Abstract
Objective:
With the widespread use of sex-steroid hormones in contraceptives and hormone replacement therapy, there is an increasing need for reliable analytical methods. We report the development of a sensitive and robust UPLC-MS/MS method for quantitation of both endogenous and synthetic sex-steroid hormones in human serum.
Study Design:
We developed and validated a UPLC-MS/MS method to quantify progestogens (etonogestrel, levonorgestrel, medroxyprogesterone acetate, norethindrone, progesterone) and estrogens (estradiol and ethinyl estradiol) with good accuracy, high sensitivity, and excellent robustness. We then applied the method to the analysis of sex-steroid hormones in serum from 451 clinical research participants.
Results:
Each UPLC-MS/MS analysis was 6.5 min. The lower limits of quantitation (LLOQs) were 25 pg/ml for the progestogens, and 2.5 and 5.0 pg/ml for estradiol and ethinyl estradiol, respectively. When estradiol was analyzed without assessment of progestogens, the LLOQ was reduced to 1 pg/ml. The calibration curves were linear from 25-50,000, 2.5-2,000 (1-2,000 for estrogens-only analysis) and 5-2,000 pg/ml, respectively. Both the accuracy and precision were below ±15% not only for routine validation (intraday and interday), but for long-term (> two years) assay robustness with external controls, thereby, demonstrating the utility of this method for multi-year clinical trial assessments of progestogens and estrogens. We applied the method to quantify sex-steroid levels in 1804 clinical samples.
Conclusions:
We successfully developed a UPLC-MS/MS method, and overcame the matrix suppression to allow sensitive quantitation of both synthetic and endogenous sex-steroid hormones in human serum.
Keywords: Sex-steroid hormones, UPLC-MS/MS, reproducibility, quantitation, clinical sample analysis
1. Introduction
Commonly used in modern contraceptives (1, 2) and hormone replacement therapy (3), sex-steroid hormones play important roles in human health and disease. Sensitive, accurate and robust methods are needed for their quantitation to support reproductive health research and clinical practice for diagnosis, treatment and disease prevention.
Steroid immunoassays such as conventional radioimmunoassay (RIA) (4) and competitive enzyme immunoassay (EIA) (sometimes referred to as enzyme-linked immunosorbent assay (ELISA) (5)) have been widely used in clinical and environmental laboratories yet they are prone to interferences, particularly at low levels of quantification (6-8). Since the first report in 1960 (9), hyphenated methods coupling chromatographic separations (i.e. gas chromatography (GC) and liquid chromatography (LC)) and tandem mass spectrometry (MS/MS) detection have gained popularity with improved selectivity, specificity, sensitivity, speed (afforded by ultra-performance liquid chromatography (UPLC)), and multiplexing capabilities (6, 7, 10-12). In the past decade, LC-MS/MS has been increasingly becoming a method of choice while limiting the time-consuming water-free derivatization and intense analyte cleanup and concentration steps required by GC-MS/MS (7, 11).
One complication of steroid hormone analysis, particularly with estradiol, is the reduced degree of ion formation during electrospray ionization, limiting the sensitivity without chemical derivatization prior to LC-MS/MS (13). Another barrier to accurate quantification is the existence of a significant known matrix effect in plasma/serum and the identification of an estrogen-free matrix source. The question of matrix reproducibility is particularly prominent in long-term and ongoing clinical studies in which samples are to be evaluated over years of acquisition, yet such long-term assessment of assay reproducibility has not been previously reported.
We aimed to develop a sensitive and robust UPLC-MS/MS method for quantitation of both synthetic and endogenous sex-steroid hormones in human serum. Five progestogens (etonogestrel, levonorgestrel, medroxyprogesterone acetate, norethindrone, progesterone) and two estrogens (estradiol and ethinyl estradiol) (Fig. 1) were included in this study to meet the requirement of our clinical applications. Our method was designed to overcome ionization and matrix interference barriers, as well as, to provide long-term assay reproducibility for clinical sample analysis.
Figure 1.
Sex-steroid hormones studied in this work. The SRM transitions used for quantitation are listed in parenthesis.
2. Materials and methods
2.1. Materials
We purchased etonogestrel, levonorgestrel, medroxyprogesterone acetate, norethindrone, progesterone, testosterone-d3, estradiol, ethinyl estradiol, dansyl chloride, sodium bicarbonate, ammonium acetate and formic acid from Sigma-Aldrich. Estradiol-d5 was purchased from CDN Isotopes. Progesterone and estradiol stock solutions were purchased from Abbott Laboratories. Water, methanol, acetonitrile, N-butylchloride, and sodium hydroxide were purchased from Fisher Scientific. We purchased double-stripped human serum from Golden West Biologicals.
2.2. Calibration and quality control standards
The progestogen panel: We prepared calibration stocks (1 mg/ml) in methanol for each of the five progestogens purchased from Sigma (etonogestrel, levonorgestrel, progesterone, norethindrone and medroxyprogesterone acetate). Calibration standards were prepared at 25, 50, 100, 250, 500, 1,000, 2,000, 10,000 and 20,000 pg/ml in double-stripped human serum. Quality contrl (QC) standards were prepared at 35, 175, 875, 10,000, 25,000 and 50,000 pg/ml from separate stock solutions. We diluted a progesterone stock solution purchased from Abbott Laboratories to make a 35 or 100 pg/ml external QC standard. A testosterone-d3 stock solution (100 μg/ml) was diluted in methanol to 20 ng/ml as an internal standard.
The estrogen panel: We prepared calibration stock solutions of estrogens (estradiol and ethinyl estradiol) from Sigma in a similar way. The calibration standards were made at 1, 2.5, 5, 10, 25, 50, 75, 100, 200, 500, 1000 and 2,000 pg/ml. QC standards were prepared at 3, 30 (or 50), 90 pg/ml for the low range or 35, 175, and 875 pg/ml for the high range samples. We prepared a 25 pg/ml external QC standard of estradiol by dilution of a stock solution purchased from Abbott Laboratories. An estradiol-d5 stock solution (1 μg/ml) was diluted to 0.5 ng/ml as an internal standard. It should be mentioned that extreme care must be taken when weighing powdered estradiol to avoid contamination of reagents and equipment near the balance. A significant portion of the powder becomes airborne. We have addressed this problem by never weighing estradiol powder in the same sample preparation room.
The progestogen and estrogen panels: We prepared a single series of calibration standards with the desired progestogen and estrogen concentrations with both internal standards present. At least eight calibration standards and three QC standards were included per sample batch, and their concentrations were chosen according to the sample source (pre- or post-menopausal women, men, or children) and expected steroid levels (low range for endogenous only or high range with hormone treatment) within the batch.
2.3. Precision, accuracy, linearity, limits of detection and quantitation
The linearity and sensitivity was evaluated by analyzing sex-steroid hormones over a broad concentration range (≥8 calibration solutions) in duplicate and spanning three days. Calibration curves were calculated based on weighted (1/Y) linear regression of peak area ratios plotted against steroid hormone concentrations in pg/ml. Intraday and interday accuracy and precision were determined by analyzing three QCs (low, medium, high) in six replicates on two different days followed by twelve replicates on a third day.
2.4. Processing efficiency, matrix effect, sample stability and specificity
Progestogens were spiked into different stages of the sample extraction procedures. All the samples were run in six replicates under each condition to determine the matrix effect and processing efficiency. In addition, we spiked the sex-steroid hormones at different concentrations into Lyphochek (prepared from human serum by BioRad Laboratories) and human plasma from central blood bank to check specificity. The sample stability was verified online by cycling the introduction of calibration solutions and QCs with each batch of clinical samples.
2.5. Robustness and reliability
To further validate our UPLC-MS/MS method and test its robustness and reliability, we purchased external quality control samples from a separate vendor (Abbott Laboratories) and analyzed them multiple times over a two-year period.
2.6. Sample preparation
Serum samples of 0.5 ml each were spiked with 10 μl of 20 ng/ml testosterone-d3 and/or 25 μl of 0.5 ng/ml estradiol-d5 as internal standard(s). The sample preparation procedures were adapted from previous work (14, 15) with slight modifications. Briefly, the samples were mixed, followed by addition of 3 ml N-butylchloride. After vortexing for 2 min and centrifugation for 10 min at full speed (Thermo Scientific CL2), approximately 2.5 ml of the organic layer was transferred to new tubes and then vaporized under a gentle nitrogen stream at 40 °C. The residue was reconstituted in 50 μl of 50:50 methanol: water. Half of the reconstituted sample (25 μl) was saved for progestogen analysis, and the remaining half (25 μl) was evaporated under nitrogen. For derivatization of estrogens, 50 μl of 50 mM bicarbonate buffer (pH adjusted to 10.5) and 50 μl of 1 mg/ml dansyl chloride in acetonitrile were added. The mixture was then heated to 60 °C for 3 min.
2.7. UPLC-MS/MS
A Waters Acquity UPLC system was used to separate the sex-steroid hormones at 55 °C with a UPLC BEH C18, 1.7 μm (2.1 mm×150 mm) reversed phase column (Waters) protected by a guard (2.1 mm×5 mm) (Waters). The injection volume was 7.5 μl. The flow rate was 0.3 ml/min. Mass spectrometric detection was conducted via a TSQ Quantum Ultra (Thermo Fisher Scientific) using a heated electrospray ionization (HESI) source in the positive mode. The collision gas pressure was kept at 1.5 mTorr and the scan time set at 0.010 s. Selected reaction monitoring (SRM) was used for quantitation (Supplemental Table S1). Two separate injections were made under different LC conditions for the progestogen and estrogen panels to optimize sensitivity.
The progestogen panel: Mobile phase A consisted of 2 mM ammonium acetate, 0.1% formic acid in water and mobile phase B contained 100% methanol. The mobile phase composition was held at 50% B for 0.5 min and then increased to 85% B in a linear fashion over 3 min. After maintaining at 85% B for 1 min, the mobile phase was returned to the initial composition in 2 min.
The estrogen panel: Mobile phases consisted of 0.1% formic acid in water (A) and 100% acetonitrile (B). The mobile phase composition was held at 50% B for 1 min and then increased to 85% B in a linear fashion over 3 min. After maintaining at 85% for 1 min, the mobile phase was returned to the initial composition in 1.5 min.
2.8. Clinical samples
We performed a Zim CHIC parallel cohort study (ClinicalTrials.gov number: NCT02038335) approved by The University of Pittsburgh Institutional Review Board and The Medical Research Council of Zimbabwe (16). Our UPLC-MS/MS assay was used to quantify progestogens and estrogens in a total of 1804 serum samples collected from 451 women.
Results
3.1. Method development
To achieve optimal chromatographic separation and adequate MS sensitivity, we found it necessary to use slightly different UPLC-MS/MS conditions for progestogens as compared to estrogens. Sample preparation included liquid-liquid extraction with N-butylchloride followed by derivatization of estrogens by dansyl chloride.
3.2. Method validation
We largely followed the 2013 FDA guidelines on bioanalytical method validation (17).
3.2.1. Precision and accuracy
Accuracy is the relative deviation in the calculated value of a standard from its true value as defined by relative error (RE). Precision is the coefficient of variation (CV) of the mean concentrations. Both intraday and interday accuracy and precision fell within ±15% for all the steroids (Table 1).
Table 1.
Accuracy and precision of our UPLC-MS/MS method.
| Sex-Steroid Hormone | Experiment | Spiked (pg/ml) |
Intraday Measured (mean±SD) (pg/ml) |
RE (%)a | CV (%)b | Interday Measured (mean±SD)(pg/ml) |
RE (%)a | CV (%)b |
|---|---|---|---|---|---|---|---|---|
| Etonogestrel | LLOQ | 25 | 25.0±1.4 | 0.0 | 5.7 | 27.0±2.0 | 8.0 | 7.4 |
| 35 | 34.0±4.1 | −2.9 | 11.9 | 36.6±3.4 | 4.6 | 9.2 | ||
| QC | 175 | 163±11.4 | −6.9 | 7.0 | 170±13.8 | −2.9 | 8.1 | |
| 875 | 776±17.8 | −11.4 | 2.3 | 827±68.4 | −5.5 | 8.3 | ||
| Levonorgestrel | LLOQ | 25 | 27.5±0.7 | 10.0 | 2.6 | 26.7±0.8 | 6.7 | 3.1 |
| 35 | 35.7±3.9 | 2.0 | 11.0 | 35.7±3.6 | 2.0 | 10.0 | ||
| QC | 175 | 185±9.7 | 5.9 | 5.2 | 176±14.4 | 0.5 | 8.2 | |
| 875 | 972±15.8 | 11.1 | 1.6 | 888±80.0 | 1.4 | 9.0 | ||
| Medroxprogesterone Acetate | LLOQ | 25 | 28.5±0.7 | 14.0 | 2.5 | 25.7±2.7 | 2.7 | 10.4 |
| 35 | 33.0±2.6 | −5.7 | 7.7 | 34.6±3.0 | −1.1 | 8.8 | ||
| QC | 175 | 154±6.4 | −12.0 | 4.2 | 170±16.3 | −3.0 | 9.6 | |
| 875 | 784±39.6 | −10.4 | 5.1 | 862±72.3 | −1.4 | 8.4 | ||
| Norethindrone | LLOQ | 25 | 25.0±2.8 | 0.0 | 11.3 | 25.2±2.6 | 0.8 | 10.3 |
| 35 | 33.8±2.8 | −3.4 | 8.3 | 35.0±3.3 | 0.0 | 9.3 | ||
| QC | 175 | 154±2.6 | −12.0 | 1.7 | 164±12.2 | −6.5 | 7.5 | |
| 875 | 783±35.3 | −10.5 | 4.5 | 843±65.9 | −3.7 | 7.8 | ||
| Progesterone | LLOQ | 25 | 23.0±1.4 | −8.0 | 6.1 | 24.2±3.6 | 0.0 | 14.9 |
| 35 | 31.8±1.8 | −9.1 | 5.6 | 34.9±3.1 | −0.3 | 9.0 | ||
| QC | 175 | 160±8.9 | −8.7 | 5.6 | 169±12.0 | −3.6 | 7.1 | |
| 875 | 756±29.2 | −13.7 | 3.9 | 819±69.2 | −6.4 | 8.5 | ||
| Estradiol | LLOQ | 2.5 | 2.62±0.2 | 4.6 | 5.7 | 2.71±0.1 | 8.4 | 4.4 |
| 35 | 36.9±2.1 | 5.4 | 5.8 | 36.4±3.1 | 4.0 | 8.4 | ||
| QC | 175 | 180±16.4 | 2.7 | 9.1 | 175±19.3 | 0.1 | 11.0 | |
| 875 | 969.±43.4 | 10.7 | 4.5 | 902±81.6 | 3.1 | 9.1 | ||
| Ethinyl Estradiol | LLOQ | 5 | 4.75±0.4 | −5.0 | 8.0 | 5.00±0.5 | 0.1 | 9.7 |
| 35 | 31.8±1.9 | −9.1 | 6.1 | 35.8±3.6 | 2.3 | 10.1 | ||
| QC | 175 | 189±10.2 | 8.0 | 5.4 | 170±18.9 | −3.0 | 11.1 | |
| 875 | 992±33.1 | 13.4 | 3.3 | 840±97.5 | −4.0 | 11.6 |
RE=relative error=(calculated value-true value)/true value *100.
CV=relative standard deviation=sample standard deviation/sample mean*100.
Intraday RE ranged from −13.7% to 14.0%, interday RE ranged from −6.5% to 8.4%.
Intraday CV ranged from 1.6 to 11.9%, interday CV ranged from 3.1 to 14.9%.
3.2.2. Linearity and sensitivity
The linear ranges were 25-50,000 pg/ml (8 calibration standards between 25-2,000 pg/ml and validated with additional QC standards at 10,000, 25,000 and 50,000 pg/ml) for progestogens, 2.5-2,000 and 5-2,000 pg/ml for estradiol and ethinyl estradiol, respectively. It should be noted that a wider linear range from 1-2,000 pg/ml was achieved when estradiol was analyzed without progestogen assessment. All the calibration standards fell within 15% RE of back-calculated amounts from nominal spiked amounts for all analytes.
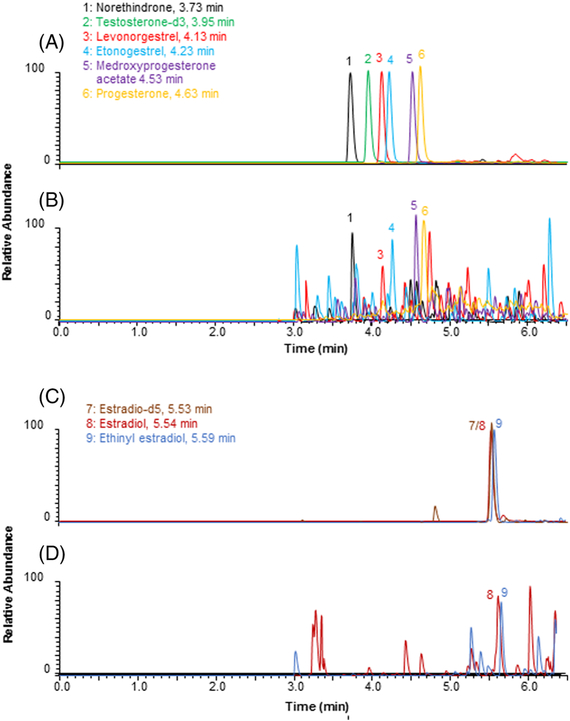
Lower limit of quantitation (LLOQ) was defined as the lowest concentration that could be measured with RE < 20% and CV <20%. LLOQs were 25 pg/ml for progestogens, 2.5 pg/ml (1 pg/ml without progestogen assessment) and 5.0 pg/ml for estradiol and ethinyl estradiol, respectively (Fig. 2, Table 1).
Figure 2.
Representative UPLC-MS/MS chromatograms of sex-steroid hormones spiked to double stripped human serum. (A) progestogens at 1,000 pg/ml and internal standard testosterone-d3 at 400 pg/ml, (B) progestogens at LLOQs (25 pg/ml), (C) estrogens at 100 pg/ml and internal standard estradiol-d5 at 25 pg/ml, (D) estrogens at LLOQs (2.5 and 5.0 pg/ml for estradiol and ethinyl estradiol, respectively).
3.2.3. Processing efficiency, matrix effect, sample stability and specificity
Matrix effect was the relative difference in measured concentrations of sex-steroid hormones between samples spiked into the blank matrix after extraction and those spiked into solvents. We found matrix effect to be between −8% and 37% at 35 pg/ml, and between −15% and −8% at 875 pg/ml, respectively.
Processing efficiency was defined as the ratio of measured sex-steroid hormone concentrations between samples spiked into the blank matrix before extraction and those spiked into solvents. Therefore, processing efficiency accounted for both the recovery and matrix effect. We found processing efficiency to be between 65% - 83% at 35 pg/ml, and 53% - 63% at 875 pg/ml, respectively. Similarly, the matrix effect and processing efficiency for estradiol at 100 pg/ml was 48% and 64%, respectively. All the REs were between −1.2% and 13.7%. Double-stripped human serum was used as the matrix for all the calibration standards and QCs to compensate for the matrix effect and processing efficiency.
For the steroids spiked into Lyphochek and human plasma, all the measured amounts were found to agree with the spiked amounts with REs ≤15%, demonstrating the good recovery, selectivity and specificity of our method. The latter two characteristics were also made possible by the inherent specificity of the triple quadrupole mass spectrometer, which allowed us to monitor the specific transitions for the analytes of interest. Though estradiol and ethinyl estradiol share the same fragment ion at m/z 171, their precursor ions have different m/z values (506 and 530, respectively). Therefore, two distinct transitions (506 → 171, 530 ₒ 171) could be monitored to distinguish the two estrogens.
Fresh standards were constantly compared with those having undergone up to four freeze-thaw cycles. No noticeable change in stability was observed.
3.3. Robustness and reliability
For the external QCs assayed over a two-years, the differences between the measured and true values were very small and the REs were all below 4%. The CVs fell below 13% for both progesterone and estradiol (Table 2).
Table 2.
Robustness and reliability of our UPLC-MS/MS method.
| Sex-Steroid Hormone | External QC (pg/ml) |
Measured (mean±SD) (pg/ml) |
# of Measurements |
Time Period | RE (%)a | CV (%) b |
|---|---|---|---|---|---|---|
| Progesterone | 35 | 34.4±3.8 | 46 | 02/25/2015 - 07/07/2016 | 1.7 | 11.1 |
| 100 | 103±7.3 | 18 | 07/14/2016 - 02/22/2017 | 3.1 | 7.1 | |
| Estradiol | 25 | 24.5±3.2 | 61 | 01/13/2015 - 02/22/2017 | 2.0 | 13.0 |
RE=relative error=(calculated value-true value)/true value *100.
CV=relative standard deviation=sample standard deviation/sample mean*100.
3.4. Quantitation of sex-steroid hormones in clinical samples
The sex-steroid hormones were quantified for all the 1804 serum samples collected from the Zim CHIC parallel cohort study. All samples that were positive for a sex-steroid hormone are reported here for each hormone as median with interquartile range (IQR) (Table 3). The progestogen and estrogen levels in these clinical samples spread over three and two orders of magnitude, respectively, yet all were within our linear dynamic ranges.
Table 3.
Summary of sex-steroid hormone levels (pg/ml) in clinical serum samples for the Zim CHIC parallel cohort study.
| Sex-Steroid Hormone | Etonogestrel | Levonorgestrel | Medroxprogesterone Acetate |
Norethindrone | Progesterone | Estradiol | Ethinyl Estradiol |
|---|---|---|---|---|---|---|---|
| # of Samples | 186 | 391 | 467 | 211 | 1239 | 1475 | 147 |
| Minimum Value | 26 | 25 | 26 | 29 | 25 | 10 | 10 |
| Maximum Value | 2249 | 23839 | 3358 | 4952 | 36956 | 833 | 1245 |
| IQRa 25th Percentile | 241 | 440 | 182 | 771 | 33 | 29 | 20 |
| Median | 309 | 722 | 278 | 1156 | 46 | 48 | 39 |
| IQRa 75th Percentile | 389 | 2620 | 553 | 1636 | 87 | 94 | 97 |
IQR=Interquartile range.
4. Discussion
We developed a fast, sensitive and selective UPLC-MS/MS method for the detection of five progestogens and two estrogens (Fig. 1). Good accuracy and precision (Table 1) were achieved with the utilization of a single deuterated internal standard per panel, making our method cost effective. The specific sex-steroid hormones included in the panels were selected to cover the full spectrum of regionally available contraceptives at the time of the Zim CHIC parallel cohort study for which these panels were developed. The 25 and 1 pg/ml LLOQs were among the lowest compared to what has been reported by others for progestogens (15, 18-22) and estrogens (11, 15, 18, 20, 23), respectively. It should be noted that the higher sensitivity we achieved in estrogen LLOQs compared to what Blue et al. recently reported (24) might be attributable to the derivatization we have used to enhance the ionization efficiency of estradiol and ethinyl estradiol (13).
Among the numerous reports on UPLC-MS/MS analysis of steroids in circulation, many quantified only one or two analytes and/or multiple analytes within the same class. Endogenous steroids were often the sole focus even for multi-panel multiple steroid analysis (11, 18, 25-35), while only a few included both endogenous and synthetic compounds (19, 22). One notable exception is a paper published by Blue et al. (24) (see discussions above), otherwise, we found no previous publications that include sensitive assessment of both synthetic and endogenous steroids in both progestogen and estrogen panels.
The robustness and reproducibility of our assay was demonstrated with the measured accuracy (REs < 4%) and precision (CVs <13%) for our external quality control samples over a period of two years (Table 2). This is an objective assessment of our UPLC-MS/MS method, demonstrating the robustness and reliability with good accuracy, precision and reproducibility over time. This is critical for clinical research sample analysis that often extends multiple years with combined sample analysis for interpretation. Such long-term assessment of assay reproducibility has not been previously reported.
The double-stripped human serum (Golden West Biologicals) we used as matrix was deprived of endogenous estrogens. It still contains trace amount of progesterone (Fig. 2), which didn’t interfere with sex-steroid hormone analysis when the actual level of progesterone was much higher for clinical sample analysis. Furthermore, our method was fully validated with 0.2% cyclodextrin as a true blank.
Our UPLC-MS/MS method has been optimized in response to clinical and research needs and thus has evolved over time. It has allowed us to accurately and precisely quantify estrogen and/or progestogen levels in pre- peri- and post-menopausal women (16, 36-39), men (36), children and young female rats (40). It is also notable that our method is not limited to the sex-steroid hormones reported in this manuscript. It is likely to be extended to other steroid metabolites as well since we have also adapted this method for the analysis of estrone (36).
Clinical studies have largely relied on participant self-report for important variables including last menstrual period (LMP) and contraceptive use to critically classify them into analysis cohorts. This self-reported status has likely often resulted in mixed analysis cohorts and published outcome data from studies accessing use of hormonal contraception and HIV acquisition risk have been similarly mixed and difficult to interpret (41). To this point, our recent Zim CHIC parallel cohort study demonstrated that only 64% of the overall samples were associated with accurate reporting of contraceptive use (16). This work is considered broadly applicable to contraceptive researchers as studies that rely on self-reporting to identify contraceptive hormone exposure could suffer from significant misclassification. Thus, the methodology described here is likely widely applicable to studies that include women using contraceptive hormones.
In conclusion, a sensitive and robust UPLC-MS/MS method for quantitation of serum progestogens and estrogens was developed and validated. The sensitivity, specificity and longterm assay reproducibility of our method makes it possible to verify and closely monitor sex-steroid hormone concentrations of participants in ongoing clinical studies and patients on hormonal contraception or hormone replacement therapy.
Supplementary Material
IMPLICATIONS.
We developed a sensitive and robust UPLC-MS/MS method to accurately measure the levels of sex-steroid hormones in serum. The method overcame matrix interference barriers and achieved excellent long-term stability and reproducibility (≥96.9% accuracy; ≤13.0% relative variability measured with external controls over 2 years), demonstrating its utility in clinical sample analysis.
Acknowledgement
We would like to thank Dr. Leslie Meyn for statistical support and Ms. Lorna Rabe for sample coordination and support while developing this assay and the associated hormone panels.
Funding
This work was supported by the NIH grant S10RR023461 (to S.M.P.) and the Bill & Melinda Gates Foundation OPP1055833 (to S.L.A.).
Footnotes
Declaration of Interest
The authors declare that there is no conflict of interest that could affect the impartiality of the research reported.
Supplemental Information
The mass spectrometric conditions for quantitation of the sex-steroid hormones are listed in Supplemental Table S1, which is freely available online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Chao JH, Page ST. The current state of male hormonal contraception. Pharmacol Ther 2016;163:109–17. [DOI] [PubMed] [Google Scholar]
- 2.De Leo V, Musacchio MC, Cappelli V, Piomboni P, Morgante G. Hormonal contraceptives: pharmacology tailored to women's health. Hum Reprod Update 2016;22(5):634–46. [DOI] [PubMed] [Google Scholar]
- 3.Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol 2017;13(4):220–31. [DOI] [PubMed] [Google Scholar]
- 4.Abraham GE. Solid-Phase Radioimmunoassay of Estradiol-17β. The Journal of Clinical Endocrinology & Metabolism 1969;29(6):866–70. [DOI] [PubMed] [Google Scholar]
- 5.Hanquez C, Urios P, Desfosses B, Samake H, Lince E, Rajkowski KM, et al. Enzyme-linked immunosorbent assay (ELISA) for steroid hormones with polyclonal and monoclonal antibodies: an assay for urinary aldosterone. Clin Chim Acta 1987;164(1):71–82. [DOI] [PubMed] [Google Scholar]
- 6.Soldin SJ, Soldin OP. Steroid hormone analysis by tandem mass spectrometry. Clin Chem 2009;55(6):1061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanczyk FZ, Clarke NJ. Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol 2010;121(3-5):491–5. [DOI] [PubMed] [Google Scholar]
- 8.Stanczyk FZ, Jurow J, Hsing AW. Limitations of direct immunoassays for measuring circulating estradiol levels in postmenopausal women and men in epidemiologic studies. Cancer Epidemiol Biomarkers Prev 2010;19(4):903–6. [DOI] [PubMed] [Google Scholar]
- 9.Sweeley CC, Horning EC. Microanalytical Separation of Steroids by Gas Chromatography. Nature 1960;187(4732):144–5. [DOI] [PubMed] [Google Scholar]
- 10.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev 2007;16(9):1713–9. [DOI] [PubMed] [Google Scholar]
- 11.Ke Y, Bertin J, Gonthier R, Simard JN, Labrie F. A sensitive, simple and robust LC-MS/MS method for the simultaneous quantification of seven androgen- and estrogen-related steroids in postmenopausal serum. J Steroid Biochem Mol Biol 2014;144 Pt B:523–34. [DOI] [PubMed] [Google Scholar]
- 12.Keevil BG. LC-MS/MS analysis of steroids in the clinical laboratory. Clin Biochem 2016;49(13-14):989–97. [DOI] [PubMed] [Google Scholar]
- 13.Anari MR, Bakhtiar R, Zhu B, Huskey S, Franklin RB, Evans DC. Derivatization of Ethinylestradiol with Dansyl Chloride To Enhance Electrospray Ionization: Application in Trace Analysis of Ethinylestradiol in Rhesus Monkey Plasma. Analytical Chemistry 2002;74(16):4136–44. [DOI] [PubMed] [Google Scholar]
- 14.Nelson RE, Grebe SK, DJ OK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem 2004;50(2):373–84. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Li YH, Li AC, Zhou S, Naidong W. Simultaneous determination of norethindrone and ethinyl estradiol in human plasma by high performance liquid chromatography with tandem mass spectrometry--experiences on developing a highly selective method using derivatization reagent for enhancing sensitivity. J Chromatogr B Analyt Technol Biomed Life Sci 2005;825(2):223–32. [DOI] [PubMed] [Google Scholar]
- 16.Achilles SL, Mhlanga FG, Musara P, Poloyac SM, Chirenje ZM, Hillier SL. Misreporting of contraceptive hormone use in clinical research participants. Contraception 2018;97(4):346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food and Drug Adminstration guidance for industry-bioanalytical method validation. 2013. [Google Scholar]
- 18.Koal T, Schmiederer D, Pham-Tuan H, Rohring C, Rauh M. Standardized LC-MS/MS based steroid hormone profile-analysis. J Steroid Biochem Mol Biol 2012;129(3-5):129–38. [DOI] [PubMed] [Google Scholar]
- 19.Methlie P, Hustad SS, Kellmann R, Almas B, Erichsen MM, Husebye E, et al. Multisteroid LC-MS/MS assay for glucocorticoids and androgens, and its application in Addison's disease. Endocrine connections 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi A, Guttikar S, Trivedi P. High-sensitivity simultaneous liquid chromatography–tandem mass spectrometry assay of ethinyl estradiol and levonorgestrel in human plasma. Journal of Pharmaceutical Analysis 2015;5(5):316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, et al. Liquid Chromatography–Tandem Mass Spectrometry Assay for Androstenedione, Dehydroepiandrosterone, and Testosterone with Pediatric and Adult Reference Intervals. Clinical Chemistry 2010;56(7):1138–47. [DOI] [PubMed] [Google Scholar]
- 22.Guedes-Alonso R, Ciofi L, Sosa-Ferrera Z, Santana-Rodriguez JJ, Bubba MD, Kabir A, et al. Determination of androgens and progestogens in environmental and biological samples using fabric phase sorptive extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry. J Chromatogr A 2016;1437:116–26. [DOI] [PubMed] [Google Scholar]
- 23.Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, et al. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. American journal of clinical pathology 2008;129(4):530–9. [DOI] [PubMed] [Google Scholar]
- 24.Blue SW, Winchell AJ, Kaucher AV, Lieberman RA, Gilles CT, Pyra MN, et al. Simultaneous quantitation of multiple contraceptive hormones in human serum by LC-MS/MS. Contraception 2018;97(4):363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao M, Baker SD, Yan X, Zhao Y, Wright WW, Zirkin BR, et al. Simultaneous determination of steroid composition of human testicular fluid using liquid chromatography tandem mass spectrometry. Steroids 2004;69(11-12):721–6. [DOI] [PubMed] [Google Scholar]
- 26.Guo T, Taylor RL, Singh RJ, Soldin SJ. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin Chim Acta 2006;372(1-2):76–82. [DOI] [PubMed] [Google Scholar]
- 27.Regal P, Vazquez Bl, Franco CM, Cepeda A, Fente C. Quantitative LC-MS/MS method for the sensitive and simultaneous determination of natural hormones in bovine serum. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877(24):2457–64. [DOI] [PubMed] [Google Scholar]
- 28.Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, et al. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids 2011;76(1-2):177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koren L, Ng ES, Soma KK, Wynne-Edwards KE. Sample preparation and liquid chromatography-tandem mass spectrometry for multiple steroids in mammalian and avian circulation. PLoS One 2012;7(2):e32496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moeller BC, Stanley SD. The development and validation of a turbulent flow chromatography-tandem mass spectrometry method for the endogenous steroid profiling of equine serum. J Chromatogr B Analyt Technol Biomed Life Sci 2012;905:1–9. [DOI] [PubMed] [Google Scholar]
- 31.Keefe CC, Goldman MM, Zhang K, Clarke N, Reitz RE, Welt CK. Simultaneous measurement of thirteen steroid hormones in women with polycystic ovary syndrome and control women using liquid chromatography-tandem mass spectrometry. PLoS One 2014;9(4):e93805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisser JJ, Hansen CH, Poulsen R, Larsen LW, Cornett C, Styrishave B. Two simple cleanup methods combined with LC-MS/MS for quantification of steroid hormones in in vivo and in vitro assays. Anal Bioanal Chem 2016;408(18):4883–95. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhofer G, Peitzsch M, Kaden D, Langton K, Pamporaki C, Masjkur J, et al. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: Impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin Chim Acta 2017;470:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hines JM, Bancos I, Bancos C, Singh RD, Avula AV, Young WF, et al. High-Resolution, Accurate-Mass (HRAM) Mass Spectrometry Urine Steroid Profiling in the Diagnosis of Adrenal Disorders. Clin Chem 2017. [DOI] [PubMed] [Google Scholar]
- 35.McCulloch RD, Robb DB. Field-Free Atmospheric Pressure Photoionization-Liquid Chromatography-Mass Spectrometry for the Analysis of Steroids within Complex Biological Matrices. Anal Chem 2017;89(7):4169–76. [DOI] [PubMed] [Google Scholar]
- 36.Crago EA, Sherwood PR, Bender C, Balzer J, Ren D, Poloyac SM. Plasma Estrogen Levels Are Associated With Severity of Injury and Outcomes After Aneurysmal Subarachnoid Hemorrhage. Biol Res Nurs 2015;17(5):558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thurston RC, Chang Y, Barinas-Mitchell E, Jennings JR, Landsittel DP, Santoro N, et al. Menopausal Hot Flashes and Carotid Intima Media Thickness Among Midlife Women. Stroke 2016;47(12):2910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thurston RC, Chang Y, Barinas-Mitchell E, Jennings JR, von Kanel R, Landsittel DP, et al. Physiologically assessed hot flashes and endothelial function among midlife women. Menopause 2017;24(8):886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chappell CA, Lamorde M, Nakalema S, Chen BA, Mackline H, Riddler SA, et al. Efavirenz decreases etonogestrel exposure: a pharmacokinetic evaluation of implantable contraception with antiretroviral therapy. AIDS 2017;31(14):1965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Oberly PJ, Poloyac SM, Gibbs RB. A microsomal based method to detect aromatase activity in different brain regions of the rat using ultra performance liquid chromatography-mass spectrometry. J Steroid Biochem Mol Biol 2016;163:113–20. [DOI] [PubMed] [Google Scholar]
- 41.Polis CB, Curtis KM, Hannaford PC, Phillips SJ, Chipato T, Kiarie JN, et al. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS 2016;30(17):2665–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.