Abstract
Aims
To determine whether a restrictive strategy of red blood cell (RBC) transfusion at lower haemoglobin concentrations is inferior to a liberal strategy of RBC transfusion at higher haemoglobin concentrations in patients undergoing cardiac surgery.
Methods and results
We conducted a systematic review, meta-analysis, and trial sequential analysis of randomized controlled trials of the effect of restrictive and liberal RBC transfusion strategies on mortality within 30 days of surgery as the primary outcome. Secondary outcomes were those potentially resulting from anaemia-induced tissue hypoxia and transfusion outcomes. We searched the electronic databases MEDLINE, EMBASE, and the Cochrane Library until 17 November 2017. Thirteen trials were included. The risk ratio (RR) of mortality derived from 4545 patients assigned to a restrictive strategy when compared with 4547 transfused according to a liberal strategy was 0.96 [95% confidence interval (CI) 0.76–1.21, I2 = 0]. A restrictive strategy did not have a statistically significant effect on the risk of myocardial infarction (RR 1.01, 95% CI 0.81–1.26; I2=0), stroke (RR 0.93, 95% CI 0.68–1.27, I2 = 0), renal failure (RR 0.96, 95% CI 0.76–1.20, I2 = 0), or infection (RR 1.12, 95% CI 0.98–1.29, I2 = 0). Subgroup analysis of adult and paediatric trials did not show a significant interaction. At approximately 70% of the critical information size, the meta-analysis of mortality crossed the futility boundary for inferiority of the restrictive strategy.
Conclusion
The current evidence does not support the notion that restrictive RBC transfusion strategies are inferior to liberal RBC strategies in patients undergoing cardiac surgery.
Keywords: Cardiac surgery , Blood transfusion , Thresholds
See page 1089 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy505)
Introduction
Anaemia is common in patients undergoing cardiac surgery.1 Perioperative anaemia in patients undergoing cardiac surgery has been associated with a significant increase in cardiac (myocardial infarction) and non-cardiac (renal failure and stroke) adverse events and mortality, which is attributed to impaired oxygen delivery and tissue hypoxia, particularly if the anaemia is acute or severe.2,3 Accordingly, these patients often receive red blood cell (RBC) transfusions in an attempt to increase tissue oxygen delivery.
Red blood cell transfusion, however, is also associated with adverse events. Mortality in transfused patients has been demonstrated to be higher than non-transfused patients in observational studies.4,5 The increased mortality may be subject to confounding by indication, but there are a number of complications related to transfusion that are associated with considerable morbidity and mortality. Non-infectious risks from transfusion such as transfusion related acute lung injury tend to occur more frequently in patients having cardiac surgery,6 which may be due to increased systemic and pulmonary inflammation with cardiac surgery and has a high case fatality rate,7 5–13%.8,9 Pulmonary oedema, due to the volume of RBCs transfused, has also been shown to occur more frequently in patients with critical illness and cardiovascular disease who were transfused at a haemoglobin concentration of 100 g/L compared with 70 g/L.10 It is not known at what haemoglobin threshold a balance between risk of anaemia and risk of transfusion is achieved.4
Two large randomized controlled trials (RCTs) have recently been conducted to address the risks associated with anaemia and RBC transfusion with discordant results. The Transfusion Indication Threshold Reduction (TITRe2) trial randomized 2007 patients undergoing cardiac surgery, with a post-operative haemoglobin level less than 90 g/L to maintain a haemoglobin concentration of more than 75 g/L (restrictive strategy) or more than 90 g/L (liberal strategy).11 A statistically significant difference was not apparent in the primary composite outcome of infection and ischaemic events 3 months following surgery (35.1% of the patients in the restrictive threshold group and 33.0% of the patients in the liberal-threshold group) [odds ratio (OR) 1.11, 95% confidence interval (CI), 0.91–1.34]; but there was a 1.6% absolute difference in a secondary outcome of mortality favouring the liberal strategy. In this study, however, patients were randomized following surgery and thus after the time when a considerable proportion of RBC transfusions occur (25% in TITRe2) and when potentially there is the highest risk of adverse events secondary to acute haemodilution.
The Transfusion Requirements in Cardiac Surgery III (TRICS III) trial randomized patients undergoing cardiac surgery with cardiopulmonary bypass to a restrictive strategy of a haemoglobin transfusion threshold of less than 75 g/L intra-operatively and post-operatively, or a liberal strategy of a haemoglobin threshold less than 95 g/L intra-operatively and post-operatively, while in an intensive care unit (ICU) and less than 85 g/L on a non-ICU ward.12 Non-inferiority was established in the primary composite outcome of mortality and renal/myocardial or neurological events [11.4% of the restrictive group and 12.5% of the liberal group (absolute risk difference −1.11%; 95% CI −2.93 to 0.72%)], and no significant differences were found for 28 day mortality [3.0% in the restrictive group and 3.6% in the liberal group (OR 0.85, 95% CI 0.62–1.16)].
Because of the uncertainty regarding RBC transfusion thresholds in patients undergoing cardiac surgery, we undertook a systematic review and meta-analysis to assess the effect of restrictive transfusion strategies compared with liberal strategies on all-cause mortality. Secondary outcomes included stroke, myocardial infarction, renal failure, infections, arrhythmias, bleeding outcomes, duration of hospital and ICU length of stay, proportion of patients transfused, and the number of units transfused.
Methods
We previously conducted a systematic review of transfusion thresholds in cardiac and vascular surgery.13 This current systematic review incorporates RCTs focusing on transfusion thresholds in cardiac surgery and includes recent significant trials. The systematic review was reported according to PRISMA guidelines.14
Study identification
Three databases were searched for relevant studies from 1 March 2012, the Cochrane Central Register of Controlled Trials (CENTRAL) The Cochrane Library until 17 November 2017, Issue 10; MEDLINE (Ovid) from 1950 to 17 November 2017; and EMBASE [Ovid] from 1950 to 17 November 2017. The search was not restricted for trials by date, language, or publication status. We also searched the reference lists of relevant reviews, published articles, available online conference proceedings (Annual Meetings of the American Society of Anesthesiologists, Canadian Anesthesiologists Society, European Society of Anaesthesiology, American Society of Hematology, European Hematology Association, Society of Critical Care Medicine, Society of Cardiovascular Anesthesiologists, and European Association of Cardiothoracic Anaesthesiology), and practice guidelines (American Society of Anesthesiologists, National Institute for Clinical Excellence, and Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists), as well as the reference lists of all included trials for further studies. The full search criteria can be found in the Supplementary material online.
Eligibility criteria
Randomized controlled trials were included if all the following criteria were fulfilled: included patients undergoing cardiac surgery whereby patients received RBCs at a lower haemoglobin concentration or haematocrit level (restrictive group), and were compared with patients who received RBCs at a higher haemoglobin concentration or haematocrit (liberal group); and included one of the primary or secondary outcomes. Trials were excluded if the effect of the transfusion strategy could not be elicited due to multiple interventions or if none of the outcomes of interest were reported.
Study selection and data collection
Two reviewers (N.M. and N.S.) independently screened citations to select trials that met inclusion criteria and abstracted data including funding, patient characteristics, the transfusion thresholds, and outcomes. Disagreements were resolved by consensus. Studies were not blinded to author, journal or institution.
Risk of bias assessment
Individual studies were assessed in duplicate using the Cochrane Collaboration’s risk of bias tool.15 We assessed the following domains for each study: sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. Risk of bias was considered ‘low’, ‘unclear’ (indicating unclear or unknown risk of bias), and ‘high’ risk of bias.
Data synthesis
Data were combined from all studies to estimate the pooled risk ratio (RR) and associated 95% CIs for the binary outcomes of all-cause mortality, myocardial infarction, stroke, acute renal failure, infections, arrhythmias, and transfusion. Mortality data were based on hospital deaths or deaths up to 30 days of follow-up. Definitions for the secondary outcomes were those reported by the authors in each trial.
We used the weighted mean difference (WMD) with 95% CIs to estimate the effect on the continuous outcomes of units of blood transfused, blood loss, and ICU and hospital length of stay. When not reported, we imputed sample means and standard deviations using the available summary statistics described in each study according to an approximate Bayesian computation model (see Supplementary material online).16 Primary analyses were based on inverse variance fixed effect models, results from DerSimonian–Laird random-effects models17 are provided for comparison. A continuity correction of 0.5 was used in case of zero events in one group. We constructed funnel plots, plotting log RRs of mortality against their standard errors, to examine small-study effects, including reporting biases.18 Statistical heterogeneity was assessed by the Cochran's Q-test and I2, which is the percentage of total between-study variability due to heterogeneity rather than chance. Substantial estimable heterogeneity was a priori considered to be present when I2 was more than 50%.19 We then conducted a trial sequential analysis20 to determine whether the results of our meta-analysis on all cause-mortality (in-hospital and up to 30 days) were futile as to the inferiority of the restrictive strategy when compared with liberal strategy and conclusive as to the non-inferiority of the restrictive strategy, using an event rate of 3%, a relative risk increase of 30%, power of 80%, and a one-sided alpha of 0.025 for both one-sided questions (see Supplementary material online).
Subgroup and sensitivity analysis
Subgroup analyses were planned a priori to examine potential differences in adult vs. paediatric trials. As intra-operative blood loss and/or haemodilution results in an acute reduction in haemoglobin levels, which is considered to be potentially harmful to patients with cardiac disease, the time-point of randomization could be associated with estimates of treatment effects. We, therefore, also conducted a pre-specified sensitivity analyses to compare estimates from trials that assessed transfusion triggers during and after surgery compared with trials where transfusion triggers were only applied post-operatively.
All analyses were conducted using Review Manager software (Version 5.3, The Cochrane Collaboration, Oxford, UK) and Stata (Release 14, College Station, TX, USA).
Results
Search results
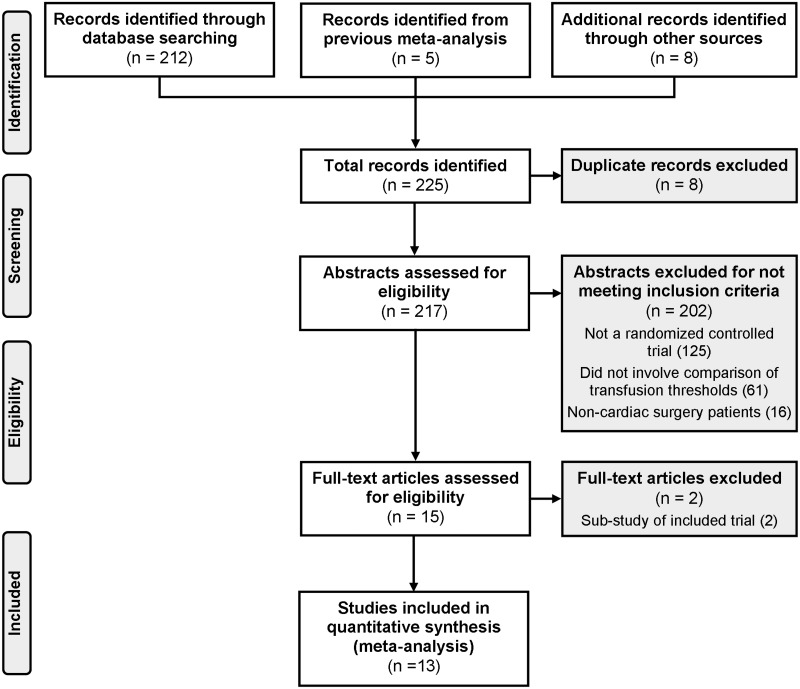
We identified an additional 220 citations in the literature since our prior systematic review (Figure 1). Six additional studies and two conference proceedings fulfilled inclusion criteria11,12,21–31 for a total of 13 studies that were included—eight focused on adults and five included paediatric patients.
Figure 1.
Study selection.
Study characteristics
Supplementary material online, Table S1 describes the study characteristics. The sample sizes ranged from 50–5092 adults to 43–162 neonates and children. Two studies in adults were above 1000 patients.11,12 The entire perioperative period was considered for haemoglobin thresholds in five trials focusing on adult cardiac patients,12,21,24,26,31 whereas the remainder compared post-operative haemoglobin thresholds. Only one paediatric trial compared perioperative haemoglobin thresholds.23 Eleven trials had haemoglobin thresholds that differed by approximately 20 g/L, whereas two adult RCTs had a 10 g/L difference between restrictive and liberal thresholds.21,28 Five adult trials included patients undergoing elective cardiac surgery only.11,21,26,28,31
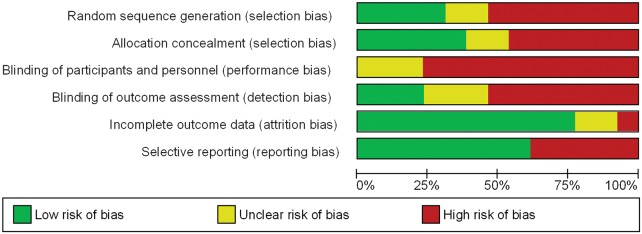
Risk of bias
Figures 2and3 describe the risk of bias according to each study and a summary of the risk of bias, respectively. Supplementary material online, Table S2 provides the rationale for the risk of bias assessment. Twenty-three percent of trials used blinded outcome assessment, 31% random sequence generation, and 38% allocation concealment. None of the RCTs were blinded to both clinical personnel and participants.
Figure 2.
Risk of bias summary.
Figure 3.
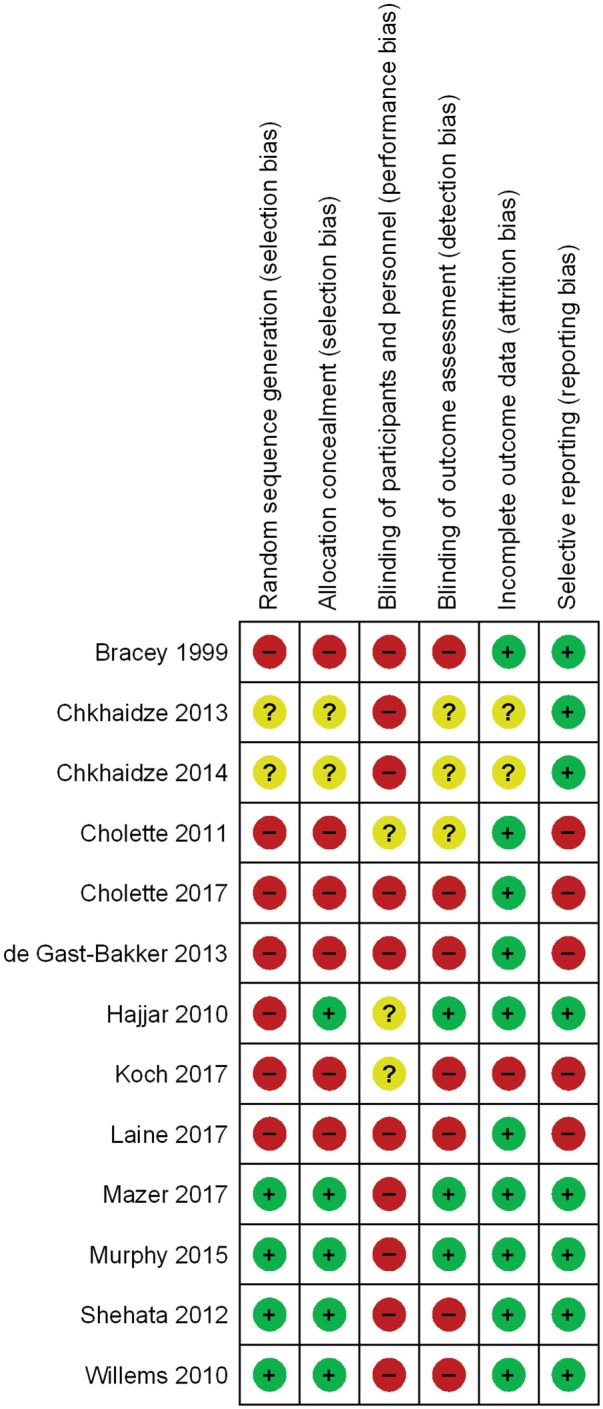
Risk of bias for each study.
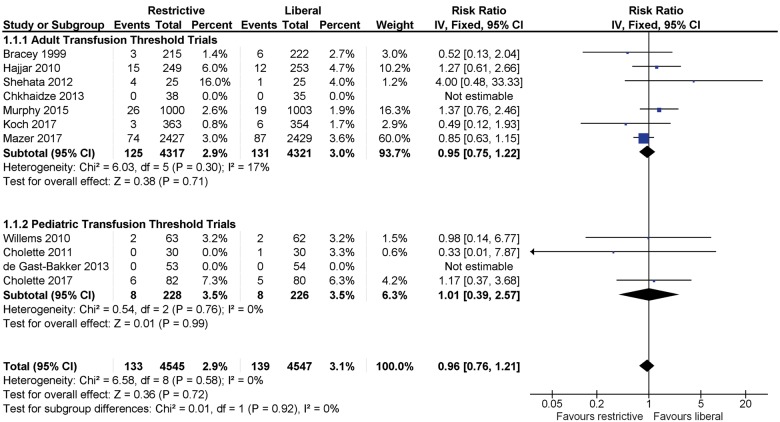
Mortality
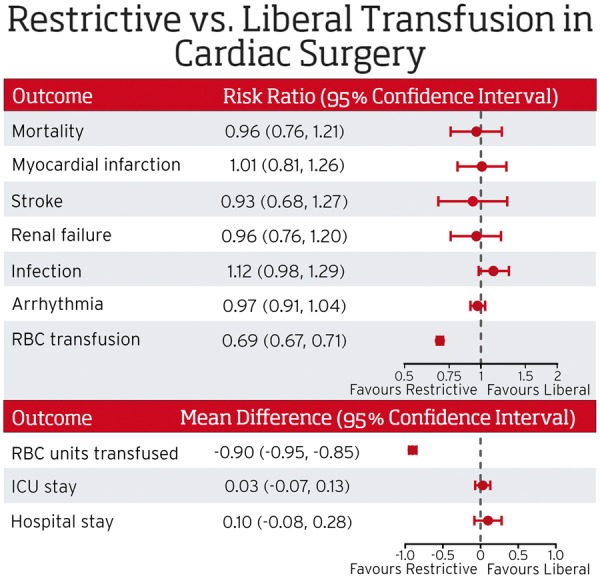
Data for mortality were provided by 11 trials, but two studies had zero events in both arms and could not be included in the meta-analysis (Figure 4). The funnel plot did not show evidence of asymmetry (Supplementary material online, Figure S1). The pooled estimate included 4545 patients receiving restrictive transfusion and 4547 transfused according to a liberal threshold. No difference in mortality within 30 days of surgery was observed between groups (RR 0.96, 95% CI 0.76–1.21; I2 = 0), with 133 (2.9%) deaths in the restrictive arm, and 139 (3%) deaths in the liberal arm. One RCT contributed to 60% of the weight in the analysis.12
Figure 4.
Mortality within 30 days of surgery in randomized controlled trials of adult and paediatric cardiac surgery patients. Fixed-effects meta-analysis.
Cardiovascular, renal, and infection-related events
Transfusion strategy did not have a statistically significant effect on the risk of myocardial infarction (RR 1.01, 95% CI 0.81–1.26, I2 = 0), stroke (RR 0.93, 95% CI 0.68–1.27, I2 = 0), renal failure (RR 0.96, 95% CI 0.76–1.20, I2 = 0), infection (RR 1.12, 95% CI 0.98–1.29, I2 = 0), or arrhythmia (RR 0.97, 95% CI 0.91–1.04, I2 = 31%) (Supplementary material online, Figures S2–S6).
Resource utilization
The mean number of units transfused was lower in the restrictive transfusion approach compared with the liberal counterpart (WMD −0.90 units, 95% CI −0.95 to −0.85, I2 = 93%). Similarly, the probability of receiving a transfusion was approximately 30% lower with the restrictive compared with the liberal approach (RR 0.69, 95% CI 0.67–0.71, I2 = 81%) (Supplementary material online, Figures S7, S8).
Blood loss did not differ between groups (WMD 5.61 mL, 95% CI −18.18 to 29.40, I2 = 0) neither did the duration of hospitalization (WMD 0.10 day, 95% CI −0.08 to 0.28, I2 = 72%) or length of stay in an ICU (WMD 0.03 days, 95% CI −0.07 to 0.13, I2 = 27%) (Supplementary material online, Figures S9–S11). Results from the random-effects provided similar results (Supplementary material online, Table S2).
Subgroup analysis: adult vs. paediatric patients
Subgroup analyses of adult compared with paediatric cardiac patients for all-cause mortality, infections, blood loss, the proportion of patients transfused RBCs, the mean number of RBCs, and duration of hospitalization and length of stay in an ICU are presented in Figure 4 and Supplementary material online, Figures S5, S7–S11. Risk ratios demonstrated a lack of difference between adult and paediatric patients except for the proportion transfused.
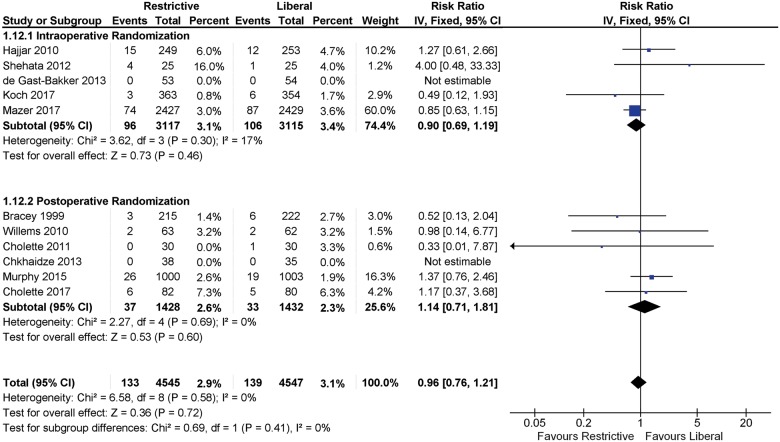
Sensitivity analysis according to whether patients were assigned to a transfusion strategy intra-operatively and post-operatively or only post-operatively did not show any statistically significant differences in mortality (P = 0.41) (Figure 5).
Figure 5.
Sensitivity analysis for mortality according to intra-operative or post-operative randomization. Fixed-effects meta-analysis.
Trial sequential analysis
Figures 6and7 present trial sequential analyses for fixed-effect meta-analyses of mortality in all patients and adult patients only, respectively. Having reached approximately 70% of the critical information size of 12 904 patients, meta-analyses had crossed the futility boundary for inferiority of the restrictive strategy, but had narrowly missed the boundary required to be crossed to conclusively establish non-inferiority of the restrictive strategy. Supplementary material online, Figures S12 and S13 present trial sequential analyses for random-effects meta-analyses of all patients and adult patients. Having reached approximately 50% of the critical information size of 18 434 patients, meta-analyses again both crossed the futility boundary for inferiority of the restrictive strategy, but now clearly missed the boundary required to be crossed to conclusively establish non-inferiority of the restrictive strategy.
Figure 6.
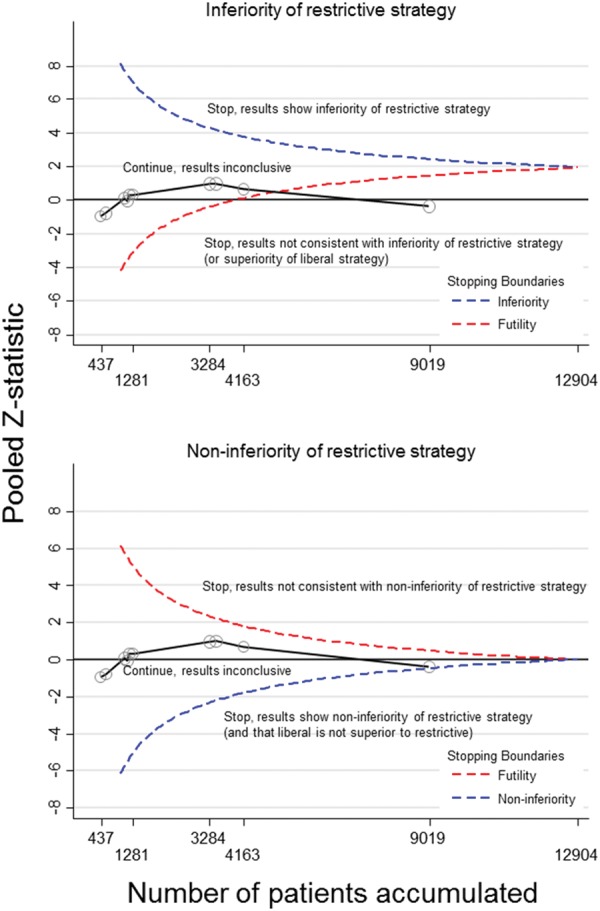
Trial sequential analysis for mortality within 30 days of surgery for adult and paediatric patients undergoing cardiac surgery using a fixed-effects model. Trials are added in chronological order and the most recent studies were the largest studies published. The information size (9019 patients) was adequate to demonstrate that the restrictive strategy was not inferior to the liberal strategy (and that the liberal strategy was not superior to restrictive) as the futility boundary was crossed (upper panel).
Figure 7.
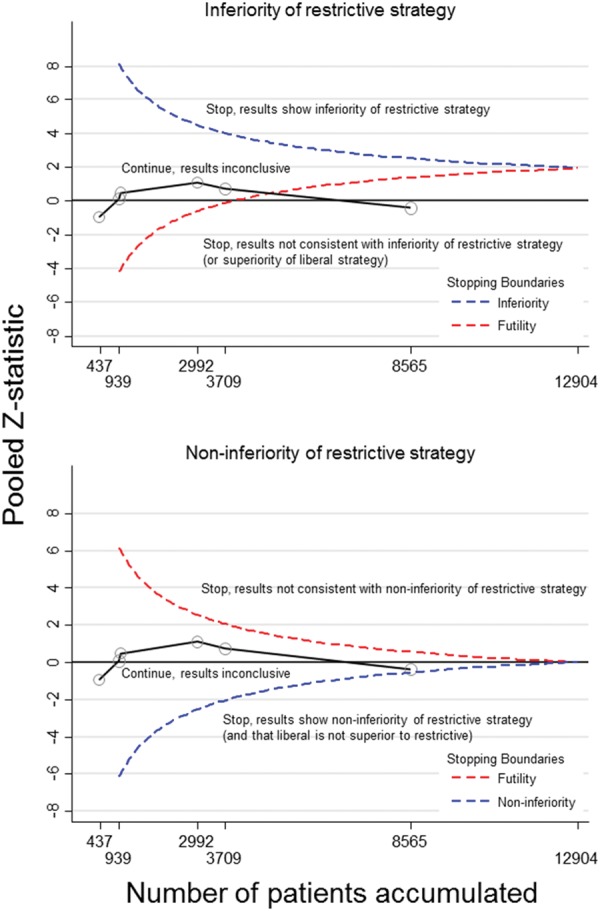
Trial sequential analysis for mortality within 30 days of surgery for adult patients undergoing cardiac surgery using a fixed effects model. Trials are added in chronological order and the most recent studies were the largest studies published. The information size (8565 patients) was adequate to demonstrate that the restrictive strategy was not inferior to the liberal strategy (and that the liberal strategy was not superior to restrictive) as the futility boundary was crossed (upper panel).
Take home figure.

The current evidence suggests restrictive transfusion strategies are not inferior to liberal transfusion strategies in adult and pediatric patients undergoing cardiac surgery. RBC indicates red blood cell; ICU indicates intensive care unit. ICU stay and hospital stay are reported as days.
Discussion
This meta-analysis and trial sequential analysis of RCTs comparing restrictive and liberal RBC transfusion strategies in patients undergoing cardiac surgery demonstrated that restrictive transfusion strategies did not result in a statistically significant increased risk of mortality within 30 days of surgery, myocardial infarction, renal failure, stroke, infections, or arrhythmias, but restrictive strategies reduced the exposure of patients to RBCs. Based on the currently available evidence, we detected statistical heterogeneity for the proportion of patients transfused, mean number of units transfused, ICU length of stay, and hospital length of stay. The statistical heterogeneity in the proportion transfused and the mean number of units transfused is not unexpected but does not detract from the anticipated finding that restrictive strategies reduce the percentage of patients transfused.
Comparison to previous reviews
A recent systematic review and meta-analysis of observational cohort studies suggested that RBC transfusion compared with no transfusion was associated with higher mortality (OR 2.72, 95% CI 2.11–3.49; I2 = 93%). The pooled estimate of RCTs appeared to favour liberal transfusion strategies, but not significantly so (OR 0.70, 95% CI 0.49–1.02; I2 = 0).32 There were no statistically significant differences in pulmonary injury, renal injury, or infections. A previous sequential analysis of restrictive compared with liberal transfusion strategies did not find a difference in mortality (RR 0.86, 95% CI 0.74–1.01) but suggested that the required information size was not reached.33 Our pooled results included the addition of two RCTs in adult cardiac surgery patients,11,12 which lead to a robust pooled estimate that did not favour either a restrictive or a liberal RBC transfusion. The trial sequential analysis demonstrated that the required information size was reached for combined and adult and paediatric trials and was nearly attained in adult cardiac surgery trials using non-inferiority margin of 3%.
Limitations of this systematic review and meta-analysis are acknowledged. We analysed mortality within 30 days of surgery and not longer term mortality. The absence of definitive long-term data does not exclude a potential later difference between groups and is thus a limitation of this analysis. We did not conduct a sensitivity analysis according to the risk of bias. We combined adult and paediatric RCTs for some analyses. We did not conduct a subgroup analysis comparing high risk and low risk cardiac surgery patients. Nonetheless the estimable statistical heterogeneity was not considered high for all-cause mortality, our primary outcome despite including these studies, and a statistically significant difference between the adult and paediatric patients was not detected. In addition, we used in-hospital mortality which limited missing data, and mortality is a well and easily defined outcome.
A restrictive RBC transfusion strategy was not found to be inferior to a liberal strategy for all-cause mortality. Although the required information size (i.e. sample size) in the trial sequential analysis was not reached, the z-curve crossed the futility boundary for inferiority of the restrictive strategy, which indicates that our results are definitely not consistent with a 30% relative risk increase of the restrictive strategy when compared with the liberal strategy. However, our analysis is not yet conclusive regarding non-inferiority of the restrictive strategy, i.e. the boundary to conclusively establish that the restrictive strategy is non-inferior was narrowly missed, even though the 95% CI of the pooled risk ratio excluded the pre-specified non-inferiority margin of 1.30 for overall mortality. Restrictive transfusion strategies were also not associated with an increased risk of myocardial infarction, stroke, infection, or renal failure. Restrictive transfusion strategies have the potential to reduce the proportion of patients transfused and the mean number of units transfused reducing the cost associated with blood transfusion. For example, assuming that 78% of patients in the liberal approach receive at least one transfusion (the weighted mean probability across trials), our results imply that, on average, replacement of the liberal transfusion by the restrictive approach would result in a reduction of approximately 242 transfused patients per 1000 surgeries (95% CI 226–259). Even under more conservative assumptions (i.e. random-effects models), we found the restrictive transfusions strategy did not show any differences in clinical outcomes compared with the liberal approach, and our results appear to be consistent across different scenarios of sensitivity analyses considered.
Conclusion
In conclusion, the current evidence does not support the notion that restrictive RBC transfusion strategies are inferior to liberal RBC transfusion strategies in adult and paediatric patients undergoing cardiac surgery. Reduction in allogeneic RBC exposure with a restrictive transfusion strategy has clear resource and economic benefits, and further economic analyses may also be useful to guide clinical decision-making.
Supplementary Material
Acknowledgements
The authors thank Drs. Colleen Koch and Ludhmila Hajjar for providing the individual components for some of the composite outcomes reported in their trials.
Funding
This work was supported by the Canadian Institutes of Health Research [232416 and 301852] and Canadian Blood Services/Health Canada.
Conflict of interest: N.S., N.M., R.W., G.M.T. H.G., P.J., C.D.M. are co-authors of the TRICS III trial. N.S. is a consultant for Canadian Blood Services. P.J. is a Tier 1 Canada Research Chair in clinical epidemiology of chronic diseases; this research was completed, in part, with funding from the Canada research chairs programme. P.J. has received research grants to the institution from Astra Zeneca, Biotronik, Biosensors International, Eli Lilly and The Medicines Company and serves as unpaid member of the steering group of trials funded by Astra Zeneca, Biotronik, Biosensors, St. Jude Medical and The Medicines Company.
References
- 1. Hung M, Ortmann E, Besser M, Martin-Cabrera P, Richards T, Ghosh M, Bottrill F, Collier T, Klein AA.. A prospective observational cohort study to identify the causes of anaemia and association with outcome in cardiac surgical patients. Heart 2015;101:107–112. [DOI] [PubMed] [Google Scholar]
- 2. Karkouti K, Wijeysundera DN, Beattie WS.. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation 2008;117:478–484. [DOI] [PubMed] [Google Scholar]
- 3. Hare GM, Freedman J, Mazer CD.. Review article: risks of anemia and related management strategies: can perioperative blood management improve patient safety? Can J Anaesth 2013;60:168–175. [DOI] [PubMed] [Google Scholar]
- 4. Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, Blackstone EH.. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med 2006;34:1608–1616. [DOI] [PubMed] [Google Scholar]
- 5. Koch CG, Li L, Duncan AI, Mihaljevic T, Loop FD, Starr NJ, Blackstone EH.. Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann Thorac Surg 2006;81:1650–1657. [DOI] [PubMed] [Google Scholar]
- 6. Silliman CC, Ambruso DR, Boshkov LK.. Transfusion-related acute lung injury. Blood 2005;105:2266–2273. [DOI] [PubMed] [Google Scholar]
- 7. Vlaar AP, Hofstra JJ, Determann RM, Veelo DP, Paulus F, Levi M, Zeerleder S, Vroom MB, Schultz MJ, Juffermans NP.. Transfusion-related acute lung injury in cardiac surgery patients is characterized by pulmonary inflammation and coagulopathy: a prospective nested case-control study. Crit Care Med 2012;40:2813–2820. [DOI] [PubMed] [Google Scholar]
- 8. Vlaar AP, Hofstra JJ, Determann RM, Veelo DP, Paulus F, Kulik W, Korevaar J, de Mol BA, Koopman MM, Porcelijn L, Binnekade JM, Vroom MB, Schultz MJ, Juffermans NP.. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: a prospective nested case-control study. Blood 2011;117:4218–4225. [DOI] [PubMed] [Google Scholar]
- 9. Vamvakas EC, Blajchman MA.. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood 2009;113:3406–3417. [DOI] [PubMed] [Google Scholar]
- 10. Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E.. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–417. [DOI] [PubMed] [Google Scholar]
- 11. Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, Reeves BC.. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015;372:997–1008. [DOI] [PubMed] [Google Scholar]
- 12. Mazer CD, Whitlock RP, Fergusson DA, Hall J, Belley-Cote E, Connolly K, Khanykin B, Gregory AJ, de Médicis É, McGuinness S, Royse A, Carrier FM, Young PJ, Villar JC, Grocott HP, Seeberger MD, Fremes S, Lellouche F, Syed S, Byrne K, Bagshaw SM, Hwang NC, Mehta C, Painter TW, Royse C, Verma S, Hare GMT, Cohen A, Thorpe KE, Jüni P, Shehata N.. Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med 2017;377:2133–2144. [DOI] [PubMed] [Google Scholar]
- 13. Curley GF, Shehata N, Mazer CD, Hare GM, Friedrich JO.. Transfusion triggers for guiding RBC transfusion for cardiovascular surgery: a systematic review and meta-analysis. Crit Care Med 2014;42:2611–2624. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269, w64. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA.. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwon D, Reis IM.. Simulation-based estimation of mean and standard deviation for meta-analysis via Approximate Bayesian Computation (ABC). BMC Med Res Methodol 2015;15:61.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 18. Sedgwick P. Meta-analyses: how to read a funnel plot. BMJ 2013;346:f1342. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wetterslev J, Thorlund K, Brok J, Gluud C.. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64–75. [DOI] [PubMed] [Google Scholar]
- 21. Koch CG, Sessler DI, Mascha EJ, Sabik JF, Li L, Duncan AI, Zimmerman NM, Blackstone EH.. A randomized clinical trial of red blood cell transfusion triggers in cardiac surgery. Ann Thorac Surg 2017;104:1243–1250. [DOI] [PubMed] [Google Scholar]
- 22. Cholette JM, Swartz MF, Rubenstein J, Henrichs KF, Wang H, Powers KS, Daugherty LE, Alfieris GM, Gensini F, Blumberg N.. Outcomes using a conservative versus liberal red blood cell transfusion strategy in infants requiring cardiac operation. Ann Thorac Surg 2017;103:206–214. [DOI] [PubMed] [Google Scholar]
- 23. de Gast-Bakker DH, de Wilde RB, Hazekamp MG, Sojak V, Zwaginga JJ, Wolterbeek R, de Jonge E, Gesink-van der Veer BJ.. Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients: a randomized controlled trial. Intensive Care Med 2013;39:2011–2019. [DOI] [PubMed] [Google Scholar]
- 24. Shehata N, Burns LA, Nathan H, Hebert P, Hare GM, Fergusson D, Mazer CD.. A randomized controlled pilot study of adherence to transfusion strategies in cardiac surgery. Transfusion 2012;52:91–99. [DOI] [PubMed] [Google Scholar]
- 25. Cholette JM, Rubenstein JS, Alfieris GM, Powers KS, Eaton M, Lerner NB.. Children with single-ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red-cell transfusion strategy. Pediatr Crit Care Med 2011;12:39–45. [DOI] [PubMed] [Google Scholar]
- 26. Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil Filho R, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leao WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NA, Auler JO Jr.. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–1567. [DOI] [PubMed] [Google Scholar]
- 27. Willems A, Harrington K, Lacroix J, Biarent D, Joffe AR, Wensley D, Ducruet T, Hebert PC, Tucci M.. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Crit Care Med 2010;38:649–656. [DOI] [PubMed] [Google Scholar]
- 28. Bracey AW, Radovancevic R, Riggs SA, Houston S, Cozart H, Vaughn WK, Radovancevic B, McAllister HA Jr, Cooley DA.. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion 1999;39:1070–1077. [DOI] [PubMed] [Google Scholar]
- 29. Chkhaidze M, Metreveli I, Tsintsadze A.. Comparison of two RBC transfusion strategies in pediatric cardiac surgery patients: 6AP2-3. Eur J Anaesthesiol 2014;31:92. [Google Scholar]
- 30. Chkhaidze M, Metreveli I, Tsintsadze A.. Optimal strategy of RBC transfusion for patients after cardiac surgery. Int Care Med 2013;39:S388. [Google Scholar]
- 31. Laine A, Niemi T, Schramko A.. Transfusion threshold of hemoglobin 80 g/L is comparable to 100 g/L in terms of bleeding in cardiac surgery: a prospective randomized study. J Cardiothorac Vasc Anesth 2018;32:131–139. [DOI] [PubMed] [Google Scholar]
- 32. Patel NN, Avlonitis VS, Jones HE, Reeves BC, Sterne JA, Murphy GJ.. Indications for red blood cell transfusion in cardiac surgery: a systematic review and meta-analysis. Lancet Haematol 2015;2:e543–e553. [DOI] [PubMed] [Google Scholar]
- 33. Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J.. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015;350:h1354.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.