Abstract
Purpose
The significance of neuroendocrine differentiation (NED) in gastric carcinoma (GC) is controversial, leading to ambiguous concepts in traditional classifications. This study aimed to determine the prognostic threshold of meaningful NED in GC and clarify its unclear features in existing classifications.
Materials and Methods
Immunohistochemical staining for synaptophysin, chromogranin A, and neural cell adhesion molecule was performed for 945 GC specimens. Survival analysis was performed using the log-rank test and univariate/multivariate models with percentages of NED (PNED) and demographic and clinicopathological parameters.
Results
In total, 275 (29.1%) cases were immunoreactive to at least 1 neuroendocrine (NE) marker. GC-NED was more common in the upper third of the stomach. PNED, and Borrmann's classification and tumor, lymph node, metastasis stages were independent prognostic factors. The cutoff PNED was 10%, beyond which patients had significantly worse outcomes, although the risk did not increase with higher PNED. Tumors with ≥10% NED tended to manifest as Borrmann type III lesion with mixed/diffuse morphology and poorer histological differentiation; the NE components in this population mainly grew in insulae/nests, which differed from the predominant growth pattern (glandular/acinar) in GC with <10% NED.
Conclusions
GC with ≥10% NED should be classified as a distinct subtype because of its worse prognosis, and more attention should be paid to the necessity of additional therapeutics for NE components.
Keywords: Stomach neoplasms, Adenocarcinoma, Neuroendocine tumors, Prognosis, Immunohistochemistry
INTRODUCTION
Gastric carcinoma with neuroendocrine differentiation (GC-NED) is defined as a heterogeneous entity of neoplasms, in which both the adenocarcinoma and neuroendocrine (NE) component exist. The incidence of NED in GC varies, ranging from 3% to 64.0% worldwide [1,2,3,4,5,6,7,8,9] and 21.3% to 39.6% in China [10,11,12]. GC-NED was previously reported to be associated with more malignant biological behaviors, such as deeper invasion, higher clinical stages, and poorer prognosis, than pure GC (PGC) [5,13].
In the latest World Health Organization (WHO) classification of tumors of the digestive system established in 2010 [14], neoplasms with NE features are classified into 3 major categories: NE tumor (NET), NE carcinoma (NEC), and mixed adenoneuroendocrine carcinoma (MANEC). NET and NEC are completely/mostly composed of NE components but have different morphology and proliferation index. MANEC contains both exocrine and NE elements, with either element exceeding 30%. However, <30% NED was not rare in GC patients in previous studies [2,4,5,15], which challenged the above classification with a terminological gap left between 0% and 30%. This situation is challenging for pathologists in making a diagnosis and for oncologists in deciding the necessity of a chemotherapy regimen for NE components. Furthermore, the cutoff percentage of 30% remains controversial because it was not an appropriate prognostic threshold in GC-NED patients according to some previous study [4,5,13,15].
Therefore, in this study, whole-tissue sections of 945 GC cases with follow-up data were immunostained for synaptophysin (Syn), chromogranin A (CgA), and neural cell adhesion molecule (CD56), and for every positive case, the percentages of NED (PNED) was calculated. The prognostic significance of NED was evaluated based on continuous PNED and clinicopathological parameters. The morphology of NE components was also observed and compared.
MATERIALS AND METHODS
Specimens
In total, 945 GC patients undergoing gastrectomy were enrolled. All specimens were histologically diagnosed as gastric adenocarcinomas or mixed exocrine-endocrine carcinoma/MANEC/gastric adenocarcinomas with NED at The First Affiliated Hospital of Fujian Medical University from 2001 to 2012. Of 945 patients, 726 (76.8%) were male and 219 (23.2%) were female, and their age ranged from 20 to 88 years (mean, 60.91±11.17 years; median, 61 years). All patients did not undergo preoperative chemotherapy or radiotherapy. Clinicopathological data, including tumor locations, Bormann's classification, Lauren's classification, tumor size, histologic differentiation, depth of invasion, lymph node and distant metastases, and tumor, node, metastasis (TNM) stages (American Joint Committee on Cancer 8th), were obtained from archived pathological reports.
Based on the 2010 WHO classification, tumors were classified as 48 early GCs and 897 advanced GCs; furthermore, 317 cases were esophagogastric junction adenocarcinomas, 269 were gastric corpus carcinoma, 332 were gastric antral carcinomas, 21 were gastric remnant carcinomas, and 6 were carcinomas invading the whole stomach. In addition, 427 cases had moderate-to-well differentiation and 518 had poor to undifferentiation. It was also noted that 389 cases were intestinal-type, 299 were diffuse-type, and 257 were mixed-type tumors (containing the 2 types equally). Moreover, 0 cases were Borrmann type I (polypoid), 94 were Borrmann type II (fungating), 720 were Borrmann type III (ulcerated), and 131 were Borrmann type IV (infiltrative). Next, 671 cases had regional lymph node metastasis and 7 had distant metastasis. Patients with stage II or above underwent postoperative adjuvant chemotherapy with 5-fluorouracil/cisplatin-based regimens.
Immunohistochemistry (IHC)
All available hematoxylin and eosin (H&E)-stained slides for each case were reviewed, and the representative section of each case was selected for immunohistochemical staining based on the following criteria: the section had to contain 1) normal tissue, tumor tissue, and transition zone and 2) the full-thickness gastric wall had to have less necrotic tissue and proper size.
The corresponding formalin-fixed paraffin-embedded tissue blocks were cut into 2.5-μm-thick sections using a semi-automatic rotary paraffin microtome (RM2245; Leica Biosystems, Wetzlar, Germany) after refrigeration for 30 minutes. They specimens were then mounted on positively charged adhesive microscope slides for subsequent staining. Sections were baked at 60°C for 2 hours before dewaxing and hydration, and then they were immersed in 3 jars of xylene for 15 minutes each and in 2 jars of anhydrous, 95%, 80%, and 70% alcohol solution for 5 minutes and each slide was subsequently flushed with distilled water. The ElivisionTM plus Polyer HRP (mouse/rabbit) IHC Kit (Lab Vision & NEOMARKERS, Fremont, CA, USA) was used for immunohistochemical staining of all 3 NE markers (the details of antibodies are shown in Supplementary Table 1). Sections for Syn and CD56 detection were pretreated by microwave in Tris-EDTA solution (pH=9.0) for 20 minutes, and those for CgA detection were pretreated by high pressure steam in citrate buffer (pH=6.0) for 5 minutes to retrieve antigenicity. For blocking the endogenous peroxidase activity, tissue on the slides were covered with 3% H2O2 solution for 10 minutes and then flushed using tris-buffered saline (TBS) and incubated with primary antibodies for 60 minutes. Next, after several TBS flushes and a 20-minute incubation with an enhancer, the secondary antibody was added for a 30-minute incubation. Then, a 3,3′-diaminobenzidine incubation was performed for staining after TBS flushes, and the immunohistochemical staining was completed using a 0.1% hematoxylin counterstain. Appropriate positive and negative control sections were also simultaneously prepared. The temperature of the laboratory was controlled at 25°C–28°C with a humidity >80%.
A case was defined as GC-NED when ≥1% of tumor cells showed immunoreactivity to at least 1 NE marker [16,17]. To calculate the PNED, 10 high-power fields (×400) containing tumor cells were selected randomly, with at least 100 tumor cells counted in each field [5].
According to Measures for the Ethical Review of Biomedical Research Involving Humans of China, as this study was retrospective based on archived materials/information, causing no harm to patients' health; patients' identifiers were protected by hospital infor-system and replaced by serial numbers during the study, informed consent was waived. This study was approved by the Ethical Committee of The First Affiliated Hospital of Fujian Medical University (ECFAH of FMU: 19023).
Statistical analysis
The χ2 test or Fisher's exact test was used to analyze the difference between demographic and clinicopathological ratios, and analysis of variance was used to compare the quantitative data. Overall survival (OS) was calculated from the time of surgery to death or the last follow-up. Survival analyses were calculated with the Kaplan-Meier method, and survival curves were compared using the log-rank test. Multivariate analysis was performed using Cox proportional hazards regression model. Receiver operating characteristic (ROC) curves were used to determine the cutoff of PNED.
A P-value <0.05 was considered statistically significant, which was adjusted according to Bonferroni correction in pairwise comparison. Statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and R software (R Foundation, Vienna, Austria).
RESULTS
Case distributions of GC-NED
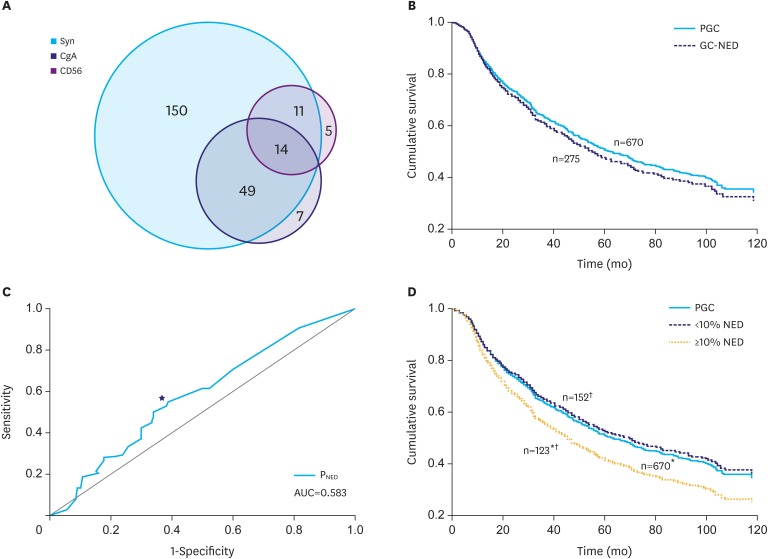
Of 945 cases, 275 (29.1%) cases were immunopositive to at least 1 NE marker in ≥1% of tumor cells, and the positive rates of Syn, CgA, and CD56 were 23.7%, 7.4%, and 3.2%, respectively (Fig. 1A). Significant correlations existed between the expression of Syn and CgA (r=0.632, P=0.000) or between Syn and CD56 (r=0.414, P=0.035), but not between CD56 and CgA.
Fig. 1.
GC-NED distribution by NE marker and grade and survival analysis. (A) Syn is the most sensitive marker, followed by CgA. CD56 shows low immunoreactivity to NE components in GC-NED. (B) In the Kaplan-Meier survival analysis, there was no significant difference between PGC and overall GC-NED (P=0.369). (C) The 8% is the point (★) of convergence that maximized both sensitivity and specificity in the ROC curve (AUC=0.583; P=0.014). (D) GC with ≥10% NED has significantly worse survival rates than PGC (*) and GC with <10% NED (†), but GC with <10% NED has similar outcomes to PGC (P=0.618).
Syn = synaptophysin; CgA = chromogranin A; CD56 = neural cell adhesion molecule; GC-NED = gastric carcinoma with neuroendocrine differentiation; NE = neuroendocrine; NED = neuroendocrine differentiation; PGC = pure gastric carcinoma; ROC = receiver operating characteristic; AUC = area under the curve.
*P=0.027; †P=0.020.
The age of GC-NED patients ranged from 33 to 85 years (mainly in patients aged >50 years), mean age was 61.63±10.33 years, and median was 62.00 years. Male predominance was prominent (male/female: 3.23) among GC-NED patients, although no significant sex difference existed among PGC, <10% NED and ≥10% NED, or between the latter 2. (Table 1).
Table 1. Clinicopathological characteristics of PGC and GC-NED.
| Characteristics | P-value* | PGC | <10% NED | ≥10% NED | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 0.658 | 516 (77.0) | 113 (74.3) | 97 (78.9) | |
| Female | 0.396 | 154 (23.0) | 39 (25.7) | 26 (21.1) | |
| Age (yr) | |||||
| <30 | 0.360 | 4 (0) | 0 (0) | 0 (0) | |
| ≥30 and <50 | 0.656 | 108 (16.1) | 19 (12.5) | 11 (8.9) | |
| ≥50 and <70 | - | 390 (58.2) | 97 (63.8) | 80 (65.0) | |
| ≥70 and <90 | - | 168 (25.1) | 36 (23.7) | 32 (26.0) | |
| Location | |||||
| Upper 1/3 | 0.017 | 200 (29.9) | 60 (39.5) | 57 (46.3) | |
| Middle 1/3 | 0.526 | 197 (29.4) | 39 (25.7) | 33 (26.8) | |
| Lower 1/3 | - | 251 (37.5) | 50 (32.9) | 31 (25.2) | |
| Whole stomach | - | 6 (0.9) | 0 (0) | 0 (0) | |
| Gastric remnant | - | 16 (2.4) | 3 (2.0) | 2 (1.6) | |
| Tumor size (cm) | |||||
| <5 | 0.942 | 370 (55.2) | 82 (53.9) | 66 (53.7) | |
| 5–10 | 0.988 | 265 (39.6) | 62 (40.8) | 52 (42.3) | |
| 10–15 | - | 26 (3.9) | 7 (4.6) | 5 (4.1) | |
| ≥15 | - | 9 (1.3) | 1 (0.7) | 0 (0) | |
| Borrmann's classification classification | |||||
| I | 0.147 | 0 (0) | 0 (0) | 0 (0) | |
| II | 0.036 | 66 (9.9) | 19 (12.5) | 9 (7.3) | |
| III | - | 510 (76.1) | 107 (70.4) | 103 (83.7) | |
| IV | - | 94 (14.0) | 26 (17.1) | 11 (8.9) | |
| Lauren's classification | |||||
| Intestinal | 0.000 | 288 (43.0) | 74 (48.7) | 27 (22.0) | |
| Diffuse | 0.000 | 211 (31.5) | 42 (27.6) | 46 (37.4) | |
| Mixed | - | 171 (25.5) | 36 (23.7) | 50 (40.7) | |
| Histological differentiation | |||||
| Well | 0.016 | 31 (4.6) | 16 (10.5) | 2 (1.6) | |
| Moderate | 0.005 | 266 (39.7) | 66 (43.3) | 46 (37.4) | |
| Poor | - | 354 (52.8) | 65 (42.8) | 70 (56.9) | |
| Undifferentiated | - | 19 (2.8) | 5 (3.3) | 5 (3.1) | |
| TNM stage | |||||
| I | 0.406 | 70 (10.4) | 19 (12.5) | 7 (5.7) | |
| II | 0.126 | 104 (15.5) | 17 (11.2) | 22 (17.9) | |
| III | - | 328 (49.0) | 79 (52.0) | 61 (49.6) | |
| IV | - | 168 (25.1) | 37 (24.3) | 33 (26.8) | |
| Growth pattern | |||||
| Insular/nested | - | - | 20 (13.2) | 55 (44.7) | |
| Trabecular | 0.000 | - | 2 (1.3) | 5 (4.1) | |
| Acinar | - | - | 79 (52.0) | 27 (22.0) | |
| Poorly | - | - | 49 (32.2) | 21 (17.1) | |
| Mixed | - | - | 2 (1.3) | 15 (12.2) | |
Values are presented as number (%). The bold numbers in the tables are P values with statistical significance (<0.05).
PGC = pure gastric carcinoma; GC-NED = gastric carcinoma with neuroendocrine differentiation; NED = neuroendocrine differentiation; TNM = tumor, node, metastasis.
*P-values in the upper rows: among PGC, <10% NED and ≥10% NED; values in the lower rows: <10% NED vs. ≥10% NED.
Prognostic analysis
The follow-up period ranged from 1 to 132 months (median, 54 months). The 5-year OS rate and mean survival time were 16.1% and 33.32±1.33, respectively, for PGC and 13.2% and 31.47±1.88, respectively, for GC-NED; however, there was no statistical difference between these 2 groups (Fig. 1B).
In the survival ROC analysis, the point of convergence that maximized both sensitivity and specificity was 8% (area under the curve=0.583; P=0.014; Fig. 1C), and 10% was considered the prognostic cutoff for convenience. The Kaplan-Meier method revealed that the prognosis of GC with ≥10% NED was significantly worse than that of PGC and GC with <10% NED; however, the latter 2 showed similar survival (Fig. 1D). Moreover, the Cox proportional hazards regression model was used to correct confounders and confirm the prognostic significance of NED (variables: sex, age, location, tumor size, Borrmann's classification, Lauren's classification, histologic differentiation, TNM stage, and logarithm of PNED), and it revealed that PNED, Bormann's classification, and TNM stage were independent prognostic factors (Table 2). In addition, we could not determine a higher prognostic cutoff in GC with ≥10% NED, which was confirmed by the result that PNED was kicked out by the Cox regression model for GC with ≥10% NED (the same variables).
Table 2. Survival analysis.
| Factors (No. of cases) | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P-value | HR | 95% CI | P-value | HR | 95% CI | ||||
| Upper | Lower | Upper | Lower | ||||||
| Sex | N | ||||||||
| Male (726) | R | 1 | - | ||||||
| Female (219) | 0.758 | 1.032 | 0.846 | 1.259 | |||||
| Age | N | ||||||||
| CV (945) | 0.608 | 1.002 | 0.995 | 1.009 | |||||
| Location | N | ||||||||
| Upper (317) | R | 1 | - | ||||||
| Middle (269) | 0.072 | 1.219 | 0.982 | 1.513 | |||||
| Lower (332) | 0.244 | 1.130 | 0.920 | 1.387 | |||||
| Whole (6) | 0.030 | 2.465 | 1.091 | 5.670 | |||||
| Remnant (21) | 0.501 | 0.829 | 0.481 | 1.429 | |||||
| Tumor size | N | ||||||||
| CV (945) | 0.478 | 1.009 | 0.985 | 1.034 | |||||
| PNED* | |||||||||
| CV (945) | 0.025 | 1.319 | 1.037 | 1.680 | 0.014 | 1.367 | 1.065 | 1.754 | |
| Borrmann's classification | |||||||||
| Type I (0) | No case | No case | |||||||
| Type II (94) | R | 1 | - | R | 1 | - | |||
| Type III (720) | 0.354 | 1.150 | 0.856 | 1.544 | 0.153 | 0.679 | 0.399 | 1.155 | |
| Type IV (131) | 0.667 | 1.080 | 0.760 | 1.536 | 0.002 | 0.220 | 0.196 | 0.704 | |
| Lauren's classification | N | ||||||||
| Intestinal (389) | R | 1 | - | ||||||
| Diffuse (299) | 0.193 | 1.143 | 0.935 | 1.398 | |||||
| Mixed (257) | 0.001 | 1.434 | 1.159 | 1.775 | |||||
| Histologic differentiation | N | ||||||||
| Well (49) | R | 1 | - | ||||||
| Moderate (378) | 0.550 | 0.871 | 0.555 | 1.368 | |||||
| Poor (489) | 0.732 | 0.925 | 0.501 | 1.442 | |||||
| Undifferentiated (29) | 0.699 | 0.879 | 0.372 | 1.687 | |||||
| TNM stage | |||||||||
| I (96) | R | 1 | - | No dead GC-NED case | |||||
| II (143) | 0.610 | 0.830 | 0.407 | 1.695 | R | 1 | - | ||
| III (468) | 0.302 | 1.418 | 0.731 | 2.751 | 0.030 | 1.965 | 1.067 | 3.619 | |
| IV (238) | 0.762 | 1.110 | 0.565 | 2.179 | 0.288 | 1.422 | 0.743 | 2.724 | |
The bold numbers in the tables are P values with statistical significance (<0.05).
HR = hazard ratio; CI = confidence interval; CV = continuous variable; R = reference; N = not in equation; PNED = percentages of neuroendocrine differentiation; GC-NED = gastric carcinoma with neuroendocrine differentiation; TNM = tumor, node, metastasis.
*With logarithm transformation.
Hence, 10% was determined to be the cutoff which categorized GC-NED into 2 parts with different outcomes. Beyond this threshold, NE components negatively affected the prognosis of GC, and the increase in PNED was no longer prognostically significant above this threshold.
Clinicopathological and morphological characteristics
Comparisons of clinicopathological characteristics revealed that significant differences were found among PGC, GC with <10% NED, and GC with ≥10% NED or between the latter 2 with respect to location, Borrmann's classification, Lauren's classification, and histological differentiation. Furthermore, GC with ≥10% NED tended to occur in the upper third of the stomach, manifesting as Borrmann type III lesion with diffuse/mixed morphology and poorer histological differentiation than GC with <10% NED (Table 1).
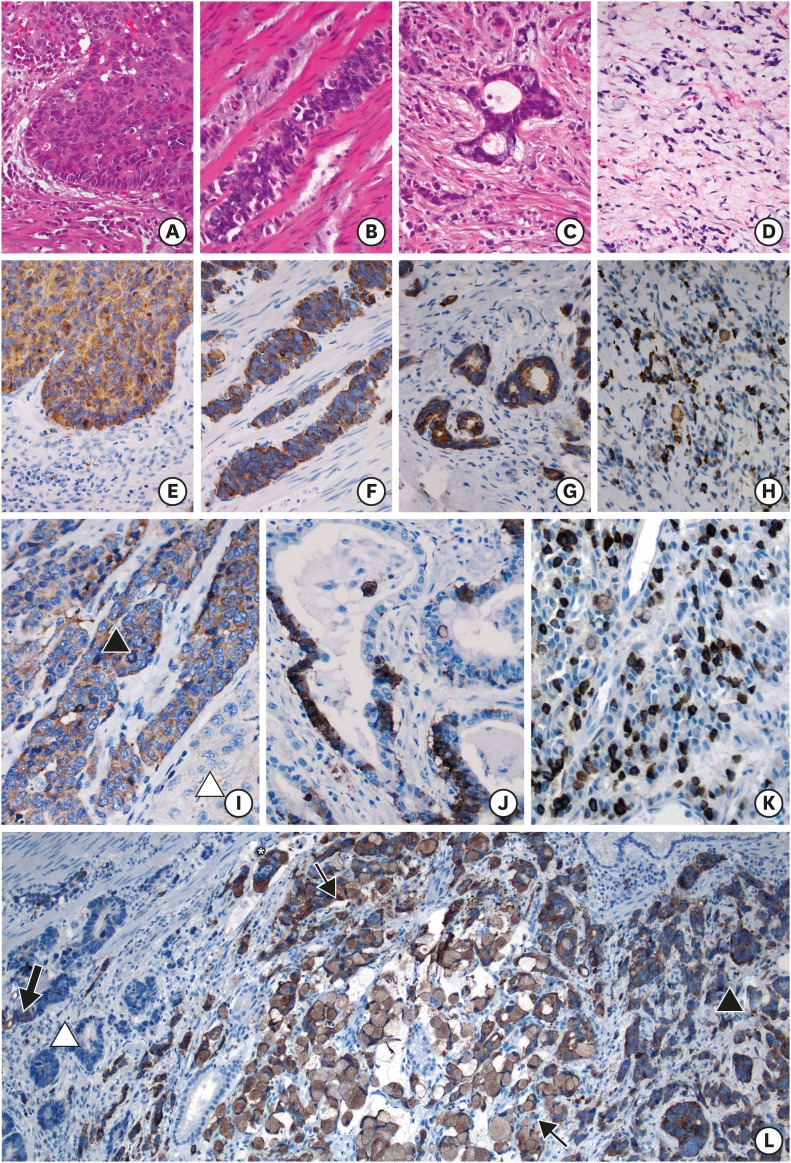
NE components grew in insular/nested, trabecular, glandular/acinar, poorly differentiated, or mixed patterns, of which the glandular/acinar type was predominant in GC with <10% NED and insular/nested type was predominant in GC with ≥10% NED (P=0.000; Table 1 and Fig. 2A-H). The following 5 coexistence patterns of NE and adenocarcinoma components were observed: collision tumors, composite tumors, tumors with NED/adenocarcinoma interspersing, amphicrine tumors, and combinations of the above (Fig. 2I-L).
Fig. 2.
Morphology of GC-NED. (A) (H&E×400) and (E) (Syn×400): NE cells stack together and form a tumor nest with apparent peripheral palisading cells. (B) (H&E×400) and (F) (Syn×400): NE components grow in long cords among the muscle bundles in the muscularis propria. (C) (H&E×400) and (G) (Syn×400): NE cells line the pseudo-glands in the cancerous stroma. (D) (H&E×400) and (H) (Syn×400): NE cells are spread in poorly differentiated carcinoma. (I) (Syn×400): a clear boundary exists between the NE component (▲) and adenocarcinoma (△) in a collision tumor. (J) (Syn×400): the pseudo-glands in a composite tumor are lined by NE and adenocarcinoma cells continuously. (K) (Syn×400): NE cells are dispersed in the background of the adenocarcinoma. (L) (Syn×100): the 3 distribution patterns above, as well as the amphicrine pattern (↑), can be observed simultaneously. The NED components (▲) show 2 growth patterns, namely small nests and signet ring cells, and form a cancer embolus (*). The adjacent adenocarcinoma (△) contains scattered or fragmental NE components (bold arrow) inside the tumor nests or pseudo-glands.
GC-NED = gastric carcinoma with neuroendocrine differentiation; H&E = hematoxylin and eosin stain; Syn = synaptophysin; NE = neuroendocrine.
DISCUSSION
The significance of NED in GC remains ambiguous, leading to unclear features in the classification of gastric NE neoplasms [18]. In this study, whole-tissue sections of 945 GC cases were immunostained for NE markers, and 10% was determined to be the cutoff of PNED, which divided GC into 2 entities with different outcomes.
In 1987, Lewin [19] introduced the first classification of mixed (composite) glandular-endocrine cell carcinomas, namely neoplasms with both exocrine and endocrine components (with each element exceeding 1/3), neoplasms with mixed differentiation at the cytological level, and neoplasms with juxtaposition of the 2 components. Then, the “1/3” (nearly 30%) was retained and adopted by WHO to define MANEC in 2010. However, the prognostic significance of “30%” is controversial. Jiang et al. [4] analyzed the survival outcome of 86 patients with GCs with >1% NED and found that GC with >20% NED had worse survival than GC with <20% NED, while no difference existed between GC with <20% NED and PGC. In Canzonieri's study [5], higher mortality and recurrence rates were also reported for GC with >20% NED. Park et al. [15] evaluated the prognosis of 88 GC-NEDs, and the results showed that the survival rate of GC with ≥10% NED was significantly poorer than that of GC with <10% NED.
In our study, 10% cutoff was found by the ROC curve and was confirmed by survival analysis; this is consistent with Park's [15] findings and reflects the significance of the NE component even as a minority in GC-NED. In addition, among the cases with ≥10% NED, higher cutoff was determined to define another entity with worse prognosis, indicating that the NE component in GC did not act in a dose-dependent manner above the threshold. Considering that the glandular/acinar pattern was the main morphology of GC with <10% NED, these cases might still possess the nature of adenocarcinoma and have similar biological behaviors to PGC.
Because the NE component would function at 10%, it is important to accurately and stably identify NED in GC. However, in previous studies, the detection rates of NED strikingly varied (3%–64%) [1,2,3,4,5,6,7,11]. The case selection procedure might be an important factor for the difference. Some large-scale study only performed IHC staining for putative cases screened based on H&E morphology [4]; however, according to our observation, atypical manifestation (glandular/acinar, poorly differentiated, or mixed) of NE components appeared at considerable rates in GC with ≥10% NED. Therefore, IHC staining for NE markers should be performed for all cases regardless of the morphology. CgA is a specific marker for NED; however, its sensitivity is low, especially when few secretory granules area present [20]. In our study, only 25.5% of GC-NED were CgA positive, whereas >80% of GC-NED immunoreacted with Syn. Thus, the combination of these 2 markers would be more appropriate. For CD56, the positivity was only 10.9%, and only 4 cases (1.45%) reacted to it solely, which lowers its importance for NED detection in GC. In addition, the heterogeneity of patients might be a possible factor for different NED incidence because the epidemiological features of GC or NE neoplasms could be affected by ethnicity [21,22,23].
Clinically, there are no published guidelines for chemotherapy regimens for GC with different proportions of NED, and the accepted strategy is “to kill the predominance,” which has proven to be clinically appropriate for colorectal MANEC [24]. However, in the study of gastric MANEC, NE components were more likely to metastasize to regional lymph nodes [25], and the predominance of NEC rather than that of adenocarcinoma was an independent risk factor (hazard ratio=2.208) [26]. These findings indicate that the NE component might play a more important role in higher malignancy than adenocarcinoma in MANEC. Some clinical oncologists attempted using a combined neoadjuvant chemotherapeutic regimen (VP-16, cisplatin and TS-1) for both NEC and adenocarcinoma in advanced MANEC and reported excellent response; after subsequent curative resection, postoperative chemotherapy (the same regimen), and radiotherapy, the patient survived for 5 years without recurrence and metastasis [27]. Hence, combining with the negative effect of NED from a low percentage in our and former study, more attention should be paid to NE components when selecting a treatment regimen, and the adaptability of “to kill the predominance” in GC-NED should be further discussed.
In summary, based on our findings, IHC staining for NE markers should be performed for every GC case in clinical practice to ensure the detection of low NED. Furthermore, the proportion of NED should be reported, especially when it is >10%. In the future, it is worth evaluating the necessity of therapeutics for NED in GC patients with low but significant NED.
ACKNOWLEDGMENTS
We are grateful to Pro. Lirong Chen who provided inspiring advice on revising the manuscript; Pro. Jun Tian, Dr. Zheyuan Ding, Dr. Fayang Lian, and Dr. Rongquan Wu for their help in data analysis; and the colleagues at Department of Pathology, The First Affiliated Hospital of Fujian Medical University and The Second Affiliated Hospital of Zhejiang University, School of Medicine.
Footnotes
- Conceptualization: Z.S., C.L., W.X.
- Data curation: Z.Y., W.L., H.S., L.S., H.M.
- Formal analysis: Z.Y., Z.S.
- Investigation: Z.Y., W.L., H.S., H.M.
- Methodology: C.Y., Z.Y., H.L., Z.S., W.P., J.L., L.G.
- Project administration: Z.S.
- Resources: Z.S.
- Software: Z.Y.
- Supervision: Z.S.
- Validation: Z.S.
- Visualization: Z.Y.
- Writing - original draft: Z.Y.
- Writing - review & editing: Z.S., Z.Y.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIAL
The detail of antibodies
References
- 1.Ooi A, Mai M, Ogino T, Ueda H, Kitamura T, Takahashi Y, et al. Endocrine differentiation of gastric adenocarcinoma. The prevalence as evaluated by immunoreactive chromogranin A and its biologic significance. Cancer. 1988;62:1096–1104. doi: 10.1002/1097-0142(19880915)62:6<1096::aid-cncr2820620612>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 2.Qvigstad G, Sandvik AK, Brenna E, Aase S, Waldum HL. Detection of chromogranin A in human gastric adenocarcinomas using a sensitive immunohistochemical technique. Histochem J. 2000;32:551–556. doi: 10.1023/a:1004102312006. [DOI] [PubMed] [Google Scholar]
- 3.Sentani K, Oue N, Noguchi T, Sakamoto N, Matsusaki K, Yasui W. Immunostaining of gastric cancer with neuroendocrine differentiation: Reg IV-positive neuroendocrine cells are associated with gastrin, serotonin, pancreatic polypeptide and somatostatin. Pathol Int. 2010;60:291–297. doi: 10.1111/j.1440-1827.2010.02519.x. [DOI] [PubMed] [Google Scholar]
- 4.Jiang SX, Mikami T, Umezawa A, Saegusa M, Kameya T, Okayasu I. Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am J Surg Pathol. 2006;30:945–953. doi: 10.1097/00000478-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Canzonieri V, Colarossi C, Del Col L, Perin T, Talamini R, Sigon R, et al. Exocrine and endocrine modulation in common gastric carcinoma. Am J Clin Pathol. 2012;137:712–721. doi: 10.1309/AJCPM13KVNCZQBUV. [DOI] [PubMed] [Google Scholar]
- 6.Eren F, Celikel C, Güllüoğlu B. Neuroendocrine differentiation in gastric adenocarcinomas; correlation with tumor stage and expression of VEGF and p53. Pathol Oncol Res. 2004;10:47–51. doi: 10.1007/BF02893409. [DOI] [PubMed] [Google Scholar]
- 7.Blumenfeld W, Chandhoke DK, Sagerman P, Turi GK. Neuroendocrine differentiation in gastric adenocarcinomas. An immunohistochemical study. Arch Pathol Lab Med. 1996;120:478–481. [PubMed] [Google Scholar]
- 8.Waldum HL, Aase S, Kvetnoi I, Brenna E, Sandvik AK, Syversen U, et al. Neuroendocrine differentiation in human gastric carcinoma. Cancer. 1998;83:435–444. [PubMed] [Google Scholar]
- 9.Fujii A, Kamiakito T, Takayashiki N, Fujii T, Tanaka A. Neuroendocrine tissue-specific transcription factor, BETA2/NeuroD, in gastric carcinomas: a comparison with chromogranin A and synaptophysin expressions. Pathol Res Pract. 2003;199:513–519. doi: 10.1078/0344-0338-00456. [DOI] [PubMed] [Google Scholar]
- 10.Wang LL, Yao GY, Zhao ZS, Wei XL, Xu RJ. Clonality analysis of neuroendocrine cells in gastric adenocarcinoma. World J Gastroenterol. 2013;19:5340–5346. doi: 10.3748/wjg.v19.i32.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao GY, Zhou JL, Lai MD, Chen XQ, Chen PH. Neuroendocrine markers in adenocarcinomas: an investigation of 356 cases. World J Gastroenterol. 2003;9:858–861. doi: 10.3748/wjg.v9.i4.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T, Su D, Mao Z, Guo X, Wang L, Bai L. Prognostic role of neuroendocrine cell differentiation in human gastric carcinoma. Int J Clin Exp Med. 2015;8:7837–7842. [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JJ, Kim JY, Hur H, Cho YK, Han SU. Clinicopathologic significance of gastric adenocarcinoma with neuroendocrine features. J Gastric Cancer. 2011;11:195–199. doi: 10.5230/jgc.2011.11.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 15.Park JY, Ryu MH, Park YS, Park HJ, Ryoo BY, Kim MG, et al. Prognostic significance of neuroendocrine components in gastric carcinomas. Eur J Cancer. 2014;50:2802–2809. doi: 10.1016/j.ejca.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Kim TY, Chae HD. Composite neuroendocrine carcinoma with adenocarcinoma of the stomach misdiagnosed as a giant submucosal tumor. J Gastric Cancer. 2011;11:126–130. doi: 10.5230/jgc.2011.11.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granberg D, Wilander E, Stridsberg M, Granerus G, Skogseid B, Oberg K. Clinical symptoms, hormone profiles, treatment, and prognosis in patients with gastric carcinoids. Gut. 1998;43:223–228. doi: 10.1136/gut.43.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006;449:499–506. doi: 10.1007/s00428-006-0306-2. [DOI] [PubMed] [Google Scholar]
- 19.Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987;11(Suppl 1):71–86. doi: 10.1097/00000478-198700111-00007. [DOI] [PubMed] [Google Scholar]
- 20.Chang S, Choi D, Lee SJ, Lee WJ, Park MH, Kim SW, et al. Neuroendocrine neoplasms of the gastrointestinal tract: classification, pathologic basis, and imaging features. Radiographics. 2007;27:1667–1679. doi: 10.1148/rg.276075001. [DOI] [PubMed] [Google Scholar]
- 21.Leoncini E, Carioli G, La Vecchia C, Boccia S, Rindi G. Risk factors for neuroendocrine neoplasms: a systematic review and meta-analysis. Ann Oncol. 2016;27:68–81. doi: 10.1093/annonc/mdv505. [DOI] [PubMed] [Google Scholar]
- 22.Gill S, Shah A, Le N, Cook EF, Yoshida EM. Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a Canadian cancer center. J Clin Oncol. 2003;21:2070–2076. doi: 10.1200/JCO.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 23.Ng CJ, Teo CH, Abdullah N, Tan WP, Tan HM. Relationships between cancer pattern, country income and geographical region in Asia. BMC Cancer. 2015;15:613. doi: 10.1186/s12885-015-1615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka T, Kaneko M, Nozawa H, Emoto S, Murono K, Otani K, et al. Diagnosis, assessment, and therapeutic strategy for colorectal mixed adenoneuroendocrine carcinoma. Neuroendocrinology. 2017;105:426–434. doi: 10.1159/000478743. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Shu M, Chen S, Xue L, Lin Y. Clinicopathological features and lymph node metastatic patterns of gastric mixed adenoneuroendocrine carcinoma. Histol Histopathol. 2018 doi: 10.14670/HH-18-045. [DOI] [PubMed] [Google Scholar]
- 26.Xie JW, Lu J, Wang JB, Lin JX, Chen QY, Cao LL, et al. Prognostic factors for survival after curative resection of gastric mixed adenoneuroendocrine carcinoma: a series of 80 patients. BMC Cancer. 2018;18:1021. doi: 10.1186/s12885-018-4943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang G, Li D, Zheng F, Yang L. Long-term disease free survival of gastric mixed adenoneuroendocrine carcinoma treated with multimodality therapy: a case report. Mol Clin Oncol. 2018;8:653–656. doi: 10.3892/mco.2018.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The detail of antibodies