Abstract
Exposure to inorganic arsenic (InAs) via drinking water and/or food is a considerable worldwide problem. Methylation of InAs generates monomethyl (MMAsIII+V)- and dimethyl (DMAsIII+V)-arsenical species in a process that facilitates urinary As elimination; however, MMA is considerably more toxic than either InAs or DMAs. Emerging evidence suggests that incomplete methylation of As to DMAs, resulting in increased MMAs, is associated with increased risk for a host of As-related health outcomes. The biochemical pathway that provides methyl groups for As methylation, one-carbon metabolism (OCM), is influenced by folate and other micronutrients, including choline and betaine. Individuals and species differ widely in their ability to methylate As. A growing body of research, including cell-culture, animal-model, and epidemiological studies, has demonstrated the role of OCM-related micronutrients in As methylation. This review examines the evidence that nutritional status and nutritional interventions can influence the metabolism and toxicity of As, with a primary focus on folate.
Keywords: folate, one-carbon metabolism, arsenic, arsenic methylation, nutrition
1. INTRODUCTION
Arsenic (As) is a naturally occurring element that is relatively ubiquitous in insoluble forms in soil. However, As in soil can be mobilized, leading to enrichment of As in groundwater (160). Ingested inorganic As (InAs) undergoes hepatic methylation in a process that relies on one-carbon metabolism (OCM), a biochemical pathway that is dependent on folate and other micronutrients. This article provides a brief overview of As exposure and As metabolism, including what is known about the influence of As methylation on As elimination, As toxicity, and risk for As-related health outcomes. We then focus on summarizing the evidence from human studies indicating that nutritional status and nutritional interventions that influence OCM can increase As methylation capacity and potentially reduce As toxicity in at-risk populations, with a primary emphasis on folate.
1.1. Arsenic Exposure
The World Health Organization (WHO) estimates that over 200 million people in more than 70 countries are exposed to As in drinking water at concentrations greater than 10 μg/L, the WHO guideline and US Environmental Protection Agency (EPA) maximum contaminant level (165). Among the most severely affected countries are Bangladesh, Cambodia, China, India, Myanmar, Nepal, Pakistan, Taiwan, Argentina, and Vietnam (125). Collectively, these countries bear a high proportion of the global burden of As-related morbidity and mortality. In Bangladesh, exposure to As through drinking water began in the 1960s, when UNICEF and other nongovernmental organizations advocated a massive switch from drinking microbially contaminated surface water to groundwater in an effort to reduce infant mortality due to diarrheal disease. Unfortunately, 20 years passed before it was discovered that the groundwater is contaminated with naturally occurring As, with concentrations ranging from nondetectable to levels exceeding 1,000 μg/L (132).
In the United States, As concentrations in groundwater are elevated in areas of the West, Midwest, and Northeast (7). It is estimated that at least five million people are exposed to As-contaminated water in the United States owing to technical challenges that prevent implementation of the drinking water standard and to the use of private wells, which are not covered by the EPA regulations (44).
While the predominant source of exposure to As worldwide is through drinking water, the ingestion of As through As-contaminated beverages (such as apple juice and some red wines), and foods (particularly rice and rice products) can contribute to a relatively large proportion of exposure in regions where drinking water As concentrations are low (34, 162). Arsenic concentrations in selected foods and beverages surveyed by the US Food and Drug Administration (FDA) and EPA are presented in Table 1 (for a more comprehensive list of As concentrations in common foods, see 45–47).
Table 1.
Summary of selected dietary sources of arsenic exposure
| Arsenic source | Reference | Sample | Arsenic concentration range |
|---|---|---|---|
| Brown rice (includes basmati, jasmine, long/medium/short grain, instant) | FDA (45) | Commercially available and supplied by the USA Rice Federation, FDA sampling, 2013 (N = 144) | Total As: 57–854 ppb InAs: 20–249 ppb (mean, weighted market share: 153.8 ppb) MMAs: <LOD–25 ppb DMAs: 11–568 ppb |
| White rice (includes basmati, jasmine, long/medium/short grain, instant) | FDA (45) | Commercially available and supplied by the USA Rice Federation, FDA sampling, 2013 (N = 429) | Total As: 40–776 ppb InAs: 20–196 ppb (mean, weighted market share: 92.3 ppb) MMAs: <LOD–23 ppb DMAs: 10–687 ppb |
| Infant brown rice cereal | FDA (45) | Commercially available, FDA sampling, 2013 and 2016 (N = 59) | InAs: 30–254 ppb (mean: 119.9 ppb) |
| Infant white rice cereal | FDA (45) | Commercially available, FDA sampling, 2013 and 2016 (N = 86) | InAs: 21–151 ppb (mean: 105.3 ppb) |
| Toddler/infant rice cereal | FDA (45) | Commercially available, FDA sampling, 2016 (N = 82) | Total As: 18.0–224 ppb (mean: 124 ppb) InAs: 20.8–126 ppb (mean: 97.8 ppb) MMAs: 4.4–4.8 ppb (mean: 4.6 ppb) DMAs: 3.3–94.2 ppb (mean: 30.5 ppb) |
| Infant formula | FDA (45) | Commercially available, FDA sampling, 2013 (N = 10) | Total As: 1–2 ppb (mean: 1 ppb) InAs: 0–1 ppb (mean: 1 ppb) |
| Rice cereal (includes hot and ready-to-eat) | FDA (45) | Commercially available, FDA sampling, 2013 (N = 110) | Total As: 50–810 ppb (mean: 176 ppb) InAs: 20–545 ppb (100 ppb) MMAs: 3–14 ppb (6 ppb) DMAs: 7–493 ppb (63 ppb) |
| Rice cakes | FDA (45) | Commercially available, FDA sampling, 2013 (N = 59) | Total As: 32–620 ppb (255 ppb) InAs: 23–273 ppb (145 ppb) MMAs: 5–17 ppb (9 ppb) DMAs: 15–477 (123 ppb) |
| Meal replacement/energy bars | FDA (45) | Commercially available, FDA sampling, 2013 (N = 29) | Total As: 10–130 ppb (mean: 68 ppb) InAs: 5–98 ppb (mean: 50 ppb) MMAs: <LOD DMAs: 6–28 ppb (mean: 13 ppb) |
| Rice beverages | FDA (45) | Commercially available, FDA sampling, 2013 (N = 42) | Total As: 6–320 ppb (mean: 109 ppb) InAs: 1–278 ppb (mean: 60 ppb) MMAs: <LOD DMAs: 5–85 ppb (mean: 28 ppb) |
| Apple juice | FDA (47) | EPA Toxic Elements Program, 2005–2011; Apple Juice Survey, 2011 (N = 144) | Total As: <LOD–45 ppb (mean: 7.2 ppb) |
| Apple juice concentrate | FDA (47) | EPA Toxic Elements Program 2005–2011 (N = 103) | Total As: <LOD–34 ppb (mean: 2.6 ppb) |
| Red wine | FDA (46) | FDA Total Diet Study program, 2006–2013 (N = 32) | Total As: <LOD–24 ppb (mean: 8 ppb) |
Abbreviations: DMA, dimethyl-arsenical species; FDA, US Food and Drug Administration; EPA, US Environmental Protection Agency; InAs, inorganic arsenic; LOD, limit of detection; MMAs, monomethyl-arsenical species.
In the United States, As contamination in rice is primarily the result of growing rice in fields that were previously used to grow cotton treated with InAs as a pesticide to eliminate cotton-eating boll weevils (11, 122). Rice species growing in these fields were selected for their ability to grow heartily in As-pesticide-treated soil (161).
Exposure to As through food varies between ethnicities, largely depending on ethnic patterns of rice consumption (45). The concentration of As in rice varies widely, ranging from less than the limit of detection to as high as 1.8 μg/g in uncooked rice (45, 64, 67, 102). Most As in rice is InAsIII+V or dimethylarsinic acid (DMAsV). The latter is a relatively less toxic form of As understood to arise from soil microbiotic processes (161). Rice-based products are also sources of As, including cereals, crackers, rice drinks, and rice syrup-sweetened products such as baby formulas and energy bars (76, 148). Brown rice and brown rice products have higher concentrations of InAs because it accumulates in the aleurone layer of the grain, which is removed when rice is refined. In contrast, DMAs can pass into the rice grain directly (21). When rice or rice bran is cooked in an excess of As-free water and then drained, up to 80% of the InAs may be removed, though if As-contaminated water is used, the As burden may be increased (22, 64, 130).
There is particular concern regarding As exposure in infants and children through food and beverages. Risk assessments conducted by the FDA found average InAs concentrations of 105 ppb in infant white rice cereal and average total As concentrations of 5 μg/L in apple juice (45). Due to a higher per-body-mass food intake and a more limited diet, infants and young children have significantly higher relative InAs intake than adults (45). While there are currently no FDA regulations regarding As concentrations in food, the FDA monitors As concentrations and has proposed an action level for InAs in infant rice cereal of 100 ppb (45).
Additional food-based sources of exposure may include chicken grown with the feed additive Roxarsone (currently banned in the United States) (108), as well as wheat (176). Although seaweed and seafood, including shellfish, are also sources of exposure, As from these sources is largely in the form of arsenobetaine and arsenocholine, which are nontoxic arsenicals (16, 110).
1.2. Health Effects of Arsenic
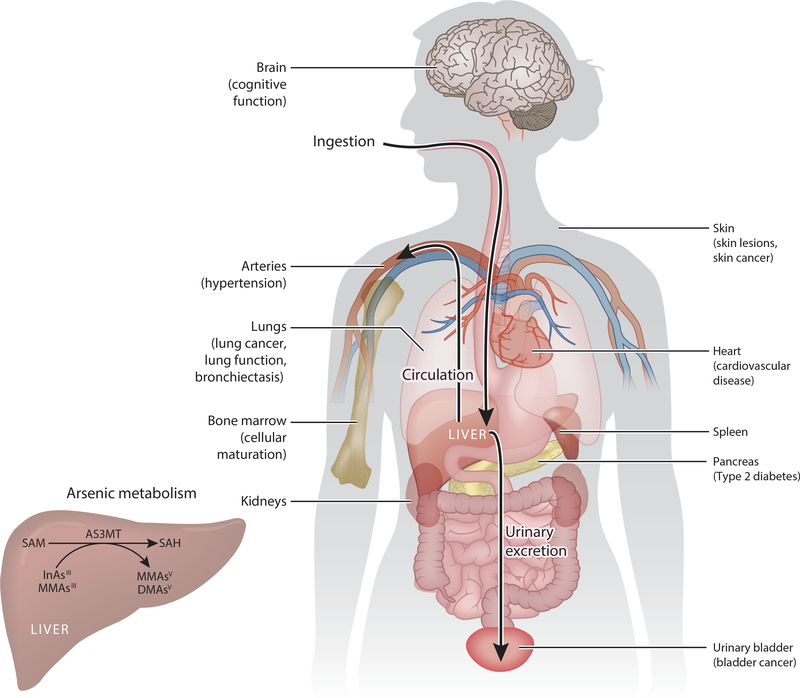
Arsenic is a Group 1 human carcinogen and toxicant that affects numerous organs and systems (3). Chronic As exposure increases the risk of health conditions including skin lesions (melanosis, leukomelanosis, and keratosis); cardiovascular disease; impaired intellectual function; inflammation; diabetes; and cancers of the bladder, lung, kidney, liver, skin (19), and possibly prostate (Figure 1) (12). In Taiwan, chronic As exposure has been associated with blackfoot disease, a peripheral vascular disease linked to systemic atherosclerosis and dry gangrene (154). Arsenic-related health risks persist after exposure has ended or been reduced. Several studies in Northern Chile, where people were exposed to highly As-contaminated drinking water for a very distinct temporal period, have demonstrated that exposure isolated to infancy or early life results in an elevated lifetime risk of As-related health outcomes such as lung and bladder cancer (133, 142, 145).
Figure 1.
Arsenic metabolism, target tissues, and comorbidities. Chronic As exposure has been associated with increased risk of skin lesions (melanosis, leukomelanosis, and keratosis), cardiovascular disease, hypertension (91), impaired intellectual function, inflammation, diabetes, and cancers. Ingested As accumulates in multiple tissues, including the spleen, liver, lungs, kidneys, bladder, skin, and bone marrow. (Inset) AS3MT is predominantly expressed in the liver, although AS3MT mRNA has also been detected in the kidneys, adrenal gland, bladder, heart, and brain (92). Abbreviations: AS3MT, arsenic-3-methyltransferase; DMAsV, dimethylarsinic acid; InAsIII, arsenite; MMAsIII, monomethylarsonous acid; MMAsV, monomethylarsonic acid; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
1.3. Arsenic Methylation and Elimination
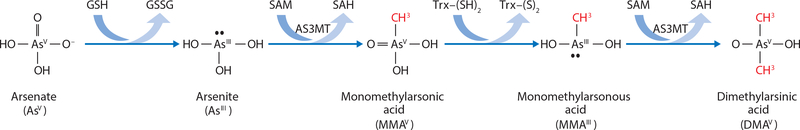
Arsenic in drinking water is predominantly inorganic arsenate (InAsV) or arsenite (InAsIII), but once ingested, it undergoes methylation in a process that facilitates urinary As elimination (20). In 1945, Challenger reported a model in which InAs undergoes alternate reduction and oxidative methylation reactions (24), illustrated in Figures 1 and 2. Briefly, InAsIII is methylated by arsenic-3-methyltransferase (AS3MT) (92), using S-adenosylmethionine (SAM) as the methyl donor, to form monomethylarsonic acid (MMAsV) (24, 151). MMAsV is then reduced to monomethylarsonous acid (MMAsIII), an intermediate with very high cytotoxicity and genotoxicity (117, 118, 147). MMAsIII is subsequently methylated by AS3MT to form dimethylarsinic acid (DMAsV) (24, 92, 151). DMAsV is rapidly excreted in urine and is considerably less toxic than MMAsIII, InAsIII, or InAsV (147). While other identified methyltransferase enzymes are capable of methylating As (171, 172), AS3MT catalyzes these methylation reactions with a Km in the nanomolar range, indicating that it is the most physiologically relevant enzyme for As methylation (92). Glutathione (GSH) may increase the speed of the reduction steps, influence the activity of AS3MT, and sequester As. Hayakawa et al. (62) proposed a pathway in which As–GSH complexes are substrates for AS3MT. Using a mathematical model based on the known biochemistry of As derived from cellular and experimental studies, our group found that the Challenger pathway of As methylation, along with the GSH effects, is sufficient to understand and predict experimental data (87). Additional experimental studies are needed to determine the relative roles that alternative pathways play in As methylation.
Figure 2.
Arsenic metabolism. According to the Challenger pathway (24), AS3MT catalyzes the oxidative methylation of arsenite using SAM as the methyl donor, forming MMAsV and SAH. MMAsV is then reduced to MMAsIII before a subsequent oxidative methylation step, yielding DMAsV and SAH. Abbreviations: AS3MT, arsenic-3-methyltransferase; DMAsV, dimethylarsinic acid; MMAsIII, monomethylarsonous acid; MMAsV, monomethylarsonic acid; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
AS3MT is predominantly expressed in the liver, but AS3MT mRNA has also been detected in the kidneys, adrenal gland, bladder, heart, and brain (92). Work by Thomas’s group (39, 74) using AS3MT knockout mice illustrates the profound role of AS3MT in As elimination, as mice deficient in AS3MT accumulate a body burden of As that is 16–20 times greater than wild-type mice and exhibit severe systemic toxicity and early death.
There are controversies surrounding the influence of As metabolism on toxicity owing in part to analytical challenges. For example, because trivalent arsenicals are readily oxidized to pentavalent forms by environmental oxygen, it is difficult to distinguish between the valence states of As metabolites, and the potential for artifact is high. Because of this limitation, most human studies report the percentages of total As %InAsIII+V, %MMAsIII+V, and %DMAsIII+V. Some studies have provided conflicting information about the portion of DMAsV versus DMAsIII that may be present in urine (36, 98, 157). This reported discrepancy may be due to the high reactivity of DMAsIII, which can be rapidly oxidized to DMAsV (82). Additionally, one chromatographic protocol that treats DMAsV with metabisulfite and thiosulfate can inadvertently generate thio-DMAsV (61), a relatively minor arsenical species (5% of total As) that has been identified in human urine (124).
1.4. Arsenic Methylation and Toxicity
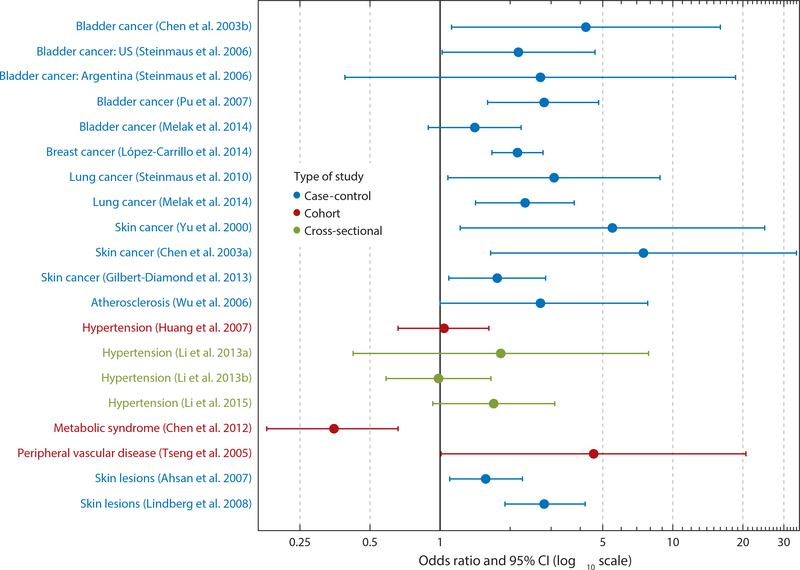
Arsenic metabolites vary considerably in their toxicity. Trivalent arsenicals have greater cytotoxicity and genotoxicity than pentavalent forms. Using real-time cell sensing in two human cell lines, Le’s group (105), in agreement with the work of others (117), reported that MMAsIII is the most cytotoxic As metabolite, followed by DMAsIII, InAsIII, InAsV, MMAsV, DMAsV, and the chicken feed additive Roxarsone. Studies utilizing hamster cells from a variety of tissues including kidney and heart demonstrate that MMAsIII is the most cytotoxic arsenical species in all cell types tested (118). In epidemiological studies, a higher percentage of MMAsIII+V in urine has been associated with increased risk for bladder, breast, lung, and skin cancer, as well as skin lesions, peripheral vascular disease, hypertension, atherosclerosis (Figure 3), and decreased birthweight (2, 27, 28, 54, 70–72, 85, 94, 97, 103, 123, 140, 144, 153, 166, 170). In contrast, the risk of metabolic syndrome and diabetes has been negatively associated with %MMAs in urine (26, 83). Most, but not all, of these studies used prevalent cases and therefore cannot establish temporality or rule out the possibility of reverse causation, i.e., that having a disease influences the ability to methylate As. However, in a recent nested case-control study of As-induced skin lesions, we found that participants falling into the lowest tertile of %DMAs in urine were at higher risk for development of skin lesions 2–7 years later (113).
Figure 3.
Summary plot of odds ratios and 95% CI for health outcomes reported to be associated with %MMAs in urine (2, 26–28, 54, 72, 89–91, 94, 97, 103, 123, 140, 144, 153, 166, 170). Abbreviations: %MMAs, percent monomethyl-arsenical species; CI, confidence interval.
Arsenic methylation capacity differs between species, individuals, and populations. In Bangladesh, members of our group have found that genetic variation in the AS3MT gene is a strong genetic predictor of As methylation capacity (52, 77). Single-nucleotide polymorphisms (SNPs) in AS3MT have also been associated with the proportion of As metabolites in urine in other populations (1, 9, 41, 65, 66), as well as As-related health outcomes such as skin lesions and skin cancer (43, 52). In addition, epigenetic regulation may influence As methylation capacity. In a study of Argentinean women, the AS3MT haplotype was found to be associated with the methylation status of the AS3MT gene—which influenced AST3MT gene expression— and with As methylation capacity (42).
1.5. Tissue Distribution of Arsenic
Early studies in rats, rabbits, and mice have shown that As accumulates in the spleen, liver, lungs, kidneys, bladder, skin, and bone marrow when administered orally (Figure 1) (14, 35, 75, 99, 146). While tissue As concentrations are difficult to study in humans, a case study of suicide by As trioxide poisoning demonstrated that following an acute high oral dose of As, concentrations were highest in the liver and kidneys; moderate in the muscles, heart, spleen, pancreas, lungs, and brain; and lowest in the skin (13). Under steady-state conditions of low-dose chronic exposure, As distribution is likely different. In a postmortem study of human tissues from individuals living in Bombay, Dang et al. found As concentrations to be more than 20 times higher in hair than in other tissues including the liver, kidneys, brain, lungs, and spleen (33). Yamauchi & Yamamura reported As concentrations from human cadavers in Japan that lacked high premortal occupational or environmental As exposure; they found concentrations in the liver, kidneys, pancreas, and skin slightly elevated relative to the lungs, muscles, and spleen (169). The accumulation of As in hair and skin is due to the affinity of As for keratin, a cysteine-rich protein that is abundant in squamous epithelia (95).
Arsenic exposure and methylation patterns in human studies are most often assessed in urine because As concentrations in urine are an order of magnitude higher than in blood, but over the past decade, analytical advances have allowed our group’s Trace Metals Core laboratory to measure As and its methylated metabolites in blood. While the concentrations of As metabolites in blood are highly correlated with those in urine (Spearman correlation coefficients range from ρ = 0.68 to ρ = 0.81), the relative distribution is strikingly different (51). Because DMAs is more rapidly excreted in urine, the %DMAs in blood is lower and the %MMAs is higher than in urine. For example, in baseline samples from our first folic acid (FA) trial, the mean distributions of As in urine versus blood were 15% versus 26% for InAs, 13% versus 40% for MMAs, and 72% versus 34% for DMAs (51). Maternal concentrations of As and As metabolites in blood are highly correlated with those of umbilical cord blood, with Spearman correlation coefficients ranging from ρ = 0.80 to ρ = 0.94 (P < 0.0001), indicating that fetal As exposure mimics that of the mother (57). The extent to which urinary and/or blood As metabolite distributions reflect their distributions in target tissues in humans is unknown. The use of in silico experiments (described in Section 3.5) can begin to give insight on As distribution in tissue compartments.
1.6. Mechanisms of Action of Arsenic
The carcinogenic and noncarcinogenic mechanisms of action of As are incompletely understood and are likely multifactorial. InAs and its metabolites are poor mutagens (81). The carcinogenic mechanisms of As may involve inhibition of DNA repair, disruption of the cell cycle, alterations in gene expression via DNA methylation and histone modifications, inhibition of thymidylate synthesis, endocrine disruption, and/or oxidative stress (55, 78, 107, 114, 127, 167). A similar battery of mechanisms is likely involved in the etiology of other As-induced diseases (131). Here, we briefly summarize those mechanisms having the most supporting evidence.
1.6.1. Protein binding.
Arsenic is known to bind to and inhibit many enzymes (25, 177). InAs is an extremely reactive metalloid, and toxicity of AsIII is in part attributable to its ability to react with critical sulfhydryl groups of many enzymes. Arsenic metabolites differ in their protein binding capacity; there are three coordination sites on InAsIII, two on MMAsIII, and only one on DMAsIII (4). Binding of As to two sulfhydryl groups in the active site of a single protein can yield a stable structure and lead to irreversible enzyme inhibition (4).
1.6.2. Oxidative stress.
Reactive oxygen species (i.e., oxygen-containing radicals, oxidizing agents, and species readily transformed into radicals) contribute to cancer development by producing DNA alterations and interfering with signal transduction and protein activity (163). In animal models and studies of human cell cultures, As exposure has been associated with the formation of 8-hydroxy-2′-deoxyguanosine, a marker of DNA oxidative stress (168). Effects differ between As species and valence states; e.g., treatment of human keratinocytes with InAsIII generated reactive oxygen species to a greater extent than treatment with InAsV (38). Furthermore, in the Folate and Oxidative Stress (FOX) study, a cross-sectional study of As-exposed adults in Bangladesh, our group demonstrated that water As was associated with significant decreases in blood GSH, a strong endogenous antioxidant (60).
1.6.3. Epigenetic dysregulation.
Many of the health effects of As exposure persist even after exposure has ended, and exposure in early life increases the risk of diseases later in life (142, 143, 145). Evidence from studies in animals and human cells strongly suggests that epigenetic dysregulation is involved in the etiology of diseases associated with As exposure (e.g., 32, 158). The association between As exposure and epigenetics has been reviewed elsewhere (5, 68, 127). Briefly, cell-culture studies show that As exposure induces genome-wide DNA hypomethylation, which likely contributes to As-induced genomic instability (100). Arsenic exposure has also been associated with changes in global DNA methylation, loci-specific DNA methylation, global 5-hydroxymethylcytosine (an intermediate product of DNA demethylation), and histone modifications in animal models and epidemiological studies (69, 112, 155, 175). Arsenic affects methylation patterns of genes associated with chronic illnesses (such as diabetes), suggesting that As exposure may influence gene expression involved in the onset of these diseases (8). In several studies of As-exposed adults in Bangladesh, we have found that As was positively associated with global DNA methylation of peripheral blood mononuclear cell DNA, and the association between As exposure and global DNA methylation was modified by folate, a potential compensatory mechanism to prevent hypomethylation (111, 119, 120). Limited research has evaluated the association between As exposure and epigenome-wide blood DNA methylation profiles using the Illumina 450K BeadChip (6, 17, 80, 129). The largest study to date, conducted on skin lesion cases in Bangladesh (N = 400), showed that As exposure was associated with four CpG sites that passed the Bonferroni significance threshold (P < 1 × 10−7) and were replicated in a second smaller Bangladeshi study population (6).
2. NUTRITIONAL INFLUENCES ON ONE-CARBON METABOLISM
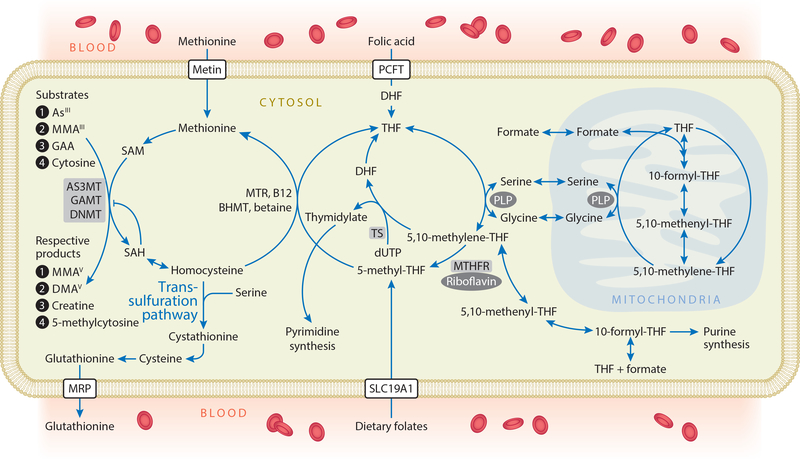
Methylation reactions are dependent on the methyl donor SAM, a critical cosubstrate in OCM (Figure 4). Several micronutrients including folate, vitamin B12 (cobalamin), betaine, choline, riboflavin, and vitamin B6 (pyridoxal phosphate) play critical roles in OCM. FA is a synthetic form of folate used in food fortification. FA must be reduced to 5-methyltetrahydrofolate (5-methyl-THF) by dihydrofolate reductase to become metabolically active in OCM. In the folate cycle, a one-carbon unit is transferred from serine to tetrahydrofolate (THF) to form 5,10-methylene-THF, which is subsequently reduced to 5-methyl-THF by methylene tetrahydrofolate reductase (MTHFR). 5-methyl-THF is the predominant naturally occurring form of folate from dietary sources (30). In a reaction catalyzed by methionine synthase, the methyl group of 5-methyl-THF is transferred to homocysteine (Hcy) utilizing B12 as a cofactor, to generate methionine and THF. Betaine is an alternative methyl donor that can be obtained in the diet or synthesized from choline. Methionine adenosyltransferase enzymes activate methionine to form SAM, the universal methyl donor for hundreds of transmethylation reactions (40). All of these methylation reactions generate the methylated product and S-adenosylhomocysteine (SAH), which is hydrolyzed to Hcy in a reaction that is readily reversible. This point is important because SAH is a potent product inhibitor of most methylation reactions, and 5-methyl-THF plays an essential role in pulling the pathway forward. Despite the fact that multiple micronutrients influence OCM, folate nutritional status appears to be the primary determinant of circulating Hcy concentrations in most studies of adults. For example, in a cross-sectional survey, we observed a high prevalence of high Hcy in Bangladesh, and plasma folate and B12 were found to explain 15% and 5% of the variance in Hcy, respectively (48).
Figure 4.
One-carbon metabolism. FA, arising from fortified foods or nutritional supplements, is reduced to DHF and THF by dihydrofolate reductase. Serine hydroxymethyl-transferase transfers one-carbon units from serine to THF, with PLP as a coenzyme, forming 5,10-methylene-THF. This is either used for the synthesis of thymidylate or reduced to 5-methyl-THF. Dietary folates can enter one-carbon metabolism as 5-methyl-THF. The methyl group of 5-methyl-THF is transferred to homocysteine in a reaction catalyzed by MTR and utilizing B12 as a cofactor, generating methionine and THF. Alternatively, in the liver, betaine can donate a methyl group for the remethylation of homocysteine in a reaction catalyzed by BHMT. Methionine adenosyltransferase enzymes activate methionine to form SAM—the methyl donor for numerous acceptors, including arsenicals, GAA (the precursor to creatine), and DNA—in reactions that involve substrate-specific methyltransferase enzymes. These methylation reactions generate the methylated products and SAH, a potent product inhibitor of most methyltransferases. SAH is hydrolyzed to generate homocysteine, which is either remethylated to regenerate methionine or directed to the transsulfuration pathway and ultimately glutathionine synthesis. Abbreviations: AS3MT, arsenic-3-methyltransferase; BHMT, betaine homocysteine methyltransferase; DHF, dihydrofolate; DMAsV, dimethylarsinic acid; DNMT, DNA methyltransferases; FA, folic acid; GAA, guanidinoacetate; GAMT, guanidinoacetate-N-methyltransferase; InAsIII, arsenite; MMAsIII, monomethylarsonous acid; MMAsV, monomethylarsonic acid; MRP, multidrug resistance proteins; MTR, methionine synthase; PCFT, proton coupled folate transporter; PLP, pyridoxal phosphate; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SLC19A1, solute carrier family 19 (folate transporter), member 1, also known as RFC1; THF, tetrahydrofolate; TS, thymidylate synthetase.
3. NUTRITIONAL INFLUENCES ON ARSENIC METHYLATION
3.1. OCM and Arsenic Metabolism in Animal Models
Early studies in animal models provided experimental evidence that nutritional regulation of OCM influences the methylation and toxicity of As. In 1987, Vahter & Marafante (156) reported that rabbits fed diets deficient in methyl donors (methionine, choline, or protein) had significantly lower urinary excretion of total As and DMAs, and increased As retention in tissues; similar results were reported by others in mice (152). Finnell’s group conducted an elegant series of studies on neural tube defects (NTDs) that employed mice nullizygous for several folate-binding proteins involved in cellular uptake of folate from the circulation (e.g., Folbp-1 and −2) and/or enterocytes (reduced folate carrier). Mice were injected with sodium arsenate early in gestation during critical periods for neural tube closure. For all genotypes studied, dietary folate deficiency caused a reduction in urinary excretion of DMAs, and Folbp-2 null mice were more susceptible to As-induced NTDs (134–136, 164). While As exposure has not yet been solidly linked to NTD risk in humans, studies are under way, and a recent study in Bangladesh reported that As exposure reduced the efficacy of FA in preventing NTDs (101).
The utility of rodent models in understanding the effects of OCM-related micronutrients on As methylation capacity is limited by the fact that (a) there are profound differences between species in As metabolism efficiency (both mice and rats are extremely efficient in As methylation); (b) animals are less prone to developing As-related cancers than are humans; and (c) rodents are much less prone to developing folate deficiency than are humans due to coprophagia. In addition, it is challenging to mimic chronic, often decades-long, lower-dose population-based As exposure levels using rodent models.
3.2. Human Studies on Folate and Arsenic Metabolism and Toxicity
Early human data suggesting a role for folate in As toxicity came in the form of isolated case reports. For example, an interesting case study of a girl deficient in MTHFR, the enzyme responsible for the reduction of 5,10-methylene-THF to 5-methyl-THF, developed severe symptoms of As toxicity following exposure to an As-containing pesticide, while no other exposed family members were affected (19). Several studies on nutrition and As are summarized in Table 2. For example, in 2002, Smith’s group (29) reported a cross-sectional study of 11 families exposed to As-contaminated drinking water in Chile (N = 44) in which the correlations of As methylation patterns between fathers and mothers were low, but they increased substantially with adjustment for folate and Hcy, indicating that folate and Hcy are sources of variation in As methylation. Two years later, Smith’s group (104) reported a nested case-control study of As-related skin lesions (keratosis and melanosis) in a population with very high water As concentrations in West Bengal, India. While they did not measure As metabolites, there was a positive trend in risk for skin lesions associated with lower quintiles of folate intake (P for trend = 0.006). In a subsequent case-control study of this population, diet was assessed using a 24-h recall, and the concentrations of selected micronutrients were measured in serum; %MMAs was observed to be significantly lower among participants in the highest tertile of serum folate compared to the lowest tertile (10). This same group conducted another study employing dietary questionnaires in As-exposed regions of the western United States, in which they found that participants falling in the lower quartiles for dietary protein, iron, zinc, and niacin had higher %MMAs and lower %DMAs in urine compared to the highest quartile (141). While they found no significant associations with dietary folate intake, the study was conducted years after mandatory fortification of cereals, breads, pastas, flours, and other grain products in the US food supply with FA; therefore, all of the study participants likely had fairly high folate intake.
Table 2.
Summary of epidemiological studies on folate and arsenic methylation
| Study | Design | Population | Measure of folate status | Summary of results related to folatea | Summary of results related to other OCM micronutrients* |
|---|---|---|---|---|---|
| Mitra et al. (104), Basu et al. (10) | Case-control study for As-induced skin lesions | Individuals in West Bengal, India, with drinking water As <500 μg/L (N = 405) | 24-h dietary recall; serum folate | Quintile of folate intake not significantly associated with skin lesions Significant positive trend in OR for skin lesions with highest to lowest quintile of folate intake Lowest tertile of serum folate associated with lower urine %InAs and higher %MMAs compared to the highest tertile |
Lowest quintile of animal protein intake associated with skin lesions Significant positive trend in OR for skin lesions with highest to lowest quintile of animal protein intake Lowest tertile of urine creatinine associated with higher urine %InAs, and lower %MMAs and %DMAs compared to highest tertile Lowest tertile of riboflavin intake associated with lower urine %InAs and higher %DMAs compared to highest tertile Lowest tertile of plasma Hcy associated with lower urine %MMAs compared to highest tertile Lowest tertile of animal protein intake associated with lower urine %MMAs compared to highest tertile |
| Steinmaus et al. (141) | Cross-sectional study | Individuals in Western United States (N = 87) | Dietary questionnaire | Folate intake not significantly associated with urine As metabolite proportions | Lowest quartile of protein intake associated with higher urine %MMAs and lower %DMAs compared to highest quartile |
| Gamble et al. (50) | Cross-sectional study | Individuals in Bangladesh (N = 300) | Plasma folate | Plasma folate associated with lower urine %InAs and %MMAs, and higher %DMAs | Total Hcy associated with higher urine %MMAs and lower %DMAs Cysteine associated with lower urine %InAs and higher %MMAs Urinary creatinine associated with lower urine %InAs and higher %DMAs |
| Gamble et al. (49, 51) | Randomized controlled trial | Folate-deficient individuals in Bangladesh (N = 300) | Supplementation of 400-μg FA per day for 12 weeks | FA treatment associated with greater decrease in urine %InAs and %MMAs, and increase in %DMAs compared to placebo FA treatment associated with greater decrease in total blood As and blood MMAs concentrations compared to placebo |
Urinary creatinine associated with lower urine %InAs and higher %DMAs |
| Li et al. (88) | Cohort study | Pregnant women in Bangladesh (N = 864) | Plasma folate at gestational week 14 | Among women with urine As >209 μg/L, lowest tertile of plasma folate associated with higher urine %InAs compared to highest tertile Lowest tertile of plasma folate, B12, and zinc associated with higher urine %InAs and primary methylation index compared to highest tertile of plasma folate, B12, and zinc |
Urinary creatinine associated with lower %InAs, and higher %DMAs, primary methylation index, and SMI |
| Pilsner et al. (120) | Nested case-control study for As-induced skin lesions | Individuals in Bangladesh (N = 548) | Plasma folate | Low folate (<9 nmol/L) associated with increased risk of skin lesions compared to high folate As compared with the referent group of low Hcys + high folate, the group with low Hcy + low folate, the group with hyperhomocysteinemia + high folate, and the group with hyperhomocysteinemia + low folate had an increased risk of skin lesions |
Hyperhomocysteinemia associated with increased risk of skin lesions compared to low homocysteine Urinary creatinine (fold increase) associated with decreased risk of skin lesions Second and third quartile of urinary creatinine associated with decreased risk of skin lesions compared to lowest quartile |
| Gardner et al. (53) | Cohort study | Pregnant women in Bangladesh (N = 324) | Plasma folate | Plasma folate not associated with change in urine As proportions during pregnancy in multivariate mixed effects linear models | Plasma B12 not associated with change in urine As proportions during pregnancy in multivariate mixed effects linear models |
| Peters et al. (115), Bozack et al. (15) | Randomized controlled trial | Individuals in Bangladesh (N = 610) | Supplementation of 400-μg FA per day, 800-μg FA per day, or 400-μg FA + 3-g creatine per day for 12 weeks | 800-μg FA per day treatment associated with greater decrease in total blood As compared to placebo FA treatment associated with greater decrease in urine %InAs and %MMAs, and increase in %DMAs compared to placebo |
Creatine treatment associated with greater decrease in urine %MMAs compared to placebo |
| López-Carrillo et al. (96) | Cross-sectional study | Women in Northern Mexico (N = 1,027) | Dietary questionnaire | High folate intake ≥ 400 μg/day associated with lower urine %InAs and higher DMAs/InAs compared to low folate intake | B12 intake associated with lower urine %InAs and higher %DMAs, DMAs/MMAs, and DMAs/InAs Choline intake associated with lower urine %InAs and higher %DMAs, DMAs/MMAs, and DMAs/InAs Methionine intake associated with lower urine %InAs and higher %DMAs, DMAs/MMAs, and DMAs/InAs |
| Kurzius-Spencer et al. (84) | Cross-sectional study | Adults and children in United States (N = 2,420) | Red blood cell and serum folate; 24-h dietary recall | Folate intake associated with lower urine %InAs and higher %DMAs in adults Red blood cell and serum folate not associated with urine As metabolite proportions Red blood cell folate associated with lower urine %InAs in multivariate models Folate intake and serum folate not associated with urine As metabolite proportions or SMI in multivariate models |
Dietary B6 associated with lower urine %InAs in adults Plasma Hcy associated with higher urine %MMAs and lower SMI in children Urinary creatinine associated with lower urine %InAs and %MMAs, and higher %DMAs and SMI in multivariate models B12 intake associated with higher urine %InAs and lower %DMAs in multivariate models B6 intake associated with lower urine %InAs in multivariate models Plasma Hcy associated with higher urine %MMAs, and lower %DMAs and SMI in multivariate models |
| Spratlen et al. (137) | Cohort study | American Indians (N = 405) | Dietary questionnaire | Folate intake not associated with urine As metabolite proportions in fully adjusted models High folate intake and high B6 intake associated with lower urine %InAs and higher %DMAs compared to low folate intake and low B6 intake |
Highest tertile of B2 intake associated with lower urine %MMAs and higher %DMAs compared to lowest tertile Highest tertile of B6 intake associated with lower urine %InAs and %MMAs, and higher %DMAs compared to lowest tertile First principal component of OCM nutrients (representing intake of all OCM nutrients) associated with lower urine %InAs and %MMAs, and higher %DMAs |
Except where otherwise indicated, all results were significant at P < 0.05.
Abbreviations: %DMAs, percent dimethyl-arsenical species; FA, folic acid; Hcy, homocysteine; %InAs, percent inorganic arsenic; %MMAs, percent monomethyl-arsenical species; OCM, one-carbon metabolism; OR, odds ratio; SMI, secondary methylation index.
Our group has conducted a series of studies in Bangladesh, a population with chronic As exposure, to better characterize the interconnection between folate, As metabolism, and As toxicity.
We first conducted a cross-sectional study of As-exposed adults in Bangladesh, in which we observed that plasma folate was negatively associated with %MMAs and positively associated with %DMAs (50). We then studied the effect of 400-μg FA per day, the US recommended daily allowance, on As metabolism in a randomized, double-blind, placebo-controlled trial among 200 folate-deficient (plasma folate <9 nmol/L) Bangladeshi adults. After 12 weeks of supplementation, the treatment group had a significantly larger increase in %DMAs and decreases in %InAs and %MMAs in urine relative to the placebo group; treatment effects were observed as early as one week post-intervention (49). In addition, FA supplementation lowered blood As by 14% and blood MMAs by 22% (51). More recently, in the Folic Acid and Creatine Trial (FACT) conducted among 622 As-exposed Bangladeshi adults selected independent of folate status, a larger decrease in blood As was observed in the treatment group receiving 800-μg FA per day for 12 weeks relative to the placebo group (115). In addition, increases in As methylation capacity were observed among the treatment groups receiving 800-μg FA per day and 400-μg FA per day relative to placebo as measured by the change in %InAs, %MMAs, and %DMAs in urine between baseline and week 12 (15). FACT included a 12-week wash-out period during which half of the participants in the FA treatment groups were switched to placebo to examine the effects of cessation of supplementation on As methylation. Arsenic metabolites reverted to pre-intervention levels 12 weeks after FA supplementation was discontinued, highlighting the importance of maintaining adequate folate nutritional status over time. This has important policy implications, as prolonged maintenance of FA effects may be more readily achieved through food FA-fortification programs than through recommendations for over-the-counter FA supplements, as the latter has limited long-term compliance.
In 2009, we reported the results of a nested case-control study of skin lesions (274 cases and 274 controls) in Bangladesh in which we found that low folate, hyperhomocysteinemia, and low urinary creatinine were associated with risk for subsequent development of skin lesions after controlling for age, urinary As, and use of betel nut (a mild stimulant and known carcinogen commonly chewed by Bangladeshi adults) (120). These findings are consistent with those of an analysis of dietary folate and skin lesions in India by Smith and colleagues (104). Similar results were found in relation to urothelial carcinoma. In a case–control study of 177 cases and 488 controls in a population in Taiwan exposed to low concentrations of As in drinking water, higher %DMA in urine and higher plasma folate concentrations were associated with a decreased risk for urothelial carcinoma. Furthermore, a significant interaction was observed between urinary As methylation profiles and plasma folate in affecting urothelial carcinoma risk (73). More recently, in a larger nested case-control study (N = 876 cases and 876 controls) of gene × nutrition × environment interactions, hyperhomocysteinemia and lower %DMAs in urine were both associated with increased risk for development of skin lesions 2–7 years later (113). We also found TYMS rs1001761 was associated with increased skin lesion risk at water As exposure >50 μg/L. The latter finding highlights a potential role of OCM in As toxicity independent of As methylation. The TYMS gene encodes thymidylate synthetase, which utilizes 5,10-methylene-THF for the methylation of 2-deoxy-uridine-5-monophosphate (dUMP) to 2-deoxy-thymidine-5-monophosphate (dTMP) and is critical for DNA synthesis and repair (Figure 4) (23). These findings raise the possibility that DNA damage involving thymidylate synthetase may be a mechanism of As toxicity at higher As concentrations. Consistent with this hypothesis, a recent study from the Stover group identified thymidylate biosynthesis as a sensitive target for As at levels observed in human populations (As trioxide at 0.5 μM, equivalent to 75-μg/L As in water) (78). The study found that As exposure to cell cultures impaired folate-dependent dTMP biosynthesis, resulting in uracil misincorporation into DNA and genomic instability. Further, folate deficiency exacerbated the impact of As on uracil misincorporation and genomic instability, providing a potential additional mechanism linking folate deficiency to skin lesion risk (78).
Recent, large epidemiological studies have confirmed our findings relating folate to As methylation, including populations with lower As exposure. In a cross-sectional study of 1,027 women in Mexico with a median urinary As of 25.9 μg/g creatinine, micronutrient intake was estimated using a food frequency questionnaire; folate intake was associated with significantly lower %InAs and higher methylation ratio of DMAs/InAs (9). In addition, in an analysis of the 2003–2004 National Health and Nutrition Examination Survey, dietary folate intake was negatively associated with urinary %InAs and positively associated with %DMAs in unadjusted models, and red blood cell folate was negatively associated with %InAs in adjusted models (8). In the Strong Heart Study, a cohort study of American Indian adults with low to moderate As exposure, high combined intake of folate and B6, as estimated by a food frequency questionnaire, was negatively associated with %InAs in urine and positively associated with %DMAs (137). The significant associations between dietary intake of folate and As metabolism in these studies differ from the results of a dietary intake analysis by members of our group that reported no association with dietary folate intake in Bangladesh (63). However, the prolonged cooking times traditionally used in Bangladesh can degrade folate in foods, making it more difficult to accurately measure true folate intake. Such considerations highlight the importance of using circulating folate concentrations, particularly in this region of the world.
Several studies relating OCM to As methylation have been conducted in pregnant women. During pregnancy, OCM influences exposure of the fetus to As, as InAs, MMAs, and DMAs are all transported through the placenta. Furthermore, As concentrations and As metabolites are similar in maternal and umbilical cord blood (31, 57). OCM is altered during pregnancy owing to the demands of fetal development. For example, maternal plasma folate levels change dramatically over the course of pregnancy. Additionally, endogenous choline synthesis is induced by estrogen and upregulated during pregnancy and lactation (173).
The relationship between OCM-related micronutrients and As metabolism in pregnant women has been investigated in several studies. In a cross-sectional study of women at 14 weeks gestation in Bangladesh (N = 753), Vahter’s group reported an inverse association between plasma folate tertiles and urinary %InAs. Women who were deficient in folate, vitamin B12, and zinc had significantly higher %InAs and lower primary methylation index (MMAs/InAs) compared to women who were not deficient in any of the three nutrients (88). Vahter’s group conducted a subsequent longitudinal study of Bangladeshi women (N = 324) assessed at gestational weeks 8, 14, and 30. They observed significant negative associations between gestational week and urinary %InAs and %MMAs, and a significant positive association between gestational age and %DMAs; however, neither plasma folate nor B12 were associated with the proportions of As metabolites (53). The conclusion that these micronutrients have little effect on As methylation during pregnancy may be complicated by changes in OCM during pregnancy. In addition, all women received a daily supplement of 400-μg FA beginning at week 14, which may have impacted As metabolism.
3.3. Impact of Other OCM-Related Nutrients on Arsenic Methylation
Although the association between folate and As methylation has been broadly studied, other micronutrients involved in OCM (Figure 4) may influence As methylation capacity. Below, we summarize research on the associations between creatine, vitamin B12, choline, and betaine and As methylation profiles in humans. These OCM micronutrients are less widely examined than folate in terms of their relationship to As methylation, and are potential avenues for future research directions.
3.3.1. Creatine.
Creatine is a nitrogenous organic acid that is present in foods and is also synthesized endogenously. Our group (15, 49, 50, 59) and others (10, 79) have consistently reported that urinary creatinine, a degradation product of creatine, is a strong predictor of As methylation capacity; it is positively associated with %DMAs in urine and negatively associated with %InAs. The synthesis of creatine, the precursor of creatinine, consumes approximately 50% of all SAM-derived methyl groups (106, 139). In omnivores, roughly half of creatine requirements are met through dietary intake of creatine, primarily from meat (18). Urinary creatinine concentrations therefore reflect both dietary creatine intake and endogenous creatine synthesis (18). Creatinine is also commonly used in urinalyses to adjust for hydration status. Increases in circulating creatine concentrations, e.g., from dietary intake, lowers creatine biosynthesis by inhibiting synthesis of guanidinoacetate (GAA), the precursor of creatine; in rodents, this has been shown to spare methyl groups and lower Hcy (37, 56, 138, 149). We hypothesized that creatine supplementation may also spare methyl groups and thereby facilitate the methylation of As, and may underlie the observed associations between the proportion of urinary As metabolites and urinary creatinine.
We tested this hypothesis in our FACT study. Creatine supplementation for 12 weeks at 3 g per day (roughly 1.5 times the normal daily creatine turnover for a 70-kg male) lowered GAA as predicted, illustrating that creatine supplementation inhibited GAA synthesis (116). Also, the mean decrease in urinary %MMAs in the creatine treatment group exceeded that of the placebo group at weeks 6 and 12 (P < 0.05); however, creatine supplementation did not affect the change in %InAs or %DMAs (15). Creatine treatment effects may have been tempered by long-range allosteric regulation of OCM (see Section 3.5). Further research is needed to understand the strong cross-sectional associations between urinary creatinine and As methylation in previous studies. One possibility is that urinary creatinine is somehow related to renal tubular reabsorption of InAs, a topic that is understudied in the scientific literature.
3.3.2. Vitamin B12 (cobalamin).
Vitamin B12 has been inconsistently linked to As metabolism in human studies, but has not been studied in animal models. In an early cross-sectional study in Bangladesh in which we oversampled B12-deficient individuals, we found plasma B12 concentrations to be inversely associated with %InAs in urine and positively associated with %MMAs, with no association with %DMAs (58). We found it difficult to reconcile a mechanism whereby B12 would facilitate mono- but not dimethylation of As. To test whether this might have been a spurious finding, we subsequently analyzed the relationship between B12 and As metabolites in blood and urine in the FACT and FOX studies. In baseline samples from the FACT study, there were no significant associations between B12 and As metabolites in urine (nonsignificant correlations were negative for %InAs and %MMAs and positive for %DMAs), and all associations with blood As metabolite concentrations were negative. In FOX, while B12 was positively associated with %MMAs in urine (Spearman correlation coefficient ρ = 0.12, P = 0.02) the correlation with MMAs concentrations in blood was null (Spearman correlation coefficient ρ = −0.02, P = 0.76) (M.V. Gamble, unpublished data). In an analysis of the 2003–2004 National Health and Nutrition Examination Survey, in adjusted models, dietary B12 intake was positively associated with urinary %InAs and negatively associated with %DMAs (84), but other studies have reported null results (137) or contrasting directions of association (96). To date, no studies investigating the treatment effects of B12 supplementation on As methylation have been published. Additional studies are needed to better understand the relationship between B12 and As methylation.
3.3.3. Choline and betaine.
The remethylation of homocysteine to methionine, critical for the synthesis of SAM, can be catalyzed either by methionine synthase, which receives a methyl group from 5-methyl-THF, or by betaine homocysteine methyltransferase, which utilizes a methyl group from betaine. Thus, folate and betaine can be used interchangeably for the remethylation of homocysteine. Betaine can be obtained through the diet or synthesized from choline. Choline can also be obtained from the diet or it can be synthesized endogenously. Choline may serve as a methyl donor through its conversion to betaine. However, endogenous choline synthesis, like creatine, is another significant consumer of SAM, as roughly 30% is synthesized de novo through a pathway that involves three sequential SAM-dependent methylation reactions catalyzed by phosphatidylethanolamine N-methyltransferase (159).
Epidemiological studies of choline and As methylation are few, but their results are generally consistent with animal studies (described in Section 3.1). In a cross-sectional analysis (N = 1,016) nested within the Health Effects of Arsenic Longitudinal Study (HEALS), an ongoing cohort in Bangladesh, multiple nutrients were found to be associated with As methylation. In multivariate adjusted models, dietary choline intake was positively associated with the secondary methylation index (DMAs/MMAs) (63). In a subsequent study of women in Mexico (described in Section 3.2), López-Carrillo et al. (96) found that higher dietary choline is inversely associated with %MMAs in urine and positively associated with %DMAs and secondary methylation index. However, results from epidemiological studies indicate that dietary betaine has a weaker association with As methylation than choline has. In the HEALS cohort as well as López-Carrillo et al.’s study of Mexican women, betaine intake was not associated with indicators of As methylation capacity; however, both studies relied on estimates of betaine intake from food frequency questionnaires without biological assessment of betaine status (63, 96).
Our group conducted an 8-week pilot intervention of choline (700 mg/day), betaine (1,000 mg/day) or choline + betaine supplementation in Bangladesh (N = 60). Within-participant changes in %MMAs and %DMAs in urine were significantly different between groups receiving choline, betaine, and choline + betaine (P < 0.05) as compared to placebo (M.N. Hall & M.V. Gamble, unpublished data). Although the sample size was small, the data suggest that choline + betaine supplementation resulted in the largest decrease in %MMAs and increase in %DMAs, supporting the hypothesis that both dietary choline and betaine impact As methylation. Enthusiasm for larger intervention studies with choline and betaine has been tempered by the finding that choline supplementation also increased plasma tri-methylamine oxide (TMAO). While TMAO has been linked to risk for cardiovascular disease (150), it is unclear whether or not this relationship is causal (174).
3.4. Impact of OCM-Related Polymorphisms
There is some evidence that polymorphisms in genes involved in OCM affect As methylation capacity. Our group has conducted several studies investigating the effect of OCM-related variants on As methylation. As part of a case-control study of skin lesions in Bangladesh (594 cases and 1,041 controls), members of our group investigated the effect of two SNPs in MTHFR (rs1801133 and rs1801131). Neither As methylation profiles, nor risk for skin lesions, differed significantly between haplotypes or diplotypes (2). In a subsequent nested case-control study of incident skin lesions in Bangladesh, we genotyped 26 SNPs in 13 OCM-related genes (N = 876 matched pairs) (113). No SNPs achieved a statistically significant association with urine As metabolite proportions at a false discovery rate (FDR)-corrected P < 0.05, although SNPs in MTHFR, methionine synthase (MTR), and serine hydroxymethyltransferase 1 (SHMT1) were nominally significant. The MTHFR rs1801133 T allele was associated with higher %MMAs and lower %DMAs, and the MTR rs1805087 G allele and SHMT1 rs1979277 A allele were associated with higher %InAs. In addition, three SNPs [rs1540087 and rs7109250 in folate receptor 1 (FOLR1) and rs1801394 in methionine synthase reductase (MTRR)] had nominally significant interactions with water As in logistic regression models predicting skin lesion risk; however, these interactions were not significant after FDR correction. Our colleagues also genotyped 19,992 variants in 4,939 Bangladeshi individuals who were enrolled in the HEALS cohort and the Bangladesh Vitamin E and Selenium Trial (BEST). The SNP rs61735836, located in the FTCD gene encoding formimidoyltransferase cyclodeaminase (a bifunctional enzyme that channels one-carbon units from formiminoglutamate to the folate pool), was associated with urinary %InAs, %MMAs, and %DMAs, and the A allele was associated with an increased risk of skin lesions (B.L. Pierce, L. Tong, M. Argos, F. Jasmine, M. Rakibuz-Zaman, et al., manuscript in review).
The association between OCM-related variants and As methylation capacity has also been investigated by other groups. In 2007, Lindberg et al. also reported that the MTHFR rs1801133 T allele was associated with higher %MMAs and lower %DMAs in urine (93). In a study of women in Argentina, SNPs in MTHFR were not associated with As methylation. However, rs9001 located in CHDH (encoding choline dehydrogenase) was significantly associated with %MMAs in urine (128). Smith’s group (121) also investigated the association between OCM-related SNPs and As methylation in their case-control study of lung cancer in Argentina. This study found that polymorphisms in MTHFR, MTR, SHMT1, TYMS, and DHFR (encoding dihydrofolate reductase) were not associated with As metabolite proportions (121). One SNP in CBS (encoding cystathionine-β-synthase), rs4920037, was significantly associated with %MMAs in urine.
3.5. Mathematical Models of OCM and Arsenic Methylation
Our collaborators, Michael C. Reed and H. Frederik Nijhout at Duke University, developed a whole-body mathematical model of OCM that includes As absorption, storage, methylation, and excretion to study and interpret the data on the effects of FA supplementation on As methylation and excretion in our clinical trials in Bangladesh (https://sites.duke.edu/metabolism/arsenic/). Parameters for hepatic As methylation were taken from the biochemical literature. The model has compartments for the binding of arsenicals to intracellular proteins, which improve the fits to data. Because transport parameters between tissues are largely unknown, they were adjusted so that the model accurately predicted the urine excretion rates of InAs, MMAs, and DMAs in single-dose experiments on human subjects. We then demonstrated that, with no further changes in parameters, the model accurately predicts the time courses of urinary As excretion in multiple-dose experiments conducted in human subjects. In our first trial of folate-deficient individuals, FA supplementation decreased total blood As by 14%. This result was also produced by the model. Furthermore, the model predicts that As in the liver will decrease by 19% and As in other body stores by 26% (86).
We have also used the mathematical model of OCM to better understand the modest changes observed with creatine supplementation in our FACT study. We asked the question: If guanidinoacetate-N-methyltransferase flux goes down, how much might hepatic SAM increase, and how much will that increase the flux through AS3MT? When one methyltransferase enzyme is downregulated, there are several long-range interactions that buffer the flux through other methyltransferases. Thus, to increase As methylation, e.g. with creatine supplementation, it would also be necessary to break one or more of those regulatory mechanisms. Because glycine-N-methyltransferase (GNMT) regulates hepatic SAM concentrations and is inhibited by binding to 5-methyl-THF, increasing hepatic 5-methyl-THF concentrations should allow creatine supplementation to increase SAM by inhibiting GNMT. However, while plasma folate concentrations increase substantially with FA supplementation, it is unknown to what extent this supplementation causes liver folate and liver SAM to increase in folate-sufficient individuals (126). Thus, the mathematical models of OCM have helped us to better understand why the effects of creatine supplementation on As methylation—while statistically significant—were modest (15). These models can also be used to inform future nutritional interventions.
4. CONCLUSIONS AND FUTURE DIRECTIONS
Arsenic exposure via drinking water and food is a worldwide problem linked to a variety of adverse health outcomes, including skin lesions, cardiovascular disease, impaired intellectual function, inflammation, diabetes, and cancers of numerous organs. Methylation of As is dependent on OCM, which facilitates urinary As excretion. A number of micronutrients play important roles in OCM, including folate, vitamin B12, pyridoxal phosphate, riboflavin, choline, and betaine. There is strong evidence from epidemiological studies and randomized controlled trials to indicate that folate status and FA supplementation influence As methylation capacity, enhance As elimination, and lower blood As concentrations, particularly in populations living in regions without FA-fortification programs. However, the literature is currently lacking sufficient information on the influences of other micronutrients involved in OCM on As methylation, elimination, and toxicity. Perhaps the most salient need for future research is to definitively establish that increasing As methylation through nutritional interventions such as FA supplementation reduces risk for As-related health outcomes. While results of nested case-control studies are highly suggestive that this is true for As-related skin lesion risk, the data are not as definitive as randomized controlled trial results. Furthermore, future studies are needed to establish possible protective effects of FA for other As-related adverse health outcomes.
Currently, more than 85 countries in the world have mandatory food FA-fortification programs. As has been demonstrated in Western countries such as the United States and Canada, folate fortification can nearly eradicate folate deficiency and many of its associated consequences. Yet of those countries suffering the most widespread problems of As-contaminated drinking water, none have mandatory FA-fortification programs. Clearly, access to safe drinking water is the highest priority. However, in As-endemic countries, FA fortification not only may help to eradicate folate deficiency, but may have the additional benefit of facilitating a partial reduction in the public health consequences of As exposure.
ACKNOWLEDGMENTS
We thank Michael C. Reed, Department of Mathematics, Duke University, and H. Frederik Nijhout, Department of Biology, Duke University, who have collaborated in modeling OCM and assisted in creating Figure 4.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Agusa T, Iwata H, Fujihara J, Kunito T, Takeshita H, et al. 2009. Genetic polymorphisms in AS3MT and arsenic metabolism in residents of the Red River Delta, Vietnam. Toxicol. Appl. Pharmacol 236:131–41 [DOI] [PubMed] [Google Scholar]
- 2.Ahsan H, Chen Y, Kibriya MG, Slavkovich V, Parvez F, et al. 2007. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol. Biomark. Prev 16:1270–78 [DOI] [PubMed] [Google Scholar]
- 3.Am. Cancer Soc. 2016. Known and probable human carcinogens. Am. Cancer Soc., Atlanta, GA. https://www.cancer.org/cancer/cancer-causes/general-info/known-and-probable-human-carcinogens.html [Google Scholar]
- 4.Aposhian HV, Aposhian MM. 2006. Arsenic toxicology: five questions. Chem. Res. Toxicol 19:1–15 [DOI] [PubMed] [Google Scholar]
- 5.Argos M 2015. Arsenic exposure and epigenetic alterations: recent findings based on the Illumina 450K DNA Methylation Array. Curr. Environ. Health Rep 2(2):137–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argos M, Chen L, Farzana J, Lin T, Pierce BL, et al. 2015. Gene-specific differential DNA methylation and chronic arsenic exposure in an epigenome-wide association study of adults in Bangladesh. Environ. Health Perspect 123:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayotte JD, Gronberg JM, Apodaca LE. 2011. Trace elements and radon in groundwater across the United States, 1992–2003. US Geol. Surv. Sci. Investig. Rep. 2011–5059, US Geol. Surv., Reston, VA [Google Scholar]
- 8.Bailey KA, Wu MC, Ward WO, Smeester L. 2013. Arsenic and the epigenome: interindividual differences in arsenic metabolism related to distinct patterns of DNA methylation. J. Biochem. Mol. Toxicol 27:106–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balakrishnan P, Vaidya D, Franceschini N, Voruganti VS, Gribble MO, et al. 2017. Association of cardiometabolic genes with arsenic metabolism biomarkers in American Indian communities: the Strong Heart Family Study (SHFS). Environ. Health Perspect 125:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu A, Mitra S, Chung J, Mazumder DNG, Ghosh N, et al. 2011. Creatinine, diet, micronutrients, and arsenic methylation in West Bengal, India. Environ. Health Perspect 119:1308–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bednar AJ, Garbarino JR, Ranville JF, Wildeman TR. 2002. Presence of organoarsenicals used in cotton production in agricultural water and soil of the southern United States. J. Agric. Food Chem 50:7340–44 [DOI] [PubMed] [Google Scholar]
- 12.Benbrahim-Tallaa L, Waalkes MP. 2008. Inorganic arsenic and human prostate cancer. Environ. Health Perspect 116:158–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benramdane L, Accominotti M, Fanton L, Malicier D, Vallon JJ. 1999. Arsenic speciation in human organs following fatal arsenic trioxide poisoning: a case report. Clin. Chem 45:301–6 [PubMed] [Google Scholar]
- 14.Bertolero F, Marafante E, Rade JE, Pietra R, Sabbioni E. 1981. Biotransformation and intracellular binding of arsenic in tissues of rabbits after intraperitoneal administration of 74As labelled arsenite. Toxicology 20:35–44 [DOI] [PubMed] [Google Scholar]
- 15.Bozack A, Hall MN, Liu X, Ilievski V, Lomax-Luu AM, et al. 2017. Folic acid supplementation enhances arsenic methylation: results from a folic acid and creatine supplementation trial in Bangladesh. Am. J. Clin. Nutr In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandon EF, Janssen PJ, de Wit-Bos L. 2014. Arsenic: bioaccessibility from seaweed and rice, dietary exposure calculations and risk assessment. Food Addit. Contam. Part A Chem. Anal. Control Exposure Risk Assess 31:1993–2003 [DOI] [PubMed] [Google Scholar]
- 17.Broberg K, Ahmed S, Engstrom K, Hossain MB, Jurkovic Mlakar S, et al. 2014. Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys. J. Dev. Orig. Health Dis 5:288–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brosnan JT, da Silva RP, Brosnan ME. 2011. The metabolic burden of creatine synthesis. Amino Acids 40:1325–31 [DOI] [PubMed] [Google Scholar]
- 19.Brouwer OF, Onkenhout W, Edelbroek PM, de Kom JFM, de Wolff FA, Peters ACB. 1992. Increased neurotoxicity of arsenic in methylenetetrahydrofolate reductase deficiency. Clin. Neurol. Neurosurg 94:307–10 [DOI] [PubMed] [Google Scholar]
- 20.Buchet JP, Lauwerys R, Rooels H. 1981. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsenate in man. Int. Arch. Occup. Environ. Health 48(1):71–79 [DOI] [PubMed] [Google Scholar]
- 21.Carey A-M, Norton GJ, Deacon C, Scheckel KG, Lombi E, et al. 2011. Phloem transport of arsenic species from flag leaf to grain during grain filling. N. Phytologist 192:87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carey M, Jiujin X, Gomes Farias J, Meharg AA. 2015. Rethinking rice preparation for highly efficient removal of inorganic arsenic using percolating cooking water. PLOS ONE 10:e0131608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carreras CW, Santi DV. 1995. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 64:721–62 [DOI] [PubMed] [Google Scholar]
- 24.Challenger F 1945. Biological methylation. Chem. Rev 36:315–61 [Google Scholar]
- 25.Chang Y-Y, Kuo T-C, Hsu C-H, Hou D-R, Kao Y-H, Huang R-N. 2012. Characterization of the role of protein–cysteine residues in the binding with sodium arsenite. Arch. Toxicol 86:911–22 [DOI] [PubMed] [Google Scholar]
- 26.Chen J-W, Wang S-L, Wang Y-H, Sun C-W, Huang Y-L, et al. 2012. Arsenic methylation, GSTO1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern Taiwan. Chemosphere 88:432–38 [DOI] [PubMed] [Google Scholar]
- 27.Chen Y-C, Guo Y-LL, Su H-JJ, Hsueh Y-M, Smith TJ, et al. 2003a. Arsenic methylation and skin cancer risk in southwestern Taiwan. J. Occup. Environ. Med 45:241–48 [DOI] [PubMed] [Google Scholar]
- 28.Chen Y-C, Su H-JJ, Guo Y-LL, Hsueh Y-M, Smith TJ, et al. 2003b. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control 14:303–10 [DOI] [PubMed] [Google Scholar]
- 29.Chung JS, Kalman DA, Moore LE, Kosnett MJ, Arroyo AP, et al. 2002. Family correlations of arsenic methylation patterns in children and parents exposed to high concentrations of arsenic in drinking water. Environ. Health Perspect 110:729–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combs GF, Gerald FC, Combs GF Jr. 2012. The Vitamins. New York: Academic [Google Scholar]
- 31.Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. 1998. Exposure to inorganic arsenic metabolites during early human development. Toxicol. Sci 44:185–90 [DOI] [PubMed] [Google Scholar]
- 32.Cui X, Wakai T, Shirai Y, Hatakeyama K, Hirano S. 2006. Chronic oral exposure to inorganic arsenate interferes with methylation status of p16INK4a and RASSF1A and induces lung cancer in A/J mice. Toxicol. Sci 91:372–81 [DOI] [PubMed] [Google Scholar]
- 33.Dang HS, Jaiswal DD, Somasundaram S. 1983. Distribution of arsenic in human tissues and milk. Sci. Total Environ 29:171–75 [DOI] [PubMed] [Google Scholar]
- 34.Davis MA, Signes-Pastor AJ, Argos M, Slaughter F, Pendergrast C, et al. 2017. Assessment of human dietary exposure to arsenic through rice. Sci. Total Environ 586:1237–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Kimpe J, Cornelis R, Wittevrongel L, Vanholder R. 1999. Dose dependent changes in 74As-arsenate metabolism of Flemish Giant rabbits. J. Trace Elements Med. Biol 12:193–200 [DOI] [PubMed] [Google Scholar]
- 36.Del Razo LM, Styblo M, Cullen WR, Thomas DJ. 2001. Determination of trivalent methylated arsenicals in biological matrices. Toxicol. Appl. Pharmacol 174:282–93 [DOI] [PubMed] [Google Scholar]
- 37.Deminice R, Portari GV, Vannucchi H, Jordao AA. 2009. Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. Br. J. Nutr 102:110–16 [DOI] [PubMed] [Google Scholar]
- 38.Ding W, Hudson LG, Liu KJ. 2005. Inorganic arsenic compounds cause oxidative damage to DNA and protein by inducing ROS and RNS generation in human keratinocytes. Mol. Cell. Biochem 279:105–12 [DOI] [PubMed] [Google Scholar]
- 39.Drobna Z, Naranmandura H, Kubachka KM, Edwards BC, Herbin-Davis K, et al. 2009. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem. Res. Toxicol 22:1713–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ducker GS, Rabinowitz JD. 2017. One-carbon metabolism in health and disease. Cell Metab. 25:27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engstrom K, Vahter M, Mlakar SJ, Concha G, Nermell B, et al. 2011. Polymorphisms in arsenic(+III oxidation state) methyltransferase (AS3MT) predict gene expression of AS3MT as well as arsenic metabolism. Environ. Health Perspect 119:182–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engström KS, Hossain MB, Lauss M, Ahmed S, Raqib R, et al. 2013. Efficient arsenic metabolism—the AS3MT haplotype is associated with DNA methylation and expression of multiple genes around AS3MT. PLOS ONE 8:e53732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engstrom KS, Vahter M, Fletcher T, Leonardi G, Goessler W, et al. 2015. Genetic variation in arsenic (+3 oxidation state) methyltransferase (AS3MT), arsenic metabolism and risk of basal cell carcinoma in a European population. Environ. Mol. Mutagen 56:60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Environ. Prot. Agency (EPA). 2001. National primary drinking water regulations; arsenic and clarifications to compliance and new cource contaminants: final rule. Fed. Regist 66:6996–706 [Google Scholar]
- 45.Food Drug Admin. (FDA). 2016. Arsenic in rice and rice products risk assessment report. Cent. Food Saf. Appl. Nutr, Food Drug Adm., US Dep. Health Hum. Serv., College Park, MD. [Google Scholar]
- 46.Food Drug Admin. (FDA). 2017. Total Diet Study: elements results summary statistics—market baskets 2006 through 2013. Cent. Food Saf. Appl. Nutr, Food Drug Adm., US Dep. Health Hum. Serv., College Park, MD; https://www.fda.gov/downloads/food...totaldietstudy/ucm184301.pdf [Google Scholar]
- 47.Food Drug Admin. (FDA), Carrington CD, Murray C, Tao S. 2013. A quantitative assessment of inorganic arsenic in apple juice. Draft Rep., Cent. Food Saf. Appl. Nutr., Food Drug Adm., US Dep. Health Hum. Serv., College Park, MD; https://www.fda.gov/downloads/food/foodscienceresearch/risksafetyassessment/ucm360016.pdf [Google Scholar]
- 48.Gamble MV, Ahsan H, Liu XH, Factor-Litvak P. 2005. Folate and cobalamin deficiencies and hyperhomocysteinemia in Bangladesh. Am. J. Clin. Nutr 81:1372–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, et al. 2006. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am. J. Clin. Nutr 84:1093–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, et al. 2005. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ. Health Perspect 113:1683–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, et al. 2007. Folic acid supplementation lowers blood arsenic. Am. J. Clin. Nutr 86:1202–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao J, Tong L, Argos M, Scannell Bryan M, Ahmed A, et al. 2015. The genetic architecture of arsenic metabolism efficiency: a SNP-based heritability study of Bangladeshi adults. Environ. Health Perspect 123:985–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gardner RM, Nermell B, Kippler M, Grandér M, Li L, et al. 2011. Arsenic methylation efficiency increases during the first trimester of pregnancy independent of folate status. Reprod. Toxicol 31:210–18 [DOI] [PubMed] [Google Scholar]
- 54.Gilbert-Diamond D, Li Z, Perry AE, Spencer SK, Gandolfi AJ, Karagas MR. 2013. A population-based case-control study of urinary arsenic species and squamous cell carcinoma in New Hampshire, USA. Environ. Health Perspect 121:1154–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gosse JA, Taylor VF, Jackson BP, Hamilton JW, Bodwell JE. 2014. Monomethylated trivalent arsenic species disrupt steroid receptor interactions with their DNA response elements at non-cytotoxic cellular concentrations. J. Appl. Toxicol 34:498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guthmiller P, Van Pilsum JF, Boen JR, McGuire DM. 1994. Cloning and sequencing of rat kidney L-arginine:glycine amidinotransferase. Studies on the mechanism of regulation by growth hormone and creatine. J. Biol. Chem 269:17556–60 [PubMed] [Google Scholar]
- 57.Hall M, Gamble M, Slavkovich V, Liu X, Levy D, et al. 2007. Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ. Health Perspect 115:1503–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall MN, Liu X, Slavkovich V, Ilievski V, Mi Z, et al. 2009. Influence of cobalamin on arsenic metabolism in Bangladesh. Environ. Health Perspect 117:1724–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall MN, Liu X, Slavkovich V, Ilievski V, Pilsner JR, et al. 2009. Folate, cobalamin, cysteine, homocysteine, and arsenic metabolism among children in Bangladesh. Environ. Health Perspect 117:825–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, et al. 2013. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ. Health Perspect 121:1068–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen HR, Raab A, Jaspars M, Milne BF, Feldmann J. 2004. Sulfur-containing arsenical mistaken for dimethylarsinous acid (DMAIII) and identified as a natural metabolite in urine: major implications for studies on arsenic metabolism and toxicity. Chem. Res. Toxicol . 17:1086–91 [DOI] [PubMed] [Google Scholar]
- 62.Hayakawa T, Kobayashi Y, Cui X, Hirano S. 2005. A new metabolic pathway of arsenite: Arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch. Toxicol . 79:183–91 [DOI] [PubMed] [Google Scholar]
- 63.Heck JE, Gamble MV, Chen Y, Graziano JH, Slavkovich V, et al. 2007. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am. J. Clin. Nutr . 85:1367–74 [DOI] [PubMed] [Google Scholar]
- 64.Heikens A 2006. Arsenic contamination of irrigation water, soil and crops in Bangladesh: risk implications for sustainable agriculture and food safety in Asia. Rep., Food Agric. Organ. United Nations, Reg. Off. Asia Pac., Bangkok, Thail: http://www.fao.org/docrep/009/ag105e/AG105E00.HTM [Google Scholar]
- 65.Hernandez A, Xamena N, Sekaran C, Tokunaga H, Sampayo-Reyes A, et al. 2008. High arsenic metabolic efficiency in AS3MT287Thr allele carriers. Pharmacogenetics Genom. 18:349–55 [DOI] [PubMed] [Google Scholar]
- 66.Hernandez A, Xamena N, Surralles J, Sekaran C, Tokunaga H, et al. 2008. Role of the Met(287)Thr polymorphism in the AS3MT gene on the metabolic arsenic profile. Mutat. Res 637:80–92 [DOI] [PubMed] [Google Scholar]
- 67.Hojsak I, Braegger C, Bronsky J, Campoy C, Colomb V, et al. 2015. Arsenic in rice: a cause for concern. J. Pediatric Gastroenterol. Nutr 60:142–45 [DOI] [PubMed] [Google Scholar]
- 68.Howe CG, Gamble MV. 2016. Influence of arsenic on global levels of histone posttranslational modifications: a review of the literature and challenges in the field. Curr. Environ. Health Rep 3:225–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howe CG, Liu X, Hall MN, Slavkovich V, Ilievski V, et al. 2016. Associations between blood and urine arsenic concentrations and global levels of post-translational histone modifications in Bangladeshi men and women. Environ. Health Perspect 124:1234–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsueh YM, Chiou HY, Huang YL, Wu WL, Huang CC, et al. 1997. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol. Biomark. Prev 6:589–96 [PubMed] [Google Scholar]
- 71.Huang Y-K, Huang Y-L, Hsueh Y-M, Yang M-H, Wu M-M, et al. 2008. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control 19:829–39 [DOI] [PubMed] [Google Scholar]
- 72.Huang Y-K, Tseng C-H, Huang Y-L, Yang M-H, Chen C-J, Hsueh Y-M. 2007. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol. Appl. Pharmacol 218:135–42 [DOI] [PubMed] [Google Scholar]
- 73.Huang YK, Pu YS, Chung CJ, Shiue HS, Yang MH, et al. 2008. Plasma folate level, urinary arsenic methylation profiles, and urothelial carcinoma susceptibility. Food Chem. Toxicol 46:929–38 [DOI] [PubMed] [Google Scholar]
- 74.Hughes MF, Edwards BC, Herbin-Davis KM, Saunders J, Styblo M, Thomas DJ. 2010. Arsenic (+3 oxidation state) methyltransferase genotype affects steady-state distribution and clearance of arsenic in arsenate-treated mice. Toxicol. Appl. Pharmacol 249:217–23 [DOI] [PubMed] [Google Scholar]
- 75.Hughes MF, Kenyon EM, Edwards BC, Mitchell CT, Razo LMD, Thomas DJ. 2003. Accumulation and metabolism of arsenic in mice after repeated oral administration of arsenate. Toxicol. Appl. Pharmacol 191:202–10 [DOI] [PubMed] [Google Scholar]
- 76.Jackson BP, Taylor VF, Karagas MR, Punshon T, Cottingham KL. 2012. Arsenic, organic foods, and brown rice syrup. Environ. Health Perspect 120:623–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jansen RJ, Argos M, Tong L, Li J, Rakibuz-Zaman M, et al. 2016. Determinants and consequences of arsenic metabolism efficiency among 4,794 individuals: demographics, lifestyle, genetics and toxicity. Cancer Epidemiol. Biomark. Prev 25(2):381–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamynina E, Lachenauer ER, DiRisio AC, Liebenthal RP, Field MS, Stover PJ. 2017. Arsenic trioxide targets MTHFD1 and SUMO-dependent nuclear de novo thymidylate biosynthesis. PNAS 114:E2319–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kile ML, Hoffman E, Hsueh Y-M, Afroz S, Quamruzzaman Q, et al. 2009. Variability in biomarkers of arsenic exposure and metabolism in adults over time. Environ. Health Perspect 117:455–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kile ML, Houseman EA, Baccarelli AA, Quamruzzaman Q, Rahman M, et al. 2014. Effect of prenatal arsenic exposure on DNA methylation and leukocyte subpopulations in cord blood. Epigenetics 9:774–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klein CB, Leszczynska J, Hickey C, Rossman TG. 2007. Further evidence against a direct genotoxic mode of action for arsenic-induced cancer. Toxicol. Appl. Pharmacol 222:289–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobayashi Y, Hirano S. 2008. Effects of endogenous hydrogen peroxide and glutathione on the stability of arsenic metabolites in rat bile. Toxicol. Appl. Pharmacol 232:33–40 [DOI] [PubMed] [Google Scholar]
- 83.Kuo C-C, Moon KA, Wang S-L, Silbergeld E, Navas-Acien A. 2017. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ. Health Perspect 125:087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurzius-Spencer M, da Silva V, Thomson CA, Hartz V, Hsu C-H, et al. 2017. Nutrients in one-carbon metabolism and urinary arsenic methylation in the National Health and Nutrition Examination Survey (NHANES) 2003–2004. Sci. Total Environ 607–608:381–90 [DOI] [PubMed] [Google Scholar]
- 85.Laine JE, Bailey KA, Rubio-Andrade M, Olshan AF, Smeester L, et al. 2015. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ. Health Perspect 123:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lawley SD, Cinderella M, Hall MN, Gamble MV, Nijhout HF, Reed MC. 2011. Mathematical model insights into arsenic detoxification. Theor. Biol. Med. Model 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lawley SD, Yun J, Gamble MV, Hall MN, Reed MC, Nijhout HF. 2014. Mathematical modeling of the effects of glutathione on arsenic methylation. Theor. Biol. Med. Model 11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li L, Ekstr E-C, Goessler W, Lonnerdal B, Nermell B, et al. 2008. Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Environ. Health Perspect 116:315–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X, Li B, Xi S, Zheng Q, Lv X, Sun G. 2013a. Prolonged environmental exposure of arsenic through drinking water on the risk of hypertension and type 2 diabetes. Environ. Sci. Pollut. Res. Int 20:8151–61 [DOI] [PubMed] [Google Scholar]
- 90.Li X, Li B, Xi S, Zheng Q, Wang D, Sun G. 2013b. Association of urinary monomethylated arsenic concentration and risk of hypertension: a cross-sectional study from arsenic contaminated areas in northwestern China. Environ. Health 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, Wang D, Li X, Zheng Q, Sun G. 2015. A potential synergy between incomplete arsenic methylation capacity and demographic characteristics on the risk of hypertension: findings from a cross-sectional study in an arsenic-endemic area of inner Mongolia, China. Int. J. Environ. Res. Public Health 12:3615–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin S, Shi Q, Nix FB, Styblo M, Beck MA, et al. 2002. A novel S-adenosyl-L-methionine:arsenicIII methyltransferase from rat liver cytosol. J. Biol. Chem 277:10795–803 [DOI] [PubMed] [Google Scholar]
- 93.Lindberg A-L, Kumar R, Goessler W, Thirumaran R, Gurzau E, et al. 2007. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ. Health Perspect 115:1081–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindberg A-L, Rahman M, Persson L-A, Vahter M. 2008. The risk of arsenic induced skin lesions in Bangladeshi men and women is affected by arsenic metabolism and the age at first exposure. Toxicol. Appl. Pharmacol 230:9–16 [DOI] [PubMed] [Google Scholar]
- 95.Lindgren A, Vahter M, Dencker L. 2009. Autoradiographic studies on the distribution of arsenic in mice and hamsters administered 74As-arsenite or -arsenate. Acta Pharmacol. Toxicol 51:253–65 [DOI] [PubMed] [Google Scholar]
- 96.López-Carrillo L, Gamboa-Loira B, Becerra W, Hernández-Alcaraz C, Hernández-Ramírez RU, et al. 2016. Dietary micronutrient intake and its relationship with arsenic metabolism in Mexican women. Environ. Res 151:445–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.López-Carrillo L, Hernandez-Ramirez RU, Gandolfi AJ, Ornelas-Aguirre JM, Torres-Sanchez L, Cebrian ME. 2014. Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol. Appl. Pharmacol 280:53–59 [DOI] [PubMed] [Google Scholar]
- 98.Mandal BK, Ogra Y, Suzuki KT. 2001. Identification of dimethylarsinous and monomethylarsonous acids in human urine of the arsenic-affected areas in West Bengal, India. Chem. Res. Toxicol 14:371–78 [DOI] [PubMed] [Google Scholar]
- 99.Markowski VP, Currie D, Reeve EA, Thompson D, Wise JP Sr. 2011. Tissue-specific and dose-related accumulation of arsenic in mouse offspring following maternal consumption of arsenic-contaminated water. Basic Clin. Pharmacol. Toxicol 108:326–32 [DOI] [PubMed] [Google Scholar]
- 100.Mauro M, Caradonna F, Klein CB, Dolinoy D. 2016. Dysregulation of DNA methylation induced by past arsenic treatment causes persistent genomic instability in mammalian cells. Environ. Mol. Mutagen 57:137–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mazumdar M, Ibne Hasan MOS, Hamid R, Valeri L, Paul L, et al. 2015. Arsenic is associated with reduced effect of folic acid in myelomeningocele prevention: a case control study in Bangladesh. Environ. Health 14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meharg AA, Rahman MM. 2003. Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic contamination. Environ. Sci. Technol 37:229–34 [DOI] [PubMed] [Google Scholar]
- 103.Melak D, Ferreccio C, Kalman D, Parra R, Acevedo J, et al. 2014. Arsenic methylation and lung and bladder cancer in a case-control study in northern Chile. Toxicol. Appl. Pharmacol 274:225–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitra SR, Mazumder DNG, Basu A, Block G, Haque R, et al. 2004. Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environ. Health Perspect 112:1104–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moe B, Peng H, Lu X, Chen B, Chen LWL, et al. 2016. Comparative cytotoxicity of fourteen trivalent and pentavalent arsenic species determined using real-time cell sensing. J. Environ. Sci. (China) 49:113–24 [DOI] [PubMed] [Google Scholar]
- 106.Mudd SH, Poole JR. 1975. Labile methyl balances for normal humans on various dietary regimens. Metab. Clin. Exp 24:721–35 [DOI] [PubMed] [Google Scholar]
- 107.Muenyi CS, Ljungman M, States JC. 2015. Arsenic disruption of DNA damage responses—potential role in carcinogenesis and chemotherapy. Biomolecules 5:2184–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nachman KE, Baron PA, Raber G, Francesconi KA, Navas-Acien A, Love DC. 2013. Roxarsone, inorganic arsenic, and other arsenic species in chicken: a U.S.-based Market Basket sample. Environ. Health Perspect 121:818–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Naujokas MF, Anderson B, Ahsan H, Aposhian V, Graziano JH, et al. 2013. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ. Health Perspect 121:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. 2011. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the U.S. population. Environ. Res 111:110–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Niedzwiecki MM, Hall MN, Liu X, Oka J, Harper KN, et al. 2013. A dose-response study of arsenic exposure and global methylation of peripheral blood mononuclear cell DNA in Bangladeshi adults. Environ. Health Perspect 121:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Niedzwiecki MM, Liu X, Hall MN, Thomas T, Slavkovich V, et al. 2015. Sex-specific associations of arsenic exposure with global DNA methylation and hydroxymethylation in leukocytes: results from two studies in Bangladesh. Cancer Epidemiol. Biomark. Prev 24:1748–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niedzwiecki MM, Liu X, Zhu H, Hall MN, Slavkovich V, et al. 2018. Serum homocysteine, arsenic methylation, and arsenic-induced skin lesion incidence in Bangladesh: a one-carbon metabolism candidate gene study. Environ. Int 113:133–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pelch KE, Tokar EJ, Merrick BA, Waalkes MP. 2015. Differential DNA methylation profile of key genes in malignant prostate epithelial cells transformed by inorganic arsenic or cadmium. Toxicol. Appl. Pharmacol 286:159–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peters BA, Hall MN, Liu X, Faruque P, Sanchez TR, et al. 2015. Folic acid and creatine as therapeutic approaches to lower blood arsenic: a randomized controlled trial. Environ. Health Perspect 123:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peters BA, Hall MN, Liu X, Parvez F, Siddique AB, et al. 2015. Low-dose creatine supplementation lowers plasma guanidinoacetate, but not plasma homocysteine, in a double-blind, randomized, placebo-controlled trial. J. Nutr 145:2245–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. 2000. Monomethylarsonous acid (MMAIII) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol 163:203–7 [DOI] [PubMed] [Google Scholar]
- 118.Petrick JS, Jagadish B, Mash EA, Aposhian HV. 2001. Monomethylarsonous acid (MMAIII) and arsenite: LD50 in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem. Res. Toxicol 14:651–56 [DOI] [PubMed] [Google Scholar]
- 119.Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, et al. 2007. Genomic methylation of peripheral blood leukocyte DNA: influences of arsenic and folate in Bangladeshi adults. Am. J. Clin. Nutr 86:1179–86 [DOI] [PubMed] [Google Scholar]
- 120.Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, et al. 2009. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ. Health Perspect 117:254–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Porter KE, Basu A, Hubbard AE, Bates MN, Kalman D, et al. 2010. Association of genetic variation in cystathionine-β-synthase and arsenic metabolism. Environ. Res 110:580–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Potera C 2007. Food safety: U.S. rice serves up arsenic. Environ. Health Perspect 115:A296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pu Y-S, Yang S-M, Huang Y-K, Chung C-J, Huang SK, et al. 2007. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicol. Appl. Pharmacol 218:99–106 [DOI] [PubMed] [Google Scholar]
- 124.Raml R, Rumpler A, Goessler W, Vahter M, Li L, et al. 2007. Thio-dimethylarsinate is a common metabolite in urine samples from arsenic-exposed women in Bangladesh. Toxicol. Appl. Pharmacol. 222:374–80 [DOI] [PubMed] [Google Scholar]
- 125.Ravenscroft P, Brammer H, Richards K. 2009. Arsenic Pollution: A Global Synthesis. New York: Wiley-Blackwell [Google Scholar]
- 126.Reed MC, Gamble MV, Hall MN, Nijhout HF. 2015. Mathematical analysis of the regulation of competing methyltransferases. BMC Syst. Biol 9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reichard JF, Puga A. 2010. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics 2:87–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schlawicke Engstrom K, Nermell B, Concha G, Stromberg U, Vahter M, Broberg K. 2009. Arsenic metabolism is influenced by polymorphisms in genes involved in one-carbon metabolism and reduction reactions. Mutat. Res 667:4–14 [DOI] [PubMed] [Google Scholar]
- 129.Seow WJ, Kile ML, Baccarelli AA, Pan W-C, Byun H-M, et al. 2014. Epigenome-wide DNA methylation changes with development of arsenic-induced skin lesions in Bangladesh: a case-control follow-up study. Environ. Mol. Mutagen 55:449–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Signes-Pastor AJ, Carey M, Meharg AA. 2017. Inorganic arsenic removal in rice bran by percolating cooking water. Food Chem 234:76–80 [DOI] [PubMed] [Google Scholar]
- 131.Singh AP, Goel RK, Kaur T. 2011. Mechanisms pertaining to arsenic toxicity. Toxicol. Int 18:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smith AH, Lingas EO, Rahman M. 2000. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull. World Health Organ 78:1093–103 [PMC free article] [PubMed] [Google Scholar]
- 133.Smith AH, Marshall G, Liaw J, Yuan Y, Ferreccio C, Steinmaus C. 2012. Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ. Health Perspect 120:1527–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spiegelstein O, Gould A, Wlodarczyk B, Tsie M, Lu X, et al. 2005. Developmental consequences of in utero sodium arsenate exposure in mice with folate transport deficiencies. Toxicol. Appl. Pharmacol 203:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spiegelstein O, Lu X, Le XC, Troen A, Selhub J, et al. 2003. Effects of dietary folate intake and folate binding protein-1 (Folbp1) on urinary speciation of sodium arsenate in mice. Toxicol. Lett 145(2):167–74 [DOI] [PubMed] [Google Scholar]
- 136.Spiegelstein O, Lu X, Le XC, Troen A, Selhub J, et al. 2005. Effects of dietary folate intake and folate binding protein-2 (Folbp2) on urinary speciation of sodium arsenate in mice. Environ. Toxicol. Pharmacol 19:1–7 [DOI] [PubMed] [Google Scholar]
- 137.Spratlen MJ, Gamble MV, Grau-Perez M, Kuo C-C, Best LG, et al. 2017. Arsenic metabolism and one-carbon metabolism at low-moderate arsenic exposure: evidence from the Strong Heart Study. Food Chem. Toxicol 105:387–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stead LM, Au KP, Jacobs RL, Brosnan ME, Brosnan JT. 2001. Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am. J. Physiol. Endocrinol. Metab 281:E1095–100 [DOI] [PubMed] [Google Scholar]
- 139.Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. 2006. Is it time to reevaluate methyl balance in humans? Am. J. Clin. Nutr 83:5–10 [DOI] [PubMed] [Google Scholar]
- 140.Steinmaus C, Bates MN, Yuan Y, Kalman D, Atallah R, et al. 2006. Arsenic methylation and bladder cancer risk in case-control studies in Argentina and the United States. J. Occup. Environ. Med 48:478–88 [DOI] [PubMed] [Google Scholar]
- 141.Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH. 2005. Dietary intake and arsenic methylation in a U.S. population. Environ. Health Perspect 113:1153–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Steinmaus C, Ferreccio C, Acevedo J, Yuan Y, Liaw J, et al. 2014. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol. Biomark. Prev 23:1529–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Steinmaus C, Ferreccio C, Yuan Y, Acevedo J, Gonzalez F, et al. 2014. Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am. J. Epidemiol 180:1082–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Steinmaus C, Yuan Y, Kalman D, Rey OA, Skibola CF, et al. 2010. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol. Appl. Pharmacol 247:138–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Steinmaus CM, Ferreccio C, Romo JA, Yuan Y, Cortes S, et al. 2013. Drinking water arsenic in Northern Chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol. Biomark. Prev 22:623–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stevens JT, Hall LL, Farmer JD, DiPasquale LC, Chernoff N, Durham WF. 1977. Disposition of 14C and/or 74As-cacodylic acid in rats after intravenous, intratracheal, or peroral administration. Environ. Health Perspect 19:151–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, et al. 2000. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol 74:289–99 [DOI] [PubMed] [Google Scholar]
- 148.Sun G-X, Williams PN, Zhu Y-G, Deacon C, Carey A-M, et al. 2009. Survey of arsenic and its speciation in rice products such as breakfast cereals, rice crackers and Japanese rice condiments. Environ. Int 35:473–75 [DOI] [PubMed] [Google Scholar]
- 149.Taes YEC, Delanghe JR, De Vriese AS, Rombaut R, Van Camp J, Lameire NH. 2003. Creatine supplementation decreases homocysteine in an animal model of uremia. Kidney Int 64:1331–37 [DOI] [PubMed] [Google Scholar]
- 150.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, et al. 2013. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med 368:1575–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thomas DJ, Waters SB, Styblo M. 2004. Elucidating the pathway for arsenic methylation. Toxicol. Appl. Pharmacol 198:319–26 [DOI] [PubMed] [Google Scholar]
- 152.Tice RR, Yager JW, Andrews P, Crecelius E. 1997. Effect of hepatic methyl donor status on urinary excretion and DNA damage in B6C3F1 mice treated with sodium arsenite. Mutat. Res 386(3):315–34 [DOI] [PubMed] [Google Scholar]
- 153.Tseng CH, Huang YK, Huang YL, Chung CJ, Yang MH, et al. 2005. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease–hyperendemic villages in Taiwan. Toxicol. Appl. Pharmacol 206:299–308 [DOI] [PubMed] [Google Scholar]
- 154.Tseng WP. 1977. Effects and dose-response relationships of skin cancer and blackfoot disease with arsenic. Environ. Health Perspect 19:109–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Uthus EO, Davis C. 2005. Dietary arsenic affects dimethylhydrazine-induced aberrant crypt formation and hepatic global DNA methylation and DNA methyltransferase activity in rats. Biol. Trace Element Res 103:133–45 [DOI] [PubMed] [Google Scholar]
- 156.Vahter M, Marafante E. 1987. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol. Lett 37:41–46 [DOI] [PubMed] [Google Scholar]
- 157.Valenzuela OL, Borja-Aburto VH, Garcia-Vargas GG, Cruz-Gonzalez MB, Garcia-Montalvo EA, et al. 2005. Urinary trivalent methylated arsenic species in a population chronically exposed to inorganic arsenic. Environ. Health Perspect 113:250–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van Breda SGJ, Claessen SMH, Lo K, van Herwijnen M, Brauers KJ, et al. 2015. Epigenetic mechanisms underlying arsenic associated lung carcinogenesis. Arch. Toxicol. 89:1959–69 [DOI] [PubMed] [Google Scholar]
- 159.Vance JE, Vance DE. 2004. Phospholipid biosynthesis in mammalian cells. Biochem. Cell Biol 82:113–28 [DOI] [PubMed] [Google Scholar]
- 160.Welch AH, Stollenwerk KG, eds. 2002. Arsenic in Ground Water: Geochemistry and Occurrence. New York: Kluwer Acad. [Google Scholar]
- 161.Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA. 2005. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ. Sci. Technol 39:5531–40 [DOI] [PubMed] [Google Scholar]
- 162.Wilson D, Hooper C, Shi X. 2012. Arsenic and lead in juice: apple, citrus, and apple-base. J. Environ. Health 75:14–20 [PubMed] [Google Scholar]
- 163.Wiseman H, Halliwell B. 1996. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J 313:17–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wlodarczyk B, Spiegelstein O, Gelineau-van Waes J, Vorce RL, Lu X, et al. 2001. Arsenic-induced congenital malformations in genetically susceptible folate binding protein-2 knockout mice. Toxicol. Appl. Pharmacol 177:238–46 [DOI] [PubMed] [Google Scholar]
- 165.World Health Organ. (WHO). 2008. Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda, Vol. 1 Geneva: World Health Organ; 3rd ed. [Google Scholar]
- 166.Wu M-M, Chiou H-Y, Hsueh Y-M, Hong C-T, Su C-L, et al. 2006. Effect of plasma homocysteine level and urinary monomethylarsonic acid on the risk of arsenic-associated carotid atherosclerosis. Toxicol. Appl. Pharmacol 216:168–75 [DOI] [PubMed] [Google Scholar]
- 167.Xu Y, Tokar EJ, Waalkes MP. 2014. Arsenic-induced cancer cell phenotype in human breast epithelia is estrogen receptor-independent but involves aromatase activation. Arch. Toxicol 88:263–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yamanaka K, Takabayashi F, Mizoi M, An Y, Hasegawa A, Okada S. 2001. Oral exposure of dimethylarsinic acid, a main metabolite of inorganic arsenics, in mice leads to an increase in 8-Oxo-2’-deoxyguanosine level, specifically in the target organs for arsenic carcinogenesis. Biochem. Biophys. Res. Commun 287:66–70 [DOI] [PubMed] [Google Scholar]
- 169.Yamauchi H, Yamamura Y. 1983. Concentration and chemical species of arsenic in human tissue. Bull. Environ. Contam. Toxicol 31:267–70 [DOI] [PubMed] [Google Scholar]
- 170.Yu RC, Hsu KH, Chen CJ, Froines JR. 2000. Arsenic methylation capacity and skin cancer. Cancer Epidemiol. Biomark. Prev 9:1259–62 [PubMed] [Google Scholar]
- 171.Zakharyan R, Wu Y, Bogdan GM, Aposhian HV. 1995. Enzymatic methylation of arsenic compounds: assay, partial purification, and properties of arsenite methyltransferase and monomethylarsonic acid methyltransferase of rabbit liver. Chem. Res. Toxicol 8:1029–38 [DOI] [PubMed] [Google Scholar]
- 172.Zakharyan RA, Ayala-Fierro F, Cullen WR, Carter DM, Aposhian HV. 1999. Enzymatic methylation of arsenic compounds. VII. Monomethylarsonous acid (MMAIII) is the substrate for MMA methyltransferase of rabbit liver and human hepatocytes. Toxicol. Appl. Pharmacol 158:9–15 [DOI] [PubMed] [Google Scholar]
- 173.Zeisel SH. 2009. Importance of methyl donors during reproduction. Am. J. Clin. Nutr 89:673S–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zeisel SH, Warrier M. 2017. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr 37:157–81 [DOI] [PubMed] [Google Scholar]
- 175.Zhang J, Mu X, Xu W, Martin FL, Alamdar A, et al. 2014. Exposure to arsenic via drinking water induces 5-hydroxymethylcytosine alteration in rat. Sci. Total Environ. 497–498:618–25 [DOI] [PubMed] [Google Scholar]
- 176.Zhao F, Stroud JL, Eagling T, Dunham SJ, McGrath SP, Shewry PR. 2010. Accumulation, distribution, and speciation of arsenic in wheat grain. Environ. Sci. Technol 44(14):5464–68 [DOI] [PubMed] [Google Scholar]
- 177.Zhao L, Chen S, Jia L, Shu S, Zhu P, Liu Y. 2012. Selectivity of arsenite interaction with zinc finger proteins. Metallomics 4:988–94 [DOI] [PubMed] [Google Scholar]