Abstract
Mammary lesions in sows can prevent suckling piglets from consuming colostrum that provides fundamental nutrients and protective immunity. Although mammary gross lesions are frequently found in sows at farms or slaughterhouses, with the exception of mastitis, they have received little research attention. In this study, we investigated mammary lesions observed in South Korean sows between 2015 and 2016. Mammary tissue samples of 82 sows showing gross lesions during meat inspection were histologically classified and immunohistochemical analysis was conducted to assess the expression of estrogen receptor (ER)-α, ER-β, and progesterone receptor (PR) for mammary hyperplastic lesions as well as that of cluster of differentiation (CD) 3, CD79a, interleukin (IL)-1α, IL-1β, IL-6, and IL-8 for mastitis. Furthermore, 20 swab samples were cultured, and the isolated bacteria were identified using polymerase chain reactions for 16S ribosomal RNA genes. The lesions were classified as hyperplasia, mastitis, or hyperplasia with mastitis. Immunohistochemistry results revealed that there was neither expression of ER-α nor of ER-β, but all examined hyperplastic samples expressed PR. In addition, there was a significant correlation between CD3 and IL-1β expressions, as well as between IL-1β and IL-6 expressions. Regarding the identity of the isolated bacteria, Pseudomonas spp. were most frequently detected. The results of this study have revealed the incidence and characteristics of porcine mammary lesions.
Keywords: Swine, mammary gland, mastitis, bacterial infection, cytokines
INTRODUCTION
Gross lesions at or near the mammary area in sows are common and are generally detected during meat inspections or audits of breeding conditions; in living animals, they are largely neglected and without treatment provided. However, such lesions potentially negatively affect the animals' quality of life, endanger animal welfare, and cause farrowing which could limit colostrum delivery and consequentially lead to defects in fundamental protective immunity among piglets [1,2].
The prevalence of gross lesions of mammary tissues depends on inspection methods and regulations in each country. According to a gross inspection report from the United States, 3.3% of slaughterhouse sows exhibit abnormalities at the udder [3]. In contrast, by adopting observation and palpation as their diagnostic method, researchers in Sweden reported that 16% to 19% of sows at 76 farms had granulomatous mastitis in 1999 [4]. In addition, a study performed in Denmark revealed that, at the time of meat inspection, and using inspection and palpation with incision performed if the nature of the lesion was uncertain, 10.5% of slaughterhouse sow carcasses had udder lesions [5].
Although the majority of lesions are found to be mastitis, hyperplasia can develop and neoplasm can also occur; however, cases of neoplasm have been extremely rare [6]. The etiology of mastitis in sows has been extensively studied, with Staphylococcus spp., Streptococcus spp., Actinomyces spp., and Escherichia coli being the primary pathogens isolated from such lesions [7,8]. Research investigating immunological responses against these bacteria has revealed that specific cytokines have a protective role against and are involved in the clinical signs of mastitis. Studies have reported that when sows were inoculated intramammarily with E. coli, animals with clinical signs had low basal levels of interleukin (IL)-1β and increased expressions of IL-1β, IL-6, and IL-10 compared with those in non-inoculated animals [9,10].
Morphological changes resulting from mammary gland hyperplasia can develop from physiological responses involving secretory activity [11] but are also triggered by other pathological responses to continuous stimulation, such as infection or duct obstruction, and can form adenoses as a reaction to adjacent benign/malignant neoplasms as has been reported in canines [12]. The pathophysiology of mammary gland lesions in some species, such as canines or felines, has been extensively investigated, in particular, that of mammary gland tumors. Estrogen receptor (ER) is one of the key factors in the formation of mammary gland lesions [13,14]. Although some studies have explored the physiological development of the porcine mammary gland [15,16], those investigating the characteristics of hyperplastic lesions in this gland are scarce.
In the present study, we aimed to histopathologically categorize porcine mammary lesions detected in South Korea in 2015–2016 and to investigate the features of these lesions by applying bacteriological and immunohistochemical methods.
MATERIALS AND METHODS
Tissue samples and histological analysis
Eighty-two sow mammary tissue specimens exhibiting gross lesion(s) detected during meat inspection were acquired from slaughterhouses in Incheon and Gyeonggi (South Korea), in 2015 and 2016. Swab samples were obtained from 20 tissue specimens associated with suppurative exudates after incision. The samples were taken from sows which had experienced parturition more than 6 times (approximately 4 to 5 years old). The sows had been slaughtered because of their low reproduction rate due to poor milk secretion caused by the prolonged presence of mammary lesions; regardless, they did not exhibit any signs of systemic illness. Tissue specimens were fixed in 10% neutral buffered formalin, trimmed, embedded in paraffin, and sectioned (4 μm thick) per routine laboratory protocols [17]. From each sow, 1 or 2 sections of the mammary tissue specimen were stained with hematoxylin and eosin and histologically analyzed to categorize mastitis and hyperplasia in the lesions.
Preparation for immunohistochemistry
To characterize the lesions, the expressions of ER-α, ER-β, and the progesterone receptor (PR) in mammary hyperplastic lesions and the cluster of differentiation (CD) 3 and CD79a, as well as IL-1α, IL-1β, IL-6, and IL-8, in inflammatory lesions were analyzed by immunohistochemical assay using specific primary antibodies for each of these investigated targets. Briefly, sectioned tissues were dried for 1 day, followed by dewaxing in xylene, and then rehydrated through a succession of graded ethanol baths. Tissue sections were treated for 20 min at room temperature with 3% hydrogen peroxide in phosphate-buffered saline (PBS; pH 7.4, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4) to inhibit endogenous peroxidase activity. Subsequently, heat-induced epitope retrieval was performed by boiling the section submerged in Tris-EDTA (pH 9.0; for PR, CD3, IL-6, and IL-8) or citric acid (pH 6.0; for ER-α, ER-β, CD79a, IL-1α, and IL-1β) in a microwave oven; the sections were then cooled in cold water. For each step, 3 PBS washes were performed. Normal goat serum (5%) or normal horse serum (2.5%, for IL-6) diluted with PBS were used as blocking agents, and primary antibodies were sequentially applied as described in Table 1. After washing off unattached primary antibodies with PBS, tissue sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (DAKO REAL Envision kit; DAKO, Denmark) for 40 min or biotinylated anti-goat serum for 15 min and conjugated with streptavidin-HRP (R.T.U. Vectastain kit; Vector Laboratories, USA) for 30 min (for IL-6) at room temperature. To detect immunolabeled proteins, diaminobenzidine was used as a visualizing agent. Tissue sections were then counterstained with Gill's hematoxylin, dehydrated in ethanol, cleared with xylene, and coverslipped. To confirm the specificity of the primary antibodies, normal mammary tissue and spleen and tonsil tissues from sows were used as positive controls for the target hormone receptors CD3/CD79a and IL-1α/IL-1β/IL-6/IL-8, respectively. Isotype-matched immunoglobulins were used as negative controls.
Table 1. Primary antibodies and protocols for immunohistochemical analysis.
| Antibody | Clone | Isotype | Manufacturer | Antigen retrieval | Dilution | Incubation |
|---|---|---|---|---|---|---|
| ER-α | 1D5 | Mouse IgG1 | DAKO | Citric acid, 20 min | 1:100 | 4°C, overnight |
| ER-β | PPG5/10 | Mouse IgG2a | Bio-Rad | Citric acid, 10 min | 1:80 | 4°C, overnight |
| PR | PR10A9 | Mouse IgG2a | Immunotech | Tris-EDTA, 15 min | 1:500 | 4°C, overnight |
| CD3 | Polyclonal | Rabbit polyclonal | DAKO | Tris-EDTA, 5 min | 1:250 | RT, 1h 30min |
| CD79a | HM57 | Mouse IgG1k | DAKO | Citric acid, 10 min | 1:600 | RT, 1h 30min |
| IL-1α | B-7 | Mouse IgG2b | Santa Cruz Biotechnology | Citric acid, 15 min | 1:600 | RT, 1h 30min |
| IL-1β | H-153 | Rabbit polyclonal IgG | Santa Cruz Biotechnology | Citric acid, 20 min | 1:200 | RT, 2h |
| IL-6 | M-19 | Goat polyclonal IgG | Santa Cruz Biotechnology | Tris-EDTA, 15 min | 1:400 | RT, 1h 30min |
| IL-8 | 807 | Mouse IgG1 | Abcam | Tris-EDTA, 5 min | 1:250 | RT, 1h 30min |
ER, estrogen receptor; Ig, immunoglobulin; PR, progesterone receptor; CD, cluster of differentiation; IL, interleukin; RT, room temperature.
Immunohistochemical evaluation
Nuclear expressions of ER-α, ER-β, and PR were considered to be positive when present in >10% of mammary gland epithelial cells [18]. For those factors expressed in the cytoplasm of inflammatory cells, including CD3, CD79a, IL-1α, IL-1β, IL-6, and IL-8, semi-quantitative morphological analyses were performed using the image analysis software ImageJ (version 1.50b) downloaded from the National Institutes of Health website (http://rsb.info.nih.gov/ij). One image at 40× (for CD3 and CD79a) or 5 images at 200× (for IL-1α, IL-1β, IL-6, and IL-8) magnification were acquired at the site of inflammation in each section. The mean of the assessed integrated density (the sum of the multiplied values of an area and the average gray value of the pixels in the image) divided by the tissue area in each image was calculated.
Bacterial culture and identification
Isolation of causative bacteria was achieved by streaking the 20 swab samples on blood agar and incubating those in various environments viz. aerobic, anaerobic, and 5% carbon dioxide for >18 h at 37°C. Subsequently, the 16S ribosomal RNA (rRNA) genes of the isolated bacteria were amplified by performing polymerase chain reaction (PCR) with 27F/1492R universal primers under the following cycling conditions: 94°C for 5 min (pre-denaturation); 30 cycles at 94°C for 1 min (denaturation); 55°C for 1 min (annealing); 72°C for 1 min (extension); and 72°C for 10 min (final extension) [19]. The sequences of the PCR products were analyzed to identify species by using BLAST as provided by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). Homology was accepted at the genus level of the isolated bacteria at the criterion of > 97% identity [20].
Statistical analysis
Pearson's correlation analyses were performed to validate the relevancy between immunoreactivity of the investigated cytokines in the inflammatory lesions; p < 0.05 was considered to indicate a statistically significant difference. SPSS version 17.0 (IBM, USA) was used for statistical analysis.
RESULTS
Gross mammary lesions and their histopathological classification
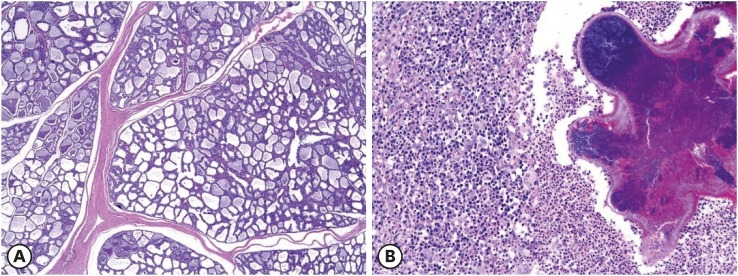
Eighty-two specimens were obtained from pigs that had experienced more than 6 parturitions and exhibited macroscopic, focally swollen, and raised mammary lesions with or without erosion or ulceration. Histopathological findings included mammary gland hyperplasia (n = 46), mammary gland hyperplasia with duct epithelial hyperplasia (n = 3), mastitis (n = 32), and mammary gland hyperplasia with mastitis (n = 1) (Table 2). Mammary gland hyperplasia was diagnosed when the number of epithelial cells composing the mammary gland was increased (Fig. 1A). Ductal hyperplasia was diagnosed in cases in which the number of ductal epithelial cells had increased and had accumulated in the forward center of the duct. All samples diagnosed with mastitis exhibited more than 1 central bacterial focus with inflammatory cells in its vicinity consisting mostly of lymphocytes, macrophages, and neutrophils (Fig. 1B).
Table 2. Histopathological categorization of mammary lesions and hormone receptor expression in mammary hyperplastic lesions.
| Diagnosis | Cases (n = 82) | Cases positive to hormone receptor | ||
|---|---|---|---|---|
| ER-α | ER-β | PR | ||
| Hyperplastic lesion* | 49 (59.76) | 0 (0) | 0 (0) | 49 (100) |
| Mammary gland hyperplasia and mastitis | 1 (1.22) | 0 (0) | 0 (0) | 1 (100) |
| Mastitis† | 32 (39.02) | ND | ND | ND |
Values are presented as number (%).
ER, estrogen receptor; PR, progesterone receptor; ND, not determined.
*Including mammary gland hyperplasia and ductal epithelial hyperplasia; †Bacterial foci and infiltrations of inflammatory cells adjacent to mammary glands.
Fig. 1. Histopathological analysis of mammary lesions in sows. (A) Mammary gland hyperplasia. The number of mammary gland ducts, as well as the number of their epithelial cells, are elevated (H&E stain, 40×). (B) Mastitis. Inflammatory cells composed of lymphocytes, macrophages, and neutrophils congregate around a central bacterial focus (H&E stain, 200×).
H&E, hematoxylin and eosin.
Analysis of the immunohistochemical properties
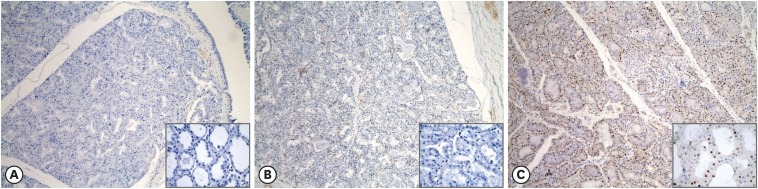
Samples of hyperplastic lesions (n = 50) were immunohistochemically analyzed with ER-α, ER-β, and PR antibodies to determine the role(s) of these factors in the emergence of hyperplasia. None of the ER-α- and ER-β-stained samples were confirmed to be positive (Fig. 2A and B). By contrast, expression of PR was evident in > 10% of the mammary gland tissue in all examined samples (Fig. 2C).
Fig. 2. Immunohistochemical expression of hormone receptors in hyperplastic lesions. ER-α (A) and ER-β (B) are expressed in < 10% of the nuclei of mammary gland epithelial cells. Brownish nucleic expression of PR (C) is present in > 10% of mammary gland epithelial cells (immunohistochemistry, 100×; inset, 600×).
ER, estrogen receptor; PR, progesterone receptor.
Analyses of T- and B-cell distributions were performed using primary antibodies against CD3 and CD79a, respectively (Fig. 3A and B). Among the 32 mastitis samples, 24 (75%) exhibited more B cells than T cells. Expression of cytokines was observed in the cytoplasm of inflammatory cells in all mastitis samples (Fig. 4); however, some epithelial cells also exhibited these cytokines. Although the level of expression varied among samples, significant correlations were identified (Table 3). In samples with an increased presence of T cells, IL-1β expression was upregulated (p < 0.01). By contrast, the B-cell distribution was not significantly correlated with the expression of any cytokines. Among the cytokines, IL-1β and IL-6 had a significantly positive relationship (p < 0.01). Although significant correlations were not detected, T-cell presence exhibited a positive trend with IL-6 expression, but negative associations with IL-1α and IL-8 expressions. The B-cell levels demonstrated positive correlational trends with IL-1α and IL-6 expressions, but negative trends with IL-1β and IL-8 expressions.
Fig. 3. Immunohistochemical expression of CD3 and CD79a in mastitis tissue. Lymphocytes gather around the central bacterial focus and exhibit cytoplasmic expression of CD3 (A) in T cells or CD79a (B) in B cells (immunohistochemistry, 40×; inset, 400×).
CD, cluster of differentiation.
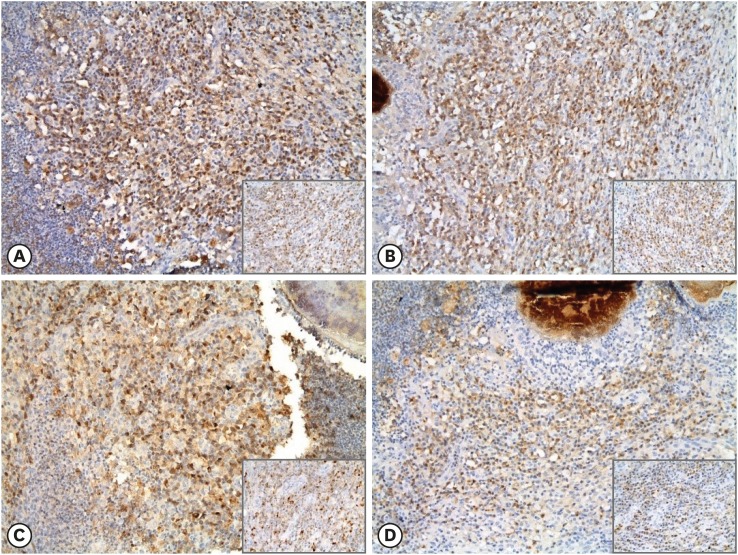
Fig. 4. Immunohistochemical expression of cytokines in mastitis tissue. Brownish cytoplasmic expressions of IL-1α (A), IL-1β (B), IL-6 (C), and IL-8 (D) in infiltrating inflammatory cells, especially lymphocytes, were observed (immunohistochemistry, 200×; inset, 400×).
IL, interleukin.
Table 3. Correlation analysis results for expression of CD3, CD79a, and selected cytokines.
| CD markers and cytokines | CD markers and cytokines | |||||
|---|---|---|---|---|---|---|
| CD3 | CD79a | IL-1α | IL-1β | IL-6 | IL-8 | |
| CD3 | - | 0.115 | −0.036 | 0.473* | 0.257 | −0.043 |
| CD79a | 0.115 | - | 0.180 | −0.008 | 0.024 | −0.097 |
| IL-1α | −0.036 | 0.180 | - | 0.041 | 0.108 | 0.059 |
| IL-1β | 0.473* | −0.008 | 0.041 | - | 0.523* | 0.051 |
| IL-6 | 0.257 | 0.024 | 0.108 | 0.523* | - | 0.250 |
| IL-8 | −0.043 | −0.097 | 0.059 | 0.051 | 0.250 | - |
Data presented as r values.
IL, interleukin; CD, cluster of differentiation.
*Statistically significant (p < 0.01).
Identification of causative bacteria
Sequencing of the 16S rRNA gene was examined to identify the causative bacteria (Table 4). Of the 20 samples, 19 bacterial isolates (demonstrating > 97% identity) were allocated to 8 genera, with Pseudomonas spp. being the most frequently detected (n = 10), followed by Escherichia spp. (n = 2), Psychrobacter spp. (n = 2), and 1 isolate each of Aeromonas spp., Proteus spp., Rahnella spp., Staphylococcus spp., and Yersinia spp.
Table 4. Genera of isolated bacteria based on 16S rRNA gene sequencing.
| Genus | No. of isolates | Identity | Accession |
|---|---|---|---|
| Pseudomonas spp. | 10 (52.63) | 100 | KY849255.1 |
| 99 | KX186955.1 | ||
| 99 | KY849255.1 | ||
| 99 | KX859167.1 | ||
| 99 | KY818010.1 | ||
| 98 | KP267700.1 | ||
| 98 | KX186962.1 | ||
| 98 | LT601008.1 | ||
| 97 | KT693288.1 | ||
| 97 | AB920824.1 | ||
| Escherichia spp. | 2 (10.53) | 98 | MF429396.1 |
| 98 | KY780352.1 | ||
| Psychrobacter spp. | 2 (10.53) | 99 | JX416703.1 |
| 99 | JX416703.1 | ||
| Aeromonas spp. | 1 (5.26) | 99 | JF928538.1 |
| Proteus spp. | 1 (5.26) | 99 | MF185140.1 |
| Rahnella spp. | 1 (5.26) | 99 | KY606575.1 |
| Staphylococcus spp. | 1 (5.26) | 98 | CP020377.1 |
| Yersinia spp. | 1 (5.26) | 98 | CP020409.1 |
Values are presented as number (%).
rRNA, ribosomal RNA.
DISCUSSION
Mammary lesions in sows have been reported with globally varying prevalences [3,4,5]; however, little attention has been devoted to the incidence and characteristics of these lesions. In this study, we used histopathological, immunohistochemical, and bacteriological methods to investigate mammary lesions in samples obtained in 2015–2016 to ascertain the fundamentals of lesion formation.
Histopathological examination of gross mammary lesions detected during visual inspections in slaughterhouses revealed hyperplastic lesions (59.8%), mastitis (39%), and hyperplastic lesions with mastitis (1.2%). This suggests that meat inspections based solely on single visual inspection can easily miss differences in lesion etiology. Even simple masses that do not show skin ulcers could have bacterial colonies, which have been associated with food spoilage [21], such as those of Pseudomonas spp. isolated in this study. Additionally, more than half of the lesions exhibited mammary gland hyperplasia located in the partial udder, especially at the hind quadrant, which could have originated from pathological sources, compared with physiological etiologies generally involving the entire udder [15]. However, in this study, the causes of the hyperplasia were not directly apparent on microscopic investigation, nor could the hyperplasia be directly linked to a specific bacterial infection, except 1 sample that exhibited hyperplasia with mastitis.
Hyperplasia of the mammary gland in mammals is commonly associated with hormonal stimuli, especially estrogen via the ER. Its aberrant expression can lead to oncogenesis [22], as has been reported in canines and felines [13,14]. However, to our knowledge, there has been little―if any―research investigating swine mammary gland tumors to date. We performed immunohistochemical analysis to determine the hormonal status of hyperplastic lesions. Nuclear staining of all samples revealed positivity of PR expression—in contrast to ER-α and ER-β expressions—compared with normal mammary glands. We hypothesize that this may reflect properties of mammary gland hyperplasia that could be induced by means other than a physiological pathway, such as hormonal stimulation, although the exact cause was not determined. An associated factor is the age of the sows; we examined sows that had experienced parturition more than 6 times, while the positive controls were from normal mammary glands of sows with weaned piglets. Further studies examining the mammary glands of sows of various ages are needed to investigate the effects of hormonal status according to age and time of parturition.
Mastitis can cause dysgalactia in lesioned mammary glands; therefore, it can affect weight gain and immune status in piglets, which in turn can lead to lower economic profits for the farm. Some reports have suggested that coliform bacteria are a major etiological agent in mastitis [23], based on immunological signaling a cytokine production under experimental conditions [9]. In the present study, we used immunohistochemical and bacteriological methods to determine the basic immunological properties and to identify the major causative bacteria of mastitis in sows in South Korea.
The most frequently isolated bacteria were Pseudomonas spp. (n = 10, 52.63%), which are gram-negative, aerobic, and ubiquitously distributed in water, soil, and moist conditions [24]. These bacteria can cause opportunistic infection, such as skin wounds and, rarely, induce mastitis and toxemia in sows in a manner similar to that in infected cows [25,26]. Although inflammatory responses were limited to the adjacent dermis and were not around the mammary glands in most cases, our results suggest that infection with Pseudomonas spp. could spread to the mammary gland, indicating it may be a more prevalent agent causing mastitis than previously estimated. The second most commonly isolated bacteria were Escherichia spp. and Psychrobacter spp. (each n = 2, 10.53%). Escherichia spp. are the most common cause of mastitis in pigs and lead to a condition known as coliform mastitis. In addition, Escherichia spp., Aeromonas spp., Proteus spp., Staphylococcus spp., and Yersinia spp. have been associated with potential food poisoning in humans; therefore, breeding management should also consider food safety [27,28,29].
The expression profiles of the cytokines evaluated in this study highlight the basic properties of the inflammatory response to bacterial infection in the skin or mammary gland of pigs. Immunolabeling of CD3, CD79a, and the cytokines investigated in this study helped us examine the relationships between immune cells, such as B and T cells, and pro-inflammatory cytokines (i.e., IL-1α, IL-1β, IL-6, and IL-8), and provided insights into the propagation of inflammation. IL-1β, which belongs to the IL-1 family, is produced by activated macrophages. This cytokine participates in pain regulation via upregulation of pro-nociceptive mediators, is involved in inflammation, regulates the autoimmune status, such as in delayed-type hypersensitivity, and can activate T-cell responses [30,31]. The results of the present study are similar; the number of T cells at the site of the bacterial infection was increased and the expression of IL-1β was significantly elevated. Whether cytoplasmic IL-1β expression was localized solely in T cells or also included B cells was not analyzed; however, trends indicated that higher levels of IL-1β may upregulate T-cell infiltration. For example, IL-1α, another member of the IL-1 family, demonstrated a tendency―albeit insignificant―to be associated with fewer T cells (in contrast to B cells), which may imply that the same IL-1 family members have different roles in attracting specific types of lymphocytes in the immunological pathways of pigs.
Among the cytokines, IL-1β exhibits a significant association with IL-6, which is increased and has a predictive potential as a biomarker in bacterial infection. IL-6 also has a role in triggering differentiation into a major pro-inflammatory T-cell population, the CD4-positive Th17 cells, and in inducing acute-phase proteins such as C-reactive protein (CRP) [32]. These actions correspond with known pathways mediated by IL-6 as a downstream target of IL-1β, thereby increasing plasma levels of CRP under inflammatory conditions. Therefore, our results suggest that the basic response to integumentary bacterial infection could involve a T-cell-mediated immune response, primarily affected by IL-1β, which would be an appropriate response to a bacterial disease of an internal organ, such as swine dysentery caused by Brachyspira hyodysenteriae [33].
In the present study, we investigated the characteristics of mammary areas exhibiting swollen or gross mass-like lesions using histological, immunohistochemical, and bacteriological methods. However, there were some limitations to our investigation: 1) The cause of mammary hyperplastic lesions was not determined, although increased PR expression was observed; 2) Inflammation did not appear to involve the mammary glands; 3) Although twenty swab samples were obtained from lesions with suppurative exudates after incision, they were not microscopically determined as mastitis; and 4) Cytokine expression was not correlated with species of isolated bacteria. Therefore, further research is needed.
In conclusion, we report that mammary lesions sampled from South Korean sows in 2015–2016 could be categorized as hyperplastic and inflammatory in nature, with the most frequently isolated causative bacteria being Pseudomonas spp. Because of bacterial infection, the development of inflammation was especially the result of lymphocyte infiltration through the IL-1β/IL-6 immune signaling pathway, partly through T cells. Hence, detailed classification and exploration of the basic nature of similar gross lesions could help in the management of other mammary lesions in sows; for instance, those caused by laceration from the teeth of suckling piglets. Therefore, sow management should deem any kind of gross lesion as a disease entity.
ACKNOWLEDGMENTS
The authors thank Ms. Eun-Mi Yu for her excellent technical assistance and the participating abattoirs of Incheon and Gyeonggi, South Korea, for providing samples of porcine mammary lesions.
Footnotes
This paper is part of the Ph.D. research of Jung-Hyung Ju.
Funding: This paper was supported by Konkuk University in 2017.
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Sur JH.
- Funding acquisition: Sur JH.
- Investigation: Ju JH, Shin JI, Lim HY, Kim HW, Seung BJ, Cho SH, Kim SH.
- Project administration: Sur JH.
- Resources: Ju JH.
- Writing - original draft: Ju JH, Shin JI.
- Writing - review & editing: Seung BJ, Sur JH.
References
- 1.Quesnel H, Farmer C, Devillers N. Colostrum intake: influence on piglet performance and factors of variation. Livest Sci. 2012;146:105–114. [Google Scholar]
- 2.Ringarp N. Clinical and experimental investigations into a post-parturient syndrome with agalactia in sows. Acta Agric Scand. 1960;Suppl 7:166. [Google Scholar]
- 3.Ritter LA, Xue J, Dial GD, Morrison RB, Marsh WE. Prevalence of lesions and body condition scores among female swine at slaughter. J Am Vet Med Assoc. 1999;214:525–528. [PubMed] [Google Scholar]
- 4.Hultén F, Persson A, Eliasson-Selling L, Heldmer E, Lindberg M, Sjögren U, Kugelberg C, Ehlorsson CJ. Clinical characteristics, prevalence, influence on sow performance, and assessment of sow-related risk factors for granulomatous mastitis in sows. Am J Vet Res. 2003;64:463–469. doi: 10.2460/ajvr.2003.64.463. [DOI] [PubMed] [Google Scholar]
- 5.Christensen RV, Aalbaek B, Jensen HE. Pathology of udder lesions in sows. J Vet Med A Physiol Pathol Clin Med. 2007;54:491–493. doi: 10.1111/j.1439-0442.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 6.Musonda MM, Une Y, Shirota K, Nomura Y, Yamaguchi G, Takahashi J. Mammary carcinoma with pulmonary metastasis in a sow. J Comp Pathol. 1990;103:229–231. doi: 10.1016/s0021-9975(08)80179-x. [DOI] [PubMed] [Google Scholar]
- 7.Hoyles L, Falsen E, Holmström G, Persson A, Sjödén B, Collins MD. Actinomyces suimastitidis sp. nov., isolated from pig mastitis. Int J Syst Evol Microbiol. 2001;51:1323–1326. doi: 10.1099/00207713-51-4-1323. [DOI] [PubMed] [Google Scholar]
- 8.Kemper N, Gerjets I. Bacteria in milk from anterior and posterior mammary glands in sows affected and unaffected by postpartum dysgalactia syndrome (PPDS) Acta Vet Scand. 2009;51:26. doi: 10.1186/1751-0147-51-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y, Fossum C, Berg M, Magnusson U. Morphometric analysis of proinflammatory cytokines in mammary glands of sows suggests an association between clinical mastitis and local production of IL-1beta, IL-6 and TNF-alpha. Vet Res. 2007;38:871–882. doi: 10.1051/vetres:2007035. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Magnusson U, Fossum C, Berg M. Escherichia coli inoculation of porcine mammary glands affects local mRNA expression of Toll-like receptors and regulatory cytokines. Vet Immunol Immunopathol. 2008;125:182–189. doi: 10.1016/j.vetimm.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Kim SW, Hurley WL, Han IK, Easter RA. Changes in tissue composition associated with mammary gland growth during lactation in sows. J Anim Sci. 1999;77:2510–2516. doi: 10.2527/1999.7792510x. [DOI] [PubMed] [Google Scholar]
- 12.Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. 2011;48:117–131. doi: 10.1177/0300985810393258. [DOI] [PubMed] [Google Scholar]
- 13.Nieto A, Peña L, Pérez-Alenza MD, Sánchez MA, Flores JM, Castaño M. Immunohistologic detection of estrogen receptor alpha in canine mammary tumors: clinical and pathologic associations and prognostic significance. Vet Pathol. 2000;37:239–247. doi: 10.1354/vp.37-3-239. [DOI] [PubMed] [Google Scholar]
- 14.Overley B, Shofer FS, Goldschmidt MH, Sherer D, Sorenmo KU. Association between ovarihysterectomy and feline mammary carcinoma. J Vet Intern Med. 2005;19:560–563. doi: 10.1892/0891-6640(2005)19[560:aboafm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Hurley WL. Mammary gland growth in the lactating sow. Livest Prod Sci. 2001;70:149–157. [Google Scholar]
- 16.Ji F, Hurley WL, Kim SW. Characterization of mammary gland development in pregnant gilts. J Anim Sci. 2006;84:579–587. doi: 10.2527/2006.843579x. [DOI] [PubMed] [Google Scholar]
- 17.Shin JI, Lim HY, Kim HW, Seung BJ, Sur JH. Analysis of hypoxia-inducible factor-1α expression relative to other key factors in malignant canine mammary tumours. J Comp Pathol. 2015;153:101–110. doi: 10.1016/j.jcpa.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Illera JC, Pérez-Alenza MD, Nieto A, Jiménez MA, Silvan G, Dunner S, Peña L. Steroids and receptors in canine mammary cancer. Steroids. 2006;71:541–548. doi: 10.1016/j.steroids.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Rahman MT, Crombie A, Chen Y, Stralis-Pavese N, Bodrossy L, Meir P, McNamara NP, Murrell JC. Environmental distribution and abundance of the facultative methanotroph Methylocella . ISME J. 2011;5:1061–1066. doi: 10.1038/ismej.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drancourt M, Berger P, Raoult D. Systematic 16S rRNA gene sequencing of atypical clinical isolates identified 27 new bacterial species associated with humans. J Clin Microbiol. 2004;42:2197–2202. doi: 10.1128/JCM.42.5.2197-2202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dogan B, Boor KJ. Genetic diversity and spoilage potentials among Pseudomonas spp. isolated from fluid milk products and dairy processing plants. Appl Environ Microbiol. 2003;69:130–138. doi: 10.1128/AEM.69.1.130-138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood CE, Hester JM, Appt SE, Geisinger KR, Cline JM. Estrogen effects on epithelial proliferation and benign proliferative lesions in the postmenopausal primate mammary gland. Lab Invest. 2008;88:938–948. doi: 10.1038/labinvest.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerjets I, Kemper N. Coliform mastitis in sows: a review. J Swine Health Prod. 2009;17:97–105. [Google Scholar]
- 24.Chen F, Xia Q, Ju LK. Aerobic denitrification of Pseudomonas aeruginosa monitored by online NAD(P)H fluorescence. Appl Environ Microbiol. 2003;69:6715–6722. doi: 10.1128/AEM.69.11.6715-6722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baer C, Bilkei G. Ultrasonographic and gross pathological findings in the mammary glands of weaned sows having suffered recidiving mastitis metritis agalactia. Reprod Domest Anim. 2005;40:544–547. doi: 10.1111/j.1439-0531.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 26.Osborne AD, Armstrong K, Catrysse NH, Butler G, Versavel L. An outbreak of Pseudomonas mastitis in dairy cows. Can Vet J. 1981;22:215–216. [PMC free article] [PubMed] [Google Scholar]
- 27.Fricker CR, Tompsett S. Aeromonas spp. in foods: a significant cause of food poisoning? Int J Food Microbiol. 1989;9:17–23. doi: 10.1016/0168-1605(89)90033-0. [DOI] [PubMed] [Google Scholar]
- 28.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Zhang S, Yu J, Zhang H, Yuan Z, Sun Y, Zhang L, Zhu Y, Song H. An outbreak of Proteus mirabilis food poisoning associated with eating stewed pork balls in brown sauce, Beijing. Food Control. 2010;21:302–305. [Google Scholar]
- 30.Nambu A, Nakae S, Iwakura Y. IL-1β, but not IL-1α, is required for antigen-specific T cell activation and the induction of local inflammation in the delayed-type hypersensitivity responses. Int Immunol. 2006;18:701–712. doi: 10.1093/intimm/dxl007. [DOI] [PubMed] [Google Scholar]
- 31.Ren K, Torres R. Role of interleukin-1β during pain and inflammation. Brain Res Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slaats J, Ten Oever J, van de Veerdonk FL, Netea MG. IL-1β/IL-6/CRP and IL-18/ferritin: distinct inflammatory programs in infections. PLoS Pathog. 2016;12:e1005973. doi: 10.1371/journal.ppat.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruse R, Essén-Gustavsson B, Fossum C, Jensen-Waern M. Blood concentrations of the cytokines IL-1beta, IL-6, IL-10, TNF-alpha and IFN-gamma during experimentally induced swine dysentery. Acta Vet Scand. 2008;50:32. doi: 10.1186/1751-0147-50-32. [DOI] [PMC free article] [PubMed] [Google Scholar]