Abstract
Background:
Previous work reported increased rates of acute cardiovascular hospitalizations associated with increased PM2.5 concentrations in the previous few days across urban centers in New York State from 2005 to 2016. These relative rates were higher after air quality policies and economic changes resulted in decreased PM2.5 concentrations and changes in PM composition (e.g. increased secondary organic carbon), compared to before and during these changes. Changes in PM composition and sources may explain this difference.
Objectives:
To estimate the rate of acute cardiovascular hospitalizations associated with increases in source specific PM2.5 concentrations.
Methods:
Using source apportioned PM2.5 concentrations at the same NYS urban sites, a time-stratified case-crossover design, and conditional logistic regression models adjusting for ambient temperature and relative humidity, we estimated the rate of these acute cardiovascular hospitalizations associated with increases in mean source specific PM2.5 concentrations in the previous 1, 4, and 7 days.
Results:
Interquartile range (IQR) increases in spark-ignition emissions (GAS) concentrations were associated with increased excess rates of cardiac arrhythmia hospitalizations (2.3%; 95% CI = 0.4%, 4.2%; IQR=2.56 μg/m3) and ischemic stroke hospitalizations (3.7%; 95% CI= 1.1%, 6.4%; 2. 73 μg/m3) over the next day. IQR increases in diesel (DIE) concentrations were associated with increased rates of congestive heart failure hospitalizations (0.7%; 95% CI = 0.2% 1.3%; 0.51 μg/m3) and ischemic heart disease hospitalizations (0.8%; 95% CI = 0.3%, 1.3%; 60 μg/m3) over the next day, as hypothesized. However, secondary sulfate PM2.5 (SS) was not. Increased acute cardiovascular hospitalization rates were also associated with IQR increases in concentrations of road dust (RD), residual oil (RO), and secondary nitrate (SN) over the previous 1, 4, and 7 days, but not other sources.
Conclusions:
These findings suggest a role of several sources of PM2.5 in New York State (i.e. traffic emissions, non-traffic emissions such as brake and tire wear, residual oil, and nitrate that may also reflect traffic emissions) in the triggering of acute cardiovascular events.
Keywords: cardiovascular hospitalizations, air pollution, source apportionment, traffic emissions
1. INTRODUCTION
We and others have reported that short term increases in ambient fine particle concentrations were associated with triggering of acute cardiovascular and cerebrovascular events (Brook et al. 2004; Brook et al. 2010; Rajagopalan et al. 2018), including myocardial infarction (Evans et al. 2017; Gardner et al. 2014; Mustafic et al. 2012; Pope et al. 2015), ischemic stroke (Shah et al. 2015), cardiac arrhythmia (Link et al. 2013; Rich et al. 2005; Rich et al. 2006a; Rich et al. 2006b), and heart failure (Shah et al. 2013). Recently, we found that shortterm increases in ambient PM2.5 concentrations were associated with small but significant increases in the rate of hospital admissions for cardiac arrhythmia, ischemic stroke, congestive heart failure, ischemic heart disease, and myocardial infarction across urban centers of New York State from 2005 to 2016 (Zhang et al. 2018). We also reported that implementation of air quality policies and economic changes occurring from 2008-2013 led to reductions of pollutant emissions and concentrations of PM2.5 and its major constituents (sulfate, nitrate, elemental carbon and organic carbon) as well as gaseous pollutants (NO2, SO2, and CO) except ozone. Further, there were increased concentrations of secondary organic carbon concentrations, but decreases in secondary inorganic species (sulfate and nitrate) and primary organic carbon concentrations (Squizzato et al. 2018a; Zhang et al. 2018). Although there were no differences in the relative rate for most disease subgroups Before (2005-2007), During (2008-2013), or After (2014-2016) these pollutant changes, the rate of hospital admissions for ischemic heart disease associated with increased PM2.5 concentration was higher in the After period (Zhang et al. 2018). This result suggests that changes in particle composition resulting from these policies and the co-occurring economic drivers may have differentially triggered these acute cardiovascular outcomes.
Specific PM2.5 sources emit particles with chemical fingerprints (profiles) that permit source identification and apportionment. Source apportionment analyses have been conducted in numerous regions and cities around the world (Belis et al. 2013; Hopke 2016a, b), and have been used to examine whether PM sources were individually associated with health events (Ebisu et al. 2018; Halonen et al. 2009; Ito et al. 2006; Krall et al. 2018; Laden et al. 2000; Mar et al. 2000; Mar et al. 2006; Ozkaynak and Thurston 1987; Thurston et al. 2005). Using positive matrix factorization (PMF) to apportion the measured mass of an atmospheric pollutant at a given site to potential emission sources by solving a mass balance equation (Hopke 2016a), we separately identified twelve PM2.5 sources at six urban sites (Buffalo, Rochester, Albany, Bronx, Manhattan, Queens) and 2 background sites in New York State (Whiteface Mountain and Pinnacle State Park) (Squizzato et al. 2018b). There were 7 PM2.5 sources common to all sites (secondary sulfate [SS], secondary nitrate [SN], biomass burning [BB], diesel [DIE], spark-ignition emissions [GAS], pyrolyzed organic rich [OP], road dust [RD]), 3 sources identified at New York City sites only (Bronx, Manhattan, Queens)(Aged sea salt [AGS], fresh sea salt [FSS], residual oil [RO]), 1 source identified in Buffalo, Rochester, and Albany only (road salt [RS]), and 1 source identified at Buffalo only (industrial [IND]). The primarily transported secondary aerosols (SS, SN, and OP) were observed at all sites since their contributions were driven by regionally distributed sources. Ubiquitous sources such as the three traffic sources (DIE, GAS, and RD) were also observed at all sites. Biomass burning was also seen at all sites due to the common practice of recreational wood burning in wood stoves and fireplaces during the winter months, occasional summer wildfire plumes, and an increasing summer use of outdoor fire pits (Zhang et al. 2018). Given the colder and snowier conditions, road salt was identified at all the upstate sites. New York City had residual oil that had been extensively used for large building space heating, and sea salt sources that were not observed elsewhere. For the “industrial” source identified in Buffalo, we could not attribute this factor to any specific activity (Squizzato et al. 2018b). We also reported that increased spark-ignition vehicle emissions were consistent with increased vehicle registrations in New York, and that increased SOC was consistent with projected increases in its formation due to reduced NOx emissions in New York State. Our previous work has also suggested higher risks/rates of myocardial infarction associated with PM2.5 when the air pollution mixture is enhanced with secondary particles, ammonium sulfate, and ammonium nitrate (Hopke et al. 2015; Rich et al. 2013), while increased mortality rates were associated with increased secondary sulfate concentrations (Ito et al. 2006).
Therefore, using the same New York State hospitalization dataset (2005-2016) as in our previous analyses (Zhang et al. 2018), and these PM2.5 sources from the six urban NYS sites (Squizzato et al. 2018b), we estimated the rates of acute cardiovascular event hospitalizations (cardiac arrhythmia, ischemic stroke, congestive heart failure, ischemic stroke, and myocardial infarction) associated with increases in the mean contributions of individual PM2.5 sources over the previous 1, 4, and 7 days. We hypothesized that increased motor vehicle emissions (diesel PM2.5 [DIE] and spark-ignition emissions PM2.5 [GAS]) as well as secondary sulfate PM2.5 (SS) would be associated with increased rates of hospitalizations for these acute cardiovascular events.
2. MATERIALS AND METHODS
2.1. Study Population and Hospital Admissions Data:
The hospital admissions used in the study have been described previously (Zhang et al. 2018). Briefly, we retained hospital admissions from the New York State Department of Health Statewide Planning and Research Cooperative System (SPARC S), a legislatively mandated database that covers ~95% of hospitals in NYS, excluding federal facilities (e.g. Veterans Affairs Hospitals) and psychiatric facilities. It contains billing and medical information on over 2.5 million discharges for New York State hospitals per year. SPARCS data include patient information on the principal diagnoses and up to 24 other diagnoses at the time of hospital admission, as well as demographic characteristics and event/hospital information. We geocoded the residential address for each hospitalization using ArcGIS 10.3.1 (©Esri, Inc. Redlands, CA). Annual geocoding success rates from 2015 to 2016 ranged from 95.0% to 95.9%. We retained those hospital admissions with a successfully geocoded address, a “principal” diagnoses of cardiovascular disease (defined using ICD-9 and ICD-10 codes), and an admission date from January 1, 2005 to December 31, 2016, for adult (⩾ 18 years of age) residents of NYS. We then used this principal diagnosis to define cardiac arrhythmias (ICD-9 = 427 and ICD-10 = I47-I49), congestive heart failure (ICD-9 = 428 and ICD-10 = I42 and I50-I51), ischemic heart disease (ICD-9 = 410-414 and ICD-10 = I20-I25), myocardial infarction (ICD-9 = 410 and ICD-10 = I21), and ischemic stroke (ICD-9 = 434 and ICD-10 = I63). We then excluded all study subjects living more than 15 miles from any our six PM2.5 monitoring sites, leaving N=1,802,836 available for analysis. This study was reviewed and approved by the Institutional Review Board at the State University of New York at Albany.
2.2. PM2.5 Composition and Weather Data:
PM2.5 compositional data were retrieved from the EPA Chemical Speciation Network (CSN; AQS, www.epa.gov/aqs), for six sites (Albany, Buffalo, Rochester, Bronx, Manhattan, and Queens). Daily (24-h) samples were collected every third or sixth day and analyzed for species that would provide mass closure. Elemental carbon (EC) and organic carbon (OC) were determined by thermo-optical analysis, major inorganic ions by ion chromatography, and elements by energy-dispersive X-ray fluorescence. Details of the sampling methods, analytical protocols, and quality assurance/quality control are summarized in Solomon et al (Solomon et al. 2014).
Positive matrix factorization (PMF) (Hopke 2016a), using U.S. EPA PMF version 5, was used to identify PM2.5 sources at each site (Squizzato et al. 2018b). PMF protocols were in compliance with guidelines and best practices reported in the literature (e.g. (Belis et al. 2014; Brown et al. 2015; Masiol et al. 2017; Paatero et al. 2014)). The best solutions were identified according to several criteria and guidelines (Belis et al. 2014; Brown et al. 2015; Hopke 2016a; Reff et al. 2007): (i) knowledge of sources affecting the study area, (ii) the Q-value with respect to the expected (theoretical) value and its stability over multiple runs (n=200), (iii) number of absolute scaled residuals greater than ±3, and (iv) finding profile uncertainties calculated by bootstrap (BS, n=200) and displacement (DISP) methods within an acceptable range (Paatero et al. 2014). Further details of methods for data handling, source apportionment analysis as well as all the source profiles and the basis of their interpretation are extensively discussed in Squizzato et al (Squizzato et al. 2018b). The trends in the apportioned source contributions are presented in Masiol et al (Masiol et al. 2019). The twelve PM2.5 sources identified were then used in the statistical analyses described below.
Hourly temperature and relative humidity data were obtained from the National Weather Service (National Climate Data Center, https://www.ncdc.noaa.gov/cdo-web/datatools/lcd) for the nearest major airport (BUF - Buffalo, ROC - Rochester, ALB - Albany, LGA - Bronx, and JFK - Queens) or the closest weather station (Central Park for Manhattan). For each study subject living within 15 miles of our six monitoring stations, we assigned PM2.5 source contributions, temperature, and relative humidity measurements from the nearest site. If a person lived <15 miles from more than one monitor (e.g. Bronx vs. Manhattan), we assigned concentrations/values from the closest monitor to that person.
2.3. Study Design and Statistical Analyses:
To estimate the relative rate of cardiac arrhythmia associated with short term increases in PM2.5 source contributions (e.g. DIE), we used the same time-stratified, case-crossover design and conditional logistic regression analyses (Levy et al. 2001; Maclure 1991) used in our previous analysis of PM2.5 and acute cardiovascular hospitalizations (Zhang et al. 2018). The case-crossover design contrasts pollutant concentrations immediately before the cardiovascular disease admission (case periods) to other times when the subject did not have a cardiovascular disease admission (control periods), matched to the case period (3-4 controls per case) by calendar month and weekday. We fit a conditional logistic regression model for cardiac arrhythmia hospitalizations and each PM2.5 source described above, in which we regressed case–control status (i.e., case = 1, control = 0) against the mean PM2.5 source concentration, mean rPM2.5 concentration (i.e. residual PM2.5 = PM2.5 mass – specific PM2.5 source mass), an indicator variable for holidays, and temperature and relative humidity (each with a natural spline with 4 degrees of freedom determined using Akaike’s Information Criterion), on the case and control days (all variables on lag day 0). As is standard in case-crossover studies, this model provided estimates of the rate ratio and its 95% confidence interval. The excess rate is the percent (%) increase in the rate per interquartile range increase in source specific PM2.5 concentration (i.e. [rate ratio – 1.0] * 100%). We then re-ran this set of models for each PM2.5 source for lag days 0-3 and then lag days 0-6, and then then again for each outcome (congestive heart failure, ischemic heart disease, myocardial infarction, and ischemic stroke). Since there were 3 lagged effects estimated for each outcome/PM2.5 source, a p<0.017 was used to define statistical significance. In exploratory analyses, we then re-ran this model with interaction terms between period (Before, During, and After) and PM2.5. All data management and statistical analyses were done using R version 3.0.1(https://www.r-project.org/).
3. RESULTS
Since some of the PMF sources identified by Squizzato et al.(Squizzato et al. 2018b) were identified at all sites, but others were only identified in New York City, upstate sites (Buffalo, Rochester, Albany), or just in Buffalo, a description of the study population for each site group is shown in Table 1. Across all sites, study subjects were predominantly female (54.2%), white (52.2%), and non-Hispanic (89.6%), with a mean (± standard deviation) age of 69 (± 15) years, and a mean (± standard deviation) hospital length of stay of 5.05 (± 7.39) days with generally larger proportions of hospitalizations at the beginning of the study period than near the end. The four site groups had similar mean length of stays, and similar age, season of admission, and year of admission distributions. However, the Upstate sites and Buffalo had substantially larger percentages of white subjects (81.0% and 82.4%, respectively) than the New York City sites and All sites (46.6% and 52.2%, respectively), and substantially smaller percentages of Hispanic subjects (2.8% and 1.3%, respectively) than the New York City sites and All sites (12.0% and 10.4%, respectively).
Table 1.
Subject and hospital admission characteristics, by site group
| All sites (N=1,802,836) |
New York City sites only (N=1,468,801) |
Buffalo, Rochester, Albany only (N=334,035) |
Buffalo only (N=151,684) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| DISEASE | ||||||||
| Cardiac arrhythmia | 321,342 | 22.9 | 259,535 | 22.5 | 61,807 | 25.1 | 27,022 | 24.5 |
| Ischemic stroke | 173,587 | 12.4 | 135,530 | 11.7 | 38,057 | 15.5 | 17,411 | 15.8 |
| Congestive heart failure | 448,222 | 32.0 | 362,788 | 31.4 | 85,434 | 34.7 | 36,389 | 33.0 |
| Ischemic heart disease | 631,193 | 45.1 | 532,520 | 46.1 | 98,673 | 40.1 | 46,989 | 42.6 |
| Myocardial infarction | 228,492 | 16.3 | 178,428 | 15.5 | 50,064 | 20.4 | 23,873 | 21.6 |
| GENDER | ||||||||
| Male | 640,977 | 45.8 | 525,790 | 45.5 | 115,187 | 46.8 | 53,080 | 48.1 |
| AGE | ||||||||
| Years: Mean (standard deviation) | 69.27 (14.56) | 68.86 (14.53) | 71.18 (14.55) | 71.24 (14.46) | ||||
| 18-39 | 34,727 | 2.5 | 29,661 | 2.6 | 5,066 | 2.1 | 2,260 | 2.1 |
| 40-49 | 99,861 | 7.1 | 84,968 | 7.4 | 14,893 | 6.1 | 6,582 | 6.0 |
| 50-59 | 229,529 | 16.4 | 193,631 | 16.8 | 35,898 | 14.6 | 15,880 | 14.4 |
| 60-69 | 311,812 | 22.3 | 263,278 | 22.8 | 48,534 | 19.7 | 21,375 | 19.4 |
| 70-79 | 333,000 | 23.8 | 275,520 | 23.9 | 57,480 | 23.4 | 26,554 | 24.1 |
| >=80 | 391,828 | 28.0 | 307,785 | 26.7 | 84,043 | 34.2 | 37,749 | 34.2 |
| RACE | ||||||||
| White | 731,723 | 52.2 | 532,662 | 46.1 | 199,061 | 81.0 | 91,014 | 82.4 |
| African American | 315,831 | 22.6 | 285,547 | 24.7 | 30,284 | 12.3 | 14,484 | 13.1 |
| Native American or Alaskan Native | 7,631 | 0.5 | 7,338 | 0.6 | 293 | 0.1 | 162 | 0.2 |
| Asian | 42,676 | 3.1 | 41,917 | 3.6 | 759 | 0.3 | 262 | 0.2 |
| Native Hawaiian or Pacific Islander | 454 | 0.0 | 392 | 0.0 | 62 | 0.0 | 8 | 0.0 |
| ETHNICITY | ||||||||
| Hispanic | 145,358 | 10.4 | 138,542 | 12.0 | 6,816 | 2.8 | 1,420 | 1.3 |
| YEAR OF ADMISSION | ||||||||
| 2005 | 141,846 | 10.1 | 116,679 | 10.1 | 25,167 | 10.2 | 11,267 | 10.2 |
| 2006 | 140,095 | 10.0 | 115,657 | 10.0 | 24,438 | 9.9 | 10,862 | 9.8 |
| 2007 | 133,093 | 9.5 | 109,797 | 9.5 | 23,296 | 9.5 | 10,308 | 9.3 |
| 2008 | 131,712 | 9.4 | 108,622 | 9.4 | 23,090 | 9.4 | 10,625 | 9.6 |
| 2009 | 129,512 | 9.3 | 107,280 | 9.3 | 22,232 | 9.0 | 9,967 | 9.0 |
| 2010 | 122,615 | 8.8 | 101,875 | 8.8 | 20,740 | 8.4 | 9,418 | 8.5 |
| 2011 | 112,586 | 8.0 | 93,136 | 8.1 | 19,450 | 7.9 | 8,646 | 7.8 |
| 2012 | 107,317 | 7.7 | 88,872 | 7.7 | 18,445 | 7.5 | 8,287 | 7.5 |
| 2013 | 102,399 | 7.3 | 84,676 | 7.3 | 17,723 | 7.2 | 8,192 | 7.4 |
| 2014 | 95,730 | 6.8 | 78,471 | 6.8 | 17,259 | 7.0 | 7,958 | 7.2 |
| 2015 | 95,406 | 6.8 | 78,135 | 6.8 | 17,271 | 7.0 | 7,754 | 7.0 |
| 2016 | 88,446 | 6.3 | 71,643 | 6.2 | 16,803 | 6.8 | 7,116 | 6.5 |
| SEASON OF ADMISSION | ||||||||
| Fall | 339,652 | 24.3 | 280,212 | 24.3 | 59,440 | 24.2 | 26,675 | 24.2 |
| Spring | 370,866 | 26.5 | 306,325 | 26.5 | 64,541 | 26.3 | 28,892 | 26.2 |
| Summer | 340,972 | 24.3 | 281,217 | 24.4 | 59,755 | 24.3 | 26,936 | 24.4 |
| Winter | 349,267 | 24.9 | 287,089 | 24.9 | 62,178 | 25.3 | 27,897 | 25.3 |
| LENGTH OF HOSPITAL STAY (DAYS) | ||||||||
| Mean (Standard Deviation) | 5.05 (7.39) | 5.07 (7.21) | 4.98 (8.17) | 5.05 (7.13) | ||||
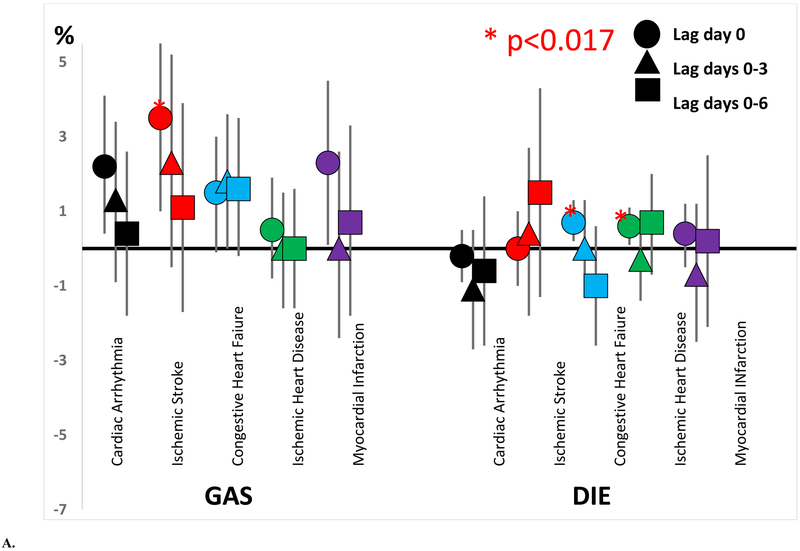
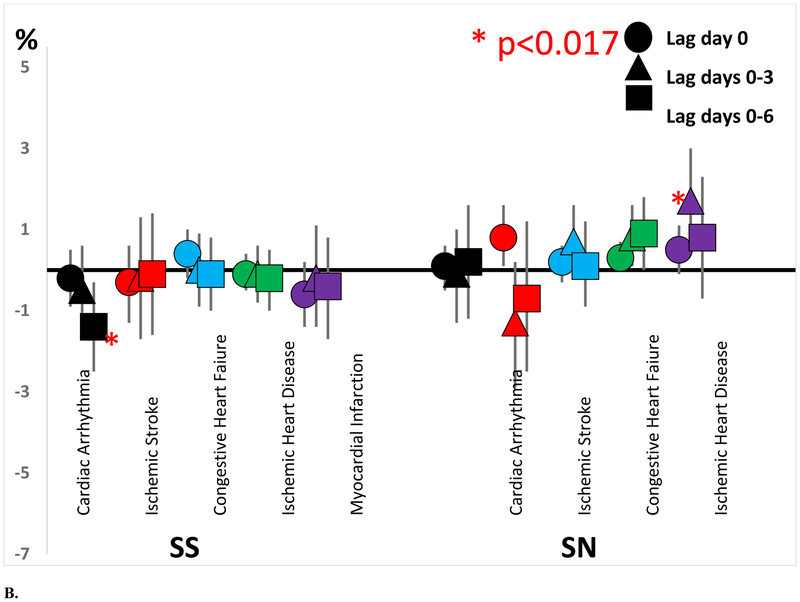
The distributions of source specific PM2.5 contributions are shown in Table 2. Detailed descriptions of each source and their chemical profiles are provided in Squizzato et al. (Squizzato et al. 2018b), while the trends in the apportioned source contributions are presented in Masiol et al. (Masiol et al. 2019). The excess rates of hospitalizations for cardiac arrhythmia, ischemic stroke, congestive heart failure, ischemic heart disease, and myocardial infarction associated with increased contribution of each PM2.5 source on the same day (lag day 0), previous 4 days (lag days 0-3), and previous 7 days (lag days 0-6) are shown in Table 3 and Figure 1. As hypothesized, we generally observed increased excess rates of hospitalizations associated with increased DIE and GAS contributions. Each interquartile range (IQR) increase in GAS contributions on the same day was associated with a 3.5% increase in the rate of ischemic stroke hospitalization (95% CI = 1.0%, 6.0%) and a 2.2% excess rate of cardiac arrhythmia hospitalization (95% CI = 0.4%, 4.1%). Although not statistically significant, most excess rates of cardiac arrhythmia, ischemic stroke, congestive heart failure, and myocardial infarction associated with increased GAS contributions on the same day and previous 4 and 7 days were also positive (i.e. >0.0%). Interquartile range increases in DIE contributions on the same day were also associated with increased excess rates of congestive heart failure (0.7%; 95% CI = 0.2%, 1.3%; IQR=0.53 μg/m3) and ischemic heart disease (0.6%; 95% CI = 0.1%, 1.1%; IQR=0.53 μg/m3) hospital admissions. However, interquartile range increases in SS contributions were generally not associated with an increased rate of hospitalization for any outcome. In fact, each 1.65 μg/m3 increase in SS contribution in the previous 7 days was associated with a −1.4% decreased excess rate of cardiac arrhythmia (95% CI = −2.5%, −0.3%).
Table 2.
Distribution of daily PM2.5 source concentrations (μg/m3) for control periods
| PM2.5 Source | Mean | Standard Deviation |
Minimum | 5th %tile | 25th %tile | 50th %tile | 75th %tile | 95th %tile | Maximum |
|---|---|---|---|---|---|---|---|---|---|
| All Sites | |||||||||
| Road Dust (RD) | 0.45 | 0.52 | −0.20 | 0.00 | 0.14 | 0.30 | 0.58 | 1.39 | 6.39 |
| Secondary Sulfate (SS) | 3.12 | 3.93 | −0.94 | −0.38 | 0.76 | 2.04 | 4.09 | 10.50 | 42.47 |
| Secondary Nitrate (SN) | 1.81 | 2.80 | −0.78 | −0.16 | 0.13 | 0.75 | 2.37 | 7.65 | 24.12 |
| Diesel (DIE) | 1.09 | 0.89 | −0.38 | 0.09 | 0.53 | 0.92 | 1.44 | 2.67 | 10.26 |
| Spark-Ignition Emissions (GAS) | 1.60 | 1.67 | −0.44 | −0.17 | 0.41 | 1.13 | 2.32 | 4.92 | 14.60 |
| Biomass Burning (BB) | 0.37 | 0.53 | −0.22 | −0.05 | 0.04 | 0.18 | 0.53 | 1.38 | 9.92 |
| Pyrolyzed Organic Rich (OP) | 1.31 | 1.81 | −0.34 | −0.18 | 0.11 | 0.83 | 1.83 | 4.35 | 20.24 |
| New York City Sites Only | |||||||||
| Fresh Sea Salt (FSS) | 0.20 | 0.66 | −0.08 | −0.01 | 0.00 | 0.02 | 0.10 | 0.98 | 10.64 |
| Aged Sea Salt (AGS) | 0.60 | 0.74 | −0.15 | −0.03 | 0.10 | 0.37 | 0.80 | 2.08 | 7.93 |
| Residual Oil (RO) | 0.63 | 0.80 | −0.20 | −0.07 | 0.11 | 0.38 | 0.85 | 2.20 | 7.17 |
| Buffalo, Rochester, and Albany Only | |||||||||
| Road Salt (RS) | 0.16 | 0.68 | −0.10 | −0.01 | 0.01 | 0.02 | 0.09 | 0.70 | 10.88 |
| Buffalo Only | |||||||||
| Industrial (IND) | 0.20 | 0.21 | −0.05 | −0.03 | 0.06 | 0.15 | 0.25 | 0.64 | 1.26 |
Table 3.
Excess rate of acute cardiovascular hospitalizations associated with each interquartile range increase in PM2.5 source contribution
| Secondary Nitrate (SN) | Secondary Sulfate (SS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Lag | N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p- value |
N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p-value |
| Cardiac arrhythmia | 0 | 63,582 | 1.18 | 0.1 (−0.5, 0.6) | 0.821 | 63,582 | 2.21 | −0.2 (−0.9, 0.5) | 0.585 |
| 0-3 | 44,582 | 1.53 | −0.1 (−1.3, 1.0) | 0.811 | 44,582 | 2.04 | −0.5 (−1.5, 0.6) | 0.404 | |
| 0-6 | 47,316 | 1.45 | 0.2 (−1.2, 1.6) | 0.767 | 47,316 | 1.65 | −1.4 (−2.5, −0.3) | 0.010 | |
| Ischemic stroke | 0 | 34,963 | 1.18 | 0.8 (0.1, 1.6) | 0.027 | 34,963 | 2.21 | −0.3 (−1.3, 0.6) | 0.484 |
| 0-3 | 24,682 | 1.53 | −1.3 (−2.9, 0.2) | 0.095 | 24,682 | 2.04 | −0.2 (−1.7, 1.3) | 0.813 | |
| 0-6 | 26,174 | 1.45 | −0.7 (−2.5, 1.2) | 0.490 | 26,174 | 1.65 | −0.1 (−1.6, 1.4) | 0.900 | |
| Congestive heart failure | 0 | 90,546 | 1.18 | 0.2 (−0.3, 0.6) | 0.478 | 90,546 | 2.21 | 0.4 (−0.2, 1.0) | 0.192 |
| 0-3 | 64,598 | 1.53 | 0.7 (−0.2, 1.6) | 0.125 | 64,598 | 2.04 | 0.0 (−0.9, 0.9) | 0.956 | |
| 0-6 | 67,626 | 1.45 | 0.1 (−0.9, 1.2) | 0.832 | 67,626 | 1.65 | −0.1 (−1.0, 0.8) | 0.872 | |
| Ischemic heart disease | 0 | 127,957 | 1.18 | 0.3 (0.0, 0.7) | 0.081 | 127,957 | 2.21 | −0.1 (−0.5, 0.4) | 0.793 |
| 0-3 | 90,443 | 1.53 | 0.8 (0.1, 1.6) | 0.024 | 90,443 | 2.04 | −0.1 (−0.8, 0.6) | 0.743 | |
| 0-6 | 96,085 | 1.45 | 0.9 (0.0, 1.8) | 0.048 | 96,085 | 1.65 | −0.2 (−1.0, 0.5) | 0.507 | |
| Myocardial infarction | 0 | 45,794 | 1.18 | 0.5 (−0.1, 1.1) | 0.135 | 45,794 | 2.21 | −0.6 (−1.4, 0.2) | 0.125 |
| 0-3 | 31,991 | 1.53 | 1.7 (0.4, 3.0) | 0.009 | 31,991 | 2.04 | −0.2 (−1.4, 1.1) | 0.779 | |
| 0-6 | 33,937 | 1.45 | 0.8 (−0.7, 2.3) | 0.290 | 33,937 | 1.65 | −0.4 (−1.7, 0.8) | 0.512 | |
| Spark-Ignition Emissions (GAS) | Diesel (DIE) | ||||||||
| Outcome | Lag | N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p- value |
N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p-value |
| Cardiac arrhythmia | 0 | 63,582 | 2.56 | 2.2 (0.4, 4.1) | 0.019 | 63,582 | 0.53 | −0.2 (−0.9, 0.5) | 0.547 |
| 0-3 | 44,582 | 1.72 | 1.3 (−0.9, 3.4) | 0.251 | 44,582 | 0.69 | −1.1 (−2.7, 0.5) | 0.174 | |
| 0-6 | 47,316 | 1.49 | 0.4 (−1.8, 2.6) | 0.726 | 47,316 | 0.71 | −0.6 (−2.6, 1.4) | 0.571 | |
| Ischemic stroke | 0 | 34,963 | 2.56 | 3.5 (1.0, 6.0) | 0.005 | 34,963 | 0.53 | 0.0 (−1.0, 1.0) | 0.971 |
| 0-3 | 24,682 | 1.72 | 2.3 (−0.5, 5.2) | 0.102 | 24,682 | 0.69 | 0.4 (−1.8, 2.7) | 0.712 | |
| 0-6 | 26,174 | 1.49 | 1.1 (−1.7, 3.9) | 0.459 | 26,174 | 0.71 | 1.5 (−1.3, 4.3) | 0.296 | |
| Congestive heart failure | 0 | 90,546 | 2.56 | 1.5 (−0.1, 3.0) | 0.067 | 90,546 | 0.53 | 0.7 (0.2, 1.3) | 0.013 |
| 0-3 | 64,598 | 1.72 | 1.8 (0.0, 3.6) | 0.050 | 64,598 | 0.69 | 0.0 (−1.3, 1.3) | 0.992 | |
| 0-6 | 67,626 | 1.49 | 1.6 (−0.2, 3.5) | 0.088 | 67,626 | 0.71 | −1.0 (−2.6, 0.6) | 0.230 | |
| Ischemic heart disease | 0 | 127,957 | 2.56 | 0.5 (−0.8, 1.9) | 0.461 | 127,957 | 0.53 | 0.6 (0.1, 1.1) | 0.012 |
| 0-3 | 90,443 | 1.72 | 0.0 (−1.6, 1.5) | 0.953 | 90,443 | 0.69 | −0.3 (−1.4, 0.7) | 0.538 | |
| 0-6 | 96,085 | 1.49 | 0.0 (−1.6, 1.6) | 0.953 | 96,085 | 0.71 | 0.7 (−0.7, 2.0) | 0.333 | |
| Myocardial infarction | 0 | 45,794 | 2.56 | 2.3 (0.1, 4.5) | 0.039 | 45,794 | 0.53 | 0.4 (−0.5, 1.2) | 0.393 |
| 0-3 | 31,991 | 1.72 | 0.0 (−2.4, 2.6) | 0.976 | 31,991 | 0.69 | −0.7 (−2.5, 1.2) | 0.497 | |
| 0-6 | 33,937 | 1.49 | 0.7 (−1.8, 3.3) | 0.572 | 33,937 | 0.71 | 0.2 (−2.1, 2.5) | 0.894 | |
| Road Dust (RD) | Biomass Burning (BB) | ||||||||
| Outcome | Lag | N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p- value |
N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p- value |
| Cardiac arrhythmia | 0 | 63,582 | 0.30 | −0.3 (−1.0, 0.5) | 0.506 | 63,582 | 0.59 | −0.2 (−1.4, 1.0) | 0.716 |
| 0-3 | 44,582 | 0.21 | 0.0 (−0.9, 0.9) | 0.997 | 44,582 | 0.42 | 0.2 (−1.4, 1.8) | 0.773 | |
| 0-6 | 47,316 | 0.24 | −0.2 (−1.4, 1.0) | 0.740 | 47,316 | 0.36 | 0.0 (−1.6, 1.7) | 0.966 | |
| Ischemic stroke | 0 | 34,963 | 0.30 | 0.3 (−0.7, 1.3) | 0.585 | 34,963 | 0.59 | 1.7 (0.0, 3.3) | 0.046 |
| 0-3 | 24,682 | 0.21 | −0.4 (−1.5, 0.8) | 0.524 | 24,682 | 0.42 | 1.3 (−0.9, 3.6) | 0.257 | |
| 0-6 | 26,174 | 0.24 | −0.5 (−2.0, 1.1) | 0.565 | 26,174 | 0.36 | 0.4 (−1.8, 2.6) | 0.747 | |
| Congestive heart failure | 0 | 90,546 | 0.30 | 0.5 (−0.2, 1.1) | 0.147 | 90,546 | 0.59 | 0.5 (−0.4, 1.5) | 0.278 |
| 0-3 | 64,598 | 0.21 | −0.1 (−0.8, 0.6) | 0.747 | 64,598 | 0.42 | 1.4 (0.1, 2.7) | 0.040 | |
| 0-6 | 67,626 | 0.24 | 0.3 (−0.7, 1.3) | 0.539 | 67,626 | 0.36 | 0.9 (−0.4, 2.3) | 0.175 | |
| Ischemic heart disease | 0 | 127,957 | 0.30 | 0.6 (0.1, 1.1) | 0.015 | 127,957 | 0.59 | −0.8 (−1.6, 0.0) | 0.057 |
| 0-3 | 90,443 | 0.21 | 0.5 (−0.1, 1.1) | 0.085 | 90,443 | 0.42 | −0.3 (−1.3, 0.7) | 0.570 | |
| 0-6 | 96,085 | 0.24 | 0.3 (−0.5, 1.0) | 0.533 | 96,085 | 0.36 | 0.4 (−0.6, 1.5) | 0.416 | |
| Myocardial infarction | 0 | 45,794 | 0.30 | 0.8 (0.0, 1.7) | 0.058 | 45,794 | 0.59 | 0.8 (−0.6, 2.1) | 0.269 |
| 0-3 | 31,991 | 0.21 | 0.8 (−0.1, 1.8) | 0.094 | 31,991 | 0.42 | 0.4 (−1.4, 2.2) | 0.672 | |
| 0-6 | 33,937 | 0.24 | 0.8 (−0.6, 2.2) | 0.280 | 33,937 | 0.36 | 0.8 (−1.0, 2.6) | 0.405 | |
| Pyrolyzed Organic Rich (OP) | Fresh Sea Salt (FSS) | ||||||||
| Outcome | Lag | N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p-value | N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p-value |
| Cardiac arrhythmia | 0 | 43,429 | 1.54 | 0.4 (−0.8, 1.7) | 0.513 | 53,931 | 0.09 | 0.1 (−0.0, 0.3) | 0.168 |
| 0-3 | 30,180 | 0.96 | 1.1 (−0.2, 2.5) | 0.085 | 40,634 | 0.14 | 0.1 (−0.3, 0.5) | 0.552 | |
| 0-6 | 32,479 | 0.93 | 1.6 (−0.0, 3.3) | 0.052 | 40,698 | 0.15 | 0.4 (−0.2, 0.9) | 0.234 | |
| Ischemic stroke | 0 | 25,074 | 1.54 | −1.0 (−2.6, 0.8) | 0.272 | 29,014 | 0.09 | 0.3 (0.0, 0.5) | 0.022 |
| 0-3 | 17,606 | 0.96 | 0.8 (−1.0, 2.6) | 0.376 | 22,309 | 0.14 | 0.0 (−0.5, 0.6) | 0.923 | |
| 0-6 | 18,842 | 0.93 | 1.7 (−0.6, 4.0) | 0.141 | 22,029 | 0.15 | −0.4 (−1.2, 0.4) | 0.295 | |
| Congestive heart failure | 0 | 59,507 | 1.54 | 0.8 (−0.2, 1.9) | 0.120 | 76,926 | 0.09 | −0.1 (−0.2, 0.0) | 0.104 |
| 0-3 | 41,892 | 0.96 | 0.6 (−0.5, 1.7) | 0.290 | 58,762 | 0.14 | 0.0 (−0.3, 0.3) | 0.995 | |
| 0-6 | 44,293 | 0.93 | 0.9 (−0.5, 2.3) | 0.189 | 58,354 | 0.15 | −0.1 (−0.5, 0.4) | 0.691 | |
| Ischemic heart disease | 0 | 77,670 | 1.54 | −0.0 (−0.9, 0.9) | 0.958 | 112,847 | 0.09 | −0.0 (−0.1, 0.1) | 0.776 |
| 0-3 | 53,781 | 0.96 | −0.5 (−1.4, 0.4) | 0.309 | 84,473 | 0.14 | 0.2 (−0.1, 0.4) | 0.169 | |
| 0-6 | 58,188 | 0.93 | −1.0 (−2.1, 0.2) | 0.105 | 85,812 | 0.15 | 0.4 (0.0, 0.8) | 0.032 | |
| Myocardial infarction | 0 | 30,249 | 1.54 | 0.1 (−1.4, 1.6) | 0.866 | 38,112 | 0.09 | 0.0 (−0.2, 0.2) | 0.963 |
| 0-3 | 20,962 | 0.96 | −0.4 (−1.9, 1.2) | 0.632 | 28,936 | 0.14 | −0.1 (−0.6, 0.4) | 0.667 | |
| 0-6 | 22,427 | 0.93 | −0.2 (−2.1, 1.8) | 0.841 | 28,668 | 0.15 | −0.2 (−0.8, 0.5) | 0.602 | |
| Aged Sea Salt (AGS) | Residual Oil (RO) | ||||||||
| Outcome | Lag | N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p- value |
N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p-value |
| Cardiac arrhythmia | 0 | 53,931 | 0.69 | 0.8 (−0.3, 2.0) | 0.167 | 53,931 | 0.71 | −0.2 (−1.5, 1.2) | 0.818 |
| 0-3 | 40,634 | 0.63 | 0.0 (−1.6, 1.7) | 0.992 | 40,634 | 0.63 | 0.1 (−1.9, 2.2) | 0.891 | |
| 0-6 | 40,698 | 0.60 | 0.5 (−1.5, 2.6) | 0.636 | 40,698 | 0.62 | −0.9 (−3.3, 1.5) | 0.450 | |
| Ischemic stroke | 0 | 29,014 | 0.69 | 0.1 (−1.4, 1.7) | 0.884 | 29,014 | 0.71 | 2.1 (0.2, 3.9) | 0.027 |
| 0-3 | 22,309 | 0.63 | −0.5 (−2.6, 1.7) | 0.675 | 22,309 | 0.63 | 0.6 (−2.1, 3.5) | 0.659 | |
| 0-6 | 22,029 | 0.60 | −1.3 (−4.0, 1.5) | 0.360 | 22,029 | 0.62 | 1.3 (−2.0, 4.6) | 0.453 | |
| Congestive heart failure | 0 | 76,926 | 0.69 | −0.5 (−1.4, 0.5) | 0.342 | 76,926 | 0.71 | −0.7 (−1.7, 0.4) | 0.215 |
| 0-3 | 58,762 | 0.63 | 0.1 (−1.3, 1.5) | 0.889 | 58,762 | 0.63 | 0.6 (−1.0, 2.2) | 0.455 | |
| 0-6 | 58,354 | 0.60 | 1.4 (−0.3, 3.2) | 0.110 | 58,354 | 0.62 | 0.8 (−1.1, 2.7) | 0.429 | |
| Ischemic heart disease | 0 | 112,847 | 0.69 | 0.0 (−0.8, 0.8) | 0.949 | 112,847 | 0.71 | 0.8 (−0.1, 1.6) | 0.092 |
| 0-3 | 84,473 | 0.63 | 0.4 (−0.8, 1.5) | 0.537 | 84,473 | 0.63 | 1.8 (0.4, 3.1) | 0.009 | |
| 0-6 | 85,812 | 0.60 | 0.4 (−1.0, 1.8) | 0.593 | 85,812 | 0.62 | 1.3 (−0.3, 2.9) | 0.116 | |
| Myocardial infarction | 0 | 38,112 | 0.69 | 0.2 (−1.1, 1.6) | 0.752 | 38,112 | 0.71 | 1.5 (−0.1, 3.0) | 0.064 |
| 0-3 | 28,936 | 0.63 | 1.3 (−0.6, 3.3) | 0.177 | 28,936 | 0.63 | 2.5 (0.1, 4.9) | 0.041 | |
| 0-6 | 28,668 | 0.60 | −0.6 (−3.0, 1.8) | 0.599 | 28,668 | 0.62 | 0.2 (−2.5, 3.0) | 0.892 | |
| Road Salt (RS) | Industrial (IND)* | ||||||||
| Outcome | Lag | N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p- value |
N Cases | IQR (μg/m3) |
Excess Rate % (95% CI) |
p-value |
| Cardiac arrhythmia | 0 | 9,651 | 0.04 | 0.0 (−0.2, 0.2) | 0.695 | 3,196 | 0.19 | −2.4 (−7.2, 2.7) | 0.359 |
| 0-3 | 3,948 | 0.08 | −0.7 (−2.1, 0.7) | 0.335 | --- | --- | --- | --- | |
| 0-6 | 6,618 | 0.07 | 0.6 (−0.1, 1.3) | 0.076 | 2,345 | 0.17 | 1.1 (−8.5, 11.8) | 0.827 | |
| Ischemic stroke | 0 | 5,949 | 0.04 | 0.1 (−0.2, 0.3) | 0.498 | 2,085 | 0.19 | 10.8 (3.9, 18.1) | 0.002 |
| 0-3 | 2,373 | 0.08 | 2.5 (0.6, 4.4) | 0.008 | --- | --- | --- | --- | |
| 0-6 | 4,145 | 0.07 | 0.5 (−0.4, 1.4) | 0.256 | 1,572 | 0.17 | −5.1 (−16.1, 7.3) | 0.405 | |
| Congestive heart failure | 0 | 13,620 | 0.04 | −0.1 (−0.2, 0.1) | 0.245 | 4,244 | 0.19 | 3.6 (−0.6, 8.0) | 0.094 |
| 0-3 | 5,836 | 0.08 | 0.1 (−0.9, 1.2) | 0.808 | --- | --- | --- | --- | |
| 0-6 | 9,272 | 0.07 | −0.4 (−0.9, 0.1) | 0.108 | 3,138 | 0.17 | 5.7 (−2.4, 14.3) | 0.172 | |
| Ischemic heart disease | 0 | 15,110 | 0.04 | −0.2 (−0.3, 0.0) | 0.019 | 5,511 | 0.19 | −1.6 (−4.9, 2.0) | 0.381 |
| 0-3 | 5,970 | 0.08 | −0.6 (−1.7, 0.6) | 0.319 | --- | --- | --- | --- | |
| 0-6 | 10,273 | 0.07 | −0.3 (−0.8, 0.1) | 0.145 | 4,015 | 0.17 | −5.9 (−12.3, 0.8) | 0.083 | |
| Myocardial infarction | 0 | 7,682 | 0.04 | −0.2 (−0.4, 0.0) | 0.066 | 2,785 | 0.19 | −0.3 (−5.3, 5.0) | 0.914 |
| 0-3 | 3,055 | 0.08 | −0.8 (−2.2, 0.7) | 0.285 | --- | --- | --- | --- | |
| 0-6 | 5,269 | 0.07 | −0.5 (−1.1, 0.2) | 0.167 | 2,043 | 0.17 | −6.0 (−14.9, 3.8) | 0.219 | |
PM2.5 filters/measurements, on which PMF Sources were identified, were only available in Buffalo every 6 days
1.
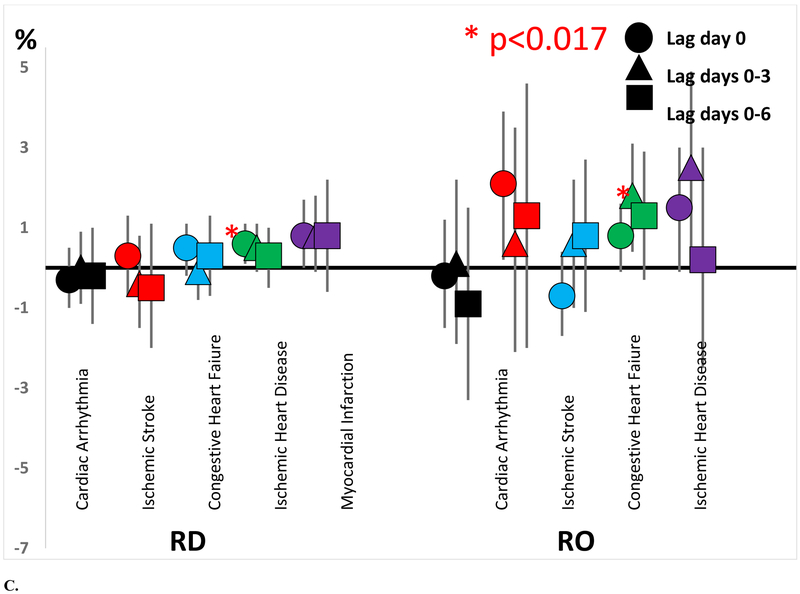
Excess rate of acute cardiovascular event hospitalization by concentrations of A) spark ignition emissions (GAS) and diesel (DIE), B) secondary sulfate (SS) and secondary nitrate (SN), and C) road dust (RD) and residual oil (RO).
Increased contributions of other PM2.5 sources (RD, SN, and RO) were also generally associated with increased rates of cardiovascular hospitalizations (Figure 1, Table 3). For example, interquartile range increases in RD contribution on the same day were associated with significantly increased excess rates of ischemic heart disease hospitalizations (0.6%; 95% CI = 0.1%, 1. 1%; IQR=0.30 μg/m3). Although not statistically significant, most excess rates of congestive heart failure, ischemic heart disease, and myocardial infarction associated with increased RD contributions on the same day and previous 4 and 7 days were positive. Each interquartile range increase in SN contribution over the previous 4 days was associated with an increased excess rate of myocardial infarction (1.7%; 95% CI = 0.4%, 3.0%; IQR=1.53 μg/m3). Again, although not statistically significant, most excess rates of congestive heart failure, ischemic heart disease, and myocardial infarction associated with increased SN contributions on the same day and previous 4 and 7 days were positive. Each 0.63 μg/m3 increase in RO contribution was associated with an increased excess rate of ischemic heart disease (1.8%, 95% CI = 0.4%, 3.1%) over the next 4 days, with most other excess rates also positive.
In contrast, there were no clear patterns of association between increased AGS or FSS contributions and rates of any outcome, while for other PM2.5 sources (OP, RS, IND, BB, OP), there were mixed results (Table 3). For example, although each IQR increase in RS contribution in the previous 4 days was associated with an increased excess rate of ischemic stroke (2.5%, 95% CI = 0.6%, 4.4%, IQR=0.08 μg/m3), most of the other excess rates associated with IQR increases in RS contribution were <0.0%. Similarly, although each IQR increase in IND contribution in the previous day was associated with a 10.8% increase in the excess rate of ischemic stroke hospitalization (95% CI =3.9%, 18.1%), many of the other excess rates associated with IQR increases in IND contributions were <0.0%. Although most excess rates of ischemic stroke, congestive heart failure, and myocardial infarction associated with IQR increases in BB contribution were >0.0%, none were statistically significant. Similarly, although rates of ischemic stroke and congestive heart failure associated with IQR increases in OP contribution were generally positive, none were statistically significant. Last, when estimating the rate of each cause specific cardiovascular hospitalization associated with increased PM source contributions in the previous 1, 4, and 7 days, separately in the Before, During, and After periods, we found little difference in the period specific relative rates (See Supplemental Tables).
4. DISCUSSION
Across urban centers in New York State from 2005 to 2016, as hypothesized, we observed increased rates of hospitalizations for acute cardiovascular events associated with increased diesel (DIE) and spark-ignition vehicle emissions (GAS) contributions, markers of traffic pollution. These effects were independent of changes in temperature and relative humidity, season, weekday, and long-term time trends. However, inconsistent with our a priori hypothesis, we generally did not find increased rates of acute cardiovascular event hospitalizations associated with increased secondary sulfate (SS) contributions. Increased rates of acute cardiovascular event hospitalizations were also generally associated with increased road dust (RD), secondary nitrate (SN), and residual oil (RO) contributions in the previous few days, but not with road salt (RS), industrial emissions (IND), biomass burning (BB), pyrolyzed organic rich emissions (OP), fresh sea salt (FSS), or aged sea salt (AGS).
Previously, using the same hospitalization data for adult New York State residents from 2005-2016 (Zhang et al. 2018), we found that interquartile range increases in ambient PM2.5 concentrations over the previous 1 to 7 days were associated with small but significant increases in the rate of hospital admissions for total cardiovascular disease (0.6%-1.2%), cardiac arrhythmia (1.0%-1.5%), ischemic stroke (1.0%-1.1%), congestive heart failure (0.9%-2.4%), ischemic heart disease (0.8%-1.3%), and myocardial infarction (0.7%-1.0%). Our findings with specific PM2.5 sources (GAS, DIE, SN, RD, and RO) are consistent with these PM2.5 findings with regard to the magnitude of relative rates and suggest that this previous PM2.5 finding was driven by these sources.
In a workshop to compare the use of resolved source apportionment methods in health effects studies Hopke et al. (Hopke et al. 2006), reported that different PM2.5 mass source apportionment methods (Unmix, Multilinear Engine, Positive Matrix Factorization, Absolute Principal Components Analysis, Principal Components Analysis), even when used by seven different user groups, generally identified the same sources and were robust for application to PM2.5 epidemiologic studies. Further, Thurston et al. reported that the PM source apportionment research groups or methods introduced little uncertainty into the assessment of PM toxicity differences across sources, adding ~ 15% to the overall source-specific mortality relative risk uncertainties (Thurston et al. 2005). Thus, use of different source apportionment methods did not prevent the consistent discernment of variations in the relative size of the source-specific PM2.5 mortality effect estimates. From that same workshop, Ito et al. summarized findings across investigative teams using data from Washington, D.C., and reported that the largest excess risk of death per 5th-95th percentile increase in source specific PM2.5 was for secondary sulfate (6.7%; 95% CI = 1.7%, 11.7%) (Ito et al. 2006). Other sources associated with an increased risk of total, cardiovascular, and respiratory mortality included primary coal-related, traffic-related, and soil-related PM2.5.
Previous studies have examined associations between acute health events (e.g. cardiorespiratory hospital admissions and emergency department visits) and short term increases in source apportioned PM concentrations (Andersen et al. 2007; Bell et al. 2014; Gass et al. 2015; Hopke et al. 2006; Ito et al. 2006; Krall et al. 2018; Laden et al. 2000; Mar et al. 2000; Ozkaynak and Thurston 1987; Sarnat et al. 2008). Sarnat et al. found associations between increased respiratory and cardiovascular ED visits and gasoline vehicle, diesel vehicle, and biomass burning PM2.5 in Atlanta (Sarnat et al. 2008). Other studies reported associations between PM10 from biomass burning and increased respiratory hospital admissions in Copenhagen, Denmark (Andersen et al. 2007), gasoline and diesel PM2.5 and increased pediatric asthma ED visits in Atlanta (Gass et al. 2015), and respiratory hospital admissions and PM2.5 from road dust in Massachusetts and Connecticut (Bell et al. 2014). Krall et al. apportioned daily PM2.5 concentrations measured in Atlanta, Birmingham, St. Louis, and Dallas into PM2.5 from biomass burning, diesel vehicles, gasoline vehicles, dust, coal combustion, and metals, but only PM2.5 from biomass burning was acutely associated with respiratory ED visits (Excess risk = 0.6%-0.8%) over the next few days (Krall et al. 2018). Although these studies examined respiratory outcomes, our finding of cardiovascular hospitalization triggering by traffic related PM2.5 sources is consistent with these studies reporting greater risks/rates associated with traffic PM sources.
Zhang et al. suggested that because only secondary organic carbon concentrations were increasing in recent years when the increased toxicity of the PM2.5 was identified, additional exposure to oxidants and the related oxidative stress and inflammation would be potential drivers of the increased rates of hospitalizations (Zhang et al. 2018). All of these factors can be associated with either the likely presence of reactive oxidative species (ROS) or the ability to induce ROS in situ. The strongest site-by-site correlations between resolved source contributions and SOC were for the spark-ignition vehicle source. GAS was the only source whose contributions increased between the three periods (Masiol et al. 2019). There was an increase in the number of registered vehicles in the state. There were also important changes in the fuel formulation between 2010 and 2014 to reduce the concentration of benzene in the fuel (Agency 2019). The shift to increasing use of gasoline direct injection (GDI) in light-duty vehicles likely also contributed to the increased contribution of GAS to the ambient PM2.5 concentrations although newer GDI vehicles certified to stricter emissions standards do not produce more SOA than older port fuel injection engines (PFI) (Zhao et al. 2018). The r2 values between SOC and GAS were 0.423, 0.548, 0.504, 0.303 0.533, 0.533 for Albany, the Bronx, Buffalo, Manhattan, Queens, and Rochester, respectively. Thus, for all but Albany and Manhattan, more than 50% of the SOC variance was related to the spark-ignition mass contributions. These results suggest that gasoline vehicles were an important source of precursor species and secondary organic aerosol (SOA) (Bahreini et al. 2012; Gordon et al. 2013; Gordon et al. 2014; Hayes et al. 2013). Fresh SOA would include peroxy radicals and peroxides and be strongly oxidizing (Chen et al. 2011; Docherty et al. 2005). Mills et al. found that even brief exposures to dilute diesel exhaust promoted myocardial ischemia and inhibited endogenous fibrinolytic capacity in men with stable coronary heart disease (Mills et al. 2007). Wang et al. showed that SOA, diesel and biodiesel exhaust particles generated significant amounts of H2O2 (Wang et al. 2012). Thus, GAS and DIE particles could contribute to both exogenous and endogenous ROS (Hopke 2015).
Road dust (RD) represents non-exhaust traffic emissions containing a number of transition metals like copper, iron, and zinc. These primary emissions result from non-exhaust emissions including brake and tire wear (Padoan and Amato 2019). Such metals can induce the formation of free radicals via Fenton-like reactions (Halliwell and Gutteridge 1984; Strlic et al. 2003). RO would also represent a source of redox-active metals such as nickel and vanadium. Lippmann et al. reported that ambient nickel concentrations were associated with acute increases in heart rate and decreases in heart rate variability in mice and with increased mortality in people (Lippmann et al. 2006).
Both SN and OP represent secondary particles with SN primarily observed in the winter when the level of photochemical activity is low and ammonium nitrate becomes the dominant particle type with respect to particle surface area on which carbonaceous species can condense. Thus, SN could just represent a surrogate for the condensed ROS being advected by the locally formed nitrate particles. OP is primarily observed during the summer months when there is sufficient photochemistry occurring to produce significant amounts of secondary sulfate and secondary organic aerosol (SOA). OP is thought to be an indicator of secondary organic aerosol (Squizzato et al. 2018b), and may represent more aged secondary organic aerosol that has been transported into the area with little associated reactive oxygen species (ROS). Chen et al. found that the lifetime of terpene producing reactive oxygen species was of the order of 6 to 7 hours, so only recently formed secondary organic carbon (SOC) would likely have significant amounts of associated reactive oxygen species (Chen et al. 2011). Thus, the lack of association with OP may reflect its low reactive oxygen species concentration. Secondary sulfate may represent more aged aerosol in which much of the initially deposited reactive oxygen species have reacted away.
The other factors such as AGS, FSS, BB, and IND would not generally include strongly oxidizing constituents. There have been some suggestions of effects of wood smoke on cardiovascular outcomes (Croft et al. 2017; Rich et al. 2018). However, a recent review concluded that the evidence base for the cardiovascular effects of wood smoke is weak and that wood smoke exposures are highly variable due to the multifactorial nature of wood smoke creation (Adetona et al. 2016).
There were several strengths of our study including a large sample size resulting in increased statistical power, uniformly collected and coded hospitalization data in New York State from 2005-2016, and a concurrent multi-year source apportionment analysis across urban centers in New York state providing an assessment of acute health associations with individual PM sources. However, there were also several limitations to consider. First, cases within 15 miles of a PM2.5 monitoring site were assigned the same values of PM2.5 source contributions for a specific day, regardless of how close they lived to the site, which likely resulted in exposure misclassification. This misclassification error is likely a combination of Berkson and classical error, resulting in a bias toward the null and underestimates of effect (Bateson et al. 2007; Zeger et al. 2000). Second, there was a change in the hospital admission diagnosis codes used in SPARCS starting October 1, 2015 (shift from ICD-9 to ICD-10). Certain ICD-9 codes could be divided into multiple more specific ICD-10 codes, possibly resulting in an undercounting of cases in our study. However, this should result in only a loss of statistical power and not bias, as the case-crossover design contrasts pollutant concentrations between two time periods within the follow-up time of each case. Third, the PMF analyses of Squizzato et al. were conducted as a single source apportionment at each site across the entire 12 years of the study period (2005-2016), with individual sources identified and named based on common chemical compositions across these 12 years (Squizzato et al. 2018b). However, it is possible that if the source apportionment was done on individual time periods separately (e.g. 2005-2007, 2008-2013, and 2014-2016), daily concentrations of individual source specific mass concentrations (e.g. SS) would be different from those used in this analysis. If we assume that this exposure misclassification is non-differential with regard to time (i.e. not different for case and control periods), then it again is likely a combination of Berkson and classical error, resulting in a bias toward the null and underestimates of effect. Fourth, although we defined statistical significance as p<0.017 based on three estimates/test per pollutant/outcome association (i.e. 3 lag days), we have 5 outcomes per PM2.5 source contribution. Thus if we made a Bonferroni correction for 15 or 5 tests per PM2.5 source contribution, we would have overestimated the p-value to define statistical significance, making far few results be statistically significant. Last, like all case-crossover designs analyzed with conditional logistic regression, adjusting for possible overdispersion is difficult if not impossible (Armstrong et al. 2014), which could result in confidence intervals that are larger than reported.
5. CONCLUSIONS
Using a large database of hospitalizations of adult New York State residents from 2005 to 2016 and concentrations of PM2.5 sources identified previously (Squizzato et al. 2018b), we found, as hypothesized, increased rates of cardiac arrhythmias and ischemic stroke associated with increased GAS concentrations in the previous few days, and increased rates of congestive heart failure and ischemic heart disease hospitalizations associated with increased DIE concentrations. We did not find any such associations with SS as hypothesized. This null SS finding may just reflect the sharp decrease in sulfate concentrations in New York State (Squizzato et al. 2018a) and its reduced role as a transport vector for reactive species condensed onto its surface. Although RD, RO, and SN concentrations were also associated with increased rates of acute cardiovascular hospitalizations, there were no such associations with other sources. These findings suggest the role of spark ignition and diesel vehicle emissions, non-traffic emissions such as tire and brake wear, ROS residual oil combustion emissions for large building heating in New York City, and advected by nitrate particles in the triggering of acute cardiovascular events. These findings are consistent with the proposed hypothesis of Zhang et al. of the triggering of acute cardiovascular events by oxidants associated with secondary organic aerosols and oxidative potential associated with transition metals (Zhang et al. 2018). However, this will require confirmation of whether these sources in locations other than New York State produce similar rates of hospitalizations for these cardiovascular outcomes.
Supplementary Material
Highlights.
Spark ignition & diesel PM associated with cardiovascular (CV) hospitalizations
Secondary nitrate, residual oi, road dust PM associated with CV hospitalizations
Associations observed over previous 1, 4, or 7 days
Suggest role of traffic & non-traffic emissions in triggering CV hospitalizations
Acknowledgments
Funding
This work was funded by the New York State Energy Research and Development Authority under agreements 59800, 59802, and 100412. Daniel Croft was funded by a National Institutes of Health training grant (T32-HL066988).
Abbreviations:
- GAS
spark ignition emissions
- DIE
diesel
- SN
secondary nitrate
- SS
secondary sulfate
- RD
road dust
- RO
residual oil
- AGS
aged sea salt
- FSS
fresh sea salt
- IND
industry
- BB
biomass burning
- OP
pyrolyzed organic rich
- RS
road salt
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adetona O, Reinhardt TE, Domitrovich J, Broyles G, Adetona AM, Kleinman MT, et al. 2016. Review of the health effects of wildland fire smoke on wildland firefighters and the public. Inhal Toxicol 28:95–139. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. 2019. Fuel trends report: Gasoline 2006 – 2016. https://www.epa.gov/fuels-registration-reporting-and-compliance-help/gasoline-properties-over-time
- Andersen ZJ, Wahlin P, Raaschou-Nielsen O, Scheike T, Loft S. 2007. Ambient particle source apportionment and daily hospital admissions among children and elderly in Copenhagen. J Expo Sci Environ Epidemiol 17:625–636. [DOI] [PubMed] [Google Scholar]
- Armstrong BG, Gasparrini A, Tobias A. 2014. Conditional poisson models: A flexible alternative to conditional logistic case cross-over analysis. BMC Med Res Methodol 14:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahreini R, Middlebrook AM, DeGouw JA, Warneke C, Trainer M, Brock CA, et al. 2012. Gasoline emissions dominate over diesel in formation of secondary organic aerosol mass. Geophysical Research Letters 39:2–7. [Google Scholar]
- Bateson TF, Coull BA, Hubbell B, Ito K, Jerrett M, Lumley T, et al. 2007. Panel discussion review: Session three--issues involved in interpretation of epidemiologic analyses--statistical modeling. J Expo Sci Environ Epidemiol 17 Suppl 2:S90–96. [DOI] [PubMed] [Google Scholar]
- Belis CA, Karagulian F, Larsen BR, Hopke PK. 2013. Critical review and meta analysis of ambient particulate matter source apportionment using receptor models in europe. Atmos Environ 69:94–108. [Google Scholar]
- Belis CA, Larsen BR, Amato F, El Haddad I, Favez O, Harrison RM, et al. 2014. European guide on air pollution source apportionment with receptor models. (JRC Reference Reports EUR26080 EN; ). [Google Scholar]
- Bell ML, Ebisu K, Leaderer BP, Gent JF, Lee HJ, Koutrakis P, et al. 2014. Associations of pm(2).(5) constituents and sources with hospital admissions: Analysis of four counties in Connecticut and massachusetts (USA) for persons >/= 65 years of age. Environ Health Perspect 122:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. 2004. Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the american heart association. Circulation 109:2655–2671. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation 121:2331–2378. [DOI] [PubMed] [Google Scholar]
- Brown SG, Eberly S, Paatero P, Norris GA. 2015. Methods for estimating uncertainty in pmf solutions: Examples with ambient air and water quality data and guidance on reporting pmf results. Sci Total Environ 518-519:626–635. [DOI] [PubMed] [Google Scholar]
- Chen X, Hopke PK, Carter WP. 2011. Secondary organic aerosol from ozonolysis of biogenic volatile organic compounds: Chamber studies of particle and reactive oxygen species formation. Environ Sci Technol 45:276–282. [DOI] [PubMed] [Google Scholar]
- Croft DP, Cameron SJ, Morrell CN, Lowenstein CJ, Ling F, Zareba W, et al. 2017. Associations between ambient wood smoke and other particulate pollutants and biomarkers of systemic inflammation, coagulation and thrombosis in cardiac patients. Environ Res 154:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty KS, Wu W, Lim YB, Ziemann PJ. 2005. Contributions of organic peroxides to secondary aerosol formed from reactions of monoterpenes with o3. Environ Sci Technol 39:4049–4059. [DOI] [PubMed] [Google Scholar]
- Ebisu K, Malig B, Hasheminassab S, Sioutas C, Basu R. 2018. Cause-specific stillbirth and exposure to chemical constituents and sources of fine particulate matter. Environ Res 160:358–364. [DOI] [PubMed] [Google Scholar]
- Evans KA, Hopke PK, Utell MJ, Kane C, Thurston SW, Ling FS, et al. 2017. Triggering of st-elevation myocardial infarction by ambient wood smoke and other particulate and gaseous pollutants. J Expo Sci Environ Epidemiol 27:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner B, Ling F, Hopke PK, Frampton MW, Utell MJ, Zareba W, et al. 2014. Ambient fine particulate air pollution triggers st-elevation myocardial infarction, but not non-st elevation myocardial infarction: A case-crossover study. Part Fibre Toxicol 11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass K, Klein M, Sarnat SE, Winquist A, Darrow LA, Flanders WD, et al. 2015. Associations between ambient air pollutant mixtures and pediatric asthma emergency department visits in three cities: A classification and regression tree approach. Environ Health 14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon TD, Tkacik DS, Presto AA, Zhang M, Jathar SH, Nguyen NT, et al. 2013. Primary gas- and particle-phase emissions and secondary organic aerosol production from gasoline and diesel off-road engines. Environ Sci Technol 47:14137–14146. [DOI] [PubMed] [Google Scholar]
- Gordon TD, Presto AA, May AA, N NT, Lipsky EM, Donahue NM, et al. 2014. Secondary organic aerosol formation exceeds primary particulate matter emissions for light-duty gasoline vehicles. Atmos Chem Phys 14:4661–4678. [Google Scholar]
- Halliwell B, Gutteridge JM. 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, Lanki T, Yli-Tuomi T, Tiittanen P, Kulmala M, Pekkanen J. 2009. Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly. Epidemiology 20:143–153. [DOI] [PubMed] [Google Scholar]
- Hayes PL, Ortega AM, Cubison MJ, Froyd KD, Zhao Y, Cliff SS, et al. 2013. Organic aerosol composition and sources in pasadena, california, during the 2010 calnex campaign. Journal of Geophysics Research and the Atmosphere 118:9233–9257. [Google Scholar]
- Hopke PK, Ito K, Mar T, Christensen WF, Eatough DJ, Henry RC, et al. 2006. Pm source apportionment and health effects: 1. Intercomparison of source apportionment results. J Expo Sci Environ Epidemiol 16:275–286. [DOI] [PubMed] [Google Scholar]
- Hopke PK. 2015. Chapter 1: Reactive ambient particles In: Air pollution and health effects, molecular and integrative toxicology, (Nadadur SS, Hollingsworth JW, eds). London:Springer-Verlag, 1–24. [Google Scholar]
- Hopke PK, Kane C, Utell MJ, Chalupa DC, Kumar P, Ling F, et al. 2015. Triggering of myocardial infarction by increased ambient fine particle concentration: Effect modification by source direction. Environ Res 142:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopke PK. 2016a. Review of receptor modeling methods for source apportionment. J Air Waste Manag Assoc 66:237–259. [DOI] [PubMed] [Google Scholar]
- Hopke PK. 2016b. Case studies of source apportionment from north am erica. Issues in Environmental Science and Technology 42:126–167. [Google Scholar]
- Ito K, Christensen WF, Eatough DJ, Henry RC, Kim E, Laden F, et al. 2006. Pm source apportionment and health effects: 2. An investigation of intermethod variability in associations between source-apportioned fine particle mass and daily mortality in Washington, dc. J Expo Sci Environ Epidemiol 16:300–310. [DOI] [PubMed] [Google Scholar]
- Krall JR, Chang HH, Waller LA, Mulholland JA, Winquist A, Talbott EO, et al. 2018. A multicity study of air pollution and cardiorespiratory emergency department visits: Comparing approaches for combining estimates across cities. Environ Int 120:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. 2000. Association of fine particulate matter from different sources with daily mortality in six u.S. Cities. Environ Health Perspect 108:941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. 2001. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology 12:186–192. [DOI] [PubMed] [Google Scholar]
- Link MS, Luttmann-Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, et al. 2013. Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol 62:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. 2006. Cardiovascular effects of nickel in ambient air. Environ Health Perspect 114:1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclure M 1991. The case-crossover design: A method for studying transient effects on the risk of acute events. Am J Epidemiol 133:144–153. [DOI] [PubMed] [Google Scholar]
- Mar TF, Norris GA, Koenig JQ, Larson TV. 2000. Associations between air pollution and mortality in phoenix, 1995-1997. Environ Health Perspect 108:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar TF, Ito K, Koenig JQ, Larson TV, Eatough DJ, Henry RC, et al. 2006. Pm source apportionment and health effects. 3. Investigation of inter-method variations in associations between estimated source contributions of pm2.5 and daily mortality in phoenix, az. J Expo Sci Environ Epidemiol 16:311–320. [DOI] [PubMed] [Google Scholar]
- Masiol M, Hopke PK, Felton HD, Frank BP, Rattigan OV, Wurth MJ, et al. 2017. Source apportionment of pm2.5 chemically speciated mass and particle number concentrations in new york city. Atmos Environ 148:215–229. [Google Scholar]
- Masiol M, Squizzato S, Rich DQ, Hopke PK. 2019. Long-term trends (2005–2016) of source apportioned pm2.5 across new york state. Atmospheric Environment 201:110–120. [Google Scholar]
- Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, et al. 2007. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med 357:1075–1082. [DOI] [PubMed] [Google Scholar]
- Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, et al. 2012. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA 307:713–721. [DOI] [PubMed] [Google Scholar]
- Ozkaynak H, Thurston GD. 1987. Associations between 1980 u.S. Mortality rates and alternative measures of airborne particle concentration. Risk Anal 7:449–461. [DOI] [PubMed] [Google Scholar]
- Paatero P, Eberly S, Brown SD, Norris GA. 2014. Methods for estimating uncertainty in factor analytic solutions. Atmospheric Measurement Techniques 7:781–797. [Google Scholar]
- Padoan E, Amato F. 2019. Vehicle non-exhaust emissions: Impact on air quality In: Non-exhaust emissions: An urban air quality problem for public health, impact and mitigation measures, (Amato F, ed). London: Academic Press, 21–65. [Google Scholar]
- Pope CA, Muhlestein JB, Anderson JL, Cannon JB, Hales NM, Meredith KG, et al. 2015. Short-term exposure to fine particulate matter air pollution is preferentially associated with the risk of st-segment elevation acute coronary events. J Am Heart Assoc 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Al-Kindi SG, Brook RD. 2018. Air pollution and cardiovascular disease: Jacc state-of-the-art review. J Am Coll Cardiol 72:2054–2070. [DOI] [PubMed] [Google Scholar]
- Reff A, Eberly SI, Bhave PV. 2007. Receptor modeling of ambient particulate matter data using positive matrix factorization: Review of existing methods. J Air Waste Manag Assoc 57:146–154. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, et al. 2005. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol 161:1123–1132. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Kim MH, Turner JR, Mittleman MA, Schwartz J, Catalano PJ, et al. 2006a. Association of ventricular arrhythmias detected by implantable cardioverter defibrillator and ambient air pollutants in the st louis, missouri metropolitan area. Occup Environ Med 63:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttmann-Gibson H, Catalano PJ, et al. 2006b. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect 114:120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Ozkaynak H, Crooks J, Baxter L, Burke J, Ohman-Strickland P, et al. 2013. The triggering of myocardial infarction by fine particles is enhanced when particles are enriched in secondary species. Environ Sci Technol 47:9414–9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Utell MJ, Croft DP, Thurston SW, Thevenet-Morrison K, Evans KA, et al. 2018. Daily land use regression estimated woodsmoke and traffic pollution concentrations and the triggering of st-elevation myocardial infarction: A case-crossover study. Air Qual Atmos Health 11:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat JA, Marmur A, Klein M, Kim E, Russell AG, Sarnat SE, et al. 2008. Fine particle sources and cardiorespiratory morbidity: An application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect 116:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, et al. 2013. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet 382:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, et al. 2015. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 350:h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PA, Crumpler D, Flanagan JB, Jayanty RKM, Rickman EE, McDade CE. 2014. Us national pm2.5 chemical speciation monitoring networks—csn and improve: Description of networks. Journal of the Air & Waste Management Association 64:1410–1438. [DOI] [PubMed] [Google Scholar]
- Squizzato S, Masiol M, Rich DQ, Hopke PK. 2018a. Pm2.5 and gaseous pollutants in new york state during 2005-2016: Spatial variability, temporal trends, and economic influences. Atmospheric Environment 183:209–224. [Google Scholar]
- Squizzato S, Masiol M, Rich DQ, Hopke PK. 2018b. A long-term source apportionment of pm2.5 in new york state during 2005–2016. Atmospheric Environment 192:35–47. [Google Scholar]
- Strlic M, Kolar J, Selih V-S, Kocar D, Pihlar B. 2003. A comparative study of several transition metals in fenton-like reaction systems at circum-neutral ph. Acta Chim Slov 50:619–632. [Google Scholar]
- Thurston GD, Ito K, Mar T, Christensen WF, Eatough DJ, Henry RC, et al. 2005. Workgroup report: Workshop on source apportionment of particulate matter health effects--intercomparison of results and implications. Environ Health Perspect 113:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Arellanes C, Paulson SE. 2012. Hydrogen peroxide associated with ambient fine-mode, diesel, and biodiesel aerosol particles in southern California. Aerosol Science and Technology 46:394–402. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. 2000. Exposure measurement error in time-series studies of air pollution: Concepts and consequences. Environ Health Perspect 108:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Lin S, Hopke PK, Thurston SW, van Wijngaarden E, Croft D, et al. 2018. Triggering of cardiovascular hospital admissions by fine particle concentrations in new york state: Before, during, and after implementation of multiple environmental policies and a recession. Environ Pollut 242:1404–1416. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Lambe AT, Saleh R, Saliba G 2018. Secondary organic aerosol production from gasoline vehicle exhaust: Effects of engine technology, cold start, and emission certification standard. Environmental Science and Technology 52:1253–1261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.