Abstract
The major challenge in treating cancers with ATRA is the limited availability inside the cell and resistance developed in prolonged treatment. We made an attempt for co-treatment of human NSCLC cell lines (A549) with ATRA and its isomeric precursor (9cisRA). In this study, the growth inhibitory effect of ATRA, 9cisRA and combination of both were tested in A549 cells by MTT and Trypan blue assays. As the effects of retinoid are mediated through their receptors, their gene expression levels were analyzed by RT-PCR. The target gene receptor, RAR-β protein expression, was analyzed by immunocytochemistry. The cancer cell (A549) growth inhibitory effect was significantly (p ≤ 0.001) enhanced in combination treatment when compared with the result of individual treatments. The mRNA expression levels of both RAR-β and RXR-β were found to be increased in co-treatment (band density of 0.75 and 0.806, respectively) when compared with 9cisRA treatment (0.25 and 0.112) and ATRA treatment (0.01 and 0.081). A concomitant enhancement in the target RAR-β protein expression was observed in co-treated cells when compared with individual treatments. We thus conclude that the co-treatment had increased the availability of ATRA, by isomerization of the 9cisRA which then resulted in an increased expression of both RAR-β and RXR-β receptors and the target protein RAR-β which in turn inhibited lung cancer cell growth. Our study results have explored the mechanism of synergistic effect of co-treatment with ATRA and 9cisRA and further preclinical studies are necessary to validate the application of co-treatment of retinoid in clinical use.
Keywords: 9-Cis retinoic acid, ATRA, Retinoic acid receptor, Retinoid X receptor, RT-PCR, Immunocytochemistry
Introduction
Lung cancer is developed by the unregulated proliferation of cells present in the lung. It is one of the deadliest diseases that affects men mostly. It is the second most cause of cancer death in women. One of the major causes of lung cancer is smoking tobacco and about 80–90% of lung cancer is due to cigarette smoking. However, only 11% of heavy cigarette smoking ultimately develops lung cancer, suggesting that there might be genetic factors which predispose to lung cancer risk. Despite improvements in therapy, 90% of lung cancer patients die (Minna et al. 2002). It urged the researchers across the globe to develop new strategies like molecular therapy to treat or at least to control the cell multiplication in lung cancer. One of the promising approaches is to regulate the gene expression, which is involved in cell growth homeostasis. Tumorigenesis is a multistep process which begins with a mutation in genes leading to the transformation of malignant derivative genes either gaining a dominant function or recessive loss of function (oncogene) of tumor suppressor genes (Hanahan and Weinberg 2000; Stewart and Wild 2017; Jemal et al. 2011).
Most of the lung cancers are caused due to mutation in various genes such as tumor suppressor gene, proto-oncogenes and by certain other epigenetic factors which are involved in the regulation of cell proliferation, apoptosis, and differentiation (Cheung et al. 2003; Moison et al. 2013). The epigenetic factors are signal and ligand-dependent molecules which are able to modulate the expression level of target genes involved in cell regulation. Certain natural compounds act by regulating proliferation and differentiation of cells. Among them, retinoid is one that is naturally occurring ligand that controls the growth of various cell types, most commonly it acts by interacting with the nuclear receptor. Natural retinoids and their synthetic derivatives act as chemoprevention agents. They are used for various cancer types and acute promyelocytic leukemia. One of the challenges faced in the chemotherapies is to develop a strategy that can control malignant disease. These retinoids treatment aims at the future therapeutic effect for the management of metastases (Dragnev et al. 2000; Pettersson et al. 2002).
Retinoids act specifically by interacting with the nuclear retinoid receptor belonging to the superfamily of steroid/thyroid hormone receptor. These receptors are retinoic acid receptors (RARs) and retinoid X receptors (RXR), each comprising 3 subtypes which are α, β, and γ. RARs and RXRs heterodimers bind to a specific DNA sequence, the RA response element, in the promoter regions of target genes to modify their expression of target genes. Several retinoid-regulated genes carrying these response elements in their regulatory regions have been identified. Each subtype of nuclear retinoid receptor is thought to regulate the expression of distinct genes and specific patterns of expression during embryonic development and different distributions in adult tissues (Dragnev et al. 2000).
All-trans retinoic acid (ATRA) and 9-cis retinoic acid (9cisRA) are the important metabolic products of Vitamin A that plays a significant role in the expression of various genes, including Retinoic Acid Receptor-β (RAR β) and Retinoid X Receptor-β (RXR-β) which play an important role in the regulation of cell cycle (Rhinn and Dollé 2012; Lotan 1999). Both ATRA and 9cisRA are reported to have therapeutic effects (Zuccari et al. 2005; Han et al. 2010; Wen et al. 2005). ATRA binds RAR only with high affinity, whereas 9cisRA binds both RAR and RXR. 9cisRA exhibited 40-fold more affinity to RXR than the RAR. However, the heterodimerization of RAR–RXR is essential for the action of retinoid (Buskohl et al. 2012). So not only ATRA but also 9cisRA are necessarily needed for efficient therapeutic effects (Bushue and Wan 2010). Currently, the ATRA therapy is in clinical use for APL and under clinical trials for other cancers. The major drawback faced in ATRA therapy is the resistance developed over the long-term treatment. This may be due to the internal regulatory mechanism through the binding proteins such as CRABP-I and II which direct the excess ATRA for degradation. Since the 9cisRA can be converted into ATRA inside the cells to meet the demand for ATRA, we intended to study the therapeutic efficacy of combinational treatment of both ATRA and 9cisRA on human lung cancer A549 cells. Several conclusions indeed recommend the therapeutic potential of combinatorial treatment of retinoids with chemotherapeutic agents, arsenic trioxide or drugs targeting epigenetic enzymes (Di Masi et al. 2015). The expression of RAR-β as well as RXR-β was reported to be downregulated in non-small cell lung cancer, which enabled the cancer cell to evade apoptosis and it was also reported that the downregulation of RAR-β expression has led to increase lung cancer risk. These receptors are suggested to act significantly in the prognosis of lung cancer and the biological aggressiveness of non-small cell lung cancer (Brabender et al. 2005). Maintaining the levels of RAR and RXR was reported to inhibit the growth of cancerous cell by regulating the cell cycle proliferation (Mi et al. 2007). It was also reported that the downregulation of RAR-β expression has led to increase in lung cancer risk (Bogos et al. 2008). The RAR-β is also known as tumor suppressor and the major target gene of retinoid action (Lei and de Thé 2003). Previous studies have noted abnormalities in RAR-β gene and its expression was depleted in human lung cancer (Soria et al. 2003).
The current investigation has been, therefore, undertaken to evaluate the synergistic therapeutic action of ATRA and 9cisRA treatments in lung adenocarcinoma cell lines and their molecular action on the expression of RAR-β and RXR-β genes by RT-PCR. The RAR-β protein expression was also analyzed in the treated cell line by immunocytochemistry using antibody. This will eventually aid to formulate an effective drug for lung cancer that can act at the molecular level, as the existing chemotherapy was reported to be ineffective to this non-small cell lung cancer. Hence, this investigation on the combinatorial drug effect on A549 cell line will lead to the development of an effective combinational drug.
Materials and methods
Chemicals and cell line
Non-small cell lung cancer cell line (A549) was obtained from NCCS, Pune and cultured in DMEM (Hi-media) containing 10% fetal bovine serum and antibiotics at 37 °C and CO2 level was maintained at 5% (Lotan 1999). All-trans retinoic acid and 9cisRA were purchased from Sigma-Aldrich, Inc.
Experimental groups
Group I: A549 cell line without treatment.
Group II: A549 cell line treated with ATRA and 9cisRA.
Group III: A549 cell line treated with 9cisRA alone.
Group IV: A549 cell line treated with ATRA alone.
Treatment of cell line
Non-small cell lung cancer (A549) cell line was seeded in 96-well flat-bottom plates (5000 cells/ well) and allowed to adhere for 24 h. Then, the cells were treated with ATRA and 9cisRA individually as well as in combination at a total concentration of 1 µM as given in the experimental groups for 24, 48 and 72 h (Szabó et al. 2014).
MTT assay
Cells were seeded in 96-well flat-bottom plates (5 × 103 cells/well) and allowed to adhere for 24 h at 37 °C in 5% CO2 atmosphere. Treatment was done as mentioned above and incubated further for different time intervals such as 24, 48 and 72 h). After incubation time, 20 μl of MTT (3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide) (5 mg/ml) was added. Then, 100 µl of DMSO was added and the absorbance was measured at 570 nm (Siddikuzzaman and Grace 2012). The percentage viability was calculated using the following formula:
Trypan blue dye exclusion method for cytotoxicity
The cells were treated as mentioned above in the experimental group for various time intervals such as 24, 48 and 72 h. Then, the cells were diluted with 0.4% trypan blue dye solution which was then counted using hemocytometer, under microscopic observation. The non-viable cells were stained blue and the viable cells remained unstained (Ramya et al. 2012). The percent viable cells were calculated as follows:
RNA isolation and quality analysis
RNA was extracted from the treated cell line using TRIzol (Invitrogen) as mentioned in the manufacturer’s instructions. The quality of the RNA was analyzed by 1% agarose gel electrophoresis and spectrophotometer readings. cDNA was generated from RNA using Superscript First-Strand cDNA Synthesis System (Invitrogen). After inactivation of the reverse transcription (RT) enzyme (15 min at 75 °C), the RNA template was hydrolyzed with 50U of RNase H for 1 h at 37 °C (Moison et al. 2013).
Retinoid receptor gene expression analysis
The synthesized cDNA was amplified using Taq polymerase (NEB, Ipswich, MA, USA) with specific primers designed for RAR-β: F-5′TCCGAAAAGCTCACCAGGAAA-3′, R-5′ GGCCAGTTCACTGAATTTGTCC-3′. RXR-β: F-5′-GCAGCCCAAATGACCCTGT-3′, R-5′CCCGCAGCAATATGACCTGA-3′ and beta-actin (internal control) F-5′CATGTACGTTGCTATCCAGG, R-5′CTCCTTAATGTCACGCACGAT′3. The PCR was carried out for 30 cycles: 94 °C for 20 s, 60–64 °C for 20 s, 72 °C for 45 s and then 10 min at 72 °C. The PCR product was then analyzed by agarose gel (1%) electrophoresis (Cheung et al. 2003). The bandwidth in the gel was measured using the software GelQuant.Net—version 1.8.2.
Immunocytochemical staining for RAR β protein expression
Coverslips were sterilized using 95% ethanol and then kept in 6 well plate. Cells were grown on a coverslip in a 6-well plate at a density of 5 × 104 cells/well and allowed to reach confluency. Cells were treated as mentioned above in the experimental group for 72 h. After the treatment, the cells were fixed using 4% paraformaldehyde and incubated overnight with primary antibody, anti-RAR β (Santa Cruz biotechnology, USA). It was incubated with IGg-HRP labeled secondary antibody (Santa Cruz biotechnology, USA) for 2 h, which was then incubated in peroxidase substrate and the cells were counter stained with Hematoxylin. The cells were then mounted on the glass slide using DPX mountant and observed under light microscope (Cras et al. 2007).
Statistical analysis
All data were expressed as mean ± SD. The statistical analysis was done using one-way analysis of variance (ANOVA) followed by a Dunnett’s test (Graph pad InStat version 3.00 for Windows 10; Graph Pad Software, Inc., La Jolla, CA, USA). A p value ≤ 0.05 is considered significant.
Results
Growth inhibitory activity of retinoid by MTT assay
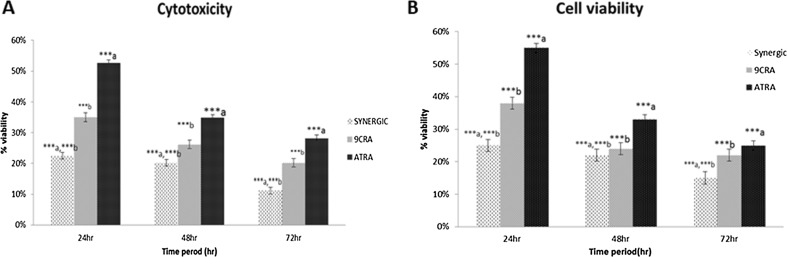
The growth inhibitory activity of 1 µM ATRA, 9cisRA and their combinational treatment on A549 cell line were expressed in terms of percent viable cells in Fig. 1a. The percent viability was found to be decreased upon increasing treatment period from 24 to 72 h in all the 3 groups. Moreover, the combination therapy of ATRA and 9cisRA has shown a significantly lower cell viability (p ≤ 0.001) when compared with individual treatments. From the observations, it was clear that A549 cells were sensitive to combinational treatment of ATRA and 9cisRA and their synergistic effect increased with the increase in treatment time period.
Fig. 1.
a Growth inhibitory effects of ATRA, 9-cis RA and its combination in A549 cell line. Values are expressed in mean ± SD (n = 6). aATRA vs combined drug (a = ***p ≤ 0.001); b9cisRA vs combined drug (b = ***p ≤ 0.001). b Cytotoxicity effects of ATRA, 9-cis RA and its combination in A549 cell line. Values are expressed in mean ± SD (n = 6). aATRA vs combined drug (a = ***p ≤ 0.001); b9cisRA vs combined drug (b = ***p ≤ 0.001)
Cytotoxicity level by trypan blue assay
Cytotoxic effect of 1 µM ATRA, 9cisRA and its combinational treatment on A549 cell line were expressed in terms of percent viable cells in Fig. 1b. The toxic effect of retinoids is time dependent. From the result, it was observed that the combinational treatment exhibited maximum toxicity towards A549 cells as indicated by significantly lower percent viability of 15% after treated for 72 h, which was considerably less in comparison to the individual treatments.
Effect of retinoid treatment on expression levels of RAR-β and RXR-β genes
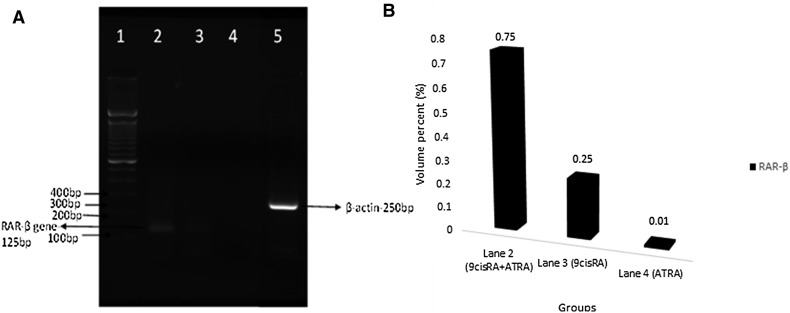
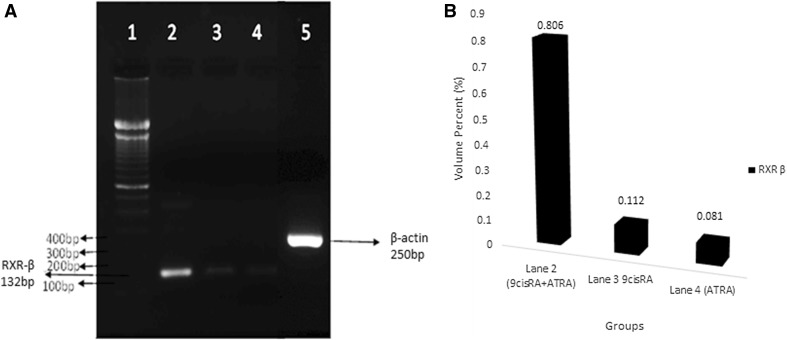
The gene expression was evaluated from the band seen at the expected molecular size of 125 bp for RAR-β and 132 bp for RXR-β for their specific primers. The RAR-β and RXR-β gene expressions were found to be upregulated in treated A549 cells, when compared with untreated control cell lines as shown in Figs. 2 and 3, respectively. Relatively, a high level of expression was found in combinatorial treatment which was indicated by the bandwidth (Figs. 2, 3). The bandwidth measurement was carried out by GelQuant.Net software and the bandwidth was given as volume percent (%) unit.
Fig. 2.
Reverse transcriptase PCR observation. a RAR-β gene expression. Lane 1 shows 100 bp DNA marker, Lane 2 corresponds to the upregulation in the expression of RAR-β in A549 cell line after combinational treatment in comparison with individual treatment 9cisRA (Lane 3) and ATRA (Lane 4) alone. Lane 5: β-actin gene expression. b RAR-β gene band density for combination treatment (0.75), 9 cis RA treatment (0.25) and ATRA treatment (0.01)
Fig. 3.
Reverse transcriptase PCR observation. a RXR-β gene expression. Lane 1 corresponds to 100 bp DNA marker, Lane 2 indicates the enhanced expression of RXR-β gene in combinatorial treatment with ATRA and 9cisRA when compared with the individual treatment with 9cisRA (Lane 3) and ATRA alone (Lane 4). Lane 5: β-actin gene expression. b RXR-β gene band density for combination treatment (0.806), 9 cis RA treatment (0.112) and ATRA treatment (0.081)
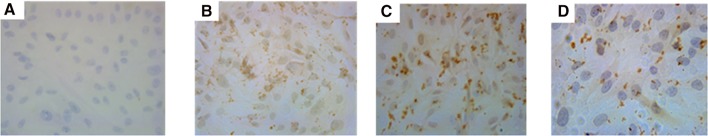
RAR-β protein expression in retinoid-treated cell lines
The expression of RAR-β protein was visualized in the nucleus as well as in the cytoplasmic region through microscope after immunocytochemistry staining in A549 cells. The expression levels correlated well with the sensitivity of the cell to RA treatment. The expression was found to be more in the co-treatment (ATRA and 9cisRA) as shown in Fig. 4 when compared with individual treatments.
Fig. 4.
Immunocytochemical observation of RAR-β protein expression in A549 cell line. a Cancer control without retinoid treatment showing very less RAR-β protein expression, b A549 cell line treated with the combination of ATRA and 9cisRA showing an upregulated expression of RAR-β protein. c, d 9cisRA and ATRA-treated cells showing enhanced RAR-β protein but in lesser level when compared with the combinatorial drug treatment
Discussions
The retinoids are a class of natural and synthetic vitamin A analogs, and some retinoids are known to play major roles in cell growth regulation and in the differentiation of normal, benign, and malignant cell type (Dragnev et al. 2000). We have already reported a promising anti-cancer effects of ATRA alone in in vivo lung cancer mice models (Siddikuzzaman and Grace 2012; Ramya et al. 2012). These retinoids act by binding to RARE via RAR/RXR heterodimer formation and eventually follows the classical pathway by which it triggers apoptosis and regulates NF-Kb, TNF-β, MAPK and chromatin remodeling. In the cancerous condition, the classical pathway is downregulated and thereby resulted in uncontrolled growth of cell. This downregulation is caused due to the lack of expression in certain types of RAR isoforms (RAR-α, β and γ) and RXR (RXR-α, β and γ) isoforms. Among the various isoforms, RAR-β and RXR-β are of importance because of their primary role as tumor suppressor (Pavan et al. 2008; Altucci et al. 2007). Altered RAR and RXR expression level was observed in premalignant oropharyngeal lesions, APL, breast, prostate, lung cancer in comparison with the normal cell. Prolonged treatment with ATRA has made the cancer cells insensitive to this drug. Hence, we made an attempt to study its therapeutic effect and its molecular response levels when co-treated with its isomeric form 9cisRA which is relatively unstable and can be converted to ATRA at demand. RAR-β protein has been reported as the responsive target of ATRA therapy by various studies (Sun et al. 2000).
In the present study, a comparative study on the effect of 1 µM ATRA, 9cisRA and its synergistic effect on non-small cell lung cancer in A549 cell line is done. As lung cancer is one of the causes of cancer death worldwide, the improvements in diagnostics and treatments are urgently needed for non-small cell lung cancer since it is referred as insensitive to chemotherapies with poor survival rate. In addition, the expression of RAR and RXR is downregulated in non-small cell lung cancer. Our data showed that ATRA, 9cisRA, and its combinational treatment could inhibit the growth of A549 cells, but the growth inhibitory effect of combinational treatment is found to be significantly (p ≤ 0.001) higher than the individual treatments. The gene expression study carried out in this research revealed that the combination treatment could enhance the mRNA expression levels of both RAR-β and RXR-β than the individual treatments did. Furthermore, the target of RAR-β protein expression has been enhanced significantly by the synergistic effect of all-trans retinoic acid and 9-cis retinoic acid treatments in our study. This RAR-β is known for its tumor suppressor activity in many cancers (Moison et al. 2013; Cras et al. 2007; Altucci et al. 2007). This combinational treatment would thus enable the treatment of retinoic acid resistant cancer cell by optimizing the levels of retinoids internally in cancer cells. However, further gene expression study is necessary to analyze the role of RAR-β protein in the lung cancer cells.
On combinatorial treatment, the RAR-β protein expression was upregulated and visualized by immunocytochemistry. We thus suggest that the 9cisRA might have been converted to ATRA by isomerization. This conversion facilitated more attachment of ATRA to RAR-β and also the 9cisRA binding to both RAR-β and RXR-β. These binding dynamics efficiently enhanced the level of RAR-β tumor suppressor protein which in turn resulted in an effective therapeutic action against human lung cancer cells.
Conclusion
The results have shown that the combinational treatment of ATRA and 9cisRA has the most impressive activity in non-small cell lung cancer cell by inducing the expression of their receptors such as RAR-β and RXR-β. This dynamic interactions lead to an enhanced level of RAR-β protein which in turn exhibited its growth inhibitory tumor suppressor action of human lung cancer cell line. Thus, the combination therapy may be proposed as an alternative molecular chemotherapy, which is efficient even in cancers known to be insensitive to ATRA. Detailed study on the mechanism of action of retinoic acid is necessary to further validate the application of co-treatment of retinoid in clinical studies.
Acknowledgements
The authors would like to acknowledge the Karunya Institute of Technology and Sciences for the financial support given through Karunya Short-Term Project Grant. We also acknowledge partial financial support shared from DST-SERB and DBT, Govt. of India. We would also like to acknowledge the valuable technical support of Dr. C. Vani, Assistant Professor and Ms. Jissin Mathew, Research Scholar, Department of Biotechnology, Karunya Institute of Technology and Sciences, Coimbatore.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Altucci L, Leibowitz MD, Ogilvie KM, De Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discovery. 2007;6(10):793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- Bogos K, Renyi-Vamos F, Kovacs G, Tovari J, Dome B. Role of retinoic receptors in lung carcinogenesis. J Exp Clin Cancer Res. 2008;27(1):18. doi: 10.1186/1756-9966-27-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabender J, Metzger R, Salonga D, Danenberg KD, Danenberg PV, Hölscher AH, Schneider PM. Comprehensive expression analysis of retinoic acid receptors and retinoid X receptors in non-small cell lung cancer: implications for tumor development and prognosis. Carcinogenesis. 2005;26(3):525–530. doi: 10.1093/carcin/bgi006. [DOI] [PubMed] [Google Scholar]
- Bushue N, Wan Y-JY. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62(13):1285–1298. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskohl PR, Gould RA, Curran S, Archer SD, Butcher JT. Multidisciplinary inquiry-based investigation learning using an ex vivo chicken culture platform: role of vitamin A on embryonic morphogenesis. Am Biol Teacher. 2012;74(9):636–643. [Google Scholar]
- Cheung B, Yan J, Smith SA, Nguyen T, Lee M, Kavallaris M, Norris MD, Haber M, Marshall GM. Growth inhibitory retinoid effects after recruitment of retinoid X receptor β to the retinoic acid receptor β promoter. Int J Cancer. 2003;105(6):856–867. doi: 10.1002/ijc.11153. [DOI] [PubMed] [Google Scholar]
- Cras A, Darsin-Bettinger D, Balitrand N, Cassinat B, Soulie A, Toubert M, Delva L, Chomienne C. Epigenetic patterns of the retinoic acid receptor β2 promoter in retinoic acid-resistant thyroid cancer cells. Oncogene. 2007;26(27):4018–4024. doi: 10.1038/sj.onc.1210178. [DOI] [PubMed] [Google Scholar]
- Di Masi A, Leboffe L, De Marinis E, Pagano F, Cicconi L, Rochette-Egly C, Lo-Coco F, Ascenzi P, Nervi C. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol Aspects Med. 2015;41:1–115. doi: 10.1016/j.mam.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Dragnev KH, Rigas JR, Dmitrovsky E. The retinoids and cancer prevention mechanisms. Oncologist. 2000;5(5):361–368. doi: 10.1634/theoncologist.5-5-361. [DOI] [PubMed] [Google Scholar]
- Han S, Fukazawa T, Yamatsuji T, Matsuoka J, Miyachi H, Maeda Y, Durbin M, Naomoto Y. Anti-tumor effect in human lung cancer by a combination treatment of novel histone deacetylase inhibitors: SL142 or SL325 and retinoic acids. PLoS ONE. 2010;5(11):e13834. doi: 10.1371/journal.pone.0013834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Lei M, de Thé H. Retinoids and retinoic acid receptor in cancer. Eur J Cancer Suppl. 2003;1(2):13–18. [Google Scholar]
- Lotan R. Aberrant expression of retinoid receptors and lung carcinogenesis. Oxford: Oxford University Press; 1999. [DOI] [PubMed] [Google Scholar]
- Mi L, Wang X, Govind S, Hood BL, Veenstra TD, Conrads TP, Saha DT, Goldman R, Chung F-L. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Can Res. 2007;67(13):6409–6416. doi: 10.1158/0008-5472.CAN-07-0340. [DOI] [PubMed] [Google Scholar]
- Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1(1):49–52. doi: 10.1016/s1535-6108(02)00027-2. [DOI] [PubMed] [Google Scholar]
- Moison C, Senamaud-Beaufort C, Fourrière L, Champion C, Ceccaldi A, Lacomme S, Daunay A, Tost J, Arimondo PB, Guieysse-Peugeot A-L. DNA methylation associated with polycomb repression in retinoic acid receptor β silencing. FASEB J. 2013;27(4):1468–1478. doi: 10.1096/fj.12-210971. [DOI] [PubMed] [Google Scholar]
- Pavan B, Dalpiaz A, Biondi C, Nieddu M, De Luca A, Prasad PD, Paganetto G, Favaloro B. An RPE cell line as a useful in vitro model for studying retinoic acid receptor β: expression and affinity. Biosci Rep. 2008;28(6):327–334. doi: 10.1042/BSR20080103. [DOI] [PubMed] [Google Scholar]
- Pettersson F, Dalgleish A, Bissonnette R, Colston K. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-γ and altered expression of Bcl-2/Bax. Br J Cancer. 2002;87(5):555–561. doi: 10.1038/sj.bjc.6600496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya D, Siddikuzzaman Manjamalai A, Berlin Grace V. Chemoprotective effect of all-trans retinoic acid (ATRA) on oxidative stress and lung metastasis induced by benzo (a) pyrene. Immunopharmacol Immunotoxicol. 2012;34(2):317–325. doi: 10.3109/08923973.2011.604087. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dollé P. Retinoic acid signalling during development. Development. 2012;139(5):843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- Siddikuzzaman, Grace VB. Inhibition of metastatic lung cancer in C57BL/6 mice by liposome encapsulated all trans retinoic acid (ATRA) Int Immunopharmacol. 2012;14(4):570–579. doi: 10.1016/j.intimp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Soria J-C, Xu X, Liu DD, Lee JJ, Kurie J, Morice RC, Khuri F, Mao L, Hong WK, Lotan R. Retinoic acid receptor β and telomerase catalytic subunit expression in bronchial epithelium of heavy smokers. J Natl Cancer Inst. 2003;95(2):165–168. doi: 10.1093/jnci/95.2.165. [DOI] [PubMed] [Google Scholar]
- Stewart B, Wild CP (2017) World cancer report 2014. Health
- Sun S-Y, Wan H, Yue P, Hong WK, Lotan R. Evidence that retinoic acid receptor β induction by retinoids is important for tumor cell growth inhibition. J Biol Chem. 2000;275(22):17149–17153. doi: 10.1074/jbc.M000527200. [DOI] [PubMed] [Google Scholar]
- Szabó DR, Baghy K, Szabó PM, Zsippai A, Marczell I, Nagy Z, Varga V, Éder K, Tóth S, Buzás EI. Antitumoral effects of 9-cis retinoic acid in adrenocortical cancer. Cell Mol Life Sci. 2014;71(5):917–932. doi: 10.1007/s00018-013-1408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Li Y, Hu K, Dai C, Liu Y. Hepatocyte growth factor receptor signaling mediates the anti-fibrotic action of 9-cis-retinoic acid in glomerular mesangial cells. Am J Pathol. 2005;167(4):947–957. doi: 10.1016/S0002-9440(10)61185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccari G, Carosio R, Fini A, Montaldo P, Orienti I. Modified polyvinylalcohol for encapsulation of all-trans-retinoic acid in polymeric micelles. J Control Release. 2005;103(2):369–380. doi: 10.1016/j.jconrel.2004.12.016. [DOI] [PubMed] [Google Scholar]