Abstract
Background:
Days of extreme temperature may be associated with transiently higher risk of preterm birth, but prior studies have been limited and results have been heterogeneous.
Objectives:
To evaluate the association between days of extreme heat and cold and risk of preterm birth among ~32 million live singleton births between 1989 and 2002 across 403 counties in the contiguous United States (US).
Methods:
We used a distributed lag nonlinear model to estimate the association between population-weighted daily mean temperature and risk of preterm birth in each county and then pooled results across geographic regions and climate zones. We defined extreme heat and cold as the 95th and 5th percentile of the county-specific temperatures, respectively.
Results:
Preterm birth accounted for 9.3% of deliveries. There was a monotonic association between ambient temperature and risk of preterm birth. Days of extreme heat and cold were associated with a relative risk of preterm birth of 1.025 (95% CI: 1.015, 1.036) and 0.985 (95% CI: 0.976, 0.993) over the subsequent four days, respectively, relative to the county-specific median temperature. If causal, the fraction of preterm births attributable to extreme heat was 0.17% (empirical 95% CI: 0.14%, 0.19%), corresponding to 154 (empirical 95% CI: 127, 173) preterm births per million births. Extreme heat was more strongly associated with preterm birth in regions with colder and drier climates, and among younger women. Days of extreme cold temperature were associated with lower rather than higher risk of preterm birth.
Conclusions:
Days of extreme heat, but not extreme cold, are associated with higher risk of preterm birth in the contiguous US. If causal, these results may have important implications for the management of pregnant women during forecasted periods of extreme heat.
Keywords: Heat, Cold, Preterm birth, Birth cohort, United States
1. Introduction
Preterm birth is one of the leading causes of neonatal death worldwide and the second most common cause of death in children under five years old (Blencowe et al., 2012; Liu et al., 2012). Additionally, preterm birth is associated with higher risk of children developing a range of complications later in life (e.g., behavioural and cardiorespiratory illness), imposing a substantial burden on healthcare delivery systems (Saigal and Doyle, 2008). In most countries the proportion of infants born preterm has increased in recent decades (Blencowe et al., 2012). Approximately 14.9 million infants were born prematurely in 2010 globally, accounting for about 11% of all livebirths worldwide (Blencowe et al., 2012). With more than 500,000 preterm births (12% of all livebirths) in 2010, the United States ranked 6th in the world with regards to absolute number of preterm births (Blencowe et al., 2012; Martin et al., 2012).
Although two recent reviews reported that heat was associated with higher risk of preterm birth (Carolan-Olah and Frankowska, 2014; Zhang et al., 2017), these reviews also highlighted the inconsistency in results across prior studies. For example, studies in Montreal (Auger et al., 2014), London (Lee et al., 2008), or Germany (Wolf and Armstrong, 2012) failed to find these associations, highlighting the potential for heterogeneity of the association by geographic region, climate zone, population characteristics (e.g., socioeconomic conditions or adaptation) or other factors. In addition, most prior studies focused on heat (Auger et al., 2014; Avalos et al., 2017; Basu et al., 2010; Vicedo-Cabrera et al., 2014), with relatively fewer studies examining the associations between cold temperatures and risk of preterm birth (Bruckner et al., 2014; Cox et al., 2016; Giorgis-Allemand et al., 2017; Guo et al., 2017; He et al., 2016).
Given the adverse health impacts and high prevalence of preterm birth, understanding potentially modifiable triggers of preterm birth is of interest to both clinicians and public health officials. Accordingly, we examined the associations between daily mean temperature and risk of preterm birth among approximately 32 million live singleton births from 1989 to 2002 across 403 counties in the contiguous US. We additionally evaluated whether these associations varied by geographic region of the country, by climate zone, and by personal characteristics.
2. Methods
2.1. Study Population
We obtained data on live births occurring in the US between 1989 and 2002 from the US Centers for Disease Control and Prevention’s National Centre for Health Statistics (Centers for Disease Control and Prevention, 2017). Data on county of residence were only available for births among women living in counties with a population of ≥100,000. We further limited the study population to singleton births in the 403 counties with data continuously available throughout the study period (Fig. 1).
Fig. 1.
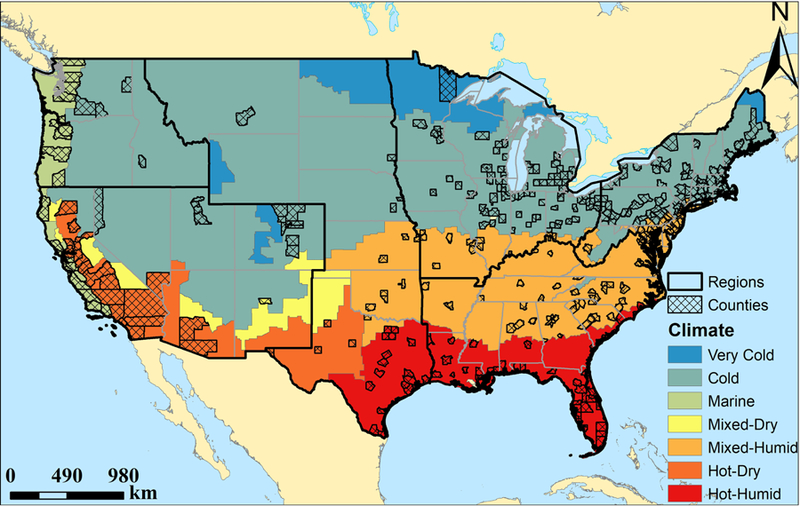
Distribution of the 403 US counties, 6 National Climate Assessment geographic regions, and 7 US Building America Climate Zones.
We applied the following additional exclusion criteria. First, we excluded births missing data on date of last normal menstrual period (LMP) or gestational age, or delivered < 20 or > 44 completed weeks of gestation. Second, to avoid artificial seasonal patterns in gestation length (“fixed cohort bias”) (Strand et al., 2011), we excluded pregnancies with a conception date more than 20 weeks before January 1, 1989, or fewer than 44 weeks before December 31, 2002. Third, we excluded births with implausible combinations of birth weight and gestational age according to the Alexander criteria (Alexander et al., 1996). Because date of birth is not directly available in these data, we imputed this variable based on LMP, completed weeks of gestation, and the recorded weekday of birth, excluding births where month of the imputed and recorded date of birth differed. The application of these exclusion criteria yielded a final analytic sample of 31,921,046 singleton births (Fig. S1).
2.2. Assessment of Ambient Temperature
We estimated daily mean ambient temperature using the Parameter-elevation Relationships on Independent Slopes (PRISM) model, a gridded climate dataset consisting of spatially interpolated weather data on a 4-km grid (Daly et al., 2008). The PRISM dataset offers a more spatially explicit representation of meteorological exposures compared to observations at individual weather stations and has been validated against station observations in both urban and rural areas (Spangler et al., 2018). To represent population exposure to temperature, we calculated a population-weighted average of daily mean temperatures for each day in each county, as previously described (Spangler et al., 2018).
2.3. Geographic Region and Climate Zone
We classified each county into one of six geographic regions as defined by the US Global Change Research Program’s Third National Climate-Assessment (Melillo et al., 2014): Northeast [number of counties included in the analyses (n)=110], Southeast (n=94), Midwest (n=99), Great Plains (n=35), Northwest (n=17), and Southwest (n=48) (Fig. 1). We also categorized each county into one of five climate zones according to the US Department of Energy’s Building America Program (US Department of Energy, 2015): Hot-Humid (n=58), Mixed-Humid (n=113), Hot-Dry/Mixed-Dry (n=26), Cold/Very Cold (n=181), and Marine (n=25).
2.4. Outcome Assessment
We defined preterm birth as births with < 37 completed weeks of gestation, calculated as the number of completed weeks between the LMP and the imputed date of birth. We further categorized preterm births into early (<34 weeks) and late preterm births (34–36 weeks) (Ha et al., 2016). For each county, we created a time-series of the number of preterm births per day. We also estimated the univariable association between each covariate and risk of preterm birth using logistic regression.
2.5. Statistical Analysis
For each county we linked time-series of the daily number of preterm births with the daily population-weighted average temperature. In the first stage of analysis we fit a distributed lag non-linear quasi-Poisson time-series model separately in each county to estimate the county-specific relative risk (RR) of preterm birth associated with daily mean temperatures (Gasparrini et al., 2015). This approach estimates the association between daily variation in temperature and daily variation in rates of preterm birth, adjusting for temporal trends and other time-varying factors (Peng et al., 2006). Under the assumption that the study population is in steady-state (conditional on the covariates in the model), individual-level covariates that do not change from day to day cannot confound the analyses. Associations between daily changes in temperature and health outcomes are typically non-linear (frequently U-, V-, or J-shaped) and delayed (lasting for several days after exposure to temperature extremes) (Gasparrini et al., 2015; Sun et al., 2016; Weinberger et al., 2017; Wellenius et al., 2017). The distributed lag non-linear model provides a flexible framework that permits non-linear exposure-response and time-response functions (Gasparrini, 2014). We modeled the association between daily mean temperature and preterm birth using a quadratic B-spline with 3 degrees of freedom (dfs) and modeled the lag function using a natural cubic B-spline with two knots placed at equal intervals on the log scale of lags up to 4 days before delivery. The dfs for temperature and lag days were chosen to minimize the Akaike Information Criterion (AIC). We adjusted all models for seasonal and long-term trends using a natural cubic spline of time with 8 dfs per year, relative humidity using a natural cubic spline with 3 dfs, day of the week, and federal holidays. We accounted for daily variation in the number of pregnancies at risk of being preterm by calculating and applying a daily offset as previously described (Vicedo-Cabrera et al., 2014). In sensitivity analyses we varied the key model assumptions to assess the robustness of our findings.
We next pooled the county-specific RR calculated from the first stage of the analysis using a multivariate random-effects meta-analytic model to estimate the overall cumulative association of temperature with preterm birth (Gasparrini and Armstrong, 2013; Gasparrini et al., 2012). We report the RRs for extreme heat (95th percentile of county-specific temperature distribution) and extreme cold (5th percentile) (Tobias et al., 2012) with reference to the median of the county-specific temperature distribution (Cox et al., 2016; Vicedo-Cabrera et al., 2014).
2.5.1. Subgroup Analyses
We evaluated whether the association between extreme temperatures and risk of preterm birth varied by the degree of prematurity (early versus late preterm births) or across subgroups defined by the following personal characteristics: infant sex (male and female), maternal age (<25, 25–34, and ≥35 years), marital status (married and unmarried), maternal race (white and non-white), maternal education (<9 and 9–17 years), and parity (0, 1, and ≥2). We also examined whether the association varied by geographic region or climate zone by pooling the county-specific relative risk estimates across these categories. We implemented a Wald statistic for testing whether the association between preterm birth and daily temperature is homogeneous across strata (Rothman et al., 2008).
2.5.2. Estimation of Attributable Risk
To assess the potential public health impact of heat on risk of preterm birth, we estimated the fraction and number of preterm births attributable to extreme heat and moderate heat (temperatures between 50th and 95th percentile) as previously described (Gasparrini and Leone, 2014). Briefly, for each county we obtained the best linear unbiased prediction of the county-specific cumulative association between temperature and risk of preterm birth (derived from the meta-analytic model) and summed the contributions from all days of extreme or moderate heat in that county. We defined the attributable fraction and attributable number as the total number of preterm births attributable to extreme or moderate heat divided by the total number of preterm births or total number of births, respectively. We used Monte Carlo simulations to estimate empirical 95% confidence interval (eCI) assuming a multivariate normal distribution of the coefficients in the best linear unbiased prediction.
All analyses were conducted in R (version 3.4.3). We used the “dlnm” package version 2.3.6 for the distributed lag non-linear time-series analysis, and the “mvmeta” package version 0.4.11 for the second-stage meta-analysis.
3. Results
Our analysis included 31,921,046 singleton births (59.2% of total singleton births in the US during the study period), of which 9.3% were preterm (Table 1). The median (interquartile range) gestational age at delivery was 35.0 (3.0) weeks for preterm births and 39.0 (2.0) weeks for term births (Table S1). At the time of delivery, most mothers were 25 to 34 years old (52.8%), married (68.9%), white (77.7%), and had at least 9 years of education (91.2%). A minority of mothers reported smoking (8.9%) or consuming alcohol (1.3%) during their pregnancy.
Table 1.
Demographics of preterm births among 403 US counties from 1989 to 2002.
| Characteristics, No. (%) | Preterm births (20–36 weeks) | OR for preterm birth (95% CI)a |
|---|---|---|
| Total birth | 2,973,909 (9.3) | — |
| Gestational age, weeks, median (IQR) | 35.0 (3.0) | — |
| Maternal age, years | ||
| <25 | 1,179,350 (10.7) | 1.34 (1.33, 1.34) |
| 25–34 | 1,390,758 (8.3) | 1 [Reference] |
| ≥35 | 403,801 (9.9) | 1.21 (1.21, 1.22) |
| Marital status | ||
| Married | 1,717,846 (7.8) | 1 [Reference] |
| Unmarried | 1,256,063 (12.7) | 1.71 (1.71, 1.72) |
| Maternal race | ||
| White | 1,999,007 (8.1) | 1 [Reference] |
| Nonwhite | 974,902 (13.7) | 1.81 (1.81, 1.82) |
| Years of education, years | ||
| ≤ 8 | 227,284 (10.9) | 1.04 (1.04, 1.04) |
| 9–12 | 1,571,182 (10.7) | 1 [Reference] |
| 13–17 | 1,103,229 (7.7) | 0.69 (0.68, 0.69) |
| Unknown | 72,214 (10.1) | 0.95 (0.94, 0.95) |
| Smoking during pregnancy | ||
| No | 1,897,344 (9.1) | 1 [Reference] |
| Yes | 344,353 (12.1) | 1.36 (1.35, 1.36) |
| Unknownb | 732,212 (8.8) | 0.96 (0.96, 0.96) |
| Alcohol consumption during pregnancy | ||
| No | 2,264,166 (9.4) | 1 [Reference] |
| Yes | 51,698 (12.4) | 1.37 (1.35, 1.38) |
| Unknownb | 658,045 (8.9) | 0.94 (0.94, 0.94) |
| Parity | ||
| 0 | 1,261,636 (9.5) | 1 [Reference] |
| 1 | 849,657 (8.3) | 0.88 (0.87, 0.88) |
| ≥ 2 | 850,362 (10.3) | 1.11 (1.11, 1.11) |
| Unknown | 12,254 (10.7) | 1.16 (1.14, 1.18) |
| Chronic hypertension | ||
| No | 2,865,650 (9.2) | 1 [Reference] |
| Yes | 43,430 (20.9) | 2.57 (2.54, 2.59) |
| Unknown | 64,829 (11.0) | 1.23 (1.22, 1.24) |
| Season of conceptionc | ||
| Spring | 690,569 (9.4) | 0.98 (0.98, 0.99) |
| Summer | 718,648 (9.3) | 0.97 (0.97, 0.98) |
| Fall | 803,703 (9.5) | 1 [Reference] |
| Winter | 760,989 (9.2) | 0.96 (0.96, 0.96) |
| Geographic region | ||
| Northeast | 715,500 (8.8) | 1.01 (1.01, 1.01) |
| Southeast | 566,639 (10.8) | 1.26 (1.25, 1.26) |
| Midwest | 591,021 (9.5) | 1.10 (1.09, 1.10) |
| Great Plains | 347,421 (10.1) | 1.19 (1.18, 1.19) |
| Northwest | 74,808 (7.4) | 0.84 (0.83, 0.84) |
| Southwest | 678,520 (8.7) | 1 [Reference] |
| Climate zone | ||
| Hot-Humid | 533,446 (10.6) | 1.26 (1.26, 1.27) |
| Mixed-Humid | 839,539 (10.0) | 1.18 (1.18, 1.18) |
| Hot-Dry/Mixed-Dry | 528,042 (9.0) | 1.06 (1.05, 1.06) |
| Cold/Very Cold | 914,837 (8.6) | 1 [Reference] |
| Marine | 158,045 (7.5) | 0.86 (0.86, 0.87) |
Abbreviations: IQR, interquartile range; OR, odds ratio; CI, confidence interval.
denotes not applicable.
Odds ratios were estimated from crude model that without including any covariates.
Smoking and drinking consumption were not recorded on California birth certificates.
Season of conception: spring (March-May), summer (June-August), fall (September-November), and winter (December-February).
Preterm birth was more common among mothers who were < 25 years of age, unmarried, non-white, smoked or consumed alcohol during pregnancy, had fewer than 8 years of education, had chronic hypertension, or lived in the Southeast US or in the Hot-Humid climate zone (Table 1). Daily mean temperature and relative humidity showed the expected geographic and climate zone variation (Fig. S2).
We observed a monotonic association between daily temperature and risk of preterm birth, with higher relative risk associated with higher temperatures (Fig. S3). Extreme heat was associated with a statistically significant 1.025 (95% CI: 1.015, 1.036) fold higher risk of preterm birth. Conversely, days of extreme cold were associated with a modestly lower relative risk of preterm birth (RR: 0.985 [95% CI: 0.976, 0.993]). The impacts of both extreme heat and cold on preterm birth risk were delayed one day, with little or no evidence of an association across the subsequent three days (Fig. S3). Results were similar in sensitivity analyses with varying dfs of the natural cubic spline of time per year or relative humidity (Table S2 and Fig. S4).
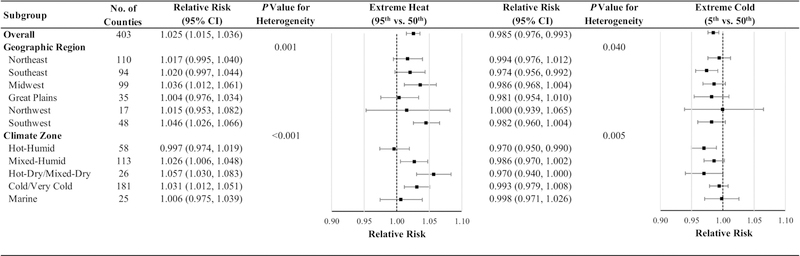
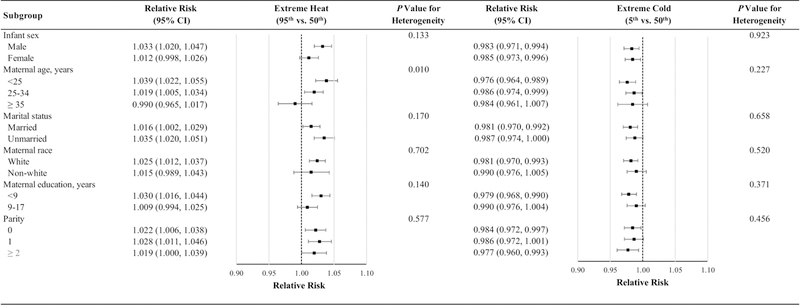
The associations between extreme temperatures and risk of preterm birth varied both by region of the country and by climate zone. The associations for extreme heat were strongest in the Southwest and in the Hot-Dry/Mixed-Dry climate zones followed by Midwest and the Cold/Very Cold climate zones (Fig. 2). The association between extreme heat and risk of preterm birth was more pronounced among mothers age < 25 years compared to older women (p for heterogeneity=0.010) (Fig. 3). Although the relative risk was somewhat higher among male infants and mothers who were unmarried or had fewer than 9 years of education, these differences were not statistically significant. The association between extreme cold and risk of preterm birth did not vary across any subgroups evaluated. We also found the association of preterm birth with extreme heat and cold was similar for early and late preterm births (Table S3 and Fig. S5).
Fig. 2. Relative risks of preterm birth associated with temperature extremes over the subsequent four days overall and stratified by geographic regions and climate zones.
Abbreviations: CI, confidence interval.
Models were adjusted for long-term and seasonal trends using a natural cubic spline of time with 8 degrees of freedom per year, relative humidity using a natural cubic spline with 3 degrees of freedom, day of the week, federal holidays, and the temporal variation of the daily expected count of preterm births.
Fig. 3. Relative risks of preterm birth associated with temperature extremes over the subsequent four days by individual characteristics.
Abbreviations: CI, confidence interval.
Models were adjusted for long-term and seasonal trends using a natural cubic spline of time with 8 degrees of freedom per year, relative humidity using a natural cubic spline with 3 degrees of freedom, day of the week, federal holidays, and the temporal variation of the daily expected count of preterm births.
Assuming that the observed association between heat and risk of preterm birth represents a causal effect, we estimated that days of extreme and moderate heat accounted for 0.17% (95% eCI: 0.14%, 0.19%) and 0.63% (95% eCI: 0.49%, 0.74%) of all preterm births, respectively, or 154 (95% eCI: 127, 173) and 586 (95% eCI: 457, 693) preterm births per million pregnancies in the study population, respectively (Table 2 and Table S4). Extreme and moderate heat accounted for a greater fraction of preterm births in the Southwest and Midwest of the country and Hot-Dry/Mixed-Dry and Cold/Very Cold climates, and a smaller fraction in the Northwest and Marine climate.
Table 2.
Fraction and number of preterm births attributable to extreme heat.
| Subgroup | Fraction of Preterm Births % (empirical CI) | Number of Preterm Births per Million Pregnancies No. (empirical CI) |
|---|---|---|
| All US | 0.17 (0.14, 0.19) | 154 (127, 173) |
| Geographic Region | ||
| Northeast | 0.13 (0.08, 0.17) | 116 (73, 153) |
| Southeast | 0.17 (0.13, 0.20) | 181 (141, 219) |
| Midwest | 0.19 (0.13, 0.23) | 176 (122, 220) |
| Great Plains | 0.14 (0.05, 0.21) | 140 (50, 216) |
| Northwest | 0.12 (0.01, 0.22) | 90 (10, 160) |
| Southwest | 0.20 (0.12, 0.26) | 174 (108, 222) |
| Climate Zone | ||
| Hot-Humid | 0.14 (0.09, 0.19) | 151 (93, 206) |
| Mixed-Humid | 0.14 (0.10, 0.18) | 145 (99, 185) |
| Hot-Dry/Mixed-Dry | 0.21 (0.13, 0.29) | 194 (113, 262) |
| Cold/Very Cold | 0.18 (0.14, 0.22) | 158 (122, 189) |
| Marine | 0.09 (0.01, 0.16) | 69 (7, 122) |
Abbreviations: CI, confidence interval.
Extreme heat was defined as temperatures above 95th percentile of county-specific temperature distributions.
4. Discussion
This retrospective observational study of nearly 32 million live singleton births across 403 counties in the contiguous United States provides evidence that days of extreme heat are associated with a higher relative risk of preterm birth. The association between extreme heat and risk of preterm birth varied by geographic regions and climate zones with stronger associations observed in the US Southwest and Midwest and in the Hot-Dry/Mixed-Dry and Cold/Very Cold climate zones. Days of extreme cold were associated with a modestly lower risk of preterm birth.
Our findings of a statistically significant positive association between extreme heat and preterm birth are broadly consistent with the findings of two recent reviews, which reported that high temperatures were associated with the occurrence of preterm births (Carolan-Olah and Frankowska, 2014; Zhang et al., 2017). For example, a study among 58,681 singleton births in California, USA, reported that a 10 oF increase in weekly average temperature before delivery was associated with an 8.6% (95% CI: 6.0%, 11.3%) higher risk of preterm birth (Basu et al., 2010).
Physiological changes during pregnancy may influence the thermoregulation efficiency of pregnant women. During pregnancy, the increase in weight may decrease the ratio of body surface area to body mass, which may limit a woman’s capacity for heat loss. In addition, heat production would increase due to fetal growth and metabolism (Wells and Cole, 2002). Thus, the ability of pregnant women to mitigate heat stress may be limited due to the increase in internal heat production and the decrease in capacity for heat loss, potentially placing them more vulnerable to heat stress.
However, the potential mechanisms by which acute exposure to heat may influence the risk of preterm birth remain unclear. Animal and human studies both suggested that heat might lead to an increase in hormones of the hypothalamic-pituitary-adrenal axis, such as cortisol and adrenocorticotropic hormone (Ansari et al., 2014; Dreiling et al., 1991; Wang et al., 2015). This is one of the primary pathways to activate myometrial function and increase uterine contractions (Finken et al., 2017; Sloboda et al., 2002), potentially leading to an early delivery. Heat may also cause dehydration, in turn increasing blood viscosity, elevating cholesterol levels, and shifting blood flow from the vital organs or the developing fetus to the skin’s surface to dissipate heat; this sequence of physiologic changes may decrease uterine oxygen and induce labor (Bouchama and Knochel, 2002; Dadvand et al., 2011; Keatinge et al., 1986).
We found that extreme cold was associated with a modestly lower risk of preterm birth. Relatively fewer prior studies have investigated the association of preterm birth with extreme cold, and the findings have not been consistent. For example, a study in Guangzhou, China (He et al., 2016) reported that extreme cold was associated with a 9% to 20% higher risk of preterm birth, whereas analyses of data from Flanders, Belgium (Cox et al., 2016), London, UK (Lee et al., 2008), and Rome, Italy (Schifano et al., 2013) did not find any statistically significant associations between extreme cold and preterm birth. Our results are consistent with a nationwide study in China among approximately 1 million singleton births from 132 Chinese cities, which found that extreme cold during the four weeks prior to delivery was associated with lower risk of preterm birth, especially in regions with warmer climates (Guo et al., 2017).
The comparatively weaker association we observed between extreme cold (versus extreme heat) and risk of preterm birth might reflect a combination of physiologic and behavioral factors. Physiologically, pregnancy-related increase in fat deposition and decrease in the ratio of body surface area to body mass might make pregnant women less vulnerable to cold versus heat. Behaviorally, the vast majority of households in the US use heating equipment during winter (Residential energy consumption survey (RECS), 2017), and people tend to alter their behavior in response to cold to limit exposure (Sheridan, 2007).
We found the association between heat and preterm birth was more pronounced in counties and regions with colder and drier climates, consistent with previous studies of preterm birth (Guo et al., 2017). This finding may be attributable to a lesser degree of physiological, behavioral, and technological adaptation to extreme heat in areas with colder and drier climates. Although we did not observe any statistically significant differences in the susceptibility to heat across subgroups defined by individual characteristics, extreme heat was more strongly associated with preterm birth among the youngest mothers, a finding that is supported by previous studies (Basu et al., 2010; Schifano et al., 2013).
Preterm birth is a complex syndrome initiated by multiple causes and the etiology of preterm birth remains poorly understood (Goldenberg et al., 2008). Despite increased efforts at prevention and intervention, the proportion of preterm births has increased in most countries in recent decades (Blencowe et al., 2012), suggesting that new risk factors may play a role. Our study found that days of extreme heat might be a novel environmental risk factor for preterm birth. Assuming that the associations observed in this study represent a causal effect, extreme heat accounted for 0.17% of all preterm births in the study population. Periods of extreme heat have occurred more frequently in recent years, and are projected to further increase in frequency, intensity, and duration due to continued climate change (Pachauri et al., 2014). Additional research is needed to identify strategies or interventions to reduce the risk of preterm births during days of extreme heat. This information is important for clinicians and public health officials who advise patients and the public.
Our study has several potential limitations. First, some degree of exposure misclassification is inevitable due to uncertainty in the imputed birth date and location of each mother on and in the days preceding her delivery. This limitation is shared by all time-series studies. On the other hand, exposure misclassification may have been lower in this study compared to many prior studies given our use of daily mean temperature estimated from a spatially refined, gridded climate dataset rather than observations from airport weather stations which typically do not represent population average exposures. Nonetheless, we expect that any remaining exposure misclassification would be non-differential and on average tend to bias our results towards the null hypothesis of no association. Second, given the lack of daily data on ambient fine particulate matter air pollution (PM2.5) during this time period, we cannot exclude the possibility of residual confounding by PM2.5, which has also been associated with higher risk of preterm birth (Shah et al., 2011). However, prior studies suggest that air pollution does not confound the short-term association between ambient temperature and risk of preterm birth (Basu et al., 2010; Cox et al., 2016; Ha et al., 2016; He et al., 2016; Vicedo-Cabrera et al., 2014). Third, we were not able to distinguish in our analyses between spontaneous and medically induced preterm deliveries as this information is not reliably available in this dataset (Schoendorf and Branum, 2006). Fourth, our analysis was limited to the 403 more populous counties of the contiguous US, accounting for nearly 60% of all births in the US during the study time period. Whether these results are generalizable to other counties with lower populations remains unknown. Similarly, whether the association would be similar today given ever-changing medical care, increasing number of days of extreme heat, and increasing prevalence of air conditioning (The U.S. Energy Information Administration, 2011) remains unknown. Nevertheless, to our knowledge, this is the largest analysis of daily ambient temperature and risk of preterm birth to date, including more than 32 million US singleton births across different geographic regions and climates.
5. Conclusions
In this large retrospective observational study, we found that days of extreme heat were associated with a higher relative risk of preterm birth. Although the pathophysiologic mechanisms by which periods of extreme heat may trigger preterm births remain unknown, this information may be useful to clinicians, public health officials, and the public in light of the rapidly changing climate.
Supplementary Material
Highlights.
We examined the association between extreme temperature and PTB in the contiguous US.
Days of extreme heat were associated with higher risk of preterm birth.
The association was stronger in regions with typically colder and drier climates.
Acknowledgements
The study was funded by the Institute at Brown for Environment and Society (IBES) and by grants R21-ES023073 and F32-ES027742 from the National Institute of Environmental Health Sciences (NIEHS, NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
REFERENCES
- Alexander GR, Himes JH, Kaufman RB, Mor J,Kogan M, 1996. A United States national reference for fetal growth. Obstet Gynecol 87, 163–168. [DOI] [PubMed] [Google Scholar]
- Ansari M, Mazloumi A, Abbassinia M, Farhang Dehghan S,Golbabaei F, 2014. Heat stress and its impact on the workers’ cortisol concentration: A case study in a metal melding industry. Health and Safety at Work 4, 59–68. [Google Scholar]
- Auger N, Naimi AI, Smargiassi A, Lo E,Kosatsky T, 2014. Extreme heat and risk of early delivery among preterm and term pregnancies. Epidemiology 25, 344–350. [DOI] [PubMed] [Google Scholar]
- Avalos LA, Chen H, Li DK,Basu R, 2017. The impact of high apparent temperature on spontaneous preterm delivery: a case-crossover study. Environ Health 16, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Malig B,Ostro B, 2010. High ambient temperature and the risk of preterm delivery. Am J Epidemiol 172, 1108–1117. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. , 2012. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172. [DOI] [PubMed] [Google Scholar]
- Bouchama A,Knochel JP, 2002. Heat stroke. N Engl J Med 346, 1978–1988. [DOI] [PubMed] [Google Scholar]
- Bruckner TA, Modin B,Vagero D, 2014. Cold ambient temperature in utero and birth outcomes in Uppsala, Sweden, 1915–1929. Ann Epidemiol 24, 116–121. [DOI] [PubMed] [Google Scholar]
- Carolan-Olah M,Frankowska D, 2014. High environmental temperature and preterm birth: a review of the evidence. Midwifery 30, 50–59. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2017. National Center for Health Statistics Available: https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm (accessed 06 November 2017).
- Cox B, Vicedo-Cabrera AM, Gasparrini A, Roels HA, Martens E, Vangronsveld J, et al. , 2016. Ambient temperature as a trigger of preterm delivery in a temperate climate. J Epidemiol Community Health 70, 1191–1199. [DOI] [PubMed] [Google Scholar]
- Dadvand P, Basagana X, Sartini C, Figueras F, Vrijheid M, De Nazelle A, et al. , 2011. Climate extremes and the length of gestation. Environ Health Perspect 119, 1449–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Halbleib M, Smith JI, Gibson WP, Doggett MK, Taylor GH, et al. , 2008. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int J Climatol 28, 2031–2064. [Google Scholar]
- Dreiling CE, Carman FS,Brown DE, 1991. Maternal endocrine and fetal metabolic responses to heat stress. J Dairy Sci 74, 312–327. [DOI] [PubMed] [Google Scholar]
- Finken MJ, van der Voorn B, Hollanders JJ, Ruys CA, de Waard M, van Goudoever JB, et al. , 2017. Programming of the hypothalamus-pituitary-adrenal axis by very preterm birth. Ann Nutr Metab 70, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, 2014. Modeling exposure–lag–response associations with distributed lag non‐ linear models. Stat Med 33, 881–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A,Armstrong B, 2013. Reducing and meta-analysing estimates from distributed lag non-linear models. BMC Med Res Methodol 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B,Kenward M, 2012. Multivariate meta‐ analysis for non‐ linear and other multi‐ parameter associations. Stat Med 31, 3821–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, et al. , 2015. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A,Leone M, 2014. Attributable risk from distributed lag models. BMC Med Res Methodol 14, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgis-Allemand L, Pedersen M, Bernard C, Aguilera I, Beelen RM, Chatzi L, et al. , 2017. The Influence of Meteorological Factors and Atmospheric Pollutants on the Risk of Preterm Birth. Am J Epidemiol 185, 247–258. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD,Romero R, 2008. Epidemiology and causes of preterm birth. Lancet 371, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Wang Y, Zhang H, Zhang Y, Zhao J, Wang Y, et al. , 2017. The association between ambient temperature and the risk of preterm birth in China. Sci Total Environ 613–614, 439–446. [DOI] [PubMed]
- Ha S, Liu D, Zhu Y, Kim SS, Sherman S,Mendola P, 2016. Ambient Temperature and Early Delivery of Singleton Pregnancies. Environ Health Perspect 125, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J-R, Liu Y, Xia X-Y, Ma W-J, Lin H-L, Kan H-D, et al. , 2016. Ambient temperature and the risk of preterm birth in Guangzhou, China (2001–2011). Environ Health Perspect 124, 1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge WR, Coleshaw SR, Easton JC, Cotter F, Mattock MB,Chelliah R, 1986. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am J Med 81, 795–800. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Hajat S, Steer PJ,Filippi V, 2008. A time-series analysis of any short-term effects of meteorological and air pollution factors on preterm births in London, UK. Environ Res 106, 185–194. [DOI] [PubMed] [Google Scholar]
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. , 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379, 2151–2161. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC,Mathews T, 2012. Births: final data for 2010. Natl Vital Stat Rep, [PubMed]
- Melillo JM, Richmond T,Yohe G Climate change impacts in the United States; 2014
- Pachauri RK, Allen MR, Barros VR, Broome J, Cramer W, Christ R, et al. Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change: IPCC; 2014 [Google Scholar]
- Peng RD, Dominici F,Louis TA, 2006. Model choice in time series studies of air pollution and mortality. J R Stat Soc Ser A Stat Soc 169, 179–203. [Google Scholar]
- Residential energy consumption survey (RECS). 2017. U.S. households’ heating equipment choices are diverse and vary by climate region (https://www.eia.gov/todayinenergy/detail.php?id=30672) [accessed on 03/27/2018].
- Rothman KJ, Greenland S,Lash TL Modern epidemiology; 2008
- Saigal S,Doyle LW, 2008. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269. [DOI] [PubMed] [Google Scholar]
- Schifano P, Lallo A, Asta F, De Sario M, Davoli M,Michelozzi P, 2013. Effect of ambient temperature and air pollutants on the risk of preterm birth, Rome 2001–2010. Environ Int 61, 77–87. [DOI] [PubMed] [Google Scholar]
- Schoendorf KC,Branum AM, 2006. The use of United States vital statistics in perinatal and obstetric research. Am J Obstet Gynecol 194, 911–915. [DOI] [PubMed] [Google Scholar]
- Shah PS, Balkhair T,births K.S.G.o.D.o.P.L., 2011. Air pollution and birth outcomes: a systematic review. Environ Int 37, 498–516. [DOI] [PubMed] [Google Scholar]
- Sheridan SC, 2007. A survey of public perception and response to heat warnings across four North American cities: an evaluation of municipal effectiveness. Int J Biometeorol 52, 3–15. [DOI] [PubMed] [Google Scholar]
- Sloboda D, Alfaidy N, Lye S, Gibb W, Patel F, Whittle W, et al. , 2002. Prostaglandins and mechanisms of preterm birth. Reproduction 124, 1–17. [DOI] [PubMed] [Google Scholar]
- Spangler KR, Weinberger KR,Wellenius GA, 2018. Suitability of gridded climate datasets for use in environmental epidemiology. J Expo Sci Environ Epidemiol, [DOI] [PMC free article] [PubMed]
- Strand LB, Barnett AG,Tong S, 2011. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol 11, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Tian L, Qiu H, Chan K-P, Tsang H, Tang R, et al. , 2016. The influence of pre-existing health conditions on short-term mortality risks of temperature: Evidence from a prospective Chinese elderly cohort in Hong Kong. Environ Res 148, 7–14. [DOI] [PubMed] [Google Scholar]
- The U.S. Energy Information Administration. Residential energy consumption survey (RECS) 2011.
- Tobias A, Armstrong B, Zuza I, Gasparrini A, Linares C,Diaz J, 2012. Mortality on extreme heat days using official thresholds in Spain: a multi-city time series analysis. BMC Public Health 12, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Energy. Volume 7.3. Guide to Determining Climate Regions by County. 2015.
- Vicedo-Cabrera AM, Iñíguez C, Barona C,Ballester F, 2014. Exposure to elevated temperatures and risk of preterm birth in Valencia, Spain. Environ Res 134, 210–217. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu F, Luo Y, Zhu L,Li G, 2015. Effect of acute heat stress on adrenocorticotropic hormone, cortisol, interleukin‑ 2, interleukin‑ 12 and apoptosis gene expression in rats. Biomed Rep 3, 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger KR, Haykin L, Eliot MN, Schwartz JD, Gasparrini A,Wellenius GA, 2017. Projected temperature-related deaths in ten large US metropolitan areas under different climate change scenarios. Environ Int 107, 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Eliot MN, Bush KF, Holt D, Lincoln RA, Smith AE, et al. , 2017. Heat-related morbidity and mortality in New England: evidence for local policy. Environ Res 156, 845–853. [DOI] [PubMed] [Google Scholar]
- Wells JC,Cole TJ, 2002. Birth weight and environmental heat load: a between‐ population analysis. Am J Phys Anthropol 119, 276–282. [DOI] [PubMed] [Google Scholar]
- Wolf J,Armstrong B, 2012. The association of season and temperature with adverse pregnancy outcome in two German states, a time-series analysis. PLoS One 7, e40228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu C,Wang L, 2017. Temperature exposure during pregnancy and birth outcomes: An updated systematic review of epidemiological evidence. Environ Pollut 225, 700–712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.