Abstract
Rationale: Mechanically ventilated patients require complex care and are at high risk for rehospitalization, but different systems of care may result in different hospital discharge practices and rates of rehospitalization.
Objectives: To compare lengths of hospitalization, discharge patterns, and rehospitalization rates in New York in the United States and Ontario in Canada.
Methods: We conducted a retrospective cohort study of mechanically ventilated patients who survived an acute care hospitalization in New York or Ontario from 2010 to 2012, using linkable administrative healthcare data.
Results: The primary outcome was the cumulative incidence of first rehospitalization within 30 days of discharge, accounting for the competing risk of death. Of 71,063 mechanically ventilated patients in New York, and 41,875 in Ontario who survived to hospital discharge, median length of initial hospital stay was similar in New York and Ontario (15 d, interquartile range = 8–28 vs. 16 d [9–30]), but was systematically shorter in New York when stratified by patient subgroups of different illness severity. Fewer patients in New York were discharged directly home (53.6% vs. 71.4%). Of patients in New York, 15,527 (cumulative incidence 21.9%) had a first rehospitalization within 30 days versus 5,580 (cumulative incidence 13.3%) in Ontario (P < 0.001). Incidence of rehospitalization was higher in New York across all subgroups assessed, with the greatest differences among patients with a tracheostomy (29.8% vs. 13.3%, P < 0.001), those who received dialysis during the hospitalization (31.9% vs. 17.4%, P < 0.001), and for patients not discharged directly home (27.6% vs. 13.3%, P < 0.001).
Conclusions: Care patterns for mechanically ventilated patients in New York and Ontario are very different; mechanically ventilated patients who survive to hospital discharge in New York have shorter hospital stays, with higher rehospitalization rates within 30 days compared with Ontario.
Keywords: mechanical ventilation, intensive care unit, patient readmission, length of stay
In the United States, preventing the need for rehospitalization is a focus of quality improvement initiatives, now including payment penalties from the Centers for Medicare and Medicaid Services (1). Current emphasis in the United States is on preventing rehospitalization for patients with congestive heart failure, acute myocardial infarction, and pneumonia. However, recent studies have assessed rehospitalization rates for a wide range of patients, including those who are critically ill (2). Within the critically ill population, patients who are mechanically ventilated typically have long initial hospitalizations (3, 4), receive complex care while in the intensive care unit (ICU), have significant post-ICU morbidity, and are at higher risk of rehospitalization (2).
One large difference between the United States and most other countries is that patients in the ICU in U.S. hospitals have relatively short hospital lengths of stay (5, 6). The U.S. practice of discharge to skilled-care facilities after hospitalization for a critical illness may be one cause of this shorter length of stay (6–9). It is unclear whether such short hospital lengths of stay occur among mechanically ventilated patients or are associated with higher rates of rehospitalization. We hypothesized that hospital lengths of stay would be shorter, and rehospitalizations more frequent in the United States among patients who received mechanical ventilation. To better understand similarities and differences in systems of care for these medically complex patients, we compared hospital lengths of stay, discharge destinations, rehospitalization rates, and outcomes for mechanically ventilated patients in New York and Ontario. To improve comparability of patient populations, we also sought to stratify patients into more homogenous groups in terms of risk of death and rehospitalization, such as those with longer duration of mechanical ventilation, or who received dialysis during the hospitalization.
Methods
Patients and Data Collection
The study protocol was reviewed and approved by the Institutional Review Board of Columbia University Medical Center (IRB-AAAJ2158; New York, New York) and the Research Ethics Board of Sunnybrook Health Sciences Center. The need for written informed consent was waived. Data for New York came from the New York Statewide Planning and Research Cooperative System for the years 2010–2012. These data capture all hospital discharges occurring within New York State, and have been used extensively for research purposes (2, 10). Within the database, each patient has a unique, encrypted identifier, allowing for linkage of hospitalizations over time. Data from the Statewide Planning and Research Cooperative System were also linked to N.Y. State Vital Records and New York City Vital Records to obtain 6-month mortality data for all patients. Data on hospitalizations in Ontario were obtained from the Canadian Institute for Health Information Discharge Abstract Database, a population-based repository of admissions to all acute care hospitals in the province. Other data sources included the Ontario Health Insurance Plan, which includes physician service billing claims and the Registered Persons Database, which provides information on demographics (age, sex, postal code) and vital status, including death date. These datasets were linked using unique, encoded identifiers and analyzed at ICES. We chose to terminate the data analysis at the end of 2012, because policy changes that instituted penalties for U.S. hospitals with high rehospitalization rates (for three specific diagnoses) may have affected rehospitalization rates after this date (1, 11).
The cohort consisted of all patients who were discharged alive after an acute care hospitalization that included an admission to an ICU (defined by ICU bed utilization billing codes in New York and special care unit codes in Ontario) and who received mechanical ventilation, defined in New York based on International Classification of Diseases (ICD)-9–Clinical Modification (CM) billing codes for invasive mechanical ventilation (96.70, 96.71, 96.72), and in Ontario based on the Canadian Classification of Health Interventions (CCI) codes (1.GZ.31.CA-ND, 1.GZ.31.CA-EP, 1.GZ.31.CR-ND, 1.GZ.31.GP-ND) (12, 13) (see Table E1 in the online supplement for details of definitions). Both sets of codes (ICD-9-CM and CCI codes) have been validated, with similarly high specificity in both countries, but lower sensitivity in the United States (12–15). We included patients who may have received noninvasive ventilation in addition to invasive mechanical ventilation at some point during the hospitalization. We excluded patients missing hospital admission or discharge dates or time to death, patients under 18 years of age, and patients whose primary residence was outside of New York or Ontario, as we did not have information regarding deaths occurring outside the state/province. We also excluded patients with human immunodeficiency virus or who had an aborted pregnancy, as these data are withheld in New York. For patients who were transferred to another acute care hospital in either database, we combined these events into a single hospitalization.
Statistical Analysis
The primary outcome was the cumulative incidence of first rehospitalization within 30 days of hospital discharge, with death modeled as a competing risk. We also assessed the percentage of rehospitalizations within 30 days that also included an ICU stay, and the percentage of rehospitalizations that resulted in death in the hospital. Finally, for each cohort, we examined hospital-free days to Day 60 and overall mortality at 30 and 180 days after hospital discharge. Hospital-free days, defined as the sum of the number of days an individual was alive and not in an acute care hospital from the date of the index admission, was calculated as 60 minus the number of days spent in a hospital. Individuals who died before Day 60 were assigned 0 hospital-free days. We included both planned and unplanned rehospitalizations, as we did not have a clear way to identify planned rehospitalizations in the Ontario data.
We summarized demographic and clinical characteristics for patients who were or were not rehospitalized within 30 days, including age, sex, quintile of income by zip code or postal code, receipt of dialysis during the index hospitalization (stratified by whether patients had new renal failure requiring dialysis, or a previous diagnosis of end-stage renal disease (ESRD) with dialysis), Charlson-Deyo comorbidity index score (16), hospital length of stay, discharge destination (home, home with care, other), and reason for rehospitalization. New York data were stratified into income quintiles by year based on the Census Fact Finder. For Ontario, income quintiles were determined by linking patient residential postal codes to Canadian census data. Due to data usage agreements that did not allow movement of data outside of each country, we did not combine datasets for statistical testing. For comparison of characteristics between New York and Ontario cohorts, we reported standardized mean differences. For outcomes, we used chi-square tests and t tests, as appropriate, on the aggregated results. However, given the size of the datasets, even small differences were statistically significant; thus, we focused on clinical interpretation of differences.
As mortality after hospital discharge precludes a subsequent rehospitalization, the Kaplan-Meier method would overestimate the rate of rehospitalizations (17). Consequently, we modeled death as a competing risk (per Fine and Gray [18]), and determined the cumulative incidence of a first rehospitalization within 30 days of hospital discharge for all survivors of critical illness who received mechanical ventilation. We chose to restrict to those mechanically ventilated to reduce heterogeneity in severity of illness in comparisons across countries. We a priori specified some subgroups for comparison of rehospitalization rates. These included patients stratified by: sex; receipt of mechanical ventilation for more or less than 96 hours; those with a new tracheostomy (received during the hospitalization); dialysis during hospitalization; Charlson comorbidity index (0; 1–2; ≥3); and discharge destination (home, home with health services, and other destination). We post hoc assessed more specific subgroups of patients who we believed were more likely to be similar with regard to severity of illness (to reduce heterogeneity of comparisons), and who have been previously shown, using New York State data, to have a high risk of rehospitalization within 30 days (2). We also examined index hospitalization length of stay for all subgroups. We report both means (SD) and medians (interquartile range), with statistical testing (t test) of the means.
Risk factors for rehospitalization within 30 days were examined using competing risk proportional hazards regression analysis. We calculated adjusted hazard ratios and 95% confidence intervals. To improve the comparability of the models, candidate predictor variables were selected based on clinical relevance and commonality between the datasets in New York and Ontario, and included baseline and clinical information during the index admission. We assessed the proportional hazards assumption for individual covariates using Schoenfeld-like residual plots, and by modeling all covariates as potentially time varying; those with significant interactions with time were modeled as time varying. Multicollinearity among covariates was assessed using the variance inflation factor and tolerance values. The final models included age, sex (modeled as time varying in New York), quintiles of household income (modeled as time varying in Ontario), presence of tracheostomy, receipt of dialysis during the hospitalization (as identified by ICD-9 procedure code 39.95 [New York] and specific physician billing codes [G and R] or CCI codes [1PZ21HQBR or 1PZ21HPD4; Ontario]), length of stay of the index hospitalization (1–6, 7–13, 14–20, 21+ days), number of comorbidities (modeled as time varying in Ontario), and discharge destination (modeled as time varying in Ontario and New York). Database management and statistical analysis were performed using SAS 9.4 and SAS Enterprise Guide 7.15 (SAS institute) and Stata 13.1 (StataCorp LP).
Results
Characteristics of Mechanically Ventilated Patients in New York versus Ontario
The cohorts consisted of 71,063 patients in New York and 41,875 patients in Ontario who received mechanical ventilation and survived to hospital discharge (Table 1). Patients were of similar age in both locations (mean = 61.8 ± 18.3 yr in New York vs. 61.3 ± 16.6 yr in Ontario), with a lower percentage of men in New York (54.0% vs. 60.9%). More patients in New York were mechanically ventilated for 96 hours or longer (27.1% vs. 19.1%), and more received a tracheostomy (14.4% vs. 9.3%). Overall rates of dialysis were similar, although more patients in New York had ESRD requiring dialysis (3.6% vs. 0.9%), as was the frequency of comorbidities (see Table E2 for full distribution of comorbidities; see Table E3 for distribution of insurance and race in New York).
Table 1.
Characteristics of mechanically ventilated patients discharged alive from acute care hospitals
| Characteristic | Entire Cohort |
Standardized Mean Difference | |
|---|---|---|---|
| New York |
Ontario |
||
| (n = 71,063) | (n = 41,875) | ||
| Age, mean (SD), yr | 61.8 (18.3) | 61.3 (16.6) | 0.03 |
| Male, n (%)* | 38,369 (54.0) | 25,484 (60.9) | 0.14 |
| Household income (quintiles), n (%)† | |||
| 1 (lowest) | 21,790 (30.7) | 9,832 (23.6) | 0.36 |
| 2 | 8,884 (12.5) | 8,960 (21.5) | |
| 3 | 9,089 (12.8) | 8,071 (19.4) | |
| 4 | 13,889 (19.5) | 7,706 (18.5) | |
| 5 (highest) | 17,411 (24.5) | 7,039 (16.9) | |
| Mechanical ventilation, n (%) | |||
| <96 h without tracheostomy‡ | 41,517 (58.5) | 29,977 (71.6) | 0.28 |
| ≥96 h without tracheostomy‡ | 19,255 (27.1) | 8,000 (19.1) | |
| Any mechanical ventilation with tracheostomy | 10,231 (14.4) | 3,898 (9.3) | |
| Dialysis during hospitalization, n (%) | |||
| No | 66,430 (93.5) | 39,195 (93.6) | 0.21 |
| Yes–acute | 2,099 (3.0) | 2,284 (5.5) | |
| Yes–ESRD | 2,534 (3.6) | 396 (0.9) | |
| Charlson comorbidity index, n (%) | |||
| 0 | 19,754 (27.8) | 12,170 (29.1) | 0.12 |
| 1–2 | 30,414 (42.8) | 15,669 (37.4) | |
| ≥3 | 20,895 (29.4) | 14,036 (33.5) | |
| Length of index hospital stay, median (IQR) | 15 (8–28) | 16 (9–30) | |
| Length of index hospital stay, mean (SD) | 23.0 (29.1) | 27.2 (39.2) | 0.12 |
| Discharge destination, n (%) | |||
| Home | 22,609 (31.8) | 20,225 (48.3) | 0.40 |
| Home with health services | 15,508 (21.8) | 9,688 (23.1) | |
| Other§ | 32,946 (46.4) | 11,962 (28.6) | |
| Percentage rehospitalized within 30 d, n (%) | 15,527 (21.8) | 5,580 (13.3) | 0.22 |
| 30-d mortality | 3,834 (5.4) | 1,012 (2.4) | 0.16 |
| 180-d mortality | 9,608 (13.5) | 3,151 (7.5) | 0.20 |
Definition of abbreviations: ESRD = end-stage renal disease; IQR = interquartile range; SD = standard deviation.
Two patients were missing data for sex in New York.
Data on household income missing for 267 patients in Ontario.
Tracheostomy performed at any time during hospitalization.
Other discharge destination in Ontario includes: long-term care (3.1%); rehabilitative care (14.3%); complex continuing care (6.0%); acute care (1.2%); facilities and others (4.0%). In New York, it includes: skilled nursing facility (34.3%); rehabilitation facility (2.7%); hospice (2.4%); other hospital (3.1%); and others (3.9%).
Overall length of hospital stay was similar for mechanically ventilated patients in New York and Ontario (median = 15 [interquartile range = 8–28] in New York vs. 16 [9–30] in Ontario; Table 1). Patients in New York were less likely to be discharged directly home (53.6% vs. 71.4% in Ontario). Overall mortality in the cohort was higher in New York than in Ontario when assessed at 30 days and out to 180 days for all patients (5.4% vs. 2.4% at 30 d, and 13.5% vs. 7.5% at 180 d; see Figure E1).
First Rehospitalizations within 30 Days of Hospital Discharge
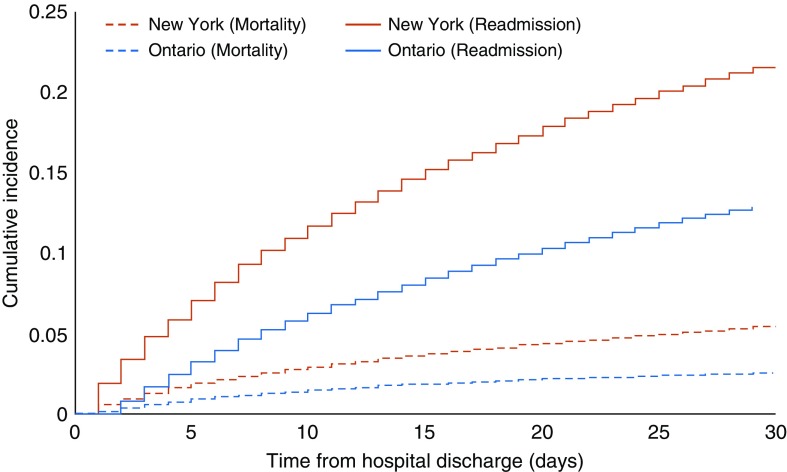
The proportion of patients who received mechanical ventilation and survived to hospital discharge who were rehospitalized within 30 days was higher in New York than in Ontario (cumulative incidence = 21.8% vs. 13.3%; Table 2; Figure 1). Stratified by individual patient characteristics, the most similar 30-day rehospitalization rates occurred in patients who were discharged home without home health services (14.0% in New York vs. 11.3% in Ontario; absolute difference = +2.7%; P < 0.001; Table 2). The greatest differences in 30-day rehospitalization rates occurred in patients who received tracheostomies (rehospitalization rate of 29.8% in New York vs. 13.6% in Ontario; absolute difference = +16.2%; P < 0.001; Table 2), who received dialysis with ESRD during the hospitalization (rehospitalization rate = 34.9% in New York vs. 21.7% in Ontario; absolute difference = +13.2%; P < 0.001), or who were not discharged directly home (rehospitalization rate = 27.6% in New York vs. 13.3% in Ontario; absolute difference = +14.3%; P < 0.001).
Table 2.
Difference between New York and Ontario in percentage of patients rehospitalized within 30 days of hospital discharge, by patient characteristics at the index hospitalization
| Characteristic | New York |
Ontario |
(New York–Ontario) |
|||
|---|---|---|---|---|---|---|
| Total(n) | One or More Rehospitalizations within 30 d n (%) | Total (n) | One or More Rehospitalizations within 30 d n (%) | Absolute Difference in Rehospitalization Proportion | P Value | |
| All | 71,063 | 15,527 (21.8) | 41,875 | 5,580 (13.3) | +8.5 | <0.001 |
| Sex | ||||||
| Male | 38,369 | 8,127 (21.2) | 25,484 | 3,232 (12.7) | +8.5 | <0.001 |
| Female | 32,692 | 7,400 (22.6) | 16,391 | 2,348 (14.3) | +8.3 | <0.001 |
| Mechanical ventilation | ||||||
| <96 h without tracheostomy* | 41,517 | 8,049 (19.4) | 29,977 | 3,904 (13.0) | +6.4 | <0.001 |
| ≥96 h without tracheostomy* | 19,255 | 4,422 (23.0) | 8,000 | 1,145 (14.3) | +8.7 | <0.001 |
| Any mechanical ventilation with tracheostomy | 10,231 | 3,046 (29.8) | 3,898 | 531 (13.6) | +16.2 | <0.001 |
| Dialysis during primary hospitalization | ||||||
| No | 66,430 | 14,050 (21.2) | 39,195 | 5,115 (13.1) | +8.1 | <0.001 |
| Yes–acute | 2,099 | 592 (28.2) | 2,284 | 379 (16.6) | +11.6 | <0.001 |
| Yes–ESRD | 2,534 | 885 (34.9) | 396 | 86 (21.7) | +13.2 | <0.001 |
| Charlson comorbidity index | ||||||
| 0 | 19,754 | 3,065 (15.5) | 12,170 | 1,056 (8.7) | +6.8 | <0.001 |
| 1–2 | 30,414 | 6,638 (21.8) | 15,669 | 1,925 (12.3) | +9.5 | <0.001 |
| ≥3 | 20,895 | 5,824 (27.9) | 14,036 | 2,599 (18.5) | +9.4 | <0.001 |
| Discharge destination | ||||||
| Home | 22,609 | 3,166 (14.0) | 20,225 | 2,276 (11.3) | +2.7 | <0.001 |
| Home with health services | 15,508 | 3,276 (21.1) | 9,688 | 1,719 (17.7) | +3.4 | <0.001 |
| Other† | 32,946 | 9,085 (27.6) | 11,962 | 1,585 (13.3) | +14.3 | <0.001 |
Definition of abbreviation: ESRD = end-stage renal disease.
Tracheostomy performed at any time during the index hospitalization.
Other discharge destination in Ontario includes: long-term care (3.1%); rehabilitative care (14.3%); complex continuing care (6.0%); acute care (1.2%) facilities and others (4.0%). In New York, it includes: skilled nursing facility (34.3%); rehabilitation facility (2.7%); hospice (2.4%); other hospital (3.1%); and others (3.9%).
Figure 1.
Cumulative incidence of 30-day rehospitalization and mortality in New York and Ontario.
To further assess rates of rehospitalization, patients were categorized into more homogeneous high-risk subgroups (Table 3). The rate of rehospitalization remained consistently higher in New York for all of these subgroups. See Table E4 for specific risk factors associated with rehospitalization in each location.
Table 3.
The 30-day rehospitalizations stratified by selected high-risk subgroups and length of hospital stay within each subgroup
| Subgroup Characteristic | New York (n = 71,063) |
Ontario (n = 41,875) |
(New York–Ontario) |
|||
|---|---|---|---|---|---|---|
| Total(n) | One or More Rehospitalizations within 30 d n (%) | Total (n) | One or More Rehospitalizations within 30 d n (%) | Absolute Difference in Rehospitalization Proportion | P Value | |
| Mechanical ventilation ≥96 h without tracheostomy & ≥3 Charlson comorbidities | 5,926 | 1,675 (28.3) | 3,127 | 587 (18.8) | +9.5 | <0.001 |
| 1–13 d* | 1,196 | 289 (24.2) | 252 | 48 (19.0) | + 5.2 | 0.08 |
| 14–20 d | 1,630 | 468 (28.7) | 503 | 89 (17.7) | +11.0 | <0.001 |
| ≥21 d | 3,100 | 918 (29.6) | 2,372 | 450 (19.0) | +10.6 | <0.001 |
| Mechanical ventilation with tracheostomy & ≥3 Charlson comorbidities | 2,885 | 964 (33.4) | 1,501 | 259 (17.3) | +16.1 | <0.001 |
| 1–13 d* | 158 | 31 (19.6) | NA† | NA† | NA† | NA† |
| 14–20 d | 273 | 90 (33.0) | NA† | NA† | NA† | NA† |
| ≥21 d | 2,454 | 843 (34.4) | 1,414 | 246 (17.4) | +17.0 | <0.001 |
| Mechanical ventilation ≥96 h without tracheostomy and ESRD requiring dialysis | 724 | 258 (35.6) | 108 | 25 (23.1) | +12.5 | 0.01 |
| 1–13 d* | 114 | 34 (29.8) | NA† | NA† | NA† | NA† |
| 14–20 d | 157 | 54 (34.4) | NA† | NA† | NA† | NA† |
| ≥21 d | 453 | 170 (37.5) | 85 | 21 (24.7) | +12.8 | 0.02 |
Definition of abbreviations: ESRD = end-stage renal disease; NA = not available.
<7 days and 7–13 days combined due to small cell size in some categories.
Suppression due to small cell size required as per ICES and Statewide Planning and Research Cooperative System policy.
Length of Hospital Stay before Initial Hospital Discharge and Hospital-Free Days
We assessed the initial hospital length of stay for patients, stratified by patient characteristics. Although overall hospital lengths of stay in the two cohorts were similar, initial hospital lengths of stay were notably shorter in New York for patient groups when stratified. In particular, length of stay was shorter in New York for patients with mechanical ventilation of 96 hours or longer without tracheostomy (mean = 26.5 ± 23.0 d vs. 37.5 ± 34.0; P < 0.001), with tracheostomies (53.4 ± 53.7 d vs. 81.5 ± 80.3 d; P < 0.001), and with acute dialysis (41.7 ± 43.0 d vs. 53.7 ± 59.3 d; P < 0.001) (Table E5). When assessed as overall acute care hospital-free days (to Day 60 after admission), patients in New York and Ontario were similar (mean = 35.4 ± 18.9 d in New York vs. 35.8 ± 18.5 d in Ontario; P < 0.001), with fewer hospital-free days in Ontario vs. New York for some subgroups (Table 4).
Table 4.
Mean (SD) number of hospital-free days* (to 60 d from the index admission) for patients in New York State and Ontario
| Characteristic | New York (n = 71,063) | Ontario (n = 41,875) | P Value |
|---|---|---|---|
| All | 35.4 (18.9) | 35.8 (18.5) | <0.001 |
| Sex | |||
| Male | 35.5 (19.0) | 36.7 (18.2) | <0.001 |
| Female | 35.2 (18.7) | 34.5 (18.9) | <0.001 |
| Mechanical ventilation | |||
| <96 h without tracheostomy | 42.5 (16.1) | 41.8 (15.1) | <0.001 |
| ≥96 h without tracheostomy | 30.6 (16.8) | 26.2 (16.9) | <0.001 |
| Any mechanical ventilation with tracheostomy | 15.3 (15.6) | 9.8 (14.1) | <0.001 |
| Dialysis during primary hospitalization | |||
| No | 36.0 (18.7) | 36.8 (18.1) | <0.001 |
| Yes–acute | 22.4 (18.4) | 21.3 (18.8) | 0.05 |
| Yes–ESRD | 30.5 (19.5) | 25.2 (19.8) | <0.001 |
| Charlson comorbidity index | |||
| 0 | 37.7 (19.0) | 42.4 (16.4) | <0.001 |
| 1–2 | 35.7 (18.6) | 36.3 (17.8) | <0.001 |
| ≥3 | 32.6 (18.9) | 29.6 (19.0) | <0.001 |
| Discharge destination | |||
| Home | 44.9 (14.5) | 44.4 (12.9) | <0.001 |
| Home with health services | 36.3 (16.9) | 32.7 (17.2) | <0.001 |
| Other | 28.4 (19.4) | 23.8 (20.1) | <0.001 |
| Mechanical ventilation ≥96 h without tracheostomy & ≥3 Charlson comorbidities | 28.5 (17.1) | 22.2 (16.7) | <0.001 |
| Mechanical ventilation with tracheostomy & ≥3 Charlson comorbidities | 16.3 (16.1) | 7.5 (12.9) | <0.001 |
| Mechanical ventilation ≥96 h without tracheostomy and ESRD requiring dialysis | 25.3 (17.2) | 19.1 (17.3) | <0.001 |
| Mechanical ventilation with tracheostomy and ESRD requiring dialysis | 7.9 (11.5) | 4.4 (9.3) | 0.03 |
Definition of abbreviations: ESRD = end-stage renal disease; SD = standard deviation.
For patients who died, hospital-free days were counted as 0.
Characteristics of Rehospitalizations in New York and Ontario
The proportion of rehospitalized patients with the same admission diagnosis as during the index hospitalization was higher in New York compared with Ontario (15.2% vs. 10.7%; P < 0.001; Table 5). The top three primary reasons for rehospitalization in New York were “septicemia,” “complications of surgical procedures or medical care,” and “congestive heart failure,” in contrast to “complications of procedures,” “congestive heart failure,” and “chronic obstructive pulmonary disease” in Ontario. More patients in New York were admitted to an ICU on rehospitalization (28.5% vs. 21.0% in Ontario; P < 0.001), and mortality during rehospitalization was slightly higher in New York (11.4% vs. 8.5%; P < 0.001).
Table 5.
Resource use and outcomes for mechanically ventilated patients rehospitalized within 30 days in the United States and Canada
| Characteristic | Rehospitalizations within 30 Days |
P Value | |
|---|---|---|---|
| New York (n = 15,527) | Ontario (n = 5,580) | ||
| Same admission diagnosis as index hospitalization | 2,355 (15.2) | 598 (10.7) | <0.001 |
| Primary reason for rehospitalization, n (%) | |||
| 1. | Septicemia: 2,540 (16.4) | Complications of procedures: 461 (8.3) | |
| 2. | Complications of surgical procedures or medical care: 1,064 (6.9) | Congestive heart failure: 364 (6.5) | |
| 3. | Congestive heart failure: 941 (6.1) | Chronic obstructive pulmonary disease: 207 (3.7) | |
| Admitted to ICU* | 4,418 (28.5) | 1,175 (21.1) | <0.001 |
| Mechanically ventilated, n (%) | 3,539 (22.8) | 549 (9.8) | <0.001 |
| ICU length of stay (for patients admitted to ICU), d | |||
| Median (IQR) | 4 (2–8) | 4 (2–8) | |
| Mean (SD) | 7.1 (11.8) | 9.0 (24.7) | 0.01 |
| Hospital length of stay, d | |||
| Median (IQR) | 7 (3–13) | 7 (4–15) | |
| Mean (SD) | 11.5 (20.3) | 15.0 (29.1) | <0.001 |
| Hospital mortality, n (%) | 1,767 (11.4) | 472 (8.5) | <0.001 |
Definition of abbreviations: ICU = intensive care unit; IQR = interquartile range; SD = standard deviation.
New York includes intensive care and coronary care; excludes intermediate intensive care. Ontario includes special care unit codes for high intensive care units.
Discussion
In a comparison of cohorts of mechanically ventilated patients admitted to the ICU in New York and Ontario, the initial hospital lengths of stay were shorter in New York than in Ontario, but rates of rehospitalization within 30 days of hospital discharge were almost double the rates in Ontario, with higher overall mortality. However, overall acute care hospital–free days were the same, suggesting overall similar use of acute care hospital resources. Although rehospitalization rates were higher in New York across all subgroups of patients assessed, we did identify a few patient groups, in particular patients with tracheostomies and those who received dialysis during the index hospitalization, who appeared to have the highest rates of rehospitalization in New York relative to Ontario. These data do not identify a “best approach” to providing care. However, given the focus on reduction of rehospitalization rates in the United States (1), these data are hypothesis generating regarding potential approaches to care that may seek to balance the costs of extended initial hospital stays against higher rehospitalization rates, particularly in higher-risk subgroups of patients.
Although the overall median hospital length of stay was similar in the two countries, this comparison alone could lead to erroneous conclusions. Stratification showed that, across all subgroups, patients in New York had a shorter hospital length of stay. The similar median was due to the fact that there was different “weighting” of patient subtypes in the two locations; in particular, a substantially larger percentage of patients in Ontario had a length of mechanical ventilation less than 96 hours (71.6% vs. 58.5% in New York), reducing the median length of stay for the group as a whole in comparison with patients in New York.
Some of the difference in rehospitalization rates seen may be accounted for by these substantially shorter initial lengths of hospital stay in New York for almost all subgroups of patients, particularly those with tracheostomies and who receive dialysis. As an example, patients with tracheostomies spent, on average, almost 3 weeks less in acute care hospitals in New York compared with Ontario. Such practices may place these patients at higher risk of needing to be readmitted to the hospital relative to counterparts in Ontario who may have longer initial hospitalizations. However, as patients had to survive to hospital discharge to be eligible for inclusion in the cohort, and given high rates of in-hospital mortality in patients with tracheostomies and who received dialysis, some of the observed differences may also be the result of survivor treatment bias. This analysis also does not account for potentially different case mix and severity of illness for any given duration of hospitalization. Thus, our findings remain speculative and an important stimulus for further explanatory studies.
Many more mechanically ventilated patients were discharged to locations other than home in New York versus Ontario. It is notable that these discharges in New York were also associated with high rehospitalization rates: 27.6% compared with 13.3% in Ontario. Comparison of care options after hospital discharge is challenging. Although New York State does not have long-term acute care hospitals (LTACs) (19), the range of facilities available for post–acute care is greater in the United States than in Canada, likely resulting in a different case mix of patients discharged to these locations.
It is notable that the rehospitalization rate for patients in Ontario discharged to “other” locations was lower than for patients discharged home with health services in Ontario, despite substantially longer lengths of hospital stay. This suggests that the group of patients who fit this category is a relatively select group of individuals, as opposed to the broader population of patients, who are often sent to different types of facilities in the United States (7). Moreover, Canadian patients are known to often spend additional days in the hospital designated as “alternative level of care,” waiting for beds in post–acute care facilities and other locations (20).
The fact that both countries may have found a similar “equilibrium” with different approaches to care is suggested by the assessment of hospital-free days; despite different initial hospital lengths of stay and different rehospitalizaton rates, the overall hospital-free days were the same. However, it is important to note that this approach does not account for days spent in other facilities, such as skilled nursing facilities or LTACs, which, as mentioned previously here, are more prevalent in the United States.
These same systems issues may explain the different observed mortality. Work by Hall and colleagues (9) has demonstrated the shift in the United States toward transfer of patients to LTACs, and the resultant lower in-hospital mortality associated with this practice; if these “same” patients remained (and died) in an acute care hospital in Ontario, they would not be included in this dataset, thus reducing the observed mortality in Ontario. An alternative (or additional) explanation may be different cultural approaches to invasive and intensive care at the end of life; in a comparison of decedents in seven countries, 9.8% of Canadians who died received intensive care in the last 30 days of life versus 27.2% in the United States (5).
Strengths of our study include the use of data from two different countries that are comparable in many ways. Population rates of mechanical ventilation have been found to be very similar in the United States and Canada (3, 21); both New York State and the Province of Ontario are large regions with a very populous city (New York City and Toronto), and both also have large rural areas. The datasets from each location are comprehensive with regard to coverage of hospitalizations, minimizing selection bias. For these reasons, these two locations have often been used to assess differences in care between Canada and the United States (22, 23). Other strengths are that we accounted for the competing risk of mortality, which provides more accurate estimates of the incidence of rehospitalization. We were also careful to harmonize definitions across the data to improve comparability.
Our study has a number of limitations. First, we chose to use a cut-off of 30 days when assessing rehospitalization rates given its wide adoption with respect to policy in the United States (24, 25). In prior work using New York data, we have demonstrated that the 30-day period captures only half of all first rehospitalizations that occur in the ICU population during the first 6 months after discharge (2). New York is known to be one of the states with a higher rehospitalization rate; as such, our estimates comparing New York rehospitalization rates with Ontario may overestimate the rates relative to other parts of the United States (24). Moreover, rehospitalization rates in the United States may be, on average, slightly lower in more recent years after the institution of the Hospital Readmission Reduction Program. However, recent studies have shown that these reductions are modest (11, 26). Mechanical ventilation coding, a requirement for inclusion in this cohort, has high specificity, but more modest sensitivity in U.S. administrative data than in Canada (12, 14, 15), and, based on information from this validation study, we may have excluded some postoperative patients in New York who received mechanical ventilation for shorter periods of time (i.e., less than 96 h); inclusion of these patients might have resulted in a lower overall rate of rehospitalization in the New York data (14). However, our stratification of data across many variables demonstrated a consistent pattern of shorter hospital length of stay and higher rehospitalization rates, which makes our finding robust to this coding concern. We also did not have information on discharge to hospice care in Ontario, and could not exclude individuals who were not considered “at risk” for rehospitalization for this reason. However, anecdotally, use of hospice is unusual in Ontario, and in New York represents a very small proportion of ICU discharges (27). Because we used administrative data, we also lacked granular clinical data that may yield additional information to understand differences in case mix, particularly regarding the choice to provide mechanical ventilation, which was part of our inclusion criteria. Finally, robust risk adjustment for rehospitalization rates, particularly related to severity of illness, was unavailable, as were comparable costs of care in each system.
International comparisons of healthcare delivery allow for reflection of different care systems, and are ultimately important for policy makers to assess current practice to identify potential alternative approaches, and for interpretation of clinical studies in the field (28). This comparison of hospitalization durations, discharge practices, and rehospitalization rates highlights some stark contrasts in care patterns between the United States and Canada, but the similarity of hospital-free days suggests some comparable balance in overall use of acute care hospital resources. Further studies are needed to fully understand the risk and benefits of each approach to care, given the differences in case mix and cost structures.
Supplementary Material
Footnotes
Supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC), and by Canadian Institutes of Health Research (CIHI) Operating grant 136,933 (H.W.) and National Institutes of Health grant K08 AG051184 (M.H.).
The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions, and statements expressed in the material are those of the authors, and not necessarily those of CIHI. Analyses were performed at Columbia University (New York, New York) and ICES/Sunnybrook Hospital (Toronto, Ontario, Canada).
Author Contributions: A.D.H. and M.H. conducted the statistical analyses; H.W. drafted the manuscript; all authors reviewed and provided critical revisions, and approved the final version of the manuscript submitted for publication. All authors contributed to the conception and design of the study, acquisition of the data, and interpretation of the findings.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345–346. doi: 10.1001/jama.2012.205110. [DOI] [PubMed] [Google Scholar]
- 2.Hua M, Gong MN, Brady J, Wunsch H. Early and late unplanned rehospitalizations for survivors of critical illness*. Crit Care Med. 2015;43:430–438. doi: 10.1097/CCM.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–1953. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 4.Moitra VK, Guerra C, Linde-Zwirble WT, Wunsch H. Relationship between ICU length of stay and long-term mortality for elderly ICU survivors. Crit Care Med. 2016;44:655–662. doi: 10.1097/CCM.0000000000001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekelman JE, Halpern SD, Blankart CR, Bynum JP, Cohen J, Fowler R, et al. International Consortium for End-of-Life Research (ICELR) Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA. 2016;315:272–283. doi: 10.1001/jama.2015.18603. [DOI] [PubMed] [Google Scholar]
- 6.Wunsch H, Angus DC, Harrison DA, Linde-Zwirble WT, Rowan KM. Comparison of medical admissions to intensive care units in the United States and United Kingdom. Am J Respir Crit Care Med. 2011;183:1666–1673. doi: 10.1164/rccm.201012-1961OC. [DOI] [PubMed] [Google Scholar]
- 7.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 8.Lilly CM, Zuckerman IH, Badawi O, Riker RR. Benchmark data from more than 240,000 adults that reflect the current practice of critical care in the United States. Chest. 2011;140:1232–1242. doi: 10.1378/chest.11-0718. [DOI] [PubMed] [Google Scholar]
- 9.Hall WB, Willis LE, Medvedev S, Carson SS. The implications of long-term acute care hospital transfer practices for measures of in-hospital mortality and length of stay. Am J Respir Crit Care Med. 2012;185:53–57. doi: 10.1164/rccm.201106-1084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gershengorn HB, Iwashyna TJ, Cooke CR, Scales DC, Kahn JM, Wunsch H. Variation in use of intensive care for adults with diabetic ketoacidosis. Crit Care Med. 2012;40:2009–2015. doi: 10.1097/CCM.0b013e31824e9eae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dharmarajan K, Wang Y, Lin Z, Normand ST, Ross JS, Horwitz LI, et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017;318:270–278. doi: 10.1001/jama.2017.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 13.Kerlin MP, Weissman GE, Wonneberger KA, Kent S, Madden V, Liu VX, et al. Validation of administrative definitions of invasive mechanical ventilation across 30 intensive care units. Am J Respir Crit Care Med. 2016;194:1548–1552. doi: 10.1164/rccm.201605-0953LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wunsch H, Kramer A, Gershengorn HB. Validation of intensive care and mechanical ventilation codes in Medicare data. Crit Care Med. 2017;45:e711–e714. doi: 10.1097/CCM.0000000000002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scales DC, Guan J, Martin CM, Redelmeier DA. Administrative data accurately identified intensive care unit admissions in Ontario. J Clin Epidemiol. 2006;59:802–807. doi: 10.1016/j.jclinepi.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland JM, Crump RT. Alternative level of care: Canada’s hospital beds, the evidence and options. Healthc Policy. 2013;9:26–34. [PMC free article] [PubMed] [Google Scholar]
- 21.Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A. Projected incidence of mechanical ventilation in Ontario to 2026: preparing for the aging baby boomers. Crit Care Med. 2005;33:574–579. doi: 10.1097/01.ccm.0000155992.21174.31. [DOI] [PubMed] [Google Scholar]
- 22.Tu JV, Naylor CD, Kumar D, DeBuono BA, McNeil BJ, Hannan EL. Coronary artery bypass graft surgery in Ontario and New York State: which rate is right? Steering Committee of the Cardiac Care Network of Ontario. Ann Intern Med. 1997;126:13–19. doi: 10.7326/0003-4819-126-1-199701010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Ko DT, Tu JV, Samadashvili Z, Guo H, Alter DA, Cantor WJ, et al. Temporal trends in the use of percutaneous coronary intervention and coronary artery bypass surgery in New York State and Ontario. Circulation. 2010;121:2635–2644. doi: 10.1161/CIRCULATIONAHA.109.926881. [DOI] [PubMed] [Google Scholar]
- 24.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 25.Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305:504–505. doi: 10.1001/jama.2011.72. [DOI] [PubMed] [Google Scholar]
- 26.Desai NR, Ross JS, Kwon JY, Herrin J, Dharmarajan K, Bernheim SM, et al. Association between hospital penalty status under the Hospital Readmission Reduction Program and readmission rates for target and nontarget conditions. JAMA. 2016;316:2647–2656. doi: 10.1001/jama.2016.18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hua M, Ma X, Morrison RS, Li G, Wunsch H. Association between the availability of hospital-based palliative care and treatment intensity for critically ill patients. Ann Am Thorac Soc. 2018;15:1067–1074. doi: 10.1513/AnnalsATS.201711-872OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papanicolas I, Jha AK. Challenges in international comparison of health care systems. JAMA. 2017;318:515–516. doi: 10.1001/jama.2017.9392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.