Abstract
Background
Elective hysterectomy is commonly performed for benign gynaecological conditions. Hysterectomy can be performed abdominally, laparoscopically, or vaginally, with or without laparoscopic assistance. Antibiotic prophylaxis consists of administration of antibiotics to reduce the rate of postoperative infection, which otherwise affects 40%‐50% of women after vaginal hysterectomy, and more than 20% after abdominal hysterectomy. No Cochrane review has systematically assessed evidence on this topic.
Objectives
To determine the effectiveness and safety of antibiotic prophylaxis in women undergoing elective hysterectomy.
Search methods
We searched electronic databases to November 2016 (including the Cochrane Gynaecology and Fertility Group Specialised Register, the Cochrane Central Register of Studies (CRSO), MEDLINE, Embase, PsycINFO, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), as well as clinical trials registers, conference abstracts, and reference lists of relevant articles.
Selection criteria
All randomised controlled trials (RCTs) comparing use of antibiotics versus placebo or other antibiotics as prophylaxis in women undergoing elective hysterectomy.
Data collection and analysis
We used Cochrane standard methodological procedures.
Main results
We included in this review 37 RCTs, which performed 20 comparisons of various antibiotics versus placebo and versus one another (6079 women). The quality of the evidence ranged from very low to moderate. The main limitations of study findings were risk of bias due to poor reporting of methods, imprecision due to small samples and low event rates, and inadequate reporting of adverse effects.
Any antibiotic versus placebo
Vaginal hysterectomy
Low‐quality evidence shows that women who received antibiotic prophylaxis had fewer total postoperative infections (risk ratio (RR) 0.28, 95% confidence interval (CI) 0.19 to 0.40; four RCTs, N = 293; I2 = 85%), less urinary tract infection (UTI) (RR 0.58, 95% CI 0.43 to 0.77; eight RCTs, N = 1473; I2 = 44%), fewer pelvic infections (RR 0.28, 95% CI 0.20 to 0.39; 11 RCTs, N = 1693; I2 = 57%), and fewer postoperative fevers (RR 0.43, 95% CI 0.34 to 0.54; nine RCTs, N = 1562; I2 = 47%) than women who did not receive such prophylaxis. This suggests that antibiotic prophylaxis reduces the average risk of postoperative infection from about 34% to 7% to 14%. Whether this treatment has led to differences in rates of other serious infection remains unclear (RR 0.20, 95% CI 0.01 to 4.10; one RCT, N = 146; very low‐quality evidence).
Data were insufficient for comparison of adverse effects.
Abdominal hysterectomy
Women who received antibiotic prophylaxis of any class had fewer total postoperative infections (RR 0.38, 95% CI 0.21 to 0.67; one RCT, N = 158; low‐quality evidence), abdominal wound infections (RR 0.51, 95% CI 0.36 to 0.73; 11 RCTs, N = 2247; I2 = 6%; moderate‐quality evidence), UTIs (RR 0.41, 95% CI 0.31 to 0.53; 11 RCTs, N = 2705; I2 = 28%; moderate‐quality evidence), pelvic infections (RR 0.50, 95% CI 0.35 to 0.71; 11 RCTs, N = 1883; I2 = 11%; moderate‐quality evidence), and postoperative fevers (RR 0.59, 95% CI 0.50 to 0.70; 11 RCTs, N = 2394; I2 = 55%; moderate‐quality evidence) than women who did not receive prophylaxis, suggesting that antibiotic prophylaxis reduces the average risk of postoperative infection from about 16% to 1% to 6%. Whether this treatment has led to differences in rates of other serious infection remains unclear (RR 0.44, 95% CI 0.12 to 1.69; two RCTs, N = 476; I2 = 29%; very low‐quality evidence).
It is unclear whether rates of adverse effects differed between groups (RR 1.80, 95% CI 0.62 to 5.18; two RCTs, N = 430; I2 = 0%; very low‐quality evidence).
Head‐to‐head comparisons between antibiotics
Vaginal hysterectomy
We identified four comparisons: cephalosporin versus penicillin (two RCTs, N = 470), cephalosporin versus tetracycline (one RCT, N = 51), antiprotozoal versus lincosamide (one RCT, N = 80), and cephalosporin versus antiprotozoal (one RCT, N = 78). Data show no evidence of differences between groups for any of the primary outcomes, except that fewer cases of total postoperative infection and postoperative fever were reported in women who received cephalosporin than in those who received antiprotozoal.
Only one comparison (cephalosporin vs penicillin; two RCTs, N = 451) yielded data on adverse effects and showed no differences between groups.
Abdominal hysterectomy
We identified only one comparison: cephalosporin versus penicillin (N = 220). Data show no evidence of differences between groups for any of the primary outcomes. Adverse effects were not reported.
Combined antibiotics versus single antibiotics
Vaginal hysterectomy
We identified three comparisons: cephalosporin plus antiprotozoal versus cephalosporin (one RCT, N = 78), cephalosporin plus antiprotozoal versus antiprotozoal (one RCT, N = 78), and penicillin plus antiprotozoal versus penicillin (one RCT, N = 18). Data were unavailable for most outcomes, including adverse effects. We found no evidence of differences between groups, except that fewer women receiving cephalosporin with antiprotozoal received a diagnosis of total postoperative infection, UTI, or postoperative fever compared with women receiving antiprotozoal.
Abdominal hysterectomy
We identified one comparison (penicillin plus antiprotozoal vs penicillin only; two RCT, N = 155). Whether differences between groups occurred was unclear. Adverse effects were not reported.
Comparison of cephalosporins in different regimens
Single small trials addressed dose comparisons and provided no data for most outcomes, including adverse effects. Whether differences between groups occurred was unclear. No trials compared route of administration.
The quality of evidence for all head‐to‐head and dose comparisons was very low owing to very serious imprecision and serious risk of bias related to poor reporting of methods.
Authors' conclusions
Antibiotic prophylaxis appears to be effective in preventing postoperative infection in women undergoing elective vaginal or abdominal hysterectomy, regardless of the dose regimen. However, evidence is insufficient to show whether use of prophylactic antibiotics influences rates of adverse effects. Similarly, evidence is insufficient to show which (if any) individual antibiotic, dose regimen, or route of administration is safest and most effective. The most recent studies included in this review were 14 years old at the time of our search. Thus findings from included studies may not reflect current practice in perioperative and postoperative care and may not show locoregional antimicrobial resistance patterns.
Plain language summary
Antibiotic prophylaxis for elective hysterectomy
Review question
Are antibiotics effective and safe for preventing postoperative infection in women undergoing elective (non‐urgent) hysterectomy?
Background
Surgical operation carried out to remove the uterus (hysterectomy) is commonly performed. Most cases are performed as non‐urgent (elective) procedures for non‐cancerous (benign) conditions affecting the uterus, such as menstrual pain or abnormal bleeding patterns. Antibiotics are usually given before the operation is performed (prophylactic antibiotics, or antibiotic prophylaxis) to prevent or reduce the occurrence of infection after the procedure. Researchers in the Cochrane Collaboration reviewed the evidence on effectiveness and safety of antibiotics used to prevent infection after non‐urgent surgical operation to remove the uterus. Evidence is current to November 2016.
Study characteristics
We identified 37 randomised controlled trials (RCTs), which included a total of 6079 women and compared 20 different types of antibiotics versus placebo (an inactive pill) or versus one another.
Key results
This review found moderate‐quality evidence showing that antibiotics appear to be effective in preventing infection in women undergoing non‐urgent surgical removal of the uterus through the vagina or abdomen. This suggests that antibiotic prophylaxis reduces the average risk of postoperative infection after vaginal hysterectomy from about 62% to 12% to 25%, and after abdominal hysterectomy from about 39% to 8% to 26%.
However, evidence is insufficient to show whether use of prophylactic antibiotics influences rates of adverse effects (side effects), or whether any one antibiotic is more effective or safer than the others.
When antibiotics are compared head‐to‐head or in combination versus single antibiotics, it is unclear which individual antibiotic was more effective and safer, or whether combined antibiotics were more effective and safer than single antibiotics. The quality of the evidence for these comparisons is very low.
It is also unclear which dose regimen or route of administration of antibiotics is safest or most effective in women undergoing elective hysterectomy.
The most recent of the studies included in this review was published 14 years ago, at the time of our search. Thus findings from the included studies may not reflect current practice in perioperative and postoperative care and may not show locoregional antimicrobial resistance patterns.
Quality of the evidence
The quality of evidence for our main comparisons ranged from very low to moderate. The main limitations of this evidence are risk of bias due to poor reporting of randomisation methods, imprecision due to small sample sizes and low event rates, and inadequate reporting of adverse effects.
Summary of findings
Background
Description of the condition
Hysterectomy is one of the most commonly performed operations, particularly in the United States, where the lifetime risk of a hysterectomy is 45% (Merrill 2013). Most hysterectomies are elective (non‐urgent) procedures for benign gynaecological conditions; the most common of these in the United States is leiomyoma (fibroids). Other common indications include endometriosis, heavy menstrual bleeding, and uterovaginal prolapse. This surgery can be performed abdominally, laparoscopically, or vaginally, with or without laparoscopic assistance (Farquhar 2002). The incidence of postoperative infection after hysterectomy was found to be 2% in a recent large cohort from the United States, in which women had surgery between 2012 and 2015 (Upall 2016). In older cohorts, this percentage is likely to be higher owing to factors such as longer hospital stay and prolonged postoperative urinary catheterisation. Some types of hysterectomy may be more susceptible to infectious complications than others, depending on the extent of the breach in body tissues and in the genital tract.
Even with the best surgical and postoperative care, hysterectomy is unavoidably associated with high risk of infection because the procedure breaches the genital tract ‐ an area commonly colonised by a wide variety and large numbers of micro‐organisms. In addition, most women undergoing hysterectomy require an indwelling urinary catheter for the first 24 hours, which increases the risk of urinary tract infection. Common sites of infection after hysterectomy include bladder, pelvic floor, the cuff of tissue at the top of the vagina (vaginal vault), and the abdominal wound; related complications include pelvic abscess, infected haematoma (accumulation of blood from the wound), septicaemia (infection of the blood), and pneumonia (Duff 1980; Faro 2001). Such infections are usually caused by a mixture of bacteria from the woman's own vaginal or urethral tissues ‐ both Gram‐positive and Gram‐negative, and both aerobic and anaerobic (these terms refer to the staining techniques used in identification, and whether the bacteria are oxygen dependent). The individual woman's susceptibility to infection depends upon the effectiveness of her immune system, the virulence of the bacteria present, and the extent of tissue trauma and fluid collection resulting from surgery (Duff 1980).
Description of the intervention
"Antibiotic prophylaxis" refers to administration of antibiotics to prevent infection: It has been used in surgery since antibiotics were introduced in the 1950s, in an attempt to reduce the rate of postoperative infection. Such infection not only causes patient morbidity but may result in additional costs, extended hospital stay, and increased antibiotic use, which promotes the emergence of antimicrobial resistant organisms (Dellinger 1994). Antibiotic prophylaxis for hysterectomy has been extensively studied, and it has been estimated that such prophylaxis has reduced the rate of postoperative infection by more than half; otherwise, about 40% to 50% of women would develop infection after vaginal hysterectomy, and more than 20% after abdominal hysterectomy (Duff 1980; Mittendorf 1993). National guidelines now recommend this practice for all types of hysterectomy (ACOG 2009; Bratzler 2013; Dellinger 1994; Nelson 2016; RCOG 1999; SIGN 2008; Van Eyk N, van Schalkwyk J 2012), although in reality, application of such guidelines is variable (Gorecki 1999).
Although various antibiotic regimens and routes of delivery have been used, the most frequent current practice consists of a single dose of antibiotic given intravenously within two hours of the surgical incision, to facilitate optimum serum antibiotic levels during the operation (Classen 1992; DiPiro 1984; Nelson 2016). A single dose has been reported to be as effective as multiple doses, although some researchers have suggested repeat dosing if surgery is long or blood loss is high (DiPiro 1986; Tanos 1994). If prophylaxis is continued postoperatively, it is recommended that the duration of therapy should not exceed 24 hours (Dellinger 1994).
The type of antibiotic most commonly used is active against a wide range of bacteria (broad spectrum); this type includes amoxicillin/clavulanic acid (Augmentin) or a cephalosporin. Cephalosporins are grouped into generations according to their antimicrobial properties, with the oldest type referred to as "first generation". Subsequent generations of these drugs have progressively widened their antibacterial coverage against Gram‐negative organisms while showing a concurrent reduction in effectiveness against Gram‐positive organisms; moreover, wide use of very broad‐spectrum antibiotics greatly increases the risk of emergence of drug‐resistant bacteria (BNF 2002). It is generally recommended that first‐ or second‐generation cephalosporins should be used for prophylaxis, as they appear to be equally effective for this purpose, less expensive than other treatments, and less likely to favour drug resistance (Fukatsu 1997; Tanos 1994; Weed 2003).
How the intervention might work
Prophylaxis works by briefly bolstering tissue defence mechanisms to promote rapid restoration of normal immune responses after the trauma of surgery.
Why it is important to do this review
A very large body of evidence on prophylactic antibiotics for hysterectomy involves hundreds of clinical trials. However, review authors have not systematically assessed this evidence in recent times. Existing meta‐analyses conducted some years back focused mainly on abdominal hysterectomy. No meta‐analysis has focused on trials involving other routes of hysterectomy.
Several Cochrane reviews of prophylactic antibiotics for elective surgery have reported mixed findings. Two of these examined the topic of caesarean section (Gyte 2014; Nabhan 2016). Gyte 2014 evaluated different classes of prophylactic antibiotics for women undergoing caesarean section and found that cephalosporins and penicillins had similar efficacy for preventing immediate postoperative infection. Investigators provided no data on late infection, nor on outcomes for the baby. Nabhan 2016 compared routes of administration of prophylactic antibiotics and concluded that data show no clear difference between irrigation and intravenous routes in rates of post‐caesarean endometritis. A review on elective endoscopic retrograde cholangiopancreatography (Brand 2010) reported that antibiotic prophylaxis appeared to reduce rates of bacteraemia, cholangitis, and septicaemia. A review of different regimens of antibiotic prophylaxis for people undergoing orthognathic surgery (Brignardello‐Petersen 2015) found that long‐term antibiotic prophylaxis decreased the risk of skin and skin structure infection compared with short‐term prophylaxis, but comparisons between short‐term prophylaxis and a single preoperative dose were inconclusive. Reviews of antibiotic prophylaxis for elective open inguinal hernia repair (Sanchez‐Manuel 2012) or for elective laparoscopic cholecystectomy (Sanabria 2010) provided no clear evidence of benefit for the intervention group.
Objectives
To determine the effectiveness and safety of antibiotic prophylaxis in women undergoing elective hysterectomy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, controlled trials (RCTs) of women having an elective total or subtotal hysterectomy by any route and comparing prophylactic antibiotics versus placebo or versus a different type, route, or timing of antibiotic. Trials were at least double‐blinded (i.e. with participants and clinicians blinded). We did not include quasi‐randomised trials (e.g. trials that allocated treatment by date of birth, day of the week, medical record number, month of the year, or the order in which participants were enrolled in the study).
We excluded from the review studies that did not analyse at least 80% of women randomised for at least one outcome. When trials analysed at least 80% of participants for some outcomes but analysed less than 80% of participants for other outcomes, we included only those outcomes analysed for at least 80% of participants. The rationale for excluding trials with high numbers of withdrawals is that attrition was unlikely to be equally distributed between trial arms: Women who did not develop infection were more likely to be lost to follow‐up than those who did develop infection.
Types of participants
Women of any age without serious comorbidity (such as cancer) undergoing an elective total or subtotal abdominal, vaginal, laparoscopic, or laparoscopically assisted hysterectomy, with or without oophorectomy, for a benign gynaecological condition such as fibroids, endometriosis, uterovaginal prolapse, or heavy menstrual bleeding.
Types of interventions
Prophylactic antibiotics versus placebo or a different type or regimen of antibiotics.
The term "prophylactic" was defined as follows. Prophylactic: antibiotic(s) given when an individual had no signs or symptoms of infection, when no antibiotics had been taken within the previous 48 hours, and when the first dose was given up to 12 hours preoperatively and the last dose was given not more than 24 hours postoperatively.
Types of antibiotics
Antibiotics were classified into the following types.
-
Cephalosporins.
First‐generation (e.g. cefazolin, cephradine, cephazolin, cephalexin, cefadroxil).
Second‐generation (e.g. cefoxitin, cefuroxime, cephamandole, cefaclor, cefprozil, loracarbef).
Third‐generation (e.g. cefotaxime, cefotetan, ceftazidime, ceftriaxone, cefixime, cefpodoxime proxetil, ceftibuten, cefdinir, cephoperazone, ceftizoxime).
Fourth‐generation (e.g. cefepime).
Penicillins (e.g. penicillin, amoxicillin).
Macrolides (e.g. erythromycin, clarithromycin, azithromycin).
Fluoroquinolones (e.g. ciprofloxacin, levofloxacin, oxfloxacin).
Sulfonamides (e.g. co‐trimoxazole, trimethoprim).
Tetracyclines (e.g. tetracycline, doxycycline).
Aminogylocosides (e.g. gentamycin, tobramycin).
Glycopeptides (e.g. vancomycin).
Antiprotozoals (e.g. metronidazole, anitroimidazole).
-
Combination drugs.
Augmentin (amoxicillin and clavulanic acid).
Other combinations of drugs (will be considered individually).
Antibiotic regimens include the following.
Route: Any systemic regimen was included, irrespective of the route of administration (e.g. intravenous, intramuscular, oral, rectal).
Number of doses (e.g. single vs repeated doses).
Types of outcome measures
We considered trials if they reported any of the following clinical outcomes.
Primary outcomes
-
Infection: measured as the proportion of women who within eight weeks of surgery developed one of the following as defined by the study.
Total postoperative infection.
Abdominal wound infection (e.g. wound cellulitis, abscess, dehiscence).
Pelvic infection (including vaginal cuff (vault) infection, pelvic inflammatory disease, pelvic abscess, infected haematoma).
Urinary tract infection.
Other serious infection or infectious complication, such as septicaemia, septic shock, distant infection (e.g. pneumonia).
Postoperative fever of > 38° on at least two occasions more than four hours apart, excluding the day of surgery.
Total adverse effects: morbidity (e.g. allergic reaction, diarrhoea, bacterial resistance, or as defined by the study) and mortality (infection‐related and all‐cause).
We classified primary outcomes as early (before discharge from hospital or within seven days of surgery), late (at follow‐up: within eight weeks of surgery), or total (early + late).
Secondary outcomes
Need for therapeutic antibiotics ‐ early (before discharge from hospital or within seven days of surgery), late (at follow‐up: within eight weeks of surgery), or total (early + late).
Length of hospital stay.
Quality of life.
Search methods for identification of studies
In consultation with the Gynaecology and Fertility Group Information Specialist, we searched the following databases for all published and unpublished RCTs.
Electronic searches
We searched the following electronic databases, trial registers, and websites up to 29 November 2016.
Gynaecology and Fertility Group (CGF) Specialised Register of Controlled Trials.
Cochrane Central Register of Studies Online (CRSO).
MEDLINE.
Embase.
PsycINFO.
-
Cumulative Index to Nursing Allied Health and Literature (CINAHL).
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.2, Chapter 6, 6.4.11). We combined Embase, PsycINFO, and CINAHL searches using trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (http://www.sign.ac.uk/methodology/filters.html#random).
-
Other electronic sources of trials included:
trial registers for ongoing and registered trials;
http://www.clinicaltrials.gov (a service of the US National Institutes of Health);
http://www.who.int/trialsearch/Default.aspx (World Health Organization International Clinical Trials Registry Platform search portal) (Note: it is now mandatory for Cochrane reviews to include searches of trial registers);
DARE (Database of Abstracts of Reviews of Effects) in the Cochrane Library (http://onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html) (for reference lists from relevant non‐Cochrane reviews);
Web of Knowledge (http://wokinfo.com/ ‐ another source of trials and conference abstracts);
OpenGrey (http://www.opengrey.eu/ ‐ for unpublished literature from Europe);
Latin American Caribbean Health Sciences Literature (LILACS database) (http://regional.bvsalud.org/php/index.php?lang=en ‐ for trials from the Portuguese‐ and Spanish‐speaking world); and
PubMed and Google Scholar (for recent trials not yet indexed in MEDLINE).
For details of search strategies, see Appendix 1,Appendix 2,Appendix 3,Appendix 4,Appendix 5, and Appendix 6.
Searching other resources
We handsearched the reference lists of articles retrieved by the search and contacted experts in the field to request additional data. We also handsearched relevant journals and conference abstracts not included in the CGF register, in liaison with the Information Specialist from the CGF Group.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, we retrieved the full texts of all potentially eligible studies. At least two review authors (of VJ, JM, and RA) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies that were eligible for inclusion in the review. We contacted study investigators as required to clarify study eligibility. We resolved disagreements regarding study eligibility by discussion or by consultation with a third review author. We documented the selection process using a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow chart.
Data extraction and management
Two review authors independently extracted data from eligible studies using a data extraction form that they had designed and pilot‐tested. We resolved disagreements by discussion or by consultation with a third review author. Data extracted included study characteristics and outcome data. When studies had multiple publications, review authors collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review, and assigned such studies a single study ID with multiple references.
We contacted study investigators to request additional data on methods and/or results, as required.
Assessment of risk of bias in included studies
Two review authors independently examined included studies for risk of bias using the Cochrane "Risk of bias" assessment tool (Higgins 2011) to assess selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias such as differences in demographic characteristics of participants. We took care to search for within‐trial selective reporting, as seen in trials failing to report obvious outcomes, or reporting them in insufficient detail to allow inclusion. We sought published protocols and compared outcomes between the protocol and the final published study.
We resolved disagreements by discussion or by consultation with a third review author. We described all judgements fully and presented conclusions in the "Risk of bias" table; we incorporated these into the interpretation of review findings by performing sensitivity analyses (see below).
Measures of treatment effect
For dichotomous data (e.g. infection rates), we used numbers of events in control and intervention groups of each study to calculate risk ratios (RRs). For continuous data (e.g. length of hospital stay), when studies reported exactly the same outcomes, we calculated mean differences (MDs) between treatment groups. We reversed the direction of effect of individual studies, if required, to ensure consistency across trials. We intended to treat ordinal data (e.g. quality of life scores) as continuous data if any included studies reported ordinal data. We presented 95% confidence intervals (CIs) for all outcomes. We compared the magnitude and direction of effects reported by studies versus how they were presented in the review, while taking account of legitimate differences.
Unit of analysis issues
The primary analysis was per woman randomised.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible and attempted to obtain missing data from the original trialists. When these were unobtainable, we analysed only available data.
When studies reported sufficient detail for calculation of mean differences but no information on associated standard deviation (SD), we assumed the outcome to have a standard deviation equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by using the I2 measurement. We took an I2 measurement greater than 50% to indicate substantial heterogeneity (Higgins 2003; Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, review authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by staying alert for duplication of data. When we included 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small‐study effects (the tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
When studies were sufficiently similar, we combined the data using a fixed‐effect model.
We graphically displayed an increase in risk of a particular outcome within meta‐analyses to the right of the centre‐line, and a decrease in risk of a particular outcome to the left of the centre‐line.
We made the following comparisons.
Any antibiotic versus placebo.
Specific antibiotics versus placebo.
Head‐to‐head comparisons of antibiotics.
Comparisons of antibiotic regimens.
We subgrouped all analyses by surgical route: vaginal or abdominal. We did not pool these subgroups.
Subgroup analysis and investigation of heterogeneity
We subgrouped our main analysis according to the surgical route used (vaginal or abdominal). We did not undertake other prespecified subgroup analyses.
When we detected substantial heterogeneity (I2 > 50%), we explored possible explanations by performing sensitivity analyses. We took any statistical heterogeneity into account when interpreting results, especially if we noted any variation in the direction of effect estimates.
Sensitivity analysis
When heterogeneity was substantial (I2 > 50%), we conducted sensitivity analysis by choosing a statistical model (fixed‐effect vs random‐effects) and an effect estimate (risk ratio vs odds ratio), regardless of the number of trials included in an analysis. We planned to explore other clinical or methodological differences between studies only if data showed variation in the direction of effect.
Overall quality of the body of evidence: "Summary of findings" table
We prepared two separate "Summary of findings" tables for vaginal hysterectomy and abdominal hysterectomy based on the review's main comparison, that is, any antibiotics versus placebo. We used GRADEPRO (GRADEPro GDT 2014) and Cochrane methods (Higgins 2011) and used these tables to evaluate the overall quality of the body of evidence for main review outcomes (total postoperative infections, abdominal wound infection, urinary tract infection, pelvic infection, other serious infection, postoperative fever, and total adverse effects) by applying GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness, and publication bias). Two review authors working independently made judgements about evidence quality (high, moderate, low, or very low) and resolved disagreements by discussion. We justified, documented, and incorporated our judgements into reporting of results for each outcome.
Results
Description of studies
Results of the search
The search produced a total of 940 titles and abstracts after duplicates were removed; we considered 149 full‐text articles for further assessment. Thirty‐seven trials in 42 reports met the eligibility criteria for inclusion, and we excluded 107 full‐text articles. See Characteristics of included studies and Characteristics of excluded studies tables. The PRISMA flow chart in Figure 1 illustrates the flow of literature throughout the search and assessment process.
1.
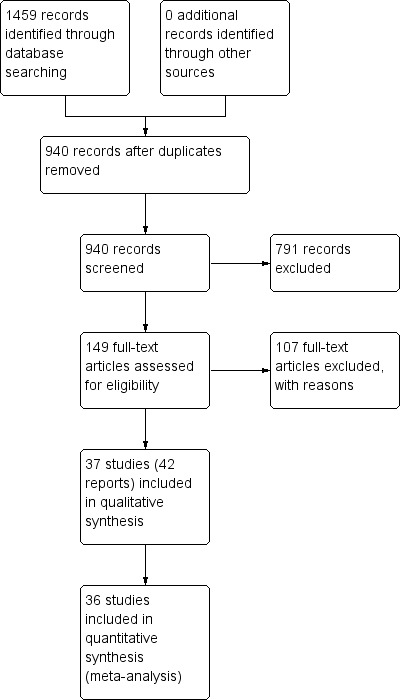
PRISMA flow chart.
Included studies
Study design and setting
We included 37 studies in this review (Benigno 1986; Boodt 1990; Chongsomchai 2002; Crosthwaite 1985; Davi 1985; Dhar 1993; Dhar 1993a; Duff 1982; Egarter 1988; Eron 1989; Faro 1988; Gall 1983; Hager 1989; Hedican 1976; Hemsell 1980; Hemsell 1983; Hemsell 1984; Hemsell 1985; Hemsell 1985a; Hemsell 1987; Hemsell 1989; Henriksson 1998; Holman 1978; Houang 1984; Houang 1984a; Jaffe 1985; Janssens 1982; Kauer 1990; Ledger 1973; Mathews 1977; Mathews 1979; Mendelson 1979; Polk 1980; Schepers 1981; Smith 1984; Stage 1982; Vincelette 1983).
The most recent study was Chongsomchai 2002, which was already 14 years old at the time of our search.
All included studies were parallel, double‐blinded, randomised controlled trials (RCTs). Twenty‐nine studies were two‐arm RCTs (Boodt 1990; Crosthwaite 1985; Davi 1985; Dhar 1993; Dhar 1993a; Duff 1982; Faro 1988; Gall 1983; Hager 1989; Hedican 1976; Hemsell 1980; Hemsell 1983; Hemsell 1984; Hemsell 1985a; Hemsell 1989; Henriksson 1998; Holman 1978; Houang 1984a; Jaffe 1985; Janssens 1982; Ledger 1973; Mathews 1977; Mathews 1979; Mendelson 1979; Polk 1980; Schepers 1981; Smith 1984; Stage 1982; Vincelette 1983). Eight studies were three‐arm RCTs (Benigno 1986; Chongsomchai 2002; Egarter 1988; Eron 1989; Hemsell 1985; Hemsell 1987; Houang 1984; Kauer 1990).
Seventeen studies were conducted in the United States (Benigno 1986; Duff 1982; Eron 1989; Gall 1983; Hager 1989; Hedican 1976; Hemsell 1980; Hemsell 1983; Hemsell 1984; Hemsell 1985; Hemsell 1985a; Hemsell 1987; Hemsell 1989; Holman 1978; Ledger 1973; Polk 1980; Stage 1982); five studies were conducted in the United Kingdom (Houang 1984; Houang 1984a; Mathews 1977; Mathews 1979; Smith 1984); two were conducted in Canada (Mendelson 1979; Vincelette 1983); and three in the Netherlands (Boodt 1990; Kauer 1990; Schepers 1981). Two studies each were conducted in Australia (Crosthwaite 1985; Egarter 1988) and India (Chandigarth) (Dhar 1993; Dhar 1993a); one study each was conducted in Belgium (Janssens 1982), Israel (Jaffe 1985), Sweden (Henriksson 1998), and Thailand (Chongsomchai 2002). The remaining two studies did not provide information on the countries in which they were conducted (Davi 1985; Faro 1988).
Six of the included studies were conducted at more than one centre: 14 centres (Stage 1982), four centres (Benigno 1986), three centres (Hager 1989; Henriksson 1998), and two centres (Chongsomchai 2002; Eron 1989); five studies did not report the number of centres (Davi 1985; Egarter 1988; Faro 1988; Hemsell 1985; Schepers 1981); and each of the remaining 26 studies was conducted at a single centre (Boodt 1990; Crosthwaite 1985; Dhar 1993; Dhar 1993a; Duff 1982; Gall 1983; Hedican 1976; Hemsell 1980; Hemsell 1983; Hemsell 1984; Hemsell 1985a; Hemsell 1987; Hemsell 1989; Holman 1978; Houang 1984; Houang 1984a; Jaffe 1985; Janssens 1982; Kauer 1990; Ledger 1973; Mathews 1977; Mathews 1979; Mendelson 1979; Polk 1980; Smith 1984; Vincelette 1983).
Participants
The 37 included studies enrolled a total of 6079 women. Seventeen studies randomised or analysed a total of 100 or fewer women (Crosthwaite 1985; Dhar 1993; Dhar 1993a; Duff 1982; Gall 1983; Hager 1989; Hedican 1976; Hemsell 1980; Hemsell 1985a; Houang 1984a; Jaffe 1985; Kauer 1990; Ledger 1973; Mathews 1977; Mathews 1979; Mendelson 1979; Smith 1984); eight studies randomised or analysed a total of 101 to 200 women (Egarter 1988; Faro 1988; Hemsell 1983; Hemsell 1984; Hemsell 1985; Janssens 1982; Schepers 1981; Vincelette 1983); five studies randomised or analysed a total of 201 to 300 women (Eron 1989; Hemsell 1987; Hemsell 1989; Holman 1978; Stage 1982); five studies randomised or analysed a total of 301 to 400 women (Benigno 1986; Chongsomchai 2002; Davi 1985; Henriksson 1998; Houang 1984); one study randomised a total of 403 women (Boodt 1990); and another randomised a total of 557 women (Polk 1980).
A common inclusion criterion was that women had to be scheduled for elective abdominal hysterectomy, vaginal hysterectomy, or both types of hysterectomy for a benign condition. Thirteeen studies included women scheduled for abdominal hysterectomy (Boodt 1990; Chongsomchai 2002; Davi 1985; Dhar 1993a; Duff 1982; Gall 1983; Hemsell 1983; Hemsell 1985; Houang 1984a; Jaffe 1985; Mathews 1977; Schepers 1981; Smith 1984); 14 studies included women scheduled for elective vaginal hysterectomy (Benigno 1986; Dhar 1993; Egarter 1988; Faro 1988; Hager 1989; Hedican 1976; Hemsell 1980; Hemsell 1984; Hemsell 1985a; Hemsell 1987; Kauer 1990; Ledger 1973; Mathews 1979; Mendelson 1979); nine studies included women scheduled for either abdominal or vaginal hysterectomy (Crosthwaite 1985; Eron 1989; Hemsell 1989; Holman 1978; Houang 1984; Janssens 1982; Polk 1980; Stage 1982; Vincelette 1983); and one study did not report the type of hysterectomy for which women were scheduled (Henriksson 1998).
No included studies focused on antibiotic prophylaxis in participants undergoing laparoscopically performed hysterectomy.
Common exclusion criteria were emergency hysterectomy; pregnancy‐related hysterectomy; hypersensitivity to antibiotics such as cephalosporin, penicillin, amoxicillin, etc.; and use of antibiotics within two to seven days before surgery.
Interventions
Included studies compared different classes of antibiotics with placebo or with each other. Included studies identified the following treatment groups.
Any antibiotic versus placebo (Boodt 1990; Chongsomchai 2002; Crosthwaite 1985; Davi 1985; Dhar 1993; Dhar 1993a; Duff 1982; Egarter 1988; Gall 1983; Hedican 1976; Hemsell 1980; Hemsell 1983; Henriksson 1998; Holman 1978; Houang 1984; Jaffe 1985; Janssens 1982; Ledger 1973; Mathews 1977; Mathews 1979; Mendelson 1979; Polk 1980; Smith 1984; Vincelette 1983).
Cephalosporin versus placebo (Chongsomchai 2002; Davi 1985; Duff 1982; Gall 1983; Hedican 1976; Hemsell 1980; Hemsell 1983; Holman 1978; Ledger 1973; Mendelson 1979; Polk 1980; Stage 1982).
Penicillin versus placebo (Chongsomchai 2002; Houang 1984).
Antiprotozoal versus placebo (Crosthwaite 1985; Dhar 1993; Dhar 1993a; Egarter 1988; Hemsell 1983; Henriksson 1998; Janssens 1982; Vincelette 1983).
Sulphonamides versus placebo (Jaffe 1985; Mathews 1977; Mathews 1979; Smith 1984).
Cephalosporin plus antiprotozoal versus placebo (Boodt 1990).
Penicillin plus antiprotozoal versus placebo (Houang 1984).
Lincosamide versus placebo (Egarter 1988).
Cephalosporin versus penicillin (Benigno 1986; Chongsomchai 2002; Faro 1988; Hager 1989).
Cephalosporin versus tetracycline (Hemsell 1985a).
Cephalosporin versus antiprotozoal (Kauer 1990).
Antiprotozoal versus lincosamide (Egarter 1988).
Cephalosporin plus antiprotozoal versus cephalosporin only (Kauer 1990).
Cephalosporin plus antiprotozoal versus antiprotozoal only (Kauer 1990).
Penicillin plus antiprotozoal versus penicillin only (Houang 1984; Houang 1984a).
Cephalosporin early administration versus usual timing (both single dose) (Eron 1989).
Cephalosporin one dose versus two doses (Hemsell 1985).
Cephalosporin one dose versus three doses (Hemsell 1984; Hemsell 1985).
Cephalosporin one dose versus multiple doses (Mendelson 1979).
Cephalosporin one gram versus two grams (Hemsell 1987).
Included studies administered antibiotics through the following routes.
Intravenous (IV) (Benigno 1986; Boodt 1990; Chongsomchai 2002; Duff 1982; Egarter 1988; Faro 1988; Gall 1983; Hager 1989; Hemsell 1985; Hemsell 1985a; Hemsell 1989; Henriksson 1998; Jaffe 1985; Kauer 1990; Mathews 1979; Mendelson 1979; Polk 1980; Schepers 1981; Stage 1982; Vincelette 1983).
Intramuscular (IM) (Davi 1985; Hemsell 1980; Hemsell 1983; Hemsell 1987; Smith 1984).
IV and IM (Eron 1989; Hedican 1976; Hemsell 1984; Holman 1978).
Oral (Crosthwaite 1985; Dhar 1993; Dhar 1993a; Janssens 1982).
IV and rectal (Houang 1984; Houang 1984a).
One of the included studies did not state the route used for administration of antibiotics (Ledger 1973).
Investigators administered antibiotics as a single dose, as multiple doses, or as single versus multiple doses in the following studies.
Single dose (Boodt 1990; Chongsomchai 2002; Crosthwaite 1985; Dhar 1993; Dhar 1993a; Duff 1982; Hager 1989; Hemsell 1987; Janssens 1982; Ledger 1973; Mathews 1977; Mathews 1979).
Multiple doses (Boodt 1990; Davi 1985; Egarter 1988; Faro 1988; Gall 1983; Hedican 1976; Hemsell 1980; Hemsell 1983; Hemsell 1984; Henriksson 1998; Holman 1978; Houang 1984; Houang 1984a; Ledger 1973; Polk 1980; Schepers 1981; Stage 1982; Vincelette 1983).
Single dose versus multiple doses (Eron 1989; Hemsell 1985; Hemsell 1985a; Hemsell 1989; Janssens 1982; Mendelson 1979).
Timing and duration of administration varied in the included studies. However, none of the included studies administered the first dose of antibiotics more than 12 hours before surgery and the last dose more than 24 hours after surgery.
Outcomes
Primary outcome measures of this review were presence of postoperative infection (total postoperative infections, abdominal wound infection, pelvic infection, urinary tract infection (UTI), other serious infection (such as pneumonia, septicaemia, septic shock), and postoperative fever), total adverse effects such as morbidity (e.g. diarrhoea, allergic reactions), and mortality. Thirty‐six included studies reported data on at least one of the review's primary outcome measures (Benigno 1986; Boodt 1990; Chongsomchai 2002; Crosthwaite 1985; Davi 1985; Dhar 1993; Dhar 1993a; Duff 1982; Egarter 1988; Eron 1989; Faro 1988; Gall 1983; Hager 1989; Hedican 1976; Hemsell 1980; Hemsell 1983; Hemsell 1984; Hemsell 1985; Hemsell 1985a; Hemsell 1987; Hemsell 1989; Henriksson 1998; Holman 1978; Houang 1984; Houang 1984a; Jaffe 1985; Janssens 1982; Kauer 1990; Ledger 1973; Mathews 1977; Mathews 1979; Polk 1980; Schepers 1981; Smith 1984; Stage 1982; Vincelette 1983); and one of the included studies did not report data on any of the review's primary outcomes (Mendelson 1979). Twenty‐five included studies reported data on adverse effects, most in narrative form (Benigno 1986; Chongsomchai 2002; Crosthwaite 1985; Davi 1985; Dhar 1993; Dhar 1993a; Duff 1982; Eron 1989; Gall 1983; Hager 1989; Hemsell 1980; Hemsell 1984; Hemsell 1985a; Hemsell 1987; Hemsell 1989; Henriksson 1998; Jaffe 1985; Kauer 1990; Mathews 1977; Mathews 1979; Polk 1980; Schepers 1981; Smith 1984; Stage 1982; Vincelette 1983). Common adverse effects included allergy reactions and diarrhoea. None of the included studies reported any incident of mortality.
Secondary outcome measures included any requirement for therapeutic antibiotics, length of hospital stay, and quality of life following surgery. Twenty‐seven included studies reported on at least one of the secondary outcome measures (Benigno 1986; Boodt 1990; Chongsomchai 2002; Dhar 1993; Dhar 1993a; Duff 1982; Egarter 1988; Eron 1989; Faro 1988; Gall 1983; Hager 1989; Hemsell 1980; Hemsell 1983; Hemsell 1984; Hemsell 1985; Hemsell 1985a; Hemsell 1987; Hemsell 1989; Holman 1978; Jaffe 1985; Kauer 1990; Ledger 1973; Mathews 1977; Mathews 1979; Polk 1980; Stage 1982; Vincelette 1983). Secondary outcome measures commonly reported were need for therapeutic antibiotics and length of hospital stay; no studies provided data on quality of life. The remaining 10 studies did not report on any of the secondary outcome measures (Crosthwaite 1985; Davi 1985; Hedican 1976; Henriksson 1998; Houang 1984; Houang 1984a; Janssens 1982; Mendelson 1979; Schepers 1981; Smith 1984).
Excluded studies
Review authors determined that 107 studies were not eligible for inclusion in this review. Common reasons for exclusion were administration of antibiotics more than 12 hours before surgery or for more than 24 hours after surgery and non‐blinding of participants and personnel. For further details on reasons for exclusion of studies, see Characteristics of excluded studies table.
Risk of bias in included studies
2.
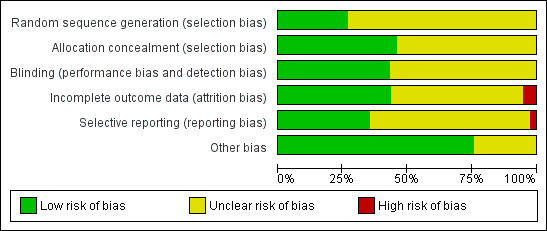
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
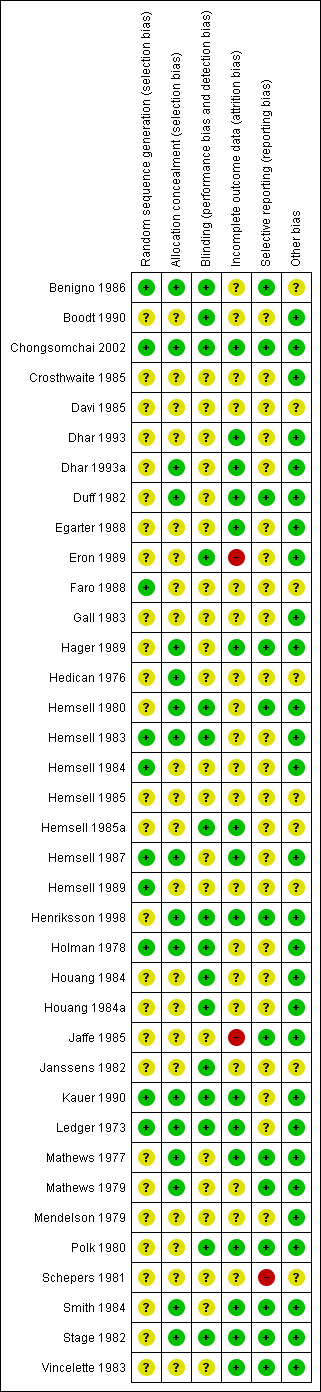
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We considered processes used in sequence generation to be adequate in 10 of the included studies because they involved the use of computers (Benigno 1986; Chongsomchai 2002; Faro 1988; Hemsell 1984; Hemsell 1987; Hemsell 1989) or random number tables (Hemsell 1983; Holman 1978; Kauer 1990; Ledger 1973). We therefore rated these studies as having low risk of bias with respect to random sequence generation. The remaining 27 studies provided insufficient information to permit conclusive judgements on the process involved in sequence generation; thus we rated them as having unclear risk of bias.
Allocation concealment
We rated 17 studies as having low risk of bias with respect to allocation concealment (Benigno 1986; Chongsomchai 2002; Dhar 1993a; Duff 1982; Hager 1989; Hedican 1976; Hemsell 1980; Hemsell 1983; Hemsell 1987; Henriksson 1998; Holman 1978; Kauer 1990; Ledger 1973; Mathews 1977; Mathews 1979; Smith 1984; Stage 1982). We considered the processes involved in concealing allocations in these studies to be adequate; these included remote or central allocation through the hospital pharmacy and use of sealed opaque envelopes. We assessed the remaining 20 studies as having unclear risk because information was insufficient to allow conclusive judgements with respect to allocation concealment.
Blinding
We considered that blinding was likely to influence findings for both primary and secondary review outcomes. Although we considered all included studies to be adequate with regard to blinding of both participants and physicians, most did not provide adequate information on how participants were evaluated postoperatively. Only 16 studies reported sufficient information on outcome assessment and/or participant follow‐up; we thus rated these studies as having low risk with respect to performance and detection bias (Benigno 1986; Boodt 1990; Chongsomchai 2002; Eron 1989; Hemsell 1980; Hemsell 1983; Hemsell 1985a; Henriksson 1998; Holman 1978; Houang 1984; Houang 1984a; Janssens 1982; Kauer 1990; Ledger 1973; Polk 1980; Stage 1982). The remaining 21 studies did not provide sufficient information on whether outcome assessors were blinded; we therefore rated these studies as having unclear risk with respect to performance and detection bias (Crosthwaite 1985; Davi 1985; Dhar 1993; Dhar 1993a; Duff 1982; Egarter 1988; Faro 1988; Gall 1983; Hager 1989; Hedican 1976; Hemsell 1984; Hemsell 1985; Hemsell 1987; Hemsell 1989; Jaffe 1985; Mathews 1977; Mathews 1979; Mendelson 1979; Schepers 1981; Smith 1984; Vincelette 1983).
Incomplete outcome data
We judged 16 studies as having low risk with respect to incomplete outcome data or attrition bias (Chongsomchai 2002; Dhar 1993; Dhar 1993a; Duff 1982; Egarter 1988; Hager 1989; Hemsell 1985a; Hemsell 1987; Henriksson 1998; Kauer 1990; Ledger 1973; Mathews 1977; Polk 1980; Smith 1984; Stage 1982; Vincelette 1983). Proportions of withdrawals/losses to follow‐up and reasons for withdrawal in these studies were fairly well balanced or similar across treatment groups, or outcome data were analysed on an intention‐to‐treat (ITT) basis by including all randomised women in data analyses. Nineteen studies provided insufficient information on the number of withdrawals/losses to follow‐up and/or on reasons for withdrawal, and data were not analysed on the basis of ITT (Benigno 1986; Boodt 1990; Crosthwaite 1985; Davi 1985; Faro 1988; Gall 1983; Hedican 1976; Hemsell 1980; Hemsell 1983; Hemsell 1984; Hemsell 1985; Hemsell 1989; Houang 1984a; Janssens 1982; Mathews 1979; Mendelson 1979; Schepers 1981). We thus rated these studies as having unclear risk with respect to attrition bias. We rated the remaining two studies as having high risk of bias: In one of these studies, proportions of withdrawals were not balanced between groups and data were not analysed on the basis of ITT (Eron 1989); in the other study, proportions of withdrawals and reasons for withdrawal were not balanced across treatment groups (Jaffe 1985).
Selective reporting
Protocols were not available for any of the included studies, and review authors could not determine whether outcomes were selectively reported. Therefore, the process of detecting selective reporting bias in included studies involved careful assessment of methods sections to determine which outcomes were prespecified and whether data were reported on all prespecified outcomes. Thirteen studies provided data on all outcomes prespecified in the methods sections; we rated these as having low risk with respect to selective reporting (within‐trial selective reporting) (Benigno 1986; Chongsomchai 2002; Duff 1982; Hager 1989; Hemsell 1980; Henriksson 1998; Jaffe 1985; Mathews 1977; Mathews 1979; Polk 1980; Smith 1984; Stage 1982; Vincelette 1983). Twenty‐three studies provided insufficient information to allow conclusive judgements with respect to selective reporting; therefore, we rated these studies as having unclear risk of selective reporting bias (Boodt 1990; Crosthwaite 1985; Davi 1985; Dhar 1993; Dhar 1993a; Egarter 1988; Eron 1989; Faro 1988; Gall 1983; Hedican 1976; Hemsell 1983; Hemsell 1984; Hemsell 1985; Hemsell 1985a; Hemsell 1987; Hemsell 1989; Holman 1978; Houang 1984; Houang 1984a; Janssens 1982; Kauer 1990; Ledger 1973; Mendelson 1979). We rated the only remaining study as having high risk of selective reporting because evidence showed selective reporting, with no data reported on some of the outcomes prespecified in the methods section (Schepers 1981).
Other potential sources of bias
We assessed other potential sources of bias with respect to whether data showed significant differences between treatment groups in terms of baseline demographic characteristics of participants, such as age and body mass index (BMI). In 28 studies, baseline demographic characteristics were similar between treatment groups; thus we rated these studies as having low risk with respect to other potential sources of bias (Boodt 1990; Chongsomchai 2002; Crosthwaite 1985; Dhar 1993; Dhar 1993a; Duff 1982; Egarter 1988; Eron 1989; Gall 1983; Hager 1989; Hemsell 1980; Hemsell 1983; Hemsell 1984; Hemsell 1987; Henriksson 1998; Holman 1978; Houang 1984; Houang 1984a; Jaffe 1985; Kauer 1990; Ledger 1973; Mathews 1977; Mathews 1979; Mendelson 1979; Polk 1980; Smith 1984; Stage 1982; Vincelette 1983). The remaining nine studies provided insufficient information to allow conclusive judgements with respect to whether significant differences in baseline demographic characteristics were evident between treatment groups; we thus rated these studies as having unclear risk with respect to other sources of bias (Benigno 1986; Davi 1985; Faro 1988; Hedican 1976; Hemsell 1985; Hemsell 1985a; Hemsell 1989; Janssens 1982; Schepers 1981).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Antibiotics compared with placebo for prophylaxis in elective vaginal hysterectomy.
| Antibiotics compared with placebo for prophylaxis in elective vaginal hysterectomy | ||||||
| Population: women having elective vaginal hysterectomy Settings: hospital Intervention: antibiotics Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Antibiotics | |||||
| Total postoperative infections ‐ early and late | Moderatea | RR 0.28 (0.19 to 0.4) | 293 (4 studies) | ⊕⊕⊕⊝ lowb,c,f | ||
| 618 per 1000 | 173 per 1000 (117 to 247) | |||||
| Urinary tract infection | Moderatea | RR 0.58 (0.43 to 0.77) | 1473 (8 studies) | ⊕⊕⊕⊝ moderateb | ||
| 127 per 1000 | 74 per 1000 (55 to 98) | |||||
| Pelvic infection | Moderatea | RR 0.28 (0.20 to 0.39) | 1693 (11 studies) | ⊕⊕⊕⊝ moderateb,d | ||
| 134 per 1000 | 38 per 1000 (27 to 52) | |||||
| Other serious infections | Moderatea | RR 0.20 (0.01 to 4.10) | 146 (1 study) | ⊕⊝⊝⊝ very lowb,e | ||
| 27 per 1000 | 5 per 1000 (0 to 111) | |||||
| Postoperative fever | Moderatea | RR 0.43 (0.34 to 0.54) | 1562 (9 studies) | ⊕⊕⊕⊝ moderateb | ||
| 219 per 1000 | 94 per 1000 (74 to 118) | |||||
| Total adverse effects ‐ not reported | This outcome was not reported | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aMedian baseline risk of control group bDowngraded one level for serious risk of bias: sequence generation and allocation concealment assessed as "unclear" in some studies owing to poor reporting cSubstantial heterogeneity for this comparison (I2 = 85%). The quality of the evidence was not downgraded for inconsistency, as the direction of effect was consistent and all inconsistency was attributable to a study that measured only early postoperative infection rates (to hospital discharge), whereas the other three studies measured both early and late infection dSubstantial heterogeneity for this comparison (I2 = 57%), but the quality of the evidence was not downgraded for inconsistency, as the direction of effect was consistent eDowngraded two levels for very serious imprecision: small sample size and effect estimate with wide confidence interval
fDowngraded two levels for serious imprecision: small sample size
Summary of findings 2. Antibiotics compared with placebo for prophylaxis in elective abdominal hysterectomy.
| Antibiotics compared with placebo for prophylaxis in elective abdominal hysterectomy | ||||||
| Population: women having elective abdominal hysterectomy Settings: hospital Intervention: antibiotics Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Antibiotics | |||||
| Total postoperative infections ‐ early and late | Moderatea | RR 0.38 (0.21 to 0.67) | 158 (1 study) | ⊕⊕⊝⊝ lowb,c | ||
| 388 per 1000 | 147 per 1000 (82 to 260) | |||||
| Abdominal wound infection | Moderatea | RR 0.51 (0.36 to 0.73) | 2247 (11 studies) | ⊕⊕⊕⊝ moderateb | ||
| 65 per 1000 | 33 per 1000 (23 to 47) | |||||
| Urinary tract infection | Moderatea | RR 0.41 (0.31 to 0.53) | 2705 (11 studies) | ⊕⊕⊕⊝ moderateb | ||
| 132 per 1000 | 54 per 1000 (41 to 70) | |||||
| Pelvic infection | Moderatea | RR 0.50 (0.35 to 0.71) | 1883 (11 studies) | ⊕⊕⊕⊝ moderateb | ||
| 83 per 1000 | 42 per 1000 (29 to 59) | |||||
| Other serious infections | Moderatea | RR 0.44 (0.12 to 1.69) | 476 (2 studies) | ⊕⊝⊝⊝ very lowb,d,e | ||
| 27 per 1000 | 12 per 1000 (3 to 46) | |||||
| Postoperative fever | Moderatea | RR 0.59 (0.50 to 0.70) | 2394 (11 studies) | ⊕⊕⊕⊝ moderateb | ||
| 242 per 1000 | 143 per 1000 (121 to 169) | |||||
| Total adverse effects | Moderatea | RR 1.80 (0.62 to 5.18) | 430 (2 studies) | ⊕⊝⊝⊝ very lowb,e | ||
| 23 per 1000 | 41 per 1000 (14 to 119) | |||||
| *The basis for assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aMedian baseline risk of control group bDowngraded one level for serious risk of bias: sequence generation and/or allocation concealment assessed as "unclear" in some studies owing to poor reporting cDowngraded one level for serious imprecision: small sample size dSubstantial heterogeneity for this comparison (I2 = 51%), but the quality of the evidence was not downgraded for inconsistency, as the direction of effect was consistent eDowngraded two levels for very serious imprecision: small sample size and effect estimate with wide confidence interval
Summary of findings 3. Head‐to‐head comparisons of antibiotics for prophylaxis in elective vaginal hysterectomy.
| Antibiotics compared with alternative antibiotics for prophylaxis in elective vaginal hysterectomy | |||||
| Population: women having elective vaginal hysterectomy Settings: hospital Intervention: antibiotics Comparison: alternative antibiotics | |||||
| Outcomes | Illustrative comparative risks | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Antibiotics vs alternative antibiotics | |||||
|
When data were available, no evidence showed a difference between any groups compared for any of our primary outcomes, except:
|
|
⊕⊝⊝⊝ very lowa,b | ||
| Total adverse effects |
|
|
⊕⊝⊝⊝ very lowa,b | ||
| CI: confidence interval; RCT: randomised controlled trial GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | |||||
aDowngraded two levels for very serious imprecision with very few events and wide confidence intervals bDowngraded one level for serious risk of bias: methods were poorly reported in most studies
Summary of findings 4. Head‐to‐head comparisons of antibiotics for prophylaxis in elective abdominal hysterectomy.
| Head‐to‐head comparisons of antibiotics for prophylaxis in elective abdominal hysterectomy | |||||
| Population: women having elective abdominal hysterectomy Settings: hospital Intervention: antibiotics Comparison: alternative antibiotics | |||||
| Outcomes | Illustrative comparative risks | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Antibiotics vs alternative antibiotics | |||||
|
|
|
⊕⊝⊝⊝ very low1,2 | ||
|
|
||||
| CI: confidence interval; RCT: randomised controlled trial GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | |||||
aDowngraded two levels for very serious imprecision with very few events and wide confidence intervals bDowngraded one level for serious risk of bias: methods were poorly reported in most studies
1. Any antibiotics versus placebo
Primary outcomes
1.1 Total postoperative infections ‐ early and late
See Analysis 1.1; Figure 4
1.1. Analysis.
Comparison 1 Any antibiotic versus placebo, Outcome 1 Total postoperative infections ‐ early and late.
4.
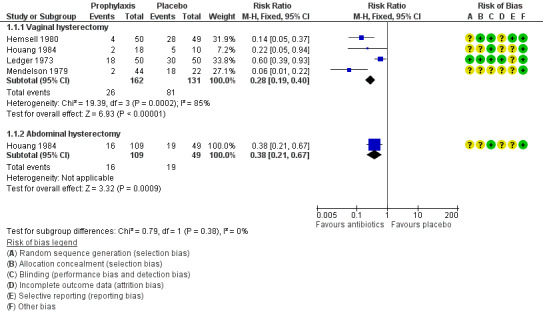
Forest plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.1 Total postoperative infections ‐ early and late.
1.1.1 Vaginal hysterectomy
The rate of postoperative infection (early or late) was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.28, 95% CI 0.19 to 0.40; four RCTs, N = 293; I2 = 85%; low‐quality evidence; Analysis 1.1). Evidence suggests that if the average risk of infection with placebo is assumed to be 62%, the risk following antibiotic prophylaxis would be between 12% and 25%. Although heterogeneity for this comparison was substantial (I2 = 85%), we did not downgrade the quality of evidence for inconsistency because the direction of effect was consistent and all inconsistency was attributable to Ledger 1973, which measured only early postoperative infection rates (to hospital discharge). The other three studies in this comparison measured both early and late infections.
On sensitivity analysis, observed evidence of a difference in the incidence of total postoperative infections between the two groups remained whether odds ratio (OR) (OR 0.13, 95% CI 0.08 to 0.23) or a random‐effects (RE) model (RR 0.19, 95% CI 0.06 to 0.67) was used.
1.1.2 Abdominal hysterectomy
The rate of postoperative infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.38, 95% CI 0.21 to 0.67; one RCT, N = 158; low‐quality evidence; Analysis 1.1). Evidence suggests that if the average risk of infection with placebo is assumed to be 39%, risk following antibiotic prophylaxis would be between 8% and 26%.
1.2 Abdominal wound infection
1.2.1 Abdominal hysterectomy
The rate of abdominal wound infection in women who received prophylactic antibiotics was lower than in those given placebo (RR 0.51, 95% CI 0.36 to 0.73; 11 RCTs, N = 2247; I2 = 6%; moderate‐quality evidence; Analysis 1.2). Evidence suggests that if the average risk of infection with placebo is assumed to be 7%, risk following antibiotic prophylaxis would be between 2% and 5%.
1.2. Analysis.
Comparison 1 Any antibiotic versus placebo, Outcome 2 Abdominal wound infection.
1.3 Urinary tract infection
1.3.1 Vaginal hysterectomy
The rate of urinary tract infection (UTI) in women who received prophylactic antibiotics was lower than in those given placebo (RR 0.58, 95% CI 0.43 to 0.77; eight RCTs, N = 1473; I2 = 44%; moderate‐quality evidence; Analysis 1.3). Evidence suggests that if the average risk of infection with placebo is assumed to be 13%, risk following antibiotic prophylaxis would be between 6% and 10%.
1.3. Analysis.
Comparison 1 Any antibiotic versus placebo, Outcome 3 Urinary tract infection.
1.3.2 Abdominal hysterectomy
The rate of UTI was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.41, 95% CI 0.31 to 0.53; 11 RCTs, N = 2705; I2 = 28%; moderate‐quality evidence; Analysis 1.3). Evidence suggests that if the average risk of infection with placebo is assumed to be 13%, risk following antibiotic prophylaxis would be between 4% and 7%.
1.4 Pelvic infection
1.4.1 Vaginal hysterectomy
The rate of pelvic infection in women who received prophylactic antibiotics was lower than in those given placebo (RR 0.28, 95% CI 0.20 to 0.39; 11 RCTs, N = 1693; I2 = 57%; moderate‐quality evidence; Analysis 1.4). Evidence suggests that if the average risk of infection with placebo is assumed to be 13%, risk following antibiotic prophylaxis would be between 3% and 5%. Heterogeneity for this comparison was substantial (I2 = 57%), but we did not downgrade the quality of the evidence for inconsistency, as the direction of effect was consistent. Evidence of a difference in reported cases of pelvic infection persisted whether sensitivity analysis was based on OR (OR 0.17, 95% CI 0.11 to 0.27) or on an RE model (RR 0.22, 95% CI 0.11 to 0.46).
1.4. Analysis.
Comparison 1 Any antibiotic versus placebo, Outcome 4 Pelvic infection.
1.4.2 Abdominal hysterectomy
The rate of pelvic infection in women who received prophylactic antibiotics was lower than in those given placebo (RR 0.50, 95% CI 0.35 to 0.71; 11 RCTs, N = 1883; I2 = 11%; moderate‐quality evidence; Analysis 1.4). Evidence suggests that if the average risk of infection with placebo is assumed to be 8%, risk following antibiotic prophylaxis would be between 3% and 6%.
1.5 Other serious infection
1.5.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups in the rate of other serious infection (RR 0.20, 95% CI 0.01 to 4.10; one RCT, N = 146; very low‐quality evidence; Analysis 1.5). Evidence suggests that if the average risk of infection with placebo is assumed to be 3%, risk following antibiotic prophylaxis would be between 0% and 11%.
1.5. Analysis.
Comparison 1 Any antibiotic versus placebo, Outcome 5 Other serious infections.
1.5.2 Abdominal hysterectomy
It is unclear whether data showed a difference between groups in the rate of other serious infection (RR 0.44, 95% CI 0.12 to 1.69; two RCTs, N = 476; I2 = 29%; very low‐quality evidence; Analysis 1.5). Evidence suggests that if the risk of other serious infection with placebo is assumed to be 3%, risk following antibiotic prophylaxis would be between 0% and 5%.
1.6. Postoperative fever
1.6.1 Vaginal hysterectomy
The rate of postoperative fever in women who received prophylactic antibiotics was lower than in those given placebo (RR 0.43, 95% CI 0.34 to 0.54; nine RCTs, N = 1562; I2 = 47%; moderate‐quality evidence; Analysis 1.6). Evidence suggests that if the average risk of postoperative fever with placebo is assumed to be 22%, risk following antibiotic prophylaxis would be between 7% and 12%.
1.6. Analysis.
Comparison 1 Any antibiotic versus placebo, Outcome 6 Postoperative fever.
1.6.2 Abdominal hysterectomy
The rate of postoperative fever in women who received prophylactic antibiotics was lower than in those given placebo (RR 0.59, 95% CI 0.50 to 0.70; 11 RCTs, N = 2394; I2 = 55%; moderate‐quality evidence; Analysis 1.6; Figure 5). Evidence suggests that if the average risk of postoperative fever with placebo is assumed to be 24%, risk following antibiotic prophylaxis would be between 12% and 17%. Heterogeneity for this comparison was substantial (I2 = 55%), but we did not downgrade the quality of the evidence for inconsistency, as the direction of effect was consistent. Evidence of a difference in reported cases of postoperative fever persisted whether sensitivity analysis was based on OR (OR 0.50, 95% CI 0.40 to 0.62) or on an RE model (RR 0.55, 95% CI 0.42 to 0.72).
5.
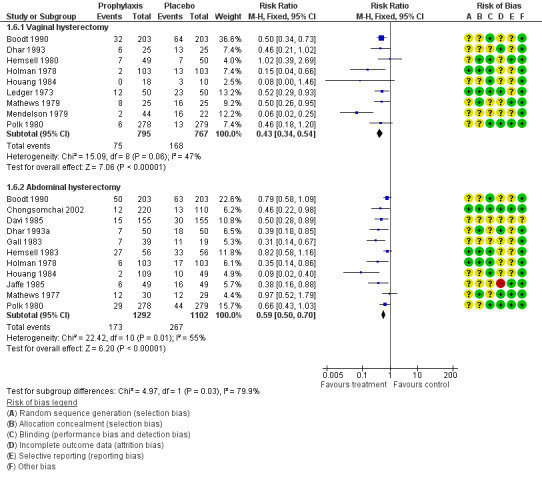
Forest plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.6 Postoperative fever.
1.7 Total adverse effects
See Analysis 1.7; Figure 6
1.7. Analysis.
Comparison 1 Any antibiotic versus placebo, Outcome 7 Total adverse effects.
6.
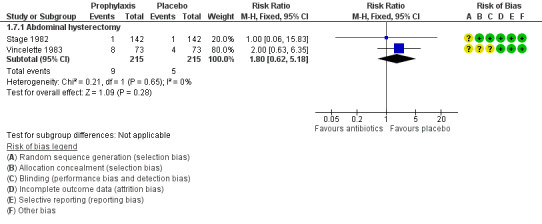
Forest plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.7 Total adverse effects.
1.7.1 Vaginal hysterectomy
Investigators provided no data for this outcome.
1.7.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups in the rate of total adverse effects (RR 1.80, 95% CI 0.62 to 5.18; two RCTs, N = 430; I2 = 0%; very low‐quality evidence; Analysis 1.7). Evidence suggests that if the average risk of total adverse effects with placebo is assumed to be 2%, risk following antibiotic prophylaxis would be between 1% and 12%.
Secondary outcomes
1.8 Need for therapeutic antibiotics
1.8.1 Vaginal hysterectomy
The rate of need for therapeutic antibiotics was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.51, 95% CI 0.37 to 0.68; six RCTs, N = 1309; I2 = 30%; Analysis 1.8).
1.8. Analysis.
Comparison 1 Any antibiotic versus placebo, Outcome 8 Need for therapeutic antibiotics.
1.8.2 Abdominal hysterectomy
The rate of need for therapeutic antibiotics was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.74, 95% CI 0.59 to 0.93; six RCTs, N = 1359; I2 = 34%; Analysis 1.8).
1.9 Length of hospital stay
1.9.1 Vaginal hysterectomy
Mean length of hospital stay was shorter in women who received prophylactic antibiotics than in those given placebo (MD ‐1.35 days, 95% CI ‐1.78 to ‐0.92; four RCTs, N = 853; I2 = 0%; Analysis 1.9).
1.9. Analysis.
Comparison 1 Any antibiotic versus placebo, Outcome 9 Length of hospital stay.
1.9.2 Abdominal hysterectomy
Mean length of hospital stay was shorter in women who received prophylactic antibiotics than in those given placebo (MD ‐0.59 days, 95% CI ‐0.76 to ‐0.43; seven RCTs, N = 1510; I2 = 87%; Analysis 1.9). We explored the presence of significant heterogeneity.
2. Cephalosporin versus placebo
Primary outcomes
2.1 Total postoperative infections ‐ early and late
2.1.1 Vaginal hysterectomy
The total postoperative infection rate was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.29, 95% CI 0.20 to 0.42; three RCTs, N = 265; I2 = 89%; Analysis 2.1; Figure 7). Although heterogeneity among studies was substantial, the directions of effect estimates for individual studies were consistent. In addition, we examined the presence of heterogeneity using sensitivity analysis. The observed difference in outcomes between the two groups remained whether sensitivity analysis was based on OR (OR 0.14, 95% CI 0.08 to 0.24) or on an RE model (RR 0.19, 95% CI 0.04 to 0.88), and more cases of total postoperative infection were reported in women in the placebo group in both analyses.
2.1. Analysis.
Comparison 2 Cephalosporin versus placebo, Outcome 1 Total postoperative infections ‐ early and late.
7.
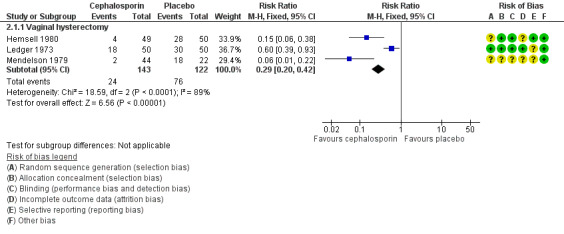
Forest plot of comparison: 2 Cephalosporin versus placebo, outcome: 2.1 Total postoperative infections ‐ early and late.
2.2 Abdominal wound infection
2.2.1 Abdominal hysterectomy
The rate of abdominal wound infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.41, 95% CI 0.25 to 0.66; seven RCTs, N = 1528; I2 = 0%; Analysis 2.2).
2.2. Analysis.
Comparison 2 Cephalosporin versus placebo, Outcome 2 Abdominal wound infection.
2.3 Urinary tract infection
2.3.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups in the rate of UTI (RR 0.71, 95% CI 0.46 to 1.08; five RCTs, N = 499; I2 = 31%; Analysis 2.3).
2.3. Analysis.
Comparison 2 Cephalosporin versus placebo, Outcome 3 Urinary tract infection.
2.3.2 Abdominal hysterectomy
The rate of UTI was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.42, 95% CI 0.31 to 0.58; six RCTs, N = 1668; I2 = 25%; Analysis 2.3).
2.4 Pelvic infection
2.4.1 Vaginal hysterectomy
The rate of pelvic infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.15, 95% CI 0.09 to 0.28; six RCTs, N = 1281; I2 = 8%; Analysis 2.4).
2.4. Analysis.
Comparison 2 Cephalosporin versus placebo, Outcome 4 Pelvic infection.
2.4.2 Abdominal hysterectomy
The rate of pelvic infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.60, 95% CI 0.39 to 0.93; seven RCTs, N = 1528; I2 = 3%; Analysis 2.4).
2.5 Other serious infection
2.5.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups in the rate of other serious infection (RR 0.20, 95% CI 0.01 to 4.12; one RCT, N = 206; Analysis 2.5).
2.5. Analysis.
Comparison 2 Cephalosporin versus placebo, Outcome 5 Other serious infections.
2.5.2 Abdominal hysterectomy
It is unclear whether data showed a difference between groups in the rate of other serious infection (RR 0.33, 95% CI 0.04 to 3.16; one RCT, N = 220; Analysis 2.5).
2.6 Postoperative fever
2.6.1 Vaginal hysterectomy
The rate of postoperative fever was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.37, 95% CI 0.25 to 0.54; five RCTs, N = 1028; I2 = 71%; Analysis 2.6; Figure 8). Direction of effect estimates in all five studies were consistent. We investigated the presence of significant heterogeneity using sensitivity analysis. The observed difference in outcomes between the two groups persisted whether sensitivity analysis was based on OR (OR 0.29, 95% CI 0.18 to 0.47) or on an RE model (RR 0.34, 95% CI 0.15 to 0.78), and more women in the placebo group were given the diagnosis of postoperative fever.
2.6. Analysis.
Comparison 2 Cephalosporin versus placebo, Outcome 6 Postoperative fever.
8.
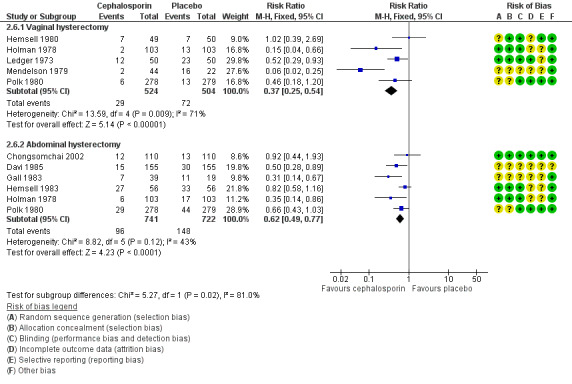
Forest plot of comparison: 2 Cephalosporin versus placebo, outcome: 2.6 Postoperative fever.
2.6.2 Abdominal hysterectomy
The rate of postoperative fever was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.62, 95% CI 0.49 to 0.77; six RCTs, N = 1463; I2 = 43%; Analysis 2.6).
2.7 Total adverse effects
2.7.1 Abdominal hysterectomy
It is unclear whether results showed a difference between groups in the rate of adverse effects (RR 1.00, 95% CI 0.06 to 15.83; one RCT, N = 284; Analysis 2.7).
2.7. Analysis.
Comparison 2 Cephalosporin versus placebo, Outcome 7 Total adverse effects.
Secondary outcomes
2.8 Need for therapeutic antibiotics
2.8.1 Vaginal hysterectomy
The rate of need for therapeutic antibiotics in women who received prophylactic antibiotics was lower than in those given placebo (RR 0.55, 95% CI 0.37 to 0.81; three RCTs, N = 863; I2 = 36%; Analysis 2.8).
2.8. Analysis.
Comparison 2 Cephalosporin versus placebo, Outcome 8 Need for therapeutic antibiotics.
2.8.2 Abdominal hysterectomy
We found no conclusive evidence of a difference between groups in the number of women requiring therapeutic antibiotics, although data suggest benefit for the antibiotic prophylaxis group (RR 0.79, 95% CI 0.61 to 1.01; four RCTs, N = 1138; I2 = 0%; Analysis 2.8).
2.9 Length of hospital stay
2.9.1 Vaginal hysterectomy
Mean length of hospital stay was shorter in women who received prophylactic antibiotics than in those given placebo (MD ‐1.30 days, 95% CI ‐1.88 to ‐0.72; two RCTs, N = 657; I2 = 0%; Analysis 2.9).
2.9. Analysis.
Comparison 2 Cephalosporin versus placebo, Outcome 9 Length of hospital stay.
2.9.2 Abdominal hysterectomy
Mean length of hospital stay was shorter in women who received prophylactic antibiotics than in those given placebo (MD ‐0.43 days, 95% CI ‐0.67 to ‐0.19; four RCTs, N = 818; I2 = 63%; Analysis 2.9). Four studies showed consistency in direction of effect estimates. In addition, we found evidence that a difference in length of hospital stay between the two groups persisted when we subjected the evidence to sensitivity analysis based on an RE model (MD ‐0.54, 95% CI ‐1.04 to ‐0.05), and that women in the placebo group stayed longer in hospital than those in the cephalosporin group.
3. Penicillin versus placebo
Primary outcomes
3.1 Total postoperative infections ‐ early and late
3.1.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups in the incidence of total postoperative infections (early and late) (RR 0.20, 95% CI 0.03 to 1.42; one RCT, N = 20; Analysis 3.1).
3.1. Analysis.
Comparison 3 Penicillin versus placebo, Outcome 1 Total postoperative infections ‐ early and late.
3.1.2 Abdominal hysterectomy
The total infection rate was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.30, 95% CI 0.13 to 0.70; one RCT, N = 100; Analysis 3.1).
3.2 Abdominal wound infection
3.2.1 Abdominal hysterectomy
The rate of abdominal wound infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.16, 95% CI 0.05 to 0.53; two RCTs, N = 320; I2 = 0%; Analysis 3.2).
3.2. Analysis.
Comparison 3 Penicillin versus placebo, Outcome 2 Abdominal wound infection.
3.3 Urinary tract infection
3.3.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups in the rate of UTI (RR 0.50, 95% CI 0.05 to 4.67; one RCT, N = 20; Analysis 3.3).
3.3. Analysis.
Comparison 3 Penicillin versus placebo, Outcome 3 Urinary tract infection.
3.3.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups in the rate of UTI (RR 0.60, 95% CI 0.21 to 1.76; two RCTs, N = 320; I2 = 0%; Analysis 3.3).
3.4 Pelvic infection
3.4.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups in the rate of pelvic infection (RR 0.14, 95% CI 0.01 to 2.45; one RCT, N = 20; Analysis 3.4).
3.4. Analysis.
Comparison 3 Penicillin versus placebo, Outcome 4 Pelvic infection.
3.4.2 Abdominal hysterectomy
The rate of pelvic infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 1.33, 95% CI 0.31 to 5.82; one RCT, N = 220; Analysis 3.4).
3.5 Other serious infection
3.5.1 Abdominal hysterectomy
It is unclear whether data showed a difference between groups in the rate of other serious infection (RR 0.14, 95% CI 0.01 to 2.73; one RCT, N = 220; Analysis 3.5).
3.5. Analysis.
Comparison 3 Penicillin versus placebo, Outcome 5 Other serious infections.
3.6 Postoperative fever
3.6.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups in the rate of postoperative fever (RR 0.14, 95% CI 0.01 to 2.45; one RCT, N = 20; Analysis 3.6; Figure 9).
3.6. Analysis.
Comparison 3 Penicillin versus placebo, Outcome 6 Postoperative fever.
9.
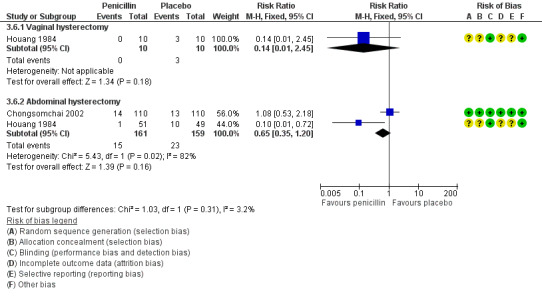
Forest plot of comparison: 3 Penicillin versus placebo, outcome: 3.6 Postoperative fever.
3.6.2 Abdominal hysterectomy
It is unclear whether data showed a difference between groups in the rate of postoperative fever (RR 0.65, 95% CI 0.35 to 1.20; two RCTs, N = 320; I2 = 82%; Analysis 3.6). Heterogeneity for this comparison was substantial (I2 = 82%) with inconsistency in the direction of effects for the two studies. Evidence of no difference in reported cases of postoperative fever persisted whether sensitivity analysis was based on OR (OR 0.61, 95% CI 0.31 to 1.22) or on an RE model (RR 0.38, 95% CI 0.03 to 4.51).
3.7 Total adverse effects
Investigators provided no data for this outcome.
Secondary outcomes
3.8 Need for therapeutic antibiotics
Investigators provided no data for this outcome.
3.9 Length of hospital stay
Investigators provided no data for this outcome.
4. Antiprotozoal versus placebo
Primary outcomes
4.1 Total postoperative infections ‐ early and late
Investigators provided no data for this outcome.
4.2 Abdominal wound infection
4.2.1 Abdominal hysterectomy
It is unclear whether results showed a difference between groups in rates of abdominal wound infection (RR 0.71, 95% CI 0.32 to 1.57; two RCTs, N = 462; I2 = 0%; Analysis 4.1).
4.1. Analysis.
Comparison 4 Antiprotozoal versus placebo, Outcome 1 Abdominal wound infection.
4.3 Urinary tract infection
4.3.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups in rates of UTI (RR 1.25, 95% CI 0.51 to 3.04; one RCT, N = 226; I2 = 75%; Analysis 4.2).
4.2. Analysis.
Comparison 4 Antiprotozoal versus placebo, Outcome 2 Urinary tract infection.
4.3.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups in rates of UTI (RR 1.00, 95% CI 0.34 to 2.96; one RCT, N = 146; Analysis 4.2).
4.4 Pelvic infection
4.4.1 Vaginal hysterectomy
The rate of pelvic infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.36, 95% CI 0.17 to 0.75; four RCTs, N = 375; I2 = 0%; Analysis 4.3).
4.3. Analysis.
Comparison 4 Antiprotozoal versus placebo, Outcome 3 Pelvic infection.
4.4.2 Abdominal hysterectomy
The rate of pelvic infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.42, 95% CI 0.22 to 0.83; four RCTs, N = 662; I2 = 0%; Analysis 4.3).
4.5 Other serious infection
4.5.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups in rates of other serious infection (RR 0.25, 95% CI 0.03 to 2.21; two RCTs, N = 246; I2 = 0%; Analysis 4.4).
4.4. Analysis.
Comparison 4 Antiprotozoal versus placebo, Outcome 4 Other serious infections.
4.5.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups in rates of other serious infection (RR 1.00, 95% CI 0.14 to 6.91; one RCT, N = 146; Analysis 4.4).
4.6 Postoperative fever
4.6.1 Vaginal hysterectomy
The rate of postoperative fever was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.45, 95% CI 0.21 to 0.97; two RCTs, N = 130; I2 = 0%; Analysis 4.5; Figure 10).
4.5. Analysis.
Comparison 4 Antiprotozoal versus placebo, Outcome 5 Postoperative fever.
10.
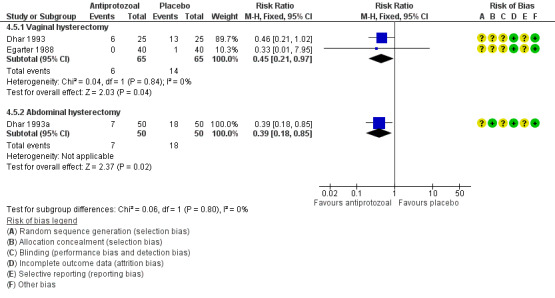
Forest plot of comparison: 4 Antiprotozoal versus placebo, outcome: 4.5 Postoperative fever.
4.6.2 Abdominal hysterectomy
The rate of postoperative fever was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.39, 95% CI 0.18 to 0.85; one RCT, N = 100; Analysis 4.5).
4.7 Total adverse effects
4.7.1 Abdominal hysterectomy
It is unclear whether results showed differences between groups in rates of adverse effects (RR 2.00, 95% CI 0.63 to 6.35; one RCT, N = 146; Analysis 4.6).
4.6. Analysis.
Comparison 4 Antiprotozoal versus placebo, Outcome 6 Total adverse effects.
Secondary outcomes
4.8 Need for therapeutic antibiotics
4.8.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups in the need for therapeutic antibiotics (RR 0.58, 95% CI 0.29 to 1.15; two RCTs, N = 196; I2 = 67%; Analysis 4.7). Findings did not change whether sensitivity analysis was based on OR (OR 0.52, 95% CI 0.24 to 1.17) or on an RE model (RR 0.55, 95% CI 0.15 to 1.95).
4.7. Analysis.
Comparison 4 Antiprotozoal versus placebo, Outcome 7 Need for therapeutic antibiotics.
4.8.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups in the need for therapeutic antibiotics (RR 0.62, 95% CI 0.36 to 1.06; two RCTs, N = 246; I2 = 78%; Analysis 4.7). Findings did not change whether sensitivity analysis was based on OR (OR 0.56, 95% CI 0.30 to 1.07) or on an RE model (RR 0.55, 95% CI 0.15 to 2.02).
4.9 Length of hospital stay
4.9.1 Vaginal hysterectomy
Mean length of hospital stay was shorter in women who received prophylactic antibiotics than in those given placebo (MD ‐0.86 days, 95% CI ‐1.22 to ‐0.49; three RCTs, N = 276; I2 = 63%; Analysis 4.8). Direction of effect estimates were consistent in the three studies. Evidence of a difference in outcome between the two groups persisted when subjected to sensitivity analysis based on an RE model (MD ‐0.97, 95% CI ‐1.72 to ‐0.23), with women in the placebo group staying longer in hospital than those in the antiprotozoal group.
4.8. Analysis.
Comparison 4 Antiprotozoal versus placebo, Outcome 8 Length of hospital stay.
4.9.2 Abdominal hysterectomy
Mean length of hospital stay was shorter in women who received prophylactic antibiotics than in those given placebo (MD ‐1.33 days, 95% CI ‐1.68 to ‐0.97; three RCTs; N = 358; I2 = 89%; Analysis 4.8). Direction of effect estimates of individual studies were not consistent. We investigated the presence of significant heterogeneity and found no evidence of a difference in outcome between the two groups when an RE model (MD ‐0.93, 95% CI ‐2.12 to 0.26) was used.
5. Sulphonamides versus placebo
Primary outcomes
5.1 Total postoperative infections ‐ early and late
Investigators provided no data for this outcome.
5.2 Abdominal wound infection
5.2.1 Abdominal hysterectomy
It is unclear whether results showed a difference between groups in rates of abdominal wound infection (RR 1.23, 95% CI 0.35 to 4.35; two RCTs, N = 119; I2 = 0%; Analysis 5.1).
5.1. Analysis.
Comparison 5 Sulphonamides versus placebo, Outcome 1 Abdominal wound infection.
5.3 Urinary tract infection
5.3.1 Vaginal hysterectomy
The rate of UTI was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.36, 95% CI 0.15 to 0.84; one RCT, N = 50; Analysis 5.2).
5.2. Analysis.
Comparison 5 Sulphonamides versus placebo, Outcome 2 Urinary tract infection.
5.3.2 Abdominal hysterectomy
The rate of UTI was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.18, 95% CI 0.06 to 0.50; two RCTs, N = 157; I2 = 0%; Analysis 5.2).
5.4 Pelvic infection
5.4.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups in rates of pelvic infection (RR 0.14, 95% CI 0.01 to 2.63; one RCT, N = 50; Analysis 5.3).
5.3. Analysis.
Comparison 5 Sulphonamides versus placebo, Outcome 3 Pelvic infection.
5.4.2 Abdominal hysterectomy
The rate of pelvic infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.11, 95% CI 0.01 to 0.84; two RCTs, N = 119; I2 = 0%; Analysis 5.3).
5.5 Other serious infection
Investigators provided no data for this outcome.
5.6 Postoperative fever
5.6.1 Vaginal hysterectomy
The rate of postoperative fever was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.50, 95% CI 0.26 to 0.95; one RCT, N = 50; Analysis 5.4).
5.4. Analysis.
Comparison 5 Sulphonamides versus placebo, Outcome 4 Postoperative fever.
5.6.2 Abdominal hysterectomy
It is unclear whether data showed a difference between groups in the numbers of women with a diagnosis of postoperative fever (RR 0.63, 95% CI 0.38 to 1.04; two RCTs, N = 157; I2 = 69%; Analysis 5.4). Direction of effect estimates were consistent across studies. We examined the presence of significant heterogeneity using sensitivity analysis; whether sensitivity analysis was based on OR (OR 0.51, 95% CI 0.25 to 1.05) or an RE model (RR 0.63, 95% CI 0.24 to 1.62) did not substantially influence the findings.
5.7 Total adverse effects
Investigators provided no data for this outcome.
Secondary outcomes
5.8 Need for therapeutic antibiotics
5.8.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups in the need for therapeutic antibiotics as the study that reported on this outcome did not find any evidence of a difference Mathews 1977 (RR 0.33, 95% CI 0.10 to 1.09; one RCT, N = 50).
5.8.2 Abdominal hysterectomy
It is unclear whether data showed a difference between groups in the need for therapeutic antibiotics as the study that reported on this outcome did not find any evidence of a difference Mathews 1977 (RR 0.97, 95% CI 0.15 to 6.41; one RCT, N = 59).
5.9 Length of hospital stay
Investigators provided no data for this outcome.
6. Cephalosporin plus antiprotozoal versus placebo
Primary outcomes
6.1 Total postoperative infections ‐ early and late
Investigators provided no data for this outcome.
6.2 Abdominal wound infection
6.2.1 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 1.00, 95% CI 0.14 to 7.03; two RCTs, N = 406; I2 = 0%; Analysis 6.1).
6.1. Analysis.
Comparison 6 Cephalosporin + antiprotozoal versus placebo, Outcome 1 Abdominal wound infection.
6.3 Urinary tract infection
6.3.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.50, 95% CI 0.24 to 1.04; one RCT, N = 406; Analysis 6.2).
6.2. Analysis.
Comparison 6 Cephalosporin + antiprotozoal versus placebo, Outcome 2 Urinary tract infection.
6.3.2 Abdominal hysterectomy
The rate of urinary tract infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.27, 95% CI 0.08 to 0.96; one RCT, N = 406; Analysis 6.2).
6.4 Pelvic infection
6.4.1 Vaginal hysterectomy
The rate of pelvic infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.05, 95% CI 0.01 to 0.37; one RCT, N = 406; Analysis 6.3).
6.3. Analysis.
Comparison 6 Cephalosporin + antiprotozoal versus placebo, Outcome 3 Pelvic infection.
6.5 Other serious infection
Investigators provided no data for this outcome.
6.6 Postoperative fever
6.6.1 Vaginal hysterectomy
The rate of postoperative fever was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.50, 95% CI 0.34 to 0.73; one RCT, N = 406; Analysis 6.4).
6.4. Analysis.
Comparison 6 Cephalosporin + antiprotozoal versus placebo, Outcome 4 Postoperative fever.
6.6.2 Abdominal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.79, 95% CI 0.58 to 1.09; one RCT, N = 406; Analysis 6.4).
6.7 Total adverse effects
Investigators provided no data for this outcome.
Secondary outcomes
6.8 Need for therapeutic antibiotics
6.8.1 Vaginal hysterectomy
The rate of need for therapeutic antibiotics was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.36, 95% CI 0.19 to 0.68; one RCT, N = 406; Analysis 6.5).
6.5. Analysis.
Comparison 6 Cephalosporin + antiprotozoal versus placebo, Outcome 5 Need for therapeutic antibiotics.
6.8.2 Abdominal hysterectomy
The rate of need for therapeutic antibiotics was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.38, 95% CI 0.15 to 0.94; one RCT, N = 406; Analysis 6.5).
6.9 Length of hospital stay
6.9.1 Abdominal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (MD ‐0.30 days, 95% CI ‐0.60 to ‐0.00; one RCT, N = 406; Analysis 6.6).
6.6. Analysis.
Comparison 6 Cephalosporin + antiprotozoal versus placebo, Outcome 6 Length of hospital stay.
7. Penicillin plus antiprotozoal versus placebo
Primary outcomes
7.1 Total postoperative infections ‐ early and late
7.1.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.25, 95% CI 0.04 to 1.73; one RCT, n 18; Analysis 7.1).
7.1. Analysis.
Comparison 7 Penicillin + antiprotozoal versus placebo, Outcome 1 Total postoperative infections ‐ early and late.
7.1.2 Abdominal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.44, 95% CI 0.23 to 0.86; one RCT, N = 107; Analysis 7.1).
7.2 Abdominal wound infection
7.2.1 Abdominal hysterectomy
The rate of abdominal wound infection was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.14, 95% CI 0.03 to 0.60; one RCT, N = 107; Analysis 7.2).
7.2. Analysis.
Comparison 7 Penicillin + antiprotozoal versus placebo, Outcome 2 Abdominal wound infection.
7.3 Urinary tract infection
7.3.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.63, 95% CI 0.07 to 5.72; one RCT, n = 18; Analysis 7.3).
7.3. Analysis.
Comparison 7 Penicillin + antiprotozoal versus placebo, Outcome 3 Urinary tract infection.
7.3.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.97, 95% CI 0.38 to 2.47; one RCT, N = 107; Analysis 7.3).
7.4 Pelvic infection
7.4.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.17, 95% CI 0.01 to 2.96; one RCT, N = 18; Analysis 7.4).
7.4. Analysis.
Comparison 7 Penicillin + antiprotozoal versus placebo, Outcome 4 Pelvic infection.
7.5 Other serious infection
Investigators provided no data for this outcome.
7.6 Postoperative fever
7.6.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.17, 95% CI 0.01 to 2.96; one RCT, N = 18; Analysis 7.5).
7.5. Analysis.
Comparison 7 Penicillin + antiprotozoal versus placebo, Outcome 5 Postoperative fever.
7.6.2 Abdominal hysterectomy
The rate of postoperative fever was lower in women who received prophylactic antibiotics than in those given placebo (RR 0.08, 95% CI 0.01 to 0.64; one RCT, N = 107; Analysis 7.5).
7.7 Total adverse effects
Researchers provided no data for this outcome.
Secondary outcomes
7.8 Need for therapeutic antibiotics
Researchers provided no data for this outcome.
7.9 Length of hospital stay
Researchers provided no data for this outcome.
8. Lincosamide versus placebo
Primary outcomes
8.1 Total postoperative infections ‐ early and late
Researchers provided no data for this outcome.
8.2 Abdominal wound infection
Researchers provided no data for this outcome.
8.3 Urinary tract infection
8.3.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups (RR 0.71, 95% CI 0.25 to 2.06; one RCT, N = 80; Analysis 8.1).
8.1. Analysis.
Comparison 8 Lincosamide versus placebo, Outcome 1 Urinary tract infection.
8.4 Pelvic infection
Researchers provided no data for this outcome.
8.5 Other serious infection
Researchers provided no data for this outcome.
8.6 Postoperative fever
8.6.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 1.00, 95% CI 0.06 to 15.44; one RCT, N = 80; Analysis 8.2).
8.2. Analysis.
Comparison 8 Lincosamide versus placebo, Outcome 2 Postoperative fever.
8.7 Total adverse effects
Researchers provided no data for this outcome.
Secondary outcomes
8.8 Need for therapeutic antibiotics
Researchers provided no data for this outcome.
8.9 Length of hospital stay
8.9.1 Vaginal hysterectomy
Evidence showed a difference in length of hospital stay between the two treatment groups, with women in the placebo group staying longer in hospital than those in the lincosamide group (MD ‐0.40, 95% CI ‐0.77 to ‐0.03; one RCT, N = 80; Analysis 8.3).
8.3. Analysis.
Comparison 8 Lincosamide versus placebo, Outcome 3 Length of hospital stay.
9. Cephalosporin versus penicillin
Primary outcomes
9.1 Total postoperative infections ‐ early and late
9.1.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 1.04, 95% CI 0.55 to 2.00; two RCTs, N = 470; I2 = 0%; Analysis 9.1; Figure 11).
9.1. Analysis.
Comparison 9 Cephalosporin versus penicillin, Outcome 1 Total postoperative infections ‐ early and late.
11.
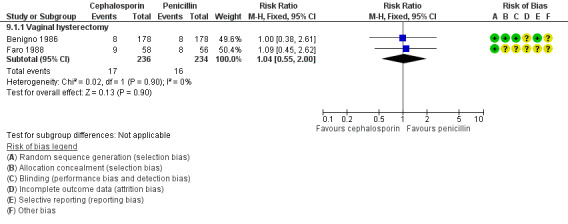
Forest plot of comparison: 9 Cephalosporin versus penicillin, outcome: 9.1 Total postoperative infections ‐ early and late.
9.2 Abdominal wound infection
9.2.1 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.33, 95% CI 0.01 to 8.09; one RCT, N = 220; Analysis 9.2).
9.2. Analysis.
Comparison 9 Cephalosporin versus penicillin, Outcome 2 Abdominal wound infection.
9.3 Urinary tract infection
9.3.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.20, 95% CI 0.01 to 3.98; one RCT, N = 95; Analysis 9.3).
9.3. Analysis.
Comparison 9 Cephalosporin versus penicillin, Outcome 3 Urinary tract infection.
9.3.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 1.00, 95% CI 0.06 to 15.79; one RCT, N = 220; Analysis 9.3).
9.4 Pelvic infection
9.4.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.88, 95% CI 0.47 to 1.64; three RCTs, N = 565; I2 = 0%; Analysis 9.4).
9.4. Analysis.
Comparison 9 Cephalosporin versus penicillin, Outcome 4 Pelvic infection.
9.4.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.50, 95% CI 0.09 to 2.67; one RCT, N = 220; Analysis 9.4).
9.5 Other serious infection
9.5.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 2.90, 95% CI 0.12 to 69.68; one RCT, N = 114; Analysis 9.5).
9.5. Analysis.
Comparison 9 Cephalosporin versus penicillin, Outcome 5 Other serious infections.
9.5.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 3.00, 95% CI 0.12 to 72.85; one RCT, N = 220; Analysis 9.5).
9.6 Postoperative fever
9.6.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.82, 95% CI 0.58 to 1.15; three RCTs, N = 565; I2 = 0%; Analysis 9.6).
9.6. Analysis.
Comparison 9 Cephalosporin versus penicillin, Outcome 6 Postoperative fever.
9.6.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.86, 95% CI 0.42 to 1.77; one RCT, N = 220; Analysis 9.6; Figure 12).
12.
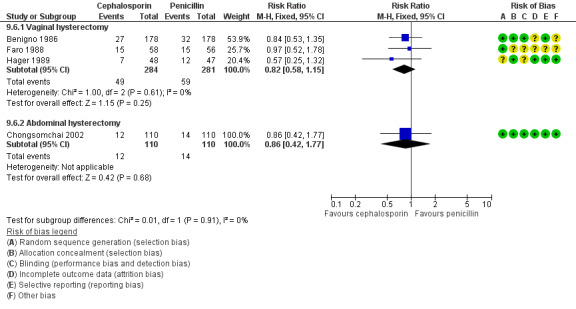
Forest plot of comparison: 9 Cephalosporin versus penicillin, outcome: 9.6 Postoperative fever.
9.7 Total adverse effects
9.7.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.95, 95% CI 0.79 to 1.14; two RCTs, N = 451; I2 = 85%; Analysis 9.7; Figure 13).
9.7. Analysis.
Comparison 9 Cephalosporin versus penicillin, Outcome 7 Total adverse effects.
13.
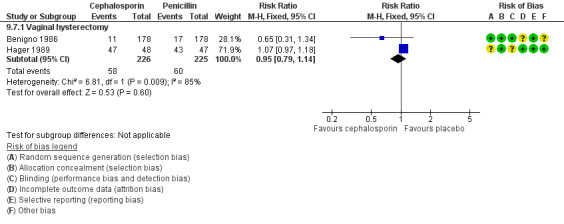
Forest plot of comparison: 9 Cephalosporin versus penicillin, outcome: 9.7 Total adverse effects.
Secondary outcomes
9.8 Need for therapeutic antibiotics
9.8.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 1.32, 95% CI 0.88 to 1.97; two RCTs, N = 470; I2 = 0%; Analysis 9.8).
9.8. Analysis.
Comparison 9 Cephalosporin versus penicillin, Outcome 8 Need for therapeutic antibiotics.
9.9 Length of hospital stay
9.9.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (MD ‐0.47, 95% CI ‐0.97 to 0.04; two RCTs, N = 209; I2 = 0%; Analysis 9.9).
9.9. Analysis.
Comparison 9 Cephalosporin versus penicillin, Outcome 9 Length of hospital stay.
10 Cephalosporin versus tetracycline
Primary outcomes
10.1 Total postoperative infections ‐ early and late
10.1.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.59, 95% CI 0.20 to 1.78; one RCT, N = 51; Analysis 10.1).
10.1. Analysis.
Comparison 10 Cephalosporin versus tetracycline, Outcome 1 Total postoperative infections ‐ early and late.
10.2 Abdominal wound infection
Researchers provided no data for this outcome.
10.3 Urinary tract infection
Researchers provided no data for this outcome.
10.4 Pelvic infection
10.4.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.83, 95% CI 0.25 to 2.75; one RCT, N = 51; Analysis 10.2).
10.2. Analysis.
Comparison 10 Cephalosporin versus tetracycline, Outcome 2 Pelvic infection.
10.5 Other serious infection
Researchers provided no data for this outcome.
10.6 Postoperative fever
10.6.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.69, 95% CI 0.13 to 3.81; one RCT, N = 51; Analysis 10.3).
10.3. Analysis.
Comparison 10 Cephalosporin versus tetracycline, Outcome 3 Postoperative fever.
10.7 Total adverse effects
Researchers provided no data for this outcome.
Secondary outcomes
10.8 Need for therapeutic antibiotics
Researchers provided no data for this outcome.
10.9 Length of hospital stay
10.9.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (MD ‐0.20 days, 95% CI ‐1.11 to 0.71; one RCT, N = 51; Analysis 10.4).
10.4. Analysis.
Comparison 10 Cephalosporin versus tetracycline, Outcome 4 Length of hospital stay.
11. Cephalosporin versus antiprotozoal
Primary outcomes
11.1 Total postoperative infections ‐ early and late
11.1.1 Vaginal hysterectomy
The rate of postoperative infection was lower in the cephalosporin group (RR 0.04, 95% CI 0.00 to 0.67; one RCT, N = 78; Analysis 11.1).
11.1. Analysis.
Comparison 11 Cephalosporin versus antiprotozoal, Outcome 1 Total postoperative infections ‐ early and late.
11.2 Abdominal wound infection
Researchers provided no data for this outcome.
11.3 Urinary tract infection
11.3.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.05, 95% CI 0.00 to 0.81; one RCT, N = 78; Analysis 11.2).
11.2. Analysis.
Comparison 11 Cephalosporin versus antiprotozoal, Outcome 2 Urinary tract infection.
11.4 Pelvic infection
11.4.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.17, 95% CI 0.01 to 4.03; one RCT, N = 78; Analysis 11.3).
11.3. Analysis.
Comparison 11 Cephalosporin versus antiprotozoal, Outcome 3 Pelvic infection.
11.5 Other serious infection
Investigators provided no data for this outcome.
11.6 Postoperative fever
11.6.1 Vaginal hysterectomy
The rate of postoperative fever was lower in women who received cephalosporin than in those given antiprotozoal (RR 0.06, 95% CI 0.01 to 0.42; one RCT, N = 78; Analysis 11.4).
11.4. Analysis.
Comparison 11 Cephalosporin versus antiprotozoal, Outcome 4 Postoperative fever.
11.7 Total adverse effects
Investigators provided no data for this outcome.
Secondary outcomes
11.8 Need for therapeutic antibiotics
11.8.1 Vaginal hysterectomy
The rate of need for therapeutic antibiotics was lower in women who received cephalosporin than in those given antiprotozoal (RR 0.03, 95% CI 0.00 to 0.44; one RCT, N = 78; Analysis 11.5).
11.5. Analysis.
Comparison 11 Cephalosporin versus antiprotozoal, Outcome 5 Need for therapeutic antibiotics.
11.9 Length of hospital stay
11.9.1 Vaginal hysterectomy
Mean length of hospital stay was shorter in women who received cephalosporin than in those given antiprotozoal (MD ‐1.90 days, 95% CI ‐3.32 to ‐0.48; one RCT, N = 78; Analysis 11.6).
11.6. Analysis.
Comparison 11 Cephalosporin versus antiprotozoal, Outcome 6 Length of hospital stay.
12. Antiprotozoal versus lincosamide
Primary outcomes
12.1 Total postoperative infections ‐ early and late
Researchers provided no data for this outcome.
12.2 Abdominal wound infection
Researchers provided no data for this outcome.
12.3 Urinary tract infection
12.3.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 4.00, 95% CI 0.47 to 34.24; one RCT, N = 80; Analysis 12.1).
12.1. Analysis.
Comparison 12 Antiprotozoal versus lincosamide, Outcome 1 Urinary tract infection.
12.4 Pelvic infection
Researchers provided no data for this outcome.
12.5 Other serious infection
Researchers provided no data for this outcome.
12.6 Postoperative fever
12.6.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.33, 95% CI 0.01 to 7.95; one RCT, N = 80; Analysis 12.2).
12.2. Analysis.
Comparison 12 Antiprotozoal versus lincosamide, Outcome 2 Postoperative fever.
12.7 Total adverse effects
Researchers provided no data for this outcome.
Secondary outcomes
12.8 Need for therapeutic antibiotics
Researchers provided no data for this outcome.
12.9 Length of hospital stay
12.9.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (MD ‐0.20 days, 95% CI ‐0.60 to 0.20; one RCT, N = 80; Analysis 12.3).
12.3. Analysis.
Comparison 12 Antiprotozoal versus lincosamide, Outcome 3 Length of hospital stay.
13. Cephalosporin plus antiprotozoal versus cephalosporin
Primary outcomes
13.1 Total postoperative infections ‐ early and late
Researchers provided no data for this outcome.
13.2 Abdominal wound infection
Researchers provided no data for this outcome.
13.3 Urinary tract infection
Researchers provided no data for this outcome.
13.4 Pelvic infection
Researchers provided no data for this outcome.
13.5 Other serious infections
Researchers provided no data for this outcome.
13.6 Postoperative fever
13.6.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.50, 95% CI 0.03 to 7.68; one RCT, N = 78; Analysis 13.1).
13.1. Analysis.
Comparison 13 Cephalosporin + antiprotozoal versus cephalosporin only, Outcome 1 Postoperative fever.
13.7 Total adverse effects
Researchers provided no data for this outcome.
Secondary outcomes
13.8 Need for therapeutic antibiotics
Researchers provided no data for this outcome.
13.9 Length of hospital stay
13.9.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (MD 0.30 days, 95% CI ‐0.43 to 1.03; one RCT, N = 78; Analysis 13.2).
13.2. Analysis.
Comparison 13 Cephalosporin + antiprotozoal versus cephalosporin only, Outcome 2 Length of hospital stay.
14. Cephalosporin plus antiprotozoal versus antiprotozoal only
Primary outcomes
14.1 Total postoperative infections ‐ early and late
14.1.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups (RR 0.04, 95% CI 0.00 to 0.67; one RCT, N = 78; Analysis 14.1).
14.1. Analysis.
Comparison 14 Cephalosporin + antiprotozoal versus antiprotozoal only, Outcome 1 Total postoperative infections ‐ early and late.
14.2 Abdominal wound infection
Researchers provided no data for this outcome.
14.3 Urinary tract infection
14.3.1 Vaginal hysterectomy
The rate of UTI was lower in women who received cephalosporin plus antiprotozoal than in those given antiprotozoal only (RR 0.05, 95% CI 0.00 to 0.81; one RCT, N = 78; Analysis 14.2).
14.2. Analysis.
Comparison 14 Cephalosporin + antiprotozoal versus antiprotozoal only, Outcome 2 Urinary tract infection.
14.4 Pelvic infection
14.4.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.17, 95% CI 0.01 to 4.03; one RCT, N = 78; Analysis 14.3).
14.3. Analysis.
Comparison 14 Cephalosporin + antiprotozoal versus antiprotozoal only, Outcome 3 Pelvic infection.
14.5 Other serious infection
Researchers provided no data for this outcome.
14.6 Postoperative fever
14.6.1 Vaginal hysterectomy
The rate of postoperative fever was lower in women who received cephalosporin plus antiprotozoal than in those given antiprotozoal only (RR 0.06, 95% CI 0.01 to 0.42; one RCT, N = 78; Analysis 14.4).
14.4. Analysis.
Comparison 14 Cephalosporin + antiprotozoal versus antiprotozoal only, Outcome 4 Postoperative fever.
14.7 Total adverse effects
Investigators provided no data for this outcome.
Secondary outcomes
14.8 Need for therapeutic antibiotics
14.8.1 Vaginal hysterectomy
The rate of need for therapeutic antibiotics was lower in women who received cephalosporin plus antiprotozoal than in those given antiprotozoal only (RR 0.03, 95% CI 0.00 to 0.44; one RCT, N = 78; Analysis 14.5).
14.5. Analysis.
Comparison 14 Cephalosporin + antiprotozoal versus antiprotozoal only, Outcome 5 Need for therapeutic antibiotics.
14.9 Length of hospital stay
14.9.1 Vaginal hysterectomy
Length of hospital stay was shorter in women who received cephalosporin plus antiprotozoal than in those given antiprotozoal only (MD ‐1.60 days, 95% CI ‐3.11 to ‐0.09; one RCT, N = 78; Analysis 14.6).
14.6. Analysis.
Comparison 14 Cephalosporin + antiprotozoal versus antiprotozoal only, Outcome 6 Length of hospital stay.
15. Penicillin plus antiprotozoal versus penicillin only
Primary outcomes
15.1 Total postoperative infections ‐ early and late
15.1.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 1.25, 95% CI 0.09 to 17.02; one RCT, N = 18; Analysis 15.1).
15.1. Analysis.
Comparison 15 Penicillin + antiprotozoal versus penicillin only, Outcome 1 Total postoperative infections ‐ early and late.
15.1.2 Abdominal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 1.47, 95% CI 0.57 to 3.75; one RCT, N = 109; Analysis 15.1).
15.2 Abdominal wound infection
15.2.1 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.94, 95% CI 0.25 to 3.59; two RCT, N = 155; I2 = 0%; Analysis 15.2).
15.2. Analysis.
Comparison 15 Penicillin + antiprotozoal versus penicillin only, Outcome 2 Abdominal wound infection.
15.3 Urinary tract infection
15.3.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 1.25, 95% CI 0.09 to 17.02; one RCT, N = 18; Analysis 15.3).
15.3. Analysis.
Comparison 15 Penicillin + antiprotozoal versus penicillin only, Outcome 3 Urinary tract infection.
15.3.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 2.00, 95% CI 0.80 to 4.97; two RCTs, N = 155; I2 = 0%; Analysis 15.3).
15.4 Pelvic infection
Investigators provided no data for this outcome.
15.5 Other serious infection
Investigators provided no data for this outcome.
15.6 Postoperative fever
15.6.1 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.96, 95% CI 0.20 to 4.50; two RCTs, N = 155; I2 = 0%; Analysis 15.4).
15.4. Analysis.
Comparison 15 Penicillin + antiprotozoal versus penicillin only, Outcome 4 Postoperative fever.
15.7 Total adverse effects
Investigators provided no data for this outcome.
Secondary outcomes
15.8 Need for therapeutic antibiotics
Investigators provided no data for this outcome.
15.9 Length of hospital stay
Investigators provided no data for this outcome.
16. Cephalosporin early administration versus usual timing (both single dose)
Primary outcomes
16.1 Total postoperative infections ‐ early and late
Researchers provided no data for this outcome.
16.2 Abdominal wound infection
16.2.1 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.50, 95% CI 0.03 to 7.90; one RCT, n = 252; Analysis 16.1).
16.1. Analysis.
Comparison 16 Cephalosporin: early administration versus usual timing (both single dose), Outcome 1 Abdominal wound infection.
16.3 Urinary tract infection
Investigators provided no data for this outcome.
16.4 Pelvic infection
16.4.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 1.50, 95% CI 0.16 to 14.20; one RCT, N = 252; Analysis 16.2).
16.2. Analysis.
Comparison 16 Cephalosporin: early administration versus usual timing (both single dose), Outcome 2 Pelvic infection.
16.4.2 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 1.50, 95% CI 0.16 to 14.20; one RCT, N = 252; Analysis 16.2).
16.5 Other serious infection
Researchers provided no data for this outcome.
16.6 Postoperative fever
Researchers provided no data for this outcome.
16.7 Total adverse effects
Researchers provided no data for this outcome.
Secondary outcomes
16.8 Need for therapeutic antibiotics
Researchers provided no data for this outcome.
16.9 Length of hospital stay
Researchers provided no data for this outcome.
17. Cephalosporin one dose versus two doses
Primary outcomes
17.1 Total postoperative infections ‐ early and late
17.1.1 Abdominal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.67, 95% CI 0.14 to 3.18; one RCT, N = 150; Analysis 17.1).
17.1. Analysis.
Comparison 17 Cephalosporin: one dose versus two doses, Outcome 1 Total postoperative infections ‐ early and late.
17.2 Abdominal wound infection
Researchers provided no data for this outcome.
17.3 Urinary tract infection
Researchers provided no data for this outcome.
17.4 Pelvic infection
Researchers provided no data for this outcome.
17.5 Other serious infection
Researchers provided no data for this outcome.
17.6 Postoperative fever
17.6.1 Abdominal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 2.00, 95% CI 0.97 to 4.13; one RCT, N = 150; Analysis 17.2).
17.2. Analysis.
Comparison 17 Cephalosporin: one dose versus two doses, Outcome 2 Postoperative fever.
17.7 Total adverse effects
Researchers provided no data for this outcome.
Secondary outcomes
17.8 Need for therapeutic antibiotics
17.8.1 Abdominal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 9.90, 95% CI 0.48 to 202.43; one RCT, N = 150; Analysis 17.3).
17.3. Analysis.
Comparison 17 Cephalosporin: one dose versus two doses, Outcome 3 Need for therapeutic antibiotics.
17.9 Length of hospital stay
Researchers provided no data for this outcome.
18. Cephalosporin one dose versus three doses
Primary outcomes
18.1 Total postoperative infections ‐ early and late
18.1.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 0.50, 95% CI 0.05 to 5.36; one RCT, N = 116; Analysis 18.1).
18.1. Analysis.
Comparison 18 Cephalosporin: one dose versus three doses, Outcome 1 Total postoperative infections ‐ early and late.
18.2 Abdominal wound infection
Investigators provided no data for this outcome.
18.3 Urinary tract infection
Investigators provided no data for this outcome.
18.4 Pelvic infection
18.4.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.50, 95% CI 0.05 to 5.36; one RCT, N = 116; Analysis 18.2).
18.2. Analysis.
Comparison 18 Cephalosporin: one dose versus three doses, Outcome 2 Pelvic infection.
18.5 Other serious infection
Investigators provided no data for this outcome.
18.6 Postoperative fever
18.6.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 0.91, 95% CI 0.42 to 1.97; one RCT, N = 116; Analysis 18.3).
18.3. Analysis.
Comparison 18 Cephalosporin: one dose versus three doses, Outcome 3 Postoperative fever.
18.7 Total adverse effects
Investigators provided no data for this outcome.
Secondary outcomes
18.8 Need for therapeutic antibiotics
Investigators provided no data for this outcome.
18.9 Length of hospital stay
It is unclear whether data showed a difference between groups for this outcome (MD ‐0.30 days, 95% CI ‐0.72 to 0.12; one RCT, N = 116; Analysis 18.4).
18.4. Analysis.
Comparison 18 Cephalosporin: one dose versus three doses, Outcome 4 Length of hospital stay.
19. Cephalosporin one dose versus multiple doses
Primary outcomes
19.1 Total postoperative infections ‐ early and late
19.1.1 Vaginal hysterectomy
We found no clear evidence of a difference between groups (RR 5.00, 95% CI 0.25 to 98.52; one RCT, N = 44; Analysis 19.1).
19.1. Analysis.
Comparison 19 Cephalosporin: one dose versus multiple doses, Outcome 1 Total postoperative infections ‐ early and late.
19.2 Abdominal wound infection
Researchers provided no data for this outcome.
19.3 Urinary tract infection
19.3.1 Vaginal hysterectomy
We found no clear evidence of a difference between groups (RR 3.00, 95% CI 0.13 to 69.87; one RCT, N = 44; Analysis 19.2).
19.2. Analysis.
Comparison 19 Cephalosporin: one dose versus multiple doses, Outcome 2 Urinary tract infection.
19.4 Pelvic infection
19.4.1 Vaginal hysterectomy
We found no clear evidence of a difference between groups (RR 3.00, 95% CI 0.13 to 69.87; one RCT, N = 44; Analysis 19.3).
19.3. Analysis.
Comparison 19 Cephalosporin: one dose versus multiple doses, Outcome 3 Pelvic infection.
19.5 Other serious infection
Researchers provided no data for this outcome.
19.6 Postoperative fever
19.6.1 Vaginal hysterectomy
We found no clear evidence of a difference between groups for this outcome (RR 5.00, 95% CI 0.25 to 98.52; one RCT, N = 44; Analysis 19.4).
19.4. Analysis.
Comparison 19 Cephalosporin: one dose versus multiple doses, Outcome 4 Postoperative fever.
19.7 Total adverse effects
Researchers provided no data for this outcome.
Secondary outcomes
19.8 Need for therapeutic antibiotics
Researchers provided no data for this outcome.
19.9 Length of hospital stay
Researchers provided no data for this outcome.
20 Cephalosporin one gram versus two grams
Primary outcomes
20.1 Total postoperative infections ‐ early and late
20.1.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 1.49, 95% CI 0.25 to 8.74; one RCT, N = 237; Analysis 20.1).
20.1. Analysis.
Comparison 20 Cephalosporin one gram versus two grams, Outcome 1 Total postoperative infections ‐ early and late.
20.2 Abdominal wound infection
Investigators reported no data for this outcome.
20.3 Urinary tract infection
Investigators reported no data for this outcome.
20.4 Pelvic infection
20.4.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 1.49, 95% CI 0.25 to 8.74; one RCT, N = 237; Analysis 20.2).
20.2. Analysis.
Comparison 20 Cephalosporin one gram versus two grams, Outcome 2 Pelvic infection.
20.5 Other serious infection
Investigators provided no data for this outcome.
20.6 Postoperative fever
20.6.1 Vaginal hysterectomy
It is unclear whether results showed a difference between groups for this outcome (RR 1.49, 95% CI 0.43 to 5.14; one RCT, N = 237; Analysis 20.3).
20.3. Analysis.
Comparison 20 Cephalosporin one gram versus two grams, Outcome 3 Postoperative fever.
20.7 Total adverse effects
Investigators provided no data for this outcome.
Secondary outcomes
20.8 Need for therapeutic antibiotics
20.8.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (RR 1.49, 95% CI 0.25 to 8.74; one RCT, N = 237; Analysis 20.4).
20.4. Analysis.
Comparison 20 Cephalosporin one gram versus two grams, Outcome 4 Need for therapeutic antibiotics.
20.9 Length of hospital stay
20.9.1 Vaginal hysterectomy
It is unclear whether data showed a difference between groups for this outcome (MD ‐0.10 days, 95% CI ‐0.60 to 0.40; one RCT, N = 237; Analysis 20.5).
20.5. Analysis.
Comparison 20 Cephalosporin one gram versus two grams, Outcome 5 Length of hospital stay.
Funnel plots
We examined the presence of publication or reporting bias by analysing funnel plots in five subgroups: 1.2.1 (Figure 14); 1.3.2 (Figure 15); 1.4.1 and 1.4.2 (Figure 16); and 1.6.2 (Figure 17). We found evidence suggesting a tendency towards publication bias; smaller studies were likely to report beneficial effects with the use of antibiotic prophylaxis.
14.
Funnel plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.2 Abdominal wound infection.
15.
Funnel plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.3 Urinary tract infection.
16.
Funnel plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.4 Pelvic infection.
17.
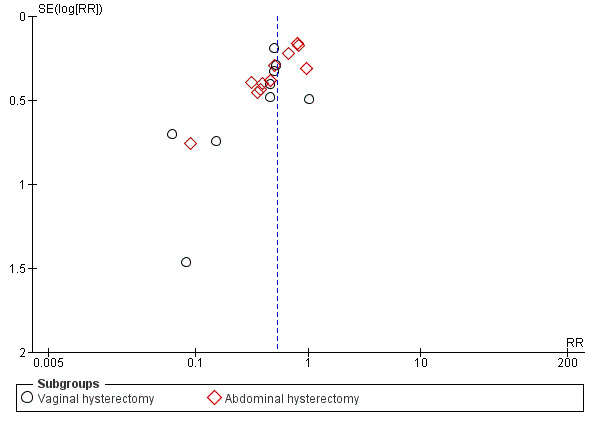
Funnel plot of comparison: 1 Any antibiotic versus placebo, outcome: 1.6 Postoperative fever.
Discussion
Summary of main results
This is the first Cochrane review to assess the effectiveness and safety of antibiotic prophylaxis for elective hysterectomy for benign disease, and to determine which, if any, prophylactic regimen is most suitable. Thirty‐seven studies met the eligibility criteria for inclusion; they compared various antibiotics with placebo and with one another in 20 comparisons involving a total of 6079 participants. Primary outcomes reported were infection (total postoperative infections ‐ early and late, abdominal wound infection, urinary tract infection, pelvic infection, other serious infection, and postoperative fever) and total adverse effects. Secondary outcomes reported were need for therapeutic antibiotics and length of hospital stay.
We subsumed the various comparisons under four broad groups as follows.
1. Any antibiotics versus placebo
Antibiotics in this case included cephalosporin, penicillin, antiprotozoal, sulphonamide, and lincosamide. Researchers compared these individually or in combination with placebo in two subgroups.
Vaginal hysterectomy
We found evidence of a difference in the incidence of postoperative infection between women who received prophylactic antibiotics and those given placebo. Researchers reported fewer cases of total postoperative infection, urinary tract infection (UTI), pelvic infection, and postoperative fever in women who were given prophylactic antibiotics of any class compared with those who received placebo. However, we found no evidence of a difference between groups in the proportions of women who developed other serious infection.
On safety, we found no available data that would allow us to properly evaluate the adverse effects associated with each group.
With regard to need for therapeutic antibiotics, fewer women in the antibiotic groups required therapeutic antibiotics postoperatively compared with those in the placebo group. Similarly, women who received prophylactic antibiotics spent fewer days in hospital than those given placebo.
Abdominal hysterectomy
As in the vaginal hysterectomy subgroup, we found evidence of a difference between groups in the proportions of women given a diagnosis of postoperative infection. Lower proportions of women who received prophylactic antibiotics received a diagnosis of total postoperative infection, abdominal wound infection, UTI, pelvic infection, and postoperative fever compared with those given placebo. However, we found no evidence of a difference between groups in reported cases of other serious infection.
With regard to safety, we found evidence of a difference between groups in the incidence of total adverse effects, with fewer cases of adverse effects reported in women who received antibiotics compared with those given placebo.
On the need for therapeutic antibiotics, fewer women in the antibiotic group required therapeutic antibiotics compared with those in the placebo group. Similarly, women who received prophylactic antibiotics spent shorter days in hospital than those given placebo.
2. Head‐to‐head comparisons between antibiotics
We identified four different head‐to‐head comparisons: cephalosporin versus penicillin, cephalosporin versus tetracycline, cephalosporin versus antiprotozoal, and antiprotozoal versus lincosamide. Investigators compared participants in two subgroups as follows.
Vaginal hysterectomy
Investigators performed all four comparisons in this subgroup. We found no evidence of a difference between groups in reported cases of total postoperative infection, abdominal wound infection, UTI, pelvic infection, other serious infection, and postoperative fever with cephalosporin versus penicillin, cephalosporin versus tetracycline, and antiprotozoal versus lincosamide, when data were available. However, researchers reported fewer cases of total postoperative infection and postoperative fever in women who received cephalosporin compared with those given antiprotozoal.
Only one comparison (cephalosporin vs penicillin) yielded data on adverse effects, and no evidence showed differences in total adverse effects between the two groups.
With regard to the need for therapeutic antibiotics and length of hospital stay, we found no evidence of a difference between groups in the proportions of women requiring therapeutic antibiotics or in the numbers of days spent in hospital with cephalosporin versus penicillin, cephalosporin versus tetracycline, and antiprotozoal versus lincosamide. However, we found evidence of a difference in the two outcomes between cephalosporin and antiprotozoal groups: Fewer women in the cephalosporin group required therapeutic antibiotics, and women in this group spent fewer days in hospital, compared with those in the antiprotozoal group.
Abdominal hysterectomy
Researchers performed only one of the comparisons (cephalosporin vs penicillin) in this subgroup. We found no evidence of a difference in reported cases of infection (total postoperative infection, abdominal wound infection, UTI, pelvic infection, other serious infection, and postoperative fever) between the two groups. Investigators provided no data on adverse effects, need for therapeutic antibiotics, and length of hospital stay.
3. Combined antibiotics versus single antibiotic
We identified three different comparisons: cephalosporin plus antiprotozoal versus cephalosporin, cephalosporin plus antiprotozoal versus antiprotozoal, and penicillin plus antiprotozoal versus penicillin. Researchers performed these comparisons in two subgroups as follows.
Vaginal hysterectomy
Investigators performed all three comparisons in this subgroup but did not provide data for most outcomes, including adverse effects. When data were available, we found no evidence of a difference in outcomes between the two groups for two of the comparisons (cephalosporin plus antiprotozoal vs cephalosporin only and penicillin plus antiprotozoal vs penicillin only). However, fewer women who received cephalosporin combined with antiprotozoal received a diagnosis of total postoperative infection, UTI, or postoperative fever compared with those who received antiprotozoal only.
Abdominal hysterectomy
Researchers performed only one comparison (penicillin plus antiprotozoal vs penicillin only) in this subgroup. They provided no data on some outcomes, including adverse effects. When data were available, we found no evidence of a difference in outcomes between the two groups.
4. Cephalosporins in different dose regimens
Investigators addressed comparisons subsumed under this broad heading most often in single small trials and did not provide data on most of the outcome measures, including total adverse effects. When outcome data were reported, we found no evidence of a difference between groups in the incidence of postoperative infection, the need for therapeutic antibiotics, and length of hospital stay for each of these comparisons.
Overall completeness and applicability of evidence
Overall, the data demonstrate that prophylactic antibiotics are more effective than placebo in preventing postoperative infection, reducing the requirement for therapeutic antibiotics, and shortening length of hospital stay in women undergoing elective vaginal or abdominal hysterectomy. However, few studies reported data on adverse effects associated with the use of antibiotic prophylaxis; therefore, we were unable to determine whether prophylactic antibiotics are associated with significant adverse effects. However, as prophylaxis is usually given as a single shot, the adverse effect rate might truly be low.
Similarly, few studies compared antibiotics head‐to‐head; thus we were unable to determine which specific antibiotic is most effective, or whether individual antibiotics are similar with respect to effectiveness and safety.
We identified few studies evaluating antibiotics in different combinations, dose regimens, and routes of administration. Thus we could not determine whether it is possible to sustain the effectiveness of antibiotics while reducing adverse effects by combining lower doses of two different antibiotics, or by using certain dose regimens or routes of administration.
None of the included studies investigated laparoscopic hysterectomy (total or subtotal laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy). Thus the findings of this review are not applicable to this type of hysterectomy, which has been performed increasingly over the past decade.
One should interpret the results on "length of hospital stay" and "urinary tract infections" with caution, as some studies reporting these outcomes were conducted decades ago. Meanwhile, hospital stay has decreased tremendously over the past few decades owing to improved knowledge of postoperative care and doctors' adaptation of the principles of "early recovery after surgery" (ERAS®). These include striving postoperatively for early mobilisation, normalisation of oral intake, and early removal of urinary catheters, thus decreasing length of hospital stay, risk of nosocomial infection, and risk of UTI. For example, it is very rare nowadays for healthy patients who undergo uncomplicated vaginal hysterectomy to be admitted to a hospital for longer than three days, whereas the studies in Analysis 1.9 show mean hospitalisation duration of 8.3 to 11.9 days.
Quality of the evidence
Most studies considered for this review were of poor quality in relation to risk of bias. We excluded many studies owing to unclear design, lack of double‐blinding, or non‐blinding. Among the included studies, very few clearly described their methods of sequence generation and allocation concealment. For most comparisons, effect estimates were associated with imprecision due to small sample sizes and wide confidence intervals.
We assessed the quality of evidence for the review's main comparison (any antibiotics vs placebo for vaginal and abdominal hysterectomy). The quality of evidence for our primary outcome ranged from very low to moderate. The main limitations in the body of evidence were risk of bias (due to poor reporting of sequence generation and allocation concealment), serious imprecision (associated with small sample size and low event rates, leading to wide confidence intervals), and inadequate reporting of adverse effects.
We rated the quality of evidence for head‐to head comparisons of antibiotics and for dose comparisons as very low owing to imprecision related to wide confidence intervals and low event rates, and to risk of bias associated with poor reporting of study methods.
We examined the presence of publication or reporting bias in a funnel plot for five subgroups in one of the comparisons (any antibiotics vs placebo) and found evidence suggesting a tendency towards publication or reporting bias, with smaller studies likely to report beneficial effects with antibiotic prophylaxis. However, we did not consider that evidence of publication bias was strong enough to necessitate downgrading the quality of evidence.
Potential biases in the review process
Although we undertook a comprehensive search to ensure that we identified potentially eligible studies, it is possible that some eligible studies might have been left out in the course of the search and selection process.
Agreements and disagreements with other studies or reviews
Clinical guidelines (ACOG 2009; Deffieux 2015; SIGN 2008) and narrative reviews (Clifford 2012; Hodges 2014; Steiner 2017) recommend antibiotic prophylaxis for women undergoing hysterectomy, and pragmatically opt to advise cephalosporins as a first choice. However, the evidence base for first‐line cephalosporins is limited by the lack of recent trials. Moreover, no randomised controlled trials (RCTs) at all examined the topic of antibiotic prophylaxis for laparoscopic hysterectomy.
Much of the evidence is very old: For example, Clifford 2012 is a narrative review that refers to old studies such as Duff 1982 and Tanos 1994 to recommend prophylactic antibiotics for hysterectomy, and Larsson 2002 to recommend preoperative treatment of bacterial vaginosis. We excluded both Larsson 2002 and Tanos 1994 from the current review because investigators utilised extended seven‐day prophylaxis as well as an historical comparison group (respectively).
A more recent review (Morrill 2013) investigated antibiotic prophylaxis in selected gynaecological surgeries, including hysteroscopic and cervical surgery, while excluding hysterectomy (Morrill 2013). Review authors concluded that evidence provides a strong case for prophylactic antibiotics for abdominal gynaecological surgery but acknowledged lack of evidence for their use in vaginal surgery. For laparoscopic surgery, we found no advantage of prophylactic antibiotics, but high‐quality evidence was lacking and results were hampered by heterogeneity of the population; women underwent widely varying surgeries, from diagnostic laparoscopy to ovarian cystectomy or extended endometriosis surgery.
A large retrospective cohort of 21,358 hysterectomies performed in the United States (Upall 2016) investigated associations between a composite outcome of "any surgical site infection" and classes of antibiotics administered preoperatively. Investigators found that women receiving beta‐lactam antibiotic regimens (i.e. first‐ or second‐generation cephalosporins, ampicillin plus sulbactam, or ertapenem) had lower risk of surgical site infection than women given a beta‐lactam alternative (i.e. clindamycin combination, gentamycin combination, metronidazole combination) or a non‐standard regimen (i.e. clindamycin, gentamycin, or aztreonam, or another antibiotic alone). We found comparable benefit for cephalosporins but only for vaginal hysterectomy when compared with antiprotozoal alone.
Several published systematic reviews and meta‐analyses of the use of antibiotics in hysterectomy have reported mainly on the same set of included RCTs.
Wttewaall‐Evelaar 1990 meta‐analysed 17 randomised blinded placebo‐controlled trials of prophylaxis for elective abdominal hysterectomy, all published between 1986 and 1988. In most cases, the antibiotics used were cephalosporins. Review authors concluded that prophylaxis significantly reduced levels of infection (p < 0.001; no odds ratio reported), and that additional placebo‐controlled trials were not warranted. Mittendorf 1993 meta‐analysed 31 English‐language RCTs published from 1972 to 1986, and concluded that antibiotic prophylaxis reduced the rate of serious infection after abdominal hysterectomy from 21.1% to 9% (P = 0.00001; no odds ratio reported in text). Trials that used different routes of administration and differing prophylaxis regimens, varying from a single dose to five days' duration, were pooled. Tanos 1994 meta‐analysed 17 "controlled or comparative" trials conducted between 1978 and 1990 to investigate single or one‐day prophylactic regimens of intravenous or intramuscular cephalosporins for abdominal hysterectomy. It is unclear whether all of the included trials were randomised, and some trials included oncology patients among their participants. Again, results clearly favoured the use of prophylaxis (odds ratio (OR) 0.35, 95% confidence interval (CI) 0.3 to 0.4).
Two of these meta‐analyses combined results from studies that included very different participants or interventions. The other (Wttewaall‐Evelaar 1990) was more rigorous but did not include any of the numerous studies carried out since 1986.
More recently, a systematic review by Costa and Krauss‐Silva meta‐analysed double‐blinded, placebo‐controlled trials on the use of antibiotic prophylaxis for elective, non‐radical abdominal hysterectomy (Costa 2004). Review authors meta‐analysed a total of 16 studies published between 1977 and 2003, but it is important to note that the most recent study was published in 1998, and the 15 remaining RCTs were published in 1988 or earlier. Review authors concluded that use of antibiotic prophylaxis is effective for prevention of postoperative infection (risk ratio (RR) 0.49, 95% CI 0.41 to 0.59). They concluded that no evidence showed benefit for multiple‐ versus single‐dose prophylaxis.
We identified no RCTs on the use of antibiotics in laparoscopic hysterectomy for inclusion in this review. A recent review by Lachiewicz on laparoscopic hysterectomy recommends use of antibiotics, with dose adjusted to body weight (increased dosage when patients weigh more than 120 kilograms), and use of antiprotozoals. The latter recommendation consists of using antiprotozoals routinely or after screening for bacterial vaginosis before surgery in which the vaginal‐abdominal barrier was breached (Lachiewicz 2015). However, arguments for these recommendations in laparoscopy derive from authority‐based guidelines or non‐randomised trials (Bratzler 2013; Soper 1993).
Findings from the studies above are consistent with the findings of this review, which found evidence that antibiotic prophylaxis is effective in preventing postoperative infection in women undergoing elective vaginal or abdominal hysterectomy.
Authors' conclusions
Implications for practice.
Antibiotic prophylaxis appears to be effective in preventing postoperative infection in women undergoing elective vaginal or abdominal hysterectomy, regardless of the dose regimen. However, evidence was insufficient to show whether their use influences rates of adverse effects. Similarly, evidence was insufficient to show which (if any) individual antibiotic, dose regimen, or route of administration is safest and most effective. In interpreting results, it is important to realise that the most recent of the included studies was published 14 years ago, at the time of our search. Thus findings from included studies might not reflect current practice in perioperative and postoperative care or might not show locoregional antimicrobial resistance patterns.
Implications for research.
More studies including large numbers of women and based on sound methods are needed to detect meaningful differences in efficacy between various antibiotics and to properly evaluate adverse effects associated with their use as prophylaxis for women undergoing elective hysterectomy. Also needed are more studies investigating various antibiotics in different combinations, dose regimens, and routes of administration to determine which combinations, dose regimens, and routes of administration are associated with better efficacy and fewer adverse effects. Laparoscopic hysterectomy is now commonly performed; thus future research should focus on the use of prophylaxis in laparoscopic hysterectomy (total or subtotal laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy).
In addition, trial publications should adequately report trial methods in accordance with the CONSORT statement.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Review declared as stable | It is unlikely that there will be any new studies for inclusion in this review, and accordingly this is now a stable review. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 6, 2017
| Date | Event | Description |
|---|---|---|
| 1 April 2019 | Amended | Correction of data errors in one included study |
| 18 March 2008 | Amended | Converted to new review format |
| 8 April 2003 | New citation required and conclusions have changed | Made substantive amendments |
Acknowledgements
The review authors would like to thank all members of the Cochrane Gynaecology and Fertility Group for valuable support during preparation of this review. The review authors also wish to thank Dr Karim Calis for contributions to the protocol, and Dr Irena Zakarija‐Grkovic for assistance in translating Vecek 1993 from Croatian and extracting data.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility database search strategy
From inception to 29.11.16
PROCITE platform
Keywords CONTAINS "Hysterectomy" or "Hysterectomy, abdominal" or "hysterectomy ‐laparoscopic" or "hysterectomy, laparoscopically assisted vaginal" or "Hysterectomy, subtotal" or "Hysterectomy, Vaginal" or "vaginal hysterectomy" or "total abdominal hysterectomy" or "total hysterectomy" or "total laparoscopic hysterectomy" or "laparoscopic hysterectomy" or "subtotal" or "abdominal hysterectomy" or Title CONTAINS "Hysterectomy" or "Hysterectomy, abdominal" or "hysterectomy ‐laparoscopic" or "hysterectomy, laparoscopically assisted vaginal" or "Hysterectomy, subtotal" or "Hysterectomy, Vaginal" or "vaginal hysterectomy" or "total abdominal hysterectomy" or "total hysterectomy" or "total laparoscopic hysterectomy" or "laparoscopic hysterectomy" or "subtotal" or "abdominal hysterectomy"
AND
Keywords CONTAINS "antibiotics" or "prophylactic" or "prophylactic antibiotics" or "prophylaxis" or "penicillin" or "penicillin G" or "amoxicillin" or "Amoxicillin‐Clavulanic Acid" or "ampicillin" or "cefamandole" or "cefamazin" or "Cefazolin" or "Cefmetazole" or "cefoperazone" or "cefonicid" or "cefotaxime" or "Cefotetan" or "Cefotiam" or "cefoxitin" or "ceftazidime" or "ceftezole" or "*Ceftizoxime" or "Ceftriaxone" or "Cefuroxime" or "cephalexin" or "cephaloride" or "cephaloride" or "cephalozin" or "cephazolin" or "Cephradine" or "Augmentin" or "erythromycin" or "Azithromycin" or "Metronidazole" or "co‐trimoxazole" or "tetracycline" or "doxycycline" or "Gentamicin" or "gentamycin" or "tobramycin" or "Metronidazole" or Title CONTAINS "antibiotics" or "prophylactic" or "prophylactic antibiotics" or "prophylaxis" or "penicillin" or "penicillin G" or "amoxicillin" or "Amoxicillin‐Clavulanic Acid" or "ampicillin" or "cefamandole" or "cefamazin" or "Cefazolin" or "Cefmetazole" or "cefoperazone" or "cefonicid" or "cefotaxime" or "Cefotetan" (263 hits)
Appendix 2. CENTRAL CRSO search strategy
From inception to 29.11.16
CRS Online platform
#1 MESH DESCRIPTOR hysterectomy EXPLODE ALL TREES 1578
#2 hysterectom*:TI,AB,KY 3458
#3 #1 OR #2 3458
#4 (anti‐bacterial agent*):TI,AB,KY 7936
#5 MESH DESCRIPTOR anti‐bacterial agents EXPLODE ALL TREES 21172
#6 MESH DESCRIPTOR Amoxicillin EXPLODE ALL TREES 2140
#7 MESH DESCRIPTOR azithromycin EXPLODE ALL TREES 718
#8 MESH DESCRIPTOR cefaclor EXPLODE ALL TREES 225
#9 MESH DESCRIPTOR cefazolin EXPLODE ALL TREES 385
#10 MESH DESCRIPTOR cefoxitin EXPLODE ALL TREES 274
#11 MESH DESCRIPTOR cephradine EXPLODE ALL TREES 75
#12 MESH DESCRIPTOR clavulanic acid EXPLODE ALL TREES 618
#13 MESH DESCRIPTOR doxycycline EXPLODE ALL TREES 741
#14 MESH DESCRIPTOR erythromycin EXPLODE ALL TREES 3258
#15 MESH DESCRIPTOR fluoroquinolones EXPLODE ALL TREES 3410
#16 MESH DESCRIPTOR gentamicins EXPLODE ALL TREES 1045
#17 MESH DESCRIPTOR ofloxacin EXPLODE ALL TREES 756
#18 MESH DESCRIPTOR Ciprofloxacin EXPLODE ALL TREES 946
#19 MESH DESCRIPTOR Penicillin G EXPLODE ALL TREES 3983
#20 MESH DESCRIPTOR Penicillin Amidase EXPLODE ALL TREES 0
#21 MESH DESCRIPTOR tetracycline EXPLODE ALL TREES 694
#22 MESH DESCRIPTOR Tobramycin EXPLODE ALL TREES 489
#23 MESH DESCRIPTOR Vancomycin EXPLODE ALL TREES 429
#24 MESH DESCRIPTOR Carboxylesterase EXPLODE ALL TREES 4
#25 MESH DESCRIPTOR Chlortetracycline EXPLODE ALL TREES 16
#26 MESH DESCRIPTOR Antiprotozoal Agents EXPLODE ALL TREES 7303
#27 metronidazole:TI,AB,KY 3304
#28 co‐trimoxazole:TI,AB,KY 359
#29 penicillin*:TI,AB,KY 3067
#30 (anti‐bacterial agent*):TI,AB,KY 7936
#31 ((antibiotic* or anti biotic*)):TI,AB,KY 17395
#32 antimicrobial*:TI,AB,KY 3993
#33 MESH DESCRIPTOR Antibiotic Prophylaxis EXPLODE ALL TREES 1001
#34 ((cefotaxime or tinidazole)):TI,AB,KY 1424
#35 (Antibiotic* adj5 Prophyla*):TI,AB,KY 2697
#36 ((cephradine or mezlocillin)):TI,AB,KY 330
#37 ((cefoxitin or clindamycin)):TI,AB,KY 1693
#38 ((cefonicid or cefoperazone)):TI,AB,KY 315
#39 ((mezlocillin or moxalactam)):TI,AB,KY 355
#40 ceftriaxone:TI,AB,KY 1156
#41 ((doxycycline or cefamandole)):TI,AB,KY 1625
#42 ((cephalosporin or piperacillin)):TI,AB,KY 1549
#43 ((cefpirome or cefazolin)):TI,AB,KY 802
#44 cefotetan:TI,AB,KY 159
#45 amoxicillin‐clavulanic:TI,AB,KY 247
#46 augmentin:TI,AB,KY 164
#47 trovafloxacin:TI,AB,KY 78
#48 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 39789
#49 #3 AND #48 327
Appendix 3. MEDLINE search strategy
From 1946 to 29.11.16
OVID platform
1 exp hysterectomy/ or exp hysterectomy, vaginal/ (28502) 2 hysterectom$.tw. (32206) 3 or/1‐2 (43888) 4 exp anti‐bacterial agents/ or exp amoxicillin/ or exp azithromycin/ or exp cefaclor/ or exp cefazolin/ or exp cefoxitin/ or exp cephradine/ or clavulanic acid/ or exp doxycycline/ or exp erythromycin/ or exp fluoroquinolones/ or exp gentamicins/ or exp ofloxacin/ or exp penicillin g/ or exp penicillin g, benzathine/ or exp penicillin g, procaine/ or exp tetracycline/ or exp tobramycin/ or exp vancomycin/ (651493) 5 exp Antiprotozoal Agents/ (159702) 6 metronidazole.tw. (13968) 7 co‐trimoxazole.tw. (2660) 8 penicillin$.tw. (52937) 9 anti‐bacterial agent$.tw. (163) 10 (antibiotic$ or anti biotic$).tw. (284453) 11 antimicrobial$.tw. (124346) 12 exp Antibiotic Prophylaxis/ (12213) 13 (cefotaxime or tinidazole).tw. (8814) 14 Prophyla$.tw. (147141) 15 (cephradine or mezlocillin).tw. (1583) 16 (cefoxitin or clindamycin).tw. (12683) 17 (cefonicid or cefoperazone).tw. (2694) 18 (mezlocillin or moxalactam).tw. (2078) 19 ceftriaxone.tw. (9034) 20 (doxycycline or cefamandole).tw. (13460) 21 (cephalosporin or piperacillin).tw. (14740) 22 (cefpirome or cefazolin).tw. (4437) 23 cefotetan.tw. (748) 24 amoxicillin‐clavulanic.tw. (1982) 25 augmentin.tw. (590) 26 trovafloxacin.tw. (756) 27 or/4‐26 (1060125) 28 3 and 27 (2208) 29 randomized controlled trial.pt. (469810) 30 controlled clinical trial.pt. (95074) 31 randomized.ab. (404455) 32 placebo.tw. (197050) 33 clinical trials as topic.sh. (189502) 34 randomly.ab. (285441) 35 trial.ti. (178951) 36 (crossover or cross‐over or cross over).tw. (76010) 37 or/29‐36 (1178869) 38 (animals not (humans and animals)).sh. (4636432) 39 37 not 38 (1085717) 40 28 and 39 (366)
Appendix 4. Embase search strategy
From 1980 to 29.11.16
OVID platform
1 exp hysterectomy/ or exp abdominal hysterectomy/ or exp vaginal hysterectomy/ or exp radical hysterectomy/ (59806) 2 hysterectom$.tw. (44636) 3 or/1‐2 (66380) 4 exp antibiotic agent/ (1133797) 5 antibiotic$.tw. (337099) 6 exp antiprotozoal agent/ (174165) 7 (metronidazole or co‐trimoxazole).tw. (21125) 8 (penicillin$ or anti‐bacterial agent$).tw. (51788) 9 (antibiotic$ or anti biotic$).tw. (337207) 10 antimicrobial$.tw. (153891) 11 (cefotaxime or tinidazole).tw. (11137) 12 Prophyla$.tw. (189472) 13 (cephradine or mezlocillin).tw. (1810) 14 (cefoxitin or clindamycin).tw. (15311) 15 (cefonicid or cefoperazone).tw. (3655) 16 (mezlocillin or moxalactam).tw. (2506) 17 ceftriaxone.tw. (12554) 18 (doxycycline or cefamandole).tw. (16045) 19 (cephalosporin or piperacillin).tw. (19481) 20 (cefpirome or cefazolin).tw. (5466) 21 cefotetan.tw. (983) 22 amoxicillin‐clavulanic.tw. (2686) 23 augmentin.tw. (3602) 24 trovafloxacin.tw. (865) 25 or/4‐24 (1569108) 26 3 and 25 (5623) 27 Clinical Trial/ (995452) 28 Randomized Controlled Trial/ (461742) 29 exp randomization/ (83639) 30 Single Blind Procedure/ (27251) 31 Double Blind Procedure/ (136941) 32 Crossover Procedure/ (53825) 33 Placebo/ (321968) 34 Randomi?ed controlled trial$.tw. (149359) 35 Rct.tw. (22353) 36 random allocation.tw. (1629) 37 randomly.tw. (338581) 38 randomly allocated.tw. (26583) 39 allocated randomly.tw. (2208) 40 (allocated adj2 random).tw. (843) 41 Single blind$.tw. (18663) 42 Double blind$.tw. (172862) 43 ((treble or triple) adj blind$).tw. (647) 44 placebo$.tw. (247461) 45 prospective study/ (386445) 46 or/27‐45 (1967839) 47 case study/ (92866) 48 case report.tw. (323319) 49 abstract report/ or letter/ (986309) 50 or/47‐49 (1393358) 51 46 not 50 (1916879) 52 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5725014) 53 51 not 52 (1793800) 54 26 and 53 (913)
Appendix 5. PsycINFO search strategy
From 1806 to 29.11.16
OVID platform
1 exp hysterectomy/ (417) 2 hysterectom$.tw. (756) 3 or/1‐2 (777) 4 exp antibiotics/ or exp drugs/ or exp amantadine/ or exp cycloheximide/ or exp penicillins/ or exp puromycin/ (277096) 5 Antiprotozoal$.tw. (1) 6 metronidazole.tw. (91) 7 co‐trimoxazole.tw. (10) 8 penicillin$.tw. (454) 9 anti‐bacterial agent$.tw. (3) 10 (antibiotic$ or anti biotic$).tw. (2276) 11 antimicrobial$.tw. (429) 12 (cefotaxime or tinidazole).tw. (14) 13 Prophyla$.tw. (6165) 14 (cephradine or mezlocillin).tw. (1) 15 (cefoxitin or clindamycin).tw. (31) 16 (cefonicid or cefoperazone).tw. (2) 17 (mezlocillin or moxalactam).tw. (0) 18 ceftriaxone.tw. (200) 19 (doxycycline or cefamandole).tw. (179) 20 (cephalosporin or piperacillin).tw. (46) 21 (cefpirome or cefazolin).tw. (6) 22 cefotetan.tw. (0) 23 amoxicillin‐clavulanic.tw. (5) 24 augmentin.tw. (2) 25 trovafloxacin.tw. (8) 26 or/4‐25 (283254) 27 3 and 26 (88)
Appendix 6. CINAHL search strategy
From inception to 29.11.16
EBSCO platform
| # | Query | Results |
| S55 | S42 AND S54 | 37 |
| S54 | S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 | 1,093,627 |
| S53 | TX allocat* random* | 5,654 |
| S52 | (MH "Quantitative Studies") | 15,044 |
| S51 | (MH "Placebos") | 9,902 |
| S50 | TX placebo* | 41,786 |
| S49 | TX random* allocat* | 5,654 |
| S48 | (MH "Random Assignment") | 41,925 |
| S47 | TX randomi* control* trial* | 114,733 |
| S46 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 863,204 |
| S45 | TX clinic* n1 trial* | 195,043 |
| S44 | PT Clinical trial | 79,858 |
| S43 | (MH "Clinical Trials+") | 206,820 |
| S42 | S3 AND S41 | 172 |
| S41 | S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 | 72,958 |
| S40 | TX trovafloxacin | 63 |
| S39 | TX augmentin | 29 |
| S38 | TX amoxicillin‐clavulanic | 142 |
| S37 | TX cefotetan | 41 |
| S36 | TX(cefpirome or cefazolin) | 468 |
| S35 | TX(cephalosporin or piperacillin) | 2,612 |
| S34 | TX(doxycycline or cefamandole) | 1,877 |
| S33 | TX ceftriaxone | 1,425 |
| S32 | TX(mezlocillin or moxalactam) | 23 |
| S31 | TX(cefonicid or cefoperazone) | 71 |
| S30 | TX(cefoxitin or clindamycin) | 1,369 |
| S29 | TX (cephradine or mezlocillin) | 22 |
| S28 | TX (antibiotic* N5 prophyla*) | 5,303 |
| S27 | TX(cefotaxime or tinidazole) | 583 |
| S26 | (MM "Antibiotic Prophylaxis") | 2,178 |
| S25 | TX antimicrobial* | 16,309 |
| S24 | TX anti‐bacterial agent* | 12 |
| S23 | TX(antibiotic* or anti biotic*) | 53,541 |
| S22 | TX penicillin | 3,530 |
| S21 | TX metronidazole | 1,864 |
| S20 | (MM "Antiprotozoal Agents") | 500 |
| S19 | (MM "Vancomycin") | 1,013 |
| S18 | (MH "Tobramycin") | 318 |
| S17 | (MM "Tetracycline") OR (MH "Tetracyclines") OR (MH "Minocycline") | 1,063 |
| S16 | (MM "Penicillin G+") OR (MH "Penicillins") OR (MH "Dicloxacillin") | 2,251 |
| S15 | (MH "Ofloxacin") | 602 |
| S14 | (MM "Gentamicins") | 626 |
| S13 | (MH "Antiinfective Agents, Fluoroquinolone") OR (MH "Trovafloxacin") OR (MH "Ofloxacin") OR (MH "Alatrofloxacin") | 1,345 |
| S12 | (MM "Erythromycin+") OR (MM "Clarithromycin") | 1,250 |
| S11 | (MM "Doxycycline") | 503 |
| S10 | (MM "Clavulanic Acid") | 41 |
| S9 | (MM "Cefoxitin") | 22 |
| S8 | (MM "Cefazolin") | 120 |
| S7 | (MM "Cefaclor") | 9 |
| S6 | (MM "Azithromycin") | 614 |
| S5 | (MM "Amoxicillin") | 461 |
| S4 | (MM "Antibiotics+") OR (MH "Antibiotics, Combined") OR (MH "Antibiotic Prophylaxis") OR (MH "Antibiotics, Lactam") OR (MH "Antibiotics, Antineoplastic") OR (MH "Antibiotics, Peptide") OR (MH "Antibiotics, Macrolide") OR (MH "Antibiotics, Antitubercular") OR (MH "Antibiotics, Antifungal") OR (MH "Aminoglycosides") | 31,924 |
| S3 | S1 OR S2 | 6,646 |
| S2 | TX hysterectom* | 6,646 |
| S1 | (MM "Hysterectomy+") OR (MM "Hysterectomy, Vaginal") | 2,727 |
Data and analyses
Comparison 1. Any antibiotic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 4 | 293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.19, 0.40] |
| 1.2 Abdominal hysterectomy | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.21, 0.67] |
| 2 Abdominal wound infection | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Abdominal hysterectomy | 11 | 2247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.36, 0.73] |
| 3 Urinary tract infection | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 8 | 1473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.43, 0.77] |
| 3.2 Abdominal hysterectomy | 12 | 2705 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.31, 0.53] |
| 4 Pelvic infection | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 11 | 1693 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.20, 0.39] |
| 4.2 Abdominal hysterectomy | 11 | 1883 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.35, 0.71] |
| 5 Other serious infections | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.10] |
| 5.2 Abdominal hysterectomy | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.12, 1.69] |
| 6 Postoperative fever | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Vaginal hysterectomy | 9 | 1562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.34, 0.54] |
| 6.2 Abdominal hysterectomy | 11 | 2394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.50, 0.70] |
| 7 Total adverse effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Abdominal hysterectomy | 2 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.62, 5.18] |
| 8 Need for therapeutic antibiotics | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Vaginal hysterectomy | 6 | 1309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.37, 0.68] |
| 8.2 Abdominal hysterectomy | 6 | 1359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.93] |
| 9 Length of hospital stay | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Vaginal hysterectomy | 4 | 853 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐1.78, ‐0.92] |
| 9.2 Abdominal hysterectomy | 7 | 1510 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.76, ‐0.43] |
Comparison 2. Cephalosporin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 3 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.20, 0.42] |
| 2 Abdominal wound infection | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Abdominal hysterectomy | 7 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.25, 0.66] |
| 3 Urinary tract infection | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.46, 1.08] |
| 3.2 Abdominal hysterectomy | 6 | 1668 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.31, 0.58] |
| 4 Pelvic infection | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 6 | 1281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.09, 0.28] |
| 4.2 Abdominal hysterectomy | 7 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.93] |
| 5 Other serious infections | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.12] |
| 5.2 Abdominal hysterectomy | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.16] |
| 6 Postoperative fever | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Vaginal hysterectomy | 5 | 1028 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.25, 0.54] |
| 6.2 Abdominal hysterectomy | 6 | 1463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.77] |
| 7 Total adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Abdominal hysterectomy | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.83] |
| 8 Need for therapeutic antibiotics | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Vaginal hysterectomy | 3 | 863 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.37, 0.81] |
| 8.2 Abdominal hysterectomy | 4 | 1138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.01] |
| 9 Length of hospital stay | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Vaginal hysterectomy | 2 | 657 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.88, ‐0.72] |
| 9.2 Abdominal hysterectomy | 4 | 818 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.67, ‐0.19] |
Comparison 3. Penicillin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.42] |
| 1.2 Abdominal hysterectomy | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.13, 0.70] |
| 2 Abdominal wound infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Abdominal hysterectomy | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.53] |
| 3 Urinary tract infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.67] |
| 3.2 Abdominal hysterectomy | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.21, 1.76] |
| 4 Pelvic infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.45] |
| 4.2 Abdominal hysterectomy | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.82] |
| 5 Other serious infections | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Abdominal hysterectomy | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.73] |
| 6 Postoperative fever | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Vaginal hysterectomy | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.45] |
| 6.2 Abdominal hysterectomy | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.35, 1.20] |
Comparison 4. Antiprotozoal versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal wound infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Abdominal hysterectomy | 2 | 462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.32, 1.57] |
| 2 Urinary tract infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.51, 3.04] |
| 2.2 Abdominal hysterectomy | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.96] |
| 3 Pelvic infection | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 4 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.17, 0.75] |
| 3.2 Abdominal hysterectomy | 4 | 662 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.22, 0.83] |
| 4 Other serious infections | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 2 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.21] |
| 4.2 Abdominal hysterectomy | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
| 5 Postoperative fever | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.97] |
| 5.2 Abdominal hysterectomy | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.18, 0.85] |
| 6 Total adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Abdominal hysterectomy | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.63, 6.35] |
| 7 Need for therapeutic antibiotics | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Vaginal hysterectomy | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.15, 1.95] |
| 7.2 Abdominal hysterectomy | 2 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.15, 2.02] |
| 8 Length of hospital stay | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Vaginal hysterectomy | 3 | 276 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.22, ‐0.49] |
| 8.2 Abdominal hysterectomy | 3 | 358 | Mean Difference (IV, Fixed, 95% CI) | ‐1.33 [‐1.68, ‐0.97] |
Comparison 5. Sulphonamides versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal wound infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Abdominal hysterectomy | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.35, 4.35] |
| 2 Urinary tract infection | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.15, 0.84] |
| 2.2 Abdominal hysterectomy | 2 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.06, 0.50] |
| 3 Pelvic infection | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.63] |
| 3.2 Abdominal hysterectomy | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.84] |
| 4 Postoperative fever | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.26, 0.95] |
| 4.2 Abdominal hysterectomy | 2 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.38, 1.04] |
| 5 Length of hospital stay | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 3 | 276 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.22, ‐0.49] |
| 5.2 Abdominal hysterectomy | 3 | 358 | Mean Difference (IV, Fixed, 95% CI) | ‐1.33 [‐1.68, ‐0.97] |
5.5. Analysis.
Comparison 5 Sulphonamides versus placebo, Outcome 5 Length of hospital stay.
Comparison 6. Cephalosporin + antiprotozoal versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Abdominal hysterectomy | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 7.03] |
| 2 Urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.24, 1.04] |
| 2.2 Abdominal hysterectomy | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.08, 0.96] |
| 3 Pelvic infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.37] |
| 4 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.34, 0.73] |
| 4.2 Abdominal hysterectomy | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.09] |
| 5 Need for therapeutic antibiotics | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.19, 0.68] |
| 5.2 Abdominal hysterectomy | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 0.94] |
| 6 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Abdominal hysterectomy | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |
Comparison 7. Penicillin + antiprotozoal versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.04, 1.73] |
| 1.2 Abdominal hysterectomy | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.23, 0.86] |
| 2 Abdominal wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Abdominal hysterectomy | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.60] |
| 3 Urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.07, 5.72] |
| 3.2 Abdominal hysterectomy | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.38, 2.47] |
| 4 Pelvic infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 2.96] |
| 5 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 2.96] |
| 5.2 Abdominal hysterectomy | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.64] |
Comparison 8. Lincosamide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.25, 2.06] |
| 2 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.44] |
| 3 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.77, ‐0.03] |
Comparison 9. Cephalosporin versus penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 2 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.55, 2.00] |
| 2 Abdominal wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Abdominal hysterectomy | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 3 Urinary tract infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 3.98] |
| 3.2 Abdominal hysterectomy | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.79] |
| 4 Pelvic infection | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 3 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.47, 1.64] |
| 4.2 Abdominal hysterectomy | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.67] |
| 5 Other serious infections | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 69.68] |
| 5.2 Abdominal hysterectomy | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.85] |
| 6 Postoperative fever | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Vaginal hysterectomy | 3 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.58, 1.15] |
| 6.2 Abdominal hysterectomy | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.42, 1.77] |
| 7 Total adverse effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Vaginal hysterectomy | 2 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.14] |
| 8 Need for therapeutic antibiotics | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Vaginal hysterectomy | 2 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.88, 1.97] |
| 9 Length of hospital stay | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Vaginal hysterectomy | 2 | 209 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.97, 0.04] |
Comparison 10. Cephalosporin versus tetracycline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.20, 1.78] |
| 2 Pelvic infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.25, 2.75] |
| 3 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.13, 3.81] |
| 4 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.11, 0.71] |
Comparison 11. Cephalosporin versus antiprotozoal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.67] |
| 2 Urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.81] |
| 3 Pelvic infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.03] |
| 4 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.42] |
| 5 Need for therapeutic antibiotics | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.44] |
| 6 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Vaginal hysterectomy | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐3.32, ‐0.48] |
Comparison 12. Antiprotozoal versus lincosamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.47, 34.24] |
| 2 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 3 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.60, 0.20] |
Comparison 13. Cephalosporin + antiprotozoal versus cephalosporin only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.03, 7.68] |
| 2 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.43, 1.03] |
Comparison 14. Cephalosporin + antiprotozoal versus antiprotozoal only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.67] |
| 2 Urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.81] |
| 3 Pelvic infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.03] |
| 4 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.42] |
| 5 Need for therapeutic antibiotics | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.44] |
| 6 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Vaginal hysterectomy | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.11, ‐0.09] |
Comparison 15. Penicillin + antiprotozoal versus penicillin only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.09, 17.02] |
| 1.2 Abdominal hysterectomy | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.57, 3.75] |
| 2 Abdominal wound infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Abdominal hysterectomy | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.25, 3.59] |
| 3 Urinary tract infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.09, 17.02] |
| 3.2 Abdominal hysterectomy | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.80, 4.97] |
| 4 Postoperative fever | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Abdominal hysterectomy | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.20, 4.50] |
| 5 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.11, ‐0.09] |
15.5. Analysis.
Comparison 15 Penicillin + antiprotozoal versus penicillin only, Outcome 5 Length of hospital stay.
Comparison 16. Cephalosporin: early administration versus usual timing (both single dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Abdominal hysterectomy | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.03, 7.90] |
| 2 Pelvic infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.16, 14.20] |
| 2.2 Abdominal hysterectomy | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.16, 14.20] |
Comparison 17. Cephalosporin: one dose versus two doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Abdominal hysterectomy | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.14, 3.18] |
| 2 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Abdominal hysterectomy | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.97, 4.13] |
| 3 Need for therapeutic antibiotics | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Abdominal hysterectomy | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.90 [0.48, 202.43] |
Comparison 18. Cephalosporin: one dose versus three doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.36] |
| 2 Pelvic infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.36] |
| 3 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.97] |
| 4 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.72, 0.12] |
Comparison 19. Cephalosporin: one dose versus multiple doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 98.52] |
| 2 Urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.87] |
| 3 Pelvic infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.87] |
| 4 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 98.52] |
Comparison 20. Cephalosporin one gram versus two grams.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total postoperative infections ‐ early and late | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal hysterectomy | 1 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.25, 8.74] |
| 2 Pelvic infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vaginal hysterectomy | 1 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.25, 8.74] |
| 3 Postoperative fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Vaginal hysterectomy | 1 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.43, 5.14] |
| 4 Need for therapeutic antibiotics | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Vaginal hysterectomy | 1 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.25, 8.74] |
| 5 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Vaginal hysterectomy | 1 | 237 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.60, 0.40] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benigno 1986.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: 356 No. analysed:298 Drop‐outs/withdrawals: 58 (27 piperacillin, 23 cephalothin, and 8 cefoxitin groups) were excluded for the following reasons: pre‐study administration of antibiotics (3), preoperative infection (15), dosage violation (11), total abdominal hysterectomy performed (8), failure to attend clinical follow‐up examination (21) Years of recruitment: not stated Setting: 7 study centres, United States |
|
| Participants | Inclusion criteria: scheduled to undergo vaginal hysterectomy Exclusion criteria: receipt of antimicrobial therapy within 7 days before entrance into study, history of hypersensitivity to cephalosporin or penicillin, renal or hepatic or both, test results significantly outside normal limits, infection at time of screening for enrolment of study Age: 19 to 80 years Type of hysterectomy: vaginal, some with associated procedures | |
| Interventions | Two protocols: piperacillin vs cephalothin, piperacillin vs cefoxitin Treatment 1: piperacillin (penicillin) Treatment 2: cephalothin (first‐generation cephalosporin) Treatment 3: cefoxitin (second‐generation cephalosporin) Dose: 3 doses of 2 grams, same regimen for all treatment groups Route: IV Single/multiple doses: multiple Duration of course of antibiotics: approx. 13 hours Timing of doses: 2 grams in first hour, then 2 grams 6‐hourly |
|
| Outcomes | Total postoperative infections Pelvic infection Postoperative fever Adverse effects Need for therapeutic antibiotics Length of hospital stay Follow up: 3 to 10 weeks |
|
| Funding | Not stated | |
| Notes | No SDs for LOS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | "Schedule maintained by hospital pharmacy" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The investigator and staff were unaware of the antibiotic assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information on proportions of withdrawals and reasons for withdrawals per treatment group |
| Selective reporting (reporting bias) | Low risk | Outcome data available on all prespecified outcomes |
| Other bias | Unclear risk | "The data were identical with regard to patient selection and criteria used for evaluation..." |
Boodt 1990.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: 406 No. analysed: 406 (reported in table of results) Drop‐outs/withdrawals: states 7 participants not evaluable (5 underwent vaginal hysterectomy with repair, 2 underwent surgery for urinary incontinence) Years of recruitment: not stated Setting: single centre, Dutch teaching hospital |
|
| Participants | Inclusion criteria: patients hospitalised for an abdominal or vaginal hysterectomy or a vaginal hysterectomy with vaginal repair, who were informed about the objective of trial in writing before the operation and gave permission to be included Exclusion criteria: emergency operation, known sensitivity to cephalosporins, preexisting infection or antibiotic therapy in the 48 hours preceding surgery Age: 41 to 59 years Type of hysterectomy: abdominal or vaginal (some vaginal with associated procedures). |
|
| Interventions | Treatment: 1500 mg cefuroxime (second‐generation cephalosporin) plus 500 mg metronidazole (antiprotozoal) Control: placebo Route: IV Single/multiple doses: single Timing of doses: 10‐minute infusion during induction of anaesthesia |
|
| Outcomes | Urinary tract infection Pelvic infection Postoperative fever Need for therapeutic antibiotics Length of hospital stay Follow up: 6 weeks |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "In view of the double blind...both the active and the placebo infusions were coloured yellow..." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information on withdrawals and reasons for withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects not systematically reported |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Chongsomchai 2002.
| Methods | Design: randomised double‐blinded No. eligible: 330 No. randomised: 330 No. analysed: 321 Drop‐outs/withdrawals: 9 did not undergo hysterectomy as planned (3 in cefazolin group, 4 in ampicillin group, 2 in placebo group) Years of recruitment: 1997 to 1999 Setting: 2 regional hospitals in Thailand |
|
| Participants | Inclusion criteria: scheduled for elective total abdominal hysterectomy Exclusion criteria: preoperative fever or infection, allergic to ampicillin or cefazolin, had received antibiotics within 48 hours of surgery, emergency cases, pregnancy‐related cases Age: mean 43 years Type of hysterectomy: abdominal | |
| Interventions | Treatment 1: 1 gram ampicillin (penicillin) Treatment 2: 1 gram cefazolin (first‐generation cephalosporin) Control: placebo Route: IV Single/multiple doses: single Timing of doses: 30 minutes before surgery |
|
| Outcomes | Postoperative infection, early and late Abdominal wound infection Urinary tract infection Pelvic infection Adverse effects (narrative data only) Other serious infection Postoperative fever Asymptomatic infection Follow‐up: 4 weeks |
|
| Funding | National Research Council, Thailand | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization" |
| Allocation concealment (selection bias) | Low risk | "Opaque sealed envelopes" ‐ probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Patients, their gynaecologists, all investigators and evaluators were blinded to the random allocation throughout the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportions of withdrawals/losses to follow‐up similar in treatment groups and < 10% in each group |
| Selective reporting (reporting bias) | Low risk | All reported outcomes were prespecified in the methods section |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Crosthwaite 1985.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: unclear, states "100 women participated" No. analysed: 100 Drop‐outs/withdrawals: none described Years of recruitment: not stated Setting: Gynaecology Unit, Royal Melbourne Hospital |
|
| Participants | Inclusion criteria: all patients undergoing hysterectomy in hospital unit Exclusion criteria: not stated Age: mean 53 years (intervention group) vs 55 years (control group) Type of hysterectomy: abdominal or vaginal | |
| Interventions | Treatment: 2 grams tinidazole (antiprotozoal): Control: placebo Route: oral Single/multiple doses: single Timing of doses: 12 hours preop |
|
| Outcomes | Postoperative infection, early Abdominal wound infection Urinary tract infection Pelvic infection Other serious infection Adverse effects (narrative data only) |
|
| Funding | Pfizer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind"; method not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not categorically stated how many women were randomised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information in the methods section to detect presence of selective reporting |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Davi 1985.
| Methods | Design: randomised double‐blinded No. randomised: not explicitly stated No. analysed:310 Drop‐outs/withdrawals: not stated Years of recruitment: not stated Setting: not stated |
|
| Participants | Inclusion criteria: not stated Exclusion criteria: not stated Age: not stated Type of hysterectomy: abdominal | |
| Interventions | Treatment: 2 grams cefoxitin (second‐generation cephalosporin) Control: placebo Route: IM Single/multiple doses: multiple Timing of doses: 20 minutes preoperatively, then 6 and 12 hours later |
|
| Outcomes | Postoperative infection, early Urinary tract infection Postoperative fever Adverse effects (narrative data only) |
|
| Funding | Not stated | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insuficient information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States "double‐blind"; no additional details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information on withdrawals and reasons for withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
Dhar 1993.
| Methods | Design: randomised double‐blinded No. randomised: 50 No. analysed: 49 Drop‐outs/withdrawals: 1 (vomited tablets) Years of recruitment: 1986 to 1988 Setting: tertiary hospital, Chandigarh |
|
| Participants | Inclusion criteria: women undergoing vaginal hysterectomy with pelvic floor repair for genital prolapse, aged 35 to 60 years Exclusion criteria: haemoglobin low, current infection, systemic disease, antimicrobial infection in past week, using corticosteroids Age: mean 49.4 years (intervention group) vs 52 years (control group) Type of hysterectomy: vaginal hysterectomy | |
| Interventions | Treatment: 2 grams tinidazole (antiprotozoal) Control: placebo Route: oral Single/multiple doses: single Timing of doses: 12 hours preoperatively |
|
| Outcomes | Postoperative infection, early Pelvic infection Postoperative fever Need for therapeutic antibiotics Adverse effects (narrative data only) Length of hospital stay Follow‐up: duration unclear |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised; no details reported. |
| Allocation concealment (selection bias) | Unclear risk | "Only the hospital pharmacist had access to the protocol code before completion of the study" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo "matched for shape, size, colour and taste" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant excluded from analysis ‐ reasons given |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Dhar 1993a.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: 100 No. analysed: 98 Drop‐outs/withdrawals: 2 (had tubo‐ovarian abscess or malignancy found at surgery) Years of recruitment: 1986 to 1988 Setting: tertiary hospital, Chandigarh |
|
| Participants | Inclusion criteria: women scheduled for abdominal hysterectomy for benign conditions Exclusion criteria: preexisting infection; diabetes; obesity; renal, hepatic, or cardiac disease; antibiotic previous week or currently using corticosteroids Age: 43 to 44 years Type of hysterectomy: abdominal | |
| Interventions | Treatment: 2 grams tinidazole (antiprotozoal) Control: placebo Route: oral Single/multiple doses: single Timing of doses: 12 hours preoperatively |
|
| Outcomes | Postoperative infection, early Pelvic infection Postoperative fever Need for therapeutic antibiotics Adverse effects (narrative data only) Follow‐up: duration unclear |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised; method not described |
| Allocation concealment (selection bias) | Low risk | "Only the hospital pharmacist had access to the drug code before completion of the trial..." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo "matched for shape, size, colour and taste" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants excluded from analysis: reasons given |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Duff 1982.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: "91 enrolled" No. analysed: 91 Drop‐outs/withdrawals: none reported Years of recruitment: 1979 to 1981 Setting: army medical centre, USA |
|
| Participants | Inclusion criteria: all women undergoing abdominal hysterectomy for benign disease
Exclusion criteria: antibiotics received in past 4 weeks, penicillin or cephalosporin allergy Age: 39 to 40 years Type of hysterectomy: abdominal |
|
| Interventions | Treatment: 1 gram cefoxitin (second‐generation cephalosporin) Control: placebo Route: IV Single/multiple doses: multiple Timing of doses: 30 minutes preoperatively and 4 hours later |
|
| Outcomes | Postoperative infection, early Abdomnal wound infection Urinary tract infection Pelvic infection Need for therapeutic antibiotics Adverse effects (narrative data only) Length of hospital stay |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" ‐ no details given |
| Allocation concealment (selection bias) | Low risk | "Only the hospital pharmacist routinely had access to the protocol code before completion of the study" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Both the patient and the attending physician were blinded as to the medication assignment"; no additional details provided with respect to outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that all participants randomised were analysed |
| Selective reporting (reporting bias) | Low risk | Outcome data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Egarter 1988.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: 120 "recruited" No. analysed: 120 Drop‐outs/withdrawals: none Years of recruitment: not stated Setting: Austria |
|
| Participants | Inclusion criteria: women having vaginal hysterectomy without a repair, with or without salpingectomy Exclusion criteria: sensitivity to antibiotics, antibiotics in previous 72 hours, current infection, impaired liver or kidney function, fever Age: 45 to 46 years Type of hysterectomy: vaginal | |
| Interventions | Treatment 1: 1800 mg clindamycin (lincosamide) Treatment 2: 1500 mg metronidazole (antiprotozoal) Control: placebo Route: IV Single/multiple doses: multiple Timing of doses: 30 to 60 minutes preoperatively, followed by 2 additional doses at 6‐hourly intervals |
|
| Outcomes | Pelvic infection Urinary tract infection Postoperative fever Hospital length of stay Duration of follow‐up: 4 to 6 weeks |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "allocated at random" ‐ no additional details |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States "in the double‐blind mode" ‐ no additional details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants recruited were analysed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Eron 1989.
| Methods | Design: randomised double‐blinded No. randomised: 252 No. analysed: 202 Drop‐outs/withdrawals: 50 (14 in treatment group 1 (see below), 18 in group 2, 19 in group 3), in most cases due to concurrent antibiotic therapy or failure to adhere to schedule Years of recruitment: not stated Setting: 2 centres, USA |
|
| Participants | Inclusion criteria: aged ≥ 18 years, scheduled for vaginal or abdominal hysterectomy with or without salpingo‐oophorectomy Exclusion criteria: preoperative fever, infection, pregnancy, lactation, hypersensitivity to antibiotics, multiple drug allergies, renal impairment, antibiotics within past 72 hours or any investigational drug within past month Age: 40 to 41 years Type of hysterectomy: vaginal or abdominal | |
| Interventions | Treatment 1: 1 gram cefocinid (second‐generation cephalosporin), 3.5 to 4 hours preoperatively, single dose Treatment 2: 1 gram cefocinid, 0.5 to 1 hour preoperatively, single dose Treatment 3: 2 grams cefoxitin (second‐generation cephalosporin), 0.5 to 1 hour preoperatively, then 6‐hourly for 4 additional doses Route: IV or IM Single/multiple doses: single vs multiple |
|
| Outcomes | Postoperative infection ‐ data not extractable for meta‐analysis Abdominal wound infection Urinary tract infection ‐ data not extractable for meta‐analysis Pelvic infection Adverse effects (narrative data only) Hospital length of stay ‐ data not extractable for meta‐analysis |
|
| Funding | Partially funded by Smith Kline & French Laboratories | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" ‐ no additional details given |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Patients and investigators (or other personnel performing patient evaluations) were not aware of which regimen was being administered" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportions of withdrawals not balanced between groups (17% vs 21% vs 23%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Faro 1988.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: 114 No. analysed: 100 Drop‐outs/withdrawals: 14 (5 in vaginal group had abdominal surgery, 1 had operation cancelled, 6 received additional antibiotics, 2 received inappropriate doses) Years of recruitment: not stated Setting: centre not stated but study took place in the United States |
|
| Participants | Inclusion criteria: women scheduled for elective vaginal hysterectomy. Exclusion criteria: not stated Age: mean 32 to 33 years Type of hysterectomy: vaginal | |
| Interventions | Treatment 1: 4 grams mezlocillin Treatment 2: 2 grams cefoxitin Route: IV Single/multiple doses: multiple Timing of doses: first dose within 1 hour of surgery, second dose on return from recovery room, and third dose 6 hours later |
|
| Outcomes | Postoperative infection, early and early + late Pelvic infection Need for therapeutic antibiotics Length of hospital stay Follow‐up: 6 weeks |
|
| Funding | Miles Laboratories | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated ... schedule" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind ‐ no details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imbalance in proportions of exclusion (10 vs 4) but reasons for exclusion not stated by treatment group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
Gall 1983.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: not stated No. analysed: 58 Drop‐outs/withdrawals: not reported Years of recruitment: not reported Setting: University Medical Centre, USA |
|
| Participants | Inclusion criteria: patients undergoing abdominal hysterectomy invited to volunteer for study Exclusion criteria: not reported Age: not stated Type of hysterectomy: abdominal | |
| Interventions | Treatment 1: cefoperazone (third‐generation cephalosporin) 2 grams up to 1 hour preoperatively, then after 12 and 24 hours (with saline at 6‐hourly intervals between doses) Treatment 2: cefamandole (second‐generation cephalosporin) 2 grams up to 1 hour preoperatively, then 6‐hourly for 4 doses Control: placebo up to 1 hour preoperatively, then 6‐hourly for 4 doses Route: IV Single/multiple doses: multiple Timing of doses: as above |
|
| Outcomes | Abdominal wound infection Pelvic infection Postoperative fever Adverse effects (narrative data only) Length of hospital stay |
|
| Funding | Cannot use LOS data ‐ unable to pool data for the 2 cephalosporin interventions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Timing of placebo infusion matched active interventions, but no details as to whether it appeared identical; also, no details provided on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not explicitly stated how many were randomised; no information about drop‐outs or withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Hager 1989.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: 95 No. analysed: 85 Drop‐outs/withdrawals: 10 (6 in treatment group 1, 4 in group 2: 3 did not have planned surgery, 3 had antibiotics within a week of surgery, 3 had antibiotics without clinical evidence of infection, 1 had inappropriate administration of a study drug, 1 had preexisting infection) Years of recruitment: not stated Setting: 3 centres, United States |
|
| Participants | Inclusion criteria: premenopausal women aged > 18 years scheduled for vaginal hysterectomy, no preexisting infection Exclusion criteria: antibiotics within past 7 days, allergy to study drugs, other conditions necessitating antibiotic prophylaxis, abnormal hepatic or renal function Age: > 18 years Type of hysterectomy: vaginal | |
| Interventions | Treatment 1: 1 gram cefotaxime (third‐generation cephalosporin) Treatment 2: 4 grams mezlocillin (penicillin) Route: IV Single/multiple doses: single Duration of course of antibiotics Timing of doses: 5 to 30 minutes preoperatively |
|
| Outcomes | Postoperative infection, early Pelvic infection Urinary tract infection Postoperative fever Adverse effects Hospital (postoperative) length of stay Follow‐up: not stated |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no details given |
| Allocation concealment (selection bias) | Low risk | "Assignment from a random code maintained in hospital pharmacy" ‐ probably remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Neither operating physician nor patient was are of which study antibiotic was used"; however, no details on outcome assessors were provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs/withdrawals appear similar across groups; reasons given |
| Selective reporting (reporting bias) | Low risk | Outcome data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Hedican 1976.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: 70 No. analysed: 70 Drop‐outs/withdrawals: none Years of recruitment: 1971 to 1972 Setting: university gynaecology and obstetrics department, USA |
|
| Participants | Inclusion criteria: women having elective vaginal hysterectomy Exclusion criteria: preoperative infection, taking antibiotics, allergy to study drugs, elevated blood urea Age: not stated Type of hysterectomy: vaginal | |
| Interventions | Treatment: cephaloridine (first‐generation cephalosporin) Control: placebo Route: IV, then IM Single/multiple doses: multiple Timing of doses: 1 gram IV at start of operation, 1 gram IM 5 hours postoperatively, 1 gram IM 12 hours postoperatively |
|
| Outcomes | Postoperative infection, early Pelvic infection |
|
| Funding | Lilly Company | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | State that "patients were numbered consecutively 1‐70 and randomly assigned..."; no additional details reported |
| Allocation concealment (selection bias) | Low risk | Appears to be remote allocation ‐ "patients were ... randomly assigned either the placebo or the study drug by the pharmacy" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Following the completion of the study ... the code was broken"; no details on outcome assessment provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appears that all participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
Hemsell 1980.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: not stated No. analysed: 99 Drop‐outs/withdrawals: not stated Years of recruitment: 1978 to 1979 Setting: hospital associated with university obstetrics and gynaecology department, United States |
|
| Participants | Inclusion criteria: premenopausal women having vaginal hysterectomy Exclusion criteria: allergy to study drugs, antibiotics within 48 hours of surgery, fever (≥ 38°) within 24 hours of surgery Age: mean 30 to 33 years Type of hysterectomy: vaginal | |
| Interventions | Treatment: 2 grams cefoxitin (second‐generation cephalosporin) Control: placebo Route: IM Single/multiple doses: multiple Timing of doses: on call to operating room, then 6 hours and 12 hours postoperatively |
|
| Outcomes | Postoperative infection, early, late, and early + late Urinary tract infection Postoperative fever Adverse effects (narrative data ‐ but only laboratory abnormalities reported, no clinical outcomes) Asymptomatic infection Hospital length of stay |
|
| Funding | Partially funded by Merck, Sharp & Dohme Research Laboratory | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no details given (see below under allocation concealment) |
| Allocation concealment (selection bias) | Low risk | States that "the women were assigned a study number upon inclusion in the study...this corresponded to that on a box containing...vials..." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The code was not broken until the woman had been classified as morbid or no‐morbid and had been examined 6 weeks after surgery" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportions of withdrawals and reasons for withdrawals not reported |
| Selective reporting (reporting bias) | Low risk | Data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Hemsell 1983.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: 112 No. analysed: 100 Drop‐outs/withdrawals: 12 (2 had positive urine cultures, 2 had no hysterectomy, 2 had vaginal hysterectomy, 5 needed antibiotics for other indications, 1 was incorrectly dosed) Years of recruitment: 1979 to 1980 Setting: Parkland Memorial Hospital, Dallas, Texas, USA |
|
| Participants | Inclusion criteria: women ≥ 18 years of age, consecutively admitted for elective abdominal hysterectomy Exclusion criteria: allergy to study drugs, antibiotics within previous 48 hours, UTI, fever (≥ 38°) in past 24 hours, gynaecological malignancy requiring radical hysterectomy, pregnancy, serious systemic disease Age: 36 years Type of hysterectomy: abdominal | |
| Interventions | Treatment: 2 grams cefoxitin (second‐generation cephalosporin) Control: placebo Route: IM Single/multiple doses: multiple Timing of doses: on call to operating theatre, then 6 hours and 12 hours later |
|
| Outcomes | Abdominal wound infection Pelvic infection Postoperative fever Asymptomatic infection Hospital length of stay |
|
| Funding | Partially funded by Merck, Sharp & Dohme Research Laboratory, sponsored by Society for Gynecologic Investigation, United States | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was generated "according to a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | States that "women were assigned consecutive numbers upon entry into the study. These corresponded to consecutively numbered kits...of study drug" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study remained blinded until all women were examined at a follow‐up clinic visit" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12 drop‐outs/withdrawals. Reasons given, but no indication which study group they were from. No ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Hemsell 1984.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: 116 No. analysed: 112 Drop‐outs/withdrawals: 4 (surgery cancelled after first dose (1), abdominal hysterectomy after examination under anaesthesia (1), inappropriate entry (2)) Year of recruitment: 1982 Setting: Parkland Memorial Hospital, United States |
|
| Participants | Inclusion criteria: premenopausal women scheduled for vaginal hysterectomy Exclusion criteria: allergy to study drugs, antibiotic therapy within 48 hours before surgery, fever (≥ 38°) in previous 24 hours, infection, any other condition that might preclude accurate evaluation of outcomes Age: mean 31 to 32 years Location: Parkland Memorial Hospital, Dallas, Texas, USA |
|
| Interventions | Treatment 1: 2 grams cefoxitin (second‐generation cephalosporin) × 3 doses Treatment 2: 2 grams cefoxitin × 1 dose, then 2 doses of placebo Single/multiple doses: single vs multiple Route: first dose intramuscular, second and third doses IV Timing of doses: first dose on call to OR, then 2 more doses 6 hours and 12 hours later Follow‐up: 3 to 6 weeks |
|
| Outcomes | Postoperative infection, early and early + late Pelvic infection Postoperative fever Adverse effects (narrative data only) Hospital length of stay |
|
| Funding | Merck, Sharp & Dohme Research Laboratories | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list sequence |
| Allocation concealment (selection bias) | Unclear risk | Reported that code not broken until last women had completed study ‐ but not stated where code was held |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States as blinded for participants, but not clear if blinded for practitioners; no information on outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Does not report withdrawal per treatment group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Hemsell 1985.
| Methods | Design: randomised blinded No. eligible: not stated No. randomised: not explicitly stated No. analysed: 150 Drop‐outs/withdrawals: not mentioned Years of recruitment: not stated Setting: United States (details not reported) |
|
| Participants | Inclusion criteria: women having elective abdominal hysterectomy "without standard exclusions" Exclusion criteria: not stated Age: not stated Type of hysterectomy: abdominal | |
| Interventions | Treatment 1: 2 grams cefoxitin (second‐generation cephalosporin) × 1 dose Treatment 2: 2 grams cefoxitin (second‐generation cephalosporin) × 2 doses Treatment 3: 2 grams cefoxitin (second‐generation cephalosporin) × 2 doses Route: IV Single/multiple doses: single vs multiple regimens Timing of doses: not stated |
|
| Outcomes | Postoperative infection, early and early + late Postoperative fever Need for therapeutic antibiotics Hospital length of stay ‐ data for each group not extractable Follow‐up: not stated, but states "no late infections observed for 149 women seen following surgery" |
|
| Funding | Drugs supplied by Merck, Sharp & Dohme | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as random ‐ no additional details |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "With placebo blinding" ‐ probably double‐blinded; no additional information on outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported on withdrawals and reasons for withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Little information about eligibility criteria ‐ study applicability unclear |
Hemsell 1985a.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: "51 women were entered" No. analysed: 51 Drop‐outs/withdrawals: none reported Years of recruitment: not stated Setting: Parkland Memorial Hospital, Dallas, United States |
|
| Participants | Inclusion criteria: premenopausal women having vaginal hysterectomy Exclusion criteria: not stated Age: 30 to 31 years Type of hysterectomy: vaginal | |
| Interventions | Treatment 1: 2 grams cefamandole (second‐generation cephalosporin) 2 hours preoperatively, then 1 gram 6‐hourly × 3 Treatment 2: 200 mg doxycycline (tetracycline) 2 hours preoperatively, then dextrose (placebo) 6‐hourly × 3 Route: IV Single/multiple doses: single vs multiple Timing of doses: as above |
|
| Outcomes | Postoperative infection, early, late, and early + late Pelvic infection Postoperative fever Adverse effects (narrative data only) Hospital length of stay Follow‐up: up to 6 weeks |
|
| Funding | Pfizer Pharmaceuticals | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The randomization code was not broken until the last woman attended the clinic" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that all women randomised were analysed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Little information about eligibility criteria ‐ study applicability unclear |
Hemsell 1987.
| Methods | Design: randomised blinded No. eligible: not stated No. randomised: 237 No. analysed: 212 Drop‐outs/withdrawals: 25 (18 did not have scheduled surgery, 6 had intraoperative antibiotics, 1 needed antibiotics postoperatively for pneumonia) Years of recruitment: 1983 to 1985 Setting: Parkland Memorial Hospital, Dallas, Texas, USA |
|
| Participants | Inclusion criteria: women having vaginal hysterectomy Exclusion criteria: antibiotic within previous 3 days, allergy to study drugs Age: 32 to 33 years Type of hysterectomy: vaginal | |
| Interventions | Treatment 1: 1 gram cephazolin (first‐generation cephalosporin) Treatment 2: 2 grams cephazolin *Study also compares cephalosporins against each other ‐ data not included Route: IM Single/multiple doses: single Timing of doses: immediately before going to operating theatre |
|
| Outcomes | Postoperative infection, late and early + late Pelvic infection Postoperative fever Adverse effects (narrative data only) Need for therapeutic antibiotics Hospital length of stay Cost of surgery (data relate only to direct healthcare costs, minus study drugs ‐ data not included in this review) |
|
| Funding | Eli Lilly and Company | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Reported that "vials completely wrapped with paper to obscure identification" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Probably double‐blinded but no additional details reported on outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportions of withdrawals and reasons for withdrawals balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Hemsell 1989.
| Methods | Design: randomised blinded No. eligible: not stated No. randomised: 214 "evaluated" No. analysed: 207 Drop‐outs/withdrawals: 7 (4 required antibiotics intraoperatively, 3 had prophylactic dose more than 10 minutes post incision) Year of recruitment: 1985 Setting: Parkland Memorial Hospital, Dallas, Texas, USA |
|
| Participants | Inclusion criteria: women scheduled for elective abdominal or vaginal hysterectomy Exclusion criteria: "routine exclusion criteria applied" Age: 36 to 39 years Type of hysterectomy: abdominal or vaginal | |
| Interventions | Treatment 1: 2 grams cefoxitin (second‐generation cephalosporin) in operating room before anaesthesia, plus 2 additional doses at 4 hours and 8 hours Treatment 2: 4 grams piperacillin (penicillin) in operating room before anaesthesia, plus 2 doses placebo at 4 hours and 8 hours Route: IV Single/multiple doses: single vs multiple Timing of doses: as above |
|
| Outcomes | Postoperative infection: early + late ‐ narrative data only Postoperative fever (narrative data only) Adverse effects (narrative data only) Length of hospital stay (narrative data only) Costs ‐ hospital costs only; data not included in this review Follow‐up: 4 to 6 weeks |
|
| Funding | Lederle Laboratories, United States | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated (separate list for each surgical approach) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Probably double‐blinded, but no details reported on outcome assessor; reported that "antibiotic...labeled only with patient's name"; unclear whether this will affect blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportions of withdrawals not given per group, although reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Little information about eligibility criteria ‐ study applicability unclear |
Henriksson 1998.
| Methods | Design: randomised double blinded No. eligible: not stated No. randomised: 316 No. analysed: 291 primary analysis, 258 secondary (per protocol) analysis Drop‐outs/withdrawals: 25 from primary analysis (15 case records not traceable, 4 hysterectomy not performed, 5 given wrong prophylaxis) Years of recruitment: not stated Setting: 3 tertiary centres, Sweden |
|
| Participants | Inclusion criteria: women scheduled for hysterectomy Exclusion criteria: antibiotics in previous 2 weeks, allergy to study drugs, taking anticoagulants or disulfiram, habitual alcohol abuse, breastfeeding Age: not stated Type of hysterectomy: abdominal | |
| Interventions | Treatment: 500 mg metronidazole (antiprotozoal) Control: placebo Route: IV Single/multiple doses: multiple Timing of doses: during induction of anaesthesia, then 8 hours later |
|
| Outcomes | Postoperative infection, early Pelvic infection Wound infection Adverse effects (narrative data only) Follow‐up: to 6 days postoperative |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Low risk | Bottles for infusion "labeled identically and only distinguished by a code number" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported that "none of the investigators knew if the patient had got metronidazole or placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analysis "based on all randomised patients from whom information was available" |
| Selective reporting (reporting bias) | Low risk | Outcome data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Holman 1978.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: not stated No. analysed: 206 Drop‐outs/withdrawals: not stated Years of recruitment: not reported Setting: Grady Memorial Hospital, United States |
|
| Participants | Inclusion criteria: all women admitted for elective vaginal or abdominal hysterectomy Exclusion criteria: allergy to study drugs, fever in past 2 weeks, fever or infection on admission, antibiotics in past 2 weeks, requirement for antibiotics for other indications Age: mean (intervention vs control): 37.8 years vs 38.5 years (abdominal hysterectomy); 27.7 years vs 30.4 years (vaginal hysterectomy) Type of hysterectomy: abdominal or vaginal | |
| Interventions | Treatment: cefazolin (first‐generation cephalosporin), Control: placebo Route: first dose IM, then IM or IV Single/multiple doses: multiple Timing of doses: first dose on call to operating room, second dose on return from recovery room, third dose 6 hours later Follow‐up: postoperative and "after discharge from the hospital" |
|
| Outcomes | Abdominal wound infection Urinary tract infection Pelvic infection Need for systemic antibiotics Hospital length of stay (no SDs) *For abdominal hysterectomy, data separated into premenopausal and postmenopausal. For vaginal hysterectomy, only premenopausal data reported for most outcomes. Therefore, data related to postmenopausal vaginal hysterectomy (n = 6) not included in this review Follow‐up: to hospital discharge |
|
| Funding | Smith Kline & French, Philadelphia, Pennsylvania, USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Reported that "patients were...assigned a study number...from a random table maintained by the pharmacy service" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported that "the code was not broken until the patient had been discharged and evaluated..." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportions of withdrawals and reasons for withdrawals not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Houang 1984.
| Methods | Design:described as randomised; medical and nursing personnel not aware of study groups No. eligible: not stated No. randomised: 345 (to four different operation groups of which only two groups (abdominal hysterectomy and vaginal hysterectomy) were relevant. Number randomised to each group at baseline not reported No. analysed: 295 (Abdominal hysterectomy group = 158; vaginal hysterectomy group = 28) Drop‐outs/withdrawals: 50 (14 in treatment group 1, 18 in the other 2 groups (see below); reasons given included required antibiotics owing to intraoperative findings, did not have planned type of surgery, data missing at follow‐up) Years of recruitment: 1982 to 1983 Setting: Chelsea Hospital for Women, London |
|
| Participants | Inclusion criteria: patients for elective vaginal or abdominal hysterectomy Exclusion criteria: not stated (2 patients with preop UTI excluded from analysis) Age: not stated Type of hysterectomy: abdominal or vaginal | |
| Interventions | Treatment 1: 500 mg ampicillin + 500 mg penicillanic acid sulphone (penicillin) (with placebo suppository) Treatment 2: 500 mg ampicillin + 1 gram metronidazole (antiprotozoal) Control: placebo suppository Route: penicillin IV, metronidazole by rectal suppository Single/multiple doses: multiple Timing of doses: suppository 2 hours preoperatively, IV penicillin(s) immediately after induction of anaesthesia |
|
| Outcomes | Postoperative infections: early and early+ late Abdominal wound infection Urinary tract infection (2 participants with preop UTI excluded from analysis) Postoperative fever Pelvic/vaginal infection Follow‐up: 6 weeks |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | States that "the study was so designed that the medical and nursing personnel would not be aware of the group allocation of the patients studied" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of withdrawals per treatment group stated but reasons for withdrawals not reported by treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Houang 1984a.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: not stated No. analysed:46 Drop‐outs/withdrawals: not reported Years of recruitment: 1983 onward Setting: Chelsea Hospital for Women, London *Study described as ongoing ‐ this is preliminary publication only |
|
| Participants | Inclusion criteria: women scheduled for elective abdominal hysterectomy Exclusion criteria: not stated Age: not stated Type of hysterectomy: abdominal | |
| Interventions | Treatment 1: piperacillin (penicillin) + placebo suppository Treatment 2: ampicillin (penicillin) + metronidazole (antiprotozoal) suppository Route: IV + rectal Single/multiple doses: multiple Timing of doses: suppository 2 hours preoperatively, followed by penicillin IV immediately after induction of anaesthesia |
|
| Outcomes | Abdominal wound infection Urinary tract infection Postoperative fever Follow‐up: 6 weeks |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | States that "the study was so designed that the medical and nursing personnel would not be aware of the group allocation of the patients studied" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportions of withdrawals and reasons for withdrawals not reported across treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Jaffe 1985.
| Methods | Design: randomised placebo‐controlled No. eligible: not stated No. randomised: 98 No. analysed:90 Drop‐outs/withdrawals: 8 (2 for positive preoperative urine culture in treatment group; 3 for positive preoperative urine culture, 2 for malignancy, and 1 for protocol mistake in placebo group) Years of recruitment: not stated Setting: Meir General Hospital, Israel |
|
| Participants | Inclusion criteria: women admitted for elective abdominal hysterectomy for benign condition Exclusion criteria: antibiotics in previous 2 weeks, allergy to study drugs Age: 46 to 48 years Type of hysterectomy: abdominal | |
| Interventions | Treatment 1: 15 mL co‐trimoxazole (antiprotozoal): 12000 mg sulphamethoxazole, 240 mg trimethoprim Control: placebo Route: IV Single/multiple doses: single Timing of doses: infused during last 30 minutes before surgery |
|
| Outcomes | Urinary tract infection Postoperative fever Adverse effects (narrative data only) Hospital length of stay |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly assigned" ‐ no other details |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States that "the placebo group received the placebo with the saline in the same manner"; no details reported on outcome assessor or evaluation of participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportions of withdrawals and reasons for withdrawals not balanced across treatment groups |
| Selective reporting (reporting bias) | Low risk | Data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Janssens 1982.
| Methods | Design: randomised double blinded. Publication reports 2 separate studies (1 and 2), for which placebo data were pooled No. eligible: not stated No. randomised: not stated No. analysed: study 1: n = 53; study 2: n = 92 Drop‐outs/withdrawals: not reported Years of recruitment: not stated Setting: St Elisabeth Hospital, Turnhout, Belgium |
|
| Participants | Inclusion criteria: "abdominal or vaginal hysterectomy patients" ‐ but also states that patients with shaving culdotomy were eligible Exclusion criteria: not stated Age: not stated Type of hysterectomy: abdominal or vaginal | |
| Interventions | Treatment 1: 1 to 2 grams tinidazole (antiprotozoal) Control: placebo Route: oral Single/multiple doses: study 1 = multiple, study 2 = single Study 1: first dose approximately 18 hours preoperatively, second dose 6 hours later, postoperative days 3, 4, and 5: 1 dose of 1 gram daily Study 2: single preoperative 2 gram dose given 6 to 8 hours preoperatively |
|
| Outcomes | Postoperative infection, early Study reports outcomes as "wound infection morbidity" (WIM). In this review, WIM grades 2 and 3 reported (i.e. those defined in review as "clinically relevant") |
|
| Funding | Not stated | |
| Notes | Publication also describes third study ‐ described as randomised with no mention of blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States that studies were randomised ‐ no additional details |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | States that "the double‐blind code was broken only after completion in each of the two studies..." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportions of withdrawals and reasons for withdrawals not reported across treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
Kauer 1990.
| Methods | Design: randomised double‐blinded No. eligible: 100 No. randomised: 78 No. analysed: 68 Drop‐outs/withdrawals: 10 (2 in treatment group 1, 4 in each of treatment groups 2 and 3: 5 had asymptomatic bacteriuria, 3 were given an incorrect antibiotic, 2 had abdominal not vaginal surgery) Years of recruitment: not reported Setting: Roman Catholic Hospital, Groningen, The Netherlands |
|
| Participants | Inclusion criteria: women ≥ 20 years of age having vaginal hysterectomy Exclusion criteria: allergy to study drugs, antibiotics within 48 hours of surgery, preexisting infection Age: mean 55 to 60 years Type of hysterectomy: vaginal | |
| Interventions | Treatment 1: 1500 mg cefuroxime (second‐generation cephalosporin) Treatment 2: 500 mg metronidazole (antiprotozoal) Treatment 3: 1500 mg cefuroxime + 500 mg metronidazole Route: IV Single/multiple doses: single Timing of doses: 15 minutes preoperatively |
|
| Outcomes | Postoperative infection, early Urinary tract infection Pelvic infection Need for therapeutic antibiotics Adverse effects (narrative data only) Hospital length of stay Follow‐up: duration not clearly stated |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States that sequence was generated through "table of random numbers" |
| Allocation concealment (selection bias) | Low risk | States that "patients were assigned by the hospital pharmacist..." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | States "vial and colour of the solution being indistinguishable...the observer was unaware of the antibiotics used..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportions of withdrawals and reasons for withdrawals fairly balanced across treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Ledger 1973.
| Methods | Design: randomised double‐blinded No. women eligible: 164 No. women randomised: 100 No. women analysed: 100 Drop‐outs/withdrawals: none Years of recruitment: 1970 to 1972 Setting: University of Michigan Medical Centre |
|
| Participants | Inclusion criteria: premenopausal women having vaginal hysterectomy Exclusion criteria: allergy to study drugs, high preoperative blood urea, already receiving prophylactic antibiotics, "vaginal approach was decided upon in the operating room" Age: mean 35 years Type of hysterectomy: vaginal |
|
| Interventions | Treatment: 1 gram cephaloridine (first‐generation cephalosporin) Control: placebo Route: not stated Single/multiple doses: multiple Timing of doses: first dose on call to operating room, second dose on return from recovery room, third dose at bedtime night of operation |
|
| Outcomes | Postoperative infection, early Urinary tract infection Pelvic infection Postoperative fever Need for therapeutic antibiotics Hospital length of stay Follow‐up: to hospital discharge *Also reports "other morbidity" ‐ no separate data for "other serious infections" |
|
| Funding | Eli Lilly Company | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | States that allocation was "assigned by the pharmacy service" ‐ probably remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | States that "the code identifying placebo or active drug was broken only after the patient had been discharged and the clinical summary sheets...completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported no drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Mathews 1977.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: "59 patients took part in the trial" No. analysed: 59 Drop‐outs/withdrawals: none reported Years of recruitment: 1975 to 1976 Setting: Sheppey Hospital, UK |
|
| Participants | Inclusion criteria: women given appointments to be admitted for abdominal hysterectomy Exclusion criteria: prophylactic antibiotics considered essential or contraindicated, allergy to study drugs Age: not stated Type of hysterectomy: abdominal | |
| Interventions | Treatment: 10 mL co‐trimoxazole (sulphonamide), containing total of 800 mg sulphamethoxazole and 160 mg of trimethoprim Control: placebo Route: IV Single/multiple doses: single Timing of dose: immediately before surgery |
|
| Outcomes | Abdominal wound infection Urinary tract infection Pelvic infection Postoperative fever Need for therapeutic antibiotics Adverse effects (narrative data only) Follow‐up: 6 weeks. However, only early data used, as unclear whether late data may overlap |
|
| Funding | One study author affiliated with Wellcome Foundation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised; no additional details |
| Allocation concealment (selection bias) | Low risk | States that "the co‐trimoxazole and placebo were supplied in random order in consecutively numbered boxes" ‐ apparently used remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States that "ampoules of apparently identical fluid..." were administered; no additional details on outcome assessor were reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Mathews 1979.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: not explicitly stated No. analysed: 50 Drop‐outs/withdrawals: none reported Years of recruitment: 1975 to 1978 Setting: All Saints' Hospital, Chatham, UK |
|
| Participants | Inclusion criteria: women given appointments to be admitted for vaginal hysterectomy Exclusion criteria: prophylactic antibiotics considered essential or contraindicated, allergy to study drugs Age: mean 56 to 61 years Type of hysterectomy: vaginal | |
| Interventions | Treatment: 10 mL co‐trimoxazole (sulphonamide), containing total of 800 mg sulphamethoxazole and 160 mg trimethoprim Control: placebo Route: IV Single/multiple doses: single Timing of dose: at beginning of operation |
|
| Outcomes | Urinary tract infection Pelvic infection Postoperative fever Need for therapeutic antibiotics Adverse effects (narrative data only) Follow‐up: 6 weeks (but only early data included in this review, as unclear whether early/late data overlap) |
|
| Funding | One study author affiliated with Wellcome Foundation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised; no additional details |
| Allocation concealment (selection bias) | Low risk | States that conduct of study was as described in Mathews 1977 (see above) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States that conduct of study was as described in Mathews 1977 (see above) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportions of withdrawals and reasons for withdrawal not reported across treatment groups |
| Selective reporting (reporting bias) | Low risk | Data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Mendelson 1979.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: not stated No. analysed: 66 Drop‐outs/withdrawals: not reported Year of recruitment: 1977 Setting: Jewish General Hospital, Montreal, Canada |
|
| Participants | Inclusion criteria: women admitted for vaginal hysterectomy Exclusion criteria: sensitivity to study antibiotics; receipt of antibiotics, anti‐infective therapy, or probenecid within past 2 weeks; autoimmune disease; impaired renal function; delivery or pregnancy termination within past 8 weeks; preexisting infection; conisation or dilatation and curettage within past 6 weeks Age: mean 53 years Type of hysterectomy: vaginal | |
| Interventions | Treatment 1: 1 gram cephradine (first‐generation cephalosporin); first dose preoperatively, then 6‐hourly for 4 doses Treatment 2: 2 grams cephradine 1 hour preoperatively Control: placebo Route: IV Single/multiple doses: single vs multiple Timing of doses: 5 to 75 minutes before initial incision |
|
| Outcomes | Early or late postoperative infection UTI Pelvic infection Postoperative fever Follow‐up: 2 to 4 weeks after discharge |
|
| Funding | ER Squibb and Sons | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States "the placebo was a...material...with the identical appearance of the active drug"; no information on outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportions of withdrawals and reasons for withdrawal not reported across treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a conclusive judgement |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Polk 1980.
| Methods | Design: randomised double‐blinded; stratified by menopausal status No. eligible: 1511 underwent non‐radical elective hysterectomy: reasons for non‐participation stated No. randomised: 557 No. analysed: 515 Drop‐outs/withdrawals: 52 (26 in each group started on therapeutic antibiotics by surgeon) Years of recruitment: 1976 to 1978 Setting: Boston Hospital for Women, Massachusetts, USA |
|
| Participants | Inclusion criteria: all women booked for elective, non‐radical, abdominal or vaginal hysterectomy Exclusion criteria: active infection, use of antibiotics within past 2 weeks, pelvic surgery within 2 weeks, sensitivity to study drugs Age: mean 41 to 42 years Type of hysterectomy: abdominal or vaginal | |
| Interventions | Treatment: cephazolin (first‐generation cephalosporin) Control: placebo Route: IM Single/multiple doses: multiple Duration of course of antibiotics Timing of doses: first dose 1 to 2 hours preoperatively, 2 more doses at 6‐hour intervals |
|
| Outcomes | Abdominal wound infection Urinary tract infection Pelvic infection Postoperative fever Need for therapeutic antibiotics Adverse effects (narrative data only) Hospital length of stay Follow‐up: 6 weeks |
|
| Funding | Eli Lilly and Company | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; stratified by menopausal status: no additional details |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | States that "participants, their physicians and all investigators were blind to the allocation throughout the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportions of withdrawals and reasons for withdrawal balanced across treatment groups |
| Selective reporting (reporting bias) | Low risk | Data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Schepers 1981.
| Methods | Design: randomised double‐blinded (abstract only) No. eligible: not stated No. randomised: 107 No. analysed: 103 Drop‐outs/withdrawals: 4 (reasons not reported) Years of recruitment: not stated Setting: The Netherlands |
|
| Participants | Inclusion criteria: premenopausal women undergoing abdominal hysterectomy Exclusion criteria: not stated Age: not stated Type of hysterectomy: abdominal | |
| Interventions | Treatment: deposition (second‐generation cephalosporin) Control: placebo Route: IV Single/multiple doses: multiple Timing of doses: first dose 30 minutes preoperatively, second dose 6 hours later |
|
| Outcomes | Postoperative infection Adverse effects (narrative data only) Follow‐up: not stated |
|
| Funding | Not stated | |
| Notes | No extractable data ‐ no denominators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no additional details |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States "double‐blind"; no additional details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportions of withdrawals and reasons for withdrawal/drop‐out not reported |
| Selective reporting (reporting bias) | High risk | Data not available on all prespecified outcomes; thus evidence of selective reporting |
| Other bias | Unclear risk | Insufficient detail to determine risk |
Smith 1984.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: 60 No. analysed: 59 Drop‐outs/withdrawals: 1 (required prophylaxis for surgical complications) Years of recruitment: not stated Setting: UK hospital |
|
| Participants | Inclusion criteria: women admitted for abdominal hysterectomy Exclusion criteria: renal disease, allergy to study drugs, malignancy suspected Age: mean 41 years; range 26 to 58 years Type of hysterectomy: abdominal | |
| Interventions | Treatment: 3 mL co‐trimoxazole (trimethoprim 160 mg, sulphamethoxazole 800 mg) Control: placebo Route: IM Single/multiple doses: single (1 ampoule) Timing of doses: 1 hour before surgery |
|
| Outcomes | Postoperative infection, early Abdominal wound infection Pelvic infection Postoperative fever Adverse effects (narrative data only) Follow‐up: 6 weeks (for UTI only) |
|
| Funding | Study author affiliation: Wellcome Foundation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised; no additional details |
| Allocation concealment (selection bias) | Low risk | Allocation concealed; "consecutively numbered envelopes" used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States "the co‐trimoxazole and placebo were supplied in ampoules containing 3 mls fluid...the placebo ampoule contained saline solution"; no information on outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 withdrawal; reason given |
| Selective reporting (reporting bias) | Low risk | Data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Stage 1982.
| Methods | Design: randomised double‐blinded No. eligible: not stated No. randomised: unclear, but appears to be 284 (see drop‐outs/withdrawals below) No. analysed: 273 Drop‐outs/withdrawals: 11 from overall study (which included 199 caesarean section patients; data not in this review) due to incomplete records Years of recruitment: 1976 to 1978 Setting: 14 centres, United Stated |
|
| Participants | Inclusion criteria: women having vaginal or abdominal hysterectomy (women having caesarean section ineligible; data not included in this review) Exclusion criteria: preoperative infection, allergy to study drugs. Age: mean 35 to 42 years Type of hysterectomy: abdominal or vaginal | |
| Interventions | Treatment: 1 gram cephradine (first‐generation cephalosporin) Control: placebo Route: IV Single/multiple doses: multiple Timing of doses: first dose within 1 hour of surgery, second dose 4 hours later |
|
| Outcomes | Postoperative infection, early Abdominal wound infection Urinary tract infection Adverse effects Need for therapeutic antibiotics Hospital length of stay (no SDs given) |
|
| Funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States that "each investigator was provided with an individually randomised block of patient numbers" |
| Allocation concealment (selection bias) | Low risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | States that "patients and investigators were blind to the allocation throughout the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although proportion of withdrawals and reasons for withdrawal were not reported for each treatment group, total withdrawals constitute a small fraction of participants randomised (4%) |
| Selective reporting (reporting bias) | Low risk | Data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
Vincelette 1983.
| Methods | Design: randomised double‐blinded No. eligible: 197 abdominal, 49 vaginal No. randomised: 108 abdominal (89 declined to take part), ? 38 vaginal (11 refused to take part) No. analysed: 106 abdominal, 38 vaginal Drop‐outs/withdrawals: 2 (1 in each abdominal group: 1 did not have hysterectomy, 1 had incorrect drug protocol) Years of recruitment: not stated Setting: Montreal General Hospital, Canada |
|
| Participants | Inclusion criteria: women consecutively admitted for elective abdominal hysterectomy Exclusion criteria: thyroid disease, antibiotics in past 2 weeks, pelvic inflammatory disease, pregnancy, physician preference for prophylaxis Age: mean 42 to 44 years Type of hysterectomy: abdominal or vaginal | |
| Interventions | Treatment: 500 mg metronidazole (antiprotozoal) Control: placebo Route: IV Single/multiple doses: multiple Timing of doses: first dose on call to operating theatre, second and third doses at 6‐hourly intervals |
|
| Outcomes | Abdominal wound infection Urinary tract infection Pelvic infection Other serious infection Postoperative fever Adverse effects Need for therapeutic antibiotics Hospital length of stay Follow‐up: 6 weeks |
|
| Funding | Medical Research Council of Canada and Rhône‐Poulenc Pharma Inc | |
| Notes | 4 had neoplasm ‐ may or may not be cervical intraepithelial neoplasia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was reported as "randomly assigned" ‐ no additional details |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States that "a double‐blind clinical evaluation was performed." No information on outcome assessor reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total withdrawals constitute a small fraction of participants randomised (2%) |
| Selective reporting (reporting bias) | Low risk | Data available on all prespecified outcomes |
| Other bias | Low risk | Baseline demographic characteristics similar between treatment groups |
ITT: intention‐to‐treat
LOS: length of stay
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adno 1979 | Antibiotics given > 12 hours preoperatively and 3 days postoperatively |
| Allen 1972 | Antibiotics given for 72 hours postoperatively |
| Appelbaum 1978 | Antibiotics given 24 hours preoperatively and 7 days postoperatively |
| Appelbaum 1980 | Prophylaxis given for up to 48 hours postoperatively |
| Batres 1980 | Prophylaxis were given for up to 4 days postoperatively. Only participants were blinded to treatment |
| Bian 1987 | Prophylactic antibiotics given 48 hours before surgery |
| Bivens 1975 | Prophylaxis given the night before surgery and postsurgical treatment continued for 48 hours |
| Britt 1978 | Antibiotics given for 48 hours post surgery |
| Brouwer WK, Hoo2 | Study methods did not indicate that blinding had been used |
| Brown 1986 | Study methods did not indicate that blinding had been used. No placebo was used for the comparative group even though different regimens were provided |
| Brown 1988 | Study not blinded for those administering treatment and for those assessing outcomes |
| Cartana J, Yarn2 | Study methods did not indicate that blinding had been used |
| Chimura 1987 | Postoperative antibiotics given for 5 days |
| Ciraru‐Vigneron 1988 | Study methods did not indicate that blinding had been used |
| de Lalla1993 | Study methods did not indicate that blinding had been used |
| Ferrari 1980 | Study methods did not indicate that blinding had been used: 1 group received no treatment and no placebo; some participants received therapeutic antibiotics during the course of the study |
| Fischbach 1988 | Study methods did not indicate that blinding had been used. No placebo was used for the control group |
| Forney 1976 | Provided antibiotics before conisation > 24 hours before hysterectomy |
| Friese 1988 | Study methods did not indicate that blinding had been used |
| Friese 1989 | Study methods did not indicate that blinding had been used |
| Fujiwara 1994 | Study methods did not indicate that blinding had been used. No placebo was used for the control group |
| Goodlin 1974 | Prophylactic given the night before surgery, then for 4 days postoperatively |
| Gordon 1982 | Study methods did not indicate that blinding had been used |
| Harms 1987 | Study methods did not indicate that blinding had been used |
| Haverkorn 1987 | Postoperative antibiotics given up to 6 days postoperatively |
| Hayashi 2000 | Postoperative antibiotics given for 2 to 3 days |
| Hemsell 1990a | No evidence of blinding |
| Huang 1987 | Study methods did not indicate that blinding had been used. No placebo was used for the control group |
| Ireland 1982 | Study was not blinded. No placebo was used for the control group |
| Jacobson 1982 | Study procedure not double‐blinded |
| Jennings 1978 | Prophylaxis administered the night before the operation, then was carried on for > 48 hours postoperatively |
| Jones RN, Wojes2 | Only a single‐blinded study. Treatment regimens differed between groups |
| Jyothi 2010 | Uncertain whether this was a true randomised controlled trial or a double‐blinded study |
| Kauppila 1983 | 23% of participants not analysed |
| Khan 1981 | More than 27% of women had repair of prolapse rather than hysterectomy |
| Knippenberger 1984 | No dose information. Significant proportion of participants received additional postoperative antibiotics because of infectious disease |
| Kunz 1982 | Quasi‐randomised (alternating days, allocation according to even/odd dates) |
| Larsson 2002 | Prophylaxis given the evening before surgery and for 7 days postoperatively |
| Littlejohn 1985 | One group received IV and the other IM; no attempt made with placebo for blinding |
| Luke 1999 | 28% of participants not analysed |
| Maki 1984 | Comparison of cephalosporins ‐ no placebo group |
| Mamsen 1992 | Participants with malignancy included ‐ no separate data |
| Mangioni 1991 | Study methods did not indicate that blinding had been used |
| Mansani 1984 | In comparative study group, antibiotics were given for 5 days postoperatively |
| Manthorpe 1982 | Antibiotics given 1 day preoperatively and 72 hours postoperatively |
| Marsden 1985 | Antibiotics given 16 hours preoperatively and 72 hours postoperatively |
| Matkaris 1991 | Comparison of cephalosporins ‐ no placebo group |
| Mattheussens 1985 | Study methods did not indicate that blinding had been used. No placebo was used for the comparative group even though different regimens were given |
| McDonald 1984 | Study not blinded |
| McDonald 1988 | Study not blinded; also, 1 of the treatment arms extended prophylactic antibiotics for 4 days |
| McGregor 1994 | More than 30% of participants not analysed |
| Mele 1985 | Prolonged antibiotic administration |
| Mele 1988 | Prophylactic antibiotics given > 48 hours postoperatively |
| Mercer 1988 | Study methods did not indicate that blinding had been used |
| Mickal 1980 | Study methods did not indicate that randomisation had been used |
| Moroni 1979 | Study methods did not indicate that blinding had been used. No placebo was used for the control group |
| Moroni 1984 | No placebo and no blinding used |
| Mozzillo 1989 | Study methods did not indicate that blinding had been used |
| Multicenter 1989 | Interventions not relevant: cephalosporin vs cephalosporin (2 different generations similar in dose and route of administration) |
| Munck 1989 | Participants included those undergoing hysterectomy for treatment of malignant disease |
| Ohm 1975 | Antibiotic prophylaxis administered 24 hours before the operation, then for up to 5 days postoperatively |
| Ohm 1976 | Treatment consisted of a 5‐day course of antibiotics |
| Ohm MJ, Galask 2 | Postoperative treatment consisted of a 5‐day course of antibiotics |
| Ohm MJ, Galask 3 | Antibiotic prophylaxis administered 24 hours before the operation, then for up to 5 days postoperatively |
| Olgiati 1980 | Study methods did not indicate that blinding had been used |
| Oliva 1990 | Study methods did not indicate that blinding had been used |
| Orr 1988 | Study methods did not indicate that blinding had been used |
| Periti 1988 | No placebo and no blinding used |
| Periti P, Mazze2 | Treatment protocols differed for the 2 drugs; no attempt was made to blind this |
| Perri 1986 | Antibiotic prophylaxis given up to 4 days postoperatively |
| Phoolcharoen 2012 | Interventions not relevant: cephalosporin vs cephalosporin (2 different generations similar in dose and route of administration) |
| Popkin 1983 | Comparison groups given prophylactic treatment the day before surgery. Blinding of treatment not attempted |
| Poulsen 1984 | No blinding; control group given no placebo treatment |
| Poulsen HK, Bor2 | Study methods did not indicate that blinding had been used |
| Queck 1991 | Control group not given placebo; therefore, no attempt to blind groups |
| Rapp 1982 | Prophylaxis administered the night before the operation, then carried on for 48 hours postoperatively |
| Rapp 1986 | Different drug administration protocols employed. Therefore, no attempt to blind treatment groups |
| Regallo 1987 | Study methods did not indicate that blinding had been used. No placebo mentioned even though different regimens were employed |
| Reggiori 1996 | Study methods did not indicate that blinding had been used |
| Reggiori A, Rav2 | In comparison group, antibiotics given for 6 days postoperatively. No attempt to blind participants or physicians |
| Regidor 2000 | Open randomised study; therefore, not double‐blinded |
| Roberts 1978 | Study methods did not indicate that randomisation had been used |
| Roy 1982 | Study methods did not indicate that blinding had been used |
| Roy 1984 | In only 1 group, antibiotics were given postoperatively. No attempt was made to blind participants or physicians by using a placebo |
| Roy 1988 | Study methods did not indicate that blinding had been used |
| Roy 1989 | Study methods did not indicate that blinding had been used |
| Roy 1990 | Study methods did not indicate that blinding had been used |
| Roy 1998 | 28% of participants not analysed |
| Santarelli 1988 | Antibiotics given for 72 hours postoperatively |
| Savage 1984 | Antibiotics given 4 to 12 hours before surgery and 3 days after surgery |
| Scarpignato 1980 | Antibiotic prophylaxis carried on for 5 days postoperatively in 1 group. No blinding was employed |
| Siekmann 1983 | Blinding status unclear |
| Simoes 2008 | Not a double‐blinded study |
| Stocklund 1980 | Antibiotics given 12 hours before surgery and 5 days after surgery |
| Sutthijumroon 1990 | Study not double‐blinded |
| Suvonnakote 1988 | Antibiotics given for > 24 hours post surgery |
| Szalay 1996 | Participants and interventions not relevant; included participants with malignancy (abdominal hysterectomy); antibiotics given to 1 group for 3 days before surgery (vaginal hysterectomy) |
| Tarczali 1997 | Study methods did not indicate that blinding had been used. No placebo was used for the control group |
| Tchabo 1985 | Study methods did not indicate that blinding had been used. No placebo was used for comparative group even though different regimens were employed |
| Turano 1992 | Open randomisation |
| van der Linden1993 | Used open randomisation technique |
| Vecek 1993 | No information on blinding |
| Voss 1989 | Not double‐blinded |
| Walker 1982 | Prophylaxis given 12 to 16 hours before the operation |
| Wideman 1982 | Blinding was not mentioned and placebo was not used |
| Zivny 1997 | Study methods did not indicate that blinding had been used. No placebo was used for the comparative group even though different regimens were given |
Differences between protocol and review
We have extensively updated the Methods of the review to reflect the latest methods, as recommended by the Cochrane Collaboration, including use of the Cochrane "Risk of bias" tool and GRADE methods to assess the quality of evidence. We have added more detail about our statistical methods (in keeping with current Cochrane recommendations and the RevMan format).
We planned to undertake subgroup analyses by surgical route, antibiotic type, and antibiotic regimen. We subgrouped our main analysis by surgical route. We decided we would not conduct the other two planned subgroup analyses but focused instead on head‐to‐head comparisons between different antibiotics and antibiotic regimens, as these are more informative than subgroup analyses, which consist of indirect comparisons.
We planned to report numbers needed to treat for an additional beneficial outcome (NNTBs) as an absolute measure but instead reported percentages, as these can be easily interpreted and are consistent with absolute measures (rates per thousand) displayed in the "Summary of findings" tables.
In our protocol, we planned to explore statistical heterogeneity when we included more than 10 trials in an analysis, by exploring methodological and clinical differences between them. In the review, we decided to explore substantial statistical heterogeneity (I2 > 50%) by conducting sensitivity analyses by choice of statistical model and effect estimate, regardless of the number of trials included in an analysis. We planned to explore other clinical or methodological differences between studies only if we noted variation in the direction of effect.
We excluded from the review the following outcomes, which we had included in the protocol ‐ asymptomatic infection, re‐admission to hospital, and costs ‐ because we decided that these three outcomes can be considered as proxies for our primary outcomes and would not be likely to assist clinical decision making.
Contributions of authors
Reuben Olugbenga Ayeleke selected studies, assessed risk of bias of included studies, extracted data, performed statistical analysis and interpreted data, and took the lead in writing the review.
Jane Marjoribanks wrote the protocol and reviewed and edited the draft of the review.
Selma Mourad selected studies, assessed risk of bias of included studies, extracted data, and contributed to writing this review.
Karim Calis reviewed drafts of the protocol, contributed to the Background section, and reviewed the draft review.
Vanessa Jordan commented on the protocol, contributed to the methods, and reviewed and edited the draft review.
Sources of support
Internal sources
University of Auckland, School of Medicine, Auckland, New Zealand.
External sources
Ministry of Health, New Zealand.
Declarations of interest
ROA, JM, KC, and VJ have no interests to declare.
SM received a travel grant from Olympus for participating in the GETUP Gynecologic Endoscopy course (Rome 2016).
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Benigno 1986 {published data only}
- Benigno BB, Evrard J, Faro S, Ford LC, LaCroix G, Lawrence WD, et al. A comparison of piperacillin, cephalothin and cefoxitin in the prevention of postoperative infections in patients undergoing vaginal hysterectomy. Surgery Gynecology & Obstetrics 1986;163(5):421‐7. [PubMed] [Google Scholar]
Boodt 1990 {published data only}
- Boodt PJ, Snijders WP, Janknegt R. Single‐dose prophylaxis in hysterectomies. An interim analysis. Pharmaceutisch Weekblad Scientific Edition 1990;12(6A):280‐3. [DOI] [PubMed] [Google Scholar]
Chongsomchai 2002 {published data only}
- Chongsomchai C, Lumbiganon P, Anansuwanchai J, Ounchai J. Prophylactic antibiotics in abdominal hysterectomy. XVI FIGO World Congress of O & G, 2000 Sept, Washington DC. Washington DC, 2000.
- Chongsomchai C, Lumbiganon P, Thinkhamrop J, Ounchai J, Vudhikamraksa N. Placebo‐controlled, double‐blind, randomized study of prophylactic antibiotics in elective abdominal hysterectomy. Journal of Hospital Infection 2002;52(4):302‐6. [DOI] [PubMed] [Google Scholar]
Crosthwaite 1985 {published data only}
- Crosthwaite AH, Hurse AB, McDonald IA, Miles HM, Pavillard ER. Single dose tinidazole prophylaxis in hysterectomy. Australian & New Zealand Journal of Obstetrics & Gynaecology 1985;25(1):55‐8. [DOI] [PubMed] [Google Scholar]
Davi 1985 {published data only}
- Davi E, Ausin J, Escofet C, et al. Cefoxitin versus placebo in the prophylaxis of postoperative infection in abdominal hysterectomy [abstract]. Archives of Gynecology 1985;237(Suppl). [Google Scholar]
Dhar 1993 {published data only}
- Dhar KK, Dhall GI, Ayyagari A. Single dose tinidazole prophylaxis in vaginal hysterectomy. International Journal of Gynaecology & Obstetrics 1993;42(2):117‐20. [DOI] [PubMed] [Google Scholar]
Dhar 1993a {published data only}
- Dhar KK, Dhall GI, Ayyagari A. Tinidazole prophylaxis in elective abdominal hysterectomy. International Journal of Gynaecology & Obstetrics 1993;42(2):121‐5. [DOI] [PubMed] [Google Scholar]
Duff 1982 {published data only}
- Duff P. Antibiotic prophylaxis for abdominal hysterectomy. Obstetrics & Gynecology 1982;60(1):25‐9. [PubMed] [Google Scholar]
Egarter 1988 {published data only}
- Egarter C, Fitz R, Brehm R, Husslein P. Prophylactic perioperative use of clindamycin and metronidazole in vaginal hysterectomy without pelvic floor repair. Archives of Gynecology & Obstetrics 1988;244(1):53‐7. [DOI] [PubMed] [Google Scholar]
Eron 1989 {published data only}
- Eron LJ, Gordon SF, Harvey LI, Sites JG. A trial using early preoperative administration of cefonicid for antimicrobial prophylaxis with hysterectomies. Annals of Pharmacotherapy 1989;23(9):655‐8. [DOI] [PubMed] [Google Scholar]
Faro 1988 {published data only}
- Faro S, Pastorek JG, Aldridge KE, Nicaud S, Cunningham G. Randomized double‐blind comparison of mezlocillin versus cefoxitin prophylaxis for vaginal hysterectomy. Surgery Gynecology & Obstetrics 1988;166(5):431‐5. [PubMed] [Google Scholar]
Gall 1983 {published data only}
- Gall SA, Hill G. Cefoperazone as a prophylactic agent in abdominal hysterectomy. Reviews of Infectious Diseases 1983;5(Suppl 1):s200‐1. [DOI] [PubMed] [Google Scholar]
Hager 1989 {published data only}
- Hager WD, Sweet RL, Charles D, Larsen B. Comparative study of mezlocillin versus cefotaxime single‐dose prophylaxis in patients undergoing vaginal hysterectomy. Current Therapeutic Research, Clinical & Experimental 1989;45(1):63‐9. [Google Scholar]
Hedican 1976 {published data only}
- Hedican RE Jr, Sarto GE. Use of prophylactic antibiotic and vaginal cuff inflammation. Journal of the Iowa Medical Society 1976;66(9):373‐4, 378. [PubMed] [Google Scholar]
Hemsell 1980 {published data only}
- Hemsell DL, Cunningham FG, Kappus S, Nobles B. Cefoxitin for prophylaxis in premenopausal women undergoing vaginal hysterectomy. Obstetrics & Gynecology 1980;56(5):629‐34. [PubMed] [Google Scholar]
Hemsell 1983 {published data only}
- Hemsell DL, Reisch J, Nobles B, Hemsell PG. Prevention of major infection after elective abdominal hysterectomy: individual determination required. American Journal of Obstetrics & Gynecology 1983;147(5):520‐8. [DOI] [PubMed] [Google Scholar]
Hemsell 1984 {published data only}
- Hemsell DL, Heard ML, Nobles BJ, Hemsell PG. Single‐dose cefoxitin prophylaxis for premenopausal women undergoing vaginal hysterectomy. Obstetrics & Gynecology 1984;63(3):285‐90. [PubMed] [Google Scholar]
Hemsell 1985 {published data only}
- Hemsell DL, Hemsell PG, Heard ML, Nobles BJ. Preoperative cefoxitin prophylaxis for elective abdominal hysterectomy. American Journal of Obstetrics & Gynecology 1985;153(2):225‐6. [DOI] [PubMed] [Google Scholar]
Hemsell 1985a {published data only}
- Hemsell DL, Hemsell PG, Nobles BJ. Doxycycline and cefamandole prophylaxis for premenopausal women undergoing vaginal hysterectomy. Surgery Gynecology & Obstetrics 1985;161(5):462‐4. [PubMed] [Google Scholar]
Hemsell 1987 {published data only}
- Hemsell DL, Bawdon RE, Hemsell PG, Nobles BJ, Johnson ER, Heard MC. Single‐dose cephalosporin for prevention of major pelvic infection after vaginal hysterectomy: cefazolin versus cefoxitin versus cefotaxime. American Journal of Obstetrics & Gynecology 1987;156(5):1201‐5. [DOI] [PubMed] [Google Scholar]
Hemsell 1989 {published data only}
- Hemsell DL, Johnson ER, Heard MC, Hemsell PG, Nobles BJ, Bawdon RE. Single‐dose piperacillin versus triple‐dose cefoxitin prophylaxis at vaginal and abdominal hysterectomy. Southern Medical Journal 1989;82(4):438‐42. [DOI] [PubMed] [Google Scholar]
Henriksson 1998 {published data only}
- Henriksson L, Colling‐Saltin AS, Frick G, Kullander S, Sandholm LE, Ursing J, et al. Metronidazole prophylaxis to prevent infections after total abdominal hysterectomy. Acta Obstetricia et Gynecologica Scandinavica 1998;77(1):116‐9. [DOI] [PubMed] [Google Scholar]
Holman 1978 {published data only}
- Holman JF, McGowan JE, Thompson JD. Perioperative antibiotics in major elective gynecologic surgery. Southern Medical Journal 1978;71(4):417‐20. [DOI] [PubMed] [Google Scholar]
Houang 1984 {published data only}
- Houang ET. The trials of antibiotic prophylaxis at Chelsea Hospital for Women. Journal of Obstetrics & Gynaecology 1984;5(Suppl 1):S20‐2. [Google Scholar]
- Houang ETW, Howell CR, Chapman M. Ampicillin combined with sulbactam or metronidazole for single‐dose chemoprophylaxis in major gynaecological surgery. Journal of Antimicrobial Chemotherapy 1984;14(5):529‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Houang 1984a {published data only}
- Houang ET. The trials of antibiotic prophylaxis at Chelsea Hospital for women. Journal of Obstetrics & Gynaecology 1984;5(Suppl 1):S20‐2. [Google Scholar]
Jaffe 1985 {published data only}
- Jaffe R, Altaras M, Fejgin M, Ben‐Aderet N. Prophylactic single‐dose co‐trimoxazole for prevention of urinary tract infection after abdominal hysterectomy. Chemotherapy 1985;31(6):476‐9. [DOI] [PubMed] [Google Scholar]
Janssens 1982 {published data only}
- Janssens D, Peeters N, Sauwaert E, Cattersel B. Tinidazole in the prevention of post‐operative wound infection after hysterectomy. Journal of Antimicrobial Chemotherapy 1982;10(Suppl. A):87‐94. [DOI] [PubMed] [Google Scholar]
Kauer 1990 {published data only}
- Kauer FM, Wijma J, Manson WL. Vaginal hysterectomy: cefuroxime, metronidazole or both?. Pharmaceutisch Weekblad Scientific Edition 1990;12(6A):284‐8. [DOI] [PubMed] [Google Scholar]
Ledger 1973 {published data only}
- Ledger WJ, Sweet RL, Headington JT. Prophylactic cephaloridine in the prevention of postoperative pelvic infections in premenopausal women undergoing vaginal hysterectomy. American Journal of Obstetrics & Gynecology 1973;115(6):766‐74. [DOI] [PubMed] [Google Scholar]
Mathews 1977 {published data only}
- Mathews DDR, Cooper J. A double‐blind trial of single‐dose chemoprophylaxis with co‐trimoxazole during total abdominal hysterectomy. British Journal of Obstetrics & Gynaecology 1977;84(12):894‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mathews 1979 {published data only}
- Mathews DDA, Argawal V, Gordon AM, Cooper J. A double‐blind trial of single‐dose chemoprophylaxis with co‐trimoxazole during vaginal hysterectomy and repair. British Journal of Obstetrics & Gynaecology 1979;86(9):737‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mendelson 1979 {published data only}
- Mendelson J, Portnoy J, Saint Victor JR, Gelfand MM. Effect of single and multidose cephradine prophylaxis on infectious morbidity of vaginal hysterectomy. Obstetrics & Gynecology 1979;53(1):31‐5. [PubMed] [Google Scholar]
Polk 1980 {published data only}
- Polk BF, Tager IB, Shapiro M, Goren‐White B, Goldstein P, Schoenbaum SC. Randomised clinical trial of perioperative cefazolin in preventing infection after hysterectomy. Lancet 1980;1(8166):437‐40. [DOI] [PubMed] [Google Scholar]
Schepers 1981 {published data only}
- Schepers JP, Merkus F. Cefoxitin sodium: double‐blind, placebo‐controlled prophylactic study in premenopausal patients undergoing abdominal hysterectomy. Clinical Pharmacology & Therapeutics 1981;29(2):230‐90. [Google Scholar]
Smith 1984 {published data only}
- Smith ARB, Stanbridge TN, Morewood GA, Cooper J. Double blind trial with a single intramuscular dose of co‐trimoxazole before total abdominal hysterectomy. Journal of Obstetrics & Gynaecology 1984;5(1):49‐52. [Google Scholar]
Stage 1982 {published data only}
- Stage AH, Glover DD, Vaughan JE. Low‐dose cephradine prophylaxis in obstetric and gynecologic surgery. Journal of Reproductive Medicine 1982;27(3):113‐9. [PubMed] [Google Scholar]
Vincelette 1983 {published data only}
- Ti TY, Vincelette J, Aoki F, et al. Randomized clinical trial of prophylactic metronidazole in vaginal hysterectomy. Clinical & Investigative Medicine (Medecine Clinique et Experimentale) 1981;4(2). [Google Scholar]
- Vincelette J, Finkelstein F, Aoki FY, Ogilvie RI, Richards GK, Seymour RJ. Double‐blind trial of perioperative intravenous metronidazole prophylaxis for abdominal hysterectomy. Canadian Medical Association Journal 1982;127(2):119‐23. [PMC free article] [PubMed] [Google Scholar]
- Vincelette J, Finkelstein F, Aoki FY, Ogilvie RI, Richards GK, Seymour RJ. Double‐blind trial of perioperative intravenous metronidazole prophylaxis for abdominal hysterectomy. Clinical & Investigative Medicine 1981;4(2):119‐23. [PMC free article] [PubMed] [Google Scholar]
- Vincelette J, Finkelstein F, Aoki FY, Ti TY, Ogilvie RI, Richard GK, et al. Double‐blind trial of perioperative intravenous metronidazole prophylaxis for abdominal and vaginal hysterectomy. Surgery 1983;93(1II):185‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Adno 1979 {published data only}
- Adno J, Cassel R. The effect of prophylactic tinidazole on the anaerobic vaginal flora in patients undergoing gynaecological surgery. South African Medical Journal 1979;56(14):565‐8. [PubMed] [Google Scholar]
Allen 1972 {published data only}
- Allen JL, Rampone JF, Wheeless CR. Use of a prophylactic antibiotic in elective major gynecologic operations. Obstetrics & Gynecology 1972;39(2):218‐24. [PubMed] [Google Scholar]
Appelbaum 1978 {published data only}
- Appelbaum PC, Moodley J, Chatterton SA, Cowan DB, Africa CW. Metronidazole in the prophylaxis and treatment of anaerobic infection. South African Medical Journal 1978;54(17):703‐6. [PubMed] [Google Scholar]
Appelbaum 1980 {published data only}
- Appelbaum PC, Moodley J, Chatterton SA, Cowan DB, Africa CW. Tinidazole in the prophylaxis and treatment of anaerobic infection. Chemotherapy 1980;26(2):145‐51. [DOI] [PubMed] [Google Scholar]
Batres 1980 {published data only}
- Batres F, Barclay DL. Minocycline (Minocin(TM)) prophylaxis in vaginal hysterectomy. Current Therapeutic Research, Clinical & Experimental 1980;28(3 I):387‐93. [Google Scholar]
Bian 1987 {published data only}
- Bian DH. Use of prophylactic antibiotics in total abdominal hysterectomy. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 1987;22(3):138‐40, 189. [PubMed] [Google Scholar]
Bivens 1975 {published data only}
- Bivens MD, Neufeld J, McCarty WD. The prophylactic use of Keflex and Keflin in vaginal hysterectomy. American Journal of Obstetrics & Gynecology 1975;122(2):169‐75. [DOI] [PubMed] [Google Scholar]
Britt 1978 {published data only}
- Britt MR, Burke JP, Weidner M, Johnson GH, Hebertson R. The use of cefamandole prophylaxis in vaginal hysterectomy patients. Clinical Research. 1978; Vol. 26:390A.
Brouwer WK, Hoo2 {published data only}
- Brouwer WK, Hoogkamp‐Korstanje JAA, Kuiper KM. Single shot ciprofloxacin, cefuroxime + metronidazole and placebo as prophylaxis in vaginal hysterectomy. International Journal of Experimental and Clinical Chemotherapy 1990;3(3):172‐7. [Google Scholar]
Brown 1986 {published data only}
- Brown E, Depares J, Robertson A. Antimicrobial prophylaxis for hysterectomy ‐ a prospective randomised trial [abstract]. 24th British Congress of Obstetrics and Gynaecology. 1986.
Brown 1988 {published data only}
- Brown EM, Depares J, Robertson AA, Jones S, Hughes AB, Coles EC, et al. Amoxicillin‐clavulanic acid (Augmentin) versus metronidazole as prophylaxis in hysterectomy: a prospective, randomized clinical trial. British Journal of Obstetrics & Gynaecology 1988;95(3):286‐93. [DOI] [PubMed] [Google Scholar]
Cartana J, Yarn2 {published data only}
- Cartana J, Yarnoz MC, Ruiz de Gopegui RM, Mascaro M, Cortes J. Antibiotic prophylaxis with piperacillin in vaginal hysterectomy. Study of 1 dose versus 3 doses. Enfermedades Infecciosas y Microbiologia Clinica 1990;8(4):218‐21. [PubMed] [Google Scholar]
Chimura 1987 {published data only}
- Chimura T, Sato S, Suzuki T, Ito K, Sumiyoshi Y, Kanasugi H, et al. Prophylactic effects of a combined use of a cephem antibiotic, latamoxef, and tobramycin on postoperative infection in obstetrics and gynecology. Japanese Journal of Antibiotics 1987;40(7):1253‐8. [PubMed] [Google Scholar]
Ciraru‐Vigneron 1988 {published data only}
- Ciraru‐Vigneron N, Engelman P, Bercau G, Sauvanet E, Bitton C. The value of antibiotic prophylaxis using cefotetan in high‐risk abdominal hysterectomy. Apropos of a prospective randomized study of 71 patients. Revue Francaise de Gynecologie et d'Obstetrique 1988;83(11):737‐40. [PubMed] [Google Scholar]
de Lalla1993 {published data only}
- Lalla F, Scalambrino S, Tassi PG, Alegente G, Conturso R, Doregatti L, et al. Piperacillin versus cefotetan as single‐dose prophylaxis in abdominal hysterectomy: a prospective, randomized, multicenter study. Journal of Chemotherapy 1993;5(2):113‐8. [DOI] [PubMed] [Google Scholar]
Ferrari 1980 {published data only}
- Ferrari A, Ferrari N, Molteni P, Sartor V, Baccolo M, Marzi MM, et al. Short‐term antibiotic prophylaxis in 665 cases of vaginal and abdominal hysterectomy: controlled clinical study. Annali di Ostetricia, Ginecologia, Medicina Perinatale 1980;101(3):149‐58. [PubMed] [Google Scholar]
Fischbach 1988 {published data only}
- Fischbach F, Voss A, Loos W, Thurmayr R, Graeff H. Peri‐operative preventive use of antibiotics in abdominal hysterectomy. Geburtshilfe und Frauenheilkunde 1988;48(12):889‐92. [DOI] [PubMed] [Google Scholar]
Forney 1976 {published data only}
- Forney JP, Morrow CP, Townsend DE, Disaia PJ. Impact of cephalosporin prophylaxis on conization‐vaginal hysterectomy morbidity. American Journal of Obstetrics & Gynecology 1976;125(1):100‐3. [DOI] [PubMed] [Google Scholar]
Friese 1988 {published data only}
- Friese S, Pricker GJ, Willems FT, Loriaux SM. Single‐dose prophylaxis in gynaecological surgery: amoxicillin/clavulanic acid versus the combination of cefuroxim and metronidazole in a randomized prospective comparison. European Journal of Obstetrics, Gynecology, & Reproductive Biology 1988;27(4):313‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Friese 1989 {published data only}
- Friese S, Willems FTC, Loriaux SM, Meewis J. Prophylaxis in gynaecological surgery: a prospective randomized comparison between single dose prophylaxis with amoxicillin/clavulanate and the combination of cefuroxime and metronidazole. Journal of Antimicrobial Chemotherapy 1989;24(Suppl B):213‐6. [DOI] [PubMed] [Google Scholar]
Fujiwara 1994 {published data only}
- Fujiwara M, Koike H, Kohno I. Evaluation of prophylactic administration of antibiotics following total abdominal hysterectomy. Chemotherapy 1994;42(6):735‐9. [Google Scholar]
Goodlin 1974 {published data only}
- Goodlin RC. Letter. Prophylactic antibiotics. Obstetrics & Gynecology 1974;44(2):310‐1. [PubMed] [Google Scholar]
Gordon 1982 {published data only}
- Gordon SF, Russell J. A randomized controlled study comparing ceftizoxime, cefamandole, and cefoxitin in obstetric and gynecological surgery: a preliminary report. Journal of Antimicrobial Chemotherapy 1982;10(Suppl C):289‐92. [DOI] [PubMed] [Google Scholar]
Harms 1987 {published data only}
- Harms E, Schumacher C. Effectiveness of perioperative prophylaxis with ceftriaxone and cefotaxime in vaginal hysterectomy with and without anterior and/or posterior colporrhaphy. Chemioterapia 1987;6(2 Suppl):622‐3. [PubMed] [Google Scholar]
Haverkorn 1987 {published data only}
- Haverkorn MJ. A comparison of single‐dose and multi‐dose metronidazole prophylaxis for hysterectomy. Journal of Hospital Infection 1987;9(3):249‐54. [DOI] [PubMed] [Google Scholar]
Hayashi 2000 {published data only}
- Hayashi H, Yaginuma Y, Yamashita T, Morizaki A, Ishiya T, Katou Y, et al. Prospective randomized study of antibiotic prophylaxis for nonlaparotomy surgery in benign conditions. Chemotherapy 2000;46(3):213‐8. [DOI] [PubMed] [Google Scholar]
Hemsell 1990a {published data only}
- Hemsell DL, Johnson ER, Hemsell PG, Nobles BJ, Heard MC. Cefazolin for hysterectomy prophylaxis. Obstetrics & Gynecology 1990;76(4):603‐6. [PubMed] [Google Scholar]
Huang 1987 {published data only}
- Huang RL. Prophylactic use of antibiotics in gynecologic abdominal surgery: a prospective study of 234 cases. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology]. 1987;22(3):134‐7, 189. [PubMed] [Google Scholar]
Ireland 1982 {published data only}
- Ireland D, Tacchi D, Bint AJ. Effect of single‐dose prophylactic co‐trimoxazole on the incidence of gynaecological postoperative urinary tract infection. British Journal of Obstetrics & Gynaecology 1982;89(7):578‐80. [DOI] [PubMed] [Google Scholar]
Jacobson 1982 {published data only}
- Jacobson JA, Hebertson R, Kasworm E. Comparison of ceforanide and cephalothin prophylaxis for vaginal hysterectomies. Antimicrobial Agents & Chemotherapy 1982;22(4):643‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennings 1978 {published data only}
- Jennings RH. Prophylactic antibiotics in vaginal and abdominal hysterectomy. Southern Medical Journal 1978;71(3):251‐4. [DOI] [PubMed] [Google Scholar]
Jones RN, Wojes2 {published data only}
- Jones RN, Wojeski W, Bakke J, Porter C, Searles M. Antibiotic prophylaxis of 1,036 patients undergoing elective surgical procedures. A prospective, randomized comparative trial of cefazolin, cefoxitin, and cefotaxime in a prepaid medical practice. American Journal of Surgery 1987;153(4):341‐6. [DOI] [PubMed] [Google Scholar]
Jyothi 2010 {published data only}
- Jyothi S, Neetha V, Pratap K, Asha K. Antibiotic prophylaxis for hysterectomy and cesarean section: amoxicillin‐clavulanic acid versus cefazolin. Journal of Obstetrics and Gynecology of India 2010;60(5):419‐23. [Google Scholar]
Kauppila 1983 {published data only}
- Kauppila A, Rautiainen H, Tuimala R. Prevention of posthysterectomy infection with a combination of preoperative vaginal and perioperative intravenous administration of metronidazole. Annales Chirurgiae et Gynaecologiae 1983;72(4):214‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Khan 1981 {published data only}
- Khan MS, Begg HB, Frampton J, Hughes TB. A comparative study of the prophylactic effect of one dose and two dose intravenous metronidazole therapy in gynaecological surgery. Scandinavian Journal of Infectious Diseases Supplementum 1981;26:115‐7. [PubMed] [Google Scholar]
Knippenberger 1984 {published data only}
- Knippenberger H, Heinrich D, Wilbrand K. Antibiotic prophylaxis in hysterectomy by means of substances with selective effects. Der Klinikarzt 1984;13:375‐84. [Google Scholar]
Kunz 1982 {published data only}
- Kunz J, Genswein A. Advantages and disadvantages of peri‐surgical antibiotic prophylaxis in routine gynecologic operations: long‐term versus short‐term prophylaxis. Schweizerische Rundsdchau fur Medizinische Praxis 1982;71(1):36‐46. [PubMed] [Google Scholar]
Larsson 2002 {published data only}
- Larsson PG, Carlsson B. Does pre‐ and postoperative metronidazole treatment lower vaginal cuff infection rate after abdominal hysterectomy among women with bacterial vaginosis?. Infectious Diseases in Obstetrics & Gynecology 2002;10(3):133‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Littlejohn 1985 {published data only}
- Littlejohn Jr TW, Evrard HM, Moonsammy G. Comparison of cefonicid and cefoxitin for prevention of infection following vaginal hysterectomy. Advances in Therapy 1985;2(5):240‐9. [Google Scholar]
Luke 1999 {published data only}
- Luke DR. Oral trovafloxacin versus intravenous cefoxitin in elective hysterectomy. Drugs 1999;58(Suppl 2):284‐5. [MEDLINE: ] [Google Scholar]
Maki 1984 {published data only}
- Maki DG, Lammers JL, Aughey DR. Comparative studies of multiple‐dose cefoxitin vs. single‐dose cefonicid for surgical prophylaxis in patients undergoing biliary tract operations or hysterectomy. Reviews of Infectious Diseases 1984;6(4):s887‐95. [DOI] [PubMed] [Google Scholar]
Mamsen 1992 {published data only}
- Mamsen A, Hansen V, Moller BR. A prospective randomized double‐blind trial of ceftriaxone versus no treatment for abdominal hysterectomy. European Journal of Obstetrics, Gynecology, & Reproductive Biology 1992;47(3):235‐8. [DOI] [PubMed] [Google Scholar]
Mangioni 1991 {published data only}
- Mangioni C, Bianchi L, Bolis PF, Lomeo AM, Mazzeo F, Ventriglia L, Scalambrino S. Multicenter trial of prophylaxis with clindamycin plus aztreonam or cefotaxime in gynecologic surgery. Reviews of Infectious Diseases 1991;13(7):s621‐5. [DOI] [PubMed] [Google Scholar]
Mansani 1984 {published data only}
- Mansani FE, Caltabiano M, Condemi V, Scarpignato C. Short‐ or long‐term antibiotic prophylaxis in obstetric and gynecologic laparotomy?. Acta Bio‐Medica de l'Ateneo Parmense 1984;55(3‐4):147‐51. [PubMed] [Google Scholar]
Manthorpe 1982 {published data only}
- Manthorpe T, Justesen T. Metronidazole prophylaxis in abdominal hysterectomy. A double‐blind controlled trial. Acta Obstetricia et Gynecologica Scandinavica 1982;61(3):243‐6. [DOI] [PubMed] [Google Scholar]
Marsden 1985 {published data only}
- Marsden DE, Cavanagh D, Wisniewski BJ, Roberts WS, Lyman GH. Factors affecting the incidence of infectious morbidity after radical hysterectomy. American Journal of Obstetrics & Gynecology 1985;152(7 Pt 1):817‐21. [DOI] [PubMed] [Google Scholar]
Matkaris 1991 {published data only}
- Matkaris M, Markantes K, Stayannis K, Iatrakis G, Kourounis G, Tzingounis V. Reduction of hospital cost and administration of prophylactic antibiotherapy in gynecological surgery. [comment]. Israel Journal of Medical Sciences 1991;27(3):134‐6. [PubMed] [Google Scholar]
Mattheussens 1985 {published data only}
- Mattheussens OJA, Goormans E, Branolte JH. Effects of two different prophylactic antibiotic regimens on infection morbidity following vaginal hysterectomy. Archives of Gynecology 1985;237(Suppl):91. [Google Scholar]
McDonald 1984 {published data only}
- McDonald PJ, Sanders T, Higgins G, Finlay‐Jones L, Hakendorf M, Turnidge J, et al. Antibiotic prophylaxis in hysterectomy ‐ cefotaxime compared to ampicillin‐tinidazole. Journal of Antimicrobial Chemotherapy 1984;14(Suppl B):223‐30. [DOI] [PubMed] [Google Scholar]
McDonald 1988 {published data only}
- McDonald PJ, Sanders R, Turnidge J, Hakendorf P, Jolley P, McDonald H, et al. Optimal duration of cefotaxime prophylaxis in abdominal and vaginal hysterectomy. Drugs 1988;35(2):216‐20. [DOI] [PubMed] [Google Scholar]
McGregor 1994 {published data only}
- McGregor JA, Phillips LE, Roy S, Dunne JT, Warwaruk AS, Johnston DW, et al. Results of a double‐blind, placebo‐controlled clinical trial program of single‐dose ceftizoxime versus multiple‐dose cefoxitin as prophylaxis for patients undergoing vaginal and abdominal hysterectomy. Journal of the American College of Surgeons 1994;178(2):123‐31. [PubMed] [Google Scholar]
Mele 1985 {published data only}
- Mele G, Greco P, Gadaleta G, et al. Clinical evaluation of short‐term prophylaxis with 2 antibiotics in gynecologic surgery. Giornale Italiano di Ostetricia e Ginecologia 1985;7(3):241‐4. [Google Scholar]
Mele 1988 {published data only}
- Mele G, Gargano G, Greco P, Tartagni M. Antimicrobial prophylaxis for vaginal hysterectomy: comparison of two regimens. Clinical & Experimental Obstetrics & Gynecology 1988;15(4):174‐7. [PubMed] [Google Scholar]
Mercer 1988 {published data only}
- Mercer LJ, Murphy HJ, Ismail MA, Hajj SN. A comparison of cefonicid and cefoxitin for preventing infections after vaginal hysterectomy. Journal of Reproductive Medicine 1988;33(2):223‐6. [PubMed] [Google Scholar]
Mickal 1980 {published data only}
- Mickal A, Curole D, Lewis C. Cefoxitin sodium: double‐blind vaginal hysterectomy prophylaxis in premenopausal patients. Obstetrics & Gynecology 1980;56(2):222‐5. [PubMed] [Google Scholar]
Moroni 1979 {published data only}
- Moroni M, Baccolo M, Cavalli G, Belloni C, Ferrari N, Sartor V. Short‐term antibiotic prophylaxis of mixed infections during hysterectomy. Progress in Clinical & Biological Research 1979;35:43‐52. [PubMed] [Google Scholar]
Moroni 1984 {published data only}
- Moroni M, Mangioni C, Ortisi G, Marca G. Thiamphenicol in the prophylaxis and treatment of mixed infections of the female genital tract. Sexually Transmitted Diseases 1984;11(4 Suppl):444‐8. [DOI] [PubMed] [Google Scholar]
Mozzillo 1989 {published data only}
- Mozzillo N, Dionigi lR, Ventriglia L. Multicenter study of aztreonam in the prophylaxis of colorectal, gynecologic and urologic surgery. Chemotherapy 1989;35(1):58‐71. [DOI] [PubMed] [Google Scholar]
Multicenter 1989 {published data only}
- The Multicenter Study Group. Single‐dose prophylaxis in patients undergoing vaginal hysterectomy: cefamandole versus cefotaxime. American Journal of Obstetrics & Gynecology. 1989;160(5 Pt 1):1198‐201. [DOI] [PubMed] [Google Scholar]
Munck 1989 {published data only}
- Munck AM, Jensen HK. Preoperative clindamycin treatment and vaginal drainage in hysterectomy. Acta Obstetricia et Gynecologica Scandinavica 1989;68(3):241‐5. [DOI] [PubMed] [Google Scholar]
Ohm 1975 {published data only}
- Ohm MJ, Galask RP. The effect of antibiotic prophylaxis on patients undergoing vaginal operations. I. The effect on morbidity. American Journal of Obstetrics & Gynecology 1975;123(6):590‐6. [DOI] [PubMed] [Google Scholar]
Ohm 1976 {published data only}
- Ohm MJ, Galask RP. The effect of antibiotic prophylaxis on patients undergoing total abdominal hysterectomy. II. Alterations of microbial flora. American Journal of Obstetrics & Gynecology 1976;125(4):448‐54. [DOI] [PubMed] [Google Scholar]
Ohm MJ, Galask 2 {published data only}
- Ohm MJ, Galask RP. The effect of antibiotic prophylaxis on patients undergoing total abdominal hysterectomy. I. Effect on morbidity. American Journal of Obstetrics & Gynecology 1976;125(4):442‐7. [DOI] [PubMed] [Google Scholar]
Ohm MJ, Galask 3 {published data only}
- Ohm MJ, Galask RP. The effect of antibiotic prophylaxis on patients undergoing vaginal operations. II. Alterations of microbial flora. American Journal of Obstetrics & Gynecology 1975;123(6):597‐604. [DOI] [PubMed] [Google Scholar]
Olgiati 1980 {published data only}
- Olgiati ML, Brignoli DM, Nencioni T. Ampicillin as antibiotic prophylaxis in gynecological surgery. Annali di Ostetricia, Ginecologia, Medicina Perinatale 1980;101(3):159‐67. [PubMed] [Google Scholar]
Oliva 1990 {published data only}
- Oliva GC, Fratoni A, Papadia L, Pieroni A, Paparella PL, Mancuso S. Antibiotic prophylaxis in gynecological surgery. Journal of Chemotherapy 1990;2(5):306‐9. [DOI] [PubMed] [Google Scholar]
Orr 1988 {published data only}
- Orr JWJ, Sisson PF, Barrett JM, Ellington JR, Jennings RH, Taylor DL. Single‐center study results of cefotetan and cefoxitin prophylaxis for abdominal or vaginal hysterectomy. American Journal of Obstetrics & Gynecology 1988;158(3 Pt 2):714‐6. [DOI] [PubMed] [Google Scholar]
Periti 1988 {published data only}
- Periti P, Mazzei T, Orlandini F, Mini E. Comparison of the antimicrobial prophylactic efficacy of cefotaxime and cephazolin in obstetric and gynaecological surgery. A randomised multicentre study. Drugs 1988;35(2):133‐8. [DOI] [PubMed] [Google Scholar]
Periti P, Mazze2 {published data only}
- Periti P, Mazzei T, Periti E. Prophylaxis in gynaecological and obstetric surgery: a comparative randomised multicentre study of single‐dose cefotetan versus two doses of cefazolin. Chemioterapia 1988;7(4):245‐52. [PubMed] [Google Scholar]
Perri 1986 {published data only}
- Perri G, D'Aloya P, Mari A, et al. Comparative prospective study on antibiotic prophylaxis in hysterectomy. Giornale Italiano di Ostetricia e Ginecologia 1986;8(1):31‐5. [Google Scholar]
Phoolcharoen 2012 {published data only}
- Phoolcharoen N, Nilgate S, Rattanapuntamanee O, Limpongsanurak S, Chaithongwongwatthana S. A randomized controlled trial comparing ceftriaxone with cefazolin for antibiotic prophylaxis in abdominal hysterectomy. International Journal of Gynecology and Obstetrics 2012;119:11‐3. [DOI: ] [DOI] [PubMed] [Google Scholar]
Popkin 1983 {published data only}
- Popkin DR, Martinez LA, Carswell GA. Metronidazole in the prophylaxis of anaerobic infections in gynecologic surgery. Surgery 1983;93(1 Pt 2):180‐4. [PubMed] [Google Scholar]
Poulsen 1984 {published data only}
- Poulsen HK, Borel J, Olsen H. Prophylactic metronidazole or suction drainage in abdominal hysterectomy. Obstetrics & Gynecology 1984;63(3):291‐4. [PubMed] [Google Scholar]
Poulsen HK, Bor2 {published data only}
- Poulsen HK, Borel J. T‐tube suction drainage and/or prophylactic two‐dose metronidazole in abdominal hysterectomy. Acta Obstetricia et Gynecologica Scandinavica 1984;63(8):711‐4. [DOI] [PubMed] [Google Scholar]
Queck 1991 {published data only}
- Queck M, Weiss E, Berle P. Local administration of metronidazole before hysterectomy ‐ effect on infectious morbidity. A prospective study. Geburtshilfe und Frauenheilkunde 1991;51(10):839‐42. [DOI] [PubMed] [Google Scholar]
Rapp 1982 {published data only}
- Rapp RP, Nagell JRJ, May JR. A double‐blind randomized study of prophylactic antibiotics in vaginal hysterectomy. Hospital Formulary 1982;17:524. [Google Scholar]
Rapp 1986 {published data only}
- Rapp RP, Connors JE, Hager WD, Donaldson ES, Nagell JR Jr. Comparison of single‐dose moxalactam and a three‐dose regimen of cefoxitin for prophylaxis in vaginal hysterectomy. Clinical Pharmacy 1986;5(12):988‐93. [PubMed] [Google Scholar]
Regallo 1987 {published data only}
- Regallo M, Scalambrino S, Landoni F, Consonni R, Mangioni C. Antibiotic prophylaxis of infection after gynecologic surgery: a prospective randomized trial between cefotetan and piperacillin. Chemioterapia 1987;6(2 Suppl):624‐6. [PubMed] [Google Scholar]
Reggiori 1996 {published data only}
- Reggiori A, Ravera M, Cocozza E, Andreata M, Mukasa F. Prophylaxis with ampicillin before surgery was more effective than penicillin after surgery in rural Africa. Therapeutics 1996;Sep/Oct:172. [MEDLINE: ] [Google Scholar]
Reggiori A, Rav2 {published data only}
- Reggiori A, Ravera M, Cocozza E, Andreata M, Mukasa F. Randomized study of antibiotic prophylaxis for general and gynaecological surgery from a single centre in rural Africa. British Journal of Surgery 1996;83(3):356‐9. [DOI] [PubMed] [Google Scholar]
Regidor 2000 {published data only}
- Regidor PA, Bier UW, Preuss MJ, Eickhoff C, Hillger H, Kienle E, et al. Efficacy and safety of two cephalosporins in the perioperative prophylaxis in patients undergoing abdominal or vaginal hysterectomies or gynaecological laparotomies: a prospective randomized study. Gynakologisch Geburtshilfliche Rundschau 2000;40(3‐4):153‐8. [DOI] [PubMed] [Google Scholar]
Roberts 1978 {published data only}
- Roberts JM, Homesley HD. Low‐dose carbenicillin prophylaxis for vaginal and abdominal hysterectomy. Obstetrics & Gynecology 1978;52(1):83‐7. [PubMed] [Google Scholar]
Roy 1982 {published data only}
- Roy S, Wilkins J. Comparison of cefotaxime with cefazolin for prophylaxis of vaginal or abdominal hysterectomy. Clinical Therapeutics 1982;5:74‐82. [PubMed] [Google Scholar]
Roy 1984 {published data only}
- Roy S, Wilkins J. Single‐dose cefotaxime versus 3 to 5 dose cefoxitin for prophylaxis of vaginal or abdominal hysterectomy. Journal of Antimicrobial Chemotherapy 1984;14:217‐21. [DOI] [PubMed] [Google Scholar]
Roy 1988 {published data only}
- Roy S, Wilkins J, Hemsell DL, March CM, Spirtos NM. Efficacy and safety of single‐dose ceftizoxime vs. multiple‐dose cefoxitin in preventing infection after vaginal hysterectomy. Journal of Reproductive Medicine 1988;33(1 Suppl):149‐53. [PubMed] [Google Scholar]
Roy 1989 {published data only}
- Roy S, Wilkins J, Galaif E, Azen C. Comparative efficacy and safety of cefmetazole or cefoxitin in the prevention of postoperative infection following vaginal and abdominal hysterectomy. Journal of Antimicrobial Chemotherapy 1989;23:109‐17. [DOI] [PubMed] [Google Scholar]
Roy 1990 {published data only}
- Roy S, Wilkins J, March CM, Solera NC, Galvan NI, Ressler RL, et al. Cefmetazole and cefonicid. Comparative efficacy and safety in preventing postoperative infections after vaginal and abdominal hysterectomy. Journal of Reproductive Medicine 1990;35(11 Suppl):1082‐90. [PubMed] [Google Scholar]
Roy 1998 {published data only}
- Roy S, Hemsell D, Gordon S, Godwin D, Pearlman M, Luke D, et al. Oral trovafloxacin compared with intravenous cefoxitin in the prevention of bacterial infection after elective vaginal or abdominal hysterectomy for nonmalignant disease. American Journal of Surgery 1998;176(6A):62s‐66s. [DOI] [PubMed] [Google Scholar]
Santarelli 1988 {published data only}
- Santarelli J, Scavizzi M, Buisson P, Jupeau‐Vessieres A. Post‐operative infections in vaginal hysterectomies and antibiotic prophylaxis. Medecine et Maladies Infectieuses 1988;18(6/7):313‐6. [Google Scholar]
Savage 1984 {published data only}
- Savage EW, Thadepalli H, Rambhatla K, Roy I, Davidson EC Jr. Minocycline prophylaxis in elective hysterectomy. Journal of Reproductive Medicine 1984;29(2):81‐7. [PubMed] [Google Scholar]
Scarpignato 1980 {published data only}
- Scarpignato C, Labruna C, Condemi V, Mansani FE. Comparative efficacy of two different regimens of antibiotic prophylaxis in total abdominal hysterectomy. Pharmatherapeutica 1980;2(7):450‐5. [PubMed] [Google Scholar]
Siekmann 1983 {published data only}
- Siekmann U, Heilmann L, Daschner F, Bornemann H. Single‐dose mezlocillin prophylaxis and postoperative morbidity after vaginal hysterectomy: pharmacokinetic and clinical results. Geburtshilfe und Frauenheilkunde 1983;43(1):20‐6. [DOI] [PubMed] [Google Scholar]
Simoes 2008 {published data only}
- Simoes JA, Discacciati MG, Poletti GB, Pereira GF. Tinidazole versus cefazolin in antibiotic prophylaxis of vaginal and abdominal hysterectomy. Revista Brasileira de Ginecologia e Obstetrícia 2008;30(11):544‐9. [DOI] [PubMed] [Google Scholar]
Stocklund 1980 {published data only}
- Stocklund KE, Kessel M, Jensen RH. Ornidazole in the prevention of infections after abdominal hysterectomy. Chemotherapy 1980;26(5):397‐401. [DOI] [PubMed] [Google Scholar]
Sutthijumroon 1990 {published data only}
- Sutthijumroon S, Chandeying V, Tungphaisal S. Efficacy of cefoxitin for the prevention of postoperative infection in abdominal hysterectomy. Asia Oceania Journal of Obstetrics & Gynaecology 1990;16(4):347‐51. [DOI] [PubMed] [Google Scholar]
Suvonnakote 1988 {published data only}
- Suvonnakote T. A comparative study of two prophylactic antibiotic regimens in abdominal hysterectomy. Journal of the Medical Association of Thailand 1988;71(8):431‐5. [PubMed] [Google Scholar]
Szalay 1996 {published data only}
- Szalay J. Ciprofloxacin prophylaxis in vaginal and abdominal hysterectomy. Magyar Noorvosok Lapja 1996;59(3):221‐5. [Google Scholar]
Tarczali 1997 {published data only}
- Tarczali D, Smid I. Prophylactic antibiotic treatment in case of abdominal and vaginal hysterectomies. Magyar Noorvosok Lapja 1997;60(1):33‐7. [Google Scholar]
Tchabo 1985 {published data only}
- Tchabo JG, Cutting ME, Butler C. Prophylactic antibiotics in patients undergoing total vaginal or abdominal hysterectomy. International Surgery 1985;70(4):349‐52. [PubMed] [Google Scholar]
Turano 1992 {published data only}
- Turano A. New clinical data on the prophylaxis of infections in abdominal, gynecologic, and urologic surgery. Multicenter Study Group. American Journal of Surgery 1992;164(4A Suppl):16s‐20s. [DOI] [PubMed] [Google Scholar]
van der Linden1993 {published data only}
- Linden MC, Erp EJ, Ruijs GJ, Holm JP. A prospective randomized study comparing amoxicillin/clavulanate with cefuroxime plus metronidazole for perioperative prophylaxis in gynaecological surgery. European Journal of Obstetrics, Gynecology, & Reproductive Biology 1993;50(2):141‐5. [DOI] [PubMed] [Google Scholar]
Vecek 1993 {published data only}
- Vecek N, Dzanic N, Pazur D, Kalenic S, Marinovic T, Ljubojevic N. A comparison of the prophylactic use of one and three cefuroxime doses in vaginal hysterectomy with correction of the bladder bolting mechanism. Gynaecologia et Perinatologia 1993;2(3):121‐2. [Google Scholar]
Voss 1989 {published data only}
- Voss A, Fischbach F, Loss W, Graeff H. Antibiotic prophylaxis in abdominal hysterectomy: a single dosis of cefotetan. Archives of Gynecology & Obstetrics 1989;245(1‐4):449‐50. [Google Scholar]
Walker 1982 {published data only}
- Walker EM, Gordon AJ, Hare MJ, Warren RE. Prophylactic single‐dose metronidazole before abdominal hysterectomy. British Journal of Obstetrics & Gynaecology 1982;89(11):957‐61. [DOI] [PubMed] [Google Scholar]
Wideman 1982 {published data only}
- Wideman GL, Matthijssen C. Comparative efficacy of cefotaxime and cefazolin as prophylaxis against infections following elective hysterectomy. Clinical Therapeutics 1982;5(Suppl A):67‐73. [PubMed] [Google Scholar]
Zivny 1997 {published data only}
- Zivny J, Mara M, Jedlickova A, Fucikova Z, Krejci V, Dohnalova J. Antibiotic prophylaxis of infectious complications in gynecologic surgery. Ceska Gynekologie 1997;62(4):204‐12. [PubMed] [Google Scholar]
Additional references
ACOG 2009
- ACOG (American College of Obstetricians and Gynecologists). Antibiotic prophylaxis for gynecologic procedures. Obstetrics & Gynecology 2009;reaffirmed 2016(113):1180‐9. [DOI] [PubMed] [Google Scholar]
BNF 2002
- British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary, 2002. 4th Edition. http://www.bnf.org/ [accessed May 2003]. [Google Scholar]
Brand 2010
- Brand M, Bizos D, O'Farrell PJR. Antibiotic prophylaxis for patients undergoing elective endoscopic retrograde cholangiopancreatography. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007345.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bratzler 2013
- Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. American Journal of Health‐System Pharmacy 2013;70(3):195–283. [DOI] [PubMed] [Google Scholar]
Brignardello‐Petersen 2015
- Brignardello‐Petersen R, Carrasco‐Labra A, Araya I, Yanine N, Cordova Jara L, Villanueva J. Antibiotic prophylaxis for preventing infectious complications in orthognathic surgery. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010266.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Classen 1992
- Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. New England Journal of Medicine 1992;326(5):281‐6. [DOI] [PubMed] [Google Scholar]
Clifford 2012
- Clifford V, Daley A. Antibiotic prophylaxis in obstetric and gynaecological procedures: a review. Australian and New Zealand Journal of Obstetrics and Gynaecology 2012;52(5):412‐9. [DOI] [PubMed] [Google Scholar]
Costa 2004
- Costa RJ, Krauss‐Silva L. Systematic review and meta‐analysis of antibiotic prophylaxis in abdominal hysterectomy [Revisão sistemática e meta‐análiseda antibioticoprofilaxiana histerectomia abdominal]. Cadernos de Saúde Pública 2004;20(Suppl 2):S175‐89. [DOI] [PubMed] [Google Scholar]
Deffieux 2015
- Deffieux X, Rochambeau B, Chêne G, Gauthier T, Huet S4, Lamblin G, et al: French College of Obstetrics and Gynaecology (CNGOF). Hysterectomy for benign pathology: guidelines for clinical practice [Hystérectomie pour pathologie bénigne: recommandations pour la pratique clinique]. Journal de Gynécologie Obstétrique et Biologie de la Reproduction 2015;44(10):1219‐27. [DOI] [PubMed] [Google Scholar]
Dellinger 1994
- Dellinger EP, Gross PA, Barrett TL, Krause PJ, Martone WJ, McGowan JE, et al. Quality standard for antimicrobial prophylaxis in surgical procedures. Clinical Infectious Diseases 1994;18:422‐7. [DOI] [PubMed] [Google Scholar]
DiPiro 1984
- DiPiro JT, Vallner JJ, Bowden TA, Clark B, Sisley JF. Intraoperative serum concentrations of cefazolin and cefoxitin administered preoperatively at different times. Clinical Pharmacy 1984;3(1):64‐7. [PubMed] [Google Scholar]
DiPiro 1986
- DiPiro JT, Cheung RP, Bowden TA, Mansberger JA. Single dose systemic antibiotic prophylaxis of surgical wound infections. American Journal of Surgery 1986;152(5):552‐9. [DOI] [PubMed] [Google Scholar]
Duff 1980
- Duff P, Park RC. Antibiotic prophylaxis in vaginal hysterectomy: a review. Obstetrics & Gynecology 1980;55(5 Suppl):193S‐202S. [DOI] [PubMed] [Google Scholar]
Faro 2001
- Faro 2001. Postoperative infections. In: Gershensen CM, DeCheney AH, Curry SL, Brubaker L editor(s). Operative Gynecology. 2nd Edition. Philadelphia: WB Saunders, 2001:123‐31. [Google Scholar]
Farquhar 2002
- Farquhar C, Steiner A. Hysterectomy rates in the United States 1990‐1997. Obstetrics & Gynecology 2002;99(2):229‐34. [DOI] [PubMed] [Google Scholar]
Fukatsu 1997
- Fukatsu K, Saito H, Matsuda T, Ikeda S, Furukawa S, Muto T. Influences of type and duration of antimicrobial prophylaxis on an outbreak of methicillin‐resistant Staphylococcus aureus and on the incidence of wound infection. Archives of Surgery 1997;132(12):1320‐5. [DOI] [PubMed] [Google Scholar]
Gorecki 1999
- Gorecki P, Schein M, Rucinski JC, Wise L. Antibiotic administration in patients undergoing common surgical procedures in a community teaching hospital: the chaos continues. World Journal of Surgery 1999;23:429‐33. [DOI] [PubMed] [Google Scholar]
GRADEPro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to November 2016. Hamilton, Ontario: GRADE Working Group, McMaster University, 2014.
Gyte 2014
- Gyte GML, Dou L, Vazquez JC. Different classes of antibiotics given to women routinely for preventing infection at caesarean section. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD008726.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. [Google Scholar]
Hodges 2014
- Hodges KR, Davis BR, Swaim LS. Prevention and management of hysterectomy complications. Clinical Obstetrics and Gynecology 2014;57(1):43‐57. [DOI] [PubMed] [Google Scholar]
Lachiewicz 2015
- Lachiewicz MP, Moulton LJ, Jaiyeoba O. Infection prevention and evaluation of fever after laparoscopic hysterectomy. Journal of the Society of Laparoendscopic Surgeons 2015;19(3):pii: e2015.00065. doi: 10.4293/JSLS.2015.00065.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merrill 2013
- Merrill RM. Hysterectomy surveillance in the United States ‐ 1997 through 2005. Medical Science Monitor 2008;14:CR24‐CR31. [PubMed] [Google Scholar]
Mittendorf 1993
- Mittendorf R, Aronson MP, Berry RE, Williams MA, Kipelnick B, Klickstein A, et al. Avoiding serious infections associated with abdominal hysterectomy: a meta‐analysis of antibiotic prophylaxis. American Journal of Obstetrics and Gynecology 1993;169:1119‐24. [DOI] [PubMed] [Google Scholar]
Morrill 2013
- Morrill MY, Schimpf MO, Abed H, Carberry C, Margulies RU, White AB, et al. Society of Gynecologic Surgeons Systematic Review Group. Antibiotic prophylaxis for selected gynecologic surgeries. International Journal of Gynaecology and Obstetrics 2013;120(1):10‐5. [DOI] [PubMed] [Google Scholar]
Nabhan 2016
- Nabhan AF, Allam NE, Hamed Abdel‐Aziz Salama M. Routes of administration of antibiotic prophylaxis for preventing infection after caesarean section. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD011876.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2016
- Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, et al. Guidelines for pre‐ and intra‐operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations ‐ PART I. Gynecologic Oncology 2016;140(2):313‐22. [DOI] [PubMed] [Google Scholar]
RCOG 1999
- Royal College of Obstetricians and Gynaecologists. Effective procedures in gynaecology suitable for audit, 1999. www.rcog.org.uk (accessed 27 May 2003).
Sanabria 2010
- Sanabria A, Dominguez LC, Valdivieso E, Gomez G. Antibiotic prophylaxis for patients undergoing elective laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD005265.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez‐Manuel 2012
- Sanchez‐Manuel FJ, Lozano‐García J, Seco‐Gil JL. Antibiotic prophylaxis for hernia repair. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD003769.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2008
- Scottish Intercollegiate Guidelines Network. Antibiotic prophylaxis in surgery. http://www.sign.ac.uk/pdf/sign104.pdf 2008, updated April 2014.
Soper 1993
- Soper DE. Bacterial vaginosis and postoperative infections. American Journal of Obstetrics and Gynecology 1993;169(2 Pt 2):467‐9. [DOI] [PubMed] [Google Scholar]
Steiner 2017
- Steiner HL, Strand EA. Surgical‐site infection in gynecologic surgery: pathophysiology and prevention. American Journal of Obstetrics and Gynecology 2017;S0002‐9378(17):30254‐5. doi: 10.1016/j.ajog.2017.02.014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Tanos 1994
- Tanos V, Rojansky N. Prophylactic antibiotics in abdominal hysterectomy. Journal of the American College of Surgeons 1994;179:593‐600. [PubMed] [Google Scholar]
Upall 2016
- Uppal S, Harris J, Al‐Niaimi A, Swenson CW, Pearlman MD, Reynolds RK, et al. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstetrics and Gynecology 2016;127(2):321‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Eyk N, van Schalkwyk J 2012
- Eyk N, Schalkwyk J, Infectious Disease Committee. Anitbiotic prophylaxis in gynaecologic procedures. Journal of Obstetrics and Gynaecology Canada 2012;34(4):382‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weed 2003
- Weed HG. Antimicrobial prophylaxis in the surgical patient. The Medical Clinics of North America 2003;87:59‐75. [DOI] [PubMed] [Google Scholar]
Wttewaall‐Evelaar 1990
- Wttewaall‐Evelaar EW. Meta‐analysis of randomized controlled trials of antibiotic prophylaxis in abdominal hysterectomy. Pharmaceutisch Weekblad Scientific 1990;12(6A):296‐9. [DOI] [PubMed] [Google Scholar]


