Abstract
Abnormal glucose metabolism causes various complications in many metabolic diseases such as obesity, hypertension, cardiovascular diseases and mainly diabetes. But commonly used oral hypoglycemic drugs cause severe side effects. Hence, there is a need to find the medicine without side effects. Compounds of medicinal plants were nontoxic, inexpensive and less or no side effects. Syzygium paniculatum Gaertn. (Magenta Cherry) is one of the important medicinal plants in the genus Syzygium. The fruit of S. paniculatum is sour apple-like flavor which tribes using for diabetes without scientific evidence. The purpose of study was to investigate the phytochemical analysis, antihyperglycemic, antihyperlipidemic, antioxidative stress and antioxidant activities of the daily oral administration of the fruit aqueous extract of S. paniculatum at a dose of 100 mg/kg bw over a period of 120 days in Streptozotocin-induced diabetic rats.
The plant material collected, shade dried and the extracts prepared in increasing order of polarity and screened for different phytoconstituents by Harborne method. The extract with significant antihyperglycemic activity evaluated for antihyperlipidemic, antioxidative stress, antioxidant activity and also for insulin resistance by OGTT. The levels of insulin, HbA1c, lipid profile, glycogen, total proteins, liver and kidney functional markers were measured. The changes in antioxidant defense system were evaluated by TBARS assay. Histopathological examination of pancreas sections carried by hematoxylin and eosin stain. The findings confirm that S. paniculatum possesses potential antihyperglycemic, antihyperlipidemic, antioxidative stress and antioxidant activity. The histological changes also coincide with its potential on insulin secretion, glycemic control, lipid metabolisms, oxidative stress and antioxidant enzyme activities. This investigation confirms the traditional use of this plant in the folk medicine.
Keywords: Biochemistry, Cell biology, Systems biology
1. Introduction
Diabetes mellitus is a progressive carbohydrate metabolic disorder and most common oxidative-stress related disease. It has overwhelmed a large number of populations all over the world. At present 2.8% of the world's population affected by diabetes, it may increase to 4.4% of the total world's population by 2030 [1]. The mechanism of pathogenesis of diabetes type 1 and type 2 are diverse, but the complications allied with hyperglycemia remain similar in both the types. In diabetes chronic hyperglycemia is main cause for the higher production of free radicals which can leads to the formation of the reactive oxygen species (ROS), intern activate oxidative stress and which measured by the index of lipid peroxidation. The upturn in lipid peroxidation leads to remarkable increase in the levels of thiobarbituric acid reactive substances (TBARS) which expressed regarding malondialdehyde (MDA) content and fatty acid hydroperoxides in diabetic condition [2]. Due to hyperglycemia, various factors like defect in the mechanism of insulin, hyperlipidemia, and oxidative stress were triggering severe complications like diabetic retinopathy, diabetic myopathy, diabetic neuropathy, cardiovascular disorders, ulcers, and amputations.
Now a days, the diabetes is controlled by using various pharmacological agents and different non-pharmacologic methods, like as diet and exercise. But, it was reported that the most commonly available oral hypoglycemic synthetic agents can generate severe adverse symptoms. In this context as a result of the serious adverse side effects associated with synthetic oral hypoglycemic drugs, compounds of medicinal plant are nontoxic, inexpensive and much productive for the management of diabetes. So that, present research is going on traditional medicinal plants, which can be applied as an alternative medicine for the treatment of diabetes. The various phytoconstituents with antidiabetic potential activity were isolated and reported from various medicinal plants. Last few years, different extracts from different traditional medicinal plants have been studied which helpful for management of diabetes mellitus.
Species of Syzygium was ethnopharmacologically reported for various medicinal properties. Moreover the fruits of Syzgium sp. edible and used for ethnobotanical practices for treating chronic diseases like cancer, diabetes and cardiovascular disorders [3]. Syzygium paniculatum Gaertn. (Syn. Eugenia paniculata (Gaertn.) Britten [Illegitimate]) one such a crucial medicinal plant in the genus Syzygium belongs to the family of Myrtaceae. Syzygium paniculatum (SP) is also known by the names of Magenta Cherry, Magenta Lilly Pilly, and Brush Cherry (Locally known as Adavi Neredu). The leaves of S. paniculatum are dark glossy above and paler below, the white color of flowers generates in the cluster and the eatable fruits are generally magenta colour but can be white, pink or purple with a pleasantly sour apple-like flavor. It is mostly edible fresh or used to cook into jams. SP has a great medicinal value and reported for various medicinal activities, as the fruit contain essential bioactive constituents with significant anti-cancer properties [4]. Tribes using the fruit of SP for diabetes without scientific evidence [5]. Therefore, this study has been aimed to assess the antihyperglycemic, antihyperlipidemic, oxidative stress status and antioxidant potential activities of the fruit aqueous extract of S. paniculatum in streptozotocin (STZ)-induced diabetic wistar rats.
2. Materials and methods
2.1. Plant material
Ripened SP fruit was collected from the foothills of the Seshachalam hill ranges of the Eastern Ghats, Tirupati. The plants were authenticated by the botanist, Sri Venkateswara University, Tirupati, Andhra Pradesh, India. A voucher specimen (Herbarium Accession Number: 1143) has kept in the library herbarium for future reference. Full-grown Syzygium paniculatum Gaertn. fruits were collected and brought to our laboratory and frozen immediately in liquid nitrogen and freeze-dried. By using a blender, the dried fruits were powdered, sieved by using a steel mesh and sealed in an airtight container for storage at 4 °C for experimentation.
2.2. Preparation of hexane, ethyl acetate, methanol and aqueous extracts
The SP fruits powder was used to prepare different solvent extracts like hexane, ethyl acetate and methanol extracts by Soxhlet extractor at 68–70 °C. The obtained filtrates further distilled and concentrated by using Buchi rotavapor R-200 under reduced pressure at low temperature (40–45 °C) and the resultant was finally freeze-dried. The yields of the hexane, ethyl acetate, and methanol extracts were 25%, 20%, and 25% w/w, respectively. Finally, the powder obtained from the fruits of SP was soaked in a glass jar for 48 h at room temperature and the solvent has been filtered. The extraction was continued 3 to 4 times repeatedly, till the colorless filtrate obtained. The filtrate was concentrated to dryness using a Buchi Rotavapor R-200 rotary at 40 °C under reduced pressure and resultant was freeze-dried. The yield of the extract was 30% (w/w). All the extracts were preserved at -20 °C in airtight containers till required.
2.3. Phytochemical analysis of solvent extracts
The freshly prepared different extracts of SP qualitatively analyzed to identify the presence of different phytochemical constituents by standard method proposed by Harborne [6].
2.4. Experimental animal model
Albino Wistar rats (Male) with the specifications of aged 3–4 months and body weights 200 ± 10g were used for the experiment. The animals have been fed with regular controlled diet and kept in a well-ventilated animal house at 25 ± 5 °C with 12 h light/dark cycle. The care and use of animals was followed by the institutional ethical committee guidelines.
2.5. Diabetes induction by STZ
Diabetes was induced by intraperitoneal administration of STZ (Sigma, Detroit, USA) with a single dose concentration of 45 mg/kg bw dissolved in freshly prepared 0.01 M citrate buffer pH 4.5. After 48 h the rat whose fasting blood glucose was higher than 250 mg/dL were used for the experiments. All the animals were permitted, free access to water and pellet diet and retained at room temperature in plastic cages, as per the advice of the IAEC.
2.6. Effect of extracts of the fruit of SP on antihyperglycemic activity
The rats divided into 6 groups, each group consisting of 6 rats.
Group 1: Control (C)
Group 2: Diabetic Control (DC)
Group 3: DC + 50 mg of Hexane extract/kg bw
Group 4: DC + 50 mg of Ethyl acetate extract/kg bw
Group 5: DC + 50 mg of Methanol extract/kg bw
Group 6: DC + 50 mg of Aqueous extract/kg bw
After, an overnight fasting, the rats of group 1 and group 2 were taken only distilled water. Whereas group 3, group 4, group 5 and group 6 diabetic rats received the fruit of hexane extract, ethyl acetate, methanol and aqueous extracts each at a dosage of 50 mg/kg bw respectively. The blood samples were collected for the analysis of fasting blood glucose (FBG) from the tail vein at 0, 1, 2, 3, 4, 5 and 6 h after feeding the extracts. The FBG levels were measured by using glucose oxidase-peroxidase reactive strips with a glucometer. The results were analyzed and compared with the control and diabetic control groups.
2.7. Effect of different doses of fruit aqueous extract of Syzygium paniculatum (FAESP); dose fixation study
The rats divided into 9 groups and each group consisting of 6 rats.
Group 1: Control (C)
Group 2: Diabetic Control (DC)
Group 3: C + 50 mg of FAESP/kg bw/day
Group 4: C + 100 mg of FAESP/kg bw/day
Group 5: C + 200 mg of FAESP/kg bw/day
Group 6: DC + 50 mg of FAESP/kg bw/day
Group 7: DC + 100 mg of FAESP/kg bw/day
Group 8: DC + 200 mg of FAESP/kg bw/day
Group 9: DC + 10 mg of glibenclamide (Gli)/kg bw/day
After the overnight fasting, the group 1 control and group 2 diabetic control rats taken only distilled water. While group 3, 4, 5 control-treated rats and group 6, 7, 8 diabetic control rats consumed FAESP at a dosage of 50, 100 and 200 mg/kg bw, respectively. But, group 9 rats received standard drug glibenclamide at a dosage of 10 mg/kg bw. The blood samples were collected for the analysis of blood glucose from the tail vein at 0, 1, 2, 3, 4, 5 and 6 h after feeding the extract. Moreover, blood glucose levels analyzed by using glucose oxidase-peroxidase reactive strips with a glucometer. The results were analyzed and compared with the control and diabetic control groups.
2.8. Effect of 120 days long-term treatment at a dose of 100 mg of FAESP/kg bw on glycemic control, glycosylated hemoglobin (HbA1c), insulin, OGTT, lipid profile, hepatic, renal function markers, oxidative stress status and antioxidant enzyme activities
The rats divided into 5 groups, and each group consisted of 6 rats.
Group 1: Control (C)
Group 2: C + 100 mg of FAESP/kg bw/day/120 days
Group 3: Diabetic Control (DC)
Group 4: DC + 100 mg of FAESP/kg bw/day/120 days
Group 5: DC + 10 mg glibenclamide/kg bw/day/120 days
The FAESP and standard drug glibenclamide (Gli) were orally administered to the animals of the respective groups in every day morning for 120 days by gastric intubation with a force-feeding needle. Before the end of the experiment, the oral glucose tolerance test (OGTT) was done in overnight fasted control and STZ-induced diabetic rats. Glucose (2 g/kg bw) was administered orally to all groups of rats using a force-feeding needle. The group 2 and group 4 rats were administered with FAESP and group 5 treated with glibenclamide respectively. Control rats (group 1) and control treated rats (group 2) were fed with distilled water alone. The blood samples were collected from the tail veins of all groups of the rats from 0 min to 180 min at every 30 min of the time interval. The blood glucose levels were determined by using dextrostix with a basic one-touch accu-chek glucometer.
The body weights of all groups rats were recorded before the treatment and during the experimental period at regular intervals. Finally, on 121st day, after an overnightfasting, all the 5 groups rats were sacrificed by cervical dislocation. The blood, liver, kidney, muscle, and pancreas were collected and stored immediately at -80oCfor further examinations.
2.9. Biochemical assessments
The fasting blood glucose was performed by glucose oxidase-peroxidase through the process of Kesari et al. [7]. Glycosylated haemoglobin (HbA1c) was assessed by the technique of Eross et al. [8]. Insulin level was estimated by the modified process of Herbert et al. [9]. Cholesterol (Chol) level was measured by method of Zlatkis et al. [10]. The levels of triglycerides (TG) were measured by the technique of Foster and Dunn [11] and high-density lipoprotein cholesterol (HDL-C) levels analyzed by Burstein technique of Burstein et al. [12]. Plasma creatinine levels were estimated by Jaffe's technique Wybenga et al. (1971) [13] and urea levels measured by diacetyl monoxime method Slot [14]. Serum alanine transaminase (ALT) and aspartate transaminase (AST) activities were analyzed by the enzymatic technique of Reitman and Frankel [15]. Serum alkaline phosphatase (ALP) activity was determined by P-Nitro Phenyl Phosphate method of Bessey et al. [16]. Oral glucose tolerance test (OGTT) was performed according to the standard method of Du Vigneaud and Karr [17].
2.10. Effect of FAESP on glycogen and protein levels in tissue homogenates
The liver and muscle tissue glycogen levels were estimated by method of Kemp and Van Hejnigen [18]. The liver and kidney tissues protein levels were estimated by the method of Lowry et al. [19].
2.11. Effect of FAESP on oxidative stress (lipid peroxidation)
The oxidative degradation of lipids in liver and kidney were determined by the method of Fraga et al. [20] through measuring the concentration of thiobarbituric acid reactive substances (TBARS) which were expressed regarding malondialdehyde (MDA) content in tissues.
2.12. Effect of FAESP on enzymatic antioxidants
The activity of catalase (CAT) was measured by the method of Sinha [21], the activity of superoxide dismutase (SOD) was measured by the method of Kakkar et al. [22], glutathione peroxidase (GPx) activity was analyzed by the method of Rotruck et al. [23], Glutathione-S-transferase (GST) activity was estimated by the method of Habig et al. [24].
2.13. Effect of FAESP on non-enzymatic antioxidants
The levels of vitamin-C were calculated according to Omaye et al. [25], vitamin-E levels were estimated according to Jachec et al. [26] and the levels of reduced glutathione (GSH) were measured according to Ellman [27].
2.14. Histopathological study
The pancreas was dissected and fixed in 10% formalin, then implanted in paraffin, and made 5μm thick sections, and finally stained with the hematoxylin and eosin [28]. The effect of FAESP on pancreatic sections was observed under light microscope.
2.15. Statistical analysis of the data
The results reported as Mean ± SEM. Statistical analysis of the results was executed by Student t-test and one-way analysis (ANOVA) followed by DMRT.
3. Results
3.1. Phytochemical analysis
The phytochemical evaluation for various extracts of the fruit of SP was showed the occurrence of steroids and triterpenes in hexane extract. Proteins, steroids and triterpenes in ethyl acetate extract. Alkaloids, carbohydrates, flavonoids, proteins and phenols in methanol extract. Presence of carbohydrates, glycosides, flavonoids, proteins, and phenols was found in aqueous extract (Table 1).
Table 1.
The Phytochemical analysis of different extracts of the fruit of Syzygium paniculatum.
| Different solvent extracts | ||||
|---|---|---|---|---|
| Phytoconstituents | Hexane | Ethyl acetate | Methanol | Aqueous |
| Alkaloids | – | – | + | – |
| Carbohydrates | – | – | + | + |
| Glycosides | – | – | – | + |
| Flavonoids | – | – | + | + |
| Proteins | – | + | + | + |
| Phenols | – | – | + | + |
| Steroids | + | + | – | – |
| Triterpenes | + | + | – | – |
+ Presence of constituents, – Absence of constituents.
3.2. Evaluation of the antihyperglycemic activity of different extracts
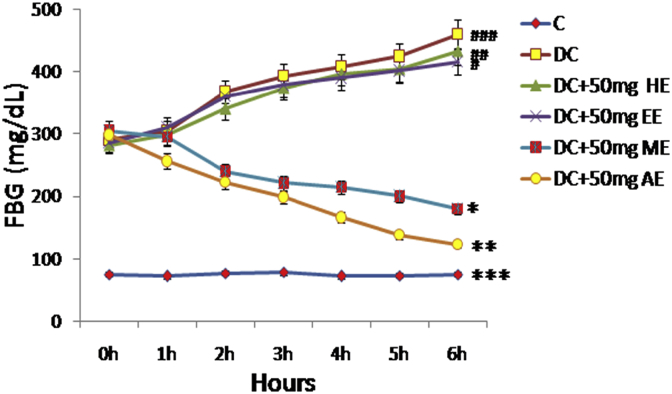
The activities of various extracts like hexane, ethyl acetate, methanol, and aqueous extracts on the FBG levels of diabetic rats (Fig. 1) were expressively greater than those of control rats (Group 1). When different extracts of the fruit of SP evaluated for their blood glucose lowering effects, the methanol and aqueous extracts each at a dose of 50 mg/kg bw revealed the maximum fall of 40% and 55% respectively in the FBG levels of diabetic rats after 6 h of treatment. Either hexane or ethyl acetate extracts did not show any antihyperglycemic activity in STZ induced diabetic rats.
Fig. 1.
Effect of different solvent extracts of the FSP in STZ-induced diabetic rats. FSP: Fruit of Syzygium paniculatum, STZ: Streptozotocin, FPG: Fasting Plasma Glucose, C: Control, DC: Diabetic Control, HE: Hexane Extract, EE: Ethyl acetate Extract, ME: Methanol Extract, AE: Aqueous Extract. Each point expressed as Mean ± SEM of each independent parameters. ***p ≤ 0.001 vs. C group, ###p ≤ 0.001 vs. DC group. *p ≤ 0.05 and ***p ≤ 0.001vs. FAESP treated group.
3.3. Effect of different doses of FAESP in STZ-induced diabetic rats
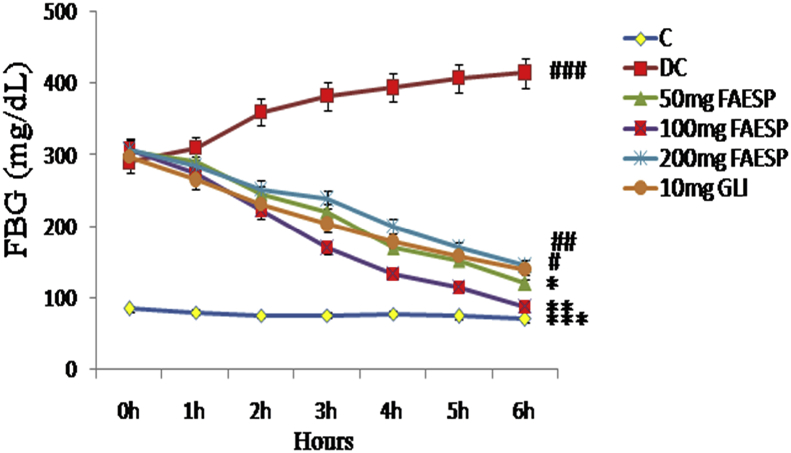
The effect of different doses 50 mg, 100 mg, and 200 mg of fruit aqueous extract of SP on the fasting blood glucose levels of diabetic rats were shown in Fig. 2, among the doses, the fruit aqueous extract at the dose of 100 mg/kg bw has revealed a maximum fall of 70% in the FBG levels of diabetic rats after 6 h of treatment, but the other doses like 50 mg and 200 mg/kg bw of aqueous extract revealed a fall of 60% and 50% respectively. The treatment with standard drug glibenclamide at a dose of 10 mg/kg bw of diabetic rats showed a 30% fall of blood glucose after 6 h of treatment.
Fig. 2.
Effect of different doses of FAESP in STZ-induced diabetic rats. FAESP: Fruit aqueous extract of Syzygium paniculatum, GLI: Glibenclamide. Each point represents the Mean ± SEM of each independent parameter. ***p ≤ 0.001 vs. C group, ###p ≤ 0.001 vs. DC group. *p ≤ 0.05; **p ≤ 0.01 and ***p ≤ 0.001 vs. FAESP treated group.
3.4. The 120 days long-termeffect of FAESP on fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1C), insulin and body weights
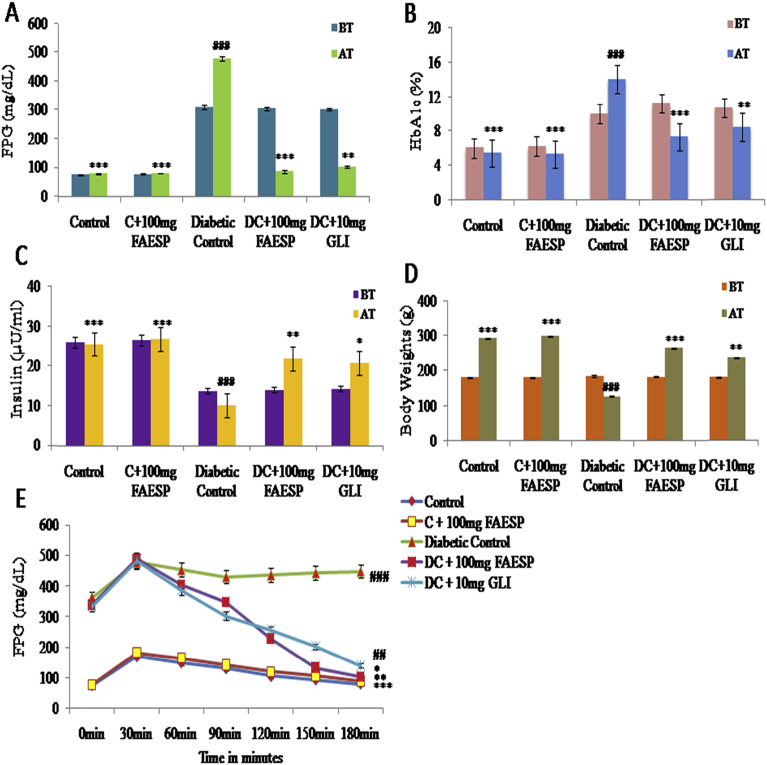
FPG levels of diabetic control rats (Group-3) were considerably greater than those of the control group (Group-1) before starting the experiment. But, at the end of 120 days of treatment, there was a 78% decrease (P < 0.001) in FPG levels of diabetic rats treated with aqueous extract, while there was a further increase in the FPG levels of diabetic control rats was observed. The treatment with glibenclamide has produced only 55% decrease in the FPG levels (Fig. 3A).
Fig. 3.
Effect of FAESP on Fasting Blood Glucose, Glycosylated hemoglobin, Insulin, Body weights and OGTT was performed to assess glucose tolerance. (A) Glucose, (B) Glycosylated hemoglobin, (C) Insulin, (D) Body weights, and (E) OGTT. FAESP: Fruit aqueous extract of Syzygium paniculatum, HbA1C: Glycosylated hemoglobin, OGTT: Oral glucose tolerance test, GLI: Glibenclamide. BT: Before treatment, AT: After treatment. Each point represents the Mean ± SEM of each independent parameter. ***p ≤ 0.001 vs. C group, ###p ≤ 0.001 vs. DC group. *p ≤ 0.05; **p ≤ 0.01 and ***p ≤ 0.001 vs. FAESP treated group.
The levels of glycosylated haemoglobin (HbA1C) (14.00 ± 0.53%) of the diabetic control group were considerably greater than those in the control group (5.47 ± 0.15%). But treatment with the FAESP in diabetic rats reduced the HbA1C significantly (Fig. 3B). Plasma insulin levels (10.17 ± 0.70μU/ml) of diabetic control rats were considerably lesser (25.6 ± 0.86μU/ml) than in control rats after 120 days. Diabetic rats treated with the FAESP have shown a significant increase in the insulin levels to 21.83 ± 1.51μU/ml. The glibenclamide treated diabetic rats also substantially upturn in the levels of insulin to 20.83 ± 1.08μU/ml. In control-treated rats, there was insignificant variation in the plasma insulin levels (Fig. 3C).
After 120 days of treatment, the body weights of the control rats, diabetic rats treated with FAESP and glibenclamide also increased considerably, however, the body weight of diabetic control group declined (Fig. 3D).
3.5. Effect of FAESP on oral glucose tolerance in control and diabetic control rats
The FPG levels of all the groups of animals calculated from 0 to 180 min. In all the groups, FPG levels were upturned after 30 min after the glucose load. Treatment with FAESP at a dose of 100 mg/kg bw along with 2g glucose/kg bw has considerably enhanced the glucose acceptance in diabetic rats. In diabetic control rats, the glucose levels stayed greater without considerably changes at 180 min after glucose load. But after treatment with FAESP and glibenclamide found to be falling in blood glucose levels started from 60 min after a glucose load, and there was a consistent reduction in the blood glucose levels till the end of 180 min after glucose load. The results are depicted in Fig. 3E.
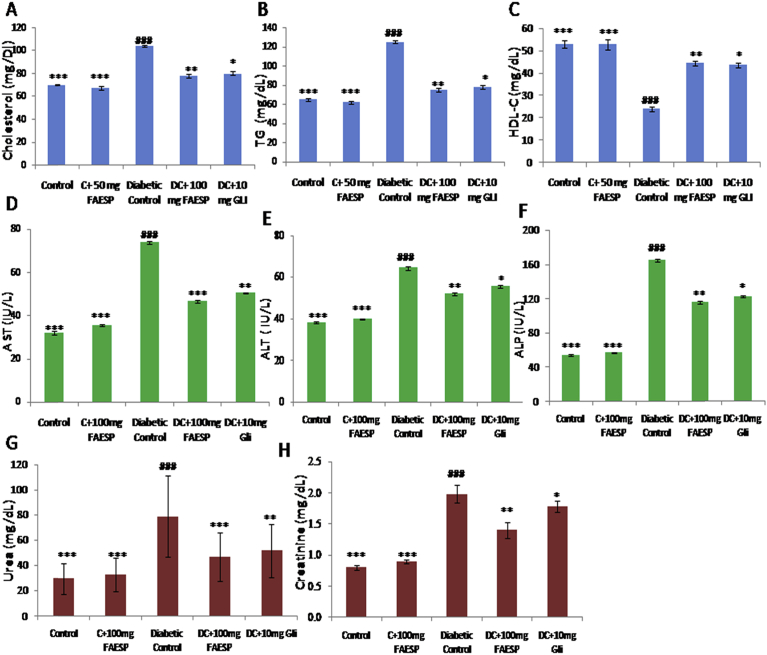
3.6. Effect of FAESP on lipid metabolism
In diabetic control rats, the levels of TC (Fig. 4A), and TG (Fig. 4B) were found to be significantly elevated; whereas the HDL-C (Fig. 4C) levels significantly reduced. But after treatment with FAESP (100 mg/kg bw) and glibenclamide, there was a substantial reduction in the levels of TC, TG and a considerable upturn in HDL-C was observed.
Fig. 4.
Effect of FAESP on lipid profile, Hepatic and Renal Functional markers. (A) Total Cholesterol, (B) TG, (C) HDL-Cholesterol, (D) AST, (E) ALT, (F) ALP, (G) Urea, and (H) Creatinine. TG: Triglycerides, HDL: High density lipoproteins, AST: Aspartate amino-transferase, ALT: Alanine aminotransferase, ALP: Alkaline phosphatase. Each point represents the Mean ± SEM of each independent parameter. ***p ≤ 0.001 vs. C group, ###p ≤ 0.001 vs. DC group. *p ≤ 0.05; **p ≤ 0.01 and ***p ≤ 0.001 vs. FAESP treated group.
3.7. Effect of FAESP on hepatic and renal functional markers
The activities of hepatic functional markers AST, ALT, and ALP are found to be increased in diabetic control rats. The treatment with FAESP and glibenclamide reversed these levels to the standard range (Fig. 4D, E, and F). Renal functional markers, urea and creatinine levels found to be increased in diabetic control rats, however, the treatment with FAESP and glibenclamide has considerably reduced these levels to the standard range (Fig. 4G and H).
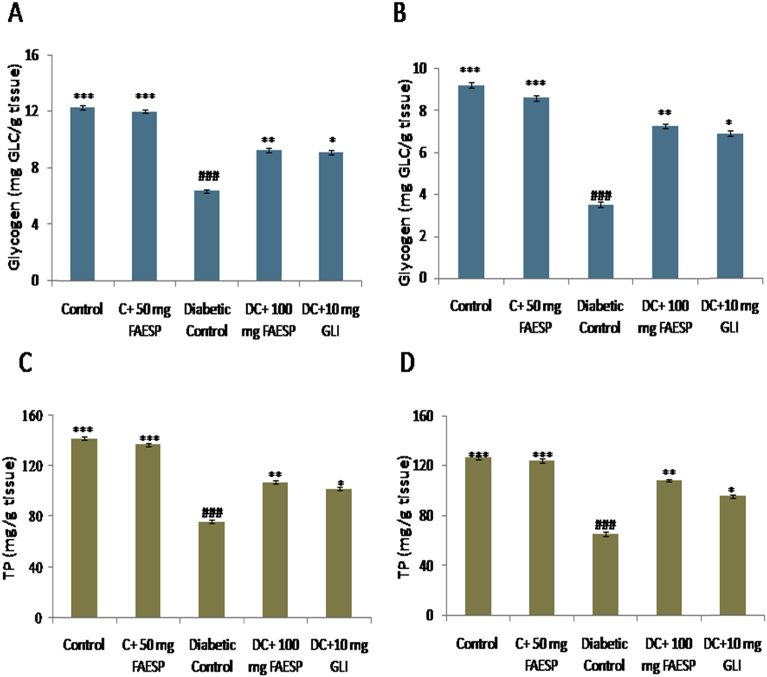
3.8. Effect of FAESP on glycogen and total protein
The levels of glycogen in both liver and muscle of diabetic control rats found to be reduced. The treatment with the FAESP (100 mg/kg bw) and glibenclamide were resulted an improvement in the liver and muscle glycogen levels (Fig. 5A and B). The total protein (TP) levels in both liver and kidney of diabetic control rats were found to be decreased. But after treatment with the FAESP (100 mg/kg bw) and glibenclamide significantly increased the levels of total proteins (Fig. 5C and D).
Fig. 5.
Effect of FAESP on Glycogen and Total protein of different tissues. (A) Glycogen in Liver, (B) Glycogen in Muscle, (C) Total Protein of liver, and (D) Total protein of kidney. Each point represents the Mean ± SEM of each independent parameter. ***p ≤ 0.001 vs. C group, ###p ≤ 0.001 vs. DC group. *p ≤ 0.05; **p ≤ 0.01 and ***p ≤ 0.001 vs. FAESP treated group.
3.9. Effect of FAESP on oxidative stress (lipid peroxidation)
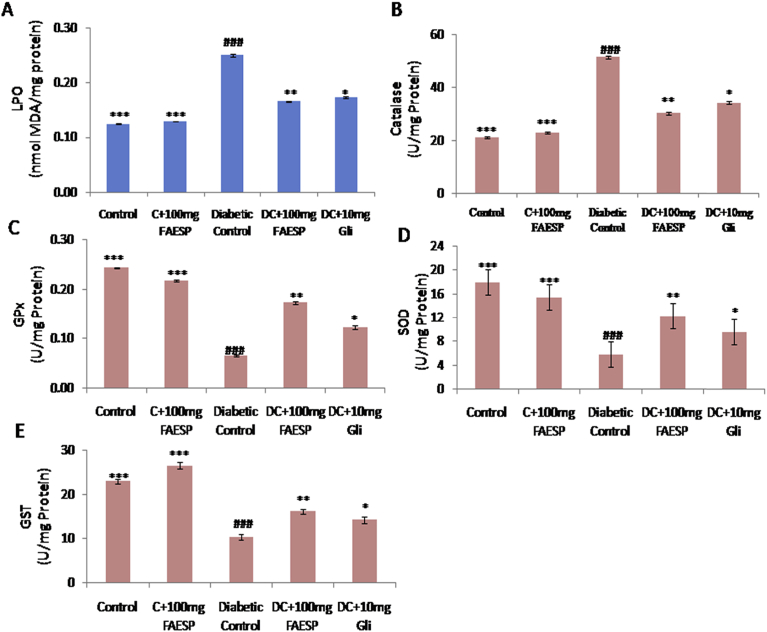
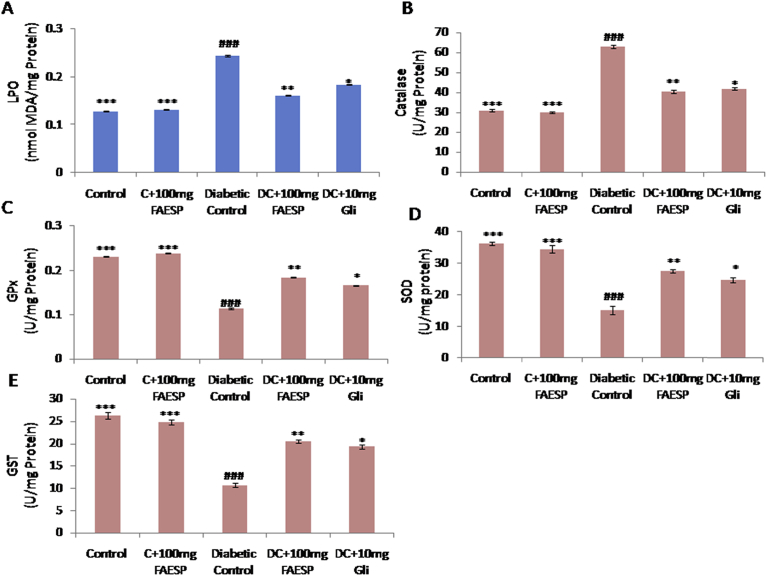
The oxidative degradation of lipids in liver and kidney were determined by evaluating the concentration of thiobarbituric acid reactive substances (TBARS) which expressed regarding malondialdehyde (MDA) content. MDA level in liver (Fig. 6A), and kidney (Fig. 7A) found to be significantly increased in the diabetic control rats in comparison to control rats, whereas FAESP (100 mg/kg bw) and glibenclamide treated groups resulted in a significant reduction (Figs. 6A and 7A).
Fig. 6.
Effect of FAESP on oxidative stress (Lipid peroxidation), TBARS levels and enzymatic antioxidant activities in Liver. (A) TBARS (LPO) in Liver, (B) Catalase in Liver, (C) GPx in Liver, (D) SOD in Liver, and (E) GST in Liver. TBARS: Thiobarbituric acid reactive substances, LPO: Lipid peroxidation, GPx: Glutathione peroxidase, SOD: Superoxide dismutase, GST: Glutathione-S-Transferase. Each point represents the Mean ± SEM of each independent parameter. ***p ≤ 0.001 vs. C group, ###p ≤ 0.001 vs. DC group. *p ≤ 0.05; **p ≤ 0.01 and ***p ≤ 0.001 vs. FAESP treated group.
Fig. 7.
Effect of FAESP on oxidative stress (Lipid peroxidation), TBARS levels and enzymatic antioxidant activities in Kidney. (A) TBARS (LPO) levels in Kidney, (B) Catalase in Kidney, (C) GPx in Kidney, (D) SOD in Kidney, and (E) GST in Kidney. Each point represents the Mean ± SEM of each independent parameter. ***p ≤ 0.001 vs. C group, ###p ≤ 0.001 vs. DC group. *p ≤ 0.05; **p ≤ 0.01 and ***p ≤ 0.001 vs. FAESP treated group.
3.10. Effect of FAESP on enzymatic antioxidants
CAT activity was found to be significantly increased (Figs. 6B and 7B), and the activities of GPx, SOD, and GST was observed to be decreased in both liver (Fig. 6C, D, and E), and kidney (Fig. 7C, D, and E) of diabetic rats. But the treatment with FAESP was decreased the CAT activity and significantly increased activities of GPx, SOD, and GST in liver (Fig. 6C, D, and E), and kidney (Fig. 7C, D, and E) of diabetic rats. Similar effects noticed with glibenclamide, but they were fewer in magnitude in comparison to those of the FAESP (Figs. 6B, C, D, E, and 7B, C, D, and E).
3.11. Effect of FAESP on non-enzymatic antioxidants
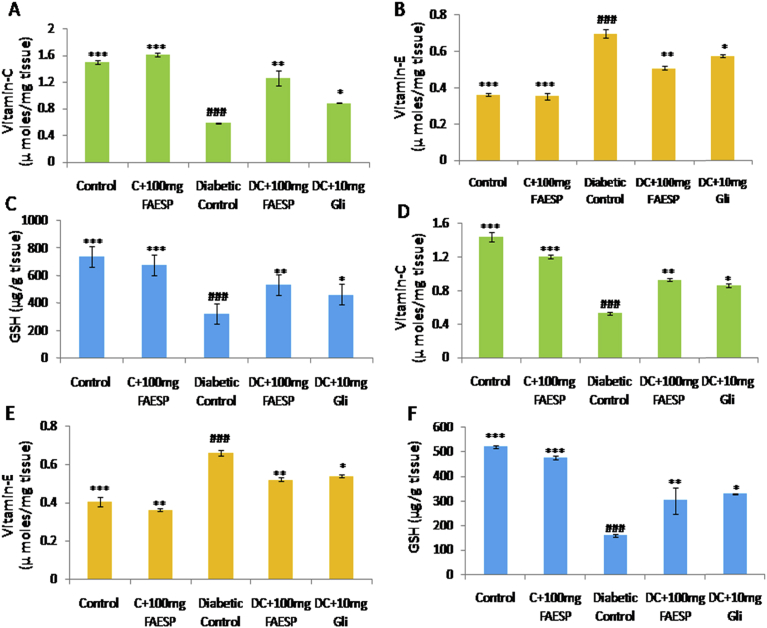
The activities of vitamin C and GSH were observed to be considerably declined (Fig. 8A, C, D, and F) while the activities of vitamin E were significantly increased (Fig. 8B and E) in both liver and kidney tissues of diabetic control rats. The treatment with FAESP (100 mg/kg bw) and glibenclamide significantly restored these values near to normal level (Fig. 8A, B, C, D, E and F).
Fig. 8.
Effect of FAESP on Non-enzymatic antioxidant activities in Liver and Kidney. (A) Vitamin-C in Liver, (B) Vitamin-E in Liver, (C) GSH, (D) Vitamin-C in Kidney, (E) Vitamin-E in Kidney, and (F) GSH in Kidney. GSH: Reduced Glutathione. Each point represents the Mean ± SEM of each independent parameter. ***p ≤ 0.001 vs. C group, ###p ≤ 0.001 vs. DC group. *p ≤ 0.05; **p ≤ 0.01 and ***p ≤ 0.001 vs. FAESP treated group.
3.12. Effect of FAESP on pancreas histopathology
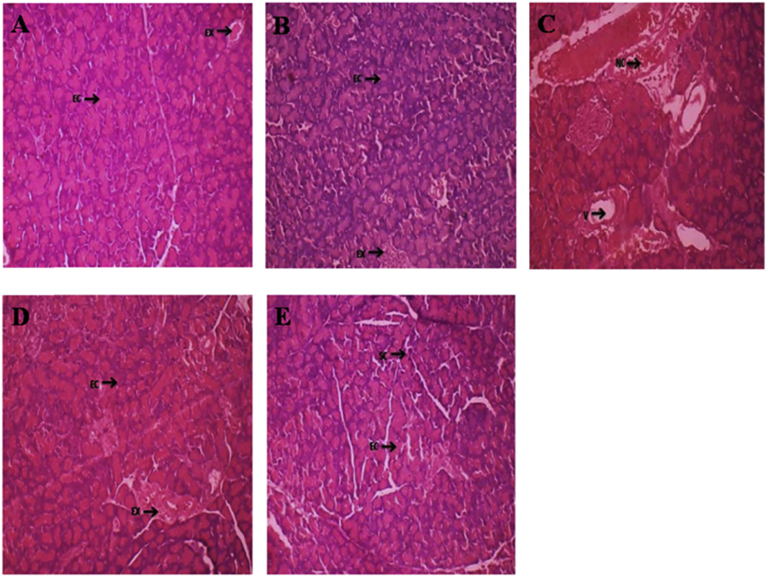
Histological changes of the pancreas from control, control and FAESP (100 mg/kg bw), diabetic control and FAESP (100 mg/kg bw) & diabetic control and glibenclamide (10 mg/kg bw) treated groups were observed. The pancreas of control and control treated rats showing the typical architecture and normal Islets of langerhans with granulated cytoplasm and acini tissues were healthy (Fig. 9A and B). Whereas pancreas of diabetic control rats exhibited irregular necrotic pancreatic acini, cytoplasmic degeneration, insulitis with lymphocytic infiltrations, atrophy and damage of β cells markedly seen. Besides, irregular outlining of the Islets of langerhans also observed (Fig. 9C). Diabetic control rats treated with FAESP resulted in gradual increase in the restoration in the exocrine pancreatic tissue with acinar cells and endocrine pancreatic tissue with Islets of langerhans by the recovery of the damaged islets and an improvement in some beta cells (Fig. 9D). Treatment with glibenclamide also resulted in restoration in the exocrine acinar cells, islets of langerhans and increased cellularity of β-cell in pancreatic cells (Fig. 9E).
Fig. 9.
The Effect of FAESP on Pancreas histopathology (H&E, Hematoxylin-Eosin Stain). (A) The typical architecture with normal Islets of Langerhans with granulated cytoplasm and acini tissues in control rats, (B) Normal Islets of Langerhans with granulated cytoplasm and acini tissues were healthy in control-treated rats, (C) Irregular necrotic pancreatic acini and cytoplasmic degeneration, insulitis with lymphocytic infiltrations, atrophy, destruction of β cells and irregular outlining of the Islets of Langerhans markedly seen in STZ-induced diabetic control rats, (D) FAESP resulted in gradual increase in the restoration in the acinar cells and Islets of Langerhans by the recovery of the damaged islets and an improvement in some beta cells, (E) Glibenclamide resulted in restoration in the exocrine acinar cells, Islets of Langerhans and increased cellularity of β-cell in pancreatic cells. IL: Islets of Langerhans, AC: Acinar cells, NC: Necrotic Change, EX: Exocrine, C: Congestion.
4. Discussion
Diabetes mellitus is very common chronic, endocrine disorder and allied with hyperglycemia, hyperlipidemia, and disorders co-occurring with obesity and hypertension. At presently available medicines for the treatment of diabetes have serious side effects. So, there is a need for medicine with less or no side effects for the management of diabetes. The natural products from medicinal plants are considered as a useful alternative treatment and management of diabetes, because of their higher efficacy and less to nil side effects. Scientific reports indicate that the fruits of Syzygium sp. used to lower the blood glucose levels during hyperglycemia three decades before the discovery of insulin. Plant based therapeutics can be a better option for the management of diabetes and related metabolic complications [29].
Our study confirms the antihyperglycemic, antihyperlipidemic, antioxidative stress and antioxidant properties of the fruit of S. paniculatum in STZ-induced diabetic rats. The fruit powder aqueous suspension resulted in a noteworthy reduction in FBG levels. So, the fruit used for preparation of different solvent extracts and evaluated for antihyperglycemic property. Among different solvent extracts, the aqueous extract at a dose of 100 mg/kg bw tended to bring FBG levels towards normal. The phytochemical investigation of FAESP revealed the presence of carbohydrates, proteins, phenols, flavonoids, and glycosides. The antihyperglycemic effect may be due to the presence of more than one antihyperglycemic principle and their synergistic characteristics. So, the aqueous extract is deliberated to have noble antihyperglycemic activity without causing any hypoglycemic effect contrasting insulin and other synthetic oral drugs.
In the long term of 120 days treatment, the simultaneous administration of the FAESP brought a significant reduction in FBG levels. This is due to improved insulin release and insulin action on potentiating glucose uptake by various tissues. HbA1C levels were checked as a consistent index of glycemic control in diabetes. Increased levels find in diabetic control rats might be due to the improved form of glycosylated haemoglobin. Long-term treatment with FAESP resulted in a substantial reduction in HbA1C levels.
This study was insulin-deficient model since at low dose it causes the partial damage of pancreatic β-cells. Therefore, insulin levels were low in diabetic control rats in comparison to control rats. The antihyperglycemic action in diabetic rats may be due to increase in the pancreatic secretion of insulin from the existing β-cells after the treatment with FAESP. The STZ induced diabetes resulted, a severe loss in body weight due to the insulin deficiency which cause deficit of structural proteins, muscle wasting and weight loss. The administration of FAESP increased insulin activity and glycemic control which prevented loss of body weight.
OGTT also resulted that, FAESP possess blood glucose lowering activity. The treatment increased glucose consumption, so blood glucose levels were considerably reduced in glucose-loaded rats. The OGTT studies also endorse 100 mg/kg bw to be the most efficient dose and the extract did not generate any hypoglycemic effect in control-treated rats. So, the present study shows that the traditional evidence is scientifically confirmed by animal trials.
It is well-known that elevated TC, TG and decreased HDL-C levels considered as the usual abnormalities in the diabetes. Here in, the decreased level of TC, TG and the increased level of HDL-C in FAESP treated groups confirm the decisive role of FAESP in increased insulin action on cholesterol, fatty acid biosynthesis and intestinal cholesterol absorption. The elevated levels of plasma AST, ALT and ALP showed that diabetes might induce hepatic dysfunction. These enzymes directly linked with the transformation of amino acids to keto acids so the levels were increased in the diabetic condition. The treatment with FAESP normalized enzyme activities and normalizing hepatocellular damage and suppression of increased protein catabolism accompanying gluconeogenesis. The plasma renal functional markers such as urea and creatinine levels found to be increase in diabetes indicate kidney destruction due to uncommon glucose regulation and hemodynamic alterations within the kidney and increased oxidative stress. However, the treatment with FAESP lowered these levels to a normal range.
The glycogen levels in liver and muscle of diabetic control rats were significantly lowered than those of control group due to influx of glucose inhibited in the absence of insulin and recovers on treatment. Our findings show that after treatment with FAESP, there was a substantial rise in the liver and muscle glycogen levels. Glibenclamide treatment also resulted in a significant enhancement in the glycogen levels. In our study, total protein reduced in diabetic control may be due to microproteinuria and improved protein catabolism during insulin deficiency. However, treatment with FAESP increased total protein level; it may be due to the effect of different phytoconstituents acting in united manner by stimulating β cells to release adequate insulin levels.
STZ induction causes tissue damage by oxidative stress, free radical and ROS generation. The increase in the level of oxidative stress marker (TBARS or MDA) disturbs the antioxidant system. Due to these events, variation in the oxidant and antioxidant defense system results changes in the activity of antioxidant enzymes like SOD, CAT, GPx, GST and impaired glutathione metabolism. In our investigation the activities of SOD, GPx, and GST were observed to be decreased in diabetic rats compared to control rats, due to the free radical-induced inactivation and glycation of the enzymes. The treatment with FAESP reversed the activities of enzymatic antioxidants that indicates the aqueous extract decreased the potential glycation of enzymes. Finally, the administration of FAESP decreased the levels of TBARS in the liver and kidney of diabetic rats. This shows that FAESP might protect the tissues (liver and kidney) against the cytotoxic action and oxidative stress of streptozotocin.
Diabetes mediated oxidative stress also correlates with a reduction in the activity of non-enzymatic antioxidants such as Vit-C, Vit-E and GSH. The levels of vitamin C and GSH found to decreased whereas the levels of vitamin E increased in the liver and kidney tissues of diabetic rats. The elevated level of Vit-E could be due to increased membrane destruction by ROS, and the subsequent release of membrane-bound α-tocopherol from the injured cell membrane. The administration of FAESP brought the levels of Vit-C and GSH near to normal by preventing the toxic effects against the increased ROS. The treatment with FAESP also brought the vitamin-E near to normal which could be as an outcome of reduced membrane destruction as demonstrated by reduced oxidative stress (lipid peroxidation).
Histopathology examination of pancreas also supports the restoration capacity in pancreatic islets. The fruits of S. paniculatum has potential to regenerate the islets of langerhans could be due to stable cells (Quiescent) which have the ability to regenerate. So, the persisting cells can proliferate to substitute the vanished cells. The restoration in the exocrine acinar cells, Islets of langerhans and increased cellularity of β-cells confirm the restoration capacity of FAESP in pancreas. Overall, this study supports the antihyperglycemic, antihyperlipidemic, antioxidative stress and antioxidant potential effect of the fruit aqueous extract of S. paniculatum because of the occurrence of phytoconstituents such as carbohydrates, proteins, phenols, flavonoids and glycosides in the aqueous extract.
5. Conclusion
The fruit aqueous extract of S. paniculatum has a potential effect on glycemic control, dyslipidemia, oxidative stress and antioxidant enzyme activities in STZ-induced diabetic rats by improving insulin secretion through β-cell restoration capacity. The active phytoconstituents such as carbohydrates, proteins, phenols, flavonoids, and glycosides in the aqueous extract are responsible for in vivo antihyperglycaemic, antihyperlipidemic, antioxidative stress and antioxidant properties. It is observed that the significant effects of FAESP on histological changes in the diabetic treated rats also coinciding with its potential on insulin secretion, glycemic control, lipid metabolism, oxidative stress and antioxidant enzyme activities in the diabetic treated rats.
However, further studies are needed for the isolation and purification of bioactive constituents from FAESP; this could be a limitation of the study. Identification of the novel and potent bioactive compounds with specific chemical moieties will provide a new potential traditional approach for the treatment and management of diabetes and its allied complications.
Declarations
Author contribution statement
Prabhakar Yellanur Konda; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sreenivasulu Dasari: Analyzed and interpreted the data; Wrote the paper.
Sreenath Konanki: Performed the experiments; Wrote the paper.
Prabhusaran Nagarajan: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest
Additional information
No additional information is available for this paper.
References
- 1.Farzaei F., Morovati M.R., Farjadmand F., Farzaei M.H. A mechanistic review on medicinal plants used for diabetes mellitus in traditional Persian medicine. Evid. Based Complement. Alternat. Med. 2017;22:944–955. doi: 10.1177/2156587216686461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prabhakar Y., Ali M.S., Kumar M.J., Tilak T.K., Rao C.A. Evaluation of antioxidant activities of aqueous extract of stem bark of Boswellia ovalifoliolata in streptozotocin induced diabetic rats. J. Pharm. Chem. 2013;7:19–24. [Google Scholar]
- 3.Reynertson K.A., Basile M.J., Kennelly E.J. Antioxidant potential of seven myrtaceous fruits. Ethnobot. Res. Appl. 2005;3:025–036. [Google Scholar]
- 4.Vuong Q.V., Hirun S., Chuen T.L., Goldsmith C.D., Bowyer M.C., Chalmers A.C., Phillips P.A., Scarlett C.J. Physicochemical composition, antioxidant and anti-proliferative capacity of a lilly pilly (Syzygium paniculatum) extract. J. Herb. Med. 2014;4:134–140. [Google Scholar]
- 5.Madhavachetty K., Sivaji K., Tulasirao K. Students Offset Printers; Tirupati: 2008. Flowering Plants of Chittoor District– Andhra Pradesh, India. [Google Scholar]
- 6.Harborne A.J. Springer science & business media; 1998. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 7.Kesari A.N., Gupta R.K., Singh S.K., Diwakar S., Watal G. Hypoglycemic and antihyperglycemic activity of Aegle marmelos seed extract in normal and diabetic rats. J. Ethnopharmacol. 2006;107:374–379. doi: 10.1016/j.jep.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Eross J., Kreutzmann D., Jimenez M., Keen R., Rogers S., Cowell C., Vines R., Silink M. Colorimetric measurement of glycosylated protein in whole blood, red blood cells, plasma and dried blood. Ann. Clin. Biochem. 1984;21:477–483. doi: 10.1177/000456328402100606. [DOI] [PubMed] [Google Scholar]
- 9.Herbert V., Lau K.S., Gottlieb C.W., Bleicher S.J. Coated charcoal immunoassay of insulin. J. Clin. Endocrinol. Metab. 1965;25:1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 10.Zlatkis A., Zak B., Boyle A.J. A new method for the direct determination of serum cholesterol. J. Lab. Clin. Med. 1953;41:486–492. [PubMed] [Google Scholar]
- 11.Foster L.B., Dunn R.T. Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clin. Chem. 1973;19:338–340. [PubMed] [Google Scholar]
- 12.Burstein M.S.H.R., Scholnick H.R., Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 1970;11:583–595. [PubMed] [Google Scholar]
- 13.Wybenga D.R., Di Giorgio J., Pileggi V.J. Manual and automated methods for urea nitrogen measurement in whole serum. Clin. Chem. 1971;17:891–895. [PubMed] [Google Scholar]
- 14.Slot C. Plasma creatinine determination a new and specific Jaffe reaction method. Scand. J. Clin. Lab. Invest. 1965;17:381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 15.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 16.Bessey O.A. A method for a rapid determination of alkaline phosphatase with five cubic millimeters of serum. J. Biol. Chem. 1954;207:19–23. [PubMed] [Google Scholar]
- 17.Du Vigneaud V., Karr W.G. Carbohydrate utilization I. Rate of disappearance of d-glucose from the blood. J. Biol. Chem. 1925;66:281–300. [Google Scholar]
- 18.Kemp A., Van Heijningen A.J.K. A colorimetric micro-method for the determination of glycogen in tissues. Biochem. J. 1954;56:646–648. doi: 10.1042/bj0560646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Fraga C.G., Leibovitz B.E., Toppel A.L. Lipid peroxidation measured as TBARS in tissue slices. Characterisation and comparison with homogenate and microsome. Free Radic. Biol. Med. 1988;4:155–161. doi: 10.1016/0891-5849(88)90023-8. [DOI] [PubMed] [Google Scholar]
- 21.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 22.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130–132. http://nopr.niscair.res.in/handle/123456789/19932 [PubMed] [Google Scholar]
- 23.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 24.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 25.Omaye S.T., Turnbull J.D., Sauberlich H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 26.Jacheć W., Tomasik A., Tarnawski R., Chwalińska E. Evidence of oxidative stress in the renal cortex of diabetic rats: favourable effect of vitamin E. Scand. J. Clin. Lab. Invest. 2002;62:81–88. doi: 10.1080/003655102753517244. [DOI] [PubMed] [Google Scholar]
- 27.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Stevens A. sixth ed. Churchill Livingstone/Elsevier; Philadelphia (PA): 1994. Connective Tissues and Stains, Theory and Practice of Histological Techniques; pp. 129–134. [Google Scholar]
- 29.Zaid H., Tamrakar A.K., Razzaque M.S., Efferth T. Diabetes and metabolism disorders medicinal plants: a glance at the past and a look to the future. Evid. Based Complement. Alternat. Med. 2018:1–3. doi: 10.1155/2018/5843298. [DOI] [PMC free article] [PubMed] [Google Scholar]