Abstract
Despite the overlap between schizophrenia and bipolar disorder, neurodevelopmental abnormalities are thought to be associated primarily with schizophrenia. Transdiagnostic and empirical identification of subgroups based on premorbid adjustment (PMA) may enhance understanding of illness trajectories. 160 patients with bipolar I or II disorder (BD; n = 104) or schizophrenia or schizoaffective disorder (SZ; n = 56) were assessed on PMA course from childhood to late adolescence and current symptoms and functioning. A hierarchical cluster analysis was performed using social and academic PMA scores, resulting in three optimal clusters. Cluster 1 (n = 28 SZ, 65 BD) had normal social and academic PMA, the most education, and mildest current symptoms. Cluster 2 (n = 15 SZ, 24 BD) had normal social PMA but an impaired-declining academic course and had a greater proportion of males than Cluster 1. Cluster 3 (n = 13 SZ, 15 BD) had an impaired-stable social PMA and an impaired-declining academic course and the most severe current negative symptoms and childhood trauma. The proportions of SZ and BD diagnoses, current neurocognition, and functioning did not differ between clusters. These findings suggest shared neurodevelopmental abnormalities between SZ and BD, with subgroups exhibiting distinct PMA trajectories that cut across disorders.
1. Introduction
A comprehensive understanding of illness trajectories of psychiatric disorders is critical for tailoring prevention and treatment efforts. Schizophrenia (SZ) is now widely considered a neurodevelopmental disorder with impairments in cognition, language, motor, and behavioral functioning evident in childhood (Murray and Lewis, 1987; Owen et al., 2011; Weinberger, 1987). From this perspective, the period prior to disease onset is important for identifying illness-related neurodevelopmental factors. For SZ, where the onset of frank psychotic symptoms is typically in late adolescence or early adulthood, measures of premorbid adjustment (PMA) includes performance during childhood through adolescence in prominent developmental domains such as social and academic functioning (Cannon-Spoor et al., 1982).
SZ and bipolar disorder (BD) exhibit within-disorder heterogeneity and cross-disorder overlap. Both disorders are heterogeneous in symptomology (American Psychiatric Association, 2013), cognitive functioning (Burdick et al., 2014; Holthausen et al., 2002), and etiology (Faraone and Tsuang, 2003; Takahashi, 2013). Moreover, SZ and BD share common genetic contributions (Lichtenstein et al., 2009), clinical symptoms including psychosis and affective dysregulation (American Psychiatric Association, 2013), and cognitive impairment (Bora et al., 2009). Despite increasingly strong evidence against the traditional Kraepelinian dichotomy between SZ and BD, SZ is still considered to have a stronger neurodevelopmental component than BD (Murray et al., 2004).
Studies have consistently shown that poor PMA is predictive of later development of SZ when compared with a number of other psychiatric disorders including affective disorders (Schmael et al., 2007). In SZ, poor PMA has been linked to male sex, earlier age of onset, greater symptom severity, and poorer outcome, global functioning, and quality of life (MacBeth and Gumley, 2008; Schmael et al., 2007). In contrast, better PMA in adolescence was shown to predict functional improvement in an early psychosis treatment program (Minor et al., 2015). Premorbid functioning trajectory is also informative of later illness characteristics. Rabinowitz et al. (2002) reported that first episode psychotic patients with stable-poor PMA performed worse on cognitive measures than those with PMA deterioration. Furthermore, declining PMA from childhood through adolescence has been associated with more severe negative symptoms (Kelley et al., 1992). In a study of first episode non-affective psychotic patients, Larsen et al. (2004) found that declining social adjustment was associated with having fewer friends and more negative symptoms than a stable course.
The literature on PMA in BD has been mixed. While one study found that BD patients, including a subsample with a psychosis history, outperformed healthy controls on premorbid sociability and adaptation to school in adolescence (Rietschel et al., 2009), another study reported that both a BD and a SZ sample had significantly poorer social PMA in adolescence than healthy controls (Cannon et al., 1997). Although the level of adjustment difficulties in BD was not to the extent seen in SZ, the two patient groups did not differ from each other (Cannon et al., 1997). Among other studies that directly compared SZ and BD, Uzalec et al. (2006) showed that BD and SZ PMA trajectories diverged such that SZ began exhibiting deterioration in late adolescence whereas BD patients remained stable across all developmental periods. Another study found differences between SZ and psychotic mood disorder earlier, reporting that SZ showed poorer social PMA than psychotic mood disorder in childhood and again in late adolescence (Tarbox et al., 2012).
Cluster analyses performed in nonaffective psychotic disorders reveal heterogeneity in PMA trajectories, with distinct clusters showing unique clinical, cognitive, and functioning profiles after disease onset. Larsen et al. (2004) performed separate cluster analyses on social and academic domains in a large sample of first episode non-affective psychosis patients. Those with a declining social course had fewer friends and greater negative symptoms after disease onset, and those with worse childhood academic adjustment achieved less education and had poorer working memory. Quee et al. (2014) identified six clusters based on social and academic PMA, ranging from “normal” to “overall impaired.” Horton et al. (2015) found three clusters of PMA trajectories in a sample of first-episode patients. The “stable poor” group had lower overall functioning at baseline and more severe negative symptoms at 1-year follow-up than the “stable good” group, and the “deteriorating” group did not differ from either of the other groups (Horton et al., 2015). To our knowledge, there has not been a cluster analysis performed on a BD sample.
To date, research on PMA in psychiatric disorders has primarily characterized and distinguished PMA based on diagnoses. However, focusing on diagnoses may obscure the potentially diverse trajectories that led to the individual’s current clinical and functional status. We aimed to elucidate the early course of SZ and BD by identifying patterns of premorbid social and academic adjustment trajectories in a transdiagnostic sample of patients with schizophrenia, schizoaffective, or bipolar disorder. Empirically derived PMA subgroups agnostic to diagnosis may provide insight into pathophysiologic overlap among major psychiatric disorders and delineate an intermediate phenotype that could help to increase homogeneity in biological studies (Schmael et al., 2007). Furthermore, it will provide information on the early course of psychiatric illness for specific interventions. We hypothesized that there would be distinct clusters of PMA trajectories independent of diagnostic category, and that poorer PMA trajectory would be associated with greater symptoms and worse functional outcome. Furthermore, we hypothesized that clusters will differ on factors associated with severe mental illness including childhood trauma and neurocognitive functioning.
2. Methods
2.1. Participants
All study procedures were approved by the appropriate Institutional Review Boards. Participants were recruited through hospital referrals and community advertisements. Informed consent was obtained from all participants. A total of 160 outpatients with a diagnosis of schizophrenia (n = 27), schizoaffective disorder (n = 29), bipolar I disorder (n = 89), or bipolar II disorder (n = 15), were enrolled through a local medical school (n = 125) and a local veterans affairs medical center (n = 35). Exclusion criteria aimed to reduce factors that may confound results of neurocognitive testing and functional measures, and consisted of: (1) history of central nervous system trauma or neurological disorder; (2) diagnosis of substance abuse or dependence within the past 3 months; (3) electroconvulsive therapy within the past 12 months; (4) estimated premorbid IQ of <70 using the Wide Range Achievement Test-3 (Wilkinson, 1993), and (5) attention deficit hyperactivity disorder or current use of medications that are known to significantly alter cognition (e.g., dextroamphetamine). See Table 1 for demographic and clinical characteristics based on diagnosis. SZ had a greater proportion of males, more overall symptoms, more negative symptoms, and less education than BD.
Table 1.
Demographic and clinical characteristics based on diagnosis, mean (SD) unless otherwise noted.
| BD | SZ | Statistics | |
|---|---|---|---|
| n = 104 | n = 56 | ||
| Diagnosis, n | – | ||
| ⬛Schizophrenia | – | 27 | |
| ⬛Schizoaffective disorder | – | 29 | |
| ⬛Bipolar I disorder | 89 | – | |
| ⬛⬛n (%) with psychosis history | 51 (57.3) | – | |
| ⬛Bipolar II disorder | 15 | – | |
| ⬛⬛n (%) with psychosis history | 5 (33.3) | – | |
| Age | 46.95 (11.35) | 48.75 (11.95) | t(158) = −0.938, p = 0.350 |
| Sex, n (%) | X2(1) = 6.291, p = 0.017 | ||
| ⬛Male | 57 (54.8) | 42 (75.0) | |
| ⬛Female | 47 (45.2) | 14 (25.0) | |
| Race, n (%) | X2(1) = 3.357, p = 0.082 | ||
| ⬛Caucasian | 41 (39.4) | 14 (25.0) | |
| ⬛Non Caucasian | 63 (60.6) | 42 (75.0) | |
| Ethnicity | X2(1) = 0.069, p = 0.832 | ||
| ⬛Hispanic | 83 (81.4) | 43 (79.6) | |
| ⬛Non Hispanic | 19 (18.6) | 11 (20.4) | |
| WRAT-3, standard score | 100.49 (14.89) | 99.87 (12.24) | t(158) = 0.265, p = 0.792 |
| Education, years | 14.63 (2.53) | 13.34 (1.93) | t(158) = 3.315, p = 0.001 |
| Handedness, n (%) | X2(1) = 0.391, p = 0.631 | ||
| ⬛Left | 13 (12.5) | 9 (16.1) | |
| ⬛Right | 91 (87.5) | 47 (83.9) | |
| Age of onset, years | 26.3 (7.59) | 25.39 (6.55) | t(158) = 0.753, p = 0.452 |
| BPRS | 22.61 (4.78) | 25.53 (5.45) | t(154) = −3.441, p = 0.001 |
| SANS total composite score | 5.71 (8.10) | 13.08 (9.85) | t(154) = −4.992, p < 0.001 |
| HAMD | 7.37 (6.28) | 8.41 (6.04) | t(158) = −1.018, p = 0.310 |
| YMRS | 2.94 (3.46) | 2.21 (3.95) | t(158) = 1.208, p = 0.229 |
SZ = schizophrenia/schizoaffective disorder group; BD = bipolar I and bipolar II disorder group; WRAT-3 = wide range achievement test – 3; BPRS = brief psychiatric rating scale; SANS = scale for the assessment of negative symptoms; HAMD = Hamilton rating scale for depression; YMRS = Young mania rating scale.
2.2. Clinical and functional measures
Trained postdoctoral fellows and research assistants administered the clinical and functional assessments. The measures in this study have been validated psychometrically, are widely used, and are generally considered in the field to be reliable and valid for the assessment of their respective constructs. The Structured Clinical Interview for DSM-IV (First et al., 2002) or DSM-5 (First et al., 2015) was used to determine DSM-5 diagnosis and age of onset. For SZ, age of onset was defined by the initial presence of psychotic symptoms that were significantly distressing or impairing. For BD, onset was defined by the initial presence of mood symptoms that met criteria for a major depressive or manic episode.
Premorbid adjustment was assessed using the Premorbid Adjustment Scale (PAS); (Cannon-Spoor et al., 1982), a widely-used retrospective rating scale that measures functioning across four developmental periods prior to the onset of psychiatric illness: childhood (up to 11 years), early adolescence (12–15 years), late adolescence (16–18 years), and adulthood (19 + years). The five subscales are sociability and withdrawal, peer relationships, socio-sexual aspects of life (beginning in early adolescence), scholastic performance, and adaptation to school. The PAS demonstrates good concurrent and predictive validity (Brill et al., 2008), discriminate validity (Cannon-Spoor et al., 1982; Krauss et al., 1998), and reliability across informant types including self-report, first-degree relative report, and combined data from all available sources including written records (Levitt et al., 1994). A personal interview was conducted using the PAS Structured Interview (Rabinowitz et al., 2007) to inform the ratings. Ratings range from 0 indicating no problems with adjustment to 6 indicating severe maladjustment. The PAS has been shown by principal component analysis to have two distinct factors: social and academic (Allen et al., 2001; Cannon et al., 1997; van Kammen et al., 1994). The social domain was calculated by summing the subscale scores for sociability and withdrawal, peer relationships, and socio-sexual aspects of life and dividing by the total possible score. The academic domain was similarly computed and included the subscales scores for scholastic performance and adaptation to school. Thus, higher percentages indicate worse adjustment. Furthermore, trajectories such as stable, improving, or declining can be identified by examining trends across developmental periods for a more dynamic understanding of PMA (Larsen et al., 2004). We only rated periods that were premorbid (i.e., prior to age of onset) and we did not use the adulthood period (19+) due to problems with its validity (van Mastrigt and Addington, 2002) and the limited number of patients with data in that developmental period.
Positive, negative, and affective symptoms were assessed using the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962), Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983), Hamilton Rating Scale for Depression (HAMD; Hamilton, 1960), and Young Mania Rating Scale (YMRS; Young et al., 1978). Self-reported substance use was measured with the Alcohol Use Disorder Identification Test-Consumption (AUDIT-C; Bush et al., 1998), and the Drug Abuse Screening Test (DAST-10; Bohn et al., 1991). Childhood trauma was assessed with the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 1994) with subscales including emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect.
The patients’ ability to perform basic living skills was assessed using the UCSD Performance-Based Skills Assessment-Brief (Mausbach et al., 2007), a role-play functional capacity assessment. Their adjustment in social roles was measured with the Social Adjustment Scale – Self-report (SAS-SR; Weissman, 1999). Their ability to perform everyday tasks was measured with the World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0; Üstün et al., 2010).
2.3. Neurocognitive measures
Neurocognitive functioning was assessed using the MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al., 2008). The subtests of the MCCB were selected by a committee of experts in the field and the psychometric properties of the MCCB have been extensively studied (Green et al., 2004). It is composed of 10 standardized cognitive tests used to assess seven cognitive domains: (1) processing speed; (2) attention and vigilance; (3) working memory; (4) verbal learning; (5) visual learning; (6) reasoning and problem solving; and (7) social cognition. The MCCB domain scores are expressed as t-scores with a mean of 50 and standard deviation of 10, normed based on the MCCB normative sample with age and gender corrections (Kern et al., 2008). The mean of the seven domain t-scores serve as a composite score. For the sample recruited at the local medical school, the verbal learning assessment was replaced with the California Verbal Learning Test – Second Edition (CVLT-II; Delis et al., 2000)) because of its demonstrated sensitivity in detecting difficulties in more mildly impaired bipolar disorder patients (Martínez-Arán et al., 2004; Yatham et al., 2010). For the 125 participants enrolled through the local medical school who received the CVLT-II instead of the MCCB verbal learning assessment, the verbal learning domain was calculated as the average of the trial 1 through 5 t-score and the short delay free recall t-score. While we acknowledge that using the immediate and delayed recall scores from the CVLT to calculate the verbal learning domain score differs from the MCCB which uses only the immediate recall score from the HVLT, we chose to obtain more information on both verbal learning and memory where available.
2.4. Statistical analysis
All statistical analyses were conducted using SPSS Statistics 23 and figures were created using R 3.2.3. In order to identify clusters of patients, regardless of diagnosis, based on their PMA, a single hierarchical cluster analysis was conducted using social and academic PMA scores for childhood, early adolescence, and late adolescence. Similarity between cases was computed with the squared Euclidean distance metric and agglomerative clustering was performed using Ward’s Method. Since the PAS scores were all on the same metric (i.e., percentage of possible score), no pre-standardization was necessary. Visual inspection of the dendogram and the plot of coefficients against the stage of cluster were used to determine the optimal number of clusters. Then, the cluster analysis was repeated with the pre-determined number of clusters and cluster membership was saved as a grouping variable. Stability of the clusters was verified by repeating the hierarchical cluster analysis on a random split half of the sample.
After the cluster analysis, the social and academic premorbid trajectories of each cluster were compared using a mixed ANOVA. The between-subjects factor was cluster and the within-subjects factors were domain (social, academic) and time (childhood, early adolescence, late adolescence). The Greenhouse-Geisser correction was applied when the assumption of sphericity was violated. Post hoc analyses examined differences between clusters as well as trajectories within clusters. Adjustment for the false discovery rate (FDR); (Benjamini and Hochberg, 1995) was used to correct for multiple comparisons (q = 0.05). We found that all results considered significant according to an alpha of 0.05 were also significant after correcting the FDR. We also examined differences between clusters on demographic, clinical, neurocognitive, and functional characteristics.
3. Results
3.1. Cluster analysis
The hierarchical cluster analysis revealed that the 160 patients were optimally clustered into three groups based on their premorbid social and academic adjustment trajectories. The first cluster consisted of 93 individuals (69.9% BD, 30.1% SZ), the second consisted of 39 individuals (61.5% BD, 38.5% SZ), and the third consisted of 28 individuals (53.6% BD, 46.4% SZ). The proportion of BD versus SZ diagnoses did not differ among the clusters, X2(2) = 2.79, p = 0.248, Cramer’s V = 0.132. Regarding demographic variables, cluster 2 had a significantly greater proportion of males than cluster 1 and cluster 1 had greater education than clusters 2 and 3. The three clusters did not differ age, race, ethnicity, handedness, or premorbid IQ (Table 2).
Table 2.
Demographic, clinical, and functional characteristics by Cluster, mean (SD) unless otherwise noted.
| Cluster 1 | Cluster 2 | Cluster 3 | Statistics | Post hoc (LSD) | |
|---|---|---|---|---|---|
| n = 93 | n = 39 | n = 28 | |||
| Age, years | 47.11 (11.43) | 49.74 (11.58) | 46.14 (11.96) | F(2, 157) = 0.98, p = 0.379 | – |
| Sex, n (%) | X2(2) = 8.88, p = 0.012 | 2 had greater proportion of males than 1 | |||
| ⬛Male | 49 (52.7) | 31 (79.5) | 19 (67.9) | ||
| ⬛Female | 44 (47.3) | 8 (20.5) | 9 (32.1) | ||
| Race, n (%) | X2(2) = 4.11, p = 0.128 | – | |||
| ⬛Caucasian | 35 (37.6) | 15(38.5) | 5 (17.9) | ||
| ⬛Non Caucasian | 58 (62.4) | 24 (61.5) | 23 (82.1) | ||
| Ethnicity, n (%) | X2(2) = 2.69, p = 0.260 | – | |||
| ⬛Hispanic | 75 (81.5) | 32 (86.5) | 19 (70.4) | ||
| ⬛Non Hispanic | 17 (18.5) | 5 (13.5) | 8 (29.6) | ||
| Site, n (%) | X2(2) = 2.79, p = 0.248 | – | |||
| ⬛Medical school | 75 (80.7) | 31 (79.5) | 19 (67.9) | ||
| ⬛Veterans affairs medical center | 18 (19.4) | 8 (20.5) | 9 (32.1) | ||
| WRAT-3 (premorbid IQ) | 101.43 (14.08) | 98.10 (14.08) | 99.46 (13.60) | F(2, 157) = 0.833, p = 0.437 | – |
| Education, years | 14.76 (2.41) | 13.31 (2.44) | 13.43 (1.81) | F(2, 157) = 7.141, p = 0.001 | 1 > 2, 3 |
| Handedness, n (%) | X2(2) = 2.60, p = 0.272 | – | |||
| ⬛Left | 13 (14.0) | 3 (7.7) | 6 (21.4) | ||
| ⬛Right | 80 (86.0) | 36 (92.3) | 22 (78.6) | ||
| Age of onset, years | 25.44 (6.79) | 27.13 (7.57) | 26.18 (8.24) | F(2, 157) = 0.757, p = 0.471 | – |
| History of psychosis, n (%) | X2(2) = 3.65, p = 0.161a | – | |||
| X2(2) = 1.74, p = 0.418b | |||||
| ⬛Yes | 60 (64.5) | 29 (74.4) | 23 (82.1) | ||
| ⬛No | 33 (35.5) | 10 (25.6) | 5 (17.9) | ||
| BPRS (clinical symptoms) | 22.22 (4.04) | 25.03 (5.00) | 26.11 (7.12) | F(2, 153) = 8.664, p < 0.001 | 1 < 2, 3 |
| SANS (negative symptoms) | F(2, 153) = 11.33, p < 0.001 | 1, 2 < 3 | |||
| ⬛Total composite | 5.82 (6.86) | 9.11 (10.173) | 14.79 (12.05) | ||
| HAMD (depressive symptoms) | 6.35 (5.58) | 9.26 (6.32) | 10.18 (6.91) | F(2, 157) = 6.018, p = 0.003 | 1 < 2, 3 |
| YMRS (manic symptoms) | 2.20 (3.33) | 3.51 (3.31) | 3.14 (4.80) | F(2, 157) = 2.067, p = 0.130 | – |
| CTQ (childhood trauma) | |||||
| ⬛Emotional abuse | 11.37 (5.80) | 11.11 (4.18) | 13.44 (5.89) | F(2145) = 1.636, p = 0.198 | |
| ⬛Physical abuse | 8.68 (4.62) | 9.67 (4.26) | 10.74 (5.42) | F(2, 148) = 2.146, p = 0.121 | |
| ⬛Sexual abuse | 7.86 (5.20) | 7.92 (4.84) | 9.75 (5.81) | F(2, 152) = 1.466, p = 0.234 | |
| ⬛Emotional neglect | 11.12 (5.42) | 11.53 (4.33) | 14.64 (4.88) | F(2, 153) = 5.228, p = 0.006 | 1, 2 < 3 |
| ⬛Physical neglect | 7.88 (3.69) | 9.14 (3.93) | 9.37 (4.25) | F(2, 150) = 2.342, p = 0.100 | |
| AUDIT-C (alcohol abuse) | 2.21 (2.15) | 1.79 (2.04) | 2.81 (2.16) | F(2, 116) = 1.393, p = 0.252 | – |
| DAST (drug abuse) | 1.99 (2.68) | 2.38 (2.75) | 1.21 (2.35) | F(2, 154) = 1.626, p = 0.200 | – |
| SAS-SR (social adjustment) | 2.15 (0.55) | 2.22 (0.57) | 2.40 (0.52) | F(2, 154) = 2.106, p = 0.125 | – |
| UPSA-B (functional capacity) | 80.12 (13.82) | 76.85 (11.78) | 77.02 (13.68) | F(2, 150) = 1.085, p = 0.340 | – |
| WHODAS (disability) | 21.63 (15.47) | 22.12 (15.72) | 27.04 (18.77) | F(2, 149) = 1.125, p = 0.327 | – |
SZ = schizophrenia/schizoaffective disorder group; BD = bipolar I and bipolar II disorder group; WRAT-3 = wide range achievement test – 3; BPRS = brief psychiatric rating scale; SANS = scale for the assessment of negative symptoms; HAMD = Hamilton rating scale for depression; YMRS = Young Mania rating scale; CTQ = childhood trauma questionnaire; AUDIT-C = alcohol use disorder identification test-consumption; DAST = drug abuse screening test; SAS = social adjustment scale-self report; UPSA-B = UCSD performance-based skills assessment-brief; WHODAS = World Health Organization Disability Assessment Schedule.
in full sample of SZ and BD;
in BD alone.
3.2. Between-subjects analysis
PAS scores by cluster in each domain are presented in Fig. 1. A control group from Radoeva et al. (2017) was used as comparison, as they also reported PAS scores separately for the social and academic domains in each developmental period. To characterize the three groups, a mixed ANOVA was conducted with the between-subjects factor of cluster (1, 2, 3) and within-subjects factors of domain (social, academic) and time (childhood, early adolescence, and late adolescence). Given that sex differed between the clusters, it was added as a covariate. Even though education differed between clusters, it was not included as a covariate because it comes temporally after the premorbid period and was a variable of interest. The analysis revealed a significant group × domain × time interaction, F(3.75, 288.85) = 6.14, p < 0.001. Post hoc analyses examined the group × time interaction in the two domains separately. In the social domain, the interaction was significant, F(3.49, 268.91) = 7.62, p < 0.001. During childhood and early adolescence, all 3 groups differed significantly from each other with cluster 2 exhibiting the lowest scores (best adjustment), followed by cluster 1, and cluster 3 having the highest scores (worst adjustment). During late adolescence, clusters 1 and 2 did not differ from each other and were both significantly lower than cluster 3. In the academic domain, the group × time interaction was also significant, F(3.69, 283.96) = 8.71, p < 0.001. Clusters 2 and 3 did not differ from each other at any developmental period and both were significantly higher than cluster 1 at all times (Table 3 and Fig. 1).
Fig. 1.
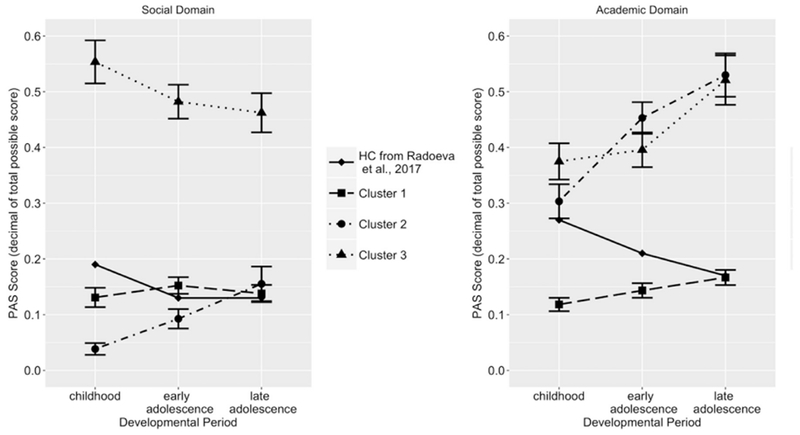
Premorbid adjustment scores in each cluster by domain, Mean ± SE. PAS = Premorbid Adjustment Scale. Higher PAS score = worse adjustment.
Table 3.
Between- and within-subjects analyses.
| Cluster 1 | Cluster 2 | Cluster 3 | Between-subjects statistics | |
|---|---|---|---|---|
| Social domain | ||||
| Childhood (ch) | 1 vs 2 p = 0.002* | |||
| 0.13 (0.17) | 0.04 (0.07) | 0.55 (0.20) | 1 vs 3 p < 0.001* | |
| 2 vs 3 p < 0.001* | ||||
| Early adolescence (ea) | 1 vs 2 p = 0.026* | |||
| 0.15 (0.14) | 0.09 (0.11) | 0.48 (0.16) | 1 vs 3 p < 0.001* | |
| 2 vs 3 p < 0.001* | ||||
| Late adolescence (la) | 1 vs 2 p = 0.590 | |||
| 0.14 (0.15) | 0.16 (0.19) | 0.46 (0.19) | 1 vs 3 p < 0.001* | |
| 2 vs 3 p < 0.001* | ||||
| Within-subjects statistics | ch vs ea p = 0.002* | |||
| N/A – omnibus F | ch vs la p < 0.001* | N/A – omnibus F | ||
| not significant | ea vs la p = 0.068 | not significant | ||
| Academic domain | ||||
| Childhood (ch) | 1 vs 2 p < 0.001* | |||
| 0.12 (0.12) | 0.30 (0.19) | 0.38 (0.17) | 1 vs 3 p < 0.001* | |
| 2 vs 3 p = 0.052 | ||||
| Early adolescence (ea) | 1 vs 2 p < 0.001* | |||
| 0.14 (0.13) | 0.45 (0.18) | 0.40 (0.17) | 1 vs 3 p < 0.001* | |
| 2 vs 3 p = 0.117 | ||||
| Late adolescence (la) | 1 vs 2 p < 0.001* | |||
| 0.17 (0.13) | 0.53 (0.24) | 0.52 (0.23) | 1 vs 3 p < 0.001* | |
| 2 vs 3 p = 0.842 | ||||
| Within-subjects statistics | ch vs ea p = 0.021* | ch vs ea p < 0.001* | ch vs ea p = 0.225 | |
| ch vs la p < 0.001* | ch vs la p < 0.001* | ch vs la p < 0.001* | ||
| ea vs la p = 0.094 | ea vs la p = 0.188 | ea vs la p = 0.003* |
Note: All statistically significant results remain significant after false discovery rate correction.
3.3. Within-subjects analysis
To examine trajectory within clusters, repeated measures ANOVAs were performed in the three clusters separately (Table 3), with the within-subjects factor as time. In cluster 1, there was a significant domain × time interaction, F(1.66, 151.21 = 3.34, p = 0.047). In the social domain, the overall ANOVA was not significant, p > 0.3, indicating no difference in PAS scores among the developmental periods. In the academic domain, the overall ANOVA revealed a significant effect of time, F(1.87, 170.51) = 7.19, p = 0.001. Follow-up pairwise comparisons indicated that the score in childhood was significantly lower than early and late adolescence, while early and late adolescence did not differ from each other. In cluster 2, there was a significant domain × time interaction, F(2, 74) = 3.331, p = 0.041. In the social domain, there was a significant effect of time, F(1.61, 59.50) = 9.95, p < 0.001. Follow-up pairwise comparisons indicated that the score in childhood was significantly lower than both early and late adolescence and that early and late adolescence did not differ from one another. In the academic domain, there was also a significant effect of time, F(2, 74) = 11.85, p < 0.001. Follow-up pairwise comparisons indicated that the score in childhood was lower than both early and late adolescence and that early and late adolescence did not differ from one another. In cluster 3, there was a significant domain × time interaction, F(2, 52) = 10.56, p < 0.001. In the social domain, there was a trend effect of time after correcting for sphericity, F(1.49, 38.68) = 3.19, p = 0.066. In the academic domain, there was a significant effect of time, F(2, 52) = 11.12, p < 0.001. Follow-up pairwise comparisons indicated that childhood and early adolescence did not differ, and they were both lower than late adolescence (Table 3 and Fig. 1).
Based on the above analyses and comparisons with a healthy control group from Radoeva et al. (2017) and original control data in Cannon-Spoor et al. (1982), the three clusters were characterized as follows: cluster 1 was labeled as “overall normal,” cluster 2 was labeled as “normal social/impaired declining academic,” and cluster 3 was labeled as “impaired stable social/impaired declining academic.”
3.4. Clinical, neurocognitive, and functional characteristics by cluster
The normal overall cluster (cluster 1) had less overall symptoms and less depressive symptoms than the other groups. The impaired stable social/impaired declining academic group (cluster 3) had more negative symptoms and more childhood trauma, specifically childhood emotional neglect, than the other two groups. The three groups did not differ significantly on age of onset, manic symptoms, or drug/alcohol use. They also did not differ in the proportion of patients with a history of psychosis in the entire sample (X2(2) = 3.65, p = 0.161) or in the BD patients alone (X2(2) = 1.74, p = 0.418; Table 2).
Given that clinical symptoms differed between clusters, the BPRS was used as a covariate when examining cluster differences in neurocognition and functioning in basic living skills, social roles, and everyday tasks. The BPRS was chosen because it is a measure of general psychopathology and crosses diagnostic relevance. The clusters did not significantly differ on the MCCB composite score, F(l, 137) = 0.51, p > 0.4; (Fig. 2). Furthermore, a 3 (cluster) × 7 (neurocognitive domain) mixed ANCOVA covarying for BPRS did not reveal any significant interactions or main effect involving cluster (p > 0.7; Fig. 2). The clusters also did not differ on the measures of community and social functioning (Table 2).
Fig. 2.
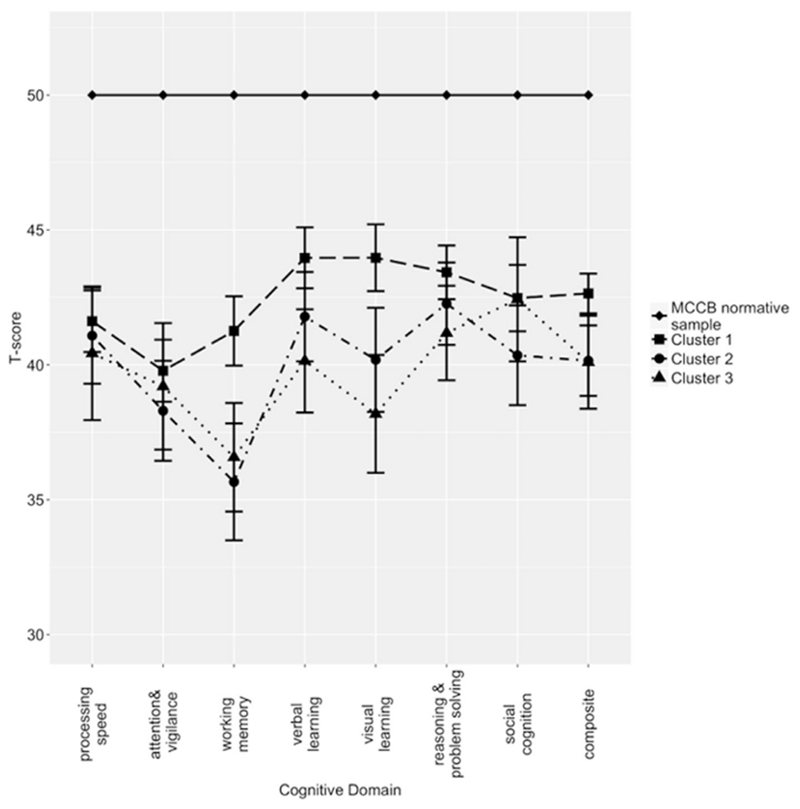
Neurocognitive performance among the clusters on the MATRICS Consensus Cognitive Battery.
4. Discussion
Major psychiatric disorders are generally defined as discrete, categorical entities; however, biological and phenomenological overlap between disorders is common. For SZ and BD in particular, diagnostic group level comparisons may be overly simplistic. However, studies that examine the potential heterogeneity and overlap between premorbid functioning in SZ and BD agnostic to diagnosis are scarce. We conducted a transdiagnostic cluster analysis and identified empirically three distinct PMA trajectory patterns among patients with SZ and BD. Each cluster contained similar proportions of SZ and BD diagnoses, which supports the hypothesis that PMA is not directly related to diagnosis and that there is substantial overlap in the two diagnostic categories. Our findings challenge the notion that, by definition, SZ and BD have different PMA trajectories, and suggest that there are shared neurodevelopmental abnormalities with subgroups of differential PMA trajectories that cut across diagnoses.
The three clusters also did not differ in the proportions of patients who has a history of psychosis, whether in the entire sample or in the BD patients alone. This suggests that there is also substantial overlap in PMA trajectories between BD patients with and without psychosis. In fact, follow-up analyses revealed that BD patients with and without a history of psychosis did not significantly differ on any of the PMA scores (all ps > 0.05), further supporting the idea that the clusters based on premorbid adjustment do not simply recapitulate existing clinical subtypes/diagnosis but rather, provide a different unique classification.
The normal overall cluster (cluster 1) consisted of patients who had no functioning difficulties prior to adulthood. Based on Fig. 1 and numerical data, this cluster appears to be comparable to a healthy control group in another study (Radoeva et al., 2017) in the social domain, and to outperform them in the academic domain. This finding is consistent with a study in BD showing that students with excellent performance in school were at heightened risk for bipolar disorder (Monte et al., 2008). The normal social/impaired declining academic cluster (cluster 2) showed an interesting dissociation between the social and academic domains in that they exhibited no difficulties with social PMA but had impaired and deteriorating academic PMA. Our findings are consistent with those of previous studies that showed social and academic domains to be distinct in both level of impairment and trajectory (Larsen et al., 2004; Monte et al., 2008). Finally, the impaired stable social/impaired declining academic cluster (cluster 3) exhibited premorbid maladjustment in both academic and social domains beginning in childhood, with the academic domain declining through development.
According to the historical perspective that premorbid maladjustment is predominantly associated with SZ, we would expect most or all of the normal overall cluster (cluster 1) to be comprised of BD patients and most or all of the impaired stable social/impaired declining academic cluster (cluster 3) to be comprised of SZ patients. However, the major finding in this study was that SZ and BD had a substantial presence in each of the clusters, with no single diagnostic category dominating a cluster by more than 70%. The relatively more balanced distribution of diagnoses demonstrates the heterogeneity in PMA in both disorders, as well as overlap across disorders, and provides information independent of diagnosis.
In partial support of our hypothesis, the impaired stable social/impaired declining academic cluster (cluster 3) had more negative symptoms than the other clusters, although there was no difference in manic symptoms, substance use, and whether there was a history of psychosis. Individuals who later develop more severe negative symptoms may be more likely to have exhibited these abnormalities prior to illness onset, which could adversely affect social functioning. Cluster 3 also had more severe childhood emotional neglect and negative symptoms than the other clusters. There is some evidence that childhood trauma may be associated with poorer premorbid functioning in patients with psychosis (Stain et al., 2014) and there is abundant literature implicating childhood trauma, including neglect, to later development of psychiatric illness including psychotic (Read et al., 2005) and mood disorders (Dvir et al., 2014). However, emotional neglect was only one of five categories of childhood trauma assessed that differed between clusters in our sample. Future studies may wish to examine the impact of specific types of childhood trauma, for example, whether emotional neglect is particularly detrimental to premorbid social and academic functioning.
These findings are important for increasing clinical awareness that there is overlapping PMA trajectories between SZ and BD. Recognizing PMA patterns early and in combination with other clinical and cognitive information (e.g., cognitive performance) can begin to inform personalized prevention and treatment efforts in the time prior to disease onset. Although we found three distinct clusters of PMA trajectories among our sample, contrary to our hypothesis, they did not differ in current neurocognitive or everyday and social functioning. It is possible that inadequate, poorly-timed intervention, or standard intervention applied uniformly, limited the potential mitigation of neurocognitive and functional decline of even the most premorbidly well-adjusted individuals. Moreover, if PMA is considered an intermediate phenotype that reflects genetic risk (Schmael et al., 2007), then environmental factors and gene-environment interactions may help to explain the course to fairly homogeneous outcomes. Our findings lay the groundwork for determining subgroups across disorders with common neurodevelopmental pathophysiology. This would help identify biomarkers that may predict specific profiles and early interventions that could alter the course of the illness.
We included scores on each domain in each developmental period in the cluster analysis for a more holistic picture of PMA trajectory. However, this also makes our study difficult to compare with others that identified PMA profiles by combining the social and academic domains (Addington and Addington, 2005; Cole et al., 2012; Horton et al., 2015) or clustering on the social and academic domains separately (Larsen et al., 2004; Trauelsen et al., 2016). Our methods were most comparable to those of Quee et al. (2014) in that each domain in each developmental period was used separately to establish clusters, although they only examined nonaffective psychotic patients. Our normal overall, normal social/impaired declining academic, and impaired stable social/impaired declining academic clusters parallel findings of a “normal” group, an “academic decline” group, and “overall impaired” group in Quee et al. (2014). Our clusters performed 0.5–1.5 SD below the MCCB normative sample even though there was no difference between clusters. Quee et al. (2014) also found few differences in cognitive performance between their clusters. They did, however, report some differences between clusters on functional outcomes including percentage of patients who manage finances independently and on a clinician-rated measure of functioning. Our results did not support differences by cluster on the measures of functioning that we used, which may reflect differences in sample characteristics or psychometric properties of the scales employed.
Another limitation to this study is the small sample size and the greater number of BD than SZ patients in the sample. However, the primary analysis was the 2 (diagnosis) × 3 (cluster) chi-square test which does not require equal sample sizes (McHugh, 2013), and the result was nonsignificant with a small effect, which suggests we would likely not detect group differences even with a larger sample size. Nevertheless, future studies using larger sample sizes with greater statistical power is needed to replicate the current findings. Additionally, the PAS was rated based on retrospective report, as was our method for determining age of onset, which may introduce recall bias. We did not have our own control PAS data and were only able to compare our clusters’ PMA with a control group from another published study (Radoeva et al., 2017), the data of which were very similar to that of the original validation study (Cannon-Spoor et al., 1982). Furthermore, we only included patients who had complete PAS data from childhood to late adolescence because we were interested in PMA trajectories. As a result, those with onset prior to late adolescence were not included. Future prospective studies that examine potential differences in PMA trajectories in patients with earlier versus later onset would help to address these limitations. Nonetheless, our study contributes to the literature by being the first to identify PMA trajectory clusters using a transdiagnostic approach in BD and SZ. A more transdiagnostic perspective of premorbid functioning focused on differential PMA trajectory patterns agnostic to diagnosis may inform early intervention efforts and reduce heterogeneity in future studies.
Acknowledgments
Role of the funding source
This work was supported by the National Institute of Mental Health [R01MH100125] and a VA Merit Award [I01CH0000995] to KEB. Writing of this manuscript was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.
Footnotes
Conflict of interest
None.
References
- Addington J, Addington D, 2005. Patterns of premorbid functioning in first episode psychosis: relationship to 2-year outcome. Acta Psychiatr. Scand 112, 40–46. [DOI] [PubMed] [Google Scholar]
- Allen DN, Kelley ME, Miyatake RK, Gurklis JA, van Kammen DP, 2001. Confirmation of a two-factor model of premorbid adjustment in males with schizophrenia. Schizophr. Bull 27, 39–46. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental disorders: DSM-5. American Psychiatric Association, Washington, D.C. [Google Scholar]
- Andreasen NC, 1983. Scale For the Assessment of Negative Symptoms (SANS). University of Iowa, Iowa City, Iowa. [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Babor T, Kranzler HR, 1991. Validity of the drug abuse screening test (DAST-10) in inpatient substance abusers. Probl. Drug Depend 119, 233–235. [Google Scholar]
- Bora E, Yucel M, Pantelis C, 2009. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. Br. J. Psychiatry 195, 475–482. [DOI] [PubMed] [Google Scholar]
- Brill N, Reichenberg A, Weiser M, Rabinowitz J, 2008. Validity of the premorbid adjustment scale. Schizophr. Bull 34, 981–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Russo M, Frangou S, Mahon K, Braga RJ, Shanahan M, Malhotra AK, 2014. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychol. Med 44, 3083–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, 1998. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med 158, 1789–1795. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, Murray RM, 1997. Premorbid social functioning in schizophrenia and bipolar disorder: similarities and differences. Am. J. Psychiatry 154, 1544–1550. [DOI] [PubMed] [Google Scholar]
- Cannon-Spoor HE, Potkin SG, Wyatt RJ, 1982. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr. Bull 8, 470. [DOI] [PubMed] [Google Scholar]
- Cole VT, Apud JA, Weinberger DR, Dickinson D, 2012. Using latent class growth analysis to form trajectories of premorbid adjustment in schizophrenia. J. Abnorm. Psychol 121, 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH, Ober RA, 2000. The California Verbal Learning Test—2nd Edition, Adult Version: A Comprehensive Assessment of Verbal Learning and Memory. The Psychological Corportion, San Antonio, TX. [Google Scholar]
- Dvir Y, Ford JD, Hill M, Frazier JA, 2014. Childhood maltreatment, emotional dysregulation, and psychiatric comorbidities. Harv. Rev. Psychiatry 22, 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Tsuang MT, 2003. Heterogeneity and the genetics of bipolar disorder. Am. J. Med. Genet 123C, 1–9. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB, 2002. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research. New York State Psychiatric Institute, New York. [Google Scholar]
- First MB, Williams JB, Karg RS, Spitzer RL, 2015. User’s Guide for the Structured Clinical Interview for DSM-5 Disorders, Research Version (SCID-5-RV). American Psychiatric Association, Arlington, VA. [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RSE, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR, 2004. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiatry 56, 301–307. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthausen EA, Wiersma D, Sitskoorn MM, Hijman R, Dingemans PM, Schene AH, van den Bosch RJ, 2002. Schizophrenic patients without neuropsychological deficits: subgroup, disease severity or cognitive compensation. Psychiatry Res 112, 1–11. [DOI] [PubMed] [Google Scholar]
- Horton LE, Tarbox SI, Olino TM, Haas GL, 2015. Trajectories of premorbid childhood and adolescent functioning in schizophrenia-spectrum psychoses: a first-episode study. Psychiatry Res 227, 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley ME, Gilbertson M, Mouton A, Van Kammen DP, 1992. Deterioration in premorbid functioning in schizophrenia: a developmental model of negative symptoms in drug-free patients. Am. J. Psychiatry 149, 1543–1543. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, 2008. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am. J. Psychiatry 165, 214–220. [DOI] [PubMed] [Google Scholar]
- Krauss H, Marwinski K, Held T, Rietschel M, Freyberger HJ, 1998. Reliability and validity of the premorbid adjustment scale (PAS) in a German sample of schizophrenic and schizoaffective patients. Eur. Arch. Psychiatry Clin. Neurosci 248, 277–281. [DOI] [PubMed] [Google Scholar]
- Larsen TK, Friis S, Haahr U, Johannessen JO, Melle I, Opjordsmoen S, Rund BR, Simonsen E, Vaglum PV, McGlashan TH, 2004. Premorbid adjustment in first-episode non-affective psychosis: distinct patterns of pre-onset course. Br. J. Psychiatry 185, 108–115. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Shenton ME, McCarley RW, Faux SF, Ludwig AS, 1994. Premorbid adjustment in schizophrenia: implications for psychosocial and ventricular pathology. Schizophr. Res 12, 159–168. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM, 2009. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 373, 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacBeth A, Gumley A, 2008. Premorbid adjustment, symptom development and quality of life in first episode psychosis: a systematic review and critical reappraisal. Acta Psychiatr. Scand 117, 85–99. [DOI] [PubMed] [Google Scholar]
- Martínez-Arán A, Vieta E, Reinares M, Colom F, Torrent C, Sánchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M, 2004. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am. J. Psychiatry 161, 262–270. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL, 2007. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr. Bull 33, 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh ML, 2013. The chi-square test of independence. Biochem Med 23, 149–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor KS, Friedman-Yakoobian M, Leung YJ, Meyer EC, Zimmet SV, Caplan B, Monteleone T, Bryant C, Guyer M, Keshavan MS, 2015. The impact of premorbid adjustment, neurocognition, and depression on social and role functioning in patients in an early psychosis treatment program. Aust. N. Z. J. Psychiatry 49, 444–452. [DOI] [PubMed] [Google Scholar]
- Monte RC, Goulding SM, Compton MT, 2008. Premorbid functioning of patients with first-episode nonaffective psychosis: a comparison of deterioration in academic and social performance, and clinical correlates of premorbid adjustment scale scores. Schizophr. Res 104, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Lewis SW, 1987. Is schizophrenia a neurodevelopmental disorder. Br. Med. J. Clin. Res. Ed 295, 681–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C, 2004. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr. Res 71, 405–416. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry 165, 203–213. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR, 1962. The brief psychiatric rating scale. Psychol. Rep 10, 799–812. [Google Scholar]
- Owen MJ, O’Donovan MC, Thapar A, Craddock N, 2011. Neurodevelopmental hypothesis of schizophrenia. Br. J. Psychiatry 198, 173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quee PJ, Meijer JH, Islam MA, Aleman A, Alizadeh BZ, Meijer CJ, van den Heuvel ER, GROUP Investigators, 2014. Premorbid adjustment profiles in psychosis and the role of familial factors. J. Abnorm. Psychol 123, 578–587. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, De Smedt G, Harvey PD, Davidson M, 2002. Relationship between premorbid functioning and symptom severity as assessed at first episode of psychosis. Am. J. Psychiatry 159, 2021–2026. 10.1176/appi.ajp.159.12.2021. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, Levine S, Brill N, Bromet E, 2007. The premorbid adjustment scale structured interview (PAS-SI): preliminary findings. Schizophr. Res 90, 255–257. [DOI] [PubMed] [Google Scholar]
- Radoeva PD, Fremont W, Antshel KM, Kates WR, 2017. Longitudinal study of premorbid adjustment in 22qll.2 deletion (velocardiofacial) syndrome and association with psychosis. Dev. Psychopathol 29, 93–106. [DOI] [PubMed] [Google Scholar]
- Read J, Os J, Morrison AP, Ross CA, 2005. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr. Scand 112, 330–350. [DOI] [PubMed] [Google Scholar]
- Rietschel M, Georgi A, Schmael C, Schirmbeck F, Strohmaier J, Boesshenz KV, Schwarz M, Nöthen MM, Schulze TG, 2009. Premorbid adjustment: a phenotype highlighting a distinction rather than an overlap between schizophrenia and bipolar disorder. Schizophr. Res 110, 33–39. [DOI] [PubMed] [Google Scholar]
- Schmael C, Georgi A, Krumm B, Buerger C, Deschner M, Nöthen MM, Schulze TG, Rietschel M, 2007. Premorbid adjustment in schizophrenia—an important aspect of phenotype definition. Schizophr. Res 92, 50–62. [DOI] [PubMed] [Google Scholar]
- Stain HJ, Bronnick K, Hegelstad WTV, Joa I, Johannessen JO, Langeveld J, Mawn L, Larsen TK, 2014. Impact of interpersonal trauma on the social functioning of adults with first-episode psychosis. Schizophr. Bull 40, 1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, 2013. Heterogeneity of schizophrenia: genetic and symptomatic factors. Am. J. Med. Genet. B Neuropsychiatr. Genet 162, 648–652. [DOI] [PubMed] [Google Scholar]
- Tarbox SI, Brown LH, Haas GL, 2012. Diagnostic specificity of poor premorbid adjustment: comparison of schizophrenia, schizoaffective disorder, and mood disorder with psychotic features. Schizophr. Res 141, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauelsen AM, Bendall S, Jansen JE, Nielsen H-GL, Pedersen MB, Trier CH, Haahr UH, Simonsen E, 2016. Childhood adversities: social support, premorbid functioning and social outcome in first-episode psychosis and a matched case-control group. Aust. N. Z. J. Psychiatry 50, 770–782. [DOI] [PubMed] [Google Scholar]
- Üstün TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, Saxena S, van Korff M, Pull C, in collaboration with WHO/NIH Joint Project, 2010. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull. World Health Org 88, 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzelac S, Jaeger J, Berns S, Gonzales C, 2006. Premorbid adjustment in bipolar disorder: comparison with schizophrenia. J. Nerv. Ment. Dis 194, 654–658. [DOI] [PubMed] [Google Scholar]
- van Kammen DP, Kelley ME, Gilbertson MW, Gurklis J, O’Connor DT, 1994. CSF dopamine beta-hydroxylase in schizophrenia: associations with premorbid functioning and brain computerized tomography scan measures. Am. J. Psychiatry 151, 372–378. [DOI] [PubMed] [Google Scholar]
- van Mastrigt S, Addington J, 2002. Assessment of premorbid function in first-episode schizophrenia: modifications to the premorbid adjustment scale. J. Psychiatry Neurosci 27, 92–101. [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, 1987. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44, 660–669. [DOI] [PubMed] [Google Scholar]
- Weissman M, 1999. Social Adjustment Scale – Self-Report (SAS-SR) User’s Manual. Multi-Health Systems, Inc, New York. [Google Scholar]
- Wilkinson GS, 1993. WRAT-3: Wide Range Achievement Test Administration Manual. Wide Range, Inc, Wilmington Del. [Google Scholar]
- Yatham LN, Torres IJ, Malhi GS, Frangou S, Glahn DC, Bearden CE, Burdick KE, Martínez-Arán A, Dittmann S, Goldberg JF, Ozerdem A, Aydemir O, Chengappa KNR, 2010. The International Society for Bipolar Disorders-Battery for Assessment of Neurocognition (ISBD-BANC). Bipolar Disord 12, 351–363. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry J. Ment. Sci 133, 429–435. [DOI] [PubMed] [Google Scholar]


