Abstract
Combinational antibiotic formulations have emerged as an important strategy to combat antibiotic resistance. The main objective of this study was to examine effects of individual components on the antimicrobial activity, physico-chemical properties, aerosolization and dissolution of powder aerosol formulations when three synergistic drugs were co-spray dried. A ternary dry powder formulation consisting of meropenem (75.5 %w/w), colistin (15.1 %w/w) and rifampicin (9.4 %w/w) at the selected ratio was produced by spray drying. The ternary formulation was characterized for in-vitro antibacterial activity, physico-chemical properties, surface composition, aerosol performance and dissolution. All of the formulations demonstrated excellent aerosolization behavior achieving a fine particle fraction of >70%, which was substantially higher than those for the Meropenem-SD and Colistin-Meropenem formulations. The results indicated that rifampicin controlled the surface morphology of the ternary and binary combination formulations resulting in the formation of highly corrugated particles. Advanced characterization of surface composition by XPS supported the hypothesis that rifampicin was enriched on the surface of the combination powder formulations. All spray-dried formulations were amorphous and absorbed substantial amount of water at the elevated humidity. Storage at the elevated humidity caused a substantial decline in aerosolization performance for the Meropenem-SD and Colistin-Meropenem, which was attributed to increased inter-particulate capillary forces or particle fusion. In contrast, the ternary combination and binary Meropenem-Rifampicin formulations showed no change in aerosol performance at the elevated storage humidity conditions; attributable to the enriched hydrophobicity of rifampicin on the particle surface that acted as a barrier against moisture condensation and particle fusion. Interestingly, in the ternary formulation rifampicin enrichment on the surface did not interfere with the dissolution of other two components (i.e. meropenem and colistin). Our study provides an insight on the impact of each component on the performance of co-spray dried combinational formulations.
Keywords: Dry powder inhaler, aerosol performance, spray drying, ternary combination, solubility, dissolution
Graphical Abstract
Introduction
Respiratory infections due to multi-drug resistant (MDR) Gram-negative pathogens are associated with high mortality and morbidity rates, which represent an unmet healthcare problem worldwide (Mizgerd, 2006; WHO, May 2014). Traditionally, antibiotics are administered via oral or parenteral routes; but for some systemically administered antibiotics only a small amount/proportion of drugs can reach the site of infections in the deep lungs which can compromise treatment efficacy (Cheah et al., 2015; Cipolla et al., 2016; Ritrovato and Deeter, 1991; Yapa et al., 2014). Simply increasing the dose of oral and parenteral administrations is often unfeasible because of dose limiting systemic toxicity, as is the case with the polymyxins (i.e. polymyxin B and colistin) (Bergen et al., 2012; Hartzell et al., 2009).
Antimicrobial resistance has become a serious global health challenge (Boucher et al., 2009; Payne et al., 2007). In the absence of antibiotics with novel mechanisms of action (Boucher et al., 2009; Payne et al., 2007), synergistic combinations of antibiotics with different mechanisms are the most economic and effective strategy to ward off an impending post-antibiotic era (Mouton, 1999). For such combinations to be optimally effective in-vivo, both drugs should localize and accumulate at the infections site concomitantly (Mukker et al., 2015). However, drugs often exhibit variable pharmacokinetic profiles, for example the rate and extent of drug absorption in the lungs following systemic administration is highly dependent on biological factors and drug properties (Levison and Levison, 2009). This raises concerns regarding the effectiveness of systemic therapies of combinational antibiotics against lower respiratory tract infections.
Inhalation therapies have advantages for the treatment of respiratory tract diseases (Frijlink and De Boer, 2004). Drugs can be delivered directly to the lungs via inhalation in order to achieve high drug concentrations at the infection site and reduced systemic exposure, as compared with oral and parenteral delivery (Hickey et al., 2016). The achievement of higher drug concentrations in the airways and lowering the systemic exposure often translates into enhanced antimicrobial activity, minimized systemic toxicity and reduces the emergence of resistance (Cipolla and Chan, 2013; Lu et al., 2012). Dry powder inhalers are portable, possess better chemical stability and higher delivery efficiencies in comparison to the traditional nebulization (Zhou et al., 2015a).
Nevertheless, inhalation of large amount of drug/excipient powders may cause local adverse events including bronchospasm and coughing, which may compromise patient compliance (Claus et al., 2014; Zhou et al., 2015a). Such adverse reactions are more prevalent with the high-dose medications such as inhaled antibiotics, likely attributable to large amount of powders deposited in the upper respiratory tracts and the inhalation maneuver of the patients (Claus et al., 2014; Velkov et al., 2015; Weers, 2015). Thus, it is important to minimize the deposition of inhaled powder in the upper respiratory tract and maximize aerosol performance to reduce the mass of the drug powders to be inhaled for improved patient compliance and adherence (Zhou et al., 2014b).
In inhalation industrial practice, raw drug materials are jet milled to obtain small particle sizes with aerodynamic diameters 1–5 μm (Lin et al., 2015). Such fine milled particles are inherently cohesive and difficult to disperse into discrete particles upon inhalation, which result in low aerosol efficiencies (Buttini et al., 2012). Controlling the powder cohesion via particle engineering methods is a straightforward approach to improve the aerosolization performance of dry powder inhaler (DPI) formulations (Buttini et al., 2012; Qu et al., 2015). One of the popular approach of particle engineering is spray drying (Bohr et al., 2014; Son et al., 2013; Vehring, 2008). Our research has been focused on developing antibiotic combinations, which not only exhibit synergistic antibacterial activity but also augment the aerosol and dissolution performance when combined into DPI formulations (Mangal et al., 2018a; Mangal et al., 2019; Shetty et al., 2018a; Wang et al., 2016; Zhou et al., 2014a; Zhou et al., 2015b; Zhou et al., 2016). For instance, a DPI formulation containing amorphous colistin and crystalline rifapentine was developed by spray drying rifapentine suspension in colistin aqueous solution (Zhou et al., 2015b). The formulation has shown synergistic antimicrobial activities against planktonic and biofilm of P. aeruginosa. In addition, crystalline rifapentine particles act as carriers that prevented moisture-induced deterioration in aerosol performance for hygroscopic colistin (Zhou et al., 2015b). In other studies, co-spray drying colistin with hydrophobic azithromycin or rifampicin was shown to form a hydrophobic coating on the surface of co-spray dried composite particles, which improved the stability of colistin against moisture-induced particle fusion/agglomeration at elevated humidity (Zhou et al., 2014a; Zhou et al., 2016).
Ternary antibiotic combinations are becoming an important avenue for eradication of MDR ‘superbugs’ that have developed resistance to almost all clinically available antibiotics (Urban et al., 2010). The literature evidence has shown the potential of ternary combinations of polymyxin B, carbapenem (or its family antibiotics) and rifampicin for rapid killing against pan-drug resistant (PDR) A. baumannii (Yoon et al., 2004), K. pneumoniae (Diep et al., 2017) and P. aeruginosa (Urban et al., 2010). Thus far there has been one attempt to develop DPI formulations of ternary antibiotic combinations (Lee et al., 2016). However, the effects of each component and surface composition on the aerosol performance at elevated humid conditions were not been investigated; this is important as the latter has been demonstrated to critically affect the aerosol stability of DPIs containing hygroscopic compounds such as colistin (Zhou et al., 2014a; Zhou et al., 2016; Zhou et al., 2013).
The aim of this study was to examine the role of colistin and rifampicin on the antimicrobial activity, physico-chemical properties, aerosolization and dissolution of meropenem as co-spray dried formulations. We employed colistin for this study, and not polymyxin B, given the potential toxic effects of polymyxin B on lung epithelial cells noted in our previous report (Ahmed et al., 2017).
Materials and Methods
Materials
Meroepenem trihydrate, colistin sulphate and rifampicin were purchased from Beta Pharma (Beta Pharma (Shanghai) Co. Ltd, Wujiang City, China). Acetonitrile (HPLC grade) was purchased from Merck (Fair Lawn, New Jersey, USA).
MDR P. aeruginosa 20143 n/m and A. baumannii 03–149.2 (colistin-resistant) were clinical samples from respiratory infections. The bacterial strains were maintained at −80°C in tryptone soy broth containing glycerol (20% v/v).
Static time-kill experiment
Antimicrobial activities were examined by static time-kill tests for colistin (8 mg/L), meropenem (40 mg/L) and rifampicin (5 mg/L) monotherapy and its combinations against P. aeruginosa 20143 n/m and A. baumannii 03–149.2. The composition of each component is considered based on the previous antimicrobial results (Diep et al., 2017) with slight modifications. All experiments were performed with an initial inoculum of ~106 CFU/mL in 20 mL of Cation-Adjusted Mueller-Hinton Broth (CAMHB) in 50 mL pyrogen-free and sterile polypropylene tubes. An aliquot of 50 μL was collected at 0, 0.5, 1, 2, 4, 6, and 24 h and to quantify bacteria a ProtoCOL automated colony counter (Synbiosis, Cambridge, United Kingdom) was employed. The limit of detection was 110 CFU/mL.
Spray-drying
A Büchi 290 spray drier (Büchi Labortechnik AG, Falwil, Switzerland) was employed to produce the powder formulations. Briefly, the antibiotic(s) were weighed (based on formulation composition mentioned in Table 1) and dissolved in water. The drug ratio was considered based on the literature (Diep et al., 2017) with slight modifications. The resultant solution was spray-dried under following conditions: inlet temperature 110°C; outlet temperature 62 ± 3°C; aspirator 35 m3/h; atomizer setting 700 L/h; feed rate 2 mL/min (Zhou et al., 2014a). The total solid load of the feed solution was kept constant (10 mg/mL) amongst different formulations. The spray-dried formulations were stored in a desiccator with silica gel at 20 ± 3°C. The spray-dried formulations were also stored at the moderate (55% RH) and elevated humidity (75% RH) at room temperature for one week to determine the aerosolization stability at the elevated humidity.
Table 1.
Compositions of the spray-dried formulations.
| Concentration (% w/w) | ||
|---|---|---|
| Meropenem | Colistin | Rifampicin |
| 100 | 0 | 0 |
| 83.3 | 16.7 | 0 |
| 88.9 | 0 | 11.1 |
| 75.5 | 15.1 | 9.4 |
Scanning electron microscopy (SEM)
Morphology of the spray-dried formulations was visualized under a field emission scanning electron microscope (NOVA nanoSEM, FEI Company, Hillsboro, Oregon, USA). Briefly, powders were dispersed on aluminum stubs with an adhesive tape and pressurized air was used to remove the access powder. The stubs were then platinum coated using a sputter coater (208 HR, Cressington Sputter Coater, England, UK) at 40 mA for 1 min and the images were captured.
Particle size distribution
Given the difficulty in measuring particle size of combinational formulations using laser diffraction, particle size was measured based on the scanning electron microscopy images (Shekunov et al., 2007; Wan et al., 2013). Briefly, the diameters of ~150 randomly selected particles (50 particles each from 3 different images with a magnification of 10,000×) were measured by the distance of the longest edge of each particle in a fixed downwards direction using the ImageJ software. The D10, D50 and D90 were calculated. Given the relatively narrow distribution of the spray dried particles, measurements of 150 particles are sufficient for the particle sizing purpose here.
Water content
Karl Fischer analysis was performed using the coulometric system with diaphragm coupled with 703 Ti Stand (831 KF Coulometer, Brinkman/Metrohm, Westbury, NY) for water content determination. Around 50 mg of each samples were weighed accurately and dissolved in 10g of methanol. Sample solution was injected into the titration cell filled with Hydranal@-Coulomat AD (Sigma–Aldrich, St. Louis, Missouri) and titrated coulometrically for water. The water content of blank methanol was also measured prior to sample injection. The water content of blank methanol was subtracted from that of samples. All measurements were triplicated.
Powder X-ray diffraction (P-XRD)
Crystallinity was determined by X-ray diffraction with a Rigaku Smartlab™ diffractometer (Rigaku Americas, Texas, USA). The diffraction was acquired at 5°/min and a step of 0.02° in the range of 5–40° 2θ.
Dynamic vapor sorption
The water sorption behavior of the spray-dried formulations was examined using a dynamic vapor sorption (DVS-Intrinsic, Surface Measuremsent Systems Ltd., London, UK). Briefly, a small amount of powder sample (15 ±5 mg) was placed on a pan. The powder sample was equilibrated with increasing relative humidity ranging from 0–90% with 10 % interval. Then, the sample was equilibrated with decreasing humidity ranging from 90–0% with 10% interval. The change in mass with time dm/dt of less than 0.002% /min was defined as the equilibrium.
X-ray photoelectron spectroscopy (XPS)
Surface composition of the spray-dried powders was quantified using X-ray photoelectron spectroscopy (XPS) (AXIS Ultra DLD spectrometer, Kratos Analytical Inc., Manchester, UK). For better resolution, a commercial Kratos charge neutralizer was used. Samples were placed on a sample holder mounted with a copper tape. XPS data were analysed using with CasaXPS software version 2313 Dev64. Surface composition of each composite formulation was determined by N 1s curve-fitting using the peaks from pure compounds as described previously (Mangal et al., 2018b).
In-vitro aerosol performance
The spray dried formulations were stored at the desiccated condition (DS), 55% RH and 75% RH for 1-week to test the in-vitro aerosolization performance and the stability. In-vitro aerosol performance was evaluated by a Multi-Stage Liquid Impinger (MSLI) (Copley Scientific Limited, Nottingham, UK) using a standard dispersion procedure (USP 38). Briefly, twenty milliliter water was filled in each of the stages 1–4 of the MSLI. Two capsules with each containing 10 ± 2 mg of the powder formulation were dispersed through a RS01 DPI device (Plastiape S.p.A., Osnago, Italy; it has a similar design and characteristics to the Osmohaler device) at an airflow of 100 L/min for 2.4 s. These experimental conditions allowed a pressure drop of approximately 4 kPa across the device. The drugs deposit in the capsule, device, USP induction port, Stages 1–4 and filter paper was collected with 20 mL of water. The amount of drug was quantified using an established HPLC method as described below. Fine particle fraction (FPF) was calculated as the fraction of drug deposited on the stage 3–4 and the filter over the total recovered doseEmitted dose (ED) represents the amount of drug emitted from the capsule and inhaler device.
Drug quantification
The concentrations of colistin sulfate and meropenem were determined using established HPLC methods as slightly modified from the previous studies (Mangal et al., 2018b). The mobile phase consisted of 30 mM sodium sulfate solution (adjusted to pH 2.5 with H3PO4) (A) and acetonitrile (B). The isocratic elution program used for colistin and meropenem detection was 76% (v/v) A and 24% (v/v) B for 7 min at 1.0 mL/min. The retention time colistin was 3.2 and 5.2 min for colistin A and colistin B (two major components of colistin) and for meropenem was 2 min. For rifampicin a gradient program was used, set as: 76 % A to 55% B in 9 min, 55 % A to 45 % B till 17 min. The retention time for rifampicin was 15 min. A calibration curve was prepared for colistin (0.002–0.25 mg/mL), meropenem (0.002–0.25mg/mL) and rifampicin (0.001–0.1 mg/mL), which was linear in the required concentration range (r 2 >0.999).
In-vitro dissolution
To date, there is no regulatory guideline for dissolution testing of DPI formulations. Franz cell method has been widely used in the literature for evaluating dissolution of inhalation formulations (Buttini et al., 2014; May et al., 2012; Salama et al., 2008); though it has several limitations including the difficulty to differentiate dissolution from diffusion, and may not exactly mimic in vivo environments regarding airway liquid volume, thickness and composition. Nevertheless, Franz cell method was used here as a simplified approach to evaluate dissolution kinetics of the spray-dried formulations with minor modifications (May et al., 2012; Wang et al., 2016).
Briefly, two capsules (size 3 hydroxypropyl methylcellulose capsules, Qualicaps, Whitsett, NC, USA) each containing 15 ± 2 mg of the spray-dried formulation were dispersed through an RS01 DPI device at an airflow of 100 L/min for 2.4 s into the Next Generation Impactor (NGI). A filter disc membrane (Whatman® Grade 2 filter paper, pore size 5 μm, GE Healthcare) was placed under in the stage 3 (effective cut-off aerodynamic diameter is 2.18 μm) to collect aerosol powders. Franz cell (PermeGear Inc., Hellertown, PA, USA) reservoirs were filled with 20 mL phosphate buffer saline (PBS, pH 7.4) at 37 °C. Given there is no regulatory guideline on the formula of lung simulated fluid, phosphate buffers have been widely used as the simple dissolution media for inhalation formulations to reflect the buffering capability of the lung fluid (May et al., 2012; Salama et al., 2008). The filter disc from the stage 3 of NGI was carefully placed on the Franz cells, being in contact with the dissolution media. The dissolution media was stirred constantly at 600 rpm (fixed speed by the supplier) with a magnetic bar (12.5 mm in diameter and 3 mm in width). An aliquot of 0.3 mL sample was collected at the selected time intervals (5, 10, 20, 30, 60, 120 and 180 min), and an equal volume of fresh PBS was added immediately after sample collection. Total amount of each drug loaded on the filter disc was determined by adding 5 mL ethanol in the donor compartment and ensure all drugs are dissolved. Percentages of drug released were calculated by dividing the amount of drug released by the total amount of drug loaded on the filter disc.
Statistical analysis
Data were presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with a Tukey–Kramer post-hoc test was performed using a GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). The asterisks over graphs denote as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 and NS denotes not significant (p > 0.05).
Results
Antimicrobial activity
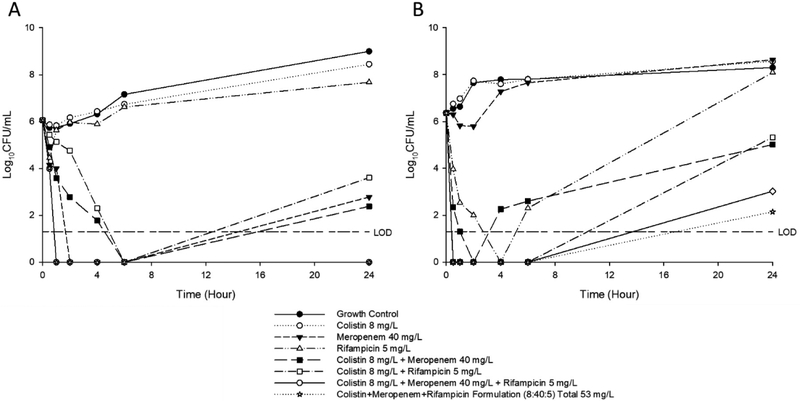
Time-kill profiles for colistin, meropenem, and rifampicin per se and in binary and ternary formulations against A. baumannii 03–149.2 and P. aeruginosa 20143 n/m are shown in Figure 1. Colistin monotherapy produced no bacterial killing against both strains, which was similar to the behaviour of the bacteria-only control; this is in line with the polymyxin resistant nature of both strains. Meropenem monotherapy, produced substantial bacterial killing within 4–6 h against A. baumannii 03–149.2 and P. aeruginosa 20143 n/m with a minimum of 2-Log10 and 4-Log10 reduction in CFU/mL, respectively. Rifampicin monotherapy was only effective against A. baumannii 03–149.2, with a > 2 log reduction in CFU/mL observed at 4 h followed by regrowth. For the various antibiotic combinations, synergistic killing was observed across 24 h; however, regrowth was evident for all combinations after 6 h. The ternary combination exhibited excellent bacterial killing against isolate P. aeruginosa 20143 n/m with no viable cell counts up to 24 h. Similarly, the ternary combination maintained good bacterial killing against A. baumannii 03–149.2 up to 6 h. At 24 h, despite regrowth to ~4 log10 CFU/mL, a 6-Log10 CFU/mL difference was evident versus colistin monotherapy. The time-kill data showed the effective and synergistic in vitro antimicrobial activities of our formulation against MDR Gram-negative bacteria.
Figure 1.
Time-kill kinetics of colistin (8 mg/L), meropenem (40 mg/L) and rifampicin (5 mg/L) mono-drug and combinations as well as the spray dried ternary formulations against (A) P. aeruginosa 20143 n/m and (B) A. baumannii 03–149.2.
Scanning electron microscopy (SEM)
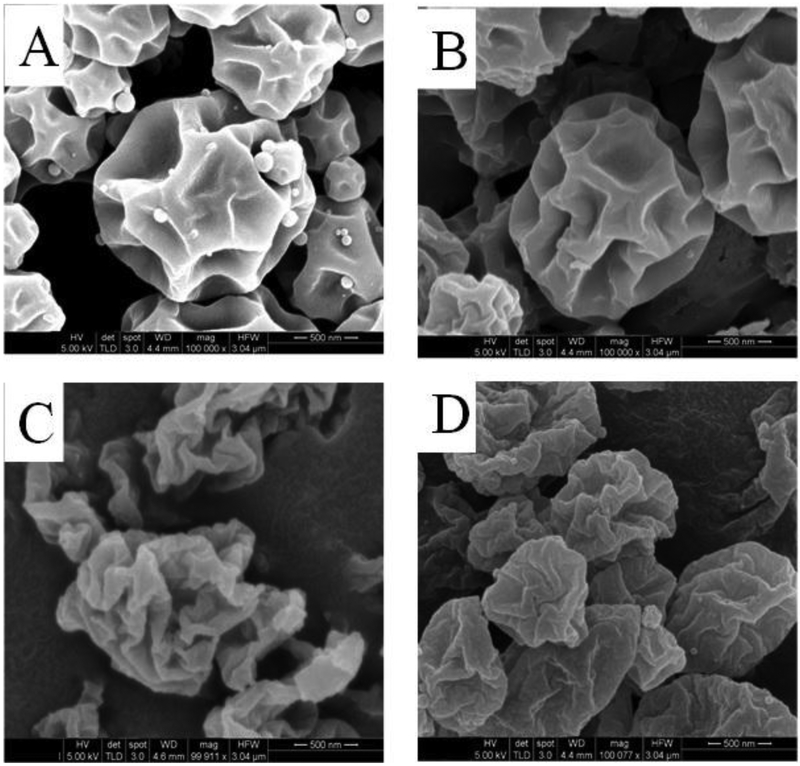
Representative SEM images of the spray dried formulations are shown in Figure 2. SEM images showed that Meropenem-SD particle had spherical particles with rough surfaces, which remained largely unchanged after co-spraying with colistin (Figure 2A and 2B). Co-spraying of meropenem with rifampicin substantially altered the morphology of meropenem with Meropenem-Rifampicin particles appearing more corrugated (Figure 2C). Particles of ternary combination i.e., Colistin-Meropenem-Rifampicin showed similar morphological features as that of the Meropenem-Rifampicin. The SEM results suggests that rifampicin enrichment on the surface controls the morphological appearance of the resulting spray-dried particles (Zhou et al., 2014a).
Figure 2.
Representative scanning electron microscopy images of (A) Meropenem-SD, (B) Colistin-Meropenem, (C) Meropenem-Rifampicin, and (D) Colistin-Meropenem-Rifampicin formulations.
Particle size
Particle sizes of the spray-dried formulations are presented in Table 2. D50 values of the spray-dried formulations were selected < 2 μm and D90 were < 3 μm.
Table 2.
Particle sizes of spray-dried powder formulations.
| Formulations | Particle size (μm) | Water content (%) w/w | ||
|---|---|---|---|---|
| D10 | D50 | D90 | ||
| Meropenem-SD | 0.5 ± 0.1 | 1.1 ± 0.1 | 1.9 ± 0.2 | 8.8 ± 0.2 |
| Colistin-Meropenem | 0.9 ± 0.0 | 1.4± 0.1 | 2.2 ± 0.3 | 5.1 ± 0.2 |
| Meropenem-Rifampicin | 0.6 ± 0.1 | 0.9 ± 0.1 | 1.6 ± 0.3 | 4.4 ± 0.3 |
| Colistin-Meropenem-Rifampicin | 0.8 ± 0.1 | 1.2 ± 0.08 | 2.1 ± 0.2 | 3.0 ± 0.3 |
The Meropenem-SD has the highest water content of 8.8 ± 0.2 % w/w right after spray while the triple combination formulation has the lowest water content of 3.0 ±0.3.
X-ray photoelectron spectroscopy (XPS)
Surface compositions of the formulations were quantified by XPS (Table 3). The XPS data demonstrated a higher colistin concentration than the theoretical value in the Colistin-Meropenem formulation, indicating surface enrichment of colistin. In the Meropenem-Rifampicin and Colistin-Meropenem-Rifampicin formulations, surface concentration of rifampicin was substantially higher than its theoretical value, indicating enrichment of rifampicin on the surface. These data also confirmed that rifampicin on the particle surface controlled the morphology of these formulations, leading to formation of corrugated particles.
Table 3.
Surface composition of the selected spray-dried formulations as measured by XPS (N 1s curve-fits).
| Formulation | Theoretical Surface Composition (atomic nitrogen percentage) | Measured Surface Composition (atomic nitrogen percentage) | ||||
|---|---|---|---|---|---|---|
| Colistin | Meropenem | Rifampicin | Colistin | Meropenem | Rifampicin | |
| Colistin-Meropenem | 7.0 | 93.0 | 0.0 | 51.7 | 42.9 | - |
| Meropenem-Rifampicin | 0.0 | 97.8 | 2.2 | - | 31.5 | 68.5 |
| Colistin-Meropenem-Rifampicin | 6.8 | 91.2 | 2.0 | 30.4 | 20.3 | 49.3 |
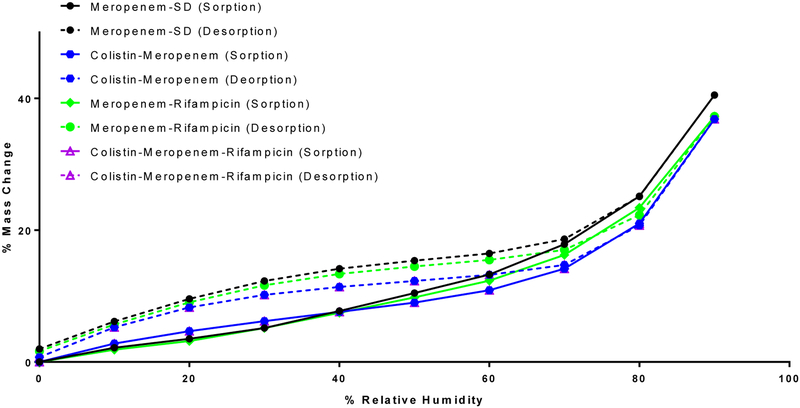
Dynamic water vapor sorption
Moisture sorption behavior of the spray-dried formulations is shown in Figure 3. All formulations absorbed significant amounts of water at the elevated RH. This suggests that all the spray dried formulations were highly hygroscopic like spray dried colistin particles (Zhou et al., 2013); and addition of colistin and rifampicin in the formulation had no substantial effect on moisture sorption of spray dried meropenem. All samples showed a sorption-desorption hysteresis due to slower escape of water molecules during desorption from the invaginations of particles (Zhu et al., 2008). Mass change of all formulations was zero or near zero at the end of desorption, indicating absence of moisture-induced crystallization or permanent water absorption.
Figure 3.
Dynamic vapor sorption behavior of the spray dried powder formulations.
Powder x-ray diffraction (P-XRD)
P-XRD diffractograms show that raw colistin powder was amorphous as identified by the absence of crystalline peaks (Figure 4A). The supplied rifampicin and meropenem were crystalline as exemplified by the sharp crystalline peaks. All spray-dried powder formulations showed no crystalline peak. This suggests that meropenem and rifampicin were amorphous after spray drying. All the spray dried formulation retained their amorphous nature after storage at 55% RH (Figure 4B) and 75% RH (Figure 4C) for a week, showing short-term physical stability.
Figure 4.
X-ray diffraction patterns of (A) raw materials and spray dried powder formulations, (B) formulations stored at 55% RH for a week, (C) formulations stored at 75% RH for a week.
In-vitro aerosol performance
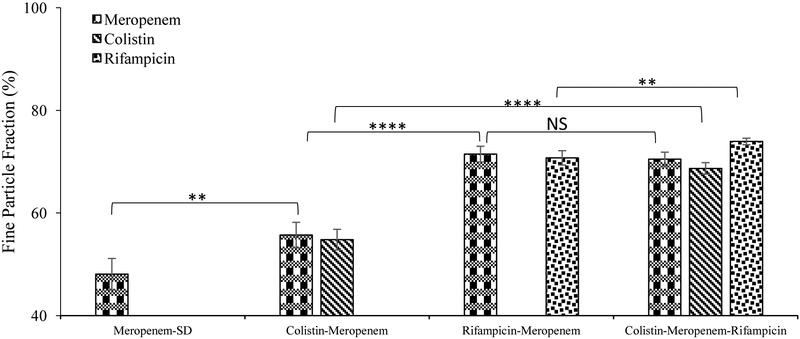
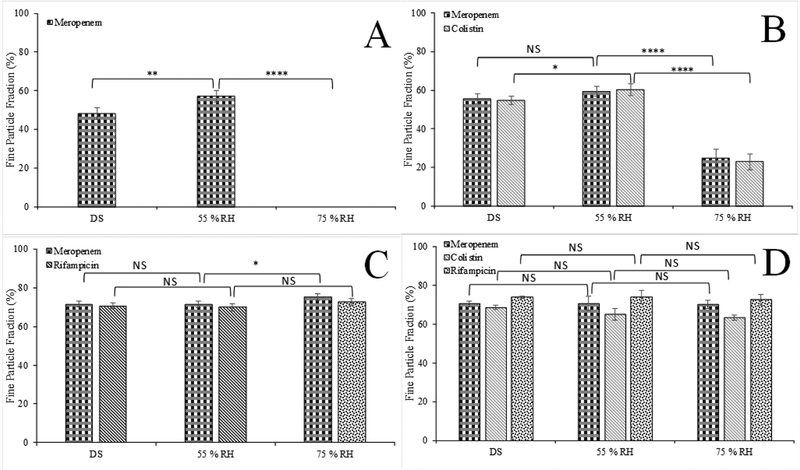
In-vitro aerosolization performance of the selected formulations stored under desiccated humidity conditions (DS, RH 20%) is presented in Figure 5. The Meropenem-SD formulation showed a relatively low FPF (48.0 ± 3.1%). Co-spray drying of meropenem with colistin and rifampicin substantially improved the FPF of meropenem compared with the Meropenem-SD formulation (p<0.05). Binary combination formulation co-spray drying with rifampicin exhibited a greater increase in FPF (71.5 ± 1.5%), when compared with the binary formulation with colistin (55.7 ± 2.5 %). FPF values of the ternary combination formulation was similar to those of Rifampicin-Meropenem but substantially higher than those of the Meropenem-SD and Colistin-Meropenem (p<0.0001). Interestingly, a higher percentage of rifampicin deposited on the filter stage for the ternary combination formulation, when compared with the colistin and meropenem in that formulation (Appendix A1). This phenomenon was observed in our previous studies, which could be attributed to the fragmentation of particle surfaces (rifampicin was enriched on the surface) during the aerosolization (Wang et al., 2016; Zhou et al., 2014a).
Figure 5.
Fine Particle Fraction of the spray-dried formulations stored in the desiccated chamber. The data are presented as mean ± SD (n=4).
Effects of storage relative humidity on aerosolization
Since the spray-dried formulations can absorb significant amounts of water upon storage at the elevated RH, the aerosol performance could be compromised due to increased inter-particulate capillary forces (Young et al., 2004; Zhou et al., 2013). Therefore, we measured the in-vitro aerosolization performance of the spray-dried formulations stored in the desiccator with silica gel (DS, RH 20%, the data is the same as shown in Figure 5), 55% and 75% RH for a week (Figure 6). Based on the DVS isotherms, storage of the formulations for a week is sufficient for the equilibrium of moisture absorption. FPF of the Meropenem-SD formulation stored at 55% RH was slightly higher than that stored at RH 20%, which may be attributed to the neutralization of electrostatic charging (Zhu et al., 2008). Storage at 75% RH resulted in intense particle fusion (Figure 7), increasing particle sizes to beyond those regarded as fine particles (i.e., <5 μm aerodynamic diameter) or inhalable fraction for the Meropenem-SD (Figure 6A). The Colistin-Meropenem formulation stored at 75% RH had a remarkable lower FPF as compared with those stored under the lower RH (Figure 6B). The FPF of Meropenem-Rifampicin and Colistin-Meropenem-Rifampicin formulations remained largely unaffected by the storage conditions indicating improved aerosolization stability against elevated humidity (Figure 6C and 6D). This is highly likely attributed to the presence of rifampicin on the surface of ternary formulation (Figure 6C and 6D).
Figure 6.
Fine particle fraction (% FPF) of the spray-dried formulations stored under 55% and 75% RH for a week: (A) Meropenem-SD formulation, (B) Meropenem-Colistin, (C) Meropenem-Rifampicin formulation, and (D) Colistin-Meropenem-Rifampicin formulation. The data are presented as mean ± SD (n=4).
Figure 7.
Representative scanning electron microscopy images of the spray-dried formulations after storage at 75% RH for 1-week: (A) Meropenem-SD, (B) Colistin-Meropenem, (C) Meropenem-Rifampicin, and (D) Colistin-Meropenem-Rifampicin.
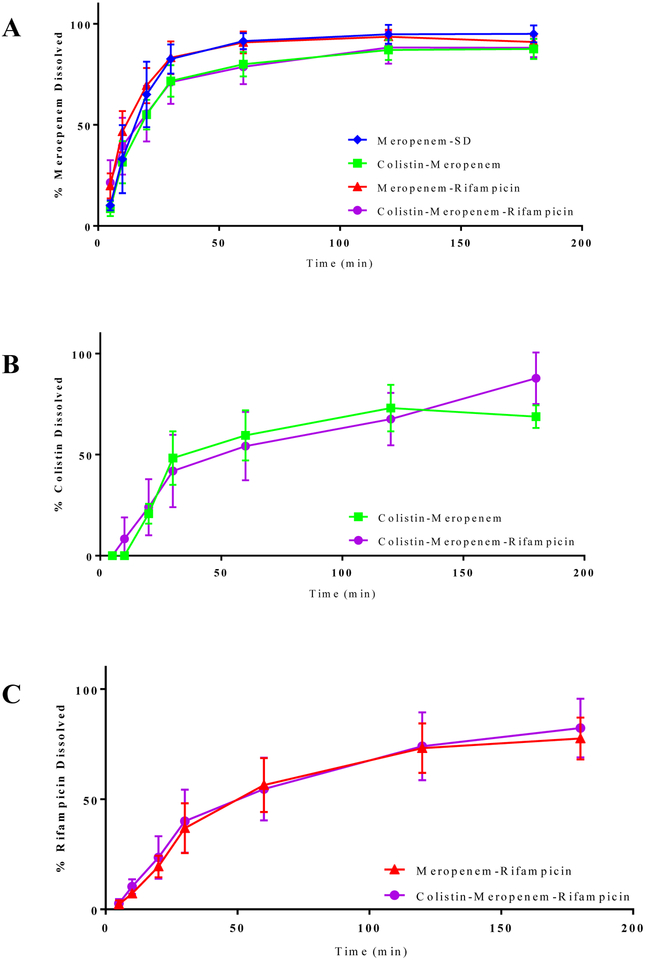
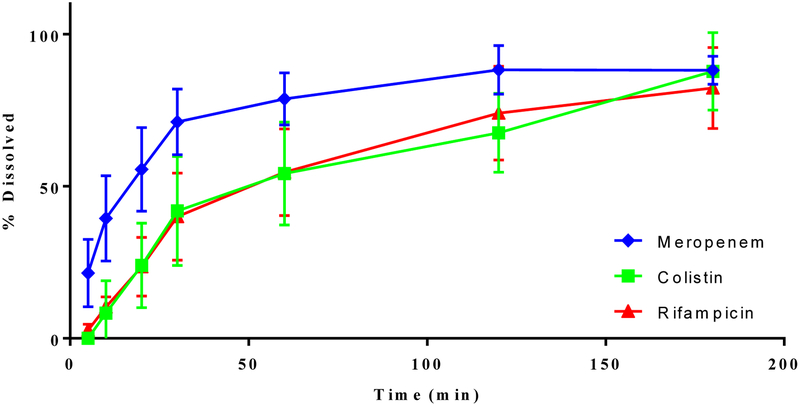
In-vitro dissolution
Meropenem-SD exhibited a rapid dissolution with ~80% of the total drug being released within the first 30 min (Figure 8). In the composite formulations, dissolution profiles of meropenem were similar to that in the Meropenem-SD, indicating that rifampicin and/or colistin in the composition formulations did not interfere with the release of meropenem (Figure 8A). In the ternary formulations, colistin and rifampicin showed a slower dissolution rate as compared with meropenem (Appendix A2). However, the drug release rates for colistin and rifampicin in the binary formulation were comparable to those dissolution rates for each corresponding drug in the ternary combination indicating that no interference in dissolution rate between different drugs (Figure 8B and 8C).
Figure 8.
In-vitro dissolution of meropenem (A), colistin (B) and rifampicin (C) from the composite formulations. The data are presented as mean ± SD (n=4).
Discussion
Inhalation is an promising route for anti-infective drug delivery targeting respiratory tract infections, as it offers direct access of the drugs to the airway surfaces, limits the systemic exposure and hence unwanted toxicity to the off-target sites (Bruinenberg et al., 2009; Ritrovato and Deeter, 1991). Inhaled antibiotics achieve relatively high drug concentrations in the lungs quickly (Gontijo et al., 2014; Yapa et al., 2013), thus reducing the risk of sub-optimal exposure which promotes the emergence of resistance. Polymyxins remain an effective treatment option for MDR Gram-negative lung infections (Levin et al., 1999). However, there has been some concern in view of increasing reports of outbreaks of polymyxin-resistant infections in recent years (Paterson and Harris, 2016). Clearly, we must look towards the future and pre-emptively develop new treatment strategies using rational combinations to rescue this important last-line class of antibiotics.
In our previous reports, we demonstrated that binary polymyxin-antibiotic combination formulations improved not only antibacterial activities but also performance of DPI formulations in terms of physical stability and aerosolization (Wang et al., 2016; Zhou et al., 2014a; Zhou et al., 2015b; Zhou et al., 2016). In the present study, we have progressed this field of study and demonstrated that a ternary combinational formulation of meropenem, rifampicin and colistin achieved rapid killing and prevention of re-growth against colistin-resistant A. baumannii and P. aeruginosa clinical isolates. The combination of these three antibiotics is ideal as they have different bacterial killing mechanisms: colistin acts by interacting with the outer membrane lipopolysaccharide of the Gram-negative bacteria and disrupting the outer membrane (Velkov et al., 2013); rifampicin inhibits RNA synthesis by inhibiting bacterial DNA-dependent RNA polymerase and thus preventing synthesis of host bacterial proteins (Campbell et al., 2001); meropenem binds the membrane-associated bacterial enzymes involved in the cell wall formation and facilitates bacterial cell lysis (Kitzis et al., 1989). When combined, these three antibiotics showed superior antimicrobial activity compared to the individual and binary formulations (Figure 1).
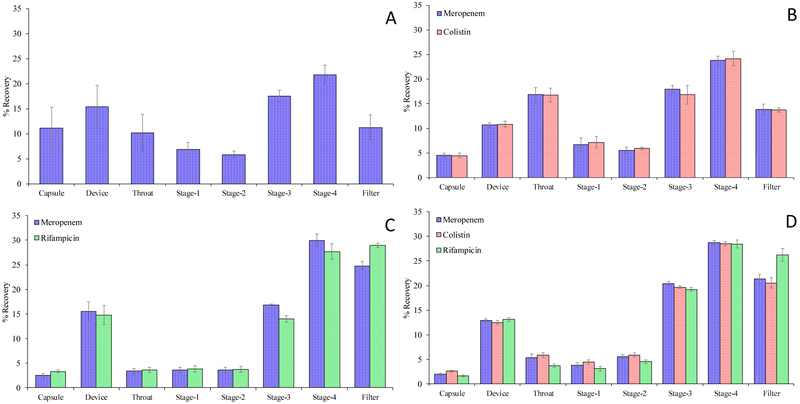
One of the key challenges for systemic administrations of combinational antibiotics is the varying PK and pharmacodynamics (PD) behaviors for different drugs. Such differences in PK and PD behaviors will lead to very varying drug concentrations at the site of infection on the airway surfaces, which likely lead to compromised synergy. Co-spray drying provides an opportunity to incorporate all three drugs in one particle at the optimized ratio, which can be delivered to the target site for maximized synergistic antimicrobial effects (Zhou et al., 2014a). Our in-vitro deposition data showed that three drugs had very similar deposition profiles (except for rifampicin in the filter) (Appendix A1), suggesting a homogenous distribution of three antibiotics at the particular level.
It is interesting to note that rifampicin-containing formulations had markedly higher FPF than the Meropenem-SD and Meropenem-Colistin formulations (Figure 5). We hypothesize that such improvements in aerosol performance is due to the enrichment of rifampicin on the particle surface since spray dried pure rifampicin showed a high FPF of > 68% in our previous study (Wang et al., 2016). The enrichment of rifampicin on the surface of the composite particles is likely attributed to its low water solubility. It has been reported that poor soluble substances precipitates early in the drying phase, which result in surfaces that are enriched with the poorly soluble materials (Vehring, 2008).It is well recognized that surface properties play a critical role in aerosolization of fine inhalable particles (Zhou and Morton, 2012). Our XPS results demonstrated that in this three-component system, rifampicin, a relatively poorly water-soluble molecule, enriched on the surface of the co-sprayed formulations. In addition, the rifampicin-containing formulations had a more corrugated surface (Figure 2) which is similar to those spray dried pure rifampicin (Wang et al., 2016), providing an evidence that surface enrichment of rifampicin controls the particle morphology. Such corrugated morphology can decrease the contact area between particles, reducing inter-particle forces and consequently result in better aerosolization performance (Chew and Chan, 2001).
Furthermore, co-spray drying also enables engineering of particulate properties for improved stability such as prevention of moisture-induced deterioration in aerosolization (Zhou et al., 2016). Many spray dried particles are amorphous and hygroscopic such as colistin and meropenem. Upon the exposure to the elevated RH, capillary forces are strong between these hygroscopic particles and even form fused lumps (Zhou et al., 2013). Such strong inter-particulate capillary forces may lead to reduction in aerosolization such as for spray dried colistin, or even no aerosolized particles due to particle fusion such as the spray dried meropenem formulation (Figure 6A). Typically, poorly water soluble molecules tend to enrich on the surface of particles after spray drying (Vehring, 2008; Vehring et al., 2007), and such hydrophobic surfaces may exhibit protection against moisture-induced particle agglomeration or particle fusion. L-leucine has exhibited such surface-active properties during spray drying (Mangal et al., 2015; Shetty et al., 2018b) and successfully inhibited the moisture-induced reduction in FPF (Li et al., 2016; Shetty et al., 2018b). In this study, enrichment of hydrophobic rifampicin on the surface of ternary combinational particles also protected the aerosolization against moisture. Using synergistic antibiotic (i.e. rifampicin) instead of excipients (e.g. L-leucine) not only improves the antimicrobial activities, but also reduce the mass of total powder for the high-dose antibiotic inhalation formulation, which likely minimize the respiratory adverse effects caused by inhalation of high-mass powders (Velkov et al., 2015; Zhou et al., 2015a).
Inhalable antibiotics are supposed to dissolve in the epithelium lining fluid in airways for effective antimicrobial activities; while solid undissolved particles are unable to enter the bacterial cell. Thus, dissolution profile of inhaled powders in airways may play a vital role in determining their efficacy (Riley et al., 2012). As there is a concern that surface modification with hydrophobic materials may retard wetting and hence the dissolution of drugs (Iranloye and Parrott, 1978), we also investigated the effect of hydrophobic rifampicin on in-vitro dissolution behavior of the ternary combinational formulation. Our results indicated that coating with rifampicin did not retard the dissolution of meropenem, attributable to partial/incoherent coating with rifampicin (Wang et al., 2016). Such incoherent coating may allow dissolution of the soluble drug (i.e., meropenem and colistin) hence does not retard dissolution. Interestingly, the coverage of these incoherent coating is sufficient to offer complete protection against moisture-induced deterioration in aerosolization given rifampicin was only less than 10% w/w in the formulation.
Conclusions
This study has examined effects of individual components on the antimicrobial activity, physico-chemical properties, aerosolization and dissolution of triple antibiotics when co-spray dried. The combination of the three antibiotics with varying antimicrobial mechanisms showed synergistic antimicrobial activities against colistin-resistant Gram-negative bacteria that may cause fatal respiratory tract infections. It is noteworthy that formulation process of spray drying did not compromise the antimicrobial activities. Incorporation of colistin in the spray drying solution resulted in an increase in FPF for meropenem. Addition of a ternary component, rifampicin, in the spray drying solution further improved the FPF to >70% and protected the hygroscopic formulation from moisture-induced deterioration in aerosolization. Such aerosolization improvements and moisture protection were attributed to the enrichment of rifampicin on the particle surface as indicated by the XPS data, even when rifampicin concentration in the formulation was less than 10% w/w. Moreover, such enrichment of hydrophobic rifampicin did not retard the dissolution of other two components, meropenem and colistin. Similar deposition profiles in the stages of impinger for three components suggested all components will likely deposit at the same sites of infection within the lungs enabling maximized synergy in antimicrobial efficacy against lung infections. Future studies may focus on the in-vivo PK and PD performance of such formulation using our established animal models (Lin et al., 2017a; Lin et al., 2017b).
Acknowledgement
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI132681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Qi (Tony) Zhou is partically supported by the Ralph W. and Grace M. Showalter Research Trust Award. Jiayang Huang was supported by Purdue College of Pharmacy Dean’s Undergraduate Research Fellowship. Jian Li is an Australian NHMRC Senior Research Fellow and Tony Velkov is an NHMRC Career Development Industrial Fellow. Kind donations of RS01 DPI device from Plastiape S.p.A. and HPMC capsules from Qualicaps, Inc are acknowledged.
Appendix
Appendix A1:
Aerosol deposition profiles of the different spray dried formulations (A) Meropenem-SD, (B) Colistin-Meropenem, (C) Meropenem-Rifampicin, and (D) Colistin-Meropenem-Rifampicin. The data are presented as mean ± SD (n=4).
Appendix A2:
In-vitro dissolution profile of ternary antibiotic formulation. The data are presented as mean ± SD (n=4).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed MU, Velkov T, Lin Y-W, Yun B, Nowell CJ, Zhou F, Zhou QT, Chan K, Azad MA, Li J, 2017. Potential toxicity of polymyxins in human lung epithelial cells. Antimicrobial Agents and Chemotherapy 61, e02690–02616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose PG, Bhavnani SM, Grosse EJE, Drusano GL, 2010. Pharmacokinetic-Pharmacodynamic Considerations in the Design of Hospital-Acquired or Ventilator-Associated Bacterial Pneumonia Studies: Look before You Leap! Clinical Infectious Diseases 51, S103–S110. [DOI] [PubMed] [Google Scholar]
- Bergen PJ, Landersdorfer CB, Zhang J, Zhao M, Lee HJ, Nation RL, Li J, 2012. Pharmacokinetics and pharmacodynamics of ‘old’ polymyxins: what is new? Diagnostic microbiology and infectious disease 74, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr A, P Boetker J, Rades T, Rantanen J, Yang M, 2014. Application of spray-drying and electrospraying/electospinning for poorly watersoluble drugs: A particle engineering approach. Current pharmaceutical design 20, 325–348. [DOI] [PubMed] [Google Scholar]
- Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J, 2009. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clinical Infectious Diseases 48, 1–12. [DOI] [PubMed] [Google Scholar]
- Bruinenberg P, Otulana B, Blanchard J, Cipolla D, Wilson J, Serisier D, 2009. Pharmacokinetics and antibacterial activity of inhaled liposomal ciprofloxacin hydrochloride in healthy volunteers and in cystic fibrosis (CF) patients. Journal of Cystic Fibrosis 8, S49. [Google Scholar]
- Buttini F, Colombo P, Rossi A, Sonvico F, Colombo G, 2012. Particles and powders: tools of innovation for non-invasive drug administration. Journal of controlled release 161, 693–702. [DOI] [PubMed] [Google Scholar]
- Buttini F, Miozzi M, Balducci AG, Royall PG, Brambilla G, Colombo P, Bettini R, Forbes B, 2014. Differences in physical chemistry and dissolution rate of solid particle aerosols from solution pressurised inhalers. International journal of pharmaceutics 465, 42–51. [DOI] [PubMed] [Google Scholar]
- Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, Darst SA, 2001. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104, 901–912. [DOI] [PubMed] [Google Scholar]
- Cheah SE, Wang J, Nguyen VT, Turnidge JD, Li J, Nation RL, 2015. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. The Journal of antimicrobial chemotherapy 70, 3291–3297. [DOI] [PubMed] [Google Scholar]
- Chew NY, Chan H-K, 2001. Use of solid corrugated particles to enhance powder aerosol performance. Pharmaceutical Research 18, 1570–1577. [DOI] [PubMed] [Google Scholar]
- Cipolla D, Blanchard J, Gonda I, 2016. Development of liposomal ciprofloxacin to treat lung infections. Pharmaceutics 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla D, Chan H-K, 2013. Inhaled antibiotics to treat lung infection. Pharmaceutical Patent Analyst 2, 647–663. [DOI] [PubMed] [Google Scholar]
- Claus S, Weiler C, Schiewe J, Friess W, 2014. How can we bring high drug doses to the lung? European Journal of Pharmaceutics and Biopharmaceutics 86, 1–6. [DOI] [PubMed] [Google Scholar]
- Diep JK, Jacobs DM, Sharma R, Covelli J, Bowers DR, Russo TA, Rao GG, 2017. Polymyxin B in Combination with Rifampin and Meropenem against Polymyxin B-Resistant KPC-Producing Klebsiella pneumoniae. Antimicrobial agents and chemotherapy 61, e02121–02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijlink H, De Boer A, 2004. Dry powder inhalers for pulmonary drug delivery. Expert opinion on drug delivery 1, 67–86. [DOI] [PubMed] [Google Scholar]
- Gontijo AV, Gregoire N, Lamarche I, Gobin P, Couet W, Marchand S, 2014. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 2. Colistin. Antimicrob Agents Chemother 58, 3950–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G, 2009. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 48, 1724–1728. [DOI] [PubMed] [Google Scholar]
- Heyder J, Gebhart J, Rudolf G, Schiller CF, Stahlhofen W, 1986. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J Aerosol Sci 17, 811–825. [Google Scholar]
- Hickey A, Durham P, Dharmadhikari A, Nardell E, 2016. Inhaled drug treatment for tuberculosis: past progress and future prospects. Journal of Controlled Release 240, 127–134. [DOI] [PubMed] [Google Scholar]
- Iranloye TA, Parrott EL, 1978. Effects of compression force, particle size, and lubricants on dissolution rate. Journal of Pharmaceutical Sciences 67, 535–539. [DOI] [PubMed] [Google Scholar]
- Kitzis MD, Acar JF, Gutmann L, 1989. Antibacterial activity of meropenem against Gram-negative bacteria with a permeability defect and against staphylococci. Journal of Antimicrobial Chemotherapy 24, 125–132. [DOI] [PubMed] [Google Scholar]
- Lee SH, Teo J, Heng D, Ng WK, Zhao Y, Tan RBH, 2016. Tailored Antibiotic Combination Powders for Inhaled Rotational Antibiotic Therapy. Journal of Pharmaceutical Sciences 105, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Levin AS, Barone AA, Penço J, Santos MV, Marinho IS, Arruda EAG, Manrique EI, Costa SF, 1999. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clinical Infectious Diseases 28, 1008–1011. [DOI] [PubMed] [Google Scholar]
- Levison ME, Levison JH, 2009. Pharmacokinetics and Pharmacodynamics of Antibacterial Agents. Infectious disease clinics of North America 23, 791–vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Sun S, Parumasivam T, Denman JA, Gengenbach T, Tang P, Mao S, Chan H-K, 2016. l-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. European Journal of Pharmaceutics and Biopharmaceutics 102, 132–141. [DOI] [PubMed] [Google Scholar]
- Lin Y-W, Wong J, Qu L, Chan H-K, Zhou QT, 2015. Powder production and particle engineering for dry powder inhaler formulations. Current pharmaceutical design 21, 3902–3916. [DOI] [PubMed] [Google Scholar]
- Lin Y-W, Zhou QT, Han M-L, Onufrak NJ, Chen K, Wang J, Forrest A, Chan H-K, Li J, 2017a. Mechanism-based Pharmacokinetic/Pharmacodynamic Modeling of Aerosolized Colistin in a Mouse Lung Infection Model. Antimicrobial agents and chemotherapy, AAC. 01965–01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-W, Zhou QT, Hu Y, Onufrak NJ, Sun S, Wang J, Forrest A, Chan H-K, Li J, 2017b. Pulmonary Pharmacokinetics of Colistin following Administration of Dry Powder Aerosols in Rats. Antimicrobial agents and chemotherapy 61, e00973–00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MDPDQ, Luo MDR, Bodin MDL, Yang MDJ, Zahr PDN, Aubry MDPDA, Golmard MDPDJ-L, Rouby MDPDJ-J, 2012. Efficacy of High-dose Nebulized Colistin in Ventilator-associated Pneumonia Caused by Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii Anesthesiology 117, 1335–1347. [DOI] [PubMed] [Google Scholar]
- Mangal S, Meiser F, Tan G, Gengenbach T, Denman J, Rowles MR, Larson I, Morton DAV, 2015. Relationship between surface concentration of L-leucine and bulk powder properties in spray dried formulations. Eur J Pharm Biopharm 94, 160–169. [DOI] [PubMed] [Google Scholar]
- Mangal S, Park H, Zeng L, Heidi HY, Lin Y. w., Velkov T, Denman JA, Zemlyanov D, Li J, Zhou QT, 2018a. Composite particle formulations of colistin and meropenem with improved in-vitro bacterial killing and aerosolization for inhalation. International journal of pharmaceutics 548, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangal S, Park H, Zeng L, Yu HH, Lin Y. w., Velkov T, Denman JA, Zemlyanov D, Li J, Zhou Q, 2018b. Composite particle formulations of colistin and meropenem with improved in-vitro bacterial killing and aerosolization for inhalation. International journal of pharmaceutics 548, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangal S, Xu R, Park H, Zemlyanov D, Shetty N, Lin Y-W, Morton D, Chan H-K, Li J, Zhou QT, 2019. Understanding the Impacts of Surface Compositions on the In-Vitro Dissolution and Aerosolization of Co-Spray-Dried Composite Powder Formulations for Inhalation. Pharmaceutical research 36, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S, Jensen B, Wolkenhauer M, Schneider M, Lehr CM, 2012. Dissolution Techniques for In Vitro Testing of Dry Powders for Inhalation. Pharmaceutical Research 29, 2157–2166. [DOI] [PubMed] [Google Scholar]
- Mizgerd JP, 2006. Lung infection--a public health priority. PLoS medicine 3, e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy BS, Schultz SG, Kim SG, Topp EM, 2014. Predicting Protein Aggregation during Storage in Lyophilized Solids Using Solid State Amide Hydrogen/Deuterium Exchange with Mass Spectrometric Analysis (ssHDX-MS). Molecular Pharmaceutics 11, 1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton JW, 1999. Combination therapy as a tool to prevent emergence of bacterial resistance. Infection 27, S24–S28. [DOI] [PubMed] [Google Scholar]
- Mukker JK, Singh RSP, Derendorf H, 2015. Pharmacokinetic and pharmacodynamic implications in inhalable antimicrobial therapy. Advanced Drug Delivery Reviews 85, 57–64. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Harris PNA, 2016. Colistin resistance: a major breach in our last line of defence. The Lancet Infectious Diseases 16, 132–133. [DOI] [PubMed] [Google Scholar]
- Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL, 2007. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6, 29–40. [DOI] [PubMed] [Google Scholar]
- Pilcer G, Amighi K, 2010. Formulation strategy and use of excipients in pulmonary drug delivery. International journal of pharmaceutics 392, 1–19. [DOI] [PubMed] [Google Scholar]
- Qu L, Morton DA, Zhou QT, 2015. Particle engineering via mechanical dry coating in the design of pharmaceutical solid dosage forms. Current pharmaceutical design 21, 5802–5814. [DOI] [PubMed] [Google Scholar]
- Rabbani NR, Seville PC, 2005. The influence of formulation components on the aerosolisation properties of spray-dried powders. Journal of controlled release 110, 130–140. [DOI] [PubMed] [Google Scholar]
- Riley T, Christopher D, Arp J, Casazza A, Colombani A, Cooper A, Dey M, Maas J, Mitchell J, Reiners M, Sigari N, Tougas T, Lyapustina S, 2012. Challenges with Developing In Vitro Dissolution Tests for Orally Inhaled Products (OIPs). AAPS PharmSciTech 13, 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritrovato CA, Deeter RG, 1991. Respiratory tract penetration of quinolone antimicrobials: a case in study. Pharmacotherapy 11, 38–49. [PubMed] [Google Scholar]
- Salama RO, Traini D, Chan H-K, Young PM, 2008. Preparation and characterisation of controlled release co-spray dried drug–polymer microparticles for inhalation 2: Evaluation of in vitro release profiling methodologies for controlled release respiratory aerosols. European Journal of Pharmaceutics and Biopharmaceutics 70, 145–152. [DOI] [PubMed] [Google Scholar]
- Shekunov BY, Chattopadhyay P, Tong HHY, Chow AHL, 2007. Particle Size Analysis in Pharmaceutics: Principles, Methods and Applications. Pharmaceutical Research 24, 203–227. [DOI] [PubMed] [Google Scholar]
- Shetty N, Ahn P, Park H, Bhujbal S, Zemlyanov D, Cavallaro A, Mangal S, Li J, Zhou QT, 2018a. Improved physical stability and aerosolization of inhalable amorphous ciprofloxacin powder formulations by incorporating synergistic colistin. Molecular pharmaceutics 15, 4004–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty N, Park H, Zemlyanov D, Mangal S, Bhujbal S, Zhou QT, 2018b. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. International journal of pharmaceutics 544, 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y-J, Worth Longest P, Hindle M, 2013. Aerosolization characteristics of dry powder inhaler formulations for the excipient enhanced growth (EEG) application: Effect of spray drying process conditions on aerosol performance. International journal of pharmaceutics 443, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sou T, Kaminskas LM, Nguyen T-H, Carlberg R, McIntosh MP, Morton DA, 2013. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. European Journal of Pharmaceutics and Biopharmaceutics 83, 234–243. [DOI] [PubMed] [Google Scholar]
- Urban C, Mariano N, Rahal JJ, 2010. In vitro double and triple bactericidal activities of doripenem, polymyxin B, and rifampin against multidrug-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli. Antimicrobial agents and chemotherapy 54, 2732–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehring R, 2008. Pharmaceutical particle engineering via spray drying. Pharm Res 25, 999–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehring R, Foss WR, Lechuga-Ballesteros D, 2007. Particle formation in spray drying. J. Aerosol Sci. 38, 728–746. [Google Scholar]
- Velkov T, Abdul Rahim N, Zhou Q, Chan H-K, Li J, 2015. Inhaled anti-infective chemotherapy for respiratory tract infections: Successes, challenges and the road ahead. Advanced Drug Delivery Reviews 85, 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velkov T, Deris ZZ, Huang JX, Azad MAK, Butler M, Sivanesan S, Kaminskas LM, Dong Y-D, Boyd B, Baker MA, Cooper MA, Nation RL, Li J, 2013. Surface changes and polymyxin interactions with a resistant strain of Klebsiella pneumoniae. Innate Immunity 20, 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F, Bohr A, Maltesen MJ, Bjerregaard S, Foged C, Rantanen J, Yang M, 2013. Critical Solvent Properties Affecting the Particle Formation Process and Characteristics of Celecoxib-Loaded PLGA Microparticles via Spray-Drying. Pharmaceutical Research 30, 1065–1076. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhou QT, Sun S-P, Denman JA, Gengenbach TR, Barraud N, Rice SA, Li J, Yang M, Chan H-K, 2016. Effects of Surface Composition on the Aerosolisation and Dissolution of Inhaled Antibiotic Combination Powders Consisting of Colistin and Rifampicin. The AAPS journal 18, 372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weers J, 2015. Inhaled antimicrobial therapy - barriers to effective treatment. Adv Drug Deliv Rev 85, 24–43. [DOI] [PubMed] [Google Scholar]
- WHO, May 2014. The 10 leading causes of death in the world, 2000 and 2012. who.int/mediacentre/factsheets/fs310/en/. [Google Scholar]
- Yapa SWS, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJH, Nation RL, McIntosh MP, 2014. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: Targeting advantage of inhalational administration. Antimicrobial Agents and Chemotherapy 58, 2570–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapa SWS, Li J, Porter CJH, Nation RL, Patel K, McIntosh MP, 2013. Population pharmacokinetics of colistin methanesulfonate in rats: Achieving sustained lung concentrations of colistin for targeting respiratory infections. Antimicrobial Agents and Chemotherapy 57, 5087–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Urban C, Terzian C, Mariano N, Rahal JJ, 2004. In vitro double and triple synergistic activities of polymyxin B, imipenem, and rifampin against multidrug-resistant Acinetobacter baumannii. Antimicrobial agents and chemotherapy 48, 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PM, Price R, Tobyn MJ, Buttrum M, Dey F, 2004. The influence of relative humidity on the cohesion properties of micronized drugs used in inhalation therapy. Journal of pharmaceutical sciences 93, 753–761. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Lin X, Liu X, Tian B, Tang X, 2010. High azithromycin loading powders for inhalation and their in vivo evaluation in rats. International journal of pharmaceutics 395, 205–214. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Gengenbach T, Denman JA, Yu HH, Li J, Chan HK, 2014a. Synergistic Antibiotic Combination Powders of Colistin and Rifampicin Provide High Aerosolization Efficiency and Moisture Protection. The AAPS journal 16, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Leung SSY, Tang P, Parumasivam T, Loh ZH, Chan H-K, 2015a. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Advanced Drug Delivery Reviews 85, 83–99. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Sun S-P, Chan JGY, Wang P, Barraud N, Rice SA, Wang J, Li J, Chan H-K, 2015b. Novel Inhaled Combination Powder Containing Amorphous Colistin and Crystalline Rifapentine with Enhanced Antimicrobial Activities against Planktonic Cells and Biofilm of Pseudomonas aeruginosa for Respiratory Infections. Molecular Pharmaceutics 12, 2594–2603. [DOI] [PubMed] [Google Scholar]
- Zhou QT, Loh ZH, Yu J, Sun SP, Gengenbach T, Denman JA, Li J, Chan HK, 2016. How Much Surface Coating of Hydrophobic Azithromycin Is Sufficient to Prevent Moisture-Induced Decrease in Aerosolisation of Hygroscopic Amorphous Colistin Powder? The AAPS journal 18, 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QT, Morton DA, 2012. Drug–lactose binding aspects in adhesive mixtures: controlling performance in dry powder inhaler formulations by altering lactose carrier surfaces. Advanced drug delivery reviews 64, 275–284. [DOI] [PubMed] [Google Scholar]
- Zhou QT, Morton DA, Yu HH, Jacob J, Wang J, Li J, Chan HK, 2013. Colistin powders with high aerosolisation efficiency for respiratory infection: preparation and in vitro evaluation. Journal of pharmaceutical sciences 102, 3736–3747. [DOI] [PubMed] [Google Scholar]
- Zhou QT, Tang P, Leung SSY, Chan JGY, Chan H-K, 2014b. Emerging inhalation aerosol devices and strategies: where are we headed? Advanced drug delivery reviews 75, 3–17. [DOI] [PubMed] [Google Scholar]
- Zhu K, Tan RBH, Kiong Ng W, Shen S, Zhou Q, Heng PWS, 2008. Analysis of the influence of relative humidity on the moisture sorption of particles and the aerosolization process in a dry powder inhaler. Journal of Aerosol Science 39, 510–524. [Google Scholar]