Abstract
Rationale: Survivorship from critical illness has improved; however, factors mediating the functional recovery of persons experiencing a critical illness remain incompletely understood.
Objectives: To identify groups of acute respiratory failure (ARF) survivors with similar patterns of physical function recovery after discharge and to determine the characteristics associated with group membership in each physical function trajectory group.
Methods: We performed a secondary analysis of a randomized controlled trial, using group-based trajectory modeling to identify distinct subgroups of patients with similar physical function recovery patterns after ARF. Chi-square tests and one-way analysis of variance were used to determine which variables were associated with trajectory membership. A multinomial logistic regression analysis was performed to identify variables jointly associated with trajectory group membership.
Results: A total of 260 patients enrolled in a trial evaluating standardized rehabilitation therapy in patients with ARF and discharged alive (NCT00976833) were included in this analysis. Physical function was quantified using the Short Physical Performance Battery at hospital discharge and 2, 4, and 6 months after enrollment. Latent class analysis of the Short Physical Performance Battery scores identified four trajectory groups. These groups differ in both the degree and rate of physical function recovery. A multinomial logistic regression analysis was performed using covariates that have been previously identified in the literature as influencing recovery after critical illness. By multinomial logistic regression, age (P < 0.001), female sex (P = 0.001), intensive care unit (ICU) length of stay (LOS) (P = 0.003), and continuous intravenous sedation days (P = 0.004) were the variables that jointly influenced trajectory group membership. Participants in the trajectory demonstrating most rapid and complete functional recovery consisted of younger females with fewer continuous sedation days and a shorter LOS. The participant trajectory that failed to functionally recover consisted of older patients with greater sedation time and the longest LOS.
Conclusions: We identified distinct trajectories of physical function recovery after critical illness. Age, sex, continuous sedation time, and ICU length of stay impact the trajectory of functional recovery after critical illness. Further examination of these groups may assist in clinical trial design to tailor interventions to specific subgroups.
Keywords: critical illness, ARDS, intensive care unit, mechanical ventilation, skeletal muscle
There are more than 5.7 million intensive care unit (ICU) admissions annually in the United States, with acute respiratory failure (ARF) being one of the most common conditions requiring critical care management (1). ARF is associated with prolonged functional impairment in many individuals (2–5). With advances in critical care, short-term mortality from ARF has improved (3, 6); however, recovery from critical illness is fraught with challenges. Previous work has shown that survivors of critical illness have persistent physical function impairments for months to years (7–12). For example, even 5 years after surviving severe acute respiratory distress syndrome, young, previously employed patients with few comorbidities continue to have reduced performance on the 6-minute-walk distance (4). These impairments persist despite recovery of pulmonary function (8). Despite this finding of persistent weakness in many ICU survivors, physical function outcomes are understudied in critical care trials, and factors mediating physical function recovery after critical illness remain poorly understood.
A previous study suggested that ICU survivors identify physical strength, fatigue, and decreased walking distance as their three most important outcomes after critical illness (13). Prior work has shown that age, hospital length of stay (LOS), sex, ethnicity, and prior smoking status may influence physical function recovery after critical illness (9, 12). Further improvement in our understanding of the factors that influence physical function recovery after critical illness may help inform survivors of the challenges and milestones of their recovery period.
The purpose of this study was to determine whether there were common patterns of physical function recovery over a 6-month time period after a critical illness and to evaluate patient-level characteristics associated with specific trajectory groups. We performed a secondary analysis on a cohort of critically ill patients enrolled in a clinical trial that included objective physical function assessments measured through 6 months after study enrollment.
Methods
Study Data
We conducted a secondary analysis of a randomized controlled clinical trial evaluating standardized rehabilitation therapy among patients with acute respiratory failure (14) (NCT00976833). The study protocol has been previously described. In brief, 300 previously independently ambulating patients aged 18 years or older with ARF were recruited for this single-center trial at Wake Forest Baptist Medical Center in North Carolina between October 2009 and May 2014. The patients could not have been mechanically ventilated for more than 80 hours or hospitalized for more than 7 days. The requirement for independent ambulation allowed for use of walker or cane and was reported by either patient or family member during evaluation for enrollment in the study. Patients underwent physical function testing by blinded assessors at hospital discharge and were subsequently followed for 6 months with repeat in-person objective evaluations of function performed at 2, 4, and 6 months after enrollment. The objective physical function evaluation included the Short Physical Performance Battery (SPPB).
Outcome Variable
The SPPB, an objective physical function measurement designed for low-functioning individuals, was chosen as the outcome variable for this trajectory analysis. The SPPB assesses gait speed, balance, and lower extremity strength with scores ranging from 0 to 12 (15, 16). Scores between 0 and 3 denote significant physical function disability, 4 to 6 low function, 7 to 9 intermediate function, and 10 to 12 high function (15). SPPB scores have been previously shown to be highly predictive of disability, hospitalization, institutionalization, and mortality in older patients (15–17). Although primarily used in geriatric populations, the SPPB has also been applied to other low-functioning populations, including patients with chronic obstructive pulmonary disease, human immunodeficiency virus, and chronic kidney disease (18–20).
Statistical Analysis
Group-based trajectory modeling (GBTM) was performed using the Statistical Analysis Software (SAS 9.4, Cary, NC) finite mixture model procedure PROC TRAJ to identify distinct subgroups of patients who followed similar trajectories of SPPB recovery over time (21, 22). All trajectories were modeled as a quadratic function of time from hospital discharge. SPPB was assumed to follow a censored normal distribution. Models containing two to six trajectory groups were tested. A combination of the Bayesian Information Criterion (BIC) and judgement (minimal observed group size of 5% and/or substantially different trajectories) were used to select the number of trajectory groups. All subjects included in this analysis were assigned a posterior probability of group membership by the TRAJ procedure, and these probabilities provided a specific participant’s likelihood of belonging to each of the model’s trajectory groups (22). The maximum posterior probability was used to determine the correct trajectory group for each participant.
For our data, the BIC increased by less than one point in going from four to five groups (see Table E1 in the online supplement). The posterior probabilities associated with group assignment in the four-trajectory model ranged from 0.44 to 1.0. The posterior probability of group assignment was greater than 50% for 93% of patients and greater than 75% for 72% of patients. The median posterior probability of group membership was 96%.
Chi-square tests and one-way analysis of variance were used to determine which variables had an association with trajectory group membership in the univariate analysis. A P value < 0.05 was considered statistically significant for all analyses (two-tailed). Subsequently, a multinomial logistic regression analysis was performed to identify variables jointly associated with group membership. All of the covariates included in the logistic regression model have been previously identified in the literature as clinically relevant to recovery after critical illness, suggesting that there may be a relationship to physical function recovery as well.
Addressing Missing Data
Patients known to be deceased were assigned scores of zero for all planned visits after their deaths. Some other patients were lost to follow-up, and data from these patients were missing after their last known visit. GBTM provides asymptotically unbiased estimates when these missing data are missing at random (22). We performed additional trajectory analyses after imputing SPPB values for the missing data to examine how sensitive the results were to assumptions regarding the missing data. Multiple imputation using the SAS Procedure MI was used to generate 10 datasets. Markov chain Monte Carlo methods were used initially to generate a monotone missing data pattern. Missing data at each visit were then imputed using regression methods on the data from the previous visits. GBTM was then performed on each of these datasets to determine the number of groups and the concordance between our results and those of the four-group models for each of the imputed datasets. We also imputed data using low (mean of 5), intermediate (mean of 8), and high (mean of 11) values for the missing data and repeated the GBTM analysis on these datasets, again noting the number of groups determined and the concordance with our results.
Results
Participants
Of the 300 patients who were randomized in the parent trial, 260 patients were discharged alive and had at least one SPPB data point available for the analysis. Baseline characteristics of the 260 patients are described in Table 1. The study population was predominantly non-Hispanic white with a mean (± standard deviation) age of 56.4 ± 15.3 years, slight predominance of women (53%), and a mean Acute Physiology and Chronic Health Evaluation (APACHE) III score of 72.8 ± 25.5 at the time of study enrollment. Before admission, 21% of patients required home oxygen and 8% required chronic renal replacement therapy.
Table 1.
Baseline characteristics of study participants at enrollment
| Variable | No. (%) |
|---|---|
| N | 260 |
| Female | 137 (53) |
| Race/ethnicity | |
| Non-Hispanic white | 200 (77) |
| Black/African American/Hispanic/Latino | 60 (23) |
| Home oxygen | 55 (21) |
| Prehospital dialysis | 20 (8) |
| Mean (SD) | |
| Age, yr | 56.4 (15.3) |
| APACHE III score | 72.8 (25.5) |
| Mean arterial blood pressure, mm Hg | 76.8 (22.9) |
| PaCO2, mm Hg | 44.6 (16.9) |
| PaO2:FiO2 | 182.8 (84.2) |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; FiO2 = fraction of inspired oxygen; PaCO2 = arterial carbon dioxide pressure; PaO2 = arterial oxygen tension pressure; SD = standard deviation.
Description of Trajectories
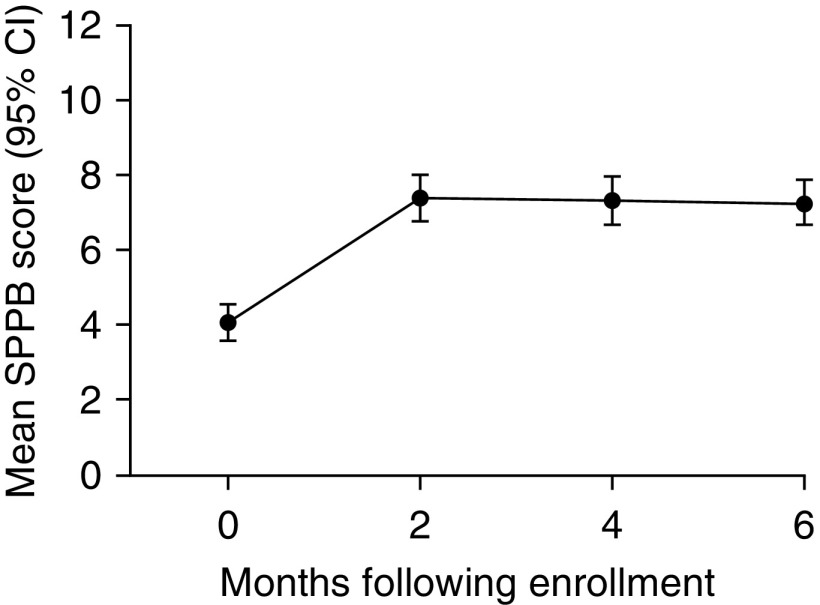
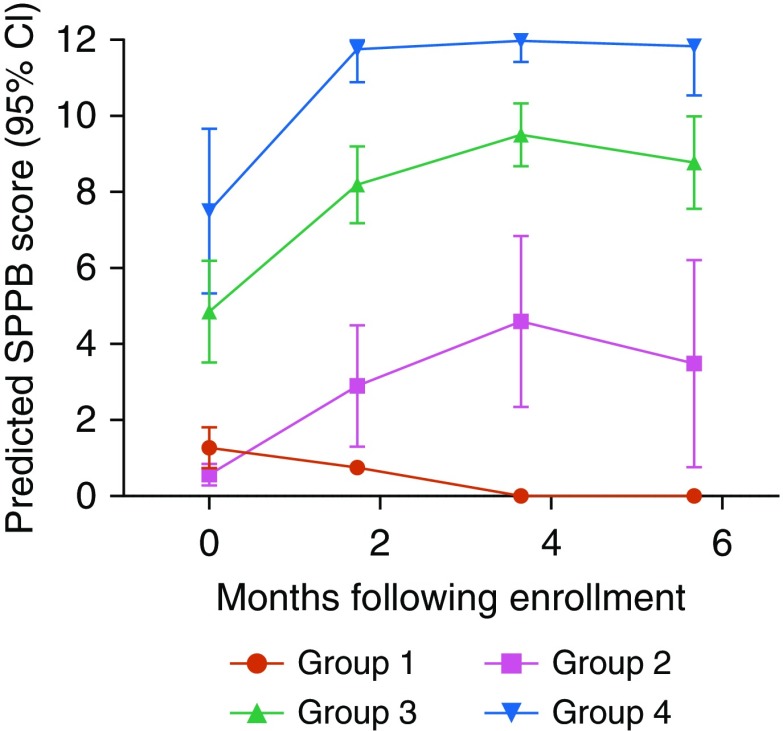
The mean SPPB score (±95% confidence interval) over the 6 months after critical illness of the study population is shown in Figure 1. The mean SPPB score at hospital discharge was in the “low functioning” category and improved by 2 months to “intermediate function,” where it remained through 6 months (Figure 1). Using the PROC TRAJ procedure on the SPPB scores, four distinct trajectory groups emerged to characterize physical function recovery in the 6 months after hospital discharge (Figure 2). The four-trajectory quadratic model was chosen as the best fit on the basis of BIC values and observation of distinctive trajectories.
Figure 1.
Mean Short Physical Performance Battery (SPPB) Score during 6-month follow-up period. SPPB evaluates gait speed, balance, and lower extremity strength, with scores ranging from 0 to 12. Scores of 0 to 3 denote physical function disability, 4 to 6 low physical function, 7 to 9 intermediate physical function, and 10 to 12 high physical function. CI = confidence interval.
Figure 2.
Trajectories of physical function recovery based on Short Physical Performance Battery (SPPB) scores over 6 months after critical illness. Four distinct recovery trajectories are identified. The rate and degree of physical function recovery is highly variable in the first 6 months after critical illness. CI = confidence interval.
As seen in Figure 2, SPPB scores are highly variable at the time of hospital discharge in patients recovering from critical illness. Recovery trajectories differ in both the degree and rate of physical function recovery in these groups. Group 1 consisted of patients who were discharged with physical function disability that did not improve by 6 months. Group 2 was discharged with physical function disability and showed minimal improvement initially but remained functionally disabled by 6 months. Patients in Group 3 had low physical function at discharge and improved to intermediate physical function. Group 4 had intermediate physical function at discharge with rapid improvement to high physical function by 2 months, which was sustained at 6 months. The greatest change in physical function appears to occur in the first 2 months after discharge.
Associations with Trajectory Group Membership
Table 2 shows the results of univariate analyses using chi-square tests and one-way analysis of variance. It describes the characteristics of the patients in each trajectory group. The multinomial logistic regression model consisted of variables that have been suggested in prior literature to influence recovery after critical illness and were therefore of interest in the investigation of physical function recovery in this population (Table 2). We excluded variables that were highly correlated with another variable of interest. For example, ICU LOS was highly correlated with ventilator days and hospital LOS. All three have been previously implicated in recovery after critical illness. We included only ICU LOS in the final model. On the basis of multinomial nominal regression, age (P < 0.001), female sex (P = 0.001), ICU LOS (P = 0.003), and continuous intravenous sedation days (P = 0.004) were the variables that jointly influence trajectory group membership.
Table 2.
Univariate and multinomial analysis to determine characteristics associated with trajectory group membership
| Characteristic | Group 1 (n = 38) | Group 2 (n = 48) | Group 3 (n = 131) | Group 4 (n = 43) | Univariate P Value | Multinomial P Value |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||
| Female sex | 23 (61) | 12 (25) | 72 (55) | 30 (70) | <0.001 | 0.001 |
| Non-Hispanic white | 35 (92) | 36 (75) | 97 (74) | 32 (74) | 0.122 | — |
| Prehospital dialysis | 2 (5) | 4 (8) | 11 (8) | 3 (7) | 0.970 | — |
| Home NIPPV | 4 (11) | 2 (4) | 13 (10) | 1 (2) | 0.269 | — |
| Received standardized rehabilitation therapy | 14 (37) | 21 (44) | 70 (53) | 24 (56) | 0.207 | 0.428 |
| Home oxygen | 11 (29) | 12 (25) | 30 (23) | 2 (5) | 0.028 | 0.467 |
| |
Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
|
|
| Age | 63.3 (11.6) | 65.0 (12.4) | 56.0 (13.7) | 42.4 (15.3) | <0.001 | <0.001 |
| PaO2:FiO2* | 175.3 (81.0) | 172.7 (93.6) | 188.4 (82.8) | 183.9 (81.4) | 0.370 | — |
| APACHE III* | 78.8 (23.8) | 79.4 (22.4) | 69.8 (24.6) | 69.4 (30.8) | 0.015 | 0.092 |
| Hospital LOS (d) | 21.5 (19.1) | 16.1 (23.4) | 11.7 (8.4) | 8.8 (5.1) | <0.001 | — |
| ICU LOS (d) | 11.2 (11.1) | 6.8 (5.9) | 6.1 (5.4) | 4.9 (3.4) | 0.004 | 0.003 |
| Ventilator days | 9.5 (12.2) | 5.4 (5.7) | 4.8 (5.9) | 3.8 (3.0) | 0.066 | — |
| Restraint days | 5.6 (8.9) | 3.0 (5.2) | 2.2 (3.4) | 2.0 (2.6) | 0.145 | 0.422 |
| Continuous IV sedation days | 4.3 (5.3) | 2.2 (2.8) | 3.0 (4.1) | 3.0 (2.6) | 0.086 | 0.004 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; FiO2 = fraction of inspired oxygen; LOS = length of stay; ICU = intensive care unit; IV = intravenous; NIPPV = noninvasive positive pressure ventilation; PaO2 = arterial oxygen tension pressure; SD = standard deviation.
Measured at enrollment.
Missing Data
After assigning SPPB scores of 0 for patients known to be deceased at a follow-up time point, 19% of possible SPPB values were missing. Of the 10 datasets generated using multiple imputation, 8 resulted in four-group solutions, 1 resulted in a five-group solution, and 1 resulted in a six-group solution. Using the four-group solution for each analysis, the group assignment average concordance with our results was 89%. The datasets generated by assigning low, intermediate, and high SPPB scores to missing values all resulted in four-group solutions and showed concordance of 82% to 85% with our results.
Discussion
In critical illness research, the role of patient heterogeneity remains underappreciated. The diagnosis of acute respiratory failure encompasses a heterogeneous group of patients, and patients differ in their trajectory of physical function recovery after critical illness. Trajectory analysis is one method of discriminating subpopulations of patients who behave or respond similarly. In this analysis, we identify four patient subgroups that demonstrate differing patterns of physical function recovery in the 6 months after hospital discharge after ARF. Both the rate and degree of physical function recovery are highly variable and differ based on trajectory group membership. Group 4, with the highest physical function, consists primarily of younger women with less continuous sedation time and shorter ICU LOS. In contrast, Group 1, with persistent physical function disability, consists primarily of older patients with longer sedation time and longer ICU LOS. Trajectory analysis discriminates these distinct subgroups of patients with physical function outcomes that are not captured by a simple analysis of the means. The variables that influence trajectory group membership are age, sex, intravenous sedation time, and ICU LOS. Patients value physical function, and functional independence is of great importance to their well-being (23). Understanding the role of the aforementioned variables in influencing recovery trajectories may help identify patients who are at greater risk for physical function disability after critical illness.
Age has been frequently associated with increased risk of acquired disability and mortality after critical illness (9, 24, 25); however, there is a paucity of data evaluating long-term physical function outcomes in older patients. It should be noted that age alone, though, is not a determinant of physical function outcome after critical illness. For example, in our analysis, Group 4 had rapid recovery from intermediate physical function disability at the time of hospital discharge to high physical function by 2 months. Half of the patients in this group are older than 45 years of age, and the others are younger. The oldest patient in this group is 71 years. Although age range also varies in group 1, this group contains no patients younger than age 40 years. One recent study of adult ICU survivors who required at least 48 hours of mechanical ventilation found that the number of preexisting comorbidities was associated with health-related quality of life and physical symptoms in the first year of recovery after critical illness (26). Older patients who exhibit improved functional recovery may reflect those who were less frail and had fewer comorbidities before their critical illness. There are limited data available about prehospital functional status in our patient cohort. APACHE III score was included as a surrogate marker for prehospital illness. In our analysis, prehospital oxygen use, dialysis, or APACHE III score were not associated with recovery trajectory.
Several studies have shown that, at least in the United States, mortality from critical illness is higher in female than male patients and that this occurs despite male patients being more likely to be admitted to the ICU and receiving more aggressive interventions (27–33). These results contradict rodent studies, which consistently show a survival advantage for females after polymicrobial sepsis (34, 35). In addition, female sex has been previously identified as a possible risk factor for ICU-acquired weakness (36, 37). By contrast, in our study female sex has an advantage for long-term physical function recovery. Explanations for these findings may be related to hormone differences. One prior study of elderly patients with infection-associated critical illness showed that mortality was not dependent on sex but correlated with elevated levels of 17B-estradiol in male and female subjects, elevated progesterone in men, and elevated testosterone in women (38). In addition, one study that also stratified patients by age demonstrated a survival advantage for women younger than 50 years of age (28). Additional studies of long-term physical function after critical illness are needed to better understand the sex-related differences in physical function recovery and the underlying factors that mediate these differences.
Longer ICU LOS is associated with reduced physical function recovery in our analysis. No patients in the group that demonstrated high physical function throughout the study period (Group 4) had a LOS longer than 15 days. Longer ICU LOS has been previously associated with higher disability at 1 year after critical illness (9). In contrast to our analysis, a recent secondary analysis of physical function recovery after ARF found that although patients with prolonged hospital course after ARF had worse physical function at 6 months than those discharged home, the rate of physical function improvement was similar between the two groups (39). Interestingly, in our analysis, physical function shows a slight decline in Groups 2 and 3 at 6 months. Iwashyna and colleagues have previously shown that in patients beyond Day 10 of ICU stay, admission diagnosis and severity are no longer more predictive of outcome than antecedent patient characteristics (40). A recent secondary analysis has also shown prehospitalization comorbidity counts to be the strongest predictor of health-related quality of life in the year after critical illness (26). Furthermore, pre-ICU impairments in hearing and vision among older adults have been associated with poor physical function recovery at 6 months (41). Whether this influence of pre–critical illness variables on LOS and their role in physical function recovery can be mitigated by targeted interventions during ICU care remains to be further elucidated.
The only modifiable factor shown to influence physical function recovery trajectory in our analysis was time receiving continuous intravenous sedation. In this population, intravenous sedation days were defined as any part of a day with continuous intravenous delivery of morphine, fentanyl, midazolam, lorazepam, propofol, or dexmedetomidine. Multiple prior studies have shown that continuous intravenous sedation and depth of sedation have a negative impact on outcomes in critically ill patients (42–44). Again, there is variability in continuous intravenous sedation time in each recovery group, as it is the interplay of these four variables and not just one that influences physical function recovery. Our data support the need to minimize continuous sedation time in critically ill patients.
Randomization to standardized rehabilitation therapy or usual care was not associated with trajectory group membership; however, the prespecified secondary analysis of the parent study did show improvement in physical function outcomes, including SPPB (14). Multiple reasons may explain this finding. Our analysis was not designed to assess the impact of standardized rehabilitation therapy on physical function recovery trajectory. Prospective studies designed for enrichment of different trajectory groups would be needed to adequately assess for the effect of rehabilitation therapy.
There are limitations to our study. One limitation is missing SPPB data due to death and missingness. To address this, multiple sensitivity analyses were performed, as described in Methods. Despite this missingness, the concordance of 82% to 89% between our model with missing data and those with imputations suggests that the four-group solution is fairly robust. As with many critical illness studies, this sample is a single-center population in the United States and is predominantly white, which may limit the generalizability of results. Another limitation is that prehospitalization functional status is not well defined for this population and may play an important role in physical function outcomes. Furthermore, long-term outcome may be significantly impacted by ICU organ failures (45), which was not assessed by this study. In addition, the follow-up time is only 6 months, and patients had a maximum of four measurements, limiting the complexity of the regression models.
There are only a few prior studies that aim to identify functional trajectories of recovery after critical illness. Herridge and colleagues evaluate a population of critically ill patients requiring mechanical ventilation for at least 7 days, whereas the course of ARF in many of our patients was considerably shorter (9). Our patient cohort is also unique from both studies by Ferrante and colleagues, which use the Precipitating Events Cohort, with patients older than 70 years of age and many with high levels of preexisting disability (41, 46). We demonstrate that even in a relatively younger population that was independent in ambulation before critical illness, recovery after ARF has very high interindividual variability. This information contributes to the field, informing the need to account for patient heterogeneity in critical care trials of physical function recovery, as patients in these recovery groups may respond differently to interventions aimed at improving physical function recovery after critical illness
Patients have distinct physical function recovery trajectories after critical illness. Physical function at hospital discharge as well as the rate and degree of physical function recovery in the subsequent 6 months are highly variable. Age, sex, continuous intravenous sedation time, and ICU length of stay jointly influence the trajectory of physical function recovery after critical illness from acute respiratory failure. Further examination of these groups may assist in clinical trial design to tailor interventions to specific subgroups and may also inform prognosis.
Supplementary Material
Footnotes
Supported by the National Institutes of Health, National Institute of Nursing Research, and National Heart, Lung, and Blood Institute.
Author Contributions: Study concept and design: S.G., J.L., D.C., D.C.F., and P.E.M.; acquisition, analysis, or interpretation of data: all authors; drafting of the manuscript: S.G., J.L., and D.C.F.; critical revision of the manuscript for important intellectual content: S.G., D.C., R.N.B., K.G., M.B., D.C.F., and P.E.M.; statistical analysis: S.G., J.L., and D.C.; study supervision: D.C.F. and P.E.M.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Barrett ML, Smith MW, Elixhauser A, Honigman LS, Pines JM. Utilization of intensive care services, 2011. Agency for Healthcare Research and Quality: statistical briefs #185. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 2.Wang CY, Calfee CS, Paul DW, Janz DR, May AK, Zhuo H, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med. 2014;40:388–396. doi: 10.1007/s00134-013-3186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 4.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 5.Herridge MS, Batt J, Santos CD. ICU-acquired weakness, morbidity, and death. Am J Respir Crit Care Med. 2014;190:360–362. doi: 10.1164/rccm.201407-1263ED. [DOI] [PubMed] [Google Scholar]
- 6.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD NIH NHLBI ARDS Network. Recent trends in acute lung injury mortality: 1996-2005. Crit Care Med. 2009;37:1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 8.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42:849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herridge MS, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, et al. RECOVER Program Investigators (Phase 1: towards RECOVER); Canadian Critical Care Trials Group. The RECOVER program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med. 2016;194:831–844. doi: 10.1164/rccm.201512-2343OC. [DOI] [PubMed] [Google Scholar]
- 10.Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, et al. NIH NHLBI ARDS Network. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188:567–576. doi: 10.1164/rccm.201304-0651OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland A, Dawson NV, Altmann I, Thomas CL, Phillips RS, Tsevat J, et al. SUPPORT Investigators. Outcomes up to 5 years after severe, acute respiratory failure. Chest. 2004;126:1897–1904. doi: 10.1378/chest.126.6.1897. [DOI] [PubMed] [Google Scholar]
- 12.Brown SM, Wilson EL, Presson AP, Dinglas VD, Greene T, Hopkins RO, et al. Understanding patient outcomes after acute respiratory distress syndrome: identifying subtypes of physical, cognitive and mental health outcomes. Thorax. 2017;72:1094–1103. doi: 10.1136/thoraxjnl-2017-210337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nedergaard HK, Haberlandt T, Reichmann PD, Toft P, Jensen HI. Patients’ opinions on outcomes following critical illness. Acta Anaesthesiol Scand. 2018;62:531–539. doi: 10.1111/aas.13058. [DOI] [PubMed] [Google Scholar]
- 14.Morris PE, Berry MJ, Files DC, Thompson JC, Hauser J, Flores L, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA. 2016;315:2694–2702. doi: 10.1001/jama.2016.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 18.Walker SR, Brar R, Eng F, Komenda P, Rigatto C, Prasad B, et al. Frailty and physical function in chronic kidney disease: the CanFIT study. Can J Kidney Health Dis. 2015;2:32. doi: 10.1186/s40697-015-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene M, Covinsky K, Astemborski J, Piggott DA, Brown T, Leng S, et al. The relationship of physical performance with HIV disease and mortality. AIDS. 2014;28:2711–2719. doi: 10.1097/QAD.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernabeu-Mora R, Medina-Mirapeix F, Llamazares-Herrán E, García-Guillamón G, Giménez-Giménez LM, Sánchez-Nieto JM. The Short Physical Performance Battery is a discriminative tool for identifying patients with COPD at risk of disability. Int J Chron Obstruct Pulmon Dis. 2015;10:2619–2626. doi: 10.2147/COPD.S94377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- 22.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 23.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 24.Heyland DK, Garland A, Bagshaw SM, Cook D, Rockwood K, Stelfox HT, et al. Recovery after critical illness in patients aged 80 years or older: a multi-center prospective observational cohort study. Intensive Care Med. 2015;41:1911–1920. doi: 10.1007/s00134-015-4028-2. [DOI] [PubMed] [Google Scholar]
- 25.Combes A, Costa MA, Trouillet JL, Baudot J, Mokhtari M, Gibert C, et al. Morbidity, mortality, and quality-of-life outcomes of patients requiring >or=14 days of mechanical ventilation. Crit Care Med. 2003;31:1373–1381. doi: 10.1097/01.CCM.0000065188.87029.C3. [DOI] [PubMed] [Google Scholar]
- 26.Griffith DM, Salisbury LG, Lee RJ, Lone N, Merriweather JL, Walsh TS RECOVER Investigators. Determinants of health-related quality of life after ICU: importance of patient demographics, previous comorbidity, and severity of illness. Crit Care Med. 2018;46:594–601. doi: 10.1097/CCM.0000000000002952. [DOI] [PubMed] [Google Scholar]
- 27.Lipes J, Mardini L, Jayaraman D. Sex and mortality of hospitalized adults after admission to an intensive care unit. Am J Crit Care. 2013;22:314–319. doi: 10.4037/ajcc2013225. [DOI] [PubMed] [Google Scholar]
- 28.Mahmood K, Eldeirawi K, Wahidi MM. Association of gender with outcomes in critically ill patients. Crit Care. 2012;16:R92. doi: 10.1186/CC11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nachtigall I, Tafelski S, Rothbart A, Kaufner L, Schmidt M, Tamarkin A, et al. Gender-related outcome difference is related to course of sepsis on mixed ICUs: a prospective, observational clinical study. Crit Care. 2011;15:R151. doi: 10.1186/cc10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg. 1999;134:1342–1347. doi: 10.1001/archsurg.134.12.1342. [DOI] [PubMed] [Google Scholar]
- 31.Pietropaoli AP, Glance LG, Oakes D, Fisher SG. Gender differences in mortality in patients with severe sepsis or septic shock. Gend Med. 2010;7:422–437. doi: 10.1016/j.genm.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kollef MH, O’Brien JD, Silver P. The impact of gender on outcome from mechanical ventilation. Chest. 1997;111:434–441. doi: 10.1378/chest.111.2.434. [DOI] [PubMed] [Google Scholar]
- 33.Combes A, Luyt CE, Trouillet JL, Nieszkowska A, Chastre J. Gender impact on the outcomes of critically ill patients with nosocomial infections. Crit Care Med. 2009;37:2506–2511. doi: 10.1097/CCM.0b013e3181a569df. [DOI] [PubMed] [Google Scholar]
- 34.Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25:106–110. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Diodato MD, Knöferl MW, Schwacha MG, Bland KI, Chaudry IH. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine. 2001;14:162–169. doi: 10.1006/cyto.2001.0861. [DOI] [PubMed] [Google Scholar]
- 36.De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30:1117–1121. doi: 10.1007/s00134-004-2174-z. [DOI] [PubMed] [Google Scholar]
- 37.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, et al. Groupe de Réflexion et d’Etude des Neuromyopathies en Réanimation. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 38.Angstwurm MW, Gaertner R, Schopohl J. Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit Care Med. 2005;33:2786–2793. doi: 10.1097/01.ccm.0000190242.24410.17. [DOI] [PubMed] [Google Scholar]
- 39.Neumeier A, Nordon-Craft A, Malone D, Schenkman M, Clark B, Moss M. Prolonged acute care and post-acute care admission and recovery of physical function in survivors of acute respiratory failure: a secondary analysis of a randomized controlled trial. Crit Care. 2017;21:190. doi: 10.1186/s13054-017-1791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwashyna TJ, Hodgson CL, Pilcher D, Bailey M, van Lint A, Chavan S, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016;4:566–573. doi: 10.1016/S2213-2600(16)30098-4. [DOI] [PubMed] [Google Scholar]
- 41.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors associated with functional recovery among older intensive care unit survivors. Am J Respir Crit Care Med. 2016;194:299–307. doi: 10.1164/rccm.201506-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 43.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168:1457–1461. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 44.Shehabi Y, Bellomo R, Kadiman S, Ti LK, Howe B, Reade MC, et al. Sedation Practice in Intensive Care Evaluation (SPICE) Study Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality: a multinational prospective longitudinal cohort study. Crit Care Med. 2018;46:850–859. doi: 10.1097/CCM.0000000000003071. [DOI] [PubMed] [Google Scholar]
- 45.Lone NI, Walsh TS. Impact of intensive care unit organ failures on mortality during the five years after a critical illness. Am J Respir Crit Care Med. 2012;186:640–647. doi: 10.1164/rccm.201201-0059OC. [DOI] [PubMed] [Google Scholar]
- 46.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175:523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.