Abstract
The Authors reported a retrospective study of 100 Leiomyosarcoma (LMS), evaluating factors that may influence Overall Survival (OS), Local Recurrence Free Survival (LRFS), Metastasis Free Survival (MFS). Tumor Size (P = 0,0009), Local Recurrence (P = 0,0487), Distant relapse (P < 0,0001), Type of Presentation (P = 0,0213) were significant risk factors affecting overall survival (OS). Tumor Size (P = 0.024), age at diagnosis (P = 0,0086), type of presentation (P < 0,0001) and Local Recurrence (P = 0.0152) affected metastasis free survival (MFS). Type of presentation (P = 0,001) was an independent prognostic factor of local recurrence-free survival (LRFS).
Keywords: Leiomyosarcoma, Size, Age, Recurrence, Metastasis
1. Introduction
Leiomyosarcoma is a malignant neoplasm showing pure smooth-muscle differentiation and is an excellent example of tumor with different biology and prognosis depending on anatomic site. LMS is one of the most representative histology of retroperitoneal (including pelvic) sarcomas and is the predominant histology arising from large blood vessels. It is less common, accounting for 10–15% of limb sarcoma. LMS of the extremities arise more frequently in the lower limbs, but may develop elsewhere. LMS usually have intramuscular and subcutaneous localizations in approximately equal proportions, and few times originate from a small to medium-sized vein1.
The typical histological pattern of LMS is that of intersecting, sharply marginated fascicles of spindle cells. This pattern may be less well-defined in some tumours, and occasionally there is a focal storiform, palisaded, or haemangiopericytoma-like arrangement.1 LMS of soft tissue are capable of both local recurrence and distant metastasis. The therapy of choice involves surgical resection with negative margins. The most important prognostic factors affecting clinical outcome are tumour location, size, histological grading, margin, age, clinical presentation, local recurrence and metastasis.1, 2, 3, 4, 5, 6
The aim of our retrospective study was to evaluate factors that may influence Overall Survival (OS), Local Recurrence Free Survival (LRFS) and Metastasis Free Survival (MFS) in a series of patients diagnosed with LMS of the limbs treated in a single centre.
2. Materials and methods
We retrospectively reviewed histological and clinical records of 100 patients treated between 1990 and 2015. Mean age was 61,5 years (range = 28–84), 98 (98%) LMS localized in the limbs and 2 (2%) in the dorsal muscle.
All data collected included patient characteristics (age, gender), tumor characteristics (site, size, type of presentation, clinical symptoms, stage, histology), diagnostic and therapeutic procedures (type of biopsy, type of surgery, margins, neoadjuvant and adjuvant therapy) and clinical outcome. This research has been approved by the IRB of the authors’ affiliated institutions.
The data were obtained from the patient's medical records. Local recurrence and distant metastasis were recorded. Each patient underwent anamnestic collection of his medical history, physical examination and routine blood tests, electrocardiogram and chest X-ray were obtained. Considering that plain x-ray or CT were not useful to identify the features and the edges of the primary tumor, MRI was performed in most patients. MRI was particularly useful in defining certain characteristics such as homogeneity, necrosis, haemorrhagic areas, the local spread of the disease (size) and tumor stage. To complete the diagnostic work up Chest CT scan, bone scan or PET (from 2009) were performed preoperatively.
Type of presentations were divided in primary lesions (VG), local recurrence (LR), radicalization or additional wide excision of LMS managed with inadequate excision in other centers (RSD) and in patients with metastases at onset (MET)) (Tabe1). All patients with a local recurrence or inadequate excision were originally treated in other Institutions.
Histological diagnosis was confirmed by open biopsy, ultrasound-guided needle biopsy or previous inadvertent excision performed at other centres.
Microscopical surgical margins were classified in wide and radical, marginal and intralesional. The surgical approach was the main treatment that attempt to get wide margins. When the tumor was adjacent to critical structures such as nerves, blood vessels or bones, a planned marginal surgery has been accepted.
Radiotherapy (RT) in preoperative or postoperative setting was performed in patients with high grade disease or tumor size > 5 cm, deep sited tumors or in case of close/positive margins.
External beam radiotherapy was delivered with 6–10 MeV photons, 3DCRT (3D conformal radiotherapy) was performed before 2011, thereafter all treatments were delivered by Helical Tomotherapy. GTV (Gross Tumor Volume) was obtained contouring the surgical bed or the gross tumor in case of preoperative RT on T1 weighted MRI images, CTV (Clinical Target Volume) derived from an expansion of 1.5 cm radially and 4 cm longitudinally from the GTV, and finally 0.5 cm were added to the CTV to obtain the PTV (Planning Target Volume). A total dose of 50 Gy and 60–66 Gy in preoperative and postoperative setting, respectively. A standard fraction schedule was used: 2Gy per fraction, 5 days a week.
Chemotherapy was performed in patients with more than two of these unfavourable prognostic factors: high grade disease, tumor size >5 cm, deep sited tumours, positive surgical margins. It consisted of 3 or 5 cycles of Epirubicine (60 mg/m2 Day 1–2) and Ifosfamide (3 g/m2 Day 1–3) administered every 21 days.7 Granulocyte colony-stimulating factor was administered to prevent neutropenia. The patients were followed every 3 months for the first 3 years, every 6 months for the following 2 years and annually thereafter.
The statistical analysis was performed with MedCalc software version 16.8.4. Values of P ≤ 0,05 were considered statistically significant. All variables were analyzed for their impact on overall survival, local recurrence-free survival and metastasis-free survival with a follow-up of 5 and 10 years from surgery time. In univariate analysis of the overall survival estimates, local recurrence-free survival and metastasis-free survival were calculated according to the method of Kaplan-Meier.
The comparison of survival curves calculated was performed by the long-rank test media. The hazard ratios and confidence intervals (95%) were calculated using the Cox hazard test. Significant results parameters univariate analysis were included in a multivariate Cox regression model.
3. Results
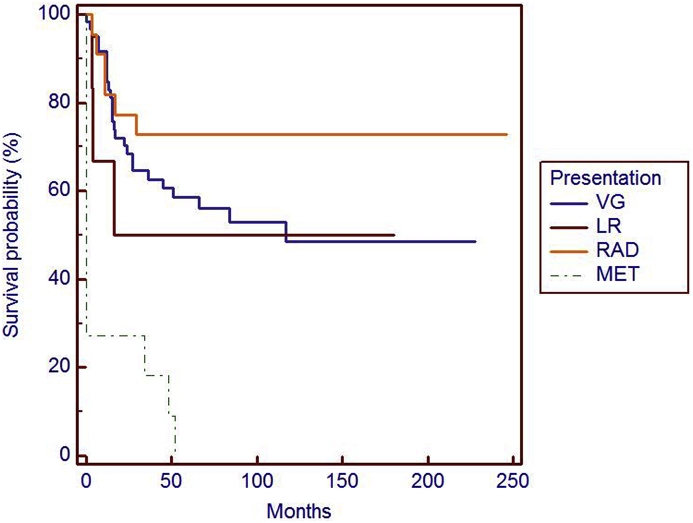
Our data included 61 (61%) primitive LMS, 6 (6%) local recurrences of primitive LMS, 22 (22%). Tumor sites were: lower extremities in 77 (77%) cases, upper limbs in 21 (20%) cases and trunk in 2 (2%) cases (Table 1). Specifically 6 LMS (6%) were localized at shoulder muscles, 10 (10%) at the arm, 5 (5%) at the elbow and distal to the elbow, 6 (6%) in pelvic muscles, 46 (46%) in the thigh and 25 (25%) in the knee and distal to the knee. Two LMS (2%) were localized in the muscle of the trunk. The preoperative MRI showed size >10 cm in 18 (18%) patients, between 5 and 10 cm in 38 (38%) patients, and <5 cm in 44 (44%) patients (Table 1). 13 (13%) tumors were classified low grade and 87 (87%) high grade (Table 1). At the final histology 87 (87%) LMS were treated with radical or wide excision, 13 (13%) with marginal excision (Table 2). 46 cases received postoperative radiotherapy (7 cases with size > 10 cm, 20 cases between 5 and 10 cm, 19 high grade LMS with dimensions < 5 cm), of which 5 patients had not adequate margins at histological examination and in 40 patients with high-grade LMS (FNCLCC grading system) (Table 2).
Table 1.
Main features.
| Characteristics | N° | % |
|---|---|---|
| Patients | 100 | 100 |
| Presentation: | ||
| Primary (VG) | 61 | 61 |
| Local recurrence (LR) | 6 | 6 |
| Radicalization (RAD) | 22 | 22 |
| Metastatic LMS (MET) | 11 | 11 |
| Grading (FNCLCC): | ||
| Low grade | 13 | 13 |
| High grade | 87 | 87 |
| Site: | ||
| Lower limb | 77 | 77 |
| Upper limb | 21 | 21 |
| Trunk | 2 | 2 |
| Size: | ||
| >10 cm | 18 | 18 |
| 5–10 cm | 38 | 38 |
| <5 cm | 44 | 44 |
Table 2.
Margin, radiotherapy, chemotherapy, local recurrence, metastasis.
| Wide/Radical | Marginal | Intralesional | |
|---|---|---|---|
| Margin | 87 | 13 | 0 |
| Preoperative (50 Gy) | Postoperative (60–66 Gy) | |
|---|---|---|
| Radiotherapy | 0 | 46 |
| Chemotherapy | Neoadjuvant | Adjuvant |
| 1 | 27 | |
| Local recurrence | 20 (16%) | 16 (wide/radical excision), 4 (marginal excision) |
| Metastasis | 42 (42%) | 11 onset mts, 24(wide/radical surgery), 7 (marginal surgery) |
| Site metastasis | 25 pulmonary, 7 bone, 11 multiple site |
Chemotherapy was administered in 27 patients with high grade LMS, 1 patient received neoadjuvant chemotherapy and 26 postoperative chemotherapy (Table 2).
The mean follow-up was 74 months (range 1–246 months), 51 patients had a follow up longer than 5-years.
3.1. Local recurrence
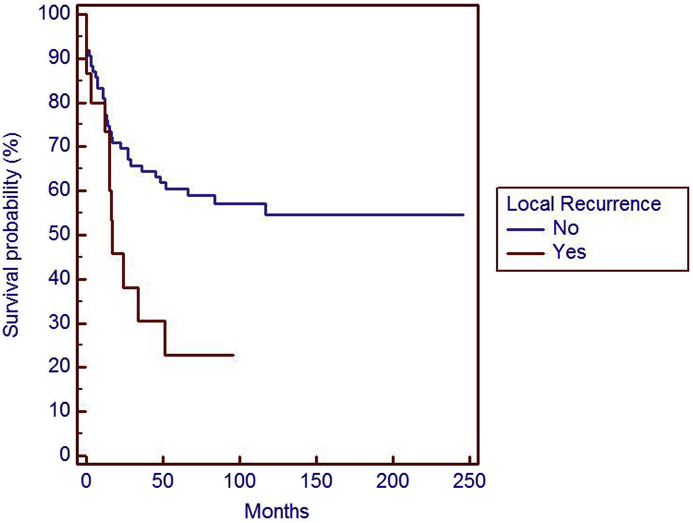
We observed 20 (20%) local recurrences with mean disease free interval of 63 months (range 1–207 months) (Table 2). LRFS was 88% at 5 years and 84% at 10 years.
Sixteen LMS treated with radical or wide excision developed local recurrence, 6 with size >10 cm, 6 with size between 5 and 10 cm and only 4 with sizes <5 cm. Seven patients with local recurrence underwent amputation for involvement of neurovascular bundle, nine patients were treated with excision.
Four LMS treated with marginal excision developed local recurrence, 2 with size >10 cm, 2 with size between 5 and 10 cm. All local recurrences were treated with amputation for involvement of neurovascular bundle.
At univariate analysis only type of presentation (local recurrence of tumors treated in non-specialized cancer center had a higher risk to develop new local recurrences) (P = 0,001) is risk factor for local recurrence-free survival (LRFS) (Fig. 1), while at multivariate analysis we did not observed significative factor.
Fig. 1.
Presentation represent a significant risk factor (P = 0,001) in local recurrence free survival (LRFS).
3.2. Distant metastasis
We observed forty-two (42%) patients with metastases, of which 11 had metastatic disease at the onset, while 31 developed metastases after surgery (Table 2). 24 primitive LMS (39%) that developed metastases were treated with wide resection and 7 (11%) with marginal surgery. The site of metastases were lungs in 25 (60%) patients, bones (above all spine) in 7(16%) and multiple sites in 11(26%) patients, respectively. Seven (35%) local recurrences developed distant metastases thereafter.
MFS was 55% and 50% at 5 and 10 years, respectively.
At univariate analysis Size (p = 0.024) (Fig. 2), age (p = 0,0086) (Fig. 3), type of presentation (p < 0,0001) (Fig. 5) and Local Recurrent disease (0.0152) (Fig. 4) were prognostic factors affecting metastasis free survival (MFS).
Fig. 2.
Size is a significant risk factor (p = 0,024) in metastasis free survival (MFS).
Fig. 3.
Age is a significant risk factor (p = 0,0086) in metastasis free survival (MFS).
Fig. 5.
Type presentation is a higher significant risk factor (p < 0,0001) in metastasis free survival (MFS).
Fig. 4.
Local Recurrence is a significant risk factor (p = 0,0152) in metastasis free survival (MFS).
In multivariate analysis for MFS size (p = 0.0051), age (p = 0,0165) and presentation (p = 0,0168) were statistically significant, unlike the event local recurrence (p = 0.0742).
3.3. Overall survival
OS was 69% and 60% at 5 and 10 years, respectively.
At univariate statistical analysis Size (p = 0,0009) (Fig. 6), recurrent disease (p = 0,0487) (Fig. 7), distant Metastases (p < 0,0001) (Fig. 8), presentation (p = 0,0213) (Fig. 9) were prognostic factors affecting Overall Survival.
Fig. 6.
Size is a significant risk factor (p = 0,0009) in overall survival (OS).
Fig. 7.
Local recurrence is a significant risk factor (p = 0,0487) in overall survival (OS).
Fig. 8.
Metastasis is a higher significant risk factor (p < 0,0001) in overall survival (OS).
Fig. 9.
Type of presentation is a significant risk factor (p = 0,0213) in overall survival (OS).
At the multivariate analysis for OS, size (p = 0,0071) was significative and metastasis is higher significative (p < 0,0001).
4. Discussion
LMS are uncommon tumors and with a well known poor long-term prognosis. In our report overall suvival was 69% and 60% at 5 and 10 years, respectively. Svarvar et al. reported a series from Scandinavian Sarcoma Group of 225 LMS patients; the authors recorded a cumulative survival of 49% at 10 years.2 Our metastasis incidence was 42%. Previous analyses from other institutions reported a higher incidence of metastases from 29.4% to 44.7%.2, 3, 4, 5
Size was a negative prognostic factor, in our experience, affecting OS (p = 0,0009) and MFS (p = 0,024). LMS with size < 5 cm had a better prognosis than LMS with size between 5 and 10 cm and >10 cm at 5 and 10 years. The importance of large tumor size as an adverse prognostic factor on OS and MFS was in line with earlier studies on soft tissue LMS and other histopathologic sarcoma types. Different Authors described a correlation between size, metastasis and survival and these data could be explained with a higher possibility of micrometastasis.2,3,5,6,8
We observed better results on OS, LRFS and MFS in LMS performed the initial operation at our institute than local recurrences after a surgery in other not specialized centres and metastatic LMS at the onset. Additional wide excision of LMS managed with inadequate excision in other centres had better LFRS (94% at 5 and 10 years). Surgical excision should be carefully planned by experienced surgeons considering the areas in proximity of vascular structures, nerves and bone.9,10 The treatment of LMS in facilities no-specialized in cancer care is an important risk factor for local recurrence. Chandrasekhar et al. reported 59% of local recurrences on 363 cases of soft tissue sarcoma treated inadequately.10 This finding is also confirmed by our data: local recurrence of tumors treated in non-specialized cancer center had a higher risk to develop new local recurrences (p = 0,001), distant metastases (p < 0,0001) and reduced overall suvival (p = 0,0213).
In contrast with other studies surgical margins in our experience are not negative prognostic factors on survival, even if we treated 87% of LMS with adequate surgery vs 13% with no-adequate margins (marginal surgery). Harati et al. did not observe in their series of 164 patients correlation of margins with LRFS, DSS, e OS.11 In Memorial Sloan-Kettering Cancer Center series, including abdominal and retroperitoneal LMS, positive margins did not have adverse impact on OS, but were associated with reduced LRFS.4 Several retrospective studies on extremity soft tissue sarcomas presented similar results and could not reveal a prognostic significance of negative margins on survival.12,13 Kandel et al. published a meta-analysis including 32 retrospective and prospective studies, most studies failed to establish a strong correlation between surgical margins and OS suggesting that tumour characteristics other than margin status are important.14 Neverthless, these findings are in contradiction to several large studies as well that determined a beneficial prognostic impact of negative margins on LRFS and OS.15, 16, 17 Scandinavia Sarcoma Group and Abraham reported that positive margins adversely affected the local outcome but did not influence OS.2,18
The cumulative incidence of our local recurrence is 16% with a mean disease free interval of 63 months (range 1–207 months). This is consistent with previous study of similar primary sites. Gladdy observed 10% of incidence of LR in extremity and truncal patients, all of which occurred within 5 years of resection.4 Miyajima et al. observed 8% LR rate and all recurrences occurred within 2 years from resection.6 We observed higher incidence of metastases in forty-two (42%) patients, of which 11 were really metastatic at the onset, while 31 developed metastases after surgery. Lung was the most common site of metastasis, a finding that has been previously reported.3,19,20 The second most common site of metastasis was bone in seven cases and 11 in multiple site. Local recurrence was a negative prognostic factor in our series on OS (p = 0,0487) and MFS (p = 0,0152). The strongest determining factor related to death was the presence of metastases in our series (p > 0,0001), as reported by other Authors.2
In one series, elderly patients with age >60 had a much higher incidence of metastasis during follow-up, however, the reasons for this observation are unclear. Surely physiological changes associated with aging, comorbid medical conditions, psychosocial factors, functional and nutritional status, and polypharmacy are several key issues that require careful consideration in elderly patients with cancer.21
In several studies histologic grading was a negative prognostic factor.3,5,22 Abraham et al. identified the histologic grade and tumor depth as independent factors predicting OS.18 We did not observe a correlation between grading and survival, even if 87% LMS were high grade, and only 13% low grade.
Radiation and chemotherapy had not showed beneficial prognostic effects or did not assess its impact, in accordance to other studies on somatic LMS.2,4,11,18 These data are according with our experience however adjuvant radiation has been determined to control local disease in non-visceral soft tissue sarcomas in general. In a prospective study of 2014 conducted by the National Cancer Institute in Bethesda 141 patients with extremity STS were included, it was found that patients who underwent limb-sparing surgery with adjuvant radiation had an improved LRFS compared to patients who underwent surgery without radiation.23
Doxorubicin or the association Doxorubicin/Ifosfamide are the backbone of first line treatment for advanced inoperable and metastatic disease, so a number of agents have emerged as effective second and third line options including gemcitabine/docetaxel, trabectedin and pazopanib,24,25 but the role of adjuvant chemotherapy remains controversial.
This study has some limitations: 1) it is a retrospective study, even though performed on a consecutive series of patients treated at a single Institution by the same surgical team; 2) This study includes patients treated over the long term of 25 years during which chemotherapy and radiotherapy therapies have changed drastically, but despite the evolution of therapies wide surgical excision remains the treatment of choice, particularly in non-metastatic forms 3) Our series included primary LMS together with local recurrence, radicalization after inadequate surgery performed elsewhere, metastatic LMS and type of presentation resulted correlate with the outcome.
In conclusion, this study provides data that may help clinicians estimate the prognosis of patients with extremity LMS. Adverse prognostic features included elder age, large tumour size, presentation of LMS, local recurrence and metastasis. The data from this study could not underscore the benefit of negative margins achieved at the resection of the primary tumour, while the local recurrence at presentation of LMS treated in no-specialized cancer center seems to have worse results and prognosis. Radicalization of LMS treated in other centre before knowing diagnosis showed better prognosis. Chemotherapy and radiotherapy do not seem have beneficial prognostic effects.
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., Mertens F., editors. World Health Organization Classification of Tumours of Soft Tissue and Bone. fourth ed. IARC Press; Lyon: 2013. [Google Scholar]
- 2.Svarvar C., Böhling T., Berlin Ö. Clinical course of nonvisceral soft tissue leiomyosarcoma in 225 patients from the scandinavian sarcoma group. Cancer. 2007;109(2):282–291. doi: 10.1002/cncr.22395. [DOI] [PubMed] [Google Scholar]
- 3.Farshid G., Pradhan M., Goldblum J., Weiss S.W. Leiomyosarcoma of somatic soft tissues. A tumor of vascular origin with multivariate analysis of outcome in 42 cases. Am J Surg Pathol. 2002;26(1):14–24. doi: 10.1097/00000478-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Gladdy R.A., Qin L.X., Moraco N., Agaram N.P., Brennan M.F., Singer S. Predictors of survival and recurrence in primary leiomyosarcoma. Ann Surg Oncol. 2013;20(6):1851–1857. doi: 10.1245/s10434-013-2876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mankin H.J., Casas-Ganem J., Kim J.I., Gebhardt M.C., Hornicek F.J., Zeegen E.N. Leiomyosarcoma of somatic soft tissues. Clin Orthop Relat Res. Apr 2004;(421):225–231. doi: 10.1097/01.blo.0000119250.08614.82. [DOI] [PubMed] [Google Scholar]
- 6.Miyajima K., Oda Y., Oshiro Y. Clinicopathological prog-nostic factors in soft tissue leiomyosarcoma: a multivariate analysis. Histopathology. 2002;40(4):353–359. doi: 10.1046/j.1365-2559.2002.01361.x. [DOI] [PubMed] [Google Scholar]
- 7.Gronchi A., Frustaci S., Mercuri M. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol. 2012 Mar 10;30(8):850–856. doi: 10.1200/JCO.2011.37.7218. [DOI] [PubMed] [Google Scholar]
- 8.Massi D., Beltrami G., Mela M.M., Pertici M., Capanna R., Franchi A. Prognostic factors in soft tissue leiomyosarcoma of the extremities: a retrospective analysis of 42 cases. Eur J Surg Oncol. 2004;30(5):565–572. doi: 10.1016/j.ejso.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Zagars G.K., Ballo M.T., Pisters P.W.T. Prognostic factors for patients with localized soft tissue sarcoma trated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003 May 15;97(10):2530–2543. doi: 10.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekar C.R., Wafa H., Grimer R.J., Carter S.R., Tillman R.M., Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br. 2008 Feb;90(2):203–208. doi: 10.1302/0301-620X.90B2.19760. [DOI] [PubMed] [Google Scholar]
- 11.Harati K., Daigeler A., Lange K. Somatic leiomyosarcoma of the soft tissues: a single institutional analysis of factors predictive of survival in 164 patients. World J Surg. 2017 Jun;41(6):1534–1541. doi: 10.1007/s00268-017-3899-5. [DOI] [PubMed] [Google Scholar]
- 12.Willeumier J., Fiocco M., Nout R. High-grade soft tissue sarcomas of the extremities: surgical margins influence only local recurrence not overall survival. Int Orthop. 2015 May;39(5):935–941. doi: 10.1007/s00264-015-2694-x. [DOI] [PubMed] [Google Scholar]
- 13.McKee M.D., Liu D.F., Brooks J.J., Gibbs J.F., Driscoll D.L., Kraybill W.G. The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol. 2004 Feb;85(2):68–76. doi: 10.1002/jso.20009. [DOI] [PubMed] [Google Scholar]
- 14.Kandel R., Coakley N., Werier J., Engel J., Ghert M., Verma S. Sarcoma disease site group of cancer care Ontario's program in evidence-based care. Surgical margins and handling of soft-tissue sarcoma in extremities: a clinical practice guideline. Curr Oncol. 2013 Jun;20(3):e247–e254. doi: 10.3747/co.20.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stojadinovic A., Leung D.H., Hoos A., Jaques D.P., Lewis J.J., Brennan M.F. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002 Mar;235(3):424–434. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novais E.N., Demiralp B., Alderete J., Larson M.C., Rose P.S., Sim F.H. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res. 2010 Nov;468(11):3003–3011. doi: 10.1007/s11999-010-1471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter B.K., Hwang P.F., Forsberg J.A. Impact of margin status and local recurrence on soft-tissue sarcoma outcomes. J Bone Joint Surg Am. 2013 Oct 16;95(20):e151. doi: 10.2106/JBJS.L.01149. [DOI] [PubMed] [Google Scholar]
- 18.Abraham J.A., Weaver M.J., Hornick J.L., Zurakowski D., Ready J.E. Outcomes and prognostic factors for a consecutive case series of 115 patients with somatic leiomyosarcoma. J Bone Joint Surg Am. 2012 Apr 18;94(8):736–744. doi: 10.2106/JBJS.K.00460. [DOI] [PubMed] [Google Scholar]
- 19.Clary B.M., DeMatteo R.P., Lewis J.J., Leung D., Brennan M.F. Gastrointestinal stromal tumors and leiomyosarcoma of the abdomen and retroperitoneum: a clinical comparison. Ann Surg Oncol. 2001 May;8(4):290–299. doi: 10.1007/s10434-001-0290-3. [DOI] [PubMed] [Google Scholar]
- 20.Weiss S.W., Goldblum J.R. Mosby; St. Louis: 2008. Enzinger & Weiss's Soft Tissue Tumors. [Google Scholar]
- 21.Balducci L. Studying cancer treatment in the elderly patient population. Cancer Control. 2014 Jul;21(3):215–220. doi: 10.1177/107327481402100306. [DOI] [PubMed] [Google Scholar]
- 22.Radkowski C.A., Dodd L.G., Johnson J.L., Harrelson J.M., Brigman B.E. Leiomyosarcoma of the somatic soft tissues. J Surg Orthop Adv. 2012 Summer;21(2):96–101. doi: 10.3113/jsoa.2012.0096. [DOI] [PubMed] [Google Scholar]
- 23.Beane J.D., Yang J.C., White D., Steinberg S.M., Rosenberg S.A., Rudloff U. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol. 2014 Aug;21(8):2484–2489. doi: 10.1245/s10434-014-3732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Judson I., Verweij J., Gelderblom H. European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014 Apr;15(4):415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 25.Noujaim J., Thway K., Sheri A., Keller C., Jones R.L. Histology-driven therapy: the importance of diagnostic accuracy in guiding systemic therapy of soft tissue tumors. Int J Surg Pathol. 2016 Feb;24(1):5–15. doi: 10.1177/1066896915606971. [DOI] [PubMed] [Google Scholar]