Abstract
Background
Left atrial volume (LAV) is an independent prognosticator of cardiovascular events. We investigated whether LAV could be accurately and reliably measured using coronary calcium score (CAC) scan.
Methods
We retrospectively selected consecutive patients that underwent coronary CT angiography (CCTA) and CAC scans. A standardized approach to calculate LAV on images was implemented. The measurements of the LAV on CAC scans and CCTA were performed one to three weeks apart in a random fashion by two readers blinded to the results of each other. The LAV measurements from CAC scan were compared to those from CCTA using correlation analysis. Inter-observer and intra-observer agreement of LAV measurement using CAC scan was evaluated.
Results
Final analysis included one hundred subjects, mean age 52 ± 12 years, 48% male. There was a trend of a marginally larger, albeit not clinically significant, mean LAV calculated using CAC scan compared to that using CCTA: 74.3 vs. 71.0 mL: p < 0.001; for reader 1, and 71.7 vs. 71.2 mL p = 0.06 for reader 2, respectively. LAV using CAC scan and CCTA were highly correlated (R = 0.954, p < 0.001 for reader1 and R = 0.945, p < 0.001 for reader 2). There was high reproducibility within each reader with ICC of 0.951 and 0.989 for readers 1 and 2, respectively (p < 0.001). Finally, there was high inter-observer agreement as indicated by R of 0.97 and ICC of 0.96 (p < 0.001 for both).
Conclusions
Quantification of LAV from CAC scan using the proposed standardized approach is feasible, highly reliable and reproducible as compared to CCTA.
Highlights
-
•
Left atrial volume (LAV) measurement from coronary calcium (CAC) scan is feasible.
-
•
The ECG gating of CAC scan improves delineation of the left atrium.
-
•
LAV measurement from CAC scan is highly accurate and reproducible.
-
•
Inclusion of LAV from CAC scan can potentially improve risk prediction.
1. Introduction
Left atrial (LA) volume is an independent predictor of adverse cardiovascular (CV) outcomes [1]. Increased LA volume (LAV) is associated with higher risk of atrial fibrillation [[2], [3], [4], [5]], incident heart failure [4,6,7] and CV mortality [[8], [9], [10]]. Further, LAV has become an essential component for evaluation of diastolic function [11]. LAV is most commonly measured during transthoracic echocardiograms (TTE) using equations that adopt certain geometric shapes and assumptions about the LA [12]. LAV as measured by TTE is highly dependent on image quality. Additionally, TTE based LAV measurement underestimates the LAV when compared to LAV as measured by contrast-enhanced computed tomography (CT) or cardiac magnetic resonance (CMR) imaging [[13], [14], [15], [16], [17], [18]].
Coronary CT angiography (CCTA) is a rapidly evolving imaging modality to evaluate coronary artery disease in symptomatic patients [19]. CCTA provides excellent spatial resolution and superb endocardial border definition due to high contrast to noise ratio from the use of iodinated contrast. Additionally, CCTA provides full volumetric data of the whole cardiac chambers including the LA, which makes measurement of the LAV possible without the need for any geometric assumption of the LA shape. LAV measurements using CCTA has been shown to be very accurate when compared to LAV by CMR [13].
Similarly, coronary artery calcium (CAC) scan is used to obtain CAC score which is becoming widely used to improve CV risk prediction [[20], [21], [22], [23]]. CAC scan is obtained using ECG-gated, non-enhanced cardiac CT scanning. The use of ECG-gating significantly reduces the motion artifact from cardiac motion enabling the definition of the LA borders with high confidence on CAC scan even without the use of iodinated contrast. Additionally, like CCTA, CAC scan provides full volumetric data of the whole cardiac chambers enabling the measurement of the true LAV without the need for any geometric assumption. However, the accuracy of LAV measurement from CAC scan has not been evaluated before.
The objective of our study was to investigate the accuracy of LAV measurement from CAC scan using a standardized approach as compared to that obtained from CCTA. Additionally, we sought to evaluate the intra-observer and inter-observer agreement of LAV measurement on CAC scan.
2. Material and methods
We retrospectively selected consecutive patients that underwent clinically indicated CCTA and CAC scans between November 2017 and February 2018. CCTA acquisition was performed using contrast enhanced and, depending on patient heart rate, either prospective or retrospective ECG-gating (field of view [FOV] 200 × 200 mm, slice thickness 0.63 cm, 120 kV). Images of CCTA were analyzed at cardiac phase of 70 ± 2%. CAC scans were obtained using ECG-prospective gating at 70% phase (FOV 200 × 200 mm, slice thickness 2.5 mm, 120 kV). All images were obtained using a 64-slice Discovery CT750 HD (GE Healthcare, Chicago, Illinois). Beta blockers were used to achieve target HR 60–70 bpm. Sublingual nitroglycerin was administered prior to CCTA scan. Relevant demographic and clinical information were also recorded.
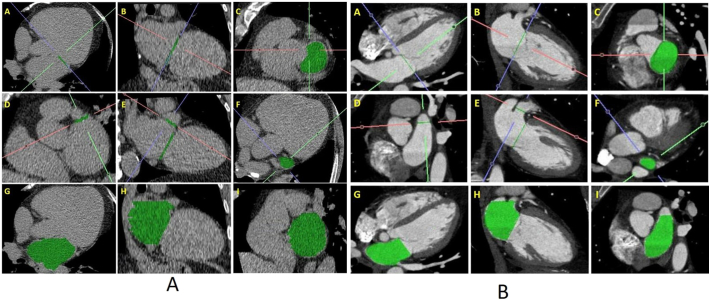
We developed the following standardized approach to calculate LAV from both CAC scans and CCTA. The LA/LV separation plane (mitral valve annular plane) was identified by connecting mitral valve leaflet insertion points in 2 orthogonal planes. In CAC scan, when uncertainty limited visualization of the MV leaflet insertion, then mitral annular plane was defined by a plane that was created to connect the anterior and posterior borders of the AV groove in 4- and 2-chamber views. Afterward, the neck of the LA appendage was identified using a reconstructed 2-chamber view. Following identification of the mitral annular plane and LAA neck, the LAV was calculated by manually tracing the contours of the LA on the axial plane, excluding the LAA at its neck and the pulmonary veins at their ostia (Fig. 1A and B). The volume of the LA was calculated as the true volume using the image processing software.
Fig. 1.
(A) Standardized approach for LAV quantification on CAC scan.
Note: Panels A–C: Identification of LA/LV separation plane (mitral valve annular plane) by connecting mitral valve leaflet insertion points in 2 orthogonal planes. Panels D–F: Identification of the neck of the LA appendage using a reconstructed 2-chamber view. Panels G–I: Following the identification of the mitral annular plane and LAA neck, the LA volume is calculated by manual tracing the endocardial contours of the LA on the axial plane (G) excluding the LAA at its neck and the pulmonary veins at their ostia. The volume of the LA was calculated as the true LA volume using the image processing software.
(B) Standardized approach for LAV quantification on contrast CT scan.
Note: Panels A–C: Identification of LA/LV separation plane (mitral valve annular plane) by connecting mitral valve leaflet insertion points in 2 orthogonal planes. Panels D–F: Identification of the neck of the LA appendage using a reconstructed 2-chamber view. Panels G–I: Following the identification of the mitral annular plane and LAA neck, the LA volume is calculated by manual tracing the endocardial contours of the LA on the axial plane (G) excluding the LAA at its neck and the pulmonary veins at their ostia. The volume of the LA was measured as the true volume using the image processing software.
Two expert readers performed all the measurements: reader 1 (TTH) and reader 2 (AC) with 5 and 3 years of experience, respectively. Using the above described method, each reader performed measurements of LAV on CAC scan and CCTA 1–3 weeks apart (described as time 1 and time 2), in a random fashion. To assess intra-observer variability of readers, each reader repeated LAV measurements on both CAC and CCTA scans on a randomly selected subset of the total study population. Readers were blinded to the results of each other and to their own results. All image analysis was done using Aquarius iNtuition software (Version 4.4.7, TeraRecon, Inc., San Mateo, CA, USA). LAV quantified by CCTA (LAV-CCTA) was used as the reference standard to which LAV measured on CAC scan (LAV-CAC) was compared. Image quality was assessed as “poor” if LA border delineation was not possible, otherwise, image quality was considered “good”.
Data are presented as mean ± standard deviation (SD) for continuous variables and as proportions for categorical variables. Paired t-test was used to compare the mean values of continuous variables of repeated measurements. To determine accuracy and agreement of LAV-CAC compared to LAV-CCTA, we compared LAV-CAC with LAV-CCTA using Pearson correlation analysis, absolute agreement intra-class correlation (ICC) analysis, and Bland-Altman plot for both reader 1 and reader 2 (Fig. 2). To assess intra-observer reproducibility of LAV-CAC, absolute agreement intra-class correlation analysis was performed for both readers. Inter-observer reproducibility of LAV-CAC was tested with two-way mixed effect intra-class correlation agreement analysis on randomly selected readings (either time 1 or time 2 readings) of CAC-CT by reader 1 (TH) and reader 2 (AC). Statistical significance was set at two tailed p < 0.05. IBM SPSS Statistic 21.0 (Chicago, IL, USA) was used for all statistical analyses. The study was approved by the Institutional Review Board.
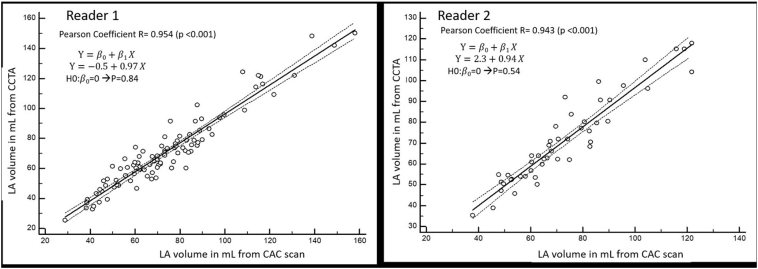
Fig. 2.
Correlation analysis of LAV-CAC vs. LAV-CCTA.
Note: Correlation of the left atrial volume as measured from CCTA (y-axis) compared to LAV as measured from CAC scan (x-axis) for reader 1 (left side of the figure) and reader 2 (right side of the figure). CCTA: coronary CT angiogram; CAC: coronary artery calcium: LA: left atrium.
3. Results
3.1. Patient characteristics and feasibility
One-hundred eleven subjects were screened. Six subjects were excluded because of poor CAC scan image quality due to motion artifacts or poor signal to noise ratio. Three subjects were excluded because CAC scan was not performed. Two more subjects were excluded as they were considered outliers, being the LAV > 3SD (99.7%) of the mean value for the entire cohort. A total of 100 subjects were included in the final analysis, mean age 52.1 ± 12.2 years, 48% male. Cohort characteristics are shown in Table 1. Measurement of the LAV from CAC scan was feasible with minimal additional time. On average, it required 2–3 min of additional time of tracing the LA border using the described standardized protocol.
Table 1.
Characteristics of the study cohort.
| Whole cohort (N = 100) |
|
|---|---|
| Demographic data | |
| Age, years | 52.1 ± 12.2 |
| Male gender, N (%) | 48 (48) |
| BMI, kg/m2 | 29.4 ± 7.2 |
| HTN, N (%) | 55 (55%) |
| DM, N (%) | 17 (17%) |
| History of CAD, N (%) | 3 (3%) |
| Congestive heart failure | 4(4%) |
| GFR, mL/min | 83.4 ± 23.8 |
| LVEF, % | 57.8 ± 7.1 |
| Coronary CT findings | |
| Mean Agatston CAC score | 124 ± 409 |
| DLP, mGy-cm | 464 ± 294 |
| No coronary artery disease | 56 (56%) |
| Mild coronary artery disease (<50% stenosis) | 33 (33%) |
| Moderate coronary artery disease (50–69% stenosis) | 6 (6%) |
| Severe coronary artery disease (≥70% stenosis) | 5 (5%) |
Notes: Data are presented as mean ± SD, or N (%). BMI: body mass index; DM: diabetes mellitus; HLP: hyperlipidemia; SBP: systolic blood pressure; DBP: diastolic blood pressure; CAD: coronary artery disease; LVEF: left ventricular ejection fraction; CAC: coronary artery calcium; HU: Hounsfield units; DLP: dose length product.
3.2. Left atrial volume quantification
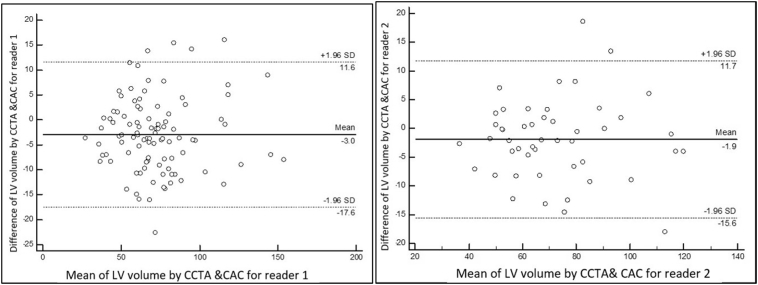
There was a very small difference between the LAV as measured from CAC scan compared to that measured from CCTA. The mean LAV measured from CAC scan was marginally larger using CAC scan compared to CCTA (74.3 mL vs. 71.0, p < 0.001, for reader 1 and 71.7 vs. 71.2; p < 0.062 for reader 2) respectively (Table 2). LAV-CAC was strongly correlated with LAV-CCTA for both reader 1 (R = 0.95) and reader 2 (R = 0.94), Table 2. The ICC agreement between LAV-CAC and LAV-CCTA for reader 1 was 0.95 (95%CI 0.91–0.97, p < 0.001) and 0.94 (95%CI 0.90–0.97, p < 0.001) for reader 2 (Table 2). The Bland-Altman analysis demonstrated good accuracy of LAV-CAC compared to LAV-CCTA yielding an arithmetic mean difference between LAV-CCTA and LAV-CAC of −2.98 mL (95%CI −4.48 to −1.49 mL) for reader 1, and −1.92 mL (95%CI −3.94 to −0.10 mL) for reader 2 (Fig. 3).
Table 2.
Left atrial volumes with intra-observer and inter-observer reproducibility of LAV-CAC.
| Left atrial volumes⁎ | ||||
|---|---|---|---|---|
| LAV CAC (mL) |
LAV CCTA (mL) |
Correlation coefficient | ICC; 95%CI | |
| Reader 1 (TH) | 74.3 ± 24.2 | 71.0 ± 24.6 | 0.954 (p < 0.001) |
0.947; 0.909–0.968 (p < 0.001) |
| Reader 2 (AC) | 71.7 ± 22.2 | 71.2 ± 20.8 | 0.944 (p < 0.001) |
0.941; 0.895–0.967 (p < 0.001) |
| Intra-observer reproducibility# | ||||
|---|---|---|---|---|
| LAV CAC (mL) Time 1 |
LAV CAC (mL) Time 2 |
Correlation coefficient | ICC; 95%CI | |
| Reader 1 (TH) | 75.5 ± 23.3 | 71.2 ± 23.9 | 0.968 (p < 0.001) |
0.951; 0.818–0.980 (p < 0.001) |
| Reader 2 (AC) | 71.7 ± 22.2 | 73.1 ± 21.1 | 0.989 (p < 0.001) |
0.989; 0.980–0.994 (p < 0.001) |
| Inter-observer reproducibility# | ||||
|---|---|---|---|---|
| Reader 1, LAV CAC, mL | Reader 2, LAV CAC, mL | Correlation coefficient | ICC; 95%CI | |
| LAV CAC | 72.9 ± 24.5 | 71.6 ± 22.1 | 0.97 (p < 0.001) |
0.958; 0.937–0.972 (p < 0.001) |
Reader 2 (AC) performed 100 LAV-CAC measurements and 50 LAV-CCTA measurements (at time 1, one to three weeks apart). For this table, only values for the 50 matched subjects are shown for reader 2.
Descriptive statistics is based on readings (performed at time 1, one-three week apart) of 100 measurements by reader 1 (TTH) both for LAV-CAC and LAV-CCTA.
To assess intra-observer reproducibility of LAV-CAC, absolute agreement intra-class correlation analysis was performed on 65 and 48 randomly selected repeated readings of LAV-CAC by reader 1 (TTH) and reader 2 (AC), respectively. To assess inter-observer reproducibility of LAV-CAC, two-way mixed intra-class correlation agreement analysis was performed on 100 randomly selected readings (either time 1 or time 2 readings) of LAV-CAC by reader 1 (TTH) and reader 2 (AC).
Fig. 3.
Bland-Altman plots of LA volume by CAC vs CCTA for two readers.
Note: Left plot: reader 1 LAV quantifications (100 readings). Arithmetic mean difference between CCTA and CAC = −2.98; 95% CI −4.48 to −1.49. Right plot: reader 2 LAV quantifications (48 readings). Arithmetic mean difference between CCTA and CAC = −1.92; 95%CI −3.94 to −0.10.
LAV-CAC demonstrated excellent intra-observer reproducibility. The ICC agreement based on 65, randomly selected, repeated readings of LAV-CAC by reader 1 was 0.95 (95%CI 0.82–0.98, p < 0.001). The two measurements of LAV-CAC at time 1 and time 2 were also highly correlated (R = 0 0.97, p < 0.001, Table 2). Similarly, there was excellent intra-observer reproducibility for reader 2 (Table 2).
LAV-CAC showed also excellent inter-observer reproducibility. The ICC agreement performed on 100 randomly selected LAV-CAC readings (either time 1 or time 2 readings) between reader 1 and reader 2 was 0.96 (95%CI 0.94–0.97, p < 0.001). LAV-CAC between readers was also highly correlated (R = 0.97, p < 0.001) (Table 2, Fig. 4).
Fig. 4.
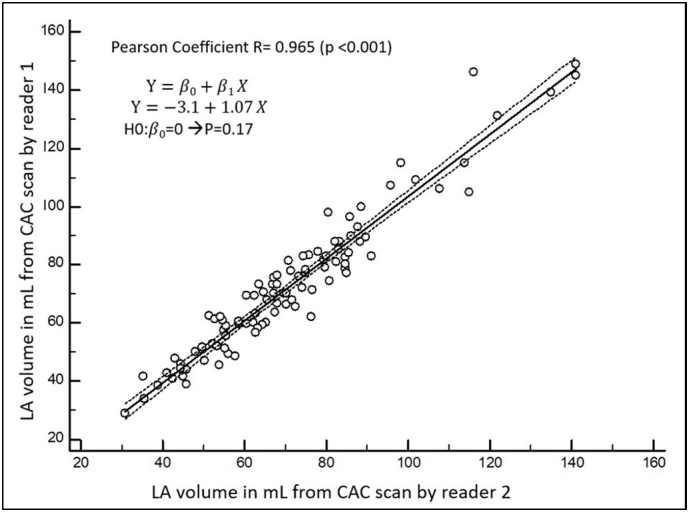
Correlation analysis between readers for LA volume from CAC scan readings.
Note: Data from a random subset of readings performed either at time 1 or time 2 by reader 1 and reader 2.
Finally, based on 65 repeated quantifications of LAV by reader 1 both for LAV-CAC and LAV-CCTA (quantifications performed at time 1 and time 2 for both methods), we found that the intra-observer variability of LAV quantification using CAC scans was not significantly different from that using CCTA. The arithmetic mean difference between repeated LAV-CAC readings and between repeated LAV-CCTA readings equal to 4.5 ± 5.9 mL, and 4.1 ± 5.7 mL, respectively (p = 0.712).
4. Discussion
Our study shows that, using a standardized protocol, quantification of LAV using CAC scan is feasible and highly accurate when compared to CCTA. Additionally, LAV quantification using CAC scan demonstrated excellent interobserver and intra-observer reproducibility.
There are 3 major findings of our study. First, it was possible to measure LAV using CAC scan in the vast majority of cases. Only 5.4% (about 1/20) of CAC scans were deemed unsuitable for analysis mainly because of significant motion artifacts. Second, the LAV measured from CAC scan had excellent correlation with that measured from CCTA. The small difference in LAV as measured from CAC scans of 3.3 mL compared to that of CCTA, although statistically significant, has no meaningful clinical implication as it represents only 4% of the average volume of the calculated LAV. Third, the near perfect interobserver and intra-observer reproducibility provided further assurance of the robustness of the measurement of LAV by CAC.
The ability to measure LAV from non-contrast-enhanced cardiac images such as those of CAC scan is attributed to several factors. The ECG-gated nature of CAC scan significantly improves the image quality by reducing motion artifact resulting in improved visualization of the left atrial borders. Additionally, the LA is naturally bordered by structures that are easily identifiable on non-contrast CT images. The LA is bounded by the mitral annulus which can be identified by the atrio-ventricular “wasting” and the fatty tissue of the atrioventricular groove where the left circumflex and coronary sinus course. Moreover, the interatrial septum can be identified by its fatty tissue that has characteristic CT density that is different from that of the blood and myocardial tissue. Inferiorly, the LA border is identified by the coronary sinus which can be seen running in the inferior AV groove separated from the LA by fatty tissue. Identification of the mitral annulus and the LA/LAA junction are known challenges in measuring the LAV in any cardiac imaging modalities and are major sources of measurement variation in calculating the LAV. The real 3D nature of the CAC scan data makes it possible to identify these anatomical landmarks with relative ease using multiplanar reformatting images. Our protocol clearly defined the method of identifying the mitral annulus and LA/LAA junction. Prior methods of LAV measurements using contrast enhanced CT scan included tracing the LA endocardial border on axial slices without clear definition of the mitral annular plane or LAA neck [13,17]. Our protocol dictated that these two areas are identified before the full, manual tracing of the LA borders (Fig. 1A and B). Once mitral annulus and LA/LAA junction are defined further tracking of the LA border on multiple axial slices becomes a straightforward process and less liable to individual variation or visual guessing. Worth noting that the posterior border of the LA frequently comes in direct contact with the esophagus. This makes it challenging to identify the LA wall from the esophageal tissue on CAC scans especially when there is no air in the esophagus. It is possible that the inclusion of part of the esophageal tissue in LAV measurement on CAC scans could have led to the slight overestimation of the LAV on CAC scan compared to CCTA. Additionally, the lower spatial resolution of CAC images from using thicker image slices than CCTA (2.5 mm for CAC scan vs. 0.64 mm for CCTA) could have also contributed to the small difference in LAV estimation. Finally, lack of contrast use on CAC scan makes it impossible to separate the endocardial from the pericardial borders of the LA. Therefore, the inclusion of the LA wall in LAV measurement on CAC scan could have also contributed to the higher LAV compared to CCTA.
This is the first report to describe a systematic method to calculate the LAV from CAC scan images with high accuracy and reproducibility compared to CCTA. The LAV is a known independent risk factor for adverse CV outcomes such as heart failure, atrial fibrillation, stroke and CV mortality. Despite that, the LAV has not been used to improve CV risk prediction in asymptomatic individuals likely because all currently available methods of LAV measurements including transthoracic echo, CMR or contrast enhanced CT, are not indicated for asymptomatic population. The ability to accurately measure the LAV from CAC scan images open the possibility of the inclusion of such robust marker of CV health to improve CV risk prediction in asymptomatic individuals. CAC scan is indicated to improve risk prediction in selected groups of asymptomatic individuals [20]. The 2018 guideline on the management of blood cholesterol has given class IIa recommendation for the use of CAC scanning for primary prevention in adults 40 to 75 years of age where LDL-c levels 70 to 198 mg/dL and where risk benefit of statin therapy is uncertain [22]. Such recommendation will likely lead to even wider use of CAC scanning. The findings of our study open the door for further studies to evaluate the effectiveness of using LAV to improve risk prediction of relevant CV outcomes. Pending such studies, including the LAV on CAC scan reports might become routine and essential component in risk stratification especially when such information does not require any additional radiation or protocol modification on the CAC scan acquisition protocol.
Our study has several strengths including the relatively large sample size, the simultaneous acquisition of the CAC scan and CCTA and the blinded nature of the readers to their own and each other's measurements. Furthermore, the LAV from CAC scan did not require any modification to the CAC scan acquisition protocol.
One limitation of our study is that the LAV measured from CAC scans and CCTA is obtained in mid diastole and does not reflect the maximum left atrial size frequently used in published literature. However, end systolic acquisition is commonly used in multiple cardiac CT laboratories and is routine in pre-atrial fibrillation ablation cardiac CT. Such acquisition could be adopted easily for CAC scan acquisition protocol to capture the maximum LAV.
5. Conclusion
Measurement of the LAV with CAC scan using the proposed standardized approach is feasible, accurate and highly reproducible when compared to LAV measured from contrast-enhanced coronary CT angiography.
Footnotes
Authors have no relevant conflict of interest/financial relationship to disclose.
Contributor Information
Andrea Cardona, Email: Andrea.cardona@osumc.edu.
Vincenzo Trovato, Email: Vincenzo.Trovato@osumc.edu.
Haikady N. Nagaraja, Email: Nagaraja.1@osu.edu.
Subha V. Raman, Email: Subha.raman@osumc.edu.
Thura T. Harfi, Email: Thura.harfi@osumc.edu.
References
- 1.Tsang T.S., Barnes M.E., Gersh B.J. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J. Am. Coll. Cardiol. 2003;42(7):1199–1205. doi: 10.1016/s0735-1097(03)00943-4. [DOI] [PubMed] [Google Scholar]
- 2.Losi M.A., Betocchi S., Aversa M. Determinants of atrial fibrillation development in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2004;94(7):895–900. doi: 10.1016/j.amjcard.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Tsang T.S., Barnes M.E., Bailey K.R. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76(5):467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 4.Tsang T.S., Barnes M.E., Gersh B.J., Bailey K.R., Seward J.B. Risks for atrial fibrillation and congestive heart failure in patients >/=65 years of age with abnormal left ventricular diastolic relaxation. Am. J. Cardiol. 2004;93(1):54–58. doi: 10.1016/j.amjcard.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Tani T., Tanabe K., Ono M. Left atrial volume and the risk of paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2004;17(6):644–648. doi: 10.1016/j.echo.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Gottdiener J.S., Kitzman D.W., Aurigemma G.P., Arnold A.M., Manolio T.A. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or = 65 years of age (the cardiovascular health study) Am. J. Cardiol. 2006;97(1):83–89. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 7.Takemoto Y., Barnes M.E., Seward J.B. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am. J. Cardiol. 2005;96(6):832–836. doi: 10.1016/j.amjcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Moller J.E., Hillis G.S., Oh J.K. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107(17):2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 9.Leung D.Y., Boyd A., Ng A.A., Chi C., Thomas L. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am. Heart J. 2008;156(6):1056–1064. doi: 10.1016/j.ahj.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Beinart R., Boyko V., Schwammenthal E. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J. Am. Coll. Cardiol. 2004;44(2):327–334. doi: 10.1016/j.jacc.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 11.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Agner B.F.R., Kühl J.T., Linde J.J. Assessment of left atrial volume and function in patients with permanent atrial fibrillation: comparison of cardiac magnetic resonance imaging, 320-slice multi-detector computed tomography, and transthoracic echocardiography. Eur. Heart J. Cardiovasc. Imaging. 2013;15(5):532–540. doi: 10.1093/ehjci/jet239. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins C., Bricknell K., Marwick T.H. Use of real-time three-dimensional echocardiography to measure left atrial volume: comparison with other echocardiographic techniques. J. Am. Soc. Echocardiogr. 2005;18(9):991–997. doi: 10.1016/j.echo.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Maceira A.M., Cosín-Sales J., Roughton M., Prasad S.K., Pennell D.J. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2010;12(1):65. doi: 10.1186/1532-429X-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodevand O., Bjornerheim R., Ljosland M., Maehle J., Smith H., Ihlen H. Left atrial volumes assessed by three-and two-dimensional echocardiography compared to MRI estimates. Int. J. Card. Imaging. 1999;15(5):397–410. doi: 10.1023/a:1006276513186. [DOI] [PubMed] [Google Scholar]
- 17.Stojanovska J., Cronin P., Patel S. Reference normal absolute and indexed values from ECG-gated MDCT: left atrial volume, function, and diameter. Am. J. Roentgenol. 2011;197(3):631–637. doi: 10.2214/AJR.10.5955. [DOI] [PubMed] [Google Scholar]
- 18.Ujino K., Barnes M.E., Cha S.S. Two-dimensional echocardiographic methods for assessment of left atrial volume. Am. J. Cardiol. 2006;98(9):1185–1188. doi: 10.1016/j.amjcard.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 19.Taylor A.J., Cerqueira M., Hodgson J.M. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122(21):e525–e555. doi: 10.1161/CIR.0b013e3181fcae66. [DOI] [PubMed] [Google Scholar]
- 20.Stone N.J., Robinson J.G., Lichtenstein A.H. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63(25 Part B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Goff D.C., Lloyd-Jones D.M., Bennett G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63(25 Part B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy S.M., Stone N.J., Bailey A.L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary. Circulation. 2018 [Epub ahead of print] [Google Scholar]
- 23.Hecht H., Blaha M.J., Berman D.S. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the society of cardiovascular computed tomography. J. Cardiovasc. Comput. Tomogr. 2017;11(2):157–168. doi: 10.1016/j.jcct.2017.02.010. [DOI] [PubMed] [Google Scholar]