±3.33 | |
| Retention | SAL | 8.00±1.90 | 96.50±1.13 | 0.53±0.18 | 4.06±1.16 | 55.63±2.05 |
| mAMPHsingle | 7.00±2.65 | 93.94±2.36 | 0.51±0.23 | 6.14±3.32 | 50.93±4.97 | |
| mAMPHescal | 4.36±0.87 | 93.68±1.14 | 0.786±0.24 | 2.63±0.46 | 57.71 ±1.71 | |
| Reversal | SAL | 12.50±2.05 | 61.81±3.62 | 9.60±1.62 | 4.38±0.51 | 55.80±4.46 |
| mAMPHsingle | 21.29±3.39 | 30.46*±9.40 | 7.91±2.37 | 8.07±1.44 | 28.63*±8.55 | |
| mAMPHescal | 19.64±2.93 | 39.45**±7.03 | 8.95±1.68 | 6.08±1.07 | 36.02**±7.01 |
Treatment group means±SEM over first 14 sessions for discrimination learning and 20 sessions for retention and reversal learning phases. Data do not include the two sessions of 85 % correct trials (criterion run)
p≤0.05,
p≤0.08, different from SAL; mAMPH-treated groups were not significantly different from each other
Number of sessions until performance criterion was met
Percent of correct trials of total trials
Number of consecutive errors
Number of trials initiated with no nose poke
Total number of nose pokes, correct or incorrect
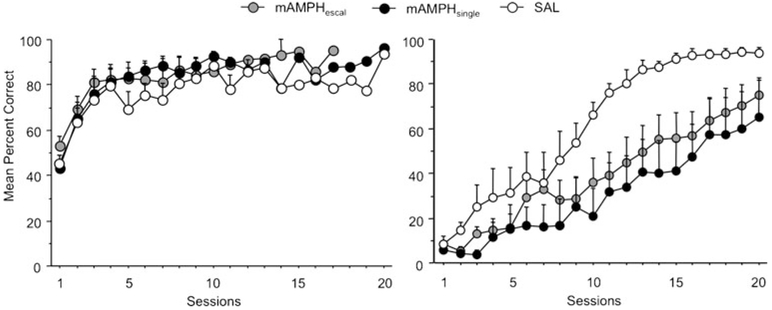
There were also no differences in learning according to stimulus assignment (i.e., which of the two stimuli is rewarded) (mean sessions to criterion: SA+SB−=5.13±1.33, SA−SB+=05.47±1.41). Thus, data for stimulus assignment were collapsed for all subsequent analyses. Initial visual discrimination learning curves for destined treatment groups are shown in Fig. 1a.
Fig. 1.
Mean percent correct by session for pretreatment discrimination learning and posttreatment reversal learning. Left panel: There were no significant destined group differences in pretreatment discrimination learning accuracy. Right panel: Both mAMPH-treated groups performed similarly on posttreatment reversal learning, exhibiting attenuated accuracy (n=16 mAMPHescal, n=9 mAMPHsingle, n=6 SAL)
Body weight during treatment
Rats quickly gained weight during intermittent periods of free feeding. However, rats receiving extended mAMPH treatment displayed attenuated body weight over the period of treatment (average weight in grams: SAL=499.25±6.06 and mAMPHescal=461.7±4.90). A rmANOVA revealed a significant interaction of treatment × day on body weight (F19, 342=9.31, p<0.01). No significant effect of treatment was observed, though there was a significant effect of day on body weight (F19, 342=56.99, p<0.01). Despite the difference in body weight across day, treatment groups did not differ in food consumption, nor was there an interaction of treatment × day on food consumption. Because the mAMPHsingle group was treated across 19 days of SAL and only 1 day of mAMPH, they constituted part of the SAL group for the repeated-measures analysis. Data are shown in Fig. 2.
Fig. 2.
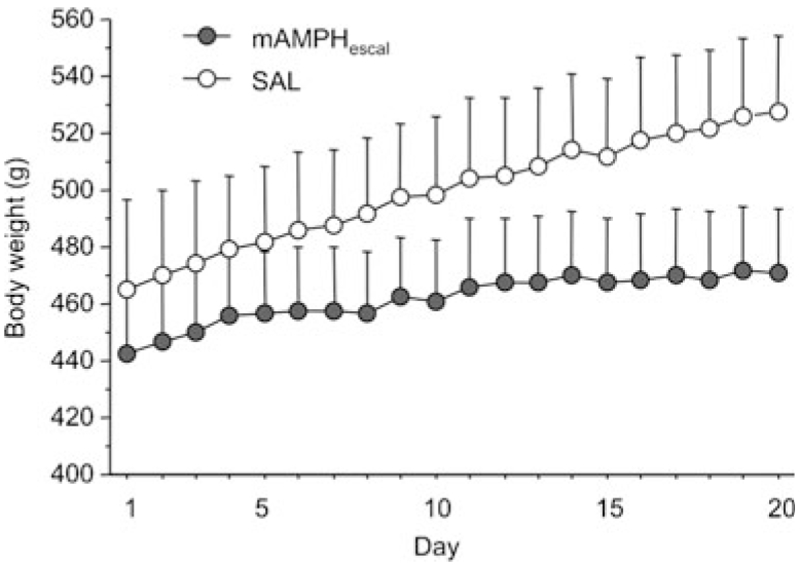
Mean body weight (in grams) during treatment. There was a significant interaction of treatment group by day on body weight. Because the mAMPHsingle group was treated across 19 days of SAL and only 1 day of mAMPH, they constituted part of the SAL group for this comparison (n=10 mAMPHescal, n=10 SAL)
Effect of mAMPH on retention of visual discrimination
Upon resuming behavioral testing after treatment, rats were tested on their retention of the original stimulus assignment. Rats performed the retention test to criterion in an average of 6.04 (±0.99) sessions. An ANOVA revealed no treatment group differences in sessions to criterion (SAL=8.00±1.90, mAMPHsingle=7.00±2.64, and mAMPHescal=4.36±2.87). There were also no treatment group differences on any other performance measure including percent correct, correction trials, omissions, or total trials committed. Similarly, rmANOVAs across 20 sessions of retention testing revealed no significant group differences or interactions on these measures. Treatment group means are shown in Table 1.
Effect of mAMPH on reversal learning
Upon reaching criterion on the original stimulus assignment, rats were tested on a reversal of the reward contingency. The rats reached the reversal learning criterion in an average of 18.33 (±1.82) sessions. An ANOVA revealed no treatment group differences in sessions to criterion. However, ANOVAs resulted in significant differences in percent correct (F2, 14=5.26, p=0.02) and total trials committed (F2, 14=4.74, p=0.03). Bonferroni post hoc tests with a corrected alpha level of 0.02 revealed differences between the mAMPHsingle and SAL groups (p<0.01) for both of these measures with a trend toward a significant difference between the mAMPHescal and SAL groups (p≤0.08). MAMPH-treated groups were not, however, significantly different from each other.
A rmANOVA across 20 sessions of reversal learning revealed a significant main effect of treatment group on percent correct (F2, 21=3.69, p=0.04). Bonferroni post hoc tests with corrected alpha level of 0.02 revealed a significant difference between the mAMPHsingle and SAL groups (p<0.02), with a trend towards a significant difference between the mAMPHescal and SAL groups (p=0.05). Here again, no significant differences were observed between mAMPHescal and mAMPHsingle groups, indicating that mAMPH-treated rats displayed lower percent correct than SAL rats (Fig. 1b). There was no significant treatment group × testing session interaction. A rmANOVA revealed a trend toward significance on total trials (F2, 21=2.94, p<0.08), suggesting a difference between mAMPHsingle and SAL rats with both mAMPH groups performing worse than SAL, but no interaction with session. Additional rmANOVAs revealed no significant group differences on correction trials and omissions or a significant treatment × session interaction for either of these measures. Treatment group means are shown in Table 1.
Effect of mAMPH on early reversal learning
Consistent with previous studies in our lab (Izquierdo et al. 2010), we explored differences in early reversal learning for three reasons: (1) initial or “early” reversal learning requires greater inhibitory control (Jones and Miskin 1972); (2) DA manipulations selectively affect early reversal learning using touchscreen response methodology in rodents (Izquierdo et al. 2006); and (3) DA changes as a result of mAMPH administration are more likely to be pronounced in earlier testing sessions, with some recovery occurring as time-since-treatment increases (Cass and Manning 1999; Friedman et al. 1998; Melega et al. 1997).
As expected in reversal learning, rats initially (preferentially) select the stimulus associated with reward according to the previous reward contingency, as evidenced by their below-chance performance. A rmANOVA on the first three sessions of reversal learning accuracy revealed a significant effect of treatment group (F2, 21=4.31p<0.03) and a significant interaction of treatment group × session (F4, 42=2.65p<0.05). Bonferroni post hoc tests with corrected alpha level of 0.02 confirmed a significant difference between mAMPHsingle and SAL rats (p<0.01) and a trend towards a difference for mAMPHescal and SAL rats (p=0.07), with both mAMPHsingle and mAMPHescal performing worse than SAL, and that the mAMPH treatment groups were not significantly different from each other. Individual ANOVAs for the first three sessions revealed differences only on day 3 (F2, 14=3.66, p=0.05), but were not present on session 1 or 2. Post hoc tests reveal only a trend towards significance between mAMPHsingle and SAL (p=0.03) and mAMPHsingle and mAMPHescal (p=0.03). Data are shown in Fig. 3.
Fig. 3.
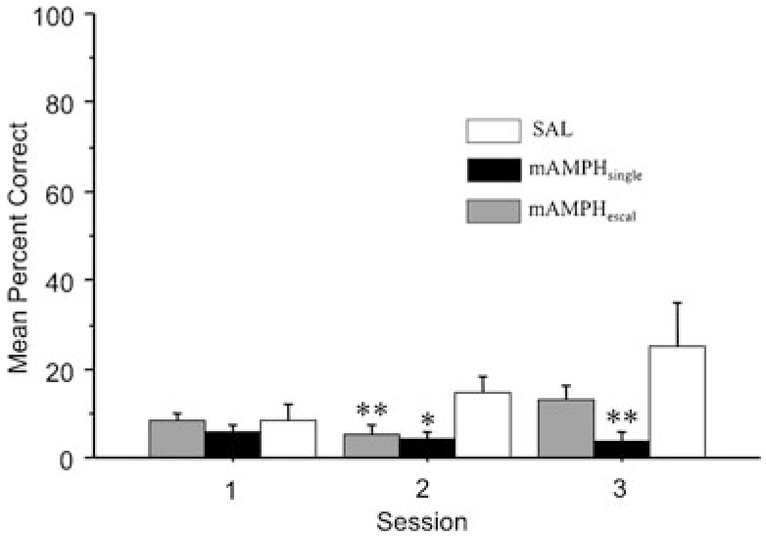
Mean percent correct for early (posttreatment) reversal learning differs between mAMPH- and SAL-treated groups. Both mAMPH-pretreated groups scored lower percent correct on early (sessions 1–3) reversal phase compared to SAL (n=16 mAMPHescal, n=9 mAMPHsingle, n=6 SAL). Bars represent group means±SEM; *p=0.05 and **p=0.01 versus SAL
Similarly, rmANOVAs on the first 3 days of reversal learning revealed no effect of treatment for number of correction trials or number of omissions, but there was a trend towards significance for total trials committed (F2, 21=2.93, p<0.08). Bonferroni post hoc tests suggest both mAMPH groups committed fewer trials than SAL rats. There were no interactions of session × treatment group for number of correction trials, number of omissions, or total trials committed.
Effects of mAMPH on [125I]RTI-55 binding to DAT and SERT
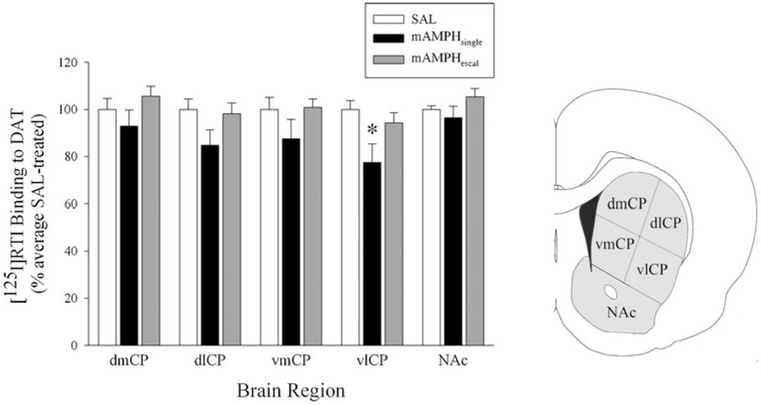
A rmANOVA revealed no overall treatment group differences for striatal DAT binding (mean levels of DAT, as a percent of SAL control values; mAMPHsingle=87.48±2.82, mAMPHescal=99.38±1.64, and SAL+100.00±1.58), but the interaction of treatment group × subregion was highly significant (F10, 105=3.73, p<0.01). Bonferroni follow-up tests (with a corrected alpha level of p<0.02) surprisingly show the primary difference to be between mAMPHsingle and mAMPHescal, though the difference did not reach statistical significance (p<0.09). A follow-up ANOVA examining each striatal subregion individually revealed a significant group difference only in the ventrolateral CP (Fig. 4) (F2, 21=4.15, p=0.03; mean DAT levels: mAMPHsingle=77.44±7.86, mAMPHescal=95.58±4.31, and SAL=100.00±3.77). Bonferroni follow-up tests suggest a trend towards significant differences between mAMPHsingle and mAMPHescal (p<0.03) and a significant difference between mAMPHsingle and SAL (p<0.02) in this region. All other striatal subregions showed no significant group differences. A rmANOVA revealed no group differences for frontoparietal SERT (mean levels of SERT: mAMPHsingle=101.07±4.03, mAMPHescal=105.44±2.37, and SAL=100.00±1.87), nor a significant group × region interaction.
Fig. 4.
Mean [125I]RTI binding to DAT in striatal subregions. Values are percent of SAL values. Coronal section (right) shows areas sampled. Bars represent group means±SEM; *p<0.03 relative to both SAL and mAMPHescal groups. CP caudate–putamen; dl dorsolateral; dm dorsomedial; NAc nucleus accumbens septi; vl ventrolateral; vm ventromedial
Discussion
We report the novel finding that single and escalating doses of mAMPH exert similar effects on reversal learning. Performance of both mAMPH groups was statistically indistinguishable for measures of accuracy (percent correct) and effort (total trials committed), with a more pronounced impairment in the rats receiving a single injection of mAMPH. This impairment was specific to reversal learning: retention of the pretreatment discrimination was unaffected after either brief or extended treatment. Our findings also substantiate the view that cognitive deficits can occur after mAMPH even in the absence of appreciable decreases in DAT and SERT binding. Interestingly, even though mAMPHescal rats perform as poorly as the mAMPHsingle group on reversal learning, only the mAMPHsingle rats demonstrated any measured DAT changes.
Single-dose versus single-day binge
Several previous experiments from our laboratories have examined the cognitive and neurochemical sequelae of single-day mAMPH binge administration (Belcher et al. 2008; Izquierdo et al. 2010; Kosheleff et al. 2011), but to our knowledge, this is the first study to examine such effects in rats receiving a single mAMPH injection. The 4×2-mg/kg binge mAMPH regimen used previously differs from the present mAMPHsingle regimen because it delivered a larger total quantity of mAMPH over a protracted period (during binge, doses are separated by 2 h). Despite these differences, measures of reversal learning, effortful behavior, and striatal DAT after a single mAMPH dose collected in the present study appear to be quite similar to measures obtained after a 4×2-mg/kg binge (Izquierdo et al. 2010; Kosheleff et al. 2011).
Reversal learning
Until recently, many rodent studies of mAMPH exposure (Marshall et al. 2007; Schröder et al. 2003) and self-administration (Reichel et al. 2011) have pointed to greater specificity for this drug's effects on recognition memory. Our observation that both brief and extended mAMPH selectively affect reversal learning provides additional evidence to that already gathered in monkeys (Groman et al. 2012) that this task may be a particularly sensitive assay for mAMPH effects on an aspect of cognition phenotypically related to compulsive drug use (Izquierdo and Jentsch 2012; Lucantonio et al. 2012).
During reversal learning, we also observed behavior indicative of diminished effort, as assessed by fewer overall trials committed or “nose pokes” on the touchscreen by the mAMPH-treated groups compared to SAL. A pattern of fewer attempts at reward during reversal learning is consistent with previous results from our lab where rats treated with a 4×2-mg/kg binge regimen were found to be work averse on an effort discounting task at increased work demands (Kosheleff et al. 2011). Analogously on the reversal task in the present study, we showed no deficits on the familiar retention phase of the task, but upon reversal of the reward contingencies (i.e., upon increasing difficulty), mAMPHsingle rats (and to a slightly lesser degree, mAMPHescal rats) show reduced effort to initiate trials and attempt to procure the food reward.
Monoaminergic markers
Animal studies have clearly established that single-day binge administration of moderate-to-high doses of mAMPH decreases striatal DA content, DAT protein and binding, as well as other markers of dopaminergic terminal integrity (e.g., tyrosine hydroxylase protein) (Cadet et al. 2009; Fleckenstein et al. 2009; Yamamoto et al. 2010). The dosing regimens employed here were below the range typically used to achieve dopaminergic neurotoxicity (three or four injections of 4–10 mg/kg, spaced at ca 2-h intervals). Nevertheless, our finding of a small but significant DAT loss in the mAMPHsingle dose group stands in contrast to the complete absence of DAT loss in the mAMPHescal group. In addition, frontal cortical SERT was not affected in either group, suggesting that (1) frontocortical SERT systems are more resistant than striatal DAT to damage by the dosing regimens employed and (2) other neural components not measured here may be involved in the impairment. The relatively low expression of SERT in the striatum and DAT in frontoparietal cortex precluded their quantification in these experiments. However, the present data suggest that cognitive changes (attenuated reversal learning) can occur in the absence of robust monoaminergic changes in either mAMPH treatment group and instead may reflect DA dysregulation (Cools et al. 2009; Jocham et al. 2009). Our present findings are consistent with a recent study showing that individual variation on reversal learning performance in mAMPH-treated monkeys could be accounted for by D2 dysregulation (Groman et al. 2012).
Conclusion
Our report of impaired reversal learning following brief and extended mAMPH adds to the list of cognitive impairments observed in the absence of appreciable monoaminergic toxicity. It is likely that mAMPH can affect cognition via mechanisms other than neurotoxicity (Belcher et al. 2006, 2008; Reichel et al. 2011; Rogers et al. 2008), and individual cognitive measures likely differ in their sensitivity to neuroplastic changes after mAMPH. Reversal learning may be particularly sensitive to mAMPH-induced neuroplasticity, whereas other tasks may be more resilient to perturbation. Future studies should be directed at discerning the etiology of mAMPH effects on reversal learning, specifically whether the primary impairment stems from reduced motivation (work aversion) or impaired reward learning.
Acknowledgments
This work was supported by 1SC2MH087974 (Izquierdo) and 1RO1 DA012204 (Marshall). We thank the CSULA Animal Care staff for their excellent support. We are grateful to Millie Grimes, Vivian Zeng, Chelsi Darling, and Aviva Sterns for their help with behavioral testing and data collection.
Footnotes
Conflict of interest There is nothing to disclose nor are there any conflicts of interest.
Contributor Information
Alisa R. Kosheleff, Department of Psychology, California State University, Los Angeles, 5151 State University Drive, Los Angeles, CA 90032, USA, aizquie@calstatela.edu
Danilo Rodriguez, Department of Psychology, California State University, Los Angeles, 5151 State University Drive, Los Angeles, CA 90032, USA, aizquie@calstatela.edu.
Steve J. O’Dell, Department of Neurobiology and Behavior, University of California, Irvine 92617, USA
John F. Marshall, Department of Neurobiology and Behavior, University of California, Irvine 92617, USA
Alicia Izquierdo, Department of Psychology, California State University, Los Angeles, 5151 State University Drive, Los Angeles, CA 90032, USA, aizquie@calstatela.edu.
References
- Belcher AM, O’Dell SJ, Marshall JF (2006) A sensitizing regimen of methamphetamine causes impairments in a novelty preference task of object recognition. Behav Brain Res 170:167–172 [DOI] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O’Dell SJ, Marshall JF (2008) Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology 33:1453–1463 [DOI] [PubMed] [Google Scholar]
- Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH, Abraham P, Kuhar MJ (1992) High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse (N Y) 12:27–36 [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Ladenheim B, Cai N-S, McCoy MT, Atianjoh FE (2009) Methamphetamine preconditioning: differential protective effects on monoaminergic systems in the rat brain. Neurotox Res 15:252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Manning MW (1999) Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J Neurosci 19:7653–7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R-K, Etchegaray M, Meck WH (2007) Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Res 1186:255–266 [DOI] [PubMed] [Google Scholar]
- Clark RE, Kuczenski R, Segal DS (2007) Escalating dose, multiple binge methamphetamine regimen does not impair recognition memory in rats. Synapse (N Y) 61:515–522 [DOI] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M (2009) Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci 29:1538–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA (2008) Effect of methamphetamine neurotoxicity on learning-induced Arc mRNA expression in identified striatal efferent neurons. Neurotox Res 14:307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Kapur S, Fletcher PJ (2008) A sensitizing regimen of amphetamine that disrupts attentional set-shifting does not disrupt working or long-term memory. Behav Brain Res 189:170–179 [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR (2009) Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology 56:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S (2007) A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology 32:1122–1132 [DOI] [PubMed] [Google Scholar]
- Friedman SD, Castañeda E, Hodge GK (1998) Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav 61:35–44 [DOI] [PubMed] [Google Scholar]
- Fukumura M, Cappon GD, Pu C, Broening HW, Vorhees CV (1998) A single dose model of methamphetamine-induced neurotoxicity in rats: effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res 806:1–7 [DOI] [PubMed] [Google Scholar]
- Graham DL, Noailles P-AH, Cadet JL (2008) Differential neurochemical consequences of an escalating dose-binge regimen followed by single-day multiple-dose methamphetamine challenges. J Neurochem 105:1873–1885 [DOI] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD (2012) Dysregulation of D2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci 32:5843–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH (2006) Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology 188:162–170 [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD (2012) Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology 219:607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, Holmes A (2006) Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res 171:181–188 [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF (2010) Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology 35:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Neumann J, von Cramon Y, Reuter M, Ullsperger M (2009) Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J Neurosci 29:3695–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Miskin M (1972) Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurol 36:362–377 [DOI] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC (2011) An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem 96:417–431 [DOI] [PubMed] [Google Scholar]
- Kosheleff AR, Grimes M, O’Dell SJ, Marshall JF, Izquierdo A (2011) Work aversion and associated changes in dopamine and serotonin transporter after methamphetamine exposure in rats. Psychopharmacology 219:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G (2012) The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci 15:358–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden LJ, Flynn CT, Zandonatti MA, May M, Parsons LH, Katner SN, Henriksen SJ, Fox HS (2005) Modeling human methamphetamine exposure in nonhuman primates: chronic dosing in the rhesus macaque leads to behavioral and physiological abnormalities. Neuropsychopharmacology 30:350–359 [DOI] [PubMed] [Google Scholar]
- Marshall JF, Belcher AM, Feinstein EM, O’Dell SJ (2007) Methamphetamine-induced neural and cognitive changes in rodents. Addict 102:61–69 [DOI] [PubMed] [Google Scholar]
- Melega WP, Raleigh MJ, Stout DB, Lacan G, Huang SC, Phelps ME (1997) Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res 766:113–120 [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ (2005) Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatr 13:141–154 [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies (2011) Guide for the care and use of laboratory animals, 8th edn. The National Academies Press, Washington [Google Scholar]
- Nordahl TE, Salo R, Leamon M (2003) Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 15:317–325 [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Galvez BA, Ball AJ, Marshall JF (2012) Running wheel exercise ameliorates methamphetamine-induced damage to dopamine and serotonin terminals. Synapse (N Y) 66:71–80 [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Lavin A, See RE (2011) Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry 69:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elsevier, London: [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME (2008) The role of impulsive behavior in drug abuse. Psychopharmacology 200:1–26 [DOI] [PubMed] [Google Scholar]
- Ragozzino ME (2002) The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem 9:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJY, Kesner RP (2002) Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci 116:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE (2011) Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36:782–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS (1980) Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res 193:153–163 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2003) Addiction. Annu Rev Psychol 54:25–53 [DOI] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE (2008) Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology 199:615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possinc K, Leamon M, Gibsond DR, Galloway GP, Flynnd NM, Henikf A, Pfefferbaum A, Sullivang EV (2002) Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res 111:65–74 [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW (1985) Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther 233:539–544 [PubMed] [Google Scholar]
- Schröder N, O’Dell SJ, Marshall JF (2003) Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse (N Y) 49:89–96 [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R (1997) Repeated binge exposures to amphetamine and methamphetamine: behavioral and neurochemical characterization. J Pharmacol Exp Ther 282:561–573 [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK (2003) Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology 28:1730–1740 [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y (2004) The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 55:1082–1089 [DOI] [PubMed] [Google Scholar]
- Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W (2002) A comparison of patterns of methamphetamine and cocaine use. J Addict Dis 21:35–44 [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M (2006) Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc 12:405–415 [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J (1980) Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res 181:151–160 [DOI] [PubMed] [Google Scholar]
- White IM, Minamoto T, Odell JR, Mayhorn J, White W (2009) Brief exposure to methamphetamine (METH) and phencyclidine (PCP) during late development leads to long-term learning deficits in rats. Brain Res 1266:72–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA (2010) Amphetamine toxicities. Ann N Y Acad Sci 1187:101–121 [DOI] [PMC free article] [PubMed] [Google Scholar]