Abstract
The existing techniques for the removal of heavy metals are expensive and frequently inefficient. Thus the application of biosorbents has arisen as an alternative, this being emergent technology that must be studied and explored, with the aim of promoting better environmental and human life quality. The objective of this study was to verify the capacity of active and inactive Pleurotus ostreatus fungal biomass in removing Cr(VI) ions by biosorption from synthetic aqueous solutions of these ions at concentrations of 10, 25, 50, 75, 100, 125 and 150 mg L−1. When using active biomass, the kinetic studies showed that 100% of biosorption was reached from the 25 mg L−1Cr(VI) solution in 360 hours, equivalent to the removal of 169.84 mg g−1 of total Cr. On the other hand the inactive biomass reached 100% of its saturation capacity in 22 minutes for a concentration of 50 mg L−1 of Cr(VI), equivalent to the removal of 368.21 mg g−1 of total Cr. The kinetic study was shown to be highly effective, presenting an efficiencies of times 500 and 750 for the active and inactive P. ostreatus biomasses, respectively, when compared to the limit of 0.1 mg L−1 of Cr(VI) for industrial effluents described in CONAMA resolution n° 430/2011.
Keywords: Environmental science, Natural hazards, Geochemistry
1. Introduction
The accelerated industrial growth that occurred in the last few decades has aggravated the situation of environmental degradation and disequilibrium of natural ecosystems, due to the progressive increase in pollution arising from this industrialization. Hence the population is exposed daily to these pollutants by diverse means, including inhaling from the air, the consumption of contaminated food and drinking water, and exposure to soils and industrial residues (Leite et al., 2015; Tomasella et al., 2015).
Inevitably the challenges of the environmental problem permeate all activities related to industrial processes, and thus the disposal of heavy metals, resulting from diverse industrial operations, into the environment, has caused extreme concern, since these pollutants are frequently found in concentrations above the limits permitted by law, representing risks to the environment and ecosystem (Arantes et al., 2016; Mattos et al., 2016).
Heavy metals are potential sources of environmental degradation, since they produce alterations in the qualities of the water and soil, and these alterations have a direct impact on the equilibrium of environmental ecosystems, presenting adverse effects on human health, due principally to bioaccumulation and their long permanence in the medium (Oves et al., 2013; Lima et al., 2015). Chromium stands out amongst the heavy metals, being a highly water-soluble metal with bio-accumulative capacity, showing low degradability and remaining active in the environment for many years (Coreño-Alonso et al., 2014; Gutiérrez-Corona et al., 2016; Souza et al., 2016). The main source of chromium release into the environment is related to industrial applications, such as the steel and alloy, cement, electroplating and tanning industries, amongst others (Banerjee et al., 2016; Kahraman, 2017). The chromium is transported within the ecosystem by way of the soil and water, and released in very low concentrations, but with time its concentration increases to toxic levels.
All the forms of chromium can be toxic in determined concentration of the metal (Coreño-Alonso et al., 2014). Hexavalent chromium Cr(VI) is one of the forms normally found in nature, and presents itself as chromates and dichromates (CrO42- and Cr2O72-), being soluble at a wide range of pH values and generally transportable in the soil/water system. These ions are extremely toxic and mutagenic, and their ingestion can be fatal when consumed in small amounts. Trivalent chromium presents itself in the form of Cr(III), and is considerably less toxic and less mobile due to its precipitation as oxides and hydroxides at pH values above 5.0. It is also considered to be an essential element for human metabolism, and its deficiency can cause diverse diseases such as diabetes, due to a reduction in the production of insulin, and problems in the regulation of carbohydrates and lipids and protein metabolism (Leite et al., 2015; Paredes-Carrera et al., 2015).
The removal of heavy metals from liquid effluents can be done by various physicochemical processes, including conventional methods such as chemical precipitation, ion exchange, filtration, membrane technologies and electrochemical treatments. However these methods have disadvantages such as high cost, low metal removal and a high reagent demand, and may also be inefficient, especially when dealing with large volumes and low concentrations (Farhan and Khadom, 2015).
Thus the search for new technologies for the treatment of effluents, presenting advantages with respect to the cost-benefit of the process, has led to increasing interest in the biosorption process, which consists of the absorption of metals by biomass by way of active (active biosorption) or inactive (passive biosorption) microorganisms. This technique has been shown to be a good alternative, since it is of low cost, elevated removal efficiency, passive to regeneration, shows selectivity when faced with different metal species, and the metal can be recovered. It is also less aggressive to the environment (Buratto et al., 2012; Vendruscolo et al., 2016). The diverse existing biomasses under study include microorganisms (bacteria, microalgae, yeasts and fungi), macroscopic vegetables (algae, grasses, aquatic plants) and parts or specific tissues of vegetable matter that are agricultural or industrial byproducts (skin, peel, bagasse, seeds).
The subjacent mechanisms of biosorption depend on various factors such as the chemical composition of the cell wall of the biosorbent, the physicochemical conditions of the external medium and the chemical properties of the metal (Silva et al., 2014; Vendruscolo et al., 2016).
Fungi are microorganisms suitable for use in the treatment of residual waters, since they grow easily and produce elevated amounts of biomass. In addition they are considered to be good biosorbents due to the chemical composition of their cell walls, composed of various chemical groups responsible for attracting and retaining metals in the biomass, such as chitin acetamide, structural fungal polysaccharides, amino acids, phosphate groups in the nucleic acids, amides, amines, carboxyl groups on proteins, and the hydroxyl groups of polysaccharides amongst others, where the metals are adsorbed by way of electrostatic interactions (Arbanah et al., 2013).
According to thermodynamic principles, the distribution of a metal ion between a liquid and a solid phase involves phase equilibrium. Thus in order to carry out a qualitative analysis of the process, the biosorption equilibrium of the metals can be described by adsorption isotherm models. These models describe the amount of metal adsorbed per unit mass of biosorbent and the metal concentration in solution at equilibrium at a determined constant temperature (Cheng et al., 2016). Two widely accepted models exist, that of Langmuir and that of Freundlich, both used to model the biosorption equilibrium in the presence of a metal (Saini and Melo, 2015).
Considering the great variety of polluting sources and generators of effluents containing heavy metals, and due to their proven deleterious potential for human health, the objective of the present study was to study the capacity of active and inactive Pleurotus ostreatus to remove Cr(VI) ions from synthetic aqueous solutions by biosorption.
2. Material and methods
2.1. Cr(VI) solution
The stock solution of Cr(VI) was prepared using K2Cr2O7 (brand Synth, 99% pure) at a concentration of 1000 mg L−1. The pH value was adjusted to 5.6 with 1.0 mol L−1 HCl and 1.0 mol L−1 NaOH. Cr(VI) was quantified using diphenylcarbazide (brand Synth, 100% pure) using the methodology presented in the Standard Methods for the Examination of Water and Wastewater (APHA, 1999).
2.2. Microorganism
Pleurotus ostreatus, conceded by the microbiology laboratory of the Food Engineering School of the Federal University of Goias (EA/UFG), Brazil was replicated in test tubes containing potato dextrose agar (PDA) slopes with the pH value adjusted to 5.6. The tubes were incubated in a BOD (Tecnal, TE 371) at 28 ± 2 °C for 7 days, and after colonization in the culture medium, were stored in a refrigerator at 4 °C for later use.
2.3. Biosorption in a solid medium
The P. ostreatus strain maintained in test tubes was replicated in Petri dishes containing PDA with the pH value adjusted to 5.6. The Petri dishes were incubated in a BOD (Tecnal, TE 371) at 28 ± 2 °C for 7 days. Using a cutting instrument with a diameter of 4 mm, fungal colonies cultivated in the PDA were removed and inoculated into Petri dishes containing agar contaminated with Cr(VI) at concentrations of 10, 25, 50, 75, 100, 125 and 150 mg L−1. Three streaks were drawn on the bottom of each plate, and the plates then incubated in a BOD (Tecnal, TE 371) at 28 ± 2 °C for 10 days. The experiments were carried out in quintuplicate, and every 24 hours, with the aid of a scaler, the radii of the colonies were measured as a function of cultivation time. The control samples consisted of PDA plates without Cr(VI). The radial growth velocity was determined using Eq. (l):
| r (t) = a + VRG × t | (1) |
where r(t) is the radius of the colony in mm, a is the linear regression coefficient (in this case 2.0 mm – half the PDA cylinder used in the inoculation), VRG is the radial growth velocity in mm h−1 and t is the cultivation time in h. Eq. (2), described by Edgington et al. (1971) and modified by Menten et al. (1976), was used to estimate the inhibition of microbial growth (IG).
| IG = ((GRC − GRT)/GRC) × 100 | (2) |
where IG is defined as the percentage inhibition of microbial growth, GRC is the radial growth index of the control (mm) and GRT is the radial growth index of the treatment (mm).
2.4. Biosorption in a liquid medium by active and inactive biomass
P. ostreatus was cultivated in Roux jars containing PDA with the pH adjusted to 5.6, and incubated in a BOD (Tecnal, TE 371) at 28 ± 2 °C for 14 days. The biomass produced was divided into two parts, and one part used in the biosorption study as from an active fungus, and the other part for the biosorption study as from an inactive fungus. The biomass was filtered and washed with distilled water to produce the inactive fungus. After washing, the biomass was dried in an oven at 80 ± 2 °C for 24 hours, ground in a knife mill and sieved through a 36-mesh sieve.
Biosorption by active and inactive fungal biomass occurred in the liquid phase in conical flasks containing 100 mL medium composed of the following components (in g L−1): 22 g of glucose, 13.2 g of NaNO3, 3.3 g of KH2PO4, 3.3 g of KCl, 1.1 g of MgSO4.7H2O, 0.0022 g of FeSO4 and 0.0022 g of ZnSO4. Each flask received the determined aliquot to make up the Cr(VI) concentrations of 10, 25, 50, 75, 100, 125 and 150 mg L−1, together with the control sample. The pH value was adjusted to 5.6 using 1.0 mol L−1 HCl and 1.0 mol L−1 NaOH and sterilized in an autoclave at 121 °C for 30 minutes. For the active biomass, each conical flask received a 1 mL aliquot of fungal suspension obtained from the Roux jar containing sterile distilled water. For the inactive biomass, each conical flask received 0.2 g of fungal biomass.
In both cases the flasks were incubated in a refrigerated incubator with orbital shaking at a temperature of 28 ± 2 °C and rotation of 150 rpm. For the active fungal biomass study, 2 mL samples were taken every 24 hours for 15 days for all concentrations, and for the inactive fungal biomass, 2 mL samples were taken every 2 minutes for 22 minutes. All samples were centrifuged at 2000 rpm and 1 mL aliquots of the supernatants transferred to appropriate volumetric flasks according to the need for dilution. Absorbance was read using a UV-visible spectrophotometer at a wavelength of 540 nm with the aid of a quartz cuvette.
2.5. Study of biosorption efficiency
The efficiency of the biosorbent in biosorbing Cr(VI) (Ef Biosorption) was determined using Eq. (3):
| EfBiosorption = (Ci – Cf)/Ci) × 100 | (3) |
where Ef Biosorption is the biosorption efficiency in %, Ci s the initial concentration of Cr(VI) ions in solution (mg L−1) and Cf is the final concentration of Cr(VI) ions in solution (mg L−1) at time t.
2.6. Atomic adsorption spectrometry – AAS
The fungal pellets obtained in the Cr(VI) biosorption study were filtered, washed with distilled water and dried in a drying oven at 60 °C for 8 hours. The dry biomass was extracted with HCl/HNO3 (1:1), filtered and transferred to an appropriate volumetric flask according to the need for dilution. The total chromium content was determined in an atomic absorption spectrometer (Varian, Spectraa 200).
2.7. Scanning electron microscopy – SEM
The fungal pellets obtained in the Cr(VI) biosorption study in a liquid medium were analyzed by SEM in a JEOL microscope (JSM 6610) equipped with EDS Thermo scientific NSS special imaging. The samples were submitted to special preparation techniques, such as fixing in glutaraldehyde, dehydration in increasing acetone concentrations, Autosamdri® CO2 critical point drying, and finally mounted on stubs and submitted to gold sputtering (Denton Vaccum, Desk V) before observing the external morphological characteristics of the fungus P. ostreatus, with image capture.
2.8. Transmission electronic microscopy – TEM
Consecutively with the Cr(VI) biosorption study, fungal fragments were collected in the form of pellets and analyzed by TEM using a JEOL microscope (JEM 2100) operating at 100 keV, and equipped with EDS (Thermo Scientific). For the TEM analyses the samples were submitted to special preparation techniques such as fixing in glutaraldehyde, post-fixing in osmium tetroxide, dehydration in increasing acetone concentrations, infiltration through resin and finally polymerization, before being examined by TEM aiming to observe the internal morphology of the fungus P. ostreatus, with image capture.
3. Results and discussion
3.1. Biosorption in a solid medium
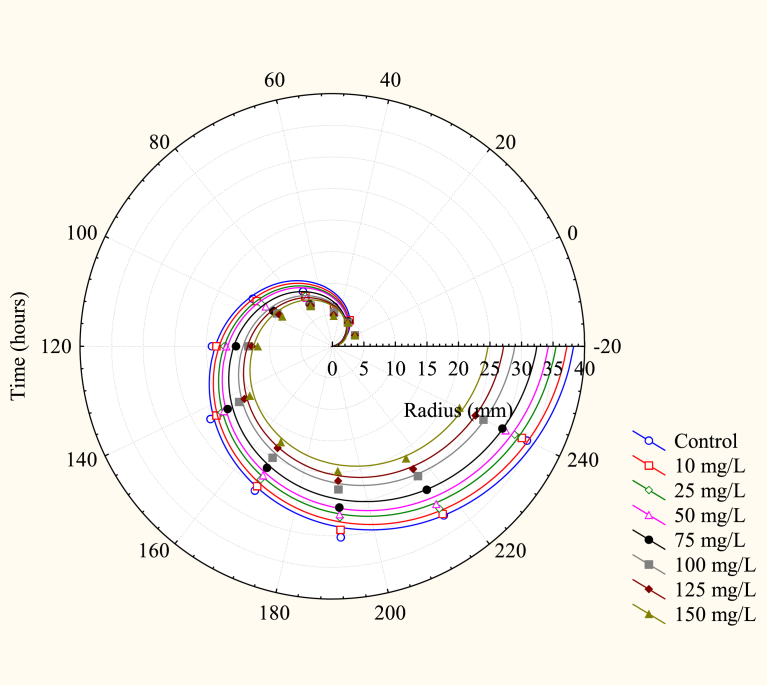
Fig. 1 provides evidence of the radial growth of the colonies, and even under conditions of metal intolerance, the fungus showed growth capacity, although the greater the concentration of Cr(VI) in the medium, the smaller the developmental capacity of the fungus. The inhibition of microbial growth was evident for all concentrations of Cr(VI), inhibition progressively increasing with increase in Cr(VI) concentration. It was evident that the presence of Cr(VI) caused a reduction in the substrate assimilation capacity of the fungus in relation to the control culture (absence of Cr(VI). Table 1 shows the equations for the radius as a function of time, obtained by linear regression.
Fig. 1.
Evolution of the radii of the Pleurotus ostreatus colonies cultivated in PDA with different Cr(VI) concentrations, as a function of cultivation time.
Table 1.
Evolution of the Pleurotus ostreatus colonies after 240 hours of cultivation in PDA with different Cr(VI) concentrations.
| Cr(VI) (mg L−1) | Equation | R2 | VRG (mm h−1) | % IG |
|---|---|---|---|---|
| 0 | r = 0.1268 × t + 2 | 0.9653 | 0.1268 | – |
| 10 | r = 0.1223 × t + 2 | 0.9590 | 0.1223 | 2.6 |
| 25 | r = 0.1156 × t + 2 | 0.9593 | 0.1156 | 6.2 |
| 50 | r = 0.1110 × t + 2 | 0.9541 | 0.1110 | 10.9 |
| 75 | r = 0.1034 × t + 2 | 0.9402 | 0.1034 | 12.6 |
| 100 | r = 0.0906 × t + 2 | 0.9415 | 0.0906 | 22.3 |
| 125 | r = 0.0842 × t + 2 | 0.9438 | 0.0842 | 26.4 |
| 150 | r = 0.0755 × t + 2 | 0.9449 | 0.0755 | 34.6 |
VRG: velocity of radial growth; % IG: percent of inhibition growth.
The tolerance of the fungus to Cr(VI) depends on both the metal concentration and on its capacity to adapt to the medium, which can be perceived from the decrease in tolerance of the fungus at different toxic metal concentrations, due to one or more resistance mechanisms, which inhibit colony development of the fungus Pleurotus ostreatus. The main resistance mechanisms which could occur are: production of enzymes such as reductase; metal immobilization due to the formation of complexes such as chelates; and those resulting from bioaccumulation or biosorption (Vale et al., 2011).
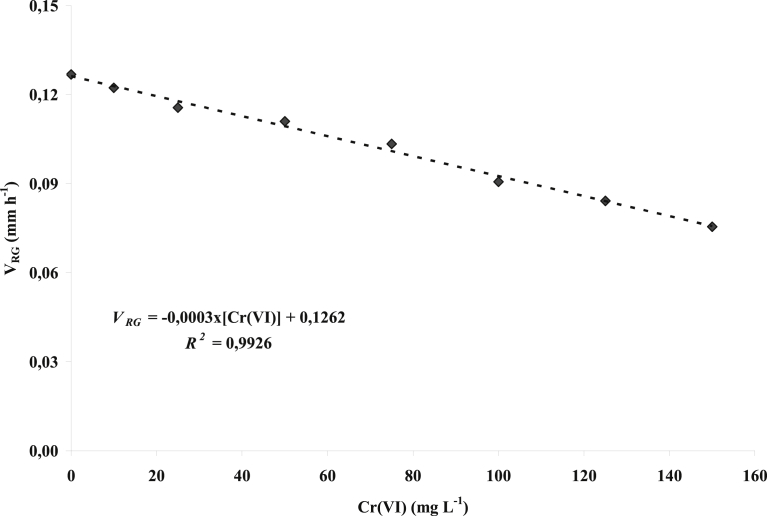
Fig. 2 demonstrates an inversely proportional behavior between the velocity of radial growth and the Cr(VI) concentration, which allowed for the prediction of radial growth at the concentrations used.
Fig. 2.
The behavior of the radial growth velocity (VRG) as a function of Cr(VI) concentration.
3.2. Biosorption of Cr(VI) in a liquid medium
The Cr(VI) biosorption study was carried out with the pH value of the medium adjusted to 5.6, creating favorable conditions for the development of the fungus P. ostreatus, and also for permanence of the equilibrium of Cr(VI) in the liquid medium with a predominance of the ion species CrO42− and Cr2O72−. As shown in Table 2, the experiments with the active P. ostreatus fungus showed a reduction in the Cr(VI) concentration in solution. The best results were obtained at the concentrations of 10 and 25 mg L−1, where the total removal of the metal by the biosorbent was observed. Significant values for the removal of Cr(VI) were also observedat the concentrations of 50 and 75 mg L−1, but the concentrations of 100, 125 and 150 mg L−1 showed reduced values for Cr(VI) removal, with the prediction that the higher the Cr(VI) concentration in a liquid medium, the lower the tolerance of the fungus in the presence of the contaminant.
Table 2.
The study of Cr(VI) biosorption in a liquid medium and the amount of total Cr biosorbed in 360 hours of incubation with active P. ostreatus.
| Cr(VI) (mg L−1) | Cr(VI) in solution (mg L−1) |
Cr(VI) in the biosorbent (mg L−1) | % Removal of Cr(VI) | aTotal Cr in the biosorbent (mg g−1) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| 10 | 10.02 ± 0.14 | nd | 10.00 ± 0.01 | 100.00 | 74.73 ± 0.17 |
| 25 | 24.98 ± 0.22 | nd | 24.98 ± 0.03 | 100.00 | 169.84 ± 0.13 |
| 50 | 49.95 ± 0.13 | 0.91 ± 0.02 | 49.04 ± 0.11 | 98.18 ± 0.12 | – |
| 75 | 75.08 ± 0.07 | 2.38 ± 0.12 | 72.70 ± 0.17 | 93.42 ± 0.21 | – |
| 100 | 99.90 ± 0.21 | 51.02 ± 0.11 | 48.88 ± 0.14 | 48.93 ± 0.15 | – |
| 125 | 125.02 ± 0.17 | 81.66 ± 0.17 | 43.36 ± 0.21 | 34.68 ± 0.14 | – |
| 150 | 150.15 ± 0.19 | 112.77 ± 0.09 | 37.38 ± 0.18 | 24.90 ± 0 12 | – |
nd: Not detected. DP: standard deviation.
Concentration of total chromium by atomic absorption spectrometry for the higher metal removal concentrations by the biosorption process. The detection limit (LD) of the atomic absorption spectrometer was 0.05 μg g−1 for Cr (VI). A UV-visible spectrophotometer obtained a limit of detection (LD) of 0.02 μg mL−1.
On the other hand, Table 3 shows the reductions in the concentration of Cr(VI) in solution for the experiments carried out with the inactive form of the fungus P. ostreatus. The best results were obtained for the concentrations of 10, 25 and 50 mg L−1, with total removal of the Cr(VI) by the biosorbent. Significant removal values were also observed for the concentrations of 75, 100, 125 and 150 mg L−1, but presenting lower values of biosorption of 73.21, 55.13, 45.89 and 39.88%, respectively, of its saturation capacity, due to the toxic effect of the metal on the fungal cells.
Table 3.
The study of Cr(VI) biosorption in a liquid medium and the amount of total Cr biosorbed in 22 minutes of incubation with inactive P. ostreatus.
| Cr(VI) (mg L−1) | Cr(VI) in solution (mg L−1) |
Cr(VI) in the biosorbent (mg L−1) | % Removal of Cr(VI) | aTotal Cr in the biosorbent (mg g−1) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| 10 | 10.00 ± 0.02 | nd | 10.00 ± 0.02 | 100.00 | 77.25 ± 0.10 |
| 25 | 25.05 ± 0.09 | nd | 25.05 ± 0.07 | 100.00 | 189.63 ± 0.14 |
| 50 | 49.95 ± 0.07 | nd | 49.95 ± 0.09 | 100.00 | 368.21 ± 0.09 |
| 75 | 74.92 ± 0.12 | 20.07 ± 0.09 | 54.85 ± 0.11 | 73.21 ± 0.12 | 409.16 ± 0.12 |
| 100 | 100.05 ± 0.07 | 44.89 ± 0.11 | 55.16 ± 0.15 | 55.13 ± 0.14 | – |
| 125 | 124.87 ± 0.15 | 67.57 ± 0.13 | 57.30 ± 0.13 | 45.89 ± 0.11 | – |
| 150 | 149.84 ± 0.11 | 90.09 ± 0.10 | 59.75 ± 0.18 | 39.88 ± 0.15 | – |
nd: Not detected. DP: standard deviation.
Concentration of total chromium by atomic absorption spectrometry for the higher metal removal concentrations by the biosorption process. The detection limit (LD) of the atomic absorption spectrometer was 0.05 μg g−1 for Cr (VI). A UV-visible spectrophotometer obtained a limit of detection (LD) of 0.02 μg mL−1.
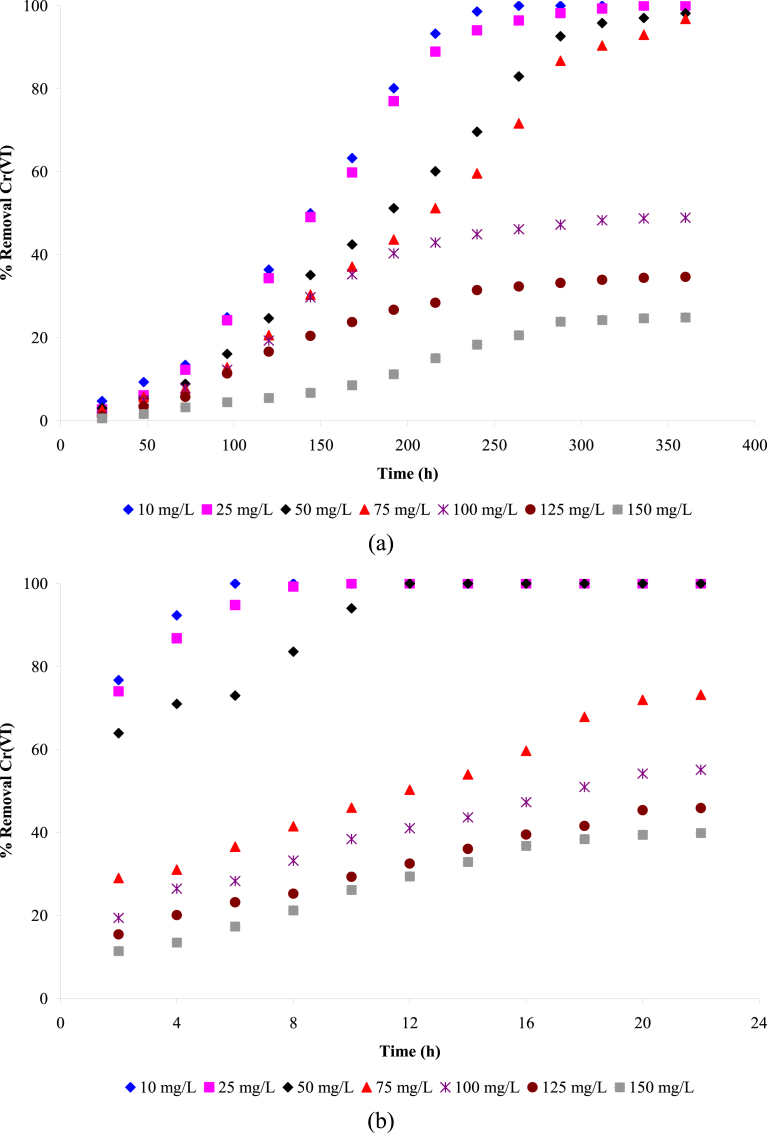
An analysis of Tables 2 and 3 shows that the inactive biomass presented greater removal of the Cr(VI) in a shorter biosorption time, corresponding to 100% removal at a concentration of 50 ppm, equivalent to 368.21 mg g−1 of total Cr in 22 minutes of the study. In comparison, the active biomass removed less toxic chromium metal in a longer biosorption time, corresponding to 100% removal at a concentration of 25 ppm, equivalent to 169.84 mg g−1 of total Cr in 360 hours of the study. The inactive biomass was shown to be more efficient, since specific culture media were not required and also because intoxication by the toxic metal did not occur, as possibly occurred with the active biomass.
Fig. 3 shows the biosorption of the ion Cr(VI) for different initial concentrations of the metal, with respect to time. A highly significant biosorption of Cr(VI) by the inactive biomass can be observed at the start of the assay, configuring a predominance of rapid physical and chemical bonding between the metal and the biomass, generating strong links and reducing the probability of desorption of the metal adhered to the biomass. On the other hand, the active biomass showed much slower removal, with a progressive increase in the Cr(VI) removal, thus contributing to the capture of the metal ion present in the synthetic solutions. In the case of biosorption by P. ostreatus in the active form, one can say that the Cr(VI) is bioavailable due to the chemical compositions present in its structure, such as amino acids and acid hydroxyl groups.
Fig. 3.
Biosorption kinetics of Cr(VI) removal by Pleurotus ostreatus (a) by active biomass and (b) by inactive biomass.
4. Conclusions
The biosorption kinetics allowed for the conclusion that the greatest Cr(VI) percent removal from a synthetic solution was accomplished by the inactive condition of the fungus in an equilibrium time of 22 minutes, reaching 100% of its saturation condition with the 50 mg L−1 concentration of Cr(VI), equivalent to 368.21 mg g−1 of total Cr. The active biomass reached 100% biosorption in 360 hours for the concentration of 25 mg L−1 of Cr(VI), equivalent to 169.84 mg g−1 of total Cr. The inactive biomass was highly efficient in the Cr(VI) biosorption process, corresponding to a value 750 times higher than the maximum concentration of Cr(VI) permitted in effluents released into fresh water according to that established by CONAMA resolution nº 430/2011, whilst the active biomass reached a value 500 times greater than the maximum Cr(VI) concentration allowed by the same resolution.
Declarations
Author contribution statement
Glalber Luiz da Rocha Ferreira: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Francielo Vendruscolo, Nelson Roberto Antoniosi Filho: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Fundação de Apoio a Pesquisa do Estado de Goiás (FAPEG) with a research scholarship.
Competing interest statement
The authors declare no conflict of interest.
Additional information
Data associated with this study has been deposited at http://repositorio.bc.ufg.br/tede/handle/tede/6711.
References
- APHA - American Public Health Association . nineteenth ed. Water Environment Federation, American Water Works Association, Water Pollution Control Federation; Washington, DC, USA: 1999. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Arantes F.P., Savassi L.A., Santos H.B., Gomes M.V.T., Bazzoli N. Bioaccumulation of mercury, cadmium, zinc, chromium, and lead in muscle, liver, and spleen tissues of a large commercially valuable catfish species from Brazil. An Acad. Bras Ciências. 2016;88:137–147. doi: 10.1590/0001-3765201620140434. [DOI] [PubMed] [Google Scholar]
- Arbanah M., Miradatul N.M.R., Halim K.K.H. Utilization of Pleurotus ostreatus in the removal of Cr(VI) from chemical laboratory waste. Int. Refreed. J. Eng. Sci. 2013;2:29–39. [Google Scholar]
- Banerjee S., Joshi S.R., Mandal T., Halder G. Insight into Cr6+ reduction efficiency of Rhodococcus erythropolis isolated from coalmine waste water. J.Chemosphere. 2016;167:269–281. doi: 10.1016/j.chemosphere.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Buratto A.P., Costa R.D., Ferreira E.S. Aplicação de biomassa fúngica de Pleurotus ostreatus em processo de biossorção de íons cobre (II) J. Eng. Sanit. Ambient. 2012;17:413–420. [Google Scholar]
- Silva J.L.B.C., Pequeno O.T.B.L., Rocha L.K.S., Araújo E.C.O., Marciel T.A.R., Barros A.J.M. Biossorção de metais pesados – uma revisão. Rev. Saúde Ciências. 2014;8:137–149. [Google Scholar]
- Cheng Y., Yang C., He H., Zeng G., Zhao K., Yan Z. Biosorption of Pb(II) Ions from aqueous solutions by waste biomass from biotrickling filters: kinetics, isotherms, and thermodynamics. J. Environ. Eng. 2016;142 [Google Scholar]
- Coreño-Alonso A., Solé A., Diestra E., Esteve I., Gutiérrez-Corona J.F., López G.E.R. Mechanisms of interaction of chromium with Aspergillus niger var tubingensis strain Ed8. Bioresour. Technol. 2014;158:188–192. doi: 10.1016/j.biortech.2014.02.036. [DOI] [PubMed] [Google Scholar]
- Edgington L.V., Khew K.L., Barron G.L. Fungitoxic spectrum of benzimidazole compounds. Phytopathology. 1971;61:42–44. [PubMed] [Google Scholar]
- Farhan S.N., Khadom A.A. Biosorption of heavy metals from aqueous solutions by Saccharomyces cerevisiae. Int. J. Ind. Chem. 2015;6:119–130. [Google Scholar]
- Gutiérrez-Corona J.F., Romo-Rodríguez P., Santos-Escobar F., Espino-Saldaña A.E., Hernández-Escoto H. Microbial interactionswith chromium: basicbiological processes and applications in environmental biotechnology. World J. Microbiol. Biotechnol. 2016;32:191. doi: 10.1007/s11274-016-2150-0. [DOI] [PubMed] [Google Scholar]
- Kahraman H.T. Development of an adsorbent via chitosan nano-organoclay assembly to remove hexavalent chromium from wastewater. Int. J. Biol. Macromol. 2017;94:202–209. doi: 10.1016/j.ijbiomac.2016.09.111. [DOI] [PubMed] [Google Scholar]
- Leite A., Silva R., Cunha E. Aplicação de um caso prático de doenças profissionais: relevância médico-legal metais pesados e carcinogénese. Arq. Med. 2015;29:93–97. [Google Scholar]
- Lima D.P., Santos C., Silva R.S., Yoshioka E.T.O., Bezerra R.M. Contaminação por metais pesados em peixes e água da bacia do rio Cassiporé, Estado do Amapá, Brasil. Acta Amazôn. 2015;45:405–414. [Google Scholar]
- Mattos A.G., Sobrinho N.M.B.A., Lima E.S.A., Sousa F.F. Sorção de Cd e Pb nos solos da região do médio Rio Paraíba-RJ, Brasil. Ver Ciência Agron. 2016;47:1–12. [Google Scholar]
- Menten J.O.M., Machado C.C., Minussi E., Castro C. Efeito de alguns fungicidas no crescimento micelial da Macrophomina phaseolina in vitro. Fitopatol. Bras. 1976;1:57–66. [Google Scholar]
- Oves M., Khan M.S., Zaidi A. Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil. Saudi J. Biol. Sci. 2013;20:121–129. doi: 10.1016/j.sjbs.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Carrera S.P., Valencia-Martínez R.F., Valenzuela-Zapata M.A., Sánches-Ochoa J.C., Castro-Sotelo L.V. Estudio de la sorcion de cromo hexavalente mediante hidrotalcitas sintetizadas utilizando irradiacíon de ultrasonido vs microondas. Rev. Mex. Ing. Quím. 2015;14:429–436. [Google Scholar]
- Saini A.S., Melo J.S. Biosorption of uranium by human black hair. J. Environ. Radioact. 2015;142:29–35. doi: 10.1016/j.jenvrad.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Souza A.M., Salviano A.M., Melo J.F.B., Felix W.P., Belém C.S., Ramos P.N. Seasonal study of concentration of heavy metals in waters from lower São Francisco River basin, Brazil. Braz. J. Biol. 2016;76 doi: 10.1590/1519-6984.05215. [DOI] [PubMed] [Google Scholar]
- Tomasella R.C., Oliveira E.G., Angelis D.F., Garcia M.L. Avaliação do potencial de compostos naturais (argila, turfa e carvão) na remoção de chumbo e toxicidade de um efluente industrial. Eng. Sanitária Ambient. 2015;20:251–258. [Google Scholar]
- Vale M.S., Abreu K.V., Gouveia S.T., Leitão R.C., Santaella S.T. Efeito da toxicidade de Cr(VI) e Zn(II) no crescimento do fungo filamentoso Aspergillus niger isolado de efluente industrial. Eng. Sanitária Ambient. 2011;16:237–244. [Google Scholar]
- Vendruscolo F., Ferreira G.L.R., Antoniosi Filho N.R. Biosorption of hexavalent chromium by microorganisms. Int. Biodeterior. Biodegrad. 2016;30:1–9. [Google Scholar]