Abstract
Key points
Spinal cord lamina I neurons receiving dense input from nociceptors and projecting to the parabrachial area at the ponto‐mesencephalic junction form the major ascending pain‐related pathway in rodents.
Lamina I spinoparabrachial (SPB) neurons have never been characterized in mice, despite the growing and extensive use of this species to understand the contribution of lamina I SPB neurons in chronic pain.
The electrophysiological properties of lamina I SPB neurons recorded here in anaesthetized mice are comparable to those of rat or cat, forming a nociceptive and thermoreceptive pathway. It was confirmed ‘on line’ that lamina I SPB neurons that normally encode noxious stimuli can receive input from low threshold mechanoreceptors in certain conditions. The present work indicates that the study of lamina I SPB neurons in vivo could take advantage of the use of genetically modified mice.
Abstract
Ongoing studies investigating the role of lamina I projection neurons in the generation of chronic pain are mainly based on the use of genetically modified mice. However, lamina I projection neurons have never been physiologically characterized in this species. The present work aimed to fill this gap, and to assess the effect of spinal ‘disinhibition’ that may occur in chronic pain states on the responses of these neurons to light touch. Seventy lamina I spinoparabrachial (SPB) neurons were characterized in anaesthetized mice. These neurons showed low central conduction velocities (<12.4 m s−1) and wide range of responses. Fifty‐six neurons responded equally to noxious mechanical and thermal (heat) stimuli (16% responded consistently to light touch). Modality‐specific neurons responded preferentially to thermal (cold) stimuli (n = 10) and pinch (n = 2), or specifically to heat (n = 2). Spinal bicuculline and strychnine application induced responses to brush in half of the neurons tested, confirming directly the potential connection between low threshold mechanoreceptors and nociceptive‐specific neurons, responsible for mechanical allodynia. Remarkably, the effect of the treatment was highly variable and apparently independent of the initial profile of the neurons. The present data confirm that mice lamina I SPB neurons have the expected characteristics to form a nociceptive and thermoreceptive pathway, but they constitute a highly heterogeneous group. The differential effect of spinal disinhibition observed here suggests that a subgroup of lamina I SPB neurons might be responsible for abnormal pain in pathological conditions, and emphasizes the importance of in vivo recording, a neglected approach.
Keywords: pain, anaesthesia, extracellular recording, allodynia
Key points
Spinal cord lamina I neurons receiving dense input from nociceptors and projecting to the parabrachial area at the ponto‐mesencephalic junction form the major ascending pain‐related pathway in rodents.
Lamina I spinoparabrachial (SPB) neurons have never been characterized in mice, despite the growing and extensive use of this species to understand the contribution of lamina I SPB neurons in chronic pain.
The electrophysiological properties of lamina I SPB neurons recorded here in anaesthetized mice are comparable to those of rat or cat, forming a nociceptive and thermoreceptive pathway. It was confirmed ‘on line’ that lamina I SPB neurons that normally encode noxious stimuli can receive input from low threshold mechanoreceptors in certain conditions. The present work indicates that the study of lamina I SPB neurons in vivo could take advantage of the use of genetically modified mice.
Introduction
Projection neurons from lamina I of the spinal cord to the parabrachial (PB) area are considered to form a major ascending pain pathway in all mammals, and the major ascending pain pathway in rodents. In rats, this is supported by solid anatomical and electrophysiological data. Retrograde labelling from supraspinal sites indicate that the PB area is the major target for lamina I projection neurons (Spike et al. 2003; Al‐Khater et al. 2008; Polgár et al. 2010). Electrophysiological recordings have shown that most lamina I spinoparabrachial (SPB) neurons respond specifically to noxious stimuli (Bester et al. 2000 a; Keller et al. 2007; Andrew, 2009), with the proportion of lamina I SPB neurons responding to innocuous stimuli ranging from 10% to 25% in these three studies. Importantly, anatomical and electrophysiological data suggest that the nociceptive SPB pathway has a significant role for the integration of the autonomic, emotional and aversive aspects of pain, since it relays noxious inputs to the amygdala, hypothalamus and bed nucleus of the stria terminalis (Gauriau & Bernard, 2002). In the mouse, the basic anatomical and electrophysiological characterization of lamina I SPB neurons has not been explored. Dense projections from lamina I in the lumbar enlargement to the parabrachial area have been described (Cameron et al. 2015), but the proportion of lamina I neurons projecting to the PB compared to other brain areas is unknown (Davidson et al. 2010; Cameron et al. 2015). In addition, responses of lamina I SPB neurons have not been characterized in the mouse in vivo. Nevertheless, the role of the SPB pathway in the affective and motivational aspect of pain has been suggested by behavioural data in genetically modified mice (Han et al. 2015).
The involvement of lamina I projection neurons in the generation of allodynia (pain elicited by low‐threshold contact) was first suggested by studies using c‐Fos as a marker of neural activation after sciatic nerve crush (Bester et al. 2000 b). The neural mechanisms leading to the activation of lamina I projection neurons by low threshold mechanoreceptors have been controversial because the latter terminate in laminae III–V, and thus have no direct connection with lamina I SPB neurons (Blomqvist & Craig, 2000). It has been suggested that in pathophysiological conditions leading to allodynia, nociceptive‐specific lamina I SPB neurons might be activated by C‐fibre low threshold mechanoreceptors that also terminate in lamina I (Andrew, 2010; Craig, 2010). Although the latter mechanism has not been ruled out, there is now evidence that demonstrates allodynia in experimental neuropathic and inflammatory conditions caused by the opening of a ventro‐dorsal spinal circuit allowing low threshold mechanoreceptive input to activate lamina I projection neurons (see Duan et al. 2018, for review). The opening of the polysynaptic pathway linking mechanoreceptors to nociceptive lamina I projection neurons can be reproduced acutely by the local application of GABAergic and glycinergic antagonists. The use of genetically modified mice (combining behavioural, anatomical and ex vivo electrophysiological experiments) has been decisive in establishing this mechanism (Duan et al. 2014; Foster et al. 2015; Peirs et al. 2015; Petitjean et al. 2015; Cui et al. 2016; Cheng et al. 2017).
Considering the importance of mice in pain research, and the importance of the lamina I SPB pathway in both acute and chronic pain, it is essential to measure the activity of lamina I SPB neurons in vivo in the mouse. The first aim of the present study was to characterize the conduction velocity and responses to thermal, mechanical and electrical stimuli of lamina I SPB neurons in the anaesthetized Swiss mouse. The second aim was to assess whether lamina I SPB neurons can be activated de novo by low threshold mechanoreceptors in the mouse, using local application of bicuculline and strychnine.
Methods
Ethical approval
Male Swiss mice, 30–40 g at the time of the experiment, were used. All experiments followed European Union (Council directive 86/609EEC) and institutional guidelines for laboratory animal care. Experiments were approved by the Auvergne‐Rhône‐Alpes regional ethical committee and the Ministère de l'Enseignement Supérieur et de la Recherche (APAFIS no. 8710‐100511051048v5).
Experimental set‐up
The procedure was performed under isoflurane anaesthesia (4% for induction, 2.5% for maintenance during the surgery). After induction of anaesthesia in a Perspex box, the animal was transferred to the operating table and fitted on a nose cone. The carotid artery and jugular vein were catheterized for blood pressure recording and for i.v. injection, respectively. The trachea was cannulated, and the animal switched to artificial ventilation. An infrared lamp and a homeothermic blanket controlled by a rectal probe were used to maintain core body temperature at 37.5°C. Mean blood pressure was measured throughout the experiment and maintained above 60 mmHg using Hemopure® i.v. injection (<200 μl; Hemoglobin Therapeutics LLC, Souderton, PA, USA) when necessary. End‐tidal CO2 partial pressure was measured with a capnograph and maintained within 25–35 mmHg. At completion of some experiments, blood gas, pH and electrolytes and glycaemia were measured using a blood analyser and a glycometer, respectively. Recordings were performed under 1.8–2.2% isoflurane (75–85 breaths min−1, 13 μl g−1 tidal volume, 1/8 O2/air mixture). Neuromuscular blockade was induced with d‐tubocurarine (boluses of 10 μg every 15–20 min). Stable blood pressure during noxious stimulation ensured that anaesthetic depth was sufficient.
The head was placed in a stereotaxic frame and a craniotomy was performed to allow the insertion of the electrode used for antidromic stimulations from the PB complex. The vertebrae were clamped with a lateral spinal fork and a laminectomy of the T13–L1 vertebrae was performed to expose the lumbo‐sacral spinal cord. A well made of agarose was set around the exposed spinal cord and filled with artificial cerebrospinal fluid (mm in distilled water: Na+, 148.2; Cl−, 156.3; K+, 2.9; Ca2+, 1.8; Mg2+, 0.8). An incision was made parallel and caudal to the femur, and the underlying muscle was opened using blunt dissection, exposing the sciatic nerve underneath. The sciatic nerve was dissected from the surrounding tissue with the epineurium left intact.
Standard elgiloy Rhodes type electrodes (SNE‐100, Microprobes Inc., Gaithersburg, MD, USA) were used for antidromic stimulations. The coordinates to target the parabrachial area were 1.1 mm lateral, 0.5–0.9 mm rostral and 4.3 mm deep from lambda, with the electrode tilted at 20° in the sagittal plane in the caudal direction. One or two electrodes stacked against each other in the coronal plane were used. Recording of spinal cord electrical activity was done with a Parylene‐coated tungsten microelectrode (2 MΩ impedance at 1 kHz, Microprobes Inc.) inserted in plastic tubing as secondary insulator (leaving 1 mm at the tip exposed). Electrical activity was acquired with a conventional set‐up, using a head‐stage preamplifier, 10–20 k amplification, 1–4 kHz band‐pass and 50 Hz notch filters. Recording and analysis were achieved with Spike2 version 2.07 (Cambridge Electronic Design, Cambridge, UK).
Recordings
The search for lamina I SPB neurons was based exclusively on antidromic stimulations from the parabrachial area (0.5 mA, 2 ms, square wave pulses at 1 Hz). Units were considered as projecting to the PB area if they responded to antidromic stimulations at 0.5 Hz with stable latency, followed a one‐for‐one train of five antidromic stimulations at 200 Hz, and the triggering of an antidromic action potential (AP) by an orthodromic AP (either ‘spontaneous’ or mechanically induced) led to systematic collision of these two APs.
Once the projection nature of the recorded unit was confirmed, the receptive field was delineated using careful pinches with blunt forceps. A series of mechanical stimuli was applied on the most responsive part of the receptive field (brush, von Frey hair applying a force of 25, 50, 100, 200 mN and pinch with haemostat clamp applying a force of 2370 mN). Further, a series of thermal stimuli was applied on the entire receptive field (10 ml water jet (WJ) at 0°C, room temperature (RT; 22–24°C), 42, 46 and 50°C). Mechanical stimuli were applied for 6 s, and a WJ, flowing by gravity from a 25 ml Pipette, lasted for approximately 3 s. Electrical stimulations were delivered on the sciatic nerve with bipolar hook silver electrodes or within the receptive field with a pair of 30 G needles. Electrical pulses of 2 ms, 0.01–10 mA and 0.2–0.5 ms, 0.01–1 mA were used for receptive field and sciatic nerve stimulations, respectively. Wind‐up consisted of four pulses at 0.05 Hz followed by 11 pulses at 1 Hz with a current intensity aiming at triggering three to five C‐fibre‐related APs. Responses to brush were measured 10 min after the wind‐up test, or 5 min after the electrical stimulations if the wind‐up test was not performed. Following baseline recording, the well over the spinal cord was filled with artificial cerebrospinal fluid containing bicuculline (100 μm) and strychnine (10 μm), and responses to brush were measured after 10 min. The recording site was marked with an electrolytic lesion (negative DC, 20 μA for 20 s). An iron depot (positive DC, 20 μA for 40 s) was made for the detection of the antidromic stimulation site. The mice were culled with an overdose of pentobarbital delivered i.v. at completion of the experiment. The spinal cord and brain were removed and fixed in paraformaldehyde overnight at 4°C, and stored in 30% sucrose. Twenty‐five‐micrometre sections were cut on a freezing microtome. The antidromic stimulation site was revealed using Perls’ Prussian blue reaction on free‐floating sections before mounting. All sections were stained using a commercial solution of haematoxylin after mounting.
Analysis
Responses to mechanical and thermal stimuli were measured over 5 s, and quantified as number of APs and peak firing frequency (over 0.1 s). Descriptive statistics were obtained using SigmaStat 4.0 software (Systat Software, Inc., San Jose, CA, USA). Non‐parametric and parametric data are presented as median (25th–75th percentile) and mean (standard deviation (SD)), respectively.
Results
Physiological parameters and blood chemistry
Blood chemistry was obtained from 23 mice. Main electrolytes concentrations (Na+, K+, Ca2+), blood gas partial pressures (O2 and CO2), haematocrit and glycaemia were directly measured (Table 1). Bicarbonate concentration ([HCO3 −]) was derived from the measured parameters. All biological and physiological parameters were distributed normally and expressed as mean ± SD. Essentially, the arterial blood CO2 partial pressure was 30.2 ± 4.1 mmHg, with a blood pH value of 7.2 ± 0.1 and a calculated [HCO3 −] of 12.5 ± 2.7 mmol l−1 (see Table 1 for all measured parameters). Blood pressure decreased from 76.4 ± 9.1 mmHg at the beginning of the recording to 71.7 ± 8.9 mmHg at completion of the recording (n = 70).
Table 1.
Physiological parameters
| pH | (mmHg) | Arterial CO2 (mmHg) | Arterial O2 (mmHg) | [Na+] (mmol l−1) | [K+] (mmol l−1) | [Ca2+] (mg dl−1) | Haematocrit (%) | Glycaemia (mmol l−1) | Initial–final BP (mmHg) |
|---|---|---|---|---|---|---|---|---|---|
| 7.2 ± 0.1 | 27 ± 3 | 30 ± 4 | 135 ± 25 | 149 ± 4 | 7.7 ± 1.1 | 5.2 ± 0.4 | 45.4 ± 4.0 | 6.3 ± 3.7 | 76 ± 9–72 ± 9 |
Results are displayed as mean ± SD. Results from blood analysis were obtained from 23 mice at completion of the experiment. , end tidal CO2; Initial–final BP: blood pressure measured at the beginning and end of the experiment.
Inclusion/exclusion of recorded neurons
It was sometimes necessary to increase the current intensity of the antidromic stimulation up to 2–3 times the threshold value to obtain the following of the train of stimulations at 200 Hz and 400 Hz. For 11 neurons, increasing current intensity of electrical stimulation over threshold led to the generation of two antidromic APs, demonstrating the presence of two branches of the axon of the lamina I SPB neuron close to the stimulating electrode. In these cases, it was not possible to follow the train of antidromic stimulations at high frequency reliably. The latter also occurred for eight neurons despite stable antidromic latency and positive collision test. These 19 neurons were not characterized further, without attempting to locate their position in the spinal cord, and the search for another neuron was pursued. Nevertheless, the inability to follow the train of antidromic stimulations at high frequency might have been due to failure of the antidromic action potential to invade the somatodendritic region rather than the lack of projection from the recorded neuron to the PB area (Lipski, 1981; Light et al. 1993).
Ultimately, 77 units were initially characterized as projection neurons according to the criteria described in the method section (Fig. 1 A–D). The median current intensity threshold for antidromic activation was 125 (82–272) μA. Location of the electrolytic lesion upon histological analysis showed that 37 and seven neurons were located in lamina I and lamina III–IV, respectively (Fig. 2 A and B and see Fig. 4 C, F, I and L). The corresponding depth of recording measured from the surface of the spinal cord was always ≤150 μm for neurons located in lamina I and ≥200 μm for neurons located in deeper laminae. No clear electrolytic lesion could be seen upon histological analysis of sections from 33 spinal cords. Since the depth of recording was always <150 μm in these latter preparations, the corresponding neurons were assumed to be located in lamina I (see Discussion). Thus, out of 77 projection neurons initially characterized, 70 and seven were located in lamina I–II and III–IV, respectively.
Figure 1. Identification of lamina I spinoparabrachial neurons.
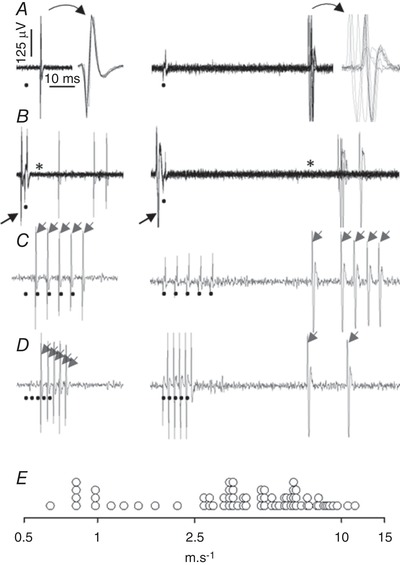
Recordings obtained from 2 units with fast (left column) or slow (right column) conduction velocity. A, superimposition of 10 successive antidromic stimulations at 1 Hz (circle indicates trigger of antidromic stimulation), with expanded time scale drawing of the superimposed antidromic AP. The antidromic latencies were 6.3 and 61.6 ms for the unit with fast (7.9 m s−1) and slow (0.8 m s−1) conduction velocity, respectively. Note on the expanded time scale the stability of the antidromic latency of the fast conducting unit compared with the slow conducting unit. B, superimposition of 10 successive antidromic stimulations triggered by an orthodromic AP generated by a careful pinch of the receptive field: there was a systematic collision between the AP that triggered the antidromic stimulation (arrow) and the antidromic AP (the asterisk indicates the missing antidromic AP). C and D, both units were able to follow a one‐for‐one train of 5 stimulations at 200 Hz (C, arrows), but only the fast conducting unit was able to follow a one‐for‐one train of 5 stimulations at 400 Hz (D, arrows). Note the slowing of conduction velocity after the first pulse of the train at 200 Hz for the slow conducting unit. E, distribution of the conduction velocity (logarithmic scale) of the 70 lamina I spinoparabrachial neurons studied.
Figure 2. Histological controls.
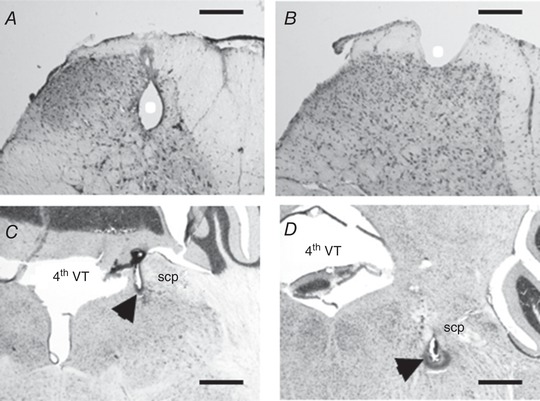
Photomicrographs of lumbar spinal cord (A and B, scale bar 200 μm) and brain (C and D, scale bar 400 μm) sections stained with haematoxylin. Recording and stimulation sites are indicated with white circle and black arrowhead, respectively. A, example of electrolytic lesion located in the deep dorsal horn spinal cord; B, the shape of the electrolytic lesion located in lamina I in the present study. C and D, antidromic stimulation sites located in the caudal (approximately 5.7 mm caudal to Bregma) and in the more rostral (approximately 5.2 mm caudal to Bregma) part of the parabrachial complex, respectively. 4th VT, fourth ventricle; scp: superior cerebellar peduncle.
Figure 4. Individual responses to thermal and mechanical stimuli.
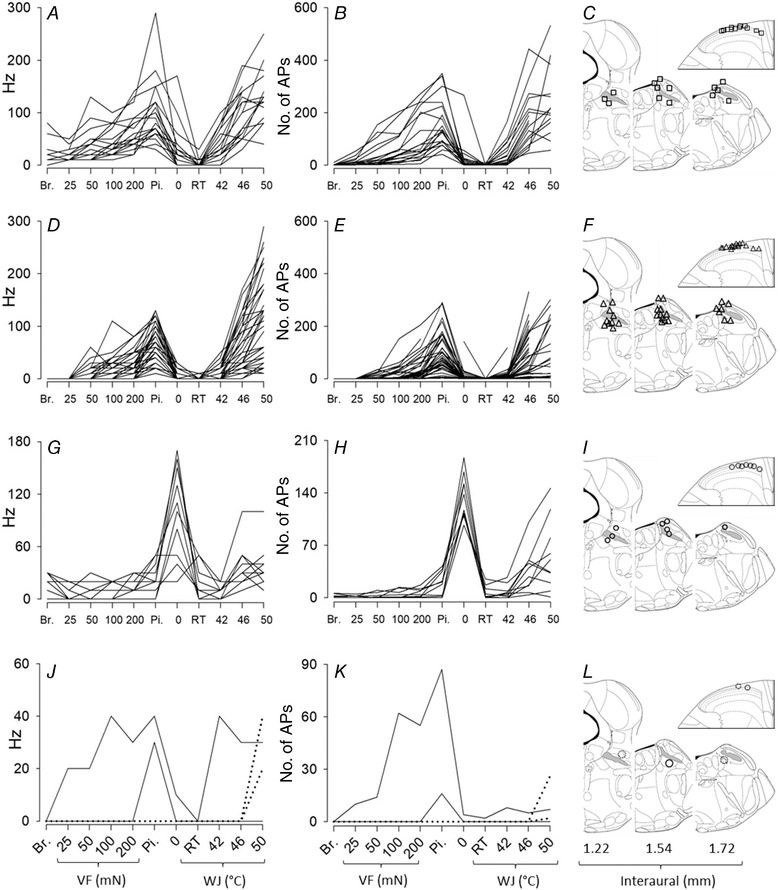
The peak firing frequency (left column, A, D, G and J) and the number of APs (middle column, B, E, H and K) of the responses to mechanical and thermal stimuli are represented for all units studied (n = 70). The right column (C, F, I and L) shows the site of stimulations (54/70) and recordings (37/70) that were recovered upon histological analysis. The superior cerebellar peduncle (or brachium conjonctivum) is highlighted in grey. Units were pooled in different groups for clarity of illustrations. A, B and C, wide dynamic range neurons (mechanical threshold corresponding to brush and/or VF at 25 mN, n = 19). D, E and F, nociceptive‐specific neurons (mechanical threshold corresponding to VF at 50, 100 and 200 mN, and pinch, n = 37). G, H and I, polymodal cold preferential neurons (n = 10). J, K and L, heat specific (dotted line, n = 2), mechanical specific (continuous line, n = 1) and polymodal mechanical preferential (continuous line, n = 1). Breaks in the line correspond to missing data, due in most cases to marked drop in the amplitude of the APs during the stimulation. Br.: brush; Pi.: pinch; RT, room temperature; VF, von Frey; WJ, water jet.
Central conduction velocity
Based on an estimated axonal length of 5 cm between recording and antidromic stimulation sites, the median conduction velocity of the 70 lamina I SPB neurons identified was 4.7 m s−1 (25th–75th percentile, 3.0–6.5 m s−1; range, 0.6–11.4 m s−1; Fig. 1 E). Thirteen and 57 units had central conduction velocities corresponding to unmyelinated fibres and thinly myelinated fibres, respectively.
Qualitative comment
The overall impression acquired during the acquisition of the recordings was that the pattern of responses of the lamina I SPB neurons was heterogeneous. Most neurons displayed no intrinsic activity during the initial antidromic stimulations, but in some cases delineation of the receptive field or triggering orthodromic APs for the collision test using careful pinches induced ongoing activity that lasted from a couple of minutes to the entire duration of the recording. The ‘hard’ pinch used for quantitative measurements as well as water jet (WJ) stimulations at 0 or 50°C could also induce prolonged activity of the recorded neuron, and waves of ‘spontaneous’ activity of unknown origin were sometimes observed. Responses to mechanical and thermal stimuli ranged from sluggish to brisk, and responses to thermal stimuli were overall more brisk than responses to mechanical stimuli. In some cases, repeated pinches applied during the receptive field delineation or because of misplacement of the clamp used for pinch quantitative measurement, decreased in effectiveness. This was especially pronounced when the responses were initially sluggish.
Lamina I SPB neurons classification
Some lamina I units could be unambiguously classified as wide dynamic range (Fig. 3 A) and some as nociceptive‐specific (Fig. 3 B). The wide dynamic range lamina I SPB neuron in Fig. 3 A responded to von Frey (VF) at 25 mN (i.e. light static touch), and the intensity of the responses increased progressively with stimuli becoming more noxious. Responses to cold (0°C) and warm (42°C) WJ were slightly greater than response to WJ at RT, whereas responses to WJ at 46 and 50°C were marked. For the nociceptive‐specific unit in Fig. 3 B, responses to mechanical stimuli were only observed for VF at 200 mN and pinch. Thermal responses to WJ at 46 and 50°C were marked compared to WJ at 42°C (almost equal to 0), and compared to WJ at 0°C and RT (equal to 0). The classification of these two units was straightforward since they represent the extreme opposite ends of a continuous distribution of mechanical and thermal thresholds within the population of neurons responding to the most noxious mechanical and thermal (heat) stimuli. The distinction between wide dynamic range and nociceptive‐specific neurons is problematic for some units falling in the median of this continuum.
Figure 3. Recordings of neuronal responses to mechanical and thermal stimuli.
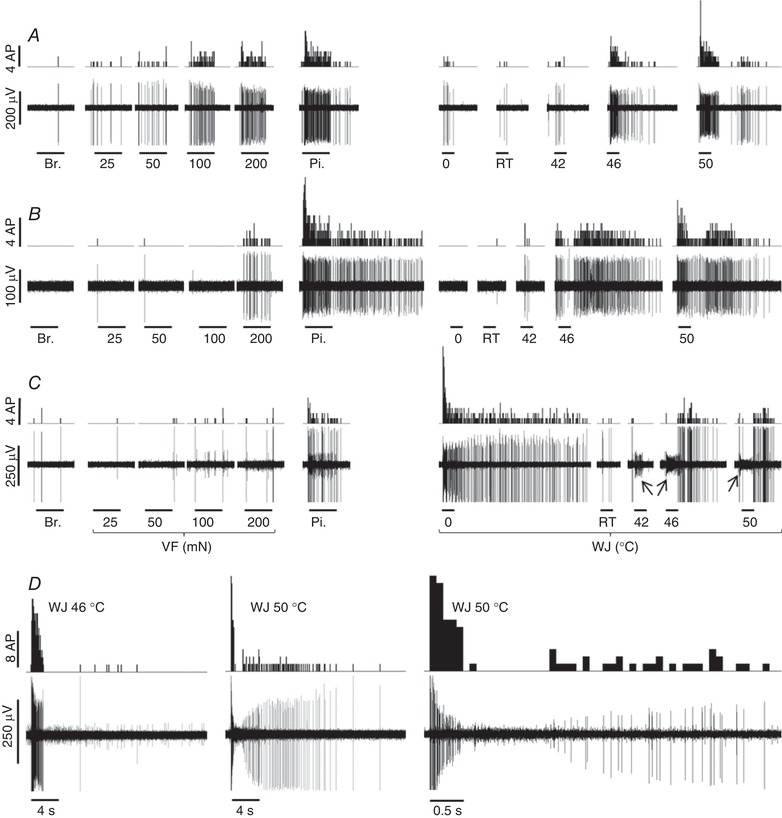
A and B, recordings of wide dynamic range and nociceptive‐specific lamina I SPB neurons, respectively. The wide dynamic range neuron in A responds to non‐noxious and noxious mechanical and thermal stimuli with increasing intensity. The nociceptive‐specific neuron in B responds exclusively to noxious mechanical stimuli (VF at 200 mN and pinch; note the prolonged response after the pinch), and to noxious heat stimuli. C, single unit characterized by a fast and prolonged response to WJ at 0°C. The response to pinch was much less pronounced. Responses to WJ at 42, 46 and 50°C appeared with delay; note in comparison the immediate response of the unit giving rise to a low amplitude signal (indicated by the arrow) during the application of WJ at 42, 46 and 50°C (the amplitude of the AP dropped during the latter application). The unit in C is considered a polymodal unit with preferential response to cold (cold preferential). The number of APs is quantified per 0.1 s bin in the peristimulus histogram. The horizontal lines below the electrical recordings correspond to 6 s for the mechanical stimulations, 3 s for the thermal stimulations for A, B and C. D, the response of a nociceptive‐specific lamina I SPB neuron to WJ at 46 and 50°C (the recording on the right corresponds to the middle recording at an expanded scale). It illustrates the decrease in amplitude of the AP during the firing at high frequency induced by WJ at 50°C. Br.: brush; Pi.: pinch; RT, room temperature; VF, von Frey; WJ, water jet.
Apart from three unambiguous modality‐specific units (2 thermal and 1 mechanical), it appeared difficult to attribute a clear‐cut phenotype to other units, such as the one shown in Fig. 3 C. This lamina I SPB neuron displayed a marked response to WJ at 0°C compared to any other stimuli. It also responded to pinch and, albeit with a noticeable delay, to noxious heat (WJ at 46 and 50°C), ruling out its strict classification as a modality‐specific cold unit. Such units showing a marked rather than an exclusive response to one modality were classified as modality preferential.
From wide dynamic range to nociceptive‐specific units
The amplitude of APs displayed a dramatic drop during the responses to WJ at 46 and 50°C for some units, most likely because of the high firing frequency induced by these stimuli (Fig. 3 D). This drop in amplitude prevented the measurement of the number of APs in response to WJ at 46 and 50°C for four and 22 lamina I SPB neurons, respectively. The measurement of the peak firing frequency was obtained in most cases, however, since the drop in amplitude occurred after the initial burst of firing (see magnification of responses to WJ at 50°C in Fig. 3 D).
A total of 56 lamina I SPB neurons responded to noxious heat and pinch without showing any preferential modality. Thirteen, 6, 11, 9, 8 and 9 had mechanical thresholds corresponding to brush, VF at 25, 50, 100 and 200 mN and pinch, respectively. These proportions are derived from a simple read out of the raw data (≥1 AP in response to 10 successive brushes or during the application of the VF or pinch stimulus for 6 s). Only nine units responded with ≥2 APs per brush and/or ≥10 APs to the application of VF at 25 mN, corresponding to clearly identified wide dynamic range neurons (13% of the entire population of lamina I SPB neurons). In addition to 26 units displaying mechanical threshold ≥VF at 100 mN, three units showed negligible response to VF at 50 mN (<3 APs), so that 29 units could be considered as being nociceptive‐specific neurons on the basis of their mechanical threshold (41% of the entire population of lamina I SPB neurons). The classification of the remaining 19 units as wide dynamic range or nociceptive specific was ambiguous and depends on the classification of VF at 50 mN as a noxious or non‐noxious stimulus. Nevertheless, for presentation of the following results, these 19 units were classified as nociceptive specific (see Discussion for further analysis). Regarding thermal stimulations, the median response of these 56 lamina I SPB neurons to WJ at 42°C was low (7 (0–18) APs at 20 (0–30) Hz). Only 18 units (32%) fired more than 10 APs in response WJ at 42°C, showing that the remaining 68% were poor encoders of warm non‐noxious stimuli.
Briefly, the magnitude of the responses of each wide dynamic range and nociceptive‐specific unit was correlated with the noxiousness intensity of the stimulus. The strongest responses were obtained with pinch and WJ at 50°C (see the overall shape of the individual responses in Fig. 4 A–F). The range of responses obtained for pinch, and to a greater extent WJ at 50°C, was wide (Fig. 4 A, B, D, E). For neurons with mechanical threshold ≥100 mN (n = 26), the peak firing frequency in response to WJ at 50°C and pinch ranged from 10 to 260 Hz (median, 60 Hz) and 10 to 130 Hz (median, 50 Hz), respectively. The corresponding number of APs ranged from 2 to 218 (median, 63) and 2 to 302 (median, 33), respectively. Finally, the distribution of the responses of wide dynamic range and nociceptive‐specific neurons indicates a predominant coding of noxious stimuli since the median responses to brush, VF at 25, 50 and 100 mN, and WJ at 0, RT and 42°C were ≤8 APs, whereas the median responses to VF at 200 mN, pinch, WJ at 46 and 50°C (i.e. all noxious stimuli) was 29, 99, 111 and 171 APs.
Modality‐specific and modality preferential units
Fourteen lamina I SPB neurons were classified as modality specific or polymodal with preferential response to one modality (modality preferential, Fig. 4 G–L). Modality‐specific neurons consisted of two units responding exclusively to WJ at 50°C and one unit responding exclusively to pinch. A focus on the number of APs was necessary to classify 11 lamina I SPB neurons as encoding preferentially mechanical (n = 1) and cold (n = 10) stimuli (Fig. 4 H and K). The lamina I SPB neurons responding preferentially to cold were characterized by a marked response to WJ at 0°C compared to any other stimuli (Fig. 4 G and H). Two of these units displayed negligible responses to WJ at 50°C and pinch compared to their response to WJ at 0°C and might have been labelled cold specific. The other eight units did respond to warm and heat stimuli in addition to cold stimuli, ruling out their classification as cold‐specific units. Interestingly, there was a noticeable delay (up to 3 s) in the appearance of the response upon application of WJ at 42 and 50°C for these eight units (see example in Fig. 3 C), suggesting that these responses might correspond to a decrease in temperature following the initial increase in temperature induced by the WJ. The classification of these neurons as cold preferential was confirmed by their prolonged discharge after WJ at 0°C compared to WJ at 50°C (the total number of APs including the post‐discharge in response to WJ at 0 and 50°C was 314 (330–1444) and 88 (40–329), respectively).
Receptive fields
Responses to pinch with hand‐held forceps were variable across the receptive field, with the whole digit, or sometimes the tip of the digit, being the most responsive. Receptive fields were usually large: 32/71 (45%) units and 19/71 (27%) units had receptive fields covering the totality or more than half (but less than the totality) of the surface of the glabrous skin of the hind paw, respectively. The remaining 20 units (28%) had receptive fields covering less than half of the surface of the glabrous skin of the hind paw. The smallest receptive field delineated covered the surface of one digit. There was no apparent relationship between receptive field size and the different subtypes of neurons defined in the study.
Responses to electrical stimulation
Electrical stimulations were attempted in 45 experiments, and quantifiable responses were obtained in 35 experiments (29 wide dynamic range and nociceptive specific, and 6 modality preferential units, see Fig. 5 A–C for examples of responses). The maximal number of A‐ and C‐fibre‐related APs obtained in response to electrical stimulation was 5 (2–8) and 4 (2–7), respectively. There was no noticeable difference in the response to electrical stimulation between the different groups apart from the proportion of units with exclusive A‐ or C‐fibre innervation. Exclusive C‐fibre innervation was observed in 3/6 modality‐specific units (1 heat specific and 2 cold preferential), and in 1/29 nociceptive‐specific units (Fig. 5 B). Exclusive A‐fibre innervation was observed in 6/29 nociceptive‐specific units (Fig. 5 C).
Figure 5. Recordings of responses to electrical stimulation.
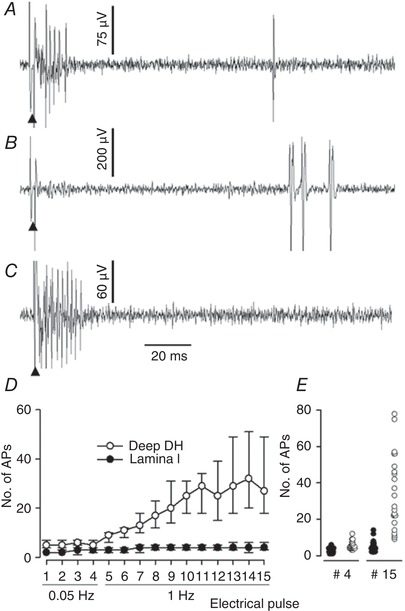
A–C, recordings of different units illustrating the various pattern of innervation of lamina I SPB neurons observed in the present study. Electrical stimulations were delivered with a pair of needles inserted in the receptive field (10 mA, 0.2 ms). A, predominant myelinated fibre innervation and reduced non‐myelinated fibre innervation (typical of nociceptive‐specific neurons in the present study). B, heat‐specific unit receiving input from non‐myelinated fibre only. C, nociceptive‐specific unit receiving input from myelinated fibre only. Arrowhead: electrical stimulation artefact. E, value of the median number of C‐fibre related APs (+75th percentile, −25th percentile) obtained during peripheral electrical stimulations at 0.05 Hz (4 pulses) and then 1 Hz (11 pulses) of lamina I SPB neurons and deep dorsal horn (DH) neuron (n = 23 in each group). Wind‐up of lamina I SPB neuron was noticeably reduced compared to that of neurons located deeper in the dorsal horn. F, dot plot individual values of the number of C‐fibre‐related APs obtained for the 4th pulse (0.05 Hz) and 15th pulse (1 Hz) during the wind‐up test for the lamina I SPB neurons (filled circles) and deep dorsal horn neurons (open circles).
Wind‐up was tested in 23 lamina I SPB neurons. The results obtained for the lamina I SPB neurons were compared with those obtained from 23 wide dynamic range and nociceptive‐specific neurons of unknown projection, located deeper in the spinal cord. The median number of C‐fibre‐related APs increased from two to three at baseline to four during the wind‐up for the lamina I SPB neurons, whereas it increased from five to six at baseline to 32 for the deep dorsal horn neurons (Fig. 5 D and E).
Pharmacological disinhibition
Responses to brush were recorded in 13 units after the application of vehicle, and in 47 units after application of bicuculline and strychnine. There was no noticeable effect after vehicle application, whereas the application of bicuculline and strychnine led to a dynamic increase in responses to brush in some units. The recordings in Fig. 6 illustrate the increase in responses (Fig. 6 A), the marked (Fig. 6 B and C) or the marginal appearance (Fig. 6 D) in responses to brush that could be observed upon spinal cord application of bicuculine and strychnine.
Figure 6. Recordings of responses to brush before and after bicuculline and strychnine (BS) application on the spinal cord.
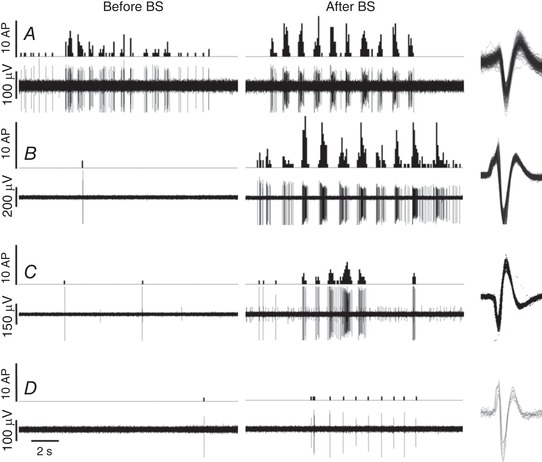
A–D, recordings (lower line) and corresponding peristimulus histogram (upper line, quantified per 0.1 s bin) of lamina I spinoparabrachial neuron responses during 10 successive brushes of the receptive field. Brushes were performed before and 10 min after the combined application of bicuculline (100 μm) and strychnine (10 μm) on the spinal cord. The effects of BS application were highly variable amongst the different units tested. The unit in A showed a robust response to brush at baseline that was enhanced by the application of BS. Units in B, C and D were not responsive to brush at baseline: application of BS resulted in the appearance of marked, moderate and minor responses to brush, respectively. All action potentials quantified are superimposed on the right hand side.
Quantification of the recordings showed that responses to brush upon vehicle application were unchanged in 12/13 and increased 1/13 units, whereas upon bicuculline and strychnine application, responses to brush were decreased in 3/47, unchanged in 12/47 and increased in 32/47 units (Fig. 7 A and C). For the 47 lamina I SPB neurons tested, the number of APs and firing frequency of responses to brush were 0 (0–0.5) AP at 0 (0–10) Hz before versus 2.5 (0–12) APs at 30 (0–100) Hz after bicuculline and strychnine application. The increase of responses obtained after bicuculline and strychnine application was highly variable, ranging from 0.1 to 83.1 APs/brush (Fig. 7 C), with increase in peak firing frequency ranging from 10 to 170 Hz (Fig. 7 A). The effect of bicuculline and strychnine application was further analysed by separating the 47 units tested as wide dynamic range and nociceptive‐specific neurons, or as modality‐preferential neurons (Fig. 7 B and D). Neurons with initially low mechanical threshold showed the greatest increase in number of APs after bicuculline and strychnine application (Fig. 7 D).
Figure 7. Quantification of the effect of bicuculline and strychnine (BS) application on neuronal responses to brush.
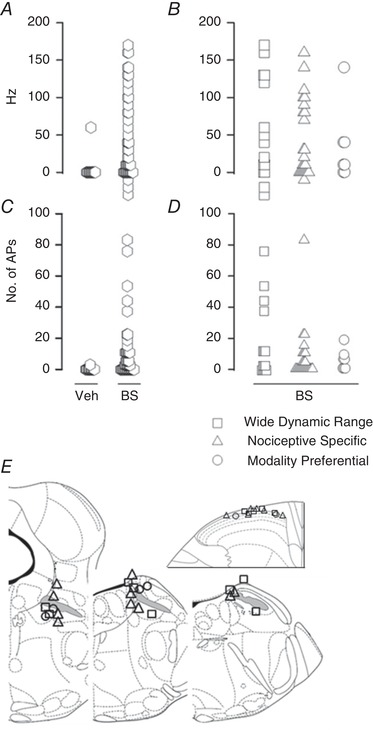
The peak firing frequency and the mean number of APs in response to 10 successive brushes were quantified before and after the application of vehicle or BS on the spinal cord. A and C show the distribution plot of the differences of the peak firing frequency (A) and number of APs (C) of the responses to brush obtained for all neurons tested, before and after spinal cord application of vehicle (n = 13) and BS (n = 47). B (peak firing frequency) and D (number of APs) focus on the responses of neurons before and after spinal cord application of BS (n = 47). B and D are a detailed view of the ‘BS column’ in A and C, the neurons being separated into 3 different groups: wide dynamic range (n = 15), nociceptive specific (n = 25), and polymodal with one preferential modality (n = 7: 6 cold preferential, 1 mechanical preferential). Overall, the greatest increase in number of APs after BS application was observed for wide dynamic range neurons. E, the location of the stimulation and recording sites for the neurons showing an increase of more than 2 APs/brush after BS application did not differ from neurons that responded less to BS application. The superior cerebellar peduncle or brachium conjonctivum is highlighted in grey.
When a threshold corresponding to a mean increase of 2 APs/brush was set to distinguish lamina I SPB neurons that responded markedly to bicuculline and strychnine application (responsive units) from those that did not (unresponsive units), 24/47 units were considered as responsive. For these responsive units, the increase of response per brush was 11 (6–22) APs and 85 (40–130) Hz. There was a similar proportion of responsive units in the different subgroups (wide dynamic range, 8/15; nociceptive specific, 12/25; modality preferential, 4/7). Within each group, there was no apparent difference between responsive and unresponsive units regarding their initial responses to mechanical and thermal stimuli, as well as their conduction velocity. Recording and antidromic stimulation sites of responsive units did not show particular locations (Fig. 7 E).
Discussion
Summary
The aim of the present study was (1) to characterize the responses of lamina I SPB neurons in the anaesthetized mouse, and (2) to assess the ability of spinal application of GABAergic and strychnine antagonists to allow the activation of these lamina I SPB neurons by low threshold mechanoreceptors. The activity of 70 lamina I SPB neurons was recorded in anaesthetized Swiss male mice. Their central conduction velocity indicated that their projection axon was predominantly thinly myelinated fibre with the minority being C‐fibre. Nociceptive‐specific, cold‐preferential and wide dynamic range neurons accounted for 41%, 14% and 13% of the population of lamina I SPB neurons. About 25% of lamina I SPB units showed a profile intermediate between true wide dynamic range and nociceptive‐specific neurons. Decreasing inhibitory tone with application of bicuculline and strychnine on the spinal cord increased or induced responses to brush in half of the neurons tested.
Location of antidromic stimulation and recording sites
Electrolytic lesion rather than Prussian blue reaction was used for the detection of the recording site since high impedance stainless steel electrodes with fine shaft diameter (125 μm) were not available. Electrolytic lesions could be recovered in lamina I in only 37/70 SPB neurons. The reason why electrolytic lesion could not be visualized in about half of the preparations is unclear. The formation of a ‘large’ bubble at the tip of the electrode was always observed when DC current was passed through, ruling out obvious misconnection or conduction failure during the lesion process. A tentative explanation is that the tip of the electrode had to be deep enough and surrounded with tissue for the electrolytic lesion to leave a marked trace. This might not have been the case for recording sites located on the lateral portion of lamina I, where the dorsal funiculus is thin or absent and lamina I is only covered by dorsal rootlets. The size of the electrodes used for antidromic stimulation (exposed active tip length × diameter, 0.25 × 0.1 mm) did not allow the stimulation of distinct nuclei within the parabrachial complex. There was no apparent relationship between the location of the antidromic stimulation site, the recording site and the profile of the recorded SPB neurons.
Physiological parameters and blood chemistry
Maintenance of all biological parameters within the physiological range during an invasive procedure under anaesthesia is essential to obtain meaningful data. Parameters usually controlled are core body temperature, blood pressure and end‐tidal CO2. Body temperature was maintained at 37.4°C, and mean arterial blood pressure was above 60 mmHg (the maintenance of cardiovascular parameters during the recordings might be improved by a more accurate control of the inspiration/expiration duration ratio; Dalkara et al. 1995).
The correspondence between the values of end tidal CO2 (measured by capnography) and arterial blood CO2 (measured with a blood gas analyser) is dependent on adequate ventilation parameters (Dalkara et al. 1995; Thal and Plesnila, 2007). Preliminary experiments using low tidal volume (<10 μl g−1) led to large discrepancies between end tidal and arterial blood CO2, and increasing tidal volume to 13–15 μl g−1 was necessary to obtain a better correspondence between the two measures. Nevertheless, end tidal CO2 underestimated arterial blood CO2 value by a mean of 3.7 mmHg.
Although arterial CO2 (30.2 ± 4.1 mmHg) was within physiological range, blood pH (7.2 ± 0.1) and calculated [HCO3 −] (12.5 ± 2.7 mmol l−1) were below physiological range (7.39 ± 0.03 and 18.4 ± 0.8 mmol l−1, respectively) (Lee et al. 2009; Iversen et al. 2012), indicating a state of metabolic acidosis. Metabolic acidosis has been reported in a study focusing exclusively on the maintenance of physiological parameters in isoflurane‐anaesthetized C57BL/6 mice under artificial ventilation (Zuurbier et al. 2002). Zuurbier and colleagues attributed the acidosis to hyperchloraemia, as a consequence of the i.v. administration of a large amount of saline (50 μl g−1 h−1 for 3 h). The administration of a much smaller volume of Ringer–lactate solution (about 5 μl g−1 h−1 for 2 h) in the present experiment, corresponding to a 10‐fold reduction in chloride load, led to a similar acidosis, raising doubt on exogenous chloride as a common determinant for the generation of acidosis in these two studies. The use of Ringer–lactate solution for fluid replacement and vehicle (instead of saline) might have slightly participated in blood acidosis, because the alkalinizing effect of lactate as a precursor of bicarbonates takes more than 3 h to occur, and is preceded by a mild acidifying effect (Schwarzkopf et al. 2013). There are no reports of specific changes in pain perception in patients with acute or chronic metabolic acidosis (Singh, 2010). The consequences of metabolic acidosis for pain‐related neurophysiological processes at the peripheral and spinal level have not been documented. It is noteworthy that blood pH was not controlled in any of the studies focused on the responses of spinal neurons quoted in the present paper, and the present data should serve to caution those relying on end tidal CO2 to estimate blood pH value. The supply of bicarbonates might help in obtaining pH values closer to physiological values (Hegeman et al. 2013).
The calculated concentration of calcium (5.2 ± 0.4 mg dl−1) was about half the value reported in the literature. Blood analysis performed 15–20 min after induction of isoflurane anaesthesia in control experiments (n = 3) led to similar values, suggesting that the low [Ca2+] was not due to physiological dysfunction caused by prolonged anaesthesia. Arterial blood O2 (135 ± 25 mmHg) was above physiological parameters (88 ± 3 mmHg), which is not considered an issue (Lee et al. 2009). Other blood parameters measured ([glucose], [Na+], [K+] and haematocrit) were within the physiological range (Serfilippi et al. 2003; Otto et al. 2016).
Evoked responses of lamina I SPB neurons
Studies in the rat (Bester et al. 2000 a; Keller et al. 2007; Andrew, 2009) and the cat (Hylden et al. 1985, 1986; Light et al. 1993) have shown that an essential characteristic of lamina I SPB neurons is the preferential or specific response to noxious stimuli. The results obtained in the present study in mice may appear somewhat different. There was a continuum in the distribution of threshold and intensity of responses throughout the population of lamina I SPB neurons, from non‐noxious (brush/VF at 25 mN/WJ at 42°C) to noxious (pinch/WJ at 50°C) stimuli. The determination of the exact value of the percentage of nociceptive‐specific neurons would require the precise definition of the threshold between noxious and non‐noxious for non‐damaging stimuli, as well as the number of APs and frequency of firing for a response to be considered physiologically relevant. On the conservative assumption that VF at 100 mN is a noxious stimulus in mice, and that 2 APs/brush, or >10 APs for VF at 25 mN defines a physiologically relevant response, nociceptive‐specific neurons and wide dynamic range neurons accounted for 41% and 13% of the lamina I SPB neurons recorded, respectively. In the rat, which is phylogenetically closer to the mouse than the cat, it is accepted that slightly more than 70% of the population of lamina I SPB neurons are nociceptive specific (Bester et al. 2000 a; Keller et al. 2007; Andrew, 2009).
This apparent discrepancy in the proportion of nociceptive‐specific neurons reported in rats compared to mice in the present study could be explained by a simple methodological difference. The only mechanical stimuli used in the rat studies were brush and pinch, and nociceptive‐specific neurons were identified on the basis of their response to pinch and lack of response to brush (Bester et al. 2000 a; Keller et al. 2007; Andrew, 2009). No mechanical stimuli of intermediate strength were used, which obviously ruled out the detection of neurons with corresponding intermediate mechanical thresholds. If only brush and pinch had been used as mechanical stimuli in the present study, nociceptive‐specific and wide dynamic range neurons would have accounted for 74% and 6% of the lamina I SPB neurons (without any ambiguity).
The above does not rule out the possibility that neurons classified as nociceptive specific in rats are indeed true nociceptive‐specific neurons, in the same way as in the cat, in which nociceptive‐specific neurons identified using graded mechanical stimuli ranging from non‐noxious to noxious accounted for 79–91% of the lamina I SPB neurons (Hylden et al. 1986; Light et al. 1993). The continuum of responses of the overall population of lamina I neurons observed in the present study would then be specific to mice. One explanation could be that this continuum does not reflect the presence of units responding to non‐noxious input amongst lamina I SPB neurons, but rather illustrates that nociceptors in mice are activated at much lower thresholds compared to rats. In other words, the continuum of responses of the overall population of lamina I neurons corresponds to the progressive recruitment of true nociceptive‐specific neurons with increasing mechanical threshold. The low value of the median threshold of activation of Aδ (10 mN) and C (20 mN) peripheral nociceptors in the glabrous skin of the mouse hindpaw (compared to 50 and 100 mN in rats, respectively) is not in contradiction with this hypothesis (Leem et al. 1993; Cain et al. 2001). Now, it may be that the continuum of responses of lamina I SPB neurons observed in the present experiment does correspond to the activation of a substantial portion of neurons by non‐noxious stimuli. This would be at odds with the current understanding of the pain‐related interoceptive function of the lamina I SPB pathway (Craig, 2002). The ability to differentiate noxious from non‐noxious stimuli and to determine the threshold for responses of lamina I SBP neurons to be considered physiologically relevant is essential to clarify this point.
Another expected characteristic of a population of lamina I SPB neurons is the presence of some thermoreceptive and other modality‐specific units. In rats, a minor fraction of lamina I SPB neurons tested have been reported to respond to innocuous cooling (Andrew, 2009) and noxious heat (Bester et al. 2000 a). In cats, about 10% of the lamina I SPB neurons were specifically activated by innocuous cooling and 50% by mechanical stimulations (Light et al. 1993). In the present study, two units responding exclusively to heat, two preferentially to pinch and 10 preferentially to cold were found out of 70 lamina I SPB neurons. The present result obtained in mice is in agreement with the idea that the population of lamina I projection neurons contains a sufficient number of modality‐specific or modality‐preferential units to encode specific pain‐related sensations, bearing in mind that this notion is based on the study of spinothalamic rather than SPB neurons in the cat (Andrew & Craig, 2001 a, b ; Craig & Andrew, 2002). Differentiation of lamina I SPB neurons responding to noxious and innocuous cooling was not possible with the cold stimulus used in the present study (WJ at 0°C). Additional WJ applications at 5, 10 and/or 15°C would be required to do so.
In most preparations, responses to electrical stimulations were obtained by directly stimulating the sciatic nerve with a bipolar hook electrode to ensure that all fibres innervating the recorded neuron would be recruited at maximal intensity. About 1/3 of lamina I SPB neurons tested were selectively innervated by A‐ or C‐fibres. In cats, 2/3 of lamina I SPB nociceptive‐specific neurons are innervated exclusively by Aδ fibres, and some lamina I SPB neurons are innervated by C‐fibres (Hylden et al. 1986; Light et al. 1993). In the rat, a similar distribution of myelinated versus non‐myelinated innervation was observed when using antidromic stimulation at the cervical level to identify lamina I projection neurons (Hylden et al. 1989). Intriguingly, there was no mention of lamina I SPB neurons being specifically innervated by A‐ or C‐fibres when using antidromic stimulations from the PB area (Bester et al. 2000 a; Andrew, 2009). Because virtually all lamina I projection neurons target the PB area (Todd, 2010), it is difficult to explain the discrepancy between the former and the two latter studies. The reduced wind‐up of lamina I SPB neurons observed in the present study is, however, in agreement with that reported in the rat (Bester et al. 2000 a). Appropriate repeated electrical stimulations induce a progressive increase in pain sensation in humans, and it is postulated that this may reflect the wind‐up of some projection neurons (Arendt‐Nielsen et al. 1996; Guirimand et al. 2000). Thus, the reduced wind‐up of lamina I SPB neurons might be essential for our understanding of the relative role of lamina I SPB neurons versus projection neurons located deeper in the dorsal horn in the encoding of pain intensity. Alternatively, the reduced wind‐up of lamina I SPB neurons observed in the present study might be specific to rodent, and indicates fundamental differences between rodent and human lamina I projection neurons, the latter but not the former being hypothetically subjected to wind‐up.
Conduction velocity
Most (82%) lamina I SPB neurons had central conduction velocities falling between 2.5 and 12 m s−1, corresponding to thinly myelinated axons, and the remaining 18% had central conduction velocities corresponding to unmyelinated axons (<2.5 m s−1). The latter percentage is underestimated as a result of the difficulty in obtaining recordings with acceptable signal to noise ratio and the one‐for‐one following of the train of antidromic stimulations at 200 Hz for the neurons with unmyelinated axons. The central conduction velocities obtained in the present study represent a more narrow distribution, skewed towards slow conduction velocities, compared to that obtained in the rat, which ranged from 0.6 to 30 m s−1 (Bester et al. 2000 a; Andrew, 2009). There was no relationship between the profile of the neuronal response (wide dynamic range versus nociceptive‐specific versus cold preferential) and their conduction velocities, in contrast to what has been shown in the cat ((Light et al. 1993), see also Andrew and Craig, 2001b for conduction velocity of lamina I spinothalamic neurons).
Receptive field
The relative size of the receptive fields of the lamina I SPB neurons observed in the present study was large, often encompassing the entire paw. The data available on receptive field size in the rat are controversial, with one study reporting small receptive fields (one digit or pad) and another study reporting large receptive fields (mean surface area of approximately 1 cm2) (Bester et al. 2000 a; Andrew, 2009). In the present study, the receptive field was mapped using pinch of the skin with forceps, and the site of stimulation was considered part of the receptive field as long as the stimulation elicited one AP. Responses to pinch were variable across the receptive field, and the most sensitive area could cover only one digit, or even the tip of a digit, while the entire receptive field, much less responsive, covered the totality of the glabrous skin. This might explain why large receptive fields have been reported herein. It should be noted that when using identical methodological conditions, all polymodal neurons of unknown projection located in lamina III–V used for the wind‐up comparison displayed a well‐defined, restricted receptive field (1 to 3 digits and a small part of the palm of the paw, data not shown). In rats, the small size of lamina I SPB neuron receptive fields is difficult to understand, since spatial resolution of lamina I projection neurons is lost at the level of their main target, the PB neurons, which have an extremely large receptive field (Bernard et al. 1994; Bester et al. 1995). There are collateral projections of lamina I SPB neurons to the ventral posterolateral nucleus and the posterior nuclear group of the thalamus (Todd, 2010). The latter structures are involved in the discriminative aspect of pain‐related input and may take advantage of the high spatial resolution of lamina I SPB neurons. However, these projections to the thalamus are quantitatively negligible at the level of the lumbosacral enlargement, and one can question why the vast majority of lamina I SPB neurons should have a restricted receptive field.
Disinhibition
Ex vivo electrophysiological, anatomical and behavioural experiments have established the existence of a polysynaptic pathway linking low threshold mechanoreceptors (terminating in lamina III) with lamina I projection neurons. This pathway is gated by a feed‐forward inhibition in healthy control conditions. Peripheral nerve injury, inflammation, or chemical removal of glycynergic and GABAergic inhibitory tone can open this pathway, turning low mechanoreceptive input into pain in conscious subjects. It is noteworthy that in vivo electrophysiological evidence supporting this finding is scarce. Indeed, only one in vivo study in the anaesthetized rat suggested that spinal application of bicuculline increased responses of lamina I SPB nociceptive neurons to light touch (Keller et al. 2007).
In the present experiments in the anaesthetized mouse, spinal cord application of bicuculline and strychnine induced responses, or enhanced pre‐existing responses, of lamina I SPB neurons to brush of the receptive field. There was a trend for neurons with initially low mechanical threshold to show the greatest disinhibition. The magnitude of the effect of disinhibition was highly variable between neurons, and neither the conduction velocity nor the responses to simple mechanical and thermal stimuli of the neuron could predict the magnitude of the effect of bicuculline and strychnine. Analysis of the shape of the AP might reveal intrinsic properties of the neurons that do respond to bicuculline and strychnine application, but fine analysis (such as rising and falling slope) is difficult with extracellular recording, and would have required recording at higher sampling rates than those used in the present experiments. Nevertheless, the present results demonstrate that GABAergic and glycinergic disinhibition allows de novo low threshold mechanoreceptive input to lamina I SPB neurons in vivo in mice.
The neurobiological mechanisms responsible for decreased spinal inhibitory tone in chronic neuropathic or inflammatory conditions are complex, involving notably changes in chloride homeostasis and loss of inhibitory interneurons (Scholz et al. 2005; Price et al. 2009). The application of bicuculline and strychnine on the spinal cord is in comparison an extreme and artefactual condition of disinhibition. This is demonstrated by the marked increase of brush response of lamina I SPB neurons induced by application of bicuculline on the spinal cord (from 0 to 3.3 AP) compared to that induced by the neuropathic condition (0.4–1 AP in control and neuropathic conditions, respectively) (Keller et al. 2007). It is now essential to demonstrate that there is a significant increase in the proportion of lamina I SPB neurons that are activated by low threshold mechanoreceptive input upon experimental inflammatory or neuropathic chronic conditions in the mouse. The convergence of multiple lamina I neurons onto PB neurons means that barely detectable changes in the activity of lamina I neurons might turn into significant changes of the responsiveness of PB neurons, regardless of any process of sensitization at the level of the PB. This might explain why some pathological conditions inducing dramatic changes in behaviour are correlated with much less dramatic changes in the activity of lamina I SPB neurons (Keller et al. 2007; Andrew 2009).
Additional information
Competing interests
None.
Author contributions
Sole author (the author designed the study, collected and analysed the data and drafted the article).
Funding
None.
Translational perspective.
The present data show that in the mouse, lamina I spinoparabrachial (SPB) neurons have the necessary characteristics for a role in pain and thermal perception, as has been shown in the rat and the cat. This was expected, but it had to be established. Future studies will hopefully challenge the present data, allowing for a more accurate description of this fundamental pain‐related pathway in the mouse. Activation by low threshold mechanoreceptors of neurons normally encoding noxious stimuli could be evoked after spinal application of GABAergic and strychnine antagonists, which was also expected. This is the first ‘on line’ demonstration of the potential link between low threshold mechanoreceptive input and central pain‐related output in the mouse in vivo. More importantly, it shows that lamina I SPB neurons can be pharmacologically manipulated and recorded in mice. Simple modifications of the experimental procedure should allow the correction of the metabolic acidosis observed in the present experiments, and possibly improvement of cardiovascular parameters. This in turn means that the study of lamina I SPB neurons could be performed in vivo while taking advantage of genetically modified mice.
Acknowledgements
The author is in debt to Hervé Bester and Wesley Miner for critical scientific discussions and help in writing the manuscript.
Biography
Julien Allard is the CSO of E‐Phys, a contract research organization focused on electrophysiology in anaesthetized mice. He received a MS in biochemistry at the National Institute of Applied Science (Lyon, France), and a PhD in neurosciences at the University René Descartes (Paris, France). He started pain research while at Pfizer (Sandwich, UK). His main interests are directed at understanding the mechanism of action analgesic drug candidates through measuring the activity of spinal lamina I and lamina III–VI projection neurons in experimental models of inflammatory and neuropathic pain in rodents.

Edited by: David Wyllie & Diego Contreras
References
- Al‐Khater KM, Kerr R & Todd AJ (2008). A quantitative study of spinothalamic neurons in laminae I, III, and IV in lumbar and cervical segments of the rat spinal cord. J Comp Neurol 511, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D (2009). Sensitization of lamina I spinoparabrachial neurons parallels heat hyperalgesia in the chronic constriction injury model of neuropathic pain. J Physiol 587, 2005–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D (2010). Quantitative characterization of low‐threshold mechanoreceptor inputs to lamina I spinoparabrachial neurons in the rat. J Physiol 588, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D & Craig AD (2001. a). Spinothalamic lamina I neurones selectively responsive to cutaneous warming in cats. J Physiol 537, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D & Craig AD (2001. b). Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci 4, 72–77. [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen L, Nielsen J, Petersen‐Felix S, Schnider TW & Zbinden AM (1996). Effect of racemic mixture and the (S+)‐isomer of ketamine on temporal and spatial summation of pain. Br J Anaesth 77, 625–631. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF & Besson JM (1994). The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J Neurophysiol 71, 1646–1660. [DOI] [PubMed] [Google Scholar]
- Bester H, Beggs S & Woolf CJ (2000. b). Changes in tactile stimuli‐induced behavior and c‐Fos expression in the superficial dorsal horn and in parabrachial nuclei after sciatic nerve crush. J Comp Neurol 428, 45–61. [DOI] [PubMed] [Google Scholar]
- Bester H, Chapman V, Besson JM & Bernard JF (2000. a). Physiological properties of the lamina I spinoparabrachial neurons in the rat. J Neurophysiol 83, 2239–2259. [DOI] [PubMed] [Google Scholar]
- Bester H, Menendez L, Besson JM & Bernard JF (1995). Spino (trigemino) parabrachiohypothalamic pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 73, 568–585. [DOI] [PubMed] [Google Scholar]
- Blomqvist A & Craig AD (2000). Is neuropathic pain caused by the activation of nociceptive‐specific neurons due to anatomic sprouting in the dorsal horn? J Comp Neurol 428, 1–4. [PubMed] [Google Scholar]
- Cain DM, Khasabov SG & Simone DA (2001). Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol 85, 1561–1574. [DOI] [PubMed] [Google Scholar]
- Cameron D, Polgár E, Gutierrez‐Mecinas M, Gomez‐Lima M, Watanabe M & Todd AJ (2015). The organisation of spinoparabrachial neurons in the mouse. Pain 156, 2061–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Duan B, Huang T, Zhang Y, Chen Y, Britz O, Garcia‐Campmany L, Ren X, Vong L, Lowell BB, Goulding M, Wang Y & Ma Q (2017). Identification of spinal circuits involved in touch‐evoked dynamic mechanical pain. Nat Neurosci 20, 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3, 655–666. [DOI] [PubMed] [Google Scholar]
- Craig ADB (2010). Why a soft touch can hurt. J Physiol 588, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD & Andrew D (2002). Responses of spinothalamic lamina I neurons to repeated brief contact heat stimulation in the cat. J Neurophysiol 87, 1902–1914. [DOI] [PubMed] [Google Scholar]
- Cui L, Miao X, Liang L, Abdus‐Saboor I, Olson W, Fleming MS, Ma M, Tao Y‐X & Luo W (2016). Identification of early RET+ deep dorsal spinal cord interneurons in gating pain. Neuron 91, 1413. [DOI] [PubMed] [Google Scholar]
- Dalkara T, Irikura K, Huang Z, Panahian N & Moskowitz MA (1995). Cerebrovascular responses under controlled and monitored physiological conditions in the anesthetized mouse. J Cereb Blood Flow Metab 15, 631–638. [DOI] [PubMed] [Google Scholar]
- Davidson S, Truong H & Giesler GJ (2010). Quantitative analysis of spinothalamic tract neurons in adult and developing mouse. J Comp Neurol 518, 3193–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia‐Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross S, Lowell BB, Wang Y, Goulding M & Ma Q (2014). Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Cheng L & Ma Q (2018). Spinal circuits transmitting mechanical pain and itch. Neurosci Bull 34, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, Johannssen H, Hösli L, Haenraets K, Ghanem A, Conzelmann KK, Bösl M & Zeilhofer HU (2015). Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 85, 1289–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauriau C & Bernard J‐F (2002). Pain pathways and parabrachial circuits in the rat. Exp Physiol 87, 251–258. [DOI] [PubMed] [Google Scholar]
- Guirimand F, Dupont X, Brasseur L, Chauvin M & Bouhassira D (2000). The effects of ketamine on the temporal summation (wind‐up) of the R(III) nociceptive flexion reflex and pain in humans. Anesth Analg 90, 408–414. [DOI] [PubMed] [Google Scholar]
- Han S, Soleiman MT, Soden ME, Zweifel LS & Palmiter RD (2015). Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman MA, Hemmes SNT, Kuipers MT, Bos LDJ, Jongsma G, Roelofs JJTH, van der Sluijs KF, Juffermans NP, Vroom MB & Schultz MJ (2013). The extent of ventilator‐induced lung injury in mice partly depends on duration of mechanical ventilation. Crit Care Res Pract 2013, 435236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Hayashi H, Bennett GJ & Dubner R (1985). Spinal lamina I neurons projecting to the parabrachial area of the cat midbrain. Brain Res 336, 195–198. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Hayashi H, Dubner R & Bennett GJ (1986). Physiology and morphology of the lamina I spinomesencephalic projection. J Comp Neurol 247, 505–515. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Nahin RL, Traub RJ & Dubner R (1989). Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant‐induced inflammation: the contribution of dorsal horn mechanisms. Pain 37, 229–243. [DOI] [PubMed] [Google Scholar]
- Iversen NK, Malte H, Baatrup E & Wang T (2012). The normal acid‐base status of mice. Respir Physiol Neurobiol 180, 252–257. [DOI] [PubMed] [Google Scholar]
- Keller AF, Beggs S, Salter MW & De Koninck Y (2007). Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain 3, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Woodske ME, Zou B & O'Donnell CP (2009). Dynamic arterial blood gas analysis in conscious, unrestrained C57BL/6J mice during exposure to intermittent hypoxia. J Appl Physiol 107, 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JW, Willis WD & Chung JM (1993). Cutaneous sensory receptors in the rat foot. J Neurophysiol 69, 1684–1699. [DOI] [PubMed] [Google Scholar]
- Light AR, Sedivec MJ, Casale EJ & Jones SL (1993). Physiological and morphological characteristics of spinal neurons projecting to the parabrachial region of the cat. Somatosens Mot Res 10, 309–325. [DOI] [PubMed] [Google Scholar]
- Lipski J (1981). Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods 4, 1–32. [DOI] [PubMed] [Google Scholar]
- Otto GP, Rathkolb B, Oestereicher MA, Lengger CJ, Moerth C, Micklich K, Fuchs H, Gailus‐Durner V, Wolf E & de Angelis MH (2016). Clinical chemistry reference intervals for C57BL/6J, C57BL/6N, and C3HeB/FeJ mice (Mus musculus). J Am Assoc Lab Anim Sci 55, 375–386. [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Williams S‐PG, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS & Seal RP (2015). Dorsal horn circuits for persistent mechanical pain. Neuron 87, 797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan L‐Y, Ribeiro‐da‐Silva A, Braz JM, Basbaum AI & Sharif‐Naeini R (2015). Dorsal horn Parvalbumin neurons are gate‐keepers of touch‐evoked pain after nerve injury. Cell Rep 13, 1246–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár E, Wright LL & Todd AJ (2010). A quantitative study of brainstem projections from lamina I neurons in the cervical and lumbar enlargement of the rat. Brain Res 1308, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Cervero F, Gold MS, Hammond DL & Prescott SA (2009). Chloride regulation in the pain pathway. Brain Res Rev 60, 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn D‐H, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE & Woolf CJ (2005). Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci 25, 7317–7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf TM, Horn T, Lang D & Klein J (2013). Blood gases and energy metabolites in mouse blood before and after cerebral ischemia: the effects of anesthetics. Exp Biol Med (Maywood) 238, 84–89. [DOI] [PubMed] [Google Scholar]
- Serfilippi LM, Pallman DRS & Russell B (2003). Serum clinical chemistry and hematology reference values in outbred stocks of albino mice from three commonly used vendors and two inbred strains of albino mice. Contemp Top Lab Anim Sci 42, 46–52. [PubMed] [Google Scholar]
- Singh AK (2010). Metabolic acidosis. In Decision Making in Medicine, 3rd edn, ed. Mushlin SB, & Greene HL, pp. 372–373. Mosby, Philadelphia. [Google Scholar]
- Spike RC, Puskár Z, Andrew D & Todd AJ (2003). A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci 18, 2433–2448. [DOI] [PubMed] [Google Scholar]
- Thal SC & Plesnila N (2007). Non‐invasive intraoperative monitoring of blood pressure and arterial pCO2 during surgical anesthesia in mice. J Neurosci Methods 159, 261–267. [DOI] [PubMed] [Google Scholar]
- Todd AJ (2010). Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11, 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier CJ, Emons VM & Ince C (2002). Hemodynamics of anesthetized ventilated mouse models: aspects of anesthetics, fluid support, and strain. Am J Physiol Heart Circ Physiol 282, H2099–H2105. [DOI] [PubMed] [Google Scholar]


