Abstract
Endogenous circadian oscillators regulate molecular, cellular and physiological rhythms, synchronizing tissues and organ function to coordinate activity and metabolism with environmental cycles. The technological nature of modern society with round-the-clock work schedules and heavy reliance on personal electronics has precipitated a striking increase in the incidence of circadian and sleep disorders. Circadian dysfunction contributes to an increased risk for many diseases and appears to have adverse effects on aging and longevity in animal models. From invertebrate organisms to humans, the function and synchronization of the circadian system weakens with age aggravating age-related disorders and pathologies. In this review, we highlight the impacts of circadian dysfunction on aging and longevity and the reciprocal effects of aging on circadian function with examples from Drosophila to humans underscoring the highly conserved nature of these interactions. Additionally, we review the potential for using reinforcement of the circadian system to promote healthy aging and mitigate age-related pathologies. Advancements in medicine and public health have significantly increased human lifespan in the past century. With the demographics of countries worldwide shifting to an older population, there is a critical need to understand the factors that shape healthy aging. Drosophila melanogaster, as a model for aging and circadian interactions, has the capacity to facilitate the rapid advancement of research in this area and provide mechanistic insights for targeted investigations in mammals.
Keywords: Drosophila, circadian, rhythms, biological clock
Graphical abstract
Increasing longevity necessitates understanding the factors that negatively impact healthy aging. The circadian clock weakens with age and circadian dysfunction exacerbates age-related pathologies. In this review, we highlight Drosophila as a valuable model for studying circadian-aging interactions, examine the interplay between the circadian system and aging with examples from Drosophila to humans, and review studies in which reinforcement of the circadian system promotes healthier aging.
Scope of Problem
The Aging Population
Over the past century, improvements in public health and scientific advances have significantly increased human longevity in countries around the world. In the United States, the average life expectancy for a baby born at the start of the 20th century was 47.3 years compared to recent projections of life expectancy for a child born in 2014 at 78.9 years (Crimmins, 2015; Arias et al., 2017). Globally, increases in lifespan are predicted to continue rising with life expectancy anticipated to be greater than 85 years for individuals in many countries by 2030 (Kontis et al., 2017). Consequently, the proportion of the adult population comprised of older individuals (greater than 65) has significantly increased with approximately 46 million people age 65 and older in the United States constituting 15% of the total population. By 2050, the number of older individuals is expected to almost double and will represent more than 22% of the population (Vincent & Velkoff, 2010; Ortman et al., 2014). With the increase in life expectancy and the changing demographics, the importance of age-related diseases for public health has escalated rapidly.
Broadly speaking, age-related diseases refer to those diseases that increase in prevalence with age. Extending lifespan significantly increases the prevalence of cancer, neurodegenerative and cognitive disorders including age-related dementia, Alzheimer’s and Parkinson’s Disease (White et al., 2014; Brown, 2015). In addition to the impacts on individual health, age-related diseases intensify societal burdens with increased demands on family members and caregivers as well as escalating the costs of health care services. Alzheimer’s disease alone affects more than 5.5 million Americans and represents the most economically costly disease in the United States with annual costs estimated at $277 billion (Alzheimer’s Association, 2018). By 2050, between 13.8 – 16 million individuals in the United States are predicted to develop Alzheimer’s disease (Alzheimer’s Association, 2018). With an increase in lifespan, individuals also must manage chronic conditions such as chronic inflammatory diseases, osteoarthritis and diabetes for longer periods potentially affecting the quality of life (Frasca et al., 2017; Franceschi et al., 2018). A recent study found that more older Americans suffer from three or more chronic conditions (36%) than older individuals in other developed countries including New Zealand, Australia, Norway, Switzerland and the Netherlands (rates range from 13% - 17%) with the differences attributed to lower affordability and access to medical care (Osborn et al., 2017). With lifespans increasing, the ability to manage chronic conditions poses a serious issue. Thus, there is a critical need for systems level studies to identify factors that exacerbate age-related diseases and identify therapeutic options to manage healthy aging.
The endogenous circadian clock regulates metabolic, physiological and behavioral rhythms with a 24 hour cycle providing the ability to coordinate physiological functions and anticipate regularly occurring environmental events. Evolutionarily, the light-dark cycle provides the strongest entrainment signal for the circadian clock (Roenneberg & Merrow, 2016), although feeding, activity, and social interactions can also provide input to the circadian system as well as being rhythmically regulated outputs (Webb et al., 2014). In the past two decades, the role of the circadian clock in health and disease has become increasingly clear. Disruptions or desynchronization of the circadian system increases the risk and incidence of many diseases including cancers, metabolic disorders, cardiovascular diseases, neurodegenerative and cognitive disorders, and neuropsychiatric disorders (Scheer et al., 2009; Schlosser Covell et al., 2012; Evans & Davidson, 2013; Golombek et al., 2013; James et al., 2017). In this review, we highlight the use of Drosophila as a model system for studying the interactions of the circadian clock with aging and underscore the conservation of these interactions between Drosophila and mammalian systems.
Versatility of the Drosophila model system
Drosophila is as an excellent genetic model to study the complexity of circadian and aging interactions, offering the ability to combine the characterization of single-gene mutations with circuit and system level analysis. The fruit fly has persisted for over a century as a model organism due to its conserved signaling pathways that make it applicable for the study of animal development and behavior, neurobiology, and human diseases. Logistically, the short lifespan (60–80 days post-eclosion) of Drosophila coupled with its capability of generating large populations within 12–14 days make fruit flies a practical model for studies of aging. Genetic tools available in flies permit the analysis of systems level tissue interactions, using spatial and temporal regulation of gene expression, with the effects of environmental interventions such as oxidative stress, diet, and inflammation that influence longevity. The fly genome is approximately 5% of the size of human genome (132 billion base pairs for the fly compared to 3.2 billion base pairs in human) with nearly 14,000 genes on its four chromosomes (Adams et al., 2000) compared to the estimated 22,000 genes on the 23 chromosome pairs of humans. Importantly, both flies and humans have retained homologous genes for over 60% of their genome, including genes related to disease traits (Reiter et al., 2001; Harrison et al., 2002; Lloyd & Taylor, 2010; Pandey & Nichols, 2011). Approximately 75% of genes associated with human cancers and genetic diseases have homologs in the fly genome (Chien et al., 2002; Lessing & Bonini, 2009). These factors render Drosophila an ideal “simple model system” for the analysis of aging-related diseases and pathologies.
The cost-effectiveness, genetic tractability, high throughput assays and availability of tools to manipulate neuronal properties with spatiotemporal accuracy of Drosophila have propelled it to the forefront as a competitive model for rapid identification of the cellular and molecular mechanisms underlying aging-related diseases including neurodegenerative diseases (Krishnan et al., 2009; Blake et al., 2015; Gerstner et al., 2016); cardiovascular diseases (Piazza & Wessells, 2011; Yu et al., 2013; Zarndt et al., 2015), cancer (Rudrapatna et al., 2012; Christofi & Apidianakis, 2013); diabetes and obesity (Trinh & Boulianne, 2013; Park et al., 2014; Alfa & Kim, 2016). As a model, Drosophila has also been used for evaluating the efficacy of potential therapeutic drugs to treat aging-related diseases and disorders (Das & Cagan, 2013; Palandri et al., 2018). Despite the obvious differences in anatomical structure and levels of behavioral complexity, the physiological mechanisms underlying most biological processes between Drosophila and mammals are remarkably well conserved (Pandey et al., 1998; Pandey, 1998; Pandey & Nichols, 2011). Flies have analogous organs or structures that perform the functional equivalent of mammalian organs including the heart (Seyres et al., 2012; Brandt & Paululat, 2013; Ma, 2016), lung (Roeder et al., 2012; Zuo et al., 2013), kidney (Dow & Romero, 2010; Miller et al., 2013), gut (Erkosar & Leulier, 2014; Katzenberger et al., 2015a; Katzenberger et al., 2015b; Pasco et al., 2015) and liver (Buchon et al., 2011; Maruyama & Andrew, 2012; Ugur et al., 2016).
The molecular, cellular, and electrophysiological properties underlying neuronal behavior and synaptic plasticity are remarkably well conserved between flies and higher organisms even with the considerable neuroanatomical differences (Kazama, 2015; Maximino et al., 2015). The Drosophila brain is on the order of 100,000 neurons compared to the 100 million neurons of the mouse brain. The Drosophila connectome has revealed 43 discreet neural subregions within the fly brain organized by modality into olfaction, auditory/mechanosensation, right vision, left vision and pre-motor modules (Chiang et al., 2011; Kaiser, 2015; Shih et al., 2015). Distinct neural circuits have been identified for the regulation of complex behaviors including sleep (Potdar & Sheeba, 2013; Parisky et al., 2016; Artiushin & Sehgal, 2017), learning and memory (Busto et al., 2016; Kaun & Rothenfluh, 2017), grooming and feeding (Joseph & Carlson, 2015; Landayan & Wolf, 2015), circadian rhythms (Helfrich-Förster, 2014), aggression and courtship (Yamamoto & Sato, 2013; Kravitz & Fernandez, 2015). Recently, whole brain imaging combined with targeted neurogenetics has generated neural maps of locomotor and social behaviors in Drosophila (Robie et al., 2017) facilitating systems level research.
Drosophila as a Model for the Circadian Clock
Colin Pittendrigh’s study of eclosion rates of fruit flies in the 1950s established Drosophila as a prominent model for circadian research allowing scientists to define the theoretical, molecular and genetic framework for the circadian clock (Pittendrigh et al., 1973; Konopka et al., 1989; Pittendrigh & Takamura, 1989; Pittendrigh et al., 1991). Understanding the molecular mechanisms of the circadian oscillator began with the seminal discovery by Konopka and Benzer in 1971 of the first genetic mutants (period gene) that changed the length of the circadian period. Genetic transformation of per0 arrhythmic flies using DNA encoding the wild-type period gene restored rhythms in eclosion and locomotor behavior in these flies confirming the role of period in the circadian oscillator (Bargiello et al., 1984; Zehring et al., 1984). Subsequent studies using genetic screens and analysis of locomotor activity rhythms identified numerous genes that form the core clock mechanism in flies including timeless, clock, cycle, doubletime, shaggy, casein kinase 2 subunits and cryptochrome (Sehgal et al., 1994; Reppert & Sauman, 1995; Allada et al., 1998; Kloss et al., 1998; Price et al., 1998; Rutila et al., 1998; Martinek et al., 2001; Lin et al., 2002; Akten et al., 2003; Fan et al., 2013). In the late 1990s, the per and clock genes were the first mammalian circadian genes to be identified and sequenced (King et al., 1997; Tei et al., 1997; Kloss et al., 1998).
The central pacemaker of Drosophila spans approximately 150 neurons, anatomically organized into seven neuronal groups comprising an interactive network of oscillators. The small and large ventral lateral neurons control the morning activity peak and express pigment dispersing factor (PDF; fly orthologue of mammalian neuropeptide VIP), a neuromodulator important for the internal synchronization and output pathways of the fly’s circadian clock. The lateral dorsal and dorsal neurons control the evening activity peak (Kaneko & Hall, 2000; Helfrich-Förster, 2003; Yao & Shafer, 2014; Yoshii et al., 2016).
A set of transcription-translation feedback loops drive the circadian rhythms in physiology and behavior for both mammals and flies (Ko & Takahashi, 2006; Dibner et al., 2010; Ozkaya & Rosato, 2012; Hardin & Panda, 2013). Although differences in individual genes exist between the core oscillators of Drosophila and mammals, the underlying mechanisms of clock function are similar. Transcription factors in the positive limb activate transcription of genes in the negative limb that then repress the transcriptional activators (Glossop et al., 1999; Morse & Sassone-Corsi, 2002; Reppert & Weaver, 2002). In Drosophila, clock (dClk) and cycle (dCyc) act as transcriptional activators which heterodimerize and bind to the E-box-regulated promoters of period (per) and timeless (tim) to activate transcription of the core circadian genes, per and tim (Sehgal et al., 1994; Reppert & Sauman, 1995; Rutila et al., 1998) and other clock controlled genes (Menet et al., 2010; Abruzzi et al., 2011). In the cytoplasm, PER monomers are phosphorylated by doubletime (DBT) rendering them unstable. When the levels of dPER and dTIM increase, these two proteins form a dPER/dTIM/DBT complex and translocate back to the nucleus, interrupting further transcription by dClk and dCyc (Kloss et al., 1998; Price et al., 1998; He et al., 2017). In mammals, the proteins CLOCK (CLK) and brain and muscle ARNT-like protein1 (BMAL1) are the members of the family of basic helix loop helix – PAS transcription factors that form the positive limb of the feedback loop (Vitaterna et al., 1994; Gekakis et al., 1998). CLK and BMAL1 heterodimerize and bind to the promoters of the per (mPer1, mPer2 and mPer3) and cryptochrome (mCry1 and mCry2) genes, activating their transcription along with many other clock genes (Huang et al., 2012; Partch et al., 2014). Following translation of mPER and mCRY proteins, mPER and mCRY heterodimerize and form a repressor complex, translocating to the nucleus to inhibit CLK-BMAL1-mediated activation of mPer and mCry genes (Partch et al., 2014). Unlike mammals, in the central brain pacemakers of Drosophila CRY has an important role in circadian photoreception and triggers light-induced TIM degradation (Stanewsky et al., 1998; Emery et al., 2000). In both mammals and flies, posttranscriptional and translational elements regulate the core clock proteins to influence their cellular localization and nuclear stability fine-tuning period length (Martinek et al., 2001; Ko & Takahashi, 2006; He et al., 2017). These include kinases such as doubletime (casein kinase 1 epsilon, CK1E in mammals) that regulate phosphorylation of PER that targets the protein for ubiquitination and proteasome degradation. More detailed information on the circadian clock can be found in the following recent review articles (Tataroglu & Emery, 2015; Yoshii et al., 2016; He et al., 2017; Mendoza-Viveros et al., 2017).
Aging-related behaviors and physiological changes are similar between Drosophila and mammals
Given the widespread changes that occur with aging across tissues and systems, it can be difficult to distinguish the primary impacts of aging on cellular function and tissue damage from secondary consequences and subsequent disease states. Recently, there has been growing emphasis on the use of ‘simple’ model systems for the study of systems level interactions. The fruit fly Drosophila has long been appreciated as a genetic model for the identification of highly conserved molecular and cellular signaling processes.
Although Drosophila have a relatively short life cycle with a maximum lifespan of 60 – 80 days post-eclosion depending upon the strain and development conditions, many of the physiological and molecular changes associated with aging parallel those processes in mammals. In flies as in humans, aging differentially affects behavioral responses, neural circuits and organs distinguishing age-related changes from general physiological decline. The adult Drosophila is almost entirely post-mitotic from the time of eclosion until death (Bozcuk, 1972; Ito & Hotta, 1992) making the fruit fly suitable for studies of aging and cellular senescence (Grotewiel et al., 2005). Similar to humans, fruit flies exhibit age-related declines in behavioral phenotypes from motor skills to learning and memory. In the following sections, we highlight a few age-related phenotypic changes to demonstrate equivalencies in aging from Drosophila to mammals and humans.
Senescence of muscles and motor activity
A marked feature of aging in humans and other mammals is the progressive loss of skeletal muscle mass known as sarcopenia (Fried et al., 2004). Muscle dysfunction as a result of aging has been extensively studied in Drosophila (Fisher, 2004; Augustin & Partridge, 2009; Demontis et al., 2013; Rai et al., 2014). Drosophila display muscle deterioration with increasing age (Taylor, 2006; Piccirillo et al., 2014). Older flies exhibit sarcomere-related defects including reduced length and increased disorganization of sarcomeres as well as changes in myofibrillar protein composition and function (D’Antona et al., 2007; Miller et al., 2008).
Commonly used assays to assess motor function with aging in Drosophila include negative geotaxis, exploratory walking and locomotor activity (Gargano et al., 2005; Grotewiel et al., 2005; Liao et al., 2017). Individual and longitudinal studies in Drosophila have shown that negative geotaxis declines as flies reach middle and old age reflecting age-related changes in motor function. (Miquel et al., 1976; Leffelaar & Grigliatti, 1984; Cook-Wiens & Grotewiel, 2002; Goddeeris et al., 2003; Rhodenizer et al., 2008; Ratliff et al., 2015). Walking velocity, exploratory activity, and the duration of individual motor activity bouts also decrease with age (Grotewiel et al., 2005; Liao et al., 2017). Phototactic behavior and flight ability have also been used to measure age related declines in motor activity (Leffelaar & Grigliatti, 1984; Palladino et al., 2002).
Age-related declines in learning and memory
Age-associated cognitive decline encompasses numerous memory impairments with increased forgetfulness, the inability to maintain focus and a lowered problem solving capability starting as early as age 50 in humans (Albert & Heaton, 1988; Aartsen et al., 2002; Salthouse, 2009). Studies of hippocampal function in rodent models suggest that aging induces changes in synaptic plasticity rather than neuronal loss (Burke & Barnes, 2006; Shetty et al., 2017). In Drosophila, aging has been found to impact the acquisition of learning with significant differences in the rate of habituation of the proboscis extension reflex observed between young flies (3 days old) and middle-aged flies (35 days old; Fois et al., 1991). Age-related impairments in the acquisition of learning also become apparent during middle age for associative learning paradigms including conditioned suppression of the proboscis extension reflex and classical olfactory conditioning (Brigui et al., 1990; Fois et al., 1991; Tamura et al., 2003; Grotewiel et al., 2005). Similarly, deficits in acquisition are seen when visual cues are paired with quinine during inhibitory conditioning of the proboscis extension reflex in middle aged (30 day) and older flies (50 days old) with these flies requiring extended training to learn the association (Fresquet & Médioni, 1993).
In addition to age-related deficits in learning acquisition, olfactory conditioning has been used to define age-related changes in multiple forms of memory. Middle-aged flies (20 or 30 days old) exhibit significant decrements in intermediate or middle-term memory measured one hour after training (Tamura et al., 2003; Yamazaki et al., 2007; Tonoki & Davis, 2012; Yamazaki et al., 2014). Functional imaging of neural circuit activity found that the memory trace for intermediate-term memory was impaired in 30 day old flies suggesting deficits in synaptic transmission (Tonoki & Davis, 2012). Long-term memory, dependent upon transcription and protein synthesis, is also subject to age-related impairments as observed for olfactory conditioning (Mery et al., 2007) with age-related defects apparent in synaptic connectivity (Tonoki & Davis, 2015). Aged flies demonstrate an accelerated rate of memory extinction, an active inhibition of memory processes, and impaired memory restoration (Chen et al., 2015). As in humans, the appearance and degree of age related memory impairments in Drosophila may be dependent upon the type of learning and the neural circuits involved. For example, learning and memory of conditioned courtship suppression remains robust in middle-aged flies (Savvateeva et al., 1999; Savvateeva et al., 2000). Aging also appears to have a minimal effect on a protein synthesis independent form of memory, anesthesia resistant memory, in Drosophila (Tamura et al., 2003; Mery, 2007; Tonoki & Davis, 2012). With the ability to distinguish multiple facets of learning and memory at the behavioral level, Drosophila presents an excellent model for studying age-related memory impairments.
Age-related changes in sensory systems
Olfactory sensitivity noticeably decreases with age in humans (Doty et al., 1984; Zhang & Wang, 2017). Deterioration of olfactory sensitivity has been identified as an early predictor of cognitive impairment in later years (Royall et al., 2002; Swan & Carmelli, 2002; Wilson et al., 2007; Schubert et al., 2008). Similarly, studies in Drosophila have shown that older flies exhibit a characteristic decline in olfactory avoidance behavior to aversive odors with olfactory deficits first appearing in middle age (25 days; Cook-Wiens & Grotewiel, 2002; Grotewiel et al., 2005). The decline in olfactory abilities can also be observed in middle aged males affecting nocturnal courtship activities (Ratliff et al., 2015). Age-dependent changes in the Drosophila olfactory system occur both at the molecular level with significantly decreased mRNA expression for many olfactory pathway components (Ratliff et al., 2015) and at the structural level with aging-induced structural changes within neurons of the olfactory circuit (Hussain et al., 2018). Age-related declines in sensory perception appear to be system specific as there are no observable effects of age on behavioral responses to electric shocks or the slow phototaxic attraction response to light (Cook-Wiens & Grotewiel, 2002; Tamura et al., 2003; Grotewiel et al., 2005; Simon et al., 2006; Hussain et al., 2018).
In humans, photoreception is particularly susceptible to age-related changes with changes in light sensitivity for circadian entrainment occurring with age (Zhang et al., 1996; Benloucif et al., 1997; Duffy et al., 2007; Zuurbier et al., 2015) and the development of age-related diseases such as macular degeneration (Ardeljan & Chan, 2013; DeAngelis et al., 2017). More than 20% of elderly Americans suffer from visual impairments (Klein & Klein, 2013). While several research studies concluded that aging did not affect Drosophila photoreception, more detailed analysis has found decreased fast phototactic responses in older flies (Simon et al., 2006; Hall et al., 2017). Although no significant retinal degeneration has been observed in flies up to 40 days of age (Kurada & O’Tousa, 1995; Hall et al., 2017), studies in Drosophila have identified changes in photoreceptor gene expression associated with visual senescence at the cellular level using transcriptome profiling (Hall et al., 2017).
Age-dependent changes in cardiac function
Cardiac performance declines progressively with increased age across many organisms (Ocorr et al., 2007a; Nishimura et al., 2011). In mammals, cardiac aging includes the decline in cardiomyocyte number, decreased diastolic function, fibrosis and accumulation of collagen as well as an increase in prevalence of atrial fibrillation and left ventricular hypertrophy (Olivetti et al., 1991a; Olivetti et al., 1991b; Khan et al., 2002; Chiao & Rabinovitch, 2015; Steenman & Lande, 2017). Unlike mammals, Drosophila has an open circulatory system with a simple open tube heart, composed of spirally and longitudinally oriented microtubules that pumps hemolymph to all other organs (Molina & Cripps, 2001; Dulcis & Levine, 2003; Rotstein & Paululat, 2016). Older flies have more disorganized spiral myofibrils with less compact arrangements (Mery et al., 2008; Taghli-Lamallem et al., 2008). Middle-aged (30 day old) and older flies (50 day old) exhibit decreased resting heart rates of approximately 250 and 200 beats per minute respectively and increasingly non-rhythmic contraction patterns compared to the strong rhythmic pattern and resting heart rate of 300 beats per minute observed in young flies (Paternostro et al., 2001; Fink et al., 2009). These age-dependent increases in arrhythmia and cardiac arrest in Drosophila are similar to the increased atrial fibrillation and heart failure observed in humans (Lakatta & Levy, 2003; Ocorr et al., 2007a; Ocorr et al., 2007b). Similar to mammals, cardiac performance under stressful conditions (such as electrical pacing, elevated temperature and hypoxia) also decreases with age in flies (Grotewiel et al., 2005; Nakamura et al., 2011; Nishimura et al., 2011). After electrical pacing, a greater proportion of older flies exhibit fibrillation and decreased ability to resume normal heart rhythms (Paternostro et al., 2001; Wessells & Bodmer, 2004; Wessels & Pérez-Pomares, 2004; Piazza et al., 2009). High resolution imaging studies of awake flies has shown that aging induces cardiac relaxation (diastole defects) increasing fibrillatory cardiac arrest similar to what is seen in many people with cardiac conditions (Klassen et al., 2017). However, in flies as in rodent models and humans, only a few conserved genes have been identified in cardiac aging through genetic studies suggesting that the conserved phenotypes of age-dependent cardiac changes can arise from dissimilar genetic mechanisms (Cannon et al., 2017).
Age-related changes in sleep
The striking similarities between sleep in Drosophila and mammals render flies an accepted model for the study of sleep (Hendricks et al., 2000; Shaw et al., 2000; Cirelli, 2003; Shaw, 2003; Donlea, 2017; Ly et al., 2018). Drosophila sleep comprises periods of sustained quiescence characterized by increased arousal threshold, stereotyped posture, changes in brain activity and differential expression of numerous genes (Hendricks et al., 2000; Shaw, 2003; Murphy et al., 2016; Donlea, 2017; Ly et al., 2018). Flies also exhibit multiple sleep stages characterized by differences in the intensity of sleep (van Alphen et al., 2013; Yap et al., 2017). Sleep in flies is regulated both homeostatically and by the circadian clock. Flies exhibit greater sleep during the night, although bouts of daytime sleep occur with males demonstrating significantly greater daytime sleep than females (Huber et al., 2004; Isaac et al., 2010). Following sleep deprivation, flies exhibit rebound sleep consistent with the homeostatic regulation of sleep (Hendricks et al., 2000; Shaw, 2003; Donlea, 2017; Ly et al., 2018).
Aging results in the breakdown of sleep-wake cycles in flies and higher vertebrates (Koh et al., 2006; Vienne et al., 2016). Young flies have long, uninterrupted bouts of sleep whereas older flies exhibited increased sleep fragmentation. In young female flies, sleep occurs primarily at night but in aged females, daytime sleep is increased with reductions in sleep during the night (Koh et al., 2006). Aging also increases the number of brief awakenings and the number of sleep bouts with decreased duration of sleep bouts (Mendelson & Bergmann, 1999b; a; Colas et al., 2005; Koh et al., 2006). Middle-aged flies (30 days) exhibit decreased sleep consolidation compared to younger 10 day old flies (Vienne et al., 2016). Middle-aged male flies (25–27 days old) also have significantly more sleep fragmentation during the night (increased number of sleep bouts with shorter duration per bout) compared to young males (Williams et al., 2016). Older flies exhibit decreased arousal thresholds and increased sensitivity to neuronal stimuli compared to younger flies (Vienne et al., 2016). Age-dependent changes in sleep homeostasis also occur in Drosophila with reduced recovery sleep following sleep deprivation in older flies (Vienne et al., 2016). Similarly, older people (60 years and older) exhibit decreased total sleep duration, poorer sleep quality and increased sleep latency (Pandi-Perumal et al., 2005; Ohayon, 2010). Older adults also are more easily aroused from sleep than young adults (Zepelin et al., 1984). Reduced sleep rebound with aging occurs in humans and in mammalian models (Carrier et al., 2001; Landolt & Borbély, 2001; Dijk et al., 2010; Zimmermann, 2011).
Sleep fragmentation and mis-timed sleep induces inflammatory responses and activates pathways reflecting oxidative stress in both young (5–7 days old) and middle-aged flies (25–27 days old); however, the continued elevation of reactive oxidative species occurs only in middle-aged flies and not in young flies following recovery sleep (Williams et al., 2016). ER-chaperone genes are expressed at low levels in both normal sleeping young and middle-aged flies but are significantly increased in middle-aged flies following recurrent sleep fragmentation, implying that older flies are more susceptible to neuronal stress and that aging affects the molecular consequences of sleep disturbances (Williams et al., 2016).
Understanding Circadian and Aging Interactions
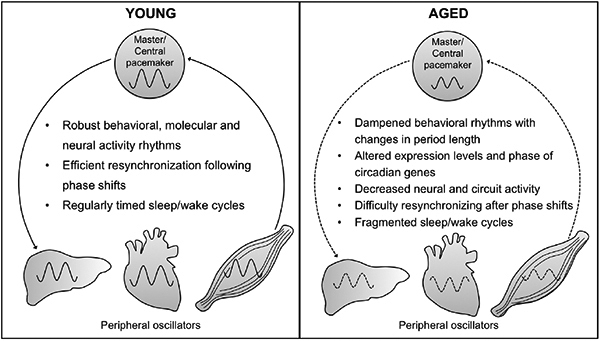
From Drosophila to humans, the weakening of the circadian system with age results in lower amplitude circadian rhythms as well as fragmentation of these rhythms (Weinert, 2000; Gibson et al., 2009; Zuurbier et al., 2015; Mattis & Sehgal, 2016). Furthermore, circadian perturbations and the weakening of the circadian system can increase sleep fragmentation with aging. Circadian dysfunction and sleep disorders aggravate many age-related cognitive and neurodegenerative disorders (Musiek & Holtzman, 2016; Liu & Chang, 2017) contributing to declining quality of life in old age. Recent research suggests that circadian rhythm fragmentation is associated with pre-clinical Alzheimer’s disease and may exacerbate disease development (Musiek et al., 2018). Disrupted circadian rhythms and sleep patterns have also been identified as a predictive factor for the development of cognitive impairment and dementia (Schlosser Covell et al., 2012; Shi et al., 2017). Below, we highlight research from Drosophila and mammalian models that has facilitated our understanding of the reciprocal interactions between the circadian system starting with examples of how aging weakens the circadian system and then examining ways through which circadian dysfunction contributes to aging pathologies. Finally, we review studies in which reinforcement or strengthening of the circadian system mitigates age-related pathologies.
Impact of Aging on Circadian Oscillators
In humans and other mammals, the suprachiasmatic nucleus (SCN) in the hypothalamus houses the ‘master’ circadian clock responsible for synchronizing many tissue specific circadian oscillators in a hierarchical manner (Albrecht, 2012). At the molecular level, aging results in changes in the phase and amplitude of rhythms in core clock genes in the SCN (Weinert et al., 2001; Kolker et al., 2003; Bonaconsa et al., 2014; Nakamura et al., 2015) and in other brain regions (Chen et al., 2016). Neural activity rhythms of SCN cells are also drastically altered by aging with significantly decreased amplitudes of the rhythms observed (Nakamura et al., 2011). In hamsters, the transplant of fetal SCN tissue to aged animals significantly increased longevity suggesting that a robust functioning circadian clock contributes to healthy aging (Hurd & Ralph, 1998).
However, aging does not uniformly affect molecular rhythms in gene expression as age-related impacts appear to differ between core circadian genes. When SCN function was analyzed in rats using a Per1-luciferase reporter, the free running period was significantly shortened in older animals although robust cycling was observed (Yamazaki et al., 2002). In Syrian hamsters, aging decreases Bmal1 expresssion in the SCN (Kolker et al., 2003; Duncan et al., 2013) and core clock gene expression of Bmal1, Clk and per2 are significantly attenuated in multiple brain regions of older mice and hamsters including the hippocampus and cingulate cortex (Duncan et al., 2001; Wyse & Coogan, 2010; Duncan et al., 2013). Similarly, age-related discrepancies in per2 mRNA rhythms in the SCN have been reported in mice (Weinert et al., 2001). However, other studies have failed to find any age-dependent weakening of the SCN molecular oscillator. In a recent gene expression profiling study of aging using a non-human primate model, no differences were found in the cycling of core clock genes or genes involved in SCN signaling between young and old animals (Eghlidi et al., 2018). When extra-SCN brain regions are examined, significant effects of age on molecular circadian rhythms can be observed similar to the impacts seen in peripheral brain oscillators in Drosophila. In a pioneering study of the effect of aging on circadian gene expression in human brain tissue, circadian rhythmicity was found to be altered in an age-dependent manner for approximately 1200 genes with changes in rhythmicity found for genes involved in cognition, sleep and mood regulation (Chen et al., 2016). More detailed reviews of changes in circadian function and their implications for health and disease in mammalian systems are available (Kondratov, 2007; Tevy et al., 2013; Arellanes-Licea et al., 2014; Banks et al., 2016; Hood & Amir, 2017).
At the behavioral level, locomotor activity rhythms are frequently used as a proxy to assess the state and function of the central brain circadian clock in Drosophila or the SCN in mammals. Aging lengthens the free-running period of locomotor activity rhythms in Drosophila and weakens rhythmicity. In addition to increasing the percentage of arrhythmic flies, more phase instability and rhythm fragmentation is observed in older flies (Koh et al., 2006; Luo et al., 2012; Rakshit et al., 2012). These changes can be observed starting in middle-age with continuous declines as flies get older (Koh et al., 2006; Luo et al., 2012; Rakshit et al., 2012; Umezaki et al., 2012). It can be difficult to distinguish the effects of aging from the adverse physiological impacts of cumulative environmental exposures to toxins. In developmental studies in which extended multi-generational selection was done to establish a population of faster developing flies, it was found that faster developing flies exhibited age-related phenotypes in circadian rhythms significantly earlier than wild-type flies including a lengthening of the circadian period and increased arrhythmicity (Yadav & Sharma, 2014). Thus, aging of the circadian system appears due to physiological and developmental mechanisms rather than chronological age or cumulative exposure to environmental factors. Deterioration of circadian rhythms with age are phylogenetically conserved with rodent models also displaying age-dependent changes in free-running period length, increased fragmentation and decreased rhythm strength (Weinert, 2000; Weinert et al., 2001; Yamazaki et al., 2002; Froy, 2011). Age-dependent changes in the free-running period of activity rhythms also occur in non-human primate models (Aujard et al., 2006).
Early molecular studies in Drosophila investigating the principles through which the core circadian oscillator functions relied upon analysis of RNA and protein levels in whole fly heads (Hardin et al., 1990; 1992), and molecular analysis of whole fly heads remains a frequent starting point for Drosophila circadian studies. With aging, a decline in the amplitude of the RNA rhythm for the per gene in whole fly heads becomes apparent starting in middle age (35 day flies) with greater suppression of the RNA rhythms seen in older flies (50 days; Krishnan et al., 2009). More widespread analysis of core circadian clock genes in aging Drosophila found that tim, cry, Pdp1€ and vri mRNA rhythms also dampened with significant reductions in the amplitude of the molecular rhythm in the heads of middle-aged and older flies (Rakshit et al., 2012; Rakshit & Giebultowicz, 2013). In contrast, aging does not alter the expression levels of Clk and cyc mRNAs with Clk mRNA remaining robustly rhythmic in older flies (Rakshit et al., 2012). In conjunction with the declining amplitude in per and tim mRNA oscillations, PER and TIM protein levels are markedly reduced in the heads of old flies compared to young flies (Rakshit et al., 2012).
Given the complexity of the circadian system even in a relatively simple model such as Drosophila, one cannot directly ascribe changes in behavioral rhythms to alterations in core oscillator function of pacemaker neurons when molecular rhythms are measured in whole fly heads. The circadian pacemaker neurons only makeup a small fraction of the neurons in the brain with the central circadian clock consisting of 150 neurons grouped into seven neuronal groups (Helfrich-Förster et al., 2011; Helfrich-Förster, 2014; Yoshii et al., 2016). As peripheral oscillators in the head, Drosophila photoreceptors comprise the largest population of rhythmic neurons when whole heads are examined. When Drosophila photoreceptors alone are examined, significant age-related declines in core circadian genes are observed (Luo et al., 2012; Rakshit et al., 2012) raising the possibility that age-related changes in photoreceptor circadian gene expression may obscure the true state of pacemaker neurons. More specific analysis of the circadian pacemaker neurons found circadian PER protein expression in key groups of clock neurons, the small ventral lateral neurons, the dorsal lateral neurons and the group 1 dorsal neurons in late middle-aged flies (40 days; Luo et al., 2012). The robust cycling of PER continued in older flies (60 days and 80 days old), although the phase was shifted compared to younger flies providing a possible mechanism for the longer free-running periods of older flies (Luo et al., 2012). It should be noted that not all studies have found similar results potentially due to genetic background differences in the fly strains examined. In a contrasting study, when the peak protein levels for PER and TIM were examined in all seven groups of pacemaker neurons in the central brain, the levels were significantly reduced in late middle age (40 days old) and older flies (50 days old; Umezaki et al., 2012). At the cellular level, aging results in the loss of structural plasticity in the pacemaker cells of the ventral lateral neurons with no increase of synaptic terminals in response to social enrichment observed in middle-aged flies (Donlea et al., 2014b). Thus, while the effects of aging on pacemaker neurons are not fully understood at the molecular level in either flies or mice, the evidence is clear that age-related changes occur in neural activity and structural plasticity in the central clock affecting circadian outputs.
Aging, Circuit Connectivity and Synchronization
Individually the neurons within the mammalian SCN maintain autonomous rhythms; however, the period and phase of the rhythms of individual cells varies significantly (Welsh et al., 1995). Synchronization and intercellular coupling between SCN neurons is necessary for robustness of the circadian clock (Aton & Herzog, 2005). During phase shifts (changes in time zones) of the circadian system, resetting to the new phase requires synchronization between central circadian pacemaker neurons and peripheral oscillators. As one ages it becomes more difficult to reset the circadian system resulting in more severe jetlag (Monk et al., 1993; Monk et al., 2000). Aging appears to impact multiple mechanisms affecting the ability of the SCN neurons to respond to phase shifts. Advancing age decreases the sensitivity of the circadian system to light resetting in humans and other animals making it more difficult for the circadian system to respond to phase shifts (Zhang et al., 1996; Benloucif et al., 1997; Duffy et al., 2007; Zuurbier et al., 2015). Furthermore, aging decreases synchronization between SCN neurons as well as affecting the neural activity of individual cells resulting in a weakening of the master pacemaker (Farajnia et al., 2012; Duffy et al., 2015; Hood & Amir, 2017).
At the molecular level, aging weakens SCN output activity with decreased rhythms in the circadian neuropeptide vasopressin (VIP) and age-dependent reductions in its receptor observed in rodent models (Kawakami et al., 1997; Krajnak et al., 1998; Duncan et al., 2001; Kalló et al., 2004; Nakamura et al., 2016; Allen et al., 2017). In non-human primates, significant age-dependent phase-shifts are also seen in VIP expression as well as in arginine-vasopressin, another neuropeptide with circadian functions, suggesting age-related deficits in transmission of circadian information from the SCN to downstream neuronal targets (Cayetanot et al., 2005; Aujard et al., 2006). In addition to synchronization within the SCN neurons, there must be a resynchronization throughout the hierarchical circadian system with peripheral oscillators becoming realigned to the new phase. Aging also impairs the resynchronization between central and peripheral oscillators following phase-shifts (Davidson et al., 2008; Sellix et al., 2012). Thus, it appears that the aging induces impairments in circadian neural output activity and circuit connectivity resulting in decreased circuit synchronization. Desynchronization between the central oscillator and peripheral oscillators can exacerbate disease pathology and accelerate mortality (Zuurbier et al., 2015; Chauhan et al., 2017).
In Drosophila, pigment dispersing factor (PDF) is the primary circadian neurotransmitter connecting the evening and morning oscillators as well as providing a mechanism for circadian output to many neurons throughout the brain (Renn et al., 1999; Taghert & Shafer, 2006; Yasuyama & Meinertzhagen, 2010) similar to the role of the VIP in the mammalian brain (Jin et al., 1999). Age-dependent changes in circadian activity rhythms in Drosophila have been correlated with reduced PDF signaling in older flies suggesting a weakening of the circadian neural circuit or weakened communication to downstream target neurons (Umezaki et al., 2012). Overexpression of PDF in older flies strengthens circadian activity rhythms and buffers the age-induced change in period length (Umezaki et al., 2012). Moreover, PDF overexpression increases TIM cycling in pacemaker neurons removing the age-related suppression of peak protein abundance in the older flies (Umezaki et al., 2012). Thus, the effect of aging on circadian output and connectivity appear conserved across species.
In addition to neuronal pacemakers, glial cells have circadian oscillators and function in the regulation of circadian rhythms in Drosophila (Ng et al., 2011; Jackson et al., 2015; You et al., 2018) and mammals (Prolo et al., 2005; Marpegan et al., 2009; Barca-Mayo et al., 2017; Brancaccio et al., 2017; Tso et al., 2017). One mechanism through which aging may modulate the circadian system is through alterations in the rhythms of the expression of clock genes in glial cells or subsets of glial cells. The GAL4/UAS system, a bipartite transgene expression system commonly used in Drosophila, permits spatial manipulation of gene expression in specific cell types (Brand & Perrimon, 1993; Osterwalder et al., 2001; Roman et al., 2001; McGuire et al., 2004) and has been used to determine the impact of aging on molecular circadian rhythms in glial cells (Long & Giebultowicz, 2017). While PER protein abundance is rhythmic in multiple glial subtypes including perineural glia, subperineural glia, cortex glia, central brain ensheathing glia and medulla giant glia in young flies (Suh & Jackson, 2007; Long & Giebultowicz, 2017), age does not affect PER protein rhythms equally in all cell types. Older flies show significantly dampened PER protein levels in all rhythmic glial populations examined with the exception of the central brain ensheathing glia and no rhythmic expression was found in either young or aged flies in central brain astrocytes (Long & Giebultowicz, 2017). Aging also affects circadian modulation of glial function in mammals. In rats, hippocampal microglia exhibit circadian mRNA rhythms in the core clock genes as well as downstream targets of the microglia oscillator TNFα and IL-1β (Fonken et al., 2016). Aged rats (25 months) display nearly flat per1 mRNA expression and low amplitude cycling of per2 in hippocampal microglia with non-rhythmic high expression levels of downstream targets (Fonken et al., 2016). Aging related changes in the circadian regulation of glial function may alter mechanisms involved in neuronal homeostasis both synaptically and non-synaptically.
Impact of Aging on Peripheral Oscillators and Tissue Function
Similar to the differential physiological impacts of aging on organ and tissue function, aging also appears to affect peripheral oscillators to varying degrees. In Drosophila, peripheral oscillators throughout the body can function independently from the central brain oscillator (Plautz et al., 1997; Giebultowicz, 2001). When an in vivo per promoter luciferase reporter system was used to assess peripheral circadian rhythms, robust circadian rhythms were present in young flies (11 days) but significantly lower expression levels of the reporter with weak cycling was observed in older flies (51 days; Luo et al., 2012). Analysis of circadian expression for per, tim, vri and Pdp1ε mRNA levels in older male bodies (50 day) found that rhythmic gene expression was apparent for all four genes, although there were lower expression levels and a seemingly reduced amplitude in the rhythms particularly for per and tim (Rakshit et al., 2012). However, PER protein levels remain rhythmic in the peripheral oscillators in the Malpighian tubules, gut and abdominal fat bodies in aged flies (50 day old) (Rakshit et al., 2012). Similarly, no differences were observed between young and aged mice in mPer1/mPer2 mRNA expression in either LD or constant darkness, or when a PER2-luciferase reporter was used to measure circadian rhythms in the kidneys, liver and submandibular gland (Tahara et al., 2017).
Age dependent changes in circadian regulation also disturb the oscillations of clock-controlled genes in peripheral tissues and target organs affecting system function. For example, the circadian clock regulates many genes involved in the responses to oxidative stress and cellular redox (Krishnan et al., 2008; Giebultowicz, 2018). Aging significantly alters the rhythms of clock-controlled genes regulating the cellular redox cycle (Klichko et al., 2015). Young flies (5 days old) display strong diurnal patterns glutathione biosynthesis with rhythms apparent in both Gclc and Gclm mRNA and protein levels, the genes encoding the catalytic and modulatory subunits of glutamate cysteine ligase (GCL; (Beaver et al., 2012). Older flies (50 days old) display an absence of diurnal glutathione rhythms and have significantly higher levels of Gclc mRNA and protein throughout the 24-hour cycle with irregular peaks and troughs as well as alterations in the expression and activity of other redox involved molecules reflecting impaired temporal redox homeostasis (Klichko et al., 2015). In mammals, oxidative stress responses are regulated by the circadian clock and deletions of Bmal1 accelerates neuronal damage and impairs the expression of genes involved in oxidative stress responses in aging mice (Musiek et al., 2013).
The circadian clock regulates drug and alcohol-induced toxicity from flies to mammals. Studies from the 1950s and 1960s demonstrated that the toxicity of alcohol in mice was dependent upon the time of delivery (Haus & Halberg, 1959) as was the toxicity of amphetamines (Scheving et al., 1968) and other drugs (Carlsson & Serin, 1950b; a). The consequences of drug and alcohol abuse appear higher in aging populations (Kendler et al., 2016) in which circadian and sleep disruption is common. Studies from our lab have shown increased behavioral sensitivity to alcohol and increased alcohol-induced toxicity correlated with decreased circadian function in Drosophila (Van der Linde & Lyons, 2011; De Nobrega & Lyons, 2016; De Nobrega et al., 2017). Aging increases the behavioral sensitivity to binge-like alcohol exposures as well as slowing recovery following alcohol exposure and increasing toxicity (De Nobrega et al., 2017). Intriguingly, increased alcohol sensitivity is phase-specific suggesting that circadian regulation provides a protective buffer to alcohol-induced toxicity that declines with aging and decreased circadian function (De Nobrega et al., 2017). Aging, in animal models and humans, has also been shown to significantly increase the sensitivity to other drugs including nicotine and benzodiazepines (Cherry & Morton, 1989; Okamoto et al., 1994; Dowling et al., 2008). Additional research is needed to identify the mechanisms through which the circadian clock mediates drug and alcohol-induced toxicity and determine the impact of these interactions on age-related increased drug susceptibility.
The core circadian clock as a mediator of lifespan
The links between the circadian clock and the processes of aging are bidirectional as circadian dysfunction appears to hasten cellular aging and age-related disease processes (Grosbellet et al., 2015). Given the high degree of circadian regulation of metabolic and physiological rhythms, one can hypothesize that alterations in the functioning of the circadian system could adversely affect an organism’s longevity. Studies in Drosophila have investigated the physiological relevance of core clock genes to lifespan and healthy aging finding that mutations in per altered lifespan with per01 flies having shorter lifespans (Ewer et al., 1990; Krishnan et al., 2012). Likewise, flies with mutations that significantly altered period length also showed reduced longevity (Klarsfeld & Rouyer, 1998). Similar observations have been made in mammals with circadian mutants exhibiting decreased longevity. For example, BMAl1 knockout mutant mice have shorter lifespans and exhibit premature aging in many systems (Kondratov et al., 2006). Mice with free-running circadian periods close to 24 hours exhibit 20% greater longevity compared to mice that had circadian periods that were longer or shorter (Libert et al., 2012). When the light-dark cycle becomes dissociated from a 24 hour period, alterations in longevity are also observed in Drosophila (Pittendrigh & Minis, 1972) and wild-type mice housed in extremely short 4 h: 4 h LD cycles exhibit increased mortality (Park et al., 2012).
The link between rhythmicity in gene function and lifespan also extends to clock controlled genes. Although the specific genes and proteins regulated by the circadian clock differ between organs and tissues, the circadian clock appears to regulate between 5 – 15% of genes at the transcriptional level or with post-transcriptional circadian regulation to regulate rhythmic translation or protein activity (Duffield et al., 2002; Panda et al., 2002). Clock-controlled genes also appear to play an important role in determining lifespan. In Drosophila, the recently characterized clock controlled gene Achilles regulates the immune system and provides a signaling link between neurons and immunological tissues (Li et al., 2017). Achilles is highly rhythmic in the brain with peak mRNA expression during the late dark period and trough expression during the late phase of the light cycle (Li et al., 2017). Even in the absence of an immune challenge, reduction of Achilles expression shortens lifespan (Li et al., 2017). In mammalian models, numerous studies have demonstrated the interrelationship between circadian function and immune system responses (Scheiermann et al., 2013; Curtis et al., 2014; Geiger et al., 2015). Research has shown distinct differences in the circadian phase and the amplitude of the rhythms for lymphocyte subsets in older adults (Mazzoccoli et al., 2011). Disrupted circadian regulation of the neuro-immune system with aging could increase disease susceptibility and affect longevity.
Circadian desynchronization or arrythmicity accelerates aging
In humans, with the exception of a few notable familial disorders such as advanced familial sleep phase syndrome (Jones et al., 2013), most individuals with circadian disorders are due to environmental perturbations rather than genetic disorders. The number of individuals affected by circadian disorders, in particular internal circadian desynchronization, has skyrocketed over the past few decades for a number of reasons. The proliferation of smartphone and personal electronic use, due to technological advances and increased affordability, has contributed to increased artificial light exposure at night weakening circadian stability (Cajochen et al., 2011; Oh et al., 2015). Longer work hours during the work week (Alterman et al., 2013) and a shift in social schedules and sleep patterns on weekends cause circadian desynchronization from the biweekly phase shifts, a condition referred to as social jet-lag (Wittmann & Werfel, 2006). Social jetlag is associated with many adverse health impacts including metabolic and cardiac diseases (Roenneberg et al., 2012; Rutters et al., 2014; Wong et al., 2015). Shiftwork also has contributed to the number of individuals suffering from circadian disorders with between 15–30% of the adult population performing shiftwork in developed countries (Drake et al., 2004a; Drake et al., 2004b; Alterman et al., 2013; Di Milia et al., 2013).
As circadian disruption and desynchronization exacerbate numerous health conditions from metabolic diseases, cancers to neuropsychiatric disorders (Khan et al., 2002; Scheer et al., 2009; Arble et al., 2010; Evans & Davidson, 2013; Golombek et al., 2013; Albrecht, 2017; James et al., 2017; Khan et al., 2018), it is important to understand the interactions and impacts that circadian dysfunction or desynchronization has on the aging process. Research in hamsters has shown that circadian disorganization resulting from a mismatch between the light-dark cycle and the free-running period results in severe cardiac pathologies and kidney disease (Martino et al., 2008). It should be noted that recent research in a mouse model of circadian desynchronization did not find a link between desynchronization and glucose intolerance or obesity suggesting that additional factors are needed to observe metabolic consequences of circadian desynchronization (van der Vinne et al., 2018). As this study was performed in young animals, it is possible that the adverse health concerns associated with circadian desynchronization in humans may be aggravated in middle age and older individuals.
In animal models, experimental paradigms employing phase shifts of the light-dark cycle to simulate acute and chronic jet lag are commonly used for studying circadian desynchronization. Experimental protocols in which the light-dark cycle is changed on a weekly basis also can be employed to mimic rotating shiftwork or social jetlag conditions. Research in Drosophila has directly addressed the interactions of circadian desynchronization with age-related impairments using a chronic jet-lag model in which flies with a short circadian period of 16 hours were maintained on a 24 hour cycle (12:12 LD cycles; (Vaccaro et al., 2016). Under these conditions, the short period flies that were constantly experiencing phase shifts exhibited accelerated declines in age related motor impairments as well as decreased longevity compared to arrhythmic per01 flies and wild-type CS flies (Vaccaro et al., 2016). However, when the short period mutants were housed with 16 hour cycles (8:8 LD schedule) the increased rate of age-related declines in motor function were mitigated while wild-type flies displayed a greater rate of decline in age-related decreases in motor function suggesting that constant phase shifts and circadian desynchronization accelerate age-related phenotypes (Vaccaro et al., 2016). Circadian disruption also negatively affects longevity in mammalian models. In one of the initial studies investigating the link between circadian function and longevity, researchers found that when mutant hamsters with a short circadian period of ~ 20 hours were maintained on a 24 hour LD cycle that was outside of the range of entrainment, these animals had significantly reduced lifespans suggesting that circadian desynchronization affected longevity (Hurd & Ralph, 1998). Similarly, aged mice placed on rotating LD schedules simulating chronic jet lag exhibit significantly higher mortality compared to younger mice on these schedules (Davidson et al., 2006). It is possible that reduced tolerance to stressors and perturbations of cellular function seen with aging make these populations more sensitive to the adverse effects of desynchronization. Circadian desynchronization using a chronic jet lag model increases cellular aging in diurnal grass rats resulting in telomere shortening (Grosbellet et al., 2015). The metabolic profiles of the desynchronized young rats were intermediate between control young and old rats, suggesting increased oxidative stress and impaired glucose tolerance due to the desynchronization (Grosbellet et al., 2015). Mammalian studies have identified correlations between disruption of core oscillator function and acceleration of aging phenotypes (Kondratov et al., 2006; Antoch et al., 2008; Vinogradova et al., 2009; Ali et al., 2015). For example, BMAL1 and CLOCK knockout mice have disrupted redox homeostasis, accelerated aging and deficits in cognition (Kondratov et al., 2006; Antoch et al., 2008; Ali et al., 2015). Similarly rats housed in constant light exhibit accelerated metabolic pathologies and tumorigenesis and significantly shortened lifespan (Vinogradova et al., 2009). In human studies, longer participation in shiftwork increases the degree of adverse health impacts and in men cognitive impairments such as cognitive efficiency and immediate recall (Rouch et al., 2005; Wirtz & Nachreiner, 2012).
Reinforcement of the Circadian System Mitigates Age-Related Pathologies
In addition to the challenges and daily perturbations of the circadian system that people face in a 24/7 society, many individuals also experience weak entrainment of the circadian system due to the urban nature of society with limited outdoor time and increased artificial light at night (Espiritu et al., 1994; Diffey, 2011; Matz et al., 2014; Smolensky et al., 2015; Falchi et al., 2016; Lunn et al., 2017). Poor functioning of the circadian system accelerates aging and aging weakens circadian function, such that these reciprocal interactions aggravate age-induced pathologies. Human longevity has significantly increased worldwide (Vaupel, 2010) with recent research suggesting that the average length of human lifespan will continue to increase significantly (Brown et al., 2017; de Beer et al., 2017; Lenart & Vaupel, 2017; Barbi et al., 2018). Consequently, it is imperative to develop new strategies for healthy aging to minimize susceptibility to age-related pathologies and increase the manageability of chronic conditions. The bidirectional interactions of the circadian clock with aging suggests that strengthening of the circadian system may mitigate age-related declines and pathologies. Indeed, there have been intriguing studies from Drosophila to humans that strongly suggest that reinforcement of the circadian clock through stronger entrainment paradigms or the use of multiple zietgebers provides health benefits in aging. Table 1 outlines genetic and environmental manipulations of the circadian clock that have been used in Drosophila to address age-related problems while Table 2 presents key studies from mammalian models and humans that employ circadian interventions to mitigate age-related declines.
Table 1:
Approaches for Reinforcement of the Circadian System in Aging Drosophila
| Genetic rescue of circadian function | ||||
| Gene | Problem | Manipulation | Outcome | Reference |
| Aging related reductions in circadian rhythmicity | Overexpression of pdf in clock neurons | Increased rhythmicity, restoration of TIM levels in older flies | Umezaki et al., 2012 | |
| per | Increased neurodegeneration in per01 flies | Rescue of per | Increased resistance to hypoxia and oxidative stress, reduction in neuronal degeneration | Krishnan et al., 2008 |
| cry | Reduced cry mRNA expression and protein levels with aging | Overexpression of cry in all clock cells in the body | Increased resistance to oxidative stress. Increased rhythmicity and lifespan | Rakshit et al., 2013 |
| tim | Age-related problems with metabolism | Overexpression of tim in peripheral tissues | Increased lifespan, metabolism and resistance to pathogens on a protein rich diet | Katewa et al., 2016 |
| Environmental reinforcement of circadian clock | ||||
| Paradigm | Problem | Manipulation | Outcome | Reference |
| Time restricted feeding | Reduced sleep in older flies and deterioration of cardiac function | Food restricted to 12 h/day during light cycle | Improved sleep/activity rhythms and decreased cardiac aging | Gill et al, 2015 |
| Temperature entrainment | Disrupted sleep-wake rhythms with aging | Coupled entrainment of light and thermal cycles | Improved sleep and locomotor activity rhythms | Luo et al., 2012 |
Table 2:
Enhancement of Circadian Function in Aging Mammals and Humans
| Genetic rescue of circadian function | ||||
| Gene | Problem | Manipulation | Outcome | Reference |
| Bmal1 | Decreased longevity in mutant mice | Restoration of Bmal1 in brain | Improved survival in 75% of mice | McDearmon et al. 2006 |
| Environmental reinforcement of circadian clock | ||||
| Paradigm | Problem | Manipulation | Outcome | Reference |
| Time restricted feeding | Disrupted entrainment to light | Restricted feeding for 2 h during the light cycle for 14 days | Aged rats entrained to feeding rhythms | Walcott and Tate, 1996 |
| Activity dependent reinforcement | Disrupted circadian rhythms in peripheral tissues | Voluntary exercise in VIP mutant mice | Improved heart rate, body temperature and locomotion rhythms | Schroeder et al., 2012 |
| Bright light therapy | Sleep disturbance and reduced performance on cognitive tests in Alzheimer’s dementia patients | Bright light exposure for 2 h during early-mid day for 4 weeks | Improved circadian rhythms and performance on cognitive tests | Yamadera et al., 2000 |
In Drosophila, several studies have analyzed the impact of overexpression of core clock genes on longevity and age-related pathologies. Researchers found that overexpression of the timeless gene throughout the body significantly increased longevity (Katewa et al., 2016). With tissue specific gene expression to manipulate individual tissue peripheral oscillators, it was found that no single tissue overexpression of tim increased longevity to the extent achieved by whole body tim overexpression suggesting that aging and longevity is influenced by the integration of multiple peripheral oscillators (Katewa et al., 2016). Reinforcement of the circadian oscillator through overexpression of cry in central and peripheral clock cells of older flies results in robust circadian oscillations of per, tim, Pdp1e and vri mRNA similar to those observed in younger flies (Rakshit & Giebultowicz, 2013). In addition to strengthening the molecular oscillator, cry overexpression suppressed the age-related locomotor activity phenotypes when cry was expressed throughout central and peripheral oscillators and decreased the age-related vulnerability to oxidative stress in older flies (Rakshit & Giebultowicz, 2013). Interestingly, cry overexpression in central brain oscillators alone was insufficient to rescue either the age-dependent motor deficits, the increase in longevity or the age-induced susceptibility to oxidative-stress (Rakshit & Giebultowicz, 2013). These studies from Drosophila emphasize the critical role of peripheral oscillators for the resilience to age-induced pathologies, although it remains to be determined whether it is the function of multiple peripheral oscillators that is crucial in healthy aging and/or the synchronization between oscillators.
Genetic strategies to enhance the output signaling activity of specific circadian neuron groups have also been employed to counter age-induced declines. In the Drosophila central brain circadian system, the small ventral lateral neurons provide signaling for the morning oscillator (Grima et al., 2004; Stoleru et al., 2004) or potentially act as the main oscillator (Rieger et al., 2006), while the large ventral lateral neurons are generally thought to provide an arousal function as part of the circadian circuit (Parisky et al., 2008; Shang et al., 2008; Sheeba et al., 2008; Potdar & Sheeba, 2012). When the activity of the ventral lateral neurons was manipulated in aging flies via input dopaminergic signaling into these neurons, age-dependent declines in neural structural plasticity were rescued as was activity dependent increases in sleep (Donlea et al., 2014a). With the exception of the 5th small ventral lateral neuron, all of the small and large ventral lateral neurons express PDF (Renn et al., 1999; Kaneko & Hall, 2000). Overexpression of PDF in these neurons rescued the age-related deficits in locomotor activity rhythms and significantly increased TIM levels in all seven groups of circadian neurons in aged flies, although only small increases were observed in PER protein levels (Umezaki et al., 2012). These studies suggest that age-dependent connectivity issues between neuronal groups in the circadian circuit and the wake-sleep/arousal circuits can also be targeted to mitigate age-related declines in behavior and neural plasticity.
Strengthening circadian entrainment through the coupling of zeitgebers enhances circadian function in both flies and mammals and has been used to mitigate age-related phenotypes. In Drosophila, temperature entrainment in sync with the light dark cycle significantly increases behavioral rhythmicity in older flies by consolidating activity to the daytime period and reducing activity during the early night resulting in longer and more consolidated sleep bouts (Luo et al., 2012). Given the numerous consequences of sleep disorders and mistimed sleep on health and cognitive function, circadian reinforcement presents a potential option to increase sleep quality and regulate the timing of sleep in older individuals to mitigate adverse health impacts. In humans, the use of bright light therapy in the morning has been used for decades to strengthen circadian entrainment for seasonal affective disorder and other mood disorders (Pail et al., 2011; Oldham & Ciraulo, 2014). Circadian genes and the circadian clock also have been implicated as a neuroprotective buffer against age-induced brain pathologies (Musiek et al., 2013; Musiek & Holtzman, 2016; Musiek et al., 2018). Weak or fragmented circadian rhythm may be a predictive biomarker for Alzheimer’s disease and increase susceptibility to age-related neurodegeneration (Musiek et al., 2013; Musiek & Holtzman, 2016; Musiek et al., 2018). Recently the potential for bright light therapy with age-related neurodegenerative disorders to reinforce circadian rhythms has also been tested. Studies in individuals suffering from Alzheimer’s and Parkinson’s disease found bright light therapy to be effective in reducing the severity of some disease symptoms. Short exposures of bright light appear to improve sleep wake rhythms as well as partially rescue the deficits in performance on cognitive and pathology tests (Yamadera et al., 2000; Skjerve et al., 2004; Videnovic et al., 2017).
The circadian system and metabolism are tightly intertwined as are the connections between metabolism and longevity. Time-restricted feeding paradigms in which food delivery is limited to the active period strengthens entrainment and synchronization of circadian oscillators. In Drosophila, seven days of time-restricted feeding in middle-aged flies (35 days old) improved sleep-wake activity cycles (Gill et al., 2015). When middle-aged flies were switched to time restricted feeing for two weeks, age-related declines in cardiac function were significantly attenuated compared to age-matched controls (Gill et al., 2015). Moreover, the suppression of cardiac aging was associated with temporal gene expression and was dependent upon a functional circadian clock (Gill et al., 2015). Time-restricted feeding, a paradigm that entrains the liver circadian clock in mammals, synchronized with activity has been used in numerous rodent studies to minimize chronic metabolic conditions often associated with aging (Manoogian & Panda, 2017). Time-restricted feeding has also been used in a mouse model of Huntington’s disease to restore molecular circadian rhythms and behavioral activity rhythms (Morton et al., 2005; Maywood et al., 2010). An interactive approach in which people used their smartphones to monitor eating patterns and then shift the timing of food consumption to avoid late night eating was found to improve sleep patterns and metabolism (Gill & Panda, 2015). These studies suggest that circadian reinforcement with time-restricted feeding may be a feasible option to mitigate age-related declines in circadian function and manage chronic conditions. Time restricted feeding has also been suggested as a possible therapeutic option with Alzheimer’s disease (Kent, 2014).
One of the earliest studies linking caloric restriction to increased longevity was published in 1947 (Ball et al., 1947) with more than 1500 papers published since then investigating the parameters and mechanisms through which dietary restriction affects longevity demonstrating the phylogenetic conservation of the advantages of caloric restrictions. Recent research in Drosophila and mammals suggests that caloric restriction enhances the core circadian oscillator function in aged animals. Young flies subject to caloric restriction displayed enhanced circadian rhythms with increased peak expression for tim, per, Pdp1ε, vrille, and Clk mRNAs as well as higher amplitude PER and TIM protein rhythms (Katewa et al., 2016). Although the oscillations were dampened with aging, middle-aged flies (33 days old) on dietary restriction exhibited significantly higher oscillations of all the clock genes compared to middle-aged flies on an ad libitum diet (Katewa et al., 2016). A functional circadian oscillator was found necessary for the benefit of dietary restriction on lifespan as flies with functional circadian clocks showed significantly greater lifespan extension compared to flies with mutations in per and tim or flies rendered arrhythmic due to constant light conditions (Katewa et al., 2016). It should be noted that the necessity of a circadian clock for longevity benefits of dietary restriction may depend upon environmental factors or microbiota as other researchers have found that dietary restriction increases lifespan even in the absence of a functional circadian clock (Ulgherait et al., 2016). In mammalian studies, mice exposed to caloric restriction were found to self-impose a temporal restriction of feeding consolidating their feeding patterns (Patel et al., 2016b; Acosta-Rodríguez et al., 2017), although differences in gene expression between caloric restriction and time-restricted feeding can be observed (Patel et al., 2016b). Caloric restriction significantly increases the rhythm amplitude and peak mRNA expression levels for several core clock genes including Bmal1, per1 and per2 (Patel et al., 2016a). Similar to what has been observed in Drosophila for core clock mutants, the efficacy of caloric restriction is also impaired in Bmal1 knockout mice (Patel et al., 2016a). Thus, caloric restrictions appear to enhance the robustness of the circadian system and this reinforcement may provide an explanation, at least in part, for how dietary restriction increases longevity. Several recent reviews provide more detailed information and comparisons of timed feeding and caloric restriction on metabolism and longevity (Froy & Miskin, 2007; Taormina & Mirisola, 2014; Longo & Panda, 2016; Kapahi et al., 2017; Manoogian & Panda, 2017; Giebultowicz, 2018).
The timing of activity and exercise has been suggested as another mechanism through which the circadian system may be potentially strengthened to mitigate age-related declines, as exercise has been shown to increase the rate of resynchronization after light-induced phase-shifts in young female mice (Castillo et al., 2011). However, the efficacy of exercise to strengthen circadian rhythms under normal physiological conditions associated with aging is unclear. In Drosophila, forced daily morning exercise, using a 180° vial rotation protocol that takes advantage of the flies’ natural negative geotaxic behavior, had no effect on age-related locomotor activity declines or longevity (Rakshit et al., 2013). In aged mice, voluntary exercise with a running wheel increased the proportion of mice exhibiting rhythmic locomotor activity reflecting a strengthening of the circadian system (Leise et al., 2013). Voluntary exercise also ameliorates some age-related impairments associated with phase-shifts of the light-dark cycle by increasing the rate of resynchronization between activity rhythms and the new light-dark cycle as well as the resynchronization of peripheral oscillators, although aged mice still take longer to phase-shift than young mice (Leise et al., 2013). In addition to the health benefits of an active lifestyle, daily exercise on a fixed schedule may promote circadian function to sustain healthy aging. Overall, the research described above highlights the promise of therapeutic options targeted to support the circadian system as viable options for the management of age-related pathologies and chronic conditions.
Future Perspective
In the past two decades, advances in circadian research have underscored the importance of circadian function for health and the consequences of circadian dysfunction in disease. With age, circadian function declines in multiple ways impacting molecular, physiological and behavioral rhythms as well as increasing disease risk and exacerbating neuropsychiatric disorders. However, we still do not understand how aging targets the circadian system at the molecular level. More research is necessary to characterize the impact of aging on the molecular rhythms of clock-controlled target genes in individual tissues to identify molecules and nodes for potential manipulation. We do not yet have a clear understanding of how aging interferes with neural circuit level communication between circadian pacemakers or at the systems level with synchronization between central and peripheral oscillators. On the reciprocal side, research defining how circadian decline contributes to cellular aging and increases disease risk and neurodegeneration is also in its early stages. Given the reciprocity between the circadian clock and healthy aging, answering these questions appears critical for improving public health.
Despite the importance of understanding the bidirectional interactions between the circadian system and aging, there are challenges for performing age-related studies including the expense and time required for studies using aged rodents or mammalian models. Drosophila as a model system, with its neurogenetic tools and ease of molecular analysis, should provide a tremendous asset in future research of circadian and aging interactions. The low cost of animal husbandry and relatively short life-span allow experimenters to conduct complex genetic experiments in a fraction of the time compared to similar experiments in vertebrate models providing a foundation for rapid translation into rodent models. The next few years will undoubtedly bring significant advances in understanding the interactions between the circadian system and healthy aging with exciting possibilities including the development of future therapies using circadian reinforcement as a potential treatment for mitigating age-related physiological vulnerabilities.
Acknowledgements:
Research in the laboratory is supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke grant NS088835.
Abbreviations:
- Bmal1
Brain and Muscle ARNT-like protein 1
- dClk or mClk
Drosophila clock or mammalian clock
- dCry or mCry (1 or 2)
Drosophila cryptochrome or mammalian cryptochrome (1 or 2)
- dCyc
Drosophila cycle
- Gcl
Glutamate cysteine ligase
- LD
Light-dark
- dPer, mPer1, mPer2
Drosophila period gene, mammalian period 1 or 2
Pigment dispersing factor
- SCN
Suprachiasmatic nucleus
- tim
timeless gene
- VIP
Vasopressin intestinal peptide
Footnotes
Conflict of Interest Statement: There are no conflicts of interest for either author.
References
- Aartsen MJ, Smits CH, van Tilburg T, Knipscheer KC & Deeg DJ (2002) Activity in older adults: cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. J Gerontol B Psychol Sci Soc Sci, 57, P153–162. [DOI] [PubMed] [Google Scholar]
- Abruzzi KC, Rodriguez J, Menet JS, Desrochers J, Zadina A, Luo W, Tkachev S & Rosbash M (2011) Drosophila CLOCK target gene characterization: implications for circadian tissue-specific gene expression. Genes Dev, 25, 2374–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodríguez VA, de Groot MHM, Rijo-Ferreira F, Green CB & Takahashi JS (2017) Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metab, 26, 267–277.e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MD & Celniker SE & Holt RA & Evans CA & Gocayne JD & Amanatides PG & Scherer SE & Li PW & Hoskins RA & Galle RF & George RA & Lewis SE & Richards S & Ashburner M & Henderson SN & Sutton GG & Wortman JR & Yandell MD & Zhang Q & Chen LX & Brandon RC & Rogers YH & Blazej RG & Champe M & Pfeiffer BD & Wan KH & Doyle C & Baxter EG & Helt G & Nelson CR & Gabor GL & Abril JF & Agbayani A & An HJ & Andrews-Pfannkoch C & Baldwin D & Ballew RM & Basu A & Baxendale J & Bayraktaroglu L & Beasley EM & Beeson KY & Benos PV & Berman BP & Bhandari D & Bolshakov S & Borkova D & Botchan MR & Bouck J & Brokstein P & Brottier P & Burtis KC & Busam DA & Butler H & Cadieu E & Center A & Chandra I & Cherry JM & Cawley S & Dahlke C & Davenport LB & Davies P & de Pablos B & Delcher A & Deng Z & Mays AD & Dew I & Dietz SM & Dodson K & Doup LE & Downes M & Dugan-Rocha S & Dunkov BC & Dunn P & Durbin KJ & Evangelista CC & Ferraz C & Ferriera S & Fleischmann W & Fosler C & Gabrielian AE & Garg NS & Gelbart WM & Glasser K & Glodek A & Gong F & Gorrell JH & Gu Z & Guan P & Harris M & Harris NL & Harvey D & Heiman TJ & Hernandez JR & Houck J & Hostin D & Houston KA & Howland TJ & Wei MH & Ibegwam C & Jalali M & Kalush F & Karpen GH & Ke Z & Kennison JA & Ketchum KA & Kimmel BE & Kodira CD & Kraft C & Kravitz S & Kulp D & Lai Z & Lasko P & Lei Y & Levitsky AA & Li J & Li Z & Liang Y & Lin X & Liu X & Mattei B & McIntosh TC & McLeod MP & McPherson D & Merkulov G & Milshina NV & Mobarry C & Morris J & Moshrefi A & Mount SM & Moy M & Murphy B & Murphy L & Muzny DM & Nelson DL & Nelson DR & Nelson KA & Nixon K & Nusskern DR & Pacleb JM & Palazzolo M & Pittman GS & Pan S & Pollard J & Puri V & Reese MG & Reinert K & Remington K & Saunders RD & Scheeler F & Shen H & Shue BC & Sidén-Kiamos I & Simpson M & Skupski MP & Smith T & Spier E & Spradling AC & Stapleton M & Strong R & Sun E & Svirskas R & Tector C & Turner R & Venter E & Wang AH & Wang X & Wang ZY & Wassarman DA & Weinstock GM & Weissenbach J & Williams SM & Woodage T & Worley KC & Wu D & Yang S & Yao QA & Ye J & Yeh RF & Zaveri JS & Zhan M & Zhang G & Zhao Q & Zheng L & Zheng XH & Zhong FN & Zhong W & Zhou X & Zhu S & Zhu X & Smith HO & Gibbs RA & Myers EW & Rubin GM & Venter JC (2000) The genome sequence of Drosophila melanogaster. Science, 287, 2185–2195. [DOI] [PubMed] [Google Scholar]
- Akten B, Jauch E, Genova GK, Kim EY, Edery I, Raabe T & Jackson FR (2003) A role for CK2 in the Drosophila circadian oscillator. Nat Neurosci, 6, 251–257. [DOI] [PubMed] [Google Scholar]
- Albert MS & Heaton RK (1988) Intelligence testing. In: Albert, MS. Guilford Press, New York. [Google Scholar]
- Albrecht U (2012) Timing to perfection: the biology of central and peripheral circadian clocks. Neuron, 74, 246–260. [DOI] [PubMed] [Google Scholar]
- Albrecht U (2017) The circadian clock, metabolism and obesity. Obes Rev, 18 Suppl 1, 25–33. [DOI] [PubMed] [Google Scholar]
- Alfa RW & Kim SK (2016) Using Drosophila to discover mechanisms underlying type 2 diabetes. Dis Model Mech, 9, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AA, Schwarz-Herzke B, Stahr A, Prozorovski T, Aktas O & von Gall C (2015) Premature aging of the hippocampal neurogenic niche in adult Bmal1-deficient mice. Aging (Albany NY), 7, 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, White NE, So WV, Hall JC & Rosbash M (1998) A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell, 93, 791–804. [DOI] [PubMed] [Google Scholar]
- Allen CN, Nitabach MN & Colwell CS (2017) Membrane Currents, Gene Expression, and Circadian Clocks. Cold Spring Harb Perspect Biol, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterman T, Luckhaupt SE, Dahlhamer JM, Ward BW & Calvert GM (2013) Prevalence rates of work organization characteristics among workers in the U.S.: data from the 2010 National Health Interview Survey. Am J Ind Med, 56, 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association (2018) Alzheimer’s Disease Facts and Figures. Alzheimer’s Dementia 2018, pp. 367–429. [Google Scholar]
- Antoch MP, Gorbacheva VY, Vykhovanets O, Toshkov IA, Kondratov RV, Kondratova AA, Lee C & Nikitin AY (2008) Disruption of the circadian clock due to the Clock mutation has discrete effects on aging and carcinogenesis. Cell Cycle, 7, 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble DM, Ramsey KM, Bass J & Turek FW (2010) Circadian disruption and metabolic disease: findings from animal models. Best Pract Res Clin Endocrinol Metab, 24, 785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeljan D & Chan CC (2013) Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res, 37, 68–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellanes-Licea E, Caldelas I, De Ita-Pérez D & Díaz-Muñoz M (2014) The circadian timing system: a recent addition in the physiological mechanisms underlying pathological and aging processes. Aging Dis, 5, 406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E, Heron M & Xu J (2017) United States Life Tables, 2014. Natl Vital Stat Rep, 66, 1–64. [PubMed] [Google Scholar]
- Artiushin G & Sehgal A (2017) The Drosophila circuitry of sleep-wake regulation. Curr Opin Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ & Herzog ED (2005) Come together, right…now: synchronization of rhythms in a mammalian circadian clock. Neuron, 48, 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin H & Partridge L (2009) Invertebrate models of age-related muscle degeneration. Biochim Biophys Acta, 1790, 1084–1094. [DOI] [PubMed] [Google Scholar]
- Aujard F, Cayetanot F, Bentivoglio M & Perret M (2006) Age-related effects on the biological clock and its behavioral output in a primate. Chronobiol Int, 23, 451–460. [DOI] [PubMed] [Google Scholar]
- Ball ZB, Barnes RH & Visscher MB (1947) The effects of dietary caloric restriction on maturity and senescence, with particular reference to fertility and longevity. Am J Physiol, 150, 511–519. [DOI] [PubMed] [Google Scholar]
- Banks G, Nolan PM & Peirson SN (2016) Reciprocal interactions between circadian clocks and aging. Mamm Genome, 27, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbi E, Lagona F, Marsili M, Vaupel JW & Wachter KW (2018) The plateau of human mortality: Demography of longevity pioneers. Science, 360, 1459–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barca-Mayo O, Pons-Espinal M, Follert P, Armirotti A, Berdondini L & De Pietri Tonelli D (2017) Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat Commun, 8, 14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargiello TA, Jackson FR & Young MW (1984) Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature, 312, 752–754. [DOI] [PubMed] [Google Scholar]
- Beaver LM, Klichko VI, Chow ES, Kotwica-Rolinska J, Williamson M, Orr WC, Radyuk SN & Giebultowicz JM (2012) Circadian regulation of glutathione levels and biosynthesis in Drosophila melanogaster. PLoS One, 7, e50454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Masana MI & Dubocovich ML (1997) Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res, 747, 34–42. [DOI] [PubMed] [Google Scholar]
- Blake MR, Holbrook SD, Kotwica-Rolinska J, Chow ES, Kretzschmar D & Giebultowicz JM (2015) Manipulations of amyloid precursor protein cleavage disrupt the circadian clock in aging Drosophila. Neurobiol Dis, 77, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaconsa M, Malpeli G, Montaruli A, Carandente F, Grassi-Zucconi G & Bentivoglio M (2014) Differential modulation of clock gene expression in the suprachiasmatic nucleus, liver and heart of aged mice. Exp Gerontol, 55, 70–79. [DOI] [PubMed] [Google Scholar]
- Bozcuk AN (1972) DNA synthesis in the absence of somatic cell division associated with ageing in Drosophila subobscura. Exp Gerontol, 7, 147–156. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Patton AP, Chesham JE, Maywood ES & Hastings MH (2017) Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron, 93, 1420–1435.e1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH & Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- Brandt R & Paululat A (2013) Microcompartments in the Drosophila heart and the mammalian brain: general features and common principles. Biol Chem, 394, 217–230. [DOI] [PubMed] [Google Scholar]
- Brigui N, Le Bourg E & Médioni J (1990) Conditioned suppression of the proboscis-extension response in young, middle-aged, and old Drosophila melanogaster flies: acquisition and extinction. J Comp Psychol, 104, 289–296. [DOI] [PubMed] [Google Scholar]
- Brown GC (2015) Living too long: the current focus of medical research on increasing the quantity, rather than the quality, of life is damaging our health and harming the economy. EMBO Rep, 16, 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NJL, Albers CJ & Ritchie SJ (2017) Contesting the evidence for limited human lifespan. Nature, 546, E6–E7. [DOI] [PubMed] [Google Scholar]
- Buchon N, Osman D, David F, Boquete J. p. & Lemaitre B (2011) Flygut: an atlass of the Drosophila adult midgut. Lemaitre Lab, EPFL, Lausanne. [Google Scholar]
- Burke SN & Barnes CA (2006) Neural plasticity in the ageing brain. Nat Rev Neurosci, 7, 30–40. [DOI] [PubMed] [Google Scholar]
- Busto GU, Guven-Ozkan T & Davis RL (2016) MicroRNA function in Drosophila memory formation. Curr Opin Neurobiol, 43, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen C, Frey S, Anders D, Späti J, Bues M, Pross A, Mager R, Wirz-Justice A & Stefani O (2011) Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol (1985), 110, 1432–1438. [DOI] [PubMed] [Google Scholar]
- Cannon L, Zambon AC, Cammarato A, Zhang Z, Vogler G, Munoz M, Taylor E, Cartry J, Bernstein SI, Melov S & Bodmer R (2017) Expression patterns of cardiac aging in Drosophila. Aging Cell, 16, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A & Serin F (1950a) Time of day as a factor influencing the toxicity of nikethamide. Acta Pharmacol Toxicol (Copenh), 6, 181–186. [DOI] [PubMed] [Google Scholar]
- Carlsson A & Serin F (1950b) The toxicity of nikethamide at different times of the day. Acta Pharmacol Toxicol (Copenh), 6, 187–193. [DOI] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ & Monk TH (2001) The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology, 38, 232–242. [PubMed] [Google Scholar]
- Castillo C, Molyneux P, Carlson R & Harrington ME (2011) Restricted wheel access following a light cycle inversion slows re-entrainment without internal desynchrony as measured in Per2Luc mice. Neuroscience, 182, 169–176. [DOI] [PubMed] [Google Scholar]
- Cayetanot F, Bentivoglio M & Aujard F (2005) Arginine-vasopressin and vasointestinal polypeptide rhythms in the suprachiasmatic nucleus of the mouse lemur reveal aging-related alterations of circadian pacemaker neurons in a non-human primate. Eur J Neurosci, 22, 902–910. [DOI] [PubMed] [Google Scholar]
- Chauhan R, Chen KF, Kent BA & Crowther DC (2017) Central and peripheral circadian clocks and their role in Alzheimer’s disease. Dis Model Mech, 10, 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Logan RW, Ma T, Lewis DA, Tseng GC, Sibille E & McClung CA (2016) Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A, 113, 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Guo A & Li Y (2015) Aging accelerates memory extinction and impairs memory restoration in Drosophila. Biochem Biophys Res Commun, 460, 944–948. [DOI] [PubMed] [Google Scholar]
- Cherry KE & Morton MR (1989) Drug sensitivity in older adults: the role of physiologic and pharmacokinetic factors. Int J Aging Hum Dev, 28, 159–174. [DOI] [PubMed] [Google Scholar]
- Chiang AS, Lin CY, Chuang CC, Chang HM, Hsieh CH, Yeh CW, Shih CT, Wu JJ, Wang GT, Chen YC, Wu CC, Chen GY, Ching YT, Lee PC, Lin HH, Hsu HW, Huang YA, Chen JY, Chiang HJ, Lu CF, Ni RF, Yeh CY & Hwang JK (2011) Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr Biol, 21, 1–11. [DOI] [PubMed] [Google Scholar]
- Chiao YA & Rabinovitch PS (2015) The Aging Heart. Cold Spring Harb Perspect Med, 5, a025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S, Reiter LT, Bier E & Gribskov M (2002) Homophila: human disease gene cognates in Drosophila. Nucleic Acids Res, 30, 149–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofi T & Apidianakis Y (2013) Drosophila and the hallmarks of cancer. Adv Biochem Eng Biotechnol, 135, 79–110. [DOI] [PubMed] [Google Scholar]
- Cirelli C (2003) Searching for sleep mutants of Drosophila melanogaster. Bioessays, 25, 940–949. [DOI] [PubMed] [Google Scholar]
- Colas D, Bezin L, Gharib A, Morales A, Cespuglio R & Sarda N (2005) REM sleep control during aging in SAM mice: a role for inducible nitric oxide synthase. Neurobiol Aging, 26, 1375–1384. [DOI] [PubMed] [Google Scholar]
- Cook-Wiens E & Grotewiel MS (2002) Dissociation between functional senescence and oxidative stress resistance in Drosophila. Exp Gerontol, 37, 1347–1357. [DOI] [PubMed] [Google Scholar]
- Crimmins EM (2015) Lifespan and Healthspan: Past, Present, and Promise. Gerontologist, 55, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P & O’Neill LA (2014) Circadian clock proteins and immunity. Immunity, 40, 178–186. [DOI] [PubMed] [Google Scholar]
- D’Antona G, Pellegrino MA, Carlizzi CN & Bottinelli R (2007) Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol, 100, 603–611. [DOI] [PubMed] [Google Scholar]
- Das TK & Cagan RL (2013) A Drosophila approach to thyroid cancer therapeutics. Drug Discov Today Technol, 10, e65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M & Block GD (2006) Chronic jet-lag increases mortality in aged mice. Curr Biol, 16, R914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Arble DM, Menaker M & Block GD (2008) Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging, 29, 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer J, Bardoutsos A & Janssen F (2017) Maximum human lifespan may increase to 125 years. Nature, 546, E16–E17. [DOI] [PubMed] [Google Scholar]
- De Nobrega AK & Lyons LC (2016) Circadian Modulation of Alcohol-Induced Sedation and Recovery in Male and Female Drosophila. J Biol Rhythms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nobrega AK, Mellers AP & Lyons LC (2017) Aging and circadian dysfunction increase alcohol sensitivity and exacerbate mortality in Drosophila melanogaster. Exp Gerontol, 97, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis MM, Owen LA, Morrison MA, Morgan DJ, Li M, Shakoor A, Vitale A, Iyengar S, Stambolian D, Kim IK & Farrer LA (2017) Genetics of age-related macular degeneration (AMD). Hum Mol Genet, 26, R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Piccirillo R, Goldberg AL & Perrimon N (2013) Mechanisms of skeletal muscle aging: insights from Drosophila and mammalian models. Dis Model Mech, 6, 1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Milia L, Waage S, Pallesen S & Bjorvatn B (2013) Shift work disorder in a random population sample--prevalence and comorbidities. PLoS One, 8, e55306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U & Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol, 72, 517–549. [DOI] [PubMed] [Google Scholar]
- Diffey BL (2011) An overview analysis of the time people spend outdoors. Br J Dermatol, 164, 848–854. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Groeger JA, Stanley N & Deacon S (2010) Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep, 33, 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea JM (2017) Neuronal and molecular mechanisms of sleep homeostasis. Curr Opin Insect Sci, 24, 51–57. [DOI] [PubMed] [Google Scholar]
- Donlea JM, Pimentel D & Miesenböck G (2014a) Neuronal machinery of sleep homeostasis in Drosophila. Neuron, 81, 860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea JM, Ramanan N, Silverman N & Shaw PJ (2014b) Genetic rescue of functional senescence in synaptic and behavioral plasticity. Sleep, 37, 1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L & Rosenberg L (1984) Smell identification ability: changes with age. Science, 226, 1441–1443. [DOI] [PubMed] [Google Scholar]
- Dow JA & Romero MF (2010) Drosophila provides rapid modeling of renal development, function, and disease. Am J Physiol Renal Physiol, 299, F1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GJ, Weiss SR & Condon TP (2008) Drugs of abuse and the aging brain. Neuropsychopharmacology, 33, 209–218. [DOI] [PubMed] [Google Scholar]
- Drake C, Richardson G, Roehrs T, Scofield H & Roth T (2004a) Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep, 27, 285–291. [DOI] [PubMed] [Google Scholar]
- Drake CL, Roehrs T, Richardson G, Walsh JK & Roth T (2004b) Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep, 27, 1453–1462. [DOI] [PubMed] [Google Scholar]
- Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ & Dunlap JC (2002) Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol, 12, 551–557. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM & Czeisler CA (2007) Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging, 28, 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Zitting KM & Chinoy ED (2015) Aging and Circadian Rhythms. Sleep Med Clin, 10, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulcis D & Levine RB (2003) Innervation of the heart of the adult fruit fly, Drosophila melanogaster. J Comp Neurol, 465, 560–578. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Herron JM & Hill SA (2001) Aging selectively suppresses vasoactive intestinal peptide messenger RNA expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res Mol Brain Res, 87, 196–203. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Prochot JR, Cook DH, Tyler Smith J & Franklin KM (2013) Influence of aging on Bmal1 and Per2 expression in extra-SCN oscillators in hamster brain. Brain Res, 1491, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghlidi D, Luna SL, Brown D, Garyfallou V, Kohama S & Urbanski HF (2018) Gene Expression Profiling of the SCN in Young and Old Rhesus Macaques. J Mol Endocrinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P, Stanewsky R, Helfrich-Förster C, Emery-Le M, Hall JC & Rosbash M (2000) Drosophila CRY is a deep brain circadian photoreceptor. Neuron, 26, 493–504. [DOI] [PubMed] [Google Scholar]
- Erkosar B & Leulier F (2014) Transient adult microbiota, gut homeostasis and longevity: novel insights from the Drosophila model. FEBS Lett, 588, 4250–4257. [DOI] [PubMed] [Google Scholar]
- Espiritu RC, Kripke DF, Ancoli-Israel S, Mowen MA, Mason WJ, Fell RL, Klauber MR & Kaplan OJ (1994) Low illumination experienced by San Diego adults: association with atypical depressive symptoms. Biol Psychiatry, 35, 403–407. [DOI] [PubMed] [Google Scholar]
- Evans JA & Davidson AJ (2013) Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci, 119, 283–323. [DOI] [PubMed] [Google Scholar]
- Ewer J, Hamblen-Coyle M, Rosbash M & Hall JC (1990) Requirement for period gene expression in the adult and not during development for locomotor activity rhythms of imaginal Drosophila melanogaster. J Neurogenet, 7, 31–73. [DOI] [PubMed] [Google Scholar]
- Falchi F, Cinzano P, Duriscoe D, Kyba CC, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA & Furgoni R (2016) The new world atlas of artificial night sky brightness. Sci Adv, 2, e1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JY, Agyekum B, Venkatesan A, Hall DR, Keightley A, Bjes ES, Bouyain S & Price JL (2013) Noncanonical FK506-binding protein BDBT binds DBT to enhance its circadian function and forms foci at night. Neuron, 80, 984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, Michel S, Deboer T, vanderLeest HT, Houben T, Rohling JH, Ramkisoensing A, Yasenkov R & Meijer JH (2012) Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J Neurosci, 32, 5891–5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Callol-Massot C, Chu A, Ruiz-Lozano P, Izpisua Belmonte JC, Giles W, Bodmer R & Ocorr K (2009) A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques, 46, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AL (2004) Of worms and women: sarcopenia and its role in disability and mortality. J Am Geriatr Soc, 52, 1185–1190. [DOI] [PubMed] [Google Scholar]
- Fois C, Médioni J & Le Bourg E (1991) Habituation of the proboscis extension response as a function of age in Drosophila melanogaster. Gerontology, 37, 187–192. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Kitt MM, Gaudet AD, Barrientos RM, Watkins LR & Maier SF (2016) Diminished circadian rhythms in hippocampal microglia may contribute to age-related neuroinflammatory sensitization. Neurobiol Aging, 47, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, Monti D, Capri M & Salvioli S (2018) The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front Med (Lausanne), 5, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB & Paganelli R (2017) Aging, Obesity, and Inflammatory Age-Related Diseases. Front Immunol, 8, 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresquet N & Médioni J (1993) Effects of ageing on visual discrimination learning in Drosophila melanogaster. Q J Exp Psychol B, 46, 399–412. [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson JD & Anderson G (2004) Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci, 59, 255–263. [DOI] [PubMed] [Google Scholar]
- Froy O (2011) Circadian rhythms, aging, and life span in mammals. Physiology (Bethesda), 26, 225–235. [DOI] [PubMed] [Google Scholar]
- Froy O & Miskin R (2007) The interrelations among feeding, circadian rhythms and ageing. Prog Neurobiol, 82, 142–150. [DOI] [PubMed] [Google Scholar]
- Gargano JW, Martin I, Bhandari P & Grotewiel MS (2005) Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol, 40, 386–395. [DOI] [PubMed] [Google Scholar]
- Geiger SS, Fagundes CT & Siegel RM (2015) Chrono-immunology: progress and challenges in understanding links between the circadian and immune systems. Immunology, 146, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS & Weitz CJ (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Science, 280, 1564–1569. [DOI] [PubMed] [Google Scholar]
- Gerstner JR, Lenz O, Vanderheyden WM, Chan MT, Pfeiffenberger C & Pack AI (2016) Amyloid-β induces sleep fragmentation that is rescued by fatty acid binding proteins in Drosophila. J Neurosci Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Williams WP & Kriegsfeld LJ (2009) Aging in the circadian system: considerations for health, disease prevention and longevity. Exp Gerontol, 44, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebultowicz JM (2001) Peripheral clocks and their role in circadian timing: insights from insects. Philos Trans R Soc Lond B Biol Sci, 356, 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebultowicz JM (2018) Circadian regulation of metabolism and healthspan in Drosophila. Free Radic Biol Med, 119, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Le HD, Melkani GC & Panda S (2015) Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science, 347, 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S & Panda S (2015) A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab, 22, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossop NR, Lyons LC & Hardin PE (1999) Interlocked feedback loops within the Drosophila circadian oscillator. Science, 286, 766–768. [DOI] [PubMed] [Google Scholar]
- Goddeeris MM, Cook-Wiens E, Horton WJ, Wolf H, Stoltzfus JR, Borrusch M & Grotewiel MS (2003) Delayed behavioural aging and altered mortality in Drosophila beta integrin mutants. Aging Cell, 2, 257–264. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Casiraghi LP, Agostino PV, Paladino N, Duhart JM, Plano SA & Chiesa JJ (2013) The times they’re a-changing: effects of circadian desynchronization on physiology and disease. J Physiol Paris, 107, 310–322. [DOI] [PubMed] [Google Scholar]
- Grima B, Chélot E, Xia R & Rouyer F (2004) Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature, 431, 869–873. [DOI] [PubMed] [Google Scholar]
- Grosbellet E, Zahn S, Arrivé M, Dumont S, Gourmelen S, Pévet P, Challet E & Criscuolo F (2015) Circadian desynchronization triggers premature cellular aging in a diurnal rodent. FASEB J, 29, 4794–4803. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Martin I, Bhandari P & Cook-Wiens E (2005) Functional senescence in Drosophila melanogaster. Ageing Res Rev, 4, 372–397. [DOI] [PubMed] [Google Scholar]
- Hall H, Medina P, Cooper DA, Escobedo SE, Rounds J, Brennan KJ, Vincent C, Miura P, Doerge R & Weake VM (2017) Transcriptome profiling of aging Drosophila photoreceptors reveals gene expression trends that correlate with visual senescence. BMC Genomics, 18, 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin PE, Hall JC & Rosbash M (1990) Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature, 343, 536–540. [DOI] [PubMed] [Google Scholar]
- Hardin PE, Hall JC & Rosbash M (1992) Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci U S A, 89, 11711–11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin PE & Panda S (2013) Circadian timekeeping and output mechanisms in animals. Curr Opin Neurobiol, 23, 724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PM, Kumar A, Lang N, Snyder M & Gerstein M (2002) A question of size: the eukaryotic proteome and the problems in defining it. Nucleic Acids Res, 30, 1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus E & Halberg F (1959) 24-Hour rhythm in susceptibility of C mice to a toxic dose of ethanol. J Appl Physiol, 14, 878–880. [DOI] [PubMed] [Google Scholar]
- He Q, Wu B, Price JL & Zhao Z (2017) Circadian Rhythm Neuropeptides in Drosophila: Signals for Normal Circadian Function and Circadian Neurodegenerative Disease. Int J Mol Sci, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C (2003) The neuroarchitecture of the circadian clock in the brain of Drosophila melanogaster. Microsc Res Tech, 62, 94–102. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C (2014) From neurogenetic studies in the fly brain to a concept in circadian biology. J Neurogenet, 28, 329–347. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Nitabach MN & Holmes TC (2011) Insect circadian clock outputs. Essays Biochem, 49, 87–101. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A & Pack AI (2000) Rest in Drosophila is a sleep-like state. Neuron, 25, 129–138. [DOI] [PubMed] [Google Scholar]
- Hood S & Amir S (2017) The aging clock: circadian rhythms and later life. J Clin Invest, 127, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Chelliah Y, Shan Y, Taylor CA, Yoo SH, Partch C, Green CB, Zhang H & Takahashi JS (2012) Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science, 337, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G & Cirelli C (2004) Sleep homeostasis in Drosophila melanogaster. Sleep, 27, 628–639. [DOI] [PubMed] [Google Scholar]
- Hurd MW & Ralph MR (1998) The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms, 13, 430–436. [DOI] [PubMed] [Google Scholar]
- Hussain A, Pooryasin A, Zhang M, Loschek LF, La Fortezza M, Friedrich AB, Blais CM, Üçpunar HK, Yépez VA, Lehmann M, Gompel N, Gagneur J, Sigrist SJ & Grunwald Kadow IC (2018) Inhibition of oxidative stress in cholinergic projection neurons fully rescues aging-associated olfactory circuit degeneration in. Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac RE, Li C, Leedale AE & Shirras AD (2010) Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc Biol Sci, 277, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K & Hotta Y (1992) Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol, 149, 134–148. [DOI] [PubMed] [Google Scholar]
- Jackson FR, Ng FS, Sengupta S, You S & Huang Y (2015) Glial cell regulation of rhythmic behavior. Methods Enzymol, 552, 45–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SM, Honn KA, Gaddameedhi S & Van Dongen HPA (2017) Shift Work: Disrupted Circadian Rhythms and Sleep-Implications for Health and Well-Being. Curr Sleep Med Rep, 3, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ & Reppert SM (1999) A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell, 96, 57–68. [DOI] [PubMed] [Google Scholar]
- Jones CR, Huang AL, Ptáček LJ & Fu YH (2013) Genetic basis of human circadian rhythm disorders. Exp Neurol, 243, 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM & Carlson JR (2015) Drosophila Chemoreceptors: A Molecular Interface Between the Chemical World and the Brain. Trends Genet, 31, 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M (2015) Neuroanatomy: connectome connects fly and mammalian brain networks. Curr Biol, 25, R416–418. [DOI] [PubMed] [Google Scholar]
- Kalló I, Kalamatianos T, Piggins HD & Coen CW (2004) Ageing and the diurnal expression of mRNAs for vasoactive intestinal peptide and for the VPAC2 and PAC1 receptors in the suprachiasmatic nucleus of male rats. J Neuroendocrinol, 16, 758–766. [DOI] [PubMed] [Google Scholar]
- Kaneko M & Hall JC (2000) Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol, 422, 66–94. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Kaeberlein M & Hansen M (2017) Dietary restriction and lifespan: Lessons from invertebrate models. Ageing Res Rev, 39, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katewa SD, Akagi K, Bose N, Rakshit K, Camarella T, Zheng X, Hall D, Davis S, Nelson CS, Brem RB, Ramanathan A, Sehgal A, Giebultowicz JM & Kapahi P (2016) Peripheral Circadian Clocks Mediate Dietary Restriction-Dependent Changes in Lifespan and Fat Metabolism in Drosophila. Cell Metab, 23, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenberger RJ, Chtarbanova S, Rimkus SA, Fischer JA, Kaur G, Seppala JM, Swanson LC, Zajac JE, Ganetzky B & Wassarman DA (2015a) Death following traumatic brain injury in Drosophila is associated with intestinal barrier dysfunction. Elife, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenberger RJ, Ganetzky B & Wassarman DA (2015b) The gut reaction to traumatic brain injury. Fly (Austin), 9, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR & Rothenfluh A (2017) Dopaminergic rules of engagement for memory in Drosophila. Curr Opin Neurobiol, 43, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami F, Okamura H, Tamada Y, Maebayashi Y, Fukui K & Ibata Y (1997) Loss of day-night differences in VIP mRNA levels in the suprachiasmatic nucleus of aged rats. Neurosci Lett, 222, 99–102. [DOI] [PubMed] [Google Scholar]
- Kazama H (2015) Systems neuroscience in Drosophila: Conceptual and technical advantages. Neuroscience, 296, 3–14. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist J & Sundquist K (2016) Alcohol Use Disorder and Mortality Across the Lifespan: A Longitudinal Cohort and Co-relative Analysis. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BA (2014) Synchronizing an aging brain: can entraining circadian clocks by food slow Alzheimer’s disease? Front Aging Neurosci, 6, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AS, Sane DC, Wannenburg T & Sonntag WE (2002) Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res, 54, 25–35. [DOI] [PubMed] [Google Scholar]
- Khan S, Nabi G, Yao L, Siddique R, Sajjad W, Kumar S, Duan P & Hou H (2018) Health risks associated with genetic alterations in internal clock system by external factors. Int J Biol Sci, 14, 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW & Takahashi JS (1997) Positional cloning of the mouse circadian clock gene. Cell, 89, 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A & Rouyer F (1998) Effects of circadian mutations and LD periodicity on the life span of Drosophila melanogaster. J Biol Rhythms, 13, 471–478. [DOI] [PubMed] [Google Scholar]
- Klassen MP, Peters CJ, Zhou S, Williams HH, Jan LY & Jan YN (2017) Age-dependent diastolic heart failure in an in vivo. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R & Klein BE (2013) The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci, 54, ORSF5-ORSF13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klichko VI, Chow ES, Kotwica-Rolinska J, Orr WC, Giebultowicz JM & Radyuk SN (2015) Aging alters circadian regulation of redox in Drosophila. Front Genet, 6, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss B, Price JL, Saez L, Blau J, Rothenfluh A, Wesley CS & Young MW (1998) The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell, 94, 97–107. [DOI] [PubMed] [Google Scholar]
- Ko CH & Takahashi JS (2006) Molecular components of the mammalian circadian clock. Hum Mol Genet, 15 Spec No 2, R271–277. [DOI] [PubMed] [Google Scholar]
- Koh K, Zheng X & Sehgal A (2006) JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science, 312, 1809–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH & Turek FW (2003) Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms, 18, 159–169. [DOI] [PubMed] [Google Scholar]
- Kondratov RV (2007) A role of the circadian system and circadian proteins in aging. Ageing Res Rev, 6, 12–27. [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV & Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev, 20, 1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka RJ, Pittendrigh C & Orr D (1989) Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J Neurogenet, 6, 1–10. [DOI] [PubMed] [Google Scholar]
- Kontis V, Bennett JE, Mathers CD, Li G, Foreman K & Ezzati M (2017) Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet, 389, 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL & Wise PM (1998) Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci, 18, 4767–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz EA & Fernandez M.e.L. (2015) Aggression in Drosophila. Behav Neurosci, 129, 549–563. [DOI] [PubMed] [Google Scholar]
- Krishnan N, Davis AJ & Giebultowicz JM (2008) Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem Biophys Res Commun, 374, 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Kretzschmar D, Rakshit K, Chow E & Giebultowicz JM (2009) The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging (Albany NY), 1, 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Rakshit K, Chow ES, Wentzell JS, Kretzschmar D & Giebultowicz JM (2012) Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol Dis, 45, 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurada P & O’Tousa JE (1995) Retinal degeneration caused by dominant rhodopsin mutations in Drosophila. Neuron, 14, 571–579. [DOI] [PubMed] [Google Scholar]
- Lakatta EG & Levy D (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation, 107, 346–354. [DOI] [PubMed] [Google Scholar]
- Landayan D & Wolf FW (2015) Shared neurocircuitry underlying feeding and drugs of abuse in Drosophila. Biomed J, 38, 496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt HP & Borbély AA (2001) Age-dependent changes in sleep EEG topography. Clin Neurophysiol, 112, 369–377. [DOI] [PubMed] [Google Scholar]
- Leffelaar D & Grigliatti TA (1984) A mutation in Drosophila that appears to accelerate aging. Development Genetics, 4, 199–210. [Google Scholar]
- Leise TL, Harrington ME, Molyneux PC, Song I, Queenan H, Zimmerman E, Lall GS & Biello SM (2013) Voluntary exercise can strengthen the circadian system in aged mice. Age (Dordr), 35, 2137–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenart A & Vaupel JW (2017) Questionable evidence for a limit to human lifespan. Nature, 546, E13–E14. [DOI] [PubMed] [Google Scholar]
- Lessing D & Bonini NM (2009) Maintaining the brain: insight into human neurodegeneration from Drosophila melanogaster mutants. Nat Rev Genet, 10, 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Terry EE, Fejer E, Gamba D, Hartmann N, Logsdon J, Michalski D, Rois LE, Scuderi MJ, Kunst M & Hughes ME (2017) Achilles is a circadian clock-controlled gene that regulates immune function in Drosophila. Brain Behav Immun, 61, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Broughton S & Nässel DR (2017) Behavioral Senescence and Aging-Related Changes in Motor Neurons and Brain Neuromodulator Levels Are Ameliorated by Lifespan-Extending Reproductive Dormancy in. Front Cell Neurosci, 11, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Bonkowski MS, Pointer K, Pletcher SD & Guarente L (2012) Deviation of innate circadian period from 24 h reduces longevity in mice. Aging Cell, 11, 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JM, Kilman VL, Keegan K, Paddock B, Emery-Le M, Rosbash M & Allada R (2002) A role for casein kinase 2alpha in the Drosophila circadian clock. Nature, 420, 816–820. [DOI] [PubMed] [Google Scholar]
- Liu F & Chang HC (2017) Physiological links of circadian clock and biological clock of aging. Protein Cell, 8, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd TE & Taylor JP (2010) Flightless flies: Drosophila models of neuromuscular disease. Ann N Y Acad Sci, 1184, e1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DM & Giebultowicz JM (2017) Age-Related Changes in the Expression of the Circadian Clock Protein PERIOD in. Front Physiol, 8, 1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD & Panda S (2016) Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab, 23, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH, Stevens RG, Turek FW, Vermeulen R, Carreón T, Caruso CC, Lawson CC, Thayer KA, Twery MJ, Ewens AD, Garner SC, Schwingl PJ & Boyd WA (2017) Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ, 607–608, 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Chen WF, Yue Z, Chen D, Sowcik M, Sehgal A & Zheng X (2012) Old flies have a robust central oscillator but weaker behavioral rhythms that can be improved by genetic and environmental manipulations. Aging Cell, 11, 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly S, Pack AI & Naidoo N (2018) The neurobiological basis of sleep: Insights from Drosophila. Neurosci Biobehav Rev, 87, 67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L (2016) Can the Drosophila model help in paving the way for translational medicine in heart failure? Biochem Soc Trans, 44, 1549–1560. [DOI] [PubMed] [Google Scholar]
- Manoogian ENC & Panda S (2017) Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev, 39, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Krall TJ & Herzog ED (2009) Vasoactive intestinal polypeptide entrains circadian rhythms in astrocytes. J Biol Rhythms, 24, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek S, Inonog S, Manoukian AS & Young MW (2001) A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell, 105, 769–779. [DOI] [PubMed] [Google Scholar]
- Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR & Sole MJ (2008) Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol, 294, R1675–1683. [DOI] [PubMed] [Google Scholar]
- Maruyama R & Andrew DJ (2012) Drosophila as a model for epithelial tube formation. Dev Dyn, 241, 119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis J & Sehgal A (2016) Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol Metab, 27, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz CJ, Stieb DM, Davis K, Egyed M, Rose A, Chou B & Brion O (2014) Effects of age, season, gender and urban-rural status on time-activity: CanadianHuman Activity Pattern Survey 2 (CHAPS 2). Int J Environ Res Public Health, 11, 2108–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximino C, Silva RX, da Silva S.e.N., Rodrigues L.o.S., Barbosa H, de Carvalho TS, Leão LK, Lima MG, Oliveira KR & Herculano AM (2015) Non-mammalian models in behavioral neuroscience: consequences for biological psychiatry. Front Behav Neurosci, 9, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Fraenkel E, McAllister CJ, Wood N, Reddy AB, Hastings MH & Morton AJ (2010) Disruption of peripheral circadian timekeeping in a mouse model of Huntington’s disease and its restoration by temporally scheduled feeding. J Neurosci, 30, 10199–10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoccoli G, Inglese M, De Cata A, Carughi S, Dagostino MP, Marzulli N, Damato M, Grilli M, Giuliani F & Greco A (2011) Neuroendocrine-immune interactions in healthy aging. Geriatr Gerontol Int, 11, 98–106. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Roman G & Davis RL (2004) Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet, 20, 384–391. [DOI] [PubMed] [Google Scholar]
- Mendelson WB & Bergmann BM (1999a) Age-related changes in sleep in the rat. Sleep, 22, 145–150. [DOI] [PubMed] [Google Scholar]
- Mendelson WB & Bergmann BM (1999b) EEG delta power during sleep in young and old rats. Neurobiol Aging, 20, 669–673. [DOI] [PubMed] [Google Scholar]
- Mendoza-Viveros L, Bouchard-Cannon P, Hegazi S, Cheng AH, Pastore S & Cheng HM (2017) Molecular modulators of the circadian clock: lessons from flies and mice. Cell Mol Life Sci, 74, 1035–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet JS, Abruzzi KC, Desrochers J, Rodriguez J & Rosbash M (2010) Dynamic PER repression mechanisms in the Drosophila circadian clock: from on-DNA to off-DNA. Genes Dev, 24, 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery A, Taghli-Lamallem O, Clark KA, Beckerle MC, Wu X, Ocorr K & Bodmer R (2008) The Drosophila muscle LIM protein, Mlp84B, is essential for cardiac function. J Exp Biol, 211, 15–23. [DOI] [PubMed] [Google Scholar]
- Mery F (2007) Aging and its differential effects on consolidated memory forms in Drosophila. Exp Gerontol, 42, 99–101. [DOI] [PubMed] [Google Scholar]
- Mery F, Belay AT, So AK, Sokolowski MB & Kawecki TJ (2007) Natural polymorphism affecting learning and memory in Drosophila. Proc Natl Acad Sci U S A, 104, 13051–13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Chi T, Kapahi P, Kahn AJ, Kim MS, Hirata T, Romero MF, Dow JA & Stoller ML (2013) Drosophila melanogaster as an emerging translational model of human nephrolithiasis. J Urol, 190, 1648–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Lekkas P, Braddock JM, Farman GP, Ballif BA, Irving TC, Maughan DW & Vigoreaux JO (2008) Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys J, 95, 2391–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel J, Lundgren PR, Bensch KG & Atlan H (1976) Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech Ageing Dev, 5, 347–370. [DOI] [PubMed] [Google Scholar]
- Molina MR & Cripps RM (2001) Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech Dev, 109, 51–59. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Carrier J & Kupfer DJ (2000) Inducing jet-lag in older people: directional asymmetry. J Sleep Res, 9, 101–116. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF & Kupfer DJ (1993) Inducing jet lag in older people: adjusting to a 6-hour phase advance in routine. Exp Gerontol, 28, 119–133. [DOI] [PubMed] [Google Scholar]
- Morse D & Sassone-Corsi P (2002) Time after time: inputs to and outputs from the mammalian circadian oscillators. Trends Neurosci, 25, 632–637. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA & Maywood ES (2005) Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci, 25, 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KR, Deshpande SA, Yurgel ME, Quinn JP, Weissbach JL, Keene AC, Dawson-Scully K, Huber R, Tomchik SM & Ja WW (2016) Postprandial sleep mechanics in. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM & Ju YS (2018) Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol, 75, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES & Holtzman DM (2016) Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science, 354, 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Giasson BI, Weaver DR, Holtzman DM & Fitzgerald GA (2013) Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest, 123, 5389–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, Colwell CS & Block GD (2015) Age-Related Changes in the Circadian System Unmasked by Constant Conditions(1,2,3). eNeuro, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS & Block GD (2011) Age-related decline in circadian output. J Neurosci, 31, 10201–10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Takasu NN & Nakamura W (2016) The suprachiasmatic nucleus: age-related decline in biological rhythms. J Physiol Sci, 66, 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng FS, Tangredi MM & Jackson FR (2011) Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol, 21, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Ocorr K, Bodmer R & Cartry J (2011) Drosophila as a model to study cardiac aging. Exp Gerontol, 46, 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Akasaka T & Bodmer R (2007a) Age-related cardiac disease model of Drosophila. Mech Ageing Dev, 128, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Perrin L, Lim HY, Qian L, Wu X & Bodmer R (2007b) Genetic control of heart function and aging in Drosophila. Trends Cardiovasc Med, 17, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JH, Yoo H, Park HK & Do YR (2015) Analysis of circadian properties and healthy levels of blue light from smartphones at night. Sci Rep, 5, 11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM (2010) Nocturnal awakenings and difficulty resuming sleep: their burden in the European general population. J Psychosom Res, 69, 565–571. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Kita T, Okuda H, Tanaka T & Nakashima T (1994) Effects of aging on acute toxicity of nicotine in rats. Pharmacol Toxicol, 75, 1–6. [DOI] [PubMed] [Google Scholar]
- Oldham MA & Ciraulo DA (2014) Bright light therapy for depression: a review of its effects on chronobiology and the autonomic nervous system. Chronobiol Int, 31, 305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivetti G, Capasso JM, Meggs LG, Sonnenblick EH & Anversa P (1991a) Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ Res, 68, 856–869. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Melissari M, Capasso JM & Anversa P (1991b) Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res, 68, 1560–1568. [DOI] [PubMed] [Google Scholar]
- Ortman JM, Velkoff VA & Hogan A (2014) An Aging Nation: The Older Population in the United States, Population estimates and projections. In Commerce D.o. (ed). U.S. Census Bureau. [Google Scholar]
- Osborn R, Doty MM, Moulds D, Sarnak DO & Shah A (2017) Older Americans Were Sicker And Faced More Financial Barriers To Health Care Than Counterparts In Other Countries. Health Aff (Millwood), 36, 2123–2132. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH & Keshishian H (2001) A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A, 98, 12596–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaya O & Rosato E (2012) The circadian clock of the fly: a neurogenetics journey through time. Adv Genet, 77, 79–123. [DOI] [PubMed] [Google Scholar]
- Pail G, Huf W, Pjrek E, Winkler D, Willeit M, Praschak-Rieder N & Kasper S (2011) Bright-light therapy in the treatment of mood disorders. Neuropsychobiology, 64, 152–162. [DOI] [PubMed] [Google Scholar]
- Palandri A, Martin E, Russi M, Rera M, Tricoire H & Monnier V (2018) Identification of cardioprotective drugs by medium-scale. Dis Model Mech. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Hadley TJ & Ganetzky B (2002) Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics, 161, 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS & Hogenesch JB (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell, 109, 307–320. [DOI] [PubMed] [Google Scholar]
- Pandey PC, Upadhyay S, Upadhyay BC & Pathak HC (1998) Ethanol biosensors and electrochemical oxidation of NADH. Anal Biochem, 260, 195–203. [DOI] [PubMed] [Google Scholar]
- Pandey SC (1998) Neuronal signaling systems and ethanol dependence. Mol Neurobiol, 17, 1–15. [DOI] [PubMed] [Google Scholar]
- Pandey UB & Nichols CD (2011) Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev, 63, 411–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Zisapel N, Srinivasan V & Cardinali DP (2005) Melatonin and sleep in aging population. Exp Gerontol, 40, 911–925. [DOI] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M & Griffith LC (2008) PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron, 60, 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisky KM, Agosto Rivera JL, Donelson NC, Kotecha S & Griffith LC (2016) Reorganization of Sleep by Temperature in Drosophila Requires Light, the Homeostat, and the Circadian Clock. Curr Biol, 26, 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park N, Cheon S, Son GH, Cho S & Kim K (2012) Chronic circadian disturbance by a shortened light-dark cycle increases mortality. Neurobiol Aging, 33, 1122.e1111–1122. [DOI] [PubMed] [Google Scholar]
- Park SY, Ludwig MZ, Tamarina NA, He BZ, Carl SH, Dickerson DA, Barse L, Arun B, Williams CL, Miles CM, Philipson LH, Steiner DF, Bell GI & Kreitman M (2014) Genetic complexity in a Drosophila model of diabetes-associated misfolded human proinsulin. Genetics, 196, 539–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Green CB & Takahashi JS (2014) Molecular architecture of the mammalian circadian clock. Trends Cell Biol, 24, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco MY, Loudhaief R & Gallet A (2015) The cellular homeostasis of the gut: what the Drosophila model points out. Histol Histopathol, 30, 277–292. [DOI] [PubMed] [Google Scholar]
- Patel SA, Chaudhari A, Gupta R, Velingkaar N & Kondratov RV (2016a) Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J, 30, 1634–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Velingkaar N, Makwana K, Chaudhari A & Kondratov R (2016b) Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms. Sci Rep, 6, 25970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternostro G, Vignola C, Bartsch DU, Omens JH, McCulloch AD & Reed JC (2001) Age-associated cardiac dysfunction in Drosophila melanogaster. Circ Res, 88, 1053–1058. [DOI] [PubMed] [Google Scholar]
- Piazza N, Gosangi B, Devilla S, Arking R & Wessells R (2009) Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS One, 4, e5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza N & Wessells RJ (2011) Drosophila models of cardiac disease. Prog Mol Biol Transl Sci, 100, 155–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo R, Demontis F, Perrimon N & Goldberg AL (2014) Mechanisms of muscle growth and atrophy in mammals and Drosophila. Dev Dyn, 243, 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Caldarola PC & Cosbey ES (1973) A differential effect of heavy water on temperature-dependent and temperature-compensated aspects of circadian system of Drosophila pseudoobscura. Proc Natl Acad Sci U S A, 70, 2037–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Kyner WT & Takamura T (1991) The amplitude of circadian oscillations: temperature dependence, latitudinal clines, and the photoperiodic time measurement. J Biol Rhythms, 6, 299–313. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS & Minis DH (1972) Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc Natl Acad Sci U S A, 69, 1537–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS & Takamura T (1989) Latitudinal clines in the properties of a circadian pacemaker. J Biol Rhythms, 4, 217–235. [PubMed] [Google Scholar]
- Plautz JD, Kaneko M, Hall JC & Kay SA (1997) Independent photoreceptive circadian clocks throughout Drosophila. Science, 278, 1632–1635. [DOI] [PubMed] [Google Scholar]
- Potdar S & Sheeba V (2012) Large ventral lateral neurons determine the phase of evening activity peak across photoperiods in Drosophila melanogaster. J Biol Rhythms, 27, 267–279. [DOI] [PubMed] [Google Scholar]
- Potdar S & Sheeba V (2013) Lessons from sleeping flies: insights from Drosophila melanogaster on the neuronal circuitry and importance of sleep. J Neurogenet, 27, 23–42. [DOI] [PubMed] [Google Scholar]
- Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B & Young MW (1998) double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell, 94, 83–95. [DOI] [PubMed] [Google Scholar]
- Prolo LM, Takahashi JS & Herzog ED (2005) Circadian rhythm generation and entrainment in astrocytes. J Neurosci, 25, 404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M, Nongthomba U & Grounds MD (2014) Skeletal muscle degeneration and regeneration in mice and flies. Curr Top Dev Biol, 108, 247–281. [DOI] [PubMed] [Google Scholar]
- Rakshit K & Giebultowicz JM (2013) Cryptochrome restores dampened circadian rhythms and promotes healthspan in aging Drosophila. Aging Cell, 12, 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshit K, Krishnan N, Guzik EM, Pyza E & Giebultowicz JM (2012) Effects of aging on the molecular circadian oscillations in Drosophila. Chronobiol Int, 29, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshit K, Wambua R, Giebultowicz TM & Giebultowicz JM (2013) Effects of exercise on circadian rhythms and mobility in aging Drosophila melanogaster. Exp Gerontol, 48, 1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff EP, Mauntz RE, Kotzebue RW, Gonzalez A, Achal M, Barekat A, Finley KA, Sparhawk JM, Robinson JE, Herr DR, Harris GL, Joiner WJ & Finley KD (2015) Aging and Autophagic Function Influences the Progressive Decline of Adult Drosophila Behaviors. PLoS One, 10, e0132768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter LT, Potocki L, Chien S, Gribskov M & Bier E (2001) A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res, 11, 1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC & Taghert PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell, 99, 791–802. [DOI] [PubMed] [Google Scholar]
- Reppert SM & Sauman I (1995) period and timeless tango: a dance of two clock genes. Neuron, 15, 983–986. [DOI] [PubMed] [Google Scholar]
- Reppert SM & Weaver DR (2002) Coordination of circadian timing in mammals. Nature, 418, 935–941. [DOI] [PubMed] [Google Scholar]
- Rhodenizer D, Martin I, Bhandari P, Pletcher SD & Grotewiel M (2008) Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol, 43, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger D, Shafer OT, Tomioka K & Helfrich-Förster C (2006) Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J Neurosci, 26, 2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robie AA, Hirokawa J, Edwards AW, Umayam LA, Lee A, Phillips ML, Card GM, Korff W, Rubin GM, Simpson JH, Reiser MB & Branson K (2017) Mapping the Neural Substrates of Behavior. Cell, 170, 393–406.e328. [DOI] [PubMed] [Google Scholar]
- Roeder T, Isermann K, Kallsen K, Uliczka K & Wagner C (2012) A Drosophila asthma model - what the fly tells us about inflammatory diseases of the lung. Adv Exp Med Biol, 710, 37–47. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M & Vetter C (2012) Social jetlag and obesity. Curr Biol, 22, 939–943. [DOI] [PubMed] [Google Scholar]
- Roenneberg T & Merrow M (2016) The Circadian Clock and Human Health. Curr Biol, 26, R432–443. [DOI] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L & Davis RL (2001) P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A, 98, 12602–12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein B & Paululat A (2016) On the Morphology of the Drosophila Heart. J Cardiovasc Dev Dis, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouch I, Wild P, Ansiau D & Marquié JC (2005) Shiftwork experience, age and cognitive performance. Ergonomics, 48, 1282–1293. [DOI] [PubMed] [Google Scholar]
- Royall DR, Chiodo LK, Polk MS & Jaramillo CJ (2002) Severe dysosmia is specifically associated with Alzheimer-like memory deficits in nondemented elderly retirees. Neuroepidemiology, 21, 68–73. [DOI] [PubMed] [Google Scholar]
- Rudrapatna VA, Cagan RL & Das TK (2012) Drosophila cancer models. Dev Dyn, 241, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutila JE, Suri V, Le M, So WV, Rosbash M & Hall JC (1998) CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell, 93, 805–814. [DOI] [PubMed] [Google Scholar]
- Rutters F, Lemmens SG, Adam TC, Bremmer MA, Elders PJ, Nijpels G & Dekker JM (2014) Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J Biol Rhythms, 29, 377–383. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2009) When does age-related cognitive decline begin? Neurobiol Aging, 30, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvateeva E, Popov A, Kamyshev N, Bragina J, Heisenberg M, Senitz D, Kornhuber J & Riederer P (2000) Age-dependent memory loss, synaptic pathology and altered brain plasticity in the Drosophila mutant cardinal accumulating 3-hydroxykynurenine. J Neural Transm (Vienna), 107, 581–601. [DOI] [PubMed] [Google Scholar]
- Savvateeva EV, Popov AV, Kamyshev NG, Iliadi KG, Bragina JV, Heisenberg M, Kornhuber J & Riederer P (1999) Age-dependent changes in memory and mushroom bodies in the Drosophila mutant vermilion deficient in the kynurenine pathway of tryptophan metabolism. Ross Fiziol Zh Im I M Sechenova, 85, 167–183. [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS & Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A, 106, 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y & Frenette PS (2013) Circadian control of the immune system. Nat Rev Immunol, 13, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheving LE, Vedral DF & Pauly JE (1968) Daily circadian rhythm in rats to D-amphetamine sulphate: effect of blinding and continuous illumination on the rhythm. Nature, 219, 621–622. [DOI] [PubMed] [Google Scholar]
- Schlosser Covell GE, Dhawan PS, Lee Iannotti JK, Hoffman-Snyder CR, Wellik KE, Caselli RJ, Woodruff BK, Wingerchuk DM & Demaerschalk BM (2012) Disrupted daytime activity and altered sleep-wake patterns may predict transition to mild cognitive impairment or dementia: a critically appraised topic. Neurologist, 18, 426–429. [DOI] [PubMed] [Google Scholar]
- Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R & Cruickshanks KJ (2008) Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc, 56, 1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A, Price JL, Man B & Young MW (1994) Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science, 263, 1603–1606. [DOI] [PubMed] [Google Scholar]
- Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, DeLisser P, Block GD, Menaker M & Davidson AJ (2012) Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci, 32, 16193–16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyres D, Röder L & Perrin L (2012) Genes and networks regulating cardiac development and function in flies: genetic and functional genomic approaches. Brief Funct Genomics, 11, 366–374. [DOI] [PubMed] [Google Scholar]
- Shang Y, Griffith LC & Rosbash M (2008) Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A, 105, 19587–19594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P (2003) Awakening to the behavioral analysis of sleep in Drosophila. J Biol Rhythms, 18, 4–11. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ & Tononi G (2000) Correlates of sleep and waking in Drosophila melanogaster. Science, 287, 1834–1837. [DOI] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK & Holmes TC (2008) Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol, 18, 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty MS, Sharma M & Sajikumar S (2017) Chelation of hippocampal zinc enhances long-term potentiation and synaptic tagging/capture in CA1 pyramidal neurons of aged rats: implications to aging and memory. Aging Cell, 16, 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Quek SI, Gao Y, Nicora CD, Nie S, Fillmore TL, Liu T, Rodland KD, Smith RD, Leach RJ, Thompson IM, Vitello EA, Ellis WJ, Liu AY & Qian WJ (2017) Multiplexed targeted mass spectrometry assays for prostate cancer-associated urinary proteins. Oncotarget, 8, 101887–101898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih CT, Sporns O, Yuan SL, Su TS, Lin YJ, Chuang CC, Wang TY, Lo CC, Greenspan RJ & Chiang AS (2015) Connectomics-based analysis of information flow in the Drosophila brain. Curr Biol, 25, 1249–1258. [DOI] [PubMed] [Google Scholar]
- Simon AF, Liang DT & Krantz DE (2006) Differential decline in behavioral performance of Drosophila melanogaster with age. Mech Ageing Dev, 127, 647–651. [DOI] [PubMed] [Google Scholar]
- Skjerve A, Holsten F, Aarsland D, Bjorvatn B, Nygaard HA & Johansen IM (2004) Improvement in behavioral symptoms and advance of activity acrophase after short-term bright light treatment in severe dementia. Psychiatry Clin Neurosci, 58, 343–347. [DOI] [PubMed] [Google Scholar]
- Smolensky MH, Sackett-Lundeen LL & Portaluppi F (2015) Nocturnal light pollution and underexposure to daytime sunlight: Complementary mechanisms of circadian disruption and related diseases. Chronobiol Int, 32, 1029–1048. [DOI] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M & Hall JC (1998) The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell, 95, 681–692. [DOI] [PubMed] [Google Scholar]
- Steenman M & Lande G (2017) Cardiac aging and heart disease in humans. Biophys Rev, 9, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J & Rosbash M (2004) Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature, 431, 862–868. [DOI] [PubMed] [Google Scholar]
- Suh J & Jackson FR (2007) Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron, 55, 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE & Carmelli D (2002) Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology, 21, 58–67. [DOI] [PubMed] [Google Scholar]
- Taghert PH & Shafer OT (2006) Mechanisms of clock output in the Drosophila circadian pacemaker system. J Biol Rhythms, 21, 445–457. [DOI] [PubMed] [Google Scholar]
- Taghli-Lamallem O, Akasaka T, Hogg G, Nudel U, Yaffe D, Chamberlain JS, Ocorr K & Bodmer R (2008) Dystrophin deficiency in Drosophila reduces lifespan and causes a dilated cardiomyopathy phenotype. Aging Cell, 7, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y, Takatsu Y, Shiraishi T, Kikuchi Y, Yamazaki M, Motohashi H, Muto A, Sasaki H, Haraguchi A, Kuriki D, Nakamura TJ & Shibata S (2017) Age-related circadian disorganization caused by sympathetic dysfunction in peripheral clock regulation. NPJ Aging Mech Dis, 3, 16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Chiang AS, Ito N, Liu HP, Horiuchi J, Tully T & Saitoe M (2003) Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron, 40, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Taormina G & Mirisola MG (2014) Calorie restriction in mammals and simple model organisms. Biomed Res Int, 2014, 308690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataroglu O & Emery P (2015) The molecular ticks of the Drosophila circadian clock. Curr Opin Insect Sci, 7, 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MV (2006) Developmental biology: micromanaging muscle growth. Curr Biol, 16, R20–23. [DOI] [PubMed] [Google Scholar]
- Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M & Sakaki Y (1997) Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature, 389, 512–516. [DOI] [PubMed] [Google Scholar]
- Tevy MF, Giebultowicz J, Pincus Z, Mazzoccoli G & Vinciguerra M (2013) Aging signaling pathways and circadian clock-dependent metabolic derangements. Trends Endocrinol Metab, 24, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoki A & Davis RL (2012) Aging impairs intermediate-term behavioral memory by disrupting the dorsal paired medial neuron memory trace. Proc Natl Acad Sci U S A, 109, 6319–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoki A & Davis RL (2015) Aging impairs protein-synthesis-dependent long-term memory in Drosophila. J Neurosci, 35, 1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh I & Boulianne GL (2013) Modeling obesity and its associated disorders in Drosophila. Physiology (Bethesda), 28, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso CF, Simon T, Greenlaw AC, Puri T, Mieda M & Herzog ED (2017) Astrocytes Regulate Daily Rhythms in the Suprachiasmatic Nucleus and Behavior. Curr Biol, 27, 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur B, Chen K & Bellen HJ (2016) Drosophila tools and assays for the study of human diseases. Dis Model Mech, 9, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulgherait M, Chen A, Oliva MK, Kim HX, Canman JC, Ja WW & Shirasu-Hiza M (2016) Dietary Restriction Extends the Lifespan of Circadian Mutants tim and per. Cell Metab, 24, 763–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezaki Y, Yoshii T, Kawaguchi T, Helfrich-Förster C & Tomioka K (2012) Pigment-dispersing factor is involved in age-dependent rhythm changes in Drosophila melanogaster. J Biol Rhythms, 27, 423–432. [DOI] [PubMed] [Google Scholar]
- Vaccaro A, Birman S & Klarsfeld A (2016) Chronic jet lag impairs startle-induced locomotion in Drosophila. Exp Gerontol, 85, 24–27. [DOI] [PubMed] [Google Scholar]
- van Alphen B, Yap MH, Kirszenblat L, Kottler B & van Swinderen B (2013) A dynamic deep sleep stage in Drosophila. J Neurosci, 33, 6917–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linde K & Lyons LC (2011) Circadian modulation of acute alcohol sensitivity but not acute tolerance in Drosophila. Chronobiol Int, 28, 397–406. [DOI] [PubMed] [Google Scholar]
- van der Vinne V, Swoap SJ, Vajtay TJ & Weaver DR (2018) Desynchrony between brain and peripheral clocks caused by CK1δ/ε disruption in GABA neurons does not lead to adverse metabolic outcomes. Proc Natl Acad Sci U S A, 115, E2437–E2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel JW (2010) Biodemography of human ageing. Nature, 464, 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, Klerman EB & Zee PC (2017) Light Therapy Promoting Dopamine Release by Stimulating Retina in Parkinson Disease-Reply. JAMA Neurol, 74, 1268–1269. [DOI] [PubMed] [Google Scholar]
- Vienne J, Spann R, Guo F & Rosbash M (2016) Age-Related Reduction of Recovery Sleep and Arousal Threshold in Drosophila. Sleep, 39, 1613–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent GK & Velkoff VA (2010) THE NEXT FOUR DECADES The Older Population in the United States: 2010 to 2050. In commerce D.o. (ed). U.S. Census Bureau, Economics and Statistics Administration [Google Scholar]
- Vinogradova IA, Anisimov VN, Bukalev AV, Semenchenko AV & Zabezhinski MA (2009) Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in rats. Aging (Albany NY), 1, 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW & Takahashi JS (1994) Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science, 264, 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC, Antle MC & Mistlberger RE (2014) Regulation of circadian rhythms in mammals by behavioral arousal. Behav Neurosci, 128, 304–325. [DOI] [PubMed] [Google Scholar]
- Weinert D (2000) Age-dependent changes of the circadian system. Chronobiol Int, 17, 261–283. [DOI] [PubMed] [Google Scholar]
- Weinert H, Weinert D, Schurov I, Maywood ES & Hastings MH (2001) Impaired expression of the mPer2 circadian clock gene in the suprachiasmatic nuclei of aging mice. Chronobiol Int, 18, 559–565. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M & Reppert SM (1995) Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron, 14, 697–706. [DOI] [PubMed] [Google Scholar]
- Wessells RJ & Bodmer R (2004) Screening assays for heart function mutants in Drosophila. Biotechniques, 37, 58–60, 62, 64 passim. [DOI] [PubMed] [Google Scholar]
- Wessels A & Pérez-Pomares JM (2004) The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol, 276, 43–57. [DOI] [PubMed] [Google Scholar]
- White MC, Holman DM, Boehm JE, Peipins LA, Grossman M & Henley SJ (2014) Age and Cancer Risk: A potentially modifiable relationship. Am J Prev Med, 46, S7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Perland E, Eriksson MM, Carlsson J, Erlandsson D, Laan L, Mahebali T, Potter E, Frediksson R, Benedict C & Schiöth HB (2016) Recurrent Sleep Fragmentation Induces Insulin and Neuroprotective Mechanisms in Middle-Aged Flies. Front Aging Neurosci, 8, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA & Bennett DA (2007) Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry, 64, 802–808. [DOI] [PubMed] [Google Scholar]
- Wirtz A & Nachreiner F (2012) Effects of lifetime exposure to shiftwork on fitness for duty in police officers. Chronobiol Int, 29, 595–600. [DOI] [PubMed] [Google Scholar]
- Wittmann M & Werfel T (2006) Interaction of keratinocytes with infiltrating lymphocytes in allergic eczematous skin diseases. Curr Opin Allergy Clin Immunol, 6, 329–334. [DOI] [PubMed] [Google Scholar]
- Wong PM, Hasler BP, Kamarck TW, Muldoon MF & Manuck SB (2015) Social Jetlag, Chronotype, and Cardiometabolic Risk. J Clin Endocrinol Metab, 100, 4612–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse CA & Coogan AN (2010) Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res, 1337, 21–31. [DOI] [PubMed] [Google Scholar]
- Yadav P & Sharma VK (2014) Circadian clocks of faster developing fruit fly populations also age faster. Biogerontology, 15, 33–45. [DOI] [PubMed] [Google Scholar]
- Yamadera H, Ito T, Suzuki H, Asayama K, Ito R & Endo S (2000) Effects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci, 54, 352–353. [DOI] [PubMed] [Google Scholar]
- Yamamoto D & Sato K (2013) [The female brain and the male brain]. Brain Nerve, 65, 1147–1158. [PubMed] [Google Scholar]
- Yamazaki D, Horiuchi J, Nakagami Y, Nagano S, Tamura T & Saitoe M (2007) The Drosophila DCO mutation suppresses age-related memory impairment without affecting lifespan. Nat Neurosci, 10, 478–484. [DOI] [PubMed] [Google Scholar]
- Yamazaki D, Horiuchi J, Ueno K, Ueno T, Saeki S, Matsuno M, Naganos S, Miyashita T, Hirano Y, Nishikawa H, Taoka M, Yamauchi Y, Isobe T, Honda Y, Kodama T, Masuda T & Saitoe M (2014) Glial dysfunction causes age-related memory impairment in Drosophila. Neuron, 84, 753–763. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M & Block GD (2002) Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A, 99, 10801–10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z & Shafer OT (2014) The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science, 343, 1516–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MHW, Grabowska MJ, Rohrscheib C, Jeans R, Troup M, Paulk AC, van Alphen B, Shaw PJ & van Swinderen B (2017) Oscillatory brain activity in spontaneous and induced sleep stages in flies. Nat Commun, 8, 1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K & Meinertzhagen IA (2010) Synaptic connections of PDF-immunoreactive lateral neurons projecting to the dorsal protocerebrum of Drosophila melanogaster. J Comp Neurol, 518, 292–304. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Hermann-Luibl C & Helfrich-Förster C (2016) Circadian light-input pathways in Drosophila. Commun Integr Biol, 9, e1102805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S, Fulga TA, Van Vactor D & Jackson FR (2018) Regulation of Circadian Behavior by Astroglial MicroRNAs in. Genetics, 208, 1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Daniels J, Glaser AE & Wolf MJ (2013) Raf-mediated cardiac hypertrophy in adult Drosophila. Dis Model Mech, 6, 964–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarndt R, Piloto S, Powell FL, Haddad GG, Bodmer R & Ocorr K (2015) Cardiac responses to hypoxia and reoxygenation in Drosophila. Am J Physiol Regul Integr Comp Physiol, 309, R1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehring WA, Wheeler DA, Reddy P, Konopka RJ, Kyriacou CP, Rosbash M & Hall JC (1984) P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell, 39, 369–376. [DOI] [PubMed] [Google Scholar]
- Zepelin H, McDonald CS & Zammit GK (1984) Effects of age on auditory awakening thresholds. J Gerontol, 39, 294–300. [DOI] [PubMed] [Google Scholar]
- Zhang C & Wang X (2017) Initiation of the age-related decline of odor identification in humans: A meta-analysis. Ageing Res Rev, 40, 45–50. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Takahashi JS & Turek FW (1996) Critical period for cycloheximide blockade of light-induced phase advances of the circadian locomotor activity rhythm in golden hamsters. Brain Res, 740, 285–290. [DOI] [PubMed] [Google Scholar]
- Zimmermann LK (2011) Chronotype and the transition to college life. Chronobiol Int, 28, 904–910. [DOI] [PubMed] [Google Scholar]
- Zuo L, Iordanou E, Chandran RR & Jiang L (2013) Novel mechanisms of tube-size regulation revealed by the Drosophila trachea. Cell Tissue Res, 354, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier LA, Luik AI, Hofman A, Franco OH, Van Someren EJ & Tiemeier H (2015) Fragmentation and stability of circadian activity rhythms predict mortality: the Rotterdam study. Am J Epidemiol, 181, 54–63. [DOI] [PubMed] [Google Scholar]


