Abstract
Objective:
Although poor sleep is often reported in adolescents with attention-deficit/hyperactivity disorder (ADHD), prior studies have been correlational. This study investigated whether sleep duration is causally linked to sleepiness, inattention, and behavioral functioning in adolescents with ADHD.
Method:
Seventy-two adolescents (ages 14–17 years) entered a three-week sleep protocol using an experimental crossover design. The protocol included a phase stabilization week, followed in randomized counterbalanced order by 1 week of sleep restriction (6.5 hours) and 1 week of sleep extension (9.5 hours). Sleep was monitored with actigraphy and daily sleep diaries, with laboratory visits at the end of each week. Analyses included 48 adolescents who had complete actigraphy data and successfully completed the sleep protocol (defined a priori as obtaining ≥1 hour actigraphy-measured sleep duration during extension compared to restriction). Parent and adolescent ratings of daytime sleepiness, ADHD symptoms, sluggish cognitive tempo (SCT), and oppositional behaviors were the primary measures. The A-X Continuous Performance Test (CPT) was a secondary measure.
Results:
Compared to the extended sleep week, parents reported more inattentive and oppositional symptoms during the restricted sleep week. Both parents and adolescents reported more SCT symptoms and greater daytime sleepiness during restriction compared to extension. Adolescents reported less hyperactivity-impulsivity during sleep restriction than extension. No effects were found for parent-reported hyperactivity-impulsivity, adolescent-reported ADHD inattention, or CPT performance.
Conclusion:
This study provides the first evidence that sleep duration is a causal contributor to daytime behaviors in adolescents with ADHD. Sleep may be an important target for intervention in adolescents with ADHD.
Keywords: adolescence, attention-deficit/hyperactivity disorder, comorbidity, sleep deprivation, sluggish cognitive tempo
Introduction
Adolescents with attention-deficit/hyperactivity disorder (ADHD) or ADHD symptoms experience more sleep problems, including shorter sleep, than their typically developing peers.1–3 It is estimated that up to 75% of youth with ADHD have sleep problems.4,5 Sleep problems are associated with more inattentive symptoms1 and oppositional behaviors6 in adolescents with ADHD. Further, daytime sleepiness is common in adolescents with ADHD5 and associated with poorer academic performance.7 These studies are important for identifying associations between sleep problems and functional impairment in adolescents with ADHD but cannot determine whether sleep is causally linked to impairment in adolescents diagnosed with ADHD.
The need for experimental research examining the impact of sleep in youth with ADHD has recently been identified as a research priority8. Only one, small-sample (n=11) study9 has manipulated sleep in children with ADHD to examine causal associations between sleep and ADHD behavior. Gruber et al. found that sleep restriction worsened attentional functioning compared to typical sleep in children with ADHD.9 A comparable study has not been performed in adolescents with ADHD nor has research been conducted examining the impact of sleep extension on functioning. Adolescents are especially prone to shortened sleep, due to a range of biological (e.g., circadian clock shifts later associated with the progression of puberty) and environmental (e.g., school start time, nighttime technology use) factors.10–12 There is also some indication that ADHD is associated with more eveningness/later chronotype,13 which may exacerbate phase delay in adolescents with ADHD. It is thus essential to document the detrimental effects of inadequate sleep as well as the beneficial effects of longer sleep if sleep is to be identified as a possible target for intervention.3,14The current study is the first to use an experimental protocol to determine whether shortened sleep is a causal contributor to daytime behaviors in adolescents with ADHD.
Studies with typically developing adolescents have found shortened sleep to be a causal contributor to increased daytime sleepiness, inattention, and oppositional defiant disorder (ODD) symptoms.15,16 In contrast, these studies have not found associations between shortened sleep and hyperactive-impulsive symptoms.15,16 Rather, because sleep restriction appears to contribute to greater hypoactivity rather than hyperactivity, Fallone and colleagues suggested over a decade ago that “researchers investigating sleep and ADHD include symptoms of sluggish cognitive tempo”.17,p. 1565 Sluggish cognitive tempo (SCT) is a set of attentional symptoms characterized by daydreaming, mental confusion, and slowed behavior/thinking that are strongly related to both ADHD inattention and daytime sleepiness.18 A recent study19 with typically developing adolescents found restricted sleep was causally related to increased SCT symptoms.
The current study used an experimental sleep restriction/extension protocol to examine shortened sleep duration as a causal contributor to poorer daytime functioning in adolescents with ADHD. Based on previous research,8,15,16,19 we hypothesized greater daytime sleepiness, inattention, SCT, and oppositional behaviors during a sleep restriction condition compared to a sleep extension condition but did not expect sleep to impact hyperactivity-impulsivity. As in previous research,15 and given limitations surrounding adolescent self-report of ADHD symptoms,20 we expected effects to be more robust when examining parent-reported as compared to adolescent-reported behavior.
Method
Participants
Participants were 72 adolescents (71% male) ages 14–17 years (M±SD=15.10±1.06) diagnosed with ADHD. All participants had an IQ≥70 (Range=79–132) based on the Kaufman Brief Intelligence Scale, Second Edition.21 Sample characteristics, including comorbid diagnoses based on the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS)22 interview conducted separately with the adolescent and parent, are provided in Table 1.
Table 1.
Sample Characteristics
| Full Sample (N = 72) |
Adherent Participants (n = 48)y |
|
|---|---|---|
| M ± SD | M ± SD | |
| Age | 15.10 ± 1.06 | 15.21 ± 1.15 |
| IQ | 102.78 ± 11.67 | 102.90 ± 11.64 |
|
N(%) |
N(%) |
|
| Sex | ||
| Male | 51 (70.8%) | 36 (75.0%) |
| Female | 21 (29.2%) | 12 (25.0%) |
| Race/Ethnicity | ||
| White | 58 (80.6%) | 37 (77.1%) |
| Black | 6 (8.3%) | 5 (10.4%) |
| Hispanic | 1 (1.4%) | 1 (2.1%) |
| Multiracial | 7 (9.7%) | 5 (10.4%) |
| Baseline Medication Statusa | ||
| Stimulant | 53 (73.6%) | 35 (72.9%) |
| Nonstimulant | 5 (6.9%) | 4 (8.3%) |
| Melatonin | 1 (1.4%) | 1 (2.1%) |
| Any medication | 56 (77.8%) | 37 (77.1%) |
| Family Incomeb | ||
| Up to $40,000 | 9 (12.5%) | 4 (8.3%) |
| $40,001 – $60,000 | 10 (14.1%) | 8 (17.0%) |
| $60,001 – $80,000 | 7 (9.9%) | 4 (8.5%) |
| Over $80,000 | 45 (63.4%) | 31 (66.0%) |
| ADHD Presentationc | ||
| Combined | 15 (20.8%) | 11 (22.9%) |
| Inattentive | 57 (79.2%) | 37 (77.1%) |
| Comorbid Diagnosesc | ||
| Depression/Dysthymia | 1 (1.4%) | 1 (2.1%) |
| GAD | 6 (8.3%) | 4 (8.3%) |
| PTSD | 1 (1.4%) | 0 (0%) |
| Mania | 0 (0%) | 0 (0%) |
| ODD | 4 (5.6%) | 2 (4.2%) |
| CD | 0 (0%) | 0 (0%) |
| Any Comorbidity | 10 (13.9%) | 6 (12.5%) |
Note: ADHD = attention-deficit/hyperactivity disorder; CD = conduct disorder; GAD = generalized anxiety disorder; ODD = oppositional defiant disorder; PTSD = posttraumatic stress disorder.
All participants were taken off any medication prior to starting the three-week sleep protocol.
One parent declined to answer the family income question.
ADHD and comorbid diagnoses established using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) conducted separately with the parent and adolescent (using an “or” rule), with ADHD diagnosis and presentation based on interview with the adolescent’s parent.
Full Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for ADHD Predominantly Inattentive or Combined Presentation on the K-SADS parent interview was required for eligibility. Exclusion criteria included autism, bipolar disorder, obsessive-compulsive disorder, or psychosis; meeting screening criteria for the possible presence of sleep-disordered breathing or restless leg syndrome using the Pediatric Sleep Questionnaire (PSQ);23,24 history of epilepsy or head trauma resulting in loss of consciousness; IQ<70; regular high caffeine use (>1 coffee/energy drink/day or 3 caffeinated soft drinks/day); highly atypical sleep duration (routinely obtaining <6 hours of >9.5 hours on school nights); or obligations that required a bedtime later than 10:00PM or waking prior to 6:00AM. Participants taking stimulant medication were allowed if the family was willing to discontinue the medication for the three-week sleep protocol during the summer. In the first year, all participants taking melatonin or a non-stimulant psychiatric medication were excluded; in the second year, these were not exclusionary but only allowable if discontinued during the summer.
Procedures
All study procedures were approved by the Institutional Review Board (IRB). Signed informed consent and assent were obtained. Recruitment materials were distributed via local schools, in the community, and at Cincinnati Children’s Hospital Medical Center where the study was conducted during the summers of 2016 and 2017. Materials described a study examining sleep in adolescents with ADHD but did not specifically mention (nor target) adolescents with sleep problems.
Sleep protocol.
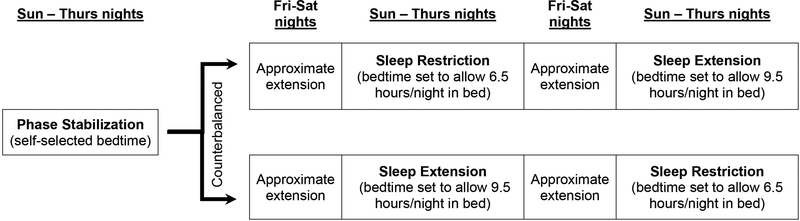
The sleep manipulation protocol used here has been used in other studies of typically developing adolescents.15,16,25 As summarized in Figure 1, study participants were involved in a three-week sleep manipulation protocol administered during the summer break from school to avoid impacting scholastic performance. All sleep occurred in the home environment and was monitored via sleep diaries and actigraphy. After a stabilization week whereby participants were asked to wake at a time that would allow them to arrive at the research location by 8:00am (see Supplement 1, available online, for additional details), participants were asked to systematically change their bedtimes to accommodate Sleep Extension (SE) and Sleep Restriction (SR) conditions. A within-subjects, crossover design was used, such that all adolescents participated in both the SR and SE conditions, with the order of conditions randomly counterbalanced across participants. During the SE condition, adolescents adjusted their bedtime to obtain 9 hours of nightly sleep (9.5 hours in bed, leaving up to ½ hour to fall asleep). A 9-hour window was selected because (a) this is how long adolescents sleep during controlled trials of sleep satiation26 and naturally on non-school nights,27 (b) 9 hours results in a well-rested state in adolescents,15 and (c) this matches clinical recommendations for adolescents.28 During the SR condition, adolescents adjusted their bedtime to allow 6.5 hours in bed, which in previous studies using this protocol resulted in an average of 6.1–6.3 hours of nightly sleep.15 This SR condition reflects a realistic dose of sleep restriction (similar to school-night sleep of 15–20% of healthy adolescents)29 that is feasible and induces daytime sleepiness, inattention, and oppositionality in typically developing adolescents.15,16 See Supplement 1, available online, for details regarding weekend sleep and caffeine use/napping during the protocol. Primary data collection occurred throughout the week (via actigraphy and daily diaries) and at a laboratory visit each Friday at the end of each condition; families were able to schedule their visit at any time during the day but were required to remain consistent across the three visits.
Figure 1:
Sleep Restriction/Extension Protocol Using a Within-Person Counterbalanced Design
Measures
Actigraphy.
Participants wore a wrist-mounted actigraph (Micro Motionlogger©, Ambulatory Monitoring, Inc.) throughout the sleep protocol to gather an objective measure of sleep. At each Friday assessment, actigraph data were downloaded and both the actigraphy data and sleep diaries (described next) were reviewed with the adolescent and their parent. In tandem with visually inspecting the sleep diaries, a validated algorithm30 was used to obtain estimates of sleep onset, sleep offset, time in bed, sleep duration, sleep efficiency, and wake after sleep onset (WASO), which were then averaged into composite sleep variables for each week.
Daily diaries.
Both adolescents and parents completed a daily diary. The adolescent diary included bedtime, sleep latency (time it took to fall asleep), rise time, napping, and caffeine consumption. Sleep onset (bedtime + latency), sleep latency, rise time, and total nocturnal sleep minutes (onset to offset) were averaged for each participant for each week. In addition, both the adolescent and parent diaries included an item assessing the adolescent’s difficulty to rise/wake up in the morning (1=very easy, 2=easy, 3=neutral, 4=difficult, 5=very difficult) and three items assessing daytime sleepiness (“felt sleepy or tired,” “had trouble staying awake,” and “yawned or put head down during day”) rated on a four-point scale (0=not at all, 1=just a little, 2=pretty much, 3=very much). Diary items were averaged into composite variables for each week (adolescent diary α=.78 and .89 across days for SE and SR, respectively; parent diary α=.89 and .96).
Daily attention and behavior.
The parent diary also included the 10-item IOWA Conners Rating Scale31 that assesses inattention (2 items), hyperactivity-impulsivity (3 items), and oppositionality (5 items) on a four-point scale (0=not at all, 3=very much). Daily items were averaged into composite variables for each week (inattention α=.96 and .93 for SE and SR, respectively; hyperactivity-impulsivity α=.87 and .86; oppositionality α=.94 and .91).
Daytime sleepiness.
At each laboratory visit, adolescents completed the 8-item Pediatric Daytime Sleepiness Scale (PDSS).32 Two items assessing sleepiness/drowsiness during the school year (i.e., during class and homework completion) were not used, resulting in six items rated on a five-point scale (0=never, 4=always). Mean scale scores were calculated (α=.65 and .70 for SE and SR, respectively).
ADHD and ODD symptoms.
At each laboratory visit, parents completed the Vanderbilt ADHD Diagnostic Parent Rating Scale (VADPRS)33 that includes the nine inattentive (ADHD-IN), nine hyperactive-impulsive (ADHD-HI) symptoms, and eight ODD symptoms of the DSM-5. Each item is rated on a four-point scale (0=never, 3=very often). Mean scale scores were calculated (ADHD-IN α=.95 and .93 for SE and SR, respectively; ADHD-HI α=.94 and .91; ODD α=.91 and .90).
Adolescent self-report of ADHD symptoms was assessed at each laboratory visit using the short version of the Conners, 3rd Edition (Conners-3),34 which includes six ADHD-IN items and five ADHD-HI items rated on a four-point scale (0=not true at all, 3=very much true). Mean scale scores were calculated (ADHD-IN α=.89 and .89 for SE and SR, respectively; ADHD-HI α=.80 and .82).
SCT symptoms.
The Child and Adolescent Behavior Inventory (CABI)35 and the Child Concentration Inventory, Second Edition (CCI-2)36 were used at each laboratory visit to assess parent- and adolescent-reported SCT symptoms, respectively. The same 15 items (e.g., daydreams, gets lost in own thoughts, slow behavior) are included on the CABI SCT module (0=almost never, 5=almost always) and CCI-2 (0=never, 3=always), are internally consistent, and demonstrate discriminant validity from ADHD symptoms.35,36 Mean scale scores were calculated (CABI α=.95 and .95 for SE and SR, respectively; CCI-2 α=.91 and .95).
Continuous Performance Test (CPT).
The A-X CPT37,38 consists of 400 letters which appear individually for 200ms, with a 1.5 second interstimulus interval and 10% target frequency. Participants were instructed to press the spacebar when they saw an “A” followed by an “X”. Accuracy, mean reaction time, number of omission errors (an index of inattention), and number of commission errors (an index of impulsivity) were calculated.
Analyses
Given the aim of this study to test whether sleep restriction and extension impacted daytime functioning in adolescents with ADHD, only adolescents adherent to the sleep protocol (defined a priori as obtaining ≥1 hour sleep duration during SE compared to SR) were included in analyses. We first confirmed that the sleep manipulation impacted sleep as designed. Next, our primary analyses examined the extent to which SR was associated with daytime sleepiness, attention, and behavior. A paired samples t-test for each outcome variable was conducted comparing the SR and SE conditions, with an alpha threshold of 0.05. Cohen’s d, corrected for dependence in within-subjects data, was computed as a measure of effect size. McNemar tests were used to examine whether participants were more likely to nap or consume caffeine during SR compared to SE.
Results
Participant Recruitment, Retention, and Adherence
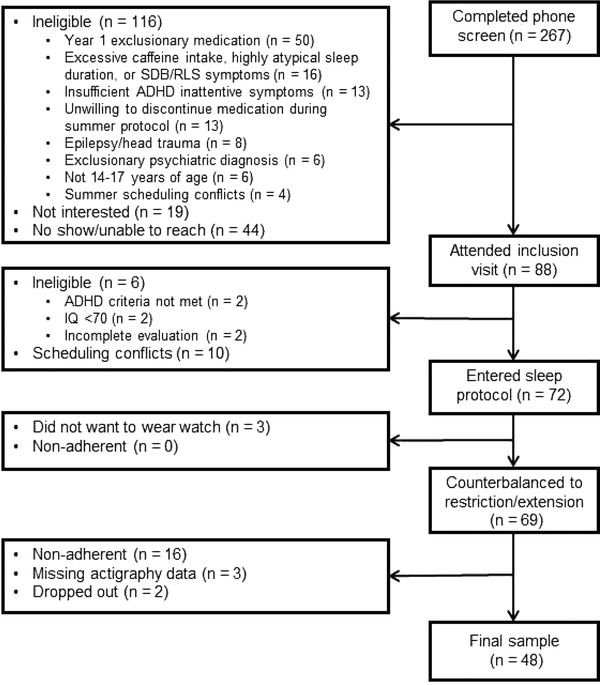
Figure 2 provides a flow diagram of study recruitment and retention Sixty-four participants completed the sleep protocol and had complete actigraph data to determine adherence. As shown in Figure 2 and detailed in Figure S1, 48 of these 64 (75%) were adherent to the sleep protocol and were included in primary analyses. Additional details regarding adherence can be found in the Supplemental Materials, available online, including analyses indicating no differences between adherent and non-adherent participants in demographic characteristics, symptom severity/comorbidity, and sleep/sleepiness (Table S1, available online) and strategies used by participants to foster adherence (Table S2, available online).
Figure 2:
Flow Diagram of Participant Recruitment and Completion of Sleep Protocol
Note: ADHD = attention-deficit/hyperactivity disorder; RLS = restless leg syndrome; SDB = sleep-disordered breathing.
Confirmation that the Sleep Protocol Impacted Sleep
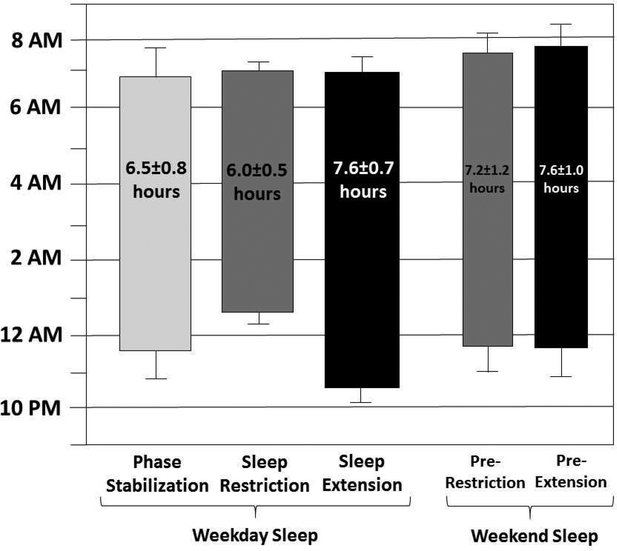
Actigraphy-measured sleep onset, offset, and duration throughout the sleep protocol for the 48 adherent participants is depicted in Figure 3. Participants averaged 1.6 hours more sleep per night during SE than SR. Table S3, available online, details effects of the sleep manipulation on actigraphy- and diary-measured sleep. Very large effects were found for both sleep duration and sleep onset time per actigraphy (ds=2.76 and 4.08) and daily diary (ds=3.76 and 4.10). Participants experienced greater actigraphy-measured sleep efficiency (d=0.63) and less actigraphy-measured waking after sleep onset (d=1.00), as well as shorter diary-reported sleep onset latency (d=0.78) and greater diary-reported difficulty waking (ds=0.75 and 0.91 for adolescent and parent diaries, respectively) during SR than SE.
Figure 3.
title: Sleep Patterns Based on Actigraphy for Each Phase of the Sleep Protocol
Note: Mean sleep onset is represented by the bottom of each bar and mean wake time by the top of each bar (error bars representing the standard deviation of each), with average sleep duration printed within each bar.
Impact on Daytime Sleepiness, Attention, and Behavior
Table 2 summarizes findings for the outcome variables (Table S4, available online, provides intercorrelations among the outcome variables during SR and SE). Greater daytime sleepiness was reported during SR compared to SE, with large effect sizes across parent- and adolescent-completed daily ratings of daytime sleepiness (ds=1.51 and 1.00, respectively) and the adolescent-completed PDSS (d=1.05). Participants were significantly more likely (p=.003) to report napping during SR (n=14) compared to SE (n=3). There was no difference (p=1.00) in report of caffeine consumption during SR (n=14) or SE (n=13).
Table 2.
Differences in Daytime Sleepiness, Attention, and Behavior Ratings and Continuous Performance Test (CPT) Performance During Sleep Restriction (SR) and Sleep Extension (SE)
| SR | SE | Paired samples t-tests comparing SR and SE | |||
|---|---|---|---|---|---|
| M ± SD | M ± SD | t | p | d | |
| Daytime sleepiness | |||||
| Sleep diary (parent)a | 1.24 ± 0.75 | 0.33 ± 0.36 | 9.19 | <.001 | 1.51 |
| Sleep diary (adolescent)a | 0.93 ± 0.68 | 0.41 ± 0.47 | 6.49 | <.001 | 1.00 |
| PDSS (adolescent) | 1.85 ± 0.78 | 1.08 ± 0.72 | 7.25 | <.001 | 1.05 |
| Attention | |||||
| IOWA-10 IN (parent)a | 1.42 ± 0.81 | 1.13 ± 0.80 | 3.66 | .001 | 0.53 |
| VADPRS ADHD-IN (parent) | 1.57 ± 0.72 | 1.25 ± 0.77 | 4.10 | <.001 | 0.60 |
| Conners-3 ADHD-IN (adolescent) | 7.48 ± 4.22 | 7.38 ± 4.67 | 0.25 | .802 | 0.04 |
| SCT (parent) | 1.50 ± 0.95 | 0.73 ± 0.74 | 6.75 | <.001 | 1.00 |
| SCT (adolescent) | 0.83 ± 0.63 | 0.64 ± 0.49 | 2.90 | .006 | 0.44 |
| Behavior | |||||
| IOWA-10 HI (parent)a | 0.66 ± 0.70 | 0.69 ± 0.69 | 0.57 | .569 | 0.08 |
| VADPRS ADHD-HI (parent) | 0.58 ± 0.63 | 0.58 ± 0.70 | 0.05 | .963 | 0.01 |
| IOWA-10 ODD (parent)a | 0.58 ± 0.56 | 0.38 ± 0.54 | 2.99 | .004 | 0.43 |
| VADPRS ODD (parent) | 0.65 ± 0.59 | 0.49 ± 0.58 | 2.24 | .030 | 0.33 |
| Conners-3 ADHD-HI (adolescent) | 4.44 ± 3.40 | 5.13 ± 3.55 | 2.82 | .007 | 0.41 |
| CPT | |||||
| Accuracy (%) | 98.68 ± 2.22 | 98.77 ± 2.05 | 1.05 | .300 | 0.17 |
| Mean reaction time (ms) | 576.93 ± 118.20 | 567.04 ± 139.06 | 0.96 | .344 | 0.15 |
| Errors of omission | 3.35 ± 5.91 | 3.30 ± 5.94 | 0.26 | .793 | 0.04 |
| Errors of commission | 1.94 ± 4.47 | 1.61 ± 4.48 | 1.80 | .079 | 0.27 |
Note: N = 48. ADHD = attention-deficit/hyperactivity disorder; CPT = continuous performance test; HI = hyperactivity-impulsivity; IN = inattention. ODD = oppositional defiant disorder; PDSS = Pediatric Daytime Sleepiness Scale; SCT = sluggish cognitive tempo; VADPRS = Vanderbilt ADHD Diagnostic Parent Rating Scale.
These measures were completed on a daily diary throughout SR and SE; all other measures completed at a laboratory visit following SR and SE.
Across both daily and weekly measures, parents reported significantly greater inattention and oppositional behaviors during SR compared to SE, with medium-sized effects for inattention (ds=0.53 and 0.60) and small effects for oppositionality (ds=0.43 to 0.33). Significant differences were not found for measures of parent-reported hyperactivity-impulsivity or for adolescent-reported inattention. However, adolescents reported significantly less hyperactivity-impulsivity during SR compared to SE (d=0.41). Both parents and adolescents reported significantly greater SCT symptoms during SR compared to SE, with a small-to-medium effect for adolescent self-reported SCT (d=0.44) and a large effect for parent-reported SCT (d=1.00). No significant effects were found when comparing CPT performance indices during SR and SE.
Discussion
This study provides the first evidence that insufficient sleep causes impairments in self- and parent-reported daytime functioning in adolescents with ADHD. Restricted sleep worsens attentional functioning and increases oppositional behaviors and daytime sleepiness in adolescents with ADHD.
The impact of restricted sleep on attention was found for parent-reported inattentive symptoms and both parent- and adolescent-reported SCT symptoms. This is robust evidence for restricted sleep worsening attentional functioning in adolescents with ADHD, though it should be noted that effects were not found for adolescent-reported inattention. There is some evidence from the broader sleep restriction literature that individuals themselves may be less aware of worsened cognitive functioning associated with restricted sleep.39 However, in typically developing adolescents, sleep restriction worsened ADHD inattentive symptoms across both parent and adolescent ratings, though effects were larger for parent ratings.15 Adolescents with ADHD may not be able to accurately report on their own ADHD symptomatology,20 and so this may have contributed to our lack of findings for adolescent-reported inattention more so than a lack of awareness of worsened cognitive functioning. Further, in contrast to ADHD inattention, there is emerging evidence that youth can provide valid self-reports of their own SCT symptoms36,40 and we found sleep restriction to worsen SCT across both parent and adolescent ratings. These multi-informant effects are important since it is estimated that 25–40% of youth with ADHD also have elevated SCT symptoms41,42 and SCT symptoms are themselves linked to a range of functional impairments such as social withdrawal and internalizing psychopathology.18
Consistent with other studies,8,15,16 parents did not report greater hyperactivity-impulsivity during sleep restriction compared to sleep extension. Rather, adolescents in our study reported less hyperactivity-impulsivity during restriction compared to extension. These findings are consistent with the hypothesis that sleep restriction induces hypoactivity rather than hyperactivity.8,17 Shortened sleep duration and sleep deprivation contribute to allostatic load throughout the human body,43 leading to a host of metabolic, hormonal, and neural responses including increased secretion of proinflammatory cytokines such as interleukin-1 (IL-6) and tumor necrosis factor-α (TNFα).44,45 These cytokines are associated with daytime sleepiness and fatigue,45–47 as well as somatic symptoms of depression such as loss of energy and psychomotor retardation.48 It would valuable for future research to directly measure cytokines and other indices of hypoactivity to further test this mechanism in adolescents following acute sleep restriction.
We did not find effects on our laboratory-based measure of attention. The literature has produced mixed findings for neurocognitive tasks in studies examining acute sleep restriction (as opposed to chronic sleep loss or sleep deprivation),49,50 and the impact of sleep restriction on neurocognition is larger in adults than in children/adolescents.51 Both of these factors may have contributed to the lack of CPT findings in our study. In addition, it would be beneficial for future studies to include other measures of neurocognition, including tasks and indicators that have shown to be sensitive at detecting ADHD-related cognitive deficits (e.g., reaction time variability).
A previous observational study found sleep problems longitudinally predicted increases in ODD symptoms over one year in young adolescents with ADHD.6 Our findings provide support for a causal link in this association. Establishing this link is theoretically and clinically important since ODD symptoms commonly co-occur with ADHD, contribute to functional impairment, and may place youth with ADHD on a trajectory for more severe conduct and substance use problems in adolescence.52 Insufficient sleep may contribute to, or exacerbate, oppositional-defiant behaviors in adolescents with ADHD and be important to include in theoretical models of ADHD comorbidity and treatments targeting externalizing behavior problems.
Also, consistent with previous research with typically developing adolescents15 and hypothesized based on physiological homeostatic mechanisms that maximize the quantity and quality of sleep in conditions of insufficient sleep,53,54 adolescents had shorter sleep onset latency, less WASO, and greater sleep efficiency during the week of sleep restriction compared to sleep extension. Both parent and adolescents also reported that it was more difficult for the adolescent to wake in the morning during restriction than extension. We also found that restricted sleep increased daytime sleepiness in adolescents with ADHD with effects were consistent across daily and end-of week measures and across adolescent and parent reports. Children and adolescents with ADHD experience more daytime sleepiness than their peers,5,55 and daytime sleepiness is specifically associated with poorer academic performance in adolescents with ADHD.7,56 Our findings indicate that shortened sleep duration is likely one mechanism contributing to elevated sleepiness in adolescents with ADHD.
Although the protocol impacted sleep as expected, the average sleep duration difference per night between the extension and restriction conditions was 1.6 hours. In contrast, studies using the same protocol with typically developing adolescents found participants to obtain an average of 2.5 hours of sleep per night during the extension condition compared to the restriction condition.15,16 In considering possibilities that may explain the larger sleep duration effect in studies of typically developing adolescents compared to our sample of adolescents with ADHD, it is important to observe that adolescents with ADHD successfully obtained the instructed sleep duration during sleep restriction but fell short of the instructed duration during sleep extension (see Figure 3). Further, as designed, the longer sleep duration during extension compared to restriction was due almost entirely to a shift the adolescents’ bedtime while maintaining a constant wake time. Adolescents with ADHD are more likely than their peers to have a delayed SOL,1,55 time-management and organization difficulties,57 conflict with parents,58 and reduced motivation,59 as well as a potentially stronger preference for eveningness.13 Any or all of these factors may have contributed to adolescents with ADHD being less successful than typically developing adolescents at going to bed earlier and/or falling asleep during the extension condition specifically. It is possible that the 1.6-hour difference in sleep duration was sufficient for producing strong effects on attention and behavior but was insufficient for producing effects on our neurocognitive outcome (i.e., CPT).
Our findings have potentially important implications for the treatment of adolescents with ADHD. If shortened sleep duration worsens daytime functioning, then the flip side of the coin is that extended sleep duration improves daytime functioning. Sleep should be assessed when treating adolescents with ADHD, and it may be important to directly target sleep in ADHD interventions. Cognitive-behavioral sleep interventions have been shown to be effective in multiple non-ADHD populations. A recent meta-analysis determined that these interventions are feasible with adolescents and result in improved sleep (e.g., overall sleep quality, total sleep time, sleep onset, sleep efficiency) and functional outcomes (e.g., daytime sleepiness, internalizing symptoms).60 However, none have been tested in adolescents with ADHD specifically, making this an important direction for future study. If a cognitive-behavioral sleep intervention can demonstrate effects similar to what we found in this brief experimental protocol, which were generally medium to large and similar if not larger in magnitude to more intensive behavioral interventions,61–63 sleep treatment would have an important place in the armamentarium of behavioral interventions for adolescents with ADHD. However, modifications to existing cognitive-behavioral sleep interventions may be needed to optimize effectiveness in adolescents with ADHD.3 Additionally, although not the focus of the current study, a public health approach to improving sleep in adolescents more broadly may also improve the daytime functioning of adolescents with ADHD. There is substantial interest in school start time for improving sleep and functioning in adolescents, with recent position statements calling for high schools to start no earlier than 8:30am.64,65 Although the effects of a later school start time are far from settled,66 the point here is that it is important to consider both individual (e.g., pharmacological and/or cognitive-behavioral treatment) and public health (e.g., school start time) approaches to improving the sleep and daytime functioning of adolescents with ADHD.
Several limitations are important to acknowledge. First, our significant findings were limited to clinical rating scales which lack objectivity and blinding to sleep conditions. Still, the fact that we found effects on hypothesized domains (e.g., inattention, oppositionality, sleepiness) and not on other hypothesized domains (e.g., hyperactivity-impulsivity) lends confidence that findings are not simply due to response bias. Second, we did not include a comparison sample of typically developing adolescents, and thus were unable to test whether the magnitude of effects differs for adolescents with and without ADHD; it appears effects may be smaller in our sample of adolescents with ADHD compared to typically developing samples.15,16 Our sample size was also modest. In addition, the level of ADHD severity in the sample was relatively mild and rates of comorbidity were relatively low. It is unknown whether our findings generalize to more diverse or more clinically impaired adolescents with ADHD. Finally, the restriction/extension protocol was conducted during the summer and was limited to five nights of each condition. Although five nights corresponds to the typical school week in U.S. schools, it would be informative for future studies to extend restriction beyond five nights to evaluate the cumulative impact of restricted sleep and to also examine the beneficial effects of extended sleep on daily life functioning during the school year among adolescents with ADHD. Administering a sleep protocol during the school year would also allow for collecting ratings from teachers, who are important informants of impairment and could be kept blind to the sleep conditions.
The current study makes a major contribution to the study of sleep and ADHD. This is the first study to demonstrate that shortened sleep duration is a causal contributor to sleepiness, inattention, SCT, and oppositionality in youth with ADHD. Conversely, extending sleep improves attentional and behavioral functioning in adolescents with ADHD, suggesting that sleep may be an important target for intervention in this population. The current study is a critical first step in showing sleep to be an important component in reducing impairment and improving the lives of adolescents with ADHD.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Mental Health (NIMH; grant R03MH109787; Dr. Becker). Dr. Becker is supported by grant K23MH108603 from the NIMH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health (NIH).
Disclosure: Dr. Becker has received research support from the Institute of Education Science (IES), the Cincinnati Children’s Research Foundation (CCRF), and the Secretariat of State for Research, Development and Innovation, Ministry of Economy, Industry and Competitiveness (Spanish Government). Dr. Epstein has received research support from the National Institutes of Health (NIH), the Agency for Healthcare Research and Quality (AHRQ), the IES, and Akili Interactive Labs. He has received royalties from Multi-Health Systems, Inc., received consulting fees from the American Academy of Pediatrics and American Board of Pediatrics, and received licensing fees from Optimal Medicine, Inc. and IXICO. Dr. Tamm has received research support from the NIH, the IES, the AHRQ, the Brady Foundation, and the CCRF. Dr. Beebe has received research support from the NIH, the IES, the American Diabetes Association, Canadian Institutes of Public Health, University of Otega, and CCRF. Ms. Tilford, Ms. Tischner, Mr. Isaacson, and Mr. Simon report no biomedical financial interests or potential conflicts of interests.
Footnotes
Clinical trial registration information: Cognitive and Behavioral Effects of Sleep Restriction in Adolescents With ADHD. https://clinicaltrials.gov/; NCT02732756
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stephen P. Becker, Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, OH, and the University of Cincinnati College of Medicine, Cincinnati, OH..
Jeffery N. Epstein, Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, OH, and the University of Cincinnati College of Medicine, Cincinnati, OH..
Leanne Tamm, Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, OH, and the University of Cincinnati College of Medicine, Cincinnati, OH..
Alina A. Tilford, Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, OH..
Clair M. Tischner, Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, OH..
Paul A. Isaacson, Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, OH..
John O. Simon, Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, OH..
Dean W. Beebe, Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, OH, and the University of Cincinnati College of Medicine, Cincinnati, OH..
References
- 1.Hysing M, Lundervold AJ, Posserud MB, Sivertsen B. Association between sleep problems and symptoms of attention deficit hyperactivity disorder in adolescence: Results from a large population-based study. Behavioral sleep medicine. 2016;14(5):550–564. [DOI] [PubMed] [Google Scholar]
- 2.Lunsford-Avery JR, Krystal AD, Kollins SH. Sleep disturbances in adolescents with ADHD: A systematic review and framework for future research. Clinical psychology review. 2016;50:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker SP. The triple threat of sleep, adolescence, and ADHD In: Hiscock H, Sciberras E, eds. Sleep and ADHD. Amsterdam: Elsevier; in press. [Google Scholar]
- 4.Sung V, Hiscock H, Sciberras E, Efron D. Sleep problems in children with attention-deficit/hyperactivity disorder: prevalence and the effect on the child and family. Archives of pediatrics and adolescent medicine. 2008;162(4):336–342. [DOI] [PubMed] [Google Scholar]
- 5.Langberg JM, Molitor SJ, Oddo LE, Eadeh HM, Dvorsky MR, Becker SP. Prevalence, patterns, and predictors of sleep problems and daytime sleepiness in young adolescents with ADHD. Journal of attention disorders. 2017:1087054717690810. [DOI] [PubMed] [Google Scholar]
- 6.Becker S, Langberg J, Evans S. Sleep problems predict comorbid externalizing behaviors and depression in young adolescents with attention-deficit/hyperactivity disorder. European child and adolescent psychiatry. 2015;24(8):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langberg JM, Dvorsky MR, Marshall S, Evans SW. Clinical implications of daytime sleepiness for the academic performance of middle school-aged adolescents with attention deficit hyperactivity disorder. Journal of sleep research. 2013;22(5):542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundahl A, Kidwell KM, Van Dyk TR, Nelson TD. A meta-analysis of the effect of experimental sleep restriction on youth’s attention and hyperactivity. Dev Neuropsychol. 2015;40(3):104–121. [DOI] [PubMed] [Google Scholar]
- 9.Gruber R, Wiebe S, Montecalvo L, Brunetti B, Amsel R, Carrier J. Impact of sleep restriction on neurobehavioral functioning of children with attention deficit hyperactivity disorder. Sleep. 2011;34(3):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker SP, Langberg JM, Byars KC. Advancing a biopsychosocial and contextual model of sleep in adolescence: a review and introduction to the special issue. Journal of youth and adolescence. 2015;44(2):239–270. [DOI] [PubMed] [Google Scholar]
- 11.National Sleep Foundation. 2006 Sleep in America Poll. Washington, D.C.: National Sleep Foundation,;2006. [Google Scholar]
- 12.Crowley SJ, Wolfson AR, Tarokh L, Carskadon MA. An update on adolescent sleep: New evidence informing the perfect storm model. Journal of adolescence. 2018;67:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coogan AN, McGowan NM. A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. Attention deficit and hyperactivity disorders. 2017;07:07. [DOI] [PubMed] [Google Scholar]
- 14.Cortese S, Brown TE, Corkum P, et al. Assessment and management of sleep problems in youths with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(8):784–796. [DOI] [PubMed] [Google Scholar]
- 15.Beebe DW, Fallone G, Godiwala N, et al. Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. Journal of child psychology and psychiatry, and allied disciplines. 2008;49(9):915–923. [DOI] [PubMed] [Google Scholar]
- 16.Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. Sleep restriction worsens mood and emotion regulation in adolescents. Journal of child psychology and psychiatry, and allied disciplines. 2014;55(2):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fallone G, Acebo C, Seifer R, Carskadon MA. Experimental restriction of sleep opportunity in children: effects on teacher ratings. Sleep. 2005;28(12):1561–1567. [DOI] [PubMed] [Google Scholar]
- 18.Becker SP, Leopold DR, Burns GL, et al. The internal, external, and diagnostic validity of sluggish cognitive tempo: A meta-analysis and critical review. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55(3):163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner AA, Hansen A, Baxley C, Becker SP, Sidol CA, Beebe DW. Effect of sleep extension on sluggish cognitive tempo symptoms and driving behavior in adolescents with chronic short sleep. Sleep medicine. 2017;30:93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibley MH, Pelham WE Jr., Molina BS, et al. Diagnosing ADHD in adolescence. Journal of consulting and clinical psychology. 2012;80(1):139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, Second Edition (KBIT-2). Pearson; 2004. [Google Scholar]
- 22.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 23.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Archives of otolaryngology--head and neck surgery. 2007;133(3):216–222. [DOI] [PubMed] [Google Scholar]
- 24.Chervin RD, Hedger KM. Clinical prediction of periodic leg movements during sleep in children. Sleep medicine. 2001;2(6):501–510. [DOI] [PubMed] [Google Scholar]
- 25.Beebe DW, Simon S, Summer S, Hemmer S, Strotman D, Dolan LM. Dietary intake following experimentally restricted sleep in adolescents. Sleep. 2013;36(6):827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2(4):453–460. [DOI] [PubMed] [Google Scholar]
- 27.National Sleep Foundation. Summary of Findings: 2006 Sleep In America Poll. Washington, DC: National Sleep Foundation;2006. [Google Scholar]
- 28.Owens JA, Adolescent Sleep Working Group. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child development. 1998;69(4):875–887. [PubMed] [Google Scholar]
- 30.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. [DOI] [PubMed] [Google Scholar]
- 31.Waschbusch DA, Willoughby MT. Parent and teacher ratings on the IOWA Conners Rating Scale. Journal of psychopathology and behavioral assessment. 2008;30(3):180–192. [Google Scholar]
- 32.Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Pietro B. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep. 2003;26(4):455–458. [PubMed] [Google Scholar]
- 33.Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. Journal of pediatric psychology. 2003;28(8):559–567. [DOI] [PubMed] [Google Scholar]
- 34.Conners CK. Conners-3. North Tonawanda, NJ: Multi-Health Systems; 2008. [Google Scholar]
- 35.Sáez B, Servera M, Becker SP, Burns GL. Optimal items for assessing sluggish cognitive tempo in children across mother, father, and teacher ratings. Journal of Clinical Child and Adolescent Psychology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sáez B, Servera M, Burns GL, Becker SP. Advancing the multi-informant assessment of sluggish cognitive tempo: Child self-report in relation to parent and teacher ratings of SCT and impairment. Journal of abnormal child psychology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halperin JM, Wolf L, Greenblatt ER, Young G. Subtype Analysis of Commission Errors on the Continuous Performance-Test in Children. Dev Neuropsychol. 1991;7(2):207–217. [Google Scholar]
- 38.Halperin JM, Wolf LE, Pascualvaca DM, et al. Differential assessment of attention and impulsivity in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27(3):326–329. [DOI] [PubMed] [Google Scholar]
- 39.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. [DOI] [PubMed] [Google Scholar]
- 40.Becker SP, Luebbe AM, Joyce AM. The Child Concentration Inventory (CCI): Initial validation of a child self-report measure of sluggish cognitive tempo. Psychological assessment. 2015;27(3):1037–1052. [DOI] [PubMed] [Google Scholar]
- 41.Barkley RA. Distinguishing sluggish cognitive tempo from ADHD in children and adolescents: Executive functioning, impairment, and comorbidity. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2013;42(2):161–173. [DOI] [PubMed] [Google Scholar]
- 42.Servera M, Sáez B, Burns GL, Becker SP. Clinical differentiation of sluggish cognitive tempo and attention-deficit/hyperactivity disorder in children. Journal of abnormal psychology. in press. doi: 10.1037/abn0000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55(10 Suppl 2):S20–23. [DOI] [PubMed] [Google Scholar]
- 44.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. The Journal of clinical endocrinology and metabolism. 2004;89(5):2119–2126. [DOI] [PubMed] [Google Scholar]
- 45.Opp MR. Cytokines and sleep. Sleep medicine reviews. 2005;9(5):355–364. [DOI] [PubMed] [Google Scholar]
- 46.Norheim KB, Jonsson G, Omdal R. Biological mechanisms of chronic fatigue. Rheumatology (Oxford, England). 2011;50(6):1009–1018. [DOI] [PubMed] [Google Scholar]
- 47.Harrington ME. Neurobiological studies of fatigue. Prog Neurobiol. 2012;99(2):93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharpley CF, Bitsika V. Differences in neurobiological pathways of four “clinical content” subtypes of depression. Behavioural brain research. 2013;256:368–376. [DOI] [PubMed] [Google Scholar]
- 49.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Paper presented at: Seminars in Neurology 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo JC, Ong JL, Leong RL, Gooley JJ, Chee MW. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: The need for sleep study. Sleep. 2016;39(3):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowe CJ, Safati A, Hall PA. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neuroscience and biobehavioral reviews. 2017;80:586–604. [DOI] [PubMed] [Google Scholar]
- 52.Molina BS, Pelham WE Jr. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of abnormal psychology. 2003;112(3):497–507. [DOI] [PubMed] [Google Scholar]
- 53.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child development. 2003;74(2):444–455. [DOI] [PubMed] [Google Scholar]
- 54.Jenni OG, Carskadon MA. Sleep behavior and sleep regulation from infancy through adolescence: Normative aspects. Sleep medicine clinics. 2007;2(3):321–329. [Google Scholar]
- 55.Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: Meta-analysis of subjective and objective studies. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(9):894–908. [DOI] [PubMed] [Google Scholar]
- 56.Langberg JM, Dvorsky MR, Becker SP, Molitor SJ. The impact of daytime sleepiness on the school performance of college students with attention deficit hyperactivity disorder (ADHD): a prospective longitudinal study. Journal of sleep research. 2014;23(3):318–325. [DOI] [PubMed] [Google Scholar]
- 57.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological psychiatry. 2005;57(11):1336–1346. [DOI] [PubMed] [Google Scholar]
- 58.Deault LC. A systematic review of parenting in relation to the development of comorbidities and functional impairments in children with attention-deficit/hyperactivity disorder (ADHD). Child psychiatry and human development. 2010;41(2):168–192. [DOI] [PubMed] [Google Scholar]
- 59.Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neuroscience and biobehavioral reviews. 2003;27(7):593604. [DOI] [PubMed] [Google Scholar]
- 60.Blake MJ, Sheeber LB, Youssef GJ, Raniti MB, Allen NB. Systematic review and meta-analysis of adolescent cognitive-behavioral sleep interventions. Clinical child and family psychology review. 2017;20(3):227–249. [DOI] [PubMed] [Google Scholar]
- 61.Daley D, van der Oord S, Ferrin M, et al. Behavioral interventions in attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials across multiple outcome domains. Journal of the American Academy of Child and Adolescent Psychiatry.2014;53(8):835–847, 847.e831–835. [DOI] [PubMed] [Google Scholar]
- 62.Sonuga-Barke EJ, Brandeis D, Cortese S, et al. Nonpharmacological interventions for ADHD: Systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. The American journal of psychiatry. 2013;170(3):275–289. [DOI] [PubMed] [Google Scholar]
- 63.Sibley MH, Kuriyan AB, Evans SW, Waxmonsky JG, Smith BH. Pharmacological and psychosocial treatments for adolescents with ADHD: an updated systematic review of the literature. Clinical psychology review. 2014;34(3):218–232. [DOI] [PubMed] [Google Scholar]
- 64.American Academy of Pediatrics. School start times for adolescents. Pediatrics. 2014;134(3):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson NF, Martin JL, Wise MS, et al. Delaying middle school and high school start times promotes student health and performance: An American Academy of Sleep Medicine Position Statement. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2017;13(4):623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marx R, Tanner-Smith EE, Davison CM, et al. Later school start times for supporting the education, health, and well-being of high school students. The Cochrane database of systematic reviews. 2017;7:CD009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.