Abstract
The temporal niche that an animal occupies includes a coordinated suite of behavioral and physiological processes that set diurnal and nocturnal animals apart. The daily rhythms of the two chronotypes are regulated by both the circadian system and direct responses to light, a process called masking. Here we review the literature on circadian regulations and masking responses in diurnal mammals, focusing on our work using the diurnal Nile grass rat (Arvicanthis niloticus) and comparing our findings with those derived from other diurnal and nocturnal models. There are certainly similarities between the circadian systems of diurnal and nocturnal mammals, especially in phase and functioning of the principal circadian oscillator within the hypothalamic suprachiasmatic nucleus (SCN). However, the downstream pathways, direct or indirect from the SCN, lead to drastic differences in the phase of extra-SCN oscillators, with most showing a complete reversal from the phase seen in nocturnal species. This reversal, however, is not universal and in some cases the phases of extra-SCN oscillators are only a few hours apart between diurnal and nocturnal species. The behavioral masking responses in general are opposite between diurnal and nocturnal species, and are matched by differential responses to light and darkness in several retinorecipient sites in their brain. The available anatomical and functional data suggest that diurnal brains are not simply a phase-reversed version of nocturnal ones, and work with diurnal models contribute significantly to a better understanding of the circadian and photic modulation of daily rhythms in our own diurnal species.
Keywords: Diurnality, circadian clock, entrainment, masking, Nile grass rats
Graphical Abstract
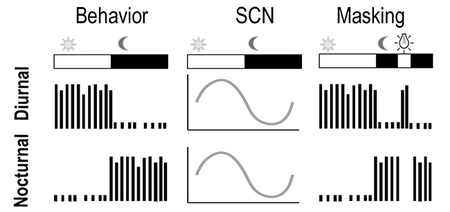
Although behavioral rhythms of diurnal and nocturnal mammals are in an anti-phase relationship, rhythms in the principal brain clock in the suprachiasmatic nucleus (SCN) oscillate in-phase in the two chronotypes. Downstream mechanisms responsible for the reversal in behavioral rhythms remain to be elucidated. Moreover, the direct behavioral response to light is very different, i.e. light increases activity in diurnal mammals but inhibits it in nocturnal ones.
Introduction
One of the most salient features of an animal’s mode of adaptation to its environment is the time of day at which it is most active. The temporal niche that an animal occupies influences a coordinated suite of behavioral and physiological processes that set diurnal and nocturnal animals apart (Smale et al., 2003; Kronfeld-Schor & Dayan, 2008; Refinetti, 2008; Cuesta et al., 2009). The basic activity patterns are heavily influenced by an internal circadian system that drives increases in activity at one time of day and decreases at another. The rhythm is generated internally, but is synchronized by environmental cues referred to as zeitgebers, with the primary one being the light/dark (LD) cycle (Daan, 1977). While this circadian system produces a drive that has a powerful influence on the daily profiles of numerous behaviors, that influence is modulated, and sometimes even blocked outright, by a variety of other factors, including the ambient light to which animals are exposed. This process is referred to as “masking” (Mrosovsky, 1999). Thus, daily rhythms in behavior and physiology are under the influence of both the circadian system and masking, as well as interactions between the two. At some times of day they reinforce each other while at others masking can block the circadian influence or the circadian system can override the processes responsible for masking (Aschoff & von Goetz, 1988; Aschoff, 1999; Redlin & Mrosovsky, 1999).
Although the vast majority of research in the field has focused on nocturnal rodents, recent development of diurnal models has enabled substantial progress to be made in our understanding of both circadian and photic modulation of activity patterns of day-active mammals (Smale et al., 2008). In general, the effects of light on the circadian timekeeping system are very similar in nocturnal and diurnal animals, whereas the effects of that system on the myriad rhythms that it controls are very different. The direct influences of light (i.e. masking) are also very different between nocturnal and diurnal species for behavioral responses, such that it increases activity in diurnal species (positive masking) and suppresses it in nocturnal ones (negative masking) (Hagenauer & Lee, 2008; Cohen et al., 2010; Shuboni et al., 2012). In the present review, we will elaborate on both of these systems and their neural mechanisms in day-active mammals. However, first, motivated by the sentiment articulated so well by Dobzhansky that “nothing makes sense in biology except in the light of evolution” (Dobzhansky, 1964), we briefly consider the pathways that have led to historical transitions from one temporal niche to another and to the emergence of diurnality along some of them.
Evolutionary pathways to mammalian diurnality
Among modern mammals, unlike fish, reptiles and birds, the predominant activity pattern is nocturnal. This nocturnal dominance reflects the evolutionary origins of this group, which began approximately 225 million years ago with entry into a “nocturnal bottleneck” (Walls, 1942). Restriction of their activity to the night through this Mesozoic Era is thought to have enabled early mammals to escape predation by the diurnal dinosaurs that dominated the landscapes (Gerkema et al., 2013). During this period, those early mammals evolved a range of adaptations that optimized survival with a night-active lifestyle, including thermoregulatory systems that support activity in a colder world and visual systems better suited for operating in the dark (Walls, 1942; Crompton et al., 1978; Gerkema et al., 2013). One of the most recent phylogenetic analysis suggests that this nocturnal bottleneck began to open up approximately 65–75 million years ago at the very end of the Mesozoic Era, when some mammals extended their active period into the daylight hours (Maor et al., 2017); the emergence of this cathemeral pattern is likely to have occurred among the shrews and moles (Soricidae). Approximately ten million years later, following the mass extinction event that wiped out all non-avian dinosaurs at the end of Cretaceous period, the mammal-dominated Cenozoic Era began. Diurnal mammals emerged at that time, and the elephant shrews (Macroscelididae) were likely to be the first to have made that move (Maor et al., 2017). Interestingly, recent analyses suggest that after a 30 million years period during which both nocturnal and diurnal mammals diversified, rising temperatures led to the extinction of most of the diurnal ones (Wu et al., 2017). It was not until the end of that second, smaller, bottleneck, that diurnality resurfaced, which it did along numerous independent evolutionary pathways, many of which involved crossing another “cathemeral bridge” (Santini et al., 2015; Maor et al., 2017). However, even today most mammals remain nocturnal (Gerkema et al., 2013).
Diurnal rodent models for the study of circadian rhythms and masking
Humans are fundamentally diurnal. Although there is considerable inter-individual variability in activity patterns, such that the so-called “early larks” or “night owls” whose sleep/wake schedules are significantly advanced or delayed, respectively, compared to the majority of the population, humans in general are most active during the day and sleep at night. Disrupting this natural diurnal pattern, e.g. with shift work or other features of a modern lifestyle, can bring with it a high risk for a multitude of pathological conditions (Nunez et al., 2018). Thus, diurnal models may make unique contributions to our understanding of the circadian and photic modulation of brain and behavior in humans.
A few diurnal rodent models have been established and have yielded important information and provided significant insight into the workings of a diurnal brain. However, each has its limitations. Ground squirrels, for example have only one very brief period of estrus per year, are extremely difficult to breed in captivity, and their circadian rhythms change on a circannual basis (Lee et al., 1986). The South American degus breeds well in captivity, but very slowly, with a 21 day estrous cycle, a three month gestation period, and a three month period to reach reproductive maturity (Palacios & Lee, 2013). These animals have been ideal for studies of how rhythms change during the adolescent period (Hagenauer & Lee, 2008), but the maintenance of a breeding colony of animals that are this slow to reproduce has limited their use.
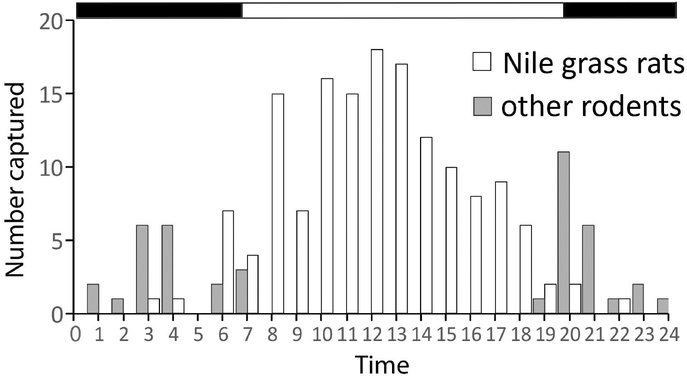
A cohort of Nile grass rats (Arvicanthis niloticus) was brought to the United States from Kenya, East Africa, twenty-five years ago and was used to develop a breeding colony that has been maintained at Michigan State University since then. Nile grass rats are members of the family Muridae, as are laboratory mice (Mus musculus), and these species are likely to have diverged from a common ancestor relatively recently. Nile grass rats, like mice, attain reproductive maturity rapidly, have a 24-day gestation period and mate on a postpartum estrus, which makes maintenance of a colony relatively straightforward (Refinetti, 2004b). In the field, Nile grass rats have a clear diurnal pattern of above-ground activity (Fig. 1 (Blanchong & Smale, 2000)). In the laboratory, these animals are diurnal with respect to patterns of sleep, general locomotor activity, mating behavior, body temperature and secretion of luteinizing hormone (McElhinny et al., 1997; McElhinny et al., 1999; Novak et al., 1999; Mahoney & Smale, 2005).
Figure 1.
Total number of Nile grass rats and other rodents captured in timer traps during each hour of a day in Kenya, the natural habitat of Nile grass rats. The Nile grass rats and the so-called “other rodents” had diurnal and nocturnal pattern of activity, respectively. Black-white bar on top indicates daily light/dark schedule with the transitioning points corresponding to the time of sunrise and sunset, respectively. Adapted from (Blanchong & Smale, 2000) with copyright permission.
The visual system has been characterized in Nile grass rats (Gaillard et al., 2008) and in a closely related species, the Sudanese grass rat (Arvicanthis. ansorgei) (Bobu et al., 2006), and many features specialized for operating in the daylight hours have been revealed in these animals. For example, 30–40% of their photoreceptors are cones compared to only 1–2% in laboratory mice or rats, and some antigens present in bipolar and amacrine cells are characteristic of grass rats and other diurnal rodents but not nocturnal ones (Bobu et al., 2006; Gaillard et al., 2008). Molecular analyses have suggested that grass rats possess blue/violet-sensitive s-cones as other diurnal rodents do (Gaillard et al., 2009), and functional specializations for diurnality have also been revealed by analyses of recordings from the electroretinogram (Gilmour et al., 2008). Furthermore, the optic tectum of a grass rat is approximately the same size as that of a laboratory rat whose body weight is 3–4 times higher, suggesting that the visual brain is also specialized for a day-active life style in these animals (Gaillard et al., 2013). Indeed, compared to other commonly used diurnal rodent models, i.e. Degus or Mongolian gerbil, the Nile grass rat has the highest diurnality index score (Refinetti, 2008). Therefore, the Nile grass rat is an ideal diurnal model and in this paper, while integrating evidence from a diverse set of diurnal species, we will highlight some work that our group has done with these rodents.
Circadian time-keeping in diurnal mammals
The circadian system of early mammals was transformed when the first ones evolved from a nocturnal ancestor such that it could now drive activity up during the day and down at night and generate physiological rhythms that could support that new pattern of behavior. This system is organized in a hierarchical manner, with the principal brain clock, the hypothalamic suprachiasmatic nucleus (SCN), coordinating the circadian rhythms of subordinate clocks in the brain and in peripheral tissues/organs (Davidson et al., 2003). So, the important question here is where and how within this system did the signals become reversed in diurnal and nocturnal animals.
The SCN, also referred as a light-entrainable oscillator, keeps time relative to the environmental LD cycle, regardless of the time of day at which animals are most active (Challet et al., 2002; Smale et al., 2003; Smale et al., 2008). The molecular mechanisms of circadian timekeeping have been described as interlocked transcriptional and translational feedback loops involving a set of so-called clock genes, a topic which has been reviewed elsewhere (Okamura, 2004; 2007; Takahashi et al., 2008). Expression of many clock genes and clock-controlled genes in the SCN has also been found to oscillate in similar phase relative to the LD cycle in diurnal and nocturnal mammals (Mrosovsky et al., 2001; Lincoln et al., 2002; Caldelas et al., 2003; Lambert et al., 2005; Ramanathan et al., 2006; Valenzuela et al., 2008; Mahoney et al., 2009; Chakir et al., 2015; Ikeno et al., 2017). There have been only subtle differences found so far between diurnal and nocturnal mammals involving the expression pattern of one clock gene, conveniently named clock. In contrast to the constitutive expression of clock mRNA in the SCN of nocturnal mammals, its expression is rhythmic in several diurnal ones including Barbary striped grass mice, sheep and capuchin monkeys, with the peak phase occurring in the late subjective day (Lincoln et al., 2002; Valenzuela et al., 2008; Chakir et al., 2015). Consistent with the similarly phased expression of other clock genes in the SCN, numerous studies measuring a range of different parameters have revealed that the phase of circadian oscillations within the SCN is the same in diurnal and nocturnal mammals. For example, its overall metabolism and electrical activity are highest during the day in both diurnal and nocturnal rodents (Inouye & Kawamura, 1979; Schwartz et al., 1983; Sato & Kawamura, 1984; Yamazaki et al., 1998). These similarities in the functioning of the SCN as a circadian timekeeper support the view that the mechanisms responsible for whether an animal is most active during the day or night most likely lies downstream of the master clock within the SCN (Smale et al., 2003; Smale et al., 2008).
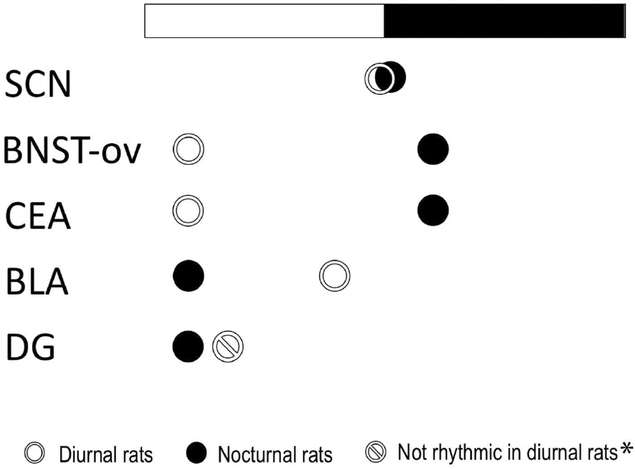
Given the fundamental differences between diurnal and nocturnal species with respect to their behavioral and physiological rhythms, one would expect the subordinate clocks directly linked to these functions to be reversed. In Nile grass rats, we have found that rhythms in many extra-SCN brain regions are in anti-phase relative to those of nocturnal rodents, however, there are other extra-SCN regions in which the phase of the rhythms differs in less extreme ways (Ramanathan et al., 2008a; Ramanathan et al., 2008b; Ramanathan et al., 2010a; Ramanathan et al., 2010b). For example, in the basolateral amygdala (BLA), the peak phase of PER2 is only a few hours apart, while in the dentate gyrus (DG), PER2 peaks in early morning in nocturnal laboratory rats but is not rhythmic in diurnal Nile grass rats (Fig. 1). The results suggest that there is no single simple switch that causes some animals to be nocturnal and others to be diurnal (Smale et al., 2008). In human brains, cortical and limbic regions have been found to show rhythms with a phase consistent with those of other diurnal mammals (Li et al., 2013).
In the periphery, circadian oscillations have been reported in various diurnal species in different tissues and organs, and the rhythms are usually reversed compared to those of nocturnal species (Andersson et al., 2005; Lambert & Weaver, 2006; Lemos et al., 2006; Valenzuela et al., 2008; Murphy et al., 2015). The most detailed and complete analysis of clock gene expression in a diurnal species comes from a recent study using our close relative, the olive baboon (Papio anubis), in which the transcriptomes were examined in 22 brain regions and 42 peripheral tissues of 12 animals, sacrificed at 2 hr intervals across the day (Mure et al., 2018). This impressive effort has revealed that over 80% of the protein-coding genes are expressed rhythmically, and most ubiquitously expressed genes exhibit rhythmic expression in a tissue-specific manner. Additionally, comparing the data from diurnal baboons and nocturnal mice, it was revealed that expression of core clock genes was in phase in the SCN, but ~12 hrs out of phase in other brain or peripheral tissues, consistent with the findings from other diurnal species discussed above.
An intriguing question that needs to be answered is how the SCN coordinates the oppositely phased extra-SCN oscillators in each chronotype (Smale et al., 2008). It is well accepted that the SCN utilizes two types of output signals: humoral ones that are sufficient for driving activity rhythms (Silver et al., 1990; Silver et al., 1996) and precise neuronal projections that are required to generate rhythms in neuroendocrine systems (Nunez & Stephan, 1977; Swann & Turek, 1985; Meyer-Bernstein et al., 1999; de la Iglesia et al., 2003). At least two potential humoral signals that regulate behavioral rhythms have been identified so far, namely PK2 and TGF-alpha (Kramer et al., 2001; Cheng et al., 2002), and these have both been examined in diurnal species. The expression of PK2 has been examined in Nile grass rats and macaque monkeys (Lambert et al., 2005; Burton et al., 2016). In both species, the daily pattern of PK2 mRNA production in the SCN is similar to that found in nocturnal species, with the peak occurring at midday. The expression of TGF-alpha in the SCN has also been compared between nocturnal and diurnal rodents (mice and Sudanese grass rats), which revealed a similar phase when animals were kept in constant darkness (Tournier et al., 2007). These results collectively suggest that those potential output signals from the SCN do not directly determine a species’ temporal pattern of behavioral rhythms. Although the pursuit of humoral signals from the SCN has provided little insight into the circadian control of diurnality, studies of the neuronal signals from the SCN have shed light on potential mechanisms underlying the switch from a nocturnal to a diurnal pattern.
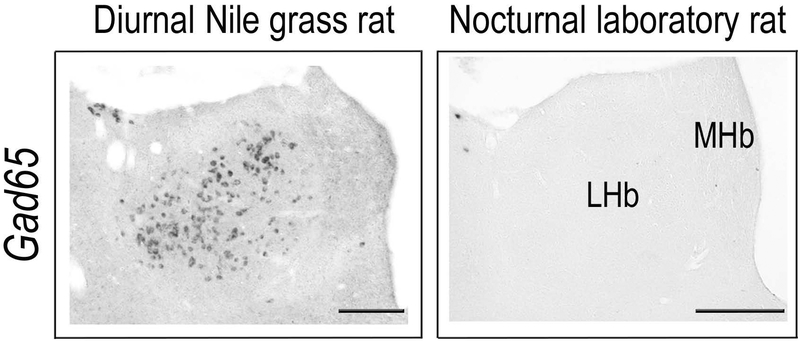
One of the best studied neuronal output pathways is the one through which the SCN regulates the daily rhythms of adrenal glucocorticoids via the neuropeptide arginine vasopressin (AVP) (Kalsbeek et al., 1992; Kalsbeek et al., 1996). Although the circadian expression of AVP mRNA within the SCN is in phase between diurnal and nocturnal rodents (Mahoney et al., 2009), the glucocorticoid rhythm is phase-reversed, with a peak occurring before lights-off in nocturnal species and before lights-on in diurnal ones (Weitzman et al., 1971; Cross & Rogers, 2004; Torres-Farfan et al., 2008). Focusing on this endocrine system, Kalsbeek et al have shown that infusing AVP into the paraventricular nucleus (PVN) promotes the release of adrenal glucocorticoids in the diurnal Sudanese grass rats, but the same treatment inhibits the glucocorticoids release in nocturnal laboratory rats (Kalsbeek et al., 2008). These findings have led to the hypothesis that a species difference in the local circuit of the PVN involving either glutamatergic or GABAergic neurons contributes to the reversal of daily rhythms in glucocorticoid secretion (Kalsbeek et al., 2008). A similar species difference in local circuitry could be responsible for the phase reversal between diurnal and nocturnal species in metabolic rhythms controlled by the autonomic nervous system (Kalsbeek et al., 2006). Our recent work comparing the distribution of GABAergic neurons of diurnal Nile grass rats and nocturnal laboratory rats provides evidence supporting a more general version of this hypothesis (Langel et al., 2018). Specifically, we found in the lateral habenula, a cluster of GABAeric neurons was present in diurnal Nile grass rats, but completely absent in nocturnal laboratory rats (Fig. 2). The lateral habenula has been implicated in regulation of circadian rhythms. Lesioning of its major efferent fiber bundle leads to an altered temporal organization of activity in animals kept in constant conditions (Paul et al., 2011) and reduces the quantity of REM sleep (Haun et al., 1992; Valjakka et al., 1998). The distinct distribution of GABAergic and glutamatergic neurons in local circuits within the habenula may contribute to the switch from a diurnal to a nocturnal pattern in extra-SCN brain brain regions.
Figure 2.
Schematic showing the peak phase of PER2 expression in the SCN, bed nucleus of the stria terminalis oval (BNST-ov), central amygdala (CEA), basolateral amygdala (BLA) and dentate gyrus (DG) in diurnal Nile grass rats and nocturnal laboratory rats. Comparing the diurnal Nile grass rats and nocturnal laboratory rats, the expression of PER2 is in-phase in the SCN, but anti-phased in the BNST-ov and CEA. In the BLA, PER2 is high in laboratory rats from late night to early morning, while in grass rats, there is an acute peak in late afternoon. In the DG of laboratory rats, PER2 peaks in early morning, while in the DG of grass rats, PER2 expression is not rhythmic (Amir et al., 2004; Lamont et al., 2005; Ramanathan et al., 2010a; Ramanathan et al., 2010b). *When Nile grass rats were exposed to running wheels, there was elevated PER2 expression in DG when animals are running on the wheels (Ramanathan et al., 2010b).
Signals from the SCN have a far-reaching impact on neuronal circuits within the brain, but they also influence oscillators throughout the body. One way that they do this is through their effects on the endocrine system, e.g. the adrenal gland, which releases its hormones in a rhythmic pattern with the rise occurring just prior to the active period of the day. This release of glucocorticoids can then set the phase of extra-SCN clocks throughout the body; the adrenal gland thus functions as something of a secondary node in the output system (Balsalobre et al., 2000; Le Minh et al., 2001; Leliavski et al., 2015). Glucocorticoid rhythms are 180° out of phase in diurnal and nocturnal species and could thus contribute to diurnality in an important way, i.e. by setting the phase of rhythms in an array of peripheral tissues and extra-SCN oscillators. Feeding and general activity patterns also play an important role in entraining rhythms of extra-SCN oscillators that are very different in diurnal and nocturnal species, as diurnal animals eat and are physically active mostly during the day and nocturnal ones eat and are active at night (Mendoza et al., 2010; Schroder & Esser, 2013). Depending on the timing of meals, restricted feeding schedules not only induce anticipatory activity during the rest phase of the species, but also shift the phase of extra-SCN oscillators in the brain and peripheral tissues (Angeles-Castellanos et al., 2007; Verwey & Amir, 2009). Meanwhile, exercise or physical activity at different time of day has also been shown to entrain circadian rhythms at least in skeletal muscles (Schroder & Esser, 2013). Thus, in addition to possible direct control of extra-SCN oscillators, the SCN exerts indirect influences on the circadian phases of other oscillators by controlling the patterns of daily feeding and physical activity differentially in diurnal and nocturnal species (Ramanathan et al., 2010a; Otalora et al., 2013).
In summary, the circadian system of diurnal mammals, which evolved from that of their nocturnal ancestors, is similar in many ways to that of their nocturnal counterpart. The anatomical loci of the master clock and the molecular machinery underlying circadian timekeeping within central and peripheral oscillators are all well conserved. However, various other mechanisms, as discussed above, changed to promote a diurnal activity pattern, under the daily 24 hr LD cycle.
Photic entrainment of daily rhythms
The endogenously generated circadian rhythms are synchronized to local geographical time, i.e. the daily 24 hr LD cycle, through the process of photic entrainment. There are two conceptual models of photic entrainment: namely non-parametric and parametric, which are associated with the effects of discrete light pulses or continuous light exposure respectively (Daan, 1977).
Following discrete light pulses, animals show phase-dependent changes in their circadian rhythms. In both diurnal and nocturnal rodents, light pulses in early night produce phase delays while light pulses in late night lead to phase advances of rhythms (Kramm, 1975; Daan & Pittendrigh, 1976; Pohl et al., 1982; Schwartz & Zimmerman, 1990; Lee & Labyak, 1997; Kas & Edgar, 2000; Mahoney et al., 2001; Caldelas et al., 2003; Lahmam et al., 2008). However, the behavioral responses of the two chronotypes differ when the light pulses are presented during the subjective day, with the nocturnal species showing a “dead zone” during which no phase shifts occur in response to light pulses, whereas diurnal ones are responsive during most, or all, of that interval. The evolution of these differences may be related to when the animals are actually exposed to light in nature. For nocturnal species that are underground or in caves during their inactive (light) phase of the day, the brief light pulses at early or late subjective night would be the only light they see. When the endogenous free-running period is shorter than 24 hours then the light at dusk, the beginning of the active period, would elicit delays, whereas if the endogenous period is longer than 24 hours light at dawn would hit the system when advances are elicited; both would entrain the rhythm to the daily LD cycle. Diurnal species on the other hand, are exposed to light continuously if they live above ground, or to discrete light pulses throughout the day if they are underground (Hut et al., 1999). Thus, light exposure during daytime would be more critical for entraining their circadian rhythms, and the responsiveness of diurnal species to light during the day or subjective day is more functionally relevant compared to that in nocturnal species.
The responses of the circadian system to light pulses have also been analyzed directly in the SCN by measuring electrical activity and clock gene expression. In contrast to nocturnal rats in which the majority of SCN neurons show increased firing rate following nighttime light exposure, in diurnal ground squirrels and degus most SCN neurons are suppressed by light (Meijer et al., 1986; Meijer et al., 1989; Jiao et al., 1999). These studies also provided some evidence that the response threshold of these cells to light of different intensities may be lower in the rats than in the diurnal rodents. On the other hand, light-induced expression of clock genes appeared to be very similar in diurnal and nocturnal rodents (Shigeyoshi et al., 1997; Yan et al., 1999; Miyake et al., 2000; Caldelas et al., 2003; Novak et al., 2006; Ramanathan et al., 2009). In both chronotypes, signficant induction of clock genes, i.e. per1 and per2, was observed following light pulses presented during the subjective night. As discussed above, behavioral rhythms of diurnal species have no, or very short, “dead zones” during the subjective day compared to nocturnal ones. Thus, studies investigating how their SCN neurons respond to light in the subjective day will help provide a more complete picture of photic entrainment in diurnal mammals (Caldelas et al., 2003). Furthermore, the SCN neurons are heterogenous in terms of their anatomical connections, neurochemical identities and photic responsiveness (Yan et al., 2007). A detailed analysis of the spatiotemporal responses of different neuronal populations in the SCN during the process of photic entrainment will also help us understand how light resets the principal brain clock and if it does this in the same manner in nocturnal and diurnal species (Yan & Okamura, 2002; Yan & Silver, 2002; 2004; Ramanathan et al., 2009; Yan, 2009).
The non-parametric paradigm has been a powerful tool for dissecting the cellular and molecular pathways involved in photic entrainment. However, in their natural environment, animals, particularly day-active ones, are often exposed to a daily extended period of light instead of only discrete pulses (Hut et al., 1999). Entrainment to the daily LD cycle is generally thought to be mediated by both nonparametric effects of light at dawn or dusk and tonic, parametric, effects that the more continuous light has throughout the day, a notion that is based primarily on findings from nocturnal species (Daan & Pittendrigh, 1976; Daan, 1977). Parametric effects are evident when there is no LD cycle and animals are free-running but the light intensities are increased or decreased. Aschoff reported that under these conditions an increase in light intensity increased the period of the rhythms of nocturnal species and decreased it in diurnal ones (Aschoff, 1960). However, this “Aschoff’s rule” has not held up over time, particularly for diurnal mammals (Moore-Ede et al., 1982). In Nile grass rats, for example, increases in light intensity lead to increases in the free-running period (Katona & Smale, 1997).
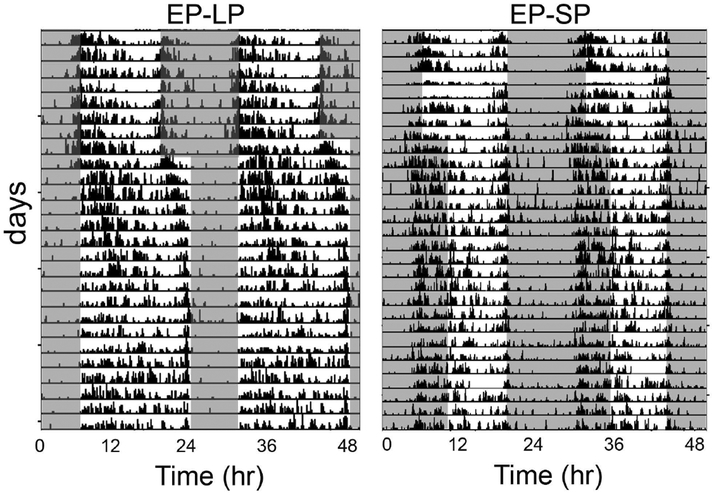
Parametric effects of light are also apparent when animals are entrained to a 24 hour cycle but the duration of the light phase is changed, i.e. under different photoperiods (day-lengths). When this happens a difference between the chronotypes becomes apparent. Nocturnal species show expansion of the active phase in short days and compression of the active phase in long day-lengths (Nuesslein-Hildesheim et al., 2000; Refinetti, 2004a; Sumova et al., 2004; VanderLeest et al., 2007). Diurnal species on the other hand, in general show the opposite responses, i.e. their active phase expands in long days and is compressed in short days (Sulzman et al., 1982; Challet et al., 2002; Lincoln et al., 2002; Lahmam et al., 2008). Interestingly, in diurnal grass rats, although their active phase is extended when day-length is increased from 12 hr to 16 hr (16:8 LD), the duration of the active phase does not change when day-length is shortened from 12 hr to 8 hr (8:16 LD) in both Nile grass rats (Refinetti, 2004a; Leach et al., 2013) and Sudanese grass rats (Itzhacki et al., 2018) (Fig. 3). The stability of entrainment, measured by the precision of activity onset and offset times, is also affected by daylength, with more stable entrainment seen in long days than in short days (Leach et al., 2013).
Figure 3.
A cluster of GABAergic neurons presents in the lateral habenula (LHb) of diurnal Nile grass rats (left panel), but absent in the same region of nocturnal laboratory rats (right panel). MHb, medial habenula. Scale bar, 200 μm.
Within the SCN, rhythms in expression of clock genes also respond to photoperiodic changes, showing expansion or compression in the duration of the peak phase, through phase dispersion or concentration of cellular oscillators along the rostral-caudal axis of the SCN in nocturnal rodents (Inagaki et al., 2007; Yan & Silver, 2008). In Nile grass rats, consistent with the behavioral response, the phase of peak PER1 expression in the SCN expanded when the animals were switched from 12:12 to 16:8 LD, but did not compress when changed from 12:12 to 8:16 LD condition (Leach et al., 2013). The diurnal grass rats are originally from areas near the equator (Nile grass rats, 3°S; Sudanese grass rats, 20°N), thus their circadian clock probably has not been shaped by a history of exposure to drastic photoperiodic changes with daylength fluctuating from 8 to 16 hr across seasons, which occurs at ~50° from the equator. However, the Nile grass rats entrain well under a 16:8 LD cycle but not to a 8:16 LD cycle, suggesting that their circadian clock is better able to adapt to long days than short days (Leach et al., 2013). This selective non-responsiveness of their circadian system to short days makes these animals a unique and important model for study of conditions related to problems associated with adaptation to fall or winter conditions, such as seasonal affective disorder (Leach et al., 2013; Itzhacki et al., 2018).
Photic entrainment is a critical process in both diurnal and nocturnal species that allows the endogenously generated circadian rhythms to be synchronized to the daily LD cycle. Another important process that regulates the temporal profile of daily rhythms in behavior and physiology is through the circadian-independent direct effects of light, namely masking.
Masking in diurnal mammals
Masking plays an important role in the regulation of the temporal niche in which a species is most active. In sharp contrast with the similarities shown across species for the effects of light on the entrainment of circadian rhythms (see above), masking of behavior by light has opposite effects. Light increases activity and promotes arousal in diurnal species while reduces activity and induces sleep in nocturnal ones (Redlin, 2001). These clear opposite effects of light on behavior raise the question of mechanisms, that is, what are the underlying causes for the species differences?
a. Differential sensitivity to wavelength between diurnal and nocturnal species?
In addition to reducing activity and inducing sleep, light exposure triggers an arousal response in nocturnal mice that is accompanied by an increase in plasma corticosterone (Ishida et al., 2005). Further, Pilorz et al. have shown that the two responses are melanopsin dependent, but can be dissociated by manipulating the wavelength of the light stimulus; blue light exposure is associated with increased plasma corticosterone and green light is associated with the induction of sleep (Pilorz et al., 2016). Functional studies also suggest that the two responses are mediated by different neural circuits (Pilorz et al. 2016). Based on these observations, Bourgin and Hubbard have proposed the hypothesis that the differential responses to polychromatic light shown by nocturnal and diurnal species stem from differential sensitivity to the effects of particular wavelengths, such that more sensitivity to green light in nocturnal species and more sensitivity to blue light in diurnal species (Bourgin & Hubbard, 2016). This interesting proposition deserves further consideration, and should be directly tested by comparing behavioral responses of diurnal and nocturnal species to light of different wavelengths. However, in diurnal species polychromatic light exposure results in a sustained increase in activity and arousal, thus it seems unlikely that correlates of a stress response (e.g., corticosterone secretion) would be present during light-induced alertness in a species adapted to be active during the day; in fact, bright light exposure reduces cortisol level in humans (Jung et al., 2010). The acute stress response to an abrupt presentation of light is likely a common immediate and short-term response of both diurnal and nocturnal species, particularly small rodents. The sustained alertness of diurnal species in response to light is probably independent of mechanisms that mediate stress responses to abrupt changes in illumination, and are likely to involve neural circuits that play different roles in diurnal and nocturnal species. Results discussed in what follows indicate that light has very different effects on several brain regions of diurnal and nocturnal rodents.
Differential cFOS responses to light by diurnal and nocturnal brains?
Although several manipulations of light exposure have been used to compare diurnal and nocturnal species (Rotics et al., 2011; Shuboni et al., 2012), one useful approach for comparative studies of masking has been to focus on methods of presenting the masking stimuli using parameters that consistently produce opposite behavioral responses in diurnal and nocturnal rodents (Shuboni et al., 2012) and then assess species differences in cFOS responses in regions of the brain that receive inputs from the intrinsic photosensitive retinal ganglion cells (ipRGCs) (Shuboni et al., 2015). That strategy revealed clear opposite effects of a 1-hr pulse of light presented 2 hours after the onset of darkness between mice and Nile grass rats, such that the same light stimulation increased general activity in Nile grass rats and reduced it in mice for the duration of the presentation while the animals were on a 12/12 hr LD cycle (Shuboni et al., 2012). The induction of cFOS expression was also very different for the two species in extra-SCN areas of the brain that receive projections from ipRGCs in both mice (Hattar et al., 2003; Hattar et al., 2006) and Nile grass rats (Langel et al., 2015). Specifically, for the lateral hypothalamus (LH), the intergeniculate leaflet (IGL), the ventral subparaventricular zone (VSPZ) and the olivary pretectal nucleus (OPT) a 1-hr pulse of light delivered 2 hours after the onset of darkness significantly increased cFOS expression in Nile grass rats, with either no change (LH, VSPZ, IGL) or the opposite response (OPT) in mice (Shuboni et al., 2015). The results for the LH may reflect the differential responses to light shown by the orexin neurons that reside there, which are activated by light in diurnal Nile grass rats (Adidharma et al., 2012), but not in nocturnal mice (Mendoza et al., 2010). In contrast, dark pulses, which represent arousal cues for nocturnal species, activate the orexin neurons of mice (Marston et al., 2008). Overall, these results are consistent with the view that functional differences in regions of the brain that receive inputs from ipRGCs mediate the divergent masking responses to light of diurnal and nocturnal species.
c. What brain regions mediate masking responses to light in diurnal species?
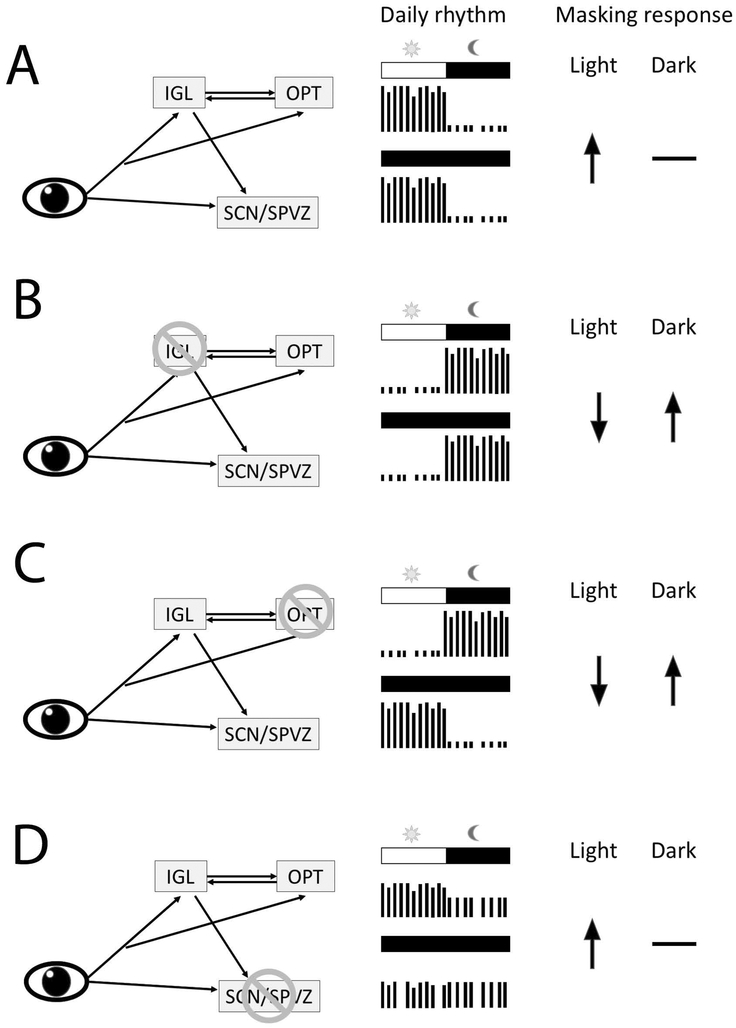
The results of experiments identifying areas of the brain that show patterns of cFOS expression that match species differences in behavior has guided lesion studies focusing on the IGL, VSPZ and OPT of Nile grass rats (Gall et al., 2013; Gall et al., 2016; Gall et al., 2017). In these animals, IGL lesions reverse the phase preference of the species; post-surgically the animals were nocturnal in their display of general activity (Gall et al., 2013). This phase reversal persisted when the animals were placed in constant darkness, during which high levels of activity were present for most of the subjective night. With respect to masking, the IGL lesions profoundly affected the responses of Nile grass rats to 1-hr pulses of light (presented 2 hrs after lights off) or darkness (presented during the first hour of the light phase). The light pulse resulted in a five-fold increase in activity in the control animals, but significantly reduced activity in the animals with IGL lesions. In contrast, the dark pulse did not affect the level of activity of the control animals, but significantly increase that of the Nile grass rats with IGL lesions. Thus, after IGL lesions, the profile of the naturally diurnal Nile grass rats was transformed to one that resembles that of nocturnal species (Gall et al., 2013). In the Octodon degus, another diurnal species, IGL lesions also increased the presence of nocturnal activity, although masking responses were not directly evaluated in that study (Goel et al., 2000). In nocturnal rodents, IGL lesions affect some circadian parameters but do not reverse the chronotype of the animals (Harrington & Rusak, 1988; Pickard, 1994), and masking responses to light typical of nocturnal species are either unaffected or enhanced by the lesions (Edelstein & Amir, 1999; Redlin et al., 1999). Thus, in the absence of the IGL, rodents display nocturnal features regardless of the predominant chronotype of the species.
IGL lesions abolished the cFOS response in the VSPZ and reversed the direction of the effect of light on cFOS expression in the OPT, following a 1-hr light pulse delivered 2 hours into the dark phase of a 12/12 hr LD cycle (Gall et al., 2014). The OPT has reciprocal connections with the IGL (Moore et al., 2000) and receives retinal inputs from ipRGCs in both nocturnal rodents (Hattar et al., 2006) and diurnal Nile grass rats (Langel et al., 2015). In Nile grass rats, lesions of the OPT also had a profound effect on masking responses (Gall et al., 2017). Like grass rats with IGL lesions, those with OPT damage showed responses to light and dark pulses that resembled those of nocturnal rodents. This resulted in enhanced nocturnal activity under a LD cycle, but different from the results with IGL lesions, the distribution of activity during the subjective night returned to normal when the animals were observed under constant darkness. Thus, both an intact OPT and IGL are needed for Nile grass rats to show their species typical masking responses, but only the IGL is necessary for the circadian regulation of diurnal behavior in these animals. OPT lesions also abolished the cFOS response in the ventrolateral geniculate nucleus (VGL) to light pulses (Gall et al., 2017). The projections from ipRGCs to the VGL are reduced in Nile grass rats (Langel et al., 2015) compared to those of nocturnal rodents (Hattar et al., 2006). Thus in Nile grass rats, connections with the OPT may be necessary for light to affect the VLG, and the reduced light responsiveness of the VLG of Nile grass rats may play a role in mediating the effects of OPT lesions on masking responses in this species.
Different from the salient effects of OPT and IGL lesions on the masking responses of Nile grass rats, lesions of the VSPZ with or without damage to the SCN, had no effects on the masking responses of these animals (Gall et al., 2016). The lesions however disrupted circadian activity rhythms and the circadian modulation of the magnitude of masking responses when light pulses are presented at different circadian times (Gall et al., 2016). The data from animals with SCN damage challenges the claim that an intact SCN is necessary for masking responses to light (Li et al., 2005), and are consistent with observations of intact masking responses in the absence of a functional SCN (Fuller et al., 1981; Mistlberger, 1992; Redlin & Mrosovsky, 1999).
Thus, from the lesion studies with Nile grass rats, the working model that emerges identifies the OPT as necessary for the display of masking responses to light typical of diurnal species, possibly via connections with the VLG and IGL (Gall et al., 2017). The IGL in turn is not only necessary for normal diurnal masking responses, but is also involved in the circadian regulation of activity (Gall et al., 2013). Finally, the SCN and VSPZ are not necessary for masking responses to light, but are involved in both the circadian regulation of activity and the circadian modulation of masking responses in Nile grass rats. It is important to test the generality of this working model using other diurnal mammals.
Temporal phenotype as a biological variable
In summary, the temporal niche in which a species is most active is shaped by its evolutionary history, and influenced by the interplay between the circadian system and the direct responses to light, i.e. masking. Although at the behavioral level, diurnal mammals are 180° out of phase from nocturnal ones, their brains are not simply operating in reverse of those of their nocturnal counterparts. There are striking anatomical differences between diurnal and nocturnal brains, including those associated with sensory adaptions, e.g. the large superior colliculi of Nile grass rats (Gaillard et al., 2013), as well as others that may be involved in circadian and photic regulation e.g. GABAergic neurons in the habenula (Fig. 2), and the differential distribution of receptors for the wakefulness promoting neuropeptide orexin (Ikeno & Yan, 2018). Particularly with respect to circadian regulation, although most of the extra-SCN oscillators in the brain and in peripheral tissues show a phase reversal between diurnal and nocturnal species, that reversal is not universal (Fig. 1). Finally, diurnal and nocturnal mammals show different behavioral responses to identical light and dark stimuli (Shuboni et al., 2012), which are matched by the distinct brain responses to the same stimuli in retinorecipient regions (Shuboni et al., 2015). These regions include the OPT, IGL and VGL that regulate masking responses (Gall et al., 2013; Gall et al., 2016).
Clearly, the normal function of diurnal brains and how they respond to perturbation involving altered light conditions cannot be fully understood based solely on studies of nocturnal models. In the biomedical field, much effort has been made to consider sex as a biological variable (Clayton, 2018). We would like to suggest that, in addition to sex differences, temporal phenotype differences, such that whether the animals are diurnal or nocturnal, present another biological variable with key implications for the translational value of research with animal models. It is important to consider how diurnal and nocturnal model species are similar and different, and how the data generated from those models should be interpreted for gaining insights into the circadian and photic modulation of daily rhythms that are unique for diurnal mammals including humans. Such knowledge will contribute to a better understanding of the neural mechanisms underlying human health problems caused by altered light conditions that are associated with shift work and modern life style in a 24-hr society.
Figure 4.
Daily rhythms of Nile grass rats following photoperiodic changes. Representative double-plotted actograms of two grass rats that were housed initially in equatorial photoperiods (EP,12:12hr light/dark) and then exposed to long photoperiods (LP 16:8hr light/dark, left panel), or to short photoperiods (SP, 8:16hr light/dark, right panel). Adapted from (Leach et al., 2013) with copyright permission.
Figure 5.
The intergeniculate leaflet (IGL), olivary pretectal nucleus (OPT), suprachiasmatic nucleus (SCN) and the ventral subparaventricular zone (VSPZ) are involved in regulating daily rhythms and masking responses in the diurnal Nile grass rats. All of these regions receive direct retinal inputs and are interconnected. Schematics depicting the daily rhythms and masking responses to light or darkness when the network is intact (A), or following the lesion of IGL (B), OPT (C) and the SCN/SPVZ (D). ↑, promotes activity; ↓, inhibits activity; -, has no effect. (Gall et al., 2013; Gall et al., 2016).
Acknowledgements
The work discussed in this review on Nile grass rats was supported by NIH R01MH53433 to LS and AAN, NSF IBN-0130977 to LS an AAN, NSF grant IOS-1051919, to LS, AAN, and LY, and NIH R03MH093760 to LY.
Abbreviations
- AVP
arginine vasopressin
- BLA
basolateral amygdala
- BNST-ov
bed nucleus of the stria terminalis oval
- CEA
central amygdala
- DG
dentate gyrus
- EP
equatorial photoperiods
- IGL
intergeniculate leaflet
- LD
light/dark
- LHb
lateral habenula
- LP
long photoperiods
- OPT
olivary pretectal nucleus
- SCN
suprachiasmatic nucleus
- SP
short photoperiods
- VGL
ventrolateral geniculate nucleus
- VSPZ
ventral subparaventricular zone
Footnotes
Conflict of Interest
The author declares that there is no potential sources of conflict of interest.
References
- Adidharma W, Leach G & Yan L (2012) Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neuroscience, 220, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S, Lamont EW, Robinson B & Stewart J (2004) A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J Neurosci, 24, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson H, Johnston JD, Messager S, Hazlerigg D & Lincoln G (2005) Photoperiod regulates clock gene rhythms in the ovine liver. Gen Comp Endocrinol, 142, 357–363. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J & Escobar C (2007) Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience, 144, 344–355. [DOI] [PubMed] [Google Scholar]
- Aschoff J (1960) Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol, 25, 11–28. [DOI] [PubMed] [Google Scholar]
- Aschoff J (1999) Masking and parametric effects of high-frequency light-dark cycles. The Japanese journal of physiology, 49, 11–18. [DOI] [PubMed] [Google Scholar]
- Aschoff J & von Goetz C (1988) Masking of circadian activity rhythms in hamsters by darkness. Journal of Comparative Physiology A, 162, 559–562. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G & Schibler U (2000) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science, 289, 2344–2347. [DOI] [PubMed] [Google Scholar]
- Blanchong JA & Smale L (2000) Temporal patterns of activity of the unstriped Nile rat, Arvicanthis niloticus. Journal of Mammalogy, 81, 595–599. [Google Scholar]
- Bobu C, Craft CM, Masson-Pevet M & Hicks D (2006) Photoreceptor organization and rhythmic phagocytosis in the nile rat Arvicanthis ansorgei: a novel diurnal rodent model for the study of cone pathophysiology. Invest Ophthalmol Vis Sci, 47, 3109–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin P & Hubbard J (2016) Alerting or Somnogenic Light: Pick Your Color. PLoS Biol, 14, e2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton KJ, Li X, Li B, Cheng MY, Urbanski HF & Zhou QY (2016) Expression of prokineticin 2 and its receptor in the macaque monkey brain. Chronobiol Int, 33, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldelas I, Poirel VJ, Sicard B, Pevet P & Challet E (2003) Circadian profile and photic regulation of clock genes in the suprachiasmatic nucleus of a diurnal mammal Arvicanthis ansorgei. Neuroscience, 116, 583–591. [DOI] [PubMed] [Google Scholar]
- Chakir I, Dumont S, Pevet P, Ouarour A, Challet E & Vuillez P (2015) The circadian gene Clock oscillates in the suprachiasmatic nuclei of the diurnal rodent Barbary striped grass mouse, Lemniscomys barbarus: a general feature of diurnality? Brain Res, 1594, 165–172. [DOI] [PubMed] [Google Scholar]
- Challet E, Pitrosky B, Sicard B, Malan A & Pevet P (2002) Circadian organization in a diurnal rodent, Arvicanthis ansorgei Thomas 1910: chronotypes, responses to constant lighting conditions, and photoperiodic changes. J Biol Rhythms, 17, 52–64. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM & Zhou QY (2002) Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature, 417, 405–410. [DOI] [PubMed] [Google Scholar]
- Clayton JA (2018) Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol Behav, 187, 2–5. [DOI] [PubMed] [Google Scholar]
- Cohen R, Smale L & Kronfeld-Schor N (2010) Masking and temporal niche switches in spiny mice. J Biol Rhythms, 25, 47–52. [DOI] [PubMed] [Google Scholar]
- Crompton AW, Taylor CR & Jagger JA (1978) Evolution of homeothermy in mammals. Nature, 272, 333–336. [DOI] [PubMed] [Google Scholar]
- Cross N & Rogers LJ (2004) Diurnal cycle in salivary cortisol levels in common marmosets. Dev Psychobiol, 45, 134–139. [DOI] [PubMed] [Google Scholar]
- Cuesta M, Clesse D, Pévet P & Challet E (2009) From daily behavior to hormonal and neurotransmitters rhythms: comparison between diurnal and nocturnal rat species. Hormones and behavior, 55, 338–347. [DOI] [PubMed] [Google Scholar]
- Daan S (1977) Tonic and phasic effects of light in the entrainment of circadian rhythms. Ann N Y Acad Sci, 290, 51–59. [DOI] [PubMed] [Google Scholar]
- Daan S & Pittendrigh CS (1976) A functional analysis of circadian pacemakers in cocturnal rodents. II. The variety of phase response curves. J. Comp. Physiol, 106, 253–266. [Google Scholar]
- Davidson AJ, Yamazaki S & Menaker M (2003) SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found Symp, 253, 110–121; discussion 121–115, 281–114. [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J & Schwartz WJ (2003) Lateralization of circadian pacemaker output: Activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci, 23, 7412–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T (1964) Biology, molecular and organismic. American Zoologist, 443–452. [DOI] [PubMed] [Google Scholar]
- Edelstein K & Amir S (1999) The role of the intergeniculate leaflet in entrainment of circadian rhythms to a skeleton photoperiod. J Neurosci, 19, 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller CA, Lydic R, Sulzman FM, Albers HE, Tepper B & Moore-Ede MC (1981) Circadian rhythm of body temperature persists after suprachiasmatic lesions in the squirrel monkey. Am J Physiol, 241, R385–391. [DOI] [PubMed] [Google Scholar]
- Gaillard F, Bonfield S, Gilmour GS, Kuny S, Mema SC, Martin BT, Smale L, Crowder N, Stell WK & Sauve Y (2008) Retinal anatomy and visual performance in a diurnal cone-rich laboratory rodent, the Nile grass rat (Arvicanthis niloticus). J Comp Neurol, 510, 525–538. [DOI] [PubMed] [Google Scholar]
- Gaillard F, Karten HJ & Sauve Y (2013) Retinorecipient areas in the diurnal murine rodent Arvicanthis niloticus: a disproportionally large superior colliculus. J Comp Neurol, 521, 1699–1726. [DOI] [PubMed] [Google Scholar]
- Gaillard F, Kuny S & Sauve Y (2009) Topographic arrangement of S-cone photoreceptors in the retina of the diurnal Nile grass rat (Arvicanthis niloticus). Invest Ophthalmol Vis Sci, 50, 5426–5434. [DOI] [PubMed] [Google Scholar]
- Gall AJ, Khacherian OS, Ledbetter B, Deats SP, Luck M, Smale L, Yan L & Nunez AA (2017) Normal behavioral responses to light and darkness and the pupillary light reflex are dependent upon the olivary pretectal nucleus in the diurnal Nile grass rat. Neuroscience, 355, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Shuboni DD, Yan L, Nunez AA & Smale L (2016) Suprachiasmatic Nucleus and Subparaventricular Zone Lesions Disrupt Circadian Rhythmicity but Not Light-Induced Masking Behavior in Nile Grass Rats. J Biol Rhythms, 31, 170–181. [DOI] [PubMed] [Google Scholar]
- Gall AJ, Smale L, Yan L & Nunez AA (2013) Lesions of the Intergeniculate Leaflet Lead to a Reorganization in Circadian Regulation and a Reversal in Masking Responses to Photic Stimuli in the Nile Grass Rat. PLoS One, 8, e67387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Yan L, Smale L & Nunez AA (2014) Intergeniculate leaflet lesions result in differential activation of brain regions following the presentation of photic stimuli in Nile grass rats. Neurosci Lett, 579, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerkema MP, Davies WI, Foster RG, Menaker M & Hut RA (2013) The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc. R. Soc. B, 280, 20130508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour GS, Gaillard F, Watson J, Kuny S, Mema SC, Bonfield S, Stell WK & Sauve Y (2008) The electroretinogram (ERG) of a diurnal cone-rich laboratory rodent, the Nile grass rat (Arvicanthis niloticus). Vision Res, 48, 2723–2731. [DOI] [PubMed] [Google Scholar]
- Goel N, Governale MM, Jechura TJ & Lee TM (2000) Effects of intergeniculate leaflet lesions on circadian rhythms in Octodon degus. Brain research, 877, 306–313. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH & Lee TM (2008) Circadian organization of the diurnal Caviomorph rodent, Octodon degus. Biological Rhythm Research, 39, 269–289. [Google Scholar]
- Harrington ME & Rusak B (1988) Ablation of the geniculo-hypothalamic tract alters circadian activity rhythms of hamsters housed under constant light. Physiol Behav, 42, 183–189. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW & Berson DM (2006) Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol, 497, 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG & Yau KW (2003) Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature, 424, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun F, Eckenrode TC & Murray M (1992) Habenula and thalamus cell transplants restore normal sleep behaviors disrupted by denervation of the interpeduncular nucleus. J Neurosci, 12, 3282–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut RA, van Oort BE & Daan S (1999) Natural entrainment without dawn and dusk: the case of the European ground squirrel (Spermophilus citellus). J Biol Rhythms, 14, 290–299. [DOI] [PubMed] [Google Scholar]
- Ikeno T, Williams CT, Buck CL, Barnes BM & Yan L (2017) Clock Gene Expression in the Suprachiasmatic Nucleus of Hibernating Arctic Ground Squirrels. J Biol Rhythms, 32, 246–256. [DOI] [PubMed] [Google Scholar]
- Ikeno T & Yan L (2018) A comparison of the orexin receptor distribution in the brain between diurnal Nile grass rats (Arvicanthis niloticus) and nocturnal mice (Mus musculus). Brain Res, 1690, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Honma S, Ono D, Tanahashi Y & Honma K (2007) Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc Natl Acad Sci U S A, 104, 7664–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye ST & Kawamura H (1979) Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A, 76, 5962–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G & Okamura H (2005) Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab, 2, 297–307. [DOI] [PubMed] [Google Scholar]
- Itzhacki J, Clesse D, Goumon Y, Van Someren EJ & Mendoza J (2018) Light rescues circadian behavior and brain dopamine abnormalities in diurnal rodents exposed to a winter-like photoperiod. Brain Struct Funct. [DOI] [PubMed] [Google Scholar]
- Jiao YY, Lee TM & Rusak B (1999) Photic responses of suprachiasmatic area neurons in diurnal degus (Octodon degus) and nocturnal rats (Rattus norvegicus). Brain Res, 817, 93–103. [DOI] [PubMed] [Google Scholar]
- Jung CM, Khalsa SB, Scheer FA, Cajochen C, Lockley SW, Czeisler CA & Wright KP Jr. (2010) Acute effects of bright light exposure on cortisol levels. J Biol Rhythms, 25, 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM, van Heerikhuize JJ, Arts M & van der Woude TP (1992) Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosteron release. Brain Res, 580, 62–67. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C & Buijs RM (2006) SCN outputs and the hypothalamic balance of life. J Biol Rhythms, 21, 458–469. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van Heerikhuize JJ, Wortel J & Buijs RM (1996) A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci, 16, 5555–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Verhagen LA, Schalij I, Foppen E, Saboureau M, Bothorel B, Buijs RM & Pevet P (2008) Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. Eur J Neurosci, 27, 818–827. [DOI] [PubMed] [Google Scholar]
- Kas MJ & Edgar DM (2000) Photic phase response curve in Octodon degus: assessment as a function of activity phase preference. Am J Physiol Regul Integr Comp Physiol, 278, R1385–1389. [DOI] [PubMed] [Google Scholar]
- Katona C & Smale L (1997) Wheel-running rhythms in Arvicanthis niloticus. Physiology & behavior, 61, 365–372. [DOI] [PubMed] [Google Scholar]
- Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC & Weitz CJ (2001) Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science, 294, 2511–2515. [DOI] [PubMed] [Google Scholar]
- Kramm KR (1975) Circadian activity of the red squirrel, Tamiasciurus hudsonicus, in continuous darkness and continuous illumination. Int J Biometeorol, 19, 232–245. [DOI] [PubMed] [Google Scholar]
- Kronfeld-Schor N & Dayan T (2008) Activity patterns of rodents: the physiological ecology of biological rhythms. Biological Rhythm Research, 39, 193–211. [Google Scholar]
- Lahmam M, El M’rabet A, Ouarour A, Pevet P, Challet E & Vuillez P (2008) Daily behavioral rhythmicity and organization of the suprachiasmatic nuclei in the diurnal rodent, Lemniscomys barbarus. Chronobiol Int, 25, 882–904. [DOI] [PubMed] [Google Scholar]
- Lambert CM, Machida KK, Smale L, Nunez AA & Weaver DR (2005) Analysis of the prokineticin 2 system in a diurnal rodent, the unstriped Nile grass rat (Arvicanthis niloticus). J Biol Rhythms, 20, 206–218. [DOI] [PubMed] [Google Scholar]
- Lambert CM & Weaver DR (2006) Peripheral gene expression rhythms in a diurnal rodent. J Biol Rhythms, 21, 77–79. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Robinson B, Stewart J & Amir S (2005) The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci U S A, 102, 4180–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langel JL, Smale L, Esquiva G & Hannibal J (2015) Central melanopsin projections in the diurnal rodent, Arvicanthis niloticus. Front Neuroanat, 9, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langel J, Ikeno T, Yan L, Nunez AA, Smale L. Distributions of GABAergic and glutamatergic neurons in the brains of a diurnal and nocturnal rodent. Brain Res. 2018. August 25 pii: S0006-8993(18)30441-4. doi: 10.1016/j.brainres.2018.08.019. [Epub ahead of print] PMID: [DOI] [PubMed] [Google Scholar]
- Le Minh N, Damiola F, Tronche F, Schutz G & Schibler U (2001) Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. Embo J, 20, 7128–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach G, Ramanathan C, Langel J & Yan L (2013) Responses of brain and behavior to changing day-length in the diurnal grass rat (Arvicanthis niloticus). Neuroscience, 234, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Carmichael MS & Zucker I (1986) Circannual variations in circadian rhythms of ground squirrels. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 250, R831–R836. [DOI] [PubMed] [Google Scholar]
- Lee TM & Labyak SE (1997) Free-running rhythms and light- and dark-pulse phase response curves for diurnal Octodon degus (Rodentia). Am J Physiol, 273, R278–286. [DOI] [PubMed] [Google Scholar]
- Leliavski A, Dumbell R, Ott V & Oster H (2015) Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J Biol Rhythms, 30, 20–34. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Downs JL & Urbanski HF (2006) Twenty-four-hour rhythmic gene expression in the rhesus macaque adrenal gland. Mol Endocrinol, 20, 1164–1176. [DOI] [PubMed] [Google Scholar]
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, Evans SJ, Choudary PV, Cartagena P, Barchas JD, Schatzberg AF, Jones EG, Myers RM, Watson SJ Jr., Akil H & Bunney WE (2013) Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A, 110, 9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gilbert J & Davis FC (2005) Disruption of masking by hypothalamic lesions in Syrian hamsters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol, 191, 23–30. [DOI] [PubMed] [Google Scholar]
- Lincoln G, Messager S, Andersson H & Hazlerigg D (2002) Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: evidence for an internal coincidence timer. Proc Natl Acad Sci U S A, 99, 13890–13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney M, Bult A & Smale L (2001) Phase response curve and light-induced fos expression in the suprachiasmatic nucleus and adjacent hypothalamus of Arvicanthis niloticus. J Biol Rhythms, 16, 149–162. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Ramanathan C, Hagenauer MH, Thompson RC, Smale L & Lee T (2009) Daily rhythms and sex differences in vasoactive intestinal polypeptide, VIPR2 receptor and arginine vasopressin mRNA in the suprachiasmatic nucleus of a diurnal rodent, Arvicanthis niloticus. Eur J Neurosci, 30, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MM & Smale L (2005) A daily rhythm in mating behavior in a diurnal murid rodent Arvicanthis niloticus. Horm Behav, 47, 8–13. [DOI] [PubMed] [Google Scholar]
- Maor R, Dayan T, Ferguson-Gow H & Jones KE (2017) Temporal niche expansion in mammals from a nocturnal ancestor after dinosaur extinction. Nat Ecol Evol, 1, 1889–1895. [DOI] [PubMed] [Google Scholar]
- Marston OJ, Williams RH, Canal MM, Samuels RE, Upton N & Piggins HD (2008) Circadian and dark-pulse activation of orexin/hypocretin neurons. Mol Brain, 1, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinny TL, Sisk CL, Holekamp KE & Smale L (1999) A morning surge in plasma luteinizing hormone coincides with elevated Fos expression in gonadotropin-releasing hormone-immunoreactive neurons in the diurnal rodent, Arvicanthis niloticus. Biol Reprod, 61, 1115–1122. [DOI] [PubMed] [Google Scholar]
- McElhinny TL, Smale L & Holekamp KE (1997) Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, Arvicanthis niloticus. Physiol Behav, 62, 91–96. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Groos GA & Rusak B (1986) Luminance coding in a circadian pacemaker: the suprachiasmatic nucleus of the rat and the hamster. Brain Res, 382, 109–118. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Rusak B & Harrington ME (1989) Photically responsive neurons in the hypothalamus of a diurnal ground squirrel. Brain Res, 501, 315–323. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Clesse D, Pevet P & Challet E (2010) Food-reward signalling in the suprachiasmatic clock. J Neurochem, 112, 1489–1499. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN & Bittman EL (1999) Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology, 140, 207–218. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE (1992) Nonphotic entrainment of circadian activity rhythms in suprachiasmatic nuclei-ablated hamsters. Behav Neurosci, 106, 192–202. [DOI] [PubMed] [Google Scholar]
- Miyake S, Sumi Y, Yan L, Takekida S, Fukuyama T, Ishida Y, Yamaguchi S, Yagita K & Okamura H (2000) Phase-dependent responses of Per1 and Per2 genes to a light-stimulus in the suprachiasmatic nucleus of the rat. Neurosci Lett, 294, 41–44. [DOI] [PubMed] [Google Scholar]
- Moore RY, Weis R & Moga MM (2000) Efferent projections of the intergeniculate leaflet and the ventral lateral geniculate nucleus in the rat. J Comp Neurol, 420, 398–418. [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC, Sulzman FM & Fuller CA (1982) The clocks that time us: physiology of the circadian timing system. Harvard Univ Pr. [Google Scholar]
- Mrosovsky N (1999) Masking: history, definitions, and measurement. Chronobiology international, 16, 415–429. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Edelstein K, Hastings MH & Maywood ES (2001) Cycle of period gene expression in a diurnal mammal (Spermophilus tridecemlineatus): implications for nonphotic phase shifting. J Biol Rhythms, 16, 471–478. [DOI] [PubMed] [Google Scholar]
- Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM & Panda S (2018) Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BA, Blake CM, Brown JA, Martin AM, Forde N, Sweeney LM & Evans AC (2015) Evidence of a molecular clock in the ovine ovary and the influence of photoperiod. Theriogenology, 84, 208–216. [DOI] [PubMed] [Google Scholar]
- Novak CM, Ehlen JC, Paul KN, Fukuhara C & Albers HE (2006) Light and GABA)(A) receptor activation alter period mRNA levels in the SCN of diurnal Nile grass rats. Eur J Neurosci, 24, 2843–2852. [DOI] [PubMed] [Google Scholar]
- Novak CM, Smale L & Nunez AA (1999) Fos expression in the sleep-active cell group of the ventrolateral preoptic area in the diurnal murid rodent, Arvicanthis niloticus. Brain Res, 818, 375–382. [DOI] [PubMed] [Google Scholar]
- Nuesslein-Hildesheim B, O’Brien JA, Ebling FJ, Maywood ES & Hastings MH (2000) The circadian cycle of mPER clock gene products in the suprachiasmatic nucleus of the siberian hamster encodes both daily and seasonal time. Eur J Neurosci, 12, 2856–2864. [DOI] [PubMed] [Google Scholar]
- Nunez AA & Stephan FK (1977) The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav Biol, 20, 224–234. [DOI] [PubMed] [Google Scholar]
- Nunez AA, Yan L & Smale L (2018) The Cost of Activity during the Rest Phase: Animal Models and Theoretical Perspectives. Front Endocrinol (Lausanne), 9, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H (2004) Clock genes in cell clocks: roles, actions, and mysteries. J Biol Rhythms, 19, 388–399. [DOI] [PubMed] [Google Scholar]
- Okamura H (2007) Suprachiasmatic nucleus clock time in the mammalian circadian system. Cold Spring Harb Symp Quant Biol, 72, 551–556. [DOI] [PubMed] [Google Scholar]
- Otalora BB, Hagenauer MH, Rol MA, Madrid JA & Lee TM (2013) Period gene expression in the brain of a dual-phasing rodent, the Octodon degus. J Biol Rhythms, 28, 249–261. [DOI] [PubMed] [Google Scholar]
- Palacios AG & Lee TM (2013) Husbandry and breeding in the Octodon degu (Molina 1782). Cold Spring Harbor Protocols, 2013, pdb. prot073577. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Indic P & Schwartz WJ (2011) A role for the habenula in the regulation of locomotor activity cycles. Eur J Neurosci, 34, 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard GE (1994) Intergeniculate leaflet ablation alters circadian rhythms in the mouse. Neuroreport, 5, 2186–2188. [DOI] [PubMed] [Google Scholar]
- Pilorz V, Tam SK, Hughes S, Pothecary CA, Jagannath A, Hankins MW, Bannerman DM, Lightman SL, Vyazovskiy VV, Nolan PM, Foster RG & Peirson SN (2016) Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol, 14, e1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl M, Mares P & Fischer J (1982) Synchronization of double asymmetrical epileptogenic foci in the cerebral cortex of the rat. Acta Neurobiol Exp (Wars), 42, 217–222. [PubMed] [Google Scholar]
- Ramanathan C, Campbell A, Tomczak A, Nunez AA, Smale L & Yan L (2009) Compartmentalized expression of light-induced clock genes in the suprachiasmatic nucleus of the diurnal grass rat (Arvicanthis niloticus). Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Nunez AA, Martinez GS, Schwartz MD & Smale L (2006) Temporal and spatial distribution of immunoreactive PER1 and PER2 proteins in the suprachiasmatic nucleus and peri-suprachiasmatic region of the diurnal grass rat (Arvicanthis niloticus). Brain Res, 1073–1074, 348–358. [DOI] [PubMed] [Google Scholar]
- Ramanathan C, Nunez AA & Smale L (2008a) Daily rhythms in PER1 within and beyond the suprachiasmatic nucleus of female grass rats (Arvicanthis niloticus). Neuroscience, 156, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Smale L & Nunez AA (2008b) Rhythms in expression of PER1 protein in the amygdala and bed nucleus of the stria terminalis of the diurnal grass rat (Arvicanthis niloticus). Neurosci Lett, 441, 86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Stowie A, Smale L & Nunez A (2010a) PER2 rhythms in the amygdala and bed nucleus of the stria terminalis of the diurnal grass rat (Arvicanthis niloticus). Neurosci Lett, 473, 220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Stowie A, Smale L & Nunez AA (2010b) Phase preference for the display of activity is associated with the phase of extra-suprachiasmatic nucleus oscillators within and between species. Neuroscience, 170, 758–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlin U (2001) Neural basis and biological function of masking by light in mammals: suppression of melatonin and locomotor activity. Chronobiol Int, 18, 737–758. [DOI] [PubMed] [Google Scholar]
- Redlin U & Mrosovsky N (1999) Masking by light in hamsters with SCN lesions. J Comp Physiol A, 184, 439–448. [DOI] [PubMed] [Google Scholar]
- Redlin U, Vrang N & Mrosovsky N (1999) Enhanced masking response to light in hamsters with IGL lesions. J Comp Physiol A, 184, 449–456. [DOI] [PubMed] [Google Scholar]
- Refinetti R (2004a) Daily activity patterns of a nocturnal and a diurnal rodent in a seminatural environment. Physiol Behav, 82, 285–294. [DOI] [PubMed] [Google Scholar]
- Refinetti R (2004b) The Nile grass rat as a laboratory animal. RESOURCE, 33. [DOI] [PubMed] [Google Scholar]
- Refinetti R (2008) The diversity of temporal niches in mammals. Biological Rhythm Research, 39, 173–192. [Google Scholar]
- Rotics S, Dayan T, Levy O & Kronfeld-Schor N (2011) Light masking in the field: an experiment with nocturnal and diurnal spiny mice under semi-natural field conditions. Chronobiol Int, 28, 70–75. [DOI] [PubMed] [Google Scholar]
- Santini L, Rojas D & Donatin G (2015) Evolving through day and night: origin and diversification of activity pattern in modern primates. Behavioral Ecology, 26, 789796. [Google Scholar]
- Sato T & Kawamura H (1984) Circadian rhythms in multiple unit activity inside and outside the suprachiasmatic nucleus in the diurnal chipmunk (Entamias sibricus). Neurosci. Res, 1, 45–52. [DOI] [PubMed] [Google Scholar]
- Schroder EA & Esser KA (2013) Circadian rhythms, skeletal muscle molecular clocks, and exercise. Exerc Sport Sci Rev, 41, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz WJ, Reppert SM, Eagan SM & Moore-Ede MC (1983) In vivo metabolic activity of the suprachiasmatic nuclei: a comparative study. Brain Res, 274, 184–187. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ & Zimmerman P (1990) Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J. Neurosci, 10, 3685–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC & Okamura H (1997) Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell, 91, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Shuboni DD, Cramm S, Yan L, Nunez AA & Smale L (2012) Acute behavioral responses to light and darkness in nocturnal Mus musculus and diurnal Arvicanthis niloticus. J Biol Rhythms, 27, 299–307. [DOI] [PubMed] [Google Scholar]
- Shuboni DD, Cramm SL, Yan L, Ramanathan C, Cavanaugh BL, Nunez AA & Smale L (2015) Acute effects of light on the brain and behavior of diurnal Arvicanthis niloticus and nocturnal Mus musculus. Physiol Behav, 138, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Lehman MN, Gibson M, Gladstone WR & Bittman EL (1990) Dispersed cell suspensions of fetal SCN restore circadian rhythmicity in SCN-lesioned adult hamsters. Brain Res, 525, 45–58. [DOI] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA & Lehman MN (1996) A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature, 382, 810–813. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T & Nunez AA (2003) Mammalian diurnality: some facts and gaps. J Biol Rhythms, 18, 356–366. [DOI] [PubMed] [Google Scholar]
- Smale L, Nunez AA & Schwartz MD (2008) Rhythms in a diurnal brain. . Biological Rhythm Research., 39, 305–318. [Google Scholar]
- Sulzman FM, Fuller CA & Moore-Ede MC (1982) Circadian entrainment of the squirrel monkey by extreme photoperiods: interactions between the phasic and tonic effects of light. Physiol Behav, 29, 637–641. [DOI] [PubMed] [Google Scholar]
- Sumova A, Bendova Z, Sladek M, Kovacikova Z & Illnerova H (2004) Seasonal molecular timekeeping within the rat circadian clock. Physiol Res, 53 Suppl 1, S167–176. [PubMed] [Google Scholar]
- Swann JM & Turek FW (1985) Multiple circadian oscillators regulate the timing of behavioral and endocrine rhythms in female golden hamsters. Science, 228, 898–900. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH & McDearmon EL (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet, 9, 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Farfan C, Valenzuela FJ, Ebensperger R, Mendez N, Campino C, Richter HG, Valenzuela GJ & Seron-Ferre M (2008) Circadian cortisol secretion and circadian adrenal responses to ACTH are maintained in dexamethasone suppressed capuchin monkeys (Cebus apella). Am J Primatol, 70, 93–100. [DOI] [PubMed] [Google Scholar]
- Tournier BB, Dardente H, Vuillez P, Pevet P & Challet E (2007) Expression of Tgfalpha in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Neuroscience, 145, 1138–1143. [DOI] [PubMed] [Google Scholar]
- Valenzuela FJ, Torres-Farfan C, Richter HG, Mendez N, Campino C, Torrealba F, Valenzuela GJ & Seron-Ferre M (2008) Clock gene expression in adult primate suprachiasmatic nuclei and adrenal: is the adrenal a peripheral clock responsive to melatonin? Endocrinology, 149, 1454–1461. [DOI] [PubMed] [Google Scholar]
- Valjakka A, Vartiainen J, Tuomisto L, Tuomisto JT, Olkkonen H & Airaksinen MM (1998) The fasciculus retroflexus controls the integrity of REM sleep by supporting the generation of hippocampal theta rhythm and rapid eye movements in rats. Brain Res Bull, 47, 171–184. [DOI] [PubMed] [Google Scholar]
- VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD & Meijer JH (2007) Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol, 17, 468–473. [DOI] [PubMed] [Google Scholar]
- Verwey M & Amir S (2009) Food-entrainable circadian oscillators in the brain. Eur J Neurosci, 30, 1650–1657. [DOI] [PubMed] [Google Scholar]
- Walls GL (1942) The vertebrate eye and its adaptive radiation.
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF & Hellman L (1971) Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab, 33, 14–22. [DOI] [PubMed] [Google Scholar]
- Wu J, Yonezawa T & Kishino H (2017) Rates of Molecular Evolution Suggest Natural History of Life History Traits and a Post-K-Pg Nocturnal Bottleneck of Placentals. Curr Biol, 27, 3025–3033 e3025. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Kerbeshian MC, Hocker CG, Block GD & Menaker M (1998) Rhythmic properties of the hamster suprachiasmatic nucleus in vivo. J. Neurosci, 18, 10709–10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L (2009) Expression of clock genes in the suprachiasmatic nucleus: effect of environmental lighting conditions. Rev Endocr Metab Disord, 10, 301–310. [DOI] [PubMed] [Google Scholar]
- Yan L, Karatsoreos I, LeSauter J, Welsh DK, Kay SA, Foley DK & Silver R (2007) Exploring spatiotemporal organizatio of the SCN circuits Clocks and Rhythms. Cold Spring Harbor Laboratory Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L & Okamura H (2002) Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur J Neurosci, 15, 1153–1162. [DOI] [PubMed] [Google Scholar]
- Yan L & Silver R (2002) Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci, 16, 1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L & Silver R (2004) Resetting the brain clock: time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur J Neurosci, 19, 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L & Silver R (2008) Day-length encoding through tonic photic effects in the retinorecipient SCN region. Eur J Neurosci, 28, 2108–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Takekida S, Shigeyoshi Y & Okamura H (1999) Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience, 94, 141–150. [DOI] [PubMed] [Google Scholar]