Abstract
Purpose of review:
Glycoimmunology is an emerging field focused on understanding how immune responses are mediated by glycans (carbohydrates) and their interaction with glycan-binding proteins called lectins. How glycans influence immunological functions is increasingly well understood. In a parallel way, in the HIV field, it is increasingly understood how the host immune system controls HIV persistence and immunopathogenesis. However, what has mostly been overlooked, despite its potential for therapeutic applications, is the role that the host glycosylation machinery plays in modulating the persistence and immunopathogenesis of HIV. Here, we will survey four areas in which the links between glycan-lectin interactions and immunology, and between immunology and HIV are well described. For each area, we will describe these links and then delineate the opportunities for the HIV field in investigating potential interactions between glycoimmunology and HIV persistence/immunopathogenesis.
Recent findings:
Recent studies show that the human glycome (the repertoire of human glycan structures) plays critical roles in driving or modulating several cellular processes and immunological functions that are central to maintaining HIV infection.
Summary:
Understanding the links between glycoimmunology and HIV infection may create a new paradigm for discovering novel glycan-based therapies that can lead to eradication, functional cure, or improved tolerance of lifelong infection.
Keywords: HIV persistence, glycosylation, galactosylation, sialylation, fucosylation, galectins
I. Introduction
The main barrier to HIV eradication is the ability of HIV to establish latent infection in long-lived CD4+ T cells, which persist in the blood and tissues [1]. These latently-infected cells are the source of viral rebound after interruption of antiretroviral therapy (ART), and their continual reactivation in vivo probably contributes, among other drivers, to the immune activation, chronic inflammation, and organ damage that persist despite long-term suppressive therapy [2, 3]. These realities have prompted a renewed interest in developing new effective and accessible therapies that can lead to eradication, functional cure, or improved tolerance of lifelong infection.
Many studies have described the important role the immune system plays in regulating HIV infection during suppressive ART [4–7]. These studies suggest that a comprehensive understanding of the host immune determinants shaping the persistence and immunopathogenesis of HIV is a critical step in developing new strategies to cure HIV and/or prevent or delay the development of inflammation-associated co-morbidities, which are more prevalent in HIV+ individuals compared to the general population, despite long-term suppressive ART [8–16].
After the initial success of the genome-wide association approach, it became evident that genetic information was only one of the layers of biologic complexity and that knowledge about several additional layers would be needed to understand life at the molecular level. A particularly important layer in this respect is glycomics. Glycobiology is an emerging field focused on defining the structures and functional roles of complex carbohydrate structures, called glycans, in biological systems. These glycan structures, composed of branched chains of monosaccharides, are added to a wide variety of biological molecules (such as proteins and lipids) through a biological process called glycosylation. Glycosylation alters not only protein/lipid structure but also their function. The specific structure of a glycan allows it to bind to a specific type of glycan-binding proteins called lectins, leading to activation of downstream signaling pathways. Glycans integrate genetic and environmental factors, contribute significantly to variability in protein structure, and function as a bridge between cells and their complex environments; thus, aberrations of glycan structures closely associate with complex diseases [17–19]. Evolutionary conservation is in the order of: genetic code ‘Genome’ > RNA sequences ‘Transcriptome’ > primary protein sequence ‘Proteome’ > metabolic pathways ‘Metabolome’ > cellular lipid composition ‘Lipidome’ > glycan structures ‘Glycome’. The reverse order generates structural diversity and richness of biological information. In other words, the genome is the most evolutionarily conserved and the least diverse, and the glycome is the least evolutionarily conserved and the most diverse, rich with biological and chemical information [20].
Recent advances in glycobiology show that the glycome (the repertoire of glycan structures of an organism) is not just a biomarker of biological functions but actually plays critical roles in modulating immune responses [21] and in cell-cell [22] and cell-pathogen interactions [23]. Since glycans affect protein structure and function, it is not surprising that they play an important role in regulating both physiological and pathophysiological processes. The recent consensus report of the National Research Council concluded that “glycans are directly involved in the pathophysiology of every major disease” … “additional knowledge from glycoscience will be needed to realize the goals of personalized medicine and to take advantage of the substantial investments in human genome and proteome research and its impact on human health” [24].
At the intersection of immunology and glycobiology is “glycoimmunology”, an emerging field focused on understanding how immune responses are mediated by glycans and glycan-lectin interactions. How glycans influence immunological functions is increasingly well understood. In a parallel way, in the HIV field, it is increasingly understood how the host immune system controls HIV infectivity, persistence, and immunopathogenesis. However, how the host glycosylation machinery may modulate the persistence and immunopathogenesis of HIV has been mostly overlooked. An association between glycomic alterations and HIV infection was suggested over two decades ago [25–27], but the precise role of the host glycome in HIV infection was never characterized due to lack of glycobiological technologies that can analyze clinical samples at a large scale. Now a wide range of advanced glycomic technologies are emerging, and we are becoming able to tackle the complexity of the host glycome. Using these new tools to understand the role of glycoimmunology in the maintenance of HIV latency, and the development of the aging- and inflammation-associated co-morbidities, may allow us to develop novel therapies that can lead to eradication, functional cure, or improved tolerance of lifelong infection.
Although the glycosylation of the HIV envelope (Env) protein and the potential for exploiting this glycan shield to elicit antibodies with broad neutralizing activity have been a key area of interest in the HIV vaccine field, here we will not discuss this area as excellent reviews have already been published [28, 29]. We rather will focus on the potential role of the host glycosylation machinery, including the binding of virus glycans to host lectins, in regulating HIV persistence and immunopathogenesis, as this area remains completely understudied, despite its potential for therapeutic applications. We will survey four areas in which the links between glycan-lectin interactions and immunology, and between immunology and HIV are well described. For each area, we will describe these links and then delineate the opportunities for the HIV field in investigating potential links between glycoimmunology and HIV persistence/immunopathogenesis.
II. The potential role of the circulating human glycome in modulating immunological responses during HIV infection
Glycomic analyses of circulating biofluids such as serum/plasma, cerebrospinal fluid (CSF), and urine have provided many biomarkers of human diseases and biological states, such as cancer progression [30–38]. These studies have suggested that the circulating glycome plays an important role in regulating the immunological responses to disease. Within the human circulating glycome, glycans on circulating immunoglobulins (Igs) are known to play an important role in regulating several immunological functions [39]. Igs are glycoproteins produced by plasma cells that contain two domains separated by a hinge region. The Fab (Fragment, antigen binding) domain determines specificity towards antigens and the Fc (Fragment, crystallizable) domain is involved in binding to Fc receptors on the surface of immune cells. The most abundant Ig in humans is immunoglobulin G (IgG), with many non-neutralizing effector functions, such as antibody-dependent cellular phagocytosis (ADCP), complement-dependent cytotoxicity (CDC), antibody-dependent cell mediated cytotoxicity (ADCC), and several pro- and anti-inflammatory activities [40–42]. The ability of IgG to function in these capacities is conferred and modulated by its glycosylation at an evolutionarily conserved N-glycosylation site at Asn-297 of the Fc domain and by variable glycosylation sites resulting from somatic hypermutation [43] in the Fab domain (15–20 % of IgG molecules) [44]. Glycans of the Fc domain are positioned in a hydrophobic pocket and quite rigid; these enable binding to the Fcγ receptors, likely by keeping the Fc domain in an open conformation [45]. On the other hand, glycans on the Fab domain are more flexible, contain more sialylated glycans and more glycans with a bisecting N-Acetylglucosamine (GlcNAc) [44] and can modulate antigen binding [45]. The next two sections will discuss the potential role of the IgG glycome in regulating both inflammatory responses and plasma-mediated innate immune effector activities during HIV infection. Note that although the glycosylation of other circulating glycoproteins in biofluids likely also plays an important role in regulating immune functions, these influences are much less well understood and will not be reviewed here.
a). Chronic inflammation has been associated with aberrant IgG glycosylation patterns and is prevalent in HIV+ individuals, despite ART
Even after long-term suppressive ART, HIV+ individuals suffer from a high incidence of diseases that are commonly associated with aging and that are caused, at least in part, by low grade, systemic, chronic inflammation, observed typically in elderly individuals, and termed inflammaging [46–49]. Examples include cardiovascular disease, cancers, neurocognitive disorders, and osteoporosis. It is hypothesized that inflammaging occurs in HIV+ individuals at younger ages than in HIV- counterparts [50]. Although considerable gaps remain in our understanding of the pathophysiological mechanisms driving the development of aging-associated co-morbidities in HIV+ individuals, the chronic inflammatory state caused by HIV infection is likely a key. Indeed, in HIV+ individuals, systemic inflammation, as measured by serum markers, can predict the incidence of mortality, cardiovascular disease, lymphoma, type 2 diabetes, cognitive dysfunction, and frailty [51–56]. Chronic inflammation likely involves multifactorial mechanisms, not all of which are well characterized. Sources of chronic inflammation in HIV+ individuals include on-going HIV production, cytomegalovirus infection, loss of regulatory T cells, and microbial translocation [57–64]. Comprehensively understanding the causes of HIV-associated chronic inflammation can lead to the development of tools to prevent it, and thereby prevent or delay the development of aging-associated co-morbidities in HIV+ individuals.
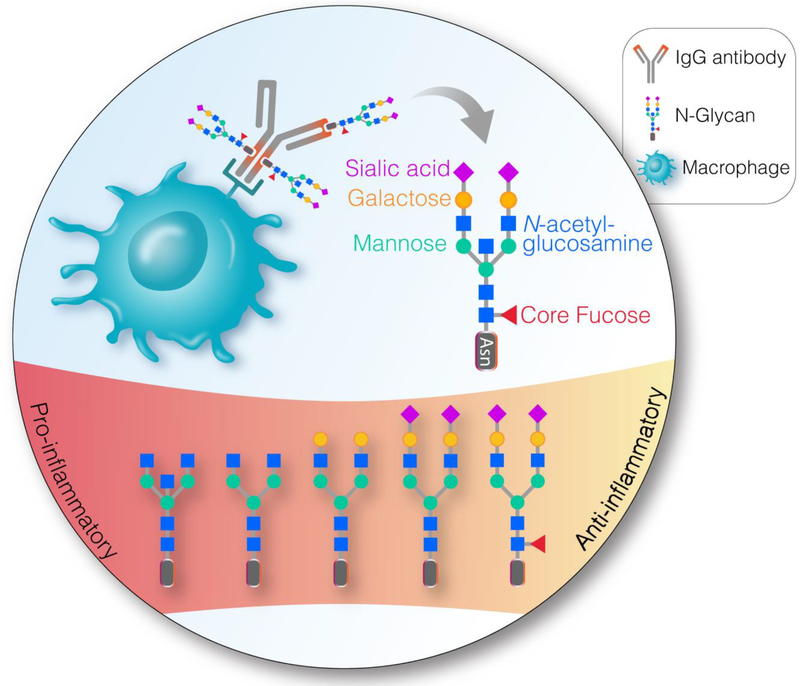
Since immunological functions are shaped by the host glycome, it is not surprising that inflammation is associated with aberrant glycosylation. A number of studies have linked altered IgG glycosylation, in particular, lowered levels of sialic acid, to systemic inflammatory responses [65–67]. A reduction in IgG sialylation, termed hypo-sialylation, increases the pro-inflammatory function of IgGs [65–68]. The exact mechanism of this action is not clear. One suggestion is that sialylation switches the antibody’s binding from classical to non-classical Fc receptors [69]; however other studies suggest that this switching is minimal [70, 71]. Another suggestion is that binding of sialic acid-containing glycans to the sialic acid binding immunoglobulin-like lectins (siglecs) on the surface of monocytes/macrophages initiates an inhibitory signal that leads to an anti-inflammatory response, through inhibition of TLR4 signal transduction. Such TLR4 inhibition reduces the production of pro-inflammatory cytokines such as TNFα and induces the production of anti-inflammatory cytokines [72–74]. This anti-inflammatory effect of sialic acid is supported by studies showing that the anti-inflammatory effect of intravenous immunoglobulin (IVIg), used to treat rheumatoid arthritis and other inflammatory conditions, is driven by sialic acid-containing N-linked glycans [75, 76, 66].
IgG glycosylation also has been closely linked to both chronological and biological age, in several large glycomic studies in the general population. Intriguingly, certain glycomic traits were found to predict chronological and biological age better than typical markers such as telomere length [77, 78]. Altered glycosylation also has been shown to associate with age-related illness: large patient cohorts showed that IgG glycosylation is significantly altered in patients with inflammatory bowel disease, systemic lupus erythematosus, cardiovascular disease (CVD), cancer, and diabetes [79–83, 17, 84, 85]. Whether IgG glycosylation is a driver or simply a biomarker of aging and aging-associated co-morbidities is still a matter of debate. However, evidence that altered glycosylation actually drives disease comes from recent studies indicating that IgG glycosylation changes years before the onset of disease [86, 87].
HIV infection causes certain IgG glycomic alterations including hypo-sialylation and agalactosylation (lack of galactose) [88]. Lower levels of galactosylation in HIV+ individuals compared to healthy controls are most pronounced in the IgG1 subclass [89]. Interestingly, agalactosylation has also been associated with pro-inflammatory functions of IgGs [90]. The pro-inflammatory action of IgG agalactosylation is thought to be conferred both indirectly, because galactose is a prerequisite for terminal sialylation, and directly, by activating the complement system through either the alternative pathway [91] or the mannose-binding lectin-dependent pathway [92]. Intriguingly, HIV-associated agalactosylation is reversible by ART, whereas hypo-sialylation is not [88]. These glycomic alterations may reflect a chronic inflammatory state as they are also observed in other inflammatory conditions [89] such as inflammatory bowel disease [79], rheumatoid arthritis [93], systemic lupus erythematosus [94], as well as with aging [77, 39].
Although it is becoming increasingly established that there is a link between the circulating glycome and the development of several pro- and anti-inflammatory responses (Fig. 1), whether the HIV-induced changes in the circulating glycome are linked to inflammaging and HIV-associated co-morbidities (during both viremic and ART-suppressed HIV infection) is less clear. Recently, plasma glycomic biomarkers were identified (using lectins) to predict HIV-associated cardiovascular events [95]. However, more work is needed in this direction. Understanding the link between circulating glycomic alterations and inflammation, during HIV infection, may provide clues about the mechanistic underpinnings of age- and inflammation-associated diseases in HIV+ individuals. This line of research might allow for discovering novel glycomic-based biomarkers of inflammaging during HIV infection or novel glycan-based interventions to prevent inflammation- and aging-associated diseases in HIV+ individuals.
Figure 1. Circulating IgG glycans mediate pro-or anti-inflammatory responses.
Sialylated and galactosylated glycans have been associated with anti-inflammatory responses while bisected N- acetylglucosamine (GlcNAc) has been associated with pro-inflammatory responses. HIV infection causes pro-inflammatory changes, e.g., ART-irreversible loss of sialic acid and ART-reversible loss of galactose. Whether the HIV-induced changes in the circulating glycome are linked to inflammaging and HIV-associated co-morbidities (such as cardiovascular diseases and neurological impairments) is not clear. Asn = Asparagine.
b). Antibody-mediated effector functions are significantly affected by changes in IgG glycosylation and are important for preventing and controlling HIV infection
The importance of the non-neutralizing Fc-mediated effector functions of antibodies (including ADCC) in preventing and controlling HIV infection has been highlighted by several studies [96–103]. In addition, the recently discovered broadly neutralizing antibodies (bNAbs) are being investigated within HIV curative strategies, especially in combination with latency reversal agents that may provoke antigen presentation [104, 103]. ADCC is one of the potential mechanisms by which bNAbs may target the latent HIV reservoirs [104, 103]. However, the molecular determinants of ADCC and other Fc-mediated effector functions (such as ADCP and CDC), especially during ART-suppressed HIV infection, are not fully characterized.
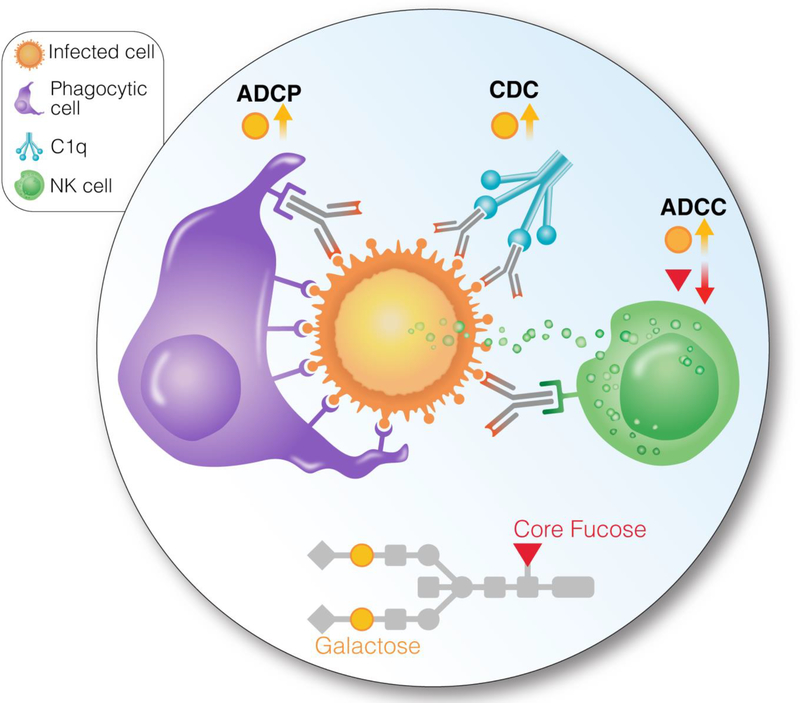
Effector and antigen-binding functions of IgG are significantly affected by changes in glycosylation. The absence of core fucose results in a stronger binding to Fcγ receptor IIIA and leads to enhanced ADCC activity, while the presence of core fucose reduces ADCC [105]. Although core fucose has the greatest impact on ADCC activity, terminal galactose also has been shown to increase ADCC [106], as well as CDC [107] and ADCP [108] (Fig. 2). Despite these results, the role of galactosylation in ADCC activity is somewhat controversial, likely because the effects of terminal galactosylation are not singular [109, 106]. For example, recent research shows that the effect on ADCC activity differs between galactose bound to antenna of the α1–3-mannose of IgG Fc-glycan (inversely correlated with ADCC activity) and galactose bound to antenna of the α1–6-mannose (directly correlated with ADCC activity) [108].
Figure 2. Antibody-mediated effector functions ADCC, ADCP, and CDC are significantly affected by changes in IgG glycosylation.
The presence of core fucose reduces ADCC, and the presence of galactose induces ADCC, ADCP, and CDC. The size of the HIV reservoir, measured using nucleic acid-based methods (CD4+ T cell-associated HIV DNA and RNA), negatively associates with levels of non fucosylated galactosylated glycans, during suppressive ART. However, it is not clear if the documented roles of non-fucosylated galactosylated glycans in promoting ADCC and ADCP impact viral control during ART. C1q = Complement component 1q.
The HIV field has started to investigate whether IgG glycosylation is important for HIV infection. Changes in global and antigen-specific antibody glycosylation have been associated with a differential ability of anti-HIV antibodies to control HIV infection [110]. Lower abundance of Fc glycans with core fucose (afucosylation) has been observed in antigen-specific anti-HIV antibodies, suggesting that there is active tuning of glycosylation by B-cells to increase antiviral control during HIV infection [110]. This afucosylation, in addition to agalactosylation, were also linked to enhanced natural killer (NK) cell activity in spontaneous controllers of HIV [110]. During suppressive ART, certain plasma and antibody glycomic traits, in particular, levels of non-fucosylated galactosylated glycans, are negatively associated with levels nucleic acid-based measures of HIV reservoir (CD4+ T cell-associated HIV DNA and RNA) [88]. These findings, during suppressive ART, are intriguing as these particular glycomic features imply higher antibody-mediated effector functions, as described above. However, it is not clear if the documented roles of non-fucosylated galactosylated glycans in promoting ADCC and ADCP activities translate into an impact on viral control during ART, because ADCC and ADCP require antigen presentation (viral production) on the cell surface, which is debatable during ART [111, 112]. Continuing this work to understand the role of antibody glycosylation in regulating HIV persistence may reveal new mechanistic underpinnings of HIV persistence, which can serve as a foundation of novel, glycomic-based HIV curative strategies. In addition, this understanding may lay the groundwork to engineer bNAbs and improve their ADCC/ADCP activities for HIV curative purposes.
III. Complex interactions between HIV glycans and host lectins modulate viral attachment, entry, and spreading
The glycosylation of HIV virions has been well described [113]. HIV gp120 is heavily N-glycosylated, with a majority of high-mannose N-glycan structures and a lower proportion of complex N-glycans carrying lactosamine residues and terminal sialic acid. HIV particles themselves contain cell-derived glycolipids, including the sialic acid-containing GM3 ganglioside [114].
These various glycan structures on HIV gp120 and HIV particles interact with a wide range of host lectins during HIV infection, promoting viral spreading or immunological responses. The dendritic cell- specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), is a member of the C-type lectin family and an HIV receptor [115]. DC-SIGN recognition of HIV high mannose glycans mediates HIV capture by dendritic cells (DC), which can subsequently lead to CD4+ T cell trans-infection. B cells also express DC-SIGN, and a similar role of HIV capture and presentation to CD4+ T cells has been described [116]. Langerin is another C-type lectin, which is expressed on Langerans cells (LCs) and binds to high-mannose HIV glycans [117]. In contrast, to capture by DC-SIGN, HIV capture by Langerin was associated with viral clearance, as LCs are mostly resistant to HIV infection and rapidly degrade the virus [118]. However, the role of Langerin in HIV clearance or spreading remains controversial [119, 120]. A third C-type lectin that can bind gp120 HIV high-mannose structures is the mannose-binding lectin (MBL) [121]. This soluble lectin has been shown to compete in vitro with DC-SIGN for HIV binding, thereby inhibiting DC-mediated CD4+ trans-infection [122]. However, in vivo, the association between HIV progression and MBL level/genotype remains elusive [123–125]. Recently, the C-type lectin L-selectin has also been described as an HIV adhesion receptor that facilitates infection of CD4+ T cells [126]. Three additional C-type lectins can bind to HIV gp120 high mannose structures: the DC-immunoreceptor (DCIR) [127], the DC-SIGN-related protein (DC-SIGNR) [128], and the surfactant protein D (SFTPD) [129]. DCIR has been shown to play a role similar to DC-SIGN. In addition, DCIR is expressed on the surface of CD4+ T cells of HIV infected patients, enhancing HIV attachment, entry, and transfer [130]. The DC-SIGN homolog DC-SIGNR is expressed mostly on endothelial cells, including in lymph nodes, and can promote viral trans-infection [128]. SFPTD is a soluble protein present in mucosal secretions [131]. The role of HIV-SFPTD binding remains unclear [129, 131].
A second layer of complexity in the interaction between HIV glycans and host lectins is conferred by sialic acid – siglec binding. Sialic acid present on gp120 complex N-glycans or HIV gangliosides is recognized by different members of the family of sialic acid-binding immunoglobulin-type lectins called siglecs. On macrophages and dendritic cells, it is siglec-1 that binds to sialic acid on HIV and mediates particle-capture and trans-infection of CD4+ T-cells [132, 133]. Interestingly, the macrophage siglec-1 is described to recognize HIV ganglioside, mostly GM3, whereas the DC siglec-1 is thought to interact with gp120. Despite these interactions, a loss-of-function mutation in siglec-1 in vivo did not significantly impact HIV prevalence and progression [134]. On monocyte/macrophage and NK cells it is siglec-7 that interacts with HIV to facilitate CD4+ T-cell infection [135]. Finally, soluble galectin-1 has been described to directly bind to HIV particles and increase HIV infectivity, apparently by interacting with CD4 glycans and gp120 lactosamine containing complex N-glycans [136].
It is unclear to what degree these complex interactions between HIV glycans and host lectins influence viral attachment, entry, and spreading in vivo, and whether they play any role during suppressive therapy, especially in tissues, where ART penetration might be suboptimal and on-going HIV replication is debatable [111, 112]. Understanding the forces that lead to HIV acquisition, pathogenesis, and persistence, especially in tissues, will be needed to develop effective therapeutic strategies to clear the infection. These glycomic interactions could be a key for this understanding and may also play a role in improving antigen presentation, which can be critical for developing effective vaccination strategies.
IV. Cell-surface glycan-lectin interactions mediate signals that define cellular processes and immunological functions; many of which are central to HIV infection
During HIV infection, the host immune system experiences several dysfunctions that are not fully recovered by ART. Several of these dysfunctions can be linked to glycan-lectin interactions. The specific structure of a glycan allows it to bind to specific lectins, leading to activation of downstream signaling pathways. These pathways are critical for a variety of cellular processes and, importantly here, immunological functions. For example, galectins (lectins that bind β-galactoside), promotes immune evasion by inducing T-cell exhaustion and apoptosis, expanding regulatory T cells, and inhibiting NK cells [137–141]. Siglecs (lectins that bind sialic acid) plays an essential role in inflammation, cell death, and immune suppression [142, 75, 143, 144, 66, 145–147, 138]. Selectins (lectins that bind fucosylated and sialylated glycans) regulate leukocyte recruitment and migration to sites of inflammation [148–150]. Fig. 3 summarizes some of the glycan-lectin interactions that regulate important cellular and immunological functions and that can be critical for HIV persistence and immunopathogenesis. After first summarizing the roles of various immune cell types in HIV infection, we will describe some of the glycan-lectin interactions that modulate function in these different cell populations.
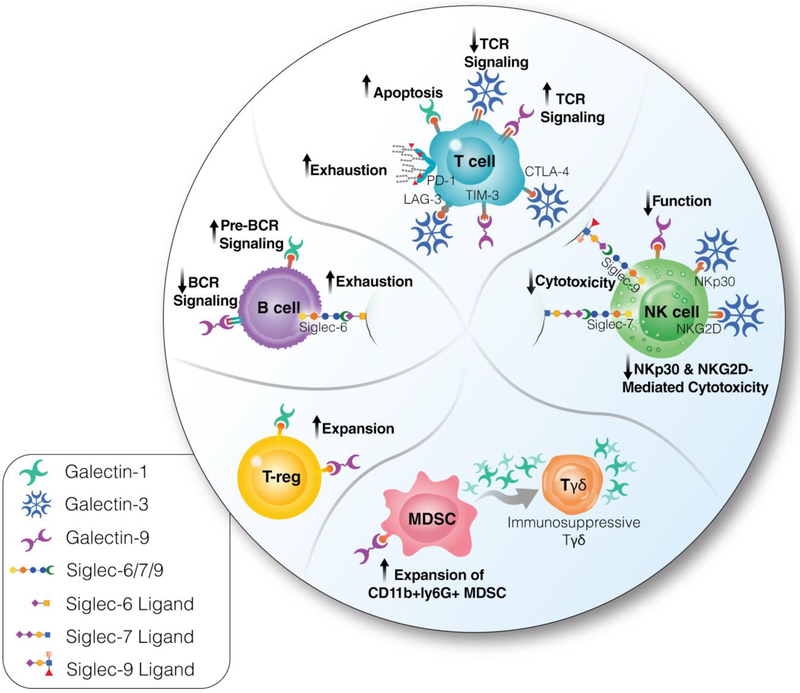
Figure 3. Cell-surface glycan-lectin interactions mediate signals that define several cellular processes and immunological functions central to HIV infection.
The specific structure of a glycan allows it to bind to specific lectins, leading to activation of downstream signaling pathways. These pathways are critical for a variety of cellular processes and immunological functions. T cells. Galectin-1 induces T cell apoptosis. Galectin-9 induces TCR signaling, while galectin-3 reduces it. Galectin-3 alters T cell function through interaction with LAG3 and other immune negative checkpoints. Last, the fucosylation of PD-1 impacts its function. NK cells. Siglecs-7 and −9 inhibit NK activity. Galectin-9 impairs NK function/cytotoxicity and cytokine production through a Tim-3 independent mechanism. Galectin-3 antagonizes NK cell-mediated antitumor immunity by diminishing the affinity of MHC I-related chain A (MICA) for the NKG2D receptor or by acting as an inhibitory ligand of the NKp30 receptor. B cells. Siglec-6 induces B-cell exhaustion. Galectin-1 is a pre-B cell receptor ligand that induces receptor clustering, leading to efficient B cell differentiation. Galectin-9 suppresses BCR signaling. T-regs. Galectins-1 and −9 can expand T-regs. Myeloid-derived suppressive cells (MDSC). The galectin-9/Tim3 interaction drives the expansion of CD11b+ly6G+ MDSC. Granulocytic MDSCs induce γδ-T cells to produce galectin-1, thus transforming them into immunosuppressive cells. These glycan-lectin interactions represent potential novel targets to enhance immune functionality during HIV infection to either cure HIV or prevent HIV-associated immune dysfunction and the subsequent development of immune dysfunction-associated diseases.
T cells.
T cell activation, CD8+ T cell dysfunction, T cell proliferation, and bystander CD4+ cell death are all critical components of HIV infection, persistence, and disease progression [151–157]. Glycan lectin interactions are known to regulate several of these functions. One class of glycan binding proteins that have been described to play critical roles in T cell function activation and apoptosis are the galectins, a family of β-galactoside-binding soluble lectins. Galectins-1 −3 and −9 induce T cell apoptosis, and increased expression a classical galectin receptor, lactosamine chains, has been associated with HIV infection [25]. This upregulation of galectin ligands has been proposed as a possible mechanism for the bystander T cell death during HIV infection. A rapid secretion of galectin-9 has been described after HIV infection, and the increased serum concentration of galectin-9 does not return to normal after ART suppression [158]. Galectin-9 has several effects on T cells in addition to inducing apoptosis; it activates cells through T cell Receptor (TCR) signaling [159], reactivates latent-HIV [6], renders CD4+ T cells less susceptible to HIV infection via induction of host restriction factors [160], and increases the cell-surface concentration of protein disulfide isomerase (PDI) that alters redox state and increases HIV entry [161]. Galectin-9 also has the ability to increase the function of regulatory T cells (T-regs) through interaction with CD44 [162]. Conversely, galectin-3 reduces T-cell activation through direct interaction with the TCR and alters TCR functional state through interaction with LAG3 and other immune negative checkpoints [163]. Intriguingly, the glycosylation of T cell immune negative checkpoints (including PD-1) significantly impacts their functions and response to cancer immunotherapy [164–166]. How glycan-lectin interactions impact these important T cell functions during HIV infection is yet not clear. Clarifying the role these interactions play during HIV infection can provide insights that may lead to the development of novel therapies.
NK cells.
NK cells are important innate effector immune cells during HIV infection [167, 168] whose functions can be influenced by glycan-lectin interactions. Altered NK function has been described for two families of lectins, the siglecs and the galectins. A decreased level of the lectin siglec-7 has been described to be a marker for a dysfunctional NK subset (CD56dim) in HIV viremic individuals [169]. Siglec-9, which is also expressed on the NK cell surface and known to play an important role in anti-tumor NK activity [170, 171] is yet to be studied in the context of HIV infection. Galectins interfere with NK cell-mediated antitumor immunity by modulating NK cell recruitment, lytic activity, and cytokine production. Galectin-9 impairs NK cytotoxicity and cytokine production through a Tim-3 independent mechanism [172]. Galectin-3 also antagonizes NK cell-mediated antitumor immunity by diminishing the affinity of MHC I-related chain A (MICA) for the NKG2D receptor [173] or by acting as an inhibitory ligand of the NKp30 receptor [174]. These glycan-lectin interactions represent potential novel targets to enhance NK functionality during HIV infection to either cure HIV or prevent immune dysfunction and the subsequent development of immune dysfunction associated diseases such as AIDS-defining and AIDS-non-defining cancers.
B cells.
B-cells are crucial for the humoral response during HIV infection. Subsets of B-cells have been described to be altered in HIV chronic infection, including exhausted tissue-like memory B-cells [175]. This exhausted phenotype has been associated with an increased expression of B-cell-inhibitory glycan receptors, including siglec-2 and siglec-6 [176, 175]. Consistently, knock-down siglec-6 in tissue-like memory B-cells restores normal function [176]. The ligands of siglec-6 in this context are not known, but the ligand is probably a sialylated glycan. In addition to siglecs, galectins can play an important role in B cell development and function. Galectin-1 is a pre-B cell receptor ligand that induces receptor clustering, leading to efficient B cell differentiation [177–179]. Recently, it was shown that galectin-9 suppresses B cell receptor signaling [180, 181]. Understating the impact of these interactions on B cell development and function, during HIV infection, could be crucial for the effective development of therapies and vaccines.
Myeloid-derived suppressive cells.
Regulatory myeloid cells, including myeloid-derived suppressive cells (MDSC), expand during chronic infections and have several immunosuppressive activities [182]. Increased levels of MDSC have been associated with HIV disease progression [183]. This MDSC expansion during HIV infection has been shown to promote the differentiation of regulatory T cells and to impair T cell function [183]. One driver of CD11b+ly6G+ MDSC expansion is the galectin-9/Tim3 interaction [184]. A second glycomic change that may augment immune suppression occurs as granulocytic MDSCs induce γδ-T cells to produce galectin-1, thus transforming them into immunosuppressive cells that abrogate protective antitumor immunity. These important roles of galectins in regulating immune responses could have a direct impact on immune functionality during HIV infection; however, they are yet to be studied.
V. The potential role of the gut glycome in regulating the homeostatic relationship between the host and its gut microbiota, during HIV infection
The gastrointestinal (GI) tract plays key roles in HIV pathogenesis and persistence during suppressive ART [185]. HIV infection is associated with changes in gut structure [186] and in a breakdown of the epithelial barrier [187, 188], which may increase permeability to gut microbial products [189]. This microbial translocation is thought to be a major cause of local and systemic immune activation and inflammation, which may further increase HIV replication (resulting in a positive feedback cycle [189–194]) and contribute to the development of non-AIDS co-morbidities [195, 196, 51, 197, 52]. In addition, the loss of cellular immune subpopulations such as Th17 and Th22 reduces mucosal immunity [198, 199]. These cells are crucial in responding to bacterial antigens and play an important role in maintaining gut epithelium integrity. Unfortunately, even with ART, the damage to the epithelial barrier caused by HIV infection is never fully repaired, allowing microbial translocation and inflammation to continue [200–202].
Gut cells are heavily glycosylated, and the intestinal epithelium is covered by a layer of mucus, which differs along the GI tract in composition, organization, and thickness. In addition, glycans expressed on gut epithelial cells have physiological, immunological and functional characteristics as they are in contact with multiple types of environmental antigens. Interestingly, the glycosylation on these cells can adapt in response to environmental stimuli including microbial stimulation [203–205]. The degree of glycosylation in the gut directly impacts the ability to maintain functional and healthy intestines. Furthermore, the availability of host and diet-provided carbohydrates in the GI tract shapes the nature and function of the gut microbiome [206, 207]. Aberrant glycosylation patterns in the gut are strongly associated with chronic inflammation. For example, a unique, inflammation-associated glycome has been described on memory CD4+ T cells in the inflamed colon [208, 209]. In addition, impaired expression of intestinal O-glycans has been observed in patients with ulcerative colitis, and deletion of intestinal core 1 O-glycans caused spontaneous colitis in mice [210].
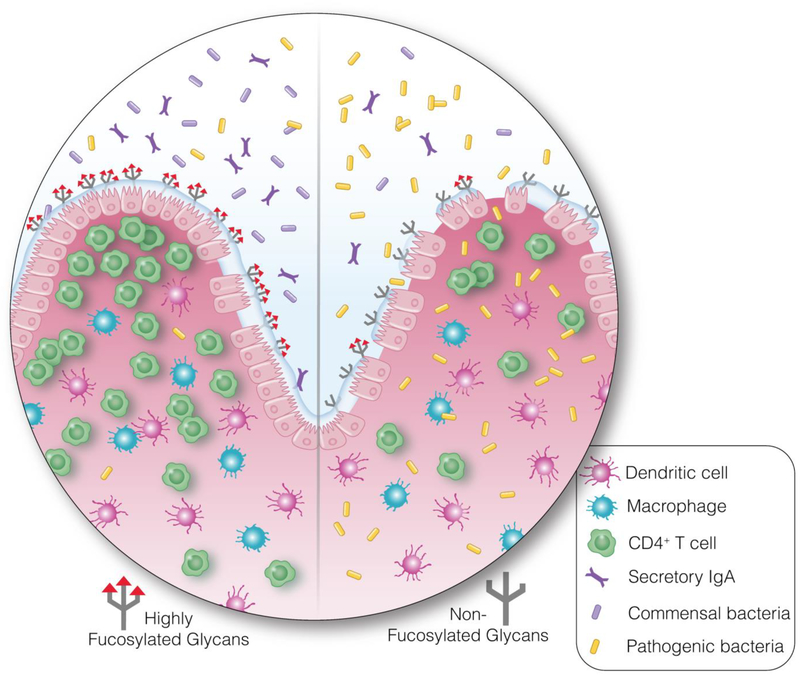
The role of the gut glycome in regulating the homeostatic relationship between the host and its gut microbiota is complex and involves multiple glycan structures. Here we will give one example by illustrating the role of gut fucosylation in the host-microbe interplay [211, 212]. Gut α1,2-fucosylation is induced by the presence of commensal and some pathogenic bacteria and acts as a food source for beneficial gut symbionts [213]. Fucosylated sugar chains are synthesized by fucosyltransferases (FUT) [214, 215]. Bacterial components, such as lipopolysaccharide (LPS), stimulate gut DCs via the TLR–Myd88 pathway [216]. IL-23 produced by gut DCs induces IL-22 production by Type 3 innate lymphoid cells (ILC3s) [203, 217]. IL-22 produced by ILC3s provides activation signals to ECs via the IL-22R–STAT3 pathway, leading to the subsequent induction of FUT2 and α1,2-fucosylation [203]. Fucose is then liberated by microbial fucosidases and becomes available for consumption by the downstream microbiota. Recent reports showed that fucose could enhance the beneficial activity of symbionts and improve colonization resistance against pathogens and pathobionts. In the absence of gut fucosylation, beneficial symbionts are weakened and decreased in abundance, and pathogenic bacteria increase, which leads to microbial translocation, inflammation, and breakdown of the epithelial barrier [211, 212] (Fig. 4). Some pathogenic microorganism can hijack epithelial fucosylation to colonize the host gastric and intestinal epithelial cells; these include Helicobacter pylori, norovirus, and rotavirus [211, 212]. Interestingly, ~20% of humans harbor homozygous loss-of-function mutations for FUT2 [218, 219]. FUT2 mutant humans are more susceptible to several inflammation-related diseases such as Crohn’s disease, Type I diabetes, and psoriasis [220–223, 219, 224, 225]. They also are more susceptible to several infections, including Candida albicans, Streptococcus pneumoniae, and urinary tract infections [226–229]. On the other hand, these individuals are more resistant to Helicobacter pylori, norovirus, rotavirus infections [230–235].
Figure 4. The gut-associated glycome is critical for maintaining a homeostatic relationship between the host and its gut microbiota.
The degree of glycosylation in the gut directly impacts the ability to maintain functional and healthy intestines. Here we give one example, by illustrating the role of gut fucosylation in the host-microbe interplay. Fucosylated glycans in the gut (left) enhance the beneficial activity of symbionts and improve resistance against colonization by pathogens and pathobionts. In the absence of gut fucosylation (right), beneficial symbionts are weakened and decreased in abundance, and pathogenic bacteria increase, which leads to microbial translocation, inflammation, and breakdown of the epithelial barrier. Fucosylated glycans are only one group out of many glycan structures composing the gut glycome. A change in the gut glycome may alter the distribution of microbial species. Therefore, it is possible that alterations in glycan metabolism may contribute to HIV-mediated intestinal damage, microbial translocation, and chronic inflammation.
Fucosylated glycans are only one group out of many glycan structures composing the gut glycome. These collective glycan structures are used as communication tools to shape the relationship between the gut and its microbiota. A change in the gut glycome, possibly induced by HIV infection and associated inflammation, may alter the distribution of microbial species. Therefore, it is possible that alterations in glycan metabolism may contribute to HIV-mediated intestinal damage, microbial translocation, and chronic inflammation. Given the importance of microbial translocation in shaping HIV disease progression, even after suppressive ART, understanding the functions of the large spectrum of glycan structures in the gut could be essential to understanding the forces that shape the microbiota during HIV infection and how to design strategies to manipulate these forces.
VI. Conclusions
The human glycome might hold the key to better understand immunological functions that are central to HIV persistence and immunopathogenesis. More studies are needed at the intersection between glycobiology, immunology, and HIV research, to take advantage of the recent advances in the emerging field of glycoimmunology. Studies to comprehensively investigate the links between host glycomic alterations and inflammation, during HIV infection, may provide novel glycomic-based diagnostic or prognostic biomarkers of HIV-associated inflammaging. These studies may also allow for the development of novel glycan-based interventions to prevent/delay the development of inflammation- and aging-associated diseases during ART-suppressed HIV infection. For example, the information to be obtained from these studies could be used to develop novel strategies to manipulate the forces that shape the gut microbiota during HIV infection and reduce the degree of microbial translocation and associated inflammation. Additional studies will be also needed to investigate the extent to which cell-surface glycans, and their interactions with host lectins, interfere with the function of the immune system, during ART-suppressed HIV infection. These studies could lead to the design of novel immunotherapies to either cure HIV or prevent HIV-associated immune dysfunction. For example, targeting siglec interactions, on NK cells, and galectin interactions, on T cells, may induce the function of these immune cells, during HIV infection.
Importantly, recent advances in the cancer field focusing on glycobiology demonstrated that the aberrant glycosylation pattern of cancer cells alters their interaction with the immune system and allows them to evade immunosurveillance [236–238]. Such advances have promoted an increasing interest in developing tools that can target the tumor “glyco-code” [238]. Recently, a number of glycan-based strategies have been tested as novel cancer immunotherapy agents, e.g., anti-glycan vaccines, glycan lectin interaction blockers, glycan-specific monoclonal antibodies, glycan-coated nanoparticles, and metabolic inhibitors for certain glycans [239–249, 171]. These, and other tools, could be used in the HIV field to lay the groundwork for discovering novel glycan-based interactions that can be targeted for novel strategies to eradicate, functionally cure, or improve tolerance of lifelong HIV infection.
Acknowledgments:
MA-M is funded by the following grants: NIH (R21 AI129636, R21 NS106970, and P30CA010815–49S2), W.W. Smith Charitable Trust (grant # A17101), Campbell Foundation award, Penn Center for AIDS Research (P30 AI 045008), and The Foundation for AIDS Research (amfAR) Impact Grant # 109840–65-RGRL. MA-M is a member of the investigation team of the NIH-funded BEAT-HIV Martin Delaney Collaboratory to cure HIV-1 infection (1UM1Al126620). Additionally, GL is supported by funding from the European Structural and Investments funds for projects “New generation of high throughput glycoanalytical services” (contract #KK.01.2.1.01.0003) and “Croatian National Centre of Research Excellence in Personalized Healthcare” (contract #KK.01.1.1.01.0010). Artwork and graphic design were provided by Marli Markovitz (https://marlimark13.wixsite.com/marlim). Rachel E. Locke, Ph.D. provided critical comments and editing.
Footnotes
COMPETING INTERESTS STATEMENT
GL declares he is a founder and owner of Genos Ltd, biotech company that specializes in glycan analysis and has several patents in the field. IT-A is an employee of Genos Ltd. Other authors have no competing interests.
HUMAN AND ANIMAL RIGHTS AND INFORMED CONSENT
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–5. [DOI] [PubMed] [Google Scholar]
- 2.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Current opinion in HIV and AIDS. 2016;11(5):492–500. doi: 10.1097/COH.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodger AJ, Lodwick R, Schechter M, Deeks S, Amin J, Gilson R et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. Aids. 2013;27(6):973–9. doi: 10.1097/QAD.0b013e32835cae9c. [DOI] [PubMed] [Google Scholar]
- 4.Graf EH, Pace MJ, Peterson BA, Lynch LJ, Chukwulebe SB, Mexas AM et al. Gag-positive reservoir cells are susceptible to HIV-specific cytotoxic T lymphocyte mediated clearance. PLoS One. 2013;8(8):e71879. doi: 10.1371/journal.pone.0071879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.*.Abdel-Mohsen M, Chavez L, Tandon R, Chew GM, Deng X, Danesh A et al. Human Galectin-9 Is a Potent Mediator of HIV Transcription and Reactivation. PLoS Pathog. 2016;12(6):e1005677. doi: 10.1371/journal.ppat.1005677.This paper suggested that host glycan-lectin interactions mediate signals that define HIV transcriptional state, as it demonstrated that the human glycan-binding protein galectin-9 regulates HIV transcription.
- 7.Abdel-Mohsen M, Wang C, Strain MC, Lada SM, Deng X, Cockerham LR et al. Select host restriction factors are associated with HIV persistence during antiretroviral therapy. Aids. 2015;29(4):411–20. doi: 10.1097/QAD.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 9.Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. Aids. 2005;19(9):953–60. [DOI] [PubMed] [Google Scholar]
- 10.Kendall CE, Wong J, Taljaard M, Glazier RH, Hogg W, Younger J et al. A cross-sectional, population-based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health. 2014;14:161. doi: 10.1186/1471-2458-14-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serrano-Villar S, Perez-Elias MJ, Dronda F, Casado JL, Moreno A, Royuela A et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PloS one. 2014;9(1):e85798. doi: 10.1371/journal.pone.0085798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(11):1130–9. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 13.Pumpradit W, Ananworanich J, Lolak S, Shikuma C, Paul R, Siangphoe U et al. Neurocognitive impairment and psychiatric comorbidity in well-controlled human immunodeficiency virus-infected Thais from the 2NN Cohort Study. J Neurovirol. 2010;16(1):76–82. doi: 10.3109/13550280903493914. [DOI] [PubMed] [Google Scholar]
- 14.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(11):1120–6. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 15.Negin J, Martiniuk A, Cumming RG, Naidoo N, Phaswana-Mafuya N, Madurai L et al. Prevalence of HIV and chronic comorbidities among older adults. Aids. 2012;26 Suppl 1:S55–63. doi: 10.1097/QAD.0b013e3283558459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo YC, Chen MY, Sheng WH, Hsieh SM, Sun HY, Liu WC et al. Risk factors for incident diabetes mellitus among HIV-infected patients receiving combination antiretroviral therapy in Taiwan: a case-control study. HIV Med. 2009;10(5):302–9. doi: 10.1111/j.1468-1293.2008.00687.x. [DOI] [PubMed] [Google Scholar]
- 17.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nature reviews Molecular cell biology. 2012;13(7):448–62. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Emerging principles for the therapeutic exploitation of glycosylation. Science. 2014;343(6166):1235681. doi: 10.1126/science.1235681. [DOI] [PubMed] [Google Scholar]
- 19.Marth JD. A unified vision of the building blocks of life. Nat Cell Biol. 2008;10(9):1015–6. doi: 10.1038/ncb0908-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varki A Evolutionary forces shaping the Golgi glycosylation machinery: why cell surface glycans are universal to living cells. Cold Spring Harb Perspect Biol. 2011;3(6). doi: 10.1101/cshperspect.a005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrera C, Espejo R, Reyes VE. Differential glycosylation of MHC class II molecules on gastric epithelial cells: implications in local immune responses. Human immunology. 2002;63(5):384–93. [DOI] [PubMed] [Google Scholar]
- 22.de Freitas Junior JC, Silva Bdu R, de Souza WF, de Araujo WM, Abdelhay ES, Morgado-Diaz JA. Inhibition of N-linked glycosylation by tunicamycin induces E-cadherin-mediated cell-cell adhesion and inhibits cell proliferation in undifferentiated human colon cancer cells. Cancer chemotherapy and pharmacology. 2011;68(1):227–38. doi: 10.1007/s00280-010-1477-8. [DOI] [PubMed] [Google Scholar]
- 23.Dwek RA, Butters TD, Platt FM, Zitzmann N. Targeting glycosylation as a therapeutic approach. Nature reviews Drug discovery. 2002;1(1):65–75. doi: 10.1038/nrd708. [DOI] [PubMed] [Google Scholar]
- 24.Walt D, Aoki-Kinoshita KF, Bendiak B, Bertozzi CR, Boons GJ, Darvill A et al. Transforming Glycoscience: A Roadmap for the Future. Washington: Nacional Academies Press; 2012. [PubMed] [Google Scholar]
- 25.Lanteri M, Giordanengo V, Hiraoka N, Fuzibet JG, Auberger P, Fukuda M et al. Altered T cell surface glycosylation in HIV-1 infection results in increased susceptibility to galectin-1-induced cell death. Glycobiology. 2003;13(12):909–18. doi: 10.1093/glycob/cwg110. [DOI] [PubMed] [Google Scholar]
- 26.Ardman B, Sikorski MA, Settles M, Staunton DE. Human immunodeficiency virus type 1-infected individuals make autoantibodies that bind to CD43 on normal thymic lymphocytes. J Exp Med. 1990;172(4):1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giordanengo V, Limouse M, Desroys du Roure L, Cottalorda J, Doglio A, Passeron A et al. Autoantibodies directed against CD43 molecules with an altered glycosylation status on human immunodeficiency virus type 1 (HIV-1)-infected CEM cells are found in all HIV-1+ individuals. Blood. 1995;86(6):2302–11. [PubMed] [Google Scholar]
- 28.Horiya S, MacPherson IS, Krauss IJ. Recent strategies targeting HIV glycans in vaccine design. Nature chemical biology. 2014;10(12):990–9. doi: 10.1038/nchembio.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward AB, Wilson IA. The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunological reviews. 2017;275(1):21–32. doi: 10.1111/imr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold JN, Saldova R, Galligan MC, Murphy TB, Mimura-Kimura Y, Telford JE et al. Novel glycan biomarkers for the detection of lung cancer. Journal of proteome research. 2011;10(4):1755–64. doi: 10.1021/pr101034t. [DOI] [PubMed] [Google Scholar]
- 31.Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, Evans R, Arnold JN et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007;17(12):1344–56. doi: 10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- 32.Gornik O, Lauc G. Glycosylation of serum proteins in inflammatory diseases. Disease markers. 2008;25(4–5):267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gornik O, Royle L, Harvey DJ, Radcliffe CM, Saldova R, Dwek RA et al. Changes of serum glycans during sepsis and acute pancreatitis. Glycobiology. 2007;17(12):1321–32. doi: 10.1093/glycob/cwm106. [DOI] [PubMed] [Google Scholar]
- 34.Keser T, Gornik I, Vuckovic F, Selak N, Pavic T, Lukic E et al. Increased plasma N-glycome complexity is associated with higher risk of type 2 diabetes. Diabetologia. 2017;60(12):2352–60. doi: 10.1007/s00125-017-4426-9. [DOI] [PubMed] [Google Scholar]
- 35.Knezevic A, Gornik O, Polasek O, Pucic M, Redzic I, Novokmet M et al. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology. 2010;20(8):959–69. doi: 10.1093/glycob/cwq051. [DOI] [PubMed] [Google Scholar]
- 36.Stanta JL, Saldova R, Struwe WB, Byrne JC, Leweke FM, Rothermund M et al. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. Journal of proteome research. 2010;9(9):4476–89. doi: 10.1021/pr1002356. [DOI] [PubMed] [Google Scholar]
- 37.Alonzi DS, Su YH, Butters TD. Urinary glycan markers for disease. Biochem Soc Trans. 2011;39(1):393–8. doi: 10.1042/BST0390393. [DOI] [PubMed] [Google Scholar]
- 38.Gizaw ST, Ohashi T, Tanaka M, Hinou H, Nishimura S. Glycoblotting method allows for rapid and efficient glycome profiling of human Alzheimer’s disease brain, serum and cerebrospinal fluid towards potential biomarker discovery. Biochimica et biophysica acta. 2016;1860(8):1716–27. doi: 10.1016/j.bbagen.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Pucic M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Molecular & cellular proteomics : MCP. 2011;10(10):M111 010090. doi: 10.1074/mcp.M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. The New England journal of medicine. 2014;370(12):1101–10. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 41.Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer research. 2010;70(11):4481–9. doi: 10.1158/0008-5472.CAN-09-3704. [DOI] [PubMed] [Google Scholar]
- 42.Sondermann P, Szymkowski DE. Harnessing Fc receptor biology in the design of therapeutic antibodies. Current opinion in immunology. 2016;40:78–87. doi: 10.1016/j.coi.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Dunn-Walters D, Boursier L, Spencer J. Effect of somatic hypermutation on potential N-glycosylation sites in human immunoglobulin heavy chain variable regions. Molecular immunology. 2000;37(3–4):107–13. [DOI] [PubMed] [Google Scholar]
- 44.Anumula KR. Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J Immunol Methods. 2012;382(1–2):167–76. doi: 10.1016/j.jim.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annual review of immunology. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 46.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69 Suppl 1:S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 47.Nasi M, De Biasi S, Gibellini L, Bianchini E, Pecorini S, Bacca V et al. Ageing and inflammation in patients with HIV infection. Clin Exp Immunol. 2017;187(1):44–52. doi: 10.1111/cei.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Negredo E, Back D, Blanco JR, Blanco J, Erlandson KM, Garolera M et al. Aging in HIV-Infected Subjects: A New Scenario and a New View. BioMed research international. 2017;2017:5897298. doi: 10.1155/2017/5897298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minciullo PL, Catalano A, Mandraffino G, Casciaro M, Crucitti A, Maltese G et al. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch Immunol Ther Exp (Warsz). 2016;64(2):111–26. doi: 10.1007/s00005-015-0377-3. [DOI] [PubMed] [Google Scholar]
- 50.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases. 2011;203(6):780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musselwhite LW, Sheikh V, Norton TD, Rupert A, Porter BO, Penzak SR et al. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. Aids. 2011;25(6):787–95. doi: 10.1097/QAD.0b013e3283453fcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown JB, Conner C, Nichols GA. Secondary failure of metformin monotherapy in clinical practice. Diabetes care. 2010;33(3):501–6. doi: 10.2337/dc09-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burdo TH, Orzechowski K, Knight HL, Miller AD, Williams K. Dorsal root ganglia damage in SIV-infected rhesus macaques: an animal model of HIV-induced sensory neuropathy. The American journal of pathology. 2012;180(4):1362–9. doi: 10.1016/j.ajpath.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. The Journal of infectious diseases. 2013;208(2):249–59. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PloS one. 2010;5(1):e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. The Journal of clinical investigation. 2002;110(8):1133–9. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14(4):195–207. doi: 10.1111/j.1468-1293.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- 60.Borges AH, O’Connor JL, Phillips AN, Ronsholt FF, Pett S, Vjecha MJ et al. Factors Associated With Plasma IL-6 Levels During HIV Infection. The Journal of infectious diseases. 2015;212(4):585–95. doi: 10.1093/infdis/jiv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freiberg MS, Bebu I, Tracy R, So-Armah K, Okulicz J, Ganesan A et al. D-Dimer Levels before HIV Seroconversion Remain Elevated Even after Viral Suppression and Are Associated with an Increased Risk of Non-AIDS Events. PloS one. 2016;11(4):e0152588. doi: 10.1371/journal.pone.0152588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamat A, Misra V, Cassol E, Ancuta P, Yan Z, Li C et al. A plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy. PloS one. 2012;7(2):e30881. doi: 10.1371/journal.pone.0030881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS. 2010;5(6):498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.So-Armah KA, Tate JP, Chang CH, Butt AA, Gerschenson M, Gibert CL et al. Do Biomarkers of Inflammation, Monocyte Activation, and Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? Journal of acquired immune deficiency syndromes. 2016;72(2):206–13. doi: 10.1097/QAI.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320(5874):373–6. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–3. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 67.Washburn N, Schwab I, Ortiz D, Bhatnagar N, Lansing JC, Medeiros A et al. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(11):E1297–306. doi: 10.1073/pnas.1422481112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bohm S, Schwab I, Lux A, Nimmerjahn F. The role of sialic acid as a modulator of the anti-inflammatory activity of IgG. Semin Immunopathol. 2012;34(3):443–53. doi: 10.1007/s00281-012-0308-x. [DOI] [PubMed] [Google Scholar]
- 69.Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(24):9868–72. doi: 10.1073/pnas.1307864110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmed AA, Giddens J, Pincetic A, Lomino JV, Ravetch JV, Wang LX et al. Structural characterization of anti-inflammatory immunoglobulin G Fc proteins. J Mol Biol. 2014;426(18):3166–79. doi: 10.1016/j.jmb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J Mol Biol. 2013;425(8):1253–8. doi: 10.1016/j.jmb.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Annals of the New York Academy of Sciences. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim YH, Min KH, Wang Z, Kim J, Jacobson O, Huang P et al. Development of Sialic Acid-coated Nanoparticles for Targeting Cancer and Efficient Evasion of the Immune System. Theranostics. 2017;7(4):962–73. doi: 10.7150/thno.19061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.*.Spence S, Greene MK, Fay F, Hams E, Saunders SP, Hamid U et al. Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci Transl Med. 2015;7(303):303ra140. doi: 10.1126/scitranslmed.aab3459.This paper highlighted the therapeutic potential of enhancing sialic-acid-siglec anti-inflammatory binding in reducing systemic inflammation.
- 75.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 2010;30 Suppl 1:S9–14. doi: 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 76.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19571–8. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kristic J, Vuckovic F, Menni C, Klaric L, Keser T, Beceheli I et al. Glycans are a novel biomarker of chronological and biological ages. J Gerontol A Biol Sci Med Sci. 2014;69(7):779–89. doi: 10.1093/gerona/glt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu X, Wang Y, Kristic J, Dong J, Chu X, Ge S et al. Profiling IgG N-glycans as potential biomarker of chronological and biological ages: A community-based study in a Han Chinese population. Medicine (Baltimore). 2016;95(28):e4112. doi: 10.1097/MD.0000000000004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trbojevic Akmacic I, Ventham NT, Theodoratou E, Vuckovic F, Kennedy NA, Kristic J et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm Bowel Dis. 2015;21(6):1237–47. doi: 10.1097/MIB.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vučković F, Krištić J, Gudelj I, Artacho MT, Keser T, Pezer M et al. Systemic lupus erythematosus associates with the decreased immunosuppressive potential of the IgG glycome. Arthritis & Rheumatology. 2015;67(11):2978–89. doi: 10.1002/art.39273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vuckovic F, Theodoratou E, Thaci K, Timofeeva M, Vojta A, Stambuk J et al. IgG Glycome in Colorectal Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(12):3078–86. doi: 10.1158/1078-0432.CCR-15-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lemmers RFH, Vilaj M, Urda D, Agakov F, Simurina M, Klaric L et al. IgG glycan patterns are associated with type 2 diabetes in independent European populations. Biochimica et biophysica acta. 2017;1861(9):2240–9. doi: 10.1016/j.bbagen.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 83.An HJ, Lebrilla CB. A glycomics approach to the discovery of potential cancer biomarkers. Methods in molecular biology. 2010;600:199–213. doi: 10.1007/978-1-60761-454-8_14. [DOI] [PubMed] [Google Scholar]
- 84.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 85.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. Journal of the American Heart Association. 2014;3(5):e001221. doi: 10.1161/JAHA.114.001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ercan A, Cui J, Chatterton DE, Deane KD, Hazen MM, Brintnell W et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis and rheumatism. 2010;62(8):2239–48. doi: 10.1002/art.27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rombouts Y, Ewing E, van de Stadt LA, Selman MH, Trouw LA, Deelder AM et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Annals of the rheumatic diseases. 2013. doi: 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- 88.*.Vadrevu SK, Trbojevic-Akmacic I, Kossenkov AV, Colomb F, Giron LB, Anzurez A et al. Frontline Science: Plasma and immunoglobulin G galactosylation associate with HIV persistence during antiretroviral therapy. Journal of leukocyte biology. 2018. doi: 10.1002/JLB.3HI1217-500R.This paper reported that levels of certain plasma and antibody glycomic alterations, in particular, non fucosylated galactosylated glycomic traits, negatively correlate with levels of cell-associated HIV DNA and RNA in ART-suppressed individuals. These glycomic traits have been previously associated with higher plasma-mediated innate immune functions (ADCC/ADCP).
- 89.Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z et al. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. Aids. 2005;19(4):381–9. [DOI] [PubMed] [Google Scholar]
- 90.Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nature medicine. 2012;18(9):1401–6. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Banda NK, Wood AK, Takahashi K, Levitt B, Rudd PM, Royle L et al. Initiation of the alternative pathway of murine complement by immune complexes is dependent on N-glycans in IgG antibodies. Arthritis and rheumatism. 2008;58(10):3081–9. doi: 10.1002/art.23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nature medicine. 1995;1(3):237–43. [DOI] [PubMed] [Google Scholar]
- 93.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452–7. [DOI] [PubMed] [Google Scholar]
- 94.Tomana M, Schrohenloher RE, Koopman WJ, Alarcon GS, Paul WA. Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis and rheumatism. 1988;31(3):333–8. [DOI] [PubMed] [Google Scholar]
- 95.Oswald DM, Sim ES, Baker C, Farhan O, Debanne SM, Morris NJ et al. Plasma glycomics predict cardiovascular disease in patients with ART-controlled HIV infections. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2018:fj201800923R. doi: 10.1096/fj.201800923R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M Jr., Carville A et al. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS pathogens. 2012;8(8):e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. Journal of immunology. 1996;157(5):2168–73. [PubMed] [Google Scholar]
- 98.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nature communications. 2016;7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chung AW, Navis M, Isitman G, Wren L, Silvers J, Amin J et al. Activation of NK cells by ADCC antibodies and HIV disease progression. Journal of acquired immune deficiency syndromes. 2011;58(2):127–31. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Isitman G, Lisovsky I, Tremblay-McLean A, Kovacs C, Harris M, Routy JP et al. Antibody-Dependent Cellular Cytotoxicity Activity of Effector Cells from HIV-Infected Elite and Viral Controllers. AIDS research and human retroviruses. 2016;32(10–11):1079–88. doi: 10.1089/AID.2016.0157. [DOI] [PubMed] [Google Scholar]
- 101.Lee WS, Kent SJ. Anti-HIV-1 antibody-dependent cellular cytotoxicity: is there more to antibodies than neutralization? Curr Opin HIV AIDS. 2018;13(2):160–6. doi: 10.1097/COH.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 102.Madhavi V, Ana-Sosa-Batiz FE, Jegaskanda S, Center RJ, Winnall WR, Parsons MS et al. Antibody-dependent effector functions against HIV decline in subjects receiving antiretroviral therapy. The Journal of infectious diseases. 2015;211(4):529–38. doi: 10.1093/infdis/jiu486. [DOI] [PubMed] [Google Scholar]
- 103.Halper-Stromberg A, Nussenzweig MC. Towards HIV-1 remission: potential roles for broadly neutralizing antibodies. J Clin Invest. 2016;126(2):415–23. doi: 10.1172/JCI80561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhiman JN, Lynch RM. Broadly Neutralizing Antibodies as Treatment: Effects on Virus and Immune System. Curr HIV/AIDS Rep. 2017;14(2):54–62. doi: 10.1007/s11904-017-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Masuda K, Kubota T, Kaneko E, Iida S, Wakitani M, Kobayashi-Natsume Y et al. Enhanced binding affinity for FcgammaRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Molecular immunology. 2007;44(12):3122–31. doi: 10.1016/j.molimm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 106.Thomann M, Reckermann K, Reusch D, Prasser J, Tejada ML. Fc-galactosylation modulates antibody-dependent cellular cytotoxicity of therapeutic antibodies. Molecular immunology. 2016;73:69–75. doi: 10.1016/j.molimm.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 107.Heyl KA, Karsten CM, Slevogt H. Galectin-3 binds highly galactosylated IgG1 and is crucial for the IgG1 complex mediated inhibition of C5aReceptor induced immune responses. Biochemical and biophysical research communications. 2016;479(1):86–90. doi: 10.1016/j.bbrc.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 108.Chung AW, Crispin M, Pritchard L, Robinson H, Gorny MK, Yu X et al. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. Aids. 2014;28(17):2523–30. doi: 10.1097/QAD.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. The Journal of biological chemistry. 2003;278(5):3466–73. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 110.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123(5):2183–92. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Pond SLK et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530(7588):51–6. doi: 10.1038/nature16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rosenbloom DIS, Hill AL, Laskey SB, Siliciano RF. Re-evaluating evolution in the HIV reservoir. Nature. 2017;551(7681):E6–E9. doi: 10.1038/nature24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Panico M, Bouche L, Binet D, O’Connor MJ, Rahman D, Pang PC et al. Mapping the complete glycoproteome of virion-derived HIV-1 gp120 provides insights into broadly neutralizing antibody binding. Scientific reports. 2016;6:32956. doi: 10.1038/srep32956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Puryear WB, Yu X, Ramirez NP, Reinhard BM, Gummuluru S. HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc Natl Acad Sci U S A. 2012;109(19):7475–80. doi: 10.1073/pnas.1201104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100(5):587–97. [DOI] [PubMed] [Google Scholar]
- 116.Rappocciolo G, Piazza P, Fuller CL, Reinhart TA, Watkins SC, Rowe DT et al. DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS Pathog. 2006;2(7):e70. doi: 10.1371/journal.ppat.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW et al. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3(10):975–83. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 118.de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13(3):367–71. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 119.Mayr L, Su B, Moog C. Langerhans Cells: the ‘Yin and Yang’ of HIV Restriction and Transmission. Trends Microbiol. 2017;25(3):170–2. doi: 10.1016/j.tim.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 120.Pena-Cruz V, Agosto LM, Akiyama H, Olson A, Moreau Y, Larrieux JR et al. HIV-1 replicates and persists in vaginal epithelial dendritic cells. J Clin Invest. 2018;128(8):3439–44. doi: 10.1172/JCI98943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saifuddin M, Hart ML, Gewurz H, Zhang Y, Spear GT. Interaction of mannose-binding lectin with primary isolates of human immunodeficiency virus type 1. J Gen Virol. 2000;81(Pt 4):949–55. doi: 10.1099/0022-1317-81-4-949. [DOI] [PubMed] [Google Scholar]
- 122.Ying H, Ji X, Hart ML, Gupta K, Saifuddin M, Zariffard MR et al. Interaction of mannose-binding lectin with HIV type 1 is sufficient for virus opsonization but not neutralization. AIDS Res Hum Retroviruses. 2004;20(3):327–35. doi: 10.1089/088922204322996563. [DOI] [PubMed] [Google Scholar]
- 123.Dzwonek A, Novelli V, Bajaj-Elliott M, Turner M, Clapson M, Klein N. Mannose-binding lectin in susceptibility and progression of HIV-1 infection in children. Antivir Ther. 2006;11(4):499–505. [PubMed] [Google Scholar]
- 124.Zinyama-Gutsire RB, Chasela C, Madsen HO, Rusakaniko S, Kallestrup P, Christiansen M et al. Role of mannose-binding lectin deficiency in HIV-1 and schistosoma infections in a rural adult population in Zimbabwe. PLoS One. 2015;10(4):e0122659. doi: 10.1371/journal.pone.0122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vallinoto AC, Freitas FB, Guirelli I, Machado LF, Azevedo VN, Cayres-Vallinoto I et al. Characterization of mannose-binding lectin plasma levels and genetic polymorphisms in HIV-1-infected individuals. Rev Soc Bras Med Trop. 2011;44(1):1–3. [DOI] [PubMed] [Google Scholar]
- 126.Mehta-D’souza P, Klopocki AG, Oganesyan V, Terzyan S, Mather T, Li Z et al. Glycan Bound to the Selectin Low Affinity State Engages Glu-88 to Stabilize the High Affinity State under Force. J Biol Chem. 2017;292(6):2510–8. doi: 10.1074/jbc.M116.767186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lambert AA, Gilbert C, Richard M, Beaulieu AD, Tremblay MJ. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans-and cis-infection pathways. Blood. 2008;112(4):1299–307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pohlmann S, Soilleux EJ, Baribaud F, Leslie GJ, Morris LS, Trowsdale J et al. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci U S A. 2001;98(5):2670–5. doi: 10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Madsen J, Gaiha GD, Palaniyar N, Dong T, Mitchell DA, Clark HW. Surfactant Protein D modulates HIV infection of both T-cells and dendritic cells. PLoS One. 2013;8(3):e59047. doi: 10.1371/journal.pone.0059047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lambert AA, Imbeault M, Gilbert C, Tremblay MJ. HIV-1 induces DCIR expression in CD4+ T cells. PLoS Pathog. 2010;6(11):e1001188. doi: 10.1371/journal.ppat.1001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pandit H, Gopal S, Sonawani A, Yadav AK, Qaseem AS, Warke H et al. Surfactant protein D inhibits HIV-1 infection of target cells via interference with gp120-CD4 interaction and modulates pro-inflammatory cytokine production. PLoS One. 2014;9(7):e102395. doi: 10.1371/journal.pone.0102395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E et al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012;10(12):e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zou Z, Chastain A, Moir S, Ford J, Trandem K, Martinelli E et al. Siglecs facilitate HIV-1 infection of macrophages through adhesion with viral sialic acids. PLoS One. 2011;6(9):e24559. doi: 10.1371/journal.pone.0024559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martinez-Picado J, McLaren PJ, Erkizia I, Martin MP, Benet S, Rotger M et al. Identification of Siglec-1 null individuals infected with HIV-1. Nat Commun. 2016;7:12412. doi: 10.1038/ncomms12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Varchetta S, Lusso P, Hudspeth K, Mikulak J, Mele D, Paolucci S et al. Sialic acid-binding Ig-like lectin-7 interacts with HIV-1 gp120 and facilitates infection of CD4pos T cells and macrophages. Retrovirology. 2013;10:154. doi: 10.1186/1742-4690-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ouellet M, Mercier S, Pelletier I, Bounou S, Roy J, Hirabayashi J et al. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J Immunol. 2005;174(7):4120–6. [DOI] [PubMed] [Google Scholar]
- 137.Mendez-Huergo SP, Blidner AG, Rabinovich GA. Galectins: emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr Opin Immunol. 2017;45:8–15. doi: 10.1016/j.coi.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 138.Zhuo Y, Bellis SL. Emerging role of alpha2,6-sialic acid as a negative regulator of galectin binding and function. The Journal of biological chemistry. 2011;286(8):5935–41. doi: 10.1074/jbc.R110.191429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269(33):20807–10. [PubMed] [Google Scholar]
- 140.Gordon-Alonso M, Hirsch T, Wildmann C, van der Bruggen P. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nature communications. 2017;8(1):793. doi: 10.1038/s41467-017-00925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.**.Smith LK, Boukhaled GM, Condotta SA, Mazouz S, Guthmiller JJ, Vijay R et al. Interleukin-10 Directly Inhibits CD8(+) T Cell Function by Enhancing N-Glycan Branching to Decrease Antigen Sensitivity. Immunity. 2018;48(2):299–312 e5. doi: 10.1016/j.immuni.2018.01.006.This paper showed that, during chronic viral infection, IL-10 promotes the production of highly branched N-glycans on the surface of CD8 T cells. These N-glycans bind to galectins, and this binding significantly impairs T-cell function and promotes viral persistence.
- 142.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475(7354):110–3. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Byrne B, Donohoe GG, O’Kennedy R. Sialic acids: carbohydrate moieties that influence the biological and physical properties of biopharmaceutical proteins and living cells. Drug discovery today. 2007;12(7–8):319–26. doi: 10.1016/j.drudis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 144.Jandus C, Simon HU, von Gunten S. Targeting siglecs--a novel pharmacological strategy for immuno-and glycotherapy. Biochemical pharmacology. 2011;82(4):323–32. doi: 10.1016/j.bcp.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 145.Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annual review of immunology. 2012;30:357–92. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rabinovich GA, Rubinstein N, Toscano MA. Role of galectins in inflammatory and immunomodulatory processes. Biochimica et biophysica acta. 2002;1572(2–3):274–84. [DOI] [PubMed] [Google Scholar]
- 147.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nature immunology. 2007;8(8):825–34. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 148.Farzi B, Young D, Scrimgeour J, Cetinkaya C. Mechanical properties of P-selectin PSGL-1 bonds. Colloids and surfaces B, Biointerfaces. 2018;173:529–38. doi: 10.1016/j.colsurfb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 149.Ley K The role of selectins in inflammation and disease. Trends Mol Med. 2003;9(6):263–8. [DOI] [PubMed] [Google Scholar]
- 150.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nature reviews Immunology. 2004;4(5):325–35. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 151.Catalfamo M, Wilhelm C, Tcheung L, Proschan M, Friesen T, Park JH et al. CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. Journal of immunology. 2011;186(4):2106–16. doi: 10.4049/jimmunol.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chavez L, Calvanese V, Verdin E. HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells. PLoS pathogens. 2015;11(6):e1004955. doi: 10.1371/journal.ppat.1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505(7484):509–14. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Freeman ML, Mudd JC, Shive CL, Younes SA, Panigrahi S, Sieg SF et al. CD8 T-Cell Expansion and Inflammation Linked to CMV Coinfection in ART-treated HIV Infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(3):392–6. doi: 10.1093/cid/civ840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khaitan A, Unutmaz D. Revisiting immune exhaustion during HIV infection. Curr HIV/AIDS Rep. 2011;8(1):4–11. doi: 10.1007/s11904-010-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kwon KJ, Siliciano RF. HIV persistence: clonal expansion of cells in the latent reservoir. The Journal of clinical investigation. 2017;127(7):2536–8. doi: 10.1172/JCI95329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Murray AJ, Kwon KJ, Farber DL, Siliciano RF. The Latent Reservoir for HIV-1: How Immunologic Memory and Clonal Expansion Contribute to HIV-1 Persistence. Journal of immunology. 2016;197(2):407–17. doi: 10.4049/jimmunol.1600343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tandon R, Chew GM, Byron MM, Borrow P, Niki T, Hirashima M et al. Galectin-9 is rapidly released during acute HIV-1 infection and remains sustained at high levels despite viral suppression even in elite controllers. AIDS Res Hum Retroviruses. 2014;30(7):654–64. doi: 10.1089/AID.2014.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lhuillier C, Barjon C, Niki T, Gelin A, Praz F, Morales O et al. Impact of Exogenous Galectin-9 on Human T Cells: CONTRIBUTION OF THE T CELL RECEPTOR COMPLEX TO ANTIGEN-INDEPENDENT ACTIVATION BUT NOT TO APOPTOSIS INDUCTION. The Journal of biological chemistry. 2015;290(27):16797–811. doi: 10.1074/jbc.M115.661272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Elahi S, Niki T, Hirashima M, Horton H. Galectin-9 binding to Tim-3 renders activated human CD4+ T cells less susceptible to HIV-1 infection. Blood. 2012;119(18):4192–204. doi: 10.1182/blood-2011-11-389585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schaefer K, Webb NE, Pang M, Hernandez-Davies JE, Lee KP, Gonzalez P et al. Galectin-9 binds to O-glycans on protein disulfide isomerase. Glycobiology. 2017;27(9):878–87. doi: 10.1093/glycob/cwx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. 2014;41(2):270–82. doi: 10.1016/j.immuni.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T et al. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol Res. 2015;3(4):412–23. doi: 10.1158/2326-6066.CIR-14-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.*.Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J et al. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer Cell. 2018;33(2):187–201 e10. doi: 10.1016/j.ccell.2018.01.009.This paper showed that the glycosylation of PD-L1 plays a critical role in immunosuppression by enhancing interactions with PD-1, and that targeting the glycosylation of immune inhibitory receptors is an effective strategy to promote the function of the immune system.
- 165.Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nature communications. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.*.Okada M, Chikuma S, Kondo T, Hibino S, Machiyama H, Yokosuka T et al. Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell reports. 2017;20(5):1017–28. doi: 10.1016/j.celrep.2017.07.027.This paper showed that loss of core fucosylation reduces cell-surface expression of PD-1 and enhances both T cell activation and T cell anti-tumor immune responses.
- 167.Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2009;265(1):29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bostik P, Takahashi Y, Mayne AE, Ansari AA. Innate immune natural killer cells and their role in HIV and SIV infection. HIV Ther. 2010;4(4):483–504. doi: 10.2217/HIV.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E et al. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood. 2009;114(18):3822–30. doi: 10.1182/blood-2009-06-226332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nature chemical biology. 2014;10(1):69–75. doi: 10.1038/nchembio.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.*.Xiao H, Woods EC, Vukojicic P, Bertozzi CR. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci U S A. 2016;113(37):10304–9. doi: 10.1073/pnas.1608069113.This paper showed that anti-HER2-sialidase conjugate reduces the inhibitory interaction between Sialic-acid and Siglec, and activate anti-tumor immunity. These results highlight the promising value of glycocalyx editing in enhancing immunological responses.
- 172.Golden-Mason L, McMahan RH, Strong M, Reisdorph R, Mahaffey S, Palmer BE et al. Galectin-9 functionally impairs natural killer cells in humans and mice. J Virol. 2013;87(9):4835–45. doi: 10.1128/JVI.01085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tsuboi S, Sutoh M, Hatakeyama S, Hiraoka N, Habuchi T, Horikawa Y et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. The EMBO journal. 2011;30(15):3173–85. doi: 10.1038/emboj.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang W, Guo H, Geng J, Zheng X, Wei H, Sun R et al. Tumor-released Galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in tumor escape from NK cell attack. The Journal of biological chemistry. 2014;289(48):33311–9. doi: 10.1074/jbc.M114.603464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205(8):1797–805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kardava L, Moir S, Wang W, Ho J, Buckner CM, Posada JG et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest. 2011;121(7):2614–24. doi: 10.1172/JCI45685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bonzi J, Bornet O, Betzi S, Kasper BT, Mahal LK, Mancini SJ et al. Pre-B cell receptor binding to galectin-1 modifies galectin-1/carbohydrate affinity to modulate specific galectin-1/glycan lattice interactions. Nat Commun. 2015;6:6194. doi: 10.1038/ncomms7194. [DOI] [PubMed] [Google Scholar]
- 178.Elantak L, Espeli M, Boned A, Bornet O, Bonzi J, Gauthier L et al. Structural basis for galectin-1-dependent pre-B cell receptor (pre-BCR) activation. J Biol Chem. 2012;287(53):44703–13. doi: 10.1074/jbc.M112.395152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci U S A. 2002;99(20):13014–9. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cao A, Alluqmani N, Buhari FHM, Wasim L, Smith LK, Quaile AT et al. Galectin-9 binds IgM BCR to regulate B cell signaling. Nature communications. 2018;9(1):3288. doi: 10.1038/s41467-018-05771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Giovannone N, Liang J, Antonopoulos A, Geddes Sweeney J, King SL, Pochebit SM et al. Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans. Nat Commun. 2018;9(1):3287. doi: 10.1038/s41467-018-05770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Macatangay BJ, Landay AL, Rinaldo CR. MDSC: a new player in HIV immunopathogenesis. AIDS. 2012;26(12):1567–9. doi: 10.1097/QAD.0b013e328355e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR et al. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS. 2012;26(12):F31–7. doi: 10.1097/QAD.0b013e328354b43f. [DOI] [PubMed] [Google Scholar]
- 184.Zhang Y, Zhang M, Li X, Tang Z, He L, Lv K. Expansion of CD11b(+)Ly-6C(+) myeloid-derived suppressor cells (MDSCs) driven by galectin-9 attenuates CVB3-induced myocarditis. Mol Immunol. 2017;83:62–71. doi: 10.1016/j.molimm.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 185.Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362(6418):355–8. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 186.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Niessen CM. Tight junctions/adherens junctions: basic structure and function. The Journal of investigative dermatology. 2007;127(11):2525–32. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 188.Turner JR. Intestinal mucosal barrier function in health and disease. Nature reviews Immunology. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 189.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 190.Chan W, Sviridov D, Dart AM. HIV, atherosclerosis and inflammation: implications for treatment. Journal of HIV therapy. 2009;14(3):61–8. [PubMed] [Google Scholar]
- 191.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Topics in HIV medicine : a publication of the International AIDS Society, USA. 2009;17(4):118–23. [PubMed] [Google Scholar]
- 192.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunological reviews. 2013;254(1):326–42. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Smith D HIV as an inflammatory disease. Journal of HIV therapy. 2009;14(3):50–1. [PubMed] [Google Scholar]
- 194.Vyboh K, Jenabian MA, Mehraj V, Routy JP. HIV and the gut microbiota, partners in crime: breaking the vicious cycle to unearth new therapeutic targets. Journal of immunology research. 2015;2015:614127. doi: 10.1155/2015/614127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annual review of immunology. 2012;30:149–73. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 197.Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. The Journal of infectious diseases. 2009;200(6):973–83. doi: 10.1086/605447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McKinnon LR, Nyanga B, Kim CJ, Izulla P, Kwatampora J, Kimani M et al. Early HIV-1 infection is associated with reduced frequencies of cervical Th17 cells. Journal of acquired immune deficiency syndromes. 2015;68(1):6–12. doi: 10.1097/QAI.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 199.Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS pathogens. 2014;10(12):e1004543. doi: 10.1371/journal.ppat.1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200(6):761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1(1):23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1(6):475–88. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 203.Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345(6202):1254009. doi: 10.1126/science.1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pham TA, Clare S, Goulding D, Arasteh JM, Stares MD, Browne HP et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe. 2014;16(4):504–16. doi: 10.1016/j.chom.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Terahara K, Nochi T, Yoshida M, Takahashi Y, Goto Y, Hatai H et al. Distinct fucosylation of M cells and epithelial cells by Fut1 and Fut2, respectively, in response to intestinal environmental stress. Biochemical and biophysical research communications. 2011;404(3):822–8. doi: 10.1016/j.bbrc.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 206.Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell host & microbe. 2011;10(5):507–14. doi: 10.1016/j.chom.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bergstrom K, Fu J, Johansson ME, Liu X, Gao N, Wu Q et al. Core 1-and 3-derived O-glycans collectively maintain the colonic mucus barrier and protect against spontaneous colitis in mice. Mucosal immunology. 2016. doi: 10.1038/mi.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nishida A, Nagahama K, Imaeda H, Ogawa A, Lau CW, Kobayashi T et al. Inducible colitis-associated glycome capable of stimulating the proliferation of memory CD4+ T cells. The Journal of experimental medicine. 2012;209(13):2383–94. doi: 10.1084/jem.20112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Srikrishna G, Turovskaya O, Shaikh R, Newlin R, Foell D, Murch S et al. Carboxylated glycans mediate colitis through activation of NF-kappa B. Journal of immunology. 2005;175(8):5412–22. [DOI] [PubMed] [Google Scholar]
- 210.Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. The Journal of clinical investigation. 2011;121(4):1657–66. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Goto Y, Uematsu S, Kiyono H. Epithelial glycosylation in gut homeostasis and inflammation. Nat Immunol. 2016;17(11):1244–51. doi: 10.1038/ni.3587. [DOI] [PubMed] [Google Scholar]
- 212.Pickard JM, Chervonsky AV. Intestinal fucose as a mediator of host-microbe symbiosis. J Immunol. 2015;194(12):5588–93. doi: 10.4049/jimmunol.1500395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Comstock LE, Kasper DL. Bacterial glycans: key mediators of diverse host immune responses. Cell. 2006;126(5):847–50. doi: 10.1016/j.cell.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 214.Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13(7):41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 215.Domino SE, Hiraiwa N, Lowe JB. Molecular cloning, chromosomal assignment and tissue-specific expression of a murine alpha(1,2)fucosyltransferase expressed in thymic and epididymal epithelial cells. The Biochemical journal. 1997;327 (Pt 1):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. Endotoxin-induced maturation of MyD88-deficient dendritic cells. Journal of immunology. 2001;166(9):5688–94. [DOI] [PubMed] [Google Scholar]
- 217.Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 2014;514(7524):638–41. doi: 10.1038/nature13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270(9):4640–9. [DOI] [PubMed] [Google Scholar]
- 219.Koda Y, Soejima M, Liu Y, Kimura H. Molecular basis for secretor type alpha(1,2)-fucosyltransferase gene deficiency in a Japanese population: a fusion gene generated by unequal crossover responsible for the enzyme deficiency. Am J Hum Genet. 1996;59(2):343–50. [PMC free article] [PubMed] [Google Scholar]
- 220.Aghdassi AA, Weiss FU, Mayerle J, Lerch MM, Simon P. Genetic susceptibility factors for alcohol-induced chronic pancreatitis. Pancreatology. 2015;15(4 Suppl):S23–31. doi: 10.1016/j.pan.2015.05.476. [DOI] [PubMed] [Google Scholar]
- 221.Folseraas T, Melum E, Rausch P, Juran BD, Ellinghaus E, Shiryaev A et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J Hepatol. 2012;57(2):366–75. doi: 10.1016/j.jhep.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42(12):1118–25. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ishitoya S, Yamamoto S, Mitsumori K, Ogawa O, Terai A. Non-secretor status is associated with female acute uncomplicated pyelonephritis. BJU Int. 2002;89(9):851–4. [DOI] [PubMed] [Google Scholar]
- 224.Svensson L, Petersson A, Henry SM. Secretor genotyping for A385T, G428A, C571T, C628T, 685delTGG, G849A, and other mutations from a single PCR. Transfusion. 2000;40(7):856–60. [DOI] [PubMed] [Google Scholar]
- 225.Yu LC, Yang YH, Broadberry RE, Chen YH, Chan YS, Lin M. Correlation of a missense mutation in the human Secretor alpha 1,2-fucosyltransferase gene with the Lewis(a+b+) phenotype: a potential molecular basis for the weak Secretor allele (Sew). Biochem J. 1995;312 (Pt 2):329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Blackwell CC, Jonsdottir K, Hanson M, Todd WT, Chaudhuri AK, Mathew B et al. Non-secretion of ABO antigens predisposing to infection by Neisseria meningitidis and Streptococcus pneumoniae. Lancet. 1986;2(8501):284–5. [DOI] [PubMed] [Google Scholar]
- 227.Blackwell CC, Jonsdottir K, Hanson MF, Weir DM. Non-secretion of ABO blood group antigens predisposing to infection by Haemophilus influenzae. Lancet. 1986;2(8508):687. [DOI] [PubMed] [Google Scholar]
- 228.Magalhaes A, Gomes J, Ismail MN, Haslam SM, Mendes N, Osorio H et al. Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa. Glycobiology. 2009;19(12):1525–36. doi: 10.1093/glycob/cwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Magalhaes A, Reis CA. Helicobacter pylori adhesion to gastric epithelial cells is mediated by glycan receptors. Braz J Med Biol Res. 2010;43(7):611–8. [DOI] [PubMed] [Google Scholar]
- 230.Azevedo M, Eriksson S, Mendes N, Serpa J, Figueiredo C, Resende LP et al. Infection by Helicobacter pylori expressing the BabA adhesin is influenced by the secretor phenotype. J Pathol. 2008;215(3):308–16. doi: 10.1002/path.2363. [DOI] [PubMed] [Google Scholar]
- 231.Boren T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262(5141):1892–5. [DOI] [PubMed] [Google Scholar]
- 232.Lewis ZT, Totten SM, Smilowitz JT, Popovic M, Parker E, Lemay DG et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome. 2015;3:13. doi: 10.1186/s40168-015-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L et al. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9(5):548–53. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 234.Rayes A, Morrow AL, Payton LR, Lake KE, Lane A, Davies SM. A Genetic Modifier of the Gut Microbiome Influences the Risk of Graft-versus-Host Disease and Bacteremia After Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(3):418–22. doi: 10.1016/j.bbmt.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 235.Smilowitz JT, O’Sullivan A, Barile D, German JB, Lonnerdal B, Slupsky CM. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr. 2013;143(11):1709–18. doi: 10.3945/jn.113.178772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nature reviews Cancer. 2015;15(9):540–55. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 237.Gubbels JA, Felder M, Horibata S, Belisle JA, Kapur A, Holden H et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer. 2010;9:11. doi: 10.1186/1476-4598-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.RodrIguez E, Schetters STT, van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol. 2018;18(3):204–11. doi: 10.1038/nri.2018.3. [DOI] [PubMed] [Google Scholar]
- 239.Danishefsky SJ, Shue YK, Chang MN, Wong CH. Development of Globo-H cancer vaccine. Accounts of chemical research. 2015;48(3):643–52. doi: 10.1021/ar5004187. [DOI] [PubMed] [Google Scholar]
- 240.Huang YL, Hung JT, Cheung SK, Lee HY, Chu KC, Li ST et al. Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(7):2517–22. doi: 10.1073/pnas.1222649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bull C, Boltje TJ, Wassink M, de Graaf AM, van Delft FL, den Brok MH et al. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol Cancer Ther. 2013;12(10):1935–46. doi: 10.1158/1535-7163.MCT-13-0279. [DOI] [PubMed] [Google Scholar]
- 242.Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4(6):477–88. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 243.Jennewein MF, Alter G. The Immunoregulatory Roles of Antibody Glycosylation. Trends Immunol. 2017;38(5):358–72. doi: 10.1016/j.it.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 244.Lisacek F, Mariethoz J, Alocci D, Rudd PM, Abrahams JL, Campbell MP et al. Databases and Associated Tools for Glycomics and Glycoproteomics. Methods Mol Biol. 2017;1503:235–64. doi: 10.1007/978-1-4939-6493-2_18. [DOI] [PubMed] [Google Scholar]
- 245.Miles D, Papazisis K. Rationale for the clinical development of STn-KLH (Theratope) and anti-MUC-1 vaccines in breast cancer. Clin Breast Cancer. 2003;3 Suppl 4:S134–8. [DOI] [PubMed] [Google Scholar]
- 246.Miles D, Roche H, Martin M, Perren TJ, Cameron DA, Glaspy J et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 2011;16(8):1092–100. doi: 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Posey AD Jr., Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 2016;44(6):1444–54. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7(10):790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 249.Unger WW, Mayer CT, Engels S, Hesse C, Perdicchio M, Puttur F et al. Antigen targeting to dendritic cells combined with transient regulatory T cell inhibition results in long-term tumor regression. Oncoimmunology. 2015;4(8):e970462. doi: 10.4161/21624011.2014.970462. [DOI] [PMC free article] [PubMed] [Google Scholar]