Sepsis—life-threatening organ dysfunction due to infection—hospitalizes an estimated 19 million patients every year, more than 5 million of whom die (1). Treatment focuses on timely antibiotic delivery, resuscitation, and source control. Yet, policies promoting early antibiotics are increasingly polarizing.
The 2016 Surviving Sepsis Campaign guidelines strongly recommend delivering antibiotics within 1 hour to all patients with sepsis (2). “Rory’s Regulations” mandate that New York State hospitals report compliance with early sepsis treatments for patients diagnosed with sepsis (3). Similarly, the Centers for Medicare & Medicaid Services’ SEP-1 performance measure requires hospitals to report compliance with an early sepsis treatment bundle for patients discharged with a diagnosis of sepsis. These initiatives have met with disparate responses—simultaneously lauded by some for reducing preventable sepsis deaths and lambasted by others as driving antibiotic overuse (4–6).
In 2018, the debate intensified. High-profile editorials have disputed the logic behind early antibiotics and raised concern about rising antimicrobial resistance and antibiotic-associated harms (7, 8). In May 2018, the Surviving Sepsis Campaign released a new 1-hour treatment bundle strengthening the recommendation that antibiotics be delivered within 60 minutes (9). Shortly thereafter, a grassroots online petition argued that the 1-hour recommendation is likely to cause “indiscriminate use of broad-spectrum antibiotics” (10). In July 2018, HospitalCompare.gov released the first public report of SEP-1 performance and revealed that less than 50% of patients with recognized sepsis received all recommended treatments within 3 hours (11).
We have observed this debate with interest as intensive care physicians and researchers and, most recently, as members of the Surviving Sepsis Campaign guideline panel and advisors to a nationwide sepsis initiative in the Veterans Affairs Healthcare System. Increasingly, we sense a disconnect between the public conversation and our notion of superb bedside care for patients with sepsis.
In contrast to some time-sensitive medical emergencies (e.g., stroke, myocardial infarction), blood work and imaging cannot reliably rule in or rule out sepsis. Sepsis diagnosis depends on clinical judgment. Sometimes it is obvious, but often, especially early in a patient’s presentation, it is unclear whether his or her illness is due to infection.
The best clinicians, we believe, simultaneously weigh the likelihood that a patient is infected, consider competing diagnoses, and assess severity of illness. Underlying such reckonings is the knowledge that the consequences of inappropriately withholding antibiotics mount for sicker patients. These subconscious calculations incorporate additional information as it becomes available, with the assessment revised over time. However, the decision to administer antibiotics must often be made when the diagnosis is still uncertain, particularly for the sickest patients.
In marked contrast to this clinical reality, sepsis performance is uniformly assessed and reported for a population knowable only in retrospect—the patients ultimately judged to have sepsis at hospital discharge. This causes substantial problems for effective audit and feedback or incentivizing clinician behavior.
To improve sepsis care, we should incorporate the following lessons learned:
-
•
Performance measures tend to encourage both appropriate and inappropriate treatment (12). Although the hazards of undertreatment in sepsis are direr for an individual patient, the opportunities for overtreatment are more numerous. Both cause harm.
-
•
The absolute benefit of treatment varies by disease severity, with sickest patients deriving the greatest benefit. For this reason, cardiovascular and diabetes guidelines stratify treatment recommendations by a patient’s risk for negative outcomes. Indeed, newer data show that the absolute benefit of earlier antibiotics is greater for sicker patients (13).
-
•
Personalized assessments of individual patients’ risks and benefits—even if imperfect—can help clinicians to tailor their care (14). Clinicians will sometimes fixate on only one side of the risk versus benefit balance, but they can be nudged toward more holistic care.
-
•
In contrast to other treatments, where risks and benefits are experienced by just the individual patient, antibiotics carry a population or societal risk of antimicrobial resistance. Indeed, recent estimates suggest that multidrug-resistant organisms contribute to 150,000 U.S. deaths annually (15), so this population risk should not be taken lightly.
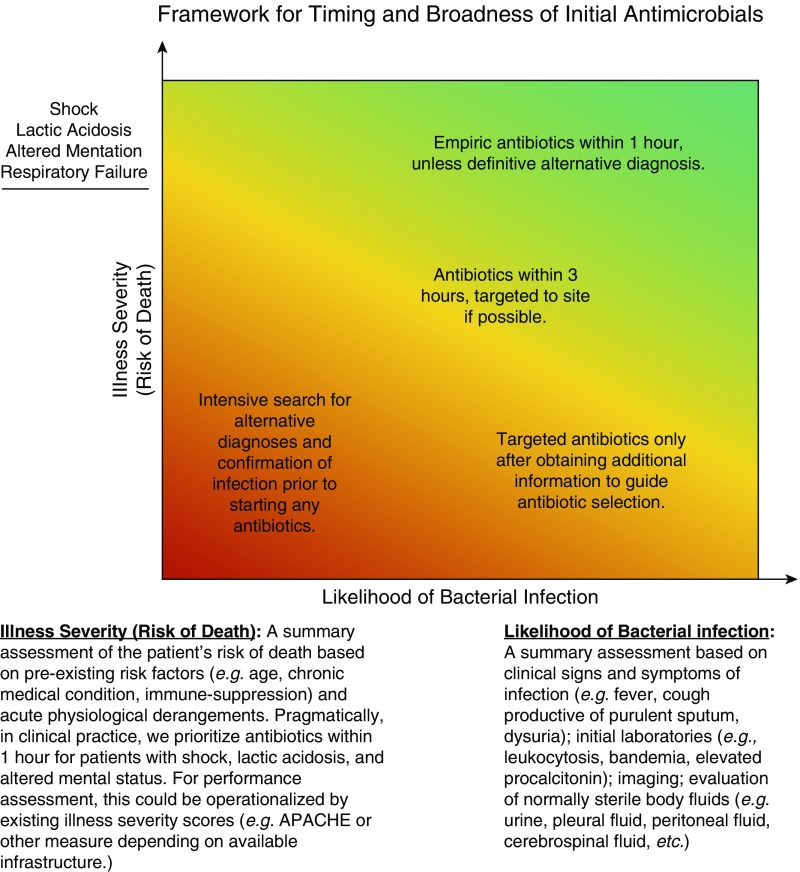
We present a broad framework to guide antibiotic prescribing for potential infection, characterizing patients across two dimensions: likelihood of infection and illness severity (Figure 1). We conceptualize illness severity as risk of death from both acute physiologic derangements and from age and chronic health conditions such as immune suppression.
Figure 1.
Framework for timing and broadness of initial antimicrobials. APACHE = Acute Physiology and Chronic Health Evaluation.
For hospitalized patients judged likely to have a bacterial infection, antibiotics are nearly always indicated. However, illness severity guides the rapidity of delivery, the broadness of the initial antibiotic regimen, and the uncertainty that one must tolerate when deciding to act. For the sickest patients, broad-spectrum antibiotics should be delivered immediately (within 1 h) and then narrowed as additional data become available. The sickest patients would include those with shock, lactic acidosis, altered mentation, or respiratory failure. For such high-risk patients, good practice involves sometimes giving antibiotics to patients later found to have a noninfectious cause of their illness.
However, for immunocompetent patients without systemic illness (e.g., with osteomyelitis), culture data should be collected first, and targeted antibiotics should be prescribed only once the infection is better characterized. Delays in antibiotics can be tolerated by such patients, and there should be a very low rate of antibiotics administered to patients who are ultimately proven to lack infection.
For patients with possible infection, additional diagnostic evaluation is necessary to make clearer the likelihood of infection and to assess for alternative diagnoses. The decision to prescribe empiric antibiotics versus awaiting results of further studies should be based on illness severity. Patients with shock, lactic acidosis, altered mentation, or respiratory failure should be given initial antibiotics on skimpier evidence—with a plan to stop them if more definitive alternative diagnostic evidence is obtained. In contrast, patients who are clinically stable (i.e., patients without shock, lactic acidosis, altered mentation, or respiratory failure) can be spared the risk of side effects until additional data to confirm the presence of infection are obtained (e.g., peritoneal fluid studies, a computed tomographic scan, a procalcitonin measurement).
When prescribing antibiotics, it is important to be clear whether they were initiated because infection is definite, infection is likely, and/or the patient is sufficiently sick that delayed treatment could have devastating consequences. These differing scenarios should imply different paths forward—monitoring for treatment response and narrowing antibiotics based on microbiological culture in definite infection versus an intensive diagnostic evaluation to confirm/refute infection and competing diagnoses when infection is unclear. In the latter group, discontinuing antibiotics is an essential part of good care once an alternative diagnosis is made.
The likelihood of infection and severity of illness occur along continuous spectra. Nonetheless, wherever boundaries are drawn, antibiotic prescribing patterns should differ across categories in meaningful and intuitive ways. In a well-functioning system, 1) the rate of antibiotic prescription should be higher in patients with greater likelihood of infection; 2) sicker patients should get more rapid and broader initial antibiotics but also should have a higher rate of early discontinuation; and 3) less ill patients should get additional diagnostic studies and, when prescribed antibiotics, should have a narrower spectrum of coverage.
This framework implies several approaches to improving care. First, a patient’s location along these axes could be defined through decision support tools that leverage clinical data accrued in real time. In the recent ProACT trial (Procalcitonin Antibiotic Consensus Trial), providing clinicians with procalcitonin assay results in isolation did not change antibiotic prescribing (16). However, integrating relevant data to provide a real-time summary assessment of infection likelihood may change physician prescribing behavior (e.g., automated calculation of an infection probability score [17], intensive care infection score [18], or similar measure).
Second, the threshold of illness severity below which it is safe to delay antibiotics for additional diagnostic studies should be empirically defined. The utility of early antibiotics for an individual patient could be estimated by weighing the absolute benefit of early antibiotics (predicted risk of death multiplied by relative risk reduction of prompter antibiotics) against the harms (rates of antibiotic-associated complications, including the incremental risk of antimicrobial resistance). By considering a range of plausible inputs, one could estimate the probable range of the net benefit of early antibiotics across patients of differing illness severities.
Third, performance measurement should align with bedside practice and support prudent decision making. We currently dichotomize patients as septic versus not septic at hospital discharge, then judge clinicians on what should have been done in retrospect. Instead, clinicians and systems should be judged on whether their responses were appropriately calibrated, given the urgency of the situation. Was the decision to prescribe antibiotics made at an appropriate interval, given the patient’s degree of illness? With the information available at the time, were the best decisions made? Did the team change their treatment plans as new data became available?
For example, healthcare systems could report rate of antibiotic delivery, median time to antibiotics, and rate of early antibiotic discontinuation for patients stratified by predicted risk of 30-day mortality (e.g., <1%, 1–10%, >10%) and a crude measure of infection likelihood (e.g., systemic inflammatory response syndrome positivity or infection probability score [17]). Even crude categorization of patients into low-, medium-, and high-risk categories has successfully improved physician behavior in other areas, such as driving selective delivery of antibleeding agents to patients at the highest risk of bleeding after heart catheterization (14) or selection of patients with atrial fibrillation for anticoagulation based on CHA2DS2-VASc (mnemonic for congestive heart failure/left ventricular dysfunction, hypertension, age ≥75 yr, diabetes mellitus, stroke/transient ischemic attack, thromboembolism, vascular disease, age 65–74 yr, sex category [i.e., female sex]) and HAS-BLED (mnemonic for hypertension, abnormal renal and liver function, stroke, bleeding, labile international normalized ratio, elderly, drugs or alcohol) scores (19, 20). Likewise, we believe that early antibiotic administration should be informed by illness severity and likelihood of infection; providing feedback to clinicians stratified by risk of death would drive clinicians to prioritize rapid delivery of antibiotics to the highest-risk patients who derive the greatest absolute benefit from early antibiotics.
Sepsis is a common and deadly condition, but diagnosis is not always knowable in real time. The optimal treatment during times of diagnostic uncertainty differs across patients. Organizing treatment recommendations and performance measurement by illness severity and likelihood of infection aligns with clinical practice and could improve sepsis care without driving inappropriate antibiotic exposure.
Supplementary Material
Footnotes
Supported by grants K08 GM115859 (H.C.P.) and K12 HL138039 (T.J.I.) from the National Institutes of Health and by grant IIR 17-219 (H.C.P.) from the U.S. Department of Veterans Affairs Health Services Research and Development Service. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the U.S. government, or the Surviving Sepsis Campaign.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 3.New York State Department of Health Sepsis regulations: guidance document 405.4(a)(4)[accessed 2017 Feb 14]. Available from: https://www.health.ny.gov/regulations/public_health_law/section/405/
- 4.Dwyer J.A boy’s life is lost to sepsis. Thousands are saved in his wake The New York Times 2017 Apr 13A18[cited 2018 Apr 21]. Available from: https://www.nytimes.com/2017/04/13/nyregion/a-boys-life-lost-to-sepsis-but-thousands-saved-in-his-wake.html
- 5.Klompas M, Rhee C. The CMS sepsis mandate: right disease, wrong measure. Ann Intern Med. 2016;165:517–518. doi: 10.7326/M16-0588. [DOI] [PubMed] [Google Scholar]
- 6.Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care—reasons for caution. N Engl J Med. 2014;370:1673–1676. doi: 10.1056/NEJMp1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer M. Antibiotics for sepsis: does each hour really count, or is it incestuous amplification? Am J Respir Crit Care Med. 2017;196:800–802. doi: 10.1164/rccm.201703-0621ED. [DOI] [PubMed] [Google Scholar]
- 8.Klompas M, Calandra T, Singer M. Antibiotics for sepsis—finding the equilibrium. JAMA. 2018;320:1433–1434. doi: 10.1001/jama.2018.12179. [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign bundle: 2018 update. Crit Care Med. 2018;46:997–1000. doi: 10.1097/CCM.0000000000003119. [DOI] [PubMed] [Google Scholar]
- 10.Aberegg S, Beck-Esmay J, Carroll S, Farkas J, Kenny JE, Koyfman A, et al. Petition to retire the Surviving Sepsis Campaign guidelines PulmCrit (EMCrit) 2018 May 2[accessed 2018 Aug 28]. Available from: https://emcrit.org/pulmcrit/ssc-petition/
- 11.U.S. Centers for Medicare & Medicaid Services Hospital compare[accessed 2018 Sep 30]. Available from: https://www.medicare.gov/hospitalcompare/search.html
- 12.Saini SD, Vijan S, Schoenfeld P, Powell AA, Moser S, Kerr EA. Role of quality measurement in inappropriate use of screening for colorectal cancer: retrospective cohort study. BMJ. 2014;348:g1247. doi: 10.1136/bmj.g1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spertus JA, Decker C, Gialde E, Jones PG, McNulty EJ, Bach R, et al. Precision medicine to improve use of bleeding avoidance strategies and reduce bleeding in patients undergoing percutaneous coronary intervention: prospective cohort study before and after implementation of personalized bleeding risks. BMJ. 2015;350:h1302. doi: 10.1136/bmj.h1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnham JP, Olsen MA, Kollef MH. Re-estimating annual deaths due to multidrug-resistant organism infections. Infect Control Hosp Epidemiol. 2019;40:112–113. doi: 10.1017/ice.2018.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang DT, Yealy DM, Filbin MR, Brown AM, Chang CH, Doi Y, et al. ProACT Investigators. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379:236–249. doi: 10.1056/NEJMoa1802670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peres Bota D, Mélot C, Lopes Ferreira F, Vincent JL. Infection Probability Score (IPS): a method to help assess the probability of infection in critically ill patients. Crit Care Med. 2003;31:2579–2584. doi: 10.1097/01.CCM.0000094223.92746.56. [DOI] [PubMed] [Google Scholar]
- 18.van der Geest PJ, Mohseni M, Linssen J, Duran S, de Jonge R, Groeneveld ABJ. The intensive care infection score - a novel marker for the prediction of infection and its severity. Crit Care. 2016;20:180. doi: 10.1186/s13054-016-1366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 20.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.