Abstract
Background:
Roux-en-Y gastric bypass surgery (RYGB) increases the rate of alcohol absorption so that peak blood alcohol concentration is two-fold higher after surgery than those reached after drinking the same amount before surgery. Because high doses of alcohol can lead to hypoglycemia, patients may be at increased risk of developing hypoglycemia after alcohol ingestion.
Objectives:
We conducted two studies to test the hypothesis that the consumption of ~2 standard drinks of alcohol would decrease glycemia more after RYGB than before surgery.
Setting:
Single-center prospective randomized trial.
Methods:
We evaluated plasma glucose concentrations and glucose kinetics (assessed by infusing a stable isotopically labelled glucose tracer) after ingestion of a non-alcoholic (placebo) or an alcoholic drink in: i) 5 women before (body mass index (BMI) = 43±5 kg/m2) and 10±2 months after RYGB surgery (BMI=31±7 kg/m2; Study 1), and ii) 8 women who had RYGB surgery 2.2±1.2 years earlier (BMI=30±5 kg/m2; Study 2)
Results:
Compared with the placebo drink, alcohol ingestion decreased plasma glucose both before and after surgery, but the reduction was greater before (glucose nadir placebo= −0.4±1.0 mg/dl vs. alcohol=−9.6±1.5 mg/dl) than after (glucose nadir placebo=−1.0±1.6 mg/dl vs. alcohol =−5.5±2.6 mg/dl; P < .001) surgery. This difference was primarily due to an alcohol-induced early increase followed by a subsequent decrease in glucose rate of appearance into the systemic circulation.
Conclusion:
RYGB does not increase the risk of hypoglycemia after consumption of a moderate dose of alcohol.
Keywords: bariatric surgery, metabolic surgery, ethanol, alcohol, glycemia, hypoglycaemia, glucose, glucose kinetics
Introduction
Roux-en-Y-gastric bypass (RYGB) is one of the most effective and widely used procedures to treat severe obesity [1] and its related comorbidities [2, 3]. Although, RYGB has marked beneficial effects on glycemic control, a subset of patients experience clinically significant postprandial hypoglycemia [4–8]
Alcohol is a dietary factor that can increase risk of hypoglycemic events, because alcohol consumption inhibits gluconeogenesis [9, 10], which can lead to hypoglycemia [11]. Our group and others have recently demonstrated that RYGB has marked effects on the pharmacokinetics of ingested alcohol [12–14]. As a result, when patients who had RYGB surgery consume two standard drinks, they reach blood alcohol concentrations (BAC) that are similar to those achieved after drinking four or five drinks before surgery [13]. However, the effect of alcohol consumption on glycemia in these patients has not been studied.
Here we tested the hypothesis that consumption of a moderate dose of alcohol would decrease plasma glucose concentrations more after RYGB than before surgery, because of surgery-induced changes in alcohol pharmacokinetics. Consequently, patients who have had RYGB surgery would be more susceptible to experiencing hypoglycemia after consuming a moderate dose of alcohol than people who had not had RYGB surgery.
Methods
Subjects.
Our study design includes both a prospective and a cross-sectional approach. The prospective part of the study involved evaluating five women who were scheduled to have RYGB surgery (RYGB before-after group). The cross-sectional part of the study involved evaluating eight women who had RYGB within the last 1–5 years (RYGB 1–5 yr. group) (Table 1). The study was approved by the Washington University Institutional Review Board, and all subjects provided written informed consent before participation.
Table 1.
Characteristics of the study participants, changes in glucose and BAC
| Mean (SD) | |||
|---|---|---|---|
| Study 1 (n=5) | Study 2 (n=8) | ||
| Characteristics | Before RYGB | After RYGB | RYGB 1–5 yr |
| Age, yr | 44.7 (4.6) | 45.5 (4.7) | 42.5 (8.0) |
| Weight, kg | 109.3 (15.0) | 79.1 (19.1)‡ | 80.8 (14.1) |
| BMI, kg/m2 | 42.9 (4.7) | 31.1 (6.9)‡ | 30.0 (5.2) |
| FFM, kg | 51.4 (5.8) | 46.6 (5.8)†‡ | 49.4 (5.7) |
| Co-morbidities (%)∥ | |||
| Hypertension | 40.0 | 20.0 | 0.0 |
| Hypercholesterolemia | 20.0 | 20.0 | 12.5 |
| Depression | 40.0 | 40.0 | 37.5 |
| Menopause Status (%)∥ | |||
| Pre-menopausal | 80.0 | 80.0 | 62.5 |
| Post-menopausal | 0.0 | 0.0 | 12.5 |
| Uncertain§ | 20.0 | 20.0 | 25.0 |
| Glucose Nadir, mg/dl | |||
| Placebo | −0.4 (1.0) | −1.0 (1.6) | −1.0 (1.0) |
| Alcohol | −9.6 (1.5)† | −5.5 (2.6)†‡ | −6.3 (3.4)† |
| Glucose Peak, mg/dl | |||
| Placebo | 5.6 (3.6) | 4.4 (2.3) | 5.8 (2.5) |
| Alcohol | 2.6 (2.5)† | 7.7 (4.6)† | 10.7 (4.6)† |
| Alcohol-related variables | |||
| Peak BAC (g/L) | 0.58 (0.09) | 1.23 (0.2)‡ | 1.10 (0.17) |
| Time to peak BAC (min)* | 36.1 (15.8) | 15.0 (0.0)‡ | 15.0 (0.0) |
| Area under the BAC-time curve (g·l−1 min) | 99.3 (6.7) | 173.5 (30.5)‡ | 151.2 (7.2) |
SD = standard deviation; RYGB = Roux-en-Y gastric bypass; BMI = Body mass index (calculated as weight in kilograms divided by height in meter squared); FFM = fat free mass. BAC = blood alcohol concentration; % = percentage
From the time of the first sip of the drink, which was ingested over 10 min.
Three patients had hysterectomy. One was on hormone replacement treatment.
The present study is underpowered to detect clinically meaningful difference
Values are represented in means (SD).
different from placebo (P < .01).
different from Before RYGB (P <.05)
Patients were recruited by reviewing their medical records followed by a personal interview conducted at the Bariatric Surgery Clinic at Barnes-Jewish Hospital in St. Louis, MO. We included only women because 81% of the patients undergoing bariatric surgery are women [15], and sex can affect the pharmacokinetics of alcohol [16].
We carried out a comprehensive medical evaluation, including blood tests, physical examination and urine pregnancy test. None of the subjects had a diagnosis of diabetes or were taking medicines to treat diabetes, but several had a history of hypertension, dyslipidemia or depression (Table1). We assessed subject’s alcohol use patterns by using the Alcohol module of the Semi-Structured Assessment for Genetics of Alcoholism (SSAGA) [17], and only regular (i.e., at least one drink per month), low risk drinkers (i.e., no more than 3 standard drinks in a 24 hour period and/or no more than 7 drinks per week [18] in the month before enrolling in the study) were eligible for participation. Those with lifetime alcohol dependence, current regular use of drugs of abuse other than alcohol, or use of any medication that interacts with alcohol pharmacokinetics or pharmacologic effects, were excluded. Additional exclusion criteria were pregnancy, breastfeeding, not using an effective birth control method, using tobacco products within the last six months, anemia, and liver disease. The study is registered with the Clinical Trials.gov identifier: NCT01843257.
Experimental Procedures
The study was conducted in the Clinical Research Unit at Washington University School of Medicine in St. Louis, MO. Using a randomized crossover design, patients were evaluated on two study visits, which took place approximately 1-week apart. During these visits, by using crossover design, patients were randomly assigned to consume either 0.5 g of alcohol per kg of fat-free mass (FFM) or a non-alcoholic beverage (placebo condition) at their first visit. The dose of alcohol was calculated based on each patient’s total FFM because FFM, not body weight, correlates closely with alcohol volume of distribution [19]. Body FFM was measured by using dual-energy X-ray absorptiometry. Patients in the RYGB before-after group repeated the two study visits 10±2 months after RYGB surgery and 28±10% weight loss.
Alcohol and placebo challenge tests
For each study visit, patients were admitted to the Clinical Research Unit after they had fasted for ~12 h overnight at home, and remained fasted during the entire challenge test. Immediately after rechecking pregnancy status by using a urine pregnancy test, two catheters were inserted. One of the catheters was inserted into an antecubital vein for glucose tracer infusion, and the second catheter was inserted into a hand-vein for blood collection to determine plasma glucose concentration, tracer-to-tracee ratio, and BAC. To obtain arterialized blood samples, we heated the patient’s hand to 55°C by using a thermostatically-controlled box [20]. A primed continuous infusion of [6,6–2H2]glucose (priming dose: 22 μmol/kg; infusion rate: 0.22 μmol/kg . min) was started and maintained until the end of the study. After 3.5 hours of tracer infusion, patients ingested a 20% v/v solution of 190 proof ethanol mixed with a fruity flavored juice containing no sugar (Kool-Aid, Kraft Heins Company, Chicago, IL) sweetened with ~1.7 grams of Splenda (Heartland Consumer Products, Carmel IN) (alcohol condition) or a non-alcoholic version of the same drink (placebo condition). The drinks were provided in two equally divided aliquots of ~76 mL (range 72–99 mL) and patients consumed each aliquot within consecutive 5-minute periods. For both conditions, 2 mL of alcohol were sprayed onto the surface of the cup to serve as a flavor mask. Blood samples were obtained immediately before starting the glucose tracer infusion, every 10 min for 30 min just before patients began drink ingestions (time 0), and then at 15, 25, 35, 45, 60, 75, 90, 105, 120, 135, 150, 180 and 210 min after initiating drinking.
Sample analyses
Plasma glucose concentration and glucose kinetics.
Glucose concentration in plasma was measured by using an automated glucose analyzer (YSI 2300 STAT plus; Yellow Spring Instrument Co.), and glucose tracer-to-tracee ratio in plasma was determined by using gas chromatography–mass spectrometry (Agilent Technologies/HP 6890 Series GC System–5973 Mass Selective Detector; Hewlett-Packard) as previously described [21].
Blood Alcohol Concentration.
BAC was measured by using headspace-gas chromatography (Agilent Technologies/HP 6890 with automated-headspace sampler) as previously described [22]. The results of these analyses have been previously reported [13] and are shown in Table 1.
Calculations
We used the Steele’s equation for non-steady-state conditions to calculate substrate kinetics. We calculated delta plasma glucose concentration and delta glucose rate of appearance (Ra) by subtraction of the value at baseline (i.e. the average of samples taken at −30, −20 and −10 min) from the values at each time point for each study visit to obtain peaks and nadirs.
Statistical analysis
The effects of alcohol consumption on time-depending changes in plasma glucose concentrations (mg/dl) and glucose kinetics (i.e., glucose endogenous production or glucose Ra (μmol/kg FFM/min) relative to placebo consumption after RYGB, and the differences between these effects before vs. after RYGB surgery were evaluated by using general linear mixed models (PROC MIXED) and repeated measure ANOVAs. Significant interactions were further analyzed using Fisher least significance difference tests. Summary residuals and fit statistics were examined for marginal and conditional raw and standardized residuals. Data in the table and figures are presented as means ± SD unless otherwise indicated. All analyses were performed with STATISTICA 13.0 (Tibco; Palo Alto, CA) and SAS 9.3 (SAS Institute Inc. Cary, NC) and criterion for statistical significance was P ≤ .05.
Results
Plasma glucose concentration
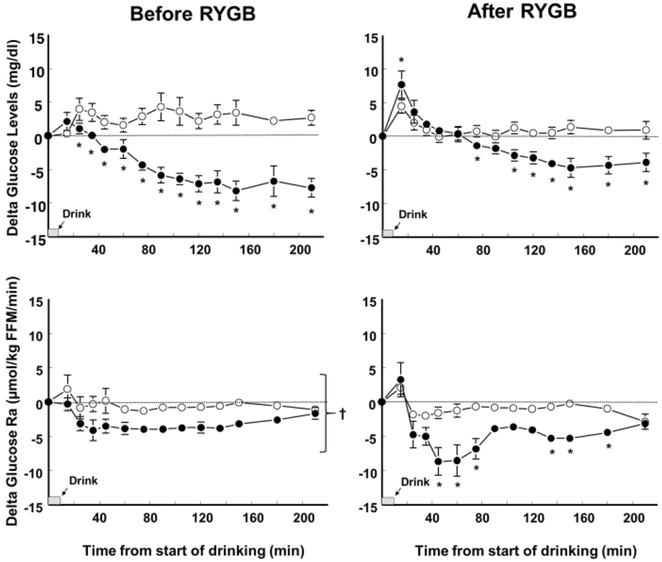
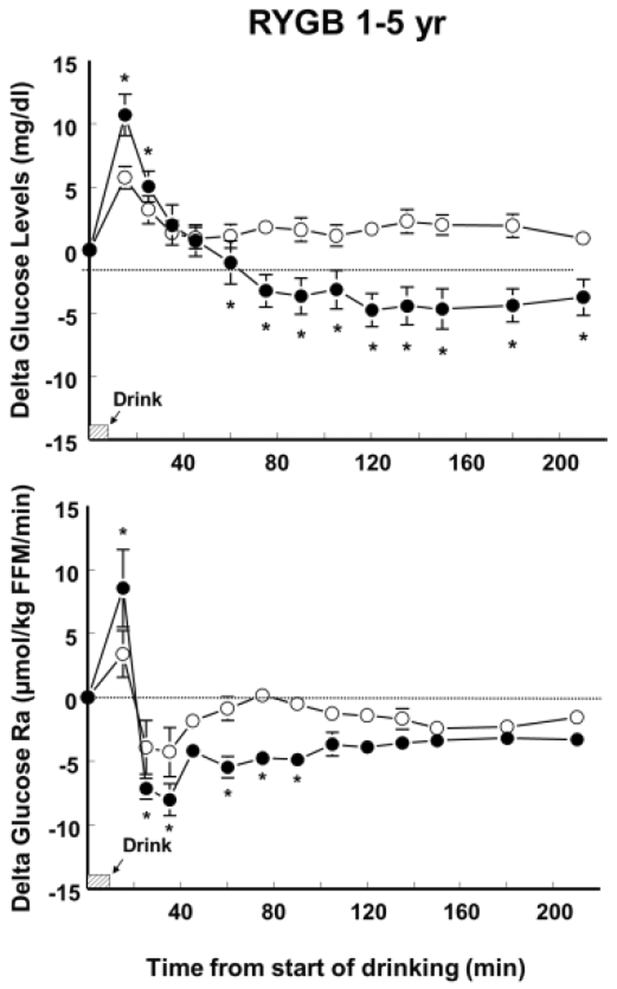
Compared with the consumption of the placebo drink, alcohol consumption decreased plasma glucose concentration both before and after surgery in the RYGB before-after group (F(1,4) = 33.9; P < .005); and in the RYGB 1–5 yr group (F(1,7) = 15.3; P < .01). However, the alcohol-induced reduction of plasma glucose concentration was smaller (F(1,4) = 10.42; P < .05) and glucose nadir was reduced by half (F(1,4) = 33.2; P < .005) after than before surgery (Figure 1 and Table 1). Before surgery, alcohol ingestion reduced plasma glucose concentration from 25 min after the start of drinking until the end of the test at 210 min. However, after surgery, alcohol consumption had a biphasic effect on plasma glucose concentration both in the RYGB before-after group (F(12,48) = 9.38; P < .0005) and the RYGB 1–5 yr group (F(12,84) = 26.4; P < .0001) such that compared with the placebo drink, alcohol caused a greater initial increase followed by a greater decrease in plasma glucose concentrations (Figures 1 and 2). Consequently, plasma glucose peaks after RYGB were greater after alcohol than placebo consumption both in the RYGB before-after group (F(1,4) = 16.6; P < .05) and the RYGB 1–5 yr. group (F(1,7)= 18.2; P < .005) (Table 1).
Figure 1.
Effect of ingesting an alcoholic drink (0.5g/kg fat-free mass –FFM-; ~ 2 standard drinks over 10 min) (closed symbols) compared with drinking a non-alcoholic version of the same drink sprayed with 2 mL of alcohol (placebo, open symbols) on plasma glucose concentrations and glucose rate of appearance (Ra) in 5 women before (left panels) and 10±2 months after RYGB surgery (right panels). The point estimates are mean values. Error bars indicate +SEM. *values different from placebo at P < .05. †main effect of alcohol.
Figure 2.
Effect of ingesting an alcoholic drink (0.5g/kg fat-free mass –FFM-; ~ 2 standard drinks over 10 min) (closed symbols) compared with drinking a non-alcoholic version of the same drink sprayed with 2 mL of alcohol (placebo, open symbols) on plasma glucose concentrations and glucose rate of appearance (Ra) in 8 women who had RYGB surgery 2.2±1.2 years earlier (RYGB 1–5 yr group). The point estimates are mean values. Error bars indicate +SEM. *values different from placebo at P < .05.
Glucose kinetics
Compared with the consumption of the placebo drink, alcohol consumption decreased glucose endogenous production both before (F(1,4) = 23.3; P < .001) and after surgery in the RYGB before-after group (F(1,4) = 25.2; P < .01); and in the RYGB 1–5 yr group (F(1,7) = 12.9; P < .01). However, while before surgery alcohol decreased glucose Ra from 15 min from start of drinking until the end of the test, after surgery, alcohol did not decrease glucose Ra until 45 min from the start of the alcohol ingestion (F(12, 48) = 2.1; P < .05) (Figure 1). Similarly, in the RYGB 1–5 yr., alcohol had a biphasic effect such that compared with the placebo drink, alcohol caused a greater increase in glucose Ra immediately after drink ingestion, and subsequently a greater decrease in glucose Ra (F(12, 84) = 2.3; P < .001) (Figure 2).
Discussion
Although the ingestion of a moderate-dose of alcohol does not affect plasma glucose concentrations, the ingestion of a high dose can lead to hypoglycemia [11]. We recently found that RYGB doubles peak BAC, such that the ingestion of a moderate dose of alcohol can result in peak BAC similar to that achieved after an episode of binge or heavy drinking [13]. The results from the present study show that despite the effect of RYGB surgery on BAC, alcohol-induced reduction of plasma glucose concentrations was smaller, not larger, after than before RYGB surgery. The cause of this reduced hypoglycemic effect of alcohol after RYGB is likely due to a unique biphasic effect of alcohol ingestion on glucose kinetics, characterized by an early increase followed by a subsequent decrease in endogenous glucose production rate.
The mechanism responsible for the observed differences in the glucose response to alcohol ingestion after than before RYGB surgery is not known. However, because the liver is the main organ responsible for both, alcohol metabolism [23] and endogenous glucose production (through glycogenolysis and gluconeogenesis) [24], we hypothesize that such changes may be due, at least in part, to the impact of faster and higher peak BAC on liver metabolism. It is well known that alcohol decreases hepatic glucose production [10, 25–27], which likely explained our finding of reduced plasma glucose concentrations for most of the time after drinking the alcoholic beverage. However, results from pre-clinical studies show that when alcohol is directly infused into the liver it increases plasma glucose by decreased glycogenesis and increased glycogenolysis [27, 28]. Changes in liver glycogen and triglyceride, like those observed after surgery in a rodent model of RYGB [29] may also play a role on the endogenous glucose response to alcohol ingestion.
Our study has several limitations. First, the small number of participants, particularly in the longitudinal study (n=5). However, convergent findings from the two small but independent groups of patients evaluated in the study suggest that the biphasic effects of alcohol consumption on plasma glucose concentrations and glucose endogenous production are likely to be a consequence of RYGB surgery. Second, we did not measure changes in insulin and other hormones that play important roles in glycemic control. Studies with larger samples, including evaluation of hormonal and neuronal factors affecting glucose control are needed to better understand underlying mechanisms of alcohol consumption effects on glucose homeostasis in RYGB patients. Third, to avoid confounding effects of sugar consumption on glucose kinetics, we prepared alcoholic drinks with a low-caloric sweetener. However, alcoholic beverages are frequently consumed along with sugars or food, which might result in different effects of alcohol on glycaemia and should be evaluated in future studies.
Conclusion
Alcohol ingestion has a unique biphasic effect on circulating glucose in people who have had RYGB surgery, manifested by an early increase and subsequent decrease in plasma glucose concentration caused primarily by an early increase followed by a subsequent decrease in endogenous glucose production rate. Despite a doubling in peak blood alcohol concentrations after than before RYGB, ingesting a moderate amount of alcohol did not cause a greater decline in plasma glucose concentration after than before RYGB surgery, even though peak BAC after surgery was double the value before surgery.
Highlights.
Alcohol ingestion has a biphasic effect on circulating glucose in RYGB patients
After RYGB, alcohol first increases, then decreases glucose rate of appearance
Having 2 standard drinks does not increase the risk of hypoglycemia after RYGB
ACKNOWLEDGMENT:
The authors thank Adewole Okunade and Jennifer Shew for technical assistance with the gas chromatographic technique, and Johanna Sonnenschein for helping with subject recruitment and testing. This study was supported by the National Institutes of Health (NIH) grants AA 020018, AA024103, DK101578, and DK 56341 (Nutrition Obesity Research Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflict of interest in relation to this article.
References
- [1].Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obesity surgery. 2015;25:1822–32. [DOI] [PubMed] [Google Scholar]
- [2].Celio AC, Wu Q, Kasten KR, Manwaring ML, Pories WJ, Spaniolas K. Comparative effectiveness of Roux-en-Y gastric bypass and sleeve gastrectomy in super obese patients. Surgical endoscopy. 2017;31:317–23. [DOI] [PubMed] [Google Scholar]
- [3].Gonzalez-Heredia R, Sanchez-Johnsen L, Valbuena VS, Masrur M, Murphey M, Elli E. Surgical management of super-super obese patients: Roux-en-Y gastric bypass versus sleeve gastrectomy. Surgical endoscopy. 2016;30:2097–102. [DOI] [PubMed] [Google Scholar]
- [4].Foster-Schubert KE. Hypoglycemia complicating bariatric surgery: incidence and mechanisms. Current opinion in endocrinology, diabetes, and obesity. 2011;18:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Millstein R, Lawler HM. Hypoglycemia after gastric bypass: An emerging complication. Cleveland Clinic journal of medicine. 2017;84:319–28. [DOI] [PubMed] [Google Scholar]
- [6].Patti ME, Goldfine AB. Hypoglycemia after gastric bypass: the dark side of GLP-1. Gastroenterology. 2014;146:605–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rariy CM, Rometo D, Korytkowski M. Post-Gastric Bypass Hypoglycemia. Current diabetes reports. 2016;16:19. [DOI] [PubMed] [Google Scholar]
- [8].Shantavasinkul PC, Torquati A, Corsino L. Post-gastric bypass hypoglycaemia: a review. Clinical endocrinology. 2016;85:3–9. [DOI] [PubMed] [Google Scholar]
- [9].Kreisberg RA, Owen WC, Siegal AM. Ethanol-induced hyperlacticacidemia: inhibition of lactate utilization. The Journal of clinical investigation. 1971;50:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Siler SQ, Neese RA, Christiansen MP, Hellerstein MK. The inhibition of gluconeogenesis following alcohol in humans. The American journal of physiology. 1998;275:E897–907. [DOI] [PubMed] [Google Scholar]
- [11].Freinkel N, Singer DL, Arky RA, Bleicher SJ, Anderson JB, Silbert CK. Alcohol hypoglycemia. I. Carbohydrate metabolism of patients with clinical alcohol hypoglycemia and the experimental reproduction of the syndrome with pure ethanol. The Journal of clinical investigation. 1963;42:1112–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Klockhoff H, Naslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. British journal of clinical pharmacology. 2002;54:587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pepino MY, Okunade AL, Eagon JC, Bartholow BD, Bucholz K, Klein S. Effect of Roux-en-Y Gastric Bypass Surgery: Converting 2 Alcoholic Drinks to 4. JAMA surgery. 2015;150:1096–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Steffen KJ, Engel SG, Pollert GA, Li C, Mitchell JE. Blood alcohol concentrations rise rapidly and dramatically after Roux-en-Y gastric bypass. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2013;9:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Martin M, Beekley A, Kjorstad R, Sebesta J. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2010;6:8–15. [DOI] [PubMed] [Google Scholar]
- [16].Baraona E, Abittan CS, Dohmen K, et al. Gender differences in pharmacokinetics of alcohol. Alcoholism, clinical and experimental research. 2001;25:502–7. [PubMed] [Google Scholar]
- [17].Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of studies on alcohol. 1994;55:149–58. [DOI] [PubMed] [Google Scholar]
- [18].Bethesda. Helping Patients Who Drink Too Much: A Clinician’s Guide. National Institute on Alcohol Abuse and Alcoholism. 2005.
- [19].Gentry RT. Determinants and analysis of blood alcohol concentrations after social drinking. Alcoholism, clinical and experimental research. 2000;24:399. [PubMed] [Google Scholar]
- [20].Sonnenberg GE, Keller U. Sampling of arterialized heated-hand venous blood as a noninvasive technique for the study of ketone body kinetics in man. Metabolism: clinical and experimental. 1982;31:1–5. [PubMed] [Google Scholar]
- [21].Magkos F, Bradley D, Eagon JC, Patterson BW, Klein S. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on gastrointestinal metabolism of ingested glucose. The American journal of clinical nutrition. 2016;103:61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pepino MY, Abate P, Spear NE, Molina JC. Disruption of maternal behavior by alcohol intoxication in the lactating rat: a behavioral and metabolic analysis. Alcoholism, clinical and experimental research. 2002;26:1205–14. [DOI] [PubMed] [Google Scholar]
- [23].Cederbaum AI. Alcohol metabolism. Clinics in liver disease. 2012;16:667–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rui L Energy metabolism in the liver. Comprehensive Physiology. 2014;4:177–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dittmar EA, Hetenyi G Jr. The effect of ethanol on glucose homeostasis. Canadian journal of physiology and pharmacology. 1978;56:54–61. [DOI] [PubMed] [Google Scholar]
- [26].Duruibe V, Tejwani GA. The effect of ethanol on the activities of the key gluconeogenic and glycolytic enzymes of rat liver. Molecular pharmacology. 1981;20:621–30. [PubMed] [Google Scholar]
- [27].Mokuda O, Tanaka H, Hayashi T, Ooka H, Okazaki R, Sakamoto Y. Ethanol stimulates glycogenolysis and inhibits both glycogenesis via gluconeogenesis and from exogenous glucose in perfused rat liver. Annals of nutrition & metabolism. 2004;48:276–80. [DOI] [PubMed] [Google Scholar]
- [28].Kubota M, Virkamaki A, Yki-Jarvinen H. Ethanol stimulates glycogenolysis in livers from fed rats. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY). 1992;201:114–8. [DOI] [PubMed] [Google Scholar]
- [29].Mu S, Liu J, Guo W, et al. Roux-en-Y gastric bypass imnproves hepatic glucose metabolism involving down-regulation involving down-regulation of protein tyrosine phosphatase 1B in obese rats. Obesity facts 2017;10:191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]