Abstract
Key points
There is clinical evidence showing that prostatic inflammation contributes to overactive bladder symptoms in male patients; however, little is known about the underlying mechanisms
In this study, we investigated the mechanism that prostatic inflammation causes detrusor overactivity by using a rat model of chemically induced prostatic inflammation.
We observed a significant number of dorsal root ganglion neurons with dichotomized afferents innervating both prostate and bladder.
We also found that prostatic inflammation induces bladder overactivity and urothelial NGF overexpression in the bladder, both dependent on activation of the pelvic nerve, as well as changes in ion channel expression and hyperexcitability of bladder afferent neurons.
These results indicate that the prostate‐to‐bladder cross‐sensitization through primary afferent pathways in the pelvic nerve, which contain dichotomized afferents, could be an important mechanism contributing to bladder overactivity and afferent hyperexcitability induced by prostatic inflammation.
Abstract
Prostatic inflammation is reportedly an important factor inducing lower urinary tract symptoms (LUTS) including urinary frequency, urgency and incontinence in patients with benign prostatic hyperplasia (BPH). However, the underlying mechanisms inducing bladder dysfunction after prostatic inflammation are not well clarified. We therefore investigated the effects of prostatic inflammation on bladder activity and afferent function using a rat model of non‐bacterial prostatic inflammation. We demonstrated that bladder overactivity, evident as decreased voided volume and shorter intercontraction intervals in cystometry, was observed in rats with prostatic inflammation versus controls. Tissue inflammation, evident as increased myeloperoxidase activity, and IL‐1α, IL‐1β, and IL‐6 levels inside the prostate, but not in the bladder, following intraprostatic formalin injection induced an increase in NGF expression in the bladder urothelium, which depended on activation of the pelvic nerve. A significant proportion (18–19%) of dorsal root ganglion neurons were double labelled by dye tracers injected into either bladder or prostate. In rats with prostatic inflammation, TRPV1, TRPA1 and P2X2 increased, and Kv1.4, a potassium channel α‐subunit that can form A‐type potassium (KA) channels, decreased at mRNA levels in bladder afferent and double‐labelled neurons vs. non‐labelled neurons, and slow KA current density decreased in association with hyperexcitability of these neurons. Collectively, non‐bacterial inflammation localized in the prostate induces bladder overactivity and enhances bladder afferent function. Thus, prostate‐to‐bladder afferent cross‐sensitization through primary afferents in the pelvic nerve, which contain dichotomized afferents, could underlie storage LUTS in symptomatic BPH with prostatic inflammation.
Keywords: prostate, bladder, inflammation, cross‐sensitization
Key points
There is clinical evidence showing that prostatic inflammation contributes to overactive bladder symptoms in male patients; however, little is known about the underlying mechanisms
In this study, we investigated the mechanism that prostatic inflammation causes detrusor overactivity by using a rat model of chemically induced prostatic inflammation.
We observed a significant number of dorsal root ganglion neurons with dichotomized afferents innervating both prostate and bladder.
We also found that prostatic inflammation induces bladder overactivity and urothelial NGF overexpression in the bladder, both dependent on activation of the pelvic nerve, as well as changes in ion channel expression and hyperexcitability of bladder afferent neurons.
These results indicate that the prostate‐to‐bladder cross‐sensitization through primary afferent pathways in the pelvic nerve, which contain dichotomized afferents, could be an important mechanism contributing to bladder overactivity and afferent hyperexcitability induced by prostatic inflammation.
Introduction
There is increasing evidence showing that inflammation of one pelvic organ such as the urinary bladder or the colon may lead to neurogenic inflammation and/or referred pain in another by means of shared neural pathways connecting different pelvic organs (Malykhina, 2007; Brumovsky & Gebhart, 2010). The cross‐sensitization between two organs could occur at three different afferent levels: an antidromic axon reflex via dichotomizing afferent neurons in dorsal root ganglia (DRG), viscero‐somatic convergence in the spinal cord (afferent‐afferent interactions via the spinal cord) or interactions of neural pathways in the brain. Previous studies have demonstrated that a significant number of dichotomized DRG neurons innervate both bladder and prostate (Chen et al. 2010; Lee et al. 2016a).
Lower urinary tract symptoms (LUTS) are commonly seen in males with benign prostatic hyperplasia (BPH), and storage LUTS such as urinary frequency and urgency overlap with those of overactive bladder. BPH‐induced bladder outlet obstruction (BOO) has been proposed as a mechanism inducing enhanced bladder afferent activity and storage LUTS due to various factors including decreased release of nitric oxide (Kim et al. 2011), increased release of prostaglandin E2 from the urothelium (Beppu et al. 2011), decreased potassium channel activity (Aydin et al. 2012), or ischaemia and oxidative stress to the detrusor (Lin et al. 2008). However, the size of prostate and LUTS severity do not always correlate and patients sometimes suffer LUTS even in the absence of prostate enlargement, which suggests that multiple factors contribute to the pathogenesis of LUTS (Funahashi et al. 2011; Chung et al. 2012). In this regard, intra‐prostatic inflammation has been proposed to be involved in the development of LUTS. There are increasing clinical reports of association between the LUTS scores and the degree of prostatic inflammation in BPH specimens (Nickel et al. 2008; Robert et al. 2009; Fibbi et al. 2010; Liao et al. 2011; Chung et al. 2012), and it is possible that afferent cross‐sensitization between the prostate and bladder due to prostatic inflammation could be another mechanism inducing storage LTUS in BPH patients.
Rodent models of non‐bacterial or bacterial prostatic inflammation show bladder overactivity evident by frequent voiding (Chen et al. 2010; Lee et al. 2015; Schwartz et al. 2016; Mizoguchi et al. 2017). However, detailed functional and molecular mechanisms underlying bladder dysfunction following prostatic inflammation remain to be elucidated. In this study, we therefore examined changes in bladder activity and molecular and functional properties of DRG neurons innervating the prostate and/or bladder in a rat model of chemically induced prostatic inflammation to investigate the inflammation‐related mechanisms underlying male LUTS due to BPH with prostatic inflammation.
Methods
Ethical approval
All animal experiments were conducted in accordance with the ARRIVE and NIH guidelines and approved by the Institutional Animal Care and Use Committees (IACUC) (Protocol approval no. 15086776). Efforts were made to minimize the suffering of the animals and the number of animals needed to obtain reliable results.
Animal surgery
Male Sprague‐Dawley rats weighing 150–200 gm (n = 110 rats in total) were anaesthetized with isoflurane and, in a supine position, the prostate and bladder were exposed with a lower abdominal incision. To produce a chemically induced prostatic inflammation model, formalin (5% in saline) or saline (vehicle control) was injected into each ventral lobe of the prostate (50 μl per lobe) as previously reported (Funahashi et al. 2014). In a separate group of rats used for analyses of the distribution of fluorescent dye‐labelled DRG cells, laser‐capture microdissection (LCM), and whole‐cell patch clamp recordings, 0.2% 1,1'‐dioctadecyl‐3,3,3',3'‐tetramethylindocarbocyanine perchlorate (DiI) in 2% dimethyl sulfoxide in 5% formalin or saline was injected into each ventral lobe of the prostate (50 μl per lobe), and 1% Fast Blue in saline (20 μl) was injected in the posterior wall of the bladder (4 sites, 5 μl per site). To decrease dye leakage, the needle tip was tunnelled 2–3 mm subserosally and the needle was held in place for 10 s after each injection. In addition, each puncture site was swabbed with fresh cotton‐tipped applicators to absorb any potential tracer leakage. The abdominal incision was closed after injection, and all experiments were performed 1 week later.
Histological analyses
The ventral lobes of prostate and the bladder were excised 1 week after surgery, embedded in OCT Tissue Tek (Sakura Finetek U.S.A, Torrance, CA, USA), frozen in liquid nitrogen, and kept at −80°C until use. Samples were serially sectioned at 8 μm thickness, and microscopic examination was conducted in tissue sections stained with Haematoxylin and Eosin.
Measurement of inflammatory markers
The prostate and bladder tissues of formalin‐ and saline‐injected rats (n = 6 per groups) were homogenized using RIPA lysis buffer. Homogenate was centrifuged at 10,000 g for 10 min and the supernatants were stored at −80°C until assayed. IL‐1α, IL‐1β, and IL‐6 levels were determined by the Luminex 200 (Bio‐Rad, Hercules, CA, USA) using a MILLIPLEX MAP Rat Cytokine/Chemokine Panel (Millipore, Billerica, MA, USA). Myeloperoxidase (MPO) concentration, which is indicative of neutrophil activity, was measured using specific enzyme‐linked immunosorbent assay (ELISA) kits (Hycult Biotech, Plymouth Meeting, PA, USA). Protein concentration was determined using a Bio‐Rad kit with bovine serum albumin as the standard. The cytokine and MPO concentrations were standardized to tissue protein levels and expressed in pico‐ or nanograms per milligram total protein, respectively.
Voiding behavioural studies
Formalin‐ or saline‐injected rats (n = 6 per groups) were placed in a metabolic cage before and 1‐week after the treatment. Voided urine was collected continuously using a cup placed beneath the metabolic cage and fitted onto a Fort 100 force displacement transducer connected to a Transbridge TBM4M amplifier (World Precision Instruments, Sarasota, FL, USA) to measure voided volume. Data were collected using a data acquisition system equipped with a Power Lab analogue‐to‐digital converter (AD Instruments, Milford, MA, USA). The overnight monitoring period started at 19.00 h and ended at 07.00 h. All rats had free access to water and food during the recording period in the cage. The variables of micturition obtained included micturition frequency, total urine output and single voided volume.
Conscious cystometry
One week after intraprostatic injection of formalin (n = 7) or saline (n = 6), rats were anaesthetized with isoflurane. Following a lower midline abdominal incision, the bladder was exposed and catheterized with PE‐50 tubing (Clay Adams Division of Becton Dickinson, Parsippany, NJ, USA) inserted through the bladder dome. After recovery from anaesthesia, conscious rats were placed in a restraining cage. After 2 h of acclimation, saline was infused into the bladder at 0.04 ml min−1, and rats voided spontaneously through the urethra. At least four reproducible micturition cycles were recorded after an initial stabilization period (60 min). A software package (Chart5 software) (ADInstrument, Milford, MA, USA) was used for data collection and analysis. Baseline pressure, pressure threshold for voiding, peak voiding pressure and intercontraction interval were evaluated.
In addition, bladder activity was evaluated in a separate group of rats with transection of the right pelvic nerve to examine whether bladder functional changes after prostatic inflammation is nerve‐mediated and dependent on pelvic nerve activation. In these animals, formalin was injected into the ipsilateral (right) or contralateral (left) side of the ventral prostate lobe following pelvic nerve transection (n = 7 and 8, respectively). Then, single cystometry was performed under an awake condition 1 week after formalin injection and the data were compared with pelvic nerve‐transected rats without formalin injection (n = 7).
Retrograde fluorescence labelling of DRG neurons
At 1 week after dye injection into the prostate or bladder, Th13 to S2 DRG of formalin‐ and vehicle‐injected rats (6 DRG from 3 rats per groups) were excised, embedded in OCT Tissue Tek, frozen in liquid nitrogen, and kept at −80°C until use. Samples were serially sectioned at 8 μm thickness, and every third section was mounted onto slides to avoid repeat counting of the cells. The slides were observed using a fluorescence microscope with appropriate filters, and the numbers of Fast Blue‐labelled, DiI‐labelled, or double‐labelled cells were counted and expressed as the average of positive cells per section in each ganglion.
Dissociation of DRG neurons and whole‐cell patch‐clamp recording
In a separate group of rats injected with dye tracers, freshly dissociated neurons from L6‐S1 DRG were prepared from formalin‐ or saline‐injected rats (n = 18 each), as described previously (Yoshimura & de Groat, 1997). L6 DRG on both sides and S1 DRG on one side were removed, minced and incubated for 25 min at 35°C in 5 ml DMEM (Sigma, St Louis, MO, USA) containing 0.5 mg/ml trypsin (Sigma), 1 mg/ml collagenase (Type I, Sigma). Trypsin inhibitor (Sigma) was then added to neutralize the enzyme activity. Individual DRG cell bodies were isolated by trituration and plated on poly‐l‐lysine‐coated 35 mm culture dishes.
Whole‐cell patch‐clamp recordings were performed at room temperature (20–22°C) on Fast Blue‐ and/or DiI‐positive neurons within 10 h of dissociation. The internal solution contained (in mm): 140 KCl, 1 CaCl2, 2 MgCl2, 11 EGTA, 10 HEPES, 2 Mg‐ATP adjusted to pH 7.4 with KOH. Patch electrodes had resistances of 2–4 MΩ when filled with the internal solution. Neurons were superfused initially at a flow rate of 2.0 ml min−1 with an external solution containing (in mm): 150 NaCl, 5 KCl, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 d‐glucose, adjusted to pH 7.4 with NaOH, to evaluate the action potential properties under the current clamp condition. Thereafter, to isolate K+ currents under the voltage‐clamp condition, as previously described (Yoshimura et al. 2006), neurons were superfused with an external solution containing 110 mm choline‐Cl, 5 mm KOH, 0.03 mm CaCl2, 3.4 mm Mg(OH)2, 10 mm HEPES and 10 mm d‐glucose, adjusted to pH 7.4 with Trizma hydrochloride (340 mOsm), which suppresses Na+ and Ca2+ currents (Yoshimura & de Groat, 1999).
After evaluation of action potential characteristics or isolation of K+ currents, capsaicin sensitivity was evaluated by direct application of capsaicin (500 nm) to the neurons in a voltage‐clamp mode with a holding potential (HP) at −60 mV. Inward shift of holding currents was observed in capsaicin‐sensitive neurons. The stock solution of capsaicin (Sigma‐Aldrich) at 5 mm containing 10% ethanol and 10% Tween 80 was prepared in the external solution described above for action potentials evaluation, and then diluted to the final concentration in the same external solution before experiments. All recordings were made with an Axopatch 700B patch‐clamp amplifier and controlled by Clampex software (Axon Instruments, Sunnyvale, CA, USA). Data were then analysed by pCLAMP software (Axon Instruments). Cell membrane capacitances were obtained by reading the value for whole‐cell input capacitance neutralization directly from the amplifier. Data are presented from neurons that exhibited resting membrane potentials greater than −40 mV and action potentials that overshot 0 mV.
LCM and RNA isolation and real‐time PCR analysis
S1 DRG from formalin‐ (n = 11) or vehicle‐injected rats (n = 10) with dye injections were embedded in Tissue Tek OCT. Sections were cut at 8 μm thickness and mounted on PEN membrane slides (Leica Microsystems, Wetzlar, Germany). LCM was performed using a Leica LMD6000 microscope (Leica Microsystems). Twenty cells that were labelled with either Fast Blue, DiI, or both, and 20 unlabelled cells from S1 DRG sections in each rat were excised and captured in 0.5 ml Eppendorf tube caps, separately. Captured specimens were lysed and RNA isolation, DNase digestion, reverse transcription and real‐time PCR were performed using the Cells Direct TM One‐Step qRT‐PCR Kit (Invitrogen, Carlsbad, CA, USA) for transient receptor potential (TRPV) 1, TRPA1, Kv1.4, P2X2 and P2X3 (Table 1). Gene‐specific primers and Taqman probes were designed with Primer 3 Software (Primer 3, Totowa, NJ, USA). All probes spanned the exon‐exon junction and contained FAM fluorophore and TAMRA quencher. The primer and probe combination were optimized within suitable ranges for efficiency and correlation coefficient using standard curve dilutions and data output on the ABI Step‐One Plus thermocycler (Applied Biosystems). Amplification of cDNA was performed under the following conditions: one cycle of 50°C for 15 min, then 95°C for 2 min, followed by 40 cycles of 15 s at 95°C, then 45 s at 60°C. The reactions were analysed in triplicate and normalized to GAPDH. Real‐time PCR data were analysed by DCp (crossing point) method as R = 2^(Cp sample − Cp control) to generate the relative expression ratio of each target gene relative to GAPDH. The mean value for each group of labelled neurons was then divided by the mean value for the non‐labelled neurons to evaluate the ratio changes in each molecule after prostatic inflammation.
Table 1.
Sequences of primers and probes
| 5ʹ primer | 3ʹ primer | Taqman probe | Efficacy (%) | |
|---|---|---|---|---|
| TRPV1 | CCGGAGAGATCTTGTCTGTG | ACCAGCATGAACAGCGACT | TATTTCCTGCAGAGGCGACCATCC | 95.7 |
| TRPA1 | ATCAGGAGACCCTGCTTCAC | GTTGATGTCTGCTCCCACTG | TGATCATCATGACCTGGCAGACTACC | 101.3 |
| Kv 1.4 | TGACCTACTGCCACAGGATG | GGTTTCGAAGCGTAGACCAG | CAGTGACTGTTGTGAACGCGTGGT | 104.4 |
| P2X2 | GCATCATCACCAGGATCGAG | GTCTTGGAGTCCCCATGGTA | TCAGACGACGACTGTATTGCCGGA | 102.2 |
| P2X3 | GGCTGGATGGAGTTTCTGAG | TTCAGGAGTGTGCGGTACTC | CCCTGGCTACAACTTCAGGTTTGCC | 104.4 |
| GAPDH | AGACAGCCGCATCTTCTTGT | GATACGGCCAAATCCGTTC | GCAGTGCCAGCCTCGTCTCA | 104.0 |
To determine the specificity of the primer/probe, we performed electrophoresis to check that the optical band was amplified without non‐specific bands. We also determined specificity to cDNA to identify that our primer/probe set did not amplify genomic DNA by electrophoresis.
Immunohistochemistry for nerve growth factor (NGF)
The bladder sections (8 μm) obtained from vehicle‐ and formalin‐injected rats (n = 3 each) were directly mounted on coated slides and fixed with 4% paraformaldehyde for 10 min, rinsed with phosphate‐buffered saline (PBS), transferred to 3% hydrogen peroxide for 10 min to block peroxidase activity, and rinsed with distilled water and PBS. Next, the sections were blocked with 10% normal goat serum (Invitrogen, Grand Island, NY, USA) for 30 min. Without rinsing, the tissues were reacted with anti‐NGF antibody (1:1000) (Abcam, Cambridge, MA, USA) for 1 h at room temperature, and washed with PBS. The tissues were then incubated for 30 min with HRP‐labelled polymer anti‐rabbit antibody (Dako, Carpinteria, CA, USA) at room temperature and rinsed with PBS. For streptavidin–horseradish peroxidase detection, the tissues were stained with avidin–biotin complex reagent (Dako) for 10 min at room temperature. Haematoxylin staining was used for counterstaining.
Western blotting for NGF
For the quantitative analysis of NGF expression changes, the bladder tissue homogenate from formalin‐ or saline‐injected rats (n = 6 per group) was dissolved in 2 × sample buffer (Bio Rad, Hercules, CA, USA) with 5% β‐mercaptoethanol and denatured at 95°C for 5 min. Bladder homogenate samples were also obtained from the bladder mucosa of right pelvic nerve‐transected rats with or without intraprostatic formalin injection (n = 7–8 per group). Equivalent amounts of protein (30 μg) were separated by SDS‐PAGE electrophoresis using 10% TBE Precast Gels (Bio Rad) and transferred onto a PVDF membrane (Millipore). Membranes were blocked with 0.5% ECL Blocking Agent (GE Healthcare, Piscataway, NJ, USA) in PBS with 0.1% Tween‐20 at 4°C overnight and incubated for 1 h at room temperature with the anti‐NGF antibody (1:250). The blots were then incubated for a further 1 h at room temperature with an HRP‐conjugated secondary antibody (1:1000; Cell Signalling Technology, Danvers, MA, USA). Specific protein signals were visualized using the ECL Plus Western Blotting Detection System (GE Healthcare). The intensity of the protein bands was semiquantitatively analysed by Image J software.
Statistics
Data are presented as mean values ± standard error of the mean. Student's unpaired t test was used to test for statistical differences between two groups. Holm‐Sidak's multiple comparisons test was used for statistical analyses among groups of more than two. All tests were two‐sided, and P values <0.05 were considered statistically significant. All statistical analyses were performed using SPSS software.
Results
Histopathology
Macroscopically, the formalin‐injected prostate showed a slightly atrophic appearance compared to vehicle‐injected prostate, and there was no difference in the ventral lobe prostate weight between vehicle‐ and formalin‐injected groups (372 ± 12 vs. 326 ± 22 mg, n = 5 each). Compared to the prostate of vehicle‐treated controls, microscopic examination of the formalin‐injected prostate demonstrated mild inflammation in most areas of the ventral lobe prostate and severe inflammation in some areas, with infiltrating leukocytes, expansion of stromal area and atrophic epithelium (Fig. 1 A–C). The bladder wall showed no evidence of inflammation in either group (Fig. 1 D and E).
Figure 1. Histological findings of bladder and prostate sections.
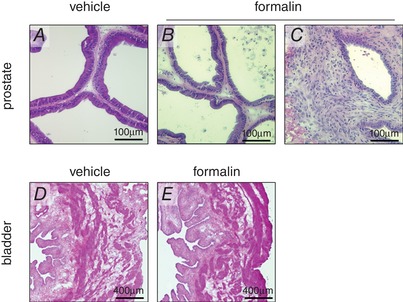
Haematoxylin and eosin staining showed that intra‐prostatic formalin injection caused either a low‐grade inflammation (B) or high‐grade inflammation (C) in the prostate compared to the vehicle‐injected prostate (A). There were no inflammatory changes in the bladder of vehicle (D) or formalin‐treated rats (E). Scale bars; 100 μm (A–C); 400 μm (D and E).
Measurement of inflammatory markers
Levels of IL‐1α, IL‐1β and IL‐6 in the formalin‐injected prostate were significantly increased by 2‐ to 3‐fold compared to vehicle‐treated controls (Fig. 2 A–C). However, in the bladder there were no significant differences between groups. Similarly, MPO activity of the prostate was increased in formalin‐injected rats compared to vehicle‐injected rats; whereas there were no significant differences in the bladder (Fig. 2 D). Taken together with histological findings, these results indicate that formalin‐induced inflammation was restricted to the prostate.
Figure 2. Inflammatory markers.
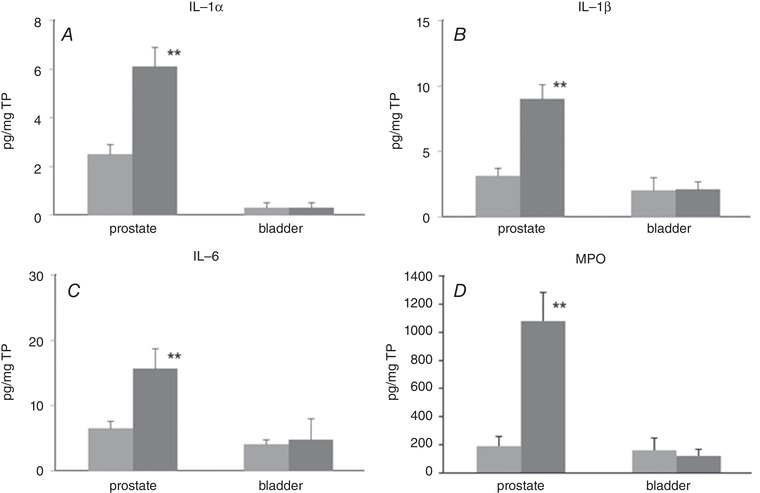
Proinflammatory cytokine levels (A, IL‐1α; B, IL‐1β; C, IL‐6) and MPO activity were measured in the prostate and bladder using ELISA. Light grey columns, vehicle‐treated rats. Dark grey columns, formalin‐treated rats. n = 6 in each group. Columns with bars represent mean ± SEM. ** P < 0.01 between groups. TP, total protein.
Voiding behavioural studies
There was no significant difference in total urine volume, the number of voids, or single voided volume between the two groups before formalin or vehicle treatment (Table 2A). None of these parameters was altered in vehicle‐treated rats at 1 week; however, in formalin‐treated rats the number of voids increased and single voided volume decreased significantly compared to pre‐treatment values.
Table 2.
Urodynamic evaluation
| Vehicle (n = 6) | Formalin (n = 6) | |||
|---|---|---|---|---|
| A. Voiding behaviour | before | After | before | after |
| Urine volume (ml) | 7.8 ± 1.3 | 7.5 ± 0.7 | 9.1 ± 2.2 | 7.9 ± 1.4 |
| No. of micturition (per day) | 18.2 ± 1.8 | 18.5 ± 1.1 | 18.7 ± 2.6 | 25.5 ± 4.3 |
| Single voided volume (ml) | 0.42 ± 0.03 | 0.41 ± 0.03 | 0.46 ± 0.08 | 0.33 ± 0.07** |
| B. Cystometry | Vehicle (n = 6) | Formalin (n = 7) |
|---|---|---|
| Baseline pressure (cmH2O) | 3.6 ± 1.5 | 5.1 ± 1.3 |
| Threshold pressure (cmH2O) | 8.4 ± 1.5 | 9.0 ± 1.2 |
| Peak pressure (cmH2O) | 39.0 ± 2.6 | 34.5 ± 2.1 |
| Intercontraction interval (s) | 1483 ± 26 | 999 ± 57* |
| No. of non‐voiding contractions per voiding cycle | 0.33 ± 0.25 | 4.25 ± 0.71* |
* P < 0.05 between vehicle and formalin groups; ** P < 0.01 between the values before and after injection.
Conscious cystometry
Cystometric parameters were measured during repetitive voiding cycles after a 60‐min acclimation period. The mean intercontraction interval was 999 ± 57 s in the formalin‐injected group vs. 1483 ± 26 s in vehicle‐injected controls (P < 0.05), and the number of non‐voiding contractions (NVC) during the storage phase was increased in formalin‐injected rats vs. vehicle‐injected rats (4.25 ± 0.71 vs. 0.33 ± 0.25/voiding cycle) (Fig. 3 and Table 2B). Baseline pressure, pressure threshold for voiding and peak voiding pressure were not different between two groups. In addition, in rats with transection of the right pelvic nerve, bladder overactivity evident as increased NVCs was not induced after formalin injection into the right ventral lobe of prostate, but was still observed after the left lobe injection (Fig. 4).
Figure 3. Continuous cystometry.
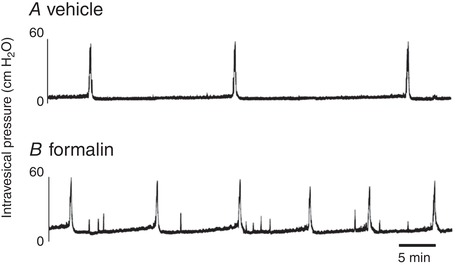
Representative traces of cystometry under an awake condition in vehicle‐ (A) or formalin‐injected rats (B). Cystometric parameter analyses showed that intercontraction intervals rats were shorter and the number of non‐voiding contraction during urine storage in formalin‐injected was greater than those in vehicle‐injected rats, indicating bladder overactivity after prostatic inflammation.
Figure 4. Single cystometry in rats with unilateral pelvic nerve transection.
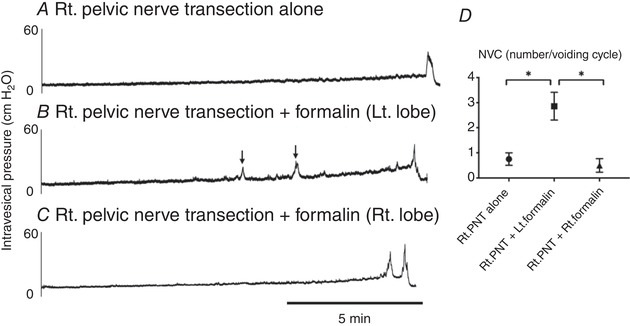
Representative traces of cystometry under an awake condition in rats with transection of the right pelvic nerve, which received no additional treatment (A), formalin injection into the contralateral side (left) of the ventral prostate lobe (B) or formalin injection into the ipsilateral side (right) of the ventral prostate lobe (C). D, the comparison of the number of non‐voiding contraction (NVC) per voiding cycle shows that NVC are more frequent (P < 0.05) in pelvic nerve‐transected rats with formalin injection into the left prostate lobe (Rt. PNT + Lt. formalin) compared to rats with Rt. PNT alone or PNT rats with the right lobe formalin injection (Rt. PNT + Rt. formalin) (n = 7–8 rats per group).
Distribution of fluorescent‐labelled neurons
Fast Blue‐labelled bladder afferent and DiI‐labelled prostate afferent neurons were identified as blue‐ and red‐coloured cells, respectively, in DRG sections using appropriate filters (Fig. 5). Some neurons were labelled with both Fast Blue and DiI, indicative of dichotomized neurons. Dye‐labelled neurons were mainly observed in L1, L6 and S1 DRG, and these distributions were similar between vehicle‐ and formalin‐injected rats, without significant differences in the number of labelled neurons per DRG section between two groups (data not shown). Double‐labelled (e.g. DiI‐ and Fast Blue‐positive) afferent neurons innervating both prostate and bladder represented 18.4% of the total number of Dil‐ and Fast Blue‐labelled neurons in thoraco‐lumbar DRG and 19.5% in lumbo‐sacral DRG.
Figure 5. Fluorescent microscopic images of DRG sections.
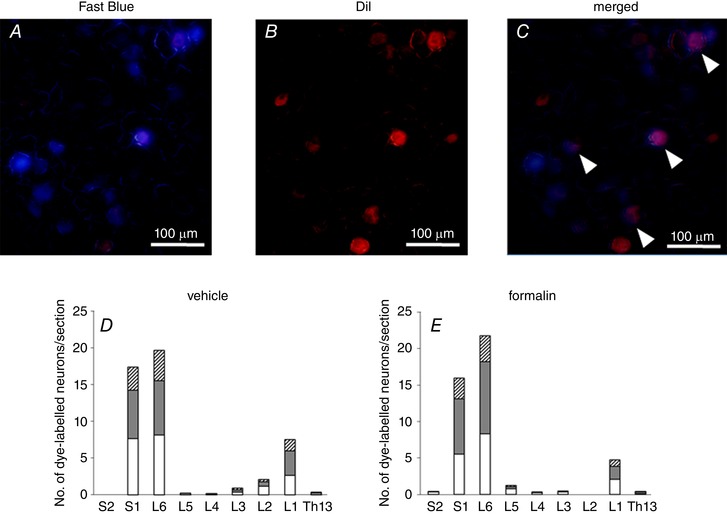
Representative fluorescent microscopic images of DRG neuron labelled with Fast Blue (A) and DiI (B) injected into the bladder wall and the ventral lobes of prostate, respectively. C, a merged image of A and B showed that some neurons were labelled with both dyes, as indicated by arrowheads. Scale bars; 100 μm. The number of neurons dye‐labelled with Fast Blue (white column), DiI (grey column) and both (hatched column) in sections of Th13 to S2 DRG from vehicle‐injected (D) and formalin‐injected rats (E) was quantified (6 DRG from 3 rats per groups). Columns represent the mean number of dye‐labelled neurons per section.
Electrophysiology
It has been reported that C‐fibre bladder afferent hyperexcitability is an important contributing factor to bladder overactivity in various pathophysiological conditions such as bladder outlet obstruction, spinal cord injury and bladder inflammation (de Groat et al. 2015). Therefore, this study focused on the changes in electrophysiological properties of the C‐fibre population of prostate and/or bladder afferent neurons. Because our previous study demonstrated that the majority (80%) of C‐fibre bladder afferent neurons are sensitive to capsaicin, while only a small population (5%) of Aδ‐fibre bladder neurons are capsaicin sensitive (Yoshimura et al. 1998), bladder afferent neurons that exhibited capsaicin (500 nm)‐induced inward currents in voltage‐clamp recordings at the end of patch‐clamp recordings are included in the analyses in the present study.
In preliminary experiments, we found similar changes in firing pattern of action potentials in DiI‐labelled prostate afferent neurons and double‐labelled prostate/bladder afferent neurons after prostatic inflammation. Therefore, we here described the electrophysiological results obtained from double‐labelled afferent neurons. In capsaicin‐sensitive, double‐labelled afferent neurons innervating both bladder and prostate, the resting membrane potential did not differ between vehicle‐ and formalin‐injected rats (Table 3 and Fig. 6). However, the mean threshold for eliciting action potentials in formalin‐injected rats (−28.3 ± 1.4 mV) was significantly lower than that in control rats (−19.5 ± 1.1 mV); and the number of action potentials during 800 ms membrane depolarization in rats with prostatic inflammation (5.1 ± 0.7 spikes) was significantly higher than that in control rats (1.2 ± 0.1 spikes), when the current intensity was set to the value just above the threshold for inducing a single action potential with a 50‐ms depolarization. Furthermore, the mean spike amplitude (46.3 ± 2.6 mV) was significantly higher and the mean spike duration (3.6 ± 0.4 ms) was significantly shorter than the measurements in control rats (36.3 ± 2.2 mV and 6.4 ± 0.9 ms, respectively). These results indicate that capsaicin‐sensitive, double‐labelled dichotomized afferent neurons become hyperexcitable in rats with prostatic inflammation. In addition, the cell input capacitance of capsaicin‐sensitive double‐labelled afferent neurons from formalin‐injected rats (37.5 ± 3.2 pF) was significantly greater than in neurons from control rats (27.0 ± 1.5 pF), indicating that prostatic inflammation induced somal hypertrophy of these neurons.
Table 3.
Action potential characteristics of capsaicin‐sensitive DRG neurons
| Double‐labelled afferent | Bladder afferent | |||
|---|---|---|---|---|
| Vehicle | Formalin | Vehicle | Formalin | |
| Total no. of neurons/rats | 8/5 | 7/5 | 8/5 | 10/5 |
| Diameter (μm) | 28.4 ± 0.8 | 30.4 ± 1.0 | 27.7 ± 1.1 | 28.9 ± 0.6 |
| Input capacitance (pF) | 27.0 ± 1.5 | 37.5 ± 3.2* | 30.8 ± 2.6 | 33.3 ± 2.4 |
| Membrane potential (mV) | ||||
| Resting | −50.3 ± 1.2 | −47.2 ± 1.4 | −50.8 ± 0.9 | −48.6 ± 0.8 |
| Spike threshold | −19.5 ± 1.1 | −28.3 ± 1.4** | −21.6 ± 2.4 | −28.7 ± 1.4* |
| Peak | 36.3 ± 2.2 | 46.3 ± 2.6* | 38.2 ± 3.7 | 39.4 ± 2.6 |
| Spike duration (ms) | 6.4 ± 0.9 | 3.6 ± 0.4* | 5.6 ± 0.7 | 4.4 ± 0.4 |
| No. of action potentials during 800 ms depolarization | 1.2 ± 0.1 | 5.1 ± 0.7** | 1.5 ± 0.3 | 3.5 ± 0.4** |
* P < 0.05; ** P < 0.01 between vehicle and formalin groups.
Figure 6. Action potential characteristics of capsaicin‐sensitive double‐labelled and Fast Blue‐labelled afferent neurons.
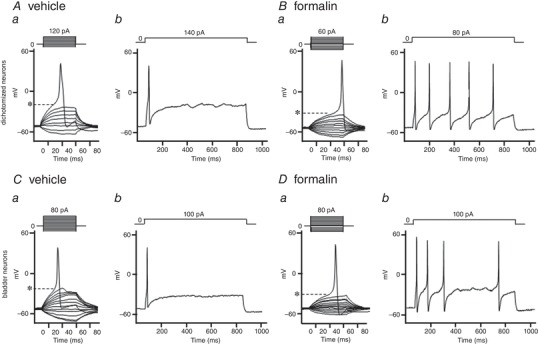
Representative recordings of action potentials in double‐labelled, dichotomized afferent neurons from vehicle‐injected (A) and formalin‐injected rats (B), and Fast Blue‐labelled bladder afferent neurons from vehicle‐injected (C) and formalin‐injected rats (D). The thresholds for eliciting action potentials (panels a of A–D) in formalin‐injected rats was lower than those in vehicle‐injected control rats (Aa vs. Ba or Ca vs. Da). The number of action potentials during 800 ms membrane depolarization (panels b of A–D) in formalin‐injected rats was greater than that in control rats (Ab vs. Bb or Cb vs. Db). *Firing thresholds of action potentials.
In capsaicin‐sensitive bladder afferent neurons that were labelled only by Fast Blue, the resting membrane potential was not affected by prostate inflammation; however, the mean threshold for eliciting action potentials in formalin‐injected rats (−28.7 ± 1.4 mV) was significantly lower than that in control rats (−21.6 ± 2.4 mV), and the number of action potentials during 800 ms membrane depolarization in formalin‐injected rats (3.5 ± 0.4 spikes) was significantly greater than that in control rats (1.5 ± 0.3 spikes).
We previously reported that the reduction of slow KA currents is involved in increased excitability of capsaicin‐sensitive bladder afferent neurons in rat models of cystitis or spinal cord injury (Yoshimura & de Groat, 1999; Hayashi et al. 2009; Takahashi et al. 2013). Because we found similar changes in firing pattern of action potentials in both prostate/bladder and bladder afferent neurons, we further evaluated the properties of potassium currents in bladder afferent neurons from vehicle‐ or formalin‐injected rats. An estimate of the density of slow KA currents in these neurons was obtained by measuring the difference in the currents activated by a depolarizing voltage pulse from −120 mV and −40 mV holding potentials (Fig. 7 A and B). This method is useful because our previous studies showed that slow KA currents in capsaicin‐sensitive C‐fibre bladder afferent neurons were activated by depolarizing voltage steps from hyperpolarized membrane potentials but were almost completely inactivated when the membrane potential was maintained at a depolarized level of more than −40 mV (Yoshimura & de Groat, 1999; Takahashi et al. 2013). As shown in Fig. 7 C, peak current densities of slow KA currents evoked by depolarization up to 30 mV, which was calculated from the difference in K+ currents activated from holding potentials of −40 and −120 mV, were significantly smaller (P < 0.05) at depolarizing pulses greater than −20 mV in neurons from formalin‐injected rats compared to those from vehicle‐injected rats. However, the peak current density of sustained KDR currents in capsaicin‐sensitive bladder afferent neurons was not different between formalin‐ and vehicle‐injected rats (Fig. 7 D). Taken together, these results indicate that capsaicin‐sensitive bladder afferent neurons become hyperexcitable at least in part due to the reduction in slow KA current activity following prostatic inflammation.
Figure 7. Changes of K+ currents in capsaicin‐sensitive bladder afferent neurons.
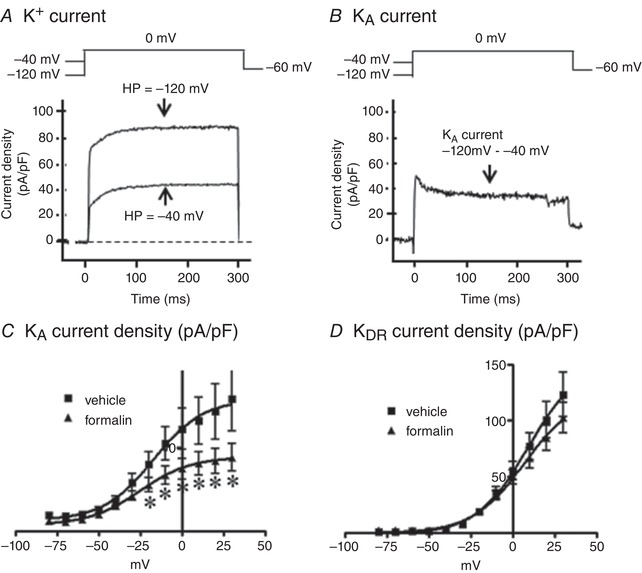
A, representative recordings in a capsaicin‐sensitive bladder afferent neuron from a control rat showing superimposed outward K+ currents evoked by depolarizing voltage pulses to 0 mV from holding potentials (HPs) of –120 and –40 mV and sustained delayed rectifier‐type K+ (sustained KDR) currents evoked by depolarization from –40 mV HP. B, representative recordings in a capsaicin‐sensitive bladder afferent neuron from a control rat showing slow decaying A‐type K+ (slow KA) currents obtained by subtracting K+ currents evoked by depolarization from HP of –40 to 0 mV from the K+ currents evoked by depolarization from HP of –120 mV to 0 mV. C, I–V relationships of slow KA currents showing that the density of KA currents in capsaicin‐sensitive bladder afferent neurons from formalin‐injected rats (n = 9 cells from 9 rats) was significantly reduced compared to that from vehicle‐injected rats (n = 9 cells from 9 rats). D, I–V relationships of sustained KDR currents showing that the density of KDR currents was not significantly different between two groups (n = 10 cells from 8 rats each). * P < 0.05 compared to vehicle‐injected rats (Student's unpaired t test).
mRNA expression in DRG neurons
We measured mRNA levels of TRPV1, TRPA1, P2X2, P2X3 and the Kv1.4 potassium channel subunit of Fast Blue‐, DiI‐, double‐labelled and non‐labelled neurons obtained by LCM from formalin‐ and vehicle‐injected rats. The Kv1.4 α‐subunit was chosen because we have previously shown that the decreased expression of this subunit is associated with increased excitability and a reduction in slow KA channel activity in bladder afferent neurons after cystitis or spinal cord injury (Hayashi et al. 2009; Takahashi et al. 2013). In vehicle‐injected rats, there were no significant changes in mRNA levels of any of these channels in dye‐labelled neurons when compared to non‐labelled neurons (Fig. 8). In contrast, TRPV1, TRPA1 and P2X2 were up‐regulated, and Kv1.4 was down‐regulated significantly in dye‐labelled cells vs. non‐labelled cells in formalin‐injected rats. The P2X3 mRNA level in labelled cells of either group was not altered compared to the level of non‐labelled cells.
Figure 8. Relative mRNA levels in the afferent neurons.
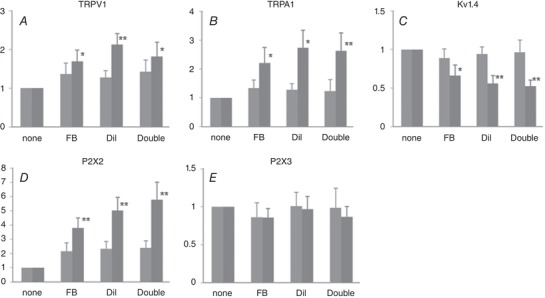
Fast Blue (FB)‐, DiI‐, and double‐labelled neurons in DRG sections were dissected by LCM, and mRNA levels of TRPV1 (A), TRPA1 (B), Kv1.4 (C), P2X2 (D) and P2X3 (E) were measured by qRT‐PCR and expressed relative to the levels expressed in non‐labelled neurons. Light grey columns, vehicle‐treated rats (n = 10). Dark grey columns, formalin‐treated rats (n = 11). Columns with bars represent means ± SEM. * P < 0.05, ** P < 0.01 compared to non‐labelled neurons.
NGF expression in the bladder
In the bladder of formalin‐injected rats, immunohistochemistry showed increased expression of NGF, which was most obvious in the urothelium, but also evident in the submucosal layer (Fig. 9 A–D). Western blotting was then performed for quantitative analyses and showed that NGF expression (normalized to β‐actin) in the whole bladder was significantly higher in the formalin‐injected rats compared to vehicle‐injected rats (2.22 ± 0.37 vs. 1.00 ± 0.23, P = 0.019). In addition, in rats with the right pelvic nerve transection (PNT), formalin injection into the left ventral lobe of prostate induced NGF upregulation in the bladder mucosa compared to PNT alone rats (1.59 ± 0.13 vs. 1.00 ± 0.04, P = 0.009), which was prevented when formalin was injected into the right ventral lobe of prostate (0.98 ± 0.09 vs. 1.00 ± 0.04, P = 0.865).
Figure 9. NGF expression in the bladder.
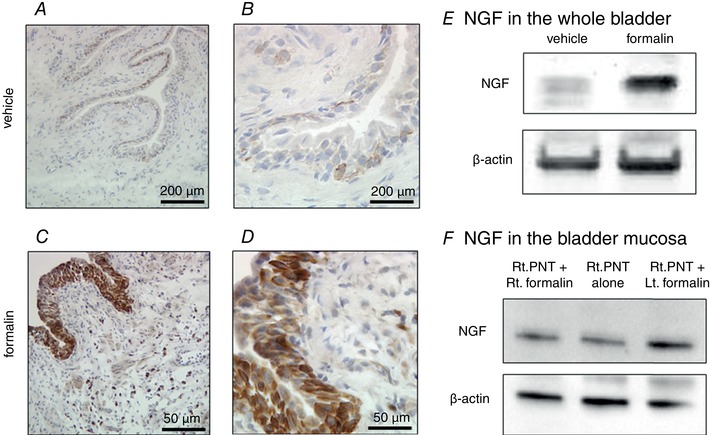
NGF expression was markedly enhanced in formalin‐injected rats, especially in the urothelium (C, D), compared to the vehicle‐injected controls (A, B). Scale bars; 200 μm (A, C) and 50 μm (B, D). Western blotting in bladder tissue extracts showed upregulation of NGF expression in the formalin‐injected compared to vehicle‐injected rats (E). F, in rats with the right pelvic nerve transection (PNT), formalin injection into the left ventral lobe of prostate induced NGF upregulation in the bladder mucosa (Rt. PNT +Lt. formalin) compared to rats with PNT alone (Rt. PNT alone); however, increased NGF expression was not seen when formalin was injected into the right ventral lobe of prostate (Rt. PNT + Rt. formalin).
Discussion
Our detailed molecular, cellular and electrophysiological analysis following intra‐prostatic injection of formalin in rats revealed a number of potential mechanisms that could contribute to various functional outcomes of the resulting prostatic inflammation. For example, formalin‐induced non‐bacterial inflammation after intra‐prostatic injection is limited to the prostate, without an extension to the bladder. Furthermore, prostatic inflammation induces bladder overactivity as evidenced by reductions in volume per void and intercontraction intervals. In addition, because the prostatic weight was not altered after formalin injection, prostate enlargement was not likely to be involved in bladder functional changes in this model. We also identified a considerable number of DRG neurons with dichotomized afferent nerves that innervate both prostate and bladder that are distinguished by a number of responses to inflammation. The proportion of DRG neurons with dichotomized afferents labelled by dye tracers from the prostate and the bladder (18–19%) in this study was comparable to those previously reported in rats (14%) (Chen et al. 2010) and mice (17%) (Schwartz et al. 2016). Specifically, prostatic inflammation induces upregulation of TRPV1, TRPA1 and P2X2 expression and downregulation of Kv1.4 not only in prostate afferent neurons, but also in dichotomized afferent neurons innervating both organs, as well as bladder afferent neurons that do not innervate the prostate. Prostatic inflammation induces functional changes such as hyperexcitability of capsaicin‐sensitive C‐fibre afferent neurons in association with downregulation of slow KA current density. Finally, NGF expression is increased especially in the bladder urothelium after prostatic inflammation. We also confirmed that bladder overactivity and NGF upregulation in the bladder mucosa after prostatic inflammation are nerve‐mediated and dependent on activation of the pelvic nerve, which contains bladder and prostate‐innervating afferents. Taken together, our results suggest that prostatic inflammation induces bladder overactivity in association with NGF upregulation in the bladder as well as changes in ion channel expression and excitability of bladder afferent neurons due to afferent cross‐sensitization from the prostate to bladder, possibly through activation of dichotomized afferent pathways that innervate both organs. The upregulation of TRPV1, TRPA1 and P2X2 channels, which are known to respond to noxious stimuli (Sun et al. 2010; Lee et al. 2016b), in combination with downregulation of the Kv1.4 potassium channel subunit, which can form slow KA channels and suppress neuronal excitability (Hayashi et al. 2009; Takahashi et al. 2013), could lead to hyperexcitability of bladder afferent pathways, thereby inducing increased urinary frequency via activation of C‐fibre afferent pathways after prostatic inflammation.
Based on these results of this study, the following mechanisms could be involved in bladder afferent activation after prostatic inflammation. When dichotomized afferents are irritated by intra‐prostatic inflammation, it is possible that afferent nerves in the bladder are also be activated via action potentials propagating antidromically into the bladder through dichotomized afferents innervating both the bladder and prostate. Neurotransmitters released from afferent nerve terminals distributed in or near the urothelium could induce NGF expression, which would then stimulate and sensitize bladder afferent pathways (Ochodnicky et al. 2012). Previous studies showed that NGF expression is significantly increased in the bladder urothelium without neutrophil infiltration following colitis (Kawamorita et al. 2016). Taken together, these sequential events including urothelial NGF upregulation through activation of dichotomized afferents innervating the prostate and the bladder may be involved in the mechanism by which prostatic inflammation contributes to storage LUTS in men with symptomatic BPH. In addition, other mechanisms such as spinal convergence of afferent nerves from different pelvic organs, increased urothelial permeability, mast cell activation in the bladder and/or neurogenic inflammation due to neuropeptide release from afferent nerve terminals have also been proposed in another model of pelvic organ cross‐sensitization induced by experimental colitis (Grundy & Brierley, 2018), although further studies are needed to investigate whether these mechanisms similarly contribute to bladder overactivity induced by prostate‐to‐bladder cross‐sensitization. In addition, the rat model of prostatic inflammation used in this study is not the direct model of BPH; however, we have shown that this rat model exhibits the upregulation of androgen receptor‐responsive genes and TGF‐β‐related genes in the prostate (Funahashi et al. 2014), which is similarly seen in human BPH tissues (O'Malley et al. 2009), and that increased IL‐18 expression induced by inflammasome activation is similarly found in the formalin‐induced rat prostatic inflammation model (Kashyap et al. 2015) and in human BPH specimens with inflammatory cell infiltration (Tyagi et al. 2017). Thus, the model of non‐bacterial prostatic inflammation used in this study seems useful for investigating inflammation‐related events that occur in human BPH with tissue inflammation.
Afferent neurons express various types of receptors and ion channels, including TRP channels, purinergic, muscarinic, endothelin, neurotrophic factor and estrogen receptors. TRPV1 and TRPA1 receptors overlap considerably in C‐fibre afferent pathways in rats (La et al. 2011), and their functional interaction has been reported (Salas et al. 2009; Staruschenko et al. 2010). Also, approximately 90% of the bladder afferent neurons are excited by ATP, which induces depolarization and firing mainly through activation of P2X2/3 receptors rather than P2X3 receptors (Zhong et al. 2003; Dang et al. 2005). Thus, the increased expression of TRPV1, TRPA1 and P2X2 receptors found in this study in both dichotomized and bladder afferent neurons in rats with prostatic inflammation, which could enhance responses of bladder afferents to noxious stimuli such as ATP, could contribute to C‐fibre bladder afferent hyperexcitability and bladder overactivity following prostatic inflammation. Only a few previous studies have reported changes in the expression of these receptors in visceral afferents, probably because only a limited number of neurons in L6‐S1 DRG innervate the pelvic viscera (Yoshimura et al. 1998; Christianson et al. 2007; Chen et al. 2010). Thus, the LCM method in combination with axonal tracing was essential in our experiments for detecting the pathology‐induced plasticity in prostate and bladder afferent neurons. However, since protein levels were not analysed in this study because of the low amount of proteins in neurons collected by LCM, further analyses using immunohistochemistry with validation of antibody specificity will be necessary.
The whole‐cell patch‐clamp recordings described herein demonstrated that prostatic inflammation induced hyperexcitability of bladder and dichotomized afferent neurons (i.e. prostate and bladder double‐innervating). Under the current‐clamp recording conditions, we observed an increase of cell input capacitance, which corresponds to cell surface area, and shortening of spike duration in the double‐labelled afferent neurons, but not the bladder afferent neurons, of the formalin‐injected rats. Thus, we speculate that the direct sensitization of double‐labelled neurons innervating the inflamed prostate leads to more profound changes in these parameters compared to bladder afferent neurons, in which sensitization was induced indirectly after prostatic inflammation. Furthermore, it is likely that changes of excitability in bladder afferent neurons are mediated at least in part by reduced expression of the Kv1.4 voltage‐gated potassium channel subunit and decreased slow KA current density. Among the many types of potassium channels, Kv1.4 is a major molecular subunit of the A‐type potassium channel, which suppresses neuronal depolarization (Zhong et al. 2003). We previously reported that a reduction in Kv1.4 subunit expression detected by immunohistochemistry is associated with reduced A‐type potassium channel activity and hyperexcitability of capsaicin‐sensitive C‐fibre bladder afferent neurons in rats with hydrochloric acid‐induced cystitis (Hayashi et al. 2009) and spinal cord injury (Takahashi et al. 2013). Furthermore, NGF application induces hyperexcitability of bladder afferent neurons by decreasing A‐type potassium channel activity in normal rats (Yoshimura et al. 2006). Thus, this study suggests that the reduction in Kv1.4 expression, which is possibly induced by NGF upregulation in the bladder, contributes directly to capsaicin‐sensitive C‐fibre bladder afferent hyperexcitability induced by prostate‐to‐bladder afferent cross‐sensitization following prostatic inflammation, although further studies are needed to confirm that NGF upregulation is a mediator in this process.
In conclusion, chemically induced inflammation localized in the prostate induces bladder overactivity and upregulation of NGF expression in the bladder urothelium. Bladder afferent function was enhanced as indicated by TRPV1, TRPA1 and P2X2 upregulation, Kv1.4 downregulation, and cell hyperexcitability. Since there is a clinical association between prostatic inflammation and BPH‐induced LUTS, the prostate‐to‐bladder cross‐sensitization through primary afferent pathways in the pelvic nerve, which contains dichotomized afferents, is a potentially important mechanism contributing to bladder overactivity and afferent hyperexcitability after prostatic inflammation, which can cause storage LUTS in symptomatic BPH patients.
Additional information
Competing interests
The authors have declared that no conflict of interest exists.
Author contributions
Y.F., R.T., Z.W., D.B.D. and N.Y. created the research design. Y.F., R.T., T.S., E.T. and S.M. performed experiments and analysed data. Y.F., R.T., T.S., P.T. and N.Y. interpreted the results of experiments. Y.F., R.T., W.C.dG. and N.Y. drafted the manuscript. Y.F., R.T., T.S., E.T., S.M., J.N., Z.W., D.B.D., W.C.dG., P.T. and N.Y. approved the final version of manuscript. All authors agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by a grant from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney (U54 DK112079).
Biography
Yasuhito Funahashi and Ryosuke Takahashi performed the experiments in the laboratory of Dr Yoshimura at the University of Pittsburgh as Research Associates. The laboratory focuses primarily on functional and physiological changes of the lower urinary tract and has published extensively on how pathophysiological conditions including spinal cord and peripheral nerve injuries, inflammation and diabetes mellitus trigger hyperexcitability of the visceral neuronal pathways innervating the lower urinary tract and how neurotrophic factors control neuronal activities.

Y. Funahashi and R. Takahashi contributed equally as co‐first authors to this study.
Edited by: Kim Barrett & Weifang Rong
This is an Editor's Choice article from the 1 April 2019 issue.
References
- Aydin M, Wang HZ, Zhang X, Chua R, Downing K, Melman A & DiSanto ME (2012). Large‐conductance calcium‐activated potassium channel activity, as determined by whole‐cell patch clamp recording, is decreased in urinary bladder smooth muscle cells from male rats with partial urethral obstruction. BJU Int 110, E402–E408. [DOI] [PubMed] [Google Scholar]
- Beppu M, Araki I, Yoshiyama M, Du S, Kobayashi H, Zakoji H & Takeda M (2011). Bladder outlet obstruction induced expression of prostaglandin E2 receptor subtype EP4 in the rat bladder: a possible counteractive mechanism against detrusor overactivity. J Urol 186, 2463–2469. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR & Gebhart GF (2010). Visceral organ cross‐sensitization – an integrated perspective. Autonom Neurosci 153, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu X, Liu J, Tang W, Zhao T & Zhang J (2010). Distribution of convergent afferents innervating bladder and prostate at dorsal root ganglia in rats. Urology 76, 764.e1–6. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO & Pezzone MA (2007). Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain 128, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JH, Yu JH, Sung LH, Noh CH & Chung JY (2012). Effect of prostatitis on lower urinary tract symptoms: retrospective analysis of prostate biopsy tissue. Korean J Urol 53, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K & Gebhart GF (2005). Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci 25, 3973–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Griffiths D & Yoshimura N (2015). Neural control of the lower urinary tract. Compr Physiol 5, 327–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibbi B, Penna G, Morelli A, Adorini L & Maggi M (2010). Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl 33, 475–488. [DOI] [PubMed] [Google Scholar]
- Funahashi Y, Hattori R, Matsukawa Y, Komatsu T, Sassa N & Gotoh M (2011). Clinical efficacy of a loading dose of naftopidil for patients with benign prostate hyperplasia. World J Urol 29, 225–231. [DOI] [PubMed] [Google Scholar]
- Funahashi Y, O'Malley KJ, Kawamorita N, Tyagi P, DeFranco DB, Takahashi R, Gotoh M, Wang Z & Yoshimura N (2014). Upregulation of androgen‐responsive genes and transforming growth factor‐beta1 cascade genes in a rat model of non‐bacterial prostatic inflammation. Prostate 74, 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy L & Brierley SM (2018). Cross‐organ sensitization between the colon and bladder: to pee or not to pee? Am J Physiol Gastrointest Liver Physiol 314, G301–G308. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Takimoto K, Chancellor MB, Erickson KA, Erickson VL, Kirimoto T, Nakano K, de Groat WC & Yoshimura N (2009). Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1.4 alpha‐subunit in rats with cystitis. Am J Physiol Regul Integr Comp Physiol 296, R1661–R1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap M, Pore S, Wang Z, Gingrich J, Yoshimura N & Tyagi P (2015). Inflammasomes are important mediators of prostatic inflammation associated with BPH. J Inflamm (Lond) 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamorita N, Yoshikawa S, Kashyap M, Tyagi P, Arai Y, Chancellor MB & Yoshimura N (2016). Liposome based intravesical therapy targeting nerve growth factor ameliorates bladder hypersensitivity in rats with experimental colitis. J Urol 195, 1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SO, Oh BS, Chang IY, Song SH, Ahn K, Hwang EC, Oh KJ, Kwon D & Park K (2011). Distribution of interstitial cells of Cajal and expression of nitric oxide synthase after experimental bladder outlet obstruction in a rat model of bladder overactivity. Neurourol Urodyn 30, 1639–1645. [DOI] [PubMed] [Google Scholar]
- La JH, Schwartz ES & Gebhart GF (2011). Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore‐domain K+ channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience 186, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yang G & Bushman W (2015). Prostatic inflammation induces urinary frequency in adult mice. PloS One 10, e0116827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yang G, Xiang W & Bushman W (2016a). Retrograde double‐labeling demonstrates convergent afferent innervation of the prostate and bladder. Prostate 76, 767–775. [DOI] [PubMed] [Google Scholar]
- Lee WC, Wu CC, Chuang YC, Tain YL & Chiang PH (2016b). Ba‐Wei‐Die‐Huang‐Wan (Hachimi‐jio‐gan) can ameliorate cyclophosphamide‐induced ongoing bladder overactivity and acidic adenosine triphosphate solution‐induced hyperactivity on rats prestimulated bladder. J Ethnopharmacol 184, 1–9. [DOI] [PubMed] [Google Scholar]
- Liao CH, Chung SD & Kuo HC (2011). Serum C‐reactive protein levels are associated with residual urgency symptoms in patients with benign prostatic hyperplasia after medical treatment. Urology 78, 1373–1378. [DOI] [PubMed] [Google Scholar]
- Lin WY, Guven A, Juan YS, Neuman P, Whitbeck C, Chichester P, Kogan B, Levin RM & Mannikarottu A (2008). Free radical damage as a biomarker of bladder dysfunction after partial outlet obstruction and reversal. BJU Int 101, 621–626. [DOI] [PubMed] [Google Scholar]
- Malykhina AP (2007). Neural mechanisms of pelvic organ cross‐sensitization. Neuroscience 149, 660–672. [DOI] [PubMed] [Google Scholar]
- Mizoguchi S, Mori K, Wang Z, Liu T, Funahashi Y, Sato F, DeFranco DB, Yoshimura N & Mimata H (2017). Effects of estrogen receptor beta stimulation in a rat model of non‐bacterial prostatic inflammation. Prostate 77, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somerville MC & Rittmaster RS (2008). The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol 54, 1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley KJ, Dhir R, Nelson JB, Bost J, Lin Y & Wang Z (2009). The expression of androgen‐responsive genes is up‐regulated in the epithelia of benign prostatic hyperplasia. Prostate 69, 1716–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochodnicky P, Cruz CD, Yoshimura N & Cruz F (2012). Neurotrophins as regulators of urinary bladder function. Nat Rev Urol 9, 628–637. [DOI] [PubMed] [Google Scholar]
- Robert G, Descazeaud A, Nicolaiew N, Terry S, Sirab N, Vacherot F, Maille P, Allory Y & de la Taille A (2009). Inflammation in benign prostatic hyperplasia: a 282 patients' immunohistochemical analysis. Prostate 69, 1774–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas MM, Hargreaves KM & Akopian AN (2009). TRPA1‐mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci 29, 1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, La JH, Young EE, Feng B, Joyce S & Gebhart GF (2016). Chronic prostatitis induces bladder hypersensitivity and sensitizes bladder afferents in the mouse. J Urol 196, 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staruschenko A, Jeske NA & Akopian AN (2010). Contribution of TRPV1‐TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem 285, 15167–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Li Q, Dong L & Rong W (2010). Ion channel and receptor mechanisms of bladder afferent nerve sensitivity. Auton Neurosci 153, 26–32. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Yoshizawa T, Yunoki T, Tyagi P, Naito S, de Groat WC & Yoshimura N (2013). Hyperexcitability of bladder afferent neurons associated with reduction of Kv1.4 alpha‐subunit in rats with spinal cord injury. J Urol 190, 2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi P, Kashyap M, Gingrich J, Wang Z & Yoshimura N (2017). Inflammasome activation leads to IL‐18 expression in prostatic inflammation associated with BPH. J Urol 197, e211–212. [Google Scholar]
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC & Seki S (2006). Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 26, 10847–10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N & de Groat WC (1997). Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J Physiol 503, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N & de Groat WC (1999). Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19, 4644–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Erdman SL, Snider MW & de Groat WC (1998). Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience 83, 633–643. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Banning AS, Cockayne DA, Ford AP, Burnstock G & McMahon SB (2003). Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience 120, 667–675. [DOI] [PubMed] [Google Scholar]


