Abstract
Fungal rhinosinusitis (FRS), once considered a rare disease, has seen a steep rise in incidence in recent times. This global rise in the burden of fungal disease is a consequence of an increment in the population with weakened immune systems. Increased life expectancy with rise in conditions like diabetes mellitus, medical advancements with invasive interventions, use of immunosuppressive drugs and chemo-radiotherapy all lead to unique risk situations. The situation becomes more alarming with the fact that there has been a significant rise in cases in immune-competent hosts with no predisposing factors. FRS represents a wide spectrum of disease ranging from the mild form of superficial colonization, allergic manifestations to life threatening extensive invasive disease. The categorization of disease into acute and chronic and invasive or noninvasive is important factor with implications in disease management and prognosis and this has been emphasized greatly in recent years. Diagnosis of FRS has been a challenge as the presenting clinical signs and symptoms and radiographic manifestations are often nonspecific. Definitive diagnosis requires direct fungi identification and hence culture and microscopic examination remain the gold standard. Availability of advanced and rapid diagnostic techniques is rare in majority of developing nations. Therapeutic dilemmas are another aspect of the management of FRS as in spite of the availability of new antifungal drugs, treatment is often empirical due to non-availability of early diagnosis, rapid disease progression and high costs of antifungal drugs. A description of the different types of FRS, their diagnosis and management has been presented in this review.
Keywords: Fungal rhinosinusitis, Mucormycosis, Invasive fungal sinusitis
Introduction
Fungi with their universal pervasive presence in the earth’s environment have complex interactions with all flora, fauna and humans alike. They have a ubiquitous distribution with a conservative estimate of 1.5 million and liberal guesstimate of over 5 million fungal species present within the earth’s ecosystems [1, 2]. Of these fungal species around 100,000 [2] have been described while roughly 300 have been linked to human diseases [3]. For reasons still largely unknown, there exists a wide variation in the geographical niches inhabited by fungi across the world. The fungal microbiota or ‘mycobiome’ is a widely researched subject and yet poorly understood. In spite of enormous advances and evolvement in the discipline of mycology over the last three decades, it remains a daunting task for any author to give a comprehensive account of all aspects ranging from disease spectrum to management.
Human exposure to indoor and outdoor fungal spores in the air is inevitable and they continuously inhale spores which deposit along the airway mucosa. Nicholas P Money states in a book chapter: “Time for a panic attack: their spores are everywhere and, depending upon your location, you may have inhaled hundreds of them since beginning to read this chapter.”
According to their pathogenic potential, fungi that cause diseases in humans are mostly opportunistic while some are true pathogens. Some factors like body temperature of 37 °C, low redox potential in tissues and natural barriers of the immune system can prevent most fungi from invading the human hosts [4]. So while most exposures are not dangerous in healthy individuals, some can be harmful to human health especially when host defenses are compromised, and the fungi have an opportunity to adapt into the changed host environment. Fungi have long been implicated as pathogens in patients of acute and chronic sinusitis (Table 1). Inhalation of fungal spores and their presence within the human sinonasal cavity triggers immunopathological processes depending on unique risk factors.
Table 1.
Brief review of historical landmarks
| 1729 | Aspergillus species first named by Italian biologist Pier Antonio Micheli |
| 1791 | Plaignaud gave first description of fungal sinusitis |
| 1893 | First citation of aspergillus infection given by Mackenzie |
| 1885 | Schubert reported more specific diagnosis of nasal and paranasal aspergillosis with account of a noninvasive aspergillosis of paranasal sinus |
| 1897 | First well documented case of invasive aspergillosis was reported by Oppe in 1897 |
| 1955 | Harris reported first survivor of Mucormycis |
| 1965 | Hora discussed the primary aspergillosis of the paranasal sinuses and classified sinus mycosis into two invasive and noninvasive |
| 1980 | Mc Gill reported 4 cases of fulminant aspergillosis of the nose and paranasal sinuses as a new clinical entity in individuals with depressed immunological responses. This variant was characterized by rapid malignant course, requiring early recognition, aggressive surgery and chemotherapy |
| 1983 | Kazenstein et al. observed the patho-physiological resemblance between Allergic broncho pulmonary aspergillosis (ABPA) and seven cases of chronic fungal sinusitis leading to identification of allergic aspergillosis of the maxillary sinus as a separate entity |
| 1997 | de-Shazo and colleagues published new classification for invasive and noninvasive fungal sinusitis |
| 2009 | The working group on Fungal sinusitis under International Society for Human and Animal Mycology proposed the classification widely used today |
Once considered a rare disease, today fungal rhinosinusitis (FRS) is being reported with increasing frequency from around the world and is an important concern in India as the subcontinent reports the highest number of mucormycosis cases in the world [5].
FRS is an important clinical entity and successful identification and management of the disease is fraught with myriad challenges. Having a high clinical index of suspicion to identify the subtle as well as obvious signs and symptoms is a paramount factor for the clinician.
Detection of fungi, assessment of host immune status and rapid categorization of disease by incorporating biopsies, radiological, microbiological, histopathological methods followed by timely surgical intervention and medical management are all crucial contributive factors if positive outcomes are to be expected. We attempt to provide an overview of the different aspects of fungal rhinosinusitis in this article.
Classification and Diagnosis of Fungal Rhinosinusitis
‘Fungal Rhino Sinusitis’ as it is better termed can be categorized along an immunologic disease spectrum and the manifestation based on this ‘tug of war’ interaction between the host and causative fungi varies from allergic reactions to superficial colonizations to noninvasive or invasive disease. The classification of FRS has gradually evolved over the last few decades amidst a backdrop of confusion. Categorization of acute or chronic and invasive or noninvasive is essential to decide appropriate management strategies and to predict prognosis [6, 7].
The diagnosis of FRS is also a challenge, as it encompasses a wide spectrum of disease conditions which present with a myriad of clinical scenarios, histopathologic appearance and radiographic manifestations. Clinical presentations are more often nonspecific in nature and a definitive diagnosis requires direct identification of fungi from infection site through diagnostic laboratory methods. Difficulty in differentiating colonization from invasive disease, false and unreliable culture results, inability of subjecting immunocompromised patients to invasive diagnostic procedures. Lack of sensitivity and specificity in the available tests are just some of the problems adding to the diagnostic dilemma.
Over the last few decades, attempts have been made to clear the controversies surrounding fungal rhinosinusitis. The utmost important factor in predicting patient prognosis and providing effective therapy is the correct classification of FRS. It is important to identify whether the disease is clinically acute or chronic and histopathologically noninvasive or invasive based on fungi invasion into tissue.
Classification
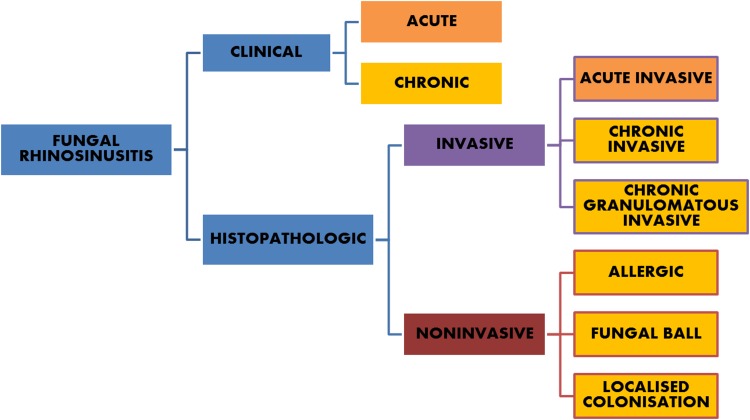
Currently, the most commonly accepted system as suggested by a consensus workshop [7, 8] (Fig. 1) divides FUNGAL RHINOSINUSITIS (FRS) into invasive or noninvasive disease based on histopathological demonstration of tissue invasion by fungal elements. Invasive diseases are differentiated into (1) acute invasive FRS, (2) chronic invasive FRS and (3) granulomatous type. The noninvasive diseases are categorized into three different clinical forms: (1) localized colonization, (2) fungal ball and (3) eosinophil-related FRS that includes AFRS.
Fig. 1.
Classification suggested by consensus workshop
Diagnostic Methods Available for Fungal Rhinosinusitis
The various diagnostic methods available include conventional microscopic examination, histopathology, culture methods, antigen antibody testing, antifungal susceptibility testing and molecular methods. But not all fungi can be identified by any one given method and appropriate diagnostic aid depends on locally prevalent fungal epidemiology and infections and the available laboratory tests (Table 2).
Table 2.
Laboratory methods available for the diagnosis of invasive fungal diseases [9]
| Conventional methods |
|---|
| Direct microscopy(KOH stain, Giemsa) |
| Histopathology |
| Culture |
| Serology |
| Galactomannan test for Aspergillus species |
| Mannan test for Candida species |
| Antibody test for Aspergillosis in immunocompetent hosts |
| β-1-3 D glucan panfungal test |
| Lateral device flow assays for Aspergillus and Cryptococcus species |
| Antigen and antibody based assays for non-European mycoses |
| Molecular methods |
| Fluorescence in situ hybridization test |
| PCR assays |
Conventional microscopic and microbiological techniques remain the cornerstone of diagnosis but lack sensitivity. Cultures are often time-consuming as fungi are slow-growing and can yield false results. Since fungi are a part of the human microbiome, antigen and antibody detection-based serological assays are also not very useful as they commonly give false positive results. Antigen assays like galactomannan and glucan tests are used but are helpful only in specific pathogens. Molecular assays like fluorescence in situ hybridization (FISH) test and polymerase chain reaction (PCR) hold a lot of promise but are very expensive and still need clinical validation [9].
Conventional microscopy is the basis on which diagnosis of fungal disease is made. Mere identification of fungal element warrants the initiation of treatment but classifying the specific variant of FRS is necessary for further management [10, 11].
Direct microscopy (using 10% Potassium hydroxide) can help in rapid detection of fungi but negative results do not rule out fungal infection.
Conventional histopathological classification is based on three important parameters
-
Identification of fungal structure:
While hematoxylin and eosin (H&E)-stained sections are usually adequate to identify fungal elements, use of special stains makes it easier.
Stains like Grocott’s and Gomori’s silver methenamine (GMS) and periodic acid schiff (PAS) make the fungal wall identifiable based on the presence of polysaccharides. These tests though sensitive are not specific and hence the fungal structures need to be carefully identified. GMS has been described to be the most sensitive stain and it has been recommended that a negative diagnosis of fungal pathology should not be given unless GMS staining is performed [12].
-
Tissue invasion
It is determined whether the disease is invasive or noninvasive based on evidence of invasion of fungal structures into the mucosa, associated vascular entities leading to thrombosis and tissue infarction and invasion into underlying bony structures.
-
Type of inflammatory reaction [13]
Based on the type of inflammatory reaction and temporal character of the pathologic process, invasive FRS can be ‘Acute invasive,’ ‘Chronic invasive’ or ‘Chronic granulomatous.’ While in the chronic disease, there is predominance of chronic inflammatory cells or there is granulomatous reaction; in the noninvasive variants, ‘Fungal ball’ shows nonspecific chronic inflammation while ‘Allergic fungal rhinosinusitis’ has a very distinctive histologic appearance which is discussed elsewhere.
Identification of the fungal species is useful in selecting appropriate antifungal therapy and immunotherapy. But this identification is a difficult task even for an experienced pathologist especially in the chronic granulomatous variant. The pathologist needs to examine multiple or complete sections as the fungal hyphae may not be present in most sections. It is important to make sure that the peripheral areas from the biopsy are carefully examined for fungal hyphae.
In difficult cases, KOH microscopy, fungal culture and PCR are often useful adjuncts highlighting the role of multiple diagnostic methods. The diagnosis of FRS though sometimes difficult can be simplified by a team approach having specialists of different fields.
Noninvasive Fungal Rhinosinusitis
I. Allergic fungal rhinosinustis (AFRS) [allergic fungal sinusitis]
AFRS described first by Safirstein in 1976 is not considered to be a true fungal infection but rather an allergic hypersensitivity reaction to extra-mucosal fungi present in the sinonasal tract. It is defined by the presence of allergic fungal mucin and some characteristic histologic findings. The pathogenesis of AFRS remains controversial and though not completely understood it is considered similar to the patients with allergic broncho pulmonary aspergillosis (ABPA) as noted by Milaar et al. in 1981.
AFRS is like one end of a spectrum of sinonasal disease defined by the presence of fungal elements and eosinophilia [14]. Extensive studies by Manning, Bent and Kuhn, Schubert and others suggest that AFRS is an IgE-mediated disease [15].
The overall prevalence of AFRS is estimated at 5–10% of all patients with chronic rhinosinusitis [7].
Recent research and publications continue to generate more questions than answers regarding this condition. Demonstration of fungal hyphae is considered important to differentiate AFRS from a recently described entity eosinophilic mucinous rhinosinusitis (EMRS) which has no identifiable fungi [16]. But whether EMRS is a distinct disease entity or not is still a widely debated topic. Common implicated fungi in the pathogenesis of AFRS include the dematiaceous fungi: Bipolaris, Alternaria, Curvularia and the hyaline molds such as Aspergillus and Fusarium [17].
Clinical Presentation
AFRS is the most common form of FRS with presentations ranging from mild to dramatic.
Typically, it is seen in immune-competent young people, with a history of atopy manifested as allergic rhinitis and/or asthma [18]. The affected nasal mucosa functions in a compromised state and a vicious cycle of chronic edema, stasis of secretions and bacterial super infection results.
Patients present with signs and symptoms of chronic nasal airway obstruction and congestion, nasal crusting, postnasal drip, rhinorrhoea and headache. Patients are not responsive to standard antihistamine therapy and mediations, which should lead to a high index of suspicion. AFRS is seen more commonly in warm, humid climates such as countries like, India and the Middle East countries, the southern parts of the USA.
It is the most common form of FRS in India accounting for 56–57% cases [19].
Imaging Features
AFRS is best visualized on non-contrast axial CT scans with coronal reformats. AFRS commonly presents with pansinusitis or multiple sinus involvement, which is often bilateral but can be unilateral and has an associated nasal component/rhinitis. Involvement of ethmoid sinus is the most common. Affected sinuses show opacification as the allergic mucin causes hyper-attenuation within the lumen of the paranasal sinus on non-contrast CT while the mucosal linings appear hypodense [20]. On administration of contrast, lack of central enhancement or in most of the sinus helps distinguish AFRS from malignant disease [21]. Local bony changes in sinus anatomy are more common in AFRS than other forms of CRS. Though not invasive if AFRS is untreated it can lead to sinus expansion and smooth bone erosion or remodeling due to the pressure effects from the presence of mucin and inflammatory changes resulting in intracranial or intra-orbital symptoms. MR images are obtained in cases where there is a concern of disease extension into the cranium or orbit [20]. High protein concentration in allergic mucin leads to low signal intensity of sinus contents on T1 images while the inflamed mucosal lining is relatively hypointense. T2-weighted images characteristically show low signal or signal void of sinus content, attributable to high metallic concentration by fungi and high protein and low free water content of mucin while the peripheral mucosal line appears hyperintense after contrast administration [22].
Diagnosis
Diagnostic criteria for AFRS vary by author, but the criteria described by Bent and Kuhn [23] in 1994 are the most widely accepted. Their five criteria include (a) nasal polyposis, (b) allergic mucin (eosinophilic mucin) with no signs of invasion, (c) hyperattenuating signal density visualized by CT scan (double density sign), (d) positive fungal stain or culture and (e) type I hypersensitivity to fungi diagnosed by history, skin testing or serology.
However, De Shazo and Swain in 1995 suggested dropping the criteria of type I hypersensitivity as they had observed in their review that one-fourth of patients diagnosed with AFRS were not atopic. This is till date a debated topic [24].
The eosinophilic mucin produced in AFRS ranges from a yellow-green, gray, brown, white-tan or blackish inspissate thick mucus material classically described as having a ‘peanut butter like’ appearance and a ‘cement/rubber’ like consistency. On histologic picture, eosinophils and eosinophil degradation products called Charcot–Leyden crystals are characteristically seen [14].
Ou et al. in his meta-analysis concluded that S. aureus super-antigen may be the risk factor for the chronicity and severity of CRS that leads to modulation of local immune response resulting in the development of AFRS. Studies suggest a strong linkage between S. aureus super-antigens and AFRS claiming S. aureus super-antigen induces an immune response that modulates eosinophil inflammation correlating with total IgE Ab levels, establishing a relationship between bacterial colonization and allergic hypersensitivity [25].
Barac et al. more recently proposed that persistent presence of sinonasal fungi and long duration of CRS are silent threat for progression of inflammation and development of FRS [17].The presence of allergy parameters and better response to corticosteroid therapy in AFRS are crucial in diagnosis.
The differential diagnosis of AFRS includes other infectious, neoplastic and inflammatory conditions leading to sinusitis. It needs to be differentiated from other entities with allergic mucin such as aspirin sensitivity syndrome. Fungal ball (FB) can show a layered appearance resembling AM, but on higher power it is easy to identify predominant fungal elements mixed with debris [26].
Treatment
Optimal management of AFRS is debatable till date but requires both surgical and medical approaches [27]. Goal of treatment is to surgically eliminate the fungal antigen and restoration of normal sinus drainage followed by control of recurrence through medication.
Surgery is the mainstay of management for AFRS and involves removal of nasal polyps complete extirpation of the allergic mucin, local secretions and fungal residues; and establishment of aeration and permanent drainage route from the sinus by opening of sinus ostia, marsupialisation of involved sinuses, while preserving the mucosal integrity. In recent times, endoscopic techniques have gained popularity.
Long-term medical therapy is essential for preventing recurrence of AFRS as surgery without postoperative medications seems to have a 100% chance of recurrence. However, the medical management for AFRS is still controversial. Combination of corticosteroids, antifungal agents, antihistamines, anti-leukotrienes and immunotherapy has been tried.
Barac et al. [17] have suggested that since fungi trigger an enhancement of IgE Ab and eosinophils inclusion of nasal lavage with hypertonic NaCl in every day hygiene routine can decrease fungal load and antigenic exposure and prevent AFRS from developing.
Antifungal agents seem to play little or no role in management, but some authors recommend use of itraconazole, topical fluconazole [28] or intranasal Amphotericin B [29] but this needs further validation.
Role of topical and systemic corticosteroids in decreasing intranasal polyposis and inflammation has been well established but there is no general consensus regarding optimum duration and dosage of therapy [30].
Some authors suggest administration of oral prednisone in dosage of 10–20 milligrams per day for a minimum of 2 weeks. Same dose is then continued on alternate days for another 2 weeks or more. Intranasal corticosteroids are also prescribed on a long-term basis [31].
Kuhn and Javer [32] recommend a long-term protocol of oral prednisone starting with a dosage of 0.4 mg/kg/day for 4 days which is then decreased by 0.1 mg/kg in 4-day cycles until a dose of 0.2 mg/kg/day or 20 mg/day is reached. They advise continuation of this maintenance dose alongside monthly nasal endoscopies and IgE-level monitoring until there is a Stage 0 appearance of mucosa (i.e., no mucosal edema or allergic mucin) as described by Kupferberg et al. [33]. If this is maintained for 4-week prednisone dosage is reduced to 0.1 mg/kg/day and intranasal corticosteroid sprays are started. If patient is asymptomatic for next 2 months, oral prednisone is stopped, and only nasal steroid spray continued for 1-year period.
| Key points for allergic fungal rhinosinusitis |
|---|
| • Associated with eosinophilic mucin (EM) containing sparse fungi |
| • Not a true fungal infection but an inflammatory reaction to fungi |
| • Common organisms associated are dematiaceous(pigmented) fungi–Bipolaris, Curvularia, Alternaria and hyaline molds like Aspergillus and Fusarium species |
| • The disease appears to be a complex interplay of IgE-mediated hypersensitivity to fungal antigens, host defense mechanisms (innate and adaptive including both T cell- and B cell-mediated immune responses), and possibly super-antigens. |
| • No uniform management protocol but surgical clearing of sinuses along with steroid therapy are commonly practiced. |
| • The role of antifungal agents, leukotriene antagonists and immunomodulators is still questionable. |
II. Fungal ball (FB) [aka sinus mycetoma/aspergilloma]
This form of fungal rhinosinusitis has been described by different terms like chronic noninvasive granulomatous, sinus mycetoma, aspergilloma [34], but sinus fungal ball is most favored [7]. Fungal ball has been defined as an accumulation of dense conglomeration of fungal hyphae within a sinus cavity, usually the maxillary sinus. This extra-mucosal form presents as an entangled mass of fungi and is associated with minimal mucosal inflammation [35].
Fungal ball of the paranasal sinus is noninvasive and usually seen in immunocompetent and non-atopic hosts but if the host becomes immune-suppressed it may rarely invade into adjacent tissues [36]. It may also coexist with other forms of fungal rhinosinusitis mostly allergic fungal rhinosinusitis [37].
The pathogenesis of FB has been attributed to three possible etiologies, i.e., airborne route, odontogenic cause and mixed etiology [38].
The odontogenic theory postulates that pathogenesis of fungal ball of maxillary sinus is based on fungal colonization due to iatrogenic oroantral communications such as extraction, periodontal destruction or endodontic treatment. The presence of zinc in endodontic sealers is thought to play a key role [39].
Clinical Presentation
Clinical manifestations are nonspecific, and diagnosis is usually suspected on imaging studies. Surgical treatment is often curative. Clinical features that should raise suspicion of FB are unilateral nature of complaint, with chronic pressure sensation involving a paranasal sinus, facial pain associated usually with complain of nasal congestion, cacosmia, dysosmia and purulent foul-smelling nasal discharge [37].
Fungal balls usually present with unilateral single sinus involvement, mostly the maxillary sinus, followed by sphenoid, rarely ethmoid and frontal sinus too [40]. Fungal balls are predominantly seen in middle-aged and elderly female patients. It is exclusively seen in adults and does not affect pediatric population. Some authors based on these two factors suggest hormonal influences on pathogenesis of FB. It has also been hypothesized that the absence of FB in young patients may be due to the rarity of endodontic treatments needed in the age group [40]. In cases with sphenoidal localization of fungal ball, complaints of vertex headache and facial pain associated with postnasal drip are common findings [41].
The clinical picture in patients with FB is most often nonspecific and confounding with similarities to antibiotic-resistant bacterial CRS. Patients commonly elicit a history of recurrent sinusitis and nasal polyps [37]. Sometimes patients present atypical symptoms like, epistaxis, seizures, fever, cough, visual impairment and proptosis. Patients can be asymptomatic or present with symptoms that are chronic and may have been present for months or years without diagnosis.
Local examination may reveal a mass within the sinus cavity associated with foul-smelling material in the sinu [18]. Nasal endoscopic examination is nonconclusive in most cases. However, sinus endoscopy, in case of maxillary fungal ball can highlight the characteristic appearance of “fungus ball” and allow harvesting of material for culture and histopathological analyses [38].
Imaging Features
In imaging studies, fungus ball appears as a rounded or ovoid mass within the lumen of a paranasal sinus presenting typically with single sinus involvement [42]. Non-enhanced CT is the imaging modality of choice with high specificity [38]. It also allows for assessment of any calcifications [14]. It appears as a hyperattenuating mass on non-contrast CT scan imaging due to the presence of dense matted hyphae. Focal hyperdense areas are noted simulating a foreign body. These actually represent calcium phosphate deposits concentrated in areas of mycelium necrosis. Punctate calcifications have also been reported [22]. The bony walls of the sinus may show osteitis, i.e., sclerosis and thickening or expansion and thinning with focal areas of erosion due to pressure necrosis. In contrast studies, fungal ball appears non-enhanced and the lining mucosa appears hyperenhancing [14]. On MRI imaging, the fungal ball demonstrates low signal on both T1- and T2-weighted images due to the absence of free water. T2-weighted images may show signal void areas due to calcifications and paramagnetic metals such as iron, magnesium and manganese deposited within the mycetoma. No tissue invasion is noted [38].
Diagnosis
The diagnosis of paranasal fungus ball is based on clinicopathological criteria as defined by deShazo and his colleagues [37]. These include:
radiological evidence of sinus opacification with or without calcifications
mucopurulent cheesy or clay-like materials within the sinus
a dense conglomeration of hyphae (= fungus ball) separate from the sinus mucosa
nonspecific chronic inflammation (lymphocytes, plasma cells, eosinophils) of the mucosa.
no predominance of eosinophils, no granuloma and no allergic mucin
no histological evidence of fungal invasion of mucosa, blood vessels or bone visualized microscopically after special stains for fungus
The definitive diagnosis of a fungal ball can only be confirmed with macroscopic and histopathological examination of the material removed in surgery. Aspergillus sp. are the most commonly isolated fungi although fungal cultures are often negative and only 23–50% cases show fungal growth [43].
Grossly, material found within the sinus is a dense chalky or friable cheesy material which can be green, yellow, brown or black and easily separated from underlying mucosa [35].
Microscopically, the fungus ball is characterized by entangled masses of mycelial elements embedded within a fibrinous and necrotic exudate [44].
Some authors describe it as an aggregate of tightly packed hyphae exhibiting alternating dense and less dense zones of fungal growth presenting concentric onion skin appearance under low-power magnification [35]. The underlying mucosa may appear normal or inflamed. Microscopic examination of the mucosa can demonstrate nonspecific chronic inflammatory change without tissue invasion by the fungi.
Treatment
Surgery is the mainstay of management of fungal ball. It involves surgical removal of the fungal aggregate and debris along with wide ventilation of involved sinus with restoration of drainage. Medical antifungal therapy is of little utility in these patients.
If a significant risk factor like oroantral fistula is noted, then surgical repair may be required to prevent recurrence. The importance of detecting precipitating antibody in follow-up is highlighted for both sinus mycetoma and chronic invasive fungal sinusitis. Precipitin antibody becomes negative or titer is reduced after surgery and it reappears in patients with recurrence or progression of lesions [45].
| Key points for fungal ball |
|---|
| • Not associated with allergic reaction |
| • Noninvasive, extra-mucosal entangled mass of fungi |
| • Commonly associated with Aspergillus sp. But cultures are usually negative |
| • Seen in elderly women, not reported in children |
| • Mostly unilateral and most commonly involves maxillary sinus, followed by sphenoid sinus |
| • Pathogenesis is debatable, role of airborne etiology and dental procedures |
| • Seen in immunocompetent hosts, rarely can progress to invasive form if immunosuppression develops |
| • Surgery mainstay of management, no role of antifungals, good prognosis |
III. Localized fungal colonization (aka saprophytic fungal colonization)
At times, mucous nasal crusts within the nasal cavity can be colonized by macroscopic fungal collections [46]. Such saprophytic fungal asymptomatic colonization of mucus within the nasal cavity is usually reported in immunocompetent patients with a history of prior sinus surgery or who have chronic rhinosinusitis [14]. The condition could lead to the formation of fungal ball in some cases. No specific signs and symptoms or radiological findings are noted. Management simply involves removal of the nasal crusts for complete resolution [34].
Invasive Fungal Rhinosinusitis
Invasive fungal sinusitis is defined as acute when the duration is less than 4 weeks, while disease of more than 4-week duration is said to be chronic [7] (Table 3).
Table 3.
Diagnostic criteria for invasive fungal sinusitis.
Adapted from deShazo et al. [10]
| 1. Mucosal thickening or air fluid levels compatible with sinusitis on radiologic imaging |
| 2. Histopathologic evidence of hyphal forms within sinus mucosa, submucosa, blood vessels, or bone |
| 3. To diagnose granulomatous invasive fungal sinusitis, histopathologic evidence of hyphal forms within sinus mucosa, submucosa, blood vessel or bone in association with granuloma containing giant cells is required. Concomitant stains for mycobacteria must be negative |
I. Acute invasive fungal rhinosinusitis (AIFRS) [aka acute fulminant/necrotizing fungal rhinosinusitis, rhinocerebral mucor mycosis]
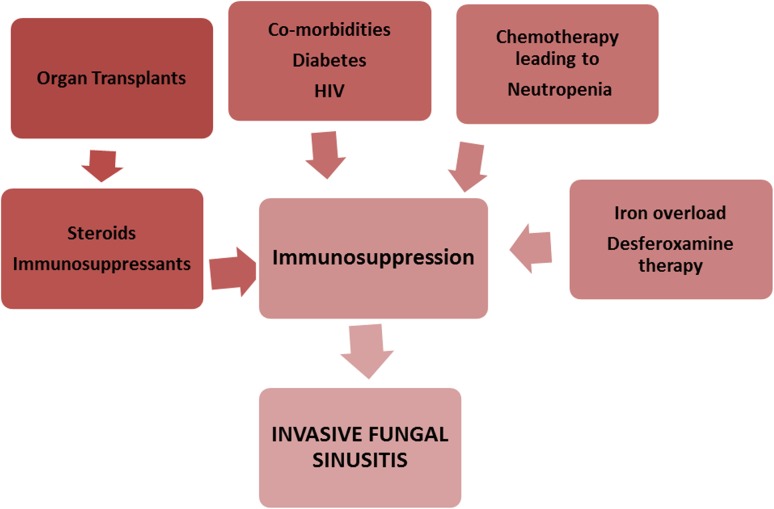
Acute invasive fungal rhinosinusitis is a relatively rare condition mostly seen in immunocompromised patients [44]. The disease can have a rapidly progressing dramatic clinical course over a few days to weeks (< 4 weeks). Rapid spread of the infection can occur into the adjacent orbits and brain parenchyma and vascular cavernous sinus pathways. This occurs as a sequela of direct invasion or vascular invasion by the fungus, leading to vascular thrombosis and tissue infarction. AIFRS is a lethal form of fungal sinusitis with reported mortality of 50–80% [22, 47]. Affected patients demonstrate some degree of compromised immune function and tend to be critically ill. Patients with decreased host cell-mediated immunity, specifically ones with impaired neutrophil function, aplastic anemia, organ transplant patients, hematologic malignancies, hemochromatosis or those undergoing immunosuppressive treatments like oral corticosteroids or chemotherapy patients, acquired immunodeficiency syndrome patients and patients with poorly controlled diabetes creates the risk group for this disease (Fig. 2).
Fig. 2.
Risk factors for invasive fungal rhinosinusitis
Hyperglycemia and acidosis create conducive environment for fungal growth and tissue invasion and ketoacidosis is shown to adversely affect phagocytosis. Infrequently, acute invasive disease has also been reported in patients with normal immune function.
Aspergillus sp. and fungi of the Mucoraceae family (Zygometes order) are implicated in most cases. In poorly controlled diabetics, especially patients with diabetic ketoacidosis and those receiving Deferoxamine, the zygomycetes fungi, including Mucor, Rhizopus, and Absidia predominate and is termed Zygomycosis or Mucormycosis while in neutropenic patients, Aspergillus sp. account for almost 80% cases [22]. Considering the high mortality rates of the condition, it is important for clinicians to maintain a high index of suspicion and awareness based on known or potential immunosuppression, and for radiologists to actively look for subtle signs that can be identified in the early stages of the disease.
Clinical Presentation
The initial presenting symptoms of the disease are nonspecific and similar to acute bacterial sinusitis. Rhinorrhea, headache, fever with spikes along with facial pain and diplopia are the most common findings. But the facial pain is often out of proportion to the physical findings noted.
In the initial stages involvement of the nasal cavity first presents with ischemia, pallor and color changes. There may be painless, necrotic nasal septal ulcer or eschar [48]. As the disease rapidly progresses, necrosis and granulation tissue may develop over the nasal mucosa, there may be loss of sensation in the oral cavity, hypesthesia, scar tissue over hard palate, peri-orbital and facial edema, diplopia and visual disturbances [48]. Spread of disease from the sinuses to orbit can present with proptosis, ophthalmoplegia, chemosis, diplopia, decreased visual acuity and even blindness. Disease invasion beyond the maxillary sinus walls can result in cheek and palatal necrosis. Sinus walls are commonly noted to be necrotic but may remain intact if fungal spread is along vascular channels. Extension of disease across the skull base or via skull base foramina indicates severe disease and presents with ominous signs of cranial nerve palsies, stroke and mental status change. Cavernous sinus thrombosis, internal carotid artery thrombosis, acute subdural hematoma may indicate rhino cerebral mucormycosis. Invasion of the carotid arteries can cause cerebral ischemia and prove fatal.
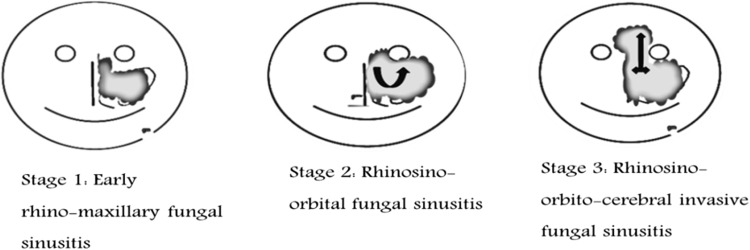
Based on disease extension disease may be clinically staged into [48, 49] (Fig. 3).
- Stage 1
Rhinomaxillary
- Stage 2
Rhino-orbital
- Stage 3
Rhino-orbito-cerebral
Fig. 3.
Clinical staging of Acute invasive fungal rhinosinusitis (AIFRS) based on disease extension
Imaging Features
CT scan of the sinus and orbits is the imaging modality of choice, but in the early stages due to the fulminant nature of disease process changes may be very subtle or may not be evident in as many as 12% patients [50].
Hypoattenuating thickened mucosa with partial or complete opacification within a unilateral nasal cavity or sinus is the most consistent but nonspecific imaging feature [46, 51, 52].
In a patient with immunosuppression, the finding of hyper-attenuation areas within opacified sinuses should raise a red flag for a possible underlying fungal cause [53].
As the disease progresses, erosion of bone may be seen and findings outside the sinonasal cavity, such as retro-antral soft tissue thickening may also be noted. These fungi also have a tendency to spread along the vascular structures, and extension beyond the sinuses may occur with intact bony walls. The areas to be meticulously examined to evaluate for soft tissue infiltration are spaces adjacent to maxillary sinuses like premaxillary and retro-antral fat, and pterygopalatine fossa areas.
While CT is ideal to assess bony changes, MRI is superior in evaluating retro-antral, intra-orbital or intracranial extension [22]. Lack of signal enhancement of the affected mucosa of the turbinates suggests necrosis due to angio-invasion and has been described as the black-turbinate sign. Obliteration of periantral fat is a subtle sign and must be diligently evaluated in patients at risk for AIFRS. Changes within the orbital fat and extraocular muscles with proptosis indicate intra-orbital invasion. Intracranial extension mostly from the sphenoid sinus [46] is seen as subtle leptomeningeal enhancement which an expert radiologist may be able to note. As invasion progresses cerebritis, cerebral abscess and granulomas may be noted. Intracranial granulomas would appear hypointense on T1/T2-weighted images with minimal enhancement on contrast CT.
Diagnosis
Rapid diagnosis and prompt treatment may save at least some of these patients. When fever with neutropenia and sinonasal symptoms are seen in patients with impaired immune function maintaining a high index of suspicion is essential, and the appropriate diagnostic work up should involve imaging studies and nasal endoscopy with a possible biopsy so as to initiate treatment in a timely manner.
On nasal endoscopy mucosa would appear pale with ulceration and tissue necrosis depending on severity. Commonly affected area is painless. Definitive diagnosis is made with microscopic identification of biopsy from suspicious tissue and culture of the tissue. The middle turbinate is the most commonly affected, hence is a high-yield target site for nasal biopsy [46].
Histopathologic evidence of invasive fungi in the mucosa, submucosa, vascular structures, bone along with necrotic tissue, infarction and inflammatory infiltrates is not difficult to identify. Simple Toluidine blue stain or H&E staining can help identify fungi. The most commonly isolated Mucor species in AIFRS are identified by their broad ribbon-like aseptate hyphae that branch at 90 degree and fold on themselves. The Aspergillus species on the other hand demonstrate acute branching patterns with narrow septate hyphae. Cultures in AİFRS have been reported to be positive in over 70% cases. There has also been an increasing trend of mucormycosis in immunocompetent individuals by Apophysomyces elegans [54].
Diagnosis-based exclusively on histopathology is not reliable and species identification condition with the use of rapid in situ hybridisation for rRNA targets may be a useful method to identify fungal species [55].
Treatment
Prompt and aggressive surgical debridement, appropriate antifungal therapy along with simultaneous elimination of underlying causes of immunosuppression and risk factors are the mainstays of AIFRS management [56, 57].
Surgery
Prompt surgical intervention is both for diagnostic biopsy and therapeutic debridement. Wide surgical debridement aimed at complete elimination of necrotic tissue with edges up to healthy bleeding tissue is necessary. Timely but difficult surgical decisions with disfiguring outcomes like maxillectomy and orbital exenteration can be lifesaving. Disease with intracranial extension can rarely be treated with direct surgical resection as prognosis is notoriously poor [46]. Intraoperative frozen section examination may provide prompt diagnosis and improve prognosis exponentially [18]. The histopathologist involved in such cases needs to be alerted by the clinician to the possibility of acute invasive fungal infection so that when frozen sections are not available then routine processing can be carried out rapidly and results promptly communicated to the treating physician without delay.
Medical Therapy
Early initiation of antifungal therapy at the very first likelihood of acute invasive fungal pathology can improve mortality rates [18]. Intravenous high-dose amphotericin B deoxycholate (dosage: 1–1.5 mg/kg daily) was widely administered but lately liposomal amphoterecin B with better tolerance and lower nephrotoxicity (dosage: 5-10 mg/kg/day) has become the empiric drug of choice in management of AIFRS generally and mucormycosis specifically. Amphoterecin B shows activity against Mucoraeles as well as many Aspergillus species but high drug costs with liposomal amphotericin B remains the biggest obstacle in its prolonged use. FDA approved promising newer azole, Posaconazole is the secondary drug of choice in Mucormycosis (also effective against Aspergillus species) as it can be taken orally (dosage: 800 mg/day in 4 divided doses) with low incidence of side effects and is hence excellent for prolonged outpatient regimes.
Caspofungin, the first member of echinocandin, a novel antifungal drug has shown no activity against mucormycosis in isolation but has synergistic activity with amphotericin B. Topical amphotericin B irrigations in the post-surgical sinonasal cavity has been recommended by some authors but dosage and efficacy remain unconfirmed.
When histopathology or culture results confirm Aspergillus, azoles like Voriconazole are the optimum drug choice. Itraconazole has also been used as alternative therapy.
The variable pharmacokinetics of triazoles including erratic absorption (itraconazole and posaconazole), drug interactions, pharmacogenetic differences(voriconazole) warrant plasma drug monitoring for optimizing the safety and efficacy. Voriconazole is extensively metabolised in the liver by enzyme CYP2C19 and 12–14% of Indians have CYP2 C19 polymorphisms which can affect optimum drug plasma levels. The dosage of Voriconazole is usually: 6 mg/kg IV 12hrly on the first day, followed by 4 mg/kg 12hrly for 7 days which may then be reduced to 200 mg oral dose twice daily.
Hyperbaric Oxygen Therapy
It has a direct fungicidal effect and has been reported as an effective adjunct in some comprehensive regimes especially in diabetic ketoacidosis-induced AIFRS.
Reversal of Risk Factors
The reversal/reduction in underlying causative immunosuppression state is of vital importance. In critically ill patients, this may not be practically achievable though and the clinician can only strive to optimize the patient condition. Controlling of diabetic ketoacidosis, iron overload states, neutropenia, discontinuation of steroid therapy all aim at normalizing immune status.
Diabetic patients benefit from reversal of diabetic ketoacidosis by aggressive insulin regimens and fluid administration.
Iron metabolism has been shown to have a central role in the pathogenesis of mucormycosis, hence correction of iron overload states and effective utilization of iron chelators like deferasirox are used to limit iron availability to the fungi preventing its proliferation.
Neutrophils play a pivotal role in antifungal immunity and neutropenic patients need to achieve near normal neutrophil counts for survival. Adjunctive therapies such as granulocyte infusion or administration of granulocyte–macrophage-colony-stimulating factor (g-csf, gm-csf) are promising. Role of IFN- γ as an adjunct to augment phagocytic function has been demonstrated in reports but needs further validation [15].
Prognosis for AIFRS remains consistently poor and restoration of neutrophil counts has been reported as the most predictive indicator of survival, while intracranial spread has the highest predictive indicator for mortality.
| Key points for Acute invasive fungal rhinosinusitis (AIFRS) |
|---|
| • Fungi of the order Mucorales and Aspergillus |
| • Rapid onset and progression |
| • Predominant in immunocompromised states with neutropenia but also seen in immune-competent |
| • Diabetes, hematologic malignancies, steroid therapy, immunosuppressive therapy, organ transplants, iron overloads are risk factors |
| • Sparse fungal elements in mucosa, submucosa, blood vessels, bone, with extensive tissue necrosis and neutrophilic inflammation |
| • Debridement, systemic antifungals, management of underlying metabolic conditions and immune suppression mainstay of treatment |
| • High rate of morbidity and mortality |
II. Chronic invasive fungal rhinosinusitis (CIFRS) [aka non-granulomatous chronic invasive fungal rhinosinusitis]
Chronic invasive fungal infection is a reportedly rare disease which can have an insidious progression over several months to years (> 12 weeks) [37]. The condition is characterized by tissue invasion and necrosis but minimal inflammatory reaction. There may be dense accumulation of fungal hyphae resembling mycetoma. It is commonly seen in patients with diabetes mellitus and corticosteroid therapy.
The disease is often associated with orbital apex syndrome in which there is extension into posterior aspect of the orbit with decreasing vision/blindness. Extension into the cranium can be fatal. [22, 37]
While some authors opine that this form has no specific geographic predilection others state that it is uncommon in the United states and is reported more from India and the Middle east [57, 58].
The most commonly reported fungal pathogen is Aspergillus fumigatus [10, 31]. CIFRS though mostly seen in patients with mild forms of immunosuppression may occur in immunocompetent individuals.
Clinical Presentation
Patients may present with a complaint of unilateral facial pain and swelling [18]. History of chronic sinusitis with nasal polyposis and complaints of nasal discharge and epistaxis are common. Symptoms may include vague sinus pain, anosmia, sero sanguinous nasal discharge, epistaxis and fever. Intracranial disease extension can present with headache followed by seizures, cranial nerve palsies, neurologic deficits [14]. Persistent oral antral fistula may be seen with exposed or necrotic bone in the maxilla and generalized mobility of maxillary teeth on the side of the affected sinus. Orbital involvement is a characteristic association signaled by proptosis, blurring vision, diplopia and ocular immobility [22].
The disease can often mimic malignant lesions. CIFRS shows slow progression but has a persistent and relapsing course.
Imaging Features
CIFRS commonly presents with hyperattenuating soft tissue collection on non-contrast CT with common involvement of one or more adjacent paranasal sinuses [22]. Involvement of ethmoid and sphenoid is common. MRI demonstrates T1 intermediate signal with low to very low T2 signal.
Due to the invasive nature of the disease presenting with sinus wall destruction and mass like presentation it can be mistaken for malignancy. However, more involvement is seen outside the sinus than within and bone erosion is usually localized to sites of extra-sinus extension [14].Orbital and intracranial involvement is common.
Diagnosis
Due to the indolent course of the disease diagnosis is often delayed. Chronic invasive fungal sinusitis is distinguished from the other two forms of invasive fungal sinusitis by its chronic course, dense accumulation of fungal hyphae resembling a fungal ball, and association with the orbital apex syndrome, diabetes mellitus, and corticosteroid treatment [8]. Endoscopic sinus evaluation can be a useful procedure to obtain biopsy tissue for culture and examine extent of sinus involvement [18]. Biopsy and orbital exploration show vascular invasion by fungal elements and minimal inflammatory infiltrate. Aspergillus fumigatus are the most commonly isolated species.
Treatment
The optimal treatment of CIFRS is highly debated with no consensus or treatment guidelines. Sinus debridement and aeration are the most important.
Surgery
Complete surgical excavation is quintessential for good prognosis. Partial/incomplete, staged or repeated debridement combined with antifungal regimes has been associated with higher failure rates as compared to radical evacuation [59]. However, extension through the sinuses is often so extensive that complete surgical removal is not amenable and medical therapy may help reduce fungal load and even be curative.
Medical Therapy
Systemic antifungal treatment is dictated by the etiologic agent responsible for disease similar to as described in AIFRS. Aspergillus species being the most common pathogens, initial therapy with Amphotericin B (dosage and forms same as AIFRS) has been suggested. High rates of relapse and inability to achieve complete infected tissue removal are indications for long-term suppressive therapy in most patients [22, 60]. Itraconazole has been used for long-term suppression at dosage of 200 mg twice daily and has been reported to reduce relapse [61].
The duration of antifungal therapy has not been well established [56]. The most effective antifungal agent and optimal duration of therapy have also not been determined even though newer azoles like Voriconazole and Posaconazole have seemed to be beneficial and are becoming drugs of choice. However, high drug costs continue to deter treatment affordability.
| Key points for CIFRS |
|---|
| • Aspergillus fumigatus is the most common pathogen |
| • No specific geographic location |
| • Immunocompetent/Mildly Immunocompromised |
| • Associated with diabetes mellitus, corticosteroids and orbital apex syndrome |
| • Dense accumulation of fungal elements forming an expansile mass |
| • Minimal inflammatory response |
| • Rhinosinusitis (often unilateral) nasal obstruction, nasal discharge |
| • Debridement, aeration mainstay of treatment |
| • Antifungals role and treatment regime not clear |
| • High rates of recurrence though prognosis good |
III. Chronic granulomatous invasive fungal rhinosinusitis (CGIFRS) [aka indolent fungal sinus rhinosinusitis/Primary paranasal granuloma]
This subtype of invasive FRS is characterized by a time course of > 12 weeks and is based on characteristic histopathology findings [7]. It is most commonly reported from countries like India, Pakistan, Saudi Arabia and Sudan though cases from the USA are also reported. [8] It was first described by Milosev et al. [62] as primary aspergilloma of paranasal sinuses.
This variant is typically seen in patients with intact cell-mediated immunity(CMI) which is essential for granuloma formation. [15] Hence it is not reported in patients with diabetes mellitus. Patients are mostly immunocompetent and Aspergillus flavus is the most isolated pathogen. Relapse rates are high.
Clinical Presentation
The clinical distinctions between the two types of chronic invasive forms are not clear as both have similar chronic presentations with orbital involvement and prognosis also is similar.
Disease course is usually indolent with most common presentation of enlarging cheek mass with involvement of orbit, nose and paranasal sinuses in an immunocompetent patient [8].
Eventual extra-sinus invasion is seen, and the patient may present with diplopia, proptosis due to the mass lesion extending into the orbit [14]. Intracranial extension may also occur rarely.
Endoscopically appears as firm to hard mass which is relatively avascular.
Imaging Features
Differentiating forms of invasive fungal rhinosinusitis based on imaging modalities alone is not possible as the imaging features are nonspecific and similar. Opacification of the involved sinuses and extra-sinus invasion with extension to adjacent soft tissues is seen. Sinus expansion is rare, and involvement of orbits and intracranial compartment may be noted in advanced cases.
Findings are analogous to those in malignant lesions.
Diagnosis
Patients usually have no predisposing factors. The diagnosis is based on histopathology only. Microscopic examination shows characteristic presence of noncaseating granulomas. Extensive granulomatous response with fungal hyphae within Langerhans-type giant cells and considerable fibrosis are common [8, 11].
This unique pathological picture is the main difference between granulomatous invasive fungal disease from the more common CIFRS seen in diabetic patients [14]. CGIFRS is considered by few authors to be part of CIFRS and not a distinct clinical entity [60]. Similar to CIFRS distinguishing CGIFRS from a malignant lesion may not be possible.
Treatment
Timely surgical intervention with complete resection of pathologic tissue is the mainstay of treatment like in the other invasive forms as any delay can worsen prognosis. However, controversy prevails regarding the role of antifungal drugs. Some authors suggest that surgery alone is sufficient while others cite a need for antifungal medication therapy [15, 63].
Regime of Amphotericin B for 6 weeks followed by Itraconazole has been recommended [60]. A decrease in high recurrence rates has been reported with the use of Itraconazole (dosage of 8–10 mg/Kilogram/day). However, the optimum duration of treatment is debatable.
| Key points for Chronic Granulomatous Invasive Fungal Rhinosinusitis (CGIFRS) |
|---|
| • Commonly seen in immunocompetent hosts |
| • Aspergillus flavus almost exclusive pathogen |
| • Predominates in India, Pakistan, Sudan |
| • Presence of noncaseating granulomas with fungal hyphae within Langerhans-type giant cells |
| • Dense fibrosis and minimal inflammatory reaction |
| • Surgery mainstay with radical debridement to histopathologically normal tissue |
| • Role of Antifungal therapy and treatment durations are controversial |
| • Prognosis is fair when limited to sinus; poor with extra-sinus invasion; relapse common. |
Conclusion
Over the last few decades, our understanding of disease caused by fungi in the nose and sinuses has improved exponentially. This may primarily be attributed to better and more systematic reporting of cases, advances in imaging techniques and the use of endoscopy in sinus surgeries.
Differentiating between the variants of fungal sinusitis and identifying the aggressive fulminant type are crucial to the management of patients of fungal sinusitis. Figure 4 (i–iii) shows some of the various clinico-radiologic presentations of FRS. Figure 5 (a–n) presents the management of a case of FRS in an immunocompetent patient. Early diagnosis is the crux of successful management and clinicians often fail in this. Increased incidence rates demand better understanding of disease. The biggest therapeutic dilemma faced in the management of FRS is judging when to stop the antifungal drug therapy. There is no fixed regime stating a defined treatment duration and the high drug costs are a big financial burden. There are no clinical markers or investigations the patient may be subjected to so as to assess if the disease is regressing or progressing. The effectiveness of therapy can only be judged by a thorough clinico-radiological assessment. Clinical features like improvement in teeth mobility, bone necrosis indicate that the patient is responding to therapy. Serial radiological assessment to note if there is any progression of necrosis can also help. We require intensive research to improve our understanding of immune-pathogenesis, risk factors, geographical and occupational predispositions and need to develop better diagnostic methods like PCR and biomarkers. Till then the disease will continue to be elusive.
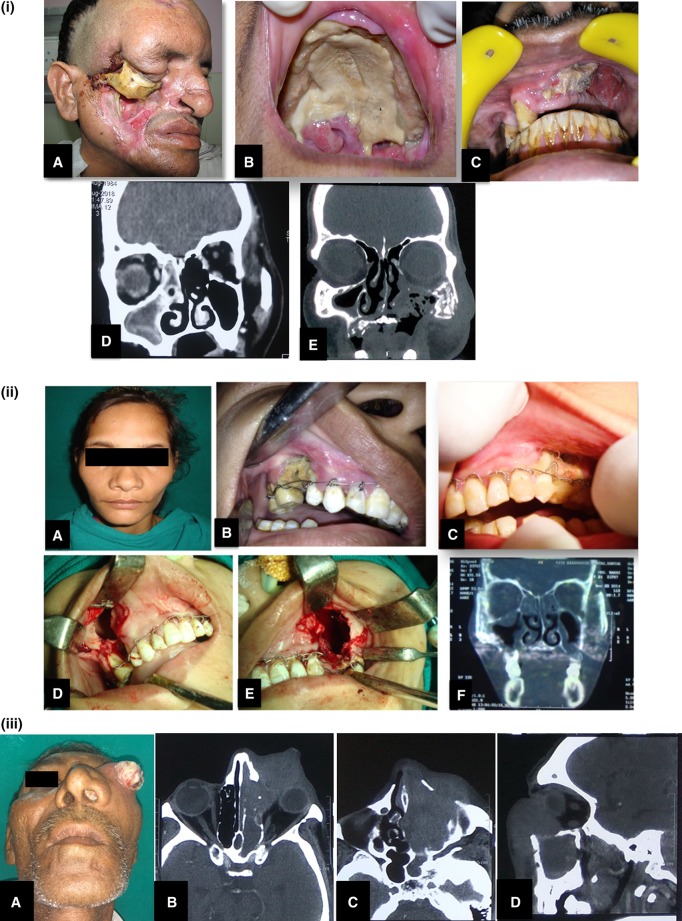
Fig. 4.
Clinico-radiologic presentations of FRS. (i) A Extensive cheek necrosis, B palatal necrosis, C exposed necrotic bone on intraoral examination, D CT scan of a patient diagnosed with Allergic FRS. Coronal section showing hyperdensity of mucin within right ethmoid and maxillary sinuses, E coronal CT image showing mixed lytic sclerotic changes with bony destruction involving left zygoma and walls of left maxillary sinus. Also there is soft tissue attenuation with air loculi. (ii) A A patient at clinical presentation with very mild diffuse bilateral swelling, B, C intraoral pictures showing exposed necrotic bone and generalized mobility of maxillary teeth stabilized with Essig’s wiring, D, E intraoperative pictures for sequestrectomy and curettage, F CT scan image shows mucosal thickening of the maxillary sinuses with erosion of bone. (iii) A A clinical presentation of case of CIFRS with extensive orbital involvement mimicking malignancy, B, C, D radiographic picture of bone erosion localized to the area of extra-sinus component of the disease more extensive than intra-sinus component
Fig. 5.
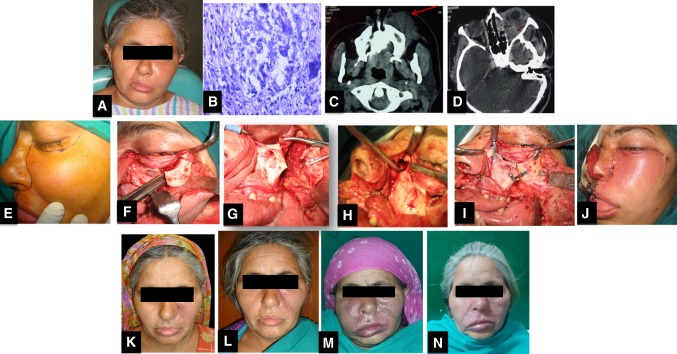
Photographic series of management of a patient with FRS. A preoperative clinical presentation of immunocompetent patient showing diffuse swelling on left side of face and orbital involvement. B Histopathology showing PAS-positive wall of a negatively stained section showing fungal hyphae(× 400), C Axial CT scan shows diffusely enhancing mass (arrow) involving the left maxillary sinus and the subcutaneous plane of the left cheek and upper lip, D Axial CT scan showing left eye proptosis caused by a mass involving the temporal fossa and orbit extending to involve the ethmoid air cells with bone erosion(arrows), E Weber- Fergusson incision, F Zygomatic swing osteotomy to get an access to orbital floor, G Zygoma swung laterally, H Surgical debridement extending to ethmoid sinus, I Realignment, J Closure, K 2 months post operative picture after a course of Amphotericin B, L 1 year postoperative patient had vision loss and was given Voriconazole after which disease no longer progressed M 5 years post treatment patient had another relapse, N Patient responded well to a course of Voriconazole again
Acknowledgements
I am extremely thankful to Dr Neha Jajodia, Senior Resident, Department of Oral and Maxillofacial Surgery, PGIDS Rohtak for her significantly critical and insightful contribution in compilation and formulation of this review article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hawksworth DL. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res. 2001;105:1422–1432. doi: 10.1017/S0953756201004725. [DOI] [Google Scholar]
- 2.Blackwell M. The fungi: 1, 2, 3… 5.1 million species? Am J Bot. 2011;98:426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- 3.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–998. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti A, Sethuraman N. Introduction to medical mycology. In: Mora-Montes H, Lopes-Bezerra L, editors. Current progress in medical mycology. Cham: Springer; 2017. [Google Scholar]
- 5.Chakrabarti A, Chatterjee SS, Shivaprakash MR. Overview of opportunistic fungal infections in India. J Med Mycol. 2008;49:165–172. doi: 10.3314/jjmm.49.165. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson BJ. Mucormycosis of the nose and paranasal sinuses. Otolaryngol Clin North Am. 2000;33:349–365. doi: 10.1016/S0030-6665(00)80010-9. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti A, Denning DW, Ferguson BJ, et al. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. The Laryngoscope. 2009;119(9):1809–1818. doi: 10.1002/lary.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarti A, Das A, Panda NK. Controversies surrounding the categorization of fungal sinusitis. Med Mycol. 2009;47(Suppl. 1):S299–S308. doi: 10.1080/13693780802213357. [DOI] [PubMed] [Google Scholar]
- 9.Lass-Flörl C. Current challenges in the diagnosis of fungal infections. In: Lion T, editor. Human fungal pathogen identification. Methods in molecular biology. New York, NY: Humana Press; 2017. [DOI] [PubMed] [Google Scholar]
- 10.deShazo RD, O’Brien M, Chapin K, et al. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1997;123:1181–1188. doi: 10.1001/archotol.1997.01900110031005. [DOI] [PubMed] [Google Scholar]
- 11.Das A, Bal A, Chakarabarti A, Panda N, Joshi K. Spectrum of fungal rhinosinusitis; a histopathologist’s perspective. Histopathology. 2009;54:854–859. doi: 10.1111/j.1365-2559.2009.03309.x. [DOI] [PubMed] [Google Scholar]
- 12.Schell WA. Histopathology of fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:251–276. doi: 10.1016/S0030-6665(00)80004-3. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande RB. Histopathology of invasive fungal rhinosinusitis. In: Mankekar G, editor. Invasive fungal rhinosinusitis. New Delhi: Springer; 2014. [Google Scholar]
- 14.Ni Mhurchu E, Ospina J, Janjua AS, Shewchuk JR, Vertinsky AT. Fungal rhinosinusitis: a radiological review with intraoperative correlation. Can Assoc J. 2017;68:178–186. doi: 10.1016/j.carj.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Thrasher RD, Kingdom TT. Fungal infections of the head and neck: an update. Otolaryngol Clin North Am. 2003;36:577–594. doi: 10.1016/S0030-6665(03)00029-X. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson BJ. Eosinophilicmucinrhinosinusitis: a distinct clinicopathological entity. Laryngoscope. 2000;110:799–813. doi: 10.1097/00005537-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Barac A, Stevanovic G, Pekmezovic M, Rakocevic Z, Stosovic R, Erovic B, et al. Study toward resolving the controversy over the definition of allergic fungal rhinosinusitis. Med Mycol. 2018;56:162–171. doi: 10.1093/mmy/myx032. [DOI] [PubMed] [Google Scholar]
- 18.Malani PN, Kauffman CA. Invasive and allergic fungal sinusitis. Curr Infect Dis Rep. 2002;4:225–232. doi: 10.1007/s11908-002-0083-2. [DOI] [PubMed] [Google Scholar]
- 19.Chakrabarti A, Rudramurthy SM, Panda N, Das A, Singh A. Epidemiology of chronic fungal rhinosinusitis in rural India. Mycoses. 2015;58:294–302. doi: 10.1111/myc.12314. [DOI] [PubMed] [Google Scholar]
- 20.Gorovoy IR, Kazanjian M, Kersten RC, Kim HJ, Vagefi MR. Fungal rhinosinusitis and imaging modalities. Saudi J Ophthalmol. 2012;26:419–426. doi: 10.1016/j.sjopt.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherji SK, Figueroa RE, Ginsberg LE, et al. Allergic fungal sinusitis: CT findings. Radiology. 1998;207(2):417–422. doi: 10.1148/radiology.207.2.9577490. [DOI] [PubMed] [Google Scholar]
- 22.Aribandi M, McCoy VA, Bazan C., III Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics. 2007;27(5):1283–1296. doi: 10.1148/rg.275065189. [DOI] [PubMed] [Google Scholar]
- 23.Bent JP, Kuhn F. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111:580–588. doi: 10.1177/019459989411100508. [DOI] [PubMed] [Google Scholar]
- 24.deShazo RD, Swain R. Diagnostic criteria for allergic fungal sinusitis. J Allergy Clin Immunol. 1995;96(1):24–35. doi: 10.1016/S0091-6749(95)70029-3. [DOI] [PubMed] [Google Scholar]
- 25.Ou J, Wang J, Xu Y, et al. Staphylococcus aureussuperantigens are associated with chronic rhinosinusitis with nasal polyps: a meta-analysis. Eur Arch Otorhinolaryngol. 2014;271:2729–2736. doi: 10.1007/s00405-014-2955-0. [DOI] [PubMed] [Google Scholar]
- 26.London NR, Reh DD. Differential diagnosis of chronic rhinosinusitis with nasal polyps. Adv Otorhinolaryngol. 2016;79:1–12. doi: 10.1159/000444957. [DOI] [PubMed] [Google Scholar]
- 27.Marple B. Allergic fungal rhinosinusitis: current theories and management strategies. Laryngoscope. 2001;111(6):1006–1019. doi: 10.1097/00005537-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Jen A, Kacker A, Huang C, Anand V. Fluconazole nasal spray in the treatment of allergic fungal sinusitis: a pilot study. Ear Nose Throat J. 2004;83:692–695. [PubMed] [Google Scholar]
- 29.Ricchetti A, Landis BN, Maffioli A, et al. Effect of anti-fungal nasal lavage with amphotericin B on nasal polyposis. J Laryngol Otol. 2002;116:261–263. doi: 10.1258/0022215021910708. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson BJ. What role do systemic corticosteroids, immunotherapy, and antifungal drugs play in the therapy of allergic fungal rhinosinusitis? Arch Otolaryngol Head Neck Surg. 1998;124(10):1174–1178. doi: 10.1001/archotol.124.10.1174. [DOI] [PubMed] [Google Scholar]
- 31.deShazo R, Chapin K, Swain R. Fungal sinusitis. N Engl J Med. 1997;337(4):254–259. doi: 10.1056/NEJM199707243370407. [DOI] [PubMed] [Google Scholar]
- 32.Kuhn FA, Javer AR. Allergic fungal rhinosinusitis: perioperative management, prevention of recurrence, and role of steroids and antifungal agents. Otolaryngol Clin North Am. 2000;33:2. doi: 10.1016/S0030-6665(00)80016-X. [DOI] [PubMed] [Google Scholar]
- 33.Kupferberg SB, Bent JP. Allergic fungal sinusitis in the pediatric population. Arch Otolaryngol Head Neck Surg. 1996;122:1381–1384. doi: 10.1001/archotol.1996.01890240087019. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson BJ. Definitions of fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;2:227–235. doi: 10.1016/S0030-6665(00)80002-X. [DOI] [PubMed] [Google Scholar]
- 35.Grosjean P, Weber R. Fungus balls of the paranasal sinuses: a review. Eur Arch Otorhinolaryngol. 2007;264:461–470. doi: 10.1007/s00405-007-0281-5. [DOI] [PubMed] [Google Scholar]
- 36.Gungor A, Adusumilli V, Corey JP. Fungal sinusitis: progression of disease in immunosuppression. Ear Nose Throat J. 1998;77:207–215. [PubMed] [Google Scholar]
- 37.deShazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Swain R, Lyons M, Bryars WC, Alsip S. Criteria for the diagnosis of sinus mycetoma. J Allergy ClinImmunol. 1997;99:475–485. doi: 10.1016/S0091-6749(97)70073-3. [DOI] [PubMed] [Google Scholar]
- 38.Cojocari L, Sandul A. Literature review. Noninvasive fungal rhinosinusitis. Romanian J Rhinol. 2017;7(26):75–81. doi: 10.1515/rjr-2017-0008. [DOI] [Google Scholar]
- 39.Mensi M, Salgarello S, Pinsi G, Piccioni M. Mycetoma of the maxillary sinus: endodontic and microbiological correlations. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2004;98:119–123. doi: 10.1016/j.tripleo.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 40.Nicolai P, Lombardi D, Tomenzoli D, et al. Fungus ball of the paranasal sinuses: experience in 160 patients treated with endoscopic surgery. Laryngoscope. 2009;119(11):2275–2279. doi: 10.1002/lary.20578. [DOI] [PubMed] [Google Scholar]
- 41.Lee TJ, Huang SF, Chang PH. Characteristics of isolated sphenoid sinus aspergilloma: report of twelve cases and literature review. Ann Otol Rhinol Laryngol. 2009;118:211–217. doi: 10.1177/000348940911800309. [DOI] [PubMed] [Google Scholar]
- 42.Gupta S, Maheshwari S. Radiology in invasive fungal sinusitis. In: Mankekar G, editor. Invasive fungal rhinosinusitis. New Delhi: Springer; 2014. [Google Scholar]
- 43.Ferguson BJ. Fungus balls of the paranasal sinuses. Otolaryngol Clin North Am. 2000;33:389–398. doi: 10.1016/S0030-6665(00)80013-4. [DOI] [PubMed] [Google Scholar]
- 44.Raz E, Win W, Hagiwara M, Lui Y, Cohen B, Fatterpekar G. Fungal sinusitis. Neuroimaging Clin N Am. 2015;25(4):569–576. doi: 10.1016/j.nic.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Chakrabarti A, Das A, Panda NK. Overview of fungal rhinosinusitis. Indian J Otolaryngol Head Neck Surg. 2004;56(4):251–258. doi: 10.1007/BF02974381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soler Z, Schlosser R. The role of fungi in diseases of the nose and sinuses. Am J Rhinol Allergy. 2012;26(5):351–358. doi: 10.2500/ajra.2012.26.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waitzman AA, Birt BD. Fungal sinusitis. J Otolaryngol. 1994;23(4):244–249. [PubMed] [Google Scholar]
- 48.Mankekar G, Chavan K. Clinical features and diagnosis. In: Mankekar G, editor. Invasive fungal rhinosinusitis. New Delhi: Springer; 2014. [Google Scholar]
- 49.Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D’Souza O. Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J Ophthalmol. 2003;51:231–236. [PubMed] [Google Scholar]
- 50.Gillespie MB, O’Malley BW, Jr, Francis HW. An approach to fulminant invasive fungal rhinosinusitis in immunocompromised host. Arch Otolaryngol Head Neck Surg. 1998;124(5):520–526. doi: 10.1001/archotol.124.5.520. [DOI] [PubMed] [Google Scholar]
- 51.DelGaudio JM, Swain RE, Jr, Kindgdom TT, et al. Computed tomography findings in patients with invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 2003;129:236–240. doi: 10.1001/archotol.129.2.236. [DOI] [PubMed] [Google Scholar]
- 52.Groppo ER, El-Sayed IH, Aiken AH, et al. Computed tomography and magnetic resonance imaging characteristics of acute invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 2011;137(10):1005–1010. doi: 10.1001/archoto.2011.170. [DOI] [PubMed] [Google Scholar]
- 53.Raz E, Win W, Hagiwara M, Lui YW, Cohen B, Fatterpekar GM. Fungal sinusitis. Neuroimaging Clin N Am. 2015;25:569–76. doi: 10.1016/j.nic.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Sridhara SR, Paragache G, Panda NK, Chakrabarti A. Mucormycosis in immunocompetent individuals: an increasing trend. J Otolaryngol. 2005;34(6):402–406. doi: 10.2310/7070.2005.34607. [DOI] [PubMed] [Google Scholar]
- 55.Wueppenhorst N, Lee M-K, Rapplod E, Kayser G, Beckervordersandforth J, de With K, Serr A. Rhino-orbito-cerebral zygomycosis caused by Conidiobolus incongruous in an immunocompromised patient in Germany. J Clin Microbiol. 2010;48(11):4322–4325. doi: 10.1128/JCM.01188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.deShazo RD. Syndromes of invasive fungal sinusitis. Med Mycol. 2009;47(Suppl I):s309–s314. doi: 10.1080/13693780802213399. [DOI] [PubMed] [Google Scholar]
- 57.Soman R, Sunavala A. Management of invasive fungal sinusitis. In: Mankekar G, editor. Invasive fungal rhinosinusitis. New Delhi: Springer; 2014. [Google Scholar]
- 58.Montone KT. Pathology of fungal rhinosinusitis: a review. Head Neck Pathol. 2016;10(1):40–46. doi: 10.1007/s12105-016-0690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clancy CJ, Nguyen MH. Invasive sinus aspergillosis in apparently immunocompetent hosts. J Infect. 1998;37:229–240. doi: 10.1016/S0163-4453(98)91921-1. [DOI] [PubMed] [Google Scholar]
- 60.Stringer SP, Ryan MW. Chronic invasive fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:375–387. doi: 10.1016/S0030-6665(00)80012-2. [DOI] [PubMed] [Google Scholar]
- 61.Gumaa SA, Mahgoub ES, Hay RJ. Post-operative responses of paranasal Aspergillus granuloma to itraconazole. Trans R Soc Trop Med Hyg. 1992;86:93–94. doi: 10.1016/0035-9203(92)90460-T. [DOI] [PubMed] [Google Scholar]
- 62.Milosev B, el-Mahgoub S, Aal OA, el-Hassan AM. Primary aspergilloma of paranasal sinuses in Sudan. A review of seventeen cases. Br J Surg. 1969;56:132–137. doi: 10.1002/bjs.1800560213. [DOI] [PubMed] [Google Scholar]
- 63.Schubert MS. Medical treatment of allergic fungal sinusitis. Ann Allergy Asthma Immunol. 2000;85:90–97. doi: 10.1016/S1081-1206(10)62445-3. [DOI] [PubMed] [Google Scholar]