Abstract
Background
Food allergy (FA) is a common public health problem that affects both children and adults. Empirical knowledge of the burden of FA in Kuwait is limited. This study sought to estimate the prevalence of FA among schoolchildren in Kuwait and assess associations between FA and the coexistence and severity of asthma, rhinitis, and eczema.
Methods
Schoolchildren aged 11–14 years (n = 3,864) were enrolled in a cross-sectional study. Parents completed questionnaires regarding their children's early life exposures and clinical history of FA and allergic diseases. Study-defined FA was ascertained by a convincing clinical history. Associations were assessed using Poisson regression with robust variance estimation, and adjusted prevalence ratios (aPRs) and 95% confidence intervals (CIs) were estimated.
Results
The 12-month prevalence of study-defined FA was estimated to be 4.1% (154/3,738), with more girls being affected than boys (aPR = 1.44, 95% CI: 1.04–1.99). Egg (2.7%), fish (1.6%), shellfish (1.3%), peanut (1.3%), and tree nut (1.2%) were the most reported offending food allergens. Underweight and adiposity, cesarean section delivery, exposure to household dogs during infancy, and parental history of doctor-diagnosed FA were associated with an increased prevalence of study-defined FA. However, later birth order was associated with a reduced prevalence of study-defined FA. The prevalence of eczema only was higher in children with study-defined FA than in those without study-defined FA (aPR = 3.49, 95% CI: 2.37–5.14). In contrast, this association was not pronounced for children who had asthma only (aPR = 1.56, 95% CI: 0.94–2.57) or rhinitis only (aPR = 1.40, 95% CI: 0.86–2.28). Study-defined FA was associated with a 9.20-fold (95% CI: 4.50–18.78) higher prevalence of coexisting asthma, rhinitis, and eczema. Moreover, study-defined FA was associated with increased severity of symptoms of asthma, rhinitis, and eczema.
Conclusions
FA affects a considerable proportion of schoolchildren in Kuwait, and the most reported offending food allergens are similar to those reported in Western countries. Study-defined FA was associated with the coexistence and increased severity of asthma, rhinitis, and eczema, indicating that FA may link the comanifestations of allergic diseases and contribute to their chronicity and severity.
Keywords: Food allergy, Prevalence, Risk factors, Asthma, Rhinitis, Eczema
Abbreviations: BMI, Body mass index; CI, Confidence interval; ETS, Environmental tobacco smoke; FA, Food allergy; ISAAC, International Study of Asthma and Allergies in Childhood; NIAID, National Institute of Allergy and Infectious Diseases; OFC, Oral food challenges; PR, Prevalence ratio; SD, Standard deviation
Background
Food allergy (FA), a global and an increasingly recognized public health concern, has detrimental clinical outcomes and prominently impacts the psychological state and the quality of life of affected individuals and their families.1, 2 An expert panel commissioned by the National Institute of Allergy and Infectious Diseases (NIAID) defined FA as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food”.3 Manifestations of FA differ in their intensity and can be graded from mild to severe and, in rare instances, can be associated with fatal anaphylaxis. In most circumstances, adverse reactions to foods are manifested within the gastrointestinal tract, the skin, and the respiratory tract.4 At this point in time, treating FA is highly challenging, and the common “therapy” remains to be the avoidance of the offending food allergen(s), which, in some instances, is not feasible and may lead to accidental adverse reactions.4, 5
Epidemiologic investigations indicate that the prevalence of FA has increased in recent decades,1, 4 an emergence that has been regarded as the “second wave” of the allergy epidemic,6 following the “first wave” of asthma,7 rhinitis,8 and eczema.9 Determining accurate prevalence estimates of FA is hampered by the varying manifestations and severity of the disease and the various definitions used and lack of standardization in the current scientific literature.10 Prevalence estimates based on self-/parent-reported FA are generally higher than estimates confirmed by the gold standard of oral food challenges (OFC). A European systematic review and meta-analysis of FA epidemiology reported overall pooled lifetime and point prevalence estimates of self-reported FA to be 17.3% and 5.9%, respectively.11 The same study reported an overall pooled point prevalence of FA, as defined by clinical history or OFC, to be 2.6%.11 Another meta-analysis, with no geographical boundaries, showed that the prevalence of self-reported FA varied from 3% to 35%,12 marking significant heterogeneity in FA prevalence that could be attributed to differences in study design and methodology, study populations, and geographic regions.
The interrelationships between allergic diseases have been explained by two concepts, namely, “allergic march” and “allergic multimorbidity”. The allergic march concept suggests a temporal progression from early-onset eczema to asthma and rhinitis13; however, the data supporting the sequential development of allergic diseases are contradictory.14, 15, 16, 17 In contrast, the allergic multimorbidity concept supports the coexistence (co-occurrence) of allergic diseases.17, 18, 19 Since FA is considered to be second wave of the allergy epidemic, understanding the role of FA in the development of allergic diseases is essential. Emerging investigations show associations between FA and the development of asthma, rhinitis, and eczema.20, 21, 22
A global survey by the World Allergy Organization on FA burden among children showed that more than one-half of the surveyed countries (52/89) had no estimates of FA prevalence.23 This finding highlights the paucity of data on FA in most countries worldwide and indicates a need to obtain information on the disease burden and its impact in different settings. Moreover, although emerging studies have indicated that rates of food-induced anaphylaxis are rapidly increasing among schoolchildren and adolescents,24, 25 few studies have described the burden of FA in this age group compared to preschool-aged children.26 To this end, due to the lack of epidemiologic data on FA in Kuwait, this large cross-sectional study sought to estimate the prevalence of FA, as defined by a convincing clinical history, among schoolchildren in Kuwait and to assess associations between FA and the coexistence (multimorbidity) and severity of asthma, rhinitis, and eczema.
Methods
Study setting, design, and participants
Kuwait is a small country overlooking the Persian (Arabian) Gulf with a total approximate area of 18,000 km2. In December 31, 2017, the total population of Kuwait was estimated to be approximately 4.5 million people, and approximately 24.6% of the population is ≤ 19 years of age. Geographically, Kuwait is divided into six governorates, and the school districts follow a similar geographic division. Education in Kuwait is mainly provided by free public schools funded by the state and, to a lesser extent, by private schools. The education system can be divided into four stages, namely, kindergarten, elementary school (1st-5th grade), middle school (6th-9th grade), and high school (10th-12th grade), and the latter three stages are segregated by gender. Schooling is compulsory for all children aged 6–14 years.
This cross-sectional study enrolled schoolchildren (n = 3,864) attending public middle schools throughout the State of Kuwait that mostly included subjects aged between 11 and 14 years. The schoolchildren were enrolled in the study during the 2016/2017 school year (September 2016 to May 2017) and the first semester of the 2017/2018 school year (September to December 2017). A stratified two-stage cluster sampling method was used to select a representative study sample of schoolchildren from a random sample of schools. At the inception of the study, there were 207 public middle schools in Kuwait enrolling approximately 106,320 students (50,655 boys and 55,665 girls). From a list obtained from the Ministry of Education, Kuwait, of all public middle schools stratified by school district and gender, schools were randomly selected using randomly generated numbers. Since the total number of students differs across the six school districts, proportional allocation was used to determine the number of participants needed from each school district by estimating gender-stratified weights relative to the student body size in each given school district. Hence, the number of schools selected in each school district was based on the number of students needed from the respective district. On average, each school enrolled around 500 students (i.e., provided a list with around 500 student names), and we have a priori decided to enroll 50% of students from each selected school. Hence, some schools might have enrolled more/less than 500 students. Once we have obtained the list of student names (sampling frame) from each school, we used simple random sampling methods to enrolled 50% of students in each selected school. Hence, in total, 21 schools served as recruitment venues for enrolling the required sample size of 4,000 students. To account for 30% refusals and absentees, we planned to invite 5,200 schoolchildren to participate in the study. Ethical approval for the current study was obtained from the Standing Committee for Coordination of Health and Medical Research, Ministry of Health, Kuwait (no. 2016/451).
The children were asked to take home the study-specific questionnaire and a standardized questionnaire (i.e., the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire27) for parental/guardian completion and return them to school. The questionnaires gathered information on the demographic data, lifestyle factors, environmental exposures, and clinical history and symptoms of FA, asthma, rhinitis, and eczema of both the children and their parents. The ISAAC questionnaire was used to obtain information on clinical history and symptoms of asthma, rhinitis, and eczema (without following the complete ISAAC methodology), whereas we constructed a FA-related module using previously published literature28, 29 to gather information on FA. Written informed consent for each enrolled child was obtained from the parents or legal guardians.
Definitions
Food allergy
We used previously published robust clinical diagnostic criteria30, 31, 32 to determine FA status among study participants in the past 12 months (i.e., current FA status). The applied a priori definition of FA was based on convincing clinical history that encompassed the following criteria:
-
1.
reporting at least one adverse reaction to a common food allergen, including cow's milk, egg, peanut, tree nut, fish, shellfish, soy, wheat, and sesame1;
-
2.reporting at least one recognized allergic symptom, including the following:
-
a.localized symptoms: itching, sting/burning of the lips/mouth or throat, urticaria/hives, angioedema;
-
b.abdominal symptoms: nausea, vomiting, crampy/colicky abdominal pain, diarrhea;
-
c.respiratory symptoms: wheeze, stridor, watery rhinitis, redness of eyes/nose;
-
d.skin symptoms: urticaria, itching, flushed skin, worsening eczema;
-
e.systemic reactions: anaphylaxis, syncope; and
-
a.
-
3.
reporting a temporal relationship of a reaction, with symptoms occurring within 2 h of food ingestion.
Children were classified as having FA if they fulfilled criteria 1, 2, and 3. This approach allowed us to estimate the 12-month (current) prevalence of FA. In the remainder of the paper, we refer to this variable as “study-defined FA”.
In addition, we estimated the lifetime prevalence of parent-reported perceived food allergy, which was defined by a positive answer to the question “Has this child ever had food allergy?“. Additionally, an affirmative response to the question “Has this child ever been diagnosed with food allergy by a doctor?” was used to estimate the lifetime prevalence of parent-reported doctor-diagnosed food allergy.
Asthma
Current asthma (i.e., asthma in the past 12 months) was defined by an affirmative response to the items “history of physician-diagnosed asthma” and “wheezing in the past 12 months” and/or “asthma treatment in the past 12 months”. The severity of asthma symptoms in the past 12 months was assessed by inquiring about the following symptoms: frequency of wheezing attacks; wheezing severe enough to limit speech to only one or two words at a time between breaths; and wheezing leading to disturbed sleep.
Rhinitis
Current rhinitis was defined as “ever doctor-diagnosed rhinitis” and “having problems with a sneezing, runny, or blocked nose in the absence of a cold or flu in the past 12 months”. The severity of rhinitis symptoms was assessed by asking about the extent to which rhinitis in the past 12 months had interfered with daily activities.
Eczema
Following the Hanifin and Rajka criteria,33 current eczema was defined as “ever doctor-diagnosed eczema” and/or “having ever had a recurrent itchy rash for at least six months” plus “having an itchy rash at any time in the past 12 months that affected the folds of the elbows or the areas behind the knees, in front of the ankles, under the buttocks, or around the neck, ears, or eyes.” The severity of eczema symptoms was assessed by asking if the itchy rash has ever cleared in the past 12 months and whether the itchy rash has caused night waking in the past 12 months.
Coexistence of allergic diseases
Combinations of current asthma, current rhinitis, and current eczema resulted in eight nonoverlapping groups of single and coexisting allergic diseases: a group with “no allergic disease”, “asthma only”, “rhinitis only”, “eczema only”, “asthma + rhinitis”, “asthma + eczema”, “rhinitis + eczema”, and “asthma + rhinitis + eczema”.
Ascertainment of exposure and covariate variables
Information regarding exposures and covariates was obtained from questionnaires completed by the parents/guardians. Since body mass index (BMI), which is a measure of general adiposity, markedly changes in children with growth, we estimated the BMI-for-age z-scores (standard deviation [SD] scores) using the WHO growth reference for those aged between 5 and 19 years.34 The BMI-for-age score was categorized as follows: underweight (thinness): <-2 SD, normal: −2 to 1 SD, overweight: >1 to 2 SD, and obese: >2 SD.34 Exposure to environmental tobacco smoke (ETS) was assessed by inquiring whether any member of the household smokes cigarettes or tobacco-related products inside the home. To ascertain exposure to household cats and dogs during infancy, the following two separate questions were asked: “Did you have a cat/dog in your home during the first year of this child's life?” Breastfeeding status was determined by asking whether the child was ever directly fed at the breast during infancy. The questionnaire also asked about the child's birth order, which was categorized as follows: first-born, second-born, third-born and higher. Moreover, information on parental history of FA was obtained by asking the following question: “Has the child's mother/father ever been diagnosed with FA by a doctor?”
Statistical analysis
All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, North Carolina, USA). The statistical significance level was set to α = 0.05 for all association analyses. Descriptive analyses were conducted to determine the frequencies and proportions of the categorical variables and the medians and 5th and 95th percentiles of the quantitative variables. To assess whether the analytical study sample (n = 3,738, i.e., participants with complete information regarding study-defined FA, asthma, rhinitis, and eczema status) was representative of the total study sample (n = 3,864), we compared proportions of categorical variables (using χ2 tests) and means of continuous variables (using t-tests) across these two samples. The 12-month prevalence of study-defined FA, lifetime prevalence of parent-reported doctor-diagnosed FA, lifetime prevalence of parent-reported perceived FA, and the 12-month prevalence of single morbidity and the coexistence of asthma, rhinitis, and eczema were estimated, along with their binomial 95% confidence intervals (CIs).
The crude and adjusted associations were assessed by applying a modified Poisson regression with robust variance estimation using the GENMOD procedure in SAS 9.4 to estimate and infer the prevalence ratios (PRs) and their 95% CIs.35 Variables that demonstrated possible association with study-defined FA (outcome variable) in the crude models (i.e., p-value ≤ 0.2, as suggested by Maldonado and Greenland36) were simultaneously entered into the multivariable regression models. Regardless of statistical significance, sex and age were included as potential confounders in all multivariable regression models. In further analysis, we considered study-defined FA as the exposure variable and assessed its association with allergic diseases occurring as a single entity or coexisting with other conditions. Moreover, we determined whether study-defined FA (the exposure variable) was associated with symptoms of the severity of asthma, eczema, and rhinitis.
Results
Description of the study sample
In total, 5,228 schoolchildren (2,483 boys and 2,745 girls) were invited to participate, and 3,864 schoolchildren (1,695 boys and 2,169 girls) were enrolled in the study (response proportion: 73.9%). The analytical sample (n = 3,738; restricted to participants with information regarding study-defined FA, asthma, rhinitis, and eczema status) and the total sample (n = 3,864) were similar in all characteristics investigated (Table 1). The median (5th, 95th percentile) age of the study participants was 12.0 (11.0, 14.0) years. The BMI-for-age groups indicated that 25.4% (929/3,663) and 28.8% (1,055/3,663) of the schoolchildren were overweight and obese, respectively. In total, 1,703 (45.7%) children were reported to have been exposed to ETS. A parental history of doctor-diagnosed FA was reported for 717 (19.5%) of the children enrolled in the study (Table 1). Prevalence estimates of maternal and paternal reported history of doctor-diagnosed FA were 12.8% (462/3,597) and 9.9% (349/3,536), respectively.
Table 1.
Characteristics of the total study sample and the analytical study sample.
| Variables | Total study sample (n = 3864) | Analytical study sample (n = 3738)a |
|---|---|---|
| Sex, n (%) | ||
| Male | 1695 (43.9) | 1652 (44.2) |
| Female | 2169 (56.1) | 2086 (55.8) |
| Age (years) | ||
| Median (5th, 95th percentile) | 12.0 (11.0, 14.0) | 12.0 (11.0, 14.0) |
| BMI-for-age groups, n (%) | ||
| Underweight (<−2 SD) | 219 (5.8) | 212 (5.8) |
| Normal (−2 to 1 SD) | 1517 (40.1) | 1467 (40.0) |
| Overweight (>1 to 2 SD) | 961 (25.3) | 929 (25.4) |
| Obese (>2 SD) | 1089 (28.8) | 1055 (28.8) |
| Missing, n | 78 | 75 |
| Mode of Birth, n (%) | ||
| Vaginal | 3106 (81.8) | 3019 (81.7) |
| Cesarean section | 692 (18.2) | 678 (18.3) |
| Missing, n | 66 | 41 |
| Birth order, n (%) | ||
| First | 1103 (28.7) | 1070 (28.7) |
| Second | 801 (20.8) | 790 (21.2) |
| ≥ Third | 1940 (50.5) | 1870 (50.1) |
| Missing, n | 20 | 8 |
| Breastfeeding ever, n (%) | ||
| Yes | 2894 (76.3) | 2812 (76.2) |
| Missing, n | 72 | 49 |
| Environmental tobacco smoke exposure, n (%) | ||
| Yes | 1755 (45.8) | 1703 (45.7) |
| Missing, n | 28 | 13 |
| Cat exposure in infancy, n (%) | ||
| Yes | 232 (6.1) | 225 (6.1) |
| Missing, n | 35 | 19 |
| Dog exposure in infancy, n (%) | ||
| Yes | 85 (2.2) | 84 (2.3) |
| Missing, n | 32 | 16 |
| Parental history of food allergyb, n (%) | ||
| Yes | 728 (19.5) | 717 (19.5) |
| Missing, n | 129 | 65 |
BMI: body mass index; SD: standard deviation.
Refers to the sample of participants with complete information regarding study-defined food allergy, asthma, rhinitis, and eczema status (i.e., excluding 126 subjects with incomplete information regarding allergic manifestations).
Maternal and/or paternal history of doctor-diagnosed food allergy.
Additional descriptive information is shown in the Online Supplementary, Table S1. In the total analytical study sample (n = 3,738), 65.9% (n = 2,465), 27.6% (n = 1,030), and 6.5% (n = 243) of the returned questionnaires were completed by mothers, fathers, and legal guardians, respectively. Majority of children's mothers (43.1%, 1,600/3,717) and fathers (36.1%, 1,338/3,701) reported having a bachelor's degree or higher. The most commonly reported (28.5%, 954/3,349) total monthly household income was between 1,000 and 1,500 Kuwaiti Dinar (∼$3,300 to $4,900). The majority of children were of Kuwaiti nationality (92.5%, 3,459/3,738), and a small proportion were of non-Kuwait nationality (7.5%, 279/3,738). Moreover, none of the previously described attributes were associated with study-defined FA (see Online Supplementary, Table S1).
Prevalence estimates of FA
The lifetime prevalence estimates of parent-reported perceived and doctor-diagnosed FA were 12.7% (469/3,692) and 8.2% (297/3,611), respectively (Table 2). The prevalence of perceived FA was higher in girls than boys (14.4% vs. 10.6%, p-value < 0.001), whereas doctor-diagnosed FA affected girls and boys equally (8.4% vs. 8.0%, p-value = 0.712). The 12-month (current) prevalence of study-defined FA was estimated to be 4.1% (154/3,738), with more girls being affected than boys (4.7% vs. 3.5%; p-value = 0.067; Table 2).
Table 2.
Prevalence estimates of study-defined food allergy, parent-reported perceived food allergy, and parent-reported doctor-diagnosed food allergy in the total analytical study sample and stratified by sex.
| Food allergy (FA) definition |
|||
|---|---|---|---|
| Current study-defined FA | Lifetime parent-reported doctor-diagnosed FA | Lifetime parent-reported perceived FA | |
| Total analytical sample | |||
| n/total | 154/3738 | 297/3611 | 469/3692 |
| Prevalence, % (95% CI) | 4.1 (3.5–4.8) | 8.2 (7.3–9.1) | 12.7 (11.6–13.8) |
| Males | |||
| n/total | 57/1652 | 128/1593 | 173/1630 |
| Prevalence, % (95% CI) | 3.5 (2.6–4.3) | 8.0 (6.7–9.4) | 10.6 (9.1–12.1) |
| Females | |||
| n/total | 97/2086 | 169/2018 | 296/2062 |
| Prevalence, % (95% CI) | 4.7 (3.8–5.6) | 8.4 (7.2–9.6) | 14.4 (12.8–15.9) |
| P-valuea | 0.067 | 0.712 | <0.001 |
FA: food allergy; CI: confidence interval.
Comparing prevalence in males and females using chi-square tests.
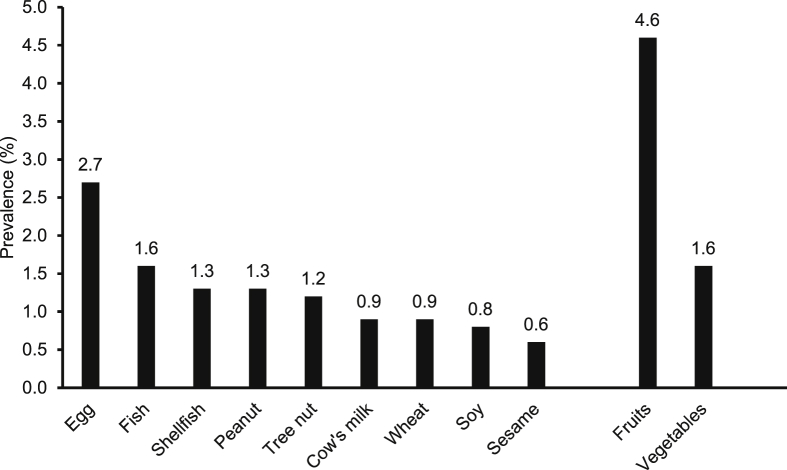
Prevalence estimates of reported allergies to common food allergens are shown in Fig. 1. Egg (2.7%), fish (1.6%), shellfish (1.3%), peanut (1.3%), and tree nut (1.2%) were the most reported food allergens (Fig. 1). Other common foods reported to cause adverse reactions included fruits (4.6%) and vegetables (1.6%). Among children with study-defined FA, majority experienced adverse reactions to a single food (59.1%, 91/154), 24.7% (38/154) to two foods, and 16.2% (25/154) to three or more foods.
Fig. 1.
Prevalence estimates of reported allergies (hypersensitivity) to individual food items and food groups. Prevalence estimates of reported allergies to common food allergens are presented in a descending manner. Reported allergies to fresh fruits and vegetables are also presented.
Reactions to foods
Table 3 presents frequencies of reported reactions to foods according to the affected organ systems. Among children with study-defined FA, 59.1% (91/154) reported two or more symptoms due to adverse reactions to foods and 50.1% (78/154) reported symptoms from two or more organ systems. Symptoms related to the ingestion of foods mainly involved the skin (72.1% for urticaria/hives, itching, or flushing), gastrointestinal tract (33.1% for itchy lips, mouth, or throat), and respiratory tract (26.6% for throat tightness).
Table 3.
Reactions to foods according to the affected organ system among children with study-defined food allergy.
| Reactions | n | % among children with study-defined food allergy (n = 154) |
|---|---|---|
| Skin reactions | ||
| Urticaria/hives, itching, or flushing | 111 | 72.1 |
| Angioedema | 33 | 21.4 |
| Gastrointestinal reactions | ||
| Itchy lips, mouth, or throat | 51 | 33.1 |
| Nausea/vomiting | 28 | 18.2 |
| Diarrhea | 16 | 10.4 |
| Abdominal pain | 30 | 19.5 |
| Respiratory reactions | ||
| Rhinorrhea/conjunctivitis | 9 | 5.8 |
| Wheeze | 23 | 14.9 |
| Shortness of breath | 36 | 23.4 |
| Throat tightness | 47 | 26.6 |
| Generalized reactions | ||
| Anaphylaxis | 6 | 3.9 |
| Syncope (fainting) | 7 | 4.6 |
Factors associated with study-defined FA
Associations between various factors and study-defined FA were explored (Table 4). Compared with the normal BMI-for-age group, individuals in the underweight (aPR = 2.13, 95% CI: 1.16–3.93), overweight (aPR = 1.63, 95% CI: 1.07–2.49), and obese (aPR = 1.93, 95% CI: 1.28–2.90) groups had a higher prevalence of study-defined FA (Table 4). Cesarean section delivery (aPR = 1.42, 95% CI: 1.05–2.16), exposure to household dogs in infancy (aPR = 3.33, 95% CI: 1.92–5.79), and parental history of doctor-diagnosed FA (aPR = 2.75, 95% CI: 2.01–3.76) were associated with increased prevalence of study-defined FA. However, children who were second-born (aPR = 0.64, 95% CI: 0.41–0.98) and third-born or higher (aPR = 0.72, 95% CI: 0.51–0.99) had a lower prevalence of study-defined FA compared with first-born children.
Table 4.
Crude and adjusted associations between personal attributes and risk factors and study-defined food allergy.
| Study-defined food allergy, % (n/total) | Crude PR (95% CI) | Adjusted PRa (95% CI) | |
|---|---|---|---|
| Sex | |||
| Male | 3.5 (57/1652) | 1.00 (Ref.) | 1.00 (Ref.) |
| Female | 4.7 (97/2086) | 1.35 (0.98–1.86) | 1.44 (1.04–1.99) |
| Age (years) | |||
| Per additional year of age | – | 1.08 (0.96–1.22) | 1.06 (0.93–1.20) |
| Dog exposure in infancy | |||
| No | 3.9 (140/3638) | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 14.3 (12/84) | 3.71 (2.15–6.42) | 3.33 (1.92–5.79) |
| Parental history of food allergyb | |||
| No | 3.0 (90/2956) | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 8.7 (62/717) | 2.84 (2.08–3.88) | 2.75 (2.01–3.76) |
| BMI-for-age groups | |||
| Underweight (<−2 SD) | 6.1 (13/212) | 2.14 (1.17–3.92) | 2.13 (1.16–3.93) |
| Normal (−2 to 1 SD) | 2.9 (42/1467) | 1.00 (Ref.) | 1.00 (Ref.) |
| Overweight (>1 to 2 SD) | 4.6 (43/929) | 1.62 (1.07–2.45) | 1.63 (1.07–2.49) |
| Obese (>2 SD) | 5.2 (55/1055) | 1.82 (1.23–2.70) | 1.93 (1.28–2.90) |
| Cat exposure in infancy | |||
| No | 4.0 (139/3494) | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 5.8 (13/225) | 1.45 (0.84–2.52) | 1.02 (0.56–1.88) |
| Mode of Birth | |||
| Vaginal | 3.8 (114/3019) | 1.00 (Ref.) | 1.00 (Ref.) |
| Cesarean section | 5.3 (36/678) | 1.41 (0.98–2.03) | 1.42 (1.05–2.16) |
| Environmental tobacco smoke exposure | |||
| No | 3.6 (73/2022) | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 4.7 (80/1703) | 1.30 (0.95–1.78) | 1.10 (0.80–1.50) |
| Breastfeeding ever | |||
| No | 3.8 (33/877) | 1.00 (Ref.) | |
| Yes | 4.2 (118/2812) | 1.12 (0.76–1.63) | |
| Birth order | |||
| First | 5.4 (58/1070) | 1.00 (Ref.) | 1.00 (Ref.) |
| Second | 3.3 (26/790) | 0.61 (0.39–0.96) | 0.64 (0.41–0.98) |
| ≥ Third | 3.7 (69/1870) | 0.68 (0.48–0.96) | 0.72 (0.51–0.99) |
PR: prevalence ratio; CI: confidence interval; BMI: body mass index; SD: standard deviation; Ref.: reference.
Variables that had a p-value ≤ 0.2 in the crude model were simultaneously included in the adjusted (multivariable) model, except for age and sex, which were included in all adjusted models.
Maternal and/or paternal history of doctor-diagnosed food allergy.
Study-defined FA and coexistence of allergic diseases
Associations between study-defined FA and the single occurrence and coexistence of asthma, rhinitis, and eczema were assessed (Table 5). The prevalence of asthma only (aPR = 1.56, 95% CI: 0.94–2.57) and rhinitis only (aPR = 1.40, 95% CI: 0.86–2.28) was increased in children with study-defined FA than in those without study-defined FA, although these associations were not statistically significant (Table 5). In contrast, study-defined FA was significantly associated with an increased prevalence of eczema only (aPR = 3.49, 95% CI: 2.37–5.14). Similarly, children with study-defined FA were more likely to have coexisting allergic diseases than those without study-defined FA. For instance, study-defined FA was associated with a 9.20-fold (95% CI: 4.50–18.78) higher prevalence of having coexisting asthma, rhinitis, and eczema.
Table 5.
Associations between study-defined food allergy and single morbidity and coexistence of current asthma, rhinitis, and eczema.
| Allergic diseases | Total analytical sample (n = 3738) |
Study-defined food allergy |
Adjusted PRa (95% CI) | ||
|---|---|---|---|---|---|
| Yes (n = 154) |
No (n = 3584) |
||||
| % (n) | 95% CI | % (n) | % (n) | ||
| None | 68.0 (2545) | 66.6–69.6 | 39.0 (60) | 69.3 (2485) | 1.00 (Ref.) |
| Asthma only | 8.9 (331) | 7.9–9.8 | 9.1 (14) | 8.9 (317) | 1.56 (0.94–2.57) |
| Rhinitis only | 9.0 (337) | 8.1–9.9 | 11.0 (17) | 8.9 (320) | 1.40 (0.86–2.28) |
| Eczema only | 6.1 (228) | 5.3–6.9 | 14.9 (23) | 5.7 (205) | 3.49 (2.37–5.14) |
| Asthma + Rhinitis | 3.8 (141) | 3.2–4.4 | 7.1 (11) | 3.6 (130) | 2.74 (1.56–4.80) |
| Asthma + Eczema | 1.9 (71) | 1.5–2.3 | 5.9 (9) | 1.7 (62) | 4.66 (2.30–9.42) |
| Rhinitis + Eczema | 1.1 (42) | 0.8–1.5 | 3.9 (6) | 1.1 (36) | 3.97 (1.63–9.66) |
| Asthma + Rhinitis + Eczema | 1.2 (43) | 0.8–1.5 | 9.1 (14) | 0.8 (29) | 9.20 (4.50–18.78) |
PR: prevalence ratio; CI: confidence interval; Ref.: reference.
Adjusted for sex, age, BMI-for-age groups, mode of birth, birth order, breastfeeding, environmental tobacco smoke exposure, cat exposure in infancy, dog exposure in infancy, and parental history of food allergy.
Study-defined FA and the severity of symptoms of allergic diseases
Associations between study-defined FA and the severity of symptoms of asthma, rhinitis, and eczema were explored (Table 6). Study-defined FA was associated with an increased prevalence of severe asthma symptoms, including 4 or more wheezing attacks (aPR = 1.63, 95% CI: 1.20–2.21), wheezing that limited speech (aPR = 1.64, 95% CI: 1.10–2.46), and wheezing that disturbed sleep during one or more nights per week (aPR = 1.56, 95% CI: 1.28–1.88). Regarding the severity of rhinitis symptoms, children with study-defined FA reported more frequently than those without study-defined FA that rhinitis moderately (aPR = 1.37, 95% CI: 0.91–2.04) and severely (aPR = 1.71, 95% CI: 0.81–3.63) affected their daily activities, although these associations did not show statistical significance. Compared to children without study-defined FA, those with study-defined FA were less likely to experience complete remission of their itchy rash (aPR = 0.74, 95% CI: 0.53–1.03), whereas they were more likely to experience one or more night wakings per week caused by their itchy rash (aPR = 2.63, 95% CI: 1.68–4.14; Table 6).
Table 6.
Associations between study-defined food allergy and severity of symptoms of current asthma, rhinitis, and eczema.
| Study-defined food allergy |
Adjusted PRa (95% CI) | ||
|---|---|---|---|
| Yes, % (n/total) | No, % (n/total) | ||
| Asthma symptomsb | |||
| Wheezing attacks in the past 12 months | |||
| 1 to 3 | 56.4 (22/39) | 71.3 (241/338) | 1.00 (Ref.) |
| ≥4 | 43.6 (17/39) | 28.7 (97/338) | 1.63 (1.20–2.21) |
| Wheezing that limited speech in the past 12 months | |||
| No | 62.0 (31/50) | 80.3 (425/529) | 1.00 (Ref.) |
| Yes | 38.0 (19/50) | 19.7 (104/529) | 1.64 (1.10–2.46) |
| Wheezing that disturbed sleeping in the past 12 months | |||
| Never | 19.5 (8/41) | 31.9 (116/364) | 1.00 (Ref.) |
| <1 night per week | 29.3 (12/41) | 39.0 (142/364) | 0.98 (0.66–1.47) |
| ≥1 nights per week | 51.2 (21/41) | 29.1 (106/364) | 1.56 (1.28–1.88) |
| Rhinitis symptomsc | |||
| Rhinitis affecting daily activities in the past 12 months | |||
| Never/little | 51.1 (23/45) | 63.7 (285/447) | 1.00 (Ref.) |
| Moderately | 33.3 (15/45) | 27.3 (122/447) | 1.37 (0.91–2.04) |
| Severely | 15.6 (7/45) | 9.0 (40/447) | 1.71 (0.81–3.63) |
| Eczema symptomsd | |||
| Rash ever cleared completely in the past 12 months | |||
| No | 54.9 (28/51) | 40.8 (135/331) | 1.00 (Ref.) |
| Yes | 45.1 (23/51) | 59.2 (196/331) | 0.74 (0.53–1.03) |
| Night awakening by rash in the past 12 months | |||
| Never | 41.2 (21/51) | 62.6 (209/334) | 1.00 (Ref.) |
| <1 night per week | 27.4 (14/51) | 24.0 (80/334) | 1.36 (0.83–2.25) |
| ≥1 nights per week | 31.4 (16/51) | 13.3 (45/334) | 2.63 (1.68–4.14) |
PR: prevalence ratio; CI: confidence interval; Ref.: reference.
Adjusted for sex, age, BMI-for-age groups, mode of birth, birth order, breastfeeding, environmental tobacco smoke exposure, cat exposure in infancy, dog exposure in infancy, and parental history of food allergy.
Associations between study-defined FA (exposure variable) and severity of asthma symptoms were assessed among subjects with current asthma.
Associations between study-defined FA (exposure variable) and severity of rhinitis symptoms were assessed among subjects with current rhinitis.
Associations between study-defined FA (exposure variable) and severity of eczema symptoms were assessed among subjects with current eczema.
Discussion
This is the first study to estimate the prevalence of FA, defined by a convincing clinical history, among schoolchildren in Kuwait. Moreover, this study assessed associations between study-defined FA with various risk factors and the coexistence and severity of asthma, rhinitis, and eczema. The 12-month prevalence of study-defined FA was estimated to be 4.1% (95% CI: 3.5–4.8). Egg, fish, shellfish, peanut, and tree nut were the most reported offending food allergens, with each affecting more than 1% of the total number of study subjects. Factors associated with the increased prevalence of study-defined FA included the female sex, underweight and adiposity, cesarean section delivery, exposure to household dogs during infancy, and parental history of doctor-diagnosed FA. However, later birth order was associated with a reduced prevalence of study-defined FA. Our study showed that study-defined FA was associated with an increased prevalence of the coexistence of asthma, rhinitis, and eczema. Moreover, study-defined FA was associated with increased severity of symptoms of allergic diseases.
Prevalence estimates of FA have mainly been based on self-/parent-reported perceived or doctor-diagnosed FA or specific immunoglobulin E (IgE) or skin prick test (SPT) sensitization to common food allergens. The aforementioned approaches are known to overestimate the true prevalence of FA.37 However, a limited number of population-based studies have estimated the prevalence of FA using the gold standard of OFC, which must be performed in a controlled clinical setting, making OFC an unfeasible option in large epidemiologic studies. An alternative is to use “convincing clinical history” to ascertain FA, a method that has provided comparable prevalence estimates to OFC-confirmed FA11 and has been shown to be crucial when assessing FA in clinical settings.38, 39 To this end, caution should be practiced when interpreting and comparing FA prevalence estimates due to the different approaches used to ascertain FA.
In the current study, we based our analysis on study-defined FA, which was ascertained using convincing clinical history that encompassed the characteristics of the reaction, timing from exposure to the development of symptoms, and exposure to common offending food allergens. This method of defining FA should identify typical IgE-mediated FA rather than food poisoning and intolerance. Using this approach yielded a prevalence estimate (4.1%) that is comparable to estimates from other reports using OFC or convincing clinical history for the diagnosis of FA among similarly aged study populations. For instance, the SchoolNuts cross-sectional study, conducted among adolescents aged 10–14 years residing in Melbourne, Australia, estimated the prevalence of FA, defined based on positive OFC or clinical history with IgE sensitization, to be 4.5% (95% CI: 3.9–5.1).26 Similarly, data from the Isle of Wight birth cohort study based on the United Kingdom indicated that 4.1% (95% CI: 3.2–5.4) of participants aged 18 years had FA, which was defined using convincing clinical history.31 In the United States, the results from the National Health and Nutrition Examination Survey 2005–2006 estimated that 3.8% (standard error: ± 0.4) of children aged 6–19 years had clinically defined FA.40 At the national level, a previous report estimated the prevalence of FA, defined by convincing clinical history, to be 5.4% among Kuwait University students (mean age ± SD: 20.7 ± 1.2 years).41
In this report, parent-reported perceived FA and parent-reported doctor-diagnosed FA presented lifetime prevalence estimates, whereas the study-defined FA estimated the prevalence in the past 12 months. As expected, parent-reported perceived FA yielded the highest prevalence estimate (12.7%) followed by parent-reported doctor-diagnosed FA (8.2%) and study-defined FA (4.1%). Our estimated parent-reported perceived FA prevalence (12.7%) is close to a pooled estimate (∼10%) from a large meta-analysis.12 Similarly, our parent-reported doctor-diagnosed FA (8.2%) is consistent with estimates among American (8.0%) and Canadian (7.14%) children.42, 43
FA is known to be a condition of early childhood (i.e., highly prevalent among preschool children). For instance, the HealthNuts cohort study in Melbourne, Australia, estimated the prevalence of challenge-confirmed FA at age 1 and 4 years to be 11.0% (95% CI: 10.1–11.9) and 3.8% (95% CI: 3.3–4.4), respectively; indicating that nearly two-thirds of FA has resolved by school age.44 Similarly, the global survey by the World Allergy Organization on FA burden showed that challenge-conformed FA is higher among infants and preschool children aged ≤5 years (range: 1%–10%) compared to schoolchildren aged > 5 years (range: 0.2%–2.5%).23 Such observations indicate that a large proportion of FA cases may resolve by school age. Hence, our estimated prevalence of study-defined FA (4.1%) among schoolchildren aged 11–14 years may not reflect the burden of FA in infancy and early childhood.
Regarding the prevalence of common food allergens, egg, fish, shellfish, peanut, and tree nut were the most reported food triggers in our study. The observed prevalence estimates in our study are comparable to estimates reported in large meta-analyses.12, 45 Surprisingly, fish (1.6%) and shellfish (1.3%) were among the most commonly reported food allergens in our study, which was not expected due to the fact that seafood forms a major food source in Kuwait. Similarly, allergy to fish/shellfish is common in coastal Asian countries where seafood consumption is also high.46, 47 Although it is suggested that the increased global consumption of seafood may have led to more frequent reporting of adverse reactions, food processing, dietary habits, and cultural practices (e.g., age of introduction of seafood) could contribute to the etiology of seafood allergy and explain the increasing trends and differences across nations.48 Nevertheless, adverse reactions to seafood may not always resemble true allergy, rather they can in fact be secondary to food contamination, toxins, or preservatives.48, 49 Moreover, a large proportion of study participants reported allergies to fresh fruits (4.6%) and vegetables (1.6%). Such allergies are mainly associated with a localized IgE-mediated reaction known as oral allergy syndrome (also called pollen-food allergy syndrome), which is caused by cross-reactivity with pollen/inhalant allergens.50 A systematic review of the literature showed that the prevalence of self-/parent-reported perceived allergy to any fruit ranged between 0.4% and 11.5% and to any vegetable varied from 0.3% to 3.3%.51 Prevalence estimates of parent-reported fruit allergy (4.6%) and vegetable allergy (1.6%) in our study sample are within the aforementioned ranges.
Multiple risk factors for FA have been identified in previous investigations and have been reviewed in detail elsewhere.1, 4, 10, 52 In our analysis, increased prevalence of study-defined FA was seen in the following subgroups: females (compared to males); underweight (thin), overweight, and obese individuals (compared to those with normal BMI-for-age); those delivered via cesarean section (compared to those delivered vaginally); those exposed to dogs in infancy; and those with a parental history of doctor-diagnosed FA. It has been suggested that the male sex in children and the female sex in adults are associated with an increased risk of FA; however, conflicting results have been reported.5, 11, 52 Our results showing increased prevalence among females are consistent with findings of a Swedish cohort study of more than 1 million children.53 The noted positive associations between adiposity, cesarean delivery, and parental history of FA and the prevalence of study-defined FA are consistent with the findings of prior investigations.53, 54, 55 Similarly, the observed inverse association between birth order and the prevalence of study-defined FA is consistent with the findings of prior studies.4, 56 Although emerging evidence suggests that exposure to dogs in early life reduces the risk of FA,4, 52 our study showed that exposure to dogs in infancy was associated with an increased prevalence of study-defined FA, an association that needs further corroboration. Similarly, the observed increased prevalence of study-defined FA among underweight children compared to those with normal BMI-for-age needs to be corroborated. However, a plausible explanation is reverse causation where malnutrition in children with FA could lead to growth impairment and underweight.57, 58 On the other hand, prior studies investigating the effect of breastfeeding on the development of allergic diseases, including FA, reported conflicting conclusions, with some studies suggesting protective effect, others showing no effect, and a few suspecting that breastfeeding could even increase the risk of developing allergic diseases.59, 60 Our report showed no association between breastfeeding and study-defined FA.
IgE-mediated inflammatory mechanisms predispose individuals to and link the development of asthma, rhinitis, eczema, and FA. Prior studies have shown that FA is associated with an increased risk of asthma, rhinitis, and eczema.20, 22 In this report, we assessed whether FA is associated with the single occurrence and the coexistence of allergic diseases. The results of this study showed that study-defined FA was not statistically significantly associated with an increased prevalence of asthma only or rhinitis only. However, study-defined FA was strongly associated with an elevated prevalence of eczema only, which further highlights that FA and eczema are closely related. On the other hand, study-defined FA showed strong associations with having more than one allergic disease, an observation that has been previously reported.61 Moreover, we demonstrated that study-defined FA is associated with increased severity of asthma, rhinitis, and eczema symptoms. Therefore, FA might contribute to the chronicity and severity of allergic diseases. Nevertheless, it is essential to indicate that our analysis aimed to assess concurrent associations rather than to determine temporal associations.
A major strength of the current study is the representative and large study sample, which allowed for the estimation of FA prevalence among schoolchildren throughout Kuwait. A limitation to our study is the lack of the use of the gold standard of OFC to ascertain FA status. However, we have applied robust symptom-based criteria that are commonly used in clinical settings. Moreover, the applied FA ascertainment criteria have been published previously30, 31, 32 and have provided results comparable to those of investigations using the gold standard for clinical diagnosis, which is a further indication of the robustness of our FA definition. The observed difference between our estimated prevalence of parent-reported anaphylaxis (3.9%) among children with study-defined FA and the estimated prevalence of anaphylaxis in the SchoolNuts study (9.7%)62 is an indication that parent-report of anaphylaxis and syncope could have led to unreliable estimates. Another potential limitation to our study is the inaccuracy of parent-/guardian-reported weight and height of their children, which could have led to the misclassification of children into BMI-for-age groups. However, the estimated prevalence of overweight (25.4%) and obesity (28.8%) in the current report did not substantially differ from that in a previous investigation conducted among schoolchildren aged 6–18 years in Kuwait that used objective weight and height measurements (overweight: 21.6% and obesity: 30.5%).63 Data on race/ethnicity of participants was not collected, which could be an important factor in FA. Rather, information on nationality (Kuwaiti vs non-Kuwaiti), a variable that could partially account for the race/ethnicity effect, was available and analysis comparing the two population groups showed that children of Kuwaiti nationality (4.1%, 142/3,459) and non-Kuwaiti nationality (4.3%, 12/379) had similar prevalence estimates of study-defined FA (see Online Supplementary, Table S1). Moreover, the determination of temporal associations between study-defined FA and the coexistence and the severity of allergic diseases was hampered by the cross-sectional nature of our study, in which concurrent data were collected. Selection bias could also be a concern in large population-based cross-sectional studies; however, the possibility of selection bias affecting the results of our study is low because the response proportion was high (i.e., 73.9%). Moreover, since this study was conducted among older children, the effect of recall bias on the evaluated associations cannot be excluded, which can either overestimate or underestimate the measure of association.
Conclusions
The findings of this study indicate that FA is common among schoolchildren in Kuwait, which mirrors prevalence estimates reported in Western nations. Common risk factors for FA identified in our study included the female sex, underweight and adiposity, cesarean section delivery, exposure to dogs in infancy, and parental history of FA, whereas increased birth order was associated with a lower prevalence of FA. Moreover, we demonstrated that FA is associated with the coexistence and the severity of asthma, rhinitis, and eczema. Therefore, FA is a common problem among schoolchildren in Kuwait that potentially links the comanifestations of asthma, rhinitis, and eczema and contributes to their chronicity and severity.
Ethics approval and consent to participate
The study was approved by the Standing Committee for Coordination of Health and Medical Research, Ministry of Health, Kuwait (no. 2016/451). Written informed consent was obtained from the parents or legal guardians to enroll children in the study.
Consent for publication
Not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AHZ conceived, designed, and planned the study; obtained funding; supervised the conduct of the research; analyzed and interpreted the data; and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to the children and their parents who participated in this study. Additionally, we sincerely appreciate the cooperation, coordination, and assistance of the staff at the different schools. We also thank Mr. Mahdi Hussain, Mr. Mohammad Ghadhanfar, Ms. Shaymaa Hussain, and Ms. Anwar Al-Baloul for their considerable assistance with data collection and data entry.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100024.
Funding
This project was funded partially by Kuwait Foundation for the Advancement of Sciences under project code: P115-13MC-05. Additionally, this work was supported and funded by Kuwait University, Research Project No. MC01/16. The funders had no role in study design, data collection, analysis, and interpretation of data and decision to publish or preparation of the manuscript.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sicherer S.H., Sampson H.A. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141:41–58. doi: 10.1016/j.jaci.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Cummings A.J., Knibb R.C., King R.M., Lucas J.S. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy. 2010;65:933–945. doi: 10.1111/j.1398-9995.2010.02342.x. [DOI] [PubMed] [Google Scholar]
- 3.Boyce J.A., Assa'ad A., Burks A.W. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol. 2010;126:1105–1118. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renz H., Allen K.J., Sicherer S.H. Food allergy. Nat Rev Dis Primers. 2018;4:17098. doi: 10.1038/nrdp.2017.98. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer S.H., Sampson H.A. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Prescott S., Allen K.J. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011;22:155–160. doi: 10.1111/j.1399-3038.2011.01145.x. [DOI] [PubMed] [Google Scholar]
- 7.Lai C.K., Beasley R., Crane J. Allergies in childhood phase three study G. Global variation in the prevalence and severity of asthma symptoms: phase three of the international study of asthma and allergies in childhood (ISAAC) Thorax. 2009;64:476–483. doi: 10.1136/thx.2008.106609. [DOI] [PubMed] [Google Scholar]
- 8.Ait-Khaled N., Pearce N., Anderson H.R. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: the international study of asthma and allergies in childhood (ISAAC) phase three. Allergy. 2009;64:123–148. doi: 10.1111/j.1398-9995.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- 9.Odhiambo J.A., Williams H.C., Clayton T.O., Robertson C.F., Asher M.I., Group I.P.T.S. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124 doi: 10.1016/j.jaci.2009.10.009. 1251-1258 e23. [DOI] [PubMed] [Google Scholar]
- 10.Savage J., Johns C.B. Food allergy: epidemiology and natural history. Immunol Allergy Clin N AM. 2015;35:45–59. doi: 10.1016/j.iac.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nwaru B.I., Hickstein L., Panesar S.S. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69:62–75. doi: 10.1111/all.12305. [DOI] [PubMed] [Google Scholar]
- 12.Rona R.J., Keil T., Summers C. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Zheng T., Yu J., Oh M.H., Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67–73. doi: 10.4168/aair.2011.3.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams H., Flohr C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J Allergy Clin Immunol. 2006;118:209–213. doi: 10.1016/j.jaci.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 15.Barberio G., Pajno G.B., Vita D., Caminiti L., Canonica G.W., Passalacqua G. Does a 'reverse' atopic march exist? Allergy. 2008;63:1630–1632. doi: 10.1111/j.1398-9995.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- 16.Goksor E., Loid P., Alm B., Aberg N., Wennergren G. The allergic march comprises the coexistence of related patterns of allergic disease not just the progressive development of one disease. Acta Paediatr. 2016;105:1472–1479. doi: 10.1111/apa.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belgrave D.C., Granell R., Simpson A. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Med. 2014;11:e1001748. doi: 10.1371/journal.pmed.1001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gough H., Grabenhenrich L., Reich A. Allergic multimorbidity of asthma, rhinitis and eczema over 20 years in the German birth cohort MAS. Pediatr Allergy Immunol. 2015;26:431–437. doi: 10.1111/pai.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballardini N., Kull I., Lind T. Development and comorbidity of eczema, asthma and rhinitis to age 12: data from the BAMSE birth cohort. Allergy. 2012;67:537–544. doi: 10.1111/j.1398-9995.2012.02786.x. [DOI] [PubMed] [Google Scholar]
- 20.Alduraywish S.A., Lodge C.J., Campbell B. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy. 2016;71:77–89. doi: 10.1111/all.12784. [DOI] [PubMed] [Google Scholar]
- 21.Foong R.X., du Toit G., Fox A.T. Asthma, food allergy, and how they relate to each other. Front Pediatr. 2017;5:89. doi: 10.3389/fped.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alduraywish S.A., Standl M., Lodge C.J. Is there a march from early food sensitization to later childhood allergic airway disease? Results from two prospective birth cohort studies. Pediatr Allergy Immunol. 2017;28:30–37. doi: 10.1111/pai.12651. [DOI] [PubMed] [Google Scholar]
- 23.Prescott S.L., Pawankar R., Allen K.J. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6:21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motosue M.S., Bellolio M.F., Van Houten H.K., Shah N.D., Campbell R.L. Increasing emergency department visits for anaphylaxis, 2005-2014. J Allergy Clin Immunol Pract. 2017;5 doi: 10.1016/j.jaip.2016.08.013. 171-175 e3. [DOI] [PubMed] [Google Scholar]
- 25.Mullins R.J., Dear K.B., Tang M.L. Time trends in Australian hospital anaphylaxis admissions in 1998-1999 to 2011-2012. J Allergy Clin Immunol. 2015;136:367–375. doi: 10.1016/j.jaci.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki M., Koplin J.J., Dharmage S.C. Prevalence of clinic-defined food allergy in early adolescence: the SchoolNuts study. J Allergy Clin Immunol. 2018;141 doi: 10.1016/j.jaci.2017.05.041. 391-398 e4. [DOI] [PubMed] [Google Scholar]
- 27.Asher M.I., Keil U., Anderson H.R. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 28.Arshad S.H., Holloway J.W., Karmaus W. Cohort profile: the Isle of Wight whole population birth cohort (IOWBC) Int J Epidemiol. 2018;47 doi: 10.1093/ije/dyy023. 1043-4i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGowan E.C., Keet C.A. Prevalence of self-reported food allergy in the national health and nutrition examination survey (NHANES) 2007-2010. J Allergy Clin Immunol. 2013;132 doi: 10.1016/j.jaci.2013.07.018. 1216-9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arshad S.H., Venter C., Roberts G., Dean T., Kurukulaaratchy R. The natural history of peanut sensitization and allergy in a birth cohort. J Allergy Clin Immunol. 2014;134 doi: 10.1016/j.jaci.2014.09.026. 1462-1463 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkataraman D., Erlewyn-Lajeunesse M., Kurukulaaratchy R.J. Prevalence and longitudinal trends of food allergy during childhood and adolescence: results of the Isle of Wight Birth Cohort study. Clin Exp Allergy. 2018;48:394–402. doi: 10.1111/cea.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkataraman D., Soto-Ramirez N., Kurukulaaratchy R.J. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J Allergy Clin Immunol. 2014;134 doi: 10.1016/j.jaci.2014.07.033. 876-882 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanifin J.M., Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl. 1980;92:44–47. [Google Scholar]
- 34.de Onis M., Onyango A.W., Borghi E., Siyam A., Nishida C., Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 36.Maldonado G., Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 37.Dunlop J.H., Keet C.A. Epidemiology of food allergy. Immunol Allergy Clin N AM. 2018;38:13–25. doi: 10.1016/j.iac.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Sicherer S.H., Wood R.A. American Academy of Pediatrics Section on A, Immunology. Allergy testing in childhood: using allergen-specific IgE tests. Pediatrics. 2012;129:193–197. doi: 10.1542/peds.2011-2382. [DOI] [PubMed] [Google Scholar]
- 39.Sicherer S.H., Allen K., Lack G., Taylor S.L., Donovan S.M., Oria M. Critical issues in food allergy: a national academies consensus report. Pediatrics. 2017;140:e20170194. doi: 10.1542/peds.2017-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu A.H., Jaramillo R., Sicherer S.H. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126 doi: 10.1016/j.jaci.2010.07.026. 798-806 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali F. A survey of self-reported food allergy and food-related anaphylaxis among young adult students at Kuwait university, Kuwait. Med Princ Pract. 2017;26:229–234. doi: 10.1159/000464361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta R.S., Springston E.E., Warrier M.R. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128 doi: 10.1542/peds.2011-0204. e9-17. [DOI] [PubMed] [Google Scholar]
- 43.Soller L., Ben-Shoshan M., Harrington D.W. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986–988. doi: 10.1016/j.jaci.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 44.Peters R.L., Koplin J.J., Gurrin L.C. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol. 2017;140 doi: 10.1016/j.jaci.2017.02.019. 145-153 e8. [DOI] [PubMed] [Google Scholar]
- 45.Nwaru B.I., Hickstein L., Panesar S.S. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992–1007. doi: 10.1111/all.12423. [DOI] [PubMed] [Google Scholar]
- 46.Chiang W.C., Kidon M.I., Liew W.K., Goh A., Tang J.P., Chay O.M. The changing face of food hypersensitivity in an Asian community. Clin Exp Allergy. 2007;37:1055–1061. doi: 10.1111/j.1365-2222.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 47.Lee A.J., Gerez I., Shek L.P., Lee B.W. Shellfish allergy--an Asia-Pacific perspective. Asian Pac J Allergy Immunol. 2012;30:3–10. [PubMed] [Google Scholar]
- 48.Thalayasingam M., Lee B.W. Fish and shellfish allergy. Chem Immunol Allergy. 2015;101:152–161. doi: 10.1159/000375508. [DOI] [PubMed] [Google Scholar]
- 49.Khora S.S. Seafood-associated shellfish allergy: a comprehensive review. Immunol Investig. 2016;45:504–530. doi: 10.1080/08820139.2016.1180301. [DOI] [PubMed] [Google Scholar]
- 50.Waserman S., Begin P., Watson W. IgE-mediated food allergy. Allergy Asthma Clin Immunol. 2018;14:55. doi: 10.1186/s13223-018-0284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuidmeer L., Goldhahn K., Rona R.J. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008;121 doi: 10.1016/j.jaci.2008.02.019. 1210-8 e4. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki M., Peters R.L., Koplin J.J. Risk factors for food allergy in early adolescence: the SchoolNuts study. J Allergy Clin Immunol Pract. 2018;6:496–505. doi: 10.1016/j.jaip.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Mitselou N., Hallberg J., Stephansson O., Almqvist C., Melen E., Ludvigsson J.F. Cesarean delivery, preterm birth, and risk of food allergy: nationwide Swedish cohort study of more than 1 million children. J Allergy Clin Immunol. 2018;142 doi: 10.1016/j.jaci.2018.06.044. 1510-1514 e2. [DOI] [PubMed] [Google Scholar]
- 54.Visness C.M., London S.J., Daniels J.L. Association of obesity with IgE levels and allergy symptoms in children and adolescents: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2009;123 doi: 10.1016/j.jaci.2008.12.1126. 1163-9, 9 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-Shoshan M., Soller L., Harrington D.W. Eczema in early childhood, sociodemographic factors and lifestyle habits are associated with food allergy: a nested case-control study. Int Arch Allergy Immunol. 2015;166:199–207. doi: 10.1159/000381829. [DOI] [PubMed] [Google Scholar]
- 56.Kikkawa T., Yorifuji T., Fujii Y. Birth order and paediatric allergic disease: a nationwide longitudinal survey. Clin Exp Allergy. 2018;48:577–585. doi: 10.1111/cea.13100. [DOI] [PubMed] [Google Scholar]
- 57.Meyer R., De Koker C., Dziubak R. Malnutrition in children with food allergies in the UK. J Hum Nutr Diet. 2014;27:227–235. doi: 10.1111/jhn.12149. [DOI] [PubMed] [Google Scholar]
- 58.Mehta H., Ramesh M., Feuille E., Groetch M., Wang J. Growth comparison in children with and without food allergies in 2 different demographic populations. J Pediatr. 2014;165:842–848. doi: 10.1016/j.jpeds.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Rajani P.S., Seppo A.E., Jarvinen K.M. Immunologically active components in human milk and development of atopic disease, with emphasis on food allergy, in the pediatric population. Front Pediatr. 2018;6:218. doi: 10.3389/fped.2018.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shroba J., Rath N., Barnes C. Possible role of environmental factors in the development of food allergies. Clin Rev Allergy Immunol. 2018 doi: 10.1007/s12016-018-8703-2. [DOI] [PubMed] [Google Scholar]
- 61.Kijima A., Murota H., Takahashi A. Prevalence and impact of past history of food allergy in atopic dermatitis. Allergol Int. 2013;62:105–112. doi: 10.2332/allergolint.12-OA-0468. [DOI] [PubMed] [Google Scholar]
- 62.McWilliam V.L., Koplin J.J., Field M.J. Self-reported adverse food reactions and anaphylaxis in the SchoolNuts study: a population-based study of adolescents. J Allergy Clin Immunol. 2018;141:982–990. doi: 10.1016/j.jaci.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 63.Elkum N., Al-Arouj M., Sharifi M., Shaltout A., Bennakhi A. Prevalence of childhood obesity in the state of Kuwait. Pediatr Obes. 2016;11:e30–e34. doi: 10.1111/ijpo.12090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.