Abstract
Triple-negative breast cancer (TNBC) is the most aggressive and prevalent subtype of breast cancer in women worldwide. Currently, chemotherapy remains the main modality for the treatment at an early stage, as there is no approved targeted therapy for early TNBC. In this review, we investigate the use of microRNAs (miRNAs), which play a key role in the post-transcriptional regulation of genes involved in the key biological processes, namely proliferation, differentiation, angiogenesis, migration, apoptosis, and carcinogenesis. Here, we emphasize the importance of the recent advances related to miRNAs, involving diagnosis, prognosis, and treatment of TNBC. We focus on the development, optimization, and stabilization of miRNA-based drugs; improvement of miRNA delivery; and control of the off-target effects of miRNA therapeutics. We speculate as to which features may present themselves as promising approaches in the treatment of TNBC.
Keywords: TNBC, miRNA, Cancer biomarkers, miRNA-based therapy
Introduction
Breast cancer accounts for 30% of cancers diagnosed in women worldwide. It comprises many biological entities with distinct pathological features and clinical implications. Breast cancer with different histopathological and biological features exhibits different responses to divergent therapeutic strategies. The common forms of breast cancer are (1) ductal carcinoma in situ (DCIS), a non-invasive breast cancer subtype which is limited to milk ducts and is characterized by development of lumps in the breast and secretion of a discharge from the nipple; (2) invasive ductal carcinoma (IDC) or infiltrating ductal carcinoma, which is characterized by invasion of lymph nodes and surrounding tissues, breast inflammation, and secretion of a discharge (Dai et al. 2016); (3) other subtypes of invasive breast cancers, most commonly with BRAC1 mutations, are medullary carcinoma, mucinous carcinoma, papillary carcinomas, and cribriform carcinoma (Dent et al. 2007).
Based on histological features, breast cancer can also be classified as (1) hormone receptor-positive (ER+, PR+), which accounts for 60–70% of cases; (2) HER2+ tumors, which cover 15–20%; and (3) triple-negative (ER−, PR−, HER2−) tumors, which account for about 10–25%. Breast cancer can be also classified using genomic miRNA profiling as luminal A (ER+ and low grade), luminal B (ER+ and high grade), HER2+, and basal-like (mainly triple negative). Table 1 summarizes the subtypes of breast cancer.
Table 1.
Subtypes of breast cancer
| Subtypes of breast cancer | Characteristic features |
|---|---|
| Ductal carcinoma in situ (DCIS) | Non-invasive, limited to milk ducts, development of lump in the breast and secretion of discharge. |
| Invasive ductal carcinoma (IDC) | Invasive, breast inflammation and secretion of discharge. |
| Medullary carcinoma | Invasive, breast inflammation and secretion of discharge. |
| Luminal A | Estrogen-receptor and/or progesterone-receptor positive, HER2 negative, and has low levels of the protein Ki-67, which helps to control cell growth. Low-grade, tend to grow slowly and have the best prognosis. |
| Luminal B | Estrogen-receptor and/or progesterone-receptor positive), and either HER2 positive or HER2 negative with high levels of Ki-67. Grow slightly faster than luminal A with poor prognosis. |
| HER2+ | Estrogen-receptor and progesterone-receptor negative and HER2 positive. Grow faster with poor prognosis. |
| Basal like | Triple-negative with estrogen/ progesterone and HER2 negative. Most prevalent with BRCA1 gene mutations. |
In what follows, we review the role played by miRNA in triple-negative breast cancer using a descriptive approach which, although not couched in terms of mathematics and physical laws typical of other articles in the Biophysical Reviews journal, will hopefully be useful to the biophysical community due to the fact that it presents components and their regulatory role in list, function, and network fashion. It is our sincere wish that the information presented in this review will both spark an interest and prove digestible/useful, to those performing biophysical modeling of breast cancer development that is inclusive of miRNA involvement.
Triple-negative breast cancer
TNBC, with significant clinical implications, overlaps “basal-like” breast cancer. It is the most prevalent and highly aggressive subtype of breast cancer among young patients (< 50 years old) and is characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) genes. Lack of these receptors makes TNBC aggressive and unresponsive to hormonal and targeted therapies. Moreover, TNBC is highly metastatic and can recur within the 3 years of treatment. Distant recurrence is seen in the brain, liver, and lungs, and less frequently in bone. In addition, only 77% of TNBC patients survive compared to the other subtypes of breast cancer, which account for 93% of survivors in the first 5 years (Dent et al. 2007).
Currently, chemotherapy remains the main modality for the treatment of TNBC at an early stage due to a lack of an approved, targeted therapy. However, various approaches are being implemented as target-based therapies for early stage TNBC, which include DNA-damage agents, immune-checkpoint inhibitors, platinum-based compounds, PI3K-pathway inhibitors, and androgen-receptor inhibitors. Due to its high aggressiveness, TNBC patients require multiple targeted drugs at different stages for acceptable outcomes to be achieved. Combinational regimens maximize clinical efficacy by minimizing the toxicity of treatment at an early stage of TNBC and thus improve the survival rate of patients approximately by 70% (Kumari et al. 2017).
Generally, diagnoses of TNBCs are based on histological grade, lymph node involvement, and ER, PR, and HER2 status (Pustylnikov et al. 2018). TNBC can also be screened and diagnosed by mammography, ultrasonography, and magnetic resonance imaging (MRI), but they cannot differentiate the central pattern of necrosis and fibrosis, which are characteristic features of TNBC (Friedman et al. 2009). Clinical diagnosis of TNBC is performed by immunohistochemistry (Le Bourgeois et al. 2018). Furthermore, the heterogeneous nature of TNBC necessitates the use of novel diagnostic approaches along with conventional methods. To improve the survival rate of TNBC patients, a greater knowledge of target pathways, development of novel drugs, and identification of biomarkers are required to facilitate development of improved treatment modalities. Based on these requirements, we believe that miRNA-based therapeutic strategies may constitute a promising approach to the treatment of TNBC and we focus the rest of the review on this aspect of the breast cancer literature.
MicroRNA
miRNAs are an evolutionarily conserved family of small non-coding RNAs that regulate a number of biological processes including proliferation, differentiation, angiogenesis, migration, apoptosis, and carcinogenesis (Ahmad et al. 2013). These single-stranded RNAs of 18–22 nucleotides were reported for the first time in Caenorhabditis elegans and later in most eukaryotes, including humans (Tang et al. 2018). Novel high-throughput sequencing techniques have identified more than 28,000 mature miRNAs, which account for 1–5% of the human genome (Cuk et al. 2013). Research in the last decade led to an understanding of miRNA-mediated regulation of gene functions. miRNAs play a key role in regulating the gene expression of the key biological processes (Klein et al. 2010). Moreover, studies have reported that dysregulated expression of miRNAs leads to the onset and progression of cancer (Klein and Dalla-Favera 2010). Recently, miRNAs are projected as potential biomarkers for diagnosis and prognosis of cancer. Furthermore, this review emphasizes on the role of miRNA in the diagnosis and prognosis as well as a therapeutic biomarker in TNBC.
Biogenesis and mechanism of regulation of miRNAs
The biogenesis of miRNA begins with the transcription of miRNA gene by the RNA polymerase II enzyme, which synthesizes a long nucleotide sequence called primary-miRNA (pri-miRNA) with a cap at its 5′ end and poly-A tail at the 3′ end (Fig. 1). This pri-miRNA forms a specific hairpin-shaped, stem–loop secondary structure, which enters a microprocessor complex (500–650 kDa) consisting a Drosha (RNase III endonuclease) and an essential cofactor DGCR8/Pasha (protein containing two double-stranded RNA binding domains) (Chan et al. 2005). The pri-miRNA is processed into a 60–70 nucleotide sequence called pre-miRNA with a 5′ phosphate group and 2 nt overhang stretch at the 3′, which is transported to the cytoplasm by Exportin-5 (Exp5), a member of the Ran transport receptor family. In the cytoplasm, pre-miRNA is further processed into a short, double-stranded miRNA:miRNA* duplex by Dicer, a second RNase III endonuclease. Later, miRNA:miRNA* duplex is unwound into a mature miRNA and miRNA* by a helicase. The mature miRNA is asymmetrically incorporated into the RNA-induced silencing complex (RISC), where it regulates gene expression by mRNA degradation or translational repression (Murakami et al. 2006).
Fig. 1.
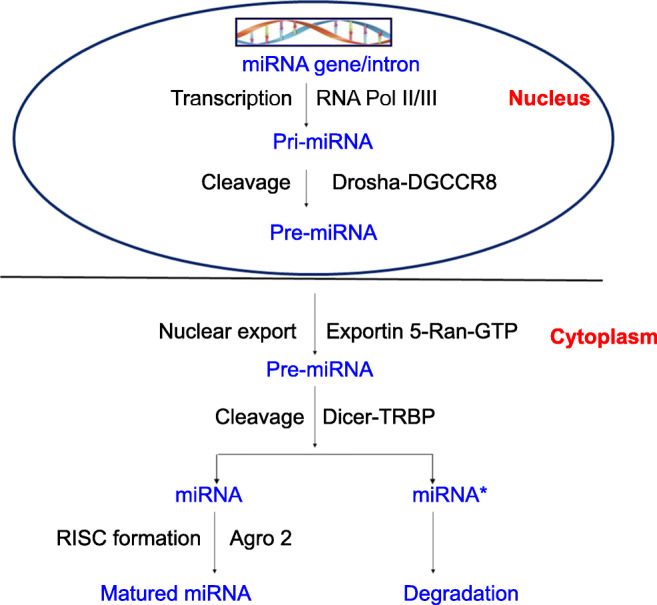
Steps involved in biogenesis of miRNA in the nucleus (synthesis of pri-miRNA and pre-miRNA). Export of pre-miRNAs by Exportin 5-Ran-GTP to the cytoplasm and its cleavage by Dicer-TRBP to yield mature miRNA and degraded miRNA* occurs in presence of RISC factor Ago2
In humans, more than 60% of protein-coding genes contain miRNA-binding sites at their 3′-untranslated region (3′-UTR) (Friedman et al. 2009). miRNAs exert their functions via direct binding to miRNA response elements (MIREs) at the target mRNAs. Each miRNA has several targets and modulates gene expression by transcript destabilization, translational repression, or by base pairing to complementary sequences at 3′-UTR. Recent studies have reported that miRNAs can modulate gene expression by binding to protein-coding exons and induce gene expression in mammalian cells (Viswanathan and Daley 2010). Proteins such as HnRNPA1, SMAD1, and SMAD5 which play an important role in cancer transformation have been shown to interact with miRNA precursors and regulate their subsequent processing (Kumari et al. 2016). Regulatory proteins can bind to mature miRNAs to direct their degradation, thus preventing their expression (Malissen and Grob 2018). Lin 28 is a regulatory protein, which binds with let-7 miRNA and targets its degradation (Choudhury et al. 2013). It is estimated that 10% of miRNA expression is controlled through DNA methylation. Additional evidence supports the regulation of miRNA in response to hypoxia and hormonal changes (Laufer and Singh 2012). Phosphatase and tensin homolog pseudogene (PTENP-1) contains many miRNA sites, which regulate PTEN levels by sequestering its regulatory miRNAs (Fish and Cybulsky 2012).
miRNAs in tumorigenesis of breast cancer
miRNAs play an important role in cancer metastasis as they are differentially expressed depending on the molecular subtypes (Blenkiron et al. 2007). Cancer-promoting miRNAs are termed as onco-miRNAs, whereas tumorigenesis-inhibiting miRNAs are called as tumor suppressor miRNAs, ts-miRNAs. Generally, such ts-miRNAs are expressed at abnormally low levels whereas onco-miRNAs are expressed at high levels in a wide range of cancers (Lal et al. 2011). A plethora of miRNAs associated with cancer has been validated using innovative clinical practices like miRNA pull-out assays. In carcinogenesis, it was noticed that either amplification of onco-miRNA expression or reduced ts-miRNA is often located in chromosomal-fragile sites (He et al. 2005).
In breast cancer, several miRNAs have been identified as tumor suppressor or oncogenic miRNAs, emphasizing miRNA-based research for early detection and therapeutic purposes (Herranz and Ruibal 2012). Yang et al. reviewed the recent literature on the role of miR-181 in breast cancer and suggested miRNA-181 as an onco-miRNA and also that its overexpression is related to cancer metastasis (Yang et al. 2017). Jiang et al. reported miRNA-155 also as an oncomiR because it regulates inflammation and tumor formation via JAK–STAT pathway in breast cancer (Jiang et al. 2010). miRNA-510 was involved in oncogenesis of breast cancer via PI3K/Akt pathway using in silico and in vitro approaches (Guessous et al. 2010). Eccles et al. (2013) confirmed, using pre-miRNA-510 or antisense miRNA-510, that miRNA-510 is a unique onco-miRNA in breast cancer reporting their role in anti-apoptosis and in drug resistance. Ward et al. (2014) established miRNA-519a as an onco-miRNA in estrogen receptor-positive and tamoxifen-resistant breast cancer. Oncogenic miRNA-10b and miRNA-155 regulate proliferation and metastasis by disrupting homeobox D10 (HOXD10), and by suppressing the expressions of a suppressor of cytokine signaling 1 (SOCS1) and forkhead box O3a (FOXO3) in breast cancer (Kong et al. 2010).
Both miRNA-630 and miRNA-133a are tumor suppressors that initiate apoptosis and block cell cycle progression in breast cancer (Zhou et al. 2017). A report has demonstrated that miRNA-21 regulates the expression of tumor suppressor proteins TIMP3, PDCD4, and tropomyosin 1 (alpha) in breast cancer (Qi et al. 2009). Clinically, it has been demonstrated that miRNA-30a negatively regulates cell proliferation, migration, and invasion of TNBC cells (De Santi et al. 2017). Expression of miRNA-203 in breast cancer reported by Ru et al. (2011) was shown to inhibit SOCS3 expression in breast cancer cells. Thus, miRNA-203 negatively regulates the expression of p53, Bax, and p2.
As stated earlier, miRNAs are master regulators of gene expression and many other cellular processes. Nonetheless, their expression is frequently improperly regulated in human cancers. Recently, elevated levels of miRNAs have been identified in serum, plasma, and other fluids of cancer patients (Kume et al. 2017). These circulating miRNAs are promising biomarkers for early-stage diagnosis, prognosis, and prediction of therapeutic response (Allen and Weiss 2010). miRNA-181a was explored as a diagnostic and prognostic biomarker for TNBC because of its altered expression and its role in oncogenesis, invasion, and metastasis (Rosi 2006). Moreover, downregulation of miRNA-329 in serum and tissue samples of breast cancer patients is associated with lymph node metastasis (Pihong et al. 2017; Procházková et al. 2017). Analysis of the expression of miRNAs as potential biomarkers in breast cancer is summarized in Table 2.
Table 2.
Expression of miRNAs in breast cancer
| S.no | Stages in breast cancer | Expression of miRs |
|---|---|---|
| 1 | Stage I and II of TNBC patients | hsa-miR-188-5p, hsa-miR-1202, hsa-miR-4281, hsa-miR-1207-5p, hsa-miR-4270, hsa-miR-1225-5p, hsa-miR-642b-3p, hsa-miR-1290, and hsa-miR-3141, miR-15a, miR-18a, miR-107, miR-133a, miR-139-5p, miR-143, miR-145, miR-365, and miR-425 |
| 2 | Stage I and II of non-TNBC cancer patients | miR-127-3p, miR-148b, miR-409-3p, miR-652, and miR-801, miR-1246, miR-1307-3p, miR-4634, miR-6861-5p, and miR-6875-5p |
Role of miRNA in TNBC tumorigenesis
Exploration of the role of miRNAs in diagnosis, prognosis, and therapy of different cancers, was conducted by Foekens and coworkers (Foekens et al. 2008). Breast cancer exhibits intrinsic heterogeneity and clinicopathological variations in terms of tumor size, vascular invasion, proliferation index, and expression of HER2, ER, and/or PR. Several attempts have been made to develop a miRNA-based marker that reflects the histopathological features of the tumor, such as the association of miRNA profiling with genomic classes. Nearly, nine miRNAs (miRNA-15b, miRNA-99a, miRNA-100, miRNA-103, miRNA-107, miRNA-126*, miRNA-130a, miRNA-136, and miRNA-146b) have been identified, which could discriminate luminal A breast cancer from luminal B breast cancer. Subsequently, 15 miRNA predictive markers for expression of ER (miRNA-135b, miRNA-190, miRNA-217, miRNA-218, miRNA-299, and miRNA-342), PR (miRNA-377, miRNA-520f, miRNA-520g, and miRNA-527-518a,), and the HER2 receptor (miRNA-30e, miRNA-181c, miRNA-320c, miRNA-376b, and miRNA-520d) were reported by Li et al. 2011. These miRNAs are secreted by a solid tumor into body fluids making miRNAs promising targets (Procházková et al. 2017). Expression profiling of miRNA in the serum of TNBC patients has demonstrated that miRNA-190a, miRNA-136-5p, miRNA-126-5p, miRNA-135b-5p, and miRNA-182-5p were downregulated compared to those of healthy controls. Furthermore, these miRNAs appear to be associated with the development and progression of TNBC. Another study suggests that low expression of miRNA-588 correlates with poor prognosis and metastasis of TNBC (Lyng et al. 2012).
miRNA-539 suppresses the expression of LAMA4, a key regulator of tumor progression, and may be an important target in the treatment of TNBC. Wu et al. (2014) suggested that miRNA-455-5p is associated with poor survival and enhancement of invasion and migration of TNBC cells. miRNA-9 is known to enhance the motility and invasive ability of TNBC cells by targeting E-cadherin and β-catenin pathways. miRNA-17-5p regulates migration and invasion by suppressing HBP1 in TNBC cells (Li et al. 2011), and it was reported that Let-7 miRNA controls metastasis and stemness of TNBC cells by regulating JAK-STAT3 and c-Myc pathways (Su et al. 2016). The role of miRNA-27 as a promoter of tumorigenesis via targeting AKT in TNBC has been documented (Kume et al. 2017).
miRNA-10a inhibits the proliferation and migration but promotes apoptosis in TNBC through PI3/AKT/mTOR pathway (Ke and Lou 2017). miRNA-29b and miRNA-17-5p suppress TNBC by inhibiting proliferation and migration by silencing the expression of the RTKN gene. Cui et al. (2018) demonstrated that miRNA-384 inhibits the progression of TNBC by targeting ACVR1. miRNA-34a is involved in the suppression of invasion and migration by inhibiting TLR signaling pathway via CXCL10 in TNBC. miRNA-212-5p suppresses the EMT in TNBC by targeting Prrx2, and miRNA-150 suppresses metastasis of TNBC through targeting HMGA2 (Tang et al. 2018).
Expression of miRNAs at different stages of TNBC
The overexpression of miRNAs (hsa-miRNA-188-5p, hsa-miRNA-1202, hsa-miRNA-4281, hsa-miRNA-1207-5p, hsa-miRNA-4270, hsa-miRNA-1225-5p, hsa-miRNA-642b-3p, hsa-miRNA-1290, hsa-miRNA-3141, miRNA-127-3p, miRNA-148b, miRNA-409-3p, miRNA-652, and miRNA-801) have been identified and validated at I and II stages in TNBC patients (Cuk et al. 2013). Similarly, Shimomura et al. (2016) reported an early overexpression of circulating miRNAs in TNBC patients (miRNA-15a, miRNA-18a, miRNA-107, miRNA-133a, miRNA-139-5p, miRNA-143, miRNA-145, miRNA-365, and miRNA-425). Also, several miRNAs were identified in the serum of early-stage TNBC patients (miRNA-1246, miRNA-1307-3p, miRNA-4634, miRNA-6861-5p, and miRNA-6875-5p). Expression patterns of miRNA-199a-5p differed in the plasma of TNBC patients and healthy controls at stages 0 and I, and a recent study reported that expression levels of miRNA-22 were higher at stages I and II of TNBC compared to stages III and IV (Zou et al. 2017).
miRNAs and the maintenance of stemness in TNBC
The phenotypic plasticity of cancer stem cells (CSCs) is an important factor for tumor malignancy (Al-Hajj et al. 2003). CSCs are enriched in TNBC tumor tissues, which exhibit a strong capacity to proliferate and induce tumors. Cancer stem cell markers, cluster of differentiation (CD44+/CD24−) levels, and aldehyde dehydrogenase 1 (ALDH1+) are enhanced in TNBC tumor tissues compared to the breast cancer subtypes and the ratio of CD44+/CD24−/low cells from TNBC tissues suggested an epithelial to mesenchymal transition (EMT) and high tumorigenicity (Sabeh et al. 2004). A remarkable link between EMT and miRNAs was reported by Li et al. indicating that miRNA-205 and five members of the miRNA-200 family are associated with EMT (Li et al. 2017). However, miRNA-103/107 induced EMT in TNBC by targeting the “Dicer” gene. Recently, Song et al. (2013) reported that the miRNA-200 family epigenetically regulates EMT via miRNA-22-mediated suppression of 10–11 translocation proteins (TET 1–3). miRNA-495 is upregulated in CD44+/CD24−/low and PROCR+/ESA+ TNBC stem cells. miRNA-205 inhibits self-renewal and expression of CD44, TAZ, and E2S.E12 in TNBC stem cells (Bertoli et al. 2015). It has been reported that miRNA-31 promotes mammary stem cell expansion and tumorigenesis by suppressing Wnt signaling antagonists (Davar et al. 2012).
miRNAs and drug resistance in TNBC
A report suggests that miRNAs can impart drug resistance through intracellular drug depletion mediated via transporters and enzymes. Several (miRNA-7, miRNA-27, miRNA-326, miRNA-328, miRNA-451, and miRNA-489) can target the ABC drug transporters and thus affect drug availability in the cell by imparting drug resistance against doxorubicin, cisplatin, or taxol (Zhou et al. 2010). miRNA-34a imparts resistance to radiation therapy in TNBC cells by impairing cell cycle, DNA damage and repair, and apoptosis (Eccles et al. 2013). However, downregulation of miRNA-373 and miRNA-302 is associated with increased DNA repair efficiency, which imparts drug resistance (Liang et al. 2013). Dysregulation of miRNA-342 and miRNA-15a/16 is related to the tamoxifen resistance in TNBC (Li et al. 2013). Table 3 summarizes the multifaceted role of miRNA in tumorigenesis.
Table 3.
Multifaceted role of miRNA in tumorigenesis
| Role of miRNA in tumorigenesis | miRNAs |
|---|---|
| Oncogenic miRNA | miRNA-181, miRNA-155, miRNA-510, miRNA-210, miRNANA-519a, miRNA-10b, miRNA-155, miRNA-21, miRNA-203, miRNA-455-5p, miRNA-17-5p, miRNA-27a |
| miRNA as biomarkers of TNBC | miRNA-15b,miRNA-99a, miRNA-100, miRNA-103, miRNA-107, miRNA-126*, miRNA-130a, miRNA-136, and miRNA-146b, miRNA-135b, miRNA-190, miRNA-217,miRNA-218, miRNA-299, and miRNA-342), PR (miRNA-377, miRNA-520f, miRNA-520g, and miRNA-527-518a, miRNA-30e, miRNA-181c, miRNA-320c, miRNA-376b, and miRNA-520d |
| Drug /radio resistant miRNA | miRNA-7, miRNA-27, miRNA-326, miRNA-328, miRNA-451, miRNA-489, miRNA-148, miRNA-152, miRNA-29, miRNA-194 and miRNA-143, miRNA-7, miRNA-345, miRNA-302 |
| Tumor suppressor miRNA | miRNA-630, miRNA-133a, miRNA-10a, miRNA-29b, miRNA-384 |
| miRNA in maintenance of stemness | miRNA-103/107, miRNA-22, miRNA-495, miRNA-205, miRNA-31 |
Several miRNAs can also impart drug resistance by inducing epigenetic changes in the genes involved in tumorigenesis. Abnormal DNA methylation, by DNA methyl transferases (DNMT)–1, –2, and –3, is a major characteristic of cancers and often, the levels of DNMTs are elevated in breast cancer subtypes (Song et al. 2013). Several miRNAs (miRNA-148, miRNA-152, miRNA-29, miRNA-194, and miRNA-143) have been shown to regulate the expression of DNMTs in TNBC, thereby inducing drug resistance (Ng et al. 2014). Recent studies clearly demonstrated that miRNA-143 is downregulated in TNBC, which in turn regulates the expression of DNMT-3. The down-regulation of miRNA-342 affects histone demethylation and is associated with cisplatin resistance, whereas miRNA-489 targets MinK-related peptide 2 (MiRP-2) and affects the efflux of this drug in breast cancer (Park et al. 2014).
Cross-talk among miRNA-200c, miRNA-203, and a stem cell transcription factor, Bmi1, has been associated with TNBC (Bertoli et al. 2015). It is often upregulated in breast cancer and involved in maintenance of stemness, which is regulated by miRNA-200c and miRNA-203. Overexpression of Bmi1 and downregulation of miRNA-200c and miRNA-203 are accompanied by a reversion of resistance to chemotherapy in TNBC. As CSCs are involved in the relapse of TNBC, modulation of miRNA in combination with therapy could decrease the possibility of its recurrence (Sahlberg et al. 2015). Reports suggest that miRNA-18b, miRNA-103, miRNA-107, and miRNA-652 signatures signal tumor relapse in TNBC patients (Xiu et al. 2013).
miRNA-based therapy for TNBC
Application of miRNA-based therapies against proliferation and metastasis of TNBC represents a challenging area, although some promising results have been obtained in both ex vivo and in vivo experiments. For instance, miRNA-145 was chosen as a target because of its multifaceted role in TNBC. The use of miRNA mimics, or inhibitor miRNA oligonucleotides, such as miRNA-21 is a promising approach for the treatment of TNBC (Sahlberg et al. 2015). Gold nanoparticles with high affinity for biomolecules, low cytotoxicity, easy size control, and well-developed surface chemistry, may be effectively used to increase the miRNA complementarity for nucleic acids, allowing the effective delivery and gene silencing inside the cells. Modulated miRNA-based therapy can affect hundreds of transcripts in different tissues, which are potentially capable of shutting down signaling pathways. MIRX34, a miRNA-34a mimic compound, is one of the first miRNA replacements to be used in clinical trials (Hamam et al. 2016).
Conclusion
This review has emphasized several recent advances in the use of miRNAs in TNBC and their utility in the diagnosis, prognosis, and treatment of patients with breast cancer. In breast cancer, changes in the expression pattern of miRNAs result from alterations in miRNA biogenesis, epigenetic control, transcription factors, and/or mutated protein controls. Consequently, prolonged aberrant expression of miRNAs can lead to oncogenesis. miRNA-9, miRNA-10b, and miRNA-17-5p have emerged as diagnostic biomarkers, and miRNA-148a and miRNA-335 are prognostic markers for TNBC. miRNA-30c, miRNA-187, and miRNA-339-5p are predictive markers of therapeutic outcomes owing to increases in their expression levels to specific treatments. Future research must be directed towards development and delivery methods of miRNA-based drugs in TNBC cases. A few of these have already shown promising results including miRNA-9, miRNA-21, miRNA34a, miRNA145, and miRNA150. Other important areas of future research include the optimization of miRNA-based drug stability and the improvement of miRNA delivery. Control of the off-target effects of miRNA therapeutics must also be improved. Moreover, combinational drug therapy including miRNA and non-miRNA-based therapy like chemotherapy should also be further investigated.
A major aim of this review was to stimulate the interest of members of the biophysics community into miRNA involvement in breast cancer development—a field which is both fascinating, in terms of its organizational complexity, and of great social importance, in the sense that breast cancer imparts terrible emotional and physical consequences onto all of its victims, their family members, and the societies where they live all around the world.
Acknowledgements
We would like to thank DST-EMR/2016/002694, DST-FIST (SR/FST/LSI-568/2013), and GITAM (Deemed to be University) for providing lab facilities.
Abbreviations
- Bmi 1
B lymphoma Mo-MLV insertion region 1 homolog
- CSC
Cancer stem cells
- ER
Estrogen receptor
- HER 2
Human epidermal growth factor receptor 2
- PR
Progesterone receptor
- TNBC
Triple-negative breast cancer
- ts-microRNA
Tumor suppressor microRNA
Authors’ contributions
RRM and SK contributed in designing of review. AKB, MM, SN participated in drafting and editing the manuscript. All authors have read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
Rama Rao Malla declares that he has no conflict of interest. Seema Kumari declares that she has no conflict of interest. Murali Mohan Gavara declares that he has no conflict of interest. Anil Kumar Badana Gavara declares that he has no conflict of interest. Shailender Gugalavath declares that he has no conflict of interest. Deepak Kakara Gift Kumar declares that he has no conflict of interest. Prasuja Rokkam declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Highlights
• Patients diagnosed with negative ER, PR, and HER 2 are the most prevalent subtypes of breast cancer with limited targeted therapy.
• miRNAs play a key role in regulating the gene expression of the key biological processes including proliferation, differentiation, angiogenesis, migration, and apoptosis. The involvement of miRNA has also been identified in carcinogenesis.
• In TNBC, miRNAs make major contributions to the diagnosis, prognosis, and treatment.
• We suggest that miRNA-based therapeutics could be a promising approach in the treatment of TNBC.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad J, Hasnain SE, Siddiqui MA, et al. MicroRNA in carcinogenesis & cancer diagnostics: a new paradigm. Indian J Med Res. 2013;137:680–694. [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KE, Weiss GJ. Resistance may not be futile: microRNA biomarkers for chemoresistance and potential therapeutics. Mol Cancer Ther. 2010;9:3126–3136. doi: 10.1158/1535-7163.MCT-10-0397. [DOI] [PubMed] [Google Scholar]
- Bertoli G, Cava C, Castiglioni I. Micrornas: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5:1122–1143. doi: 10.7150/thno.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkiron C, Goldstein LD, Thorne NP, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- Choudhury NR, Alves F de L, de Andrés-Aguayo L et al (2013) Tissue-specific control of brain-enriched miR-7 biogenesis. Genes Dev. 10.1101/gad.199190.112 [DOI] [PMC free article] [PubMed]
- Cui Y, Wu F, Tian D, et al. MiR-199a-3p enhances cisplatin sensitivity of ovarian cancer cells by targeting ITGB8. Oncol Rep. 2018;39:1649–1657. doi: 10.3892/or.2018.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuk K, Zucknick M, Madhavan D et al (2013) Plasma MicroRNA panel for minimally invasive detection of breast cancer. PLoS One 8. 10.1371/journal.pone.0076729 [DOI] [PMC free article] [PubMed]
- Dai X, Xiang L, Li T, Bai Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J Cancer. 2016;7:1281–1294. doi: 10.7150/jca.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davar D, Tarhini A, Kirkwood JM. Adjuvant therapy for melanoma. Cancer J. 2012;18:192–202. doi: 10.1097/PPO.0b013e31824f118b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi C, Melaiu O, Bonotti A et al (2017) Deregulation of miRNAs in malignant pleural mesothelioma is associated with prognosis and suggests an alteration of cell metabolism. Sci Rep 7. 10.1038/s41598-017-02694-0 [DOI] [PMC free article] [PubMed]
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- Eccles S a, Aboagye EO, Ali S, et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013;15:R92. doi: 10.1186/bcr3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Cybulsky MI. Taming endothelial activation with a microRNA. J Clin Invest. 2012;122:1967–1970. doi: 10.1172/JCI63818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foekens JA, Sieuwerts AM, Smid M, et al. Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proc Natl Acad Sci. 2008;105:13021–13026. doi: 10.1073/pnas.0803304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guessous F, Zhang Y, Kofman A et al (2010) microRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle [DOI] [PMC free article] [PubMed]
- Hamam R, Ali AM, Alsaleh KA et al (2016) microRNA expression profiling on individual breast cancer patients identifies novel panel of circulating microRNA for early detection. Sci Rep 6. 10.1038/srep25997 [DOI] [PMC free article] [PubMed]
- He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz M, Ruibal A (2012) Optical imaging in breast cancer diagnosis: the next evolution. J Oncol. 10.1155/2012/863747 [DOI] [PMC free article] [PubMed]
- Jiang S, Zhang HW, Lu MH, et al. MicroRNA-155 functions as an oncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res. 2010;70:3119–3127. doi: 10.1158/0008-5472.CAN-09-4250. [DOI] [PubMed] [Google Scholar]
- Ke K, Lou T. MicroRNA-10a suppresses breast cancer progression via PI3K/Akt/mTOR pathway. Oncol Lett. 2017;14:5994–6000. doi: 10.3892/ol.2017.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U, Dalla-Favera R. New insights into the pathogenesis of chronic lymphocytic leukemia. Semin Cancer Biol. 2010;20:377–383. doi: 10.1016/j.semcancer.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Klein U, Lia M, Crespo M, et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Kong W, He L, Coppola M, et al. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J Biol Chem. 2010;285:17869–17879. doi: 10.1074/jbc.M110.101055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kumari S, Badana A, Gayatridevi V, et al (2016) Coralyne targets proteases involved in cancer progression: an in silico study. In: SpringerBriefs in applied sciences and Technology pp 19–30
- Kumari S, Badana AK, Mohan GM et al (2017) Synergistic effects of coralyne and paclitaxel on cell migration and proliferation of breast cancer cells lines. Biomed Pharmacother 91. 10.1016/j.biopha.2017.04.027 [DOI] [PubMed]
- Kume K, Iwama H, Deguchi K et al (2017) Serum microRNA expression profiling in patients with multiple system atrophy. Mol Med Rep. 10.3892/mmr.2017.7995 [DOI] [PMC free article] [PubMed]
- Lal A, Thomas MP, Altschuler G et al (2011) Capture of microRNA-bound mRNAs identifies the tumor suppressor miR-34a as a regulator of growth factor signaling. PLoS Genet. 10.1371/journal.pgen.1002363 [DOI] [PMC free article] [PubMed]
- Laufer B, Singh S. A macro role for imprinted clusters of MicroRNAs in the brain. MicroRNA e. 2012;1:59–64. doi: 10.2174/2211536611201010059. [DOI] [PubMed] [Google Scholar]
- Le Bourgeois T, Strauss L, Aksoylar H-I et al (2018) Targeting T cell metabolism for improvement of cancer immunotherapy. Front Oncol. 10.3389/fonc.2018.00237 [DOI] [PMC free article] [PubMed]
- Li H, Bian C, Liao L, Li J, Zhao RC (2011) miR-17-5p promotes human breast cancer cell migration and invasion through suppression of HBP1. Breast Cancer Res Treat 126(3):565–575 [DOI] [PubMed]
- Li L, Yuan L, Luo J, et al. MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin Exp Med. 2013;13:109–117. doi: 10.1007/s10238-012-0186-5. [DOI] [PubMed] [Google Scholar]
- Li P, Dong J, Zhou X, Sun W, Huang H, Chen T, Ye B, Zheng Z, Lu M (2017) Expression patterns of microRNA-329 and its clinical performance in diagnosis and prognosis of breast cancer. OncoTargets and Therapy 10:5711–5718 [DOI] [PMC free article] [PubMed]
- Li Q, Li S, Wu Y, Gao F. miRNA-708 functions as a tumour suppressor in hepatocellular carcinoma by targeting SMAD3. Oncol Lett. 2017;14:2552–2558. doi: 10.3892/ol.2017.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Ahn J, Guo D, et al. MicroRNA-302 replacement therapy sensitizes breast cancer cells to ionizing radiation. Pharm Res. 2013;30:1008–1016. doi: 10.1007/s11095-012-0936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng MB, Lænkholm AV, Søkilde R et al (2012) Global microRNA expression profiling of high-risk ER+ breast cancers from patients receiving adjuvant tamoxifen mono-therapy: a DBCG study. PLoS One 7. 10.1371/journal.pone.0036170 [DOI] [PMC free article] [PubMed]
- Malissen N, Grob JJ (2018) Metastatic melanoma: recent therapeutic progress and future perspectives. Drugs [DOI] [PubMed]
- Murakami Y, Yasuda T, Saigo K, et al. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537–2545. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- Ng EKO, Li R, Shin VY, et al. MicroRNA-143 is downregulated in breast cancer and regulates DNA methyltransferases 3A in breast cancer cells. Tumor Biol. 2014;35:2591–2598. doi: 10.1007/s13277-013-1341-7. [DOI] [PubMed] [Google Scholar]
- Park EY, Chang ES, Lee EJ, et al. Targeting of miR34a-NOTCH1 axis reduced breast cancer stemness and chemoresistance. Cancer Res. 2014;74:7573–7582. doi: 10.1158/0008-5472.CAN-14-1140. [DOI] [PubMed] [Google Scholar]
- Pihong L, Jianda D, Xiang Z, et al (2017) Expression patterns of microRNA-329 and its clinical performance in diagnosis and prognosis of breast cancer. Onco Targets Ther 10:5711–5718 [DOI] [PMC free article] [PubMed]
- Procházková I, Lenčo J, Fučíková A, et al. Targeted proteomics driven verification of biomarker candidates associated with breast cancer aggressiveness. Biochim Biophys Acta - Proteins Proteomics. 2017;1865:488–498. doi: 10.1016/j.bbapap.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Pustylnikov S, Costabile F, Beghi S, Facciabene A (2018) Targeting mitochondria in cancer: current concepts and immunotherapy approaches. Transl Res [DOI] [PMC free article] [PubMed]
- Qi L, Bart J, Tan LP et al (2009) Expression of miR-21 and its targets (PTEN, PDCD4, TM1) in flat epithelial atypia of the breast in relation to ductal carcinoma in situ and invasive carcinoma. BMC Cancer 9. 10.1186/1471-2407-9-163 [DOI] [PMC free article] [PubMed]
- Rosi NL. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- Ru P, Steele R, Hsueh EC, Ray RB. Anti-miR-203 upregulates SOCS3 expression in breast cancer cells and enhances cisplatin chemosensitivity. Genes Cancer. 2011;2:720–727. doi: 10.1177/1947601911425832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlberg KK, Bottai G, Naume B, et al. A serum MicroRNA signature predicts tumor relapse and survival in triple-negative breast cancer patients. Clin Cancer Res. 2015;21:1207–1214. doi: 10.1158/1078-0432.CCR-14-2011. [DOI] [PubMed] [Google Scholar]
- Shimomura A, Shiino S, Kawauchi J, et al. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci. 2016;107:326–334. doi: 10.1111/cas.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SJ, Poliseno L, Song MS et al (2013) XMicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 154. 10.1016/j.cell.2013.06.026 [DOI] [PMC free article] [PubMed]
- Su Y, Wu H, Pavlosky A, et al. Regulatory non-coding RNA: new instruments in the orchestration of cell death. Cell Death Dis. 2016;7:e2333. doi: 10.1038/cddis.2016.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Xu P, Wang H, et al. MicroRNA-150 suppresses triple-negative breast cancer metastasis through targeting HMGA2. Onco Targets Ther. 2018;11:2319–2332. doi: 10.2147/OTT.S161996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan SR, Daley GQ. Lin28: a MicroRNA regulator with a macro role. Cell. 2010;140:445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Ward A, Shukla K, Balwierz A, et al. MicroRNA-519a is a novel oncomir conferring tamoxifen resistance by targeting a network of tumour-suppressor genes in ER+ breast cancer. J Pathol. 2014;233:368–379. doi: 10.1002/path.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RC, Wang TL, Shih IM. The emerging roles of ARID1A in tumor suppression. Cancer Biol Ther. 2014;15:655–664. doi: 10.4161/cbt.28411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu B, Zhang W, Huang B, et al. Genetic inhibition of vascular endothelial growth factor receptor-1 significantly inhibits the migration and proliferation of leukemia cells and increases their sensitivity to chemotherapy. Oncol Rep. 2013;29:2030–2038. doi: 10.3892/or.2013.2348. [DOI] [PubMed] [Google Scholar]
- Yang C, Tabatabaei SN, Ruan X, Hardy P. The dual regulatory role of MiR-181a in breast cancer. Cell Physiol Biochem. 2017;44:843–856. doi: 10.1159/000485351. [DOI] [PubMed] [Google Scholar]
- Zhou M, Liu Z, Zhao Y, et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem. 2010;285:21496–21507. doi: 10.1074/jbc.M109.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhou W, Zeng Q, Xiong J. MicroRNA-138 inhibits hypoxia-induced proliferation of endothelial progenitor cells via inhibition of HIF-1α-mediated MAPK and AKT signaling. Exp Ther Med. 2017;13:1017–1024. doi: 10.3892/etm.2017.4091. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zou Q, Tang Q, Pan Y, et al. MicroRNA-22 inhibits cell growth and metastasis in breast cancer via targeting of SIRT1. Exp Ther Med. 2017;14:1009–1016. doi: 10.3892/etm.2017.4590. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


