Abstract
Biopolymer microgels are emerging as a versatile tool for aiding in the regeneration of damaged tissues due to their biocompatible nature, tunable microporous structure, ability to encapsulate bioactive factors, and tailorable properties such as stiffness and composition. These properties of microgels, along with their injectability, have allowed for their utilization in a multitude of different tissue engineering applications. Controlled release of growth factors, antibodies, and other bioactive factors from microgels have demonstrated their capabilities as transporters for essential bioactive molecules necessary for guiding tissue reconstruction. Additionally, recent in vitro studies of cellular interaction and proliferation within microgel structures have laid the initial groundwork for regenerative tissue engineering using these materials. Microgels have even been crosslinked together in various ways or 3D printed to form three-dimensional scaffolds to support cell growth. Additionally, in vivo studies of microgels have pioneered the clinical relevance of these novel and innovative materials for regenerative tissue engineering. This review will cover recent developments and research of microgels as they pertain to bioactive factor release, cellular interaction and proliferation in vitro, and tissue regeneration in vivo.
Keywords: microgels, tissue engineering, biopolymers, regenerative medicine, drug delivery
Graphical Abstract

INTRODUCTION
Tissue engineering is a field that aims to regenerate and repair damaged tissues through the utilization of bioactive molecules and materials. A frequently used class of material for tissue engineering applications is a hydrogel: a crosslinked, porous, hydrated, tailorable scaffold constructed from hydrophilic biopolymers. Recent advances in tissue engineering have demonstrated the versatility and efficacy of hydrogel microparticles, or microgels, as drug delivery depots and scaffolds for regenerative medicine. Microgels are unique in the sense that they are injectable, and provide a highly tunable, modular microstructure, all while maintaining drug encapsulation capabilities and biocompatibility of their hydrogel analog. The large amount of tunability with microgel systems has established their applicability in a multitude of tissue engineering facets. In conjunction with microgel particles, bioactive factors or cultured cells can be encapsulated for delivery to the desired regeneration site. This review will cover microgel system properties, bioactive factor delivery with microgels, and advances in tissue engineering with microgels, highlighting both in vitro and in vivo studies conducted for various types of tissues. Synthesis, fabrication, and functionality of microgels has been discussed in detail in other reviews and will not be emphasized here. [1–6]
Inclusion of microgels into tissue engineering systems adds benefits that include unique and controlled release mechanisms that originate from the modular feature of microgels [7], regulation and control over porosity of the system[8], and tailorable mechanical properties to fit the needs of the injury site. [9,10] In this review, modularity refers to the potential combination of independently formed microgels to create a diverse population of properties, cells, or bioactive factors. Porosity refers to the void spaces between microgels depending on packing order and, sometimes, on how closely microgels are crosslinked to one another to form systems. Mechanical properties refer to the properties of the larger structures that are formed by crosslinking microgels together and vary based on the polymer substrate(s) used, type of crosslinking utilized, and in some cases the ratio between microgels and hydrogel in the system. With these features, microgels offer significant promise as a class of materials to be used in regenerative medicine.
A variety of methods are available for the formation of microgel systems such as emulsion, coacervation, gelation, spray drying, grinding, and microfluidics that have been widely recognized and discussed, and have been reviewed in [11,12]. Recently, microgel particles have been created on scales from nanoscale [13] to the more common microscale [14]. Along with this range of size scales comes a variety of morphologies such as spherical, discoidal, and even lock-and-key shapes. [15–17] Some systems can form disks, plugs, threads, or spheres based on control of microfluidic device flow rates. [14] The morphology of microgel particles has been shown to be a variable that can be readily manipulated and plays an integral role in defining the porosity of microgel systems, which include composite materials that incorporate microgels into another bulk carrier material, and microgels linked together to form scaffolds. The crosslinking of microgels can either be physical or chemical and contributes to the structure of the system but it will not be discussed in depth in this review, as it has been thoroughly detailed elsewhere [1]. The shape and morphology of microgels play an integral role in the fate of the damaged tissue, [8] and with the variety of sizes and properties that can be engineered in microgels, they can be tailored for specific tissue engineering applications. Porosity of the microgels and microgel-scaffolds also contributes to the cell and tissue interaction. The porosity allows for cellular infiltration or movement as well as for nutrient diffusion and transport. [18] Accordingly, encapsulated growth factors and bioactive agents can diffuse through these pores to the damaged tissue areas and provide guidance to the cellular growth and repair in the area. [13] In essence, tunable porosity gives control over drug delivery, nutrient diffusion, and cellular expansion.
Microgels have been manufactured from various materials from synthetic polymers such as poly(ethylene glycol) (PEG) [19] to naturally derived polysaccharides such as chitosan and alginate. [20–22] The microgel material, composition, and, if applicable, bulk matrix used to encapsulate the microgels into a system, can allow for mechanically tailorable properties of the system and gives rise to control over cellular response [23] as well as tissue property matching for a more contiguous implant in vivo. [9]
The modular feature of microgel particles is attractive for the incorporation of more than one bioactive agent or growth factor in, for example, a dual release mechanism. [24] Encapsulation of two growth factors into two different microgels will give a unique and specific drug release profile that can be engineered to coincide with cellular processes including proliferation and differentiation. There are a variety of encapsulation methods which expand even further the potential for the creation of specific drug release profiles. The modular nature of these microgel systems paves the way for a multitude of applications in the tissue engineering field as well as giving rise to complex drug delivery systems.
Another recent use for microgels is as inks in 3D printing. Novel advances in tissue engineering have revealed 3D printing as a method for formation of hydrogel and microgel systems. 3D printing systems for microgels utilize devices for the creation of uniform microparticles and even have the capabilities of functionalization of the microgels with lock and key bonds as well as addition of dangling groups. [7] The momentum for these 3D printing approaches stems from the control over the spatial and temporal organization of the system and as 3D printing develops, new methodologies are expected to emerge. Employing injectable shear thinning properties of microgel solutions and mixtures can aid in formation of highly tailorable systems that can be utilized for tissue regeneration and cellular growth.[25] 3D printed microgels and microgels that themselves comprise the ink for 3D printing larger structures are emerging as promising avenues for biopolymer scaffold formation in tissue engineering.
The versatility and utility of these microgel systems for specific applications in tissue engineering stem from the control over material, size, morphology, and porosity. The following review will cover microgels as vectors for drug and bioactive factor delivery, microgel use in vitro for cell expansion and proliferation, and microgels incorporated in vivo for tissue regeneration and reconstruction.
BIOACTIVE DRUG ENCAPSULATION AND DELIVERY
Controlled and complex release kinetics of microgel systems make them a novel platform for directing cellular pathways and regenerative tissue engineering. Microgel systems have emerged as drug and growth factor delivery systems that have tailorable release kinetics, [26] including those that possess the potential for specified release based on surrounding pH [27], and microgels can even be combined for dual drug delivery [24]. In general, hydrogels are good materials for bioactive factor encapsulation due to their hydrophilic nature and often gentle fabrication and crosslinking methods.
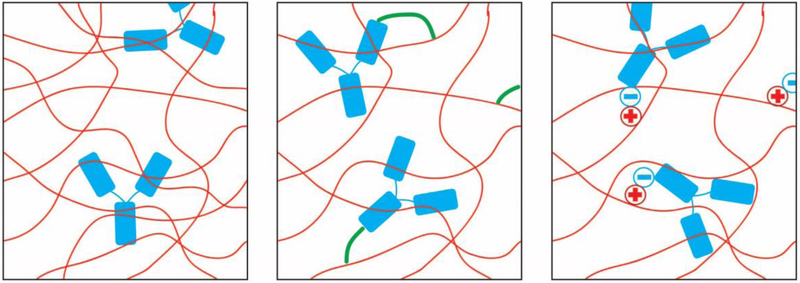
Encapsulation of bioactive factors is often pursued for tissue engineering applications to provide localized delivery of necessary biofactors for regeneration. Various methods to encapsulate bioactive factors and drugs are available by varying electrostatic interactions, covalent bonds, hydrogen bonds, or entangled in the polymer matrix. These different methods, shown in Figure 1, play a role in the release kinetics of the system and should be thoughtfully chosen when designing a microgel system. Drug delivery from bulk hydrogel systems presents some limitations in terms of release mechanics due to the hydrated microstructure which can cause a relatively rapid release of biofactors, typically on the order of only several days or sometimes up to one month. [28] Modifications to the bulk hydrogel may quell the rapid release, but often result in unwanted changes in properties. The advantage of including microgels into a system is that they can enhance bioactive factor loading as well as propagate release to more extended time periods without exaggerated changes in material properties.
Figure 1.
Encapsulation methods for bioactive factors in polymer matrices, (from left to right) encapsulation through entanglement, encapsulation through covalent bonding, and encapsulation through electrostatic interactions
Encapsulation of bioactive factors can be done utilizing covalent bonding between the polymer matrix and the factor itself. Degradation in vivo will release the encapsulated factors over time and depends heavily on the hydrolytic or enzymatic degradation rate of the polymer matrix. Faster degradation will result in a faster release rate of the bonded drug, and this can be tuned based upon the polymer used. Entanglement of drugs within the polymer matrix is another method of sequestration. Diffusion gradients play an important role in how the drug will release into the environment or the in vivo site. Larger molecules will diffuse slower based upon the permeability of the gels and can be tailored utilizing different gels selected as the vector. [29] Proteins will also adsorb to the polymer chains through electrostatic interactions. Positively charged polymers can effectively encapsulate negatively charged bioactive factors (and vice versa) and slow down release of the drug when compared to pure entanglement methods. [20] Drug release from hydrogels has been heavily researched and can be tailored based upon pH-responsive [27] or temperature-responsive [30] hydrogels to further modulate drug release to the specified area.
One type of complex and unique drug release profile was established by Richardson et. al who were one of the first to use a composite microsphere-hydrogel system. [24] Their unique poly(lactic-co-glycolic acid) (PLG)-derived system was able to quickly release vascular endothelial growth factor (VEGF) from the hydrogel scaffold and slowly release platelet-derived growth factor (PDGF) from encapsulated microspheres and paved the way for other drug and bioactive agent delivery vehicles. Although this system implemented hydrophobic microspheres rather than gels, it was a stepping stone for microgel co-delivery to expand upon. Other groups have used this idea of modular properties in polymer microgels for encapsulation and creation of chemical gradients, mineral gradients, and even co-culture of cells, expanding potential applications for microgels in a variety of areas to more than co-delivery of growth factors.
Release mechanics of peptides from poly(acrylic acid) microgels aided in understanding the effect of electrostatic interactions on release. [31] Moderately charged, short peptides interact strongly with the oppositely charged matrix which inhibits diffusive release from the microgel. Additionally, changes of pH within the system around the isoelectric point of the attached proteins can also influence the release rate. Electrostatic interactions of growth factors with negatively-charged chondroitin sulfate microgels mirrored similar results. For example, positively charged transforming growth factor-β1(TGF-β1) was effectively encapsulated while negatively charged TGF-α was not. [15] pH-responsive release of methotrexate disodium conjugated to apo-transferrin at low pH from chitosan microgels has emerged as a potential for localized cancer treatments where the pH deviates from neutral body pH. [27] These pH- and charge-based microgels are only just emerging as a platform for drug release and tissue engineering but have already shown much promise for controlled drug release applications.
Following the trend of environmentally-responsive gels, bone morphogenetic protein-2 (BMP-2)-loaded gelatin microspheres that have a release mechanism dependent on inflammatory macrophage degradation have been engineered. [32] This gel system will only release in the presence of macrophages, which are cells that respond to an injury, thus providing a selective release mechanism based upon physiologic response to an injury. Enzyme-mediated release has also been demonstrated using genipin crosslinked gelatin microspheres, where the loading of growth factors was dependent on zeta potential of the type of gelatin used, and the growth factor release was dependent on proteolytic enzymes in the area, correlating closely to inflammatory responses. [33] Tailoring gel release for a specific immune response promotes enhanced temporal control of drug delivery and adds a selective premise upon which the microgel system responds aiding in tissue regeneration only when called upon.
Another environmentally responsive hydrogel that has been demonstrated is chitosan modified with poly (N-isopropylacrylamide) (PNIPAM) and methacryloyl groups to create UV crosslinkable and thermoresponsive hydrogels. [34] The origin of the unique release of doxorubicin from these gels is based upon the shrinkage of the gel at physiological pH followed by NIR-triggered release from the system.
In summary, responsive microgels can promote increased spatial and temporal control of drug and biofactor release, cascading down to directed cellular responses in the area. With directed control of the necessary bioactive factors, cellular infiltration and differentiation can be promoted and tuned for repair, and regeneration of damaged tissues. A more detailed review of functional microgels and fabrication methods can be found in [28].
MICROGELS AND CELLS
Cellular processes and responses are highly dependent on the surrounding microenvironment, including scaffold morphology, porosity, polymer composition, and bioactive factor release. For this reason, in vitro studies serve as an initial validation of a new microgel system by allowing the study of cellular responses, but they fail to account for the highly complex nature of cellular functions in the body. In turn, in vivo studies help to illuminate organism responses to the introduced system and allow for determination of clinically viable systems. Microgels are currently being studied in vitro and in vivo for various tissue engineering applications, including bone, cartilage, vascular, cardiac, hepatic, and neuronal tissues.
I. Bone Tissue Engineering
Bone tissue engineering aims to regenerate and repair the mineralized, vascularized tissue structure in injured bones. Currently, the gold standard treatment for bone defects are autografts or allografts, but these can be associated with several drawbacks such as bleeding, inflammation, infection, prolonged pain, high cost, and a shortage of available tissue. Biomaterial scaffolds that promote cellular infiltration, differentiation, and ultimately tissue regeneration may provide an alternative solution to tissue grafts. [35] Microgels have emerged as a potential type of scaffold for osteogenic tissue reconstruction due to their high porosity and mechanical tunability which facilitates vasculature formation, mineral deposition, and tissue regeneration. [36]
Shen et. al used a naturally derived polymer for osteogenesis promoting microgels, specifically, hydroxyapatite-coated chitosan microspheres. [21] These coated microspheres were chosen due to their injectability and capability of controlled degradation. Addition of the hydroxyapatite coating helped promote attachment, proliferation, and differentiation of preosteoblastic MC3T3 cells, and additional surface area of hydroxyapatite correlated to increased cellular proliferation.
In another use of chitosan, the polymer was modified with an N-methacryloyl group to create a hydrosoluble and UV cross-linkable polymer for use in microgel fabrication. [37] Preosteoblastic MC3T3s were encapsulated into the modified chitosan and microgels formed utilizing photolithography techniques, with cell viability remaining above 90%. In vivo, mice responded with minimal inflammation.
An integral part of bone tissue engineering is the promotion of osteogenic differentiation of cells to form bone within the site, not only can microgels act as vectors for biofactors but for cells as well. Alginate microbeads were utilized as a means of controlled adipogenic stem cell delivery in vitro. [38] To promote the release of cells from the microspheres, the addition of alginate-lyase was necessary as the alginate polymer gel itself does not degrade in vivo although the polymer matrix will fall apart as the calcium crosslinker diffuses out. Cellular messenger RNA (mRNA) was monitored for insight into biological activity and showed delivery of viable cells that favored an osteogenic pathway and control of downstream osteogenic factors.
As one would expect, in vitro and in vivo osteogenic studies may have different results when it comes to cellular infiltration, regeneration, and formation of new bone due to the increased level of complexity introduced by the in vivo environment, and thus it is important to test new biomaterial systems in vivo. Some technologies rely on the recruitment of endogenous cells to the site in vivo, rather than having a cell-based technology that could be more expensive and have greater regulatory hurdles. In one cell free approach, both synthetic and natural polymers were integrated for the formation of microgels: chitosan and PLG. [39] In vivo data was collected in a critical-sized rabbit ulnar defect with BMP-2 and heparin added into the blended polymer material. The model saw complete bridging of the defect, enhancement of mechanical properties, and supported natural bone formation.
Mao et. al performed in vivo studies of high polymer to cell ratio alginate microgels intravenously in mice. [40] They were able to encapsulate single marrow stromal cells in their microgels through microfluidic techniques and demonstrated that gel stiffness and cell density play an integral role into osteogenic differentiation. This method in vivo demonstrated an increased sensitivity to exogenous stimuli as compared to bulk hydrogels from cell secreted cytokines. The intravenous injection of the encapsulated cells sustains donor originated factors and delays kinetics of degradation. This unique technique can be used for integration of multiple cell lines for the formation of the heterogenous structure that can be seen in many tissues.
Another way to implement microgels is to make a composite system with microgels incorporated into a bulk hydrogel. A composite system of vascular endothelial growth factor (VEGF)-loaded alginate microgels in a collagen-hydroxyapatite scaffold was explored for bone reconstruction. [41] A rat calvarial defect model displayed the potential of the system by exhibiting bone reformation and improved vascularization in the system compared to untreated controls.
Recently, a unique method for bone regeneration was proposed that implemented silk fibroin microgels that were subsequently crosslinked together in a dilute silk fibroin solution that exhibited mechanical properties similar to that of natural cancellous bone.[42] New Zealand white rabbit femur defect models showed packed woven bone formation throughout, but also came with a small degree of inflammation in the area after a 4-week period. The method of formation of microgels crosslinked together is emerging as a platform for control over mechanics, contiguity, and unique porosity while also allowing for modulation of various incorporated growth factors and cells.
In an approach where cells were included in the material system, collagen and chitosan microgels were used in a modular fashion to introduce two different cell types into a subcutaneous rat dorsum model. [43] The implementation of both mesenchymal stem cells (MSCs) and bone marrow mononuclear cells (BMMCs) into the model produced improved bone formation over either cell type alone. Here, the microgels allowed for modular incorporation of two different types of cells, and the high porosity provided good nutrient diffusion to maintain cell viability and activity.
As demonstrated, there are a variety of techniques to encourage the regeneration of bony tissue using growth factors, materials, and cells, and microgels show promise as a class of materials that may benefit this regenerative medicine application.
II. Osteochondral and Cartilage Tissue Engineering
One of the greater challenges within tissue engineering is promoting the regeneration of chondrogenic tissues. Cartilage is avascular with a high cell density, making it a complex tissue to regenerate and reconstruct. One group has formed polyelectrolyte complexes (PECs) using chondroitin sulfate and chitosan embedded in agarose microbeads to form a composite system that mimics the extracellular matrix seen within cartilaginous tissue.[44] MSCs were co-embedded into the agarose microbeads with the PEC complex and allowed to proliferate, and over time the sulfated glycosaminoglycan (sGAG) and collagen type II gene expression demonstrated the chondrogenic differentiation potential of the material. Co-embedding agarose microbeads with the PEC and MSCs resulted in a more homogenous microgel structure as opposed to directly implementing MSCs into the PEC. The modular feature of microgels has been exploited to form gradients of growth factors to guide cellular differentiation in a manner that fits the heterogeneous structure of osteochondral tissues. [45] Silk and PLG microspheres were encapsulated in an alginate or silk scaffold for the dual drug delivery of insulin-like growth factor-1 (IGF-1) and BMP-2. MSCs proliferated on the scaffolds, and it was demonstrated that the growth factor gradient directed stem cell differentiation toward chondrogenic or osteogenic fates.
Animal models of cartilage and osteochondral injury have been used to improve our understanding of the highly hierarchal, avascular, and heterogenous structure of chondrogenic tissue. The avascularity of cartilage poses an interesting problem when it comes to cell growth and nutrient exchange as it must perfuse through the tissue rather than through blood exchange.
Bian et. al attempted to tackle chondrogenic tissue engineering using a composite microgel system with TGF-β3 loaded alginate microspheres in a hyaluronic acid gel scaffold with MSCs. [46] A subcutaneous mouse model proved that cartilage matrix formed but there was also evidence of calcification. To prevent calcification, parathyroid hormone-related protein (PTHrP) was co-delivered with TGF-β3 using the modular feature of microgels. Some reduction of calcification was observed but additional research is needed, specifically to avert directed differentiation of MSCs down a hypertrophic phenotype.
Articular cartilage injuries can progress to cartilage degeneration over time which is often associated with joint inflammation and pain. A novel way to counteract the inflammation is to use microgels to deliver anti-inflammatory factors. In a recent study, a synthetic polymer microgel carrying the anti-inflammatory drug, tacrolimus, was tested in a middle carpal horse model. [47] Drug release investigations showed localized delivery with no systemic effects and decreased inflammation demonstrating the benefit of using microgels to deliver anti-inflammatory factors. Not only did this model provide a method of locally delivering tacrolimus but showed that microgels can be an efficient vector for delivery in this type of arthritic model. Overall, microgel therapies are slowly taking hold in the field of cartilage tissue engineering due to their easily tunable nature which is helpful in the avascular and cellularly dense environment of cartilage.
III. Endothelial and Vascular Tissue Engineering
Vasculature is essential for the regeneration of most damaged tissues as it aids in nutrient, waste, and gas exchange necessary for cellular proliferation. Endothelial cells line blood vessels and promote healthy blood flow and nutrient exchange to surrounding tissues, hence, the formation of these structures in the process of angiogenesis is vital in directing growth of tissues. Vascular endothelial growth factor (VEGF) is an essential growth factor involved in the promotion of vascularization in tissues and is found in multiple isoforms with different surface charges. Riederer, et. al, formed uniform genipin-crosslinked chitosan microgels through an emulsion method, which exhibited microgel aggregation at a pH of 7.4; the microgels could sequester both positively and negatively charged VEGF as well as promote endothelial cell proliferation. [20] In addition to growth factors, microgel orientation itself has also been shown to direct the formation of vascular structures. Researchers were able to create and orient microgels onto one another for directed formation of vascular-like tube structures with high cellular viability. [17] Additionally, this group was able to form microchannels from modular cell laden microgels that have the potential for use in directed vasculature formation. Together, these studies have carved out a path for promoting and directing endothelial cell growth using microgels as delivery mechanisms and building blocks, a fundamental step in vasculature formation in tissue engineering.
Formation of vasculature in vitro has been demonstrated in a multitude of ways but when it comes to in vivo models, vascularization adds a new level of complexity. Vascularization in vivo has been attempted using PLG / hydroxyethylmethacrylate (HEMA) and chitosan crosslinked materials. [48] These materials formed open network spheroids that allowed for cellular infiltration and nutrient exchange for human adipose-derived stem cells (hASCs). A cranial defect mouse model was used for implantation of these gels, and they were able to form vascular adipogenic tissues after a 12-week period indicating the promise of the system. Intriguingly, these materials were capable of almost matching the rate of construction of vasculature with the rate of degradation of the system.
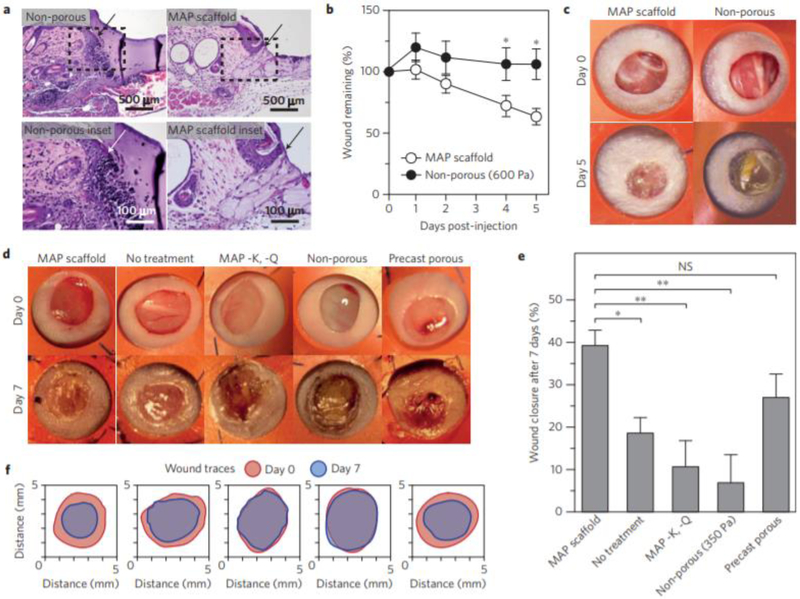
RGD (peptide sequence arginine-glycine-aspartic acid) modified PEG microgels were crosslinked to one another using transglutaminase peptide substrates forming an inter-crosslinked microgel system which was investigated as a means for improving vascularization.. [8] The system facilitated cellular migration and reformation in a mouse model. The unique aspect of this system is the importance of the annealing of the microgels to one another, as shown in Figure 2. Unannealed microgels demonstrated poor results in vivo comparably. Annealing the surface of microgels together likely adds mechanical stability to the system and consequently aided in a higher understanding of requirements for improved vascular engineering.
Figure 2.
Taken with permission from Griffin et al, “Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks”, Nat. Mater. [2] Demonstrated use of microgel scaffolds for wound healing. (a) H&E staining of tissue sections showing microgel scaffold integration with tissue; (b) Quantification of wound closure in vivo over 5 days; (c and d) Photographs of wound healing with various scaffolds after 5 and 7 days; (e) Quantification of wound closure in vivo over 7 days; (f) Traces of wound bed closure in vivofor each treatment category.
Continuing with implementation of the modular feature of microgels, both MSCs and olfactory ensheathing cells (OECs) were incorporated into RGD-modified alginate microgels for promotion of vasculature growth. [49] Prior to in vivo implementation, a human growth factor cocktail and oxygen were used to prime the system in vitro. Oxygen and necessary growth factors were perfused through the system to create an optimal environment for cell growth. A chick embryo model was utilized for the investigation and established that the system promotes vascularization and cellular interaction with the host, indicating that the inclusion of more than one cell type may promote the growth, repair, and reconstruction of tissues. However, with the inclusion of multiple cell lines, more data on cell-to-cell contact and how these cellular interactions affect complex biochemical cascades are required to better understand the impact of having multiple cell types in a system.
On the other end of vascular formation are hemostatic agents that are vitally important in prohibiting blood loss in emergency situations. Recently, Jin et. al took a triple blend of naturally derived polymers for synthesis of microgels: carboxymethyl chitosan, sodium alginate, and collagen to form microgels. [50] The materials were chosen as hemostatic agents in microgel form due to their injectability, degradability, stability, and biocompatibility. When applied to an in vivo model, wound closure and clotting were both accelerated and provided no side effects systemically. Essentially, it can be shown that microgels can be utilized in a multitude of applications not just in long term tissue regeneration.
IV. Cardiac Tissue Engineering
Regeneration of cardiac tissue comes with an added level of complexity due to the necessity of electrical conduction for heart stimulation and contraction. The added complexity comes with added exposure to stresses on cells that must be overcome such as synchronized signaling and contractions for cellular proliferation and functionality to be maintained. An outer shell on cell-encapsulating microgels is one method of protecting cells from stresses, as some researchers have demonstrated. [51] Microfluidics were employed for the creation of gelatin-core and silica-shell microgels through a photo-crosslinking method. Incorporated cardiac cells proliferated, migrated, and maintained high viability. The outer silica shell provided protection from oxidative stresses and had no effect on the cellular viability, providing a means for inclusion of cardiac cells into higher stress environments.
Tackling the problem in a different fashion, the idea of cardiomyocyte growth using PEG microgels that encapsulated HL-1 cardiomyocytes was explored. [52] Here, the microspheres were adhered to one another using a dextran solution to form a porous bulk hydrogel. The encapsulated cells proliferated and maintained their cardiomyocytic markers, as well as retained electrical activity. Synchronization of activation peaks was shown on the formed scaffold, indicating signals are being transmitted efficiently. The cellular interactions shown here are promising for the formation of electrically conductive cardiac tissues needed for integration into an in vivo model. Further exploration of electrical cell signaling should be investigated as it plays an integral role in cardiac tissue and may help progress tissue engineering in the area.
The increasing complexity of tissues comes with an increasing number of challenges for regeneration, repair, and reconstruction. In vivo studies of microgel use in cardiac tissue regeneration are not as prolific as other types of tissue, likely due to the sensitive nature of the organ, but progress in the field looks bright as novel methods for tissue engineering emerge.
V. Hepatic Tissue Engineering
Liver tissue engineering is dependent on multiple cell types, as well as growth factors for cell proliferation and tissue development. Hepatocytes, the main cells in liver, respond to cellular and biological cues from their microenvironment. To deliver hepatocyte growth factor to Hep3B cells, a double blended synthetic polymer of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) and PLG was used to manufacture microspheres. Bioactivity of the delivered growth factor was verified and was shown to be maintained over a 40 day period. [53] Additionally, the same author also established that Hep3B liver-derived cells proliferated when exposed to PHBV conjugated with collagen, laminin, and fibronectin. These three extracellular matrix components enhanced the proliferation of hepatocytes as they more closely mimic what the cells experience in an in vivo environment, indicating the important role that microgels can play in tissue engineering.
VI. Neuronal Tissue Engineering
Multiple cell types, electrical conduction, growth factor cues, and cellular orientation all make construction of neuronal tissues difficult and intricate. Patel et. al used PEG-poly(L-alanine) microspheres in a polypeptide thermogel with brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) and tonsil-derived mesenchymal stem cells (TMSCs) to explore neuronal differentiation. [30] Enhancement of neuronal differentiation was observed based on measured gene expression and morphology changes of the incorporated cells. The system properties exhibited a modulus of 800 Pa at 37 °C, similar to that of native brain tissue, and provided controlled release of necessary growth factors in the area through the integrated microgels.
Another group studied the growth of multiple cell types, including cortical neurons, PC12 cells derived from a pheochromocytoma of the rat adrenal medulla, and neuronal progenitor cells (NPCs), on PHBV microspheres. [54] All cell types proliferated, the cortical neurons created signaling pathways through dendrites and axons, and the NPCs differentiated into neurons, highlighting the promise of the system in neuronal tissue engineering. The use of microgels allows for increased cellular contact and interactions due to the large amount of surface area available, and this feature may have been beneficial in this study with multiple cell types.
The mosaic cell structure of neural tissue is highly complex, and cues need to be deconvoluted in order to gain a more thorough understanding of how to best repair the area. One group analyzed the effect of collagen cues on neural cells by decoupling chemical and mechanical cues of PC12 cells with a PEG and collagen microsphere system. [55] The addition of collagen to the system did not change the modulus of the microgels but had a large effect on the cellular activity, improving both cellular aggregation and viability in the system.
Taken altogether, microgels allow for the introduction of multiple cell lines, multiple material types, growth factors, and increases cellular surface area for growth, and all of these features may be beneficial in neuronal tissue engineering.
VII. Microgels for Drug Delivery In Vivo
Microgels have been demonstrated as a potential carrier for anti-cancer drugs and embolic agents. Schiff base chemistry was implemented to crosslink chitosan with carboxymethyl cellulose to make microgels.[26] Doxorubicin, an anticancer drug, was loaded into the microgels for further implantation into a renal embolization rabbit model. The gels demonstrated compatibility with delivery methods, occluded arteries in the tumor to starve it, and were biodegradable. In addition, the research established control over degradation via the carboxymethyl cellulose oxidation levels indicating that it can be tuned for various models.
VIII. Microgels for Other Tissue Engineering Applications
Microgels are being investigated for many other types of tissue engineering in addition to the ones described above. For example, directed differentiation of pluripotent stem cells towards ectoderm, mesoderm, and endoderm lineages have been a point of investigation. To direct the differentiation of pluripotent embryonic stem cells (ESCs), Siltanen et. al loaded the cells into heparin and PEG-based microgels that had various growth factors and assessed differentiation. [56] Specifically, growth factors Nodal and fibroblast growth factor-2 (FGF-2) were added to encourage endoderm lineages and the genes Sox17 and FoxA2 were monitored. The system facilitated approximately a 90-fold decrease in exogenous growth factor consumption compared to conventional methods. Another group used a gelatin-heparin microparticle approach to deliver pluripotent stem cell aggregates in addition to morphogenic factors to direct the cell differentiation pathways toward ectoderm and mesoderm lineages. [57] Overall, it was demonstrated that microparticle material provided control over temporal release profiles as well as the growth factor loading amount. Heparin-based microparticles have also been used to investigate the sequestration of cell-secreted proteins and the consequent controlled effect on the differentiation of cells in the localized area. [58] Omitting the need for introduction of exogenous proteins but instead regulating the naturally produced proteins is a unique and novel step for tissue engineering.
An interesting application of microgels was the use of the thermoresponsive polymer poly(N-isopropylacrylamide) (PNIPAM) to form microgels to moderate cell detachment and adhesion. [59] Microgels were applied as a coating to cell culture surfaces to provide “gentle, local lift-off” of cells that is both repeatable and reversible. The unique method was able to provide distinct boundaries between populated and unpopulated cell areas based on the coating applied to the culture dish area and demonstrates its potential utility for integration into cell assays in the future.
Another unique application of microgels was the implementation of hyaluronic acid derivatives carrying hydrazide functionalities or aldehyde functionalities in order to decouple mechanical properties with degradation effects. [60] Fibroblasts were incorporated into the gels for regeneration for vocal fold defects and injuries. Not only were these materials biocompatible, but they have the potential to smooth and heal the scar tissue in the vocal fold tissue.
In summary, microgels have been incorporated into a variety of tissue engineering applications and have seen promise in each field highlighted above. As microgels are modular versatile constructs, they can be tuned and tailored to fit the needs of different regenerative medicine applications, allowing for a wide range of potential clinical uses someday.
3D PRINTING IN TISSUE ENGINEERING
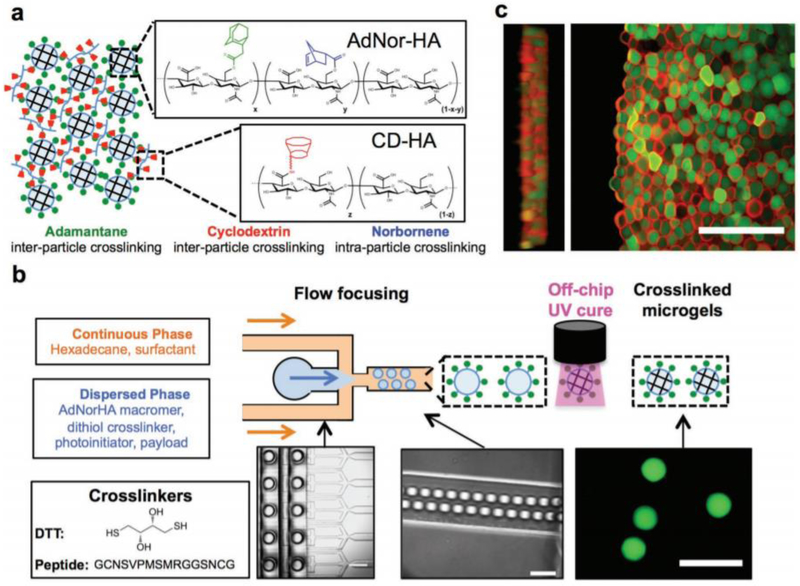
3D printing of biopolymers promises the ability to create a precise three-dimensional scaffold with specifically planned architecture for personalized medical applications, and the use of hydrogels as bio-inks are especially promising. Some groups have recently explored the use of 3D printing with microgels as a printing material due to their injectable, modular properties. Encapsulated MSCs and bovine chondrocytes within poly (ethylene glycol) diacrylate (PEGDA) microgels were studied in order to have both macro and micro-environmental control such as material and nutrient exposure. [61] These cell-encapsulating gels were then used as bio-inks for printing. The protection of cells from shearing during the printing process, as well as capability of control over the microenvironment, makes the use of microgels a promising addition to the 3D printing world. One group has created a system that can be tailored through the mixture of different microgels that interact with one another utilizing a lock and key mechanism, as shown in Figure 3. [7] The mixture of differently manufactured microgels gives rise to complex release and degradation mechanics that also allow for cellular infiltration and in turn can be utilized for specified locations.
Figure 3.
Taken with permission from Mealy et. al, “Injectable Granular Hydrogels with Multifunctional Properties for Biomedical Applications,” Adv. Mat. [1] Demonstrated use of microgels in 3D printing scaffolds for tissue engineering. (a) Method for inter- and intra-crosslinking of microgels in 3D printing; (b) Schematic of microgel printing through microfluidic devices (Scale bars from left to right: 500, 100, 100 μm, respectively.); (c) Confocal images of microgel network with rhodamine labeled CD-HA (inter-crosslinking mechanism) and fluorescein labeled microgels. (Scale bar: 200 μm)
Jammed microgel inks have recently been created with shear thinning properties that can be used in both extrusion and layer-by-layer printing of cell scaffolds. [25] These microgel inks were composed of norbornene-modified hyaluronic acid, poly(ethylene glycol) diacrylate, and agarose and were combined in various compositions to produce a variety of cell scaffolds with a range of properties. In addition, the inks can further be augmented with double-crosslinking mechanisms such as photoinitiators or dithiothretol to interconnect the jammed microgels together. The potential of 3D printing with microgels may allow for greater tuning of system properties compared to printing with polymer solutions alone.
CONCLUSIONS
Overall, the field of microgels and microspheres has demonstrated their versatility, modularity, and tunability for an abundance of applications ranging from tissue engineering to therapeutic delivery. With such promise surrounding their properties of hydrophilicity, tunability, biomimicry, and ability to encapsulate a wide variety of bioactive factors and cells, the use of microgels will likely continue to expand with combinations of various polymers, drugs, cells for these and additional regenerative medicine and therapeutic applications.
Microgels can be fabricated and used alone or in combination with each other for timed single or multiple delivery, and/or crosslinked into 3D structures for use as cell scaffolds. 3D printing with microgels as the ink is a promising avenue for building constructs for tissue engineering. All the facets described above with the precision and control associated with 3D printing point to a future where unique and complex structures can be formed. Microgels offer promise for clinical scenarios where complex biomaterial structures may be required, such as multiple bioactive factor release timings, or the incorporation of cells or bioactive factors into select portions of a 3D structure. They also enable the manufacture of 3D hydrogels that have macroscale porosity by crosslinking microgels together into a larger structure, which can facilitate cellular migration and diffusion of factors. With the variety of chemistries available to manufacture microgels, different properties can be readily incorporated into a 3D structure by mixing together different microgels. Thus, the use of microgels in regenerative medicine could be a powerful way to gain more control spatially over the composition of scaffolds that are being used to promote tissue growth or as a way to modulate the delivery of various drugs to an area.
Statement of Significance:
This review is focused on state-of-the-art microgel technology and innovations within the tissue engineering field, focusing on the use of microgels in bioactive factor delivery and as cell-interactive scaffolds, both in vitro and in vivo. Microgels are hydrogel microparticles that can be tuned based on the biopolymer from which they are derived, the crosslinking chemistry used, and the fabrication method. The emergence of microgels for tissue regeneration applications in recent years illuminates their versatility and applicability in clinical settings.
ACKNOWLEDGEMENTS
The authors acknowledge funding from NIH NIAMS R03AR068087 and R21AR071585.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Maitra J, Shukla VK, Cross-linking in Hydrogels - A Review, Am. J. Polym. Sci 4 (2014) 25–31. [Google Scholar]
- [2].Plamper FA, Richtering W, Functional Microgels and Microgel Systems, Acc. Chem. Res 50 (2017) 131–140. doi: 10.1021/acs.accounts.6b00544. [DOI] [PubMed] [Google Scholar]
- [3].Hamzah YB, Hashim S, Rahman WAWA, Synthesis of polymeric nano/microgels: a review, J. Polym. Res 24 (2017). doi: 10.1007/s10965-017-1281-9. [DOI] [Google Scholar]
- [4].Dai Z, Ngai T, Microgel particles: The structure-property relationships and their biomedical applications, J. Polym. Sci. Part Polym. Chem 51 (2013) 2995–3003. doi: 10.1002/pola.26698. [DOI] [Google Scholar]
- [5].Suzuki D, Horigome K, Kureha T, Matsui S, Watanabe T, Polymeric hydrogel microspheres: design, synthesis, characterization, assembly and applications, Polym. J 49 (2017) 695–702. doi: 10.1038/pj.2017.39. [DOI] [Google Scholar]
- [6].Saxena S, Hansen CE, Lyon LA, Microgel Mechanics in Biomaterial Design, Acc. Chem. Res 47 (2014) 2426–2434. doi: 10.1021/ar500131v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mealy JE, Chung JJ, Jeong H-H, Issadore D, Lee D, Atluri P, Burdick JA, Injectable Granular Hydrogels with Multifunctional Properties for Biomedical Applications, Adv. Mater 0 (n.d.) 1705912. doi: 10.1002/adma.201705912. [DOI] [PubMed] [Google Scholar]
- [8].Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T, Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks, Nat. Mater 14 (2015) 737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Milani AH, Freemont AJ, Hoyland JA, Adlam DJ, Saunders BR, Injectable Doubly Cross-Linked Microgels for Improving the Mechanical Properties of Degenerated Intervertebral Discs, Biomacromolecules. 13 (2012) 2793–2801. doi: 10.1021/bm3007727. [DOI] [PubMed] [Google Scholar]
- [10].Sen H, Influence of cross-link density on rheological properties of temperature-sensitive microgel suspensions, (n.d.) 11. [Google Scholar]
- [11].Hamidi M, Azadi A, Rafiei P, Hydrogel nanoparticles in drug delivery, Adv. Drug Deliv. Rev 60 (2008) 1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [12].Jiang W, Li M, Chen Z, Leong KW, Cell-laden Microfluidic Microgels for Tissue Regeneration, Lab. Chip 16 (2016) 4482–4506. doi: 10.1039/c6lc01193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang X, Lü S, Gao C, Chen C, Zhang X, Liu M, Highly stable and degradable multifunctional microgel for self-regulated insulin delivery under physiological conditions, Nanoscale. 5 (2013) 6498. doi: 10.1039/c3nr00835e. [DOI] [PubMed] [Google Scholar]
- [14].Liu EY, Jung S, Weitz DA, Yi H, Choi C-H, High-throughput double emulsion-based microfluidic production of hydrogel microspheres with tunable chemical functionalities toward biomolecular conjugation, Lab. Chip 18 (2018) 323–334. doi: 10.1039/C7LC01088E. [DOI] [PubMed] [Google Scholar]
- [15].Lim JJ, Hammoudi TM, Bratt-Leal AM, Hamilton SK, Kepple KL, Bloodworth NC, McDevitt TC, Temenoff JS, Development of Nano- and Micro-Scale Chondroitin Sulfate Particles for Controlled Growth Factor Delivery, Acta Biomater. 7 (2011) 986–995. doi: 10.1016/j.actbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang D, Liu T, Yin J, Liu S, Stimuli-Responsive Fluorescent Poly(N-isopropylacrylamide) Microgels Labeled with Phenylboronic Acid Moieties as Multifunctional Ratiometric Probes for Glucose and Temperatures, Macromolecules. 44 (2011) 2282–2290. doi: 10.1021/ma200053a. [DOI] [Google Scholar]
- [17].Du Y, Lo E, Ali S, Khademhosseini A, Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs, Proc. Natl. Acad. Sci 105 (2008) 9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nih LR, Sideris E, Carmichael ST, Segura T, Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion, Adv. Mater. Deerfield Beach Fla 29 (2017). doi: 10.1002/adma.201606471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xin S, Wyman OM, Alge DL, Assembly of PEG Microgels into Porous Cell-Instructive 3D Scaffolds via Thiol-Ene Click Chemistry, Adv. Healthc. Mater 7 (n.d.) 1800160. doi: 10.1002/adhm.201800160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Riederer MS, Requist BD, Payne KA, Way JD, Krebs MD, Injectable and microporous scaffold of densely-packed, growth factor-encapsulating chitosan microgels, Carbohydr. Polym 152 (2016) 792–801. doi: 10.1016/j.carbpol.2016.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shen S, Fu D, Xu F, Long T, Hong F, Wang J, The design and features of apatite-coated chitosan microspheres as injectable scaffold for bone tissue engineering, Biomed. Mater 8 (2013) 025007. doi: 10.1088/1748-6041/8/2/025007. [DOI] [PubMed] [Google Scholar]
- [22].Hu X, Shen H, Yang F, Liang X, Wang S, Wu D, Modified composite microspheres of hydroxyapatite and poly(lactide-co-glycolide) as an injectable scaffold, Appl. Surf. Sci 292 (2014) 764–772. doi: 10.1016/j.apsusc.2013.12.045. [DOI] [Google Scholar]
- [23].Simone A, Laura K, Samantha Z, Jo’an B, L. MP, Cell-Instructive Microgels with Tailor-Made Physicochemical Properties, Small. 11 (2015) 5647–5656. doi: 10.1002/smll.201501001. [DOI] [PubMed] [Google Scholar]
- [24].Richardson TP, Peters MC, Ennett AB, Mooney DJ, Polymeric system for dual growth factor delivery, Nat. Biotechnol. N. Y 19 (2001) 1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- [25].Highley CB, Song KH, Daly AC, Burdick JA, Jammed Microgel Inks for 3D Printing Applications, Adv. Sci 0 (n.d.) 1801076. doi: 10.1002/advs.201801076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Weng L, Rostamzadeh P, Nooryshokry N, Le HC, Golzarian J, In vitro and in vivo evaluation of biodegradable embolic microspheres with tunable anticancer drug release, Acta Biomater. 9 (2013) 6823–6833. doi: 10.1016/j.actbio.2013.02.017. [DOI] [PubMed] [Google Scholar]
- [27].Zhang H, Mardyani S, Chan WCW, Kumacheva E, Design of Biocompatible Chitosan Microgels for Targeted pH-Mediated Intracellular Release of Cancer Therapeutics, Biomacromolecules. 7 (2006) 1568–1572. doi: 10.1021/bm050912z. [DOI] [PubMed] [Google Scholar]
- [28].Sivakumaran D, Maitland D, Hoare T, Injectable Microgel-Hydrogel Composites for Prolonged Small-Molecule Drug Delivery, Biomacromolecules. 12 (2011) 4112–4120. doi: 10.1021/bm201170h. [DOI] [PubMed] [Google Scholar]
- [29].Pérez-Luna V, González-Reynoso O, Pérez-Luna VH, González-Reynoso O, Encapsulation of Biological Agents in Hydrogels for Therapeutic Applications, Gels. 4 (2018) 61. doi: 10.3390/gels4030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Patel M, Moon HJ, Jung BK, Jeong B, Microsphere-Incorporated Hybrid Thermogel for Neuronal Differentiation of Tonsil Derived Mesenchymal Stem Cells, Adv. Healthc. Mater 4 (n.d.) 1565–1574. doi: 10.1002/adhm.201500224. [DOI] [PubMed] [Google Scholar]
- [31].Bysell H, Schmidtchen A, Malmsten M, Binding and Release of Consensus Peptides by Poly(acrylic acid) Microgels, Biomacromolecules. 10 (2009) 2162–2168. doi: 10.1021/bm9003354. [DOI] [PubMed] [Google Scholar]
- [32].Annamalai RT, Turner PA, Carson WF, Levi B, Kunkel S, Stegemann JP, Harnessing macrophage-mediated degradation of gelatin microspheres for spatiotemporal control of BMP2 release, Biomaterials. 161 (2018) 216–227. doi: 10.1016/j.biomaterials.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Turner PA, Thiele JS, Stegemann JP, Growth factor sequestration and enzyme-mediated release from genipin-crosslinked gelatin microspheres, J. Biomater. Sci. Polym. Ed 28 (2017) 1826–1846. doi: 10.1080/09205063.2017.1354672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang L, Li B, Xu F, Xu Z, Wei D, Feng Y, Wang Y, Jia D, Zhou Y, UV-crosslinkable and thermo-responsive chitosan hybrid hydrogel for NIR-triggered localized on-demand drug delivery, Carbohydr. Polym 174 (2017) 904–914. doi: 10.1016/j.carbpol.2017.07.013. [DOI] [PubMed] [Google Scholar]
- [35].Amini AR, Laurencin CT, Nukavarapu SP, Bone Tissue Engineering: Recent Advances and Challenges, Crit. Rev. Biomed. Eng 40 (2012) 363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Oliveira MB, Mano JF, Polymer-based microparticles in tissue engineering and regenerative medicine, Biotechnol. Prog 27 (2011) 897–912. doi: 10.1002/btpr.618. [DOI] [PubMed] [Google Scholar]
- [37].Li B, Wang L, Xu F, Gang X, Demirci U, Wei D, Li Y, Feng Y, Jia D, Zhou Y, Hydrosoluble, UV-crosslinkable and injectable chitosan for patterned cell-laden microgel and rapid transdermal curing hydrogel in vivo, Acta Biomater. 22 (2015) 59–69. doi: 10.1016/j.actbio.2015.04.026. [DOI] [PubMed] [Google Scholar]
- [38].Leslie SK, Cohen DJ, Sedlaczek J, Pinsker EJ, Boyan BD, Schwartz Z, Controlled release of rat adipose-derived stem cells from alginate microbeads, Biomaterials. 34 (2013) 8172–8184. doi: 10.1016/j.biomaterials.2013.07.017. [DOI] [PubMed] [Google Scholar]
- [39].Jiang T, Nukavarapu SP, Deng M, Jabbarzadeh E, Kofron MD, Doty SB, Abdel-Fattah WI, Laurencin CT, Chitosan-poly(lactide-co-glycolide) microsphere-based scaffolds for bone tissue engineering: in vitro degradation and in vivo bone regeneration studies, Acta Biomater. 6 (2010) 3457–3470. doi: 10.1016/j.actbio.2010.03.023. [DOI] [PubMed] [Google Scholar]
- [40].Mao AS, Shin J-W, Utech S, Wang H, Uzun O, Li W, Cooper M, Hu Y, Zhang L, Weitz DA, Mooney DJ, Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery, Nat. Mater 16 (2017) 236–243. doi: 10.1038/nmat4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Quinlan E, López-Noriega A, Thompson EM, Hibbitts A, Cryan SA, O’Brien FJ, Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair, J. Tissue Eng. Regen. Med 11 (n.d.) 1097–1109. doi: 10.1002/term.2013. [DOI] [PubMed] [Google Scholar]
- [42].Nisal A, Sayyad R, Dhavale P, Khude B, Deshpande R, Mapare V, Shukla S, Venugopalan P, Silk fibroin micro-particle scaffolds with superior compression modulus and slow bioresorption for effective bone regeneration, Sci. Rep 8 (2018). doi: 10.1038/s41598-018-25643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wise JK, Alford AI, Goldstein SA, Stegemann JP, Synergistic enhancement of ectopic bone formation by supplementation of freshly isolated marrow cells with purified MSC in collagen-chitosan hydrogel microbeads, Connect. Tissue Res. 57 (2016) 516–525. doi: 10.3109/03008207.2015.1072519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Daley ELH, Coleman RM, Stegemann JP, Biomimetic microbeads containing a chondroitin sulfate/chitosan polyelectrolyte complex for cell-based cartilage therapy, J. Mater. Chem. B 3 (2015) 7920–7929. doi: 10.1039/C5TB00934K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL, Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering, J. Controlled Release 134 (2009) 81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA, Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo, Biomaterials. 32 (2011) 6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sandker MJ, Duque LF, Redout EM, Klijnstra EC, Steendam R, Kops N, Waarsing JH, van Weeren R, Hennink WE, Weinans H, Degradation, Intra-Articular Biocompatibility, Drug Release, and Bioactivity of Tacrolimus-Loaded Poly(d-l-lactide-PEG)-b-poly(l-lactide) Multiblock Copolymer-Based Monospheres, ACS Biomater. Sci. Eng 4 (2018) 2390–2403. doi: 10.1021/acsbiomaterials.8b00116. [DOI] [PubMed] [Google Scholar]
- [48].Xia P, Zhang K, Gong Y, Li G, Yan S, Yin J, Injectable Stem Cell Laden Open Porous Microgels That Favor Adipogenesis: In Vitro and in Vivo Evaluation, ACS Appl. Mater. Interfaces 9 (2017) 34751–34761. doi: 10.1021/acsami.7b13065. [DOI] [PubMed] [Google Scholar]
- [49].Torres AL, Bidarra SJ, Pinto MT, Aguiar PC, Silva EA, Barrias CC, Guiding morphogenesis in cell-instructive microgels for therapeutic angiogenesis, Biomaterials. 154 (2018) 34–47. doi: 10.1016/j.biomaterials.2017.10.051. [DOI] [PubMed] [Google Scholar]
- [50].Jin J, Ji Z, Xu M, Liu C, Ye X, Zhang W, Li S, Wang D, Zhang W, Chen J, Ye F, Lv Z, Microspheres of Carboxymethyl Chitosan, Sodium Alginate, and Collagen as a Hemostatic Agent in Vivo, ACS Biomater. Sci. Eng 4 (2018) 2541–2551. doi: 10.1021/acsbiomaterials.8b00453. [DOI] [PubMed] [Google Scholar]
- [51].Cha C, Oh J, Kim K, Qiu Y, Joh M, Shin SR, Wang X, Camci-Unal G, Wan K, Liao R, Khademhosseini A, Microfluidics-Assisted Fabrication of Gelatin-Silica Core–Shell Microgels for Injectable Tissue Constructs, Biomacromolecules. 15 (2014) 283–290. doi: 10.1021/bm401533y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Smith AW, Segar CE, Nguyen PK, MacEwan MR, Efimov IR, Elbert DL, Long-term culture of HL-1 cardiomyocytes in modular poly(ethylene glycol) microsphere-based scaffolds crosslinked in the phase-separated state, Acta Biomater. 8 (2012) 31–40. doi: 10.1016/j.actbio.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhu XH, Gan SK, Wang C-H, Tong YW, Proteins combination on PHBV microsphere scaffold to regulate Hep3B cells activity and functionality: a model of liver tissue engineering system, J. Biomed. Mater. Res. A 83 (2007) 606–616. doi: 10.1002/jbm.a.31257. [DOI] [PubMed] [Google Scholar]
- [54].Chen W, Tong YW, PHBV microspheres as neural tissue engineering scaffold support neuronal cell growth and axon–dendrite polarization, Acta Biomater. 8 (2012) 540–548. doi: 10.1016/j.actbio.2011.09.026. [DOI] [PubMed] [Google Scholar]
- [55].Scott RA, Elbert DL, Willits RK, Modular poly(ethylene glycol) scaffolds provide the ability to decouple the effects of stiffness and protein concentration on PC12 cells, Acta Biomater. 7 (2011) 3841–3849. doi: 10.1016/j.actbio.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Siltanen C, Yaghoobi M, Haque A, You J, Lowen J, Soleimani M, Revzin A, Microfluidic fabrication of bioactive microgels for rapid formation and enhanced differentiation of stem cell spheroids, Acta Biomater. 34 (2016) 125–132. doi: 10.1016/j.actbio.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bratt-Leal AM, Nguyen AH, Hammersmith KA, Singh A, McDevitt TC, A microparticle approach to morphogen delivery within pluripotent stem cell aggregates, Biomaterials. 34 (2013) 7227–7235. doi: 10.1016/j.biomaterials.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rinker TE, Philbrick BD, Hettiaratchi MH, Smalley DM, McDevitt TC, Temenoff JS, Microparticle-mediated sequestration of cell-secreted proteins to modulate chondrocytic differentiation, Acta Biomater. 68 (2018) 125–136. doi: 10.1016/j.actbio.2017.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Uhlig K, Wegener T, He J, Zeiser M, Bookhold J, Dewald I, Godino N, Jaeger M, Hellweg T, Fery A, Duschl C, Patterned Thermoresponsive Microgel Coatings for Noninvasive Processing of Adherent Cells, Biomacromolecules. 17 (2016) 1110–1116. doi: 10.1021/acs.biomac.5b01728. [DOI] [PubMed] [Google Scholar]
- [60].Jia X, Yeo Y, Clifton RJ, Jiao T, Kohane DS, Kobler JB, Zeitels SM, Langer R, Hyaluronic Acid-Based Microgels and Microgel Networks for Vocal Fold Regeneration, Biomacromolecules. 7 (2006) 3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- [61].Single Cell Microgel Based Modular Bioinks for Uncoupled Cellular Micro‐ and Macroenvironments - Kamperman - 2017 - Advanced Healthcare Materials - Wiley Online Library, (n.d.). https://onlinelibrary.wiley.com/doi/abs/10.1002/adhm.201600913 (accessed July 9, 2018). [DOI] [PubMed] [Google Scholar]