Abstract
CD52 is a glycoprotein expressed on normal as well as leukemic immune cells and shed as soluble CD52 (sCD52). We studied sCD52 levels in three CLL cohorts: the ‘early’, the ‘high-risk’, and the ‘ibrutinib-treated’. The ‘high-risk’ patients had significantly higher sCD52 levels than the ‘early’ patients. For the ‘early’ patients, high sCD52 levels were associated with a significantly shorter time to first treatment. Regarding prognostic factors, no clear correlations with stage, IGHV, or beta-2-microglobulin were found; in a cox multivariate analysis of the ‘early’ patients, sCD52 and IGHV both had independent prognostic value. Following chemo-immunotherapy, sCD52 decreased in parallel with leukocytes while during ibrutinib treatment and ibrutinib-induced ymphocytosis, sCD52 decreased along with lymph node reductions. In vitro IgM stimulation of CLL cells led to increased sCD52 levels in the medium. Our findings indicate that sCD52 reflects disease activity and potentially treatment efficacy in CLL.
Keywords: CD52, CLL, chemo-immunotherapy, ibrutinib
Introduction
The CD52 molecule is a glycosylphosphatidylinositol (GPI)-anchored glycoprotein of approximately 29 kDa. It is comprised of only 12 amino acids with a large carbohydrate domain [1,2]. CD52 is expressed on virtually all hematopoietic cells at different levels; low levels on stem cells and high levels on lymphocytes and neoplastic B cells [3]. The molecule can be released by phospholipase C from the surface of immune cells as soluble CD52 (sCD52), which has been shown to have inhibitory effects on T cells via Siglec-10 [4]. In chronic lymphocytic leukemia (CLL), sCD52 can be shed from CLL cells in vitro and can be bound by the anti-CD52 antibody alemtuzumab [5]. The biological function of the glycoprotein in patients with CLL remains unknown.
After induction treatment with fludarabine, cyclophosphamide and rituximab (FCR), sCD52 levels predict progression and are proposed as a marker for minimal residual disease [6]. As CD52 is targeted by alemtuzumab, a correlation between the effect of alemtuzumab and CD52 expression has been sought. In the CLL2H trial of alemtuzumab consolidation for patients with fludarabine refractory CLL, high CD52 mRNA levels predicted a shorter overall and progression-free survival upon alemtuzumab treatment [7]. The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib approved for treatment of CLL, targets the B-cell receptor (BCR) pathway. It is highly efficacious, also in patients with deletion of the short arm of chromosome 17 (del(17p)) in the front line and relapse setting [8,9]. No studies have yet investigated sCD52 levels in relation to BCR-targeted treatment. We here address the correlation of sCD52 levels with prognostic factors in patients with CLL at diagnosis/referral and at treatment baseline as well as during treatment with chemo-immunotherapy and ibrutinib.
Materials and methods
Patient cohorts
Blood samples from 111 patients with CLL and 45 healthy blood donors were included. Three CLL cohorts were studied. Cohort 1 (‘early’): 44 consecutive, unselected patients referred to Rigshospitalet, Copenhagen, Denmark in 2014, mainly at diagnosis (77%); cohort 2 (‘high-risk’): 42 patients selected for high-risk features (unmutated IGHV, and/or with del(17p), del(11q) or trisomy 12 by FISH), and in need of treatment according to IWCLL guidelines and enrolled in the HOVON68 first-line trial [10] (trialregister.nl: NTR529); cohort 3 (‘ibrutinib-treated’): 25 patients with advanced CLL in need of treatment, included in a phase II trial of single agent ibrutinib at the National Institutes of Health (NIH), Bethesda, MD(ClinicalTrials.gov: NCT01500733) [8]. Furthermore, blood samples from four additional treatment naïve patients with unmutated IGHV status from NIH were included only for in vitro stimulation assays.
The study was approved by the ethical committees in Denmark (Regional ethics committee, Capital region of Denmark), The Danish Data protection agency, all participating countries in HOVON68, and at NIH (Bethesda, MD), and was performed in accordance with the declaration of Helsinki. Anonymized blood samples from healthy blood donors were provided by the Department of Immunology at Rigshospitalet, Copenhagen, Denmark with approval for research purposes. Clinical data were collected from patient medical records at Rigshospitalet, NIH, and from the HOVON database.
Samples
Plasma from cohort 1 and 2 was stored at −20 °C, serum from cohort 3 at −80 °C. Due to differences in handling and storage of samples that may influence protein levels we abstained from comparing plasma and serum values across cohorts. Thus, serum samples from cohort 3 were only used for analyses of sCD52 during treatment (n = 11) and as baseline values (n = 25).
ELISA and other analyses
sCD52 was quantified in plasma or serum by a commercial CD52 ELISA kit (CSB-EL004943HU, Cusabio Biotech, China) following the manufacturer’s instructions. Values for sCD52 were extrapolated from a standard curve using Curve Expert Professional software version 2.0.3 (www.curveexpert.net). The kit was validated by western blot as described in the supplement (Figure S1, S2(A–C)).
Fluorescence in situ hybridization (FISH), IGHV mutational status, as well as the full protocol for affinity chromatography, anti-IgM stimulation, and Western blotting is provided in the supplement.
Statistical analyses
The statistical analyzes were performed in SAS 9.3 and SAS enterprise Guide 5.1 software (SAS institute, Cary, NC). Graphical presentations were performed in Graph Pad Prism 5 (GraphPad software Inc., La Jolla, CA). Data were log transformed to convey a normal distribution. Samples below quantification limit of the ELISA were set to half the value of the lower limit of quantification of the assay (78ng/mL) for group-wise comparisons, and were not included in linear regression analysis. If samples were below the detection limit of the ELISA, they were valued as ‘0’. The median sCD52 for each cohort was used as cutoff value for dichotomization. Linear mixed models [11] were applied for analyses of correlations over time. Cox proportional hazards models were used for testing the independent and additive effects of sCD52. For specific analyses see figure legends. p Values were two-sided and considered significant if <.05.
Results
We evaluated sCD52 as an indicator of disease activity in different cohorts of patients with CLL. The clinical characteristics of the three cohorts are summarized in Table 1. The unselected ‘early’ cohort consisted of untreated, mainly stage A, and low-risk patients with CLL. The ‘high-risk’ cohort was selected for younger patients, mainly in stage B/C, with higher white blood cell counts (WBC) and beta-2-microglobulin (β2m) levels compared to the ‘early’ cohort. The ‘ibrutinib-treated’ cohort was selected for advanced CLL with untreated or relapsed/refractory disease with either TP53 aberrations or age above 65 years.
Table 1.
Clinical characteristics of the three CLL cohorts.
| Cohort 1 (‘early’) | Cohort 2 (‘high-risk’) | Cohort 3 (‘ibrutinib-treated’) | |
|---|---|---|---|
| n | 44 | 42 | 25 |
| Treatment naïve, % | 100 | 100 | 52 |
| High-risk, % | 41 | 100 | 84 |
| Median age | 68 | 60 | 68 |
| M, % | 48 | 79 | 44 |
| Binet B + C, % | 9 | 86 | 96 |
| WHO 0, % | 80 | 57 | ND |
| M-IGHV, % | 57 | 14 | 40 |
| del(17p), % | 2 | 26 | 48 |
| del(11q), % | 7 | 15 | 24 |
| Tri12, % | 5 | 23 | 8 |
| Median β2m | 191 | 351 | 348.5 |
| Median WBC/ALC | 27 | 91 | 72 (ALC) |
%: percentage; n: number; High-risk: (del(17p), del(11q), t(12) and/or unmutated IGHV); M: male gender; M-IGHV: mutated IGHV; del(17p): deletion of the short arm of chromosome17; del(11q): deletion of the long arm of chromosome 11; Tri12: Trisomy of chromosome 12; β2m: beta 2 microglobulin; WBC: white-blood cell count; ALC: absolute lymphocyte count. FISH categories are restricted to those defining high risk CLL.
We first observed that levels of sCD52 in plasma were higher in patients with CLL (median 365 ng/mL, interquartile range (IQR) [229–617.6]) than in healthy blood donors (median 114 ng/mL, IQR [87–168]), (p < .0001, Figure 1(A)). Among patients with CLL, however, sCD52 levels were significantly higher for ‘high-risk’ patients (median 628 ng/mL, IQR [472–798]), as compared to ‘early’ patients (median 243 ng/mL, IQR [155–320]) (p < .0001, Figure 1(B)). We then assessed the correlation between sCD52 levels and known prognostic factors. Regarding clinical stage, in ‘early’ CLL, the few patients with Binet stage B/C had largely the same low sCD52 levels as those with Binet stage A, despite a statistical difference (p = .04, Figure 1(C)). In ‘high-risk’ CLL, sCD52 levels were similarly high in Binet stages A and B/C (p = .21, Figure 1(C)). Regarding IGHV mutational status, in ‘early’ CLL, no difference in sCD52 levels was found between patients with mutated (M) vs. unmutated (UM) IGHV (p = .59, data not shown). In contrast, in ‘high-risk’ CLL, sCD52 levels were significantly higher in patients with M-IGHV compared to UM-IGHV (p = .004, data not shown). However, these patients all had adverse FISH findings. In general, sCD52 levels did not correlate with cytogenetic aberrations in ‘early’ CLL (One-way ANOVA p = .20, data not shown) or in ‘high-risk’ CLL (One-way ANOVA, p = .13, data not shown). Beta2-microglobulin did not correlate with sCD52 in either ‘early’ or ‘high-risk’ patients, respectively (p = .78, p = .70 resp. data not shown) or with the WBC (p = .54, p = .22, respectively Figure 1(D)).
Figure 1.
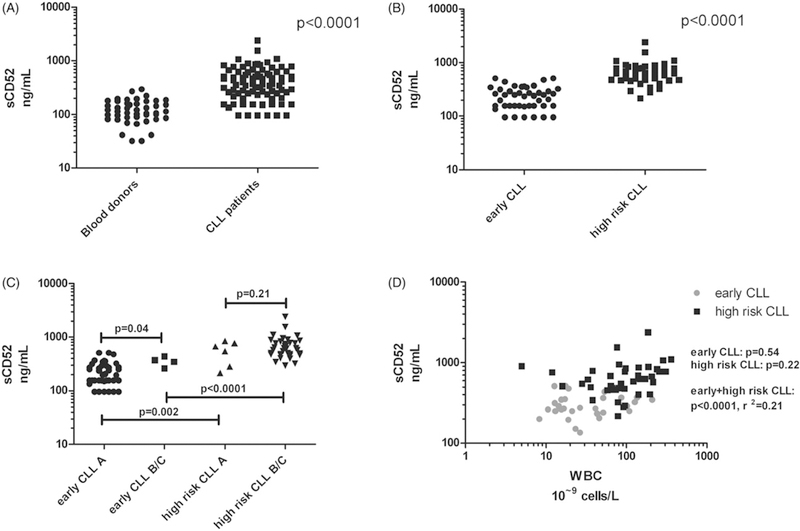
Levels of sCD52 quantified by ELISA. (A) healthy blood donors (n = 45) and patients with CLL (n = 86). (B) patients with early CLL (n = 44) and with progressive CLL (progressive CLL, n = 42). (C) patients with early CLL and progressive CLL by Binet stage grading A (n = 39 and n = 6, early CLL and progressive CLL, respectively), vs. B/C (n = 4 and n = 36, early CLL and progressive CLL, respectively). (D) Levels of sCD52 and β2-microglobulin (β2m) in patients with early CLL (n = 26) and progressive CLL (n = 30). Statistical analyses were performed as unpaired Student’s t tests and linear regression analysis, data were logarithmically transformed.
To assess whether sCD52 had prognostic impact in the unselected patients with ‘early’ CLL, we analyzed the effect on time to the first treatment (TTFT): Despite a median follow-up of only 11 months, patients with sCD52 levels above median had a significantly shorter TTFT than those with sCD52 below median (p = .02, Figure 2(A)). As expected, IGHV status predicted for TTFT as well (p = .0002, data not shown). In a model including both sCD52 levels and IGHV status, sCD52 levels retained independent significance by cox regression analysis (p = .02). Thus, the combination of sCD52 and IGHV allowed for a better discrimination between aggressive and indolent disease at diagnosis (Figure 2(B)).
Figure 2.
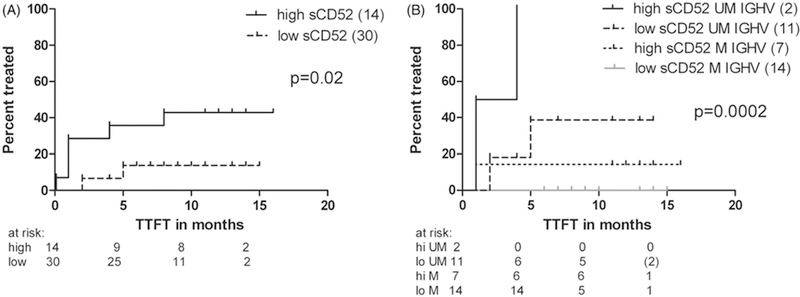
Levels of sCD52 and time to first treatment (TTFT) in ‘early’ CLL. (A) TTFT in patients with ‘early’ CLL divided by the median sCD52 level* (n = 44). (B) TTFT in patients with ‘early’ CLL at diagnosis based on sCD52 and IGHV mutational status, a sub-group of the patients shown in A (n = 34). Statistical analyses were performed as log rank test, cumulative incidence rates are shown. M-IGHV: mutated IGHV, UM-IGHV: unmutated IGHV. *The median level of sCD52 (274 ng/mL) in ‘early CLL’ patients with a sCD52 level above lower limit of quantification in the ELISA found to discriminate best between healthy donors and patients with CLL by ROC curve analysis, data not shown.
We then analyzed sCD52 levels before and during treatment with chemo-immunotherapy. Following therapy with fludarabine and cyclophosphamide alone (FC) or in combination with alemtuzumab (FCA), the WBC and sCD52 levels decreased in parallel over time (p < .0001, Figure 3(A)). To address whether sCD52 levels simply reflected the WBC, we included a cohort of ibrutinib-treated patients. During ibrutinib-induced lymphocytosis, sCD52 levels still decreased in parallel with a decrease in overall tumor burden including lymph nodes, spleen, and bone marrow [12] (Figure 3(B)). High pretreatment sCD52 levels correlated with larger LN size (p = .04, Figure S3A) and lower absolute lymphocyte counts (ALC, p = .04, Figure S3B) as well as CD38 positivity (p = .005, Figure S3C). Patients with high sCD52 levels at baseline had a more efficient LN reduction during ibrutinib treatment (p = .009, Figure 3(C)). Even when incorporating LN size pretreatment (large LN size correlating with increased LN reduction) in a linear mixed model, pretreatment sCD52 levels retained a significant correlation with LN reduction (sCD52 p = .048, LN size p = .003). Of note, only two patients had progression on ibrutinib; both harbored del(17p), had UM-IGHV, and very low levels of sCD52 pretreatment (serum sCD52 = 0ng/mL). In vitro stimulation of primary CLL cells with anti-IgM, resembling the BCR-activated state of CLL cells in the microenvironment [13], significantly increased the level of sCD52 in the supernatants as compared to untreated cells (p = . 004, Figure 3(D)). Thus, high sCD52 levels may identify patients with more active CLL that responds well to ibrutinib; while the decrease of sCD52 levels during treatment reflects treatment efficacy with both chemo-immunotherapy and ibrutinib.
Figure 3.
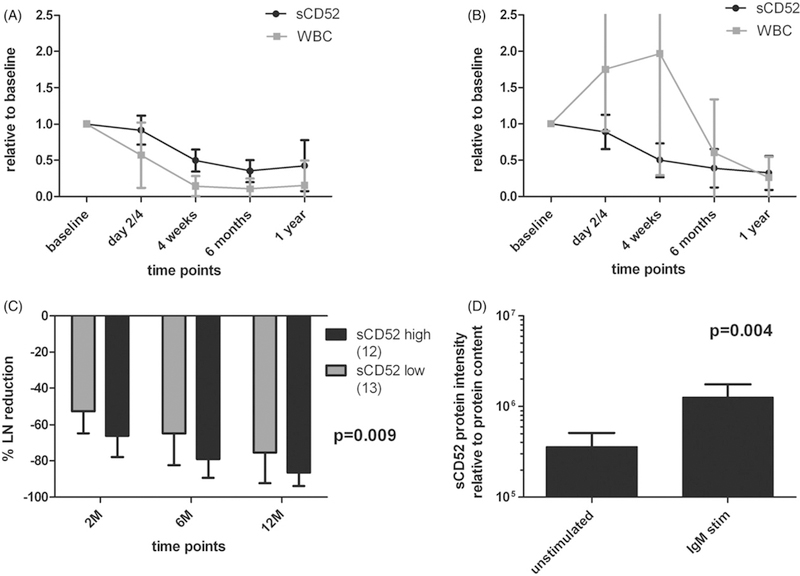
Correlation of sCD52 and WBC or lymph node reductions. (A) Before and during treatment in patients with ‘high risk’ CLL treated with chemo/chemo-immunotherapy in HOVON68 (n = 20, p < .0001). (B) In ‘ibrutinib-treated’ CLL (n = 11, p = ns). (C) Percent lymph node reduction by level of sCD52 at treatment baseline below or equal to (sCD52 low) or above (sCD52 high) sCD523 median in “ibrutinib-treated CLL” patients at 2, 6, and 12 months (n = 25). (D) Levels of sCD52 quantified by western blot in supernatants from anti-IgM bead stimulated and vehicle treated CLL PBMC (n = 4). Statistical analyses were performed by (A–C) linear mixed models and (D) paired t test, (D) data were logarithmically transformed, and (C–D) presented as normalized to pre-values and means with SD are shown. In (C) analysis was made by linear mixed models analyzing differences in lymph node reduction at all three time points in one model.
Discussion
sCD52 is a rather unnoticed tumor marker in CLL where the levels are much higher than in healthy blood donors [5,14]. Despite the recently published prognostic indices such as the CLL-IPI [15], there is still a need for biological markers of disease activity that relates to treatment effect. We here demonstrate a correlation of sCD52 levels with the aggressiveness of CLL disease and with treatment effectiveness in differing disease compartments following conventional chemo-immunotherapy as well as ibrutinib. The perspective of sCD52 as a tumor marker in the era of targeted treatment for CLL is thus broadened.
Our finding of increased sCD52 levels in ‘progressive CLL’ in need of treatment, suggest that sCD52 levels possibly reflect disease aggressiveness, progressive disease or disease burden, independently of e.g. β2m. A previous study showed the prognostic impact of sCD52 levels on overall survival in a mixed population of both treatment naïve and relapsed/refractory patients with CLL [5]. However, we found sCD52 levels also impact TTFT among unselected patients with ‘early CLL’. The same study has reported sCD52 levels to correlate with the WBC [5]. Looking at patients with either ‘early’ or ‘high-risk’ CLL separately, we found no correlation of sCD52 with WBC. However, Albitar et al. addressed a mixed population of patients with CLL; in agreement with their findings we also found a weak linear correlation with the WBC when looking at the ‘early’ and ‘high-risk’ population together. This reflects that sCD52 is a marker of progressive disease emphasizing the importance of analyzing patients at specific time points in the disease course.
The predictive impact of sCD52 levels after conventional treatment has also been investigated [6,7], however, only analyzing sCD52 levels or CD52 mRNA expression after treatment and not at treatment baseline or during treatment. By close disease monitoring during conventional as well as ibrutinib treatment, and by our in vitro experiments, we show that sCD52 is a potential marker of CLL disease activity, possibly even BCR signaling: Following conventional therapy, sCD52 levels decrease in parallel with the blood disease burden represented by the WBC, while following BCR-inhibiting treatment with ensuing lymphocytosis, it parallels the decreased activity and total decrease in LN disease burden found on ibrutinib [12]. Thus, sCD52 levels correlated with disease activity in a broader sense than just WBC, and in the particular setting of ibrutinib-induced lymphocytosis, sCD52 levels mirror the active – e.g. BCR activated – disease found in the lymph nodes. In light of this, it is not surprising that we found patients with high-sCD52 levels at baseline had the most effective lymph node reductions in contrast to the two patients who progressed on ibrutinib and had undetectable levels of sCD52 at baseline. Thus, sCD52 levels are indicative of treatment response across disease compartments, and can be used to monitor the effect of targeted treatment.
Patients with del(17p) have less effect from conventional treatment [16], but do quite well on treatment using ibrutinib [17]. Our studied cohorts were too small to allow for a reasonable analysis of the effects of del(17p). Also, patients with UM-IGHV have been shown to be more sensitive to ibrutinib both in cellular studies [18] and as a result of ibrutinib trials [19]. In the ‘ibrutinib-treated’ patients, the two patients progressing on ibrutinib both had UM-IGHV status and del(17p). Thus, if sCD52 levels reflect BCR pathway activity, then theoretically, sCD52 could predict treatment response in this subgroup of patients, but further studies are needed.
In conclusion, we confirm sCD52 as a marker for CLL with prognostic impact. We find sCD52 levels are related to CLL disease activity and possibly even BCR pathway activation. Up until now we have quantified response to conventional treatment by the IWCLL 2008 response criteria [20], updated in 2013 [21], to be able to classify patients receiving new targeted treatment. However, we are in need of better measures for treatment effect in all disease compartments as it is cumbersome and resource demanding to do both CT scans, bone marrow, and blood examinations in all patients receiving therapy. Thus, in the era of BCR-targeted therapy, sCD52 may have a new merit as a marker of disease activity.
Supplementary Material
Acknowledgements
The authors would like to thank statistician, Assoc. Prof. Lene T. Skovgaard at Copenhagen University, Prof. Niels Borregaard, and technician Charlotte Horn at the Laboratory of Granulocyte Research at Rigshospitalet, as well as the funding sources: The Danish Cancer Society, The Research Council of Rigshospitalet, The Danish Cancer Research Foundation, The Novo Nordisk Foundation, The Anders Hasselbalch Foundation, The Torben and Alice Frimodt Foundation, The Jakob Madsen and wife Olga Madsen Foundation, The Einar Willumsen Foundation, and The Eva and Henry Frænkel Foundation. Work done at NIH was supported through The Intramural Program of the National Heart Lung and Blood Institute.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at http://dx.doi.org/10.1080/10428194.2017.1285027.
References
- [1].Xia MQ, Hale G, Lifely MR, et al. Structure of the CAMPATH-1 antigen, a glycosylphosphatidylinositol-anchored glycoprotein which is an exceptionally good target for complement lysis. Biochem J 1993; 293:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Treumann A, Lifely MR, Schneider P, et al. Primary structure of CD52. J Biol Chem 1995;270:6088–6099. [DOI] [PubMed] [Google Scholar]
- [3].Klabusay M, Sukova V, Coupek P, et al. Different levels of CD52 antigen expression evaluated by quantitative fluorescence cytometry are detected on B-lymphocytes, CD34+ cells and tumor cells of patients with chronic B-cell lymphoproliferative diseases. Cytometry B 2007;72B:363–370. [DOI] [PubMed] [Google Scholar]
- [4].Bandala-Sanchez E, Zhang Y, Reinwald S, et al. T cell regulation mediated by interaction of soluble CD52 with the inhibitory receptor Siglec-10. Nat Immunol 2013;14:741–748. [DOI] [PubMed] [Google Scholar]
- [5].Albitar M, Do KA, Johnson MM, et al. Free circulating soluble CD52 as a tumor marker in chronic lymphocytic leukemia and its implication in therapy with anti-CD52 antibodies. Cancer 2004;101:999–1008. [DOI] [PubMed] [Google Scholar]
- [6].Alatrash G, Albitar M, O’Brien S, et al. Circulating CD52 and CD20 levels at end of treatment predict for progression and survival in patients with chronic lymphocytic leukaemia treated with fludarabine, cyclophosphamide and rituximab (FCR). Br J Haematol 2010;148:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bloehdorn J, Sill M, Langer C, et al. High CD52 mRNA expression is an adverse prognostic factor in fludarabine-refractory CLL treated with alemtuzumab on the Gcllsg CLL2H trial. Blood 2013;122:21 Abstract 1618. [Google Scholar]
- [8].Farooqui MZH, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol 2015;16:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014;370:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Geisler CH, van T, Veer MB, Jurlander J, et al. Frontline low-dose alemtuzumab with fludarabine and cyclophosphamide prolongs progression-free survival in high-risk CLL. Blood 2014;123:3255–3262. [DOI] [PubMed] [Google Scholar]
- [11].West B, Welch K, Galecki A. Linear mixed models: a practical guide using statistical software Boca raton (FL): Chapman and Hall/CRC Press;2008. [Google Scholar]
- [12].Herman SEM, Niemann CU, Farooqui M, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia 2014;28:2188–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011; 117:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Giles FJ, Vose JM, Do KA, et al. Circulating CD20 and CD52 in patients with non-Hodgkin’s lymphoma or Hodgkin’s disease . Br J Haematol 2003;123:850–857. [DOI] [PubMed] [Google Scholar]
- [15].Pflug N, Bahlo J, Shanafelt TD, et al. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood 2014;124:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376:1164–1174. [DOI] [PubMed] [Google Scholar]
- [17].Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2016;125:2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guo A, Lu P, Galanina N, et al. Heightened BTK-dependent cell proliferation in unmutated chronic lymphocytic leukemia confers increased sensitivity to ibrutinib. Oncotarget 2016;7:4598–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wiestner A The role of B-cell receptor inhibitors in the treatment of patients with chronic lymphocytic leukemia. Haematologica 2015;100:1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


