Abstract
Objectives:
To evaluate the pharmacokinetics (PK) of levonorgestrel-containing combined oral contraceptives (COCs) in obese women.
Study design:
We pooled and reanalyzed data from 89 women with different BMI categories from four clinical studies. The levonorgestrel (LNG) and ethinyl estradiol (EE) PK were analyzed utilizing a zero-order absorption (K0), two-compartment PK model to evaluate key PK parameters in relation to a range of weights, body mass index (BMI), and body surface area (BSA).
Results:
Increasing of body habitus metrics are correlated with decreasing Cmax (p<0.0001) and AUCτ (p<0.05) for both LNG and EE, but no correlation found for Cmin (p≥0.17). Increasing weight and BMI were associated with a modest increase (p≤0.056) of clearance (CL) and appreciable increases of central volume (V1, p<0.05), distribution clearance (CLd, p≤0.001), and peripheral volume (V2, p<0.0001) for LNG. For EE, increases in CL (p≤0.009) were found with greater weight, BMI and BSA. Values of V1, CLd, and V2 also increased (p<0.0001) in obese subjects. The half-life (T1/2) and steady-state volume (Vss) were greater among obese women (p<0.0001) for both LNG and EE. LNG and EE PK parameters correlated well (p≤0.006 for all), indicating that individual subject physiology affected both drugs similarly.
Conclusions:
The primary effects of obesity on LNG and EE were a modest increase in CL and a marked increase in distribution parameters. We observed no obesity-related differences in trough LNG and EE concentrations.
Implications:
This population PK analysis demonstrated reduced systemic exposure to LNG/EE oral contraceptives in obese subjects (Cmax and AUCτ); these particular differences are unlikely to lower contraceptive effectiveness among obese women who are correctly using LNG-containing contraceptives.
Keywords: Combined oral contraceptives, Obesity, Pharmacokinetics, Clearance, Volume of distribution
1. Introduction
Over one-third of American women 20 years and older are clinically obese, with a body mass index (BMI) greater than 30 kg/m2 [1]. Combined oral contraceptives (COCs) are one of the most widely used methods to prevent pregnancies [2, 3]. The impact of obesity on the oral contraceptive efficacy remains controversial. Obesity has been associated with altered COC efficacy in some studies [4–8], while not in others [9–11].
Drug efficacy is often linked to pharmacokinetic (PK) changes caused by genotypic and/or phenotypic differences, such as obesity, with effects at one or multiple points during absorption, distribution, metabolism and excretion. Unfortunately, PK studies of combined COCs have not brought clarity to whether and how obesity plays a role in COC efficacy. Westhoff et al. [12, 13] found lower levonorgestrel (LNG) and ethinyl estradiol (EE) concentrations in obese women compared to women with normal BMIs, but trough concentrations were similar. Edelman et al. [14, 15] also found lower LNG and EE concentrations in obese women. These small studies did not fully elaborate potentially available PK parameters related to absorption and distribution.
Given the opportunity to provide more comprehensive and definitive assessments, we examined serum concentration versus time data of LNG and EE from four PK studies performed by three independent investigators. Population modeling applying a two-compartment model allowed joint and more extensive assessments than performed in the individual studies. We estimated the PK parameters in relation to a range of weights, body mass index (BMI), and body surface area (BSA) metrics.
2. Materials and Methods
The principal investigators and their respective universities established data transfer agreements with Dr. WJ Jusko and the University of Buffalo, State University of New York (Buffalo, NY), and provided de-identified data for the pooled analysis. A brief description of the studies and their methods follows:
All study subjects received COC tablets in a 28-day regimen with day 21 being the last active tablet and days 22–28 inert tablets only. Westhoff et al. [13] administered LNG 150 mcg / EE 30 mcg (Portia, Barr Laboratories, Montvale, NJ) to healthy participants (18–35 years old) for two to three COC cycles prior to a study cycle. For the PK sub-study, 15 normal-BMI participants (BMI 19.0–24.9 kg/m2) and 15 obese participants (BMI 30.0–39.9 kg/m2) were admitted for 24 hours between cycle day 15–21. Blood samples were collected at 0,0.5,1.5,2,3,4,6,8,12,16 and 24 h. Participants ingested COCs immediately after the time zero (t0) blood draw. Two normal weight subjects were found to be non-compliant (i.e. not at steady-state) and thus data used for the modeling contains only 28 subjects.
Edelman et al. [14] administered LNG 100 mcg / EE 20 mcg (Alesse, Wyeth, Madison, WI). The modeling included PK data from 9 normal (BMI 19.0–24.9 kg/m2) and 10 obese subjects (BMI >30.0 kg/m2) participants who had undergone blood sample collection on day 21 of cycle 1 at 0,0.5,1,1.5,2,3,4,6,8, and 12 h, and day 22 at 0,4,8,12, and 24 h as well as day 28 (last day of hormone-free interval) at 0,4,8 and 12 h. Serum LNG concentration-time data used in the model were obtained on the last day of active pills (day 21), the first day of 7-day placebo tablets (day 22), and the end of the 7-day hormone-free interval (day 28). For the EE modeling, only the data from days 21 and 22 were used.
Edelman et al. [15] administered LNG 100 mcg / EE 20 mcg (Aviane, Teva, Petah Tikva, Israel). Thirty-two healthy obese (BMI>30 kg/m2, 18–35 years old) women contributed blood samples starting on day 21 (last active tablet) at 0,0.5,1,1.5,2,3,4,6,8 and 12 h, day 22 (first placebo tablet) at 0,4,8,12, and 24 h, and at a single time point on days 23, 25, and 27.
Natavio et al. [16] administered LNG 150 mcg / EE 30 mcg (Marlissa, Glenmark Pharmaceuticals, Mahwah, NJ) (ClinicalTrials.gov NCT02531321). Ten HIV positive women, aged 18–45 years, including normal (BMI 19.0–24.9 kg/m2), over-weight (BMI 25–29.9 kg/m2), and obese subjects (BMI 30.0 kg/m2-39.9 kg/m2) participated. These women were either not using anti-retroviral therapy or using regimens previously shown not to affect contraceptive steroid metabolism. Samples were collected from day 21 through day 24 at 1,2,3,4,6,8,12,24,48, and 72 h.
2.1. Laboratory assays
The Reproductive Endocrine Research Laboratory at the University of Southern California under the direction of Dr. Frank Z Stanczyk performed all study assays. The lab quantified LNG and EE in serum samples by specific and sensitive radioimmunoassays (RIAs) [13–15]. Briefly, prior to RIA, laboratory personnel extracted each analyte with ethyl acetate:hexane (3:2) to remove interfering steroids and metabolites. Procedural losses were estimated by adding titrated internal standards (3H-LNG or 3H-EE) to the serum prior to the extraction step. The losses ranged from 15–30% and the recoveries were used to correct the RIA values. Each RIA used a highly specific antiserum in conjunction with an iodinated radioligand. A second antibody achieved separation of free from antiserum-bound LNG or EE. The sensitivities of the RIAs were 0.05 ng/mL for LNG and 20 pg/mL for EE. The inter-assay coefficient of variation (CV) for LNG was 8.1% at 0.43 ng/mL, 8.8% at 3.05 ng/mL, and 7.4% at 9.79 ng/mL. The inter-assay CV for EE was 12.5% at 25 pg/mL, 8.2% at 69 pg/mL, and 7.2% at 285 pg/mL.
2.2. Noncompartmental analysis (NCA)
We pooled the PK data from four studies [13–16] first examining the Cmax (maximum concentration), Cmin (concentration at 24 h), and AUC (area under the concentration versus time curve) of both LNG and EE. The NCA was performed using Phoenix WinNonlin version 7.0 (Certara, St. Louis, MO) with the linear up - log down method.
2.3. Population PK
We utilized population models for both LNG and EE using NONMEM 7.3, Pirana 2.9.6 with the stochastic approximation expectation maximization (SAEM) by the importance sampling method (IMP) [17]. The time-course of both total LNG and EE concentrations were fitted to a two-compartment model (2CM) with zero-order absorption (K0). We needed to include a lag-time for LNG PK but not for EE. The library model (ADVAN 3 TRANS 4) was used and set to attain steady-state. The equations are:
| (1) |
| (2) |
at t = 0, A(1) = 0, A(2) = 0
| (3) |
| (4) |
| (5) |
where A(1) and A(2) are amounts in V1 and V2, Cp is measured drug concentration, Ct is assumed peripheral concentration, CL is plasma clearance, CLd is inter-compartmental distribution clearance, and V1 and V2 are central and peripheral volumes. Bioavailability of LNG and EE are not known due to absence of IV data, so estimates of CL, V1, V2 and CLd are all “apparent”.
Each model was validated by checking goodness-of-fit plots, normality of the inter-individual variability (IIV), residuals, and shrinkage. The predictive quality of the model was evaluated with visual predictive checks (VPC).
2.4. Linear regression and statistics
In additional to weight, body habitus metrics were calculated for body surface area (BSA) [18]:
| (6) |
and body mass index (BMI) [19]:
| (7) |
Subjects with BMI 18.5–24.9 kg/m2 are considered normal, BMI 25.0–29.9 kg/m2 are overweight, BMI 30.0–39.9 kg/m2 are obese, and BMI greater than 40.0 kg/m2 are extremely obese. We applied linear regression to each PK parameter (Cmax, Cmin, AUCT, Vss, T1/2, CL, V1, CLd, V2, and K0) with each body habitus metric (BW, BMI and BSA) using GraphPad Prism 7 to evaluate correlations. Regression parameters included R2, slope, intercept, and we assessed whether the slopes differ significantly from 0 for both LNG and EE. For linear regressions of Cmin and AUCT, intercepts were forced to 0. The LNG values with residuals greater than 6×SD were ignored (2 out of 89 subjects) and EE values with residuals greater than 4×SD were ignored (1 out of 89 subjects). Linear regressions of PK parameters between LNG and EE were also forced through (0, 0).
3. Results
3.1. Population PK model
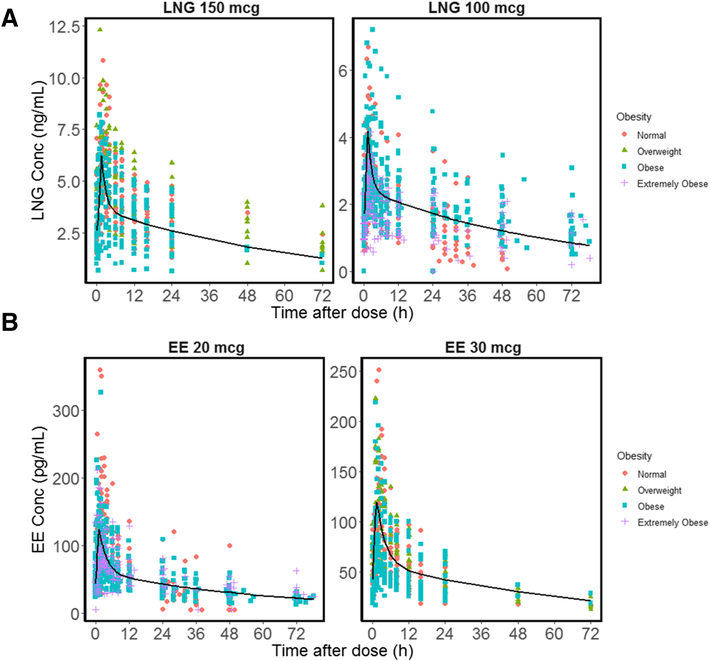
We analyzed drug concentrations from 89 subjects; one subject had missing EE data (Table 1). As shown in Figure 1, the time course of serum concentrations of both LNG and EE exhibited a rapid rise followed by a bi-exponential decline and were well-described by the two-compartment model (2CM) with zero-order absorption. The peak serum concentrations of both LNG and EE occurred within 1–2 h. A mean lag-time of 0.38 h was estimated for LNG (Table S-1). The model for LNG was the same for all studies and data were stratified by COC dose (Figure 1A) to show the population predicted profile. LNG PK were well described with the 2CM despite different doses and blood collection durations, with observed and individual predicted concentrations matching well and most conditionally weighted residual (CWRES) values symmetrically falling between −2 and +2 SD (Figure S-1). The PK parameters (CL, V1, CLd, V2, T0, Tlag) were estimated with good precision, with relative standard error (RSE) less than 30% (Table S-1). The LNG PK at 100 and 150 mcg showed good dose-proportionality (AUCT: 51.5 ±20.5, 94.5±34.2 ng/mL*h; Cmax: 3.65±1.16, 6.82±2.13 ng/mL; Cmin: 1.91±0.92, 3.35±1.82 ng/mL, Table S-2)
Table 1.
Demographic characteristics of participants in 4 COC studies combined in this analysis.
| Study | aWesthoff et al. | bEdelman et al. | cEdelman et al. | dNatavio et al. | Pooled |
|---|---|---|---|---|---|
| Number of Subjects | 28 | 19 | 32 | 10 | 89 |
| Age (years) | 25.5 ±4.5 | 29.5 ±5.1 | 29.0 ±4.7 | 35.2±3.9 | 28.7 ±5.4 |
| Weight (kg) | 77.5 ±19.4 | 83.2 ±25.7 | 106.6 ±17.7 | 71.8±9.6 | 88.5 ±23.8 |
| Height (cm) | 164.8 ±7.2 | 165.3 ±9.4 | 162.8 ±7.6 | 158.8±7.3 | 163.5 ±8.0 |
| Non-Hispanic | 20 (71.4) | 18 (94.7) | 30 (93.8) | 2(20) | 70 (79) |
| Normale | 13 (46.4) | 9 (47.4) | 0 (0) | 1(10) | 23 (26) |
| Overweighte | 0(0) | 0(0) | 0(0) | 6(60) | 6 (7) |
| Obesee | 15 (53.6) | 8 (42.1) | 23 (71.9) | 3(30) | 49 (55) |
| Extremely Obesee | 0 (0) | 2 (10.5) | 9 (28.1) | 0(0) | 11 (12) |
| BMI (kg/m2) | 28.4 (6.2) | 30.0 (9.1) | 39.4 (6.6) | 28.5(3.3) | 32.7 (8.4) |
| BSA (m2) | 1.84 (0.24) | 1.89 (0.25) | 2.09 (0.18) | 1.74(0.14) | 1.93 (0.25) |
Study from Westhoff et al.[13] administered LNG 150 mcg/EE 30 mcg.
Study from Edelman et al. [14] published in 2009 administered LNG 100 mcg/EE 20 mcg.
Another study from Edelman et al. [15] published in 2014 administered LNG 100 mcg/EE 20 mcg.
Study from Natavio et al. [16] administered LNG 150 mcg/EE 30 mcg.
Subjects with BMI 18.5–24.9 kg/m2 are considered normal, BMI 25.0–29.9 kg/m2 are overweight, BMI 30.0–39.9 kg/m2 are obese, and BMI greater than 40.0 kg/m2 are extremely obese.
All data are presented as n (%) or mean ± standard deviation.
Figure 1.
General model fittings of serum levonorgestrel (LNG) and ethinyl estradiol (EE) concentrations versus time. (A) LNG 150 mcg [13, 16] or 100 mcg PK profiles[14, 15]; (B) EE 30 mcg [13, 16] or 20 mcg PK profiles [14, 15]. Population predicted (PRED) profiles are shown as black lines. Subjects with different extent of obesity are shown using different colors and shapes. Subjects with BMI 18.5–24.9 kg/m2 are considered normal, BMI 25.0–29.9 kg/m2 are overweight, BMI 30.0–39.9 kg/m2 are obese, and BMI greater than 40.0 kg/m2 are extremely obese.
The EE concentrations did not show dose-proportionality. As shown in Table S-2, the 20 mcg dose produced Cmax values of 136 ± 64.3 pg/mL similar to those given EE 30 mcg (126 ± 50.7 pg/mL); the 20 mcg dose produced Cmin (44.1 ± 19.8 pg/mL) similar to those given EE 30 mcg (39.6 ± 15.6 pg/mL). Thus, EE data were separated into two groups due to these differences. However, when comparing PK changes across studies, EE doses from Edelman et al. [14, 15] were set to 30 mcg. The EE PK were well described by the 2CM model with zero-order absorption, with observed and individual predicted concentrations agreeing well and the CWRES symmetrically falling between −2 and +2 SD (Figure S-2). All EE PK parameters were estimated with good precision (Table S-1).
3.2. LNG PK parameters in relation to body habitus metrics
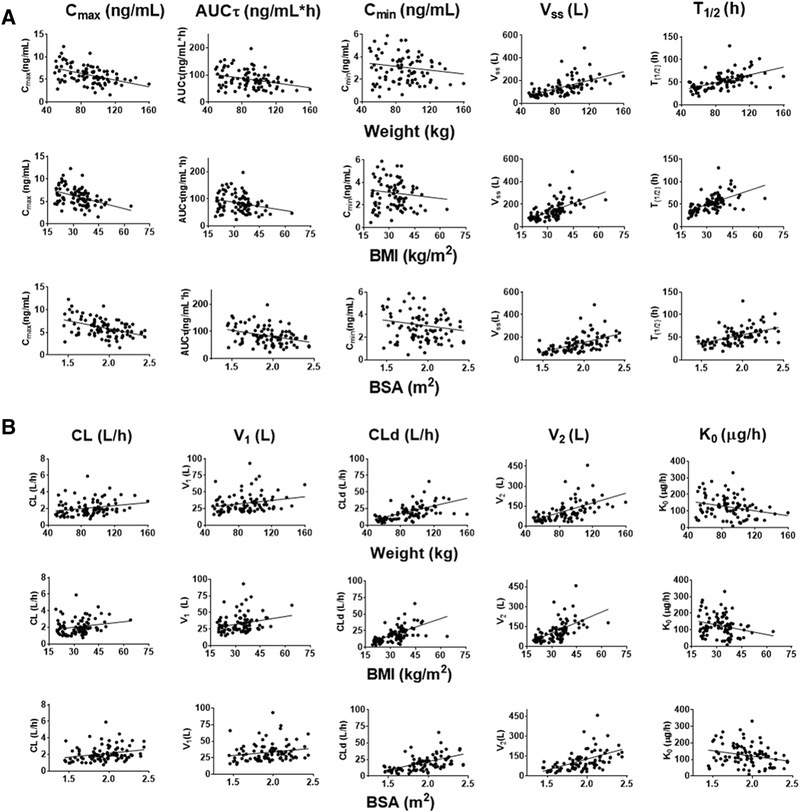
When comparing NCA parameters across the studies, we multiplied Cmax, AUCτ, and Cmin from Edelman et al. [14, 15] (LNG 100 mcg/EE 20 mcg) by 1.5 to match the dose from Westhoff et al. [13] and Natavio et al. [16] (LNG 150 mcg/EE 30 mcg) given the good dose proportionality of LNG. The Cmax of LNG decreased with increasing weight, BMI and BSA (p<0.0001 for all body metrics, Figure 2A, Table S-3). Similarly, the AUCT of LNG also decreased with increasing weight, BMI and BSA (p≤0.015 for all). However, Cmin did not correlate with any of the body habitus metrics (p ≥0.17 for all). The steady-state volume (Vss) of each individual obtained from the population analysis increased with increasing weight, BMI and BSA (p<0.0001 for all). Similarly, the individual half-life (T1/2) increased with increasing weight, BMI and BSA (p<0.0001 for all). The latter differences are consistent with the greater tissue mass for distribution of the drugs.
Figure 2.
LNG PK parameters in relation to body habitus metrics. (A) NCA parameters of individual subjects from 4 studies [13–16] in relation to weight, BMI, or BSA. (B) Two-compartment model parameters of individual subjects from 4 studies [13–16] in relation to weight, BMI, or BSA. Regression parameters and statistics are listed in Supplemental Table S-3. All linear regressions had slopes significantly different from 0 (p<0.05 or less), except for Cmin.
We obtained values of CL, V1, CLd, V2 and K0 of individual fitted parameters from the population PK analysis. Linear regression of each PK parameter with increasing body habitus metrics yielded the following results (Figure 2B, Table S-4): The LNG CL increased with BW (p=0.027) and BSA (p=0.015); CL also increased with BMI, but with marginal significance (p=0.056). V1 increased with weight (p=0.028) and BMI (p=0.022) while marginal significance related to BSA (p=0.067). The CLd increased with all body habitus metrics (p≤0.001 for all). Similarly, V2 also significantly increased with all body habitus metrics (p<0.0001 for all). Interestingly, the K0 decreased with increasing BW, BMI, and BSA (p≤0.018 for all).
3.3. EE PK parameters in relation to body habitus metrics
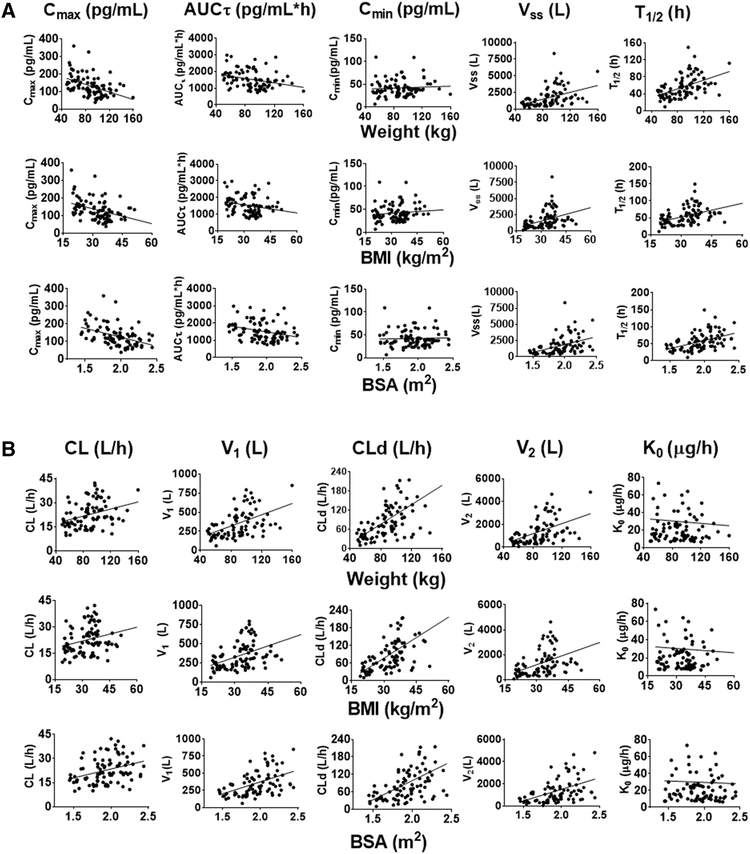
Since we found no dose proportionality between two doses, the Cmax, AUCτ, and Cmin of EE were compared directly without dose adjustment. In all four studies, Cmax and AUCT decreased with increasing weight, BMI and BSA (p<0.0001 and p≤0.018, respectively) for all body metrics (Figure 3A, Table S3). There was no correlation between Cmin and the body habitus metrics (p≥0.28 for all). Both the Vss and T1/2 increased with weight, BMI, and BSA (p<0.0001 for all). For population PK parameters, CL increased with weight, BMI, and BSA (p≤0.009 for all, Figure 3B, Table S4). The V1, CLd, and V2 also increased with BW, BMI, and BSA (p<0.0001 for all). There was no correlation between K0 values and weight, BMI, and BSA (p≥0.70 for all). As for LNG, the EE PK parameters from all studies were distributed homogeneously in relation to body metrics.
Figure 3.
EE PK parameters in relation to body habitus metrics. (A) NCA parameters for individual subjects from 4 studies [13–16] in relation to weight, BMI, or BSA. (B) Two-compartment model parameters for individual subjects from 4 studies [13–16] in relation to weight, BMI, or BSA. Regression parameters and statistics are listed in Supplemental Table S-4. All linear regressions had slopes significantly different from 0 (p<0.05 or less), except for Ko and Cmin.
3.4. Correlation of LNG and EE PK parameters
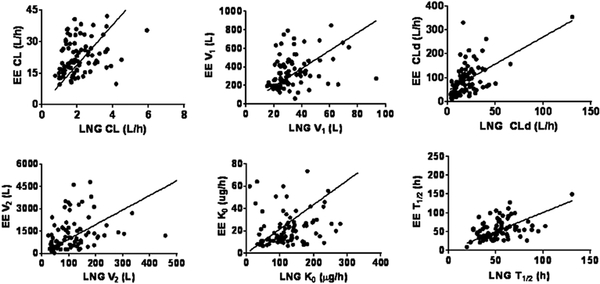
Comparisons of LNG and EE PK parameters were possible for a total of 88 subjects. As can be seen from Figure 4 and Table S-5 for each individual, the increase of LNG CL is correlated with the increase of EE CL (p<0.0001). Similarly, there were significant correlations for V1, CLd, V2, K0, and T1/2, (p≤0.0056 for all parameters). These findings suggest that the change of both LNG and EE PK is affected by the physiology of each subject where greater body mass leads to similar changes in the PK parameters for the two compounds.
Figure 4.
Correlation of LNG and EE PK parameters from 4 studies [13–16]. Lines denote linear regressions with force to 0 intercepts. Regression parameters and statistics are listed in Supplemental Table S-5. All correlations are highly significant (p<0.05 or less).
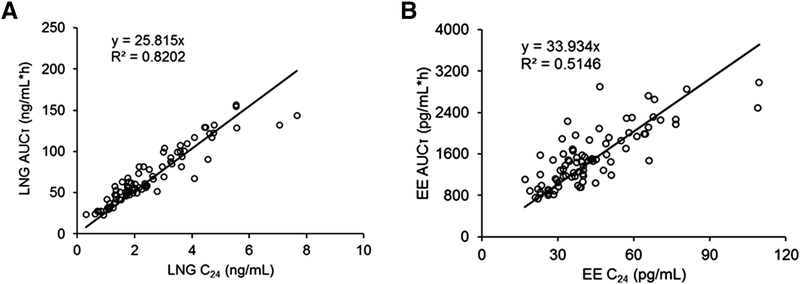
3.5. Relationships of AUCT and Cmin values
The AUCT values of LNG and EE versus the Cmin values for each subject are shown in Figure 5. For LNG, Cmin correlates well with AUCT (R2=0.82) with a slope of 25.82. For EE, Cmin also correlates well with AUCT although greater variability was seen (R2=0.51) with a slope of 33.93.
Figure 5.
Relationship between AUCT and Cmin values for LNG (left) and EE (right panel) from 4 studies [13–16]. The lines depict regressions with a force through 0. Slopes and correlations are listed on the graphs.
4. Discussion
This joint population PK analysis demonstrated reduced systemic COC exposure in obese subjects (Cmax and AUCT), but no difference in trough LNG and EE concentrations. Population PK analysis revealed markedly increased values of Vss and T1/2, which can be attributed to the increased tissue mass in obesity. Obese women exhibited an increased CL for both LNG and EE. As hepatic metabolism determines the clearances of these compounds, this result may reflect greater liver size or function with increased body mass. We do not think it is likely that these modest differences in PK translate into an increased pregnancy risk in obese women but, in combination with non-adherence, it may make them more at risk. However, these findings only apply to COCs with LNG and EE, and not for any other combinations of COCs or other progestins and etonogestrol implant. Because there are PK interactions between LNG and EE, the findings here are not relevant to EE combined with other progestins.
The present analysis offers the capabilities and advantages of joint population modeling, a highly relevant 2CM model fitting approach, and a much larger database than the previous individual studies assessing LNG and EE exposures in relation to obesity. These features allow more certainty in drawing conclusions about the role of obesity in the PK of COCs. The graphs depict the clear trends of all PK parameters in relation to the three methods of expressing body habitus in comparable groups of women taking COCs from 3 sites in the US. We found no clear advantage to using any one of the three body habitus indices.
LNG PK is known to be linear as AUC increases proportionally with dose [20]. EE should also be linear within a certain dose range; Fotherby [21] showed that EE PK is linear in the range of 20–100 mcg. However, our analysis did not demonstrate this for women of higher BMI. The EE PK data from Edelman et al. [14, 15] (EE 20 mcg) and the other studies [13, 16] (EE 30 mcg) are not dose proportional. Both Edelman studies that gave EE 20 mcg had concentrations similar to the other EE 30 mcg studies. Greater inter-individual variability was also observed in the Edelman studies, perhaps due to inconsistencies in timing of dosing of some subjects. However, factors such as enterohepatic circulation, peripheral conversion, formulation (different bioavailability and/or absorption rate), study conditions, and assay date/run differences may also occur. This complicates combining the studies for this analysis. Based on other published data with same product (Alesse), the Edelman group had EE concentrations about twice those from Koch et al. [22] (AUCT 1567 vs 596 pg/mL*h, Cmax 136 vs 66 pg/mL). The method used by the latter group was gas chromatography–mass spectrometry (GC-MS), which tends to provide greater specificity than radioimmunoassay, which was used in our studies. The Wyeth product label [23] lists the EE Cmax (82.3 pg/mL) and AUCT (776 pg/mL*h) closer to Koch et al.[22]. For the purpose of combining all 4 studies, the EE doses for the Edelman studies were set to 30 mcg. An alternative approach for analyzing the 20 and 30 mcg data separately led to similar trends (increase of CL, V1, V2, CLd, Vss and T1/2 with increase of BW, BMI, BSA for both doses), but with lesser significance due to the lower power when reducing the number of subjects.
One consistency for this analysis was that we performed all LNG and EE assays for all studies in one laboratory. The methodology involved long-established and validated radioimmunoassays. While the use of chromatography and mass spectrometry methods would offer greater possible specificity, there have not been to our knowledge any published and validated comparison of the two methods assessing the same samples from dosed subjects. On the other hand, Jusko [24] summarized the Cmax and AUC values for a large array of COC studies and found similar values for LNG exposures for studies using different analytical methods. Nevertheless, the use of RIA rather than LC-MS or GC-MS is a limitation of this study.
Obesity alters the clearance and disposition of various drugs [25, 26]. For both LNG and EE, increasing body weights correlated with lower Cmax, Cmin, and AUCT values. However, trough concentrations (Cmin) of both LNG and EE did not differ with body habitus. Both drugs are eliminated largely by metabolism. For drugs cleared primarily by hepatic metabolism, obesity can increase, decrease or have no effect on clearance. Clearances in obesity are more likely to increase for drugs that undergo conjugation reactions with glucuronic acid, sulfonates, glutathione, or amino acids [27, 28]. As EE undergoes conjugation with glucuronic acid and sulfonates [21], this could explain the increased EE clearance in obese subjects.
Obesity was found to increase the clearance of LNG given alone, likely due to the lower sex hormone binding globulin (SHBG) concentrations and binding [29]. The clearance of LNG and EE in COCs is complicated as EE induces SHBG production, which in turn reduces the clearance of LNG. The analysis of PK data from the present study shows that both LNG and EE have increased clearances in obese subjects and the increase is greater for EE. While untreated obese subjects have lower SHBG concentrations than normal women [30, 31], the present studies showed no differences in SHBG in relation to body weight during chronic COC use (Figure S-3). This implies that free LNG and EE concentrations are also likely to be proportional to their total serum concentrations.
The Edelman group obtained PK data for as long as 192 h revealing a long apparent terminal phase for EE. This is likely due to the reversible metabolism of EE sulfate, which acts as a reservoir or slow release form of parent EE [21]. To assess the PK changes consistently for the four studies, the sample times utilized were limited to 72 h. Reversible metabolism and/or consideration of enterohepatic circulation may be needed for a more complete description of EE PK. As EE was measured using RIA in all four studies, mass spectrometry assays are needed to explain the terminal EE concentrations. However, this late washout phase consists of very low apparent EE concentrations that are unlikely to contribute to either Cmax and AUCT or to the efficacy of the OC product.
Researchers have been interested in the possibility of using trough or Cmin concentrations of drugs including COCs to monitor exposure and compliance during multiple-dosing. Indeed, reasonable correlations of AUCT and Cmin were found for LNG and EE in small studies of COC products in normal weight women [12, 32]. The present study provides data from much larger numbers of subjects than previously with the addition of the disturbances in PK caused by obesity. The results in Figure 5 indicate good correlation between Cmin and AUCT, suggesting that Cmin values can be used fairly reliably as a surrogate for systemic COC exposures.
Supplementary Material
Acknowledgements/Funding:
Supported in part by NIH Grant GM24211. Edelman data obtained while supported by: HD 01243–03 Women’s Reproductive Health Research Fellow (NICHD K-12), PHS Grant 5 M01 RR000334, R03 HD 053611–01, R01 HD061582–01 NICHD, NIH NCRR 1 UL1 RR024120). Westhoff data supported by NIH grants HD04578 and RR024156.
Author Disclosures
William J Jusko has been a recent consultant for Novartis, Boehringer Ingelheim, Reveragen, and Bayer Healthcare Products. A. Edelman: consultant for World Health Organization, Gynuity Health Projects, Genzyme, Agile Therapeutics, and HRAPharma. Nexplanon trainer for Merck and the recipient of a Merck-investigator initiated grant. Author for UptoDate (Royalties received). C Westhoff: consultant for Merck and Bayer, Agile Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
William J Jusko has been a recent consultant for Novartis, Boehringer Ingelheim, Reveragen, and Bayer Healthcare Products. A. Edelman: consultant for World Health Organization, Gynuity Health Projects, Genzyme, Agile Therapeutics, and HRAPharma. Nexplanon trainer for Merck and the recipient of a Merck-investigator initiated grant. Author for UptoDate (Royalties received). C Westhoff: consultant for Merck and Bayer, Agile Therapeutics. No conflicts of interest to disclose for the other authors.
5. References
- [1].Centers for Disease Control and Prevention. http://wwwcdcgov/nchs/hus/contents2013.
- [2].Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004:1–36. [PubMed] [Google Scholar]
- [3].Skouby SO. Contraceptive use and behavior in the 21st century: a comprehensive study across five European countries. Eur J Contracept Reprod Health Care. 2004;9:57–68. [DOI] [PubMed] [Google Scholar]
- [4].Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body mass index, weight, and oral contraceptive failure risk. Obstet Gynecol. 2005;105:46–52. [DOI] [PubMed] [Google Scholar]
- [5].Holt VL, Cushing-Haugen KL, Daling JR. Body weight and risk of oral contraceptive failure. Obstet Gynecol. 2002;99:820–7. [DOI] [PubMed] [Google Scholar]
- [6].Brunner Huber LR, Hogue CJ. The association between body weight, unintended pregnancy resulting in a livebirth, and contraception at the time of conception. Matern Child Health J. 2005;9:413–20. [DOI] [PubMed] [Google Scholar]
- [7].Dinger J, Minh TD, Buttmann N, Bardenheuer K. Effectiveness of oral contraceptive pills in a large U.S. cohort comparing progestogen and regimen. Obstet Gynecol. 2011;117:33–40. [DOI] [PubMed] [Google Scholar]
- [8].Yamazaki M, Dwyer K, Sobhan M, et al. Effect of obesity on the effectiveness of hormonal contraceptives: an individual participant data meta-analysis. Contraception. 2015;92:445–52. [DOI] [PubMed] [Google Scholar]
- [9].Dinger JC, Cronin M, Mohner S, Schellschmidt I, Minh TD, Westhoff C. Oral contraceptive effectiveness according to body mass index, weight, age, and other factors. Am J Obstet Gynecol. 2009;201:263.e1–9. [DOI] [PubMed] [Google Scholar]
- [10].Vessey M Oral contraceptive failures and body weight: findings in a large cohort study. J Fam Plann Reprod Health Care. 2001;27:90–1. [DOI] [PubMed] [Google Scholar]
- [11].McNicholas C, Zhao Q, Secura G, Allsworth JE, Madden T, Peipert JF. Contraceptive failures in overweight and obese combined hormonal contraceptive users. Obstet Gynecol. 2013;121:585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Westhoff CL, Pike MC, Tang R, DiNapoli MN, Sull M, Cremers S. Estimating systemic exposure to ethinyl estradiol from an oral contraceptive. Am J Obstet Gynecol. 2015;212:614.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Westhoff CL, Torgal AH, Mayeda ER, Pike MC, Stanczyk FZ. Pharmacokinetics of a combined oral contraceptive in obese and normal weight women. Contraception. 2010;81:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Edelman AB, Cherala G, Munar MY, et al. Prolonged monitoring of ethinyl estradiol and levonorgestrel levels confirms an altered pharmacokinetic profile in obese oral contraceptives users. Contraception. 2013;87:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barcellos T, Natavio M, Luo D, Stanczyk FZ, Jusko WJ, NM B. Effects of protease inhibitor Ritonavir on combined oral contraceptive pharmacokinetics and pharmacodynamics in HIV-positive women. Contraception. 2018;In Preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM User’s Guides (1989–2009). Ellicott City, MD, USA: Icon Development Solution; 2009. [Google Scholar]
- [18].DuBois DDE. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:873–71. [Google Scholar]
- [19].Organization WH. BMI classification.
- [20].Fotherby K Levonorgestrel. Clinical pharmacokinetics. Clin Pharmacokinet. 1995;28:203–15. [DOI] [PubMed] [Google Scholar]
- [21].Fotherby K Pharmacokinetics of ethynyloestradiol in humans. Methods Find Exp Clin Pharmacol. 1982;4:133–41. [PubMed] [Google Scholar]
- [22].Koch K, Campanella C, Baidoo CA, Manzo JA, Ameen VZ, Kersey KE. Pharmacodynamics and pharmacokinetics of oral contraceptives co-administered with alosetron (Lotronex). Dig Dis Sci. 2004;49:1244–9. [DOI] [PubMed] [Google Scholar]
- [23].Wyeth Pharmaceuticals Inc. Alesse® 28 tablets (levonorgestrel and ethinyl estradiol tablets): US prescribing information 2004 Apr [online]. Available from URL: http://wwwwyethcom.
- [24].Jusko WJ. Perspectives on variability in pharmacokinetics of an oral contraceptive product. Contraception. 2017;95:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71–87. [DOI] [PubMed] [Google Scholar]
- [26].Abernethy DR, Greenblatt DJ, Divoll M, Harmatz JS, Shader RI. Alterations in drug distribution and clearance due to obesity. J Pharmacol Exp Ther. 1981;217:681–5. [PubMed] [Google Scholar]
- [27].Speerhas R Drug metabolism in malnutrition and obesity: clinical concerns. Cleve Clin J Med. 1995;62:73–5. [DOI] [PubMed] [Google Scholar]
- [28].Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51:277–304. [DOI] [PubMed] [Google Scholar]
- [29].Natavio M, Stanczyk FZ, Molins EAG, WJ J. Pharmacokinetics of 1.5 mg levonorgestrel tablets in obesity. In Revision. 2018.
- [30].Edelman AB, Cherala G, Blue SW, Erikson DW, Jensen JT. Impact of obesity on the pharmacokinetics of levonorgestrel-based emergency contraception: single and double dosing. Contraception. 2016;94:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hautanen A Synthesis and regulation of sex hormone-binding globulin in obesity. Int J Obes Relat Metab Disord. 2000;24 Suppl 2:S64–70. [DOI] [PubMed] [Google Scholar]
- [32].Basaraba CN, Westhoff CL, Pike MC, Nandakumar R, Cremers S. Estimating systemic exposure to levonorgestrel from an oral contraceptive. Contraception. 2017;95:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.