Abstract
Rationale: There is increasing evidence that aberrant processes occurring in the airways may precede the development of idiopathic pulmonary fibrosis (IPF); however, there has been no prior confirmatory data derived from imaging studies.
Objectives: To assess quantitative measures of airway wall thickness (AWT) in populations characterized for interstitial lung abnormalities (ILA) and for IPF.
Methods: Computed tomographic imaging of the chest and measures of AWT were available for 6,073, 615, 1,167, and 38 participants from COPDGene (Genetic Epidemiology of COPD study), ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study), and the Framingham Heart Study (FHS) and in patients with IPF from the Brigham and Women’s Hospital Herlihy Registry, respectively. To evaluate these associations, we used multivariable linear regression to compare a standardized measure of AWT (the square root of AWT for airways with an internal perimeter of 10 mm [Pi10]) and characterizations of ILA and IPF by computed tomographic imaging of the chest.
Results: In COPDGene, ECLIPSE, and FHS, research participants with ILA had increased measures of Pi10 compared with those without ILA. Patients with IPF had mean measures of Pi10 that were even greater than those noted in research participants with ILA. After adjustment for important covariates (e.g., age, sex, race, body mass index, smoking behavior, and chronic obstructive pulmonary disease severity when appropriate), research participants with ILA had increased measures of Pi10 compared with those without ILA (0.03 mm in COPDGene, 95% confidence interval [CI], 0.02–0.03; P < 0.001; 0.02 mm in ECLIPSE, 95% CI, 0.005–0.04; P = 0.01; 0.07 mm in FHS, 95% CI, 0.01–0.1; P = 0.01). Compared with COPDGene participants without ILA older than 60 years of age, patients with IPF were also noted to have increased measures of Pi10 (2.0 mm, 95% CI, 2.0–2.1; P < 0.001). Among research participants with ILA, increases in Pi10 were correlated with reductions in lung volumes in some but not all populations.
Conclusions: These results demonstrate that measurable increases in AWT are consistently noted in research participants with ILA and in patients with IPF. These findings suggest that abnormalities of the airways may play a role in, or be correlated with, early pathogenesis of pulmonary fibrosis.
Keywords: interstitial lung abnormalities, idiopathic pulmonary fibrosis, square root of AWT for airways with an internal perimeter of 10 mm, airways, lung capacity
Interstitial lung abnormalities (ILA) are defined as radiologic abnormalities on computed tomographic (CT) imaging of the chest suggestive of an underlying interstitial lung disease (ILD) in those without a clinical diagnosis. Previous studies have demonstrated that some research participants with ILA may experience a syndrome similar to but less severe than that noted in patients with idiopathic pulmonary fibrosis (IPF) and includes physiologic decrements (1, 2), common histopathologic findings (3), shared genetic predictors (4, 5), and adverse clinical outcomes (6–9). This information implies that assessments of research participants with ILA may help to improve understanding of the processes that lead to and characterize IPF.
Although much remains to be learned about the biological processes that result in the earliest stages of IPF development, a growing consensus (10) suggests that aberrant processes originating in the airways in general (11, 12), and alveolar epithelium in particular (13, 14), may play an important role in early IPF disease pathogenesis. In contrast to this consensus, IPF is often defined by a gross lack of airway-centered imaging abnormalities (15). However, comparisons of airway wall measurements between patients with IPF and undiagnosed research participants with ILA with airway wall measurements in those without imaging evidence of an underlying ILD have not been presented previously.
We hypothesized that quantitative measures of airway wall thickness (AWT) would be increased in research participants with ILA and in patients with IPF compared with those without imaging evidence of ILD. To evaluate this hypothesis, we assessed standardized chest CT measures of AWT in three populations also characterized for ILA and in patients with IPF.
Methods
Study Design
Protocols for study recruitment and participant phenotyping in COPDGene (Genetic Epidemiology of COPD study), ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study), and the Framingham Heart Study (FHS) and in an IPF cohort identified from a registry of patients with ILD at Brigham and Women’s Hospital (BWH) have previously been reported (1, 5, 16, 17). In brief, the COPDGene study is a multicenter longitudinal study of smokers designed to identify genetic and nongenetic risk factors for chronic obstructive pulmonary disease (COPD) that excluded participants with lung diseases other than asthma, emphysema, or COPD (18). The ECLIPSE study is a multicenter, multinational 3-year observational study of patients with COPD and control subjects (this analysis was limited to patients with COPD) (19). The FHS, which now includes several cohorts, is a longitudinal study originally designed to identify risk factors for cardiovascular disease; this analysis includes assessments of the Third Generation and Offspring cohorts (20). The IPF cohort includes a selected group of IPF cases from the David E. Herlihy Data Registry and DNA repository of ILD at the BWH as of August 15, 2015, that had a lung biopsy demonstrating usual interstitial pneumonia (17). In addition, all patients with IPF included consented to a medical history review and an analysis of their imaging data. The institutional review boards of the BWH and individual participating centers approved this study.
Chest CT Evaluation
For ILA determination, chest CT scans were evaluated (21) by up to three readers (radiologists and pulmonologists) who were blind to any patient-specific information. ILA were defined as nondependent changes affecting more than 5% of any lung zone, including any combination of nondependent ground-glass or reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, or traction bronchiectasis, as previously described (1, 7). CT scans with either focal or unilateral ground-glass attenuation, focal or unilateral reticulation, or patchy ground-glass abnormality (<5% of the lung) were considered indeterminate. Next, the scans with ILA were classified into subtypes by the type and location of radiologic densities as well as the consistency with IPF imaging patterns (1, 5, 16).
AWT
In each participant or patient, measurements of the square root of a hypothetical airway with an internal perimeter of 10 mm (Pi10) were collected using 3D Slicer (www.slicer.org) (COPDGene, FHS, IPF cohort) and VIDA software (ECLIPSE) as previously described (22–24). In brief, up to six segmental airways in COPDGene and ECLIPSE and up to three or four segmental airways in the FHS were selected for measurements of AWT. For each airway, manual tracings are obtained of the total airway (Ao) and the luminal airway (Ai), and the AWT is calculated as the result of Ao − Ai. Pi10 is then assessed from the slope of the regression line calculated by comparing the square root of the wall area measures plotted against the internal perimeter of the airway. In the BWH cohort of patients with IPF, approximately eight airway wall measurements (mean, 7.8 airways) were preferentially chosen from airways in the right and left upper lobes to minimize the selection of frankly fibrotic airways.
Statistical Analysis
Associations between pairs of variables were conducted with Fisher’s exact tests (for categorical variables) and with two-tailed t tests or Wilcoxon rank-sum tests (for continuous variables) when appropriate. In ILA cohorts, analyses were limited to participants with and without ILA, and participants with indeterminate ILA status were excluded. Multivariable linear regression (in COPDGene, ECLIPSE, and IPF cohort) and multivariable mixed effects models with a random effect for familial relatedness (FHS) analyses were performed to identify associations between AWT as measured by Pi10 and ILA, adjusting for age, sex, pack-years smoked, current smoking status, body mass index, Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification (when available and with COPD defined as those with GOLD stage 2 or higher), and measures of total lung capacity (TLC; calculated from volumetric CT measurements [COPDGene, ECLIPSE, and FHS] or plethysmography [IPF cohort]) when reported. Patients with IPF were compared with COPDGene participants without ILA who were older than 60 years of age. Additional analyses limited to the subset of participants with ILA within each cohort were also performed. All analyses were conducted using SAS software (version 9.4; SAS Institute) or R version 2.15.3 (R Foundation for Statistical Computing). Reported P values are two sided, and those less than 0.05 were considered statistically significant.
Results
Baseline Characteristics
Baseline characteristics of participants from COPDGene, ECLIPSE, and the FHS stratified by ILA status, as well as those of patients with IPF, are presented in Table 1. Similarly to prior publications (1, 5, 16), research participants with ILA tend to be older, are more likely to be current smokers, and are more likely to have a greater total smoking exposure. The patients with IPF evaluated were more likely to be male and had often smoked, but had less cumulative tobacco exposure, compared with cohorts limited to smokers.
Table 1.
Baseline characteristics of participants in each cohort, stratified by interstitial lung abnormality status when applicable
| COPDGene |
ECLIPSE |
Framingham Heart Study |
IPF Cohort (n = 38) | ||||
|---|---|---|---|---|---|---|---|
| No ILA (n = 5,375) | ILA (n = 698) | No ILA (n = 473) | ILA (n = 142) | No ILA (n = 1,062) | ILA (n = 105) | ||
| Age, yr | 59 ± 9 | 61 ± 10 | 62 ± 7 | 64 ± 8 | 55 ± 11 | 68 ± 12 | 63 ± 6 |
| Sex, female, n (%) | 2,465 (46) | 343 (49) | 167 (35) | 39 (27) | 541 (51) | 55 (52) | 10 (26) |
| Race, white, n (%) | 3,692 (69) | 481 (69) | 461 (97) | 139 (98) | 1,062 (100) | 105 (100) | 31 (82) |
| Body mass index, kg/m2 | 29 ± 6 | 29 ± 6 | 27 ± 6 | 26 ± 5 | 29 ± 6 | 28 ± 5 | 30 ± 5 |
| Current smoker, n (%) | 2,736 (51) | 432 (62) | 180 (38) | 67 (47) | 63 (6) | 13 (13) | 0 (0) |
| Ever smoker, n (%) | 5,316 (99) | 695 (99) | 473 (100) | 142 (100) | 512 (48) | 62 (60) | 30 (79) |
| Pack-years of smoking, median (IQR) | 38 (27) | 42 (28) | 45 (27) | 42 (30) | 0 (11) | 7 (20) | 25 (25) |
| History of COPD, n (%) | 1,795 (33) | 215 (31) | 473 (100) | 142 (100) | 87 (8) | 14 (13) | 0 (0) |
| TLC percent predicted, median (IQR) | 97 (21) | 91 (22) | 117 (23) | 112 (30) | 87 (19) | 78 (25) | 58 (22) |
| FVC percent predicted, median (IQR) | 90 (23) | 85 (22) | 78 (25) | 80 (28) | 101 (17) | 101 (15) | 64 (27) |
| Pi10, mm | 3.66 ± 0.13 | 3.72 ± 0.14 | 3.94 ± 0.2 | 3.99 ± 0.21 | 3.64 ± 0.24 | 3.71 ± 0.24 | 5.76 ± 0.67 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease, defined here as Global Initiative for Chronic Obstructive Lung Disease stage 2 or higher; COPDGene = Genetic Epidemiology of COPD study; ECLIPSE = Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study; FVC = forced vital capacity as a percentage of predicted value; ILA = interstitial lung abnormalities; IPF = idiopathic pulmonary fibrosis; IQR = interquartile range; Pi10 = square root of the airway wall area of a hypothetical airway with internal perimeter of 10 mm; TLC = total lung capacity as a percentage of predicted value.
Values are means and standard deviations or number (percent). Missing values: COPDGene: pack-years of smoking, n = 62; TLC, n = 1; FVC, n = 33; ECLIPSE: ever smoker, n = 7; TLC, n = 363; Framingham Heart Study, body mass index, n = 4; current smoker, n = 3; ever smoker, n = 3; pack-years of smoking, n = 4; TLC, n = 3; FVC, n = 67; IPF: body mass index, n = 1; pack-years of smoking, n = 13; TLC, n = 2.
ILA and Pi10
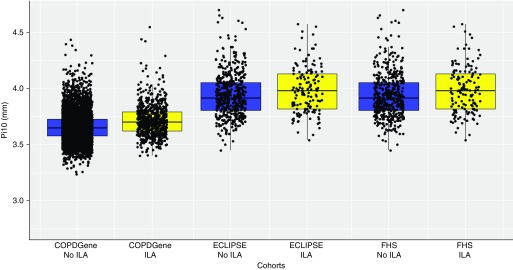
In each population, research participants with ILA had increased measures of Pi10 compared with those without ILA (see Table 1, and Figure 1). The mean measures of Pi10 among patients with IPF were even greater than those reported for research participants with ILA (see Table 1).
Figure 1.
The median, minimum, maximum, and first and third quartiles (bottom and top of boxes) of the square root of airway wall thickness (in mm) of hypothetical airways with internal perimeter of 10 mm (Pi10) are shown for each cohort, with individual data points superimposed. Blue boxes represent subjects without interstitial lung abnormalities (ILA), and yellow boxes represent subjects with ILA. COPDGene = Genetic Epidemiology of COPD; ECLIPSE = Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints; FHS = Framingham Heart Study.
In both unadjusted analyses and analyses adjusted for important covariates (e.g., age, sex, body mass index, pack-years of smoking, current smoking status, and COPD [defined by those in GOLD stage 2 or higher]), airway wall measures were thicker in those with ILA than in those without ILA (see Table 2 and Figure 2). Larger increases in airway wall measures in unadjusted and adjusted analyses were also noted in patients with IPF than in COPDGene participants without ILA who were older than 60 years of age (see Table 2 and Figure 2).
Table 2.
Associations between interstitial lung abnormalities, idiopathic pulmonary fibrosis, and Pi10 measurement
| COPDGene |
ECLIPSE |
Framingham Heart Study |
IPF Cohort |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean Difference in Pi10, mm (95% CI) | P Value | Mean Difference in Pi10, mm (95% CI) | P Value | Mean Difference in Pi10, mm (95% CI) | P Value | Mean Difference in Pi10, mm (95% CI) | P Value | |
| Unadjusted analysis | 0.029 (0.02–0.03) | <0.001 | 0.022 (0.003–0.04) | 0.02 | 0.069 (0.02–0.1) | 0.002 | 2.1 (2.07–2.15) | <0.001 |
| Adjusted* analysis | 0.026 (0.02–0.03) | <0.001 | 0.023 (0.005–0.04) | 0.01 | 0.063 (0.01–0.1) | 0.01 | 2.0 (1.96–2.07) | <0.001 |
| Adjusted† analysis | 0.018 (0.01–0.02) | <0.001 | 0.026 (0.001–0.05) | 0.04 | 0.044 (0.006–0.1) | 0.09 | 1.98 (1.92–2.05) | <0.001 |
| Adjusted† subgroup‡ analysis | 0.019 (0.01–0.02) | <0.001 | 0.03 (0.004–0.06) | 0.03 | 0.045 (0.006–0.1) | 0.09 | — | — |
Definition of abbreviations: CI = confidence interval; COPDGene = Genetic Epidemiology of COPD study; ECLIPSE = Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study; IPF = idiopathic pulmonary fibrosis (Comparison group for the IPF cohort is participants from COPDGene without interstitial lung abnormalities over the age of 60 years.); Pi10 = square root of the airway wall area of a hypothetical airway with internal perimeter of 10 mm (Mean change in Pi10 is calculated using linear regression models comparing those with interstitial lung abnormalities with those without interstitial lung abnormalities.)
Adjusted analyses are adjusted for age, sex, pack-years of smoking, current smoking status, body mass index, and Global Initiative for Chronic Obstructive Lung Disease stage (when available).
Adjusted analyses are adjusted for age, sex, pack-years of smoking, current smoking status, body mass index, Global Initiative for Chronic Obstructive Lung Disease stage (when available), and total lung capacity.
Adjusted analyses of association between Pi10 and interstitial lung abnormalities excluding subjects with predominantly centrilobular abnormalities.
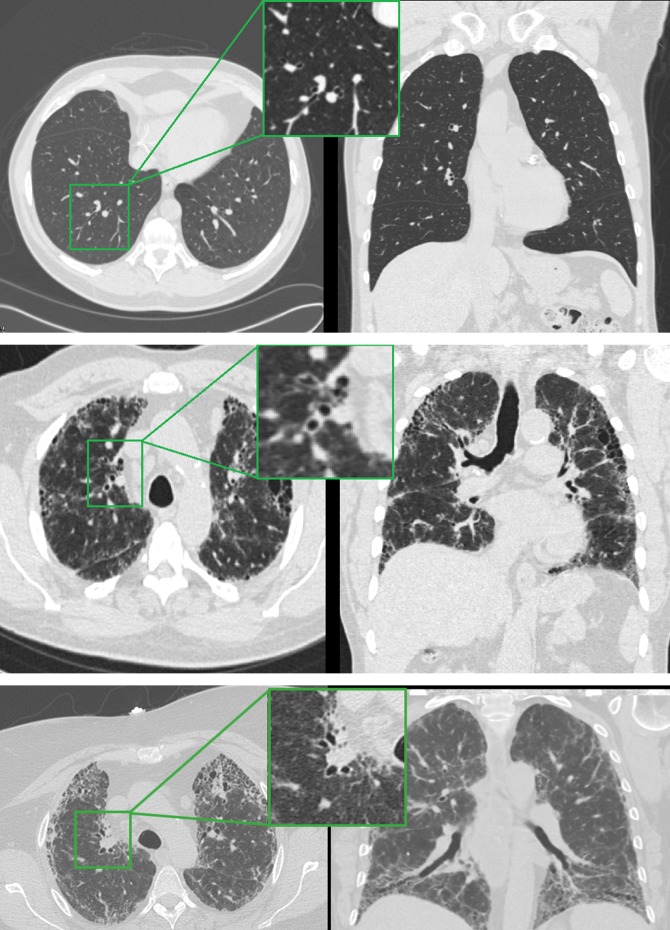
Figure 2.
Axial and coronal slices from computed tomographic scans of participants in COPDGene and David E. Herlihy Data Registry and DNA repository of interstitial lung disease. Top: Patient without interstitial lung abnormalities (ILA) from COPDGene revealing an absence of reticular changes. Top inset: Narrow airway walls of an airway with approximate internal perimeter of 10 mm. Middle: Subject from COPDGene with ILA and definite fibrosis showing subpleural reticular changes and traction bronchiectasis. Middle inset: Thickened airway walls of an airway with approximate internal perimeter of 10 mm. Bottom: Patient with IPF from David E. Herlihy Data Registry and DNA repository of interstitial lung disease with fibrotic changes showing subpleural reticular changes, honeycombing, and traction bronchiectasis. Bottom inset: Thickened airway walls of proximal right upper lobe airways.
We assessed for effect modification of the association between Pi10 and ILA by smoking and COPD status. There was no statistical evidence for differences in the association between Pi10 and ILA among current versus former smokers in the COPDGene and ECLIPSE cohorts (P > 0.3 for both cohorts) or between ever versus never smokers in the FHS and IPF cohorts (P > 0.7 for both cohorts). Although there was borderline evidence of statistically significant differences in the association between Pi10 and ILA among those with and without COPD (COPDGene, P = 0.052; FHS, P = 0.07) favoring an increased effect among those without COPD, these differences were minor (e.g., ILA resulted in a 0.005-mm greater increase in Pi10 among those without COPD than in those with COPD in COPDGene).
ILA and Pi10 Supportive Analyses
First, to exclude the possibility that these findings of association were due to an artifact of airway compression secondary to reduced lung volumes, additional analyses were performed adjusting for TLC (as a percent predicted value). There was not strong evidence that the associations between increased airway wall measures and ILA were explained by reductions in lung volumes alone in all cohorts (although some findings of association were attenuated after lung volume adjustment) (see Table 2). Compared with those without ILA (after adjusting for important covariates including measures of TLC), patients with IPF were noted to have 1.98-mm (95% confidence interval [CI], 1.92–2.05 mm; P < 0.001) increases in Pi10 measures.
Second, to exclude the possibility that these findings of association in research cohorts of smokers could be limited to those with airway-centered interstitial abnormalities, additional analyses were performed between those with and without ILA, with ILA defined by a predominance of centrilobular nodules excluded. There was not strong evidence that these exclusions substantially altered the associations between increased measures of Pi10 and ILA (see Table 2). Of note, only one research participant with ILA in the FHS was defined by the presence of centrilobular nodules, and the patients with IPF evaluated did not have centrilobular nodules on their chest CT scans. Although some difference between the associations of Pi10 and ILA exist between ILA subgroups, there was not strong evidence that these associations were being driven by the presence of pulmonary fibrosis exclusively (see also Table E1 in the online supplement).
Third, to exclude the possibility that these findings of association could be limited to alterations in airways adjacent to visually defined ILA, individual lobar measurements of airway walls were reassessed in the COPDGene cohort (where these separate measures were recorded). In COPDGene, there was evidence that right upper lobe airway measurements (as measured by airway wall area in the right upper lobe apical segment) were increased among those with ILA defined by a lower lobe subpleural distribution. After adjusting for important covariates, compared with those without ILA, research participants with ILA (in this case defined by a lower lobe subpleural distribution) were noted to have a 1.04-mm2 (95% CI, 0.4–1.7 mm2; P = 0.002) increase in airway wall area. Additional findings of association for airways in other anatomic locations are included in Tables E2A and E2B.
Finally, because Pi10 is a composite measure, we also evaluated associations between absolute wall area and airway lumen measures in research participants with ILA compared with those without ILA (in the COPDGene and FHS cohorts when this information was available). Similar increases in the adjusted changes in absolute airway wall measures were noted among those with ILA compared with those without ILA for most but not all individual airway assessments (see also Tables E2A and E2B).
Pi10 and Lung Volumes in Those with ILA
To determine the impact of increased Pi10 in these populations, the associations between lung volume reductions and Pi10 among research participants with ILA and among patients with IPF were evaluated. As noted in Table 3, although there was consistent evidence between increases in Pi10 and reduced measures of TLC across all populations characterized for ILA, after adjustment for important covariates, these associations were statistically significant only among participants with ILA in the COPDGene and FHS cohorts. There was not robust evidence that Pi10 was strongly associated with the variability of lung volume measures in this sample of patients with IPF.
Table 3.
Associations between 0.1-mm increase in Pi10 and changes in total lung capacity and forced vital capacity in participants with interstitial lung abnormalities and idiopathic pulmonary fibrosis
| COPDGene |
ECLIPSE |
Framingham Heart Study |
IPF Cohort |
|||||
|---|---|---|---|---|---|---|---|---|
| Analysis | Mean Difference in Percent Predicted Volume (95% CI) | P Value | Mean Difference in Percent Predicted Volume (95% CI) | P Value | Mean Difference in Percent Predicted Volume (95% CI) | P Value | Mean Difference in Percent Predicted Volume (95% CI) | P Value |
| TLC unadjusted | −3.4 (−4.2 to −2.6) | <0.001 | −2.7 (−5.2 to −0.2) | 0.04 | −1.5 (−2.8 to −0.2) | 0.03 | 0.07 (−0.7 to 0.8) | 0.88 |
| TLC adjusted* | −4.1 (−4.9 to −3.4) | <0.001 | −2.1 (−4.8 to 0.5) | 0.11 | −1.3 (−2.5 to −0.1) | 0.03 | −0.2 (−1.3 to 0.9) | 0.67 |
| FVC unadjusted | −4.3 (−5.1 to −3.4) | <0.001 | −2.3 (−3.9 to −0.8) | 0.004 | −0.4 (−1.5 to 0.7) | 0.47 | −0.03 (−1.0 to 1.0) | 0.95 |
| FVC adjusted | −2.2 (−2.8 to −1.5) | <0.001 | −0.9 (−2.6 to 0.7) | 0.25 | −0.08 (−1.2 to 1.1) | 0.89 | −0.3 (−1.6 to 0.9) | 0.59 |
Definition of abbreviations: CI = confidence interval; COPDGene = Genetic Epidemiology of COPD study; ECLIPSE = Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study; FVC = forced vital capacity as a percentage of predicted value; IPF = idiopathic pulmonary fibrosis; Pi10 = square root of the airway wall area of a hypothetical airway with internal perimeter of 10 mm (Mean changes in percent predicted lung volumes are calculated using linear regression models among those with interstitial lung abnormalities and IPF; the associations reflect the correlations between a 0.1-mm increase in Pi10 and the result of measures of lung volumes.); TLC = total lung capacity as a percentage of predicted value.
Adjusted analyses in participants with interstitial lung abnormalities are adjusted for age, sex, pack-years of smoking, current smoking status, body mass index, and Global Initiative for Chronic Obstructive Lung Disease stage (when available). In participants with interstitial lung abnormalities, they are adjusted for age, sex, body mass index, and pack-years of smoking.
Discussion
Our study demonstrates that measures of AWT are increased in three independent cohorts of research participants with ILA and in patients with IPF. The associations between ILA and increased measures of AWT are not explained solely by reductions in lung volumes, by airway-centered interstitial abnormalities, or by measurements obtained from airways in close proximity to the observed interstitial abnormalities. There is not strong evidence that these associations were limited to smokers. In some but not all cohorts, relative increases in AWT are associated with reduced lung volumes. In total, these findings demonstrate that detectable thickening commonly occurs in the airways in early (3) and later stages of pulmonary fibrosis and suggests that airway thickening may be implicated in or occur coincident with the pathologic processes that result in clinical disease.
Although the alveolar epithelium is most commonly implicated as the anatomic focus of IPF pathophysiology (25), a growing body of evidence derived from genetic (4, 14, 26) and histopathologic studies (27–30) has also implicated biological processes occurring in the airways as potential important drivers of IPF pathogenesis. Despite these findings, there has been no prior study that has presented imaging assessments of airway wall measures in either research participants with ILA or patients with IPF. Our findings provide further support that aberrant airway processes may contribute to or occur coincident with IPF disease pathogenesis, and they suggest that these processes may begin early in the disease.
More is known about the role of Pi10 in obstructive airway diseases (31–33) such as COPD, in which increased Pi10 measures have been associated with worsening respiratory symptoms, increased frequency of COPD exacerbations, and increased mortality risk (32–35). In addition, in patients with COPD, increased AWT is frequently correlated with the presence (36) and development (37) of airflow limitation. In contrast, our findings demonstrate that AWT can be correlated with restrictive lung physiology in some populations. This suggests that the contribution of increased AWT to the type of physiologic decrement likely also depends on the underlying disease process correlated with these changes.
To provide some context, the relative differences observed in Pi10 measures between research participants with and without ILA (0.05–0.06 mm square root of wall area) are similar to the amounts of difference in Pi10 measures that have been associated with chronic bronchitis (0.03 mm) (38), bronchodilator responsiveness (0.04 mm) (39), and increases in respiratory symptoms (0.1 mm) (32) in patients with COPD. The median value for Pi10 (derived from upper lobe airway measurements) noted in patients with biopsy-proven IPF (5.78 mm) is larger than the means commonly reported in patients with COPD (3.69–4.94 mm) (35, 39–41).
Although our study provides evidence that airway wall thickening commonly occurs in early (3) as well as in later stages of pulmonary fibrosis when airway abnormalities are commonly detected (e.g., traction bronchiectasis) (42), our findings do not allow us to comment on the specific reasons for these increased measures of Pi10. Although increased measures of Pi10 are commonly viewed as increased measures of AWT, it is important to note that Pi10 is a composite measure that results both from factors that increase thickness of the airway wall and from factors that narrow the airway lumen. This suggests that factors which result in narrowing of the airway lumen (e.g., diffuse increases in airway mucus content) (43) also may have contributed to these findings. In addition, it is important to note that Pi10 is primarily a measure of wall thickness in large airways. Although Pi10 has been correlated with histologic assessments of small airways in patients with COPD, it is unclear if correlations would be noted in the development of ILD.
Our study has a number of additional limitations. First, although increases in AWT were consistently noted in all populations, the associations between relative increases in measures of Pi10 and relative decreases in lung volume measures were not statistically significant, when adjusted for important covariates, in all cohorts. Although this reduces the confidence in these findings and suggests the possibility of true-negative associations between Pi10 and lung volume measures, it is also possible that smaller sample sizes, limited measurement variability, and unmeasured confounders limit our study’s statistical power to detect an association in some populations. Second, although substantial increases in measures of Pi10 were noted among patients with IPF, increases in Pi10 measures were not substantially greater in those with ILA imaging findings more consistent with IPF or among those with more fibrotic features alone. This suggests that increases in Pi10 among those with ILA may be a result of diverse processes other than those limited to pulmonary fibrosis. Third, because this is the first presentation of Pi10 measures in patients with IPF, we recommend caution in the interpretation of these findings until these associations can be replicated in other populations of patients with IPF, including those defined by image criteria alone. Finally, because measurements of Pi10 were available at a single time point in each of these populations, our study does not allow us to comment on associations between increasing measures of Pi10 over time and the development or progression of ILA. Future longitudinal assessments of Pi10 would be important to answering these questions.
In conclusion, our results demonstrate that there are measurable differences in AWT between research participants with ILA, as well as in patients with IPF, compared with those without ILA. In some but not all populations, these differences in AWT are correlated with reduced lung volumes. These findings suggest that abnormalities of the airways may play a role in or be correlated with early pathogenesis of pulmonary fibrosis.
Supplementary Material
Footnotes
Supported by National Institutes of Health (NIH) grant T32 HL007633 (E.R.M.); NIH grant K08 HL140087 (R.K.P.); NIH grants K01 HL118714 and R01 HL133137 (A.A.D.); NIH grants K25 HL104085 and R01 HL116473 (R.S.J.E.); NIH grant R01 CA203636 (M.N.); NIH grant K25 HL130637 (J.R.); NIH grant OT2 OD026553 (G.T.O’C.); NIH grants U01 HL089856, P01 HL114501, R01 HL113264, R01 HL133135, and R01 HL137927 (E.K.S.); NIH grant R01 HL130974, R01 HL129920, and U01 HL133232 (I.O.R.); NIH grant R01 HL122464 and R01 HL116473 (G.W.); and NIH grants R01 HL111024, R01 HL130974, and R01 HL135142 (G.M.H. and this work). The COPDGene (Genetic Epidemiology of COPD) study is supported by National Heart, Lung, and Blood Institute (NHLBI) grants U01 HL089897 and U01 HL089856. COPDGene (NCT00608764) is also supported by the COPD Foundation through contributions made to an industry advisory committee comprised of AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Sunovion. The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study (NCT00292552; GSK code SCO104960) was sponsored by GlaxoSmithKline. This work was partially supported by the NHLBI’s Framingham Heart Study contract (N01-HC-25195). This work was supported by a generous gift from the family of David E. Herlihy to the Interstitial Diseases Group at Brigham and Women’s Hospital for the creation of the David E. Herlihy Data Registry and DNA Repository, which is also supported by NHLBI grant R01 HL130974.
Author Contributions: Study design: G.M.H., E.R.M., and G.W.; acquisition, analysis, or interpretation of the data: T.A., S.P.d.F., H.C., A.A.D., J.D., H.H., T.H., G.M.H., E.R.M., M.N., G.T.O’C., R.K.P., I.O.R., J.R., R.S.J.E., E.K.S., G.W., and H.X.; critical revision of the manuscript for important intellectual content: T.A., H.C., A.A.D., J.D., H.H., T.H., G.M.H., E.R.M., M.N., G.T.O’C., R.K.P., I.O.R., J.R., R.S.J.E., E.K.S., G.W., and H.X.; statistical analysis: A.A.D., J.D., G.M.H., E.R.M., R.K.P., G.W., and H.X.; and obtained funding: G.M.H., G.T.O’C., I.O.R., E.K.S., and G.W.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) and COPDGene investigators
References
- 1.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle TJ, Hunninghake GM, Rosas IO. Subclinical interstitial lung disease: why you should care. Am J Respir Crit Care Med. 2012;185:1147–1153. doi: 10.1164/rccm.201108-1420PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller ER, Putman RK, Vivero M, Hung Y, Araki T, Nishino M, et al. Histopathology of interstitial lung abnormalities in the context of lung nodule resections. Am J Respir Crit Care Med. 2018;197:955–958. doi: 10.1164/rccm.201708-1679LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putman RK, Gudmundsson G, Araki T, Nishino M, Sigurdsson S, Gudmundsson EF, et al. The MUC5B promoter polymorphism is associated with specific interstitial lung abnormality subtypes. Eur Respir J. 2017;50:1700537. doi: 10.1183/13993003.00537-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators; COPDGene Investigators. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podolanczuk AJ, Oelsner EC, Barr RG, Bernstein EJ, Hoffman EA, Easthausen IJ, et al. High-attenuation areas on chest computed tomography and clinical respiratory outcomes in community-dwelling adults. Am J Respir Crit Care Med. 2017;196:1434–1442. doi: 10.1164/rccm.201703-0555OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putman RK, Hunninghake GM, Dieffenbach PB, Barragan-Bradford D, Serhan K, Adams U, et al. Interstitial lung abnormalities are associated with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:138–141. doi: 10.1164/rccm.201604-0818LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolters PJ, Blackwell TS, Eickelberg O, Loyd JE, Kaminski N, Jenkins G, et al. Time for a change: is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir Med. 2018;6:154–160. doi: 10.1016/S2213-2600(18)30007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryu C, Homer RJ, Herzog EL. The airway in idiopathic pulmonary fibrosis: protecting the lung or promoting disease? Am J Respir Crit Care Med. 2016;193:1081–1082. doi: 10.1164/rccm.201601-0055ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti C, Montero-Fernandez A, Borg E, Osadolor T, Viola P, De Lauretis A, et al. Mucins MUC5B and MUC5AC in distal airways and honeycomb spaces: comparison among idiopathic pulmonary fibrosis/usual interstitial pneumonia, fibrotic nonspecific interstitial pneumonitis, and control lungs. Am J Respir Crit Care Med. 2016;193:462–464. doi: 10.1164/rccm.201507-1322LE. [DOI] [PubMed] [Google Scholar]
- 13.Bueno M, Lai Y, Romero Y, Brands J, St Croix CM, Kamga C, et al. PINK1 deficiency impairs mitochondrial homeostasis promoting lung fibrosis. J Clin Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathai SK, Pedersen BS, Smith K, Russell P, Schwarz MI, Brown KK, et al. Desmoplakin variants are associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:1151–1160. doi: 10.1164/rccm.201509-1863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raghu G, Rochwerg B, Zhang Y, Garcia CAC, Azuma A, Behr J, et al. American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis: an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 16.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ash SY, Harmouche R, Vallejo DLL, Villalba JA, Ostridge K, Gunville R, et al. Densitometric and local histogram based analysis of computed tomography images in patients with idiopathic pulmonary fibrosis. Respir Res. 2017;18:45. doi: 10.1186/s12931-017-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic Epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, et al. ECLIPSE investigators. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur Respir J. 2008;31:869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 20.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. 2010;17:48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 23.Gietema HA, Edwards LD, Coxson HO, Bakke PS ECLIPSE Investigators. Impact of emphysema and airway wall thickness on quality of life in smoking-related COPD. Respir Med. 2013;107:1201–1209. doi: 10.1016/j.rmed.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Patel BD, Coxson HO, Pillai SG, Agustí AGN, Calverley PMA, Donner CF, et al. International COPD Genetics Network. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:500–505. doi: 10.1164/rccm.200801-059OC. [DOI] [PubMed] [Google Scholar]
- 25.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 26.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myre M, Allard S, Bernard C, Martin RR. Clinical, functional and pathological correspondence in early stage idiopathic pulmonary fibrosis: evidence for small airway obstruction 1-2. Respiration. 1988;53:174–186. doi: 10.1159/000195411. [DOI] [PubMed] [Google Scholar]
- 28.Figueira de Mello GC, Ribeiro Carvalho CR, Adib Kairalla R, Nascimento Saldiva PH, Fernezlian S, Ferraz Silva LF, et al. Small airway remodeling in idiopathic interstitial pneumonias: a pathological study. Respiration. 2010;79:322–332. doi: 10.1159/000235722. [DOI] [PubMed] [Google Scholar]
- 29.Plantier L, Crestani B, Wert SE, Dehoux M, Zweytick B, Guenther A, et al. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax. 2011;66:651–657. doi: 10.1136/thx.2010.151555. [DOI] [PubMed] [Google Scholar]
- 30.Polosukhin VV, Degryse AL, Newcomb DC, Jones BR, Ware LB, Lee JW, et al. Intratracheal bleomycin causes airway remodeling and airflow obstruction in mice. Exp Lung Res. 2012;38:135–146. doi: 10.3109/01902148.2012.658595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donohue KM, Hoffman EA, Baumhauer H, Guo J, Ahmed FS, Lovasi GS, et al. Asthma and lung structure on computed tomography: the Multi-Ethnic Study of Atherosclerosis Lung Study. J Allergy Clin Immunol. 2013;131:361–368.e11. doi: 10.1016/j.jaci.2012.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grydeland TB, Dirksen A, Coxson HO, Eagan TML, Thorsen E, Pillai SG, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181:353–359. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 33.Oelsner EC, Smith BM, Hoffman EA, Kalhan R, Donohue KM, Kaufman JD, et al. Prognostic significance of large airway dimensions on computed tomography in the general population: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Ann Am Thorac Soc. 2018;15:718–727. doi: 10.1513/AnnalsATS.201710-820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Tho N, Ogawa E, Trang TH, Ryujin Y, Kanda R, Nakagawa H, et al. A mixed phenotype of airway wall thickening and emphysema is associated with dyspnea and hospitalization for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12:988–996. doi: 10.1513/AnnalsATS.201411-501OC. [DOI] [PubMed] [Google Scholar]
- 35.Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187:602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 36.Aziz ZA, Wells AU, Desai SR, Ellis SM, Walker AE, MacDonald S, et al. Functional impairment in emphysema: contribution of airway abnormalities and distribution of parenchymal disease. AJR Am J Roentgenol. 2005;185:1509–1515. doi: 10.2214/AJR.04.1578. [DOI] [PubMed] [Google Scholar]
- 37.Mohamed Hoesein FAA, de Jong PA, Lammers JWJ, Mali WPTM, Schmidt M, de Koning HJ, et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J. 2015;45:644–651. doi: 10.1183/09031936.00020714. [DOI] [PubMed] [Google Scholar]
- 38.Kim V, Davey A, Comellas AP, Han MK, Washko G, Martinez CH, et al. COPDGene® Investigators. Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res. 2014;15:52. doi: 10.1186/1465-9921-15-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim V, Desai P, Newell JD, Make BJ, Washko GR, Silverman EK, et al. COPDGene Investigators. Airway wall thickness is increased in COPD patients with bronchodilator responsiveness. Respir Res. 2014;15:84. doi: 10.1186/s12931-014-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grydeland TB, Dirksen A, Coxson HO, Pillai SG, Sharma S, Eide GE, et al. Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. Eur Respir J. 2009;34:858–865. doi: 10.1183/09031936.00167908. [DOI] [PubMed] [Google Scholar]
- 41.Nambu A, Zach J, Schroeder J, Jin G, Kim SS, Kim YI, et al. Quantitative computed tomography measurements to evaluate airway disease in chronic obstructive pulmonary disease: relationship to physiological measurements, clinical index and visual assessment of airway disease. Eur J Radiol. 2016;85:2144–2151. doi: 10.1016/j.ejrad.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piciucchi S, Tomassetti S, Ravaglia C, Gurioli C, Gurioli C, Dubini A, et al. From “traction bronchiectasis” to honeycombing in idiopathic pulmonary fibrosis: a spectrum of bronchiolar remodeling also in radiology? BMC Pulm Med. 2016;16:87. doi: 10.1186/s12890-016-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickey BF, Whitsett JA. Understanding interstitial lung disease: it’s in the mucus. Am J Respir Cell Mol Biol. 2017;57:12–14. doi: 10.1165/rcmb.2017-0116ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.