Abstract
Rationale
Drug addiction can be described as aberrant allocation of effort toward acquiring drug, despite associated costs. It is unclear if this behavioral pattern results from an overvaluation of reward or to an altered sensitivity to costs.
Objective
Present experiments assessed reward sensitivity and effortful choice in rats following 1 week of withdrawal from methamphetamine (mAMPH).
Methods
Rats were treated with either saline or an escalating dose mAMPH regimen, then tested after a week without the drug. In experiment 1, rats were given a free choice between water and various concentrations of sucrose solution to assess general reward sensitivity. In experiment 2, rats were presented with a choice between lever-pressing for sucrose pellets on a progressive ratio schedule or consuming freely-available chow.
Results
In experiment 1, we found no differences in sucrose preference between mAMPH- and saline-pretreated rats. In experiment 2, when selecting between two options, mAMPH-pretreated rats engaged in less lever-pressing for sucrose pellets (p < 0.01) and switched from this preferred reward to the chow sooner than saline-pretreated rats (p < 0.05). This effect was not consistent with general reward devaluation or loss of motivation.
Conclusions
These findings demonstrate that mAMPH exposure and withdrawal lead to steeper discounting of reward value by effort, an effect that is consistent with the effect of mAMPH on discounting by delay, and which may reflect an underlying shared mechanism.
Keywords: Effort discounting, Cost-benefit, decision-making, Reward sensitivity, Psychostimulant
Introduction
A prominent feature of drug addiction is the persistent effort to seek out and acquire a drug, often at the expense of other healthy behaviors associated with natural rewards, or despite negative consequences. This aspect of the disorder likely results, in part, from alterations in neural cost-benefit decision-making mechanisms that optimize response selection and reward acquisition. A relevant metric of these mechanisms is effortful choice behavior—selecting between pursuing low effort/low value rewards and high effort/high value rewards—which has been modeled in a basic form in experimental animals, most extensively in rats, using food as an outcome (Randall et al. 2012; 2014).
We have previously reported that rats, when assessed after acute methamphetamine withdrawal, are more willing to expend effort for valued natural rewards such as food and exercise (Stolyarova et al. 2015; Thompson et al. 2015). Similarly, rats exposed to and withdrawn from a variety of drugs, including the psychostimulants amphetamine, MDMA, and cocaine, show increased lever pressing behavior for sucrose pellets in a progressive ratio task relative to saline-treated animals (Olausson et al. 2006). These findings suggest that animals that have undergone withdrawal from psychostimulants are more willing to expend effort to acquire rewards; however, it is unclear whether this effect is due to alterations in reward sensitivity or alterations in cost-benefit valuation.
Humans and nonhumans alike typically have more options available to them when making decisions in the real world than an animal in an operant chamber with a single lever-press response option, and decisions often carry other costs unrelated to effort, such as delay to reward and risk associated with pursuing the reward. In these domains, choice behavior focused on short-term reward acquisition at the expense of overall or long-term gain is often termed “impulsivity” (Jentsch et al. 2014) or “impulsive choice” (Cardinal et al. 2001). Heightened impulsive behavior is, in many cases, a predictor of drug use (Dalley et al. 2007; Belin et al. 2008; Crews et al. 2009; Dalley et al. 2011; Cervantes et al. 2013) and can also be a consequence of repeated psychostimulant exposure and withdrawal (Richards et al. 1999; Jentsch et al. 2002; Calu et al. 2007; Simon et al. 2007; Izquierdo et al. 2010; Kosheleff et al. 2012a). This suggests that psychostimulant exposure and withdrawal may not simply affect general reward sensitivity, but rather that it influences value-based decision making mechanisms in a way that biases choices toward short-term reward procurement. While impulsive choice has been studied extensively in the context of delays to reward receipt, impulsive behavior in the context of effortful choice has received comparably less attention. It has been shown that reward value is discounted by effort costs in a similar manner to delays (Prévost et al. 2010), and that subjects with low scores on a measure of self-control discount rewards more steeply by both delay and effort (Hiraoka and Kabasawa 2013). However, it is currently unknown whether mAMPH exposure and withdrawal can bias choice behavior in an impulsive-like manner toward an easier reward option at the expense of a more difficult but preferred option.
To assess this, a modification to the standard progressive ratio task may be employed. The addition of a freely available, less valued reinforcer to the task environment alters effortful choice behavior by providing the animal a choice between consuming a less valuable, but easier to acquire reward versus working for a better, but harder to acquire reward. This structure parallels tests of impulsive choice behavior in delay-related decision making, and the progressive nature of the task ensures that the animal repeatedly makes decisions between the two reward options throughout the session (Randall et al. 2012). Under these conditions, rats tend to vigorously press the lever at the beginning of the session, then switch to chow consumption once the effort requirement has increased, and spend the remainder of the session alternating between pressing the lever and eating chow. Thus, the breakpoint in this task represents the effort requirement at which the rat is roughly indifferent between the two options, that is, the value of the preferred option has been discounted by the effort cost to the value of the freely available chow. Impulsive-like choice in this task can be characterized by earlier switching from lever pressing to chow.
Though the ability of mAMPH to induce impulsive behavior in an intertemporal choice setting is well-documented, the effects of mAMPH exposure and withdrawal on long-term reward sensitivity and effortful behavior in the context of choice are still unclear. We therefore set out to assess these behaviors in rats in prolonged withdrawal from methamphetamine. We first investigated whether general reward sensitivity (or hedonic tone) is changed using a two-bottle choice sucrose preference task (experiment 1). Then, we assessed effortful choice behavior and impulsivity using a task in which rats could choose to consume freely available lab chow, or to exert increasing amounts of effort for a preferred sucrose reward (experiment 2).
Methods
Subjects
Two separate cohorts of male Long-Evans rats (Charles River Laboratories, Raleigh, NC) were used. All subjects arrived at postnatal day (PND) 70, weighing between 250 and 300 g at the beginning of the study, and were maintained under a 12-h light/12-h dark cycle under temperature- and humidity-controlled conditions. Only male rats were used as the presence of female rats in the housing and testing rooms has led to changes in reward-related decision making (unpublished observations). Lab chow and water were available ad libitum prior to the inception of operant behavioral training. After arrival in the facility, rats were left undisturbed for 3 days to acclimate to the vivarium, then handled over the next 5 days for a minimum of 10 min per rat per day. During acclimation, handling, and methamphetamine pre-exposure, rats were pairhoused. Each methamphetamine-treated rat was housed with a saline-treated rat to minimize aggression. The morning after the last day of drug treatment, all rats were singly housed for the remainder of the experiment. All behavioral testing took place during the dark phase. All procedures were conducted in accordance with the latest NIH Guide for the Care and Use of Laboratory Animals (National Institutes of Health 2011) and were approved by the Chancellor’s Animal Research Committee of UCLA.
Drug pretreatment
Rats were pretreated with d-methamphetamine hydrochloride (Sigma, St. Louis, MO), using a subchronic escalating regimen (Thompson et al. 2015) that is known to protect DA systems from neurotoxicity following subsequent binge exposures (Segal et al. 2003). In experiment 1, rats were given three daily injections (separated by 3.25 h) of methamphetamine (“mAMPH”, n = 11; 0.1–6.0 mg free base/kg, s.c., escalating in 0.1 mg/kg increments up to 2.1 mg/kg, then in 0.2 mg/kg increments from 2.1 mg/kg to 6.0 mg/kg) or physiological saline solution (“SAL”, n = 12; 1 ml/kg, s.c.) for 2 weeks. This dosing regimen resulted in the death of one rat in this experiment, leading us to use a milder regimen (shallower escalation and lower culminating dose) in experiment 2: rats were given three daily injections (separated by 3.25 h) of mAMPH (n = 16; 0.1–4.0 mg free base/kg, s.c., escalating in 0.1 mg/kg increments) or physiological saline solution (“Sal”, n = 16; 1 ml/kg, s.c.). Rats were behaviorally tested following an acute withdrawal period of at least 7 days.
Experiment 1. Sucrose preference
Behavioral testing
Testing began 7 days after the final injection of methamphetamine. Water bottles were removed from home cages for 8 h during the inactive phase. At the onset of the dark phase, two pre-weighed bottles were placed on each cage, with lab chow. One bottle contained drinking water throughout the entire experiment (bottle 2) and the other (bottle 1) contained solutions that varied in contents each day. In brief, bottle 1 contained pure drinking water every other day, and contained sucrose concentrations of 1, 2, 4, 8, 16, and 32% on alternate days (modified from Pothion et al. 2004). The locations of the two bottles were alternated every 2 days to prevent formation of a side preference. Bottles were left on the cages for 30 min then carefully removed and weighed. Rats had free access to water after testing until removal of the bottles 4 h into the light cycle on the next day.
Data analysis
Pre-post differences in weight were calculated for each bottle, and a preference score was determined by dividing the amount of fluid consumed from bottle 1 by the total volume of fluid consumed from both bottles. Days on which both bottles contained water were excluded from the analysis, and repeated measures ANOVA was used to examine between-pretreatment group differences in sucrose preference across escalating concentrations.
Experiment 2. Progressive ratio choice task
Food restriction
Two days before behavioral testing began, the amount of food given to each rat was reduced to 18 g/day, and rats were given ~60 sucrose pellets (45-mg dustless precision sucrose pellets; Bio-Serv, Frenchtown NJ) to acclimate them to the food rewards. Throughout pretraining, rats were maintained on 18 g of lab chow per day. Once rats were advanced to the progressive ratio phase of training, they were given 20 g of chow per day to maintain a healthy body weight. Finally, rats performing the choice task were given 25 g of food per day. Food was always given between 2 and 3 h after behavioral testing had concluded to prevent rats from associating the termination of testing with immediate chow availability.
Behavioral testing apparatus
Training and testing for this experiment were conducted in automated chambers fitted with a house light, a food-delivery magazine in which sucrose pellets were delivered, and a retractable lever positioned on the chamber wall opposite the magazine. Chambers were enclosed in sound-attenuating boxes and were controlled by a PC running Med-PC IV (Med-Associates, St. Albans, VT). Each time a pellet was earned, the lever was retracted for 5 s before being presented again.
Progressive ratio
Habituation
On the first day of behavioral testing, rats were placed into the operant chambers with the house light on and 5 sucrose pellets were dropped into the magazine. The rats were left in the boxes for 15 min, at which point they were removed. If a rat had eaten all five pellets during that time, they were allowed to advance to the next stage. If not, habituation was repeated daily until all the pellets were eaten.
Training
Rats were trained to press the lever on a fixed ratio schedule in which each lever response was reinforced with one sucrose pellet. Once an animal acquired 30 pellets within 30 min, it was shifted to the progressive ratio phase. In this stage, the required number of presses increased according to the formula ni = 5e(i/5)−5, where ni is equal to the number of presses required on the ith ratio, after 5 successive schedule completions. Training continued until a rat had earned 50 pellets within 30 min or 6 sessions passed, at which point a ceramic ramekin containing 18 g of lab chow was introduced (modified from Randall et al. 2012). Rats were advanced individually to each stage based on performance. Rats were free to choose between consuming freely available but less preferred chow or lever pressing for preferred sucrose pellets. The ramekin was located near the magazine in the opposite corner of box from the lever to ensure that the two activities were mutually exclusive. After every 30-min testing session, the number of presses, the number of reinforcers, the highest ratio achieved, and the amount of chow remaining (including chow dropped through the bars and collected in a tray) were recorded. Lever pressing behavior was allowed to stabilize for a week prior to behavioral or pharmacologic manipulations (4 consecutive days with <20% variation in lever pressing from the mean across the 4 days).
Sucrose pellet devaluation
Once lever pressing behavior had stabilized, rats underwent a specific outcome devaluation task. Rats were given 30 min of unrestricted sucrose pellet availability in their home cages immediately prior to behavioral testing to assess the effect of incentive state on effortful output.
Systemic CGS 21680 administration
In order to assess the role of D2-expressing medium spiny neurons in our effortful choice paradigm, a subset of rats were injected intraperitoneally with vehicle (5% DMSO) or the selective A2A agonist CGS 21680 (Bio-Techne, Abingdon, U.K.; 0.05 and 0.1 mg/kg) immediately prior to behavioral testing. Doses were given in escalating order (0, 0.05, 0.1 mg/kg) on 3 days separated by 2-day washout periods.
Free choice control
Following all behavioral and pharmacologic manipulations, rats were given 30 min of free access to pre-weighed amounts of either sucrose pellets or lab chow (~18 g each) in standard shoebox cages. Following the 30 min, leftover food was collected and weighed to determine rats’ food preferences when differential effort requirements were eliminated.
Data analysis
The number of lever presses and the grams of chow consumed were analyzed as dependent measures. Between-group differences in stable baseline performance were analyzed with unpaired t tests. The effects of chow introduction, outcome devaluation, and CGS administration were analyzed with two-way repeated measures ANOVA with pretreatment as a between- and condition as a within-subject factor. The free choice control was analyzed by two-way ANOVA. When significant interactions were detected, Bonferroni-corrected post hoc tests are reported.
Results
Experiment 1
Total consumption
There was a significant main effect of sucrose concentration on total fluid consumed on test days [F(5105) = 9.497, p < 0.0001] such that rats drank more total fluid as sucrose concentration increased. There was no main effect of pretreatment (p = 0.9071) or concentration by pretreatment interaction (p = 0.4913) on total fluid consumption. When all days (including rest days on which water was present in both bottles) were taken into account, total fluid consumption significantly increased throughout the test [F(13,273) = 16.81, p < 0.0001], but again, no main effect of pretreatment (p = 0.7674) or day by pretreatment interaction (p = 0.3637) was observed (Fig. 1a).
Fig. 1.
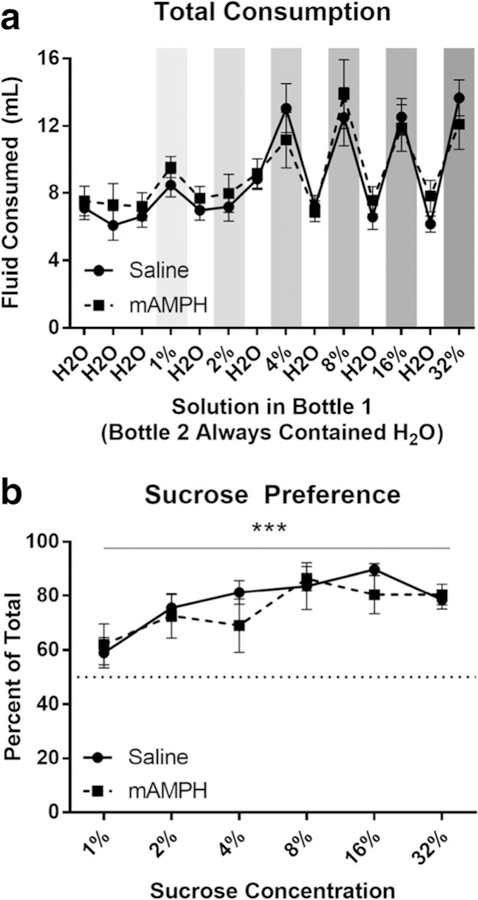
Sucrose preference. a Total fluid consumption on each day of the sucrose preference test. Days on which bottle 1 contained sucrose are shaded; darker shades indicate higher sucrose concentrations. b Percent of total fluid consumption drawn from bottle 1 on sucrose days. Figures denote mean ± SEM. ***p < 0.001
Sucrose preference
Sucrose preference score was determined by dividing the fluid consumed from the sucrose bottle by the total amount of fluid consumed and multiplying by 100. Sucrose preference significantly increased with increasing sucrose concentration [F(5105) = 5.301, p < 0.001], but no main effect of pretreatment (p = 0.5769), nor concentration by pretreatment interaction (p = 0.6243), was observed (Fig. 1b).
Experiment 2
Chow/choice introduction
Lever presses on the day before chow introduction and the first day of choice availability were analyzed. Rats significantly reduced their lever pressing in the presence of chow [F(1,18) = 79.28, p < 0.0001], but no main effect of pretreatment (p = 0.61) or chow presence by pretreatment interaction (p = 0.881) was detected (data not shown).
Stable choice performance
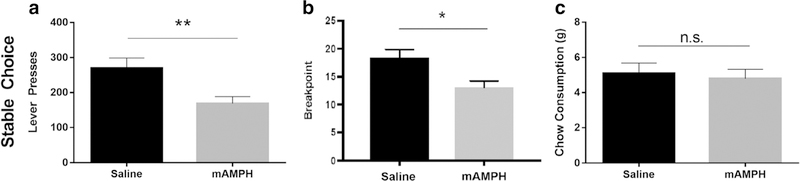
After performance had stabilized following the introduction of chow (5–9 days of choice procedure, no difference between pretreatment groups), an unpaired two-tailed t test revealed that rats pretreated with mAMPH pressed the lever significantly fewer times per session than rats pretreated with saline [t(18) = 2.888, p < 0.01] (Fig. 2a). An unpaired two-tailed t test of progressive ratio breakpoints revealed that mAMPH-pretreated rats disengaged from the lever at a significantly lower effort requirement than saline-pretreated rats [t(18) = 2.823, p < 0.05] (Fig. 2b). There was no significant difference in chow consumption between mAMPH-pretreated and saline-pretreated rats at stable performance (p = 0.7094) (Fig. 2c).
Fig. 2.
Effortful choice behavior at stable performance. a Number of lever presses in the concurrent choice task, where rats selected between lever pressing on a progressive ratio schedule for a preferred sucrose pellet option versus freely available chow. b Breakpoints in the concurrent choice task. c Chow consumption in the concurrent choice task. Bars represent mean ± SEM. *p < 0.05, **p < 0.01
Sucrose pellet devaluation
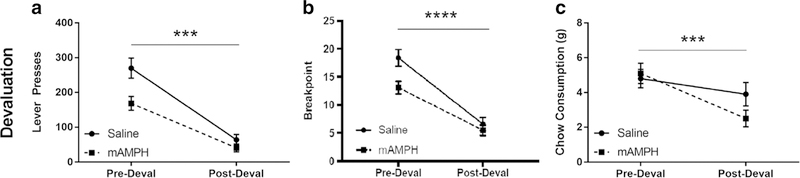
Following stabilization of choice performance, rats were subjected to an outcome devaluation protocol. Two-way repeated measures ANOVA of lever pressing revealed a significant main effect of devaluation [F(1,18) = 106.8, p < 0.0001] and of pretreatment [F(1,18) = 7.366, p < 0.05], as well as a significant devaluation by pretreatment interaction [F(1,18) = 5.853, p < 0.05]. Bonferroni-corrected post hoc tests revealed a significant decrease in lever presses between the day before devaluation and the day of devaluation for both the saline-pretreated [t(18) = 9.018] and mAMPH-pretreated [t(18) = 5.597] groups (Fig. 3a). Repeated measures ANOVA of breakpoint revealed a significant main effect of devaluation [F(1,18) = 119.25, p < 0.0001] and a significant main effect of pretreatment [F(1,18) = 4.95, p < 0.05], as well as a significant devaluation by pretreatment interaction [F(1,18) = 5.59, p < 0.05]. Bonferroni-corrected post hoc tests revealed a significant decrease in breakpoint between the day before devaluation and the day of devaluation for both the saline-pretreated [t(18) = 6.050] and mAMPH-pretreated [t(18) = 9.394] groups (Fig. 3b). Repeated measures ANOVA of chow consumption revealed a significant main effect of devaluation [F(1,18) = 16.38, p < 0.001], but no main effect of pretreatment (p = 0.4319). There was a trend toward a devaluation by pretreatment interaction [F(1,18) = 3.865, p = 0.0649], though this did not reach significance at the α = 0.05 level (Fig. 3c).
Fig. 3.
Effects of devaluation on effortful choice behavior. a Number of lever presses in the concurrent choice task (where rats selected between lever pressing on a progressive ratio schedule for a preferred sucrose pellet option versus freely available chow). Measures were taken before and after satiation with sucrose pellets (i.e., devaluation). b Breakpoints in the concurrent choice task before and after devaluation. c Chow consumption in the concurrent choice task before and after devaluation. Figures represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001
Systemic CGS 21680
One predesignated rat from each pretreatment group was removed from the study at this point. The remainder (n = 9) was given three injections of 5% DMSO containing three different doses of the A2A agonist CGS 21680 (0, 0.05, and 0.1 mg/kg, i.p.) (adapted from Mingote et al. 2008). Repeated measures ANOVA of lever pressing revealed a significant main effect of CGS 21680 dose [F(2,28) = 43.87, p < 0.0001], but no main effect of pretreatment (p = 0.1706) nor a dose by pretreatment interaction (p = 0.1665) (Fig. 4a). A repeated measures ANOVA of chow consumption revealed a similar pattern of effects: a significant main effect of CGS 21680 dose [F(2,28) = 4.065, p < 0.05], with no main effect of pretreatment (p = 0.1038) or dose by pretreatment interaction (p = 0.5281) (Fig. 4b).
Fig. 4.
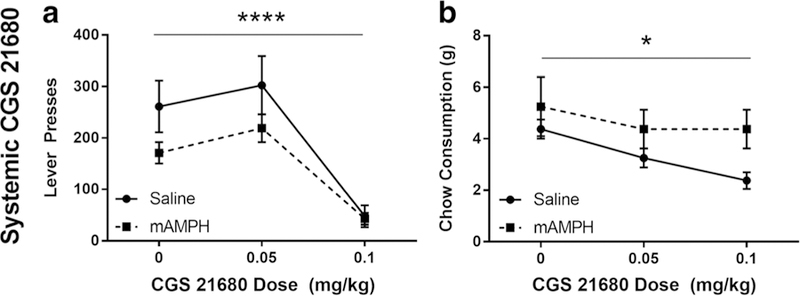
Effects of systemic CGS21680 on effortful choice behavior. a Number of lever presses in the concurrent choice task at different doses of CGS21680. b Chow consumption in the concurrent choice task at different doses of CGS21680. Figures denote mean ± SEM. *p < 0.05, ***p < 0.001
Free choice control
Following a 3-day washout period from CGS 21680 treatment, rats were given a choice between freely available chow and freely available sucrose pellets. Two-way ANOVA revealed a significant main effect of food type on consumption behavior [F(1,28) = 10.61, p < 0.01], such that all rats preferred sucrose pellets over chow when effort requirements were equalized. There was no main effect of pretreatment (p = 0.1696) or food type by pretreatment interaction (p = 0.6869), further supporting the lack of an effect of mAMPH withdrawal on free sucrose preference, as observed in experiment 1 (Fig. 5).
Fig. 5.
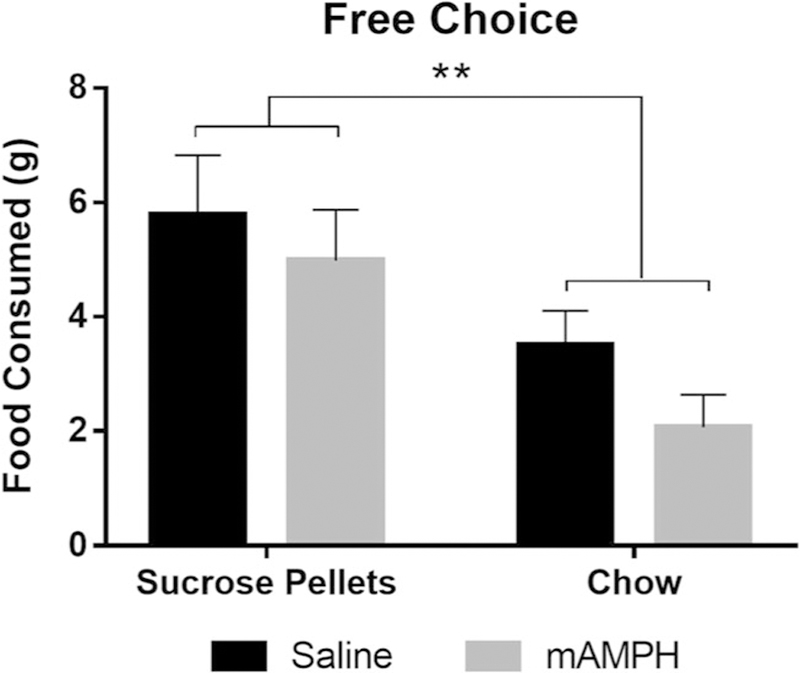
Consumption of sucrose pellets and chow in the free choice control task. Rats selected between two freely available options and exhibited a preference for the sucrose pellets under equal effort conditions. Bars represent mean ± SEM. **p < 0.01
Discussion
Methamphetamine exposure and withdrawal did not affect reward sensitivity as measured by sucrose preference. Despite this, rats in protracted withdrawal were less willing to exert effort for a preferred reward in the presence of a less-preferred but free reward. This reduced lever pressing was exhibited only in the context of choice. This pattern of findings suggests that mAMPH leads to steeper discounting of reward value by effort. This is consistent with findings that mAMPH can lead to steep discounting of reward by delay and may suggest a common underlying mechanism.
Intact reward sensitivity
Sucrose preference did not differ between mAMPH- and saline-pretreated rats, which was somewhat surprising because transient anhedonia, lasting up to 1 week after the last dose of the drug, has been reported in withdrawal from many drugs, including methamphetamine (Koob 2013). Indeed, acute withdrawal from methamphetamine is associated with anhedonia in both rodents (Jang et al. 2013) and humans (McGregor et al. 2005). Our present work showed no effect of mAMPH pretreatment on sucrose preference across a range of sucrose concentrations. Lack of sucrose preference has been observed in mice 7 days after methamphetamine exposure (Ren et al. 2015). However, there were major methodological differences between that study and the present work; notably, one sucrose concentration was used (1%) in Ren et al. (2015), and mice had free access to it for 24 h prior to the test day. Though consumption during this period was not reported, lack of sucrose preference in mAMPH-pretreated mice during the 1-h preference test may reflect a devaluation effect, rather than anhedonia. It is, however, possible that the effects of methamphetamine on sucrose preference are only present with certain sucrose concentrations presented at certain times after withdrawal of mAMPH. Due to the escalating nature of this task, withdrawal time overlapped with increasing sucrose concentration, and this possibility cannot be ruled out. However, the 1% sucrose in the present experiment was presented at approximately the same post-withdrawal day as in Ren et al. (2015), suggesting that the difference between the two findings is more likely due to methodological or species differences. These findings are similar to those reported in Kosheleff et al. (2012b), in which mAMPH- and saline-pretreated rats showed no difference in preference for a high reward arm in an effortful barrier-climbing maze task when effort demands were removed (equal). These results also suggest that the anhedonia present during early withdrawal does not persist beyond the acute phase of withdrawal.
Steep discounting of reward value by effort cost during choice
We were able to determine that sucrose pellets were the qualitatively preferred reward given results from the free choice consumption task: all rats preferred sucrose pellets over chow when effort requirements were equal. The introduction of freely available chow in the operant chamber caused a dramatic reduction in lever pressing behavior, an indication that the freely available option diverted behavior away from effortful responding for the preferred reward. The progressive nature of this task, in which effort requirements for the preferred option steadily increased throughout the session, allows rats to self-titrate their effortful output and requires the animal to continually select between the two options. All rats selected the preferred reinforcer at the beginning of the session, then become more likely to choose the chow as increasing effort cost discounts the value of the reinforcer. Once the value of the preferred reinforcer and its associated effort cost is roughly equal to the value of the free chow, the animal reaches an “indifference point” and spends the remainder of the session alternating between low levels of lever pressing and chow consumption. Lower lever pressing and breakpoints in mAMPH-pretreated rats is therefore indicative of greater discounting of reward value as a function of effort requirement, the same pattern deemed “impulsive” in intertemporal choice. It is important to note that this task necessarily conflates delay and effort, as the effortful option takes longer to acquire than the free option. However, the effects of amphetamine and related manipulations on effort discounting are also observed when equivalent delays are introduced for the low effort option, suggesting that the effect seen here on effort discounting would persist in the absence of different delays (Floresco et al. 2008; Ghods-Sharifi et al. 2009).
Importantly, methamphetamine exposure and withdrawal did not induce a shift away from chow consumption in this task. This is consistent with previous literature using a variant of this task, in which pharmacologic manipulations that affected lever pressing left chow consumption unchanged (Randall et al. 2012). This is likely due to the fact that once rats reach the indifference point, they continue to consume chow and press the lever at a low rate, resulting in a ceiling effect on chow intake. Indeed, rats given 30 min to freely consume lab chow rarely consumed more than 8 g, even in the absence of a competing reinforcer (Randall et al. 2010). By contrast, prefeeding with chow has been shown to reduce both chow consumption and lever pressing in a variant of this task (Randall et al. 2012), and the present work expands this to show that the task is also sensitive to devaluation of the preferred outcome. When rats are given 30 min of unlimited access to sucrose pellets prior to the test, both lever pressing and chow consumption are reduced.
A variant of this task has also been shown to be sensitive to systemic administration of the A2A antagonist MSX-3 (Randall et al. 2012). A similar task which used a fixed ratio 5 schedule of reinforcement rather than a progressive ratio was found to be sensitive to the A2A agonist CGS 21680 (Mingote et al. 2008), though the drug reduced both lever pressing and chow intake. We show that systemic CGS 21680 decreased both lever pressing and chow intake in the current task, which serves as confirmation of these findings. Because systemic CGS 21680 reduced both lever pressing and chow consumption in a similar manner to prefeeding, it is likely that this effect is mediated by general motivation or sedation, rather than a specific alteration to cost/benefit decision-making. By contrast, mAMPH pre-exposure specifically affected the indifference point, leaving general motivation intact.
An interesting pattern emerges when these results are compared against other studies of amphetamine withdrawal and effortful choice. Some studies have found no effect of repeated amphetamine exposure on effortful choice (Floresco and Whelan 2009), while others have reported work aversion in rats 10–11 days following an experimenter-administered binge dose of mAMPH (Kosheleff et al. 2012b). Stolyarova et al. (2015) report increased willingness to climb a 20-cm barrier to acquire a slightly larger reward following treatment with mAMPH and withdrawal. The lack of a clear effect on this measure between tasks suggests that effortful choice is highly dependent on specific task demands such as fixed effort costs (barrier heights or number of lever presses) and reward magnitudes. The progressive ratio task employed here side-steps this issue by allowing the rat to self-titrate its effort expenditure to whatever it is willing to pay for the reward. This results in a cleaner mapping of effortful output to reward value.
It is likely that mAMPH-induced alterations in D2 receptor expression may underlie the observed effects. In healthy and mAMPH-dependent humans, impulsive-like choice behavior has been linked to the function of dopamine D2-like receptors in the striatum, such that lower D2-like receptor availability predicts greater impulsivity (Lee et al. 2009; Buckholtz et al. 2010; Kohno et al. 2016), an effect confirmed in animal models (Groman et al. 2013; Dalley et al. 2007). Additional work in animal models has shown that mAMPH exposure and withdrawal reduces striatal D2-like receptor availability and increases impulsive-like responding in a reversal learning task (Groman et al. 2012). Dopamine and medium spiny neurons of the D2 pathway are also implicated in effortful choice behavior. Dopamine reactivity in the striatum predicts willingness to expend physical effort for a reward in humans (Treadway et al. 2012), and dopamine has been postulated to play a role in mitigating response costs (Salamone et al. 2007). Systemic administration of the selective D2 antagonist haloperidol reduces choice of a high magnitude reward when it is blocked by a barrier, but has no effect when the barrier is absent (Salamone et al. 1994). Similarly, haloperidol reduces lever pressing in a progressive ratio/chow choice task without affecting consumption of freely available chow (Randall et al. 2012). These findings are interpreted as a specific effect on effort allocation rather than on primary motivation, as they only occur in the context of differential effort requirements, and chow devaluation through prefeeding decreases both lever-pressing and chow consumption in these tasks.
Although the present experiments utilized experimenter-administered, noncontingent mAMPH, impulsive-like choice behavior is frequently observed in humans who have self-administered mAMPH (Lee et al. 2009; Hoffman et al. 2006; Monterosso et al. 2006), suggesting that this effect is not specific to any particular regimen of mAMPH exposure. Additionally, there is similarity in the effects of behavioral flexibility assays between self- and experimenter-administered mAMPH in male rats (Cox et al. 2016). Changes in effort allocation for rewards may relate to relapse vulnerability in protracted withdrawal from mAMPH in humans. Drug abuse can be conceptualized as a disorder of aberrant effort allocation toward acquisition of a drug, despite associated costs and at the expense of other reward sources. Similarly, maintaining sobriety following detoxification requires effort, and this effort cost may discount the value of sobriety. If mAMPH exposure and withdrawal can lead to steeper discounting of effortful rewards, the value of a relatively easy-to-acquire high may be greater than the value of working to maintain sobriety. It is likely that impulsive effortful choice interacts with steep delay discounting and risky decision-making to increase relapse vulnerability in protracted mAMPH withdrawal.
Acknowledgements
This work was supported by UCLA’s Division of Life Sciences Recruitment and Retention fund (Izquierdo), the NIH supported Training program in Translational Neuroscience of Drug Abuse (T32 DA024635, London), the ARCS Foundation, Los Angeles Founder Chapter (Thompson), the Stefan and Shirley Hatos Center for Neuropharmacology (Evans), and by the UCLA PEERS program.
Footnotes
Compliance with ethical standards All procedures were conducted in accordance with the latest NIH Guide for the Care and Use of Laboratory Animals (National Institutes of Health 2011) and were approved by the Chancellor’s Animal Research Committee of UCLA.
Conflict of interest The authors declare that they have no conflict of interest.
Competing financial interests The author(s) declare no competing financial interests.
References
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320(5881):1352–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH (2010) Dopaminergic network differences in human impulsivity. Science 329(5991):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G (2007) Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem 14(5):325–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ (2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292(5526):2499–2501 [DOI] [PubMed] [Google Scholar]
- Cervantes MC, Laughlin RE, Jentsch JD (2013) Cocaine self-administration behavior in inbred mouse lines segregating different capacities for inhibitory control. Psychopharmacology 229(3):515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Cope ZA, Parsegian A, Floresco SB, Aston-Jones G, See RE (2016) Chronic methamphetamine self-administration alters cognitive flexibility in male rats. Psychopharmacology 233(12):2319–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA (2009) Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav 93(3):237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69(4):680–694 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, Peña Y, Murphy ER, Shah Y, Brobst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron J, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315(5816):1267–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Tse MTL, Ghods-Sharifi S (2008) Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology 33(8):1966–1979 [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Onge JR St., Floresco SB (2009) Fundamental contributions by the basolateral amygdala to different forms of decision making. J Neurosci 29(16):5251–5259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD (2012) Dysregulation of D2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci 32(17):5843–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Morales AM, Lee B, London ED, Jentsch JD (2013) Methamphetamine-induced increases in putamen gray matter associate with inhibitory control. Psychopharmacology 229(3):527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka K, Kabasawa N (2013) Relationships between redressive self-control and discounting of reward with delay or effort. Shinrigaku Kenkyu 84(4):267–273 [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH (2006) Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology 188(2):162–170 [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF (2010) Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology 35(2):505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang CG, Whitfield T, Schulteis G, Koob GF, Wee S (2013) A dysphoriclike state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology 225(3): 753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, James AS, Groman SM, Pennington ZT (2014) Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci 1327:1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza RII, Taylor JR (2002) Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology 26:183–190 [DOI] [PubMed] [Google Scholar]
- Kohno M, Okita K, Morales AM, Robertson CL, Dean AC, Ghahremani G, Sabb FW, Rawson RA, Mandelkern MA, Bilder RM, London ED (2016) Midbrain functional connectivity and ventral striatal dopamine D2-type receptors: link to impulsivity in methamphetamine users. Mol Psychiatry 21(11):1554–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2013) Addiction is a reward deficit and stress surfeit disorder. Front Psych 4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosheleff AR, Rodriguez D, O’Dell SJ, Marshall JF, Izquierdo A (2012a) Comparison of single-dose and extended methamphetamine administration on reversal learning in rats. Psychopharmacology 224(3): 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosheleff AR, Grimes M, O’Dell SJ, Marshall JF, Izquierdo A (2012b) Work aversion and associated changes in dopamine and serotonin transporter after methamphetamine exposure in rats. Psychopharmacology 219(2): 411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA (2009) Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29(47):14734–14740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Songtan T, White JM (2005) The nature, time course and severity of methamphetamine withdrawal. Addiction 100(9):1320–1329 [DOI] [PubMed] [Google Scholar]
- Mingote S, Pereira M, Farrar AM, McLaughlin PJ, Salamone JD (2008) Systemic administration of the adenosine A2A agonist CGS 21680 induces sedation at doses that suppress lever pressing and food intake. Pharmacol Biochem Behav 89(3):345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED (2006) Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp 28(5):383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals, 8th edn. National Academies Press (US), Washington, DC [Google Scholar]
- Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR (2006) ΔFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci 26(36):9196–9204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothion S, Bizot J, Trovero F, Belzung C (2004) Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain res 155(1):135–136 [DOI] [PubMed] [Google Scholar]
- Prévost C, Pessiglione M, Météreau E, Cléry-Melin ML, Dreher JC (2010) Separate valuation subsystems for delay and effort decision costs. J Neurosci 30(42):14080–14090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Podurgiel SJ, Hart E, Yohn SE, Jones M, Rowland M, López-Cruz L, Correa M, Salamone JD (2014) Bupropion increases selection of high effort activity in rats tested on a progressive ratio/chow feeding choice procedure: implications for treatment of effort-related motivational symptoms. Int J Neuropsychopharmacol 18(2):pyu017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, Cruz LL, Vemuri VK, Makriyannis A, Baqi Y, Müller CE, Correa M, Salamone JD (2012) Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One 7(10):e47934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Vemuri VK, Segovia KN, Torres EF, Hosmer S, Nunes EJ, Santerre JL, Makriyannis A, Salamone JD (2010) The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacol Biochem Behav 97(1):179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q, Ma M, Yang C, Zhang JC, Yao W, Hashimoto K (2015) BDNF-TrkB signaling in the nucleus accumbens shell of mice has key role in methamphetamine withdrawal symptoms. Transl Psychiatry 5: e666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H (1999) Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology 146(4):432–439 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S (1994) Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain res 65(2):221–229 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM (2007) Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191(3):461–482 [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK (2003) Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology 28: 1730–1740 [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B (2007) Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci 121(3):543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolyarova A, Thompson AB, Barrientos RM, Izquierdo A (2015) Reductions in frontocortical cytokine levels are associated with long-lasting alterations in reward valuation after methamphetamine. Neuropsychopharmacology 40(5):1234–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AB, Stolyarova A, Ying Z, Zhuang Y, Gomez-Pinilla F, Izquierdo A (2015) Methamphetamine blocks exercise effects on Bdnf and Drd2 gene expression in frontal cortex and striatum. Neuropharmacology 99:658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH (2012) Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci 32(18):6170–6176 [DOI] [PMC free article] [PubMed] [Google Scholar]