Abstract
The recent emergence of Staphylococcus schleiferi in dogs with otitis externa or skin and soft tissue infections has become a significant zoonotic issues. In the current study, we investigated 1) the carriage rates of S. schleiferi among major staphylococci in healthy dogs and dogs with otitis externa, 2) antibiotic susceptibility profiles of S. schleiferi, particularly methicillin resistance (MR), and 3) virulence factors associated with skin and soft tissue infections such as ability to form biofilm, resistance to cationic antimicrobial peptides (CAMPs), and carriage of staphylococcal enterotoxin genes. Among the 21 S. schleiferi isolates, 5 isolates (24%) were determined to be methicillin-resistant (MRSS). Staphylococcal cassette chromosome mec (SCCmec) typing revealed the presence of SCCmec type V in 4 MRSS isolates and type VII in one MRSS. Higher levels of antibiotic resistance, especially multidrug resistance, were observed in MRSS isolates compared to the methicillin-susceptible S. schleiferi (MSSS) isolates. In addition, MRSS isolates exhibited enhanced ability to form biofilm under static condition and all the 5 MRSS isolates carried three or more enterotoxin genes. However, there were no significant differences in resistance to CAMPs between MRSS and MSSS isolates. These findings suggest that coagulase-negative S. schleiferi is becoming more prevalent in canine otitis externa cases. Our results also highlight the presence of multidrug-resistant MRSS isolates with enhanced biofilm production and carriage of multiple enterotoxins.
Keywords: Staphylococcal otitis externa, dogs, methicillin resistance, virulence factors
INTRODUCTION
Since it was first isolated from human clinical specimens by Freney et al. (1988) [1], Staphylococcus schleiferi subsp. schleiferi (S. schleiferi) has been implicated in a number of infections in humans and companion animals [1,2,3,4,5]. Although coagulase-positive Staphylococcus pseudintermedius has most frequently been recognized as a causative pathogen for canine pyoderma and otitis [6,7,8], the pathogenic potential of S. schleiferi and other coagulase-negative staphylococci (CoNS) in dogs has been underestimated. Furthermore, the recent emergence of S. schleiferi among clinically healthy dogs and dogs with otitis externa and/or pyoderma has become a significant global issues in veterinary medicine due its high antimicrobial resistance and variety of virulence factors [2,3,9].
In Korea, several reports on staphylococcal colonization in healthy and diseased dogs have been published over the last decade, mainly focusing on S. pseudintermedius isolates [8,10,11]. However, to the best of the authors' knowledge, no study has investigated the prevalence of S. schleiferi and other CoNS staphylococci in healthy dogs and dogs with otitis externa that have not received antimicrobial treatment in Korea. Thus, the aim of the current study was to investigate 1) the carriage rates of S. schleiferi among major staphylococcal species in clinically healthy dogs and dogs with otitis externa, 2) the genotypic and phenotypic correlates associated with antimicrobial resistance in S. schleiferi, including the detection of the mecA gene, staphylococcal cassette chromosome mec (SCCmec) typing, and antimicrobial resistance profiles, and 3) important virulence factors associated with skin and soft tissue infections such as biofilm formation, susceptibilities to two prototypical antimicrobial peptides (K9CATH and LL-37) of canine and human origins, and carriage of staphylococcal enterotoxins (SEs) genes.
MATERIALS AND METHODS
Bacterial isolation and identification
A total of 69 staphylococcal strains were isolated from swab samples collected from 80 dogs (42 healthy dogs and 38 dogs with otitis externa) that were referred to three different tertiary veterinary hospitals located in the cities of Seoul, Seongnam, and Yongin from 2017 to 2018. None of the dogs had prior exposure to any antimicrobial agent within the previous 30 days. Two samples were obtained from each dog (> 12 months old of both sexes) from both ears using separate sterile cotton swabs, and these were inoculated onto Baird-Parker agar plates (Difco, USA) and blood agar plates at 37°C for 24-48 h.
All staphylococcal strains were identified by using both a Vitek 2 system (BioMérieux, France) and 16S rRNA sequencing (Cosmogenetech, Korea). Sequencing of tuf gene was also performed to confirm S. schleiferi isolates [12]. Coagulase production was confirmed as described in previous research [13].
Antimicrobial susceptibility assays
Susceptibility assays were performed using the disc diffusion methods according to the 2017 Clinical and Laboratory Standards Institute guidelines [14]. The antimicrobial agents used were ampicillin (AMP, 10 μg), chloramphenicol (CHL, 30 μg), erythromycin (ERY, 15 μg), enrofloxacin (ENR, 5 μg), gentamicin (GEN, 10 μg), kanamycin (KAN, 30 μg), oxacillin (OXA, 1 μg), rifampicin (RIF, 5 μg), trimethoprim-sulfamethoxazole (SXT, 23.75–1.25 μg), and tetracycline (TET, 30 μg). In addition to the disc diffusion tests, OXA minimal inhibitory concentrations (MICs) were determined for all S. schleiferi isolates by using 2-fold broth microdilution method at concentrations ranging from 0.015 μg/mL to 32 μg/mL [14,15]. S. schleiferi strains exhibiting OXA MIC of ≥ 0.5 μg/mL were determined as methicillin-resistant S. schleiferi (MRSS) [15].
Detection of mecA gene and SCCmec typing
Because OXA-susceptible staphylococci may harbor the mecA gene [16], polymerase chain reaction (PCR) assays for the detection of mecA gene was performed on all staphylococcal isolates independent of antimicrobial susceptibility assays, as described before with minor modifications [17]. Briefly, the primers used to amplify mecA were mecA-F (5′-TGCTATCCACCCTCAAACAGG-3′) and mecA-R (5′-AACGTTGTAACCACCCCAA GA-3′). PCR amplification was carried out with an initial denaturation at 95°C for 2 min followed by 30 cycles of amplification (denaturation at 95°C for 30 sec, annealing at 57°C for 30 sec, and extension at 72°C for 2 min) and final extension at 72°C for 5 min.
SCCmec types were determined by using multiplex PCR analysis as described in previous research with minor modifications [17]. Briefly, PCR was performed to amplify cassette chromosome recombinase genes (ccrA1, ccrB1, ccrA2, ccrB2, ccrA3, ccrB3, and ccrC) and mec regulatory genes. The combinations of ccr types and classes of mec gene complexes were used to determine the SCCmec types of the S. schleiferi isolates.
Detection of SEs
Detection of 19 different SEs in S. schleiferi isolates was performed by using a multiplex PCR method as described previously [18,19] with minor modification. Briefly, four separate multiplex PCR reactions were set for the amplification of 19 SE genes: set 1 for sea, seb, sec, sed, see; set 2 for seg, seh, sei, selj, selp; set 3 for selk, selm, selo, tst1; and set 4 for sell, seln, selq, selr, selu. Mixture of genomic DNA samples from reference Staphylococcus aureus strains were used for positive controls for each multiplex PCR (FRI472: sed, seg, selj, sell, selm, seln, selo, selr, selu [20]; MW2: seh [21]; FRI913: sea, sec, see, selk, selq, tst1 [22]; COL: seb [23]; and N135: sei, selp [24]).
Biofilm formation assays
Biofilm formation under static conditions was determined on all the S. schleiferi isolates as described previously [25]. Briefly, S. schleiferi cells from overnight culture were adjusted to a density of 0.5 McFarland standard and diluted 1:100 into brain heart infusion broth (Becton Dickinson, France) supplemented with 0.5% glucose. Then, 200 μL of the staphylococcal cell suspension was transferred to 96-flat well polystyrene cell culture plates (SPL life sciences, Korea) and incubated for 48 h at 37°C as described before [25]. After 48 h incubation, wells were washed twice with phosphate-buffered saline, air dried, and stained with 5% safranin for 5 min (Sigma, USA). The dying agent adherent on the cells was dissolved by 30% acetic acid (Sigma) and the absorbance was measured at OD492nm [25]. The extent of biofilm formation in S. aureus Newman strain was normalized to 100%. A minimum of three independent biofilm assays was performed for each S. schleiferi isolate.
In vitro susceptibilities to cationic antimicrobial peptides (CAMPs)
Cathelicidins represent prototypical CAMPs and are characterized the key elements in the host defense mechanisms within epithelial cells, intestinal mucosa, and skin. Human cathelicidin (LL-37) [26] and canine cathelicidin (K9CATH) [27] were synthesized at GL Biochen (China) with purity > 95%.
Standard MIC testing in nutrient broth, such as Mueller Hinton broth, may inhibit the bactericidal activity of CAMPs [28]. Thus, in vitro susceptibility assays with LL-37 and K9CATH were carried out as previously described using the 2 h microdilution method in RPMI-1640 medium (Sigma) supplemented with 5% Luria-Bertani broth. Briefly, the assays were performed with LL-37 (1 μg/mL) and K9CATH (3 μg/mL) using an initial S. schleiferi cell inoculum of 5 × 103 CFUs. These LL-37 and K9CATH concentrations were determined through extensive preliminary assays displaying their inability to completely kill the initial inoculum of the S. schleiferi cells over the 2-h assay duration. Data represent the relative percentage of surviving CFUs (± standard deviation [SD]) of CAMP-treated versus untreated cells. At least three independent experiments were performed for each cathelicidin peptides.
Statistical analysis
The Mann-Whitney U test was used for statistical analysis of all quantitative assay data using IBM SPSS Statistics 23 software (SPSS, USA). Significance was determined at p value of < 0.05.
RESULTS
Profiles of staphylococci among dogs with otitis externa and healthy dogs
As shown in Table 1, 42 coagulase-positive staphylococci (CoPS) and 27 CoNS were isolated from ear swab samples from 80 dogs. The most common otitis externa-associated staphylococci observed in this study were S. pseudintermedius (26 isolates) and S. schleiferi (19 isolates), which were present in 50% and 39% of the dogs with otitis externa, respectively. All the 41 S. pseudintermedius and 21 S. schleiferi strains were isolates from each individual dog except for the four diseased dogs, which were positive for both S. pseudintermedius and S. schleiferi.
Table 1. Profiles of Staphylococcus spp. isolated from dogs with otitis externa and healthy dogs.
| Staphylococcal strains | No. (%) of staphylococcal isolates from | ||
|---|---|---|---|
| Dogs with otitis externa (n = 38) | Healthy dogs (n = 42) | ||
| CoPS | |||
| S. pseudintermedius | 26 (50) | 15 (31) | |
| S. aureus | 1 (3) | 0 | |
| CoNS | |||
| S. schleiferi | 19 (39) | 2 (5) | |
| S. capitis | 2 (5) | 0 | |
| S. epidermidis | 1 (3) | 0 | |
| S. haemolyticus | 0 | 1 (2) | |
| S. saprophyticus | 0 | 1 (2) | |
| S. equorum | 0 | 1 (2) | |
CoPS, coagulase-positive staphylococci; CoNS, coagulase-negative staphylococci.
Fifteen of the 42 healthy dogs (31%) were also positive for S. pseudintermedius. In contrast, only two S. schleiferi strains were isolated from two healthy dogs, indicating a lower carriage rate of S. schleiferi among healthy dogs compared to the S. pseudintermedius.
MRSS and SCCmec types
MRSS were identified by the detection of mecA and OXA resistance (≤ 17 mm of zone diameter). Overall, 5/21 S. schleiferi (24%) isolates were determined as MRSS. We then determined the SCCmec types of the 5 MRSS isolates. Interestingly, 4 of the 5 MRSS isolates were SCCmec type V and the other one MRSS isolate was SCCmec type VII. These data indicate that the methicillin resistance (MR) observed in the S. schleiferi isolates was mainly conferred by SCCmec type V.
Multidrug resistance (MDR) among S. schleiferi isolates
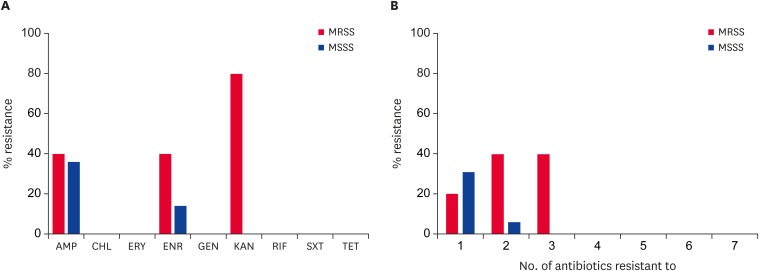
The MRSS isolates exhibited increased levels of resistance to AMP, ENR, and KAN compared to the methicillin-susceptible S. schleiferi (MSSS) isolates (Fig. 1A). Regardless of MR and disease status, all 21 of the S. shcleiferi isolates were susceptible to CHL, ERY, GEN, RIF, SXT, and TET. The two S. schleiferi isolates from healthy dogs were susceptible to all nine antibiotics tested.
Fig. 1. Antimicrobial susceptibility of S. schleiferi isolates from dogs with otitis externa (A) and frequency of multidrug resistance among the S. schleiferi isolates (B). The percentages indicate the rates of resistant isolates among the S. schleiferi isolates. Susceptibility assays were performed using the disc diffusion methods according to the 2017 Clinical and Laboratory Standards Institute guidelines [14].
AMP, ampicillin; CHL, chloramphenicol; ERY, erythromycin; ENR, enrofloxacin; GEN, gentamicin, KAN, kanamycin; RIF, rifampicin; SXT, trimethoprim-sulfamethoxazole; TET, tetracycline; MRSS, methicillin-resistant S. schleiferi; MSSS, methicillin-susceptible S. schleiferi.
As shown in Fig. 1B and Table 2, two MRSS isolates (SS4 and SS6) exhibited MDR, displaying resistance to ≥ 3 different classes of antibiotics.
Table 2. Antimicrobial resistance profiles, SCCmec types, and SE genes of Staphylococcus schleiferi isolates from dogs* .
| Samples No. | Clinical symptoms | SCCmec | mecA | Antimicrobial resistance profiles | OXA MICs† | SEs |
|---|---|---|---|---|---|---|
| SS1 | Otitis externa | - | - | ENR | 0.125 | seg, sei, selk, sell, selm, selq |
| SS2 | V | + | KAN-OXA | 4 | seg, sei, sell, selm, selq | |
| SS3 | V | + | KAN-OXA | 4 | seg, sei, sell, selm, selq | |
| SS4 | V | + | AMP-ENR-KAN-OXA | 8 | seg, sei, sell, selm, selq | |
| SS5 | - | + | - | 0.125 | seg, sei, sell, selm, selq | |
| SS6 | VII | + | AMP-ENR-KAN-OXA | 2 | seg, sei, sell, selq | |
| SS7 | - | - | - | 0.125 | - | |
| SS8 | - | + | AMP | 0.25 | seg, sei, selm | |
| SS9 | - | + | AMP-ENR | 0.25 | seg, sei, selm | |
| SS10 | V | + | OXA | 0.5 | seg, sei, selm | |
| SS11 | - | + | - | 0.125 | selm | |
| SS12 | - | - | - | 0.125 | selm | |
| SS13 | - | - | - | 0.25 | - | |
| SS14 | - | + | - | 0.19 | - | |
| SS15 | - | - | - | 0.19 | seg, sei, selm | |
| SS16 | - | - | - | 0.19 | selo | |
| SS17 | - | - | AMP | 0.25 | selk, selm | |
| SS18 | - | - | AMP | 0.25 | selm | |
| SS19 | - | - | AMP | 0.25 | selm | |
| SS20 | Healthy | - | + | - | 0.125 | sell, selm, selq, selu |
| SS21 | - | + | - | 0.125 | selm, seln, selu |
SCCmec, staphylococcal cassette chromosome mec; ENR, enrofloxacin; KAN, kanamycin; OXA, oxacillin; AMP, ampicillin; SE, staphylococcal enterotoxin; MIC, minimal inhibitory concentration.
*Methicillin-resistant Staphylococcus schleiferi isolates (OXA MICs ≥ 0.5) are represented by shading; †MICs to OXA were determined by broth microdilution method as described in Materials and Methods (the values in bold are greater than or equal to 0.5).
Biofilm formation
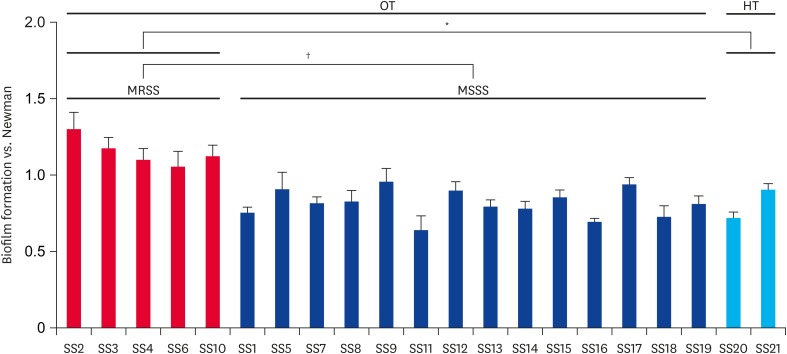
As noted in Fig. 2, the MRSS isolates exhibited significantly enhanced biofilm formation compared with the MSSS isolates (p < 0.01). Interestingly, although only two S. schleiferi strains isolated from healthy dogs were included in the biofilm assays, the MRSS isolates also showed significantly increased biofilm formation versus the two MSSS isolates (p < 0.05).
Fig. 2. Biofilm formation of S. schleiferi isolates. The data was normalized to the wild-type S. aureus Newman strain. Data represent the means (± standard deviation) from three independent experiments.
MRSS, methicillin-resistant S. schleiferi; MSSS, methicillin-susceptible S. schleiferi; OT, dogs with otitis externa; HT, healthy dogs.
*p < 0.05, †p < 0.01.
Prevalence and distribution of SEs
Of the 21 S. schleiferi isolates tested for SEs, 18 isolates (86%) carried at least one SE gene (Table 2). All the 5 MRSS isolates carried more than three SE genes (5 SE genes in SS2, SS3, and SS4 strains; 4 SE genes in SS6; and 3 SE genes in SS10). Most of the S. schleiferi isolates carried multiple SE genes regardless of MR or level of MDR. The two S. schleiferi isolates from healthy dogs, SS20 and SS21, also had 4 and 3 SE genes, respectively. Among the 19 SE genes screened, selm gene was most frequently carried by canine-associated S. schleiferi isolates (76%) followed by seg and sei genes (48% each). None of the S. schleiferi isolates carried sea, seb, sec, sed, see, tsst-1, seh, selj, selp, and selr.
In vitro susceptibilities to K9CATH and LL-37
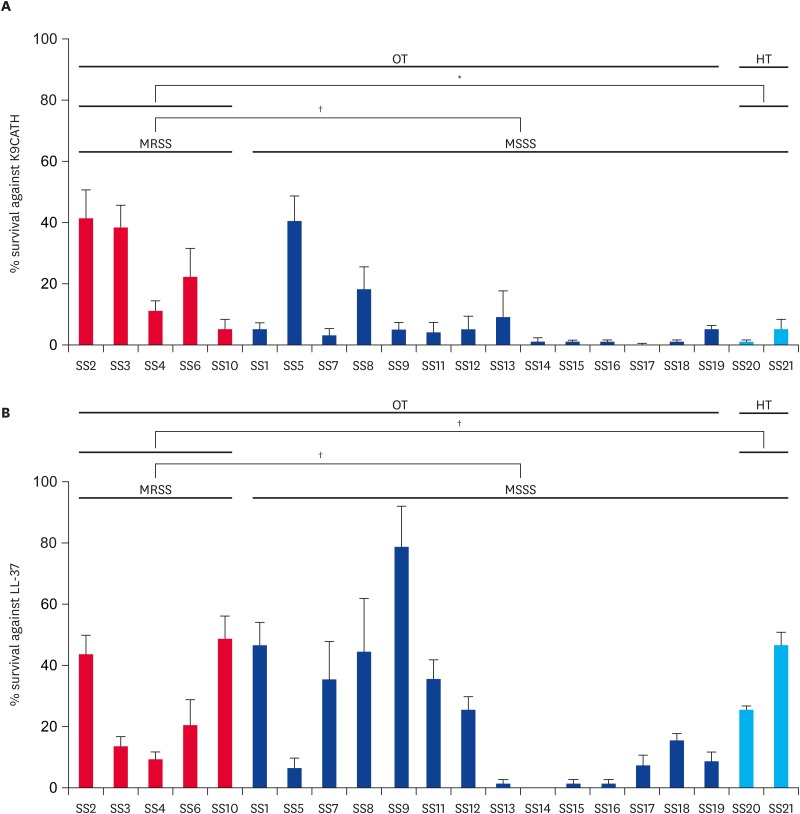
There were no significant differences in susceptibilities to K9CATH and LL-37 between MRSS and MSSS strains isolated from canine otitis externa (Fig. 3). However, the MRSS isolates from dogs with otitis externa showed significantly reduced susceptibility to killing by K9CATH as compared to the two MSSS isolates from healthy dogs.
Fig. 3. In vitro susceptibility profiles of S. schleiferi strains to K9CATH (A) and LL-37 (B). In vitro cell survival assays were performed with K9CATH (3 μg/mL) and LL-37 (1 μg/mL) as described previously [26,27]. The in vitro survival assays were performed at least three times in triplicate. Data represent means (± standard deviation).
MRSS, methicillin-resistant S. schleiferi; MSSS, methicillin-susceptible S. schleiferi; OT, dogs with otitis externa; HT, healthy dogs.
*p < 0.01; †not significant.
DISCUSSION
S. schleiferi has frequently been implicated as an opportunistic pathogen of canine skin and ear infections [9,16,29]. Although at a lower prevalence than in dogs, S. shcleiferi has also been associated with skin infections in cats and small birds [2,30]. S. pseudintermedius has been most frequently associated with canine otitis and/or other skin infections in small companion animals [8]. However, recent studies have demonstrated that CoNS [3,31], particularly S. schleiferi, are becoming an emerging zoonotic pathogen causing a growing concern in both human and veterinary medicine [1,5,30,31]. While several previous studies have investigated the prevalence and molecular characteristics of S. pseudintermedius isolates originating from dogs in Korea [8,10], the carriage of S. schleiferi among dogs, their antibiotic resistance profiles, and virulence factors associated with skin infections have not been studied in detail.
In the current investigation, in agreement with previous studies [8,10,32], S. pseudintermedius was the most frequent staphylococcal isolates from dogs with otitis externa and clinically healthy dogs (50% and 31%, respectively; Table 1). The current study, in support of those by May et al. [9] and Penna et al. [33], also demonstrated the importance of S. schleiferi in canine otitis externa, with 39% of the dogs culture-positive for S. schleiferi. However, unlike the study by Penna et al. [29], which reported a culture-positivity for S. schleiferi of ~32% among healthy dogs in Brazil, only two S. schleiferi isolates were recovered from two of the 42 healthy dogs tested in our investigation. Except for the two major staphylococci, S. pseudintermedius and S. schleiferi, only three other isolates of staphylococci (Staphylococcus capitis, Staphylococcus epidermidis, and S. aureus) were associated with canine otitis externa (Table 1).
Recent publications in Korea have reported MR rates for S. pseudintermedius isolates from clinically healthy dogs or those with skin infections ranging from 34% to 68% [8,10]. In line with these studies, our S. pseudintermedius isolates exhibited a 59% of MR rate overall (62% and 53% for the otitis externa and healthy groups, respectively; data not shown). Although the rate was lower than that of S. pseudintermedius, S. schleiferi isolates recovered from dogs with otitis externa displayed an MRSS prevalence of ~26%. Since the mecA gene lies within the SCCmec element, SCCmec typing was also conducted on all methicillin-resistant S. pseudintermedius (MRSP) and MRSS isolates. Consistent with previous findings in Korea [8,10], the majority of the MRSP isolates (22/24 isolates, 92%) harbored SCCmec V (data not shown). Importantly, 4/5 MRSS isolates (80%) also had SCCmec V present, suggesting that MR may have been disseminated within and between CoPS and CoNS in Korea.
In addition to the MR, MRSS tended to have increased levels of MDR compared to MSSS isolates (Fig. 1A and B). This association between an increased MDR phenotype with mecA-positive S. schleiferi isolates has not been reported before in Korea. Although prior antibiotic use is a major predisposing factors for the selection of antibiotic resistant S. schleiferi, the full history of antibiotic use earlier than 30 days before the ear swab, particularly in dogs with otitis externa, could not be included in the current investigation.
It has been reported that the ability of staphylococci to bind to extracellular adhesion molecules and host skin cells is an important virulence factor for inflammatory skin infections and otitis externa [34,35]. Interestingly, static biofilm assays revealed that the extent of biofilm formation in MRSS isolates were significantly enhanced compared with the MSSS isolates from dogs with otitis externa or healthy dogs (Fig. 2). Since the S. schleiferi biofilm formation during host infection can restrict the access of many clinically important antimicrobial drugs, it is conceivable that MRSS isolates with increased levels of MDR can survive even during antimicrobial treatment, especially against β-lactam antibiotics, and cause persistent infections.
Cathelicidins, typically amphipathic peptides (< 50 amino acids) with a net positive charge, are found in neutrophils, monocytes, and epithelial cells of the skin, gastrointestinal and respiratory epithelial cells. The persistence and progression of skin and soft tissue S. schleiferi infections would require the organism to resist the bactericidal action of cathelicidins. In contrast to the biofilm formation, there were no significant differences in in vitro susceptibilities to two cathelicidins of human and canine origins (LL-37 and K9CATH, respectively) between MRSS and MSSS strains isolated from dogs with otitis externa (Fig. 3A and B). However, the 5 MRSS strains isolated from dogs with otitis externa exhibited significantly higher resistance to K9CATH than the two MSSS strains from healthy dogs. Although this difference was observed in a small number of strains, it is possible that the enhanced ability to form biofilm in MRSS isolates might also reduce the exposure of S. schleiferi cell community within the biofilm mass to host defense CAMPs. Current studies are ongoing in our laboratory to address these hypothesis.
SEs and toxic shock syndrome toxin-1 were originally identified and characterized in S. aureus [18]. Five different types of classical SE genes (sea, seb, sec, sed, and see) had been identified and 19 new types of SEs (seg through sely) have been reported in S. aureus [18]. Although SE genes have frequently been studied in coagulase-positive S. aureus, recent studies demonstrated that classical and newer enterotoxin genes were also carried by CoNS [19,36,37]. In addition, it has been reported that staphylococcal skin colonization and superficial infection were highly correlated with the presence of some of the SEs, possibly through the immunomodulatory activity of the enterotoxins [38]. In the present study, most of the canine-associated S. schleiferi isolates carried SE genes, especially the newer SEs. Of note, 16 of the 21 S. schleiferi strains (76.2%) were positive for selm gene. The enterotoxin gene clusters (egc locus) have been known to harbor several SE genes (seg, sei, selm, seln, and selo) together in staphylococci [37]. In line with this report, 10/16 selm-positive S. schleiferi strains (62.5%) were also positive for seg, sei, seln, or selo (Table 2). Although the enterotoxin genes were found in S. schleiferi strains regardless of disease status or antimicrobial resistance phenotypes, it might be still possible that the immunomodulatory/superantigenic activities of the SEs contribute virulence of the MRSS isolates in combination with enhanced biofilm formation and MDR phenotype.
We recognize that there are some limitations in the current investigation. Due to the limited sample size, we examined a relatively small number of staphylococcal isolates and phenotypes. Moreover, genetic factors associated with enhanced biofilm formation in MRSS isolates were not defined in the present studies. Nonetheless, in conclusion, our results provide important information on the prevalence of S. schleiferi among CoPS and CoNS in healthy dogs and dogs with otitis externa. The widespread presence of SCCmec type V among MRSS and MRSP also suggests that the SCCmec type V could have disseminated between CoPS and CoNS, particularly between S. pseudintermedius and S. schleiferi. In addition, increased level of MDR phenotype as well as higher level of biofilm formation was observed in MRSS compared to the MSSS isolates. Finally, this is the first study to report the prevalence of S. schleiferi among dogs in Korea, particularly MRSS which harbor SCCmec type V.
Footnotes
Funding: This work was supported by Cooperative Research Program for Agriculture Science & Technology Development (grant No. PJ012811) funded by Rural Development Administration, Republic of Korea. This research was also supported by the Chung-Ang University Research Grants in 2017.
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Yang SJ, Hwang SY, Lyoo KS.
- Data curation: Yang SJ, Lyoo KS.
- Formal analysis: Yang SJ, Lee GY.
- Funding acquisition: Yang SJ.
- Investigation: Lee GY, Yang SJ.
- Methodology: Lee GY, Yang SJ, Lee HH, Hong J.
- Writing - original draft: Lee GY, Yang SJ.
- Writing - review & editing: Lee GY, Yang SJ.
References
- 1.Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont PA, Nervi C, Fleurette J. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38:168–172. [Google Scholar]
- 2.Abraham JL, Morris DO, Griffeth GC, Shofer FS, Rankin SC. Surveillance of healthy cats and cats with inflammatory skin disease for colonization of the skin by methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi ssp. schleiferi . Vet Dermatol. 2007;18:252–259. doi: 10.1111/j.1365-3164.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- 3.May ER, Hnilica KA, Frank LA, Jones RD, Bemis DA. Isolation of Staphylococcus schleiferi from healthy dogs and dogs with otitis, pyoderma, or both. J Am Vet Med Assoc. 2005;227:928–931. doi: 10.2460/javma.2005.227.928. [DOI] [PubMed] [Google Scholar]
- 4.Vandenesch F, Lebeau C, Bes M, Lina G, Lina B, Greenland T, Benito Y, Brun Y, Fleurette J, Etienne J. Clotting activity in Staphylococcus schleiferi subspecies from human patients. J Clin Microbiol. 1994;32:388–392. doi: 10.1128/jcm.32.2.388-392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yarbrough ML, Hamad Y, Burnham CA, George IA. The brief case: bacteremia and vertebral osteomyelitis due to Staphylococcus schleiferi . J Clin Microbiol. 2017;55:3157–3161. doi: 10.1128/JCM.00500-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chrobak-Chmiel D, Golke A, Dembele K, Cwiek K, Kizerwetter-Swida M, Rzewuska M, Binek M. Staphylococcus pseudintermedius, both commensal and pathogen. Med Weter. 2018;74:362–370. [Google Scholar]
- 7.Garbacz K, Zarnowska S, Piechowicz L, Haras K. Pathogenicity potential of Staphylococcus pseudintermedius strains isolated from canine carriers and from dogs with infection signs. Virulence. 2013;4:255–259. doi: 10.4161/viru.23526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang JH, Chung TH, Hwang CY. Clonal distribution of methicillin-resistant Staphylococcus pseudintermedius isolates from skin infection of dogs in Korea. Vet Microbiol. 2017;210:32–37. doi: 10.1016/j.vetmic.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 9.May ER, Kinyon JM, Noxon JO. Nasal carriage of Staphylococcus schleiferi from healthy dogs and dogs with otitis, pyoderma or both. Vet Microbiol. 2012;160:443–448. doi: 10.1016/j.vetmic.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Han JI, Rhim H, Yang CH, Park HM. Molecular characteristics of new clonal complexes of Staphylococcus pseudintermedius from clinically normal dogs. Vet Q. 2018;38:14–20. doi: 10.1080/01652176.2017.1400710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo JH, Yoon JW, Lee SY, Park HM. High prevalence of Fluoroquinolone- and Methicillin-resistant Staphylococcus pseudintermedius isolates from canine pyoderma and otitis externa in veterinary teaching hospital. J Microbiol Biotechnol. 2010;20:798–802. [PubMed] [Google Scholar]
- 12.Khosravi AD, Roointan M, Abbasi Montazeri E, Aslani S, Hashemzadeh M, Taheri Soodejani M. Application of tuf gene sequence analysis for the identification of species of coagulase-negative staphylococci in clinical samples and evaluation of their antimicrobial resistance pattern. Infect Drug Resist. 2018;11:1275–1282. doi: 10.2147/IDR.S172144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zdovc I, Ocepek M, Pirs T, Krt B, Pinter L. Microbiological features of Staphylococcus schleiferi subsp. coagulans, isolated from dogs and possible misidentification with other canine coagulase-positive staphylococci. J Vet Med B Infect Dis Vet Public Health. 2004;51:449–454. doi: 10.1111/j.1439-0450.2004.00792.x. [DOI] [PubMed] [Google Scholar]
- 14.Patel JB. Performance standards for antimicrobial susceptibility testing. 27th ed. Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 15.Huse HK, Miller SA, Chandrasekaran S, Hindler JA, Lawhon SD, Bemis DA, Westblade LF, Humphries RM. Evaluation of oxacillin and cefoxitin disk diffusion and MIC breakpoints established by the clinical and laboratory standards institute for detection of mecA-mediated oxacillin resistance in Staphylococcus schleiferi. J Clin Microbiol. 2018;56:e01653–e01617. doi: 10.1128/JCM.01653-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kania SA, Williamson NL, Frank LA, Wilkes RP, Jones RD, Bemis DA. Methicillin resistance of staphylococci isolated from the skin of dogs with pyoderma. Am J Vet Res. 2004;65:1265–1268. doi: 10.2460/ajvr.2004.65.1265. [DOI] [PubMed] [Google Scholar]
- 17.Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, Etienne J, Hiramatsu K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother. 2007;51:264–274. doi: 10.1128/AAC.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher EL, Otto M, Cheung GYC. Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front Microbiol. 2018;9:436. doi: 10.3389/fmicb.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JY, Fox LK, Seo KS, McGuire MA, Park YH, Rurangirwa FR, Sischo WM, Bohach GA. Detection of classical and newly described staphylococcal superantigen genes in coagulase-negative staphylococci isolated from bovine intramammary infections. Vet Microbiol. 2011;147:149–154. doi: 10.1016/j.vetmic.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monday SR, Bohach GA. Use of multiplex PCR to detect classical and newly described pyrogenic toxin genes in staphylococcal isolates. J Clin Microbiol. 1999;37:3411–3414. doi: 10.1128/jcm.37.10.3411-3414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 22.Bania J, Dabrowska A, Bystron J, Korzekwa K, Chrzanowska J, Molenda J. Distribution of newly described enterotoxin-like genes in Staphylococcus aureus from food. Int J Food Microbiol. 2006;108:36–41. doi: 10.1016/j.ijfoodmicro.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt KA, Donegan NP, Kwan WA, Jr, Cheung A. Influences of sigmaB and agr on expression of staphylococcal enterotoxin B (seb) in Staphylococcus aureus. Can J Microbiol. 2004;50:351–360. doi: 10.1139/w04-017. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, Lian J, Ito T, Kanamori M, Matsumaru H, Maruyama A, Murakami H, Hosoyama A, Mizutani-Ui Y, Takahashi NK, Sawano T, Inoue R, Kaito C, Sekimizu K, Hirakawa H, Kuhara S, Goto S, Yabuzaki J, Kanehisa M, Yamashita A, Oshima K, Furuya K, Yoshino C, Shiba T, Hattori M, Ogasawara N, Hayashi H, Hiramatsu K. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 25.Pompilio A, De Nicola S, Crocetta V, Guarnieri S, Savini V, Carretto E, Di Bonaventura G. New insights in Staphylococcus pseudintermedius pathogenicity: antibiotic-resistant biofilm formation by a human wound-associated strain. BMC Microbiol. 2015;15:109. doi: 10.1186/s12866-015-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouhara K, Komatsuzawa H, Kawai T, Nishi H, Fujiwara T, Fujiue Y, Kuwabara M, Sayama K, Hashimoto K, Sugai M. Increased resistance to cationic antimicrobial peptide LL-37 in methicillin-resistant strains of Staphylococcus aureus. J Antimicrob Chemother. 2008;61:1266–1269. doi: 10.1093/jac/dkn106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sang Y, Teresa Ortega M, Rune K, Xiau W, Zhang G, Soulages JL, Lushington GH, Fang J, Williams TD, Blecha F, Melgarejo T. Canine cathelicidin (K9CATH): gene cloning, expression, and biochemical activity of a novel pro-myeloid antimicrobial peptide. Dev Comp Immunol. 2007;31:1278–1296. doi: 10.1016/j.dci.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Xiong YQ, Mukhopadhyay K, Yeaman MR, Adler-Moore J, Bayer AS. Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:3114–3121. doi: 10.1128/AAC.49.8.3114-3121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penna B, Mendes W, Rabello R, Lilenbaum W. Carriage of methicillin susceptible and resistant Staphylococcus schleiferi among dog with or without topic infections. Vet Microbiol. 2013;162:298–299. doi: 10.1016/j.vetmic.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Briscoe JA, Morris DO, Rosenthal KL, Shofer FS, Rankin SC. Evaluation of mucosal and seborrheic sites for staphylococci in two populations of captive psittacines. J Am Vet Med Assoc. 2009;234:901–905. doi: 10.2460/javma.234.7.901. [DOI] [PubMed] [Google Scholar]
- 31.Davis MF, Cain CL, Brazil AM, Rankin SC. Two coagulase-negative staphylococci emerging as potential zoonotic pathogens: wolves in sheep's clothing? Front Microbiol. 2013;4:123. doi: 10.3389/fmicb.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon JW, Lee KJ, Lee SY, Chae MJ, Park JK, Yoo JH, Park HM. Antibiotic resistance profiles of Staphylococcus pseudintermedius isolates from canine patients in Korea. J Microbiol Biotechnol. 2010;20:1764–1768. [PubMed] [Google Scholar]
- 33.Penna B, Varges R, Medeiros L, Martins GM, Martins RR, Lilenbaum W. Species distribution and antimicrobial susceptibility of staphylococci isolated from canine otitis externa. Vet Dermatol. 2010;21:292–296. doi: 10.1111/j.1365-3164.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 34.Moreira CA, de Oliveira LC, Mendes MS, Santiago Tde M, Barros EB, de Carvalho CB. Biofilm production by clinical staphylococci strains from canine otitis. Braz J Microbiol. 2012;43:371–374. doi: 10.1590/S1517-838220120001000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonesson A, Przybyszewska K, Eriksson S, Mörgelin M, Kjellström S, Davies J, Potempa J, Schmidtchen A. Identification of bacterial biofilm and the Staphylococcus aureus derived protease, staphopain, on the skin surface of patients with atopic dermatitis. Sci Rep. 2017;7:8689. doi: 10.1038/s41598-017-08046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argudín MA, Mendoza MC, Rodicio MR. Food poisoning and Staphylococcus aureus enterotoxins. Toxins (Basel) 2010;2:1751–1773. doi: 10.3390/toxins2071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coldea IL, Zota L, Dragomirescu CC, Lixandru BE, Dragulescu EC, Sorokin M, Codita I. Staphylococcus aureus harbouring egc cluster coding for non-classical enterotoxins, involved in a food poisoning outbreak, Romania, 2012. Rev Rom Med Lab. 2015;23:285–294. [Google Scholar]
- 38.Verkaik NJ, Dauwalder O, Antri K, Boubekri I, de Vogel CP, Badiou C, Bes M, Vandenesch F, Tazir M, Hooijkaas H, Verbrugh HA, van Belkum A, Etienne J, Lina G, Ramdani-Bouguessa N, van Wamel WJ. Immunogenicity of toxins during Staphylococcus aureus infection. Clin Infect Dis. 2010;50:61–68. doi: 10.1086/648673. [DOI] [PubMed] [Google Scholar]