INTRODUCTION
Background
Gastric cancer is the most common cancer and the fourth most common cause of cancer death in South Korea [1]. Despite the large number of gastric cancer patients newly diagnosed and treated annually in South Korea, there has been no appropriate practice guideline for domestic medical situations. Although Korean guidelines for gastric cancer were published through interdisciplinary collaborations in 2004 and 2014 [2,3], they were not widely used in South Korea. Therefore, we have produced the present clinical practice guideline to create guidelines that can provide the standard of gastric cancer treatment in accordance with the medical reality in South Korea.
Scope
The present clinical practice guideline is intended for physicians to treat patients with gastric cancer. This guideline is specific and comprehensive for gastric cancer treatment and pathological evaluations; however, it does not address issues related to prevention, screening, diagnosis, and postoperative follow-up. It is based on domestic and overseas evidence and has been developed to be applied to Korean gastric cancer patients under the current medical situation and to ensure their widespread adoption in clinical practice.
This guideline is intended to help medical staffs and educate training physicians at secondary and tertiary care medical institutions, including endoscopists, surgeons, medical oncologists, radiology oncologists, and pathologists. Additionally, the guideline was designed to allow patients and populations to receive optimum care by providing adequate medical information. Furthermore, it is intended for widespread adoption to increase the standard of gastric cancer treatment, thereby contributing to improving patient quality of life as well as national health care.
Chronology
The present guideline was initiated by the Korean Gastric Cancer Association (KGCA) based on the consensus for national need with the associated academic societies. This guideline was prepared in an integrated and comprehensive manner through an interdisciplinary approach that included the KGCA, the Korean Society of Medical Oncology (KSMO), the Korean Society of Gastroenterology (KSG), the Korean Society for Radiation Oncology (KOSRO), and the Korean Society of Pathologists (KSP), along with the participation of experts in the methodology of guideline development (National Evidence-based Healthcare Collaborating Agency). To complete this guideline, the Guideline Committee of the KGCA established the Development Working Group and Review Panel for Korean Practice Guidelines for Gastric Cancer 2018. The members were nominated by each participant association and society. This guideline will be revised every 3 to 5 years when there is solid evidence that can affect the outcomes of patients with gastric cancer.
Method
We systematically searched published literature using databases including MEDLINE, EMBASE, and the Cochrane Library through January 2018. Manual searches were also performed to complement the results. The selection of relevant studies was performed by panels composed of pairs of clinical experts. The selection and exclusion criteria were predefined and tailored to key questions. The articles were screened by title and abstract and full texts were then retrieved for selection. In each step, 2 panels were independently selected and reached agreements.
We critically appraised the quality of the selected studies using risk-of-bias tools. We used Cochrane Risk of Bias (ROB) for randomized controlled trials (RCTs), ROB for Nonrandomized Studies for non-RCTs, Quality Assessment of Diagnostic Accuracy Studies-2 for diagnostic studies, and A Measurement Tool to Assess Systematic Reviews for systematic reviews/meta-analysis [4,5,6,7]. The panels independently assessed and reached a consensus. Disagreements were resolved by discussion and the opinion of a third member. We extracted data using a predefined format and synthesized these data qualitatively. Evidence tables were summarized according to key questions.
The levels of evidence and grading of the recommendations were modified based on the Scottish Intercollegiate Guidelines Network and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology reviews [8,9].
The evidence was classified into 4 levels. The main factors were study design and quality (Table 1). Additionally, we considered outcome consistency. The grading of the recommendations was performed according to a modified GRADE methodology into 5 levels including strong for, weak for, weak against, strong against, and inconclusive (Table 2). The recommendation factors considered evidence level, clinical applicability, and benefit and harm. The Development Working Group simultaneously reviewed the draft and discussed for consensus.
Table 1. Levels of evidence.
| Class | Explanation |
|---|---|
| High | At least 1 RCT or SR/meta-analysis with no concern regarding study quality |
| Moderate | At least 1 RCT or SR/meta-analysis with minor concern regarding study quality or at least 1 cohort/case-control/diagnostic test design study with no concern regarding study quality |
| Low | At least 1 cohort/case-control/diagnostic test study with minor concern regarding study quality or at least 1 single arm before-after study, cross-sectional study with no concern regarding study quality |
| Very low | At least 1 cohort/case-control/diagnostic test design study with serious concern regarding study quality or at least 1 single arm before-after study, cross-sectional study with minor/severe concern regarding study quality |
Table 2. Grading of recommendations.
| Grade classification | Explanation |
|---|---|
| Strong for | The benefit of the intervention is greater than the harm, with high or moderate levels of evidence. The intervention can be strongly recommended in most clinical practice. |
| Weak for | The benefit and harm of the intervention may vary depending on the clinical situation or patient/social value. The intervention is recommended conditionally according to the clinical situation. |
| Weak against | The benefit and harm of the intervention may vary depending on the clinical situation or patient/social values. The intervention may not be recommended in clinical practice. |
| Strong against | The harm of the intervention is greater than the benefit, with high or moderate levels of evidence. The intervention should not be recommended in clinical practice. |
| Inconclusive | It is not possible to determine the recommendation direction owing to a lack of evidence or a discrepancy in results. Thus, further evidence is needed. |
Review and approval process
The Review Panel examined the final version of the draft by careful expert review. Revisions were made reflecting the Review Panel's opinions. The guideline was then approved by the KSMO, the KSP, the KSG, the KOSRO, and the KGCA at a Korean Gastric Cancer Guideline Presentation Symposium held on 30th November 2018.
OVERALL TREATMENT ALGORITHM
All statements in this guideline are summarized in Table 3. The tumor description was confined to adenocarcinoma and the tumor status (TNM and stage) was based on the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control 8th edition [10].
Table 3. Summary of statements.
| No. | Recommendations | Level of evidence | Grade of recommendation |
|---|---|---|---|
| Statement 1 | Endoscopic resection is recommended for well or moderately differentiated tubular or papillary early gastric cancers meeting the following endoscopic findings: endoscopically estimated tumor size ≤2 cm, endoscopically mucosal cancer, and no ulcer in the tumor. | Moderate | Strong for |
| Statement 2 | Endoscopic resection could be performed for well or moderately differentiated tubular early gastric cancer or papillary early gastric cancers with the following endoscopic findings: endoscopically estimated tumor size >2 cm, endoscopically mucosal cancer, and no ulcer in the tumor or endoscopically estimated tumor size ≤3 cm, endoscopically mucosal cancer, and ulcer in the tumor. | Moderate | Weak for |
| Statement 3 | Endoscopic resection could be considered for poorly differentiated tubular or poorly cohesive (including signet-ring cell) early gastric cancers meeting the following endoscopic findings: endoscopically estimated tumor size ≤2 cm, endoscopically mucosal cancer, and no ulcer in the tumor. | Low | Weak for |
| Statement 4 | After endoscopic resection, additional curative surgery is recommended if the pathologic result is beyond the criteria of the curative endoscopic resection or if lymphovascular or vertical margin invasion is present. | Moderate | Strong for |
| Statement 5 | Proximal as well as total gastrectomy could be performed for early gastric cancer in terms of survival rate, nutrition, and quality of life. Esophagogastrostomy after proximal gastrectomy can result in more anastomosis-related complications including stenosis and reflux; caution is needed in the selection of reconstruction method. | Moderate | Weak for |
| Statement 6 | PPG could be performed for early gastric cancer as well as DG in terms of survival rate, nutrition, and quality of life. | Moderate | Weak for |
| Statement 7 | Gastroduodenostomy and gastrojejunostomy (Roux-en-Y and loop) are recommended after DG in middle and lower gastric cancers. There are no differences in terms of survival, function, and nutrition between the different types of reconstruction. | High | Strong for |
| Statement 8 | D1+ is recommended during the surgery for early gastric cancer (cT1N0) patients in terms of survival. | Low | Strong for |
| Statement 9 | Prophylactic splenectomy for splenic hilar LND is not recommended during curative resection for advanced gastric cancer in the proximal third stomach. | High | Strong against |
| Statement 10 | Lower mediastinal LND could be performed to improve oncologic outcome without increasing postoperative complications for adenocarcinoma of the EGJ. | Low | Weak for |
| Statement 11 | Laparoscopic surgery is recommended in early gastric cancer for postoperative recovery, complications, quality of life, and long-term survival. | High | Strong for |
| Statement 12 | Laparoscopic gastrectomy could be performed for advanced gastric cancer in terms of short-term surgical outcomes and long-term prognosis. | Moderate | Weak for |
| Statement 13 | Adjuvant chemotherapy (S-1 or capecitabine plus oxaliplatin) is recommended in patients with pathological stage II or III gastric cancer after curative surgery with D2 LND. | High | Strong for |
| Statement 14 | Adjuvant chemoradiation could be added in gastric cancer patients after curative resection with D2 lymphadenectomy to reduce recurrence and improve survival. | High | Weak for |
| Statement 15 | Neoadjuvant chemotherapy for potentially resectable gastric cancer is not conclusive if D2 LND is considered. | High | Inconclusive |
| Statement 16 | The evidence for the effectiveness of neoadjuvant chemoradiation in locally advanced gastric cancer is not conclusive if D2 LND is considered. | High | Inconclusive |
| Statement 17 | Palliative gastrectomy is not recommended for metastatic gastric cancer except for palliation of symptoms. | High | Strong against |
| Statement 18-1 | Palliative first-line combination platinum/fluoropyrimidine is recommended in patients with locally advanced unresectable or metastatic gastric cancer if the patient's performance status and major organ functions are preserved. | High | Strong for |
| Statement 18-2 | Palliative trastuzumab combined with capecitabine or fluorouracil plus cisplatin is recommended in patients with HER2 IHC 3+ or IHC 2+ and ISH-positive advanced gastric cancer. | High | Strong for |
| Statement 19 | Palliative second-line systemic therapy is recommended in patients with locally advanced unresectable or metastatic gastric cancer if the patient's performance status and major organ functions are preserved. Ramucirumab plus paclitaxel is preferably recommended and monotherapy with irinotecan, docetaxel, paclitaxel, or ramucirumab could also be considered. | High | Strong for |
| Statement 20 | Palliative third-line systemic therapy is recommended in patients with locally advanced unresectable or metastatic gastric cancer if the patient's performance status and major organ functions are preserved. | High | Strong for |
| Statement 21 | Palliative RT could be offered to alleviate symptoms and/or improve survival in recurrent or metastatic gastric cancer. | Moderate | Weak for |
| Statement 22 | Peritoneal washing cytology is recommended for staging. Advanced gastric cancer patients with positive cancer cells in the peritoneal washing cytology are associated with frequent cancer recurrence and a poor prognosis. | Moderate | Strong for |
PPG = preserving gastrectomy; DG = distal gastrectomy; LND = lymph node dissection; EGJ = esophagogastric junction; IHC = immunohistochemistry; ISH = in situ hybridization; RT = radiotherapy.
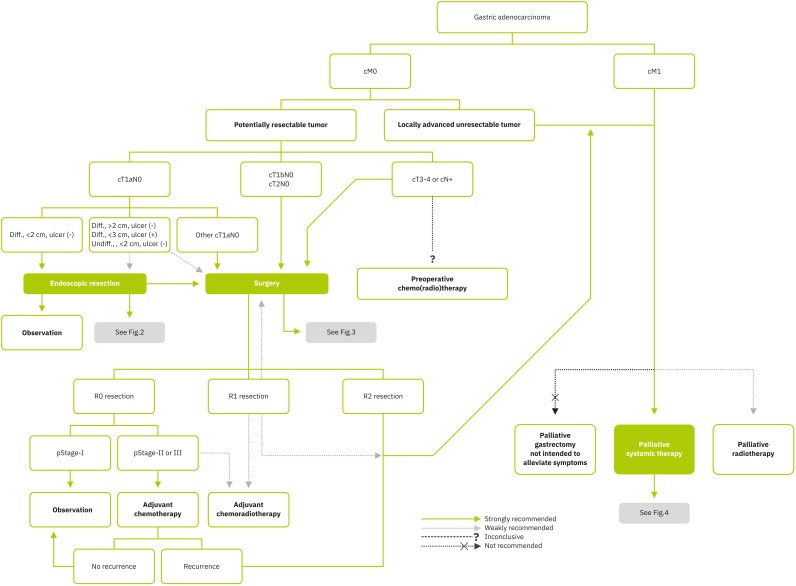
Gastric adenocarcinoma was divided into localized (non-metastatic [M0]) and metastatic-1 (M1) disease according to the status of distant metastasis (Fig. 1).
Fig. 1. Overall treatment algorithm.
Diff. = differentiated; Undiff. = undifferentiated.
In cases of M0 gastric cancer, clinical (c) T- and N-stages can be determined based on preoperative esophagogastroduodenoscopy or endoscopic ultrasound examination findings and computed tomography. Endoscopic resection can be indicated for selected cT1aN0 gastric cancer with minimal risk of lymph node (LN) metastasis (statements 1–3). The necessity of additional curative gastrectomy after endoscopic treatment is determined based on the pathologic review of the endoscopic resection specimen (statement 4).
Surgical resection is recommended if the tumor is outside of endoscopic resection indications in cT1a and ≥cT1b or cN+. The extent of gastrectomy (statements 5 and 6) and lymphadenectomy (statements 8, 9, and 10), reconstruction methods (statement 7), and approach methods (statements 11 and 12) should be considered when deciding surgical procedures.
Adjuvant chemotherapy is recommended in patients with pathological stage II or III gastric cancer after curative R0 resection with D2 LN dissection (LND) (statement 13). Adjuvant chemoradiation can be considered in patients with incomplete resection, including R1 resection and/or less than D2 LND, and after curative R0 resection with D2 LND, especially with LN metastasis (statement 14). When the result of primary gastrectomy is R1 resection, 3 treatment options can be considered, according to the location of microscopic residual tumor: re-resection, adjuvant chemoradiotherapy, or palliative therapy, depending on the clinical situation.
Although neoadjuvant chemo (radio) therapy has high levels of evidence, we did not reach a conclusion on whether to recommend it in Asian populations because the backgrounds of almost all clinical trials on preoperative therapy were not consistent with Asian situations (statements 15 and 16).
Palliative systemic therapy is the primary treatment to be considered in patients with locally advanced unresectable or those after non-curative resection or metastatic disease (M1) (statements 18–20). Palliative radiotherapy (RT) can be considered for the alleviation of tumor-related symptoms or to improve survival (statement 21); however, palliative gastrectomy not intended to alleviate tumor-related symptoms or complications (i.e., obstruction, bleeding, perforation, etc.) is not recommended for the purpose of improving overall survival (OS) (statement 17).
ENDOSCOPIC RESECTION
Statement 1. Endoscopic resection is recommended for well or moderately differentiated tubular or papillary early gastric cancers meeting the following endoscopic findings: endoscopically estimated tumor size ≤2 cm, endoscopically mucosal cancer, and no ulcer in the tumor (evidence: moderate, recommendation: strong for).
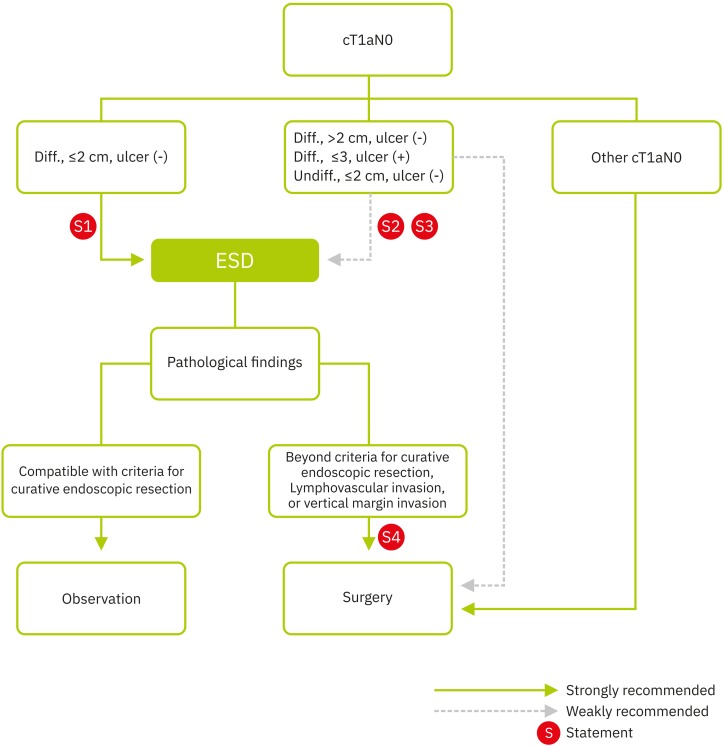
Endoscopic submucosal dissection (ESD) has been used as a minimally invasive treatment modality for early gastric cancer since the early 2000s in Korea [11,12]. A total of 7,734 early gastric cancer patients underwent ESD in 2014 [12]. Many studies have indicated that ESD should be considered as the first-line treatment modality for the early gastric cancer with well or moderately differentiated tubular adenocarcinoma or papillary adenocarcinoma with tumor size ≤2 cm, confined to the mucosal layer, and without ulcer in the tumor as these findings definitely indicated that the lesions had a very low-risk of LN metastasis [13,14] and ESD allows high rates of en bloc curative resection with low adverse event rates [11,14,15,16,17,18] (Fig. 2).
Fig. 2. Treatment algorithm for endoscopic resection.
Diff. = differentiated; Undiff. = undifferentiated; ESD = endoscopic submucosal dissection.
The 5-year OS rates of patients meeting this definite indication for ESD did not differ significantly from those of patients who received endoscopic resection (93.6%–96.4%) and surgery (94.2%–97.2%) in large retrospective cohort studies in Korea [16,17,18]. The 10-year OS rates were comparable between endoscopic resection (81.9%) and surgery (84.9%) (P=0.14) [17].
However, the 5-year cumulative metachronous recurrence rates were significantly higher after endoscopic resection (5.8%–10.9%) than those after surgery (0.9%–1.1%) [16,17,18]. Therefore, close surveillance should be performed after ESD to detect early-stage metachronous gastric cancer that can be treated with endoscopic resection. Nevertheless, endoscopic treatment for early gastric cancer can provide a better quality of life, though stomach preservation might provoke worries of metachronous cancer recurrence [19]. Moreover, ESD had lower treatment-related complication rates [17,18], shorter hospital stay, and lower costs than those of surgery [16].
In the aspect of patient preference, ESD can provide better health-related quality of life for early gastric cancer patients, especially in terms of physical function, eating limits, dyslexia, diarrhea, and body image [20].
Statement 2. Endoscopic resection could be performed for well or moderately differentiated tubular early gastric cancer or papillary early gastric cancers meeting the following endoscopic findings: endoscopically estimated tumor size >2 cm, endoscopically mucosal cancer, and no ulcer in the tumor or endoscopically estimated tumor size ≤3 cm, endoscopically mucosal cancer, and ulcer in the tumor (evidence: moderate, recommendation: weak for).
Endoscopic resection for early gastric cancer is limited in that LND cannot be performed during the procedure. Therefore, to achieve curative resection and comparable survival to that of surgery with endoscopic resection, early gastric cancers with very low-risk of LN metastasis should be carefully selected. The clinically acceptable rate of LN metastasis might be determined in the context of perioperative mortality associated with radical gastrectomy (0.1%–0.3% in a high-volume center in Korea and Japan) [21,22,23]. In addition, it is required that en bloc resection is technically feasible with endoscopic resection to avoid the possibility of remnant tumor or local recurrence after the procedure. When the following criteria 1 or 2 were met, the extragastric recurrence (LN or distant metastasis) rate after endoscopic resection was between 0 and 0.21%, which is comparable to that of perioperative mortality associated with radical gastrectomy [24,25,26,27].
Although standard gastrectomy with LND is recommended when submucosal invasion of the tumor (T1b) is suspected in preoperative evaluation, the extragastric recurrence rate after ESD ranged from 0.9% to 1.5% in large retrospective cohort studies when the pathologic specimen of ESD fulfilled criteria 3 [24,25,26]. Because the diagnosis of minute submucosal invasion (≤500 µm) of the tumor before ESD is very difficult, criteria 3 applies to post-ESD pathologic specimens.
When criteria 1, 2, or 3 were met, the OS was comparable between patients undergoing endoscopic resection and those treated with radical surgery [18,28,29,30,31,32,33,34,35,36,37,38].
Criteria 1, 2, and 3: well or moderately differentiated tubular adenocarcinoma or papillary adenocarcinoma, en bloc resection, negative lateral resection margins, negative vertical resection margin, no lymphovascular invasion (LVI), and 1) tumor size >2 cm, mucosal cancer, no ulcer in the tumor, or 2) tumor size ≤3 cm, mucosal cancer, ulcer in the tumor, or 3) tumor size ≤3 cm, submucosal invasion depth ≤500 μm from the muscularis mucosa layer.
Because many the factors of these criteria can be confirmed after ESD (i.e., en bloc resection, resection margin, LVI, and minute submucosal invasion), ESD can be considered if the early gastric cancer meets the following endoscopic findings: 1) Well or moderately differentiated tubular adenocarcinoma or papillary adenocarcinoma on forceps biopsy specimen, endoscopically estimated tumor size >2 cm, endoscopically mucosal cancer, and no ulcer in the tumor, or 2) Well or moderately differentiated tubular adenocarcinoma or papillary adenocarcinoma on forceps biopsy specimen, endoscopically estimated tumor size ≤3 cm, endoscopically mucosal cancer, and ulcer in tumor (Fig. 2).
Until now, the standard treatment for these criteria has been gastrectomy with LND. Although a number of retrospective cohort studies support these criteria, no prospective trial has compared the outcomes of endoscopic resection with those of standard operation based on these criteria. A significant portion of these criteria estimated by pre-ESD workup is confirmed to be out of criteria by the pathologic examination of ESD specimens [39,40,41,42,43]. Thus, standard operation (gastrectomy with LND) may also be considered for cases meeting these criteria.
Statement 3. Endoscopic resection could be considered for poorly differentiated tubular or poorly cohesive (including signet-ring cell) early gastric cancers meeting the following endoscopic findings: endoscopically estimated tumor size ≤2 cm, endoscopically mucosal cancer, and no ulcer in the tumor (evidence: low, recommendation: weak for).
Poorly-differentiated tubular and poorly cohesive (including signet-ring cell) early gastric cancers are associated with a higher risk of LN metastasis than those of well and moderately differentiated tubular early gastric cancer. Thus, endoscopic resection can be considered very cautiously within strict criteria. When the following criteria were fulfilled, a few retrospective cohort studies reported extragastric recurrence after endoscopic resection [24,26,44,45,46,47,48,49] and a comparable OS between patients undergoing endoscopic resection and those treated with radical gastrectomy [18,29,35,36,49].
Poorly differentiated tubular adenocarcinoma or poorly cohesive carcinoma (including signet-ring cell carcinoma), en bloc resection, negative lateral resection margins, negative vertical resection margin, no LVI, and tumor size ≤ 2 cm, mucosal cancer, and no ulcer in the tumor.
Because many factors of these criteria can be confirmed after ESD (i.e., en bloc resection, resection margin, and LVI), ESD can be considered for poorly-differentiated tubular and poorly cohesive (including signet-ring cell) early gastric cancers meeting the following endoscopic findings (Fig. 2).
Poorly differentiated tubular adenocarcinoma or poorly cohesive carcinoma (including signet-ring cell carcinoma) on forceps biopsy specimen, endoscopically estimated tumor size ≤2 cm, endoscopically mucosal cancer, and no ulcer in the tumor.
Until now, the standard treatment for these criteria has been gastrectomy with LND. A few retrospective cohort studies support these criteria for ESD and the results of prospective trials are lacking (level of evidence is low, and the level of recommendation is weak). A significant portion of these criteria estimated by pre-ESD workup is confirmed to be out of criteria by the pathologic examination of ESD specimens [39,40,41,42,43]. Thus, standard operation (gastrectomy with LND) can also be considered for cases meeting these criteria.
Statement 4. After endoscopic resection, additional curative surgery is recommended if the pathologic result is beyond the criteria of the curative endoscopic resection or if lymphovascular or vertical margin invasion is present (evidence: moderate, recommendation: strong for).
Early gastric cancer patients who received endoscopic resection could be considered as being beyond the criteria of endoscopic resection by pathologic specimen evaluation. Resected tumor characteristics beyond the following criteria are also considered for non-curative resection: 1) Differentiated (well or moderately differentiated tubular or papillary) intramucosal cancer measuring >2 cm in the long diameter without ulcer (active or scar), 2) differentiated mucosal cancer measuring <3 cm with ulcer (active or scar), 3) undifferentiated (poorly differentiated tubular or poorly cohesive) mucosal cancer measuring <2 cm without ulcer (active or scar), and 4) differentiated mucosal cancer measuring <3 cm with subtle submucosal invasion (<500 µm). LVI and positive vertical margin, which are confirmed after endoscopic resection, are also important reasons for the recommendation of rescue surgery (Fig. 2).
Many studies have investigated the long-term outcomes with or without additional surgery in patients who did not meet the curative criteria for endoscopic resection in early gastric cancer [39,40,41,42,50,51,52,53,54,55,56,57]. All studies were retrospective cohort designs and only 2 used propensity score matching analysis, which is used to minimize potential selection bias and mimic randomization in observational studies [39,50]. Although several small studies showed no difference in OS between rescue surgery and follow-up [51,52,53], most studies, including the 2 studies that used propensity score matching, showed a significant survival benefit (OS or disease-specific survival [DSS]) for additional curative surgery compared to that for follow-up [39,40,41,42,50,54,55,56,57]. Patients with LVI or deep vertical margins showed a particularly evident survival benefit for additional curative surgery [40,54,55,57].
The Japanese multicenter retrospective cohort study that used propensity score matching analysis reported 5-year DSS rates after ESD of 99.0% in the additional curative surgery group and 96.8% in the no additional curative surgery group (P=0.013). The 5-year OS rates were 91.0% and 75.5%, respectively (P<0.001) [50]. In the Korean single-center retrospective cohort study using propensity score matching analysis, the 5-year overall mortality in no additional curative surgery group (26.0%; 95% confidence interval [CI], 13.5%–49.9%) was higher than that of the matched initial standard surgery patients (14.5%; 95% CI, 6.3%–33.6%; P=0.04). The overall mortality did not differ significantly between the initial ESD with additional curative surgery group and the corresponding initial standard surgery group [39]. Thus, additional curative surgery is strongly recommended in patients undergoing non-curative endoscopic resection (exceeding the criteria of endoscopic resection) for early gastric cancer.
The survival benefit of additional curative surgery in older patients (>75 years) is controversial. Two studies showed a significant survival benefit but another study showed no difference in long-term outcomes [42,54,56]. Selection bias is inevitable in retrospective cohort designs. For example, all studies showed a younger age in the additional curative surgery group compared to that in the follow-up group, although the age difference disappeared after propensity score matching in 2 studies. Patients undergoing noncurative resection without additional curative surgery also tended to have a higher incidence of comorbidity [41,42]. Although 2 of 12 studies used propensity score matching analysis, selection and measurement biases are still possible. Additional curative surgery may be not feasible in some patients because of very old age, poorly-controlled underlying diseases, or poor general condition. In these patients, follow-up observation could be a feasible option after they are provided an explanation of the risk of recurrence.
SURGICAL THERAPY
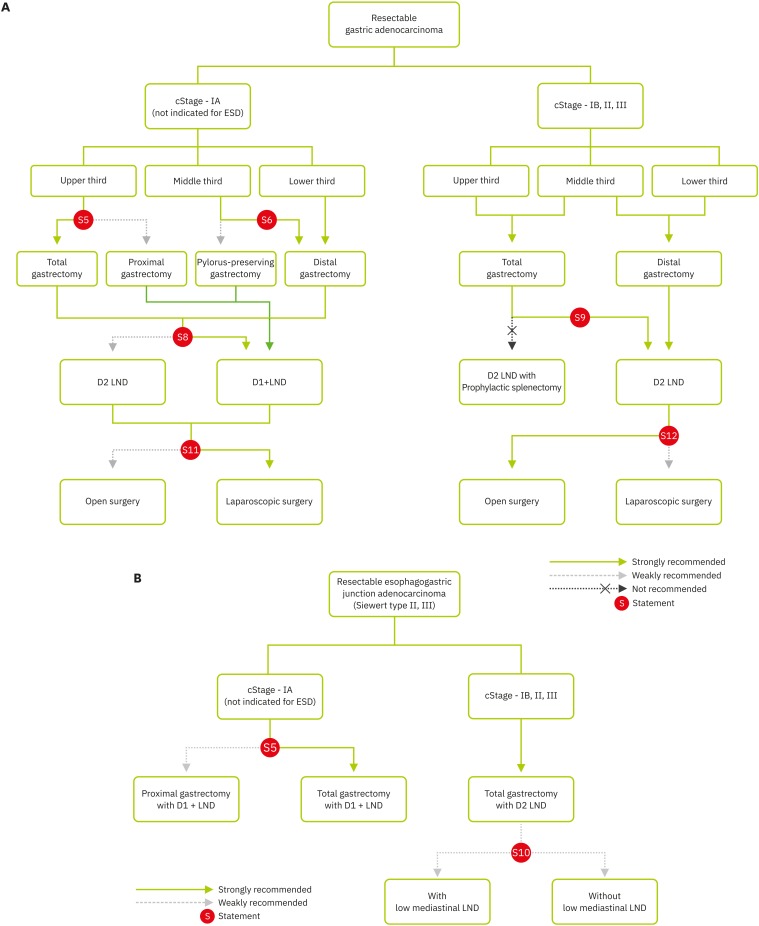
Standard surgery is recommended in cases of cT1a, which are outside of the indication for endoscopic resection, and ≥cT1b or cN+ and M0 gastric cancer (Fig. 3A).
Fig. 3. (A) Treatment algorithm for resectable gastric adenocarcinoma. (B) Treatment algorithm for resectable esophagogastric junction adenocarcinoma.
ESD = endoscopic submucosal dissection; LND = lymph node dissection.
Standard surgery is defined as total or subtotal gastrectomy with D2 LND. Subtotal gastrectomy in distal gastric cancer has been recognized as a standard surgery based on the results of 2 RCTs in which the subtotal gastrectomy group showed similar long-term oncologic results and lower morbidity and mortality rates compared to those in the total gastrectomy group [58,59,60]. Although the standard extent of LND has been debated for decades among Eastern and Western countries, there has been an international trend to accept D2 LND as a standard surgery [2,61,62,63], which was supported by results of prospective trials and meta-analyses [64,65,66]. The extent of LND in each gastrectomy was defined according to Japanese guidelines [63].
Palliative systemic therapy is the primary treatment in cases of locally advanced unresectable or cM1 gastric cancer (Fig. 1). However, conversion surgery could be considered if R0 resection is possible after palliative systemic therapy, which is currently under investigation. Surgery with curative intent could also be considered in cases of locally advanced unresectable or cM1 gastric cancer not detected in preoperative evaluation but incidentally identified during surgery and if R0 resection is possible, which should be investigated in future studies. Intraperitoneal chemotherapy with or without hyperthermia could be applied to patients with peritoneal metastasis in a clinical trial setting; however, this requires additional evidence.
Gastric resection and reconstruction
Statement 5. Both proximal and total gastrectomy could be performed for early gastric cancer in terms of survival rate, nutrition, and quality of life. Esophagogastrostomy after proximal gastrectomy can result in more anastomosis-related complications including stenosis and reflux, and caution is needed in the selection of reconstruction method (evidence: moderate, recommendation: weak for).
A prospective randomized controlled study comparing proximal and total gastrectomy with sufficient numbers of cases and power to evaluate the survival rate as the primary endpoint has not been conducted. However, several retrospective studies reported non-inferior long-term survival rates after proximal gastrectomy as compared to that of total gastrectomy [67,68,69,70,71,72,73,74]. In addition, the incidences of early postoperative complications after proximal gastrectomy were similar to those after total gastrectomy in most studies [68,70,71,73,75,76,77]; however, they have also been reported to be more [72] and less [69] frequent.
Although various reconstructions after proximal gastrectomy have been tried in order to reduce short- or long-term complications, they remain controversial. Esophagogastrostomy after proximal gastrectomy is the simplest procedure but resulted in significantly more frequent reflux esophagitis (16.2%–42.0% vs. 0.5%–3.7%) and symptoms of reflux [67,69,72,74,76,77], as well as stenosis at the anastomosis site (3.1%–38.2% vs. 0%–8.1%) [67,69,71,72,74,76,77]. An et al. [69] and Ahn et al. [70] concluded that proximal gastrectomy with esophagogastrostomy is an inferior surgical method to total gastrectomy due to the significantly higher incidence of anastomosis-related and/or postoperative complications and no nutritional benefit. However, other studies have reported that proximal gastrectomy with esophagogastrostomy can be still beneficial compared to total gastrectomy in terms of serum albumin level [71,72,77], maintenance of body weight [74,76,77,78], prevention of anemia [72,74,77], and serum vitamin B12 level [72].
Jejunal interposition could be another option after proximal gastrectomy. Anastomosis with jejunal interposition has been shown to be beneficial in the context of nutritional parameters and anemia [71,74,75,76]. Postgastrectomy syndrome including dumping syndrome occurred less frequently in patients who underwent proximal gastrectomy with jejunal interposition compared to that in patients who underwent total gastrectomy [75,76]. A study including 115 cases that received esophagogastrostomy and 78 that received jejunal interposition also reported less frequent diarrhea and dumping syndrome for proximal gastrectomy (2.0 vs. 2.3 points on a 7-point scale) [78].
Recently, double-tract reconstruction after proximal gastrectomy has been proposed as an option that did not increase the incidence of complications or reflux and showed superiority to total gastrectomy in terms of body weight, anemia, and serum vitamin B12 level [73]. A multicenter prospective randomized clinical trial was launched in 2016 in Korea based on this result (NCT02892643).
The incidence of metachronous cancer in the remnant stomach after proximal gastrectomy was 33.1% (6/192) and 6.2% (4/65) in reports by Huh et al. [72] and Ohashi et al., [76] respectively.
In conclusion, proximal gastrectomy is a surgical option with possible benefits in aspects of shorter operative time, less blood loss, better maintenance of postoperative nutrition, lower incidence of anemia, better maintenance of vitamin B12 level, and lower incidence of post-gastrectomy syndrome (Fig. 3A). However, proximal gastrectomy requires caution in the choice of reconstruction technique because of the significantly increased incidence of anastomosis-related complications and reflux to the esophagus after esophagogastrostomy.
Statement 6. Pylorus-preserving and distal gastrectomy (DG) could be performed for early gastric cancer in terms of survival rate, nutrition, and quality of life (evidence: moderate, recommendation: weak for).
Conventional DG and pylorus-preserving gastrectomy (PPG) can be performed for middle-third early gastric cancer. PPG preserves the pre-pyloric antrum and the pylorus to prevent the rapid transit of food into the duodenum and reflux of the duodenal contents. Consequently, the postoperative incidence of dumping syndrome and reflux gastritis is decreased, and a nutritional benefit is expected.
Most of the literature on PPG is retrospective studies. All studies that assessed the long-term survival concluded that there was no difference in long-term survival between conventional DG and PPG (5-year survival rates: 95% for PPG vs. 87% for DG, P=0.087 [79]; 3-year survival rates: 98.2% for PPG vs. 98.8% for DG, P=0.702 [80]; odds ratio [OR] of PPG, 0.83, 95% CI, 0.10–6.66, P=0.86 [81]; hazard ratio [HR] for recurrence in PPG, 0.393, 95% CI, 0.116–1.331, P=0.12 [82]). In addition, except for 1 report from Japan [79], most of the reports concluded that there was no difference in the incidence of postoperative complications [80,81]. As expected, after PPG, patients showed a significantly low incidence of postoperative dumping syndrome and reflux (reflux: 4% for PPG vs. 40% for DG; reflux gastritis: 8% for PPG vs. 68% for DG [83]; dumping syndrome: OR, 0.02, 95% CI, 0.10–0.41, P<0.001; bile reflux: OR, 0.16, 95% CI, 0.06–0.45, P<0.01; remnant gastritis: OR, 0.2, 95% CI, 0.08–0.50, P<0.001; reflux esophagitis: OR, 0.78, 95% CI, 0.43–1.40, P=0.41 [81]). Some studies reported significantly reduced development of gallstones after PPG [80,81]. However, significantly more patients complained of delayed gastric emptying after PPG (symptoms of delayed gastric emptying at 1 postoperative year: 15.8% for PPG vs. 0% for DG; 7.8% for PPG vs. 1.7% for DG [80]; and OR for delayed gastric emptying, 2.12, 95% CI, 1.43–3.15, P<0.001 [81]).
PPG for middle-third early gastric cancer can be performed with relatively similar incidences of surgical complications and long-term survival (Fig. 3A). Although it is evident that PPG significantly reduces the incidence of post-gastrectomy syndromes such as dumping syndrome, reflux and gallstone formation, postoperative delayed gastric emptying is not uncommon. Thus, we are awaiting the results of a large-scale, prospective randomized trial. Therefore, PPG can be performed at the surgeon's discretion with caution regarding postoperative delayed emptying.
Statement 7. Gastroduodenostomy and gastrojejunostomy (Roux-en-Y and loop) are recommended after DG in middle and lower gastric cancer. There are no differences in terms of survival, function, and nutrition between the different types of reconstruction (evidence: high, recommendation: strong for).
A variety of reconstructions after subtotal gastrectomy have been proposed, including Billroth I, Billroth II, and Roux-en-Y; however, there is still no consensus regarding a standard reconstruction method. Complications after subtotal gastrectomy, such as reflux gastritis and esophagitis, dumping syndrome, and delayed gastric emptying, could depend on the reconstruction method [84]. A recent meta-analysis of the types of reconstruction method and endoscopic findings showed no difference in the incidence of dumping syndrome. Roux-en-Y reconstruction is superior to Billroth I and Billroth II reconstruction in terms of preventing bile reflux (OR, 0.095; 95% CI, 0.010–0.63 and OR, 0.064; 95% CI, 0.0037–0.84, respectively) and remnant gastritis (OR, 0.33; 95% CI, 0.16–0.58 and OR, 0.40; 95% CI, 0.17–0.92, respectively). Meanwhile, Roux-en-Y gastric anastomosis resulted in more frequent delayed gastric emptying than did Billroth I (OR, 3.4; 95% CI, 1.1–13) [85]. However, there was no difference in patient quality of life according to the type of reconstruction (P=0.290–0.994) [86]. In addition, there were no differences in nutritional aspects among those methods [86,87]. Although no prospective study has assessed the incidence of remnant gastric cancer according to the anastomotic method, a nationwide survey in Japan reported no difference in terms of remnant gastric cancer [88].
In conclusion, there are no significant differences in the functional, nutritional, and long-term prognosis of each reconstruction method.
Lymphadenectomy
Statement 8. D1+ is recommended during surgery for early gastric cancer (cT1N0) patients in terms of survival (evidence: low, recommendation: strong for).
In gastric cancer surgery, adequate resection of regional LNs is essential along with resection of the primary lesion. It is recommended to perform modified D2, D1, or D1+ if early gastric cancer is not clinically suspected to have LN metastasis. Standard D2 is recommended for early gastric cancer with suspected LN metastasis. However, no prospective clinical trials have compared the survival of patients after modified D2 or standard D2.
The results of a retrospective study published in Italy indicated that LN metastasis was a poor prognostic factor for early gastric cancer patients, suggesting that standard D2 should be performed even for early gastric cancer [89]. Other Italian researchers have reported 10-year survival rates of 95% and 87.5% after standard D2 and D1, respectively, in early gastric cancer patients. There were no statistically significant differences in 10-year survival between groups (P=0.80) [90]. In a report from Japan, the 5- and 10-year survival rates were 97% and 91% in patients after standard D2 and 98% and 91% after modified D2 (D1+). There were no cases with metastasis to second-tier LNs in patients with cT1N0 or cT1N1 disease [91].
In conclusion, D1+ is recommended for the treatment of T1N0 gastric cancer patients, with relatively comparable oncological safety (Fig. 3A).
Statement 9. Prophylactic splenectomy for splenic hilar LND is not recommended during curative resection for advanced gastric cancer in the proximal-third stomach (evidence: high, recommendation: strong against).
The standard surgical procedure for proximal-third gastric carcinoma is total gastrectomy with proper lymphadenectomy. Therapeutic splenectomy is necessary if the tumor directly invades the spleen or if LN metastasis around the splenic hilum is suspected. However, there is debate regarding whether the spleen should be preserved or resected during total gastrectomy in patients diagnosed with proximal-third gastric cancer without a definite indication for splenectomy.
Three prospective randomized clinical trials have evaluated the survival advantage of prophylactic splenectomy in proximal-third gastric carcinoma [92,93,94]. However, no studies recommend prophylactic splenectomy to dissect macroscopically-negative LNs around the splenic hilum for proximal-third gastric cancer. The only study in Korean patients showed a slightly better 5-year OS in the splenectomy group but the difference was not statistically significant (P=0.50) [93]. A recent large-scale study showed that postoperative complications were more common for splenectomy than for spleen preservation (30.3% and 16.7%, P<0.010), without a survival advantage [94]. However, prophylactic splenectomy for patients with Borrmann type IV or tumors located in the greater curvature remains inconclusive because they were not included in the enrollment criteria of the largest randomized controlled clinical trial [94].
A systemic review of splenectomy for proximal-third gastric cancer concluded that spleen-preserving total gastrectomy decreased postoperative complications without negatively affecting the OS [95]. In addition, a meta-analysis indicated that splenectomy did not show a beneficial effect on survival rate compared to splenic preservation [96].
Therefore, prophylactic splenectomy for LND is not recommended in the curative resection for proximal-third gastric cancer without macroscopically LN metastasis near the spleen or direct invasion of the spleen or distal pancreas (Fig. 3A). This statement addresses the need for prophylactic splenectomy and does not address the need for prophylactic LN #10 dissection, which should be investigated in the future and is not conclusive in the present guideline.
Statement 10. Lower mediastinal LND could be performed to improve oncologic outcome without increasing postoperative complications for adenocarcinoma of the esophagogastric junction (EGJ) (evidence: low, recommendation: weak for).
Dissection of the lower mediastinal LN as a part of the treatment for Siewert type II or III EGJ adenocarcinoma is controversial. High level of evidence is lacking regarding the necessity for lower mediastinal LND. Although LN metastasis in the lower mediastinal LNs is frequently found in Siewert type II or III EGJ cancer, it usually indicates a poor prognosis. In a retrospective analysis conducted in Korea, the 5-year disease-free survival (DFS) rates were 62.6% and 82.5% for Siewert type II and III cancers, respectively [97]. In this study, when these cancers were early gastric cancer, survival was good and comparable (93.2% vs. 96.7% vs. 98.7% for Siewert type II, III, and upper-third gastric cancer, P=0.158); however, for advanced cancer, the survival was worse in Siewert type II than that in Siewert type III cancer (47.9% vs. 75.4% vs. 71.8% in Siewert type II, III, and upper-third gastric cancer, P<0.001).
Most randomized clinical trials on this issue have compared the surgical outcomes of transabdominal and transthoracic approaches [98,99,100,101]. However, no study has demonstrated a survival benefit of transthoracic approaches by thorough dissection of the lower mediastinal LNs and negative surgical margins over transabdominal approaches for Siewert type II and III EGJ cancer. In a Japanese phase III randomized clinical trial comparing outcomes between the left thoracoabdominal and transhiatal approaches for EGJ cancer, the 5-year OS were 37.9% and 52.3%, respectively. The HR of death for the left thoracoabdominal approach compared to the transhiatal approach was 1.36 (0.89–2.08, P=0.92).
A cohort study was also performed in the UK of Siewert type I and II EGJ cancer with data from 2 institutions [102]. In this study, the in-hospital mortality rates were 1.1% and 3.2% (P=0.110) and there were no differences in OS (HR, 1.07; 95% CI, 0.84–1.36) or time to tumor recurrence (HR, 0.99; 95% CI, 0.76–1.29) between the transhiatal and transthoracic approaches. A meta-analysis reported the transthoracic approach to be associated with higher incidences of systemic complications such as respiratory and cardiovascular problems, longer hospital stay, and early postoperative mortality compared to those in the transabdominal approach [103]. Survival did not differ between the 2 approaches.
Based on the results from these studies, lower mediastinal LND for EGJ adenocarcinoma, especially by means of transthoracic approaches, to obtain more LNs and a negative surgical margin may not be recommended (Fig. 3B).
Surgical approach
Statement 11. Laparoscopic surgery is recommended in early gastric cancer for improved postoperative recovery, complications, quality of life, and long-term survival (evidence: high, recommendation: strong for).
Laparoscopic gastrectomy is currently widely performed in the treatment of early gastric cancer. Since the first clinical trial was reported in the early 2000s [104], studies comparing laparoscopic and open surgery for early gastric cancer have proven the oncologic safety and excellence of laparoscopic gastrectomy [23,105,106,107,108,109,110,111].
The 5-year survival rates of laparoscopic gastrectomy did not significantly differ from those of open gastrectomy for early gastric cancer in a Korean single-center RCT with a large number of enrolled patients (DFS, 98.8% vs. 97.6%; P=0.514 and OS, 97.6% vs. 96.3%; P=0.721) [107]. In addition, the complication rate was significantly lower for laparoscopic than that for open gastrectomy (23.2% vs. 41.5%; P=0.012). A multicenter RCT conducted in Korea (KLASS-01) recently demonstrated better short-term outcomes of laparoscopic gastrectomy than those of open gastrectomy. In this study, the overall complication rate was significantly lower for laparoscopic than for open gastrectomy (13.0% vs. 19.9%, P=0.001) and the mortality rates did not differ between the 2 groups (0.6% vs. 0.3%, P=0.687) [23].
Therefore, laparoscopic surgery comprised of gastrectomy and adequate LND demonstrated a more beneficial effect for patients with early gastric cancer in terms of all oncologic aspects compared to those for open surgery. In early gastric cancer, laparoscopic surgery should be the first option for treatment (Fig. 3A).
Statement 12. Laparoscopic gastrectomy could be performed for advanced gastric cancer in terms of short-term surgical outcomes and long-term prognosis (evidence level: moderate, recommendation: weak for).
Most previous studies suggesting the feasibility of laparoscopic gastrectomy for locally advanced gastric cancer were small retrospective studies. Meta-analyses of those retrospective studies have demonstrated that laparoscopic gastrectomy required longer operating times but led to less operative blood loss, faster postoperative bowel recovery, and reduced hospital stay compared to those for open surgery [112,113,114,115,116,117,118]. The postoperative morbidity and mortality rates of laparoscopic gastrectomy were also lower or similar compared to those for open surgery. As for the quality of LND, most studies have reported that the number of harvested LNs during laparoscopic surgery does not differ significantly from that of open surgery. Furthermore, the long-term outcomes including OS and DFS were comparable between laparoscopic and open surgery.
Despite reports from a number of retrospective studies, the long-term outcomes of laparoscopic gastrectomy for advanced gastric cancer have rarely been investigated in prospective studies. Park et al. [119] performed a randomized phase II trial comparing non-compliance of D2 LND, short-term surgical outcomes, and 3-year DFS between laparoscopy-assisted DG (LADG) and open DG (ODG) for cT2-4/cN0-2 gastric cancer. In their study, there were no significant differences between groups in postoperative morbidity (17% in LADG vs. 18.8% in ODG, P=0.749) and hospital stay (9.8 days in LADG vs. 9.1 days in ODG, P=0.495). The non-compliance rates of D2 LND, defined as the proportion of patients with more than 1 empty LN station, were also similar between the 2 groups (47.0% in LADG vs. 43.2% in ODG, P=0.648). There was no significant difference in 3-year DFS between the 2 groups (80.1% in LADG vs. 81.9% in ODG, P=0.648). In addition, other small RCTs from Western countries have also reported that laparoscopic gastrectomy showed no significant differences in disease recurrence and OS of advanced gastric cancer compared to open surgery [120,121]. However, these studies are limited by their small sample sizes and inappropriate study designs; the final results of ongoing large multicenter randomized trials are awaited to determine the long-term outcomes of laparoscopic gastrectomy for locally advanced gastric cancer [122,123].
The short-term outcomes of laparoscopic gastrectomy with D2 LND for locally advanced gastric cancer have relatively been well demonstrated in clinical trials. Interim analysis of a large multicenter RCT in China (CLASS-01) reported no significant difference in postoperative complications between LADG and ODG (15.2% in LADG vs. 12.9% in ODG, P=0.285) [123]. In their study, patients with LADG also showed better postoperative recovery, such as faster bowel recovery and reduced hospital stay, than those with ODG (10.8 days in LADG vs. 11.3 days in ODG, P<0.001). Another small RCT reported similar numbers of harvested LNs and postoperative morbidity between open and laparoscopic gastrectomy with D2 LND [124]. More recently, a Japanese multicenter randomized trial (JLSSG 0901) reported interim results on the short-term outcomes of laparoscopic gastrectomy with D2 LND [125]. In their study, the incidence of anastomosis leakage or pancreatic fistula was 4.7%, which was within their expected target range and the study is ongoing.
In conclusion, with advances in laparoscopic surgery, short-term surgical outcomes and technical adequacy of laparoscopic gastrectomy with D2 LND have been well demonstrated both in retrospective studies and large RCTs. However, the long-term outcomes of laparoscopic gastrectomy for locally advanced gastric cancer require further investigation in large multicenter RCTs (Fig. 3A).
Robot gastrectomy
The current robotic surgical systems provide advantages such as 3-dimensional views, wristed instruments with 7 degrees of freedom, and tremor filtration, which enable surgeons to perform more accurate and thorough operations compared to that for conventional laparoscopic surgery [97,126,127,128]. Although it remains unclear whether the benefits of robotic gastrectomy outweigh the cost, the use of robotic gastrectomy has expanded gradually since the first clinical application of robotic surgery for the treatment of gastric cancer [97,129]. Robotic gastrectomy has shown several clinical benefits, including reduced blood loss and a possibly larger number of retrieved LN than those for conventional laparoscopic gastrectomy [126,130,131,132]. However, these advantages did not seem to significantly improve the short-term outcomes of patients [97,126,129]. These negative results were also demonstrated in a prospective multicenter study in Korea, although it was a non-randomized trial comparing the relatively early experience of robotic gastrectomy to well-established laparoscopic surgery [129]. The long-term oncologic outcomes of robotic gastrectomy reported by a few retrospective analyses are similar to those for laparoscopic surgery, but evidence is still lacking [126,130,131]. Overall, robotic gastrectomy seems to be feasible, safe, and easy to learn, but its advantages over laparoscopic gastrectomy are not obvious from the patient's standpoint.
ADJUVANT THERAPY
Statement 13. Adjuvant chemotherapy (S-1 or capecitabine plus oxaliplatin) is recommended in patients with pathological stage II or III gastric cancer after curative surgery with D2 LND (evidence: high, recommendation: strong for).
Surgical resection with D2 LND is the standard of care in gastric cancer. However, high rates of locoregional and distant recurrences have been reported in these cases, for which the prognosis is usually very poor [133].
European phase III studies demonstrated that perioperative chemotherapy including adjuvant chemotherapy was superior to surgery alone for patients with resectable gastroesophageal cancer [134,135]. As only 30%–50% of these European cases involved D2 LND, perioperative chemotherapy was not accepted as a treatment for such cases in East Asia.
Recently, 2 large randomized phase III trials conducted in Asian patients showed a significant survival benefit for adjuvant chemotherapy over observation after curative surgery with D2 LND in patients with resectable gastric cancer [136,137]. In the Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer (ACTS-GC) in Japan, 1,059 patients with stage II (excluding T1) or III gastric cancer (by Japanese classification, 2nd English edition [138]) following D2 gastrectomy received observation or S-1 for 1 year after surgery [136]. The rates of relapse-free survival at 3 years were 72.2% in the S-1 group and 59.6% in the surgery-only group (HR, 0.62; 95% CI, 0.50–0.77; P<0.001) and the 3-year OS rates were 80.1% and 70.1%, respectively (HR, 0.68; 95% CI, 0.52–0.87; P=0.003). In the capecitabine and oxaliplatin adjuvant study in stomach cancer (CLASSIC) conducted in South Korea, China, and Taiwan, 1,035 patients with stage II–IIIB gastric cancer (by AJCC 6th edition [139]) following D2 gastrectomy received either observation or capecitabine and oxaliplatin for 6 months [137]. The 3-year DFS rates were 74% in the chemotherapy and surgery group and 59% in the surgery-only group (HR, 0.56; 95% CI, 0.44–0.72; P<0.001). The 5-year follow-up data in these 2 studies confirmed these findings [140,141].
Based on the results of these studies, both chemotherapy regimens (S-1 or capecitabine plus oxaliplatin) are currently accepted as standard treatment in pathological stage II or III gastric cancer after D2 gastrectomy in East Asia (Fig. 1).
Statement 14. Adjuvant chemoradiation could be added for gastric cancer patients after curative resection with D2 lymphadenectomy to reduce recurrence and improve survival (evidence: high, recommendation: weak for).
High rates of loco-regional recurrence (LRR) have been reported in gastric cancer even after complete resection, especially in locally advanced stages of gastric cancer [142]. There have been attempts to minimize recurrences and improve outcomes through adjuvant RT, usually combined with chemotherapy, and several prospective or retrospective studies have shown promising outcomes of improved survival by reducing LRR [143,144,145]. In this context, a randomized phase III trial comparing surgery followed by adjuvant chemoradiation therapy (CRT) versus surgery alone in stage IB through IV (M0) gastric cancer (by AJCC 6th edition [139]) was performed (South Western Oncology Group-Directed Intergroup Study 0116 [INT-0116]) [146,147]. There was a clear advantage when adding adjuvant CRT, with a significant prolongation of survival as well as reduction of recurrences. Despite the positive outcomes of the INT-0116 study, however, several limitations were revealed. First, D2 lymphadenectomy, highly recommended as a standard surgical procedure in locally advanced gastric cancer, was performed in only 10% of the enrolled patients [65]. Second, this study was mainly conducted in gastric cancer patients from a Western population with different characteristics from those of Asian populations including Korean [148]. Because of these limitations, the necessity of adjuvant RT in completely resected stomach cancer remains controversial. Meanwhile, a retrospective pooled analysis of Dutch Gastric Cancer Group Trial reported that adjuvant CRT improved survival as well as local control in the D1 but not D2 resected subgroup [149].
However, adjuvant chemotherapy without RT showed a survival benefit over surgery alone in following randomized phase III trials and has become a standard of care [136,137,140,141]. Thus, the role of RT in addition to chemotherapy has been further questioned. Several RCTs have compared adjuvant CRT versus chemotherapy alone in gastric cancer after complete resection with D2 lymphadenectomy [150,151,152,153,154]. Among them, one trial performed by a single Korean institution (Lee et al., [153] Adjuvant Chemoradiation Therapy in Stomach Cancer [ARTIST] trial) completed the preplanned patient accrual but 3 other trials (2 Korean and 1 Greek trial) failed to complete the planned registration and were terminated prematurely [150,151,152]. There was no mention of the planned number of patients or completion of registration in the 1 remaining multicenter Chinese trial [154].
A meta-analysis of the aforementioned trials found that adjuvant CRT can improve not only LRR-free survival (LRRFS) but also DFS compared to chemotherapy alone [155,156,157,158,159,160]. However, improved OS in adjuvant CRT was not demonstrated. Furthermore, the ARTIST trial failed to confirm the superiority of adjuvant CRT over chemotherapy alone in terms of DFS as well as OS for all patients even after long-term follow-up, although it showed a significant benefit in LRRFS [153,161,162]. The beneficial effect of DFS on adjuvant CRT was limited to patients with nodal involvement. This trial is considered to be the most reliable study in terms of adjuvant RT in gastric cancer for Korean patients because it is a well-designed prospective study that was conducted and completed in Korea.
Based on the results of these studies, adjuvant CRT can be considered in gastric cancer patients with incomplete resection and/or less than D2 lymphadenectomy (Fig. 1). Adjuvant CRT could also be considered in patients with gastric cancer after complete resection with D2 lymphadenectomy, especially for those with LN metastasis.
NEOADJUVANT THERAPY
Statement 15. Neoadjuvant chemotherapy for potentially resectable gastric cancer is not conclusive if D2 LND is considered (evidence: high, recommendation: inconclusive).
European phase III studies demonstrated that perioperative chemotherapy including neoadjuvant chemotherapy was superior to surgery alone in potentially resectable gastric cancer. The outcomes of patients treated with surgery alone were compared to those of patients treated with perioperative epirubicin, cisplatin, and infusional 5-fluorouracil (5-FU) in the MAGIC trial or with perioperative cisplatin and infusional 5-FU in the FNCLCC/FFCD trial [135,163]. In these studies, perioperative chemotherapy significantly prolonged both OS and progression-free survival (PFS) or DFS. The FLOT-4 study showed that a perioperative regimen comprising 5-FU, leucovorin, oxaliplatin, and docetaxel was superior to perioperative epirubicin, cisplatin, and 5-FU or capecitabine [134]. However, as D2 LND was performed in only 30%–50% of patients in these European studies, these perioperative chemotherapeutic regimens might not be applicable to patients in Korea, where D2 LND is the standard of care. Recently, the Japanese phase III JCOG 0501 study compared 2 cycles of neoadjuvant S-1 plus cisplatin followed by D2 surgery and adjuvant S-1 to D2 surgery followed by adjuvant S-1 in far-advanced localized gastric cancer. This trial observed no statistically significant difference in OS and PFS between the 2 arms [164].
Therefore, neoadjuvant chemotherapy for potentially resectable gastric cancer is not conclusive at present in Korea except in clinical trials (Fig. 1).
Statement 16. The evidence for the effectiveness of neoadjuvant chemoradiation in locally advanced gastric cancer is not conclusive if D2 LND is considered (evidence: high, recommendation: inconclusive).
Neoadjuvant CRT is mainly studied for cancer of the esophagus, EGJ, and/or gastric cardia, where obtaining a complete R0 resection is challenging and thus, there is a higher probability of locoregional relapse. Two RCTs have been conducted and 1 trial is ongoing to compare the outcomes of neoadjuvant CRT versus neoadjuvant chemotherapy alone in resectable cancer of the EGJ or stomach [165,166,167,168].
The PreOperative therapy in Esophagogastric adenocarcinoma Trial (POET) showed a higher probability of pathologic complete response (15.6% vs. 2.0%) and pathologic N0 (64.4% vs. 37.7%) after neoadjuvant CRT compared to those for neoadjuvant chemotherapy alone [168]. Additionally, improved OS was also noticed after neoadjuvant CRT (47.4% vs. 27.7% at 3 years), although the difference failed to reach statistical significance (P=0.07). The improved OS remained in long-term analysis (39.5% vs. 24.4% at 5 years, P=0.06) with a significant benefit of LRRFS (P=0.01; HR, 0.37) [167]. Similar benefits of neoadjuvant CRT were also reported in another RCT from Sweden and Norway [165]. Neoadjuvant CRT showed higher probabilities of complete pathologic response (28% vs. 9%, P=0.002), pathologic N0 (62% vs. 35%, P=0.001), and R0 resection rate (87% vs. 74%, P=0.04) in this study. The Trial Of Preoperative therapy for Gastric and Esophagogastric junction AdenocaRcinoma (TOPGEAR) also demonstrated that neoadjuvant CRT can be safely delivered to the majority (85%) of patients without a significant increase in treatment-related toxicities or surgical morbidity [166]. Those findings have been confirmed in several meta-analyses of randomized trials [159,169,170,171].
Despite their promising outcomes, the aforementioned studies were performed mainly in patients with esophageal and/or EGJ cancer. EGJ cancer is common in Western countries [148] and most studies evaluating the efficacy of neoadjuvant CRT for gastric cancer (mainly EGJ cancer) were also performed in Western populations. Thus, it might be inappropriate to simply apply these results to Asian populations, especially to Koreans, where gastric cancer occurs mainly in the antral area [148] (Fig. 1). To evaluate the effect of neoadjuvant CRT in gastric cancer, further prospective studies targeting Asian populations with non-junction cancer are mandatory.
PALLIATIVE THERAPY
The prognosis for locally advanced unresectable or metastatic gastric cancers is dismal, and these patients have a median OS of 6–13 months. The goals of therapy for these patients are to palliate disease-related symptoms and to prolong survival. Such palliative systemic therapy also provides a greater quality of life than best supportive care. Thus, systemic therapy is the primary treatment to be considered in patients with locally advanced unresectable (unresectable T4b or extensive nodal disease) or metastatic disease or those after non-curative resection. Palliative systemic therapy for advanced gastric cancer should be determined based on patient performance status, medical comorbidities, and organ function. Furthermore, systemic therapy regimens can be individualized for each patient, with the regimen determined by the clinician according to various patient or gastric cancer-related conditions and participation in clinical trials can be actively considered. A recent study conducted in Germany reported that patients' preferences impacted the specific responses, including low toxicity of chemotherapy, self-care ability, and additional survival benefits [172]. Therefore, patient preferences should also be considered in making decisions regarding palliative therapy
Surgery
Statement 17. Palliative gastrectomy is not recommended for metastatic gastric cancer except for palliation of symptoms (evidence level: high, recommendation: strong against).
Palliative surgery is usually indicated for metastatic gastric cancer for the control of urgent symptoms such as obstruction, bleeding, or perforation. However, the effect of palliative gastrectomy on the survival of patients with metastatic gastric carcinoma has long been debated. Several retrospective studies have reported inconsistent results depending on patient population and analytic methods. Some studies have reported significantly improved patient survival for gastrectomy plus chemotherapy compared to chemotherapy alone in carefully selected patients [173,174,175,176,177,178,179]. Some reports have suggested that patients with hepatic metastasis might benefit from gastrectomy plus partial hepatectomy when no other distant metastasis existed [180,181,182,183]. In contrast, other studies have reported that gastrectomy neither prolonged patient survival nor improved the quality of life in patients with metastatic gastric carcinoma [184,185,186,187,188,189,190,191]. Meanwhile, a meta-analysis of 14 retrospective studies showed that gastrectomy followed by chemotherapy could significantly improve patient survival (median survival, 14.96 vs. 7.07 months; HR, 0.56; 95% CI, 0.39–0.80), compared to that for chemotherapy alone [192]. Another meta-analysis of 19 non-randomized studies reported that gastrectomy could improve patient survival (1-year survival: OR, 2.6; 95% CI, 1.7–4.3; P<0.001) in metastatic gastric carcinoma [193]. However, these studies are mostly biased by patient selection, in which surgery was usually indicated for patients with relatively better performance status and less advanced disease.
To investigate the survival benefit of gastrectomy for metastatic gastric carcinoma, a large international phase III trial was performed in Korea, Japan, and Singapore (REGATTA trial) [194]. In this trial, 175 advanced gastric cancers with a single non-curable factor (liver, peritoneum, or distant nodal metastasis) were randomly assigned to receive gastrectomy plus chemotherapy or chemotherapy alone. The results of an interim analysis revealed that gastrectomy prior to chemotherapy had no effect on OS (HR, 1.08; 95% CI, 0.74–1.58; P=0.66) or PFS (HR, 1.01; 95% CI, 0.74–1.37; P=0.96). Based on these findings, this trial was interrupted in 2013, concluding that gastrectomy did not show any survival benefit compared to that for chemotherapy alone in advanced gastric carcinoma with a single non-curable factor.
In conclusion, although some retrospective studies have reported a possible survival benefit of palliative gastrectomy for metastatic gastric carcinoma, a well-designed multi-institutional randomized trial proved that gastrectomy does not improve patient survival in metastatic gastric carcinoma. Therefore, gastrectomy should only be performed with a palliative intent to relieve patient symptoms (Fig. 1).
First-line systemic therapy
Statement 18-1. Palliative first-line platinum/fluoropyrimidine combination is recommended in patients with locally advanced unresectable or metastatic gastric cancer if the patient's performance status and major organ functions are preserved (evidence: high, recommendation: strong for).
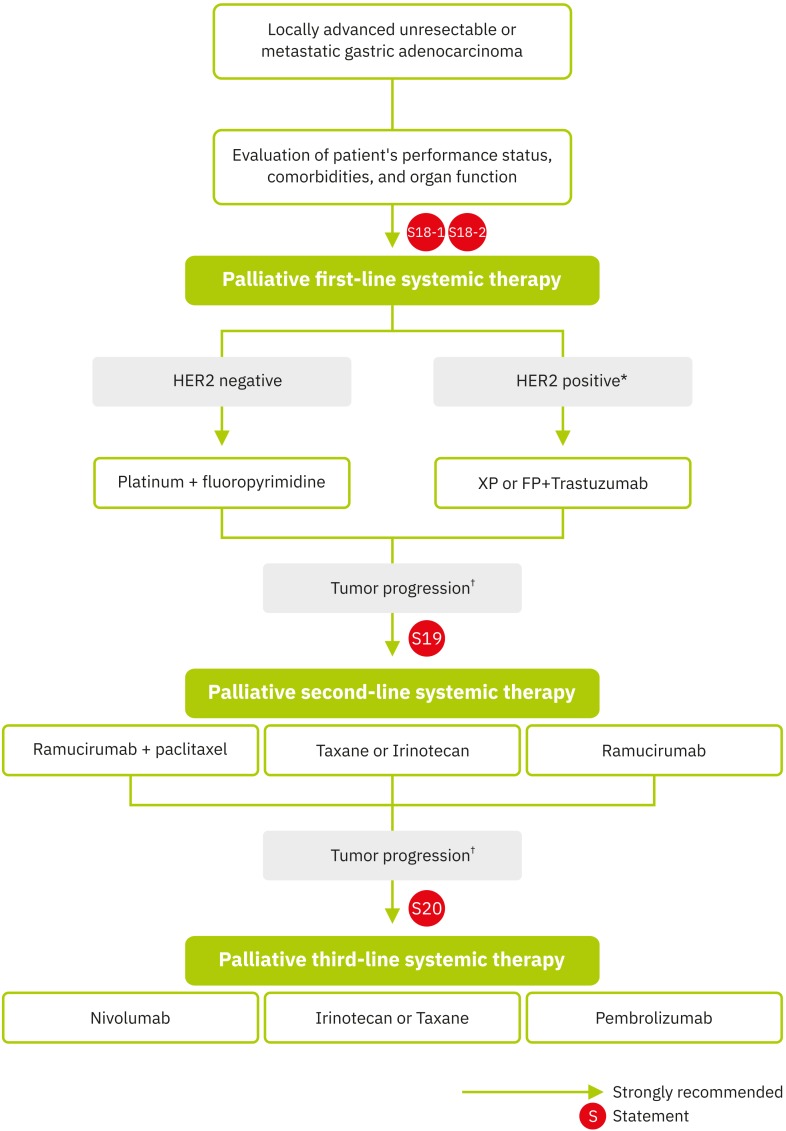
The effective cytotoxic agents for advanced gastric cancer include infusional 5-FU, oral fluoropyrimidines, platinum agents, taxanes, irinotecan, and anthracyclines. Randomized studies have evaluated various 5-FU-based regimens for the treatment of locally advanced unresectable or metastatic gastric cancer [195,196,197]. In a meta-analysis, significant OS benefits were shown for chemotherapy versus best supportive care, with increased survival of approximately 6 months. In addition, combination chemotherapy showed a statistically significant survival benefit over single-agent chemotherapy, with a difference in weighted mean average survival of approximately 1 month [198] (Fig. 4).
Fig. 4. Treatment algorithm for palliative systemic therapy.
HER2 = human epidermal growth factor receptor 2; XP = capecitabine and cisplatin; FP = fluorouracil and cisplatin; IHC = immunohistochemistry.
*HER2 IHC 3+ or IHC 2+ and in situ hybridization-positive; †Evaluation of patient performance status, comorbidities, and organ function.
Although infusional 5-FU is one of the most commonly used cytotoxic agents for advanced gastric cancer, continuous intravenous infusions can prolong hospital stays and result in thrombosis and infection. Randomized phase III studies have demonstrated that the oral fluoropyrimidines capecitabine [199,200,201] and S-1 [202,203] are as effective as infusional 5-FU. Therefore, oral fluoropyrimidines (capecitabine or S-1) are safe and convenient alternatives to 5-FU for combinations with platinum compounds in patients with advanced gastric cancer. For many years, cisplatin was the leading compound used for the treatment of patients with advanced gastric cancer. To avoid some of the associated side effects such as nausea, vomiting, nephrotoxicity, and ototoxicity, other platinum compounds were investigated. The results of the REAL-2 study suggested that pooled oxaliplatin-based regimens are not inferior to pooled cisplatin-based regimens in terms of OS [199]. A randomized trial in Germany showed that oxaliplatin had better efficacy than that of cisplatin in older adult patients and a more favorable overall toxicity profile [204]. The G-SOX study in Japan and the SOPP study in Korea showed that S-1 plus oxaliplatin is as effective as S-1 plus cisplatin for the treatment of advanced gastric cancer, with a favorable safety profile [205,206]. Therefore, oxaliplatin is at least as effective as cisplatin for prolonging survival and is generally better tolerated.
Regarding combination therapies, it remains unclear if there is a benefit from combining 3 rather than 2 cytotoxic agents. The phase III V325 study showed an increased overall response rate, PFS, and OS for docetaxel, cisplatin, 5-FU (DCF) compared to those of cisplatin/5-FU [207]. However, the implementation of DCF is difficult in clinical practice because the DCF regimen showed only a modest OS benefit (9.2 [DCF] vs. 8.6 months [CF]) but caused markedly increased hematological and gastrointestinal toxicity in this highly selected study population, with a median age of 55 years. In various clinical trials, modifications of this DCF regimen have demonstrated efficacy with improved safety profiles in patients with advanced gastric cancer. Therefore, selected patients can benefit from docetaxel-containing triplet combinations but increased side effects should be considered (high, weak for).
Statement 18-2. Palliative trastuzumab combined with capecitabine or fluorouracil plus cisplatin is recommended in patients with human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) 3+ or IHC 2+ and in situ hybridization (ISH)-positive advanced gastric cancer (evidence: high, recommendation: strong for).
Trastuzumab is a humanized anti-HER2 immunoglobulin G1 (IgG1) monoclonal antibody and the first successful biologic agent, with documented clinical activity as a first-line treatment in advanced gastric cancer (Fig. 4). The Trastuzumab for Gastric Cancer (ToGA) trial demonstrated clinically and statistically significant improvements in OS with the addition of trastuzumab to a cisplatin/fluoropyrimidine doublet (13.8 vs. 11.1 months; HR, 0.74; 95% CI, 0.60–0.91; P<0.01) [208]. A post hoc subgroup analysis revealed that the addition of trastuzumab to chemotherapy substantially improved the OS of patients whose tumors were IHC 3+ or ICH 2+ and ISH-positive (16.0 vs. 11.8 months; HR, 0.65; 95% CI, 0.51–0.83). Therefore, a trastuzumab-containing regimen is recommended in patients with HER2-positive gastric cancer and a combination of trastuzumab, cisplatin, and either capecitabine or infusional 5-FU is recommended in clinical practice based on the results of this trial.
Various agents targeting epidermal growth factor receptor, hepatocyte growth factor receptor, and vascular endothelial growth factor receptor (VEGFR) have been evaluated as first-line treatments for advanced gastric cancer; however, except for trastuzumab, none of these agents demonstrated a significant OS benefit in global phase III trials.
Second-line systemic therapy
Statement 19. Palliative second-line systemic therapy is recommended in patients with locally advanced unresectable or metastatic gastric cancer if the patient's performance status and major organ functions are preserved. Ramucirumab plus paclitaxel is preferably recommended and monotherapy with irinotecan, docetaxel, paclitaxel, or ramucirumab could also be considered (evidence: high, recommendation, strong for).
Randomized trials and a meta-analysis have demonstrated the survival benefit of second-line palliative chemotherapy (with irinotecan or taxanes) compared to best supportive care alone for patients with locally advanced unresectable or metastatic gastric cancer (HR, 0.64; 95% CI, 0.52–0.79; P<0.001) [209,210,211,212] (Fig. 4). Weekly paclitaxel resulted in a similar OS to that achieved with irinotecan in phase III trials [213,214]. In addition, ramucirumab, a monoclonal antibody targeting VEGFR-2, was shown to significantly improve survival in 2 phase III double-blind placebo-controlled trials. In the REGARD trial, patients receiving ramucirumab had improvements in both OS and PFS compared to those in patients receiving placebo [215]. Similarly, in the RAINBOW trial, the addition of ramucirumab to weekly paclitaxel significantly prolonged the median OS (9.6 vs. 7.4 months; HR, 0.807; 95% CI, 0.678–0.962; P=0.017) compared to that for paclitaxel plus placebo [216].
Based on the available data, ramucirumab in combination with paclitaxel is recommended as the most preferred second-line treatment. Irinotecan, docetaxel, paclitaxel, or ramucirumab as single agents can also be considered as a second-line option if not previously administered in the first-line treatment.
Pembrolizumab, an anti-programmed cell death 1 (PD-1) antibody, was recently approved by the Food and Drug Administration (FDA) for the treatment of unresectable or metastatic microsatellite instability-high (MSI-H) or deficient mismatch repair (MMR) solid tumors that have progressed after initial treatments, thus representing a second-line or later option for such gastric cancer cases [217].
Third-line systemic therapy
Statement 20. Palliative third-line systemic therapy is recommended in patients with locally advanced unresectable or metastatic gastric cancer if the patient's performance status and major organ functions are preserved (evidence: high, recommendation: strong for).
Despite the lack of clear evidence for third-line cytotoxic chemotherapy, data from several phase II and retrospective studies indicate a 15%–20% response rate with third-line taxane- or irinotecan-based chemotherapy [218,219,220] (Fig. 4). In a randomized phase III trial in Korea, second- or third-line salvage chemotherapy significantly prolonged patient survival compared to that for best supportive care [210]. Therefore, palliative third-line chemotherapy with cytotoxic agents (e.g., irinotecan, paclitaxel, or docetaxel) not used in second-line therapy can be recommended (moderate, strong for). Recently, a phase III study of patients with metastatic gastric cancer refractory to standard therapies showed a benefit in terms of OS with TAS-102 (trifluridine/tipiracil) compared to that for best supportive care [221]. TAS -102 can be considered if it is approved for use in gastric cancer (high, weak for).
In a randomized phase III trial, apatinib mesylate, a small-molecule inhibitor of VEGFR-2, significantly prolonged the survival of patients who experienced disease progression after 2 or more lines of systemic therapy [222]. However, with an increasing number of patients receiving ramucirumab in the second-line setting, the efficacy of apatinib mesylate in overcoming resistance to ramucirumab is unclear. Moreover, the only results with apatinib mesylate have been reported among Chinese patients; therefore, additional studies are needed to confirm these results (high, weak for).
Recently, immune checkpoint inhibitors have been shown to enhance antitumor T-cell activity via inhibition of the PD-1 receptor. Nivolumab is a humanized IgG4 anti-PD-1 monoclonal antibody. ATTRACTION-2 (ONO-4538-12), the first phase III trial of third-line or later nivolumab versus placebo, showed the efficacy and safety of nivolumab in heavily pretreated patients with advanced gastric cancer (median OS, 5.26 vs. 4.14 months; HR, 0.63; 95% CI, 0.51–0.78; P<0.001) [223]. Another such antibody, pembrolizumab, also showed promising activity and manageable safety in advanced gastric cancer patients who had received at least 2 lines of treatment in a phase Ib trial (KEYNOTE-012) (8) as well as a phase II trial (KEYNOTE-059; cohort 1), in which the overall response rates trended higher in PD-L1-positive versus PD-L1-negative tumors [224,225]. Nivolumab improves OS as third-line treatment irrespective of PD-L1 status in Asian patients with gastric cancer and is registered in Korea, Japan, and Taiwan (high, strong for). Pembrolizumab shows significant efficacy as a third-line treatment, especially in PD-L1-positive patients in whom its use is approved by the US FDA (moderate, weak for).
Radiotherapy (RT)
Statement 21. Palliative RT could be offered to alleviate symptoms and/or improve survival in recurrent or metastatic gastric cancer (evidence: moderate, recommendation: weak for).
Systemic chemotherapy is the mainstay treatment for the management of recurrent or metastatic gastric cancer, even for isolated LRR [50]. However, the addition of local modalities including RT may add a benefit over chemotherapy alone in certain situations [226,227,228,229,230,231].
Unfortunately, no prospective randomized phase III trial has evaluated the efficacy of adding RT in recurrent or metastatic gastric cancer. However, successful symptom alleviation has been reported with the addition of RT in symptomatic advanced gastric cancer [228,229,230] and prolongation of survival is suggested according to the results of several prospective and retrospective reports [226,227,228,231]. Tey et al. [230] reported improvement of symptoms such as tumor bleeding (83/103, 80.6%), obstruction (9/17, 52.9%), and pain (5/11, 45.5%) after RT, with an acceptable rate (2.6%) of grade 3 gastrointestinal toxicities [230]. Sun et al. [228] reported that clinical symptoms were relieved after RT in 19 of 21 patients (90.5%) with recurrent gastric cancer with abdominal LN metastasis. Hingorani et al. [227] reported the outcomes of a retrospective study comparing chemotherapy followed by RT to primary tumor and chemotherapy alone in metastatic EGJ cancer patients with responding or stable disease after 3 months of chemotherapy. Both OS and time to local progression were significantly improved in irradiated patients, at 23.3 vs. 14.0 months (P<0.001) and 17.3 vs. 8.3 months (P=0.006), respectively.
Despite a lack of evidence from randomized phase III trials on the efficacy of RT in recurrent or metastatic stomach cancer, RT could be used for palliation of symptoms in localized primary and/or metastatic disease and could possibly improve survival by maximizing local control in patients with responding or stable disease after chemotherapy (Fig. 1). The efficacy and necessity of RT in recurrent or metastatic stomach cancer should be evaluated in larger studies.
PATHOLOGY
This guideline only describes in detail several pathological topics important to gastric cancer treatment. For topics not described here, please refer to the existing guidelines [2,232].
Histologic classification
World Health Organization (WHO) classification is used for the pathologic classification of gastric carcinoma [233]. Lauren classification is added in resected specimens, including ESD specimens [234].
WHO classifications
1) Papillary adenocarcinoma
Although the diagnostic criteria for papillary carcinoma are not clearly defined in the 2010 WHO Blue Book, many publications define papillary carcinoma as more than 50% of the tumor present in the papillary [235]. Papillary adenocarcinoma is graded as well, moderately, or poorly differentiated [233]. Most papillary adenocarcinomas are well-differentiated. However, the prognosis is poor if the nuclear atypia is severe [236]. Therefore, such cases should be classified as poorly differentiated.
2) Tubular adenocarcinoma
Tubular adenocarcinoma, the most common histologic type of gastric carcinoma, is graded as well, moderately, or poorly differentiated. Well-differentiated tubular adenocarcinoma is composed of well-formed tubular structures. Poorly differentiated adenocarcinoma shows irregular glands with indistinct lumens. Moderately differentiated adenocarcinomas are intermediate between well- and poorly differentiated [233].
3) Mucinous adenocarcinoma
This variant of adenocarcinoma is defined by the presence of >50% of tumor lesions with an extracellular mucin pool regardless of the tumor cell type, signet ring cell or not [233].
4) Poorly cohesive carcinoma
This tumor is composed of poorly cohesive neoplastic cells that are isolated or form small aggregates [233]. This type includes signet ring cell carcinoma and other cellular variants composed of poorly cohesive neoplastic cells [233]. However, signet ring cell carcinoma is usually diagnosed separately if the signet ring cell component exceeds 50% rather than diagnosed as poorly cohesive carcinoma.
5) Mixed carcinoma
This type of carcinoma has a discrete mixture of both glandular (tubular or papillary) and signet ring/poorly cohesive components [233]. The latter component is associated with a poor prognosis [233].
Addendum: In Japan, gastric carcinomas are commonly divided into 2 major categories, differentiated and undifferentiated types, especially with respect to the indications for endoscopic resection [237,238]. Although the WHO classifications do not completely match to this classification, the differentiated type generally includes well and moderately differentiated tubular adenocarcinomas and papillary adenocarcinoma, while the undifferentiated type includes poorly differentiated tubular adenocarcinoma and poorly cohesive carcinoma. Mucinous adenocarcinoma is classified as differentiated (with tubules) or undifferentiated (with signet ring cells) according to the type of tumor cell and is sometimes also categorized as undifferentiated type.
Lauren classifications
The Lauren classification divides tumors into intestinal, diffuse, and mixed types [234]. Intestinal carcinomas form glands with various degrees of differentiation and are almost invariably associated with intestinal metaplasia and variable degrees of atrophic gastritis. Diffuse carcinoma consists of poorly cohesive cells with little or no gland formation. Tumors containing approximately equal quantities of intestinal and diffuse components are termed “mixed.”
Pathologic diagnosis of mixed histology
In early gastric cancer, the histologic type and grade of the biopsy tissue are important for determining the treatment modality. Tumor heterogeneity and inter- and intra-observer discrepancies may lead to differences in histological types before and after ESD [239,240,241]. Submucosal and even intramucosal cancer with heterogeneity have recently been reported to have a higher incidence of LN metastasis than that of homogeneously differentiated types of tumor [242,243]. Thus, there is a view that the minor component of undifferentiated histology should be reported in cases of biopsy and ESD specimens. However, this requires more discussion and consensus of clinical and pathologic departments.
Biomarkers
HER2
IHC tests should first be performed for evaluation of HER2 status. IHC results are scored as 0, 1+, 2+, or 3+ (Table 4). IHC 3+ is considered positive for HER2 overexpression, while IHC 0-1+ is considered negative. IHC 2+ is regarded as an equivocal finding and should be followed by ISH tests. The area with the strongest IHC intensity should be selected and stained for HER2 and chromosome enumeration probe (CEP) 17. The criteria for HER2 amplification is a HER2:CEP17 ratio of ≥2. If CEP17 polysomy is present and the ratio is <2, an average HER2 signal of >6 is interpreted as a positive finding. IHC 3+ or IHC 2+ and ISH-positivity are considered HER2-positive [244,245]. HER2-positivity is an indication for anti-HER2 targeted therapy in the palliative setting [208].
Table 4. Interpretation of IHC findings [244,245].
| HER2 status | Intensity | IHC staining |
|---|---|---|
| Negative | 0 | Reactivity in <10%* of tumor cells |
| Negative | 1+ | Faint membranous† reactivity in ≥10% of tumor cells; reactive only in part of their membrane |
| Equivocal | 2+ | Weak to moderate complete or basolateral membranous reactivity in ≥10% of tumor cells |
| Positive | 3+ | Strong complete or basolateral membranous reactivity in ≥10% of tumor cells |
IHC = immunohistochemistry; HER2 = human epidermal growth factor receptor 2.
*For biopsy samples, measure scores when stained tumor cell clusters (≥5 cells) are present, irrespective of the percentage of tumor cells; †Only membranous staining should be considered true reactivity.
Microsatellite instability (MSI)
MSI is assessed by polymerase chain reaction (PCR)-based tests, which can be replaced by IHC tests for the 4 MMR proteins (MMR deficient [dMMR]) [246,247]. Instability is examined by PCR of a representative panel of microsatellites. The grade of the instability is determined by the numbers of unstable microsatellites: MSI-H, MSI-low (MSI-L), or microsatellite stable (MSS) [248,249]. MSI-H is considered MSI-positive. In the IHC method, the IHC staining is done for the 4 MMR proteins: MLH1, MSH2, PMS2, and MSH6. When the expression of any one of the MMR proteins is lost, the case is considered to be dMMR. MSI positivity is the criteria for MSI-subtype gastric cancer. MSI-positive gastric cancer is classified as a separate subtype in the molecular classifications of gastric cancer and shows elevated mutation rates and distinctive patterns of methylation [250,251]. This subtype has unique clinical characteristics, including distal location, high frequency of intestinal-type histology, lower stage, and good prognosis [252]. In the palliative setting, MSI positivity is an indication for immune checkpoint inhibitor therapy (pembrolizumab) [253].
Epstein-Barr virus (EBV)
The presence of the EBV genome can be examined by several methods. The most widely used method for tissue sections, performed in almost all hospitals, is ISH to EBV-encoded RNA (EBER) [254]. When signals in the tumor cell nuclei are observed, the case is considered to be EBV-positive gastric cancer. EBV positivity is the criteria for EBV-positive gastric cancer. EBV-positive gastric cancer is classified as a separate subtype in the molecular classification of gastric cancer and shows hypermethylation different from that of the MSI subtype [250]. This subtype is distinct in its proximal location, relation to poorly differentiated histology, lower stage, and good prognosis [255,256].
PD-L1
Several systems can be used for the interpretation of IHC staining of PD-L1. The combined positive score (CPS) [224] is the number of PD-L1-stained viable tumor cells, lymphocytes, and macrophages divided by the number of viable tumor cells, multiplied by 100. Lymphocytes or macrophages are scorable when they are contiguous: intercalated within a confluent area of neoplastic cells or a part of a confluent area adjacent to neoplastic cells (within a 20× field) [257]. The tumor proportion score (TPS) is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity [258]. The criteria for PD-L1 positivity differs depending on the therapeutic agent. For pembrolizumab, PD-L1 positivity is defined as CPS ≥1. In a clinical trial that enrolled 259 patients, the objective response rates were 15.5% and 6.4% in patients with CPS ≥1 and <1, respectively [225]. For nivolumab, the cut-off for PD-L1 expression has not been established. In a clinical trial, an exploratory subgroup analysis was performed with a TPS of >1% vs. ≤1% but the median survival increased regardless of PD-L1 level [223].
Peritoneal washing cytology
Statement 22. Peritoneal washing cytology is recommended for staging. Advanced gastric cancer patients with positive cancer cells in the peritoneal washing cytology are associated with frequent cancer recurrence and poor prognosis (evidence: moderate, recommendation: strong for).
The association between peritoneal washing cytology results and prognosis in patients with advanced gastric cancer has been reported in prospective [259,260] and retrospective [261,262,263,264] studies but not in any randomized case-control studies. The exact prognostication of advanced gastric cancer patients with positive peritoneal washing cytology result is difficult due to variability in enrolled patients, peritoneal washing methods, and treatment of cancer patients in these studies. Recent systemic reviews and meta-analyses have shown that peritoneal washing cytology is useful for determining the prognosis of advanced gastric cancer patients [265,266,267,268].
Although some studies have reported that peritoneal washing cytology results are not related to prognosis, most studies and meta-analyses have observed a high recurrence rate and short survival time in advanced gastric cancer patients with positive washing cytology. In 2 meta-analyses, the HRs for survival among cytology-positive patients were 3.27 (95% CI, 2.82–3.78) [267] and 3.46 (95% CI, 2.77–4.31) [265], respectively, which was significantly higher than those for cytology-negative patients. The HR of cancer recurrence (4.15; 95% CI, 3.10–5.57) was also higher in cytology-positive patients [267].
The positivity rate of peritoneal washing cytology varies from 7% to 58% [259,260,261,263,264,265,267]; this range may be due to differences in the study populations, including early gastric cancer patients. The diverse pathologic criteria of positive cancer cells could also contribute to the wide range of positivity. Although most of the studies did not address the pathologic criteria for ‘positive’ cancer cells in peritoneal washing cytology, there is a need for further studies to establish these pathologic criteria for the clinical application of peritoneal washing cytology in advanced gastric cancer patients.
In conclusion, peritoneal washing cytology is recommended for accurate staging and prognosis for advanced gastric cancer even though there remain several controversies.
MULTIDISCIPLINARY TEAM (MDT)APPROACH
The effectiveness of MDTs in cancer treatment has been controversial because there was no strong evidence and MDT also requires significant time and resources [269,270]. However, MDT has been important for the treatment of cancer because treatment methods are diverse, complex, and specialized. The advantages of MDT include correct diagnosis, changing to a better treatment plan, and survival benefit. For these reasons, health services in several countries have produced guidelines citing MDTs as the preferred system for cancer treatment [271].
Several studies have shown the advantages of MDT in gastrointestinal malignancy. After MDT meetings, changes in diagnosis occurred in 18.4%–26.9% of evaluated patients [272,273], and the treatment plan was changed in 23.0%–76.8% of cancer patients [273,274,275]. Furthermore, the National Comprehensive Cancer Network guideline emphasizes the recommendation for MDT and the European Society for Medical Oncology and European Cancer Organization guidelines indicate that MDT before determining cancer treatment is mandatory [61,62,276].
The many types of MDT include conferences the without patient present, face-to-face with the patient, and telemedicine. There is no strong evidence regarding which type is better, although Kunkler et al. [277] reported no difference between telemedicine and face-to-face medicine. In addition, Allum et al. [61] recommended that MDTs should discuss treatment decisions with patients. The MDT members for gastric cancer treatment are recommended to include surgeons, gastroenterologist, medical and radiation oncologists, radiologists and pathologists, and other members such as those from nutritional services, social workers, nurses, and palliative care specialists [62,276,278,279].
In conclusion, MDT treatment has a benefit in the treatment of gastric cancer patients regarding patient satisfaction, change to better treatment plans, and more correct diagnosis; however, stronger evidence is required.
ACKNOWLEDGMENTS
Members of the Guideline Committee
Development Working Group
Korean Gastric Cancer Association: Keun Won Ryu (National Cancer Center), Young Suk Park (Seoul National University Bundang Hospital), Oh Kyoung Kwon (Kyungpook National University Chilgok Hospital), Jeong Oh (Chonnam National University Hwasun Hospital), Han Hong Lee (The Catholic University of Korea Seoul St. Mary's Hospital), Seong Ho Kong (Seoul National University Hospital), Taeil Son (Severance Hospital), Hoon Hur (Ajou University Hospital), Ye Seob Jee (Dankook University Hospital), Hong Man Yoon (National Cancer Center); Korean Society of Gastroenterology: Changyoo Kim (National Cancer Center), Byung-Hoon Min (Samsung Medical Center), Ho-june Song (Asan Medical Center), Woon Geon Shin (Kangdong Sacred Heart Hospital), Sang Kil Lee (Severance Hospital), Jae-Young Jang (Kyung Hee University Hospital), Hye-kyung Jung (Ewha Womans University Mokdong Hospital); Korean Society of Medical Oncology: Min-Hee Ryu (Asan Medical Center), Sun Jin Sym (Gachon University Gil Medical Center), Sangcheul Oh (Korea University Guro Hospital), Byoung Yong Shim (Catholic University of Korea St. Vincent's Hospital), Dae Young Zang (Hallym University Sacred Heart Hospital), Hye Sook Han (Chungbuk National University Hospital), Dong-Hoe Koo (Kangbuk Samsung Hospital), Hyeong Su Kim (Hallym University Kangnam Sacred Heart Hospital), Chi Hoon Maeng (Kyung Hee University Hospital), In Gyu Hwang (Chung-Ang University Hospital); Korean Society for Radiation Oncology: Jeong Il Yu (Samsung Medical Center), Eui Kyu Chie (Seoul National University Hospital); Korean Society of Pathologists: Joon Mee Kim (Inha University Hospital), Baek-Hui Kim (Korea University Guro Hospital), Myeong-Cherl Kook (National Cancer Center), Hye Seung Lee (Seoul National University Bundang Hospital); National Evidence-Based Healthcare Collaboration Agency: Miyoung Choi.
Review Panel
Korean Gastric Cancer Association: Chan-Young Kim (Chonbuk National University Hospital), Sungho Jin (Korea Institute of Radiological and Medical Sciences); Korean Society of Gastroenterology: Jae Myung Park (Seoul St. Mary's Hospital), Cheol Min Shin (Seoul National University Bundang Hospital); Korean Society of Medical Oncology: Do-Youn Oh (Seoul National University Hospital), Keun-Wook Lee (Seoul National University Bundang Hospital); Korean Society for Radiation Oncology: Tae-Hyun Kim (National Cancer Center); Korean Society of Pathologists: Kyoung-Mee Kim (Samsung Medical Center).
Footnotes
Funding: This guideline was completed with the support of the Korean Gastric Cancer Association (KGCA). The KGCA had no influence on the content of the guideline.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Flowcharts of the literature search and study selection
Selected literature lists
Quality assessment tables
Evidence tables
References
- 1.Jung KW, Won YJ, Kong HJ, Lee ES Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50:303–316. doi: 10.4143/crt.2018.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87–104. doi: 10.5230/jgc.2014.14.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Korean Gastric Cancer Association. Korean guideline for gastric cancer. J Korean Gastric Cancer Assoc. 2004;4:286–293. [Google Scholar]
- 4.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62:1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 8.Scottish Intercollegiate Guidelines Network. SIGN 50: a guideline developer's handbook [Internet] Edinburgh: Scottish Intercollegiate Guidelines Network; 2015. [cited 2018 Sep 23]. Available from: http://www.sign.ac.uk. [Google Scholar]
- 9.Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Handbook: Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. place unknown: The GRADE Working Group; 2013. [Google Scholar]
- 10.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, editors. AJCC Cancer Staging Manual. 8th ed. New York (NY): Springer International Publishing; 2017. [Google Scholar]
- 11.Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD study group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Kim SG, Lyu DH, Park CM, Lee NR, Kim J, Cha Y, et al. Current status of endoscopic submucosal dissection for early gastric cancer in Korea: role and benefits. Korean J Intern Med. 2018 doi: 10.3904/kjim.2017.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 14.Nishizawa T, Yahagi N. Long-term outcomes of using endoscopic submucosal dissection to treat early gastric cancer. Gut Liver. 2018;12:119–124. doi: 10.5009/gnl17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SG, Park CM, Lee NR, Kim J, Lyu DH, Park SH, et al. Long-term clinical outcomes of endoscopic submucosal dissection in patients with early gastric cancer: a prospective multicenter cohort study. Gut Liver. 2018;12:402–410. doi: 10.5009/gnl17414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ, Lee JY, et al. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc. 2015;81:333–341.e1. doi: 10.1016/j.gie.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 17.Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim DH, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942–948. doi: 10.1016/j.gie.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 18.Hahn KY, Park CH, Lee YK, Chung H, Park JC, Shin SK, et al. Comparative study between endoscopic submucosal dissection and surgery in patients with early gastric cancer. Surg Endosc. 2018;32:73–86. doi: 10.1007/s00464-017-5640-8. [DOI] [PubMed] [Google Scholar]
- 19.Song M, Choi JY, Yang JJ, Sung H, Lee Y, Lee HW, et al. Obesity at adolescence and gastric cancer risk. Cancer Causes Control. 2015;26:247–256. doi: 10.1007/s10552-014-0506-z. [DOI] [PubMed] [Google Scholar]
- 20.Kim YI, Kim YA, Kim CG, Ryu KW, Kim YW, Sim JA, et al. Serial intermediate-term quality of life comparison after endoscopic submucosal dissection versus surgery in early gastric cancer patients. Surg Endosc. 2018;32:2114–2122. doi: 10.1007/s00464-017-5909-y. [DOI] [PubMed] [Google Scholar]
- 21.Honda M, Hiki N, Kinoshita T, Yabusaki H, Abe T, Nunobe S, et al. Long-term outcomes of laparoscopic versus open surgery for clinical stage I gastric cancer: the LOC-1 study. Ann Surg. 2016;264:214–222. doi: 10.1097/SLA.0000000000001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32:627–633. doi: 10.1200/JCO.2013.48.8551. [DOI] [PubMed] [Google Scholar]
- 23.Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01) Ann Surg. 2016;263:28–35. doi: 10.1097/SLA.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Choi KD, Hong SM, Park SH, Gong EJ, Na HK, et al. Pattern of extragastric recurrence and the role of abdominal computed tomography in surveillance after endoscopic resection of early gastric cancer: Korean experiences. Gastric Cancer. 2017;20:843–852. doi: 10.1007/s10120-017-0691-z. [DOI] [PubMed] [Google Scholar]
- 25.Min BH, Kim ER, Kim KM, Park CK, Lee JH, Rhee PL, et al. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2015;47:784–793. doi: 10.1055/s-0034-1392249. [DOI] [PubMed] [Google Scholar]
- 26.Oda I, Oyama T, Abe S, Ohnita K, Kosaka T, Hirasawa K, et al. Preliminary results of multicenter questionnaire study on long-term outcomes of curative endoscopic submucosal dissection for early gastric cancer. Dig Endosc. 2014;26:214–219. doi: 10.1111/den.12141. [DOI] [PubMed] [Google Scholar]
- 27.Kim TS, Min BH, Kim KM, Lee JH, Rhee PL, Kim JJ. Endoscopic submucosal dissection for papillary adenocarcinoma of the stomach: low curative resection rate but favorable long-term outcomes after curative resection. Gastric Cancer. 2019;22:363–368. doi: 10.1007/s10120-018-0857-3. [DOI] [PubMed] [Google Scholar]
- 28.Chang JY, Shim KN, Tae CH, Lee KE, Lee J, Lee KH, et al. Comparison of clinical outcomes after endoscopic submucosal dissection and surgery in the treatment of early gastric cancer: a single-institute study. Medicine (Baltimore) 2017;96:e7210. doi: 10.1097/MD.0000000000007210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu PW, Teoh AY, To KF, Wong SK, Liu SY, Lam CC, et al. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc. 2012;26:3584–3591. doi: 10.1007/s00464-012-2371-8. [DOI] [PubMed] [Google Scholar]
- 30.Cho JH, Cha SW, Kim HG, Lee TH, Cho JY, Ko WJ, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc. 2016;30:3762–3773. doi: 10.1007/s00464-015-4672-1. [DOI] [PubMed] [Google Scholar]
- 31.Feng F, Sun L, Xu G, Cai L, Hong L, Yang J, et al. Is it reasonable to treat early gastric cancer with mucosal infiltration and well differentiation by endoscopic submucosal resection? J Gastrointest Surg. 2015;19:2111–2119. doi: 10.1007/s11605-015-2932-y. [DOI] [PubMed] [Google Scholar]
- 32.Fukunaga S, Nagami Y, Shiba M, Ominami M, Tanigawa T, Yamagami H, et al. Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest Endosc. 2017;85:143–152. doi: 10.1016/j.gie.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 33.Gong EJ, Kim DH, Ahn JY, Jung KW, Lee JH, Choi KD, et al. Comparison of long-term outcomes of endoscopic submucosal dissection and surgery for esophagogastric junction adenocarcinoma. Gastric Cancer. 2017;20:84–91. doi: 10.1007/s10120-016-0679-0. [DOI] [PubMed] [Google Scholar]
- 34.Kim YI, Kim YW, Choi IJ, Kim CG, Lee JY, Cho SJ, et al. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy. 2015;47:293–301. doi: 10.1055/s-0034-1391284. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Choi KD, Han M, Na HK, Ahn JY, Jung KW, et al. Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer meeting expanded indication including undifferentiated-type tumors: a criteria-based analysis. Gastric Cancer. 2018;21:490–499. doi: 10.1007/s10120-017-0772-z. [DOI] [PubMed] [Google Scholar]
- 36.Park CH, Lee H, Kim DW, Chung H, Park JC, Shin SK, et al. Clinical safety of endoscopic submucosal dissection compared with surgery in elderly patients with early gastric cancer: a propensity-matched analysis. Gastrointest Endosc. 2014;80:599–609. doi: 10.1016/j.gie.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 37.Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, et al. Long-term outcome of endoscopic resection vs. surgery for early gastric cancer: a non-inferiority-matched cohort study. Am J Gastroenterol. 2016;111:240–249. doi: 10.1038/ajg.2015.427. [DOI] [PubMed] [Google Scholar]
- 38.Shin DW, Hwang HY, Jeon SW. Comparison of endoscopic submucosal dissection and surgery for differentiated type early gastric cancer within the expanded criteria. Clin Endosc. 2017;50:170–178. doi: 10.5946/ce.2016.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eom BW, Kim YI, Kim KH, Yoon HM, Cho SJ, Lee JY, et al. Survival benefit of additional surgery after noncurative endoscopic resection in patients with early gastric cancer. Gastrointest Endosc. 2017;85:155–163.e3. doi: 10.1016/j.gie.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 40.Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol. 2017;52:175–184. doi: 10.1007/s00535-016-1210-4. [DOI] [PubMed] [Google Scholar]
- 41.Kim ER, Lee H, Min BH, Lee JH, Rhee PL, Kim JJ, et al. Effect of rescue surgery after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2015;102:1394–1401. doi: 10.1002/bjs.9873. [DOI] [PubMed] [Google Scholar]
- 42.Kusano C, Iwasaki M, Kaltenbach T, Conlin A, Oda I, Gotoda T. Should elderly patients undergo additional surgery after non-curative endoscopic resection for early gastric cancer? Long-term comparative outcomes. Am J Gastroenterol. 2011;106:1064–1069. doi: 10.1038/ajg.2011.49. [DOI] [PubMed] [Google Scholar]
- 43.Kim YI, Kim HS, Kook MC, Cho SJ, Lee JY, Kim CG, et al. Discrepancy between clinical and final pathological evaluation findings in early gastric cancer patients treated with endoscopic submucosal dissection. J Gastric Cancer. 2016;16:34–42. doi: 10.5230/jgc.2016.16.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, et al. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703–707. doi: 10.1055/s-0033-1344396. [DOI] [PubMed] [Google Scholar]
- 45.Ahn JY, Park HJ, Park YS, Lee JH, Choi KS, Jeong KW, et al. Endoscopic resection for undifferentiated-type early gastric cancer: immediate endoscopic outcomes and long-term survivals. Dig Dis Sci. 2016;61:1158–1164. doi: 10.1007/s10620-015-3988-y. [DOI] [PubMed] [Google Scholar]
- 46.Kim JH, Kim YH, Jung DH, Jeon HH, Lee YC, Lee H, et al. Follow-up outcomes of endoscopic resection for early gastric cancer with undifferentiated histology. Surg Endosc. 2014;28:2627–2633. doi: 10.1007/s00464-014-3514-x. [DOI] [PubMed] [Google Scholar]
- 47.Oka S, Tanaka S, Higashiyama M, Numata N, Sanomura Y, Yoshida S, et al. Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg Endosc. 2014;28:639–647. doi: 10.1007/s00464-013-3222-y. [DOI] [PubMed] [Google Scholar]
- 48.Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, et al. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122–127. doi: 10.1055/s-0031-1291486. [DOI] [PubMed] [Google Scholar]
- 49.Park JC, Lee YK, Kim SY, Roh Y, Hahn KY, Shin SK, et al. Long-term outcomes of endoscopic submucosal dissection in comparison to surgery in undifferentiated-type intramucosal gastric cancer using propensity score analysis. Surg Endosc. 2018;32:2046–2057. doi: 10.1007/s00464-017-5901-6. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki S, Gotoda T, Hatta W, Oyama T, Kawata N, Takahashi A, et al. Survival benefit of additional surgery after non-curative endoscopic submucosal dissection for early gastric cancer: a propensity score matching analysis. Ann Surg Oncol. 2017;24:3353–3360. doi: 10.1245/s10434-017-6039-4. [DOI] [PubMed] [Google Scholar]
- 51.Noh GY, Ku HR, Kim YJ, Park SC, Kim J, Han CJ, et al. Clinical outcomes of early gastric cancer with lymphovascular invasion or positive vertical resection margin after endoscopic submucosal dissection. Surg Endosc. 2015;29:2583–2589. doi: 10.1007/s00464-014-3973-0. [DOI] [PubMed] [Google Scholar]
- 52.Choi JY, Jeon SW, Cho KB, Park KS, Kim ES, Park CK, et al. Non-curative endoscopic resection does not always lead to grave outcomes in submucosal invasive early gastric cancer. Surg Endosc. 2015;29:1842–1849. doi: 10.1007/s00464-014-3874-2. [DOI] [PubMed] [Google Scholar]
- 53.Toya Y, Endo M, Nakamura S, Akasaka R, Kosaka T, Yanai S, et al. Clinical outcomes of non-curative endoscopic submucosal dissection with negative resected margins for gastric cancer. Gastrointest Endosc. 2017;85:1218–1224. doi: 10.1016/j.gie.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Jung DH, Lee YC, Kim JH, Lee SK, Shin SK, Park JC, et al. Additive treatment improves survival in elderly patients after non-curative endoscopic resection for early gastric cancer. Surg Endosc. 2017;31:1376–1382. doi: 10.1007/s00464-016-5123-3. [DOI] [PubMed] [Google Scholar]
- 55.Kawata N, Kakushima N, Takizawa K, Tanaka M, Makuuchi R, Tokunaga M, et al. Risk factors for lymph node metastasis and long-term outcomes of patients with early gastric cancer after non-curative endoscopic submucosal dissection. Surg Endosc. 2017;31:1607–1616. doi: 10.1007/s00464-016-5148-7. [DOI] [PubMed] [Google Scholar]
- 56.Yano T, Ishido K, Tanabe S, Wada T, Azuma M, Kawanishi N, et al. Long-term outcomes of patients with early gastric cancer found to have lesions for which endoscopic treatment is not indicated on histopathological evaluation after endoscopic submucosal dissection. Surg Endosc. 2018;32:1314–1323. doi: 10.1007/s00464-017-5809-1. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki H, Oda I, Abe S, Sekiguchi M, Nonaka S, Yoshinaga S, et al. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer. 2017;20:679–689. doi: 10.1007/s10120-016-0651-z. [DOI] [PubMed] [Google Scholar]
- 58.Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Crose N, et al. Total versus subtotal gastrectomy: surgical morbidity and mortality rates in a multicenter Italian randomized trial. The Italian Gastrointestinal Tumor Study Group. Ann Surg. 1997;226:613–620. doi: 10.1097/00000658-199711000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170–178. doi: 10.1097/00000658-199908000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gouzi JL, Huguier M, Fagniez PL, Launois B, Flamant Y, Lacaine F, et al. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg. 1989;209:162–166. doi: 10.1097/00000658-198902000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R, et al. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449–1472. doi: 10.1136/gut.2010.228254. [DOI] [PubMed] [Google Scholar]
- 62.Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi57–vi63. doi: 10.1093/annonc/mdt344. [DOI] [PubMed] [Google Scholar]
- 63.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seevaratnam R, Bocicariu A, Cardoso R, Mahar A, Kiss A, Helyer L, et al. A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer. 2012;15(Suppl 1):S60–S69. doi: 10.1007/s10120-011-0110-9. [DOI] [PubMed] [Google Scholar]
- 65.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 66.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309–315. doi: 10.1016/S1470-2045(06)70623-4. [DOI] [PubMed] [Google Scholar]
- 67.Kitamura K, Yamaguchi T, Nishida S, Yamamoto K, Ichikawa D, Okamoto K, et al. The operative indications for proximal gastrectomy in patients with gastric cancer in the upper third of the stomach. Surg Today. 1997;27:993–998. doi: 10.1007/BF02385777. [DOI] [PubMed] [Google Scholar]
- 68.Yoo CH, Sohn BH, Han WK, Pae WK. Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat. 2004;36:50–55. doi: 10.4143/crt.2004.36.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.An JY, Youn HG, Choi MG, Noh JH, Sohn TS, Kim S. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg. 2008;196:587–591. doi: 10.1016/j.amjsurg.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 70.Ahn SH, Lee JH, Park DJ, Kim HH. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer. 2013;16:282–289. doi: 10.1007/s10120-012-0178-x. [DOI] [PubMed] [Google Scholar]
- 71.Masuzawa T, Takiguchi S, Hirao M, Imamura H, Kimura Y, Fujita J, et al. Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: a multi-institutional retrospective study. World J Surg. 2014;38:1100–1106. doi: 10.1007/s00268-013-2370-5. [DOI] [PubMed] [Google Scholar]
- 72.Huh YJ, Lee HJ, Oh SY, Lee KG, Yang JY, Ahn HS, et al. Clinical outcome of modified laparoscopy-assisted proximal gastrectomy compared to conventional proximal gastrectomy or total gastrectomy for upper-third early gastric cancer with special references to postoperative reflux esophagitis. J Gastric Cancer. 2015;15:191–200. doi: 10.5230/jgc.2015.15.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung DH, Lee Y, Kim DW, Park YS, Ahn SH, Park DJ, et al. Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proximal early gastric cancer. Surg Endosc. 2017;31:3961–3969. doi: 10.1007/s00464-017-5429-9. [DOI] [PubMed] [Google Scholar]
- 74.Toyomasu Y, Ogata K, Suzuki M, Yanoma T, Kimura A, Kogure N, et al. Restoration of gastrointestinal motility ameliorates nutritional deficiencies and body weight loss of patients who undergo laparoscopy-assisted proximal gastrectomy. Surg Endosc. 2017;31:1393–1401. doi: 10.1007/s00464-016-5127-z. [DOI] [PubMed] [Google Scholar]
- 75.Yoo CH, Sohn BH, Han WK, Pae WK. Proximal gastrectomy reconstructed by jejunal pouch interposition for upper third gastric cancer: prospective randomized study. World J Surg. 2005;29:1592–1599. doi: 10.1007/s00268-005-7793-1. [DOI] [PubMed] [Google Scholar]
- 76.Ohashi M, Morita S, Fukagawa T, Oda I, Kushima R, Katai H. Functional advantages of proximal gastrectomy with jejunal interposition over total gastrectomy with Roux-en-Y esophagojejunostomy for early gastric cancer. World J Surg. 2015;39:2726–2733. doi: 10.1007/s00268-015-3180-8. [DOI] [PubMed] [Google Scholar]
- 77.Ushimaru Y, Fujiwara Y, Shishido Y, Yanagimoto Y, Moon JH, Sugimura K, et al. Clinical outcomes of gastric cancer patients who underwent proximal or total gastrectomy: a propensity score-matched analysis. World J Surg. 2018;42:1477–1484. doi: 10.1007/s00268-017-4306-y. [DOI] [PubMed] [Google Scholar]
- 78.Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18:407–416. doi: 10.1007/s10120-014-0377-8. [DOI] [PubMed] [Google Scholar]
- 79.Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukuda K, et al. Evaluation of a pylorus-preserving gastrectomy for patients preoperatively diagnosed with early gastric cancer located in the middle third of the stomach. Surg Today. 2010;40:228–233. doi: 10.1007/s00595-009-4043-4. [DOI] [PubMed] [Google Scholar]
- 80.Suh YS, Han DS, Kong SH, Kwon S, Shin CI, Kim WH, et al. Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg. 2014;259:485–493. doi: 10.1097/SLA.0b013e318294d142. [DOI] [PubMed] [Google Scholar]
- 81.Xiao XM, Gaol C, Yin W, Yu WH, Qi F, Liu T. Pylorus-preserving versus distal subtotal gastrectomy for surgical treatment of early gastric cancer: a meta-analysis. Hepatogastroenterology. 2014;61:870–879. [PubMed] [Google Scholar]
- 82.Aizawa M, Honda M, Hiki N, Kinoshita T, Yabusaki H, Nunobe S, et al. Oncological outcomes of function-preserving gastrectomy for early gastric cancer: a multicenter propensity score matched cohort analysis comparing pylorus-preserving gastrectomy versus conventional distal gastrectomy. Gastric Cancer. 2017;20:709–717. doi: 10.1007/s10120-016-0644-y. [DOI] [PubMed] [Google Scholar]
- 83.Nakane Y, Akehira K, Inoue K, Iiyama H, Sato M, Masuya Y, et al. Postoperative evaluation of pylorus-preserving gastrectomy for early gastric cancer. Hepatogastroenterology. 2000;47:590–595. [PubMed] [Google Scholar]
- 84.Smith JW, Brennan MF. Surgical treatment of gastric cancer. Proximal, mid, and distal stomach. Surg Clin North Am. 1992;72:381–399. doi: 10.1016/s0039-6109(16)45685-9. [DOI] [PubMed] [Google Scholar]
- 85.Cai Z, Zhou Y, Wang C, Yin Y, Yin Y, Shen C, et al. Optimal reconstruction methods after distal gastrectomy for gastric cancer: a systematic review and network meta-analysis. Medicine (Baltimore) 2018;97:e10823. doi: 10.1097/MD.0000000000010823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, et al. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539–1547. doi: 10.1007/s00464-011-2064-8. [DOI] [PubMed] [Google Scholar]
- 87.Inokuchi M, Kojima K, Yamada H, Kato K, Hayashi M, Motoyama K, et al. Long-term outcomes of Roux-en-Y and Billroth-I reconstruction after laparoscopic distal gastrectomy. Gastric Cancer. 2013;16:67–73. doi: 10.1007/s10120-012-0154-5. [DOI] [PubMed] [Google Scholar]
- 88.Tanigawa N, Nomura E, Lee SW, Kaminishi M, Sugiyama M, Aikou T, et al. Current state of gastric stump carcinoma in Japan: based on the results of a nationwide survey. World J Surg. 2010;34:1540–1547. doi: 10.1007/s00268-010-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Folli S, Morgagni P, Roviello F, De Manzoni G, Marrelli D, Saragoni L, et al. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC) Jpn J Clin Oncol. 2001;31:495–499. doi: 10.1093/jjco/hye107. [DOI] [PubMed] [Google Scholar]
- 90.Degiuli M, Calvo F. Survival of early gastric cancer in a specialized European center. Which lymphadenectomy is necessary? World J Surg. 2006;30:2193–2203. doi: 10.1007/s00268-006-0179-1. [DOI] [PubMed] [Google Scholar]
- 91.Shimoyama S, Yasuda H, Mafune K, Kaminishi M. Indications of a minimized scope of lymphadenectomy for submucosal gastric cancer. Ann Surg Oncol. 2002;9:625–631. doi: 10.1007/BF02574477. [DOI] [PubMed] [Google Scholar]
- 92.Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery. 2002;131:401–407. doi: 10.1067/msy.2002.121891. [DOI] [PubMed] [Google Scholar]
- 93.Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006;93:559–563. doi: 10.1002/bjs.5353. [DOI] [PubMed] [Google Scholar]
- 94.Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017;265:277–283. doi: 10.1097/SLA.0000000000001814. [DOI] [PubMed] [Google Scholar]
- 95.Brar SS, Seevaratnam R, Cardoso R, Law C, Helyer L, Coburn N. A systematic review of spleen and pancreas preservation in extended lymphadenectomy for gastric cancer. Gastric Cancer. 2012;15(Suppl 1):S89–S99. doi: 10.1007/s10120-011-0087-4. [DOI] [PubMed] [Google Scholar]
- 96.Yang K, Chen XZ, Hu JK, Zhang B, Chen ZX, Chen JP. Effectiveness and safety of splenectomy for gastric carcinoma: a meta-analysis. World J Gastroenterol. 2009;15:5352–5359. doi: 10.3748/wjg.15.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee IS, Ahn JY, Yook JH, Kim BS. Mediastinal lymph node dissection and distal esophagectomy is not essential in early esophagogastric junction adenocarcinoma. World J Surg Oncol. 2017;15:28. doi: 10.1186/s12957-016-1088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 99.Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol. 2006;7:644–651. doi: 10.1016/S1470-2045(06)70766-5. [DOI] [PubMed] [Google Scholar]
- 100.Omloo JM, Lagarde SM, Hulscher JB, Reitsma JB, Fockens P, van Dekken H, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246:992–1000. doi: 10.1097/SLA.0b013e31815c4037. [DOI] [PubMed] [Google Scholar]
- 101.Kurokawa Y, Sasako M, Sano T, Yoshikawa T, Iwasaki Y, Nashimoto A, et al. Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br J Surg. 2015;102:341–348. doi: 10.1002/bjs.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davies AR, Sandhu H, Pillai A, Sinha P, Mattsson F, Forshaw MJ, et al. Surgical resection strategy and the influence of radicality on outcomes in oesophageal cancer. Br J Surg. 2014;101:511–517. doi: 10.1002/bjs.9456. [DOI] [PubMed] [Google Scholar]
- 103.Zheng Z, Cai J, Yin J, Zhang J, Zhang ZT, Wang KL. Transthoracic versus abdominal-transhiatal resection for treating Siewert type II/III adenocarcinoma of the esophagogastric junction: a meta-analysis. Int J Clin Exp Med. 2015;8:17167–17182. [PMC free article] [PubMed] [Google Scholar]
- 104.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 105.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 106.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 107.Kim YW, Yoon HM, Yun YH, Nam BH, Eom BW, Baik YH, et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy for early gastric cancer: result of a randomized controlled trial (COACT 0301) Surg Endosc. 2013;27:4267–4276. doi: 10.1007/s00464-013-3037-x. [DOI] [PubMed] [Google Scholar]
- 108.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 109.Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Watanabe M, et al. Laparoscopy versus open distal gastrectomy by expert surgeons for early gastric cancer in Japanese patients: short-term clinical outcomes of a randomized clinical trial. Surg Endosc. 2013;27:1695–1705. doi: 10.1007/s00464-012-2658-9. [DOI] [PubMed] [Google Scholar]
- 110.Takiguchi S, Fujiwara Y, Yamasaki M, Miyata H, Nakajima K, Sekimoto M, et al. Laparoscopy-assisted distal gastrectomy versus open distal gastrectomy. A prospective randomized single-blind study. World J Surg. 2013;37:2379–2386. doi: 10.1007/s00268-013-2121-7. [DOI] [PubMed] [Google Scholar]
- 111.Yamashita K, Sakuramoto S, Kikuchi S, Futawatari N, Katada N, Hosoda K, et al. Laparoscopic versus open distal gastrectomy for early gastric cancer in Japan: long-term clinical outcomes of a randomized clinical trial. Surg Today. 2016;46:741–749. doi: 10.1007/s00595-015-1221-4. [DOI] [PubMed] [Google Scholar]
- 112.Lu C, Zhou S, Peng Z, Chen L. Quality of D2 lymphadenectomy for advanced gastric cancer: is laparoscopic-assisted distal gastrectomy as effective as open distal gastrectomy? Surg Endosc. 2015;29:1537–1544. doi: 10.1007/s00464-014-3838-6. [DOI] [PubMed] [Google Scholar]
- 113.Zou ZH, Zhao LY, Mou TY, Hu YF, Yu J, Liu H, et al. Laparoscopic vs open D2 gastrectomy for locally advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2014;20:16750–16764. doi: 10.3748/wjg.v20.i44.16750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choi YY, Bae JM, An JY, Hyung WJ, Noh SH. Laparoscopic gastrectomy for advanced gastric cancer: are the long-term results comparable with conventional open gastrectomy? A systematic review and meta-analysis. J Surg Oncol. 2013;108:550–556. doi: 10.1002/jso.23438. [DOI] [PubMed] [Google Scholar]
- 115.Chen K, Xu XW, Mou YP, Pan Y, Zhou YC, Zhang RC, et al. Systematic review and meta-analysis of laparoscopic and open gastrectomy for advanced gastric cancer. World J Surg Oncol. 2013;11:182. doi: 10.1186/1477-7819-11-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martínez-Ramos D, Miralles-Tena JM, Cuesta MA, Escrig-Sos J, Van der Peet D, Hoashi JS, et al. Laparoscopy versus open surgery for advanced and resectable gastric cancer: a meta-analysis. Rev Esp Enferm Dig. 2011;103:133–141. doi: 10.4321/s1130-01082011000300005. [DOI] [PubMed] [Google Scholar]
- 117.Wang W, Li Z, Tang J, Wang M, Wang B, Xu Z. Laparoscopic versus open total gastrectomy with D2 dissection for gastric cancer: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:1721–1734. doi: 10.1007/s00432-013-1462-9. [DOI] [PubMed] [Google Scholar]
- 118.Ding J, Liao GQ, Liu HL, Liu S, Tang J. Meta-analysis of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer. J Surg Oncol. 2012;105:297–303. doi: 10.1002/jso.22098. [DOI] [PubMed] [Google Scholar]
- 119.Park YK, Yoon HM, Kim YW, Park JY, Ryu KW, Lee YJ, et al. Laparoscopy-assisted versus open D2 distal gastrectomy for advanced gastric cancer: results from a randomized phase II multicenter clinical trial (COACT 1001) Ann Surg. 2018;267:638–645. doi: 10.1097/SLA.0000000000002168. [DOI] [PubMed] [Google Scholar]
- 120.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28:331–337. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 122.Hur H, Lee HY, Lee HJ, Kim MC, Hyung WJ, Park YK, et al. Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer. 2015;15:355. doi: 10.1186/s12885-015-1365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34:1350–1357. doi: 10.1200/JCO.2015.63.7215. [DOI] [PubMed] [Google Scholar]
- 124.Cui M, Li Z, Xing J, Yao Z, Liu M, Chen L, et al. A prospective randomized clinical trial comparing D2 dissection in laparoscopic and open gastrectomy for gastric cancer. Med Oncol. 2015;32:241. doi: 10.1007/s12032-015-0680-1. [DOI] [PubMed] [Google Scholar]
- 125.Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, et al. A multi-institutional, prospective, phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901) World J Surg. 2015;39:2734–2741. doi: 10.1007/s00268-015-3160-z. [DOI] [PubMed] [Google Scholar]
- 126.Suda K, Man-I M, Ishida Y, Kawamura Y, Satoh S, Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. 2015;29:673–685. doi: 10.1007/s00464-014-3718-0. [DOI] [PubMed] [Google Scholar]
- 127.Yang SY, Roh KH, Kim YN, Cho M, Lim SH, Son T, et al. Surgical outcomes after open, laparoscopic, and robotic gastrectomy for gastric cancer. Ann Surg Oncol. 2017;24:1770–1777. doi: 10.1245/s10434-017-5851-1. [DOI] [PubMed] [Google Scholar]
- 128.Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. 2009;249:927–932. doi: 10.1097/01.sla.0000351688.64999.73. [DOI] [PubMed] [Google Scholar]
- 129.Murphy R, Evennett NJ, Clarke MG, Robinson SJ, Humphreys L, Jones B, et al. Sleeve gastrectomy versus Roux-en-Y gastric bypass for type 2 diabetes and morbid obesity: double-blind randomised clinical trial protocol. BMJ Open. 2016;6:e011416. doi: 10.1136/bmjopen-2016-011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Noshiro H, Ikeda O, Urata M. Robotically-enhanced surgical anatomy enables surgeons to perform distal gastrectomy for gastric cancer using electric cautery devices alone. Surg Endosc. 2014;28:1180–1187. doi: 10.1007/s00464-013-3304-x. [DOI] [PubMed] [Google Scholar]
- 131.Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc. 2014;28:2606–2615. doi: 10.1007/s00464-014-3511-0. [DOI] [PubMed] [Google Scholar]
- 132.Junfeng Z, Yan S, Bo T, Yingxue H, Dongzhu Z, Yongliang Z, et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc. 2014;28:1779–1787. doi: 10.1007/s00464-013-3385-6. [DOI] [PubMed] [Google Scholar]
- 133.Lim DH, Kim DY, Kang MK, Kim YI, Kang WK, Park CK, et al. Patterns of failure in gastric carcinoma after D2 gastrectomy and chemoradiotherapy: a radiation oncologist's view. Br J Cancer. 2004;91:11–17. doi: 10.1038/sj.bjc.6601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Al-Batran SE, Homann N, Schmalenberg H, Kopp HG, Haag GM, Luley KB, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. J Clin Oncol. 2017;35(15_suppl):4004. [Google Scholar]
- 135.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 136.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 137.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 138.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma - 2nd English edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 139.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, editors. AJCC Cancer Staging Manual. 6th ed. Chicago (IL): Springer; 2002. [Google Scholar]
- 140.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 141.Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–1396. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 142.Chang JS, Lim JS, Noh SH, Hyung WJ, An JY, Lee YC, et al. Patterns of regional recurrence after curative D2 resection for stage III (N3) gastric cancer: implications for postoperative radiotherapy. Radiother Oncol. 2012;104:367–373. doi: 10.1016/j.radonc.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 143.Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1–11. doi: 10.1016/0360-3016(82)90377-7. [DOI] [PubMed] [Google Scholar]
- 144.Moertel CG, Childs DS, O'Fallon JR, Holbrook MA, Schutt AJ, Reitemeier RJ. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol. 1984;2:1249–1254. doi: 10.1200/JCO.1984.2.11.1249. [DOI] [PubMed] [Google Scholar]
- 145.Landry J, Tepper JE, Wood WC, Moulton EO, Koerner F, Sullinger J. Patterns of failure following curative resection of gastric carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:1357–1362. doi: 10.1016/0360-3016(90)90344-j. [DOI] [PubMed] [Google Scholar]
- 146.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 147.Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shim JH, Song KY, Jeon HM, Park CH, Jacks LM, Gonen M, et al. Is gastric cancer different in Korea and the United States? Impact of tumor location on prognosis. Ann Surg Oncol. 2014;21:2332–2339. doi: 10.1245/s10434-014-3608-7. [DOI] [PubMed] [Google Scholar]
- 149.Dikken JL, Jansen EP, Cats A, Bakker B, Hartgrink HH, Kranenbarg EM, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28:2430–2436. doi: 10.1200/JCO.2009.26.9654. [DOI] [PubMed] [Google Scholar]
- 150.Bamias A, Karina M, Papakostas P, Kostopoulos I, Bobos M, Vourli G, et al. A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother Pharmacol. 2010;65:1009–1021. doi: 10.1007/s00280-010-1256-6. [DOI] [PubMed] [Google Scholar]
- 151.Kim TH, Park SR, Ryu KW, Kim YW, Bae JM, Lee JH, et al. Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int J Radiat Oncol Biol Phys. 2012;84:e585–e592. doi: 10.1016/j.ijrobp.2012.07.2378. [DOI] [PubMed] [Google Scholar]
- 152.Kwon HC, Kim MC, Kim KH, Jang JS, Oh SY, Kim SH, et al. Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac J Clin Oncol. 2010;6:278–285. doi: 10.1111/j.1743-7563.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 153.Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 154.Zhu WG, Xua DF, Pu J, Zong CD, Li T, Tao GZ, et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol. 2012;104:361–366. doi: 10.1016/j.radonc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 155.Dai Q, Jiang L, Lin RJ, Wei KK, Gan LL, Deng CH, et al. Adjuvant chemoradiotherapy versus chemotherapy for gastric cancer: a meta-analysis of randomized controlled trials. J Surg Oncol. 2015;111:277–284. doi: 10.1002/jso.23795. [DOI] [PubMed] [Google Scholar]
- 155.Huang YY, Yang Q, Zhou SW, Wei Y, Chen YX, Xie DR, et al. Postoperative chemoradiotherapy versus postoperative chemotherapy for completely resected gastric cancer with D2 Lymphadenectomy: a meta-analysis. PLoS One. 2013;8:e68939. doi: 10.1371/journal.pone.0068939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Min C, Bangalore S, Jhawar S, Guo Y, Nicholson J, Formenti SC, et al. Chemoradiation therapy versus chemotherapy alone for gastric cancer after R0 surgical resection: a meta-analysis of randomized trials. Oncology. 2014;86:79–85. doi: 10.1159/000354641. [DOI] [PubMed] [Google Scholar]
- 158.Soon YY, Leong CN, Tey JC, Tham IW, Lu JJ. Postoperative chemo-radiotherapy versus chemotherapy for resected gastric cancer: a systematic review and meta-analysis. J Med Imaging Radiat Oncol. 2014;58:483–496. doi: 10.1111/1754-9485.12190. [DOI] [PubMed] [Google Scholar]
- 159.Valentini V, Cellini F, Minsky BD, Mattiucci GC, Balducci M, D'Agostino G, et al. Survival after radiotherapy in gastric cancer: systematic review and meta-analysis. Radiother Oncol. 2009;92:176–183. doi: 10.1016/j.radonc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 160.Zhou ML, Kang M, Li GC, Guo XM, Zhang Z. Postoperative chemoradiotherapy versus chemotherapy for R0 resected gastric cancer with D2 lymph node dissection: an up-to-date meta-analysis. World J Surg Oncol. 2016;14:209. doi: 10.1186/s12957-016-0957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol. 2015;33:3130–3136. doi: 10.1200/JCO.2014.58.3930. [DOI] [PubMed] [Google Scholar]
- 162.Yu JI, Lim DH, Ahn YC, Lee J, Kang WK, Park SH, et al. Effects of adjuvant radiotherapy on completely resected gastric cancer: a radiation oncologist's view of the ARTIST randomized phase III trial. Radiother Oncol. 2015;117:171–177. doi: 10.1016/j.radonc.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 163.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 164.Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer: Japan Clinical Oncology Group study (JCOG0501) J Clin Oncol. 2018;36(15_suppl):4046. doi: 10.1007/s10120-019-00941-z. [DOI] [PubMed] [Google Scholar]
- 165.Klevebro F, Alexandersson von Döbeln G, Wang N, Johnsen G, Jacobsen AB, Friesland S, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol. 2016;27:660–667. doi: 10.1093/annonc/mdw010. [DOI] [PubMed] [Google Scholar]
- 166.Leong T, Smithers BM, Haustermans K, Michael M, Gebski V, Miller D, et al. TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol. 2017;24:2252–2258. doi: 10.1245/s10434-017-5830-6. [DOI] [PubMed] [Google Scholar]
- 167.Stahl M, Walz MK, Riera-Knorrenschild J, Stuschke M, Sandermann A, Bitzer M, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183–190. doi: 10.1016/j.ejca.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 168.Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 169.Fu T, Bu ZD, Li ZY, Zhang LH, Wu XJ, Wu AW, et al. Neoadjuvant chemoradiation therapy for resectable esophago-gastric adenocarcinoma: a meta-analysis of randomized clinical trials. BMC Cancer. 2015;15:322. doi: 10.1186/s12885-015-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Lind PA, Lindblad M, et al. Survival benefit and additional value of preoperative chemoradiotherapy in resectable gastric and gastro-oesophageal junction cancer: a direct and adjusted indirect comparison meta-analysis. Eur J Surg Oncol. 2015;41:282–294. doi: 10.1016/j.ejso.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 171.Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, et al. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer. 2013;49:3149–3158. doi: 10.1016/j.ejca.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 172.Hofheinz R, Clouth J, Borchardt-Wagner J, Wagner U, Weidling E, Jen MH, et al. Patient preferences for palliative treatment of locally advanced or metastatic gastric cancer and adenocarcinoma of the gastroesophageal junction: a choice-based conjoint analysis study from Germany. BMC Cancer. 2016;16:937. doi: 10.1186/s12885-016-2975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hsu JT, Liao JA, Chuang HC, Chen TD, Chen TH, Kuo CJ, et al. Palliative gastrectomy is beneficial in selected cases of metastatic gastric cancer. BMC Palliat Care. 2017;16:19. doi: 10.1186/s12904-017-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dong Y, Ma S, Yang S, Luo F, Wang Z, Guo F. Non-curative surgery for patients with gastric cancer with local peritoneal metastasis: a retrospective cohort study. Medicine (Baltimore) 2016;95:e5607. doi: 10.1097/MD.0000000000005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang K, Liu K, Zhang WH, Lu ZH, Chen XZ, Chen XL, et al. The value of palliative gastrectomy for gastric cancer patients with intraoperatively proven peritoneal seeding. Medicine (Baltimore) 2015;94:e1051. doi: 10.1097/MD.0000000000001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Naka T, Iwahashi M, Nakamori M, Nakamura M, Ojima T, Iida T, et al. The evaluation of surgical treatment for gastric cancer patients with noncurative resection. Langenbecks Arch Surg. 2012;397:959–966. doi: 10.1007/s00423-012-0906-z. [DOI] [PubMed] [Google Scholar]
- 177.Sougioultzis S, Syrios J, Xynos ID, Bovaretos N, Kosmas C, Sarantonis J, et al. Palliative gastrectomy and other factors affecting overall survival in stage IV gastric adenocarcinoma patients receiving chemotherapy: a retrospective analysis. Eur J Surg Oncol. 2011;37:312–318. doi: 10.1016/j.ejso.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 178.Zhu G, Zhang M, Zhang H, Gao H, Xue Y. Survival predictors of patients with gastric cancer with peritoneal metastasis. Hepatogastroenterology. 2010;57:997–1000. [PubMed] [Google Scholar]
- 179.Kim KH, Lee KW, Baek SK, Chang HJ, Kim YJ, Park DJ, et al. Survival benefit of gastrectomy ± metastasectomy in patients with metastatic gastric cancer receiving chemotherapy. Gastric Cancer. 2011;14:130–138. doi: 10.1007/s10120-011-0015-7. [DOI] [PubMed] [Google Scholar]
- 180.Li SC, Lee CH, Hung CL, Wu JC, Chen JH. Surgical resection of metachronous hepatic metastases from gastric cancer improves long-term survival: a population-based study. PLoS One. 2017;12:e0182255. doi: 10.1371/journal.pone.0182255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Markar SR, Mackenzie H, Mikhail S, Mughal M, Preston SR, Maynard ND, et al. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England. Gastric Cancer. 2017;20:379–386. doi: 10.1007/s10120-016-0604-6. [DOI] [PubMed] [Google Scholar]
- 182.Saito A, Korenaga D, Sakaguchi Y, Ohno S, Ichiyoshi Y, Sugimachi K. Surgical treatment for gastric carcinomas with concomitant hepatic metastasis. Hepatogastroenterology. 1996;43:560–564. [PubMed] [Google Scholar]
- 183.Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19:1146–1153. doi: 10.1093/annonc/mdn026. [DOI] [PubMed] [Google Scholar]
- 184.Okumura Y, Yamashita H, Aikou S, Yagi K, Yamagata Y, Nishida M, et al. Palliative distal gastrectomy offers no survival benefit over gastrojejunostomy for gastric cancer with outlet obstruction: retrospective analysis of an 11-year experience. World J Surg Oncol. 2014;12:364. doi: 10.1186/1477-7819-12-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tokunaga M, Terashima M, Tanizawa Y, Bando E, Kawamura T, Yasui H, et al. Survival benefit of palliative gastrectomy in gastric cancer patients with peritoneal metastasis. World J Surg. 2012;36:2637–2643. doi: 10.1007/s00268-012-1721-y. [DOI] [PubMed] [Google Scholar]
- 186.Park SH, Kim JH, Park JM, Park SS, Kim SJ, Kim CS, et al. Value of nonpalliative resection as a therapeutic and pre-emptive operation for metastatic gastric cancer. World J Surg. 2009;33:303–311. doi: 10.1007/s00268-008-9829-9. [DOI] [PubMed] [Google Scholar]
- 187.Kokkola A, Louhimo J, Puolakkainen P. Does non-curative gastrectomy improve survival in patients with metastatic gastric cancer? J Surg Oncol. 2012;106:193–196. doi: 10.1002/jso.23066. [DOI] [PubMed] [Google Scholar]
- 188.Lupaşcu C, Andronic D, Ursulescu C, Vasiluţă C, Raileanu G, Georgescu St, et al. Palliative gastrectomy in patients with stage IV gastric cancer--our recent experience. Chirurgia (Bucur) 2010;105:473–476. [PubMed] [Google Scholar]
- 189.Yoshikawa T, Kanari M, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, et al. Should gastric cancer with peritoneal metastasis be treated surgically? Hepatogastroenterology. 2003;50:1712–1715. [PubMed] [Google Scholar]
- 190.Rafique M, Adachi W, Kajikawa S, Kobayashi M, Koike S, Kuroda T. Management of gastric cancer patients with synchronous hepatic metastasis: a retrospective study. Hepatogastroenterology. 1995;42:666–671. [PubMed] [Google Scholar]
- 191.He MM, Zhang DS, Wang F, Wang ZQ, Luo HY, Jin Y, et al. The role of non-curative surgery in incurable, asymptomatic advanced gastric cancer. PLoS One. 2013;8:e83921. doi: 10.1371/journal.pone.0083921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sun J, Song Y, Wang Z, Chen X, Gao P, Xu Y, et al. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13:577. doi: 10.1186/1471-2407-13-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lasithiotakis K, Antoniou SA, Antoniou GA, Kaklamanos I, Zoras O. Gastrectomy for stage IV gastric cancer. a systematic review and meta-analysis. Anticancer Res. 2014;34:2079–2085. [PubMed] [Google Scholar]
- 194.Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–318. doi: 10.1016/S1470-2045(15)00553-7. [DOI] [PubMed] [Google Scholar]
- 195.Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 196.Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587–591. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 198.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 199.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 200.Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 201.Okines AF, Norman AR, McCloud P, Kang YK, Cunningham D. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. 2009;20:1529–1534. doi: 10.1093/annonc/mdp047. [DOI] [PubMed] [Google Scholar]
- 202.Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–1069. doi: 10.1016/S1470-2045(09)70259-1. [DOI] [PubMed] [Google Scholar]
- 203.Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–1553. doi: 10.1200/JCO.2009.25.4706. [DOI] [PubMed] [Google Scholar]
- 204.Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 205.Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–148. doi: 10.1093/annonc/mdu472. [DOI] [PubMed] [Google Scholar]
- 206.Ryu MH, Park YI, Chung IJ, Lee KW, Oh HS, Lee KH, et al. Phase III trial of s-1 plus oxaliplatin (SOX) vs s-1 plus cisplatin (SP) combination chemotherapy for first-line treatment of advanced gastric cancer (AGC): SOPP study. J Clin Oncol. 2016;34(15_suppl):4015. [Google Scholar]
- 207.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 208.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 209.Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–2314. doi: 10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 210.Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 211.Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 212.Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW, Baek SK, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol. 2013;24:2850–2854. doi: 10.1093/annonc/mdt351. [DOI] [PubMed] [Google Scholar]
- 213.Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438–4444. doi: 10.1200/JCO.2012.48.5805. [DOI] [PubMed] [Google Scholar]
- 214.Lee KW, Maeng CH, Kim TY, Zang DY, Kim YH, Hwang IG, et al. A phase III study to compare the efficacy and safety of paclitaxel versus irinotecan in patients with metastatic or recurrent gastric cancer who failed in first-line therapy (KCSG ST10-01) Oncologist. 2019;24:18–e24. doi: 10.1634/theoncologist.2018-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 216.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 217.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee MJ, Hwang IG, Jang JS, Choi JH, Park BB, Chang MH, et al. Outcomes of third-line docetaxel-based chemotherapy in advanced gastric cancer who failed previous oxaliplatin-based and irinotecan-based chemotherapies. Cancer Res Treat. 2012;44:235–241. doi: 10.4143/crt.2012.44.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fanotto V, Uccello M, Pecora I, Rimassa L, Leone F, Rosati G, et al. Outcomes of advanced gastric cancer patients treated with at least three lines of systemic chemotherapy. Oncologist. 2017;22:1463–1469. doi: 10.1634/theoncologist.2017-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Choi IS, Choi M, Lee JH, Kim JH, Suh KJ, Lee JY, et al. Treatment patterns and outcomes in patients with metastatic gastric cancer receiving third-line chemotherapy: a population-based outcomes study. PLoS One. 2018;13:e0198544. doi: 10.1371/journal.pone.0198544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tabernero J, Shitara K, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. LBA-002 Overall survival results from a phase III trial of trifluridine/tipitacil vs. placebo in patients with metastatic gastric cancer refractory to standard therapies (TAGS) Ann Oncol. 2018;29:mdy208.001 [Google Scholar]
- 222.Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 223.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 224.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 225.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Depypere L, Lerut T, Moons J, Coosemans W, Decker G, Van Veer H, et al. Isolated local recurrence or solitary solid organ metastasis after esophagectomy for cancer is not the end of the road. Dis Esophagus. 2017;30:1–8. doi: 10.1111/dote.12508. [DOI] [PubMed] [Google Scholar]
- 227.Hingorani M, Dixit S, Johnson M, Plested V, Alty K, Colley P, et al. Palliative radiotherapy in the presence of well-controlled metastatic disease after initial chemotherapy may prolong survival in patients with metastatic esophageal and gastric cancer. Cancer Res Treat. 2015;47:706–717. doi: 10.4143/crt.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sun J, Sun YH, Zeng ZC, Qin XY, Zeng MS, Chen B, et al. Consideration of the role of radiotherapy for abdominal lymph node metastases in patients with recurrent gastric cancer. Int J Radiat Oncol Biol Phys. 2010;77:384–391. doi: 10.1016/j.ijrobp.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 229.Tey J, Back MF, Shakespeare TP, Mukherjee RK, Lu JJ, Lee KM, et al. The role of palliative radiation therapy in symptomatic locally advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2007;67:385–388. doi: 10.1016/j.ijrobp.2006.08.070. [DOI] [PubMed] [Google Scholar]
- 230.Tey J, Choo BA, Leong CN, Loy EY, Wong LC, Lim K, et al. Clinical outcome of palliative radiotherapy for locally advanced symptomatic gastric cancer in the modern era. Medicine (Baltimore) 2014;93:e118. doi: 10.1097/MD.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yoshikawa T, Tsuburaya A, Hirabayashi N, Yoshida K, Nagata N, Kodera Y, et al. A phase I study of palliative chemoradiation therapy with paclitaxel and cisplatin for local symptoms due to an unresectable primary advanced or locally recurrent gastric adenocarcinoma. Cancer Chemother Pharmacol. 2009;64:1071–1077. doi: 10.1007/s00280-009-0963-3. [DOI] [PubMed] [Google Scholar]
- 232.Kim WH, Park CK, Kim YB, Kim YW, Kim HG, Bae HI, et al. A standardized pathology report for gastric cancer. Korean J Pathol. 2005;39:106–113. [Google Scholar]
- 233.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: IARC Press; 2010. [Google Scholar]
- 234.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 235.Iacobuzio-Donahue CA, Montgomery EA. Gastrointestinal and Liver Pathology E-Book: a Volume in the Series: Foundations in Diagnostic Pathology: Elsevier Health Sciences. 2nd ed. Philadelphia (PA): Elsevier; 2012. [Google Scholar]
- 236.Nakashima Y, Yao T, Hirahashi M, Aishima S, Kakeji Y, Maehara Y, et al. Nuclear atypia grading score is a useful prognostic factor in papillary gastric adenocarcinoma. Histopathology. 2011;59:841–849. doi: 10.1111/j.1365-2559.2011.04035.x. [DOI] [PubMed] [Google Scholar]
- 237.Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968;59:251–258. [PubMed] [Google Scholar]
- 238.Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn. 1982;32(Suppl 2):329–347. [PubMed] [Google Scholar]
- 239.Kim JM, Sohn JH, Cho MY, Kim WH, Chang HK, Jung ES, et al. Pre- and post-ESD discrepancies in clinicopathologic criteria in early gastric cancer: the NECA-Korea ESD for early gastric cancer prospective study (N-Keep) Gastric Cancer. 2016;19:1104–1113. doi: 10.1007/s10120-015-0570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shibata A, Longacre TA, Puligandla B, Parsonnet J, Habel LA. Histological classification of gastric adenocarcinoma for epidemiological research: concordance between pathologists. Cancer Epidemiol Biomarkers Prev. 2001;10:75–78. [PubMed] [Google Scholar]
- 241.Flucke U, Mönig SP, Baldus SE, Zirbes TK, Bollschweiler E, Thiele J, et al. Differences between biopsy- or specimen-related Laurén and World Health Organization classification in gastric cancer. World J Surg. 2002;26:137–140. doi: 10.1007/s00268-001-0195-0. [DOI] [PubMed] [Google Scholar]
- 242.Mita T, Shimoda T. Risk factors for lymph node metastasis of submucosal invasive differentiated type gastric carcinoma: clinical significance of histological heterogeneity. J Gastroenterol. 2001;36:661–668. doi: 10.1007/s005350170028. [DOI] [PubMed] [Google Scholar]
- 243.Takizawa K, Ono H, Kakushima N, Tanaka M, Hasuike N, Matsubayashi H, et al. Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: how to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer. 2013;16:531–536. doi: 10.1007/s10120-012-0220-z. [DOI] [PubMed] [Google Scholar]
- 244.Bartley AN, Washington MK, Ventura CB, Ismaila N, Colasacco C, Benson AB, 3rd, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Arch Pathol Lab Med. 2016;140:1345–1363. doi: 10.5858/arpa.2016-0331-CP. [DOI] [PubMed] [Google Scholar]
- 245.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 246.Provenzale D, Gupta S, Ahnen DJ, Blanco AM, Bray TH. NCCN cinical practice guidelines in oncology. Genetic/familial high-risk assessment: colorectal. Version 1. 2018 [Internet] Plymouth Meeting (PA): National Comprehensive Cancer Network; 2018. [cited 2018 Jul 14]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. [DOI] [PubMed] [Google Scholar]
- 247.Funkhouser WK, Jr, Lubin IM, Monzon FA, Zehnbauer BA, Evans JP, Ogino S, et al. Relevance, pathogenesis, and testing algorithm for mismatch repair-defective colorectal carcinomas: a report of the association for molecular pathology. J Mol Diagn. 2012;14:91–103. doi: 10.1016/j.jmoldx.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 248.Serrano M, Lage P, Belga S, Filipe B, Francisco I, Rodrigues P, et al. Bethesda criteria for microsatellite instability testing: impact on the detection of new cases of Lynch syndrome. Fam Cancer. 2012;11:571–578. doi: 10.1007/s10689-012-9550-6. [DOI] [PubMed] [Google Scholar]
- 249.Buhard O, Suraweera N, Lectard A, Duval A, Hamelin R. Quasimonomorphic mononucleotide repeats for high-level microsatellite instability analysis. Dis Markers. 2004;20:251–257. doi: 10.1155/2004/159347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 252.Polom K, Marano L, Marrelli D, De Luca R, Roviello G, Savelli V, et al. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg. 2018;105:159–167. doi: 10.1002/bjs.10663. [DOI] [PubMed] [Google Scholar]
- 253.Ajani JA, D'Amico TA, Baggstrom M, Bentrem DJ, Chao J. NCCN clinical practice guidelines in oncology (NCCN guidelines). Gastric cancer, version 2. 2018 [Internet] Plymouth Meeting (PA): National Comprehensive Cancer Network; 2018. [cited 2018 Jul 13]. Available from: https://www.nccn.org. [Google Scholar]
- 254.Weiss LM, Chen YY. EBER in situ hybridization for Epstein-Barr virus. Methods Mol Biol. 2013;999:223–230. doi: 10.1007/978-1-62703-357-2_16. [DOI] [PubMed] [Google Scholar]
- 255.Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–833. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63:236–243. doi: 10.1136/gutjnl-2013-304531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Dako Agilent Pathology Solutions. PD-L1 IHC 22C3 pharmDx is FDA-Approved. Santa Clara (CA): Dako Agilent Pathology Solutions; 2018. [Google Scholar]
- 258.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 259.Kang KK, Hur H, Byun CS, Kim YB, Han SU, Cho YK. Conventional cytology is not beneficial for predicting peritoneal recurrence after curative surgery for gastric cancer: results of a prospective clinical study. J Gastric Cancer. 2014;14:23–31. doi: 10.5230/jgc.2014.14.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cotte E, Peyrat P, Piaton E, Chapuis F, Rivoire M, Glehen O, et al. Lack of prognostic significance of conventional peritoneal cytology in colorectal and gastric cancers: results of EVOCAPE 2 multicentre prospective study. Eur J Surg Oncol. 2013;39:707–714. doi: 10.1016/j.ejso.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 261.Lee SD, Ryu KW, Eom BW, Lee JH, Kook MC, Kim YW. Prognostic significance of peritoneal washing cytology in patients with gastric cancer. Br J Surg. 2012;99:397–403. doi: 10.1002/bjs.7812. [DOI] [PubMed] [Google Scholar]
- 262.Shimizu H, Imamura H, Ohta K, Miyazaki Y, Kishimoto T, Fukunaga M, et al. Usefulness of staging laparoscopy for advanced gastric cancer. Surg Today. 2010;40:119–124. doi: 10.1007/s00595-009-4017-6. [DOI] [PubMed] [Google Scholar]
- 263.Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, Strong VE. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010;17:3173–3180. doi: 10.1245/s10434-010-1183-0. [DOI] [PubMed] [Google Scholar]
- 264.La Torre M, Ferri M, Giovagnoli MR, Sforza N, Cosenza G, Giarnieri E, et al. Peritoneal wash cytology in gastric carcinoma. Prognostic significance and therapeutic consequences. Eur J Surg Oncol. 2010;36:982–986. doi: 10.1016/j.ejso.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 265.Jamel S, Markar SR, Malietzis G, Acharya A, Athanasiou T, Hanna GB. Prognostic significance of peritoneal lavage cytology in staging gastric cancer: systematic review and meta-analysis. Gastric Cancer. 2018;21:10–18. doi: 10.1007/s10120-017-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tustumi F, Bernardo WM, Dias AR, Ramos MF, Cecconello I, Zilberstein B, et al. Detection value of free cancer cells in peritoneal washing in gastric cancer: a systematic review and meta-analysis. Clinics (Sao Paulo) 2016;71:733–745. doi: 10.6061/clinics/2016(12)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Pecqueux M, Fritzmann J, Adamu M, Thorlund K, Kahlert C, Reißfelder C, et al. Free intraperitoneal tumor cells and outcome in gastric cancer patients: a systematic review and meta-analysis. Oncotarget. 2015;6:35564–35578. doi: 10.18632/oncotarget.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2012;15(Suppl 1):S27–S37. doi: 10.1007/s10120-011-0071-z. [DOI] [PubMed] [Google Scholar]
- 269.Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncol. 2006;7:935–943. doi: 10.1016/S1470-2045(06)70940-8. [DOI] [PubMed] [Google Scholar]
- 270.Taylor C, Munro AJ, Glynne-Jones R, Griffith C, Trevatt P, Richards M, et al. Multidisciplinary team working in cancer: what is the evidence? BMJ. 2010;340:c951. doi: 10.1136/bmj.c951. [DOI] [PubMed] [Google Scholar]
- 271.Lamb B, Green JS, Vincent C, Sevdalis N. Decision making in surgical oncology. Surg Oncol. 2011;20:163–168. doi: 10.1016/j.suronc.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 272.Basta YL, Baur OL, van Dieren S, Klinkenbijl JH, Fockens P, Tytgat KM. Is there a benefit of multidisciplinary cancer team meetings for patients with gastrointestinal malignancies? Ann Surg Oncol. 2016;23:2430–2437. doi: 10.1245/s10434-016-5178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Basta YL, Bolle S, Fockens P, Tytgat KM. The value of multidisciplinary team meetings for patients with gastrointestinal malignancies: a systematic review. Ann Surg Oncol. 2017;24:2669–2678. doi: 10.1245/s10434-017-5833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Du CZ, Li J, Cai Y, Sun YS, Xue WC, Gu J. Effect of multidisciplinary team treatment on outcomes of patients with gastrointestinal malignancy. World J Gastroenterol. 2011;17:2013–2018. doi: 10.3748/wjg.v17.i15.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Oxenberg J, Papenfuss W, Esemuede I, Attwood K, Simunovic M, Kuvshinoff B, et al. Multidisciplinary cancer conferences for gastrointestinal malignancies result in measureable treatment changes: a prospective study of 149 consecutive patients. Ann Surg Oncol. 2015;22:1533–1539. doi: 10.1245/s10434-014-4163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 277.Kunkler IH, Prescott RJ, Lee RJ, Brebner JA, Cairns JA, Fielding RG, et al. TELEMAM: a cluster randomised trial to assess the use of telemedicine in multi-disciplinary breast cancer decision making. Eur J Cancer. 2007;43:2506–2514. doi: 10.1016/j.ejca.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 278.Brar SS, Mahar AL, Helyer LK, Swallow C, Law C, Paszat L, et al. Processes of care in the multidisciplinary treatment of gastric cancer: results of a RAND/UCLA expert panel. JAMA Surg. 2014;149:18–25. doi: 10.1001/jamasurg.2013.3959. [DOI] [PubMed] [Google Scholar]
- 279.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowcharts of the literature search and study selection
Selected literature lists
Quality assessment tables
Evidence tables