Abstract
Objectives:
Increasing age is a well-recognized risk factor for in-hospital mortality in patients receiving extracorporeal membrane oxygenation (ECMO) for cardiogenic shock (CS), but the shape of this relationship is unknown. In addition, the impact of age on hospital length of stay (LOS), patterns of patient disposition, and costs have been incompletely characterized.
Design:
Retrospective analysis of the National Inpatient Sample.
Setting:
United States nonfederal hospitals, years 2004–2016.
Patients:
Adults with CS treated with ECMO (3,094; weighted national estimate: 15,415).
Interventions:
None.
Measurements and Main Results:
The mean age of ECMO recipients was 54.8 ± 15.4 years (range 18–90 years). Crude in-hospital mortality was 57.7%. Median time-to-death was 8 days (IQR 3–17). A linear relationship between age and in-hospital mortality was observed with a 14% increase in the adjusted odds of in-hospital mortality for every ten-year increase in age (AOR 1.14; 95% CI 1.08–1.21; p<0.0001). Thirty-four percent of patients were discharged alive at a median time of 30 days (IQR 19–48). The median LOS and total hospitalization costs were 14 days (IQR [5–29]) and $134,573 ($71,782-$239,439), respectively, both of which differed significantly by age group (LOS range 17 days [18–49 years] to 9 days [80–90 years], p<0.0001; cost range $147,548 [18–49 years] to $105,350 [80–90 years], p<0.0001).
Conclusions:
Age is linearly associated with increasing in-hospital mortality in individuals receiving ECMO for CS without evidence of a threshold effect. Median time-to-death is approximately one week. One third of patients are discharged from the hospital alive, but the median time-to-discharge is one month. Median LOS ranges from 9–17 days depending on age. Hospitalization costs exceed $100,000 in all age groups.
Keywords: Extracorporeal Membrane Oxygenation, Cardiogenic Shock, Aged, Mortality, Length of Stay, Hospital Costs
Introduction
Extracorporeal membrane oxygenation (ECMO) is a rescue therapy that can provide hemodynamic and gas exchange support to critically ill patients in refractory cardiac or respiratory failure. Use of ECMO has been increasing both nationally(1–4) and internationally,(5) particularly in the setting of cardiogenic shock (CS).(4,6)
While ECMO can provide rapid stabilization of the patient in extremis, it remains a morbid(7–9) and resource-intensive therapy.(10) Appropriate selection of patients who could derive the greatest benefit from this therapy remains a challenge prompting ongoing efforts to identify determinants of outcomes. One strong determinant of mortality in ECMO use is increasing age.(11–13) However, the presence or absence of a threshold age conferring a higher magnitude of risk has not been clearly defined in studies of ECMO including those utilizing the Extracorporeal Life Support Organization (ELSO) database(14,15), which is limited by the self-reported nature of data collection. Similarly, studies using the National Inpatient Sample (NIS), an administrative database with systematic capture of data elements, have not addressed this topic.(3,10,16,17) The presence of a threshold age may impact the futility and appropriateness of treatment.
In addition, while ECMO duration has been studied,(18) hospital length of stay (LOS) has not been clearly delineated in these patients. Understanding the average times to death or discharge may aid in contextualizing a patient’s hospitalization. Furthermore, the effect of age on discharge trends and costs has not been analyzed - information that may be helpful in quantifying the degree of resource utilization needed in the care of those receiving ECMO. For these reasons, we used the NIS to evaluate the relationship between increasing age and in-hospital mortality, LOS, patient disposition, and costs for patients with CS treated with ECMO.
Materials and Methods
Data Source
The NIS, sponsored by the Agency for Healthcare Research and Quality, is the largest publicly available all-payer inpatient administrative database in the United States.(19) The NIS collects information from 4,411 specialty and public hospitals and academic medical centers (not including federal, rehabilitation, and long-term acute care hospitals) in 45 states participating in the Healthcare Cost and Utilization Project (HCUP) and provides patient-level billing data on 7–8 million discharges each year. Studies utilizing the NIS are exempt from Institutional Review Board approval at the Beth Israel Deaconess Medical Center and the Massachusetts General Hospital.
Study Population
Adults (≥ 18 years) diagnosed with CS were included in the analysis if they had at least one procedure code for ECMO in any position. From 1/1/2004–9/30/2015, CS and ECMO were defined by the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes 785.51(6,21) and 39.65 or 39.66(10), respectively. The ICD-9-CM code for CS has high positive (78.8%) and negative (98.1%) predictive values, high specificity (99.3%), and moderate sensitivity (59.8%).(22) From 10/1/2015–12/31/2016, CS and ECMO were defined by ICD-10-CM codes R57.0 and 5A15223, respectively.
Covariates and Outcomes
Baseline patient covariates included demographics and 29 ICD-9-CM Elixhauser comorbidities, which represent covariates validated against mortality for use in administrative datasets.(16,21,23) The number of ECMO patients with CS associated with cardiac surgical procedures were assessed by defining several mutually exclusive groups with ICD-9-CM procedure codes: transcatheter aortic valve replacement (TAVR; transfemoral and transapical), heart transplant, lung transplant, durable devices (such as the HeartMate II [Abbott, St. Paul, MN]), and postcardiotomy (cardiac surgical procedures not including TAVR, heart/lung transplant, or durable devices), (Supplemental Table 1).(10) Lung transplant frequency was analyzed as a negative control assessing CS/ECMO code capture of presumed isolated respiratory failure. Outcomes included in-hospital mortality, hospital LOS, discharge disposition, and costs.
Total direct hospitalization costs and costs per day were calculated using hospital charges converted to costs using the HCUP Cost-to-Charge Ratio File.(24) All costs were indexed to 2016 dollars using the Bureau of Labor Statistics Consumer Price Index.(25)
Statistical Analysis
Categorical variables are presented as counts and percentages and continuous variables are presented as means and standard deviations (SD) or medians and interquartile ranges (IQR) as appropriate. National estimates of ECMO use were derived using survey weights provided by HCUP.(20) Common primary discharge diagnoses were determined. Cumulative incidence function plots were created to display times-to-disposition. Multi-group comparisons of the ranked medians of continuous variables were carried out using the Kruskal-Wallis rank sum test. Restricted cubic splines were used to examine potential non-linear relationships between the logarithmic odds of mortality and age. Multivariable logistic regression adjusting for gender, race, and 29 ICD-9-CM Elixhauser clinical comorbidities was used to model the association between age and in-hospital mortality. A two-sided p-value < 0.05 was used to define statistical significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Overall Results
A total of 240,367 hospitalizations with CS were identified, of which 3,094 (1.3%) received ECMO therapy (weighted national estimates of 1,190,594 and 15,415, respectively). Seventy-six percent of cases (2344/3094) occurred between 2012–2016 (Supplemental Figure 1). The mean age of ECMO recipients was 54.8 ± 15.4 years (range 18–90 years) (Table 1) and the age distribution was unimodal (Supplemental Figure 2). The majority of patients were male (67.2%) and Caucasian (57.0%). Forty percent of patients received ECMO in the postcardiotomy setting, 10.4% with durable devices, 5.2% with heart transplantation, 1.6% with TAVR, and none with lung transplantation. Common ICD-9-CM comorbidities and diagnoses associated with ECMO utilization included fluid and electrolyte disorders (68.9%) and acute anterior myocardial infarction (7.2%), respectively (Supplemental Tables 2 and 3).
Table 1:
Patient and Hospital Characteristics, Disposition, and Outcomes
| Characteristic, Disposition, and Outcomes | Frequencya |
|---|---|
| Age, years (mean ± SD) | 54.8±15.4 |
| Female Sex – no. (%) | 1014 (32.8) |
| Race – no. (%) | |
| Caucasian | 1762 (57.0) |
| African American | 393 (12.7) |
| Hispanic | 212 (6.9) |
| Asian or Pacific Islander | 88 (2.8) |
| Other | 208 (6.7) |
| Missing | 431 (13.9) |
| Primary expected payer – no. (%) | |
| Medicare | 1068 (34.5) |
| Medicaid | 450 (14.5) |
| Private Insurance | 1338 (43.2) |
| Self-pay/Other | 233 (7.5) |
| Admission characteristics – no. (%) | |
| Elective | 546 (17.7) |
| Emergency department | 855 (27.6) |
| Transfer in from acute care facility | 1227 (39.7) |
| Transfer in from another facility | 135 (4.4) |
| Hospital characteristics – no. (%) | |
| Bed sizeb | |
| Small | 66 (2.1) |
| Medium | 321 (10.4) |
| Large | 2703 (87.4) |
| Geographic region | |
| Northeast | 844 (27.3) |
| Midwest | 711 (23.0) |
| South | 1053 (34.0) |
| West | 486 (15.7) |
| Location | |
| Urban, nonteaching | 155 (5.0) |
| Urban, teaching | 2923 (94.5) |
| Procedures Occurring During Hospitalization – no. (%)c | |
| Postcardiotomy | 914 (39.6) |
| Durable devices | 241 (10.4) |
| Heart transplant | 119 (5.2) |
| Transcatheter aortic valve replacement | 37 (1.6) |
| Lung transplant | 0 |
| Discharge disposition – no. (%) | |
| Death during hospitalization | 1784 (57.7) |
| Transfer to short-term hospital | 268 (8.7) |
| Discharge home (without home care) | 247 (8.0) |
| Discharge home (with home health care) | 230 (7.4) |
| Transfer to skilled nursing facilityd | 563 (18.2) |
| Discharge alivee | 1040 (33.6) |
| LOS, days (median [IQR]) | 14 (5–29) |
| Total cost of hospitalization (median [IQR]) | $134,573 [71,782; 239,439] |
| Cost per day (median [IQR]) | $10,168 [6,737; 16,951] |
LOS: length of stay, IQR: interquartile range.
Estimates represent unweighted sample.
The definition of hospital size depends on region, rural/urban and teaching/non-teaching status, and bed size, with number of beds ranging from 1–249 (small hospitals), 25–449 (medium hospitals), and 45–450+ (large hospitals).
Data to September 2015 using ICD-9 codes.
Includes intermediate and long-term care hospitals and rehabilitation facilities.
Combination of “Discharge Home with/without home health care” and “Transfer to Skilled Nursing Facility.”
Only 17.7% of hospitalizations resulting in ECMO use were elective with admission through the emergency department in 27.6%, and transfer from another acute care facility in 39.7%. Patients were predominantly treated at urban teaching hospitals (94.5%) that were considered large (87.4%; 325+ to 450+ beds depending on region).(19) The crude in-hospital mortality rate was 57.7%. Mortality in subgroups was 58.9% (postcardiotomy), 49.0% (durable devices), 40.5% (TAVR), and 34.5% (heart transplant) (Table 2). Overall, 8.7% of patients were transferred to an acute care hospital and 33.6% were discharged alive, either to home (self-care: 8.0%; home health care: 7.4%) or to a skilled nursing facility (18.2%, inclusive of intermediate care and long-term care hospitals and rehabilitation facilities). The median LOS was 14 days (IQR 5–29). Total median hospitalization costs and median costs per day for the entire cohort were $134,573 (IQR $71,782-$239,439) and $10,168 (IQR $6,737-$16,951), respectively.
Table 2:
Mortality Rates of Subgroups
| Category | Mortalitya |
|---|---|
| Gender - no. (%) | |
| Female | 559/1014 (55.1) |
| Male | 1225/2080 (58.9) |
| Primary expected payer - no. (%) | |
| Medicare | 681/1068 (63.8) |
| Medicaid | 238/450 (52.9) |
| Private Insurance | 710/1338 (53.1) |
| Self-pay/Other | 152/233 (65.2) |
| Procedures - no. (%)b | |
| Postcardiotomy | 538/914 (58.9) |
| Durable devices | 118/241 (49.0) |
| Transcatheter aortic valve replacement | 14/37 (40.5) |
| Heart transplant | 41/119 (34.5) |
| Admission characteristics - no. (%) | |
| Elective | 336/546 (61.5) |
| Emergency department | 456/855 (53.3) |
| Transfer in from acute care facility | 723/1227 (58.9) |
| Transfer in from another facility | 88/135 (65.2) |
| Hospital characteristics - no. (%) | |
| Bed sizec | |
| Small | 28/66 (42.4) |
| Medium | 169/321 (52.7) |
| Large | 1586/2703 (58.7) |
| Geographic region | |
| Northeast | 459/844 (54.4) |
| Midwest | 423/711 (59.5) |
| South | 614/1053 (58.3) |
| West | 288/486 (59.3) |
| Location | |
| Urban, nonteaching | 68/155 (43.9) |
| Urban, teaching | 1709/2923 (58.5) |
Estimates represent unweighted sample.
Data to September 2015 using ICD-9 codes.
The definition of hospital size depends on region, rural/urban and teaching/non-teaching status, and bed size, with number of beds ranging from 1–249 (small hospitals), 25–449 (medium hospitals), and 45–450+ (large hospitals).
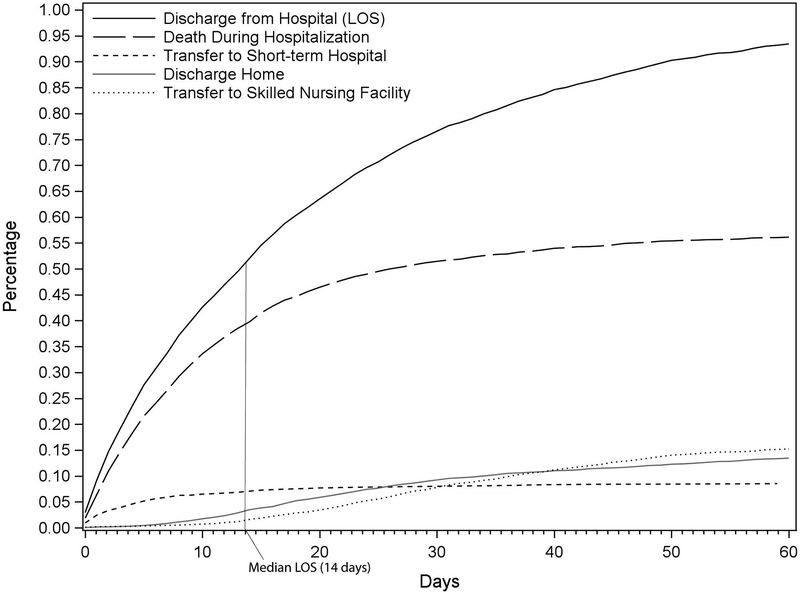
Cumulative rates of times-to-disposition were analyzed where time origin is the admission date. (Figure 1). The median time-to-death was 8 days (IQR 3–17). The median time-to-transfer to a short-term hospital was 4 days (IQR 1–10.5), to discharge home: 26 days (IQR 15.5–45), to transfer to a skilled nursing facility: 34 days (IQR 23–49), and to discharge alive (combined discharge home and transfer to a skilled nursing facility): 30 days (IQR 19–48). Ninety-five percent of all inpatient deaths and discharges alive from the hospital occurred by day 46 and 92, respectively (Supplemental Table 4).
Figure 1:
Cumulative incidence functions plotting times-to-disposition including time-to-discharge from hospital (i.e. length of stay; black line), time-to-death (long dashed line), time-to-transfer to short-term hospital (short dashed line), time-to-discharge home (self-care and home health care; grey line), and time-to-transfer to skilled nursing facility (dotted line). The “Discharge from Hospital (LOS)” curve represents the summation of patients who have experienced death, transfer, or discharge home; as such, plots of these events illustrate the contributions of these individual components to LOS. At day 14 after admission, which represents the median LOS (vertical grey line), 76.4% (1230/1609) of patients had died, 13.6% (219/1609) had been transferred to a short-term hospital, 6.7% (108/1609) had been discharged home, and 3.1% (50/1609) had been transferred to a skilled nursing facility.
Unadjusted Results by Age
Twelve percent of ECMO hospitalizations (n=372) occurred in patients between 70–79 years and 3% (n=87) occurred in patients aged 80–90 years (Table 3), the majority of which took place between 2012–16 (76/87; trend not shown secondary to HCUP restrictions(19)). Mortality was lowest in patients aged 18–49 years (49.5%) and highest in those aged 80–90 years (74.7%) (p<0.0001) (Figure 2). Consequently, discharge alive was more frequent in the group aged 18–49 years (42.1%) and lowest in the group aged 80–90 years (21.8%) (p<0.0001). ECMO in the postcardiotomy setting was least common in the youngest group (25.8%) and most common in the 80–90-year group (56.9%) (p<0.0001).
Table 3:
Discharge Disposition, Times-to-Disposition, and Costs By Age Groupa
| Characteristics, Disposition, and Costs | Age (years) | |||||
|---|---|---|---|---|---|---|
| 18–49 | 50–59 | 60–69 | 70–79 | 80–90 | P-value | |
| Number of Patients - no. (%) | 968 (31.3) |
803 (26.0) | 864 (28.0) |
372 (12.0) | 87 (2.8) |
-- |
| Postcardiotomy - no. (%)b | 192/743 (25.8) |
242/605 (40.0) |
281/621 (45.3) |
162/274 (59.1) |
37/65 (56.9) |
<0.0001 |
| Death During Hospitalization - no. (%) |
479/968 (49.5) |
462/803 (57.5) |
526/864 (60.9) |
252/372 (67.7) |
65/87 (74.7) |
<0.0001 |
| Transfer to Short-term Hospital - no. (%) | 79/968 (8.2) |
79/803 (9.8) |
80/864 (9.3) |
30/459 (6.5) |
0.20 | |
| Discharge Homec- no. (%) | 238/968 (24.6) |
127/803 (15.8) |
87/864 (10.1) |
25/459 (5.5) |
<0.0001 | |
| Transfer to Skilled Nursing Facilityd - no. (%) | 170/968 (17.6) |
135/803 (16.8) |
171/864 (19.8) |
72/372 (19.4) |
15/87 (17.2) |
0.53 |
| Discharge Alivee- no. (%) | 408/968 (42.1) |
262/803 (32.6) |
258/864 (29.9) |
93/372 (25.0) |
19/87 (21.8) |
<0.0001 |
| Discharge from Hospital (Length of Stay), days (median) | 17 | 14 | 13 | 10 | 9 | <0.0001 |
| Death During Hospitalization, days (median) | 9 | 9 | 8 | 7 | 7 | 0.13 |
| Transfer to Short-term Hospital, days (median) | 4 | 4 | 3 | 5 | --f |
0.22 |
| Discharge Home,c days (median) | 28 | 28 | 21 | 11 | --f |
<0.0001 |
| Transfer to Skilled Nursing Facility,d days (median) | 35 | 37 | 36 | 30 | 14 | 0.0001 |
| Discharge Alive,e days (median) | 30 | 33 | 31 | 25 | 13 | <0.0001 |
| Total Cost of Hospitalization (median [IQR]) |
$147,548 [77,943; 263,958] |
$140,220 [73,135; 256,368] | $134,864 [68,745; 235,138] | $118,065 [68,813; 186,828] | $105,350 [71,147; 151,906] | <0.0001 |
| Cost per Day (median [IQR]) |
$9,325 [6,305; 15,794] | $10,326 [6,771; 16,978] | $10,442 [7,010; 17,546] | $11,065 [7,070; 19,435] | $10,736 [7,558; 16,229] |
0.0006 |
Estimates represent unweighted sample.
Data to September 2015 using ICD-9 codes.
With and without home health care
Includes intermediate and long-term care hospitals and rehabilitation facilities
Combination of “Discharge Home” and “Transfer to Skilled Nursing Facility”
Insufficient number of observations
Figure 2:
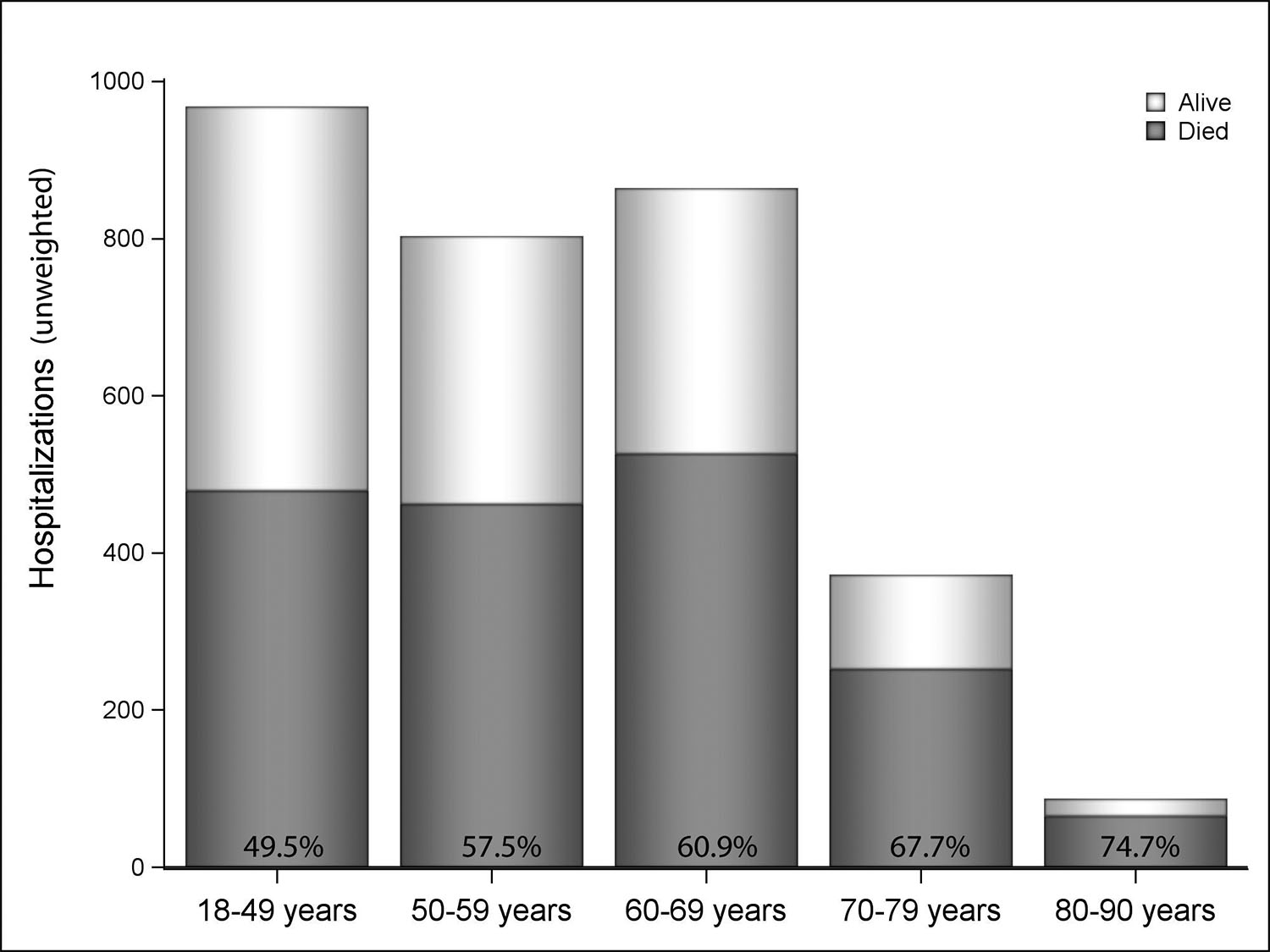
In-hospital mortality by age category.
The median LOS was longest in the youngest group (18–49 years: 17 days, IQR 6–34) and shortest in the 80–90-year group (9 days, IQR 5–15) (p<0.0001) (Supplemental Table 5). Median time-to-discharge home was longest in the youngest groups (18–49 years: 28 days, IQR 18–46; 50–59 years: 28 days, IQR 16–51) and shortest in the 70–79-year group (11 days, IQR 7–18) (p<0.0001). Additionally, median time-to-transfer to a skilled nursing facility ranged from 37 days (50–59 years; IQR 24–55) to 14 days (80–90 years; IQR 9–24) (p=0.0001). Median time-to-discharge alive was longest in the 50–59-year group (33 days, IQR 21–52) and shortest in the 80–90-year group (13 days, IQR 9–23) (p<0.0001). Median times-to-death (p=0.13) and transfer to short-term hospital (p=0.22) were not significantly different among age groups.
Total median hospitalization costs were highest in the group aged 18–49 years ($147,548, IQR $77,943-$263,958) and lowest in the group aged 80–90 years ($105,350, IQR $71,147-$151,906) (p<0.0001). Median costs per day ranged from $9,325 (18–49 years, IQR $6,305-$15,794) to $11,065 (70–79 years, IQR $7,070-$19,435) (p=0.0006).
Adjusted Results
Testing for a non-linear relationship between the log odds of mortality and age was non-significant (p = 0.09) and a linear relationship was observed. After adjustment for gender, race, and 29 ICD-9-CM Elixhauser comorbidities, the odds of in-hospital mortality were found to increase by 14.0% for every ten-year increase in age (adjusted odds ratio [AOR] 1.14; 95% CI 1.08–1.21; p<0.0001) (Supplemental Figure 3). Other predictors of in-hospital mortality included coagulopathy (AOR 1.68; 95% CI 1.33–2.12; p<0.0001) and peripheral vascular disease (AOR 1.55; 95% CI 1.09–2.20; p=0.01) (Supplemental Figure 4).
Discussion
Multiple studies have demonstrated that age is a predictor of in-hospital mortality in patients receiving ECMO,(11–14,26–33) but there are few assessments of the shape of this relationship. Our study provides the largest analysis to date showing that age is linearly associated with in-hospital mortality in recipients of ECMO for CS without evidence for a threshold effect.
One single center study by Elsharkawy et al. demonstrated a linear increase in mortality with each decade of age conferring a 52% increased risk of in-hospital death (AOR 1.52; 95% CI 1.20–1.92; p<0.001); however, the study was small (n=233) and limited to the postcardiotomy population.(34) We identified a 14% increased risk of in-hospital mortality per each additional decade of life without an inflection point above which mortality increases non-linearly. The higher mortality risk with aging found by Elsharkawy et al. may be reflective of a more severe pathophysiological process specific to patients unable to separate from cardiopulmonary bypass after cardiac surgery as this subset of patients with CS have historically demonstrated worse rates of survival to discharge (24–42%)(27,31,35–37) in comparison with other etiologies such as myocarditis (survival to discharge 60–88%).(38–40)
The in-hospital mortality of our postcardiotomy group was 58.9%, which is lower than that reported by other publications (64–75%)(31,34,35) suggesting that factors such as patient frailty or surgical case mix may be contributory to the higher mortalities seen in these single center studies. The in-hospital mortality of our transplant group was noticeably lower than the overall in-hospital mortality rate (34.5% vs. 57.7%). It is unclear whether this reflects better recovery of patients with primary graft failure or rather with another etiology of CS that was subsequently bridged to transplant through ECMO.
The decision to implement ECMO for CS can be challenging as hemodynamic instability can limit the time available for gathering and discussing information crucial to appropriate candidate selection. The linear relationship between age and mortality suggests that disqualification for ECMO should not be made on the basis of a particular age, but that an individual’s age should be factored into the decision-making process along with other crucial elements such as baseline function, etiology, comorbidities, organ dysfunction, and potential for viable exit strategies (recovery, transplant, ventricular assist device) in a case-by-case assessment of each individual.
Nonetheless, the effect of age should not be minimized. The in-hospital mortality rate for patients with CS treated with non-durable mechanical circulatory support has been reported at 32.7%.(4) In contrast, the in-hospital mortality of patients with CS treated specifically with ECMO remains ≥50% at all ages; this increased rate of mortality is observed even in the youngest cohort and rises to 75% in patients aged 80–90 years. Thus, a linear relationship between age and mortality does not preclude the presence of a prohibitively high absolute rate of death in the older decades. There is not a clear cut-point for disqualification from ECMO, but it is crucial that the significant gradient in mortality associated with increased age be recognized and factored into the decision-making process.
This study also provides in-depth analyses of the impact of age upon hospital LOS, patterns of patient disposition, and costs - topics which have not been previously elucidated. LOS is longest in the youngest group and shortest in the oldest group with a difference of more than one week. This discrepancy may be due to a propensity towards providing circulatory support to younger patients for a longer time period prior to withdrawal. As the median time-to-death across age groups only ranged from 7 to 9 days, it is possible that patients are supported with ECMO for this time period as a general practice pattern of support for one week prior to further care decisions.
The shorter LOS in the older groups is also influenced by shorter median times-to-discharge home and to transfer to a skilled nursing facility in the older groups. The quicker times-to-discharge and transfer in the older groups are somewhat counterintuitive. One possible explanation is the availability of hospice services at home or a skilled nursing facility prompting early discharge. Unfortunately, due to changes in coding, the proportion of individuals being discharged with hospice services could not be determined. However, supporting this notion, transfers-to-skilled nursing facilities accounted for a greater proportion of discharges from the hospital than discharges home in older patients.
Despite having the lowest median costs per day ($9,325, IQR $6,305-$15,794), the youngest cohort demonstrated the highest hospitalization costs ($147,548) likely due to the longest LOS (17 days, IQR 6–34). The overall median cost of hospitalization is high at $134,573. As a point of comparison, the average cost per hospital stay as assessed by HCUP is approximately ten times lower at $10,606 (2016 dollars)(25,41) and the average cost of hospitalization in CS due to ST-segment elevation myocardial infarction is still only $49,884 (2016 dollars)(25,42), which emphasizes the enormous resources required to care for a patient requiring ECMO for CS.
Our study has a number of limitations. First, procedure codes for ECMO do not differentiate between venoarterial or venovenous ECMO; however, use of venoarterial ECMO in the context of CS is most likely. Significant code capture of venovenous ECMO should have resulted in a large increase in hospitalizations during the 2009 influenza pandemic, but this was not observed in our data. Also, isolated respiratory failure requiring venovenous ECMO should be associated with lung transplant, but no cases were seen in our cohort from 1/2004–9/2015. Second, the results may be subject to errors in coding; however, billing accuracy is likely high due to the elevated severity of illness resulting in clinically prominent diagnoses and heavy resource utilization requiring appropriate reimbursement. Third, information regarding the procedure groups was limited to the 1/2004–9/2015 ICD-9-CM era due to a marked increase in complexity of ICD-10-CM coding rendering uncertain the comparability of the groups between the two periods. In addition, because the ICD-9-CM and ICD-10-CM codes for Elixhauser comorbidities differ, we could not ensure a valid risk-adjustment procedure that spanned the changeover interval and so the adjusted analysis was limited to this timeframe, as well. Fourth, the NIS lacks granularity on clinical factors that may influence mortality in CS such as blood pressure, troponin, and lactate, and subsequently precludes adjustment of results based on severity indices such as the Sequential Organ Failure Assessment score. Fifth, the temporality of coding cannot be discerned including covariates relative to ECMO insertion (e.g. timing of renal failure) and ECMO insertion relative to cardiac procedures although ECMO prior to TAVR or non-transplant procedures is likely uncommon. Sixth, time trend analyses could not be accomplished secondary to low cell counts. Seventh, while results from this study are broadly applicable to patients in general, subgroups with heterogeneous outcomes may exist including myocarditis, congenital heart disease, (5,12,29,32,43) cardiac arrest,(11,40,43–45) postcardiotomy,(11,37,45–48) and patients with certain comorbidities or organ dysfunction.(11,27,29,31,34,35,43,44,49,50) Finally, we did not compare our findings in the NIS with those that might be found in ELSO given the use of self-reported data increasing the concern for selection bias and lack of data regarding hospital LOS, disposition, and costs.
Conclusions
Age is linearly associated with increasing in-hospital mortality in individuals receiving ECMO for CS without evidence of a threshold effect. In-hospital mortality for ECMO remains high regardless of age with over half of deaths occurring at approximately one week. Median LOS ranges from 9–17 days with the longest stays in the youngest group. One third of patients are discharged from the hospital alive, but the median time-to-discharge is one month. Hospitalization costs exceed $100,000 in all age groups.
Supplementary Material
Acknowledgments
Conflicts of Interest and Source of Funding
Mabel Chung, MD-None
Yuansong Zhao, MA-None
Jordan B. Strom, MD-None
Changyu Shen, PhD-None
Robert W. Yeh, MD, MSc, MBA- Abiomed, Inc. and Abbott Vascular (funding not primarily for study under consideration)
The authors acknowledge Linda Valsdottir for her assistance with the manuscript.
Footnotes
Copyright form disclosure: Dr. Yeh’s institution received funding from Abiomed, and he has received funding not primarily for the study under consideration from Abiomed Inc, Boston Scientific, Medtronic (consulting), and Abbott Vascular.
References
- 1.Gerke AK, Tang F, Cavanaugh JE, Doerschug KC, Polgreen PM. Increased trend in extracorporeal membrane oxygenation use by adults in the United States since 2007. BMC Res Notes. 2015;8:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015;61(1):31–36. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy FH, McDermott KM, Kini V, et al. Trends in U.S. Extracorporeal Membrane Oxygenation Use and Outcomes: 2002–2012. Semin Thorac Cardiovasc Surg. 2015;27(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strom JB, Zhao Y, Shen C, et al. National Trends, Predictors of Use, and In-Hospital Outcomes in the Mechanical Circulatory Support for Cardiogenic Shock EuroIntervention. 2018. [DOI] [PubMed] [Google Scholar]
- 5.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63(1):60–67. [DOI] [PubMed] [Google Scholar]
- 6.Shah M, Patnaik S, Patel B, et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States Clin Res Cardiol. 2017. [DOI] [PubMed] [Google Scholar]
- 7.Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97(2):610–616. [DOI] [PubMed] [Google Scholar]
- 8.Xie A, Phan K, Tsai YC, Yan TD, Forrest P. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest: a meta-analysis. J Cardiothorac Vasc Anesth. 2015;29(3):637–645. [DOI] [PubMed] [Google Scholar]
- 9.Pavasini R, Cirillo C, Campo G, et al. Extracorporeal Circulatory Support in Acute Coronary Syndromes: A Systematic Review and Meta-Analysis. Crit Care Med. 2017;45(11):e1173–e1183. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell BG, Powers AJ, Sheikh AY, Lee PH, Lobato RL, Wong JK. Resource use trends in extracorporeal membrane oxygenation in adults: an analysis of the Nationwide Inpatient Sample 1998–2009. J Thorac Cardiovasc Surg. 2014;148(2):416–421 e411. [DOI] [PubMed] [Google Scholar]
- 11.Batra J, Toyoda N, Goldstone AB, Itagaki S, Egorova NN, Chikwe J. Extracorporeal Membrane Oxygenation in New York State: Trends, Outcomes, and Implications for Patient Selection. Circ Heart Fail. 2016;9(12). [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015;36(33):2246–2256. [DOI] [PubMed] [Google Scholar]
- 13.Aso S, Matsui H, Fushimi K, Yasunaga H. In-hospital mortality and successful weaning from venoarterial extracorporeal membrane oxygenation: analysis of 5,263 patients using a national inpatient database in Japan. Crit Care. 2016;20:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorusso R, Gelsomino S, Parise O, et al. Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock in Elderly Patients: Trends in Application and Outcome From the Extracorporeal Life Support Organization (ELSO) Registry. Ann Thorac Surg. 2017;104(1):62–69. [DOI] [PubMed] [Google Scholar]
- 15.Mendiratta P, Wei JY, Gomez A, et al. Cardiopulmonary resuscitation requiring extracorporeal membrane oxygenation in the elderly: a review of the Extracorporeal Life Support Organization registry. ASAIO J. 2013;59(3):211–215. [DOI] [PubMed] [Google Scholar]
- 16.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64(14):1407–1415. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy FH, McDermott KM, Spragan D, et al. Unconventional Volume-Outcome Associations in Adult Extracorporeal Membrane Oxygenation in the United States. Ann Thorac Surg. 2016;102(2):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith M, Vukomanovic A, Brodie D, Thiagarajan R, Rycus P, Buscher H. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: an analysis of the Extracorporeal Life Support Organization (ELSO) registry. Crit Care. 2017;21(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HCUP. Introduction to the HCUP National Inpatient Sample (NIS). 2014; https://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2014.jsp. Accessed 11/14/2017.
- 20.HCUP. Trend Weights for HCUP NIS Data. 2017; https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. Accessed 12/18/17.
- 21.Shaefi S, O’Gara B, Kociol RD, et al. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc. 2015;4(1):e001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert L, Blais C, Hamel D, et al. Evaluation of care and surveillance of cardiovascular disease: can we trust medico-administrative hospital data? Can J Cardiol. 2012;28(2):162–168. [DOI] [PubMed] [Google Scholar]
- 23.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 24.https://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp. Accessed 2/27/2018.
- 25.Statistics BoL. CPI Inflation Calculator. https://www.bls.gov/data/inflation_calculator.htm. Accessed 2/27/2018.
- 26.Cardarelli MG, Young AJ, Griffith B. Use of extracorporeal membrane oxygenation for adults in cardiac arrest (E-CPR): a meta-analysis of observational studies. ASAIO J. 2009;55(6):581–586. [DOI] [PubMed] [Google Scholar]
- 27.Wu MY, Lin PJ, Lee MY, et al. Using extracorporeal life support to resuscitate adult postcardiotomy cardiogenic shock: treatment strategies and predictors of short-term and midterm survival. Resuscitation. 2010;81(9):1111–1116. [DOI] [PubMed] [Google Scholar]
- 28.Sheu JJ, Tsai TH, Lee FY, et al. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38(9):1810–1817. [DOI] [PubMed] [Google Scholar]
- 29.Smedira NG, Moazami N, Golding CM, et al. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg. 2001;122(1):92–102. [DOI] [PubMed] [Google Scholar]
- 30.Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016;42(3):370–378. [DOI] [PubMed] [Google Scholar]
- 31.Rastan AJ, Dege A, Mohr M, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. 2010;139(2):302–311, 311 e301. [DOI] [PubMed] [Google Scholar]
- 32.Truby L, Mundy L, Kalesan B, et al. Contemporary Outcomes of Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock at a Large Tertiary Care Center. ASAIO J. 2015;61(4):403–409. [DOI] [PubMed] [Google Scholar]
- 33.Slottosch I, Liakopoulos O, Kuhn E, et al. Outcomes after peripheral extracorporeal membrane oxygenation therapy for postcardiotomy cardiogenic shock: a single-center experience. J Surg Res. 2013;181(2):e47–55. [DOI] [PubMed] [Google Scholar]
- 34.Elsharkawy HA, Li L, Esa WA, Sessler DI, Bashour CA. Outcome in patients who require venoarterial extracorporeal membrane oxygenation support after cardiac surgery. J Cardiothorac Vasc Anesth. 2010;24(6):946–951. [DOI] [PubMed] [Google Scholar]
- 35.Ko WJ, Lin CY, Chen RJ, Wang SS, Lin FY, Chen YS. Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg. 2002;73(2):538–545. [DOI] [PubMed] [Google Scholar]
- 36.Khorsandi M, Dougherty S, Bouamra O, et al. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Surg. 2017;12(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doll N, Kiaii B, Borger M, et al. Five-year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. Ann Thorac Surg. 2004;77(1):151–157; [DOI] [PubMed] [Google Scholar]
- 38.Cheng R, Hachamovitch R, Kittleson M, et al. Clinical outcomes in fulminant myocarditis requiring extracorporeal membrane oxygenation: a weighted meta-analysis of 170 patients. J Card Fail. 2014;20(6):400–406. [DOI] [PubMed] [Google Scholar]
- 39.Lorusso R, Centofanti P, Gelsomino S, et al. Venoarterial Extracorporeal Membrane Oxygenation for Acute Fulminant Myocarditis in Adult Patients: A 5-Year Multi-Institutional Experience. Ann Thorac Surg. 2016;101(3):919–926. [DOI] [PubMed] [Google Scholar]
- 40.Diddle JW, Almodovar MC, Rajagopal SK, Rycus PT, Thiagarajan RR. Extracorporeal membrane oxygenation for the support of adults with acute myocarditis. Crit Care Med. 2015;43(5):1016–1025. [DOI] [PubMed] [Google Scholar]
- 41.Pfuntner AWL, Steiner C. Statistical Brief #146: Costs for Hospital Stays in the United States, 2010 In. Health Care Cost and Utilization Project 2013:1–11. [Google Scholar]
- 42.Kolte D, Khera S, Aronow WS, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3(1):e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Combes A, Leprince P, Luyt CE, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36(5):1404–1411. [DOI] [PubMed] [Google Scholar]
- 44.Pontailler M, Demondion P, Lebreton G, Golmard JL, Leprince P. Experience with Extracorporeal Life Support for Cardiogenic Shock in the Older Population more than 70 Years of Age. ASAIO J. 2017;63(3):279–284. [DOI] [PubMed] [Google Scholar]
- 45.Flecher E, Anselmi A, Corbineau H, et al. Current aspects of extracorporeal membrane oxygenation in a tertiary referral centre: determinants of survival at follow-up. Eur J Cardiothorac Surg. 2014;46(4):665–671; [DOI] [PubMed] [Google Scholar]
- 46.Chen YS, Chao A, Yu HY, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41(2):197–203. [DOI] [PubMed] [Google Scholar]
- 47.Carroll BJ, Shah RV, Murthy V, et al. Clinical Features and outcomes in adults with cardiogenic shock supported by extracorporeal membrane oxygenation. Am J Cardiol. 2015;116(10):1624–1630. [DOI] [PubMed] [Google Scholar]
- 48.Chang CH, Chen HC, Caffrey JL, et al. Survival Analysis After Extracorporeal Membrane Oxygenation in Critically Ill Adults: A Nationwide Cohort Study. Circulation. 2016;133(24):2423–2433. [DOI] [PubMed] [Google Scholar]
- 49.Lee WC, Fang CY, Chen HC, et al. Associations with 30-day survival following extracorporeal membrane oxygenation in patients with acute ST segment elevation myocardial infarction and profound cardiogenic shock. Heart Lung. 2016;45(6):532–537. [DOI] [PubMed] [Google Scholar]
- 50.Saxena P, Neal J, Joyce LD, et al. Extracorporeal Membrane Oxygenation Support in Postcardiotomy Elderly Patients: The Mayo Clinic Experience. Ann Thorac Surg. 2015;99(6):2053–2060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.