Abstract
Key points
The present study aimed to determine the impact of ageing on endogenous adropin levels in human skeletal muscle feed arteries (SMFAs) and the role of adropin in age‐related vascular dysfunction.
Adropin protein expression falls progressively with advancing age in the human peripheral vasculature. Endothelial‐dependent vasodilatation, typically attenuated with age, was strongly correlated with SMFA adropin protein levels.
Adropin incubation restored age‐related endothelial‐dependent vasodilatory dysfunction and increased the phosphorylated endothelial nitric oxide synthase (eNOS)/eNOS ratio in an age‐dependent manner in the SMFAs. The role of nitric oxide bioavailability was additionally indicated by NOS blockade ablating both the positive vascular effects of adropin incubation and the relationship between endothelial function and adropin protein expression.
Additional evidence of a mechanistic link between declining adropin and age‐related endothelial dysfunction was documented by a progressively increasing magnitude of effect of adropin‐induced eNOS‐mediated vasodilatation with ageing.
Adropin appears to be a novel therapeutic target for facilitating the restoration of endothelial function with ageing.
Abstract
The present study aimed to determine the impact of advancing age on endogenous adropin levels in human skeletal muscle feed arteries (SMFAs) and the role of adropin in age‐related vascular dysfunction. Adropin protein expression and vasodilatory capacity was assesed in SMFAs from Young (27 ± 2 years, n = 10), Middle Aged (54 ± 2 years, n = 10) and Old (75 ± 2 years, n = 16) subjects. Endothelial‐dependent vasodilatation, with and without adropin incubation, was assessed in response to flow‐induced shear stress and ACh. Both SMFA adropin protein expression and endothelial‐dependent vasodilatory function exhibited a progressive, age‐related, reduction (Flow: Y: 65 ± 3%; Middle Aged: 36 ± 3%; Old: 15 ± 2%; ACh: Young: 63 ± 2%, Middle Aged: 34 ± 3%; Old: 23 ± 3%, P < 0.05). There was a strong positive correlation between SMFA adropin protein expression and both flow (r = 0.81, P < 0.05) and ACh (r = 0.78, P < 0.05). Adropin incubation in the Middle Aged and Old SMFAs restored the vasodilatory response to flow (Middle Aged + Adropin: 59 ± 3%; Old + Adropin: 47 ± 3%, P < 0.05) and ACh (Middle Aged + Adropin: 59 ± 3%; Old + Adropin: 49 ± 2%, P < 0.05). A mechanistic link between adropin and nitric oxide (NO) biovavailabilty was supported by (i) increased phosphorylated endothelial NO synthase (eNOS)/eNOS protein expression with adropin incubation only in the Middle Aged and Old SMFAs; (ii) eNOS blockade ablating both the positive vascular effects of adropin incubation and the relationship between endothelial function and adropin protein expression and (iii) a progressive increase in the magnitude of effect of adropin‐induced eNOS‐mediated vasodilatation with advancing age. Adropin could be a novel therapeutic target for facilitating the restoration of endothelial function via increased NO bioavailability, with advancing age.
Keywords: human skeletal muscle feed artery, shear stress, endothelium‐dependent vasodilation, NO bioavailability, ageing
Key points
The present study aimed to determine the impact of ageing on endogenous adropin levels in human skeletal muscle feed arteries (SMFAs) and the role of adropin in age‐related vascular dysfunction.
Adropin protein expression falls progressively with advancing age in the human peripheral vasculature. Endothelial‐dependent vasodilatation, typically attenuated with age, was strongly correlated with SMFA adropin protein levels.
Adropin incubation restored age‐related endothelial‐dependent vasodilatory dysfunction and increased the phosphorylated endothelial nitric oxide synthase (eNOS)/eNOS ratio in an age‐dependent manner in the SMFAs. The role of nitric oxide bioavailability was additionally indicated by NOS blockade ablating both the positive vascular effects of adropin incubation and the relationship between endothelial function and adropin protein expression.
Additional evidence of a mechanistic link between declining adropin and age‐related endothelial dysfunction was documented by a progressively increasing magnitude of effect of adropin‐induced eNOS‐mediated vasodilatation with ageing.
Adropin appears to be a novel therapeutic target for facilitating the restoration of endothelial function with ageing.
Introduction
Worldwide, cardiovascular disease (CVD) is a primary cause of both mortality and morbidity (Yazdanyar & Newman, 2009; North & Sinclair, 2012). Advancing age is considered to be the major risk factor for the progression of CVD (Niccoli & Partridge, 2012), which is accelerated by a progressive decline in physical capacity across the lifespan (Strait & Lakatta, 2012). Vascular ageing has been implicated as a contributor to the decline in physical capacity by decreasing blood flow and therefore oxygen delivery to skeletal muscle (Wahren et al. 1974; Muller‐Delp, 2016; Park et al. 2016). Several potential factors have been suggested to be responsible for this age‐associated reduction in oxygen delivery to skeletal muscle, which include both central (e.g. reduced cardiac output, diminished pulmonary function) and peripheral mechanisms [e.g. impaired endothelium function, reduced capacity to release ATP from erythrocytes, lower nitric oxide (NO) bioavailability, etc.] and, in combination, they result in attenuated vascular conductance and blood flow capacity (Calbet & Lundby, 2012; Hellsten, 2016). However, given the extraordinary complexity of the multitude of mechanisms underlying blood flow regulation, the age‐related alterations in these processes are still under investigation and novel therapeutic targets probably still remain to be discovered.
Adropin, a recently identified protein, initially associated with the maintenance of energy homeostasis, was first identified by Kumar et al. (2008). Although adropin does play a critical role in maintaining energy balance via glucose and fat homeostasis, recent indirect evidence suggests that adropin may also have an important impact on cardiovascular dysfunction and disease (Lovren et al. 2010). For example, the elderly, as well as patients with myocardial infarction, cardiac syndrome X and heart failure, exhibit attenuated serum adropin levels (Celik et al. 2013; Wu et al. 2014; Yu et al. 2014) (Butler et al. 2012). Additionally, Lovren et al. (2010) reported that endothelial cells incubated with adropin exhibit significantly greater proliferation, migration and capillary‐like tube formation, implicating a role for adropin in angiogenesis. Furthermore, the expression of endothelial nitric oxide synthase (eNOS), the main enzyme responsible for maintaining NO bioavailability, was increased with adropin incubation (Lovren et al. 2010). Specifically, adropin upregulates vascular endothelial growth factor receptor 2 (VEGFR2) which stimulates both threonine kinase (Akt) (Ser473) and extracellular signal‐regulated kinases 1/2 (ERK1/2), ultimately resulting in endothelial NO synthase (Ser1177) phosphorylation (Lovren et al. 2010). Accordingly, it appears that adropin is expressed in vascular endothelial cells and regulates NO bioavailability in vascular tissue by the upregulation of eNOS. Given the recognized link between attenuated NO bioavailability in the vasculature and the development of endothelial dysfunction with age (Trott et al. 2009; Sindler et al. 2013; Park et al. 2016), the concomitant decline in adropin may play a significant role in the development of endothelium‐dependent vasodilatory dysfunction in the elderly. However, to date, the role of adropin and mechanism of action, with respect to changes in endothelial function across the human lifespan, still remains entirely unknown.
Therefore, utilizing isolated human skeletal muscle feed arteries (SMFAs) and the pressure myography technique, the present study aimed to determine the impact of advancing age on endogenous adropin levels in the vasculature and the role of adropin in age‐related vascular dysfunction. Specifically, we tested three hypotheses: (i) both SMFA adropin protein expression and endothelial‐dependent vasodilatory function would exhibit a progressive, age‐related, reduction; (ii) adropin incubation would restore age‐related vasodilatory dysfunction in human SMFAs and (iii) this restoration of age‐related vasodilatory dysfunction by adropin in human SMFAs is mediated by improved NO bioavailability.
Methods
Subjects and general procedures
In total, 36 SMFAs from the axillary and inguinal regions were obtained from Young (27 ± 2 years, n = 10), Middle Aged (54 ± 2 years, n = 10) and Old (75 ± 2 years, n = 16) subjects during melanoma‐related surgeries. All subjects were free from metastatic cancer and not undergoing chemotherapy, although there were no other specific exclusion criteria for the study; however, all medical conditions and medications were noted. All procedures were approved by the Institutional Review Board (IRB) of the University of Utah and Salt Lake City VA Medical Center (IRB # 32786), carried out in accordance with the Declaration of Helsinki, and written informed consent was obtained from all subjects prior to surgery.
Vessel harvest and preparation
Briefly, as described previously (Ives et al. 2012; Park et al. 2016; Park et al. 2018), SMFAs were obtained during a sentinel node biopsy for melanoma surgery at the Huntsman Cancer Hospital. Specifically, SMFAs were harvested during the dissection to locate sentinel lymph nodes. SMFAs were ligated, excised, immediately placed on iced normal physiological saline solution (PSS) (in mm: 145.0 NaCl, 4.7 KCL, 2.0 CaCl2, 1.17 MgSO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 3.0 Mops buffer and 10 g L–1 BSA at pH 7.4) and transferred to the laboratory within 15 min of harvesting.
Immunoblotting
The proteins of interest included adropin, and biomarkers of NO bioavailability which were measured, before and after a 30 min of adropin incubation of the SMFAs of Young, Middle Aged and Old subjects, by western blot analysis. Briefly, SMFAs were homogenized in a lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) supplemented with a protease inhibitor cocktail, 10 mm sodium fluoride and 1 mm phenylmethylsulphonyl fluoride (all listed chemicals were obtained from Santa Cruz Biotechnology). Protein concentration was determined using the Bradford technique. Next, 50 μg of homogenate was separated by polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and incubated with primary and secondary antibodies directed against the proteins of interest. Membranes were imaged on a ChemiDoc XRS (Bio‐Rad, Hercules, CA, USA) and quantified with Image Lab software (Bio‐Rad) (Park et al. 2018). The specific antibodies used to detect SMFA proteins included: anti‐adropin antibody (dilution 1:500; ab213634; Abcam, Cambridge, MA, USA), phospho‐eNOS at Ser1177 (dilution 1:1000; #9570; Cell Signaling, Boston, MA, USA) and total eNOS (dilution 1:1000; #610296; BD Transduction, San Jose, CA, USA). Protein abundance was normalized to beta‐actin (dilution 1:1000; ab8227; Abcam), which served as a loading control. Note that the vessel samples used for these analyses had not been exposed to any other assessments.
Titrating the adropin concentration for incubation
To determine the optimal adropin concentration to improve vasodilatation, an adropin‐titration, using 12 Old SMFAs (63 ± 8 years) was performed. Specifically, based on the results from Lovren et al. (2010), the initial 30 min incubations were performed at concentrations of 10 and 25 ng mL–1, with increments of 10 ng mL–1 beyond the concentration of 25 ng mL–1, up to 75 ng mL–1. Following each 30 min incubation, the SMFAs were studied to determine which adropin incubation concentration elicited the peak vasodilatation in response to flow (45 μL min–1) and ACh (10−3 m).
Vasodilatation assessments and adropin incubation
Briefly, as described previously (Ives et al. 2012; Park et al. 2016; Park et al. 2018), cleaned SMFAs were cannulated at both ends with micropipette tips and placed in the pressure myography organ baths (110p; DMT Systems, Aarhus, Denmark) containing PSS. Maximal outer diameter was measured by incubating the SMFAs in Ca2+‐free PSS for 30 min (unpressurized). With two pieces of the same vessel, in separate baths, both were left for 30 min, either with or without the addition of adropin (55 ng mL–1). The vessel outer diameters were then recorded under an inverted microscope with a video camera (TS100; Nikon Eclipse, Melville, NY, USA). Percentage possible vasodilatation was assessed in response to three stimuli. First, the endothelium‐mediated vasodilatory response to flow‐induced shear stress was measured following pre‐constriction with phenylephrine (PE) (∼7–10 μm) (Ives et al. 2012; Park et al. 2016; Park et al. 2018). This was achieved by altering the heights of the independent fluid reservoirs, contiguous with the SMFAs, in equal and opposite directions, so that a pressure difference was developed across the vessel without altering mean intraluminal pressure. Three pressure differences of 15, 30 and 40 mmHg, which yielded an approximate flow rate of 15, 30 and 45 μL min–1, assessed with a flow meter (162FM; DMT Systems), were utilized for the flow experiments. Second, to assess endothelium‐dependent vasodilatation pharmacologically, an ACh dose–response curve (10−7 to 10−3 m) was performed following pre‐constriction with PE (∼7–10 μm, to achieve ∼60–70% vasoconstriction). Third, to assess endothelium independent vasodilatation, a sodium nitroprusside (SNP) dose–response curve was performed (10−9 to 10−4 m) following pre‐constriction with PE (∼7–10 μm). Additionally, to determine the contribution of eNOS and NO bioavailability to endothelium‐mediated vasodilatation with adropin incubation, flow and ACh dose–response curves were performed in SMFAs from Young, Middle Aged and Old subjects in the presence and absence of N‐monomethyl‐l‐arginine (l‐NMMA) (10−3 m, 30 min incubation), as described previously (Park et al. 2018).
Calculations
The vasodilator response was assessed as percent possible vasodilatation calculated using the following equation (Hotta et al. 2017) (Lesniewski et al. 2009):
where D Dose is the vessel diameter at a given drug dose/flow rate, D P is the pre‐constricted diameter with PE (immediately prior to dose‐response curves) and D M is the maximal diameter assessed in Ca2+ free PSS. Note that each of these diameters was assessed with the vessel pressurized at 60 mmHg, which, when immersed in a shallow, unpressurized bath, yields a transmural pressure of 60 mmHg. The magnitude of effect of adropin‐induced NOS‐mediated vasodilatation was calculated as the difference in the effect of l‐NMMA on flow‐ and ACh‐mediated percentage possible vasodilatation with and without adropin incubation.
Statistical analysis
Statistical analyses were performed using Prism, version 7 (GraphPad Software Inc., San Diego, CA, USA). Two‐way repeated measures ANOVA was used to assess relative changes in vessel diameter with and without adropin in response to flow, ACh and SNP. Two‐way repeated measures ANOVA were used to assess changes in vessel diameter with and without adropin and with and without l‐NMMA in response to flow and ACh. When necessary, a Tukey's post hoc test was used to identify significant differences. For all other comparisons, one‐way ANOVAs were used and, if necessary, a Tukey's post hoc test was employed to identify significant differences. Correlations between variables were assessed with Pearson product‐moment correlations. For all analyses, P < 0.05 was considered statistically significant. All data are reported as the mean ± SEM.
Results
Subject characteristics
The subject characteristics, obtained from preoperative examination medical records, are presented in Table 1. Note that subjects were predominantly not taking cardiovascular (β‐blocker, angiotensin‐converting enzyme inhibitor, diuretic, Ca2+ channel blocker, etc.) and diabetic (insulin, metformin, etc.) medications, whereas subjects taking cancer‐related medications were excluded from the study. It should also be recognized that all the blood chemistry and complete blood count results (Table 1) were within the normal range, suggesting that the subjects who participated in the present study were relatively healthy.
Table 1.
Subject characteristics
| Young (n = 10) | Middle Aged (n = 10) | Old (n = 16) | |
|---|---|---|---|
| Age (years) | 27 ± 2 | 54 ± 2* | 75 ± 2* , # |
| Male (male/total n) | 6/10 | 5/10 | 10/16 |
| Female (female/total n) | 4/10 | 5/10 | 6/16 |
| Height (cm) | 180 ± 9 | 179 ± 9 | 175 ± 13 |
| Body mass (kg) | 70.5 ± 12 | 73 ± 11 | 77.1 ± 13 |
| Body mass index (kg m−2) | 20.6 ± 6 | 21 ± 5 | 24.5 ± 5 |
| Systolic blood pressure (mmHg) | 120 ± 6 | 122 ± 4 | 129 ± 9 |
| Diastolic blood pressure (mmHg) | 79 ± 5 | 76 ± 5 | 80 ± 8 |
| Glucose (mg dL−1) | 109 ± 5.1 | 109.9 ± 5.7 | 108 ± 7.6 |
| Blood urea nitrogen (mg dL−1) | 18 ± 3.0 | 19.3 ± 3.0 | 20.5 ± 4.0 |
| Creatinine (mg dL−1) | 1 ± 0.3 | 0.9 ± 0.4 | 1.2 ± 0.5 |
| Albumin (g dL−1) | 4.0 ± 0.3 | 4.0 ± 0.3 | 4.1 ± 0.5 |
| Lactate dehydrogenase (U L−1) | 497.4 ± 42.8 | 502.6 ± 30.1 | 505 ± 40.1 |
| Hemoglobin (g dL−1) | 15.6 ± 0.5 | 15.1 ± 0.9 | 14.1 ± 0.5 |
| White blood cells (thousands per microlitre, K μL−1) | 4.0 ± 1.0 | 4.9 ± 2.0 | 7.3 ± 0.9 |
| Red blood Cells (millions per microlitre, M μL−1) | 5.2 ± 0.9 | 4.7 ± 0.6 | 4.6 ± 0.9 |
| Lymphocytes (%) | 36.3 ± 5.0 | 31.5 ± 3.5 | 28 ± 7.4 |
| Monocytes (%) | 8.5 ± 1.0 | 8.5 ± 1.8 | 8 ± 3.3 |
| Medications (users/n) | |||
| Diuretics | 0/10 | 0/10 | 1/16 |
| Angiotensin‐ converting enzyme inhibitors | 0/10 | 0/10 | 1/16 |
| Diabetic drugs | 0/10 | 0/10 | 1/16 |
| Statins | 0/10 | 0/10 | 0/16 |
Data are expressed as the mean ± SE or number of subjects (of the total number; n). *Significantly different from Young subjects, P < 0.05. ≠Significantly different from Middle Aged subjects, P < 0.05
Adropin protein expression in SMFAs
Adropin protein expression in the SMFAs exhibited a progressive, age‐related, reduction. Example western blots and quantification of the signal intensity for the Young, Middle Aged and Old subjects are provided in Fig. 1 A.
Figure 1. Relative abundance of adropin protein and the visualization of the locus of adropin.
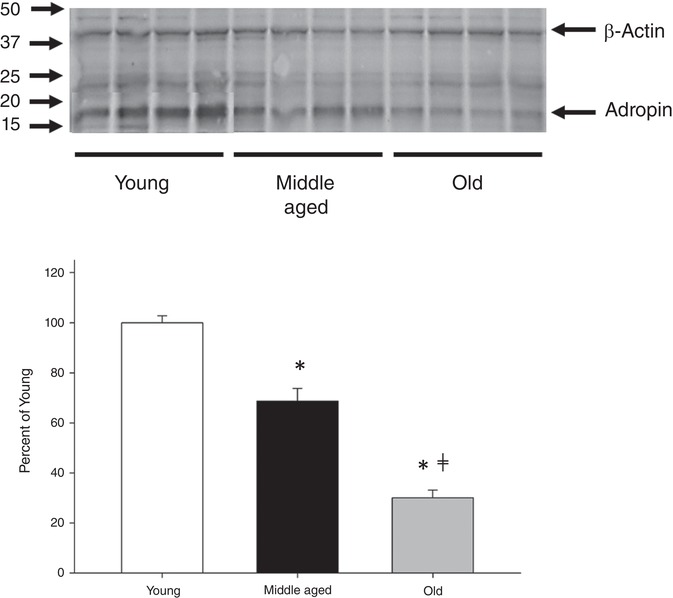
Representative blots for the relative abundance of adropin protein (A) and the visualization of the locus of adropin (B) in the skeletal muscle of feed arteries of Young, Middle Aged and Old subjects. Adropin protein expression was normalized by β‐actin protein expression. To date, there have not any prior publications utilizing the antibody utilized in the western blot analysis (ab213634; Abcam), although the manufacturer has characterized this antibody with the positive control HEK‐293T whole cell lysate overexpressing human adropin. Data are expressed as the mean ± SE. n = 10 Young, 10 Middle Aged and 13 Old subjects. *Significantly different from Young P<0.05. ≠Significantly different from Middle Aged P<0.05.
Adropin concentration for incubation
The results of the preliminary studies titrating the concentration of adropin for the 30 min incubation and the effect of peak vasodilatation are shown in Fig. 2. For both flow and ACh, the greatest vasodilatation was achieved following incubation in adropin at a concentration of 55 ng mL–1 and a greater concentration (65 and 75 ng mL–1) did not yield an enhanced response. Thus, a concentration of 55 ng mL–1 of adropin was utilized for all further incubations.
Figure 2. Titration of adropin concentration and peak vasodilatation to determine optimal adropin incubation.
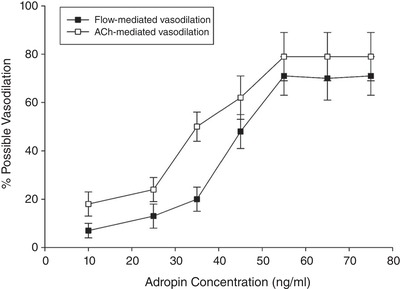
A series of SMFAs was studied to determine the optimal adropin incubation concentration to elicit peak vasodilatation in response to flow (45 μL min–1) and ACh (10−3 m) (n = 12, age = 63 ± 8 years).
Vessel diameters with and without adropin incubation
Unpressurized, outer diameter of the SMFAs was not statistically different in Young, Middle Aged and Old with and without adropin incubation (Young: 530 ± 20 μm; Middle Aged: 522 ± 18 μm; Old: 527 ± 14 μm, Young + Adropin: 535 ± 30 μm; Middle Aged + Adropin: 525 ± 21 μm; Old + Adropin: 533 ± 20 μm). Additionally, maximal outer diameter of the SMFAs, achieved by Ca2+ free PSS incubation (unpressurized), in Young, Middle Aged and Old with and without adropin incubation was not statistically different (Young: 818 ± 19 μm; Middle Aged: 822 ± 14 μm; Old: 820 ± 15 μm, Young + Adropin: 823 ± 20 μm; Middle Aged + Adropin: 820 ± 22 μm; Old + Adropin: 823 ± 24 μm). After being pressurized, the diameter of the SMFAs was significantly larger in Young compared to Old, although it was not statistically different in the Young, Middle Aged and Old as a result of adropin incubation. It should be noted that, in all assessments, the control and adropin incubated samples was performed on paired samples from the same subject.
Flow‐induced vasodilatation with and without adropin incubation
In control conditions, the SMFAs exhibited a progressive, age‐related, reduction in flow‐induced, endothelial‐dependent, vasodilatation. Indeed, the greatest vasodilatation in response to the maximal intraluminal flow of 45 ± 5 μL min–1 was significantly attenuated from the Young to the Middle Aged to the Old (Young: 65 ± 3%; Middle Aged: 36 ± 3%; Old: 15 ± 2%, P < 0.05) (Fig. 3 A–C). This trend was consistent in response to the lower intraluminal flows of 15 ± 5 and 30 ± 3 μL min–1 (Fig. 3 A–C). Following 30 min of incubation with adropin (55 μL min–1), the response of the young to the flow was not different (Fig. 3 A). However, adropin incubation in the Middle Aged and Old SMFAs improved the maximal vasodilatory response to flow to be more similar to that of the Young (Middle Aged + Adropin: 59 ± 3%; Old + Adropin: 47 ± 3%) (Fig. 3 A–C). This trend was also consistent in response to the lower intraluminal flows of 15 ± 5 and 30 ± 3 μL min–1 (Fig. 3 A–C).
Figure 3. Skeletal muscle feed arteries with and without adropin incubation evoked by flow and ACh.
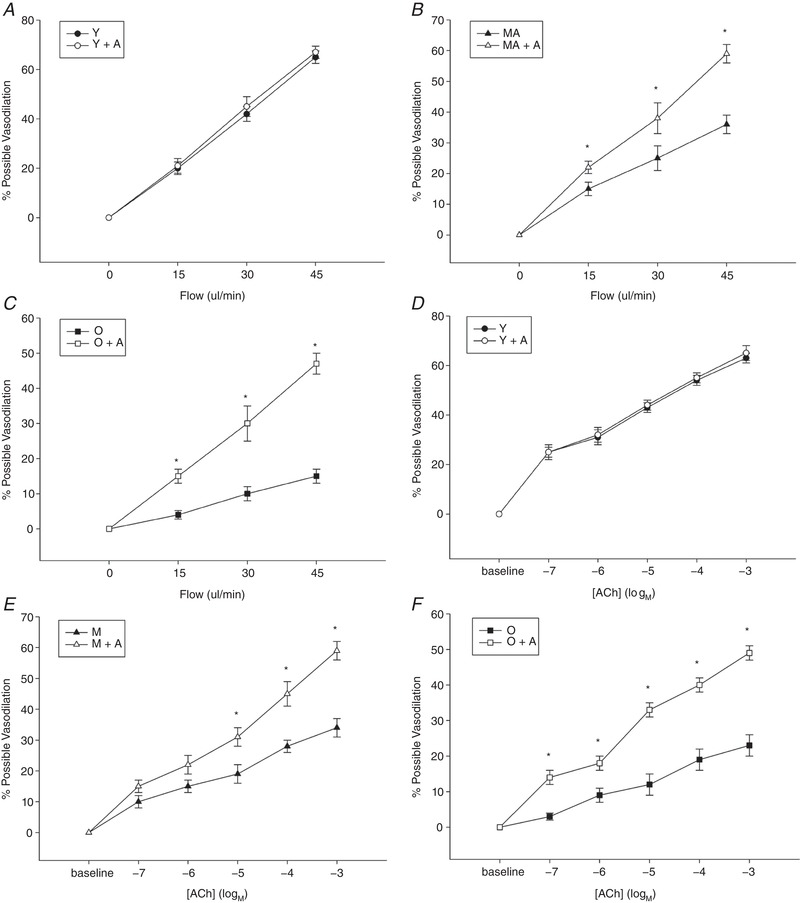
The vasodilator dose–response curves of skeletal muscle feed arteries from Young, Middle Aged and Old subjects with and without adropin incubation evoked by flow (A–C) and ACh (D–F). Data are expressed as the mean ± SE. n = 10 Young, 10 Middle Aged and 16 Old subjects. *Significant difference between with and without adropin incubation, P < 0.05.
ACh‐induced vasodilatation with and without adropin incubation
In control conditions, the SMFAs exhibited a progressive, age‐related, reduction in ACh‐induced, endothelial‐dependent, vasodilatation. Indeed, the greatest vasodilatation in response to the maximal ACh dose of 10−3 m was significantly attenuated from the Young to the Middle Aged to the Old (Young: 63 ± 2%, Middle Aged: 34 ± 3%; Old: 23 ± 3%, P < 0.05) (Fig. 4 A). This trend was consistent in response to the lower ACh doses (Fig. 3 D–F). Following 30 min of incubation with adropin (55 ng mL–1), the response of the Young to the ACh was not different (Fig. 3 D–F). However, adropin incubation in the Middle Aged and Old SMFAs improved the maximal vasodilatory response to ACh to be more similar to that of the Young (Middle Aged + Adropin: 59 ± 3%; Old + Adropin: 49 ± 2%, P < 0.05) (Fig. 4 A). This trend was also consistent in response to the lower ACh doses (Fig. 4 D–F).
Figure 4. Skeletal muscle feed arteries with and without adropin incubation.
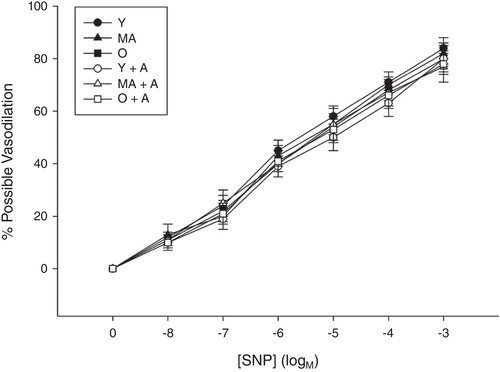
The vasodilator dose–response curves of skeletal muscle feed arteries from Young, Middle Aged and Old subjects with and without adropin incubation evoked by SNP. Data are expressed as the mean ± SE. n = 10 Young, 10 Middle Aged and 16 Old subjects.
SNP‐induced vasodilatation with and without adropin incubation
The vasodilatory response to the maximal SNP dose of 10−4 m, endothelial independent vasodilatory function, was similar among Young, Middle Aged and Old with or without adropin incubation (Young: 84 ± 4%, Middle Aged: 82 ± 4%; Old: 80 ± 3%, P < 0.05, Young + Adropin: 80 ± 14%; Middle Aged + Adropin: 77 ± 6%; Old + Adropin: 78 ± 4%) (Fig. 4). This finding was consistent in response to the lower SNP doses (Fig. 4).
eNOS, adropin and NO bioavailability
The initial relative abundance of total eNOS was not significantly different in the Young, Middle Aged and Old subjects; furthermore, 30 min of adropin incubation had no significant effect on total eNOS in the Young, Middle Aged and Old subjects. The initial relative abundance of phosphorylated (p‐)eNOS was significantly higher in the Young compared to the Middle Aged and Old subjects. Although the 30 min of adropin incubation had no effect on p‐eNOS in the Young, this treatment significantly increased p‐eNOS in both the Middle Aged and Old subjects. Example blots of the relative abundance of protein for eNOS and p‐eNOS at Ser1177 from SMFAs of Young, Middle Aged and Old subjects with and without adropin incubation are shown in Fig. 5 A. As also illustrated in Fig. 5 A, the ratio of p‐eNOS to eNOS was significantly attenuated in both the Middle Aged and Old SMFAs, with a clear trend toward a progressive age‐related decline. The 30 min of adropin incubation of the Middle Aged and Old SMFAs restored the p‐eNOS/eNOS ratio to that of the Young and had no effect on this ratio in the Young SMFAs (Fig. 5 A). Basal SMFA p‐eNOS/NOS ratio was strongly correlated with adropin protein expression and exhibited a clear influence of advancing age (Fig. 5 B). The adropin‐induced (30 min of incubation) change in p‐eNOS/NOS ratio was also strongly negatively related to SMFA adropin protein expression (Fig. 5 C). l‐NMMA, a NOS inhibitor, ablated the previously strong, and statistically significant, relationship between maximal percentage possible vasodilatation, evoked by both flow and ACh, and adropin protein expression (Fig. 6 A and B). Of note, there was no relationship between adropin protein expression and endothelium independent vasodilatation (Fig. 6 C). Furthermore, l‐NMMA ablated the adropin‐induced recovery of both flow‐ and ACh‐mediated endothelial‐dependent vasodilatation in the Old and, in both cases, reduced the vasodilatory response in the Young and Middle Aged SMFAs to that of the Old vessels (Fig. 7 A and D). The impact of l‐NMMA both with and without adropin incubation on percentage maximal possible flow‐ and ACh‐induced vasodilatation is shown in Fig. 7 B and E, revealing an attenuated effect without adropin in the Middle Aged and Old subjects that was augmented following adropin incubation. Finally, as a result of the inhibitory effect of l‐NMMA on eNOS, and the subsequent calculation of the difference in the effect of l‐NMMA on flow‐ and ACh‐mediated percentage possible vasodilatation with and without adropin incubation, this allowed the determination of the magnitude of effect of adropin‐induced NOS‐mediated vasodilatation, revealing a clear effect of advancing age (Fig. 7 C and F).
Figure 5. eNOS and p‐eNOS at Ser1177 from skeletal muscle feed arteries.
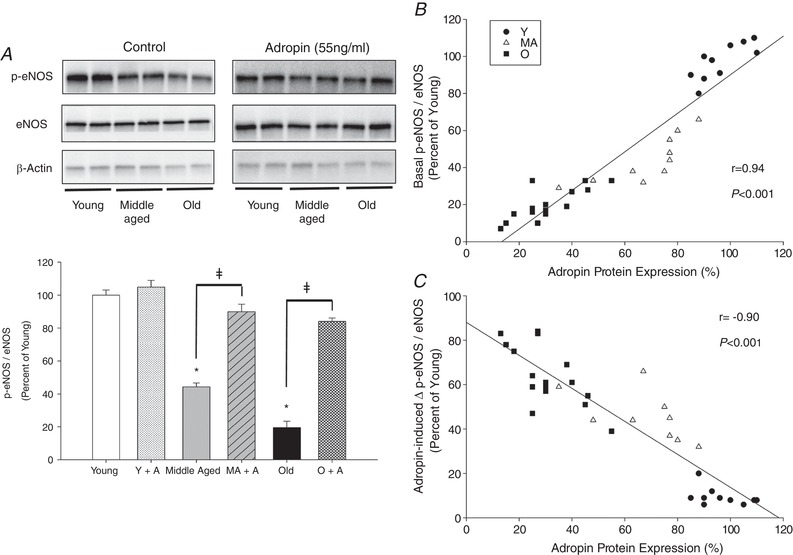
A, representative blots for eNOS and p‐eNOS at Ser1177 from skeletal muscle feed arteries of Young (Y), Middle Aged (MA) and Old (O) subjects with and without adropin (A) incubation. Note, the vessel samples used for these analyses had not been exposed to any other assessments. Data are expressed as the mean ± SE. n = 10 Young, 10 Middle Aged and 16 Old subjects. *Significant difference between the Middle Aged and Old without adropin compared to young both with and without adropin, P < 0.05. ǂSignificant difference between with and without adropin in the Middle Aged and the Old, P < 0.05. B, the relationship between basal p‐eNOS / eNOS protein expression and adropin protein expression in skeletal muscle feed arteries from Young (Y), Middle Aged (MA), and Old (O) subjects. C, the relationship between adropin‐induced delta p‐eNOS / eNOS protein expression and adropin protein expression in skeletal muscle feed arteries from Young (Y), Middle Aged (MA), and Old (O) subjects.
Figure 6. The relationship between adropin protein expression and vasodilatory function.
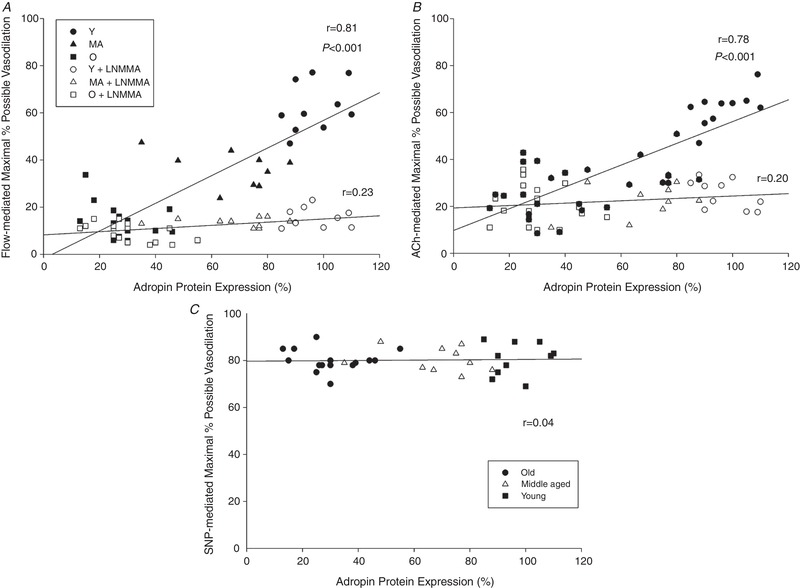
The relationship between adropin protein expression and vasodilatory function evoked by flow (A), ACh (B) and SNP (C) in skeletal muscle feed arteries from Young, Middle Aged and Old subjects.
Figure 7. The vasodilator dose–response curves of skeletal muscle feed arteries.

The vasodilator dose–response curves of skeletal muscle feed arteries from Young (Y), Middle Aged (MA) and Old (O) subjects both with and without adropin (A) incubation, and with and without nitric oxide synthase blockade (l‐NMMA) evoked by both flow and ACh (A and D), the quantification of the effect of l‐NMMA with and without adropin incubation (B and E) and the magnitude of effect of adropin‐NOS mediated vasodilatation (C and F). Data are expressed as the mean ± SE. n = 10 Young, 10 Middle Aged and 16 Old subjects. A, *significant difference between Y, Young with adropin incubation (Y+A) and Middle Aged with adropin incubation (MA+A) compared to MA and Old with adropin incubation (O+A), P < 0.05; †significant difference between MA and O+A compared to O, Young with adropin and LNMMA incubation, Middle Aged with adropin and LNMMA incubation (MA+A+LNMMA) and O+A+LNMMA, P < 0.05; ǂsignificant difference between Y, Y+A and MA+A compared to O, Y+A+LNMMA, MA+A+LNMMA and O+A+LNMMA, P < 0.05. D, *significant difference between Y, Y+A, MA+A and O+A compared to MA, P < 0.05; †significant difference between MA compared to O, Y+A+LNMMA, MA+A+LNMMA and O+A+LNMMA, P < 0.05; ǂsignificant difference between Y, Y+A, MA+A and O+A compared to O, Y+A+LNMMA, MA+A+LNMMA and O+A+LNMMA, P < 0.05. B and E, *significantly different from Y, P < 0.05; ǂsignificant difference between with and without adropin, P < 0.05; †significantly different from Y+A, P < 0.05). C and F, *significantly different from Y, P < 0.05; ǂsignificantly different from MA, P < 0.05.
Discussion
The present study aimed to determine the impact of advancing age on endogenous adropin levels in human SMFAs and the role of adropin in age‐related vascular dysfunction. There are several novel and important findings of this study. First, as hypothesized, adropin protein expression was, for the first time, documented to fall progressively with advancing age in the human peripheral vasculature and this was related to the now commonly recognized age‐related reduction in endothelial‐dependent vasodilatory function. Additionally, both assessments of endothelial‐dependent vasodilatation (flow and ACh), but not endothelial independent vasodilatation (SNP), revealed a strong correlation with SMFA adropin protein levels. Second, again for the first time, this study revealed that adropin incubation restored age‐related endothelial‐dependent vasodilatory dysfunction in human vessels. Third, this adropin facilitated restoration of age‐related vasodilatory dysfunction was mediated by NO bioavailability, as indicated by an adropin‐induced activation of p‐eNOS. Furthermore, and of significant importance, NOS blockade ablated both the positive vascular effects of adropin in the older subjects and the significant relationship between endothelial function and adropin protein expression. Finally, additional support for a mechanistic link between declining adropin and age‐related endothelial dysfunction was indicated by a progressively increasing magnitude of effect of adropin‐induced eNOS‐mediated vasodilatation with advancing age. In combination, these findings show that adropin plays a role in age‐related vascular dysfunction via a decrease in NO bioavailability, and it appears to be a novel therapeutic target to facilitate the restoration of endothelial function with advancing age.
Adropin and adropin abundance
Adropin is a 43 amino acid peptide fragment encoded by the energy homeostasis associated transcript, expressed in the plasma membrane. Adropin was first identified using microarrays to investigate fatty liver and insulin resistance in genetically induced obese mice (Kumar et al. 2008). In terms of metabolic homeostasis, adropin is considered to increase glucose oxidation and decrease fat oxidation, thereby playing a role in regulating glucose homeostasis independently of changes in body weight, food intake and whole body energy expenditure (Kumar et al. 2008). Although adropin does play a critical role in maintaining energy balance and insulin resistance, adropin has recently also been implicated in cardiovascular dysfunction and CVD (Lovren et al. 2010). With adropin expression now documented in liver, brain, heart, small intestine and skeletal muscle (Aydin et al. 2013; Wong et al. 2014) (Lovren et al. 2010), as well as human umbilical vein and coronary artery endothelial cells (Lovren et al. 2010), this peptide appears to be fairly ubiquitous. As shown in Fig. 1, adropin protein abundance in the SMFAs, as assessed by western blotting, exhibits a progressive decline with advancing age. It should be noted that adropin is also found in the blood and appears to decline here also with age, with our group measuring serum levels (using an enzyme‐linked immunosorbent assay) of ∼15 ng mL–1 in a group of seven young subjects (<30 years) and an attenuated concentration in their older counterparts (∼5 ng mL–1, n = 7, > 65 years) (OS Kwon, RS Richardson; unpublished observations). These serum adropin levels are certainly much lower than the concentration of 55 ng mL–1 in which the SMFAs were incubated in the present study, which may raise concerns about physiological relevance; however, currently, the concentration of adropin in the vasculature, itself, is unknown. Finally, with respect to this point, as shown in Fig. 2, in a dose–response for adropin, the lowest concentration of adrophin that resulted in the greatest vasodilatation in the SMFAs was 55 ng mL–1, supporting the use of this concentration.
Adropin, ageing and vascular function
Growing evidence, driven by the role of adropin in insulin resistance and the close relationship between insulin resistance and endothelial function, suggests that adropin may be involved in cardiovascular dysfunction and CVD (Lovren et al. 2010). Indeed, several recent studies have determined that circulating adropin levels are decreased with age and this altered adropin level is closely related to the prevalence of CVD (Butler et al. 2012; Celik et al. 2013; Wu et al. 2014; Yu et al. 2014). Of note, in the original work proposing a link between adropin and CVD, by Lovren et al. (2010), it was determined that adropin is expressed in endothelial cells and regulates NO bioavailability by eNOS expression. Therefore, this implies that adropin may be a regulator of endothelial function, which is recognized as a precursor and mediator of CVD (Seals et al. 2011). However, although impactful in terms of the potential link between adropin and vascular function, the previous study used commercially available human endothelial cells in culture and did not perform vessel function studies to translate these findings. Hence, verification of the link between adropin and vascular function was not achieved.
Recently, utilizing, in vivo, relatively non‐invasive methods, Topuz et al. (2013) reported correlations between circulating adropin levels and vascular function in patients with type 2 diabetes. Specifically, a positive correlation was revealed between plasma adropin levels and brachial artery flow‐mediated dilatation (Topuz et al. 2013). Furthermore, Fujie et al. (2015) reported that aerobic exercise increases serum adropin levels and this increase appears to be linked to reduced arterial stiffness in middle aged and older adults. In combination, these studies bolster the concept that adropin probably plays a role in regulating vascular function and therefore CVD, although definitive conclusions were somewhat limited by the relatively simple experimental design of these studies. By contrast, the present study, utilizing both flow and ACh to stimulate endothelial‐dependent vasodilatation, SNP to assess endothelial independent vasodilatation, adropin protein expression and immunofluorescence, and adropin incubation, comprehensively assessed the link between adropin and vascular function in human SMFAs. The present study not only provides additional correlative evidence that adropin is strongly related to endothelial‐dependent vasodilatation, but also documents that there is no such relationship with endothelial independent vasodilatation (Fig. 6). Furthermore, adropin protein levels fall progressively with advancing age (Fig. 1) and so too does endothelial‐mediated vasodilatation (Fig. 3), but not endothelial independent vasodilatation (Fig. 4). Finally, and most convincingly in terms of linking adropin to vascular function, as shown in Fig. 7, adropin incubation restored the attenuated endothelial dependent vasodilatation exhibited by the Middle Aged and Old SMFAs, with no impact on endothelial dependent vasodilatation, and no impact on the Young SMFAs. Indeed, a mechanistic link between declining adropin and age‐related endothelial dysfunction was documented by a progressively increasing magnitude of effect of adropin‐induced eNOS‐mediated vasodilatation with advancing age (Fig. 7 C and F). Hence, for the first time, the present study clearly documents the link between adropin and vascular function and, more specifically, the promotion of endothelial‐dependent vasodilatation by adropin.
Endothelial function, adropin and NO bioavailability
The present study contributes significantly to the evidence indicating that adropin is a novel regulator of endothelial function and this is important because endothelial dysfunction is recognized as a precursor and mediator of CVD (Seals et al. 2011; Widmer & Lerman, 2014). Healthy endothelial function is dependent upon the anti‐atherogenic molecule NO, which, in addition to simulating vasodilatation (Taddei et al. 2001; Su, 2015) has anti‐oxidant properties, making NO very important for vascular function. NO production, and therefore bioavailability, in the vasculature is predominantly dependent upon eNOS in the vascular endothelial cells. Thus, eNOS also plays a critical role in arterial endothelial function (Taddei et al. 2001; Crecelius et al. 2010; Su, 2015; Trinity et al. 2016). Indeed, utilizing similar techniques to those of the present study, our group recently documented the importance of these processes in explaining the mechanisms responsible for endothelial‐dependent vasodilatory dysfunction with advancing age in the skeletal muscle resistance vasculature (Park et al. 2016). Therefore, eNOS and NO bioavailability are two of the most important components of maintaining healthy endothelial function.
Recently, it was determined that adropin increases eNOS mRNA and eNOS protein expression in endothelial cells via VEGFR2 and ultimately Akt and ERK1/2 (Lovren et al. 2010). However, because these studies were performed in plated endothelial cells, there was no opportunity to assess the practical implications of this apparent adropin‐driven phosphorylation of eNOS and subsequent increase in NO bioavailability. An additional study, performed in vivo, utilizing brachial artery flow‐mediated vasodilatation as an indicator of endothelial‐mediated vascular function (Topuz et al. 2013), supported this postulate, with a positive correlation between plasma adropin and vascular function in patients with type 2 diabetes. Therefore, building upon and extending this evidence, the present study initially demonstrated a relationship between adropin protein content and endothelial‐dependent vascular function (Fig. 6) and the restoration of endothelial‐mediated vascular function by incubation of the Middle Aged and Old SMFAs in adropin (Fig. 3). These data document that adropin is not only linked to endothelial‐mediated vascular function, but also appears to directly regulate it. However, to clarify the effect of adropin on eNOS and NO bioavailability, p‐eNOS and eNOS protein content were assessed with and without adropin incubation and this revealed that Middle Aged and Old SMFAs increased p‐eNOS/eNOS levels in response to this treatment, whereas there was no such effect in the Young SMFAs (Fig. 5 A). Additionally, both basal p‐eNOS/eNOS ratio (Fig. 5 B) and the adropine incubation‐induced change in p‐eNOS/eNOS (Fig. 5 C) were strongly correlated with adropin protein expression in the SMFAs. Although it should be acknowledged that these changes in p‐eNOS/eNOS induced by adropin incubation were only assessed in basal conditions, and not during flow and ACh treatments, this molecular evidence of a significant role of adropin in phosphorylating eNOS was translated by functional vessel studies, performed in Young, Middle Aged and Old SMFAs. Specifically, the restoration of the Middle Aged and Old SMFA endothelial‐mediated vasodilatation in response to flow and ACh, achieved by the incubation of the vessels in adropin (Fig. 3), was ablated by the NOS blocker, l‐NMMA (Fig. 7 A and B).
The impact of blocking the vascular mechanism of action of adropin (eNOS phosphorylation) with l‐NMMA across the lifespan are illustrated in Fig. 7 B, C, E and F. These panels clearly indicate that, with advancing age, the effect of NOS blockade on endothelial‐mediated vascular function falls precipitously as a result of a fall in age‐related eNOS‐mediated NO bioavailability. However, following incubation in adropin, this fall in eNOS‐mediated vasodilatation is greatly ameliorated, which, as illustrated in Fig. 7 C and F, reveals a progressively increasing magnitude of effect of adropin‐induced NOS‐mediated vasodilatation with advancing age. Furthermore, and as additional evidence of a mechanistic link between declining adropin and age‐related endothelial dysfunction, blocking the vascular mechanism of action of adropin (eNOS phosphorylation) with l‐NMMA ablated the aforementioned significant relationship between vascular function (flow‐ and ACh‐induced) and adropin protein expression (Fig. 6 A and B). In combination, this comprehensive series of studies demonstrates that the mechanism of action for adropin on endothelial function comprises an increase in p‐eNOS and, ultimately, an increase in NO bioavailability, and also that this effect of adropin translates into improved endothelial‐dependent vascular function.
Conclusions
The findings of the present study document that adropin plays a role in age‐related vascular dysfunction via an eNOS‐mediated decrease in NO bioavailability. Adropin could therefore be a novel therapeutic target to facilitate the restoration of endothelial function with advancing age.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
OSK and RSR designed and conducted the experiments, analysed the data and wrote the manuscript. RHIA and JRH received the consents from patients and biopsied skeletal muscle feed arteries. RHIA, JZ and JRH helped with the data analysis.
Funding
This work was funded, in part, by the National Heart, Lung and Blood Institute at the National Institute of Health (PO1 HL1091830 and T32 HL139451) and the Veteran's Administration Rehabilitation Research and Development Service (E6910‐R, E1697‐R, E1433‐P, E9275‐L and E1572‐P).
Acknowledgements
The authors wish to thank all the subjects who partook in this study.
Biography
Oh Sung Kwon received his PhD in exercise physiology (2013) from the University of Florida under the supervision of Professor Scott K. Powers. Subsequently, Oh Sung recently completed a Postdoctoral Fellow at the Utah Vascular Research Laboratory in the University of Utah under the guidance of Professor Russell Richardson. He is currently an Assistant Professor at the University of Connecticut. His research focus is on the integration of ageing, skeletal muscle and cardiovascular function, especially the role of mitochondria and free radical production in the attenuation of endothelial function with advancing age.

Edited by: Michael Hogan & Russell Hepple
References
- Aydin S, Kuloglu T, Aydin S, Eren MN, Yilmaz M, Kalayci M, Sahin I, Kocaman N, Citil C & Kendir Y (2013). Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin‐induced diabetes. Mol Cell Biochem 380, 73–81. [DOI] [PubMed] [Google Scholar]
- Butler AA, Tam CS, Stanhope KL, Wolfe BM, Ali MR, O'Keeffe M, St‐Onge MP, Ravussin E & Havel PJ (2012). Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J Clin Endocrinol Metab 97, 3783–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA & Lundby C (2012). Skeletal muscle vasodilatation during maximal exercise in health and disease. J Physiol 590, 6285–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik A, Balin M, Kobat MA, Erdem K, Baydas A, Bulut M, Altas Y, Aydin S & Aydin S (2013). Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc Ther 31, 174–178. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF & Dinenno FA (2010). Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299, H1633–H1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujie S, Hasegawa N, Sato K, Fujita S, Sanada K, Hamaoka T & Iemitsu M (2015). Aerobic exercise training‐induced changes in serum adropin level are associated with reduced arterial stiffness in middle‐aged and older adults. Am J Physiol Heart Circ Physiol 309, H1642–H1647. [DOI] [PubMed] [Google Scholar]
- Hellsten Y (2016). Limitations of skeletal muscle oxygen supply in ageing. J Physiol 594, 2259–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K, Chen B, Behnke BJ, Ghosh P, Stabley JN, Bramy JA, Sepulveda JL, Delp MD & Muller‐Delp JM (2017). Exercise training reverses age‐induced diastolic dysfunction and restores coronary microvascular function. J Physiol 595, 3703–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives SJ, Andtbacka RH, Park SY, Donato AJ, Gifford JR, Noyes RD, Lesniewski LA & Richardson RS (2012). Human skeletal muscle feed arteries: evidence of regulatory potential. Acta Physiol (Oxf) 206, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN, Kousoulas KG, Rogers PM, Kesterson RA, Thearle M, Ferrante AW, Jr. , Mynatt RL, Burris TP, Dong JZ, Halem HA, Culler MD, Heisler LK, Stephens JM & Butler AA (2008). Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab 8, 468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ & Seals DR (2009). B6D2F1 Mice are a suitable model of oxidative stress‐mediated impaired endothelium‐dependent dilation with aging. J Gerontol A Biol Sci Med Sci 64, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta M, Al‐Omran M, Teoh H & Verma S (2010). Adropin is a novel regulator of endothelial function. Circulation 122, S185–S192. [DOI] [PubMed] [Google Scholar]
- Muller‐Delp JM (2016). Heterogeneous ageing of skeletal muscle microvascular function. J Physiol 594, 2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T & Partridge L (2012). Ageing as a risk factor for disease. Curr Biol 22, R741–R752. [DOI] [PubMed] [Google Scholar]
- North BJ & Sinclair DA (2012). The intersection between aging and cardiovascular disease. Circ Res 110, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Ives SJ, Gifford JR, Andtbacka RH, Hyngstrom JR, Reese V, Layec G, Bharath LP, Symons JD & Richardson RS (2016). Impact of age on the vasodilatory function of human skeletal muscle feed arteries. Am J Physiol Heart Circ Physiol 310, H217–H225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kwon OS, Andtbacka RHI, Hyngstrom JR, Reese V, Murphy MP & Richardson RS (2018). Age‐related endothelial dysfunction in human skeletal muscle feed arteries: the role of free radicals derived from mitochondria in the vasculature. Acta Physiol (Oxf) 222, 10.1111/apha.12893. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL & Donato AJ (2011). Aging and vascular endothelial function in humans. Clin Sci (Lond) 120, 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Reyes R, Chen B, Ghosh P, Gurovich AN, Kang LS, Cardounel AJ, Delp MD & Muller‐Delp JM (2013). Age and exercise training alter signaling through reactive oxygen species in the endothelium of skeletal muscle arterioles. J Appl Physiol (1985) 114, 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait JB & Lakatta EG (2012). Aging‐associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin 8, 143–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JB (2015). Vascular endothelial dysfunction and pharmacological treatment. World J Cardiol 7, 719–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A & Salvetti A (2001). Age‐related reduction of NO availability and oxidative stress in humans. Hypertension 38, 274–279. [DOI] [PubMed] [Google Scholar]
- Topuz M, Celik A, Aslantas T, Demir AK, Aydin S & Aydin S (2013). Plasma adropin levels predict endothelial dysfunction like flow‐mediated dilatation in patients with type 2 diabetes mellitus. J Investig Med 61, 1161–1164. [DOI] [PubMed] [Google Scholar]
- Trinity JD, Wray DW, Witman MA, Layec G, Barrett‐O'Keefe Z, Ives SJ, Conklin JD, Reese V, Zhao J & Richardson RS (2016). Ascorbic acid improves brachial artery vasodilation during progressive handgrip exercise in the elderly through a nitric oxide‐mediated mechanism. Am J Physiol Heart Circ Physiol 310, H765–H774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Gunduz F, Laughlin MH & Woodman CR (2009). Exercise training reverses age‐related decrements in endothelium‐dependent dilation in skeletal muscle feed arteries. J Appl Physiol (1985) 106, 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J, Saltin B, Jorfeldt L & Pernow B (1974). Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest 33, 79–86. [DOI] [PubMed] [Google Scholar]
- Widmer RJ & Lerman A (2014). Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract 2014, 291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CM, Wang Y, Lee JT, Huang Z, Wu D, Xu A & Lam KS (2014). Adropin is a brain membrane‐bound protein regulating physical activity via the NB‐3/Notch signaling pathway in mice. J Biol Chem 289, 25976–25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Fang J, Chen L, Zhao Z, Luo Y, Lin C & Fan L (2014). Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non‐diabetic patients. Clin Chem Lab Med 52, 751–758. [DOI] [PubMed] [Google Scholar]
- Yazdanyar A & Newman AB (2009). The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med 25, 563–577, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HY, Zhao P, Wu MC, Liu J & Yin W (2014). Serum adropin levels are decreased in patients with acute myocardial infarction. Regul Pept 190–191, 46–49. [DOI] [PubMed] [Google Scholar]


