Abstract
Background
How sex and age influence post-myocardial infarction (post-MI) outcomes remains unclear. This study evaluated the influence of sex and age on drug therapy, echocardiographic parameters, and outcomes in post-MI patients undergoing percutaneous coronary intervention (PCI).
Methods
We retrospectively enrolled 643 patients with first acute MI who underwent successful PCI and two echocardiographic examinations within 1 year after MI. Clinical characteristics and 4-year follow-up outcomes were compared between sexes and age groups. Primary endpoints were cardiovascular mortality and hospitalization for heart failure (HF).
Results
Compared with males, female patients with MI, particularly older females, had more systemic diseases. Younger females received fewer guideline-directed therapies. Older patients presented with higher left ventricular volume and mass index but no significant differences in left ventricular ejection fraction. The Kaplan–Meier analysis revealed increased mortality in both younger and older females. Elderly patients, particularly older females, exhibited significantly higher post-MI HF incidence but no difference in recurrent MI, ventricular arrhythmia, or revascularization.
Conclusions
In MI patients receiving PCI, outcome differences between sexes are age-dependent. Age influences outcome more heavily in females than in males. Females are likely to exhibit worse overall survival, and older females are at higher risk of post-MI HF.
Keywords: MI, Age, Gender, Cardiac structure
1. Introduction
Myocardial infarction (MI) remains the leading cause of death among both females and males worldwide [1]. With the advancement of percutaneous coronary intervention (PCI), the incidence of MI seems to be declining among the younger population in Western countries, but it remains high among Asians, especially in the older generations [[1], [2], [3]]. Multiple risk factors for MI have been identified and validated, including hypertension, diabetes mellitus (DM), hyperlipidemia, and smoking [4]. However, sex and age are also clinically important prognostic factors for MI and subsequent outcome. Although females are less likely to develop acute MI than males at any given age, due to the protective effects of female hormones, females with acute MI exhibit a higher incidence of several cardiovascular risk factors [5,6]. In addition, females with MI who received PCI were less likely than males to be prescribed guideline-directed therapies [1,[5], [6], [7], [8]]. Most previous studies on post-MI outcome focused on survival but rarely examined post-MI cardiac recovery due to sex-related differences [1,6]. Therefore, in this study, we comprehensively investigated the influence of sex and age on drug therapy, post-MI short- and long-term cardiac structure, cardiac function, and outcomes among MI patients receiving PCI.
2. Methods
2.1. Study design
From January 2014 to December 2017, we retrospectively enrolled 643 hospitalized patients with acute MI validated by two of the following three criteria: [1] history of prolonged ischemic chest pain, [2] evolutionary changes of the ST segment and/or T wave, and [3] dynamic elevation of serum cardiac enzymes such as cardiac troponin and/or creatine kinase-muscle/brain (MB). All enrolled patients received PCI as reperfusion therapy and were divided into young (<65 years) and older (≥65 years) groups of males and females. Clinical information on comorbidities, type of MI, and prescriptions for cardiovascular medications were also obtained. Smoking was defined as current or former and hyperlipidemia as receiving lipid-lowering therapy or either total cholesterol >200 mg/dl or serum triglycerides >150 mg/dl. Hypertension and DM were defined according to previous diagnosis by a physician and chronic kidney disease (CKD) as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 by Cockcroft-Gault equation. Angiographic stenosis was defined as >50% stenosis and indication for PCI as >70% narrowing of the coronary artery luminal diameter. Patients with histories of previous MI, heart failure (HF), significant structural heart disease, or valvular disease severity above moderate were excluded. The study was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the Review Board of Chi-Mei Hospital (CV code: 10410-002).
2.2. Echocardiographic parameters
In accordance with the recommendations of the American Society of Echocardiography [9], echocardiography was performed with a 3.5-MHz multiphase-array probe and GE Vivid E9 system (Vingmed Ultrasound AS, Horten, Norway) by the cardiologists in charge. The chamber dimensions and left ventricular (LV) mass were measured using the two-dimensionally guided M-mode method, and LV ejection fraction was measured using the biplane Simpson's method. In addition, LV diastolic function-associated parameters, including trans-mitral early filling velocity (E) to atrial velocity (A) ratio, were also measured. Echocardiography was performed during hospitalization and within 1 year post-MI to evaluate the post-MI cardiac functional and structural changes.
2.3. Outcomes
According to medical review, the following clinical outcomes were analyzed: mortality, HF requiring hospitalization, recurrent MI, ventricular arrhythmia, and revascularization.
2.4. Statistical analysis
Continuous data are presented as mean ± standard deviation or median (interquartile range), depending on the distribution. Dichotomous data is presented as numbers and percentages. The chi-squared test or Fisher's exact test was used to compare categorical variables as appropriate. Group differences were compared using analysis of variance. The Kaplan–Meier method with log-rank test was used to compare event-free rates between groups. A univariate Cox regression analysis was performed to evaluate factors associated with mortality. Factors with p < 0.1 based on univariate analysis were included in multivariate Cox regression analysis to identify independent risk factors for endpoints. All analyses were performed using SPSS, version 18 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Baseline characteristics of MI patients receiving PCI
Among patients with MI, the majority were male (76%) and mean age was younger in males than in females (Table 1). There were more obese young males, whereas more females had comorbid DM and hypertension. CKD incidence was higher in the older population (≥65 years), whereas hyperlipidemia was more frequent in the younger population (<65 years). Regarding the coronary interventions, there were neither significant differences of numbers of treated vessels nor specific locations of lesions among groups. Nevertheless, most patients were treated for one vessel disease, which was mainly in the left anterior descending (LAD) artery.
Table 1.
The baseline clinical characteristics and sequential echocardiographic parameters of total 643 patients between young men, old man, young women and old women.
| Men |
Woman |
p-Value |
|||
|---|---|---|---|---|---|
| Young (<65 y/o) N = 256 | Old (≥65 y/o) N = 229 | Young (<65 y/o) N = 48 | Old (≥65 y/o) N = 110 | ||
| Age (y/o) | 54.0 ± 7.9a, c | 74.4 ± 6.8b, d | 55.8 ± 7.7a, c | 77.5 ± 7.6b, d | <0.001 |
| BMI (kg/M2) | 27.6 ± 12.1a | 23.9 ± 4.3d | 22.8 ± 5.2 | 23.5 ± 4.1 | 0.011 |
| CV risk factors, n (%) | |||||
| DM | 78 (30.4) | 96 (41.9) | 26 (54.1) | 57 (51.8) | 0.001 |
| HTN | 78 (30.4) | 88 (38.4) | 15 (31.2) | 47 (42.7) | 0.001 |
| CKD | 64(25) | 180(78.6) | 25(52) | 93(84.5) | 0.001 |
| Hyperlipidemia | 208(81.2) | 159(69.4) | 33(78.6) | 70(68) | 0.029 |
| Stroke | 5 (1.9) | 21 (9.1) | 1(2.1) | 8(7.2) | 0.072 |
| Smoking | 45 (17.6) | 46 (20) | 6(12.5) | 3(2.7) | 0.12 |
| PCI for, n (%) | |||||
| LAD | 154 (60.1) | 128 (55.8) | 28 (58.3) | 72(65.5) | 0.23 |
| LCX | 75 (29.2) | 97(42.3) | 14(29.1) | 45(40.9) | 0.06 |
| RCA | 140 (54.7) | 110(48) | 23 (47.9) | 56(50.9) | 0.31 |
| One vessel | 162 (63.2) | 136(59.4) | 34 (70.8) | 58(52.7) | 0.19 |
| Two vessels | 75 (29.2) | 80(34.9) | 11(22.9) | 41(37.3) | 0.42 |
| Three vessels | 19 (7.4) | 13(5.6) | 3 (6.2) | 11(10) | 0.58 |
| Echocardiographic parameters | |||||
| Post MI (during hospitalization) |
|||||
| LVEF (%) | 60.6 ± 38.5 | 55.4 ± 21.4 | 54.4 ± 21.2 | 59.8 ± 13.9 | 0.168 |
| LVEDVi (mL/M2) | 73.7 ± 29.7a, c | 83.1 ± 36.6d | 70.9 ± 25.4c | 90.1 ± 44.4b, d | <0.001 |
| LVESVi (mL/M2) | 31.2 ± 20.8a | 37.3 ± 25.0d | 31.5 ± 16.1 | 37.9 ± 26.1 | 0.009 |
| LVMi (g/M2) | 129.3 ± 59.1c | 137.7 ± 72.1c | 129.4 ± 70.9 | 160.5 ± 84.7a, d | 0.002 |
| E/A | 8.19 ± 6.84 | 9.37 ± 8.63 | 9.45 ± 7.84 | 10.29 ± 9.91 | 0.21 |
| E/e′ | 12.44 ± 5.34 | 14.2 ± 6.78 | 12.25 ± 6.20 | 14.95 ± 5.86 | 0.08 |
| Post MI (within one year) |
|||||
| LVEF (%) | 61.4 ± 13.6 | 58.8 ± 14.8 | 60.8 ± 15.7 | 60.8 ± 14.2 | 0.233 |
| LVEDVi (mL/M2) | 75.7 ± 30.9 | 80.9 ± 38.4 | 73.2 ± 41.3 | 83.5 ± 43.4 | 0.151 |
| LVESVi (mL/M2) | 31.6 ± 22.5 | 36.1 ± 25.0 | 31.7 ± 28.1 | 37.5 ± 28.0 | 0.099 |
| LVMi (g/M2) | 114.4 ± 56.3a, c | 135.9 ± 73.4d | 125.4 ± 65.6 | 150.3 ± 72.4d | <0.0001 |
| E/A | 0.92 ± 0.46 | 1.12 ± 1.13 | 0.79 ± 0.50 | 1.01 ± 0.57 | 0.2 |
| E/e′ | 12.14 ± 5.77 | 12.57 ± 5.97 | 13.18 ± 7.97 | 15.04 ± 5d | 0.05 |
| Changes of LVEDVi (mL/M2) | 2.0 ± 1.6 | −2.2 ± 2.3 | 0.6 ± 5.5 | −6.5 ± 3.4 | 0.11 |
| Changes of LVESVi (mL/M2) | 0.6 ± 1.1 | −1.2 ± 1.5 | 0.2 ± 3.7 | −0.4 ± 2.5 | 0.82 |
| Changes of LVMi (g/M2) | −14.6 ± 3.2 | −1.7 ± 5.6 | −6.9 ± 10.4 | −10.1 ± 8.3 | 0.28 |
| Changes of LVEF (%) | 0.68 ± 2.37 | 3.43 ± 1.33 | 6.40 ± 3.08 | 1.32 ± 1.43 | 0.5 |
DM = diabetes mellitus; HTN = hypertension; CKD = chronic kidney disease; LAD = left anterior descending artery; LCX = left circumflex artery; RCA = right coronary artery; LVEF = left ventricular ejection fraction; LVEDVi = left ventricular end diastolic volume index; LVESVi = left ventricular end systolic volume index; LVMi = left ventricular mass index; E/A = trans-mitral valve E to A velocity ratio; E/e′ = mitral early filling velocity to early diastolic mitral annular velocity ratio.
p < 0.05, compared with old men.
p < 0.05, compared with young women.
p < 0.05, compared with old women.
p < 0.05, compared with young men.
3.2. The post-MI prescriptions among patients with different age and sex
Usage of aspirin was similar among groups, but there were differences in other post-PCI prescriptions. The prescription rates of clopidogrel and other guideline-directed treatments such as angiotensin-converting-enzyme inhibitor/angiotensin II receptor blocker and statins were significantly lower in younger females (Supplement Table 1). Conversely, the usage of diuretics was significantly higher in older females than among all other groups. Only a small proportion of patients received β-blockers, despite recommendation for post-MI treatment.
3.3. The post-MI cardiac structural and functional changes among patients with different age and sex
During hospitalization, LV volume and LV mass were higher in older patients than in younger patients, especially in the older females (Table 1). In early-stage post-MI, despite slightly elevated LV end diastolic pressures (E/e′) among the older patients, the LV ejection fractions were similar among groups. One year after MI, there were no significant changes of LV volume, mass, and ejection fraction in each group. In the logistic regression, increased LV volumes at the acute stage post-MI predicted a worse outcome even after age and sex were adjusted (Table 2). In the multivariate regression, left ventricular end diastolic volume index (LVEDVi) measured during hospitalization was still significantly correlating to the cardiovascular mortality (Table 2). Collectively, our findings indicated that although there were no significant changes of LV geometries and functions during the post-MI recovery phase, an increased LV volume during MI may imply a potentially increased risk of cardiovascular mortality.
Table 2.
Crude and adjusted hazard ratios of cardiovascular mortality in myocardial infarction (MI) patients receiving percutaneous coronary intervention (PCI).
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| Crude HR (95% CI) | p | Adjusted HRa (95% CI) | p | Adjusted HRa (95% CI) | p | |
| Age | 1.027 (0.99–1.05) | 0.09 | – | – | ||
| Female | 2.456 (1.15–5.24) | 0.02 | – | – | ||
| BMI | 0.93 (0.87–0.99) | 0.03 | 0.942(0.87–1.01) | 0.11 | ||
| DM | 4.39 (1.85–10.38) | 0.001 | 3.893(1.63–9.28) | 0.002 | 2.08 (0.68–6.33) | 0.19 |
| HTN | 2.99 (1–8.88) | 0.04 | 2.474(0.81–7.53) | 0.11 | ||
| CKD | 8.22 (1.94–34.77) | 0.004 | 7.981(1.68–37.73) | 0.009 | 6.34(1.33–30.19) | 0.02 |
| Hyperlipidemia | 0.88 (0.35–2.23) | 0.8 | 1.02(0.40–2.62) | 0.95 | ||
| PCI for LAD | 6.67 (2–22.14) | 0.002 | 6.048(1.81–20.14) | 0.003 | 3.39(0.76–15.07) | 0.11 |
| PCI for LCX | 1.16 (0.53–2.5) | 0.7 | 1.06(0.49–2.30) | 0.86 | ||
| PCI for RCA | 1.04 (0.49–2.24) | 0.9 | 1.16(0.54–2.49) | 0.69 | ||
| LVEDVi (during hospitalization) |
1.01 (1.00–1.02) | <0.001 | 1.011(1.00–1.02) | 0.008 | 1.02(1.00–1.03) | 0.04 |
| LVESVi (during hospitalization) |
1.01 (1.00–1.02) | 0.01 | 1.014(1.00–1.02) | 0.03 | 0.99(0.96–1.01) | 0.53 |
| LVMi (during hospitalization) |
0.99 (0.99–1.003) | 0.28 | 0.99(0.99–1.00) | 0.12 | ||
| LVMi (within one year) |
1.00(0.9–1.00) | 0.51 | 1.00 (0.99–1.00) | 0.91 | ||
| E/e′ (within one year) |
1.05(0.95–1.15) | 0.29 | 1.03(0.93–1.15) | 0.52 | ||
Abbreviation as Table 1.
Adjusted with age and sex.
3.4. The survival and cardiovascular outcomes among patients of different age and sex
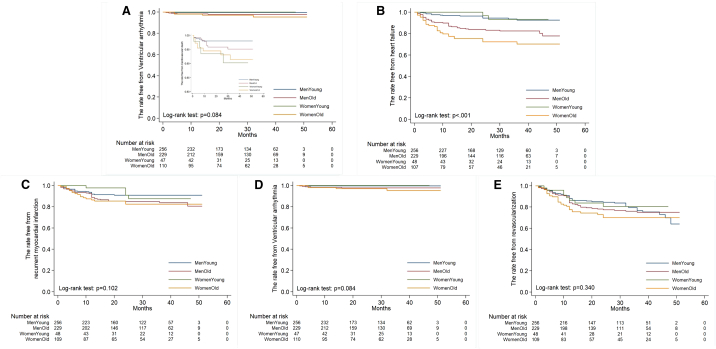
To investigate long-term outcomes further, Kaplan–Meier plots were constructed. These revealed increased cardiovascular mortality in both younger and older females compared with males (Fig. 1A). In contrast, the older group, especially older females, exhibited a higher incidence of post-MI HF (Fig. 1B) but not of recurrent MI (Fig. 1C), ventricular arrhythmia (Fig. 1D), or revascularization (Fig. 1E). In the univariate regression, female sex indicated worse survival (HR: 2.45, 95% confidence interval [CI]: 1.15–5.24, p = 0.02) (Table 2). In addition, cardiovascular risk factors, including DM, HTN, CKD, and PCI for LAD, contributed to a higher cardiovascular mortality. After adjustment for age and sex, DM, CKD, and PCI for LAD remained significantly associated with increasing cardiovascular mortality. Furthermore, in the multivariate regression, only CKD and LVEDVi measured during hospitalization significantly correlated with the cardiovascular mortality. This finding suggests that among patients with MI receiving PCI, older females in particular should be closely monitored for the development of HF. Conversely, other potential post-MI complications may have less influence on post-MI survival in older females than in younger females and males regardless of age.
Fig. 1.
The Kaplan–Meier plot of (A) accumulating survival, (B) free from heart failure, (C) free from recurrent myocardial infarction, (D) free from ventricular arrhythmia, and (E) free from revascularization among young men (<65 y/o), old men (≥65 y/o), young women, and old women post–acute myocardial infarction and receiving percutaneous intervention.
3.5. Risk factors contributing to post-MI mortality in the younger and older populations
As separately discussed according to age, among the younger population, young women with MI were prone to be at higher cardiovascular risk. Up to half of the younger women had DM and CKD although there were no significant changes of cardiac geometries in the post-MI stage (Supplement Table 2). On the contrary, as we focused on the older population, despite a slightly increasing incidence of DM in the older women, there were significant elevations of the left ventricular mass index and LV end diastolic pressure compared with those in the older men (Supplement Table 3). This finding implied that in addition to the innate cardiovascular risks prior to MI, cardiac hypertrophy in the post-MI period also indicated a worse outcome. To investigate the risk factors contributing to a higher mortality in the older women (Supplement Table 4) further, the logistic regression identified some echocardiographic parameters, including LV dimension and E/A ratio during hospitalization or post-MI within one year. However, none of them was statistically significant in the older women with MI.
4. Discussion
According to our findings, among MI patients receiving PCI, females demonstrate larger age-dependent differences in outcome than males. Specifically, older age is more strongly predictive of poor outcome in female than male MI patients receiving PCI. In addition, females in general are likely to realize shorter overall survival. Older females in our study were more prone to develop post-MI HF than were younger females or males in general.
Although advanced age is likely associated with unfavorable post-MI cardiac structural changes, it is generally accepted that sex hormones such as estrogen protect against myocardial injury and delay the occurrence of MI in females by approximately 10 years compared with males [10,11]. Furthermore, females are believed to be protected from apoptosis and to experience less-severe cardiac remodeling than males, whereas high testosterone levels in males enhance acute myocardial inflammation, adversely affecting myocardial healing and promoting early remodeling [10,11]. In contrast, our findings indicate that despite these advantages, females are at increased risk for post-MI HF. Correspondingly, in a previous study in Spain, females with MI were older on average than males and had a worse cardiovascular risk profile; further, female sex was an independent predictor of both short- and long-term mortality [12]. Another retrospective study reported that guideline-directed medications for MI were underused for females [1]. The authors concluded that females with MI were treated less aggressively than males, possibly due to the atypical clinical presentation of MI in woman and potential bias of physicians regarding the relative mortality risks of male and female MI patients. Several previous reports focusing on patients with ST-elevated MI also support our findings of worse long-term outcome in females than in males [6,7,13]. In the Netherlands, among 59,534 patients with MI, medical adherence was lower in women, young patients, and elderly patients, specifically in non-ST-elevation myocardial infarction (NSTEMI) patients [8]. According to the CONCORDANCE Acute Coronary Syndrome Registry from 41 Australian hospitals, among 2898 patients with MI, women were less likely to receive invasive management or preventive medication at discharge [7]. Also, in the Korean registry, among 85,329 new patients admitted for MI, the in-hospital mortality rate was higher among female patients although it was associated with a lower procedure rate. Evidence highlights the need for health policies and education in awareness of sex-related differences in early MI management [14].
Given that female patients with MI have the highest incidence of cardiovascular risk factors, including hypertension and diabetes, enrolled females with MI were likely to have an inherently higher risk of MI, resulting in greater subsequent mortality [13]. Although female sex hormones protect against remodeling, females are more likely to present HF with preserved systolic function [15,16]. Likewise, the older women in our study presented with significantly higher rates of cardiac hypertrophy, which is a risk factor for HF and mortality. Indeed, in previous studies, MI was associated with a higher risk of HF with reduced ejection fraction (HFrEF) rather than with HF with preserved ejection fraction (HFpEF) [17]. However, diastolic dysfunction was also pivotal in patients post-MI., Lavine et al., using echocardiography, concluded that in 109 patients the development of heart failure following their first MI was associated with a moderately reduced ejection fraction (EF) and pseudo-normal diastolic dysfunction [18].
4.1. Study limitations
This study has some limitations. First, most of the patients were referred from local clinics for the intervention of acute coronary syndrome. Thus, the records of previous medications for the control of cardiovascular risks such as hypertension, diabetes, and hyperlipidemia were incomplete. Second, given that most studied patients were referred, it is difficult to identify the duration of myocardial ischemic symptoms. Third, the absence of baseline echocardiographic findings prior to MI made differentiating the higher mortality in older females caused by either the post-MI HF or the underlying comorbidities difficult. Fourth, because the incidence of DM, HTN, and CKD were higher in old women, whether the higher mortality and HF are owing to the innate risks per se instead of to MI requires more investigations. Nevertheless, according to our findings, patients such as older females with increased LV volumes during MI may encounter potentially higher risks of cardiovascular mortality.
5. Conclusions
Collectively, given the higher incidence of cardiovascular risks, females with MI (especially old females) demonstrate higher probabilities of subsequent cardiovascular mortality and HF.
Funding
This research is funded by Ministry of Science and Technology (105-2628-B-384-001-MY3) and Chi-Mei Medical Center.
Acknowledgments
Acknowledgments
This study was supported by the Chi-Mei Medical Center.
Conflicts of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2019.100350.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Lee C.H., Cheng C.L., Yang Y.H. Trends in the incidence and management of acute myocardial infarction from 1999 to 2008: get with the guidelines performance measures in Taiwan. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzuhairi K.S., Sogaard P., Ravkilde J., Gislason G., Kober L., Torp-Pedersen C. Incidence and outcome of first myocardial infarction according to gender and age in Denmark over a 35-year period (1978–2012) Eur. Heart J. Qual. Care Clin. Outcomes. 2015;1:72–78. doi: 10.1093/ehjqcco/qcv016. [DOI] [PubMed] [Google Scholar]
- 3.Ohira T., Iso H. Cardiovascular disease epidemiology in Asia: an overview. Circ J. 2013;77:1646–1652. doi: 10.1253/circj.cj-13-0702. [DOI] [PubMed] [Google Scholar]
- 4.Piro M., Della Bona R., Abbate A., Biasucci L.M., Crea F. Sex-related differences in myocardial remodeling. J. Am. Coll. Cardiol. 2010;55:1057–1065. doi: 10.1016/j.jacc.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 5.Blomkalns A.L., Chen A.Y., Hochman J.S. Gender disparities in the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: large-scale observations from the CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the American College of Cardiology/American Heart Association guidelines) National Quality Improvement Initiative. J. Am. Coll. Cardiol. 2005;45:832–837. doi: 10.1016/j.jacc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 6.Jneid H., Fonarow G.C., Cannon C.P. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118:2803–2810. doi: 10.1161/CIRCULATIONAHA.108.789800. [DOI] [PubMed] [Google Scholar]
- 7.Khan E., Brieger D., Amerena J. Differences in management and outcomes for men and women with ST-elevation myocardial infarction. Med. J. Aust. 2018;209:118–123. doi: 10.5694/mja17.01109. [DOI] [PubMed] [Google Scholar]
- 8.Eindhoven D.C., Hilt A.D., Zwaan T.C., Schalij M.J., Borleffs C.J.W. Age and gender differences in medical adherence after myocardial infarction: women do not receive optimal treatment - the Netherlands claims database. Eur. J. Prev. Cardiol. 2018;25:181–189. doi: 10.1177/2047487317744363. [DOI] [PubMed] [Google Scholar]
- 9.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Cavasin M.A., Tao Z.Y., Yu A.L., Yang X.P. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am. J. Phys. Heart Circ. Phys. 2006;290:H2043–H2050. doi: 10.1152/ajpheart.01121.2005. [DOI] [PubMed] [Google Scholar]
- 11.Biondi-Zoccai G.G., Baldi A., Biasucci L.M., Abbate A. Female gender, myocardial remodeling and cardiac failure: are women protected from increased myocardiocyte apoptosis? Ital. Heart J. 2004;5:498–504. [PubMed] [Google Scholar]
- 12.Degano I.R., Elosua R., Marrugat J. Epidemiology of acute coronary syndromes in Spain: estimation of the number of cases and trends from 2005 to 2049. Rev. Esp. Cardiol. 2013;66:472–481. doi: 10.1016/j.rec.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Kyto V., Sipila J., Rautava P. Gender, age and risk of ST segment elevation myocardial infarction. Eur. J. Clin. Investig. 2014;44:902–909. doi: 10.1111/eci.12321. [DOI] [PubMed] [Google Scholar]
- 14.Hong J.S., Kang H.C. Sex differences in the treatment and outcome of Korean patients with acute myocardial infarction using the Korean National Health Insurance Claims Database. Medicine. 2015;94 doi: 10.1097/MD.0000000000001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sofia R.R., Serra A.J., Silva J.A., Jr. Gender-based differences in cardiac remodeling and ILK expression after myocardial infarction. Arq. Bras. Cardiol. 2014;103:124–130. doi: 10.5935/abc.20140113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrasekhar J., Mehran R. Sex-based differences in acute coronary syndromes: insights from invasive and noninvasive coronary technologies. JACC Cardiovasc. Imaging. 2016;9:451–464. doi: 10.1016/j.jcmg.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Borlaug B.A., Redfield M.M. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–2013. doi: 10.1161/CIRCULATIONAHA.110.954388. (discussion 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavine S.J. Prediction of heart failure post myocardial infarction: comparison of ejection fraction, transmitral filling parameters, and the index of myocardial performance. Echocardiography. 2003;20:691–701. doi: 10.1111/j.0742-2822.2003.02156.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables