Abstract
Openness to experience has been found to be a correlate of successful aging outcomes yet also has been found to decline from middle age onward. We hypothesized that decline in openness would be associated with death. Using longitudinal data from the Swedish Adoption/Twin Study of Aging (SATSA), the analytic sample encompassed 1954 individuals, approximately two-thirds of whom were deceased. We tested whether openness declines across late adulthood and, central to our hypothesis, whether the decline correlated with age at death. Multivariate modeling adjusted for age at study entry, sex, education, as well as the time-varying effects of physical illness, depressive symptoms, and cognitive ability. Correlations between change in neuroticism and extraversion and death were modeled for comparison. A follow-up co-twin control analysis adjusted for genetic and environmental familial confounders. Significant mean-level change was identified in all personality traits, but only for openness was change correlated with age at death, in support of our hypothesis. The findings were not explained by health factors or cognition. Co-twin control analyses indicated that the twin who died earlier showed a greater drop in openness prior to death, compared to their co-twin measured at the same time points. There was no co-twin finding for neuroticism or extraversion. We suggest that declines in openness may reflect a change in goal orientation due to the experience of a shortened time horizon, leading to an optimized selection of experiences as people approach the end of life.
Keywords: openness to experience, extraversion, mortality, neuroticism, personality
No longer considered to be unchanging throughout adulthood, personality development occurs across the lifespan (see Roberts, Walton, & Viechtbauer, 2006). Openness to experience (Openness; O), characterized by intellect, knowledge seeking, engagement with new experiences, and curiosity (McCrae, 1987), has been found to decline across older adulthood (Pedersen & Reynolds, 1998; Roberts & Mroczek, 2008; Schwaba & Bleidorn, 2018; Wagner, Ram, Smith, & Gerstorf, 2016). Additionally, lower levels of openness at a single measurement point have been found to correlate with mortality risk (Ferguson & Bibby, 2012; Iwasa et al., 2008; Jonassaint et al., 2007; Turiano, Spiro, & Mroczek, 2012). Unaddressed in the literature is whether declines in openness are associated with impending death. As proposed below, the correlation between decline in openness and death matters in understanding the role of openness in the lives of older adults.
The primary purpose of this study was to examine whether openness declines in advance of death. Two other traits were available longitudinally in the current study: neuroticism (N) and extraversion (E). These traits were modeled for comparison. We predicted that decline in openness would correlate with death as compared to neuroticism and extraversion, and that the association would not be explained by other factors, including poor physical and psychological function.
Openness to Experience
Openness has been conceptualized within a framework of intellectual plasticity, curiosity, behavioral flexibility, and social engagement (Hirsh, DeYoung, & Peterson, 2009; McCrae & Sutin, 2009), such that individuals reporting higher openness have been found to engage more in physical, cognitive, and social activities (Barnett, 2006; Ihle, Oris, Fagot, Maggiori, & Kliegel, 2016; Stephan, Boiché, Canada, & Terracciano, 2014) and feel better about engaging in these activities (Kahlbaugh & Huffman, 2017). Studies of the oldest old, for example, have demonstrated that centenarians high in openness reported a more engaged lifestyle (Martin, Baenziger, MacDonald, Siegler, & Poon, 2009) and had higher cognitive functioning and engagement in volunteer work (Baek, Martin, Siegler, Davey, & Poon, 2016). Both openness and activity engagement have been found to be associated with better cognitive ability in older adults (Hogan, Staff, Bunting, Deary, & Whalley, 2012). Similarly, in a prior separate SATSA study openness measured at baseline correlated with better cognitive functioning in the second half of the lifespan, adjusting for effects of education, activities of daily living, and presence of cardiovascular disease (Sharp, Reynolds, Pederson, & Gatz, 2010). Taken together, characteristics associated with openness – intelligence, curiosity, behavioral flexibility, and an active lifestyle – correlate with better outcomes across biological, social, and cognitive domains in older adulthood (Rowe & Kahn, 1997; 2015). Yet, despite higher openness predicting better outcomes, openness is consistently found to decline after midlife (Roberts et al., 2006). Other prior work (Iwasa et al., 2008; Jonassaint et al., 2007) has shown that lower baseline openness scores predicted earlier age at death. The motivation for the current study, thus, is to extend previous work and test whether decline in openness was associated with death.
Theoretical Framework
The selective optimization with compensation model (SOC; P.B. Baltes, 1987; P.B. Baltes and M.M. Baltes, 1990) provides a useful framework for our prediction that declines in openness occur in advance of death, in contrast to what we would expect for other personality traits, like neuroticism and extraversion. SOC is a general life-span developmental framework that specifies processes through which people maximize gains relative to losses across the life span (P.B. Baltes, 1987). A core feature of SOC is that, in the face of age-related cognitive and physical losses, people make “selective and compensatory efforts dealing with evolving deficits for the purpose of life mastery and effective aging” (P.B. Baltes, 1987; p. 616). SOC explains how older adults conserve biological and social resources for essential goals as normative aging-related losses increase relative to gains (see also McAdams & Olson, 2010). With aging, people narrow their goals, select the most important goals, focus their resources on those goals, and enlist compensatory strategies toward meeting those goals and avoiding further loss (Nikitin & Freund, 2018). While not tested directly in the current study, we suggest that these SOC processes may explain decline in openness scores in advance of death as decline in openness reflects focusing on familiar goals and activities as opposed to seeking new goals (e.g., new knowledge and new experiences).
Subsequent theorists have expanded upon the SOC framework and describe related processes that lead to similar predictions, including socioemotional selectivity theory (SST; Carstensen, 1992) and terminal decline (Gerstorf & Ram, 2013; Schilling, Wahl, & Wiegering, 2013). SST draws on the idea that conscious or unconscious awareness of reduced time horizons, i.e., fewer years remaining, lead people to invest in only the most socially rewarding relationships as opposed to exploring new relationships and information (Carstensen, 1992; Charles & Carstensen, 2010). Terminal decline theorists suggest that the successful navigation of terminal decline also entails change in goal orientation, noting that “it is also conceivable that goal disengagement becomes increasingly relevant at the end of life because disengagement allows people to let go of the goals that are not attainable anymore” (Gerstorf & Ram, 2013, p. 734). These authors likened shifts in goals to secondary control. Along these lines, Worsch and Heckhausen (1999) observed that late middle-aged older adults, compared to younger adults, engaged in greater loss avoidant relationship goals, fewer gain-oriented relationship goals, and more compensatory secondary control strategies (e.g., goal disengagement and downward comparisons such as comparing current well-being to less fortunate people). Notably, greater use of compensatory secondary control strategies was positively correlated with positive affect in older adults but negatively correlated in younger adults.
These alternative end-of-life formulations complement SOC in suggesting that selection and optimization of one’s goals—which we argue would be reflected in decline in openness scores—may serve to maintain sense of well-being. Although all personality traits may fluctuate with age, including extraversion and neuroticism (Roberts & Mroczek, 2008), loss avoidance and goal disengagement that naturally occur with age should be uniquely associated with declines in openness in the years prior to death.
In the remainder of the introduction, we briefly review the literature on change with age in O, N, and E, their relationship with death, and potential health mediators of this relationship to frame the current investigation.
Openness and Death
Openness has been found to be generally stable through middle adulthood, followed by decline through late adulthood (Mõttus, Johnson, Starr, & Deary, 2012; Roberts et al., 2006), a finding also observed in a previous study using SATSA data (Pedersen & Reynolds, 1998). There is support in the literature for an inverse relationship between mean openness and mortality, although findings have been mixed. In a large sample of men from the Veterans Affairs Normative Aging Study, low scores on two facets of openness—intellect and creativity— were associated with increased mortality risk (Turiano et al., 2012). Similarly, higher openness scores were associated with decreased mortality risk in a community-based Japanese sample (Iwasa et al., 2008) as well as in a sample of cardiac disease patients (Jonassaint et al., 2007). Further, a meta-analysis of 11 studies examining openness and all-cause mortality, lower openness scores predicted increased mortality risk (Ferguson & Bibby, 2012). We note, however, that other studies have not observed a significant relationship between openness and mortality (Christensen et al., 2002; Turiano, Chapman, Gruenewald, & Mroczek, 2015; Weiss & Costa, 2005; Wilson, Mendes de Leon, Bienias, Evans, & Bennett, 2004). A recent longitudinal investigation, for example, found that openness declined approximately one-half standard deviation over the last ten years of life in a decedent population from the Berlin Study of Aging and this decline was related to poor health; yet neither the mean-level (intercept) nor slope (change) of openness was moderated by a time to death index (Wagner et al., 2016).
Neuroticism and Death
Change in neuroticism in late adulthood has also been demonstrated (e.g., Small, Hertzog, Hultsch, & Dixon, 2003): both to decrease with age (Bleidorn, Kandler, Riemann, Spinath, & Angleitner, 2009; Pedersen & Reynolds, 1998) and to increase with age (Mroczek & Spiro, 2007; Wagner et al., 2016). Findings from studies examining the relationship between neuroticism and mortality have also been mixed. In a large study of older Catholic clergy members (Wilson et al., 2005), individuals with high neuroticism (90th percentile) had significantly increased mortality compared to individuals with low neuroticism (10th percentile). Similar results were observed when examining effects of neuroticism on risk of cardiovascular mortality (Shipley, Weiss, Der, Taylor, & Deary, 2007). Increases in neuroticism scores over age also have been found to predict mortality (Mroczek & Spiro, 2007; Wagner et al., 2016). Yet, other studies have shown that higher baseline neuroticism correlates with a reduction in mortality risk (Friedman, Kern, & Reynolds, 2010; Gale et al., 2017; Korten et al., 1999; Weiss & Costa, 2005). Further, results from a 42-year follow-up period found no significant relationship between baseline neuroticism and all-cause mortality (Batty, Jokela, Kivimaki, & Shipley, 2016).
Extraversion and Death
Extraversion also has been observed to change as well as remain constant over age (Bleidorn et al., 2009; Roberts et al., 2006), and findings regarding the association between extraversion and mortality have also been mixed. Higher extraversion across adulthood has been found to predict decreased mortality risk, including after adjusting for possible mediators such as health and well-being (Fry & Debats, 2009; Iwasa et al., 2008; Read, Vogler, Pedersen, & Johansson, 2006; Wilson et al., 2004). Other large cohort investigations, however, did not report a relationship between extraversion and death (see Batty et al., 2016; Shipley et al., 2007; Wagner et al., 2016). Chapman, Roberts, and Duberstein (2011) suggested that the mixed findings for extraversion might be partially explained by historical changes in scale items. Specifically, whether extraversion is defined by positive mood and sociability versus impulsivity and sensation seeking may result in different relationships with age, health, and death.
Personality, Health, and Death
Health factors may explain change in personality in advance of death. Both protective factors like health care seeking and risky behaviors like smoking may moderate or mediate the correlation between personality and death (Friedman et al., 2010; Martin, Friedman, & Schwartz, 2007; Mroczek, Spiro, & Turiano, 2009; Turiano et al., 2012; Turiano et al., 2015). For example, negative components of extraversion (e.g., impulsivity) were found to positively correlate with negative health behaviors known to increase mortality risk (Chapman et al., 2011). Yet, the association between health and personality varies between and within populations. For example, high neuroticism has been found to be a risk factor for cardiovascular mortality in low SES women, whereas in high SES women, high neuroticism was associated with decreased mortality risk (Hagger-Johnson et al., 2012).
Low openness scores have been found to correlate with high blood pressure, stroke, heart conditions, and arthritis—factors that increase the risk of death (Weston, Hill, & Jackson, 2015). The relationship between openness and health also may be mediated by cognitive ability. For example, greater intellectual abilities—a factor associated with openness—may promote better health behaviors and/or better capacity for navigating healthcare, which in turn may lower mortality risk (see Jonassaint et al., 2007). Finally, higher self-reported disabilities were related to steeper declines in openness, particularly within the last seven years of life (Wagner et al., 2016). For these reasons, we include measures of physical illness, depressive symptoms, and cognitive ability in our study to examine the possibility that health factors explain declines in openness.
The Current Study and Hypotheses
The central purpose of this study was to examine whether openness declines in advance of death. Further, we hypothesized the association would hold after adjusting for time-varying effects of physical illness, depressive symptoms, and cognitive ability on the change in openness.
First, we examined differences in survival estimates as a function of differences in baseline openness scores, expecting to confirm prior results that lower openness would be associated with death. Second, we fit latent growth models to repeated measures of openness scores, expecting to replicate past findings that openness would decline in late adulthood. Third, we used a simultaneous growth-survival model (Ghisletta, 2008; Ghisletta, McArdle, & Lindenberger, 2006) to test our hypothesis that differences in rates of decline in openness would correlate with age at death, above and beyond baseline openness scores. Fourth, we adjusted our models for effects of time-constant demographic variables as well as time-varying physical illness, depressive symptoms, and general cognitive ability covariates to examine whether health decline and/or disability account for declines in openness in advance of death. Finally, we leveraged the genetically informed design of our sample, hypothesizing that twins who died earlier would be associated with greater change in levels of openness from baseline to final measurement preceding death compared to their co-twins who died later (McGue, Osler, & Christensen, 2010).
For comparison, we conducted analogous analyses with neuroticism and extraversion, where we expected to replicate prior findings that neuroticism would decline with age and that extraversion would remain stable (Bleidorn et al., 2009; Pedersen & Reynolds, 1998). Given the conflicting literature, we did not make a specific prediction about the association between neuroticism and death. We did not expect baseline extraversion to be related to mortality risk. We predicted that change in neither neuroticism nor extraversion would correlate with death.
Method
Participants.
Participant data were drawn from the questionnaire-based portion of the Swedish Adoption/Twin Study of Aging (SATSA; Pedersen et al., 1991). Participants must have completed at least one personality measurement to be included in the current sample (McArdle & Anderson, 1990; McArdle & Hamagami, 1992). The total sample for the openness analysis included 1,954 participants while the total sample for the neuroticism and extraversion analyses included 2,149 participants. The first wave of measurement (Time 1: T1) was in 1984, with T2 in 1987, T3 in 1990, T4 in 1993, T5 in 2004, T6 in 2007, and T7 in 2010. Each questionnaire was sent out to the entire sample of still living participants regardless of whether they responded to the prior wave. The number of participants included in each wave fluctuates based on participant attrition, inaccessibility at specific waves, refusal, and death (Finkel & Pedersen, 2004). Table 1 shows samples sizes across all seven waves.
Table 1.
Participation sample sizes and personality means and standard deviations across waves of measurement (T).
| Openness | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|
| N | 1428 | 1486 | 1364 | 1351 | 748 | 610 | 505 |
| MeanOpen | 17.84 | 17.79 | 17.90 | 17.86 | 18.11 | 18.15 | 18.20 |
| SDOpen | 4.08 | 4.29 | 4.44 | 4.41 | 4.21 | 4.16 | 4.26 |
| MeanAge | 59.02 | 61.37 | 63.03 | 64.58 | 69.44 | 71.45 | 73.16 |
| SDAge | 13.48 | 13.53 | 12.84 | 12.88 | 10.89 | 10.32 | 9.64 |
| # died between previous & current T | - | 95 | 86 | 126 | 516 | 150 | 117 |
| Neuroticism | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
| N | 1925 | 1575 | 1438 | 1407 | 772 | 633 | 539 |
| MeanNeuroticism | 2.81 | 2.43 | 2.43 | 2.30 | 2.37 | 2.27 | 2.26 |
| SDNeuroticism | 2.36 | 2.22 | 2.16 | 2.18 | 2.11 | 2.14 | 2.15 |
| MeanAge | 59.71 | 61.91 | 63.35 | 64.80 | 69.73 | 71.66 | 73.22 |
| SDAge | 13.95 | 13.63 | 12.94 | 12.92 | 11.04 | 10.33 | 9.66 |
| # died between previous & current T | - | 145 | 117 | 141 | 565 | 158 | 123 |
| Extraversion | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
| N | 1934 | 1576 | 1436 | 1410 | 772 | 635 | 541 |
| MeanExtraversion | 4.81 | 5.07 | 5.09 | 5.09 | 5.20 | 5.17 | 5.25 |
| SDExtraversion | 2.29 | 2.25 | 2.23 | 2.25 | 2.25 | 2.26 | 2.18 |
| MeanAge | 59.76 | 61.99 | 63.42 | 64.92 | 69.74 | 71.74 | 73.25 |
| SDAge | 13.99 | 13.63 | 12.94 | 12.96 | 11.03 | 10.37 | 9.62 |
| # died between previous & current T | - | 146 | 119 | 143 | 563 | 158 | 123 |
Note. Years of measurement: T1 = 1984; T2 = 1987; T3 = 1990; T4 = 1993; T5 = 2004; T6 = 2007; and T7 = 2010. Of the total analytic samples, 60.75% (n = 1,187) were deceased for openness (97 more died after T7); 63.05% (n = 1,355) were deceased for neuroticism (106 more died after T7); and 63.15% (n = 1,357) were deceased for extraversion (105 more died after T7). We also note that there is an increase in participants who provided openness scores from T1 to T2 because the T1 openness measure was included in a second booklet that was sent to participants after they had returned their first booklet. The first booklet contained the Eysenck Personality Inventory.
Mortality data were obtained for all decedents from the Swedish Cause of Death Register (CDR). For nondecedents, age was calculated from the censoring date (01 April 2014). This censoring date was selected because it was three years following the last wave of personality data collection (T7 in 2010). The survival period was from study entry to either death or study end (max survival = 29.41 years). Of the total analytic samples for openness, neuroticism, and extraversion, 60.75% (n = 1,187) were deceased for openness; 63.05% (n = 1,355) were deceased for neuroticism; and 63.15% (n = 1,357) were deceased for extraversion. Decedents’ mean age was 67.23 (SD = 10.35) at baseline measurement, 75.28 (SD = 9.44) at last measurement, and the mean age-at-death was 82.12 (SD = 9.44). The mean interval between baseline measurement and death was 14.89 years (SD = 7.84), and the mean interval between the last measurement and death was 6.84 years (SD = 5.61). The modal number of measurement occasions completed was four. The most common pattern of attrition was participant death or drop out during the 10.5-year gap between waves 4 (1993) and 5 (2004).
Measures
Personality Traits.
Openness, neuroticism, and extraversion data were collected via questionnaires mailed to all eligible participants. Openness was measured with a 6-item scale derived by factor analyzing the 26-item scale of the NEO Personality Inventory (Costa & McCrae, 1985; Pedersen, Plomin, McClearn, & Friberg, 1988) and selecting the 6 items with the highest factor loadings (see Bergeman et al., 1993). The items tapped both the intellectual curiosity and engagement in new experiences aspects associated with openness (e.g., pondering ideas, engagement in hobbies, exploring new foods). Openness items were measured in the traditional NEO-PI fashion using a 5-point Likert scale ranging from 1 = strongly disagree to 5 = strongly agree. Neuroticism and extraversion were measured using a 9-item short form of the Eysenck Personality Inventory (Floderus, 1974). Neuroticism consists of items that measure anxiety, dysphoria, obsessiveness, and sensitivity; and extraversion consists of items that measure sociability, impulsivity, and sensation seeking. Extraversion and neuroticism items were scored based on “yes-no” response categories. All items were scored so that higher values indicate more of the trait. Items were summed to create trait scores for openness, neuroticism, extraversion; openness ranged from 6–30; neuroticism and extraversion ranged from 0–9. Across the 7 waves, reliabilities (coefficient alphas) ranged from .62–.69 for openness, .66–.69 for extraversion, and .71–.76 for neuroticism. Personality traits were measured with the same items across all longitudinal measurement occasions. Conscientious and agreeableness were not available longitudinally.
Physical Illness.
A cumulative illness variable was created to index physical disorders and conditions (Harris, Pedersen, McClearn, Plomin, & Nesselroade, 1992; Rowe, 1985). Thirteen items were used to measure a range of health problems covered in a standard review of systems (e.g., cardiovascular disease, respiratory disorders, musculoskeletal disorders, allergies, vision disorders, metabolic disorders, gastrointestinal disorders, and urological disorders). All items were endorsed using “yes-no” response categories and summed to create an overall index of physical illness at each measurement occasion (range 0–13). Higher scores indicate worse health problems. Physical illness, as indexed by cumulative illness, was included as a time-varying covariate in the latent growth curve models, except at wave 6.
Depressive Symptoms.
The Center for Epidemiological Studies Depression scale (CESD; Radloff, 1977) was used to measure depressive symptoms beginning at the second wave of measurement. The CESD was not administered at wave 1 (1984). Participants endorsed items using the original response categories (0 = “rarely or none of the time” to 3 = “most or all of the time”). Items were summed to create an overall depressive symptom score. CESD sum scores were included as a time-varying covariate in the latent growth curve models, except at wave 1.
General Cognitive Ability.
A subset of participants in SATSA aged 50 and older was selected for in-person cognitive testing in the years between waves of measurement when personality data were collected (n1986 = 587, n1989 = 469, n1992 = 453, n2002 = 349, n2006 = 263, n2009 =207). The 10 cognitive tests included verbal and performance measures and were designed to assess intellectual abilities (e.g., not clinical measures). Principal components analysis was performed on the 10 subtests to derive the first principal component at each wave (Pedersen, Plomin, Nesselroade, & McClearn, 1992). The factor structure of these individual cognitive tests has previously been shown not to vary systematically across age or time (Finkel, Reynolds, McArdle, & Pedersen, 2005). Scores from the first principal component were used as the measure of cognitive ability and were included as a time-varying covariate in the latent growth curve models.
Education.
Participants were asked their highest educational attainment: elementary school; lower secondary or vocational school, upper secondary education, and tertiary education. In the current sample, the majority (60%) had an elementary education (6 to 7 years), which was the obligatory number of years of education required to complete formal education in Sweden during this time period. Education was collected at wave 1 and was available for greater than 90% of the sample.
Entry Age.
This covariate indicated the age at which participants entered SATSA.
Data Analysis
Descriptive analyses (means, standard deviations, and correlations) are presented to describe mean levels of O, N, and E traits over time.
Survival analysis.
Kaplan-Meier survival curve plots are presented to examine differences in survival rates as a function of differences in baseline O, N, and E traits. Survival curves for high and low baseline O, N, and E trait score groups were constructed using the survival package in R 3.3.1 (Therneau, 2013). For descriptive purposes, participants were categorized based on whether their O, N, and E trait scores were +1 SD above baseline means (i.e., high openness, neuroticism, and extraversion) or −1 SD below baseline means (i.e., low openness, neuroticism, and extraversion).
Latent Growth Curve Modeling.
We next used latent growth curve modeling (LGCM) to examine patterns of stability and change in O, N, and E traits longitudinally. The purpose of this analysis is to quantify and test change on traits across late adulthood. LGCMs allow for comparisons of individual trajectories of decline as well as an average trajectory of decline across the entire sample where complete data for all participants are not available (Bryk & Raudenbush, 1987; Finkel, Reynolds, McArdle, Gatz, & Pedersen, 2003; McArdle & Anderson, 1990; McArdle & Hamagami, 1992). In the current study, data from individuals with only one measurement occasion is included to stabilize both mean and variance estimates (Finkel, Reynolds, McArdle, Gatz, & Pedersen, 2003; McArdle & Anderson, 1990; McArdle & Hamagami, 1992). A basic statistical assumption of LGCMs is that missing data are missing at random (MAR). Latent growth curve models allow for missing data by giving more weight to individuals with the most measurement occasions. Full information maximum likelihood (FIML) was used to handle missingness under the MAR assumption (Enders, 2010; Little, 1995).
A common issue in longitudinal studies of aging is that missing data patterns are nonignorable. While FIML assumes that data are missing at random (MAR), missingness often is correlated with the outcome of interest and so fails to meet the MAR assumption. In our preliminary data analyses, we tested the hypothesis that missingness was truly random (i.e., missing completely at random (MCAR)) using a test of MCAR (Jamshidian & Jalal, 2010), and found that missingness was not random for all personality traits. In order to understand missing data mechanisms underlying missingness for each trait, we explored whether the number of completed assessments correlated with death status, gender, education, and zygosity. The number of completed assessments significantly correlated with death status, and education, but not gender or zygosity. Further analysis suggested no statistically significant differences on mean baseline openness, neuroticism, and extraversion scores between still living participants who completed all assessments and still living participants who only completed a baseline assessment, which suggests attrition due to death only partly explains missingness. As mortality is the outcome of interest and education and gender are included as covariates in our analyses, we proceeded with performing all multivariate analyses assuming missing data were MAR. Even though missingness may be nonignorable, FIML estimates are likely to be unbiased assuming MAR (Schafer & Graham, 2002), especially when demographic covariates correlated with missingness are included (Enders, 2010).
Latent growth curve models include the following parameters: a random intercept (I) that quantifies the level of a trait at the first wave of measurement; a random linear slope (S) that quantifies the mean rate of change across the sample; and a random quadratic slope (Q) that quantifies the mean nonlinear rate of change, i.e., acceleration or deceleration. The intercept and slopes are estimated for each participant and then aggregated across the sample and consist of fixed effects (means) and random effects (variances and covariances) that describe the distributions of each LGCM parameter based on chronological age (McArdle, Ferrer-Caja, Hamagami, & Woodcock, 2002). Significant random effects indicate individual differences in intraindividual change.
In the current study, age was centered at the sample mean age at first measurement occasion (65 years) and divided by 10 to evaluate change per decade. Thus, for example, the intercept represents the sample-level mean openness score at age 65; the linear slope represents the amount of per decade linear change in openness; and the quadratic slope represents nonlinear change per decade in openness. Indices of physical illness, depressive symptoms, and cognitive ability were included as time-varying covariates, meaning that these effects were regressed out of observed O, N, and E scores in the growth curve estimation. Time invariant covariates, including education level, sex, and entry age, were included to adjust the parameter estimates for individual differences in the random intercept and slopes. For educational attainment, three dummy variables were entered into the model to account for effects of lower secondary, upper secondary, and tertiary education on the intercepts and slopes with elementary education as the reference category. Sex was effect-coded (males = −0.5, females = +0.5). For each trait, in this example, openness (O), the LGCM is defined as:
| (1) |
Oij represents personality trait scores for the ith individual at measurement j; Ageij is the ith individual’s age at measurement j; Wi is a general term that represents the covariates for the ith individual (i.e., education, sex, and entry age); γ00 reflects the average intercept at age 65 and average education score; γ10 represents the linear rate of change at age 65 at the average education level score by decade; γ20 represents the quadratic rate of change at the average education level score by decade; ζ 0i, ζ 1i and ζ 2i reflect the ith individual’s deviations from the mean intercept, slope, and quadratic parameter estimates, respectively; γ01 is the effect of education level on the intercept; γ11 is the effect of education level on the slope; γ21 is the effect of education level on the quadratic slope; and εij reflects the deviation of the ith individual’s score at measurement j from their expected linear trajectory.
Initial LGCMs were fit to identify whether an intercept only, intercept and linear slope, or intercept, linear slope, and quadratic slope provided the best fit to each trait. All models were adjusted for effects of sex, education level, and entry age into the SATSA study. We summarize the results from the best fitting model prior to reporting results from the growth survival models (described below). Model fitting was computed in Mplus 8.0 (Muthén & Muthén, 1998–2017) using full-information maximum likelihood with robust standard errors as clustering via twin pair was accounted for in the model, as well as violations of the multivariate normality. Standard errors and chi-square tests of model fit were computed to take into account the non-independence of observations (i.e., twins nested within family).
Simultaneous growth-survival models.
Next, we fit latent growth curve-survival models to test the main hypothesis that openness declines in advance of death. For this analysis, years between study entry and death or study end describe the survival period during which death could occur. In this model, individuals’ age at death is regressed on their growth parameters of openness while accounting for censoring in a single analysis (Guo & Carlin, 2004). Figure 1 presents a path model of the simultaneous growth and survival model within a quadratic LGCM framework, adjusting for effects of the time constant covariates (see McArdle, Small, Bäckman, & Fratiglioni, 2005). The following semi-parametric Cox regression model using maximum likelihood with robust standard errors to estimate the likelihood values and chi-square statistic was fit to participants’ age of death scores, where differences in death, t, were modeled as a continuous-time hazard process (“failure” after entry into the SATSA study):
| (2) |
Cox regression parameter estimates are the natural log odds of individual i’s death (nested in family j). First, βh,C represents the p x 1 vector of effects of the time-invariant covariates, xT. including sex, education level, and entry age. Entry age adjusts for potential selection effects on study entry and was centered at age 65. Next, bh,I and bh,S represent the effects of the random intercept and linear slope effects, respectively, estimated in the latent growth curve models. In the next step, time-varying indices of physical illness, depressive symptoms, and cognitive ability were included as covariates to test the robustness of effects of the random intercept and linear slope of openness on death. A statistically significant bh,S parameter for openness provides support for the hypothesis that openness declines in advance of death. Odds ratios (OR) are estimated by exponentiating the log odds regression coefficient. An OR represents the factor increase (or decrease) in death associated with each predictor variable.
Figure 1.
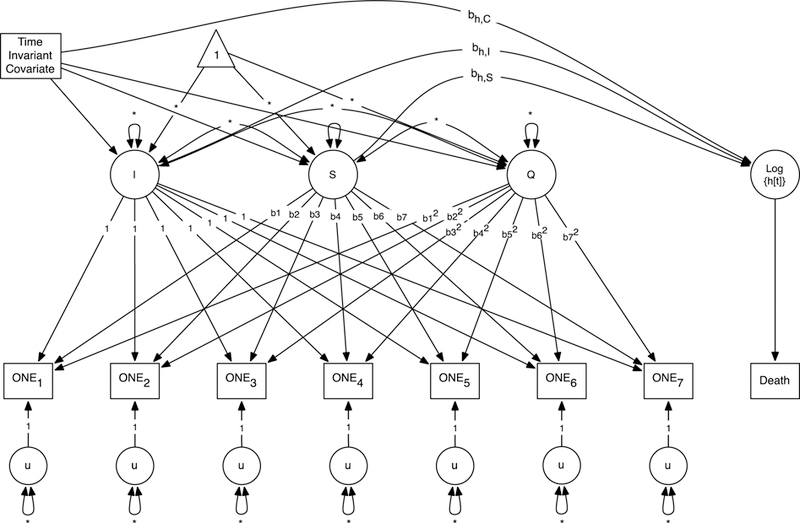
Simultaneous latent growth model and Cox regression model. Simultaneous growth and survival model is an expansion of the quadratic growth model for O, N, and E personality traits (ONE). Growth parameters – intercept (I), linear slope (S) and quadratic slope (Q) – correlate with hazard rates (Log{h[t]}). The hazard is indicated by a binary outcome of death at a particular age (adapted from McArdle, et al., 2005). Time-invariant covariates include sex, education level, age of entry into SATSA study. Time-varying physical illness, depressive symptoms, and general cognitive ability covariates (not depicted for clarity of presentation), but included in secondary analysis (see Supplemental Materials).
All growth-survival models were estimated in Mplus 8.0 (Muthén & Muthén, 1998–2017). All models were estimated using full-information maximum likelihood with robust standard errors so that all available data (twins from families both with complete and incomplete data) could be used. Standard errors and chi-square tests of model fit were computed to take into account the non-independence of observations. The Satorra–Bentler scaled chi-square (S-Bχ2) difference test was used to calculate a chi-square distributed test statistic to compare nested models (Satorra & Bentler, 2001). The Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) were used to evaluate relative model fit (Burnham & Anderson, 2004), where values nearer to zero indicate better model fit based on model parsimony and model complexity.
Sample sizes available for the multivariate analyses described above varied with each O, N, and E trait and model selection. Not all participants completed all trait measures within each wave of measurement. Kaplan-Meier survival plots were estimated using one twin randomly selected from each pair (n = 879) for the reason that family membership could not be adjusted for in these plotting models. In the simultaneous growth and survival models, sample sizes varied among personality trait (nOpenness= 1,762, nNeuroticism = 1,939, nExtraversion = 1,938).
Co-twin control analysis.
Finally, an exploratory analysis was undertaken using co-twin control methods to evaluate whether O, N, and E traits decline in advance of death. Twins are matched for age and gender, as well as for additive effects of genotype (completely matched in the case of monozygotic [MZ] twins, and partially matched in dizygotic [DZ] twins) and early life environmental influences (completely matched for pairs reared together), thereby controlling for a wealth of measured and unmeasured genetic and shared environmental influences on the trait. In this way, co-twin control designs are useful for strengthening conclusions about whether openness declines in advance of death by testing whether the twin in the pair who dies first shows the greater decrease in openness (McGue et al., 2010). Both MZ and DZ twin pairs were identified for who were discordant for death, i.e., one twin was deceased prior to the other twin. Within discordant pairs, matched t-tests were used to compare the intrapair difference at first time of measurement to the intrapair difference at the last time of measurement. The first time of measurement was the first wave at which both members of the pair completed each scale, and the last time of measurement was the last wave at which both members of the pair responded prior to the first twin’s death, with the second twin living at least to the next wave of measurement, (nOpenness= 207 pairs, nExtraversion = 274 pairs, nNeuroticism = 274 pairs).
Results
Descriptive Statistics
Table 1 presents the means and standard deviations for each O, N, and E trait and age at each wave of measurement.
Our initial predictions were supported by a graphical illustration (Figure 2), where participant attrition due to death is depicted by comparing the trait means of participants who died prior to the next measurement occasion, died after a later measurement occasion, or were still alive as of the censoring date. Participants’ neuroticism and extraversion scores did not change before their final measurement occasion prior to death compared to participants who continued to complete subsequent measurements. Clearly depicted, however, participants’ openness scores declined before their final measurement occasion prior to death compared to participants who continued to complete subsequent measurements. Post-hoc follow-up analyses of drop out indicated that the openness scores in the last measurement prior to a participant dropping out tended to remain flat, suggesting that openness scores declined in specific relation to death rather than attrition due to drop out.
Figure 2.
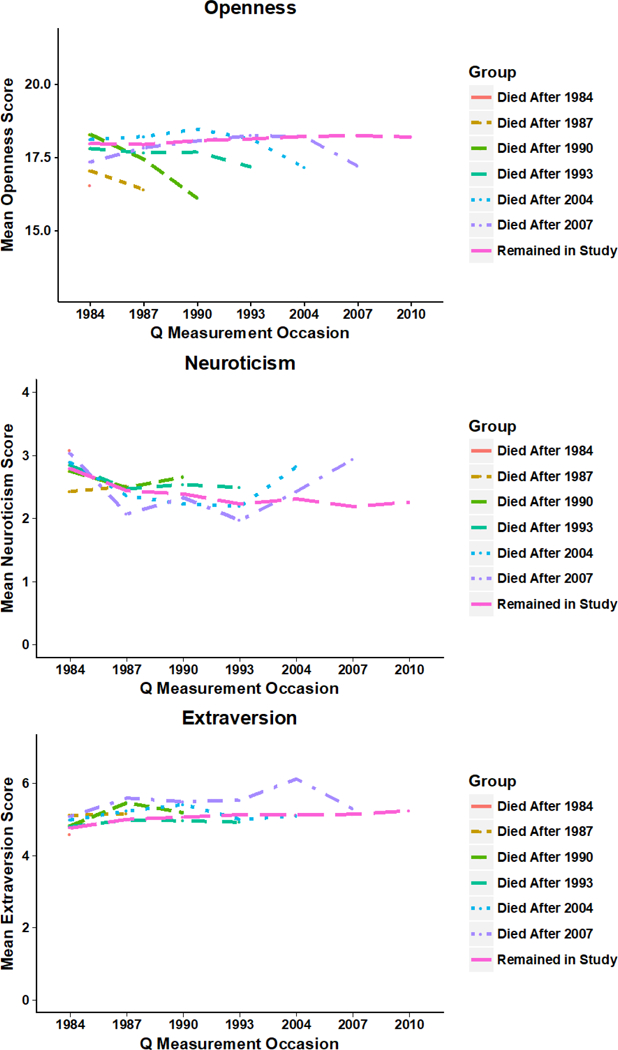
Longitudinal scores on O, N, and E for participants who died prior to the next measurement occasion compared to participants who remained in the study.
Survival Analyses
Kaplan-Meier survival curves (Figure 3) demonstrate that participants with high baseline openness had greater rates of survival compared to participants with low baseline scores. There was a significant difference in survival rates, as indicated by the nonoverlapping 95% confidence intervals of the survival curves of participants high on openness and the curves of participants low on openness. Survival rates were nearly equal for participants with high and low neuroticism and high and low extraversion scores, suggesting that baseline neuroticism and extraversion did not differentially predict survival rates.
Figure 3.
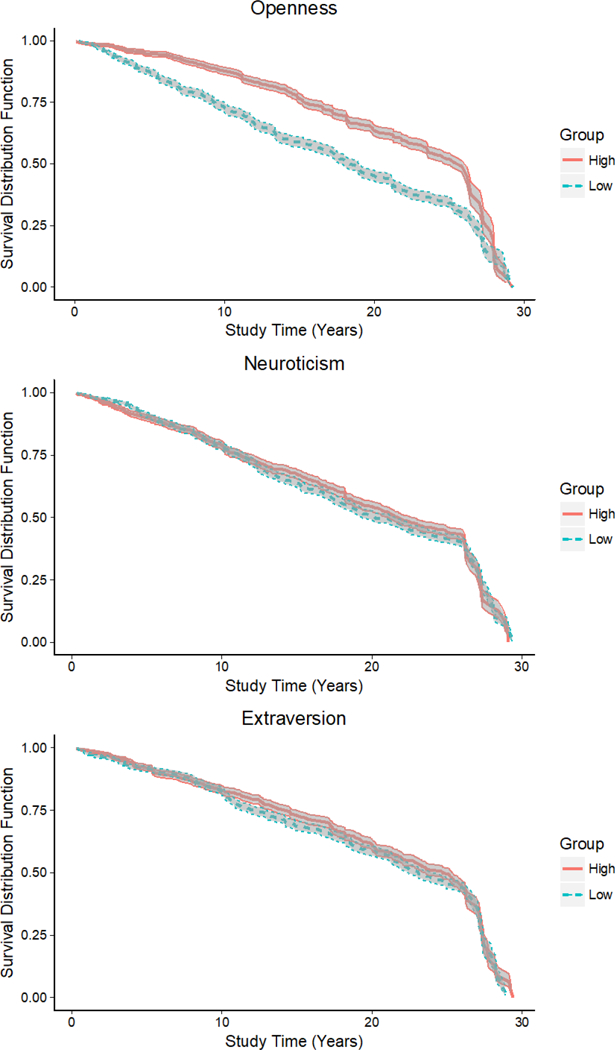
Kaplan-Meier survival plot by baseline O, N, and E scores.
Note. High scores =1 standard deviation above; Low scores = 1 standard deviation below the mean. Shaded regions indicate the 95% confidence interval for each group.
Latent Growth Curve Models
LGCMs were fit to O, N, and E traits to determine whether each trait demonstrated change over time. The best fitting model is subsequently used in the growth-survival models. Table 2 presents the model fit results. The best fitting LGCM for openness (indicated by significant improvement in the S-Bχ2, AIC, and BIC values) was Model 3 and was characterized by an intercept (MI = 16.98, t = 126.93; σ2I = 11.50, t = 21.41), linear slope (MS = −0.41, t = −6.28; σ2S = 0.77, t = 5.02), and quadratic slope (MQ = −0.24, t = −3.24; σ2Q = 0.37, t = 3.61). Openness decreased linearly for every year of age after 65 and the quadratic effect accelerated the decline.
Table 2.
Model fit results from the latent growth models for O, N, and E personality traits.
| Model | Description | -LL | Comparison | ΔS-Bχ2 | Δdf | p | AIC | BIC |
|---|---|---|---|---|---|---|---|---|
| Openness | ||||||||
| 1 | Intercept only | −17896.38 | - | - | - | - | 35806.75 | 35845.07 |
| 2 | Linear slope | −17822.35 | 1 | 142.01 | 5 | 0.000 | 35672.69 | 35749.33 |
| 3 | Quadratic slope | −17762.53 | 2 | 111.01 | 6 | 0.000 | 35569.07 | 35689.50 |
| Neuroticism | ||||||||
| 1 | Intercept only | −15307.88 | - | - | - | - | 30629.76 | 30668.74 |
| 2 | Linear slope | −15204.79 | 1 | 186.07 | 5 | 0.000 | 30437.57 | 30515.54 |
| 3 | Quadratic slope | −15138.10 | 2 | 118.35 | 6 | 0.000 | 30320.19 | 30442.72 |
| Extraversion | ||||||||
| 1 | Intercept only | −14993.48 | - | - | - | - | 30000.97 | 30039.96 |
| 2 | Linear slope | −14908.04 | 1 | 170.57 | 5 | 0.000 | 29844.07 | 29922.05 |
| 3 | Quadratic slope | −14858.13 | 2 | 102.98 | 6 | 0.000 | 29760.26 | 29882.80 |
Note. All models include time-invariant sex and education level covariates. -LL= -Log Likelihood; ΔS-Bχ2 = Satorra-Bentler chi-square difference test of nested models; AIC = Akaike Criterion Index; BIC = Bayesian Criterion Index.
For neuroticism, the best fitting LGCM also was Model 3 and was characterized by an intercept (MI = 2.49, t = 33.74; σ2I = 3.57, t = 19.98), linear slope (MS = −0.04, t = −1.08; σ2S = 0.28, t = 5.25), and quadratic slope (MQ = 0.15, t = 3.78; σ2Q = 0.09, t = 2.73). Neuroticism linearly decreased for every unit increase above age 65, but the quadratic effect attenuated the decline at older ages.
For extraversion, the best fitting LGCM was Model 3 for extraversion and was characterized by an intercept (MI = 4.96, t = 73.84; σ2I = 3.59, t = 27.17), linear slope (MS = 0.07, t = 2.21; σ2S = 0.27, t = 7.76), and quadratic slope (MQ = −0.05, t = −1.27; σ2Q = 0.05, t = 2.45). Extraversion increased linearly for every unit increase above age 65, but the quadratic effect attenuated this increase.
Simultaneous Growth Curve Survival Models
Growth-survival models were fit to test our central hypothesis that openness declines in advance of death. Here we report whether linear change in O, N, and E traits significantly correlates with death. In the first model (labeled Model 1 in Table 3), age at death was regressed on the latent intercept and latent linear slope of O/N/E trait, as well as sex and educational level. The second model (labeled Model 2 in Table 3) added age of entry into SATSA to the covariates to adjust for selection effects. For all personality traits, the full model (Model 2) provided the best fit, as demonstrated by the significant improvement in the S-Bχ2, AIC, and BIC values. Table 4 presents the parameter estimates from Model 2 for all 3 traits. Supplemental Table S1 presents the growth model fixed effects for the growth-survival model. For openness, even after adjusting for effects of the time-invariant covariates, the linear slope (bh,S) significantly correlated with age at death. Because the slope effect was negative, steeper slope scores (i.e., greater decline) were correlated with earlier age at death. In contrast, for neuroticism and extraversion, neither the intercept nor the slope correlated with death.
Table 3.
Model fit results from the simultaneous latent growth models and Cox proportional hazards regression model for O, N, and E personality traits.
| Model | -LL | ΔS-Bχ2 | Δdf | p | AIC | BIC |
|---|---|---|---|---|---|---|
| Openness | ||||||
| Model 1 | −22176.77 | - | - | - | 44409.54 | 44562.82 |
| Model 2 | −21645.94 | 833.79 | 4 | 0.000 | 43355.88 | 43531.05 |
| Neuroticism | ||||||
| Model 1 | −20145.35 | - | - | - | 40346.70 | 40502.64 |
| Model 2 | −19528.76 | 768.19 | 4 | 0.000 | 39121.52 | 39299.74 |
| Extraversion | ||||||
| Model 1 | −19878.94 | - | - | - | 39813.89 | 39969.84 |
| Model 2 | −19257.69 | 748.18 | 4 | 0.000 | 38579.38 | 38757.62 |
Note. Model 1 included sex and educational attainment covariates. Model 2 further included the age of entry into the SATSA study covariate. -LL= -Log Likelihood; ΔS-Bχ2 = Satorra-Bentler chi-square difference test of nested models; AIC = Akaike Criterion Index; BIC = Bayesian Criterion Index.
Table 4.
Log odds effects of O, N, and E personality traits and covariates on age of death from simultaneous latent growth model and Cox proportional hazard model.
| Openness | Neuroticism | Extraversion | ||||
|---|---|---|---|---|---|---|
| Model Parameters | Estimate | SE | Estimate | SE | Estimate | SE |
| Survival Estimates | ||||||
| Intercept bh,I | −0.01 | 0.01 | 0.04 | 0.02 | 0.002 | 0.02 |
| Slope bh,S | −0.30* | 0.11 | −0.06 | 0.24 | −0.30 | 0.23 |
| Sex bh,sex | −0.44* | 0.08 | −0.46* | 0.07 | −0.45* | 0.07 |
| Primary education (Ref.) | - | - | - | - | - | - |
| Lower secondary bh,educ | −0.11 | 0.09 | −0.14 | 0.08 | −0.15 | 0.08 |
| Upper secondary bh,educ | 0.04 | 0.15 | 0.07 | 0.13 | −0.03 | 0.16 |
| Tertiary bh,educ | −0.24 | 0.17 | −0.18 | 0.14 | −0.25 | 0.15 |
| Entry Age bh,age | 0.11* | 0.01 | 0.10* | 0.01 | 0.11* | 0.01 |
Note. Asterisk (*) indicates statistically significant estimate (p < .05).
In Figure 4, we present odds ratios (ORs) and 95% confidence intervals for the likelihood of death associated with a unit increase in the slopes of each trait. ORs are the odds of event occurrence and are more easily understood than their associated log odds, which are presented in Table 4. Confidence intervals of ORs are not symmetric about maximum likelihood point estimates, as antilogging the log odds confidence intervals produces limits on a scale that are necessarily nonlinear and assymmetric about the point estimate (Singer & Willett, 2003). An OR is statistically significant if the confidence interval does not include 1.0. For openness, less steep decline over time represents lower odds of death (OR: 0.74, 95% CI 0.60, 0.92). The ORs for N and E were non-significant.
Figure 4.
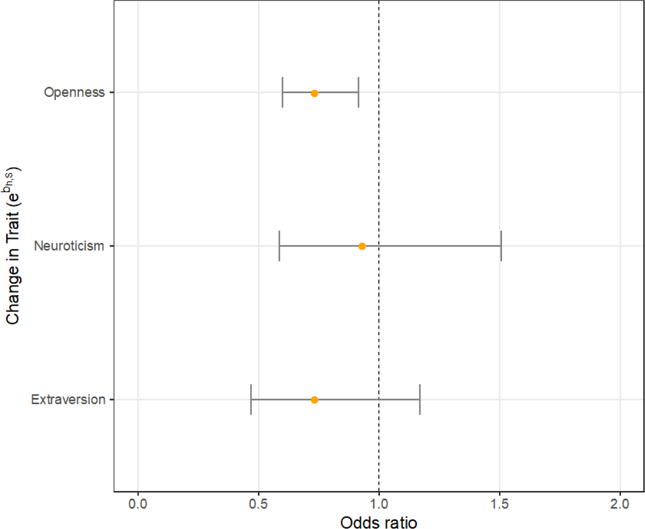
Forest plot of fitted odds ratios for effect of change in trait (slope) of O, N, and E scores on survival.
Note. Odds ratios and 95% confidence intervals correspond to coefficients labeled bh,S in Figure 1 and Table 4.
Potentially Confounding Factors
Sensitivity analyses were carried out to test whether results from the simultaneous latent growth-survival models held adjusting for time-varying effects of physical illness, depressive symptoms, and cognitive ability covariates on observed trait scores. Time-varying covariates were not included in the main analysis for the reason that only a subset of SATSA participants completed the cognitive ability measures. While modern missing data analysis recommends using FIML (Enders, 2010), a conservative approach was taken to estimate the growth-survival models with and without the time-varying covariates and compare the results. Model fitting results and parameter estimates for time-varying covariates are presented in the online Supplemental Materials (Table S2). Overall, the pattern of results for the association between decline in openness and death was unchanged by inclusion of covariates.
Co-Twin Control Analysis
For openness, 207 twin pairs were identified who were discordant for death and had openness data from both twins. The first deceased twin died on average three years after completing their last openness measure. At baseline, openness was not significantly different within discordant twin pairs (mean difference = 0.17, 95% CI = −0.52, 0.87). However, openness scores at the last time of measurement at which both members of the pair responded were significantly lower for the twin who would die before the next measurement occasion than for the longer surviving twin (mean difference = 0.82, 95% CI = 0.10, 1.54). The difference between the intrapair difference at baseline and the intrapair difference at last time of measurement was significant, t = 2.15, p = .033, providing additional evidence for change in openness with age, particularly in advance of death.
By comparison, co-twin control results did not support a change in extraversion or neuroticism prior to death. For extraversion, in 274 twin pairs who were discordant for death, there were no significant differences between twins in a pair on either baseline extraversion or extraversion scores at the last time of measurement before the first twin’s death. The difference between the intrapair difference at baseline and the intrapair difference at last time of measurement for extraversion was not statistically significant, t = −0.38, p = .70. For neuroticism, in 274 twin pairs, there was a consistent, although non-significant, pattern of intrapair differences with the earlier deceased twin scoring higher on neuroticism both at baseline (M = −0.32, 95% CI = −0.64, 0.01) and at last measurement (M = −0.33, 95% CI = −0.67, 0.02). The difference between the intrapair difference at baseline and the intrapair difference at last time of measurement for neuroticism was not statistically significant, t = −0.08, p = .94.
Discussion
Results from the current study replicate previous findings, supporting that openness declines over the second half of the lifespan (Bleidorn et al., 2009; Roberts & Mroczek, 2008; Schwaba & Bleidorn, 2018) and that mean openness is inversely related to mortality (Ferguson & Bibby, 2012; Turiano et al., 2012). The current study is the first to document that late life declines in openness are correlated with age of death. Further, this result was not explained by an overall worsening of health in late adulthood (Wagner et al., 2016; Weston, et al., 2015), as the association between decline in openness and death remained significant after adjusting for time-varying effects of cumulative physical illness, depressive symptoms, and cognitive ability, as well as time-constant effects of sex, education level, and age of entry into SATSA. Exploratory co-twin-control analyses, moreover, suggest that openness was significantly lower for the twin who died first than for the surviving twin, despite non-significant difference in openness scores between twins at their baseline measurement. This is a unique contribution to the literature as co-twin control analyses adjust for genetic and environmental confounders shared between twins. In contrast, neither baseline nor change in extraversion and neuroticism were found to be associated with death, adjusting for time-varying covariates, time-constant, or shared genetic and environmental effects.
The primary contribution of the current study is the finding that openness declines in advance of death, above and beyond baseline measures of openness as previously reported (Iwasa et al., 2008; Jonassaint et al., 2007; Turiano et al., 2012). While higher openness tends to correlate with successful aging outcomes, including well-being and cognitive functioning (Hill, Turiano, Mroczek, & Roberts, 2012; Sharp et al., 2010), openness declines after midlife and appears to do so in advance of death. The association may reflect unique processes above and beyond the measured influence of physical illness, depressive symptoms, and cognitive ability on openness scores across age. We suggest that openness declines in advance of death because of late life developmental shifts in goal orientation. That is, changes in openness could reflect a process of optimization in the face of late life functional losses.
Under the SOC model of aging (P. B. Baltes & M.M. Baltes, 1990; Freund & Baltes, 2007), shortened time horizons and age-related limitations are expected to lead to changes in goal orientation to optimize functioning (e.g., focusing on one area of expertise rather than many). SOC helps put into perspective the mechanism through which decline in openness correlates with death. Following the SOC model, decline in openness may reflect normative social and emotional change in response to a narrowing of goals in older adulthood (Ebner, Freund, & Baltes, 2006; Gerstorf & Ram, 2013). The realization that one has fewer years ahead in life than behind may lead to an audit of one’s values and activities. Such an audit in response to shortened time horizons may cause individuals to carefully adjust their life goals, which in turn may lead to lower levels of openness with age (Carstensen, 2006; Charles & Carstensen, 2010) but not necessarily reduced well-being (Worsch & Heckhausen, 1999). Revised selection of goals, for example, may depend on a shift in values away from gathering information, experiencing novelty, and expanding breadth of knowledge that were important in young adulthood to optimizing existing relationships, pursuing experiences trusted to bring enjoyment and comfort, and sharing knowledge and wisdom accrued throughout life (Carstensen, 2006; Charles & Carstensen, 2010). Over time, knowledge and experience seeking goals might be reduced further, accounting for decline in openness in advance of death.
Although we could not directly test SOC in this study, our findings are in keeping with other recent studies of differential goal selection based on variable time horizons. For example, participants assigned to limited time horizon paradigm recalled more positive images compared to those assigned to an expansive time horizon group (Barber, Opitz, Martins, Sakaki, & Mather, 2016). As death nears, individuals may select activities that aid life mastery, well-being, and positive affect over novel experiences (Kahlbaugh & Huffman, 2017). People’s awareness of limited time might translate into less novelty seeking and lower openness. Even within short periods of time, older adults were found to actively manage selection and optimization strategies in relation to their current feelings of happiness (Teshale & Lachman, 2016). Similarly, when young individuals were asked to write about their own death, individuals were found to change their previously identified goal structure to focus on intrinsic values and shift away from extrinsic values (e.g., money; status) and this change was moderated by degree of reported openness (Prentice, Kasser, & Sheldon, 2018). Declines in openness, in other words, might reflect a change in goal orientation as a function of increased awareness of limited time horizons, including nearness to death. Future research could test whether individuals of all ages newly experiencing limited time horizons subsequently have declines in their openness scores compared to an equivalent group not encountering limited time horizons.
This theoretical framework would explain why openness declines in advance of death whereas change in neuroticism and extraversion were uncorrelated with death. We propose that change in openness may reflect restructuring of goals such that individuals are less likely to focus on seeking out new knowledge and experiences. Change on other personality traits, like neuroticism and extraversion, may be important but not explained by these processes.
Strengths and Limitations
The advantages of this study include the use of a large well-established longitudinal sample of participants aged 60 and older, a large proportion of that sample being deceased, a large proportion of individuals with 4 or more longitudinal measurements, and a genetically informed sample allowing for a co-twin control analysis. Additionally, the SATSA sample is demographically representative of the Swedish population (Cederlöf, Friberg, & Lundman, 1977). Further, we used both invariant demographic variables and time-varying covariates to adjust for potential confounding variables that might explain the relationship between O, N, and E traits and death. We used an advanced statistical technique—a latent growth-survival model— that permitted examination of change in openness, neuroticism, and extraversion in advance of death. This analytical approach of modeling longitudinal change to predict an event (e.g., illness; death) advances the literature beyond examinations of single-point (baseline) personality measurement and death while allowing for adjustments for demographic variables but also, importantly, time-varying and age-related covariates (e.g., physical illness). We, thus, suggest that modeling personality change in advance of death may provide new insights into understanding lifespan development in old age.
The study results are limited by the use of archival data and by personality measures initially selected in 1984 for longitudinal follow-up. We also note that the interval of time between wave T4 and T5 was 11 years and not three years as it was between all other waves. Had personality measures been collected between T4 and T5 on individuals who died nearer to T5 (2004), we would have expected to observe greater decline in openness in these individuals. As a result, the greater interval of time between T4 and T5 may have introduced error in our model and therefore underestimated the association between decline in openness and death. Further, while the co-twin control analysis supported the association between declines in openness and impending death, more extensive investigation of the longitudinal genetic and environmental contributions to declines in openness in advance of death should be pursued in future studies.
We note that the openness scale used in the current study was created by factor analyzing the original 26-item NEO-PI inventory questions and selecting the six highest loading items. We cannot conclude that the results would be identical if the original scale had been used. The shortened scale also precluded an investigation of whether different facets of openness differentially correlated with death. Related to this limitation, we note that there have been historical changes in scale content for extraversion that could not be addressed in SATSA (see Chapman et al., 2011). For example, this study included an extraversion scale that focused on sociability, impulsivity, and sensation seeking. It is possible that an extraversion scale characterized by sociability or positivity items might show a different course of stability and change as well as a different association with death.
Finally, we only considered all causes of death in the current study. Openness might have a different relationship with specific causes of death (e.g., cardiovascular disease, cancer, Alzheimer’s disease), although there is no clear evidence in the literature to suggest differential associations with death. Future research may help clarify systematic patterns of association between openness and specific causes of death as well as the moderating effects of various biomarkers (Čukić & Bates, 2014).
Overall, the current study advances the literature on personality and mortality and utilizes an advanced statistical method to explore these complex associations. This was the first study to identify a relationship between late life longitudinal change in openness and age at death. We posit that the relationship between openness and death that we observed could be explained by a shift in goals in older adults away from external pursuits of knowledge and experience to other activities more valued at this time in life, rather than explained by health-related disability. Given that health-related mediators did not explain the unique relationship between openness and death, we encourage future research to explore the relationships among future time awareness, decline in openness, use of SOC behaviors, and death.
Supplementary Material
Acknowledgments
Data were collected under the aegis of the National Institute on Aging (NIA) grants AG04563 and AG10175. Initial analyses were supported by in part by a grant from the National Institute of Aging: Multidisciplinary Research Training in Gerontology (5T32AG00037; Emily Schoenhofen Sharp and Christopher Beam) and from a K. Patricia Cross dissertation grant awarded by Road Scholar (Emily Schoenhofen Sharp). We thank John J. McArdle for his consultation on the statistical approach and analyses. Simultaneous Growth Survival modeling analyses were made possible by the High Performance Computing Center at the University of Southern California.
Initial findings from this study have been presented at two conferences: Emily Schoenhofen Sharp, Chandra A. Reynolds, Nancy L. Pedersen, and Margaret Gatz (November 2012). Individual differences in openness and risk for death. Poster presented at the annual meeting of the Gerontological Society of America in San Diego, California. Updated findings and analytical approach were presented by Christopher Beam, Emily Sharp, Chandra Reynolds, and Margaret Gatz (June 2017). Personality change in late adulthood: A reciprocal effects modeling approach. Poster presented at the annual meeting of the Behavior Genetics Association in Oslo, Norway.
SATSA data have been reposited in the National Archive of Computerized Data on Aging (NACDA) housed by the Inter-university Consortium for Political and Social Research (ICPSR) at the University of Michigan: https://www.icpsr.umich.edu/icpsrweb/NACDA/studies/3843
Contributor Information
Emily Schoenhofen Sharp, Yale School of Medicine.
Christopher R. Beam, University of Southern California
Chandra A. Reynolds, University of California, Riverside
Margaret Gatz, University of Southern California.
References
- Baek Y, Martin P, Siegler IC, Davey A, & Poon LW (2016). Personality Traits and Successful Aging: Findings from the Georgia Centenarian Study. The International Journal of Aging and Human Development, 83, 207–227. [DOI] [PubMed] [Google Scholar]
- Baltes PB (1987). Theoretical propositions of life-span developmental psychology: On the dynamics between growth and decline. Developmental Psychology, 23, 611–626. 10.1037//0012-1649.23.5.611 [DOI] [Google Scholar]
- Baltes PB, & Baltes MM (1990). Psychological perspectives on successful aging: The model of selective optimization with compensation. In Baltes PB & Baltes MM (Eds.), Successful aging: Perspectives from the behavioral sciences (pp. 1–34). Cambridge: Cambridge University Press. [Google Scholar]
- Barber SJ, Opitz PC, Martins B, Sakaki M, & Mather M (2016). Thinking about a limited future enhances the positivity of younger and older adults’ recall: Support for socioemotional selectivity theory. Memory & Cognition, 44, 869–882. 10.3758/s13421-016-0612-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett LA (2006). Accounting for leisure preferences from within: The relative contributions of gender, race or ethnicity, personality, affective style, and motivational orientation. Journal of Leisure Research, 38, 445–474. [Google Scholar]
- Batty GD, Jokela M, Kivimaki M, & Shipley M (2016). Examining the long-term association of personality with cause-specific mortality in London: Four decades of mortality surveillance in the original Whitehall Smoking Cessation Trial. American Journal of Epidemiology, 184, 436–441. 10.1093/aje/kwv454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeman CS, Chipuer HM, Plomin R, Pedersen NL, McClearn GE, Nesselroade JR, … McCrae RR (1993). Genetic and environmental effects on openness to experience, agreeableness, and conscientiousness: An adoption/twin study. Journal of Personality, 61, 159–179. 10.1111/j.1467-6494.1993.tb01030.x [DOI] [PubMed] [Google Scholar]
- Bleidorn W, Kandler C, Riemann R, Spinath FM, & Angleitner A (2009). Patterns and sources of adult personality development: Growth curve analyses of the NEO PI-R scales in a longitudinal twin study. Journal of Personality and Social Psychology, 97, 142–155. 10.1037/a0015434 [DOI] [PubMed] [Google Scholar]
- Bryk AS, & Raudenbush SW (1987). Application of hierarchical linear models to assessing change. Psychological Bulletin, 101, 147–158. 10.1037//0033-2909.101.1.147 [DOI] [Google Scholar]
- Burnham KP, & Anderson DR (2004). Multimodel inference: Understanding AIC and BIC in model selection. Sociological Methods & Research, 33, 261–304. 10.1177/0049124104268644 [DOI] [Google Scholar]
- Carstensen LL (1992). Social and emotional patterns in adulthood: Support for socioemotional selectivity theory. Psychology and Aging, 7, 331–338. [DOI] [PubMed] [Google Scholar]
- Carstensen LL (2006). The influence of a sense of time on human development. Science, 312(5782), 1913–1915. 10.1126/science.1127488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederlöf R, Friberg L, & Lundman T (1977). The interactions of smoking, environment and heredity and their implications for disease etiology. Acta Medica Scandinavia, 612, 1–128. [PubMed] [Google Scholar]
- Chapman BP, Roberts B, & Duberstein P (2011). Personality and longevity: Knowns, unknowns, and implications for public health and personalized medicine. Journal of Aging Research, 2011, 1–24. 10.4061/2011/759170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, & Carstensen LL (2010). Social and emotional aging. Annual Review of Psychology, 61, 383–409. 10.1146/annurev.psych.093008.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AJ, Ehlers SL, Wiebe JS, Moran PJ, Raichle K, Ferneyhough K, & Lawton WJ (2002). Patient personality and mortality: A 4-year prospective examination of chronic renal insufficiency. Health Psychology, 21, 315–320. 10.1037//0278-6133.21.4.315 [DOI] [PubMed] [Google Scholar]
- Costa PT, & McCrae RR (1985). The NEO personality inventory: Manual, form S and form R Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Čukić I, & Bates TC (2014). Heart rate variability and adult personality: A nationally representative study. Personality and Individual Differences, 60, S31. [Google Scholar]
- Ebner NC, Freund AM, & Baltes PB (2006). Developmental changes in personal goal orientation from young to late adulthood: From striving for gains to maintenance and prevention of losses. Psychology and Aging, 21, 664–678. 10.1037/0882-7974.21.4.664 [DOI] [PubMed] [Google Scholar]
- Enders CK (2010). Applied missing data analysis New York, NY: The Guilford Press. [Google Scholar]
- Ferguson E, & Bibby PA (2012). Openness to experience and all-cause mortality: A meta-analysis and r equivalent from risk ratios and odds ratios. British Journal of Health Psychology, 17, 85–102. 10.1111/j.2044-8287.2011.02055.x [DOI] [PubMed] [Google Scholar]
- Finkel D, & Pedersen NL (2004). Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology, and Cognition, 11, 325–345. 10.1080/13825580490511152 [DOI] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Gatz M, & Pedersen NL (2003). Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Developmental Psychology, 39, 535–550. 10.1037/0012-1649.39.3.535 [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, & Pedersen NL (2005). The longitudinal relationship between processing speed and cognitive ability: Genetic and environmental influences. Behavior Genetics, 35, 535–549. [DOI] [PubMed] [Google Scholar]
- Floderus B (1974). Psycho-social factors in relation to coronary heart disease and associated risk factors. Nordisk Hygienisk Tidskrift, 6, 7–148 [Google Scholar]
- Freund AM, & Baltes PB (2007). Toward a theory of successful aging: Selection, optimization, and compensation. In Fernández-Ballesteros R (Ed.), Geropsychology: European perspectives for an aging world (pp. 239–254). Boston, MA: Hogrefe & Huber. [Google Scholar]
- Friedman HS, Kern ML, & Reynolds CA (2010). Personality and health, subjective well-being, and longevity. Journal of Personality, 78, 179–216. 10.1111/j.1467-6494.2009.00613.x [DOI] [PubMed] [Google Scholar]
- Fry PS, & Debats DL (2009). Perfectionism and the five-factor personality traits as predictors of mortality in older adults. Journal of Health Psychology, 14, 513–24. 10.1177/1359105309103571 [DOI] [PubMed] [Google Scholar]
- Gale CR, Čukić I, Batty D, Mcintosh AM, Weiss A, & Deary IJ (2017). When is higher neuroticism protective against premature death? Findings from UK Biobank. Psychological Science, 28, 1345–1357. 10.1177/0956797617709813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, & Ram N (2013). Inquiry into terminal decline: Five objectives for future study. The Gerontologist, 53, 727–737. 10.1093/geront/gnt046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P (2008). Application of a joint multivariate longitudinal-survival analysis to examine the terminal decline hypothesis in the Swiss Interdisciplinary Longitudinal Study on the Oldest Old. The Journals of Gerontology. Series B: Psychological Sciences and Social Sciences, 63, 185–192. 10.1093/geronb/63.3.P185 [DOI] [PubMed] [Google Scholar]
- Ghisletta P, McArdle JJ, & Lindenberger U (2006). Longitudinal cognition-survival relations in old and very old age: 13-year data from the Berlin Aging Study. European Psychologist, 11(3), 204–223. 10.1027/1016-9040.11.3.204 [DOI] [Google Scholar]
- Guo X, & Carlin BP (2004). Separate and joint modeling of longitudinal and event time data using standard computer packages. The American Statistician, 58, 16–24. 10.1198/0003130042854 [DOI] [Google Scholar]
- Hagger-Johnson G, Roberts B, Boniface D, Sabia S, Batty D, Elbaz A, … Deary I (2012). Neuroticism and cardiovascular disease mortality: Socioeconomic status modifies the risk in women (UK Health and Lifestyle Survey). Psychosomatic Medicine, 74, 596–603. 10.1097/PSY.0b013e31825c85ca [DOI] [PubMed] [Google Scholar]
- Harris JR, Pedersen NL, McClearn GE, Plomin R, & Nesselroade JR (1992). Age differences in genetic and environmental influences for health from the Swedish Adoption/Twin Study of Aging. Journal of Gerontology: Psychological Sciences, 47, 213–220. 10.1093/geronj/47.3.P213 [DOI] [PubMed] [Google Scholar]
- Hill PL, Turiano NA, Mroczek DK, & Roberts BW (2012). Examining Concurrent and longitudinal relations between personality traits and social well-being in adulthood. Social Psychological and Personality Science, 3, 698–705. 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh JB, DeYoung CG, & Peterson JB (2009). Metatraits of the Big Five differentially predict engagement and restraint of behavior. Journal of Personality, 77, 1085–1102. http://doi:10.1111/j.1467-6494.2009.00575 [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Staff RT, Bunting BP, Deary IJ, & Whalley LJ (2012). Openness to experience and activity engagement facilitate the maintenance of verbal ability in older adults. Psychology and Aging, 27, 849–854. [DOI] [PubMed] [Google Scholar]
- Ihle A, Oris M, Fagot D, Maggiori C, & Kliegel M (2016). The association of educational attainment, cognitive level of job, and leisure activities during the course of adulthood with cognitive performance in old age: the role of openness to experience. International Psychogeriatrics 28, 733–740. [DOI] [PubMed] [Google Scholar]
- Iwasa H, Masui Y, Gondo Y, Inagaki H, Kawaai C, & Suzuki T (2008). Personality and all-cause mortality among older adults dwelling in a Japanese community: A five-year population-based prospective cohort study. The American Journal of Geriatric Psychiatry, 16, 399–405. 10.1097/JGP.0b013e3181662ac9 [DOI] [PubMed] [Google Scholar]
- Jamshidian M and Jalal S (2010). Tests of homoscedasticity, normality, and missing at random for incomplete multivariate data. Psychometrika, 75, 649–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassaint CR, Boyle SH, Williams RB, Mark DB, Siegler IC, & Barefoot JC (2007). Facets of openness predict mortality in patients with cardiac disease. Psychosomatic Medicine, 69(4), 319–322. 10.1097/PSY.0b013e318052e27d [DOI] [PubMed] [Google Scholar]
- Kahlbaugh P, & Huffman L (2017). Personality, emotional qualities of leisure, and subjective well-being in the elderly. The International Journal of Aging and Human Development, 85, 164–184. [DOI] [PubMed] [Google Scholar]
- Korten AE, Jorm AF, Jiao Z, Letenneur L, Jacomb PA, Henderson AS, … Rodgers B (1999). Health, cognitive, and psychosocial factors as predictors of mortality in an elderly community sample. Journal of Epidemiology and Community Health, 53, 83–88. 10.1136/jech.53.2.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJ (1995). Modeling the drop-out mechanism in repeated-measures studies. Journal of the American Statistical Association, 90, 1112–1121. [Google Scholar]
- Martin LR, Friedman HS, & Schwartz JE (2007). Personality and mortality risk across the life span: The importance of conscientiousness as a biopsychosocial attribute. Health Psychology, 26(4), 428–436. 10.1037/0278-6133.26.4.428 [DOI] [PubMed] [Google Scholar]
- Martin P, Baenziger J, MacDonald M, Siegler IC, & Poon LW (2009). Engaged lifestyle, personality, and mental status among centenarians. Journal of Adult Development, 16(4), 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams DP, & Olson BD (2010). Personality development: Continuity and change over the life course. Annual Review of Psychology, 61, 517–542. 10.1146/annurev.psych.093008.100507 [DOI] [PubMed] [Google Scholar]
- McArdle JJ, & Anderson E (1990). Latent variable growth models for research on aging. In Birren JE & Schaie KW (Eds.), Handbook of the psychology of aging (Third, pp. 21–44). San Diego, CA: Academic Press. [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, & Woodcock RW (2002). Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology, 38, 115–142. 10.1037/0012-1649.38.1.115 [DOI] [PubMed] [Google Scholar]
- McArdle JJ, & Hamagami F (1992). Modeling incomplete longitudinal and cross-sectional data using latent growth structural models. Experimental Aging Research, 18, 145–166. 10.1080/03610739208253917 [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Small BJ, Bäckman L, & Fratiglioni L (2005). Longitudinal models of growth and survival applied to the early detection of Alzheimer’s disease. Journal of Geriatric Psychiatry and Neurology, 18, 234–241. 10.1177/0891988705281879 [DOI] [PubMed] [Google Scholar]
- McCrae RR (1987). Creativity, divergent thinking, and openness to experience. Journal of Personality and Social Psychology, 52, 1258–1265. [Google Scholar]
- McCrae RR, & Sutin AR (2009). Openness to experience. In Leary MR & Hoyle RH (Eds.), Handbook of individual differences in social behavior (pp. 257–273). New York, NY: Guilford Press. [Google Scholar]
- McGue M, Osler M, & Christensen K (2010). Causal Inference and Observational Research: The Utility of Twins. Perspectives on Psychological Science, 5, 546–556. 10.1177/1745691610383511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mõttus R, Johnson W, Starr JM, & Deary IJ (2012) Correlates of personality trait levels and their changes in very old age: The Lothian Birth Cohort 1921. Journal of Research in Personality, 46, 271–278. 10.1016/j.jrp.2012.02.004. [DOI] [Google Scholar]
- Mroczek DK, & Spiro A (2007). Personality change influences mortality in older men. Psychological Science, 18, 371–376. 10.1111/j.1467-9280.2007.01907.x.Personality [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A, & Turiano NA (2009). Do healthy behaviors explain the effect of neuroticism on mortality? Longitudinal findings from the VA Normative Aging Study. Journal of Research in Personality, 43, 653–659. 10.1016/j.pestbp.2011.02.012.Investigations [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2017). Mplus user’s guide Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nikitin J, & Freund AM (2018). Feeling loved and integrated or lonely and rejected in everyday life: The role of age and social motivation. Developmental Psychology, 54(6), 1186–1198. 10.1037/dev0000502 [DOI] [PubMed] [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, & DeFaire U (1991). The Swedish Adoption Twin Study of Aging: An update. Acta Geneticae Medicae et Gemellologiae, 40, 7–20. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, McClearn GE, & Friberg L (1988). Neuroticism, extraversion, and related traits in adult twins reared apart and reared together. Journal of Personality and Social Psychology, 55, 950–957. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, Nesselroade JR, & McClearn GE (1992). A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychological Science, 3, 346–352. 10.1111/j.1467-9280.1992.tb00045.x [DOI] [Google Scholar]
- Pedersen NL, & Reynolds CA (1998). Stability and change in adult personality: Genetic and environmental components. European Journal of Personality, 386, 365–386. [Google Scholar]
- Prentice M, Kasser T, & Sheldon KM (2018). Openness to experience predicts intrinsic value shifts after deliberating one’s own death. Death studies, 42(4), 205–215. 10.1080/07481187.2017.1334016 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Read S, Vogler GP, Pedersen NL, & Johansson B (2006). Stability and change in genetic and environmental components of personality in old age. Personality and Individual Differences, 40, 1637–1647. 10.1016/j.paid.2006.01.004 [DOI] [Google Scholar]
- Roberts BW, & Mroczek DK (2008). Personality trait change in adulthood. Current Directions in Psychological Science, 17, 31–35. 10.1016/j.pestbp.2011.02.012.Investigations [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, Walton KE, & Viechtbauer W (2006). Patterns of mean-level change in personality traits across the life course: A meta-analysis of longitudinal studies. Psychological Bulletin, 132, 1–25. 10.1037/0033-2909.132.1.1 [DOI] [PubMed] [Google Scholar]
- Rowe JW (1985). Health care of the elderly. New England Journal of Medicine, 312, 827–835. [DOI] [PubMed] [Google Scholar]
- Rowe JW, & Kahn RL (1997). Successful aging. The Gerontologist, 37, 433–440. [DOI] [PubMed] [Google Scholar]
- Rowe JW, & Kahn RL (2015). Successful aging 2.0: Conceptual expansions for the 21str century. Journal of Gerontology. Series B: Psychological Sciences and Social Sciences, 70, 593–596. doi:10/1093/geronb/gbv025. [DOI] [PubMed] [Google Scholar]
- Satorra A, & Bentler PM (2001). A scaled difference chi-square test statistic for moment structure analysis. Psychometrika, 66, 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: our view of the state of the art. Psychological Methods, 7, 147–177. [PubMed] [Google Scholar]
- Sharp ES, Reynolds CA, Pedersen NL, & Gatz M (2010). Cognitive engagement and cognitive aging: Is openness protective? Psychology and Aging, 25, 60–73. 10.1037/a0018748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley BA, Weiss A, Der G, Taylor MD, & Deary IJ (2007). Neuroticism, extraversion, and mortality in the UK Health and Lifestyle Survey: A 21-year prospective cohort study. Psychosomatic Medicine, 69, 923–931. 10.1097/PSY.0b013e31815abf83 [DOI] [PubMed] [Google Scholar]
- Schwaba T, & Bleidorn W (2018). Individual differences in personality change across the adult life span. Journal of Personality, 86(3), 450–464. 10.1111/jopy.12327 [DOI] [PubMed] [Google Scholar]
- Schilling OK, Wahl H-W, & Wiegering S (2013). Affective development in advanced old age: Analyses of terminal change in positive and negative affect. Developmental Psychology, 49, 1011–1020. doi: 10.1037/a0028775 [DOI] [PubMed] [Google Scholar]
- Small BJ, Hertzog C, Hultsch DF, & Dixon RA (2003). Stability and change in adult personality over 6 years: Findings from the Victoria Longitudinal Study. The Journals of Gerontology. Series B: Psychological Sciences and Social Sciences, 58, 166–176. 10.1093/geronb/58.3.P166 [DOI] [PubMed] [Google Scholar]
- Stephan Y, Boiché J, Canada B, & Terracciano A (2014). Association of personality with physical, social, and mental activities across the lifespan: Findings from US and French samples. British Journal of Psychology, 105, 564–580. [DOI] [PubMed] [Google Scholar]
- Teshale SM, & Lachman ME (2016). Managing daily happiness. The relationship between selection, optimization, and compensation strategies and well-being in adulthood. Psychology and Aging, 31, 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau TM (2013). A package for survival analysis in S
- Turiano NA, Chapman BP, Gruenewald TL, & Mroczek DK (2015). Personality and the leading behavioral contributors of mortality. Health Psychology, 34, 51–60. 10.1037/hea0000038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turiano NA, Spiro A, & Mroczek DK (2012). Openness to experience and mortality in men: Analysis of trait and facets. Journal of Aging and Health, 24, 654–672. 10.1016/j.pestbp.2011.02.012.Investigations [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Ram N, Smith J, & Gerstorf D (2016). Personality Trait Development at the End of Life: Antecedents and Correlates of Mean-Level Trajectories. Journal of Personality and Social Psychology, 111, 411–429. 10.1037/pspp0000071 [DOI] [PubMed] [Google Scholar]
- Weiss A, & Costa PT (2005). Domain and Facet Personality Predictors of All-Cause Mortality Among Medicare Patients Aged 65 to 100. Psychosomatic Medicine, 67, 724–733. 10.1097/01.psy.0000181272.58103.18 [DOI] [PubMed] [Google Scholar]
- Weston SJ, Hill PL, & Jackson JJ (2015). Personality Traits Predict the Onset of Disease. Social Psychological and Personality Science, 6, 309–317. 10.1177/1948550614553248 [DOI] [Google Scholar]
- Wilson RS, Krueger KR, Gu L, Bienias JL, Mendes de Leon CF, & Evans DA (2005). Neuroticism, extraversion, and mortality in a defined population of older persons. Psychosomatic Medicine, 67, 841–845. 10.1097/01.psy.0000190615.20656.83 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Bienias JL, Evans DA, & Bennett DA (2004). Personality and mortality in old age. Journal of Gerontology. Series B: Psychological Sciences and Social Sciences, 59, 110–116. 10.1093/geronb/59.3.P110 [DOI] [PubMed] [Google Scholar]
- Worsch C, & Heckhausen J (1999). Control processes before and after passing a developmental deadline: Activation and deactivation of intimate relationship goals. Journal of Personality and Social Psychology, 77, 415–427. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


