Abstract
Foot-and-mouth disease (FMD) is one of the most important livestock diseases in East Africa with outbreaks reported annually that cause severe economic losses. It is possible to control disease using vaccination, but antigenic matching of the vaccine to circulating strains is critical. To determine the relationship between foot-and-mouth disease viruses circulating in districts along the Uganda and Tanzanian border between 2016 and 2017 and currently used vaccines, phylogenetic analysis of the full VP1 virus sequences was carried out on samples collected from both sides of the border. A total of 43 clinical samples were collected from animals exhibiting signs of FMD and VP1 sequences generated from 11 of them. Eight out of the 11 sequences obtained belonged to serotype O and three belonged to serotype A. The serotype O sequences obtained showed limited nucleotide divergence (average of 4.9%) and belonged to topotype East Africa-2, whereas the most common O-type vaccine strain used in the region (O/KEN/77/78) belonged to East Africa-1. The serotype A viruses belonged to topotype Africa-G1 (average nucleotide divergence 7.4%), as did vaccine strain K5/1980. However, vaccine strain K35/1980 belonged to Africa G VII with an average sequence divergence of 20.5% from the study sequences. The genetic distances between current vaccine strains and circulating field strains underscores the crucial need for regular vaccine matching and the importance of collaborative efforts for better control of FMD along this border area.
Keywords: Foot-and-mouth disease, topotype, virus, sequences, East Africa
INTRODUCTION
Foot-and-mouth disease (FMD) is an important livestock disease that can affect all cloven-hooved animals. The disease is ranked among 117 notifiable diseases by the World Organisation for Animal Health and requires immediate action for controlling its spread. Although the disease causes low mortality in adult animals, the economic effects from an outbreak include loss of income and food availability, and increased expenditure at both the individual and national level [1,2].
The foot-and-mouth disease virus (FMDV) is the aetiological agent of FMD with a genome length of approximately 8400 nucleotides. The non-enveloped positive sense RNA virus spreads through both direct contact and airborne transmission [3]. The virus belongs to the Picornaviridae family and Aphthovirus genus with seven serotypes existing globally (O, A, C, Asia 1, and Southern African Territories [SAT] 1, 2, and 3). The non-structural proteins of FMDV are involved mainly in virus replication and coded for by the regions 2A, 2B, 2C, 3A, 3B, 3Cpro and 3Dpol [4] while the structural proteins (SP) VP1, VP2, VP3 and VP4 are coded for by the regions, ID, IB, IC, and IA, respectively. The ID region that encodes VP1 is considered to be the most antigenically variable region of the FMDV and widely used to determine epidemiology and evolutionary relationships between FMDV strains and serotypes [5]. FMD viruses have therefore been grouped into epidemiological and genetic clusters called topotypes based on VP1 region sequences [4,5,6].
Out of 11 topotypes for serotype O viruses, four have been documented to circulate in Uganda, Kenya and Tanzania. These include; East Africa- 1 (EA-1), EA-2, EA-3 and EA-4. Topotype EA-1 has primarily circulated in Uganda and Kenya between 1964 and 1996, but recent studies have revealed that it is currently restricted to Kenya whereas EA-2 is the most recently dominant topotype in the region. Topotype EA-3 and EA-3 are mostly restricted to Ethiopia and Kenya [7]. Serotype A viruses have been grouped into three major topotypes with over 26 genotypes of the virus. In East Africa, four recognized groupings (Africa-G-I, Africa G-II, Africa G-IV and Africa-VII) have been in circulation [8].
All seven serotypes, except for Asia 1, have been reported in East Africa [1,9,10,11,12,13,14,15,16,17], making it the region with the highest diversity of FMDV serotypes in the world. This high diversity has implications for FMD control through vaccination, with a need for frequent and rigorous comparisons between vaccines and circulating strains as well as the development of additional multivalent vaccines. Regular surveillance and characterisation of circulating serotypes is critical for selection of appropriate vaccine strains and to ensure the effectiveness of investments in FMD control. FMD vaccines are commonly used in Uganda with the country's annual expenditure on vaccination control ranging from US $58,000 to $1,088,820 [18]. The eastern Africa border areas have previously been implicated as risk areas for FMD circulation [19], making them important regions for FMD surveillance and control. The aim of this study was to determine the within- and between-topotype genetic diversity of FMD viruses isolated from cattle in the districts along the Uganda-Tanzania border and compare them with current vaccines in use.
MATERIALS AND METHODS
Sample collection and study areas
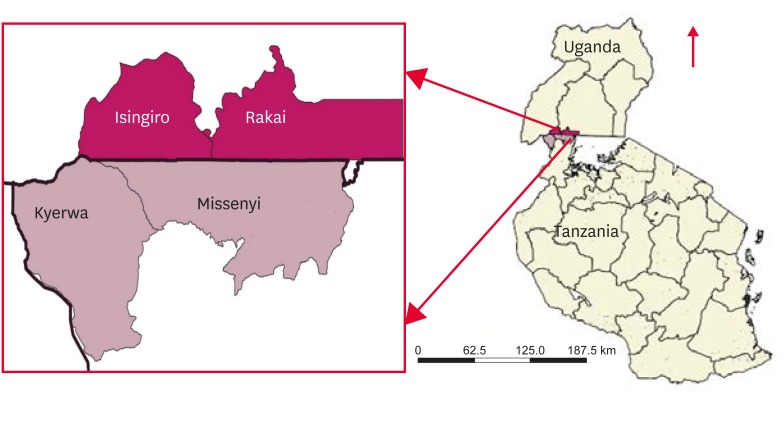
The sampling was approved by the Ministry of Agriculture Animal Industry and Fisheries (Letter No: LHE 138/1) and by the Tanzania Commission for Science and Technology (Permit No: 2016-277-NA-2016-214), and farmers all provided consent before sampling was performed. During 2016-2017, a total of 43 clinical samples were collected from cattle on farms that reported FMD outbreaks in the districts of Isingiro and Rakai located in the western part of Uganda, and the Missenyi and Kyerwa districts located in the north-western part of Tanzania (Fig. 1). Samples were stored in duplicate, one in phosphate buffered saline (PBS) in liquid nitrogen, the other in RNAlater (Ambion, USA). Samples collected included probang tissue, mouth and foot lesion swabs, epithelial tissue, blood, and saliva from animals that exhibited clinical signs of the disease.
Fig. 1. Study districts. Map showing border districts of Isingiro and Rakai in Uganda along with Missenyi and Kyerwa in Tanzania.
RNA extraction and one-step real time reverse transcriptase-polymerase chain reaction (rRT-PCR)
Extraction of total viral RNA was performed using Invitrogen PureLink RNA Mini Kits (USA). The samples were diagnosed by rRT-PCR using Invitrogen SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase (USA) and a Stratagene Mx3000P thermocycler (2009, USA). The 25 µL reaction mix consisted of 2 µL of each primer with a concentration of 10 pmol, 1.5 µL of the probe, 1.5 µL of nuclease free water, 12.5 µL of buffer, 0.5 µL of Supercript III RT/Platinum Taq mix and 5 µL of template. The cDNA synthesis and PCR were run under the following conditions: 50oC for 30 min, 95oC for 2 min, followed by 40 amplification cycles of 95oC for 15 sec, 56oC for 30 sec, 72oC for 30 sec and final extension at 72oC for 3 min. The sequences of the primers and probe set targeted the 3D open reading frame (ORF) of the FMDV genome and as described by [20] (Table 1).
Table 1. Primer and probe sequences used for real time reverse transcriptase-polymerase chain reaction.
| Primers/probe | Sequence | Gene |
|---|---|---|
| Forward primer | 5′-ACT GGG TTT TAC AAA CCT GTG A-3′ | 3D |
| Reverse primer | 5′-TCC TTT GCA CGC CGT GGG AC-3′ | 3D |
| Probe (FAM-TAMRA) | 5′-GCG AGT CCT GCC ACG GA-3′ | 3D |
Antigen enzyme linked immune sorbent assay (Ag ELISA)
The 35 samples that were positive by rRT-PCR were processed and subjected to antigen ELISA that was performed using the IZSLER Antigen ELISA kit obtained from the Pirbright Institute, Pirbright, UK. Small pieces of epithelial and probang tissues were cut using sterile forceps and scissors and placed in separate sterile mortars, 10 mL of PBS was added, the tissues were crushed using sterile pestles and another 5 mL of PBS was added. The suspension was centrifuged at 2,000 g for 10 min, and the supernatants were subjected to the assay procedure following the manufacturer's instructions. The test included a positive control with optical density (OD) > 0.1 and a negative control with OD < 0.1. All samples with OD ≥ 0.1 were considered positive for FMDV while those with OD < 0.1 were considered negative.
Amplification of the VP1 coding region using conventional PCR
The samples that were positive by rRT-PCR were subjected to conventional PCR using Invitrogen SuperScript III One-Step PCR System with Platinum Taq DNA Polymerase. Serotype-specific primers targeting VP1 were selected based on the antigen ELISA results (results not shown). The primers were as described by [20] and are presented in Table 2. For each sample, the reaction master mix was prepared by adding 9.2 µL of nuclease-free water, 1.6 µL of each primer (reverse and forward), 0.8 µL of dNTPs, 4 µL of buffer, 0.8 µL of Qiagen One–step RT-PCR enzyme and 2.0 µL of template, leading to a final 20 µL volume. The four serotype-specific PCRs included nuclease-free water as a negative control and known positive controls that were supplied by the National Animal Disease Diagnostic Centre as RNA extracts that had previously been stored at −80oC. The PCR was run in a Techne TC-412 thermocycler (Techne, USA) using the following cycle conditions; 50oC for 30 min and 95oC for 15 min, 95oC for 10 sec, followed by 35 cycles of 30 sec at 60oC for serotype O, 30 sec at 55°C for serotype A and 30 sec at 50°C for SAT 1, SAT 2 and SAT 3 with extensions of 72oC for 30 sec and 72oC for 10 min as modified from a protocol described by Knowles et al. [20].
Table 2. Serotype primers were used to perform conventional reverse transcriptase-polymerase chain reaction.
| Serotype | Name | Sequence | Direction | Gene | Size |
|---|---|---|---|---|---|
| O | O–1C244F | GCAGCAAAACACATGTCAAACACCTT | + | VP3 | 1,165 |
| O/A/C/Asia | EUR–2B52R | GACATGTCCTCCTGCATCTGGTTGAT | − | 2B | |
| A | A–1C562F | TACCAAATTACACACGGGAA | + | VP3 | 866 |
| SAT 1 | SAT1–1C559F | GTGTATCAGATCACAGACACACA | + | VP3 | 1,043 |
| SAT 1–3 | SAT–2B208R | ACAGCGGCCATGCACGACAG | − | 2B | |
| SAT2 | SAT2 P1–1223F | TGAACTACCACTTCATGTACACAG | + | VP3 | 1,279 |
| SAT3 | SAT3–P1–1222F | AATCTGCATTTCATGTACAC | + | VP3 | 1,277 |
SAT, Southern African Territories.
Gel electrophoresis and cleaning of PCR products
The PCR products were analysed on a 2% agarose Tris/borate/EDTA gel stained with 1% ethidium bromide including a 1 kb Gene ruler DNA ladder (Fermentas, USA). Removal of excess primers and nucleotides was undertaken using the PureLink PCR Purification kit according to the manufacturer's instruction. Elution was achieved with 50 µL of elution buffer.
Sequencing of PCR products
The PCR amplicons were sent to Macrogen (Korea) for sequencing using the BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies, USA). Universal reverse primer NK72 was employed for all samples whereas specific primers (Table 2) were used for each of the different serotypes as described by Knowles et al. [20].
Sequence analysis
The nucleotide sequences obtained were edited using CLC Work Bench version 9.5.3 (Qiagen, USA). The generated sequences were run through the Basic Local Alignment Search Tool (BLAST) [21] in order to retrieve related sequences from Genbank. The serotype O VP1 sequences were aligned using ClustalW as implemented in Molecular Evolutionary Genomic Analysis (MEGA) version 7 [22] and trimmed down to 639 nucleotides. Serotype A VP1 sequences obtained were trimmed to 640 nucleotides after alignment with other selected serotype A sequences from Genbank. Phylogenetic analysis was performed with the neighbour-joining tree method in MEGA 7 based on the Kimura 2-model parameter for nucleotide substitution [23]. The robustness of the phylogeny was assessed with 1,000 bootstrap replicates. Sequences from this study were compared with selected virus sequences derived from cattle from Uganda, Tanzania, Malawi, Zambia, Burundi and Kenya (Table 3). Sequences from Hong Kong, Vietnam and Turkey were employed as out-groups.
Table 3. List of the foot-and-mouth disease virus serotype O and A VP1 sequences employed in the study.
| SN | Country | Year | Serotype | Accession Number | Sequence Name | Reference |
|---|---|---|---|---|---|---|
| 1 | Tanzania | 2016 | O | Not yet available | TAN/02/O/2016 | This study |
| 2 | Tanzania | 2016 | O | Not yet available | TAN/07/O/2016 | This study |
| 3 | Tanzania | 2017 | O | Not yet available | TAN/04/O/2017 | This study |
| 4 | Tanzania | 2017 | O | Not yet available | TAN/08/O/2017 | This study |
| 5 | Uganda | 2017 | O | Not yet available | UG/13/O/2017 | This study |
| 6 | Uganda | 2017 | O | Not yet available | UG/03/O/2017 | This study |
| 7 | Uganda | 2017 | O | Not yet available | UG/09/O/2017 | This study |
| 8 | Uganda | 2017 | O | Not yet available | UG/11/O/2017 | This study |
| 9 | Uganda | 2005 | O | HM756628 | U25/06 | [9] |
| 10 | Tanzania | 2008 | O | KF561684 | TAN/16/2008 | [12] |
| 11 | Uganda | 2004 | O | HM756621 | U20B/04 | [9] |
| 12 | Tanzania | 2002 | O | MF592671 | O/TAN/10/2014 | [1] |
| 13 | Zambia | 2006 | O | KU821591 | O/ZAM/14/2010 | [25] |
| 14 | Uganda | 2009 | O | JN974308 | OUGA2009LIRA | [10] |
| 15 | Uganda | 2011 | O | KF478938 | U04/11 | [13] |
| 16 | Tanzania | 2009 | O | KF561685 | TAN/5/2009 | [12] |
| 17 | Tanzania | 2013 | O | MF592650 | O/TAN/10/2013 | [1] |
| 18 | Tanzania | 2012 | O | MF592623 | O/TAN/38/2012 | [1] |
| 19 | Malawi | 1998 | O | DQ165074 | O/MAL/1/98 | Unpublished |
| 20 | Ethiopia | 2007 | O | FJ798138 | ETH/26/2007 | [28] |
| 21 | Tanzania | 1998 | O | KF561677 | TAN/9/98 | [12] |
| 22 | Tanzania | 2004 | O | KF561682 | TAN/14/2004 | [12] |
| 23 | Uganda | 1996 | O | EU919247 | O/UGA/5/96 | [29] |
| 24 | Kenya | 1978 | O | HM756588 | O/KEN/77/78 | [9] |
| 25 | Kenya | 1995 | O | HM756601 | K56/95 | [9] |
| 26 | Kenya | 2009 | O | KR149720 | KEN/62/2009 | [16] |
| 27 | Kenya | 2011 | O | KF135292 | K91/11 | [16] |
| 28 | Hong Kong | 2002 | O | AY317098 | HKN/2002 | [30] |
| 29 | Tanzania | 2017 | A | Not yet available | TAN/10/A/2017 | This study |
| 30 | Tanzania | 2017 | A | Not yet available | TAN/12/A/2017 | This study |
| 31 | Uganda | 2017 | A | Not yet available | UGA/5/A/2017 | This study |
| 32 | Uganda | 2013 | A | KP089985 | U75/13 | [13] |
| 33 | Kenya | 2009 | A | KF561703 | KEN/22/2009 | [12] |
| 34 | Tanzania | 2009 | A | KF561697 | TAN/47/2009 | [12] |
| 35 | Kenya | 2008 | A | KF561702 | KEN/28/2008 | [12] |
| 36 | Tanzania | 2009 | A | KF561693 | TAN/9/2009 | [12] |
| 37 | Tanzania | 2008 | A | KF561690 | TAN/11/2008 | [12] |
| 38 | Kenya | 2008 | A | KF561701 | KEN/8/2008 | [12] |
| 39 | Kenya | 1966 | A | KF561699 | KEN/42/66 | [12] |
| 40 | Tanzania | 1968 | A | KF561688 | TAN/3/68 | [12] |
| 41 | Tanzania | 1980 | A | KF561689 | TAN/4/80 | [12] |
| 42 | Kenya | 1980 | A | KJ440846 | K35/1980 | [17] |
| 43 | Kenya | 1980 | A | KJ440848 | K5/1980 | [17] |
| 44 | Uganda | 1966 | A | KF112925 | A/UGA/13/66 | [31] |
| 45 | Ghana | 1973 | A | KF561698 | GHA/16/73 | [12] |
| 46 | Nigeria | 1973 | A | KF561704 | NGR/2/73 | [12] |
| 47 | Turkey | 2005 | A | FJ755100 | A/TUR/12/2005 | [32] |
| 48 | Vietnam | 2010 | A | JQ070332 | VIT/1/2010 | [33] |
RESULTS
The FMDV genome was successfully amplified in 35/43 samples using rRT-PCR with cycle threshold (CT) values of less than 35. VP1 sequences were only recovered from 11 of these samples by serotype-specific conventional PCR. The obtained band sizes were as expected; 1,065 base pairs (bp) for serotype O and 866 bp for serotype A. BLAST analysis of 11 VP1 sequences, two from 2016 and nine from 2017, showed that eight were serotype O and three were serotype A. Four serotype O sequences were from the Isingiro district (Uganda) and four from Tanzania (one from Kyerwa and three from the Missenyi districts). For the serotype A sequences, one was from the Rakai district and two from Missenyi, all collected in 2017. Additionally, one of the epidemiological units (farms) in Tanzania had both O and A FMD viruses (TAN/08/O/2017 and TAN/10/A/2017) detected during a single outbreak that occurred in July 2017. Antigen ELISA results showed that epithelial samples were positive for serotypes O, A, and SAT 2.
Analysis of the serotype O VP1 nucleotide sequences
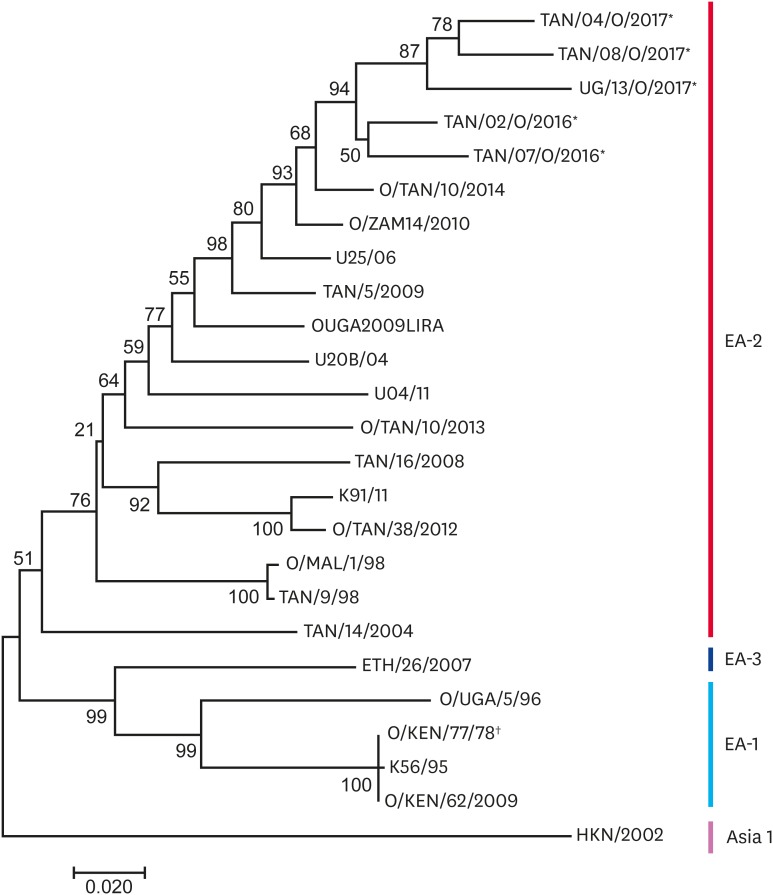
The eight Ugandan and Tanzanian serotype O VP1 sequences obtained from this study had an average nucleotide divergence of 4.9% and grouped together into one clade as shown in the neighbour-joining tree (Fig. 2). The four sequences from Isingiro, Uganda had 100% nucleotide similarity and so only one sequence was used to represent them all in the neighbour-joining tree. The O-type sequences were closest to sequences from Tanzania obtained in 2014 (O/TAN/10/2014) and from Zambia in 2010 (O/ZAM14/2010) with an average intra-serotype nucleotide divergence of 7%. The O-type sequences that were generated in this study belonged to topotype East Africa-2 (EA-2). The study sequences grouped differently from the vaccine strain, O/KEN/77/78, which belonged to topotype EA-1.
Fig. 2. The serotype O neighbour-joining tree. The probability of trees in which the associated taxa clustered together is shown next to the branches. *Study strains; †Vaccine strains.
Analysis of the serotype O VP1 amino acid sequences
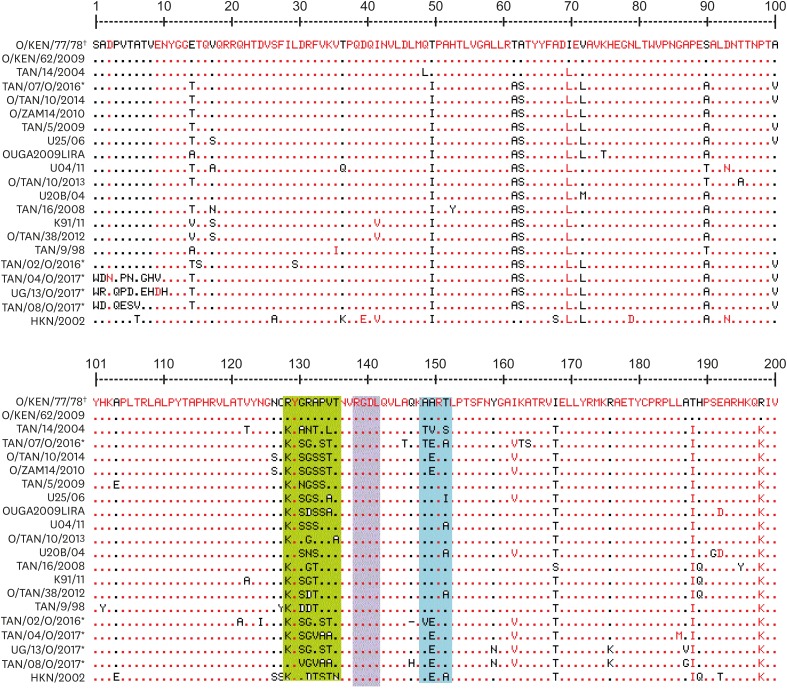
Twenty 200-amino-acid-long sequences were aligned and compared to the vaccine strain, O/KEN/77/78, currently used in East Africa. The Arginine (R) Glycine (G) Aspartate (D) (RGD) motif at positions 139–142 was maintained, while the flanking region upstream of the RGD (positions 129–137) showed high variability between the vaccine strain and field strains from this study (Fig. 3). At the upstream −10 RGD motif, there were up to seven amino acid changes. Study viruses exhibited little variation −10 downstream of the RGD motif, whereas there was considerable variation +10 up stream of the motif. Viruses TAN/07/O/2016, TAN/02/O/2016 and UG/13/O/2017 were more closely aligned in their amino acid sequences compared to the others. Non-synonymous mutations occurred at position 131 in TAN/08/O/2017 where G was changed to V and position 135 in sequences TAN/04/O/2017 and TAN/08/O/2017, where A was changed to V. Other non-synonymous changes were observed in sequences TAN/04/O/2017 and TAN/08/O/2017, where P was changed to A and V was changed to A, respectively. Non synonymous changes were also seen in positions 129 (R to L), 131 (G to S), 132 (R to G), 133 (A to T), 134 (P to S), 138 (V to T) and 136 (T to A). While amino acid alignments between UG/13/O/2017 from Uganda and Tanzanian strain TAN/02//2016 were a close match, the average amino acid difference between sequences obtained from this study and the serotype O vaccine strain O/KEN/77/78 was 22.04%.
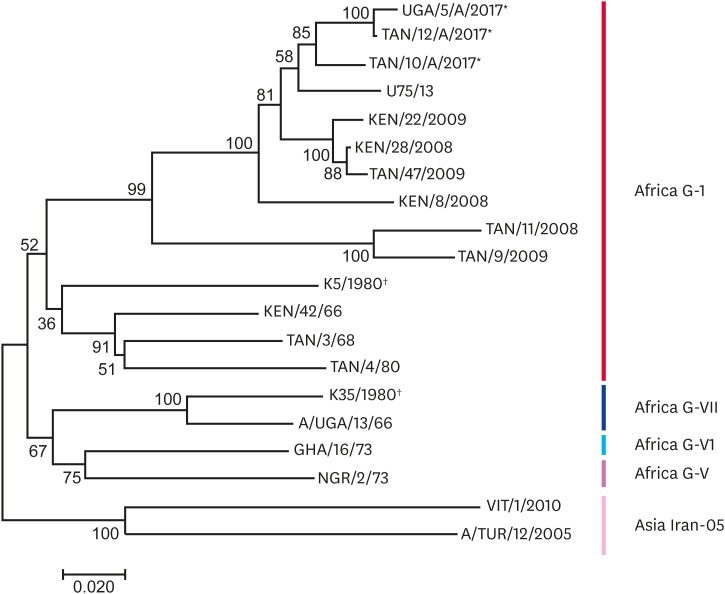
Fig. 3. The serotype A neighbour-joining tree showing 20 nucleotide sequences. The probability of trees in which the associated taxa clustered together is shown next to the branches. *Study sequences; †The vaccine strain in use.
Analysis of the serotype A VP1 nucleotide sequences
The three serotype A viruses from this study grouped together with the Ugandan sequence that was obtained in 2013 from the Isingiro district [13] (Fig. 4). These viruses belonged to topotype Africa G-I and had an overall 7.4% sequence divergence between each other. The vaccine strain, K5/1980, belonged to the same Africa G-I topotype, but was 16.4% different from the study strains, whereas the vaccine strain, K35/1980, belonged to topotype, Africa G-VII.
Fig. 4. Amino acid sequence alignment for serotype O sequences inferred from the study nucleotide sequences (*) with the reference vaccine strain O/O/KEN/77/78 (†) currently incorporated in the vaccines used in Uganda. The conserved RGD motif is within the purple rectangle. The dots represent identical regions to the reference strain, O/O/KEN/77/78, while the green and blue rectangles indicate the changes upstream and downstream of the RGD region.
Analysis of the serotype A VP1 amino acid sequences
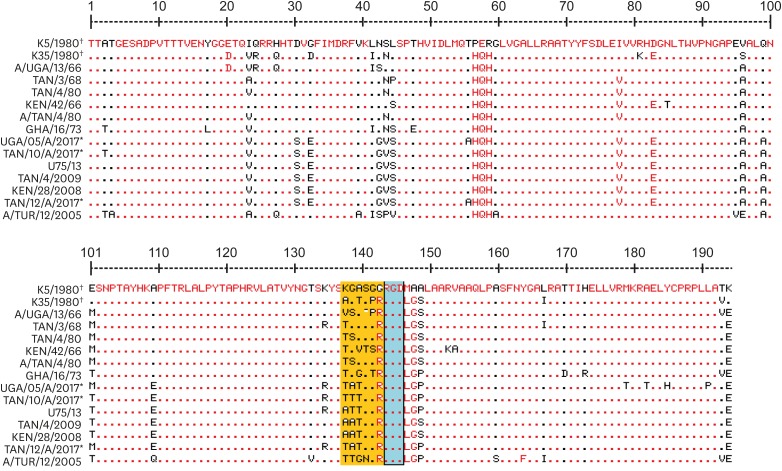
The vaccine strain, K5/1980, which belonged to the same topotype as the study strains (Africa G-I) was used as reference strain for amino acid alignment alongside selected sequences. The results showed that there were 50 variable positions out of the 196 positions, and that the study strains were different from the reference strain at the 124–130 positions (Fig. 5). The RGD motif (144–146) for receptor-binding proteins was conserved across all viruses included in the alignment. However, flanking regions upstream of the RGD motif (from −1 to −6) were highly variable, with major changes from 138–143. Two amino acid changes were observed downstream +2 from the RGD motif. Non-synonymous mutations occurred at position 138 (K to T) and position 140 (A to T). Other non-synonymous changes were observed at position 141 (G to T and G to R). The average amino acid difference between sequences obtained from this study and the serotype A vaccine strain K5/80 was 15.9%.
Fig. 5. Alignment of serotype A partial VP1 amino acid sequences inferred from the study nucleotide sequences (*) with the K5/1980 vaccine sequence (†) and selected serotype A sequences. The conserved RGD motif is within the blue rectangle. The dots represent identical regions to the reference strain, K5/1980. The orange rectangle shows a portion of the upstream region of the RGD motif.
DISCUSSION
The main aim of this work was to compare FMDV isolates from Uganda and Tanzania following outbreaks that occurred in the districts situated along the border of Uganda and Tanzania between 2016 and 2017. The study also sought to compare the variability between the vaccine strains and viruses obtained. The presence of two major outbreaks in this area and circulation of two FMDV serotypes, A and O, within a span of roughly 8 months in this border area highlights the high frequency of outbreaks. The reappearance of serotype A and high similarity between virus strains circulating between the two countries suggests the border is readily permeable to livestock movements and spread of FMD viruses. The presence of two serotypes on the same farm at the same time demonstrates the need for more appropriate multivalent vaccines given vaccines have to be antigenically similar to circulating viral strains [24].
Although analysis of whole genomes is now widely used in epidemiological and evolutionary studies, allowing for high-resolution comparison of viruses, sequencing and analysis of the sub-genomic VP1 region of the FMDV provides a fast, accessible and affordable method that can generate valuable evolutionary and epidemiological insights. Therefore, the strains of FMDV are organised into major groups called topotypes based on phylogenetic analysis of the VP1 region [5,6].
The eight serotype O viruses obtained in this study belonged to the same topotype, EA-2, consistent with other recent studies on FMD in Uganda and Tanzania, which have shown FMD viruses clustering within the EA-2 topotype [5,13,15,25]. Conversely, in the neighbouring country, Kenya, recent serotype O viruses that were responsible for outbreaks between 2010 and 2011 belonged to two topotypes, EA-2 and EA-4 [17].
All serotype O sequences from this study belonged to same topotype, EA-2, with an overall nucleotide sequence divergence of 4.9%. This low intra-serotype difference between Ugandan and Tanzanian sequences suggests that the viruses were responsible for outbreaks in this study had a similar origin. This reinforces results of previous work [9,10,12,13] that demonstrated a close relationship between FMD viruses responsible for outbreaks in Uganda and Tanzania. This study provides new insights into the relationships between trans-border outbreaks and emphasizes border areas as important for FMD epidemiology. The four districts that were included in this study each share the Tanzanian-Ugandan border. With this, the work presented here further supports the view that back and forth human-livestock movement is playing a significant role in trans-boundary disease spread [19,26]. This study additionally provides new insights into relationships between trans-boundery outbreak viruses and vaccine strains. The serotype O vaccine strain (O/KEN/77/78) that is incorporated in the trivalent vaccine currently utilised in East Africa belongs to a different topotype, EA-1, from the serotype O virus isolated from outbreaks and is divergent at over 20% of amino acid positions. A recent vaccine-matching study also demonstrated that the O/KEN/77/78 vaccine strain had a low in vitro match to recent circulating strains in East Africa belonging to EA-2 and EA-3 [7]. Furthermore, alignment of the deduced amino acid sequences shows a number of differences between the reference vaccine strain and circulating strains, confirming that the currently used vaccine may not provide protection against current serotype O FMDVs. Non-synonymous changes observed in the study sequences downstream and upstream of the RGD motif are likely to be antigenically significant and have been reported to be significantly involved in determining receptor specificity for FMDV [27].
Prior to this study, serotype A viruses were most recently isolated from Uganda in 2013 from the Wakiso district and before then in 2002 [18]. The low frequency of detection of serotype A in Tanzania has also been highlighted and was attributed to either poor surveillance systems or certain epidemiological factors associated with the serotype [12]. This study [13] further implicates cross-border movement as a possible factor in the reappearance of type A viruses as it may not be endemic to Tanzania. A recent study by Casey-Bryars [1] in Tanzania showed that serotype outbreaks appeared in waves and a particular serotype may take a while to reappear after an outbreak. This could also be the case for serotype A viruses in Uganda. Serotype A sequences obtained in this study were closely related to viral sequences obtained from the 2013 outbreak from the Wakiso district in Uganda. Namatovu et al. [13] suggested that the origin of the serotype A virus isolated in 2013 could have been from another country given that it was genetically different from previously isolated serotype A viruses from Uganda. However, the similarity between the 2013 and 2016/2017 viruses detected in our study suggests that the serotype A virus may now be circulating as an endemic infection in Uganda with frequent cross-border transmission between Tanzania and Uganda. Although the vaccine strain K5/1980 belongs to the same topotype as the circulating serotype A strains, it is important to note that there were a number of non-synonymous differences in amino acid alignment. Thus, vaccine performance studies must also be carried out. However, the vaccine strain, K35/1980, belongs to a different topotype, Africa G-V11, casting doubt on the effectiveness of this vaccine against A-type viruses presently circulating.
The results from this study show that the isolated serotype O viruses were all closely related and belonged to the EA-2 topotype, which is different from the topotype EA-1 vaccine strain that is currently being used in Uganda and Tanzania. The presence of serotype A both in Uganda and Tanzania is clearly shown in this study and the isolated serotype A viruses were demonstrated to belong to topotype Africa-GI, to which the vaccine strain, K5/1980, also belongs, whereas the vaccine strain, K35/1980, belongs to a different topotype, Africa-GVII.
There is a need for active routine surveillance systems and collaboration efforts between Tanzania and Uganda so that both governments have up-to-date information on currently circulating viruses to enable effective decision-making on vaccine strains to be deployed during vaccination campaigns. Moreover, vaccine-matching studies are paramount for determining how effective the current vaccines are for protection against circulating strains.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Kerfua SD, Kusiluka L, Ayebazibwe C, Cleaveland S, Haydon DT.
- Data curation: Kerfua SD.
- Formal analysis: Kerfua SD.
- Funding acquisition: Kusiluka L.
- Investigation: Kerfua SD.
- Methodology: Kerfua SD, Martin E, Arinaitwe E.
- Project administration: Shirima G, Kusiluka L.
- Resources: Shirima G, Kusiluka L.
- Software: Kerfua SD.
- Supervision: Shirima G, Kusiluka L, Ayebazibwe C, Cleaveland S, Haydon DT.
- Validation: Shirima G, Kusiluka L, Ayebazibwe C, Cleaveland S, Haydon DT.
- Visualization: Kerfua SD.
- Writing - original draft: Kerfua SD.
- Writing - review & editing: Kusiluka L, Cleaveland S, Haydon DT.
References
- 1.Casey-Bryars M, Reeve R, Bastola U, Knowles NJ, Auty H, Bachanek-Bankowska K, Fowler VL, Fyumagwa R, Kazwala R, Kibona T, King A, King DP, Lankester F, Ludi AB, Lugelo A, Maree FF, Mshanga D, Ndhlovu G, Parekh K, Paton DJ, Perry B, Wadsworth J, Parida S, Haydon DT, Marsh TL, Cleaveland S, Lembo T. Waves of endemic foot-and-mouth disease in eastern Africa suggest feasibility of proactive vaccination approaches. Nat Ecol Evol. 2018;2:1449–1457. doi: 10.1038/s41559-018-0636-x. [DOI] [PubMed] [Google Scholar]
- 2.Knight-Jones TJ, Rushton J. The economic impacts of foot and mouth disease - what are they, how big are they and where do they occur? Prev Vet Med. 2013;112:161–173. doi: 10.1016/j.prevetmed.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandersen S, Zhang Z, Donaldson AI, Garland AJ. The pathogenesis and diagnosis of foot-and-mouth disease. J Comp Pathol. 2003;129:1–36. doi: 10.1016/s0021-9975(03)00041-0. [DOI] [PubMed] [Google Scholar]
- 4.Jamal SM, Belsham GJ. Foot-and-mouth disease: past, present and future. Vet Res (Faisalabad) 2013;44:116. doi: 10.1186/1297-9716-44-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowles NJ, Samuel AR. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003;91:65–80. doi: 10.1016/s0168-1702(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 6.Samuel AR, Knowles NJ. Foot-and-mouth disease type O viruses exhibit genetically and geographically distinct evolutionary lineages (topotypes) J Gen Virol. 2001;82:609–621. doi: 10.1099/0022-1317-82-3-609. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones K, Mahapatra M, Upadhyaya S, Paton DJ, Babu A, Hutchings G, Parida S. Genetic and antigenic characterization of serotype O FMD viruses from East Africa for the selection of suitable vaccine strain. Vaccine. 2017;35:6842–6849. doi: 10.1016/j.vaccine.2017.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bari FD, Parida S, Tekleghiorghis T, Dekker A, Sangula A, Reeve R, Haydon DT, Paton DJ, Mahapatra M. Genetic and antigenic characterisation of serotype A FMD viruses from East Africa to select new vaccine strains. Vaccine. 2014;32:5794–5800. doi: 10.1016/j.vaccine.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balinda SN, Sangula AK, Heller R, Muwanika VB, Belsham GJ, Masembe C, Siegismund HR. Diversity and transboundary mobility of serotype O foot-and-mouth disease virus in East Africa: implications for vaccination policies. Infect Genet Evol. 2010;10:1058–1065. doi: 10.1016/j.meegid.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Kasambula L, Belsham GJ, Siegismund HR, Muwanika VB, Ademun-Okurut AR, Masembe C. Serotype identification and VP1 coding sequence analysis of foot-and-mouth disease viruses from outbreaks in eastern and northern Uganda in 2008/9. Transbound Emerg Dis. 2012;59:323–330. doi: 10.1111/j.1865-1682.2011.01276.x. [DOI] [PubMed] [Google Scholar]
- 11.Kasanga CJ, Wadsworth J, Mpelumbe-Ngeleja CA, Sallu R, Kivaria F, Wambura PN, Yongolo MG, Rweyemamu MM, Knowles NJ, King DP. Molecular characterisation of foot-and-mouth disease viruses collected in Tanzania between 1967 and 2009. Transbound Emerg Dis. 2015;62:e19–e29. doi: 10.1111/tbed.12200. [DOI] [PubMed] [Google Scholar]
- 12.Kasanga CJ, Sallu R, Kivaria F, Mkama M, Masambu J, Yongolo M, Das S, Mpelumbe-Ngeleja C, Wambura PN, King DP, Rweyemamu MM. Foot-and-mouth disease virus serotypes detected in Tanzania from 2003 to 2010: conjectured status and future prospects. Onderstepoort J Vet Res. 2012;79:462. doi: 10.4102/ojvr.v79i2.462. [DOI] [PubMed] [Google Scholar]
- 13.Namatovu A, Tjørnehøj K, Belsham GJ, Dhikusooka MT, Wekesa SN, Muwanika VB, Siegismund HR, Ayebazibwe C. Characterization of foot-and-mouth disease viruses (FMDVs) from Ugandan cattle outbreaks during 2012–2013: evidence for circulation of multiple serotypes. PLoS One. 2015;10:e0114811. doi: 10.1371/journal.pone.0114811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sangula AK, Siegismund HR, Belsham GJ, Balinda SN, Masembe C, Muwanika VB. Low diversity of foot-and-mouth disease serotype C virus in Kenya: evidence for probable vaccine strain re-introductions in the field. Epidemiol Infect. 2011;139:189–196. doi: 10.1017/S0950268810000580. [DOI] [PubMed] [Google Scholar]
- 15.Swai ES, Mrosso A, Masambu JI. Occurrence of foot and mouth disease serotypes in Tanzania: a retrospective study of tongue epithelial tissue samples. Tanz Vet J. 2009;26:7–12. [Google Scholar]
- 16.Wekesa SN, Muwanika VB, Siegismund HR, Sangula AK, Namatovu A, Dhikusooka MT, Tjørnehøj K, Balinda SN, Wadsworth J, Knowles NJ, Belsham GJ. Analysis of recent serotype O foot-and-mouth disease viruses from livestock in Kenya: evidence of four independently evolving lineages. Transbound Emerg Dis. 2015;62:305–314. doi: 10.1111/tbed.12152. [DOI] [PubMed] [Google Scholar]
- 17.Wekesa SN, Sangula AK, Belsham GJ, Muwanika VB, Heller R, Balinda SN, Masembe C, Siegismund HR. Genetic diversity of serotype A foot-and-mouth disease viruses in Kenya from 1964 to 2013; implications for control strategies in eastern Africa. Infect Genet Evol. 2014;21:408–417. doi: 10.1016/j.meegid.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Muleme M, Barigye R, Khaitsa ML, Berry E, Wamono AW, Ayebazibwe C. Effectiveness of vaccines and vaccination programs for the control of foot-and-mouth disease in Uganda, 2001–2010. Trop Anim Health Prod. 2013;45:35–43. doi: 10.1007/s11250-012-0254-6. [DOI] [PubMed] [Google Scholar]
- 19.Di Nardo A, Knowles NJ, Paton DJ. Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub-Saharan Africa, the Middle East and Southeast Asia. Rev Sci Tech. 2011;30:63–85. doi: 10.20506/rst.30.1.2022. [DOI] [PubMed] [Google Scholar]
- 20.Knowles NJ, Wadsworth J, Bachanek-Bankowska K, King DP. VP1 sequencing protocol for foot and mouth disease virus molecular epidemiology. Rev Sci Tech. 2016;35:741–755. doi: 10.20506/rst.35.3.2565. [DOI] [PubMed] [Google Scholar]
- 21.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 24.Kitching P, Hammond J, Jeggo M, Charleston B, Paton D, Rodriguez L, Heckert R. Global FMD control--is it an option? Vaccine. 2007;25:5660–5664. doi: 10.1016/j.vaccine.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 25.Van Borm S, Rosseel T, Haegeman A, Fana ME, Seoke L, Hyera J, Matlho G, Vandenbussche F, De Clercq K. Complete genome sequences of three African foot-and-mouth disease viruses from clinical samples isolated in 2009 and 2010. Genome Announc. 2016;4:pii: e00326-e16. doi: 10.1128/genomeA.00326-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fèvre EM, Bronsvoort BM, Hamilton KA, Cleaveland S. Animal movements and the spread of infectious diseases. Trends Microbiol. 2006;14:125–131. doi: 10.1016/j.tim.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burman A, Clark S, Abrescia NG, Fry EE, Stuart DI, Jackson T. Specificity of the VP1 GH loop of foot-and-mouth disease virus for alphav integrins. J Virol. 2006;80:9798–9810. doi: 10.1128/JVI.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayelet G, Mahapatra M, Gelaye E, Egziabher BG, Rufeal T, Sahle M, Ferris NP, Wadsworth J, Hutchings GH, Knowles NJ. Genetic characterization of foot-and-mouth disease viruses, Ethiopia, 1981–2007. Emerg Infect Dis. 2009;15:1409–1417. doi: 10.3201/eid1509.090091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chitray M, de Beer TA, Vosloo W, Maree FF. Genetic heterogeneity in the leader and P1-coding regions of foot-and-mouth disease virus serotypes A and O in Africa. Arch Virol. 2014;159:947–961. doi: 10.1007/s00705-013-1838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Q, Yu H, Liu Y, He C, Hu J, Sang H, Ding N, Ding M, Fung YW, Lau LT, Yu AC, Chen J. Genome comparison of a novel foot-and-mouth disease virus with other FMDV strains. Biochem Biophys Res Commun. 2004;323:254–263. doi: 10.1016/j.bbrc.2004.08.086. [DOI] [PubMed] [Google Scholar]
- 31.Ludi A, Ahmed Z, Pomeroy LW, Pauszek SJ, Smoliga GR, Moritz M, Dickmu S, Abdoulkadiri S, Arzt J, Garabed R, Rodriguez LL. Serotype diversity of foot-and-mouth disease virus in livestock without history of vaccination in the far north region of Cameroon. Transbound Emerg Dis. 2016;63:e27–e38. doi: 10.1111/tbed.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knowles NJ, Nazem Shirazi MH, Wadsworth J, Swabey KG, Stirling JM, Statham RJ, Li Y, Hutchings GH, Ferris NP, Parlak U, Ozyörük F, Sumption KJ, King DP, Paton DJ. Recent spread of a new strain (A-Iran-05) of foot-and-mouth disease virus type A in the Middle East. Transbound Emerg Dis. 2009;56:157–169. doi: 10.1111/j.1865-1682.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- 33.Knowles NJ, He J, Shang Y, Wadsworth J, Valdazo-González B, Onosato H, Fukai K, Morioka K, Yoshida K, Cho IS, Kim SM, Park JH, Lee KN, Luk G, Borisov V, Scherbakov A, Timina A, Bold D, Nguyen T, Paton DJ, Hammond JM, Liu X, King DP. Southeast Asian foot-and-mouth disease viruses in Eastern Asia. Emerg Infect Dis. 2012;18:499–501. doi: 10.3201/eid1803.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]