Abstract
Turbidity is a characteristic impurity of groundwater in Pakistan. Turbid water is not suitable for drinking purposes. The main objective of this study is to reduce water turbidity using natural coagulant, extracted from pine cones. The coagulation activity of coagulant is tested using synthetic turbid water. Coagulant activity is affected by various factors such as coagulant dose, water turbidity, pH, extract density and settling time. The optimum coagulant dose and water turbidities are fixed; 0.5 ml/L, 67, and 75 NTU, respectively. The highest coagulation activities are observed at pH values 2 and 12. Further, coagulation activity of pine cone extract is maximized to 82% when its density is 1.8 g/cm3. Moreover, most of the coagulation activity takes place in the first hour. The results recommend the potential use of pine cone extract for turbid water purification.
Keywords: Environmental science, Natural hazards, Biochemistry, Natural product chemistry
1. Introduction
Ground water is often contaminated by suspended solid particles. Purification of such turbid water generally requires the use of coagulants. A number of inorganic and synthetic polymeric coagulants are available for water treatment. Inorganic coagulants such as alums are widely used for removing turbidity from water. Synthetic polymers have also found application in water treatment. However, these coagulants generate non-biodegradable sludge, which cannot be recycled easily [1]. Moreover, traces of polymeric coagulants left in water, may engender serious health hazards [2]. Therefore, the inorganic and synthetic coagulants cannot provide an environment friendly and sustainable solution for turbid water treatment.
Over the past few years natural coagulants have gained interest among researchers for treatment of turbid water. These coagulants can be extracted either from different plant or animal sources. However, natural coagulants obtained from plant sources such as Moringa Oleifera [3], Maize [4], and Cactus Latifaria [5] etc., are more widely studied by researchers. Feculent water can be treated effectively by utilizing these natural coagulants, with ease of regeneration and lower stress on environment [6, 7].
Water turbidity is a major issue associated with ground water in Pakistan and it is not suitable for drinking purposes. Further, drinking of turbid water is causing several health issues among people of Pakistan. Pine cone trees are often referred as Pinus Gerardiana. These trees are abundantly available in Quetta and northern areas of Pakistan. Since, Pine cones can be used for the preparation of natural and cost-effective coagulant. Therefore, treatment of turbid water using pine cone is better option. Coagulant extract from pine cone is prepared using distilled water. Coagulation activity of pine cone extract is probed with synthetic turbid water. It has been observed that the coagulation activity of pine cone extract is either better or comparable to other natural coagulants and alum.
Several explanations have been suggested in literature regarding the coagulation activity of natural coagulant extracts. Ndabigengesere et. al proposed that the proteins present in plant extracts are active components for coagulation [3]. Some authors have suggested that the active coagulation components in plant extracts are not proteins but are some sort of organic polyelectrolyte [8, 9]. Since, the chemical composition of extracts obtained from solid coagulants do not explain clearly the coagulation activity of natural coagulants [6]. Therefore, the coagulation activity of natural coagulants can only be justified by experimental results.
Moving on to the mechanism, by which natural coagulants such as pine cones perform their activity, can be explained in two ways. First, the pine cone extract neutralizes oppositely charged particles in the aqueous solution. Second, the polymeric structure of active proteins in pine cone extract binds the dispersed particles in aqueous solution [10].
In previous studies, researchers have employed coagulant extracts immediately after preparing, without alteration [11]. However, in this work we not only have prepared the coagulant extract but also concentrated it. The aim of this step was to investigate the effect of coagulant density over water treatment efficiency. Since, only an optimized protein concentration in coagulant extract, can remove turbidity from water effectively. Therefore, it is critically important that coagulant extract is concentrated to an optimum density. Apart from coagulant density, a number of other parameters like coagulation dose, initial water turbidity, and pH affect the coagulation process and activity of coagulant. The coagulation efficiency is highly dependent upon coagulant itself, i.e., the extent to which crude extract mixes with turbid water and make better flocks of agglomerates.
2. Materials and methods
2.1. Materials
Kaolin (CAS 1332-58-7, Sigma Aldrich), HCl (CAS 7647-01-0, Fluka, 30–35%), NaOH (CAS 1310-73-2, Sigma-Aldrich, 99.99%), Distilled water, and Pine cones obtained from Quetta City of Pakistan are used in this study.
2.2. Procedure
2.2.1. Coagulant preparation
Pine cones were washed with adequate tap water thrice to remove any impurities that may reduce coagulating properties of the material. The cones were then put in oven for drying at 80 °C for 24 h. The dried cones were manually chopped off for grinding process. The chopped cone pieces were again washed with tap water and allowed to dry in oven at 200 °C for 2 h. This biomass was first subjected to coarse grinding using hammer mill followed by ball mill for fine grinding. The ground material was then sieved through 100 mesh sieve in order to obtain particle sizes below 0.15 mm. The powder was washed with distilled water till the removal of color and acidity to yield biomass with pH 7.0.
50 gm/l of pine cone powder was mixed with water and stirred at 400 rpm using magnetic stirrer for 60 min at room temperature. The suspension was allowed to settle for 30 min. The supernatant solution which contained the active coagulant was decanted off. The mother liquor was centrifuged for 2 h using Kubota 6500 centrifuge with operating conditions of 9000 rpm, and 8 °C. The supernatant solution was filtered through vacuum filter and the filtrate was initially stored at 4 °C in a refrigerator. Higher density coagulant extract was obtained by heating it at 60 °C, to evaporate the water content in it. Pine cone extract density was measured after every 30 min using Anton Paar DMA 4500 M digital densitometer. Samples with various densities ranging 1.2–2.0 g/cm3 were stored in 5 ml glass vials.
2.2.2. Synthetic turbid water preparation
10g of kaolin was dried in an oven at 105 °C for 5 h. It was then added into 1 liter of distilled water to prepare stock kaolin solution. Synthetic turbid water with initial turbidities 67, 69, 71, and 75 NTU was prepared by adding 9.5, 9.8, 10.1, and 10.7 ml of kaolin suspension in 1 liter of distilled water respectively. This synthetic turbid water was used for coagulation tests. These turbidities were chosen because different samples of ground water obtained from Quetta City had similar turbidities. 1 molar solution of HCl and NaOH were added into the synthetic turbid water to adjust the pH values.
2.2.3. Coagulation test
The coagulation activity of pine cone extract was determined by Jar test. 100 ml of Synthetic turbid water of different initial turbidities was filled into 500 ml beakers. Pine cone extracts with variable doses and densities were added to these beakers and mixed at 100 rpm for 30 min using mechanical stirrer. The suspensions were then allowed to settle down. After 4 h of sedimentation, clarified samples from beakers were collected and their residual turbidities were measured using turbidity meter as . Similar test was conducted with the absence of coagulant and turbidity was reported as .Coagulation activity was calculated as:
Further, coagulation activity and efficiency are used interchangeably in this study.
3. Results and discussion
3.1. Effect of coagulant dose and initial turbidity
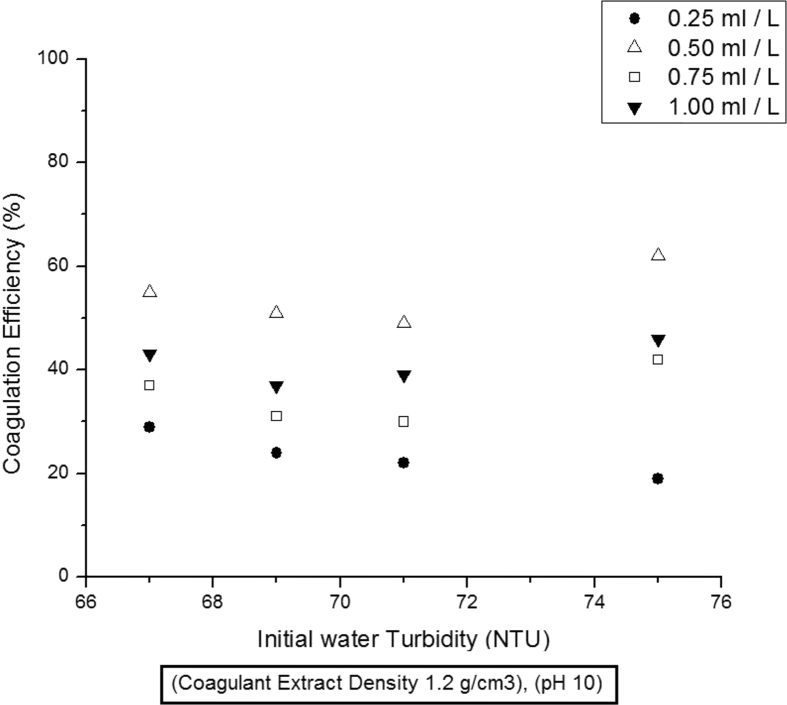
In order to investigate the effect of initial turbidity and coagulant dose on coagulation efficiency, aqueous solutions with turbidities 67 NTU, 69 NTU, 71 NTU and 75 NTU, were treated with different coagulant doses of 0.25, 0.5, 0.75 and 1 ml/L and the results are presented in Fig. 1. Coagulant dose of 0.25 ml/L imparted the least coagulation activity and a continuous decline in efficiency was observed with increasing initial turbidity. The lower concentration of active components i.e. proteins in coagulant extract, is the main reason of reduced coagulation efficiency. Since, the amount of proteins needed to bind together all the dispersed particles in the aqueous solution, increases with increased turbidities. Therefore, 0.25 ml/L coagulant dose is insufficient to provide that much amount of proteins and coagulation efficacy has decreased with increasing turbidities. The highest coagulation efficiencies are observed when coagulant dose of 0.5 ml/L was used with initial turbidities of 67 and 75 NTU. However, reduced coagulation activities are noticed with turbidities 69 and 71 NTU. This validates the assumption of Okuda et. al. that maximum coagulation efficiency can only be achieved when both coagulant dose and initial turbidities are at optimum levels [9]. Similar coagulation activity trends have been observed with coagulant doses of 0.75 ml/L and 1.0 ml/L, Fig. 1. Since the best coagulation efficiencies of 55 and 62 % have been obtained with coagulant dose of 0.5 ml/L and initial turbidities of 67 and 75 NTU respectively. This indicates that a coagulant dose more than 0.5 ml/L, may itself contribute towards the generation of turbidity in aqueous solution. Therefore, rest of the coagulation tests were carried out at these optimum conditions.
Fig. 1.
Effect of Coagulant dose and Initial turbidity on coagulation activity.
3.2. Effect of pH
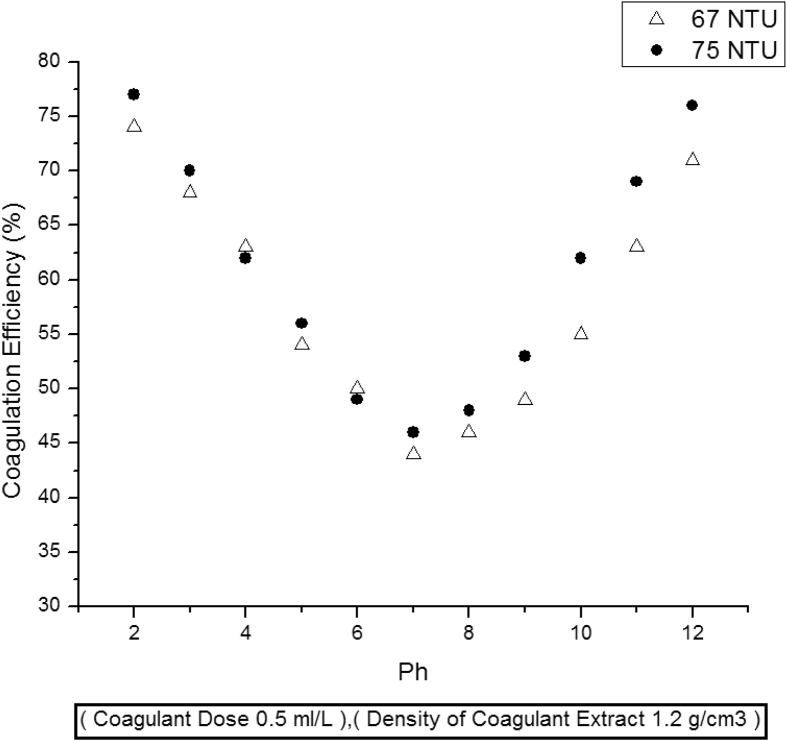
Coagulation activity is greatly influenced by pH of water because active components in coagulant extract often carry a charge. Okudaet al. and Diazet al. suggested that coagulation activity is enhanced at extreme pH values i.e. lower and higher pH values [5, 9]. To monitor the effect of pH, the coagulation tests were conducted at pH values ranging from 2 to 12. It has been observed that best coagulation activities have been obtained either at extremely acidic or basic pH values. For instance, 74 and 77 % coagulation efficiency has been obtained at pH value 2 and initial water turbidities of 67 and 75 NTU respectively. Similarly, at pH value of 12, 71 and 76 % coagulation efficiencies have been observed. This is in accordance with the findings of Okuda et al. and Diaz et al. Moreover, these results indicate that pine cone extract is comprised of both cationic and anionic coagulating components. At lower pH values the anionic components of coagulant are responsible for turbidity removal. Similarly, at higher pH values cationic protein components get activated. However, poor coagulation activity has been noticed near neutral pH zone i.e. at values 6–8, Fig. 2. Although conducting coagulation tests at extreme pH values may violate the guidelines of EPA, only higher pH values give excellent results in terms of coagulation activity [12]. The pH values of clarified water are adjusted by adding molar quantities of acid or base at the end of each coagulation test.
Fig. 2.
Effect of pH on coagulation activity.
3.3. Effect of extract's density
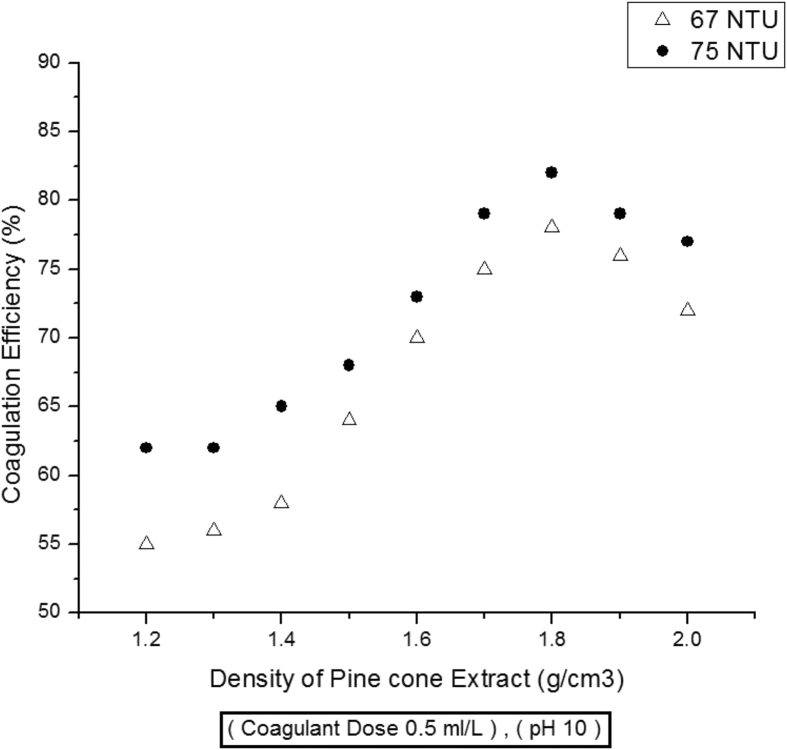
The density of coagulant extract also contributes towards coagulation activity. To investigate the effect of coagulant extract's density on coagulation activity, variable densities ranging from 1.2 to 2.0 g/cm3are used for treatment of water. It has been observed that increased density of extract generally has a positive impact on coagulation efficiency. As evident from Fig. 3, coagulation activity shows an increasing trend with the rising density values of coagulant extract. The highest coagulation efficiencies of 78 and 82 % have been obtained when extract's density was 1.8 g/cm3. It implies that with increased densities of extract, concentration of active components in coagulant extract also increases, which maximizes coagulation activity. However, beyond a certain point of optimum density value i.e. 1.8 g/cm3, the coagulation activity of extract starts declining. It is possible that by further increasing the density, pine cone extract self contributes towards turbidity and ultimately results in reduction of coagulation efficiency.
Fig. 3.
Effect of Extract's Density on Coagulation activity.
3.4. Effect of settling time
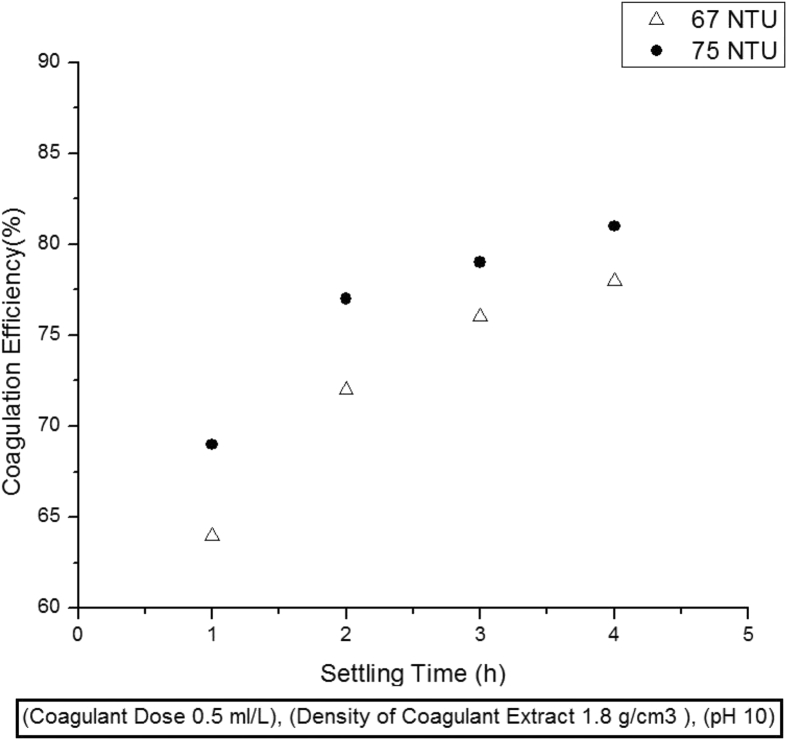
Settling time has also a considerable and quantitative effect on coagulation efficiency. In order to observe the effect of settling time on water treatment efficiency, coagulant dose of 0.5 ml/L was used in water samples having initial turbidities of 67, and 75 NTU and allowed to settle down from 1 h to 4 h. It has been observed that maximum turbidity of 64 and 67% was removed in the first hour. It is quite obvious that at first optimum conditions i.e. initial turbidity and extract dose for coagulation are available. Moreover, in the first hour of treatment, maximum amount of active proteins are available in the aqueous solution causing enhanced activity. However, the removal efficiency decreases dramatically in 2nd, 3rd and 4th hours respectively. It is because of reduction in the concentration of both, the leftover suspended solid particles causing turbidity and active coagulating proteins in the extract; requiring more time for the active components of coagulant to bind together those suspended particles in water Fig. 4.
Fig. 4.
Effect of settling time on coagulation activity.
3.5. Coagulation activity comparison of various coagulants
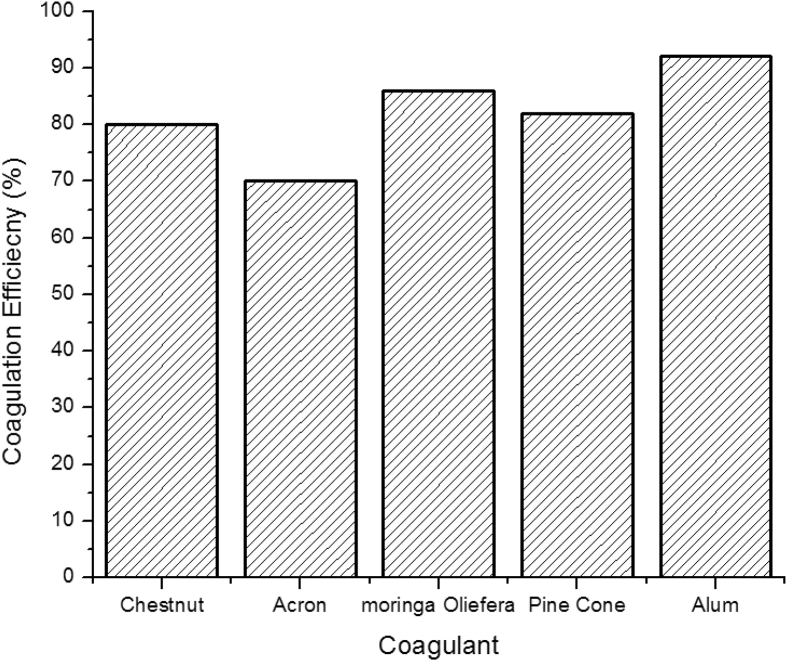
Coagulation activity of 0.5 ml/L of coagulant dose (pine cone extract) for treatment of turbid water has been compared with other natural coagulant and alum. It has been observed that its coagulation efficiency is higher as compared to European Chestnut and Common oak Acron by 2 and 12% respectively [6]. However, coagulation efficiency of pine cone coagulant is less than Moringa Oliefera and Alum by 4 and 10% respectively Fig. 5.
Fig. 5.
Coagulation efficiency comparison of Pine cone with other coagulants.
4. Conclusion
This study has shown the potential of pine cone extract for the treatment of turbid water. Favorable results of coagulation activity have been observed with moderate coagulant dose, indicating that only moderate doses provide sufficient proteins which can bind all the suspended particles in turbid water. Moreover, only highly acidic or basic pH values can activate the proteins in the coagulant to achieve maximum activity. Apart from coagulant dose and pH, the optimized density of coagulant extract is very important for maximum turbidity removal. The highest coagulation efficiency of 82% was achieved by optimizing the controlling parameters. Hence, it is deduced that, pine cone extract can be used as a natural and sustainable source for purification of turbid water.
Declarations
Author contribution statement
Sajid Hussain: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Awais Sattar Ghouri: Analyzed and interpreted the data.
Ashfaq Ahmad: Conceived and designed the experiments; Performed the experiment.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors of this work acknowledge the technical and financial support provided by Pakistan Council of Scientific & Industrial Research (PCSIR) and Higher Education Commission of Pakistan (HEC).
References
- 1.Ramavandi B. Treatment of water turbidity and bacteria by using a coagulant extracted from Plantago ovata. Water Resour. Ind. 2014;6:36–50. [Google Scholar]
- 2.Mallevialle J., Bruchet A., Fiessinger F. How safe are organic polymers in water treatment? J AWWA (Am Water Works Assoc) 1984;76(6):87–93. [Google Scholar]
- 3.Ndabigengesere A., Narasiah K.S., Talbot B.G. Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 1995;29:703–710. [Google Scholar]
- 4.Raghuwanshi P.K., Mandloi M., Sharma A.J., Malviya H.S., Chaudhari S. Improving filtrate quality using agrobased materials as coagulant aid. Water Qual. Res. J. Can. 2002;37:745–756. [Google Scholar]
- 5.Diaz A F.C., Rincon N., Escorihuela A., Fernandez N., Chacin E. A preliminary evaluation of turbidity removal by natural coagulants indegeneous to Venezuela. Process Biochem. 1999;35:391–395. [Google Scholar]
- 6.Šćiban M., Klašnja M., Antov M., Škrbić B. Removal of water turbidity by natural coagulants obtained from chestnut and acorn. Bioresour. Technol. 2009;100:6639–6643. doi: 10.1016/j.biortech.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 7.Narasiah K.S., Vogel A., Kramadhati N.N. Coagulation of turbid waters using Moringa oleifera seeds from two distinct sources. Water Sci. Technol. Water Supply. 2002;2:83–88. [Google Scholar]
- 8.Sanghi R., Bhatttacharya B., Singh V. Cassia angustifolia seed gum as an effective natural coagulant for decolourisation of dye solutions. Green Chem. 2002;4:252–254. [Google Scholar]
- 9.Okuda T., Baes A.U., Nishijima W., Okada M. Coagulation mechanism of salt solution-extracted active component in Moringa oleifera seeds. Water Res. 2001;35:830–834. doi: 10.1016/s0043-1354(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 10.Ravikovitch P.I., Vishnyakov A., Russo R., Neimark A.V. Unified approach to pore size characterization of microporous carbonaceous materials from N 2 , Ar, and CO 2 adsorption isotherms. Langmuir. 2000;16:2311–2320. [Google Scholar]
- 11.Altenor S., Gaspard S. Royal Society of Chemistry; Cambridge: 2013. Biomass for Sustainable Applications. [Google Scholar]
- 12.United States Environmental Protection Agency Drinking water contaminants. Am. Child. Environ. 2015:21–23. [Google Scholar]